Validation Sample Set Generator
|
|
|
- Deborah Holmes
- 5 years ago
- Views:
Transcription
1 Validation Sample Set Generator General Information This tool is an example program which demonstrates some capabilities of the Empower Toolkit. It was not created using Waters Software Development Lifecycle. This tool was tested in a limited fashion and is not structurally validated by Waters Corporation. This tool is open source and Waters freely distributes the source code for use and modification by customers that have purchased the Empower Toolkit Option. This source code was created using Visual Basic version 6.0. For more information on use of the Toolkit, see the documentation provided with the Empower 2 Toolkit option. The Validation Sample Set Generator (VSG) tool is intended to be used in conjunction with the Empower 2 Method Validation Manager option to create the necessary instrument methods, method sets, and sample set methods to satisfy the robustness experimental plan specified for a particular robustness test in an MVM validation protocol method. The definition of robustness of an analytical procedure is the measure of its capacity to remain unaffected by small, but deliberate, variations in method parameters and provides an indication of its reliability during normal usage. Hence, in a robustness experiment the user deliberately varies specified parameters, referred to as factors. The variation is performed at a high value, a low value and a centerpoint, or nominal value (the normal value specified by the method you are validating). These factors can include sample and reagent preparation variations such as sonication time, shaking time, extraction time, sample ph, and so on. These factors can also include instrument method variations such as column temperature, sample compartment temperature, flow rate, mobile phase composition, detector wavelength, and so on. The Empower 2 Method Validation Manager (MVM) software provides a robustness experimental plan, based on user input of what factors are to be varied and what type of plan to be used. This experimental plan dictates the combination of factor variations (experiments) necessary for a set of injections (i.e. a sample set) on an injection-byinjection basis; an example experimental plan is shown below. The factor values are represented either by -1 (the low value of the factor) and +1 (the high value of the factor), or by specifying the factor and values explicitly (both representations are available in MVM). The VSG tool requires user inputs to specify which factors map to sample, preparation, or instrument variations. The VSG tool also requires a sample set method which specifies the method set containing the nominal instrument method. The tool then translates the experimental plan into the necessary instrument methods, method sets and sample set(s). Page 1 of 10
2 ½ Fraction (2 4-1 ) Experimental Plan example (without centerpoints): Experiment Factor 1 Factor 2 Factor 3 Factor This tool was not developed using Waters software development lifecycle and has not been structurally validated. It is the user s responsibility to validate this tool, if validation is necessary. Hardware and Software Requirements The Validation Sample Set Generator tool supports the same computer hardware/software configuration requirements as the base Empower 2 application. The Validation Sample Set Generator tool supports the following chromatographic systems: Alliance (at firmware supported by Empower 2) ACQUITY Binary Solvent Manager, Sample Manager, Column Heater, Tunable UV + Photodiode Array (at firmware supported by Empower 2) A1100 Isocratic Pump, Quaternary Pump, Binary Pump, Auto Injector, Auto Injector With Heater, VW Detector, RI Detector, Column heater, MW Detector, DA Detector (at firmware supported by Empower 2) Installation of the Validation Sample Set Generator Installing the Validation Sample Set Generator requires the use of a privileged account with permission to write to the system resources. Please ensure that the account used to perform the installation has sufficient privileges. Note that a Domain Administrator account may not have the same privileges or permissions as the Local Administrator account. Page 2 of 10
3 1. Navigate to and double-click on the "Setup.exe" file. 2. Follow all prompts. If you receive the following message during installation of this tool, select Yes. Use of the Validation Sample Set Generator Use the Start menu to navigate to the Validation Sample Set Generator. (For example, Start > All Programs > Empower > Validation Sample Set Generator.) Login using the following login dialog: Page 3 of 10
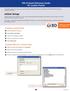
4 The following describes the different fields used by the Validation Sample Set Generator to determine how to create the required sample sets. Refer to Figure 1: Validation Sample Set Generator User Interface. Creation Parameters Validation Method Robustness Test No. of Preparations Use a unique vial for each line Use this dropdown list to specify the MVM validation protocol method which contains the desired robustness test. Use this dropdown list to specify the MVM robustness test for which you want the Validation Sample Set Generator to automatically create instrument methods and sample set methods. Use this selection to specify how many sample preparations will be performed for each sample (experiment). When you specify a number of Preparations greater than 1, it is recommended to also select Use a unique vial for each line so that each sample (experiment) in the resulting sample set method(s) will be duplicated the specified number of times. Note: This setting does not affect the Sample Preparation field in the resulting sample set method. The user will need to manually specify the Sample Preparation value for each line in the sample set method. By selecting this you turn off intelligent vial allocation. Every new sample set line will use its own unique vial (and so be assigned a unique vial location depending upon your current Page 4 of 10
5 No. of Injections Include Standards No. of Centerpoints Template Sample Set Method instrument plate). This selection specifies the number of injections for each sample (experiment) in the resulting sample set method(s). This selection specifies that the Validation Sample Set Generator should place standard injections in the resulting sample set method(s). These standards will be created using perturbations of the factors defined as Instrument Factor Types but will hold all other Factor Types at the Nominal value. (If you need to run standards using perturbations of all factor types, you will need to manually enter standards in the resulting sample set method. This can be easily accomplished by highlighting an appropriate sample row and selecting Edit > Insert Row.) Note: The Robustness test checkbox field will be enabled for any standard lines in the resulting sample set method. This check box will need to be manually disabled. This selection specifies that the Validation Sample Set Generator should place the specified number of Centerpoint injections in the resulting sample set method(s). The centerpoint injections are run at the nominal factor conditions and are placed in appropriate locations in the resulting sample set method(s). The position is determined using the same criteria as standard lines, this is based upon the instrument method factors equilibration time. Note: The Sample Preparation field for the centerpoint injections in the resulting sample set method(s) will be specified as Reference. This field will need to be set appropriately (to Preparation 1, Preparation 2, and so on.) The first line of the template SampleSet Method is used as the template for all lines to be created. No other lines are examined/required within the template sample set method. All of the parameters in the template sample set line are copied exactly unless they are altered by a validation factor or they represent a key field e.g Sample Name. The first line in the template sample set method must contain a valid method set which specifies an appropriate instrument method. This instrument method should contain the nominal instrument parameters (the nominal parameter settings per the method that you are validating) and will be used as the starting point by the Validation Sample Set Generator tool. When instrument factors require changes, in order to affect these changes new instrument methods must be created. For each instrument factor change the tool will create a new Page 5 of 10
6 Reset instrument method and a new Method Set containing this instrument method. The Method set will then be assigned to the appropriate Sample Set Line. Instrument methods and method sets are reused where possible. Selecting this button clears the following fields so that you can initiate the Validation Sample Set Generator process with a different robustness test: Validation Method Robustness Test Template Sample Set Method Vial Information Maximum Extraction Volume from each Vial (ul) Sample Sets have unique vials Sample Sets share vials Specify the maximum volume that can be injected from each vial so that the Validation Sample set Generator knows when to specify a new vial for the same sample. (Other factors such as vial dead volume, needle overfill volume, and syringe volume also need to be considered and subtracted from the total available volume.) This selection specifies that if multiple sample set methods are created, the sample vials in one sample set method are not reused in the other sample set method(s). This selection specifies that if multiple sample set methods are created, the sample vials in one sample set method are to be reused in the sample set method(s). Method Names to Use Sample Sets Method Sets Instrument Methods Add method type prefixes (SS, MS, IM) Specify the root name of the resulting sample set method(s) using alphanumeric characters. Do not use special characters such as /, %, +, #, and so on. Specify the root name of the resulting method set(s) using alphanumeric characters. Do not use special characters such as /, %, +, #, and so on. Specify the root name of the resulting instrument method(s) using alphanumeric characters. Do not use special characters such as /, %, +, #, and so on. Specify that the resulting methods should contain an identifying prefix: SS = sample set method MS = method set IM = instrument method Note: The resulting method will also contain an incrementing numeric suffix. Keep in mind that the maximum number of Page 6 of 10
7 characters that can be used in a method name is limited to 30 characters in Empower 2. Factor Definitions Factor Name Factor Type The Factor Name (custom field) specified in the MVM robustness acquisition parameters. Use this selection when the factor is a sample set Sample Set parameter (for example, Analyst). The factor values in the corresponding field in the sample set method will be set according to the robustness experimental plan in Method Validation Manager. Instrument Use this selection when the factor is an instrument method parameter, for example Flow Rate. The Validation Sample Set generator will modify the appropriate instrument methods according to the robustness experimental plan in Method Validation Manager and specify them in the appropriate sample lines in the sample set method(s). The factor values in the corresponding field in the sample set method will be set according to the robustness experimental plan in Method Validation Manager. Ignore Preparation Custom The following fields will be enabled and must be set appropriately: Instrument Type Instrument Method Parameter Relative Equilibration Time Use this selection when a factor in the MVM robustness parameters should be ignored. Use this selection when the factor is a sample preparation parameter, for example Sonication Time of sample. The Validation Sample Set generator will change to a new sample vial in the resulting sample set for each factor perturbation. The User Set Up Information documents a mapping of which vial numbers are set to which factor perturbations in the resulting sample set method(s). The factor values in the corresponding field in the sample set method will be set according to the robustness experimental plan in Method Validation Manager. Use this selection when you will manually set up this factor in the resulting instrument methods and sample set methods. After the sample sets have been created an information box displays which sample set lines (and if relevant, Page 7 of 10
8 instrument methods) are associated with each factor setting. The display allows the user to manually assign the correct values to sample set lines and instrument methods. For example, this tool does not have the capability to set changing gradient percentages or slopes. These settings can be manually changed by the user once the Validation Sample Set Generator creates the sample set method(s). Minimum Value Nominal Value Maximum Value Requires Own Sample Set Generate Instrument Method Instrument Type Instrument Method Parameter Relative Equilibration Time The relevant factors values will be specified in each sample set line. The Lower Value (-1) specified in the MVM robustness acquisition parameters. The Nominal Value (0) specified in the MVM robustness acquisition parameters. The Upper Value (+1) specified in the MVM robustness acquisition parameters. For use with Sample Set, Instrument, Preparation and Custom Factor Types. Specifies that when the factor is varied, a new sample set method must be created. (For example, a factor of Analyst would require each perturbation of Analyst to be placed in a separate sample set method.) For use with a Custom Factor Type only. Specifies that a separate instrument method should be created for each perturbation of this factor. It is up to the user, however, to set the corresponding parameter in the resulting instrument method. For use with an Instrument Factor Type only. Select the instrument to which the factor applies. For use with an Instrument Factor Type only. Select the instrument parameter to which the factor applies. For use with an Instrument Factor Type only. Select from: N/A Short Medium Long For any factors which will require equilibration in between injections, this setting allows you to specify the equilibration time relative to the other factors which also require an equilibration time. This setting affects the order of instrument methods in the resulting sample set method(s). For example, if you are using 2 factors which require an equilibration time such as column temperature and flow rate, the temperature change requires a longer equilibration time than the flow rate change. Hence, the oven factor should be set as Long and the flow rate should be set as Short. The Validation Sample Set Generator tool will attempt to order the sample set method lines so that they are ordered from the lowest to the highest Page 8 of 10
9 oven temperature. Secondarily, the tool will attempt to order the sample set method lines from the lowest to the highest flow. Once the sample set method(s) is created, the user must add the appropriate Equilibration lines. (Select a row and choose Edit > Insert Row. Set the Function field to Equilibrate and the Run Time equal to the time required for the equilibration. Note: This functionality works best when the factor values are numeric, rather than alpha-numeric strings. Figure 1: Validation Sample Set Generator User Interface After specifying the required information, click the Create SampleSets button. When the sample set methods are complete, a SampleSets Created Successfully message is displayed. Upon successful completion, the User Setup Information window will be displayed. This window provides a mapping of the Preparation type factors to the vials listed in the sample set method(s) as well as a mapping of the custom field value to each sample set line. In the example User Setup Information below, Sonication Time has been set as a Preparation factor type so a mapping of the appropriate factor value to use for each vial is Page 9 of 10
10 provided. Additionally, StrokeVol was set as a custom factor type, so a mapping of the custom field value to each sample set line is displayed with the name of the relevant line and the name of the method set that contains the instrument parameters for this line. Example User Setup Information: **SS_Alpha_1** SS_Alpha_1 : VIAL INFORMATION Vial Id : 1 Sonication time: 15 Vial Id : 2 Sonication time: 10 Vial Id : 3 Sonication time: 20 SS_Alpha_1 : CUSTOM FIELD INFORMATION Line Name = Sample0 - StrokeVol : 50, Method Set Name : MS_Alpha_2 Line Name = Sample1 - StrokeVol : 130, Method Set Name : MS_Alpha_2 Line Name = Sample2 - StrokeVol : 50, Method Set Name : MS_Alpha_2 Line Name = Sample3 - StrokeVol : 130, Method Set Name : MS_Alpha_2 Line Name = Sample4 - StrokeVol : 50, Method Set Name : MS_Alpha_2 Line Name = Sample5 - StrokeVol : 130, Method Set Name : MS_Alpha_2 Line Name = Sample6 - StrokeVol : 50, Method Set Name : MS_Alpha_2 Line Name = Sample7 - StrokeVol : 130, Method Set Name : MS_Alpha_2 Line Name = Centerpoint - StrokeVol : 100, Method Set Name : MS_Alpha_3 Line Name = Sample8 - StrokeVol : 50, Method Set Name : MS_Alpha_1 Line Name = Sample9 - StrokeVol : 130, Method Set Name : MS_Alpha_1 Line Name = Sample10 - StrokeVol : 50, Method Set Name : MS_Alpha_1 Line Name = Sample11 - StrokeVol : 130, Method Set Name : MS_Alpha_1 Line Name = Sample12 - StrokeVol : 50, Method Set Name : MS_Alpha_1 Line Name = Sample13 - StrokeVol : 130, Method Set Name : MS_Alpha_1 Line Name = Sample14 - StrokeVol : 50, Method Set Name : MS_Alpha_1 Line Name = Sample15 - StrokeVol : 130, Method Set Name : MS_Alpha_1 If further modifications of the sample set method(s) are required, you can do so in the Sample Set Editor. To add lines, select an appropriate row and select Edit > insert row. To re-order lines, use drag and drop functionality. Page 10 of 10
For additional information, see these documents on the Breeze 2 software DVD:
 R E L E A S E N O T E S Waters Breeze 2 Read these release notes before you install and operate Breeze 2. They contain information necessary to ensure successful liquid chromatography results, including:
R E L E A S E N O T E S Waters Breeze 2 Read these release notes before you install and operate Breeze 2. They contain information necessary to ensure successful liquid chromatography results, including:
Chromatography Software Training Materials. Contents
 Chromatography Software Training Materials This document contains information on how to build a method, start the instrument to acquire data, and then process the data using the Galaxie Program. You will
Chromatography Software Training Materials This document contains information on how to build a method, start the instrument to acquire data, and then process the data using the Galaxie Program. You will
Agilent Instrument Control Framework (ICF) Update A Driver Update 1 (DU1) Hot Fix 2 (HF2)
 R E L E A S E N O T E S Agilent Instrument Control Framework (ICF) Update A.02.03 Driver Update 1 (DU1) Hot Fix 2 (HF2) These release notes explain the installation of the updated Agilent Instrument Control
R E L E A S E N O T E S Agilent Instrument Control Framework (ICF) Update A.02.03 Driver Update 1 (DU1) Hot Fix 2 (HF2) These release notes explain the installation of the updated Agilent Instrument Control
Manual HPLC-4 Alliance-UV
 Manual HPLC-4 Alliance-UV MAKE SURE NEVER TO: 1. pump particles or air through the column. 2. inject when the injector is dry. 3. inject solutions containing particles. 4. apply large (sudden) pressure
Manual HPLC-4 Alliance-UV MAKE SURE NEVER TO: 1. pump particles or air through the column. 2. inject when the injector is dry. 3. inject solutions containing particles. 4. apply large (sudden) pressure
HITACHI LACHROM ELITE
 HITACHI LACHROM ELITE Clarity Control Module ENG Code/Rev.: M133/60D Date: 7/16/2018 Phone: +420 251 013 400 DataApex Ltd. Fax: +420 251 013 401 Petrzilkova 2583/13 clarity@dataapex.com 158 00 Prague 5
HITACHI LACHROM ELITE Clarity Control Module ENG Code/Rev.: M133/60D Date: 7/16/2018 Phone: +420 251 013 400 DataApex Ltd. Fax: +420 251 013 401 Petrzilkova 2583/13 clarity@dataapex.com 158 00 Prague 5
Streamline the Chromatographic Method Validation Process using Empower 2 Method Validation Manager
 Streamline the Chromatographic Method Validation Process using Empower 2 Method Validation Manager Table of Contents Introduction... 3 Optimize your chromatographic method validation workflow... 4 Efficiently
Streamline the Chromatographic Method Validation Process using Empower 2 Method Validation Manager Table of Contents Introduction... 3 Optimize your chromatographic method validation workflow... 4 Efficiently
Release Note for Agilent LC and CE Drivers Revision A.02.16
 Release Note for Agilent LC and CE Drivers Revision A.02.16 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.16. The Agilent LC and CE Driver
Release Note for Agilent LC and CE Drivers Revision A.02.16 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.16. The Agilent LC and CE Driver
What s New in Empower 3
 What s New in Empower 3 Revision A Copyright Waters Corporation 2010 All rights reserved Copyright notice 2010 WATERS CORPORATION. PRINTED IN THE UNITED STATES OF AMERICA AND IN IRELAND. ALL RIGHTS RESERVED.
What s New in Empower 3 Revision A Copyright Waters Corporation 2010 All rights reserved Copyright notice 2010 WATERS CORPORATION. PRINTED IN THE UNITED STATES OF AMERICA AND IN IRELAND. ALL RIGHTS RESERVED.
Release Note for Agilent LC and CE drivers Revision A.02.14
 Release Note for Agilent LC and CE drivers Revision A.02.14 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.14. The 1260 Infinity II LC is
Release Note for Agilent LC and CE drivers Revision A.02.14 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.14. The 1260 Infinity II LC is
3 Starting the chiral HPLC and logging into LC Real Time Analysis.
 Edition: 1.2 Date: 7-Oct-11 Page 1 of 5 1 Responsibilities. First person responsible: Mariëlle Delville. Second person responsible: Helene I.V. Amatdjais-Groenen. 2 Definitions. Read the information on
Edition: 1.2 Date: 7-Oct-11 Page 1 of 5 1 Responsibilities. First person responsible: Mariëlle Delville. Second person responsible: Helene I.V. Amatdjais-Groenen. 2 Definitions. Read the information on
ACQUITY UPLC Systems with 2D Technology Capabilities Guide
 ACQUITY UPLC Systems with 2D Technology Capabilities Guide Revision A Copyright Waters Corporation 2012 All rights reserved Copyright notice 2012 WATERS CORPORATION. PRINTED IN THE UNITED STATES OF AMERICA
ACQUITY UPLC Systems with 2D Technology Capabilities Guide Revision A Copyright Waters Corporation 2012 All rights reserved Copyright notice 2012 WATERS CORPORATION. PRINTED IN THE UNITED STATES OF AMERICA
MassLynx 4.2 SCN 989 Release Notes
 MassLynx 4.2 SCN 989 Release Notes Page 1 of 20 Waters, THE SCIENCE OF WHAT S POSSIBLE., ACQUITY, Alliance, MassLynx, nanoacquity, and Xevo are registered trademarks and OpenLynx, Quanpedia, and TargetLynx
MassLynx 4.2 SCN 989 Release Notes Page 1 of 20 Waters, THE SCIENCE OF WHAT S POSSIBLE., ACQUITY, Alliance, MassLynx, nanoacquity, and Xevo are registered trademarks and OpenLynx, Quanpedia, and TargetLynx
Steps for LCMS-8040 Triple Quadrupole
 Steps for LCMS-8040 Triple Quadrupole June 2017 Version 2 A. Data Acquisition 1. Check lights on MS. There should be a total of three lights lit by LED. One of them is blinking. Power Blinking Gas Status
Steps for LCMS-8040 Triple Quadrupole June 2017 Version 2 A. Data Acquisition 1. Check lights on MS. There should be a total of three lights lit by LED. One of them is blinking. Power Blinking Gas Status
Waters Driver Pack 2016 Release 1
 Waters Driver Pack 2016 Release 1 Installation and Configuration Guide 715005197 Revision A Copyright Waters Corporation 2016 All rights reserved General information Copyright notice 2016 WATERS CORPORATION.
Waters Driver Pack 2016 Release 1 Installation and Configuration Guide 715005197 Revision A Copyright Waters Corporation 2016 All rights reserved General information Copyright notice 2016 WATERS CORPORATION.
Sciex QTrap Operational Steps for Trained Personnel
 Sciex 6500+ QTrap Operational Steps for Trained Personnel Last Updated 09172017 1. If any of the following instructions does not make sense to you or was not covered during your hand-on training, stop
Sciex 6500+ QTrap Operational Steps for Trained Personnel Last Updated 09172017 1. If any of the following instructions does not make sense to you or was not covered during your hand-on training, stop
Release Notes for Agilent Drivers for Thermo Scientific Chromeleon 7 (Rev. 1.1)
 Release Notes for Agilent Drivers for Thermo Scientific Chromeleon 7 (Rev. 1.1) Release Notes for Agilent Drivers for Thermo Scientific Chromeleon 7 (Rev. 1.1) This document provides information on the
Release Notes for Agilent Drivers for Thermo Scientific Chromeleon 7 (Rev. 1.1) Release Notes for Agilent Drivers for Thermo Scientific Chromeleon 7 (Rev. 1.1) This document provides information on the
Operating Manual. High Performance Liquid Chromatograph. Scientific Equipment Center, Prince Of Songkla University
 Operating Manual High Performance Liquid Chromatograph Agilent 1100 ; VWD, DAD, FLD and RID Scientific Equipment Center, Prince Of Songkla University Operating Manual High Performance Liquid Chromatograph
Operating Manual High Performance Liquid Chromatograph Agilent 1100 ; VWD, DAD, FLD and RID Scientific Equipment Center, Prince Of Songkla University Operating Manual High Performance Liquid Chromatograph
Impact Analysis for Software changes in OpenLAB CDS A.01.04
 Impact Analysis for Software changes in OpenLAB CDS A.01.04 Document Information: Filename Product Identifier Product Revision Project Identifier Document Revision Impact_Analysis_OpenLAB_CDS_A0104 OpenLAB
Impact Analysis for Software changes in OpenLAB CDS A.01.04 Document Information: Filename Product Identifier Product Revision Project Identifier Document Revision Impact_Analysis_OpenLAB_CDS_A0104 OpenLAB
The instrument drivers and firmware in Driver Pack 4 support these ACQUITY UPLC systems: ACQUITY UPLC Systems with 2D Technology
 R E L E A S E N O T E S Waters Driver Pack 4 Driver Pack 4 includes instrument drivers and firmware required to control Waters instruments, as well as helpful utility applications. The driver pack is compatible
R E L E A S E N O T E S Waters Driver Pack 4 Driver Pack 4 includes instrument drivers and firmware required to control Waters instruments, as well as helpful utility applications. The driver pack is compatible
A. About this Manual
 Section A. About this Manual A. About this Manual 1. Table of Contents 1. Table of Contents... 1 2. How to Use this User Manual... 2 3. General Introduction to the PAL3 System... 3 4. PAL3 System Specifications...
Section A. About this Manual A. About this Manual 1. Table of Contents 1. Table of Contents... 1 2. How to Use this User Manual... 2 3. General Introduction to the PAL3 System... 3 4. PAL3 System Specifications...
UniPoint System Software User s Guide
 UniPoint System Software User s Guide LT2137 1998 Gilson, Inc. All rights reserved Exercise 3-Creating operations list... 4-22 Create and name operations list... 4-22 Set up steps... 4-23 Identify sample
UniPoint System Software User s Guide LT2137 1998 Gilson, Inc. All rights reserved Exercise 3-Creating operations list... 4-22 Create and name operations list... 4-22 Set up steps... 4-23 Identify sample
Agilent Instrument Control Framework A for Agilent LC/CE and GC Instruments in Non-Agilent Chromatography Data Systems Release Notes
 Contents Agilent Instrument Control Framework A.02.01 for Agilent LC/CE and GC Instruments in Non-Agilent Chromatography Data Systems Release Notes Instrument Control Framework (ICF) Revision A.02.01 [085]
Contents Agilent Instrument Control Framework A.02.01 for Agilent LC/CE and GC Instruments in Non-Agilent Chromatography Data Systems Release Notes Instrument Control Framework (ICF) Revision A.02.01 [085]
Agilent MassHunter Workstation Software 7200 Accurate-Mass Quadrupole Time of Flight GC/MS
 Agilent MassHunter Workstation Software 7200 Accurate-Mass Quadrupole Time of Flight GC/MS Familiarization Guide Before you begin 3 Prepare your system 3 Prepare the samples required for data acquisition
Agilent MassHunter Workstation Software 7200 Accurate-Mass Quadrupole Time of Flight GC/MS Familiarization Guide Before you begin 3 Prepare your system 3 Prepare the samples required for data acquisition
ANALYTICAL SYSTEMS. L3320 Autosampler with excellent accuracy and linearity. System configuration examples
 ANALYTICAL SYSTEMS Modular HPLC system can be configured according to customer needs from components as follows: PDA Detector with wavelength range 200-800 nm with scanning possibility or UV-VIS Detector
ANALYTICAL SYSTEMS Modular HPLC system can be configured according to customer needs from components as follows: PDA Detector with wavelength range 200-800 nm with scanning possibility or UV-VIS Detector
ChromQuest 5.0 Quick Reference Guide
 ChromQuest 5.0 Quick Reference Guide This guide contains an overview of the ChromQuest chromatography data system, with topics organized by workflow. For more information, refer to the ChromQuest User
ChromQuest 5.0 Quick Reference Guide This guide contains an overview of the ChromQuest chromatography data system, with topics organized by workflow. For more information, refer to the ChromQuest User
Alliance e2695 Separations Module Firmware Version 3.04
 *716005261* *Rev.A* Alliance e2695 Separations Module Firmware Version 3.04 Release Notes 716005261 Revision A Copyright Waters Corporation 2018 All rights reserved General information Copyright notice
*716005261* *Rev.A* Alliance e2695 Separations Module Firmware Version 3.04 Release Notes 716005261 Revision A Copyright Waters Corporation 2018 All rights reserved General information Copyright notice
Agilent ICF Support v3.0
 *716004817* *Rev.A* Agilent ICF Support v3.0 Release Notes 716004817 Revision A Copyright Waters Corporation 2018 All rights reserved General information Copyright notice 2018 WATERS CORPORATION. PRINTED
*716004817* *Rev.A* Agilent ICF Support v3.0 Release Notes 716004817 Revision A Copyright Waters Corporation 2018 All rights reserved General information Copyright notice 2018 WATERS CORPORATION. PRINTED
Enhancing your Lab Workflow with NuGenesis 8 ELN and Empower Waters Corporation 1
 Enhancing your Lab Workflow with NuGenesis 8 ELN and Empower 2013 Waters Corporation 1 Agenda Browse Empower Data Using ELN Documents to Submit Samples and retrieve Results 2013 Waters Corporation 2 Agenda
Enhancing your Lab Workflow with NuGenesis 8 ELN and Empower 2013 Waters Corporation 1 Agenda Browse Empower Data Using ELN Documents to Submit Samples and retrieve Results 2013 Waters Corporation 2 Agenda
PurityChrom Bio 5 Software Manual V2650
 PurityChrom Bio 5 Software Manual V2650 Introduction 3 Table of Contents Note: For your own safety, read the manual and always observe the warnings and safety information on the device and in the manual!
PurityChrom Bio 5 Software Manual V2650 Introduction 3 Table of Contents Note: For your own safety, read the manual and always observe the warnings and safety information on the device and in the manual!
Agilent G2727AA LC/MS Data Browser Quick Start
 What is Data Browser? Agilent G2727AA LC/MS Data Browser Quick Start A guide to get started with Data Browser Use this quick reference guide as a road map for your first steps with the Data Browser software,
What is Data Browser? Agilent G2727AA LC/MS Data Browser Quick Start A guide to get started with Data Browser Use this quick reference guide as a road map for your first steps with the Data Browser software,
Waters 2707 Autosampler ICS v1.20 Installer
 R E L E A S E N O T E S Waters 2707 Autosampler ICS v1.20 Installer Read these release notes before you install and operate the Waters 2707 autosampler instrument control software (ICS). They contain information
R E L E A S E N O T E S Waters 2707 Autosampler ICS v1.20 Installer Read these release notes before you install and operate the Waters 2707 autosampler instrument control software (ICS). They contain information
Empower Data Exporter. The Empower Data Exporter is for use with Empower 2154 and requires the Microsoft.NET Framework version 2.0.
 Empower Data Exporter This tool is an example program which demonstrates some capabilities of the Empower Toolkit. It was not created using Waters Software Development Lifecycle. This tool was tested in
Empower Data Exporter This tool is an example program which demonstrates some capabilities of the Empower Toolkit. It was not created using Waters Software Development Lifecycle. This tool was tested in
Galaxie Photodiode Array Software
 Varian, Inc. 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Galaxie Photodiode Array Software User s Guide Varian, Inc. 2002-2006 03-914950-00:6 Table of Contents Introduction... 3 General... 3 PDA
Varian, Inc. 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Galaxie Photodiode Array Software User s Guide Varian, Inc. 2002-2006 03-914950-00:6 Table of Contents Introduction... 3 General... 3 PDA
Release Note for Agilent LC and CE Drivers Revision A.02.17
 Release Note for Agilent LC and CE Drivers Revision A.02.17 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.17. The Agilent LC and CE Driver
Release Note for Agilent LC and CE Drivers Revision A.02.17 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.17. The Agilent LC and CE Driver
C h r o m S t a r 7. M a n u a l
 C h r o m S t a r 7 M a n u a l ÿþýü Software für Chromatographie und Prozess-Analytik GmbH Am Weidufer 32, D-28844 Weyhe Tel.: 0421-802806, Fax: 0421-890346 E-mail: info@scpa.de, Internet: www.scpa.de
C h r o m S t a r 7 M a n u a l ÿþýü Software für Chromatographie und Prozess-Analytik GmbH Am Weidufer 32, D-28844 Weyhe Tel.: 0421-802806, Fax: 0421-890346 E-mail: info@scpa.de, Internet: www.scpa.de
ACQUITY UPLC Systems June 2011 Driver Pack and Firmware Updates
 R E L E A S E N O T E S ACQUITY UPLC Systems June 2011 Driver Pack and Firmware Updates What s new in this release... 3 Reasons for installing this software release... 3 Installation Notes... 3 Recommended
R E L E A S E N O T E S ACQUITY UPLC Systems June 2011 Driver Pack and Firmware Updates What s new in this release... 3 Reasons for installing this software release... 3 Installation Notes... 3 Recommended
Using the Chromeleon Summit 2 Tandem Operation Wizard
 Technical Note 702 Using the Chromeleon Summit 2 Tandem Operation Wizard 1 INTRODUCTION New Summit 2 Tandem Operation support of the Program Wizard is available in Chromeleon version 6.70. The purpose
Technical Note 702 Using the Chromeleon Summit 2 Tandem Operation Wizard 1 INTRODUCTION New Summit 2 Tandem Operation support of the Program Wizard is available in Chromeleon version 6.70. The purpose
MassLynx 4.1 SCN 804. Release Notes. SQ Detector 2
 R E L E A S E N O T E S MassLynx 4.1 SCN 804 Release Notes SQ Detector 2 43464 Rev 01 Copyright Waters Corporation 2011. All rights reserved. Copyright 2011 Waters Corporation 43464_1. Waters is a registered
R E L E A S E N O T E S MassLynx 4.1 SCN 804 Release Notes SQ Detector 2 43464 Rev 01 Copyright Waters Corporation 2011. All rights reserved. Copyright 2011 Waters Corporation 43464_1. Waters is a registered
Waters Driver Pack 2018 Release 1
 Waters Driver Pack 2018 Release 1 Installation and Configuration Guide 715005659 Revision B Copyright Waters Corporation 2018 All rights reserved General information Copyright notice 2018 WATERS CORPORATION.
Waters Driver Pack 2018 Release 1 Installation and Configuration Guide 715005659 Revision B Copyright Waters Corporation 2018 All rights reserved General information Copyright notice 2018 WATERS CORPORATION.
EASY-nLC Series User Guide for the Xcalibur Data System (Xcalibur version 2.1 or later)
 Thermo EASY-nLC Series User Guide for the Xcalibur Data System (Xcalibur version 2.1 or later) P/N 60053-97230 Revision B January 2013 2013 Thermo Fisher Scientific Inc. All rights reserved. EASY-nLC,
Thermo EASY-nLC Series User Guide for the Xcalibur Data System (Xcalibur version 2.1 or later) P/N 60053-97230 Revision B January 2013 2013 Thermo Fisher Scientific Inc. All rights reserved. EASY-nLC,
Agilent OpenLAB Chromatography Data System
 Agilent OpenLAB Chromatography Data System EZChrom and ChemStation Editions Supported Instruments and Firmware Guide Notices Agilent Technologies, Inc. 2012 No part of this manual may be reproduced in
Agilent OpenLAB Chromatography Data System EZChrom and ChemStation Editions Supported Instruments and Firmware Guide Notices Agilent Technologies, Inc. 2012 No part of this manual may be reproduced in
Thermo. EASY-nLC User Guide for LC Devices
 Thermo EASY-nLC 1200 User Guide for LC Devices 60053-97273 Revision A September 2015 2015 Thermo Fisher Scientific Inc. All rights reserved. AFC, EASY-nLC, EASY-Column, EASY-Spray, Foundation, LC TriPlus,
Thermo EASY-nLC 1200 User Guide for LC Devices 60053-97273 Revision A September 2015 2015 Thermo Fisher Scientific Inc. All rights reserved. AFC, EASY-nLC, EASY-Column, EASY-Spray, Foundation, LC TriPlus,
Fusion AE LC Method Validation Module. S-Matrix Corporation 1594 Myrtle Avenue Eureka, CA USA Phone: URL:
 Fusion AE LC Method Validation Module S-Matrix Corporation 1594 Myrtle Avenue Eureka, CA 95501 USA Phone: 707-441-0404 URL: www.smatrix.com Regulatory Statements and Expectations ICH Q2A The objective
Fusion AE LC Method Validation Module S-Matrix Corporation 1594 Myrtle Avenue Eureka, CA 95501 USA Phone: 707-441-0404 URL: www.smatrix.com Regulatory Statements and Expectations ICH Q2A The objective
Data Acquisition with CP-2002/2003 Micro-GC Control
 Varian Analytical Instruments 2700 Mitchell Drive Walnut Creek, CA 94598 Star Chromatography Workstation Version 6 Data Acquisition with CP-2002/2003 Micro-GC Control Operation Manual Varian, Inc. 2002
Varian Analytical Instruments 2700 Mitchell Drive Walnut Creek, CA 94598 Star Chromatography Workstation Version 6 Data Acquisition with CP-2002/2003 Micro-GC Control Operation Manual Varian, Inc. 2002
Agilent 7890 GC Instrument Control Software Version 2.01
 R E L E A S E N O T E S Agilent 7890 GC Instrument Control Software Version 2.01 These release notes explain how to install Waters Instrument Control Software (ICS), version 2.01, for the Agilent 7890
R E L E A S E N O T E S Agilent 7890 GC Instrument Control Software Version 2.01 These release notes explain how to install Waters Instrument Control Software (ICS), version 2.01, for the Agilent 7890
StarFinder. Operation Manual. Star Chromatography Workstation Version 6
 Varian Analytical Instruments 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Star Chromatography Workstation Version 6 StarFinder Operation Manual Varian, Inc. 2002 03-914723-00:Rev. 2 Trademark Acknowledgment
Varian Analytical Instruments 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Star Chromatography Workstation Version 6 StarFinder Operation Manual Varian, Inc. 2002 03-914723-00:Rev. 2 Trademark Acknowledgment
Varian Modular HPLC System Galaxie Driver Installation and Configuration Instructions
 Varian Modular HPLC System Galaxie Driver Installation and Configuration Instructions These instructions, and the Varian Modular HPLC System Galaxie Driver, are for use with a Varian Modular HPLC System
Varian Modular HPLC System Galaxie Driver Installation and Configuration Instructions These instructions, and the Varian Modular HPLC System Galaxie Driver, are for use with a Varian Modular HPLC System
Agilent OpenLAB CDS. Version 2.2. Release Notes. OpenLAB CDS Release Notes 1
 Agilent OpenLAB CDS Version 2.2 Release Notes OpenLAB CDS Release Notes 1 Introduction This document provides a listing of the major feature modifications made in each release of the Agilent OpenLAB Chromatography
Agilent OpenLAB CDS Version 2.2 Release Notes OpenLAB CDS Release Notes 1 Introduction This document provides a listing of the major feature modifications made in each release of the Agilent OpenLAB Chromatography
Agilent 1290 Infinity with ISET
 Agilent 1290 Infinity with ISET User Manual Agilent Technologies Notices Agilent Technologies, Inc. 2012, 2013 No part of this manual may be reproduced in any form or by any means (including electronic
Agilent 1290 Infinity with ISET User Manual Agilent Technologies Notices Agilent Technologies, Inc. 2012, 2013 No part of this manual may be reproduced in any form or by any means (including electronic
Agilent 1290 Infinity with ISET
 Agilent 1290 Infinity with ISET User Manual Agilent Technologies Notices Agilent Technologies, Inc. 2011 No part of this manual may be reproduced in any form or by any means (including electronic storage
Agilent 1290 Infinity with ISET User Manual Agilent Technologies Notices Agilent Technologies, Inc. 2011 No part of this manual may be reproduced in any form or by any means (including electronic storage
Startup Guide. Version 1.7
 Startup Guide 1 INTRODUCTION 3 COMPANIES & USERS 4 Companies & Users Licensee Offices 4 Companies & Users Insurers 6 Companies & Users Distributors 7 Companies & Users Users 8 Reset Password 10 Companies
Startup Guide 1 INTRODUCTION 3 COMPANIES & USERS 4 Companies & Users Licensee Offices 4 Companies & Users Insurers 6 Companies & Users Distributors 7 Companies & Users Users 8 Reset Password 10 Companies
Aqueous GPC Instructions - Detailed
 Aqueous GPC Instructions - Detailed Waste Bottle Columns PL Datastream Injection Port GPC Computer UV Detector GPC Pump Eluent Bottle The Aqueous GPC Set Up RI Detector Important The points below must
Aqueous GPC Instructions - Detailed Waste Bottle Columns PL Datastream Injection Port GPC Computer UV Detector GPC Pump Eluent Bottle The Aqueous GPC Set Up RI Detector Important The points below must
Agilent Instrument Control Framework for Agilent LC/CE and GC instruments in Non-Agilent Chromatography Data Systems A.02.
 Agilent Instrument Control Framework for Agilent LC/CE and GC instruments in Non-Agilent Chromatography Data Systems A.02.02 Release Notes Contents Instrument Control Framework (ICF) Revision A.02.02 4
Agilent Instrument Control Framework for Agilent LC/CE and GC instruments in Non-Agilent Chromatography Data Systems A.02.02 Release Notes Contents Instrument Control Framework (ICF) Revision A.02.02 4
Standard Operating Procedure for the HPLC-ICP-MS System By Celina Dozier Fall 2017
 Standard Operating Procedure for the HPLC-ICP-MS System By Celina Dozier Fall 2017 Introduction This document is only for operation of the HPLC and ICP-MS instruments to obtain data. It contains no maintenance
Standard Operating Procedure for the HPLC-ICP-MS System By Celina Dozier Fall 2017 Introduction This document is only for operation of the HPLC and ICP-MS instruments to obtain data. It contains no maintenance
Automating HPLC Analytical Methods Development
 Automating HPLC Analytical Methods Development By Graham Shelver, Varian Inc, Walnut Creek, CA and Richard Verseput, S-Matrix Corp, Eureka, CA Abstract There are many different ways that chromatographer
Automating HPLC Analytical Methods Development By Graham Shelver, Varian Inc, Walnut Creek, CA and Richard Verseput, S-Matrix Corp, Eureka, CA Abstract There are many different ways that chromatographer
Standard Operating Procedure for the Horiba FluroMax-4
 Standard Operating Procedure for the Horiba FluroMax-4 Adapted from Horiba Operations Manual Created by Michael Delcau, Modified by Brian Lamp The Fluoromax is capable of making a variety of measurements.
Standard Operating Procedure for the Horiba FluroMax-4 Adapted from Horiba Operations Manual Created by Michael Delcau, Modified by Brian Lamp The Fluoromax is capable of making a variety of measurements.
Standard: 2 x 500 ml and 2 x 250 ml (safety) bottles with shut-off valve and 2 µm in-line filter. Built-in 4 channel He degasser.
 The UltiMate Solvent Organizer 3.9 Specifications 3.9.1 Solvent Organizer Reservoirs Solvent Degassing Standard: 2 x 500 ml and 2 x 250 ml (safety) bottles with shut-off valve and 2 µm in-line filter.
The UltiMate Solvent Organizer 3.9 Specifications 3.9.1 Solvent Organizer Reservoirs Solvent Degassing Standard: 2 x 500 ml and 2 x 250 ml (safety) bottles with shut-off valve and 2 µm in-line filter.
Waters Headspace Control Option, Version 3.0
 R E L E A S E N O T E S Waters Headspace Control Option, Version 3.0 Version 3.0 of the Headspace Control Option (HCO) contains instrument drivers for Agilent Headspace Samplers and a toolkit program that
R E L E A S E N O T E S Waters Headspace Control Option, Version 3.0 Version 3.0 of the Headspace Control Option (HCO) contains instrument drivers for Agilent Headspace Samplers and a toolkit program that
Release Note for Agilent LC and CE Drivers Revision A SR1
 Release Note for Agilent LC and CE Drivers Revision A.02.19 SR1 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.19 SR1. Service Release A.02.19
Release Note for Agilent LC and CE Drivers Revision A.02.19 SR1 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.19 SR1. Service Release A.02.19
Agilent GC/MSD Instructions
 Agilent GC/MSD Instructions GC/MS SAMPLE PREPARATION: Sample Components to Avoid Completely: The following should never be injected: metals, strong acids or bases, salts, oligomeric and polymeric material.
Agilent GC/MSD Instructions GC/MS SAMPLE PREPARATION: Sample Components to Avoid Completely: The following should never be injected: metals, strong acids or bases, salts, oligomeric and polymeric material.
TA Instruments New Features in ITCRun TM Software
 TA Instruments New Features in ITCRun TM Software Notice The material contained in this manual, and in the online help for the software used to support TA Instruments products, is believed adequate for
TA Instruments New Features in ITCRun TM Software Notice The material contained in this manual, and in the online help for the software used to support TA Instruments products, is believed adequate for
Release Note for Agilent LC and CE Drivers Revision A.02.19
 Release Note for Agilent LC and CE Drivers Revision A.02.19 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.19. The Agilent LC and CE Driver
Release Note for Agilent LC and CE Drivers Revision A.02.19 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.19. The Agilent LC and CE Driver
SHIMADZU LC-10/20 SYSTEM
 SHIMADZU LC-10/20 SYSTEM Clarity Control Module ENG Code/Rev.: M108/70C Date: 10/2/2017 Phone: +420 251 013 400 DataApex Ltd. Fax: +420 251 013 401 Petrzilkova 2583/13 clarity@dataapex.com 158 00 Prague
SHIMADZU LC-10/20 SYSTEM Clarity Control Module ENG Code/Rev.: M108/70C Date: 10/2/2017 Phone: +420 251 013 400 DataApex Ltd. Fax: +420 251 013 401 Petrzilkova 2583/13 clarity@dataapex.com 158 00 Prague
EASY-nLC Virtual Instrument for Xcalibur 2.1.x Quick Reference Guide
 EASY-nLC Virtual Instrument for Xcalibur 2.1.x Quick Reference Guide This guide describes how to install, configure, and use the Thermo EASY-nLC Virtual Instrument (VI) software with the Thermo Xcalibur
EASY-nLC Virtual Instrument for Xcalibur 2.1.x Quick Reference Guide This guide describes how to install, configure, and use the Thermo EASY-nLC Virtual Instrument (VI) software with the Thermo Xcalibur
Marketer's Guide. User guide for marketing analysts and business users
 Marketer's Guide Rev: 18 November 2014 Email Campaign Manager 2.2 for Sitecore CMS 7.5 Marketer's Guide User guide for marketing analysts and business users Table of Contents Chapter 1 Introduction...
Marketer's Guide Rev: 18 November 2014 Email Campaign Manager 2.2 for Sitecore CMS 7.5 Marketer's Guide User guide for marketing analysts and business users Table of Contents Chapter 1 Introduction...
Huqun Liu, Varian Inc., Lake Forest CA
 Rapid development of an LC method for separating high molecular weight degradants from a biopharmaceutical product using an automated Design of Experiments (DOE) approach. Introduction Huqun Liu, Varian
Rapid development of an LC method for separating high molecular weight degradants from a biopharmaceutical product using an automated Design of Experiments (DOE) approach. Introduction Huqun Liu, Varian
Waters Empower 2 Service Pack G
 R E L E A S E N O T E S Waters Empower 2 Service Pack G Empower 2 Service Pack G enhances LDAP capabilities. These release notes also address the following information and instructions: System and software
R E L E A S E N O T E S Waters Empower 2 Service Pack G Empower 2 Service Pack G enhances LDAP capabilities. These release notes also address the following information and instructions: System and software
Guidebook for Installation and Familiarization. HP 1100 Series Combinatorial Chemistry Analysis System
 Guidebook for Installation and Familiarization HP 1100 Series Combinatorial Chemistry Analysis System Copyright Hewlett- Packard Company 1997 All rights reserved. Reproduction, adaption, or translation
Guidebook for Installation and Familiarization HP 1100 Series Combinatorial Chemistry Analysis System Copyright Hewlett- Packard Company 1997 All rights reserved. Reproduction, adaption, or translation
Release Note for Agilent LC and CE Drivers Revision A.02.15
 Release Note for Agilent LC and CE Drivers Revision A.02.15 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.15. The Agilent LC and CE Driver
Release Note for Agilent LC and CE Drivers Revision A.02.15 Introduction This release note provides important information for the release of Agilent LC and CE drivers A.02.15. The Agilent LC and CE Driver
Optimizing System Dispersion on the Agilent 1290 Infinity II LC
 Optimizing System Dispersion on the Agilent 1290 Infinity II LC The Agilent Ultralow Dispersion Kit Technical Overview Authors Bettina Schuhn and Sonja Schneider Agilent Technologies, Inc. Waldbronn, Germany
Optimizing System Dispersion on the Agilent 1290 Infinity II LC The Agilent Ultralow Dispersion Kit Technical Overview Authors Bettina Schuhn and Sonja Schneider Agilent Technologies, Inc. Waldbronn, Germany
Breeze 2 Installation and Configuration Guide
 Breeze 2 Installation and Configuration Guide 715001951/Revision B Copyright Waters Corporation 2008 2009 All rights reserved Copyright notice 2008 2009 WATERS CORPORATION. PRINTED IN THE UNITED STATES
Breeze 2 Installation and Configuration Guide 715001951/Revision B Copyright Waters Corporation 2008 2009 All rights reserved Copyright notice 2008 2009 WATERS CORPORATION. PRINTED IN THE UNITED STATES
Unscrew and remove the cap from the waste tank
 Stanford Cytoflex HTS User Guide 11232015 Starting up 1. Check the sheath fluid level every time you use the cytometer. This ensures that you do not run out of sheath fluid during an experiment. Replenish
Stanford Cytoflex HTS User Guide 11232015 Starting up 1. Check the sheath fluid level every time you use the cytometer. This ensures that you do not run out of sheath fluid during an experiment. Replenish
Introduction to the HPLC ChemStation and Acquisition
 Introduction to the HPLC ChemStation and Acquisition In This Section, We Will Discuss: How to work in the Microsoft Windows Environment The structure of the ChemStation Software. How to set up an acquisition
Introduction to the HPLC ChemStation and Acquisition In This Section, We Will Discuss: How to work in the Microsoft Windows Environment The structure of the ChemStation Software. How to set up an acquisition
Agilent 1100 HPLC ChemStation 1) Turn on all the modules for HPLC system (CapPump G1376A, DAD G1315B) and allow for system to initialize.
 Standard Operating Procedure Remcho Research Group P a g e 1 Agilent 1100 HPLC ChemStation 1) Turn on all the modules for HPLC system (CapPump G1376A, DAD G1315B) and allow for system to initialize. 2)
Standard Operating Procedure Remcho Research Group P a g e 1 Agilent 1100 HPLC ChemStation 1) Turn on all the modules for HPLC system (CapPump G1376A, DAD G1315B) and allow for system to initialize. 2)
LIMS QUICK START GUIDE. A Multi Step Guide to Assist in the Construction of a LIMS Database. Rev 1.22
 LIMS QUICK START GUIDE A Multi Step Guide to Assist in the Construction of a LIMS Database Rev 1.22 Contents Contents...1 Overview - Creating a LIMS Database...2 1.0 Folders...3 2.0 Data Fields...3 2.1
LIMS QUICK START GUIDE A Multi Step Guide to Assist in the Construction of a LIMS Database Rev 1.22 Contents Contents...1 Overview - Creating a LIMS Database...2 1.0 Folders...3 2.0 Data Fields...3 2.1
Agilent OpenLAB Chromatography Data System A SR1 - Updates
 Agilent OpenLAB Chromatography Data System A.02.02 SR1 - Updates Sequence Table updates In OpenLAB CDS ChemStation version C.01.06 and C.01.07, the sequence table is updated with new features. Introduced
Agilent OpenLAB Chromatography Data System A.02.02 SR1 - Updates Sequence Table updates In OpenLAB CDS ChemStation version C.01.06 and C.01.07, the sequence table is updated with new features. Introduced
Agilent MassHunter Easy Access Software
 Agilent MassHunter Easy Access Software Quick Start Guide Where to Find More Information 3 Installation 4 Sharing Configurations with Multiple Instruments 10 Installed Files 11 Using Easy Access Software
Agilent MassHunter Easy Access Software Quick Start Guide Where to Find More Information 3 Installation 4 Sharing Configurations with Multiple Instruments 10 Installed Files 11 Using Easy Access Software
Quick Start Guide. Introduction. Importing Raw Data. Method 1: Use Open Data Panel
 ACD/Spectrus Processor: Chromatography Data Irina Oshchepkova and Laura Zepeda Advanced Chemistry Development, Inc. Toronto, ON, Canada www.acdlabs.com Introduction The following document outlines how
ACD/Spectrus Processor: Chromatography Data Irina Oshchepkova and Laura Zepeda Advanced Chemistry Development, Inc. Toronto, ON, Canada www.acdlabs.com Introduction The following document outlines how
Empower Software Release Notes Appendix Changes Implemented through Service Packs
 Empower Software Release Notes Appendix Changes Implemented through Service Packs Problems Fixed with Empower Service Pack A PCS #: 28564 In certain circumstances, the Server is unable to access data files
Empower Software Release Notes Appendix Changes Implemented through Service Packs Problems Fixed with Empower Service Pack A PCS #: 28564 In certain circumstances, the Server is unable to access data files
Release Notes. Analyst TF Software
 Release Notes Analyst TF 1.5.1 Software Release Date: April 2011 This document is provided to customers who have purchased AB SCIEX equipment to use in the operation of such AB SCIEX equipment. This document
Release Notes Analyst TF 1.5.1 Software Release Date: April 2011 This document is provided to customers who have purchased AB SCIEX equipment to use in the operation of such AB SCIEX equipment. This document
Agilent 7890 GC Instrument Control Software Version 2.5
 R E L E A S E N O T E S Agilent 7890 GC Instrument Control Software Version 2.5 The Agilent 7890 GC Instrument Control Software version 2.5 enables dual-tower injection capability and provides support
R E L E A S E N O T E S Agilent 7890 GC Instrument Control Software Version 2.5 The Agilent 7890 GC Instrument Control Software version 2.5 enables dual-tower injection capability and provides support
New features and Workflow Enhancements Empower 3 Software
 New features and Workflow Enhancements Empower 3 Software Mogens Hallas Nordic User Training 2011 Långvik, September 6th to 9th, 2011 2011 Waters Corporation 1 Empower 3 Architecture and operating systems
New features and Workflow Enhancements Empower 3 Software Mogens Hallas Nordic User Training 2011 Långvik, September 6th to 9th, 2011 2011 Waters Corporation 1 Empower 3 Architecture and operating systems
Agilent 971-FP. Software Installation Instructions
 Software Installation Instructions This document contains installation and upgrade procedure for the Agilent 971-FP Flash Purification Software v3.2. Introduction This document describes how to install
Software Installation Instructions This document contains installation and upgrade procedure for the Agilent 971-FP Flash Purification Software v3.2. Introduction This document describes how to install
vbound User Guide vbound User Guide Version Revised: 10/10/2017
 vbound User Guide Version 4.1.1 Revised: 10/10/2017 Copyright 2014-2017 FFL Solutions Inc. Page 1 of 87 Table of Contents Using vbound...5 Starting vbound... 5 Bound Book List... 6 vbound Ribbon Menu...
vbound User Guide Version 4.1.1 Revised: 10/10/2017 Copyright 2014-2017 FFL Solutions Inc. Page 1 of 87 Table of Contents Using vbound...5 Starting vbound... 5 Bound Book List... 6 vbound Ribbon Menu...
This chapter provides information about managing end user directory information.
 End user setup This chapter provides information about managing end user directory information. About end user setup, page 1 End user deletion, page 2 End user settings, page 3 Create Cisco Unity Connection
End user setup This chapter provides information about managing end user directory information. About end user setup, page 1 End user deletion, page 2 End user settings, page 3 Create Cisco Unity Connection
Joomla Pre-install Tasks
 Joomla 3.0.1 Pre-install Tasks Before commencing the actual installation of Joomla CMS on your webhost you have to create: A MySQL database A MySQL user ( with password based access to the MySQL database
Joomla 3.0.1 Pre-install Tasks Before commencing the actual installation of Joomla CMS on your webhost you have to create: A MySQL database A MySQL user ( with password based access to the MySQL database
Spark Holland introduces
 BETTER SAMPLE CARE Spark Holland introduces ALIAS All-round, state-of-the-art autosampler for HPLC Spark Holland introduces ALIAS All-round, state-of-the-art autosampler for HPLC ALIAS All-round, state-of-the-art
BETTER SAMPLE CARE Spark Holland introduces ALIAS All-round, state-of-the-art autosampler for HPLC Spark Holland introduces ALIAS All-round, state-of-the-art autosampler for HPLC ALIAS All-round, state-of-the-art
Agilent OpenLAB Chromatography Data System (CDS) ChemStation Edition
 Agilent OpenLAB Chromatography Data System (CDS) ChemStation Edition Supported Instruments and Firmware Guide Notices Agilent Technologies. 2010, 2011-2017 No part of this manual may be reproduced in any
Agilent OpenLAB Chromatography Data System (CDS) ChemStation Edition Supported Instruments and Firmware Guide Notices Agilent Technologies. 2010, 2011-2017 No part of this manual may be reproduced in any
Data Acquisition with 3800 GC Control
 Varian, Inc. 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Star Chromatography Workstation Version 6 Data Acquisition with 3800 GC Control Operation Manual Varian, Inc. 2005 03-914731-00:Rev. 6 Trademark
Varian, Inc. 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Star Chromatography Workstation Version 6 Data Acquisition with 3800 GC Control Operation Manual Varian, Inc. 2005 03-914731-00:Rev. 6 Trademark
SPA III Quick Reference Guide for Custom Panels
 SPA III Quick Reference Guide for Custom Panels This guide contains instructions for using the BD FACS Sample Prep Assistant III (SPA III) with BD FACS SPA software version.0 and later. Initial Setup Custom
SPA III Quick Reference Guide for Custom Panels This guide contains instructions for using the BD FACS Sample Prep Assistant III (SPA III) with BD FACS SPA software version.0 and later. Initial Setup Custom
Waters Empower Software Build 1154 Toolkit Service Pack Release Notes
 Waters Empower Software Build 1154 Toolkit Service Pack Release Notes These release notes are intended as supplemental information and should be used in conjunction with the Empower Software Release Notes.
Waters Empower Software Build 1154 Toolkit Service Pack Release Notes These release notes are intended as supplemental information and should be used in conjunction with the Empower Software Release Notes.
Ansur Index 2XL. Users Manual. Plug-In
 Ansur Index 2XL Plug-In Users Manual April 2010, Rev. 1 2010 Fluke Corporation. All rights reserved. All product names are trademarks of their respective companies. Table of Contents Chapter Title Page
Ansur Index 2XL Plug-In Users Manual April 2010, Rev. 1 2010 Fluke Corporation. All rights reserved. All product names are trademarks of their respective companies. Table of Contents Chapter Title Page
Interfacing with the Biacore instrument
 Interfacing with the Biacore instrument Different ways of interfacing with the instrument Manual run Wizards Method Builder methods 2 1 Manual Run 3 Application Wizards Guides you through each step in
Interfacing with the Biacore instrument Different ways of interfacing with the instrument Manual run Wizards Method Builder methods 2 1 Manual Run 3 Application Wizards Guides you through each step in
Agilent G2070BA GC ChemStation
 Agilent G2070BA GC ChemStation Familiarization Guide Agilent Technologies Notices Agilent Technologies, Inc. 2011 No part of this manual may be reproduced in any form or by any means (including electronic
Agilent G2070BA GC ChemStation Familiarization Guide Agilent Technologies Notices Agilent Technologies, Inc. 2011 No part of this manual may be reproduced in any form or by any means (including electronic
Imagine. Create. Discover. User Manual. TopLine Results Corporation
 Imagine. Create. Discover. User Manual TopLine Results Corporation 2008-2009 Created: Tuesday, March 17, 2009 Table of Contents 1 Welcome 1 Features 2 2 Installation 4 System Requirements 5 Obtaining Installation
Imagine. Create. Discover. User Manual TopLine Results Corporation 2008-2009 Created: Tuesday, March 17, 2009 Table of Contents 1 Welcome 1 Features 2 2 Installation 4 System Requirements 5 Obtaining Installation
Waters Alliance GPC 2000 Series System
 Waters Alliance GPC 2000 Series System Quick Start Guide 34 Maple Street Milford, MA 01757 71500021503, Revision A NOTICE The information in this document is subject to change without notice and should
Waters Alliance GPC 2000 Series System Quick Start Guide 34 Maple Street Milford, MA 01757 71500021503, Revision A NOTICE The information in this document is subject to change without notice and should
Standard Operating Procedure for the Beckman PA/CE MDQ Electrophoresis System. Last Modified October 19, 2012
 Standard Operating Procedure for the Beckman PA/CE MDQ Electrophoresis System Last Modified October 19, 2012 Considerations Prior to Beginning Experiment All solutions must be filtered (using 0.45 m or
Standard Operating Procedure for the Beckman PA/CE MDQ Electrophoresis System Last Modified October 19, 2012 Considerations Prior to Beginning Experiment All solutions must be filtered (using 0.45 m or
Data Acquisition with 3400/3600 GC Control
 Varian, Inc. 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Star Chromatography Workstation Version 6 Data Acquisition with 3400/3600 GC Control Operation Manual Varian, Inc. 2002 03-914730-00:Rev.
Varian, Inc. 2700 Mitchell Drive Walnut Creek, CA 94598-1675/USA Star Chromatography Workstation Version 6 Data Acquisition with 3400/3600 GC Control Operation Manual Varian, Inc. 2002 03-914730-00:Rev.
MY MEDIASITE. https://mediasite.ecu.edu/ms/mymediasite
 MY MEDIASITE https://mediasite.ecu.edu/ms/mymediasite My Mediasite provides tools for managing your recordings. All faculty have access to My Mediasite and the ability to download the Desktop Recorder.
MY MEDIASITE https://mediasite.ecu.edu/ms/mymediasite My Mediasite provides tools for managing your recordings. All faculty have access to My Mediasite and the ability to download the Desktop Recorder.
Dissolution Automation: Off line Systems
 Dissolution Automation: Off line Systems Designed specifically for Tablet Dissolution testing, this unique versatile system allows samples to be collected over very short Time Intervals with additional
Dissolution Automation: Off line Systems Designed specifically for Tablet Dissolution testing, this unique versatile system allows samples to be collected over very short Time Intervals with additional
