From raw data to submission: A metadata-driven, repository-based process of data conversion to CDISC models
|
|
|
- Dwayne Perkins
- 5 years ago
- Views:
Transcription
1 Paper CD08 From raw data to submission: A metadata-driven, repository-based process of data conversion to CDISC models Dimitri Kutsenko, Entimo AG, Berlin, Germany ABSTRACT The paper presents a visionary framework to solve selected practical problems when converting data to CDISC models. It will guide the reader through the steps of a data conversion process from raw data to SDTM conformant data and show through practical examples how efficient metadata management in a repository based environment supported by smart tools helps speed up the whole workflow. The examples will touch on generation of mapping specifications and mapping programs. Special focus will be placed on such issues as quality control, validation and reusability of work results all supported by metadata-driven mechanisms. The article is intended to be a top-level guideline for organizations which are just starting or have been involved in data conversion to CDISC models and are looking for innovative ways to design a flexible data conversion workflow developed with a metadata-based vision. INTRODUCTION Good news - with every new version, the CDISC SDTM model becomes more stable and robust. Bad news - numerous mapping projects set up to transform data to SDTM-conformant structures are still suffering from significant inefficiencies along the entire data conversion workflow: A typical mapping process separates distinct phases of mapping specification and mapping program development. Mapping programs are manually coded based on the mapping specification which is provided as Excel sheets. Due to the fact that models are dynamic and change over time, mapping programs as well as specifications are difficult to maintain due to the need of interlinked information and are error-prone if model adjustments are necessary. The degree of reusability for structure definitions and program code is minimal: For every study the structure definitions and code need to be created from scratch or are re-used by copy and paste automation in manual replacements. Moreover, the phases are often carried out by different organizational roles. Communication-related friction at the interfaces between organizational roles and units is pre-programmed in such a process. Without doubt, projects with a few team members are easily manageable in terms of coordination efforts and communication flows. However, even small teams would benefit from a regulatory compliant and traceable process. With increasing project team size and number of studies to be processed, especially when teams are distributed in space and time zones, the management of such projects becomes a challenge. Imagine a world where everything is possible. What would the mapping process look like? What smart tools could make the process more efficient and release you from routine tasks by replacing them with automation? As an agile software R&D company and technology provider, Entimo has been able to absorb enormous know-how in different areas over the years and has stayed at the forefront of radical changes in technology, adapting them in development processes and implementing them in its products. In this paper, I would like to share with you our vision of the data conversion process. We will use CDISC SDTM as an example model for transformations (CDISC 2005b). However, SDTM is just a metadata model in a general sense. If metadata handling is flexible enough, the same process and tools can be used to support any target model be it specific or standardized. Certainly, organizational contexts differ from company to company as well as in terms of SOPs, experience of people involved, and their technical background. For this reason, concrete implementations of the mapping process including steps and roles may take various forms with different names. However, 1
2 looking at the process, we can crystallize out key elements and tasks which are generic in their nature. To analyze them, we will break down the whole process into parts, try to detect possible inefficiencies in the separate process steps, look for means of eliminating these inefficiencies and, finally, reintegrate the parts into a continuous process. Our additional goal is to find out how organization-wide initiatives may help to increase efficiency even further in the long run. DELIVERABLES Following the trend, and for a number of other reasons, big pharmaceutical companies are on the way to partly or completely outsourcing study conversion to SDTM, especially with regards to the large stock of legacy studies available in every house. (The reasons for this are outside the scope of this paper and shall not be investigated here). On the other side, to cope with a growing workload and enjoy globalization advantages, CROs and conversion specialists who typically take over the task have experienced a rapid and large-scale concentration wave through numerous M&As and have gone global. Serving the customers at their best, many CROs often find themselves in a situation where they have to maintain several EDC systems simultaneously differing not only in current technological capabilities with regards to SDTM support, but also in terms of future potential. In such a situation CROs face a dilemma: to wait until all EDC are upgraded and natively support SDTM, a process which might last years (if realistic at all, as some of the EDC systems are not being actively developed and will hardly survive). On the other hand, mastering all in-house EDC systems in order to be time and cost efficient in comparison with the competition, is expensive due to significant training needs among other things. In addition to the ongoing studies, numerous legacy studies need to be brought into a standardized form (SDTM or SDTM+, for example) for pooling or other reasons. Is there an alternative option to waiting or working with many processes simultaneously? One answer is to find an optimum point where all the EDC systems cross and set up from this point a unified, company-wide mapping process for which training is required only once and which makes the task cost and time efficient through use of intelligent tools. Such a process would allow the user to be competitive and fast, which is especially important considering the tough project timelines shortly before submissions. We begin our journey from raw to SDTM conformant data with a minimal set of deliverables including raw datasets and CRF and will put all other information sources aside for simplification reasons (however, for legacy studies, even such minimal set is possible). Already the procedure of deliverables transfer reveals some potential for improvement. Due to the fact that source data does not need to be changed, the question arises if it is necessary to migrate the data from one system to another? In the best case, the data will be kept in one place, but be accessible for transformation to SDTM from other tools. Further, data might come from customers or older legacy studies stored in SAS datasets or other formats. So the process should provide means to access external data sources on the one hand, and on the other, to import and store datasets, metadata descriptions and specifications ready to be used for transformations. MAPPING SPECIFICATION The first stop on our journey is the creation of the mapping specification a crucial part of the whole process. Classically, it begins with annotation of the CRF according to target SDTM structures. The data manager who is typically responsible for this task uses PDF commenting tools and a solid knowledge of CDISC models and raw data. This process is time consuming, so innovation is advancing into this area: Next generation EDC systems can natively generate target annotated CRF - which is obvious as they support the study from the very beginning - which will simplify this step in the future. However, older legacy studies are excluded from this convenient situation due to the fact that CRFs are stored in different formats, either scanned or as searchable content. For this reason, a certain amount of manual work will remain here in spite of the technical progress, even if some technical aid can be imagined. Once the CRF is target-annotated, it is passed along the workflow to the role creating the mapping specification (let us call this role mapper for convenience reasons). Let us have a look at the source side first. Classical tools (e.g. SAS PROC CONTENTS) certainly deliver an exact description of the raw datasets. Though very useful, this information cannot be directly used for 2
3 mapping. With every dataset, the mapper needs to create source structures manually from scratch as they are different from study to study, from dataset to dataset. Simple multiplication of the number of studies with the number of datasets in a study shows that already at this stage some automation would save a significant amount of time and routine work. A more user-friendly solution would provide the possibility of selecting the attributes from a list, thus at least eliminating the need to type in attribute names manually and reducing the number of errors. In our ideal world, a tool is available which allows us to automatically extract metadata definitions from raw datasets and use them as data definition elements in mapping specifications and programs (we will see later that both outcomes don t need to be considered separately). In addition the source metadata definitions can be used for quality checks before carrying out the transformation: while appending several datasets, for example, incoming datasets can be checked for conformance with the metadata definitions to eliminate such basic errors as deviations in attribute types or in the number of attributes. The target side looks slightly differently. The structure definitions for CDISC SDTM are publicly available on the CDISC web page ( As a harmonization model, the SDTM structures do not completely reflect the clinical reality of particular studies by default. Typically, sponsors collect more information than required for submission. When converting studies to SDTM, this information should not get lost and it might be necessary in the future. Some studies might not contain all attributes available in SDTM. The term SDTM+ incorporates company- or study-specific adjustments of the standard SDTM structures - be it extension or reduction. The SDTM model allows model-specific modifications with regards to domains and attributes as long as certain rules apply. Tracing the usage of SDTM structures on the target side, we can see that they are used or referenced in several outcomes of the process: in the target-annotated CRF, mapping specification, mapping programs and define.xml. In addition, the submission information spans several interconnected dimensions (or multi level metadata, in other words, which are linked together). With increasing complexity, its consistent management becomes a challenge. Considering the fact that the core structures do not change very often, the management of target metadata reveals a great deal of potential for reusability within organizations and studies. However, for this reason, the metadata management mechanism must remain flexible to address tailoring and future model changes. Imagine how comfortable and time-saving it would be to define the core target metadata only once and then reuse it in different mapping projects and studies to create the required outcomes! This is exactly the approach our metadata driven world is built on: to begin the mapping process with the definition of metadata templates for target structures. Such an approach brings enormous synergies: Following its internal SOPs, an organization can create a set of predefined templates with company-wide standards, possibly going beyond pure SDTM and containing all relevant attribute definitions. The templates contain all core information such as domain and attribute level metadata as well as links to controlled terminology and/or dictionaries already pre-configured. Promulgated to the study level or other intermediate organizational levels, the templates can be adjusted to concrete needs: not available (and optional) attributes can be removed from the target metadata definitions; additional study specific elements can be added. This approach reduces the work in a mapping project to small adjustments, minimal in comparison with setting up the structures from scratch. Certainly it means more work initially, but allows users to benefit later from reusable templates. The templates can be used in many ways: in mapping specifications and programs, for SDTM compliance checks as well as for creation of define.xml. These four advantages are a powerful reason to invest more time in the beginning in order to have time and resource savings at the end when time is usually critical. Certainly, deploying such an approach requires a central metadata repository (for an overview of general requirements to the metadata management see Kutsenko 2009). If implemented in an organization, the repository would provide controlled access to templates for the roles involved in the mapping process and would support means to work flexibly with reusable templates at the project and study level with version control and configurable workflows. CDISC has also been actively working on its Metadata Repository (CMDR) to present a pilot in the coming winter (CDISC 2008). Once the target structures are fixed, they can be related to the available sources, with conversion functions, if necessary. 3
4 What sounds easy might turn out to be an extremely time consuming task which might even involve different organizational roles and knowledge domains in resolving ambiguities. In the best case an attribute required or expected in the target structure is directly available in the source definition (even if with a different name). In this case a simple 1:1 assignment can be done (as practice shows, simple assignments account for the largest part of all assignments and transformations to SDTM). If you consider, for example, the LB domain with 44 attributes in the standard CDISC structure (CDISC 2005c), a little help for the mapping definition (which might consist of several transformation steps built on each other and include transpositions) would save a great deal of time for this routine job and reduce the number of typing errors. In our environment, automations are available for such tasks starting with a drag-and-drop interface for attributes with different names (unfortunately even the smartest tools can t interpret the data - the human brain still is and will be required) up to automatic assignment of attributes with the same names. More difficult cases (e.g. unclear or unavailable sources which happen often in older legacy studies or specific transformation functions) may require the involvement of data managers and programmers who might be globally distributed at several company locations. Imagine a situation where a mapper has to resolve some ambiguous source-target relation and to create consequently a complex conversion algorithm. The mapper would need to specify the problem to solve (unclear source for the attribute A), contact a data manager or another person who would help with the source side (let s assume, for example, that the attribute A requires a complex transformation based on several source datasets). This person would need to store comments on the attribute which would help the programmer to develop an efficient algorithm. Then all the information is passed to the programmer who can develop an algorithm for this particular conversion (in our example, the mapper can not program SAS very well). In order to make the process more efficient, communication between the team mates should be supported by tools which would help them find their way in complex structures and focus on problem zones, and to work according to specified workflows and processes. In our visionary world, communication between all roles involved would happen in an environment which provides effective collaboration tools and automatically documents communication flows. In such an encapsulated environment it is an easy task: you can send a role-bound notification (via or as an internal system message) to your colleagues pointing to specific problems in a mapping definition. They can review the specification online, provide additional information as attribute comments and forward the issue back and forth for resolution according to the defined workflow. You can even start a discussion in a linked forum or discussion group. All communication flows are stored related to the mapping definition, so the communication and decisions are transparent even later for new personnel. For numerous standard transformation algorithms, it is even not necessary to involve programmers drag-and-drop GUIs are available (e.g. for selection of available attributes and formats) and can be assigned even by users who are not very familiar with SAS. To deal with terminology mapping, a GUIdriven profiling tool helps us to compare several datasets and to detect differences in terminology used and consequent mapping needs. The mapping rules are stored in the mapping definition (also supported by drag-and-drop GUIs) and used later on. The principle of visual definition helps to reduce the SAS skills required for mapping to an absolute minimum. At the end of this key process step, a well-defined mapping specification is available. It is stored in the repository as a metadata-based mapping definition of sources, targets and their relations including conversion algorithms, is accessible in a dedicated view to all users with access rights online, and is upto-date and exportable in different formats (as an overview or full mapping report, for example). MAPPING PROGRAM DEVELOPMENT Now we have reached the stage with the highest inefficiency in the whole mapping process: In a classical process, after the mapping specification is finalized, programmers have to implement the conversions in mapping programs based on the developed mapping specification. Looking at the mapping specification from the abstract perspective, you can ask yourself the following question: if the mapping specification is supposed to be a 1:1 documentation of the sources, targets and algorithms, why can t I automate this task? At this point, we have the exact descriptions of the sources 4
5 (derived from the raw datasets) and targets (adjusted standard templates); we defined all assignments and standard conversion algorithms (supported by GUIs - so standardized). The programmers were involved during the specification phase to develop specific conversions algorithms. These algorithms were stored using the syntax of the mapping program s target language directly in the mapping specification. It would be obvious to generate mapping programs from the specification! You re absolutely right! In our visionary process, programs don t need to be manually developed. All inputs described above are sufficient to automatically create the mapping programs (e.g. SAS) based on the mapping specification. Just one button click is required when we are supported by a smart program generator! This frees your programming resources for more complex tasks than programming of 1:1 assignments and standard algorithms. DATA CONVERSION As you noticed above, we haven t mentioned real data yet (except for the first step to create source metadata from raw data). Real data only needs to come into play at this step when the generated mapping programs are executed with real data to be converted to the target SDTM structures. To keep the generated mapping programs flexible and reusable with different datasets, no hard-coded information about the location and names of the datasets to be transformed is stored in programs. They contain only parameters for all objects involved. While running the mapping program, the user simply assigns real objects to the program parameters (drag-and-drop support is also available in the system for this purpose, together with the storage of used parameter values). By default, generated mapping programs contain built-in checks for source data: existence of the attributes and their conformance with the metadata definitions used in the specification. These checks are the first step of the quality control. If the source checks have been passed and there are no logical errors in the definition ( human error ), datasets compliant with the mapping specification are available after the mapping program execution. An important question arises at this step: Are my converted datasets conformant with SDTM? How could this process be made efficient? One solution is to use a SAS check program and to integrate this program into the conversion workflow. This brings several advantages: not only can I launch the check program directly after the conversion and so see the results of the quality checks on the converted datasets immediately; I can also, with a stand alone check program, obtain the flexibility to enhance the checks with my specific ones. (Many organizations have already implemented own checks. In addition, CROs may need to execute specific checks required by their customers.) Integration of standard and specific checks into the workflow makes the whole process flexible, convenient and fast. DEFINE.XML We are approaching the last stop of the mapping process define.xml (CDISC 2005a). In order to get the submission bundle ready, just a few more actions are required - time consuming in the technologyconservative world, a few mouse clicks away in the technology-driven one. There are certainly many ways to create define.xml. At this stage in the process, we already have target metadata of SDTM domains which we also used for the creation of the mapping specification and generation of the mapping programs. Controlled terminology and value level metadata need to be created - be it manually or with a mapping program (this program follows the same principles as described above for the SDTM target datasets). Transport files are created from target datasets with dedicated format converters. The newest version of the official CDISC style sheet for define.xml supports page references to annotated CRFs and allows navigation from define.xml directly to the pages of the annotated CRF were the attributes appear. This means that the full annotated CRF needs to be scanned for attributes! For this task, we developed a tool which automatically scans annotated CRFs for domain attributes used, extracts page numbers and writes them into the attribute level metadata. It s as easy as this define.xml requires only a few mouse clicks! With such automation, define.xml can be created within the shortest time - from less than an hour up to several hours. It means a significant improvement in comparison with manual programming of define.xml. In addition, the whole process can be easily repeated to update define.xml with changed content. 5
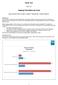
6 FINAL NOTE By the way, the process described above is not a vision; it is a reality with Entimo s tools! entimice DARE (Data Analysis and Reporting Environment) provides a collaboration environment with a central repository, configurable workflows, notifications, versioning support and many other useful features. A smart mapping tool (entimice Mapping) creates mapping specifications and programs from metadata definitions stored in the repository. Define.xml Generator bundles define.xml and SDTM Checker validates the conformance of datasets with SDTM. Finally, the illustration below depicts the described process as an overview with the stages of our journey from raw to SDTM conformant data. Illustration 1: From raw data to submission with entimice tools REFERENCES CDISC 2005a, Case Report Tabulation Data Definition Specification (define.xml) Version 1.0, viewed 2 June CDISC 2008, CDISC Meta data Repository. Enrichment & integration of CDISC Data Standards toward semantic interoperability. Business Requirements, viewed 2 June CDISC 2009, CDISC Meta data Repository. Enrichment & integration of CDISC Data Standards toward semantic interoperability. Pilot Specification: Business Scenarios (working document), _PilotSpecification_v0.4.doc, viewed 10 July CDISC 2005b, Study Data Tabulation Model (SDTM) Final Version 1.1, viewed 2 June CDISC 2005c, Study Data Tabulation Model (SDTM) Implementation Guide Final Version 3.1.1, viewed 2 June Kutsenko, D 2009, An analysis of requirements for metadata in metadata driven mapping projects and organizations, Data Basics (SCDM), vol.15, no. 2, pp
7 CONTACT INFORMATION Your comments and questions are valued and encouraged. Contact the author at: Dimitri Kutsenko Entimo AG Stralauer Platz Berlin / Germany Work Phone: Fax: Web: Brand and product names are trademarks of their respective companies. 7
Taming Rave: How to control data collection standards?
 Paper DH08 Taming Rave: How to control data collection standards? Dimitri Kutsenko, Entimo AG, Berlin, Germany Table of Contents Introduction... 1 How to organize metadata... 2 How to structure metadata...
Paper DH08 Taming Rave: How to control data collection standards? Dimitri Kutsenko, Entimo AG, Berlin, Germany Table of Contents Introduction... 1 How to organize metadata... 2 How to structure metadata...
Why organizations need MDR system to manage clinical metadata?
 PharmaSUG 2018 - Paper SS-17 Why organizations need MDR system to manage clinical metadata? Abhinav Jain, Ephicacy Consulting Group Inc. ABSTRACT In the last decade, CDISC standards undoubtedly have transformed
PharmaSUG 2018 - Paper SS-17 Why organizations need MDR system to manage clinical metadata? Abhinav Jain, Ephicacy Consulting Group Inc. ABSTRACT In the last decade, CDISC standards undoubtedly have transformed
Taming Rave: How to control data collection standards?
 Taming Rave: How to control data collection standards? Dimitri Kutsenko (Entimo AG - Berlin/Germany) Agenda Project Initiation How to: Organize metadata Structure metadata Manage metadata Check metadata
Taming Rave: How to control data collection standards? Dimitri Kutsenko (Entimo AG - Berlin/Germany) Agenda Project Initiation How to: Organize metadata Structure metadata Manage metadata Check metadata
How a Metadata Repository enables dynamism and automation in SDTM-like dataset generation
 Paper DH05 How a Metadata Repository enables dynamism and automation in SDTM-like dataset generation Judith Goud, Akana, Bennekom, The Netherlands Priya Shetty, Intelent, Princeton, USA ABSTRACT The traditional
Paper DH05 How a Metadata Repository enables dynamism and automation in SDTM-like dataset generation Judith Goud, Akana, Bennekom, The Netherlands Priya Shetty, Intelent, Princeton, USA ABSTRACT The traditional
Lex Jansen Octagon Research Solutions, Inc.
 Converting the define.xml to a Relational Database to enable Printing and Validation Lex Jansen Octagon Research Solutions, Inc. Leading the Electronic Transformation of Clinical R&D PhUSE 2009, Basel,
Converting the define.xml to a Relational Database to enable Printing and Validation Lex Jansen Octagon Research Solutions, Inc. Leading the Electronic Transformation of Clinical R&D PhUSE 2009, Basel,
PharmaSUG 2014 PO16. Category CDASH SDTM ADaM. Submission in standardized tabular form. Structure Flexible Rigid Flexible * No Yes Yes
 ABSTRACT PharmaSUG 2014 PO16 Automation of ADAM set Creation with a Retrospective, Prospective and Pragmatic Process Karin LaPann, MSIS, PRA International, USA Terek Peterson, MBA, PRA International, USA
ABSTRACT PharmaSUG 2014 PO16 Automation of ADAM set Creation with a Retrospective, Prospective and Pragmatic Process Karin LaPann, MSIS, PRA International, USA Terek Peterson, MBA, PRA International, USA
Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata
 Paper SD02 Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata Dr. Philippe Verplancke, XClinical GmbH, Munich, Germany ABSTRACT define.xml is often created at the end of
Paper SD02 Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata Dr. Philippe Verplancke, XClinical GmbH, Munich, Germany ABSTRACT define.xml is often created at the end of
The development of standards management using EntimICE-AZ
 Paper PP19 The development of standards management using EntimICE-AZ Shyamprasad Perisetla, AstraZeneca, Cambridge, UK Per-Arne Stahl, AstraZeneca, Mölndal, Sweden INTRODUCTION Historically, using excel
Paper PP19 The development of standards management using EntimICE-AZ Shyamprasad Perisetla, AstraZeneca, Cambridge, UK Per-Arne Stahl, AstraZeneca, Mölndal, Sweden INTRODUCTION Historically, using excel
The Wonderful World of Define.xml.. Practical Uses Today. Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008
 The Wonderful World of Define.xml.. Practical Uses Today Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008 Agenda Introduction to Formedix What is Define.xml? Features and Benefits
The Wonderful World of Define.xml.. Practical Uses Today Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008 Agenda Introduction to Formedix What is Define.xml? Features and Benefits
Customizing SAS Data Integration Studio to Generate CDISC Compliant SDTM 3.1 Domains
 Paper AD17 Customizing SAS Data Integration Studio to Generate CDISC Compliant SDTM 3.1 Domains ABSTRACT Tatyana Kovtun, Bayer HealthCare Pharmaceuticals, Montville, NJ John Markle, Bayer HealthCare Pharmaceuticals,
Paper AD17 Customizing SAS Data Integration Studio to Generate CDISC Compliant SDTM 3.1 Domains ABSTRACT Tatyana Kovtun, Bayer HealthCare Pharmaceuticals, Montville, NJ John Markle, Bayer HealthCare Pharmaceuticals,
CDISC Variable Mapping and Control Terminology Implementation Made Easy
 PharmaSUG2011 - Paper CD11 CDISC Variable Mapping and Control Terminology Implementation Made Easy Balaji Ayyappan, Ockham Group, Cary, NC Manohar Sure, Ockham Group, Cary, NC ABSTRACT: CDISC SDTM (Study
PharmaSUG2011 - Paper CD11 CDISC Variable Mapping and Control Terminology Implementation Made Easy Balaji Ayyappan, Ockham Group, Cary, NC Manohar Sure, Ockham Group, Cary, NC ABSTRACT: CDISC SDTM (Study
Main challenges for a SAS programmer stepping in SAS developer s shoes
 Paper AD15 Main challenges for a SAS programmer stepping in SAS developer s shoes Sebastien Jolivet, Novartis Pharma AG, Basel, Switzerland ABSTRACT Whether you work for a large pharma or a local CRO,
Paper AD15 Main challenges for a SAS programmer stepping in SAS developer s shoes Sebastien Jolivet, Novartis Pharma AG, Basel, Switzerland ABSTRACT Whether you work for a large pharma or a local CRO,
Managing your metadata efficiently - a structured way to organise and frontload your analysis and submission data
 Paper TS06 Managing your metadata efficiently - a structured way to organise and frontload your analysis and submission data Kirsten Walther Langendorf, Novo Nordisk A/S, Copenhagen, Denmark Mikkel Traun,
Paper TS06 Managing your metadata efficiently - a structured way to organise and frontload your analysis and submission data Kirsten Walther Langendorf, Novo Nordisk A/S, Copenhagen, Denmark Mikkel Traun,
Now let s take a look
 1 2 3 4 Manage assets across the end to end life cycle of your studies This includes forms, datasets, terminologies, files, links and more, for example: - Studies may contain the protocol, a set of Forms,
1 2 3 4 Manage assets across the end to end life cycle of your studies This includes forms, datasets, terminologies, files, links and more, for example: - Studies may contain the protocol, a set of Forms,
SAS offers technology to facilitate working with CDISC standards : the metadata perspective.
 SAS offers technology to facilitate working with CDISC standards : the metadata perspective. Mark Lambrecht, PhD Principal Consultant, Life Sciences SAS Agenda SAS actively supports CDISC standards Tools
SAS offers technology to facilitate working with CDISC standards : the metadata perspective. Mark Lambrecht, PhD Principal Consultant, Life Sciences SAS Agenda SAS actively supports CDISC standards Tools
Standards Driven Innovation
 Standards Driven Innovation PhUSE Annual Conference 2014 Frederik Malfait IMOS Consulting GmbH, Hoffmann-La Roche AG Managing Standards 2 Data Standards Value Proposition Standards are increasingly mandated
Standards Driven Innovation PhUSE Annual Conference 2014 Frederik Malfait IMOS Consulting GmbH, Hoffmann-La Roche AG Managing Standards 2 Data Standards Value Proposition Standards are increasingly mandated
Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library
 PharmaSUG 2018 - Paper SS-12 Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library Veena Nataraj, Erica Davis, Shire ABSTRACT Establishing internal Data Standards helps companies
PharmaSUG 2018 - Paper SS-12 Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library Veena Nataraj, Erica Davis, Shire ABSTRACT Establishing internal Data Standards helps companies
SAS Clinical Data Integration 2.4
 SAS Clinical Data Integration 2.4 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2013. SAS Clinical Data Integration 2.4: User's Guide.
SAS Clinical Data Integration 2.4 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2013. SAS Clinical Data Integration 2.4: User's Guide.
Beyond OpenCDISC: Using Define.xml Metadata to Ensure End-to-End Submission Integrity. John Brega Linda Collins PharmaStat LLC
 Beyond OpenCDISC: Using Define.xml Metadata to Ensure End-to-End Submission Integrity John Brega Linda Collins PharmaStat LLC Topics Part 1: A Standard with Many Uses Status of the Define.xml Standard
Beyond OpenCDISC: Using Define.xml Metadata to Ensure End-to-End Submission Integrity John Brega Linda Collins PharmaStat LLC Topics Part 1: A Standard with Many Uses Status of the Define.xml Standard
Automated Creation of Submission-Ready Artifacts Silas McKee, Accenture, Pennsylvania, USA Lourdes Devenney, Accenture, Pennsylvania, USA
 Paper DH06 Automated Creation of Submission-Ready Artifacts Silas McKee, Accenture, Pennsylvania, USA Lourdes Devenney, Accenture, Pennsylvania, USA ABSTRACT Despite significant progress towards the standardization
Paper DH06 Automated Creation of Submission-Ready Artifacts Silas McKee, Accenture, Pennsylvania, USA Lourdes Devenney, Accenture, Pennsylvania, USA ABSTRACT Despite significant progress towards the standardization
CDASH MODEL 1.0 AND CDASHIG 2.0. Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams
 CDASH MODEL 1.0 AND CDASHIG 2.0 Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams 1 What is CDASH? Clinical Data Acquisition Standards Harmonization (CDASH) Standards for the collection
CDASH MODEL 1.0 AND CDASHIG 2.0 Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams 1 What is CDASH? Clinical Data Acquisition Standards Harmonization (CDASH) Standards for the collection
esource Initiative ISSUES RELATED TO NON-CRF DATA PRACTICES
 esource Initiative ISSUES RELATED TO NON-CRF DATA PRACTICES ISSUES RELATED TO NON-CRF DATA PRACTICES Introduction Non-Case Report Form (CRF) data are defined as data which include collection and transfer
esource Initiative ISSUES RELATED TO NON-CRF DATA PRACTICES ISSUES RELATED TO NON-CRF DATA PRACTICES Introduction Non-Case Report Form (CRF) data are defined as data which include collection and transfer
How to write ADaM specifications like a ninja.
 Poster PP06 How to write ADaM specifications like a ninja. Caroline Francis, Independent SAS & Standards Consultant, Torrevieja, Spain ABSTRACT To produce analysis datasets from CDISC Study Data Tabulation
Poster PP06 How to write ADaM specifications like a ninja. Caroline Francis, Independent SAS & Standards Consultant, Torrevieja, Spain ABSTRACT To produce analysis datasets from CDISC Study Data Tabulation
Implementing CDISC Using SAS. Full book available for purchase here.
 Implementing CDISC Using SAS. Full book available for purchase here. Contents About the Book... ix About the Authors... xv Chapter 1: Implementation Strategies... 1 The Case for Standards... 1 Which Models
Implementing CDISC Using SAS. Full book available for purchase here. Contents About the Book... ix About the Authors... xv Chapter 1: Implementation Strategies... 1 The Case for Standards... 1 Which Models
Pharmaceuticals, Health Care, and Life Sciences. An Approach to CDISC SDTM Implementation for Clinical Trials Data
 An Approach to CDISC SDTM Implementation for Clinical Trials Data William T. Chen, Merck Research Laboratories, Rahway, NJ Margaret M. Coughlin, Merck Research Laboratories, Rahway, NJ ABSTRACT The Clinical
An Approach to CDISC SDTM Implementation for Clinical Trials Data William T. Chen, Merck Research Laboratories, Rahway, NJ Margaret M. Coughlin, Merck Research Laboratories, Rahway, NJ ABSTRACT The Clinical
Submission-Ready Define.xml Files Using SAS Clinical Data Integration Melissa R. Martinez, SAS Institute, Cary, NC USA
 PharmaSUG 2016 - Paper SS12 Submission-Ready Define.xml Files Using SAS Clinical Data Integration Melissa R. Martinez, SAS Institute, Cary, NC USA ABSTRACT SAS Clinical Data Integration simplifies the
PharmaSUG 2016 - Paper SS12 Submission-Ready Define.xml Files Using SAS Clinical Data Integration Melissa R. Martinez, SAS Institute, Cary, NC USA ABSTRACT SAS Clinical Data Integration simplifies the
SAS Clinical Data Integration Server 2.1
 SAS Clinical Data Integration Server 2.1 User s Guide Preproduction Documentation THIS DOCUMENT IS A PREPRODUCTION DRAFT AND IS PROVIDED BY SAS INSTITUTE INC. ON AN AS IS BASIS WITHOUT WARRANTY OF ANY
SAS Clinical Data Integration Server 2.1 User s Guide Preproduction Documentation THIS DOCUMENT IS A PREPRODUCTION DRAFT AND IS PROVIDED BY SAS INSTITUTE INC. ON AN AS IS BASIS WITHOUT WARRANTY OF ANY
Harmonizing CDISC Data Standards across Companies: A Practical Overview with Examples
 PharmaSUG 2017 - Paper DS06 Harmonizing CDISC Data Standards across Companies: A Practical Overview with Examples Keith Shusterman, Chiltern; Prathima Surabhi, AstraZeneca; Binoy Varghese, Medimmune ABSTRACT
PharmaSUG 2017 - Paper DS06 Harmonizing CDISC Data Standards across Companies: A Practical Overview with Examples Keith Shusterman, Chiltern; Prathima Surabhi, AstraZeneca; Binoy Varghese, Medimmune ABSTRACT
SDTM Implementation Guide Clear as Mud: Strategies for Developing Consistent Company Standards
 Paper CD02 SDTM Implementation Guide Clear as Mud: Strategies for Developing Consistent Company Standards Brian Mabe, UCB Biosciences, Raleigh, USA ABSTRACT Many pharmaceutical companies are now entrenched
Paper CD02 SDTM Implementation Guide Clear as Mud: Strategies for Developing Consistent Company Standards Brian Mabe, UCB Biosciences, Raleigh, USA ABSTRACT Many pharmaceutical companies are now entrenched
Introduction to Define.xml
 Introduction to Define.xml Bay Area CDISC Implementation Network 4 April 2008 John Brega, PharmaStat LLC Presentation Objectives 1. Introduce the concept and purpose of define.xml 2. Introduce the published
Introduction to Define.xml Bay Area CDISC Implementation Network 4 April 2008 John Brega, PharmaStat LLC Presentation Objectives 1. Introduce the concept and purpose of define.xml 2. Introduce the published
SAS Application to Automate a Comprehensive Review of DEFINE and All of its Components
 PharmaSUG 2017 - Paper AD19 SAS Application to Automate a Comprehensive Review of DEFINE and All of its Components Walter Hufford, Vincent Guo, and Mijun Hu, Novartis Pharmaceuticals Corporation ABSTRACT
PharmaSUG 2017 - Paper AD19 SAS Application to Automate a Comprehensive Review of DEFINE and All of its Components Walter Hufford, Vincent Guo, and Mijun Hu, Novartis Pharmaceuticals Corporation ABSTRACT
Streamline SDTM Development and QC
 Paper SI09 Streamline SDTM Development and QC Stephen Gormley, Amgen, United Kingdom ABSTRACT Amgen s Global Statistical Programming ( GSP ) function have one centralised team (The CDISC Consultancy and
Paper SI09 Streamline SDTM Development and QC Stephen Gormley, Amgen, United Kingdom ABSTRACT Amgen s Global Statistical Programming ( GSP ) function have one centralised team (The CDISC Consultancy and
CDISC SDTM and ADaM Real World Issues
 CDISC SDTM and ADaM Real World Issues Washington DC CDISC Data Standards User Group Meeting Sy Truong President MXI, Meta-Xceed, Inc. http://www.meta-x.com Agenda CDISC SDTM and ADaM Fundamentals CDISC
CDISC SDTM and ADaM Real World Issues Washington DC CDISC Data Standards User Group Meeting Sy Truong President MXI, Meta-Xceed, Inc. http://www.meta-x.com Agenda CDISC SDTM and ADaM Fundamentals CDISC
SAS Clinical Data Integration 2.6
 SAS Clinical Data Integration 2.6 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2015. SAS Clinical Data Integration 2.6: User's Guide.
SAS Clinical Data Integration 2.6 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2015. SAS Clinical Data Integration 2.6: User's Guide.
Lex Jansen Octagon Research Solutions, Inc.
 Converting the define.xml to a Relational Database to Enable Printing and Validation Lex Jansen Octagon Research Solutions, Inc. Leading the Electronic Transformation of Clinical R&D * PharmaSUG 2009,
Converting the define.xml to a Relational Database to Enable Printing and Validation Lex Jansen Octagon Research Solutions, Inc. Leading the Electronic Transformation of Clinical R&D * PharmaSUG 2009,
ODM The Operational Efficiency Model: Using ODM to Deliver Proven Cost and Time Savings in Study Set-up
 ODM The Operational Efficiency Model: Using ODM to Deliver Proven Cost and Time Savings in Study Set-up Mark Wheeldon, CEO, Formedix Bay Area User Group Meeting, 15 th July 2010 Who are we? Proven Business
ODM The Operational Efficiency Model: Using ODM to Deliver Proven Cost and Time Savings in Study Set-up Mark Wheeldon, CEO, Formedix Bay Area User Group Meeting, 15 th July 2010 Who are we? Proven Business
Material covered in the Dec 2014 FDA Binding Guidances
 Accenture Accelerated R&D Services Rethink Reshape Restructure for better patient outcomes Sandra Minjoe Senior ADaM Consultant Preparing ADaM and Related Files for Submission Presentation Focus Material
Accenture Accelerated R&D Services Rethink Reshape Restructure for better patient outcomes Sandra Minjoe Senior ADaM Consultant Preparing ADaM and Related Files for Submission Presentation Focus Material
Advantages of a real end-to-end approach with CDISC standards
 Advantages of a real end-to-end approach with CDISC standards Dr. Philippe Verplancke CEO XClinical GmbH 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna Austria Disclaimer The views and opinions expressed
Advantages of a real end-to-end approach with CDISC standards Dr. Philippe Verplancke CEO XClinical GmbH 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna Austria Disclaimer The views and opinions expressed
Doctor's Prescription to Re-engineer Process of Pinnacle 21 Community Version Friendly ADaM Development
 PharmaSUG 2018 - Paper DS-15 Doctor's Prescription to Re-engineer Process of Pinnacle 21 Community Version Friendly ADaM Development Aakar Shah, Pfizer Inc; Tracy Sherman, Ephicacy Consulting Group, Inc.
PharmaSUG 2018 - Paper DS-15 Doctor's Prescription to Re-engineer Process of Pinnacle 21 Community Version Friendly ADaM Development Aakar Shah, Pfizer Inc; Tracy Sherman, Ephicacy Consulting Group, Inc.
An Efficient Solution to Efficacy ADaM Design and Implementation
 PharmaSUG 2017 - Paper AD05 An Efficient Solution to Efficacy ADaM Design and Implementation Chengxin Li, Pfizer Consumer Healthcare, Madison, NJ, USA Zhongwei Zhou, Pfizer Consumer Healthcare, Madison,
PharmaSUG 2017 - Paper AD05 An Efficient Solution to Efficacy ADaM Design and Implementation Chengxin Li, Pfizer Consumer Healthcare, Madison, NJ, USA Zhongwei Zhou, Pfizer Consumer Healthcare, Madison,
From ODM to SDTM: An End-to-End Approach Applied to Phase I Clinical Trials
 PhUSE 2014 Paper PP05 From ODM to SDTM: An End-to-End Approach Applied to Phase I Clinical Trials Alexandre Mathis, Department of Clinical Pharmacology, Actelion Pharmaceuticals Ltd., Allschwil, Switzerland
PhUSE 2014 Paper PP05 From ODM to SDTM: An End-to-End Approach Applied to Phase I Clinical Trials Alexandre Mathis, Department of Clinical Pharmacology, Actelion Pharmaceuticals Ltd., Allschwil, Switzerland
How to handle different versions of SDTM & DEFINE generation in a Single Study?
 Paper CD15 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai,
Paper CD15 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai,
Managing CDISC version changes: how & when to implement? Presented by Lauren Shinaberry, Project Manager Business & Decision Life Sciences
 1 Managing CDISC version changes: how & when to implement? Presented by Lauren Shinaberry, Project Manager Business & Decision Life Sciences 2 Content Standards Technical Standards SDTM v1.1 SDTM IG v3.1.1
1 Managing CDISC version changes: how & when to implement? Presented by Lauren Shinaberry, Project Manager Business & Decision Life Sciences 2 Content Standards Technical Standards SDTM v1.1 SDTM IG v3.1.1
Document, presentation and spreadsheet applications To support your business objectives. Why IBM Lotus Symphony? Why free?
 Document, presentation and spreadsheet applications To support your business objectives Why IBM Lotus Symphony? Why free? 2 Follow your IT budget follow the numbers Let s face it, in most organizations,
Document, presentation and spreadsheet applications To support your business objectives Why IBM Lotus Symphony? Why free? 2 Follow your IT budget follow the numbers Let s face it, in most organizations,
Programming checks: Reviewing the overall quality of the deliverables without parallel programming
 PharmaSUG 2016 Paper IB04 Programming checks: Reviewing the overall quality of the deliverables without parallel programming Shailendra Phadke, Baxalta US Inc., Cambridge MA Veronika Csom, Baxalta US Inc.,
PharmaSUG 2016 Paper IB04 Programming checks: Reviewing the overall quality of the deliverables without parallel programming Shailendra Phadke, Baxalta US Inc., Cambridge MA Veronika Csom, Baxalta US Inc.,
Helping The Define.xml User
 Paper TT01 Helping The Define.xml User Dave Iberson-Hurst, Assero Limited, Teignmouth, United Kingdom ABSTRACT The FDA often comment at industry gatherings on the quality of define.xml files received as
Paper TT01 Helping The Define.xml User Dave Iberson-Hurst, Assero Limited, Teignmouth, United Kingdom ABSTRACT The FDA often comment at industry gatherings on the quality of define.xml files received as
PhUSE EU Connect Paper PP15. Stop Copying CDISC Standards. Craig Parry, SyneQuaNon, Diss, England
 Paper PP15 Abstract Stop Copying CDISC Standards Craig Parry, SyneQuaNon, Diss, England We repeatedly see repositories which require a large amount of front loading, a lot of duplicating of the Clinical
Paper PP15 Abstract Stop Copying CDISC Standards Craig Parry, SyneQuaNon, Diss, England We repeatedly see repositories which require a large amount of front loading, a lot of duplicating of the Clinical
From Implementing CDISC Using SAS. Full book available for purchase here. About This Book... xi About The Authors... xvii Acknowledgments...
 From Implementing CDISC Using SAS. Full book available for purchase here. Contents About This Book... xi About The Authors... xvii Acknowledgments... xix Chapter 1: Implementation Strategies... 1 Why CDISC
From Implementing CDISC Using SAS. Full book available for purchase here. Contents About This Book... xi About The Authors... xvii Acknowledgments... xix Chapter 1: Implementation Strategies... 1 Why CDISC
Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai, India
 Paper CD15 PhUSE 2016 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences,
Paper CD15 PhUSE 2016 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences,
The Submission Data File System Automating the Creation of CDISC SDTM and ADaM Datasets
 Paper AD-08 The Submission Data File System Automating the Creation of CDISC SDTM and ADaM Datasets Marcus Bloom, Amgen Inc, Thousand Oaks, CA David Edwards, Amgen Inc, Thousand Oaks, CA ABSTRACT From
Paper AD-08 The Submission Data File System Automating the Creation of CDISC SDTM and ADaM Datasets Marcus Bloom, Amgen Inc, Thousand Oaks, CA David Edwards, Amgen Inc, Thousand Oaks, CA ABSTRACT From
Harnessing the Web to Streamline Statistical Programming Processes
 Paper TT03 Harnessing the Web to Streamline Statistical Programming Processes Daniel Boisvert, Biogen, Cambridge MA, USA Pankaj Kumar, Biogen, Cambridge MA, USA Gordon Schantz, Biogen, Cambridge MA, USA
Paper TT03 Harnessing the Web to Streamline Statistical Programming Processes Daniel Boisvert, Biogen, Cambridge MA, USA Pankaj Kumar, Biogen, Cambridge MA, USA Gordon Schantz, Biogen, Cambridge MA, USA
Legacy to SDTM Conversion Workshop: Tools and Techniques
 Legacy to SDTM Conversion Workshop: Tools and Techniques Mike Todd President Nth Analytics Legacy Data Old studies never die Legacy studies are often required for submissions or pharmacovigilence. Often
Legacy to SDTM Conversion Workshop: Tools and Techniques Mike Todd President Nth Analytics Legacy Data Old studies never die Legacy studies are often required for submissions or pharmacovigilence. Often
Sandra Minjoe, Accenture Life Sciences John Brega, PharmaStat. PharmaSUG Single Day Event San Francisco Bay Area
 Sandra Minjoe, Accenture Life Sciences John Brega, PharmaStat PharmaSUG Single Day Event San Francisco Bay Area 2015-02-10 What is the Computational Sciences Symposium? CSS originally formed to help FDA
Sandra Minjoe, Accenture Life Sciences John Brega, PharmaStat PharmaSUG Single Day Event San Francisco Bay Area 2015-02-10 What is the Computational Sciences Symposium? CSS originally formed to help FDA
Cloud Computing: Making the Right Choice for Your Organization
 Cloud Computing: Making the Right Choice for Your Organization A decade ago, cloud computing was on the leading edge. Now, 95 percent of businesses use cloud technology, and Gartner says that by 2020,
Cloud Computing: Making the Right Choice for Your Organization A decade ago, cloud computing was on the leading edge. Now, 95 percent of businesses use cloud technology, and Gartner says that by 2020,
SDTM Automation with Standard CRF Pages Taylor Markway, SCRI Development Innovations, Carrboro, NC
 PharmaSUG 2016 - Paper PO21 SDTM Automation with Standard CRF Pages Taylor Markway, SCRI Development Innovations, Carrboro, NC ABSTRACT Much has been written about automatically creating outputs for the
PharmaSUG 2016 - Paper PO21 SDTM Automation with Standard CRF Pages Taylor Markway, SCRI Development Innovations, Carrboro, NC ABSTRACT Much has been written about automatically creating outputs for the
CDASH Standards and EDC CRF Library. Guang-liang Wang September 18, Q3 DCDISC Meeting
 CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
PharmaSUG. companies. This paper. will cover how. processes, a fairly linear. before moving. be carried out. Lifecycle. established.
 PharmaSUG 2016 - Paper PO17 Standards Implementationn & Governance: Carrot or Stick? Julie Smiley, Akana, San Antonio, Texas Judith Goud, Akana, Bennekom, Netherlands ABSTRACT With the looming FDA mandate
PharmaSUG 2016 - Paper PO17 Standards Implementationn & Governance: Carrot or Stick? Julie Smiley, Akana, San Antonio, Texas Judith Goud, Akana, Bennekom, Netherlands ABSTRACT With the looming FDA mandate
define.xml: A Crash Course Frank DiIorio
 sponsor requests ODM extensions XSL-FO schema/xsd define.xml define.xml: A Crash Course metadata tables XML4Pharma metadata interface (the other) define.pdf ODM itext Frank DiIorio CodeCrafters, Inc. Philadelphia
sponsor requests ODM extensions XSL-FO schema/xsd define.xml define.xml: A Crash Course metadata tables XML4Pharma metadata interface (the other) define.pdf ODM itext Frank DiIorio CodeCrafters, Inc. Philadelphia
SDTM Attribute Checking Tool Ellen Xiao, Merck & Co., Inc., Rahway, NJ
 PharmaSUG2010 - Paper CC20 SDTM Attribute Checking Tool Ellen Xiao, Merck & Co., Inc., Rahway, NJ ABSTRACT Converting clinical data into CDISC SDTM format is a high priority of many pharmaceutical/biotech
PharmaSUG2010 - Paper CC20 SDTM Attribute Checking Tool Ellen Xiao, Merck & Co., Inc., Rahway, NJ ABSTRACT Converting clinical data into CDISC SDTM format is a high priority of many pharmaceutical/biotech
Tips on Creating a Strategy for a CDISC Submission Rajkumar Sharma, Nektar Therapeutics, San Francisco, CA
 PharmaSUG 2015 - Paper IB09 Tips on Creating a Strategy for a CDISC Submission Rajkumar Sharma, Nektar Therapeutics, San Francisco, CA ABSTRACT A submission to FDA for an NDA (New Drug Application) or
PharmaSUG 2015 - Paper IB09 Tips on Creating a Strategy for a CDISC Submission Rajkumar Sharma, Nektar Therapeutics, San Francisco, CA ABSTRACT A submission to FDA for an NDA (New Drug Application) or
Study Data Reviewer s Guide
 Revision History Date Study Data Reviewer s Guide Completion Guideline: Nonclinical (nnsdrg) Version Summary V1.1 03 March 2016 1.0 First Public Version: posted for Public Comment 1.1 Update from Public
Revision History Date Study Data Reviewer s Guide Completion Guideline: Nonclinical (nnsdrg) Version Summary V1.1 03 March 2016 1.0 First Public Version: posted for Public Comment 1.1 Update from Public
BUSINESS-BASED VALUE IN AN MDR
 MERCK METADATA REPOSITORY: BUSINESS-BASED VALUE IN AN MDR A. Brooke Hinkson Manori Turmel Karl Konrad PhUSE Connect Conference, Raleigh NC, 4-6 June 2018 2 Business Problems to Address Current information
MERCK METADATA REPOSITORY: BUSINESS-BASED VALUE IN AN MDR A. Brooke Hinkson Manori Turmel Karl Konrad PhUSE Connect Conference, Raleigh NC, 4-6 June 2018 2 Business Problems to Address Current information
Aligned Elements The professional Product Suite built to keep the Design History Files complete and consistent at all times, using minimal effort and
 Aligned Elements The professional Product Suite built to keep the Design History Files complete and consistent at all times, using minimal effort and tying up a minimum of resources Aligned Elements will
Aligned Elements The professional Product Suite built to keep the Design History Files complete and consistent at all times, using minimal effort and tying up a minimum of resources Aligned Elements will
PhUSE Giuseppe Di Monaco, UCB BioSciences GmbH, Monheim, Germany
 PhUSE 2014 Paper PP01 Reengineering a Standard process from Single to Environment Macro Management Giuseppe Di Monaco, UCB BioSciences GmbH, Monheim, Germany ABSTRACT Statistical programming departments
PhUSE 2014 Paper PP01 Reengineering a Standard process from Single to Environment Macro Management Giuseppe Di Monaco, UCB BioSciences GmbH, Monheim, Germany ABSTRACT Statistical programming departments
Click4Assistance - Features List. Important Extras. Chat Facilities. UK Based Support. Help & Advice. Branding / Customisation.
 Important Extras UK Based Support Help & Advice Branding / Customisation Developed, Supported and Located in the UK. Speak to your own dedicated account manager based in our UK offices. Let our experienced
Important Extras UK Based Support Help & Advice Branding / Customisation Developed, Supported and Located in the UK. Speak to your own dedicated account manager based in our UK offices. Let our experienced
The main website for Henrico County, henrico.us, received a complete visual and structural
 Page 1 1. Program Overview The main website for Henrico County, henrico.us, received a complete visual and structural overhaul, which was completed in May of 2016. The goal of the project was to update
Page 1 1. Program Overview The main website for Henrico County, henrico.us, received a complete visual and structural overhaul, which was completed in May of 2016. The goal of the project was to update
Planning to Pool SDTM by Creating and Maintaining a Sponsor-Specific Controlled Terminology Database
 PharmaSUG 2017 - Paper DS13 Planning to Pool SDTM by Creating and Maintaining a Sponsor-Specific Controlled Terminology Database ABSTRACT Cori Kramer, Ragini Hari, Keith Shusterman, Chiltern When SDTM
PharmaSUG 2017 - Paper DS13 Planning to Pool SDTM by Creating and Maintaining a Sponsor-Specific Controlled Terminology Database ABSTRACT Cori Kramer, Ragini Hari, Keith Shusterman, Chiltern When SDTM
Examining Rescue Studies
 White Paper Examining Rescue Studies Introduction The purpose of this White Paper is to define a Rescue Study, outline the basic assumptions, including risks, in setting up such a trial based on DATATRAK
White Paper Examining Rescue Studies Introduction The purpose of this White Paper is to define a Rescue Study, outline the basic assumptions, including risks, in setting up such a trial based on DATATRAK
CDISC Migra+on. PhUSE 2010 Berlin. 47 of the top 50 biopharmaceu+cal firms use Cytel sofware to design, simulate and analyze their clinical studies.
 CDISC Migra+on PhUSE 2010 Berlin 47 of the top 50 biopharmaceu+cal firms use Cytel sofware to design, simulate and analyze their clinical studies. Source: The Pharm Exec 50 the world s top 50 pharmaceutical
CDISC Migra+on PhUSE 2010 Berlin 47 of the top 50 biopharmaceu+cal firms use Cytel sofware to design, simulate and analyze their clinical studies. Source: The Pharm Exec 50 the world s top 50 pharmaceutical
Data Consistency and Quality Issues in SEND Datasets
 Data Consistency and Quality Issues in SEND Datasets PointCross has reviewed numerous SEND datasets prepared for test submissions to the FDA and has worked with the FDA on their KickStart program to ensure
Data Consistency and Quality Issues in SEND Datasets PointCross has reviewed numerous SEND datasets prepared for test submissions to the FDA and has worked with the FDA on their KickStart program to ensure
CDISC Standards End-to-End: Enabling QbD in Data Management Sam Hume
 CDISC Standards End-to-End: Enabling QbD in Data Management Sam Hume 1 Shared Health and Research Electronic Library (SHARE) A global electronic repository for developing, integrating
CDISC Standards End-to-End: Enabling QbD in Data Management Sam Hume 1 Shared Health and Research Electronic Library (SHARE) A global electronic repository for developing, integrating
Product Features. Web-based e-learning Authoring
 Web-based e-learning Authoring Product Features Composica Enterprise is an advanced web-based e-learning authoring system offering high flexibility and an abundance of features to collaboratively create
Web-based e-learning Authoring Product Features Composica Enterprise is an advanced web-based e-learning authoring system offering high flexibility and an abundance of features to collaboratively create
How to clean up dirty data in Patient reported outcomes
 Paper DH02 How to clean up dirty data in Patient reported outcomes Knut Mueller, UCB Schwarz Biosciences, Monheim, Germany ABSTRACT The current FDA Guidance for Industry - Patient Reported Outcome Measures
Paper DH02 How to clean up dirty data in Patient reported outcomes Knut Mueller, UCB Schwarz Biosciences, Monheim, Germany ABSTRACT The current FDA Guidance for Industry - Patient Reported Outcome Measures
Creating Define-XML v2 with the SAS Clinical Standards Toolkit 1.6 Lex Jansen, SAS
 Creating Define-XML v2 with the SAS Clinical Standards Toolkit 1.6 Lex Jansen, SAS Agenda Introduction to the SAS Clinical Standards Toolkit (CST) Define-XML History and Background What is Define-XML?
Creating Define-XML v2 with the SAS Clinical Standards Toolkit 1.6 Lex Jansen, SAS Agenda Introduction to the SAS Clinical Standards Toolkit (CST) Define-XML History and Background What is Define-XML?
It s All About Getting the Source and Codelist Implementation Right for ADaM Define.xml v2.0
 PharmaSUG 2018 - Paper SS-15 It s All About Getting the Source and Codelist Implementation Right for ADaM Define.xml v2.0 ABSTRACT Supriya Davuluri, PPD, LLC, Morrisville, NC There are some obvious challenges
PharmaSUG 2018 - Paper SS-15 It s All About Getting the Source and Codelist Implementation Right for ADaM Define.xml v2.0 ABSTRACT Supriya Davuluri, PPD, LLC, Morrisville, NC There are some obvious challenges
The Benefits of Traceability Beyond Just From SDTM to ADaM in CDISC Standards Maggie Ci Jiang, Teva Pharmaceuticals, Great Valley, PA
 PharmaSUG 2017 - Paper DS23 The Benefits of Traceability Beyond Just From SDTM to ADaM in CDISC Standards Maggie Ci Jiang, Teva Pharmaceuticals, Great Valley, PA ABSTRACT Since FDA released the Analysis
PharmaSUG 2017 - Paper DS23 The Benefits of Traceability Beyond Just From SDTM to ADaM in CDISC Standards Maggie Ci Jiang, Teva Pharmaceuticals, Great Valley, PA ABSTRACT Since FDA released the Analysis
Standardising The Standards The Benefits of Consistency
 Paper DH06 Standardising The Standards The Benefits of Consistency Nathan James, Roche Products Ltd., Welwyn Garden City, UK ABSTRACT The introduction of the Study Data Tabulation Model (SDTM) has had
Paper DH06 Standardising The Standards The Benefits of Consistency Nathan James, Roche Products Ltd., Welwyn Garden City, UK ABSTRACT The introduction of the Study Data Tabulation Model (SDTM) has had
SDD Unleashed - Extending the Capabilities of SAS Drug Development
 SDD Unleashed - Extending the Capabilities of SAS Drug Development Stephen Baker d-wise Technologies Raleigh, NC ABSTRACT SAS Drug Development is well known as a compliant, hosted repository for storing
SDD Unleashed - Extending the Capabilities of SAS Drug Development Stephen Baker d-wise Technologies Raleigh, NC ABSTRACT SAS Drug Development is well known as a compliant, hosted repository for storing
Standardizing FDA Data to Improve Success in Pediatric Drug Development
 Paper RA01 Standardizing FDA Data to Improve Success in Pediatric Drug Development Case Study: Harmonizing Hypertensive Pediatric Data across Sponsors using SAS and the CDISC Model Julie Maddox, SAS Institute,
Paper RA01 Standardizing FDA Data to Improve Success in Pediatric Drug Development Case Study: Harmonizing Hypertensive Pediatric Data across Sponsors using SAS and the CDISC Model Julie Maddox, SAS Institute,
PhUSE Paper SD09. "Overnight" Conversion to SDTM Datasets Ready for SDTM Submission Niels Mathiesen, mathiesen & mathiesen, Basel, Switzerland
 Paper SD09 "Overnight" Conversion to SDTM Datasets Ready for SDTM Submission Niels Mathiesen, mathiesen & mathiesen, Basel, Switzerland ABSTRACT This demonstration shows how legacy data (in any format)
Paper SD09 "Overnight" Conversion to SDTM Datasets Ready for SDTM Submission Niels Mathiesen, mathiesen & mathiesen, Basel, Switzerland ABSTRACT This demonstration shows how legacy data (in any format)
Converting Data to the SDTM Standard Using SAS Data Integration Studio
 Paper RA01 Converting Data to the SDTM Standard Using SAS Data Integration Studio Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT The CDISC Study Data Tabulation Model (SDTM) is becoming
Paper RA01 Converting Data to the SDTM Standard Using SAS Data Integration Studio Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT The CDISC Study Data Tabulation Model (SDTM) is becoming
Paper DS07 PhUSE 2017 CDISC Transport Standards - A Glance. Giri Balasubramanian, PRA Health Sciences Edwin Ponraj Thangarajan, PRA Health Sciences
 Paper DS07 PhUSE 2017 CDISC Transport Standards - A Glance Giri Balasubramanian, PRA Health Sciences Edwin Ponraj Thangarajan, PRA Health Sciences Agenda Paper Abstract CDISC Standards Types Why Transport
Paper DS07 PhUSE 2017 CDISC Transport Standards - A Glance Giri Balasubramanian, PRA Health Sciences Edwin Ponraj Thangarajan, PRA Health Sciences Agenda Paper Abstract CDISC Standards Types Why Transport
ebook library PAGE 1 HOW TO OPTIMIZE TRANSLATIONS AND ACCELERATE TIME TO MARKET
 ebook library PAGE 1 HOW TO OPTIMIZE TRANSLATIONS AND ACCELERATE TIME TO MARKET Aligning people, process and technology to improve quality and speed to market To succeed in the global business arena, companies
ebook library PAGE 1 HOW TO OPTIMIZE TRANSLATIONS AND ACCELERATE TIME TO MARKET Aligning people, process and technology to improve quality and speed to market To succeed in the global business arena, companies
Paper FC02. SDTM, Plus or Minus. Barry R. Cohen, Octagon Research Solutions, Wayne, PA
 Paper FC02 SDTM, Plus or Minus Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT The CDISC Study Data Tabulation Model (SDTM) has become the industry standard for the regulatory submission
Paper FC02 SDTM, Plus or Minus Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT The CDISC Study Data Tabulation Model (SDTM) has become the industry standard for the regulatory submission
Migrating NetBackUp Data to the Commvault Data Platform
 Migrating NetBackUp Data to the Commvault Data Platform LEGACY MIGRATION OVERVIEW Legacy backup data migrations are typically perceived to be high-cost, take a long time to complete, and prone to error
Migrating NetBackUp Data to the Commvault Data Platform LEGACY MIGRATION OVERVIEW Legacy backup data migrations are typically perceived to be high-cost, take a long time to complete, and prone to error
SDTM-ETL 3.2 User Manual and Tutorial
 SDTM-ETL 3.2 User Manual and Tutorial Author: Jozef Aerts, XML4Pharma Last update: 2017-07-29 Adding mappings for the Vital Signs domain After loading and validating the ODM file with metadata, your load
SDTM-ETL 3.2 User Manual and Tutorial Author: Jozef Aerts, XML4Pharma Last update: 2017-07-29 Adding mappings for the Vital Signs domain After loading and validating the ODM file with metadata, your load
A smart and simple solution to improve personal productivity
 Personal and Co l l a b o r a t i v e Workspace Solution A smart and simple solution to improve personal productivity The combination of Canon s iw Document Manager software, range of Multi-Function Devices,
Personal and Co l l a b o r a t i v e Workspace Solution A smart and simple solution to improve personal productivity The combination of Canon s iw Document Manager software, range of Multi-Function Devices,
Cost-Benefit Analysis of Retrospective vs. Prospective Data Standardization
 Cost-Benefit Analysis of Retrospective vs. Prospective Data Standardization Vicki Seyfert-Margolis, PhD Senior Advisor, Science Innovation and Policy Food and Drug Administration IOM Sharing Clinical Research
Cost-Benefit Analysis of Retrospective vs. Prospective Data Standardization Vicki Seyfert-Margolis, PhD Senior Advisor, Science Innovation and Policy Food and Drug Administration IOM Sharing Clinical Research
embedded hmi system zenon Operator Make the best out of your plant: Easy and intuitive handling, secure operation and ergonomic control
 embedded hmi system Make the best out of your plant: Easy and intuitive handling, secure operation and ergonomic control zenon everything under control from sensor to erp zenon Supervisor Independent SCADA
embedded hmi system Make the best out of your plant: Easy and intuitive handling, secure operation and ergonomic control zenon everything under control from sensor to erp zenon Supervisor Independent SCADA
An introduction to Headless Content Management Systems
 WHITEPAPER An introduction to Headless Content Management Systems John Winter, Co-Founder, Content Bloom Introduction Surfing web content used to be limited to desktop computers. This has drastically changed
WHITEPAPER An introduction to Headless Content Management Systems John Winter, Co-Founder, Content Bloom Introduction Surfing web content used to be limited to desktop computers. This has drastically changed
Xyleme Studio Data Sheet
 XYLEME STUDIO DATA SHEET Xyleme Studio Data Sheet Rapid Single-Source Content Development Xyleme allows you to streamline and scale your content strategy while dramatically reducing the time to market
XYLEME STUDIO DATA SHEET Xyleme Studio Data Sheet Rapid Single-Source Content Development Xyleme allows you to streamline and scale your content strategy while dramatically reducing the time to market
Clinical Data Model and FDA Submissions
 Clinical Data Model and FDA Submissions Shy Kumar, Datafarm, Inc., Marlboro, MA Gajanan Bhat, Boston Scientific Corporation, Natick, MA ABSTRACT Development of data model in clinical trial management environment
Clinical Data Model and FDA Submissions Shy Kumar, Datafarm, Inc., Marlboro, MA Gajanan Bhat, Boston Scientific Corporation, Natick, MA ABSTRACT Development of data model in clinical trial management environment
From SDTM to displays, through ADaM & Analyses Results Metadata, a flight on board METADATA Airlines
 From SDTM to displays, through ADaM & Analyses Results Metadata, a flight on board METADATA Airlines Omar SEFIANI - Stéphane BOUGET, Boehringer Ingelheim DH13, PhUSE Barcelona 2016, October, 12 th Outline
From SDTM to displays, through ADaM & Analyses Results Metadata, a flight on board METADATA Airlines Omar SEFIANI - Stéphane BOUGET, Boehringer Ingelheim DH13, PhUSE Barcelona 2016, October, 12 th Outline
Content. 4 What We Offer. 5 Our Values. 6 JAMS: A Complete Submission System. 7 The Process. 7 Customizable Editorial Process. 8 Author Submission
 Content 4 What We Offer 5 Our Values 6 JAMS: A Complete Submission System 7 The Process 7 Customizable Editorial Process 8 Author Submission 9 In-Built Email Templates 10 Editor Decision 11 Production
Content 4 What We Offer 5 Our Values 6 JAMS: A Complete Submission System 7 The Process 7 Customizable Editorial Process 8 Author Submission 9 In-Built Email Templates 10 Editor Decision 11 Production
pre- & post-processing f o r p o w e r t r a i n
 pre- & post-processing f o r p o w e r t r a i n www.beta-cae.com With its complete solutions for meshing, assembly, contacts definition and boundary conditions setup, ANSA becomes the most efficient and
pre- & post-processing f o r p o w e r t r a i n www.beta-cae.com With its complete solutions for meshing, assembly, contacts definition and boundary conditions setup, ANSA becomes the most efficient and
A Macro to replace PROC REPORT!?
 Paper TS03 A Macro to replace PROC REPORT!? Katja Glass, Bayer Pharma AG, Berlin, Germany ABSTRACT Some companies have macros for everything. But is that really required? Our company even has a macro to
Paper TS03 A Macro to replace PROC REPORT!? Katja Glass, Bayer Pharma AG, Berlin, Germany ABSTRACT Some companies have macros for everything. But is that really required? Our company even has a macro to
Making your information system simple again
 Making your information system simple again Critical applications: the key to your business success Bringing together an organization s core expertise, its most sensitive data, and critical applications
Making your information system simple again Critical applications: the key to your business success Bringing together an organization s core expertise, its most sensitive data, and critical applications
HABS1 Business Suite on HANA
 Business Suite on HANA SAP HANA Course Version: 08 Course Duration: 3 Day(s) Publication Date: 2014 Publication Time: Copyright Copyright SAP AG. All rights reserved. No part of this publication may be
Business Suite on HANA SAP HANA Course Version: 08 Course Duration: 3 Day(s) Publication Date: 2014 Publication Time: Copyright Copyright SAP AG. All rights reserved. No part of this publication may be
Secure Messaging as a Service
 Security Infrastructure Management the way we do it Secure Messaging as a Service in collaboration with Insert partner logo Messaging as a Service (MaaS) In today s complex technological society, diversity
Security Infrastructure Management the way we do it Secure Messaging as a Service in collaboration with Insert partner logo Messaging as a Service (MaaS) In today s complex technological society, diversity
User Guide 16-Mar-2018
 16-Mar-2018 This document is freely distributable Identification Authors Name Organization Title TMF User Guide and Implementation Guide Sub-Team Version History Version Steering Committee Approval Date
16-Mar-2018 This document is freely distributable Identification Authors Name Organization Title TMF User Guide and Implementation Guide Sub-Team Version History Version Steering Committee Approval Date
