An informatics framework for testing data integrity and correctness of federated biomedical databases.
|
|
|
- Valentine White
- 6 years ago
- Views:
Transcription
1 An informatics framework for testing data integrity and correctness of federated biomedical databases. Mijung Kim, Georgia Institute of Technology Tahsin Kurc, Emory University Alessandro Orso, Georgia Institute of Technology Jake Cobb, Georgia Institute of Technology David Gutman, Emory University Mary Jean Harrold, Georgia Institute of Technology Andrew Post, Emory University Ashish Sharma, Emory University Joel Saltz, Georgia Institute of Technology Journal Title: AMIA Joint Summits on Translational Science Proceedings Volume: Volume 2011 Publisher: American Medical Informatics Association 2011, Pages Type of Work: Article Final Publisher PDF Permanent URL: Copyright information: 2011 AMIA. This is an Open Access article: verbatim copying and redistribution of this article are permitted in all media for any purpose. Accessed January 29, :36 AM EST
2 An Informatics Framework for Testing Data Integrity and Correctness of Federated Biomedical Databases Mijung Kim, 1 Tahsin Kurc, 2 Alessandro Orso, 1 Jake Cobb, 1 David Gutman 2, Mary Jean Harrold, 1 Andrew Post, 2 Ashish Sharma, 2 Joel Saltz 2 1 College of Computing, Georgia Institute of Technology, Atlanta, GA 2 Center for Comprehensive Informatics, Emory University, Atlanta, GA Abstract Clinical research is increasingly relying on information gathered and managed in different database systems and institutions. Distributed data collection and management processes in such settings can be extremely complex and lead to a range of issues involving the integrity and accuracy of the distributed data. To address this challenge, we propose a middleware framework for assessing the data integrity and correctness in federated environments. The framework has two main elements: (1) a test model describing the dependencies between and constraints on data sources and datasets, and (2) a family of testing techniques that create and execute test cases based on the model. 1. Introduction A growing number of studies gather and manage information from multiple data types and sources. Logistics, regulations, and data-analysis requirements may not always allow for centralized data gathering and management. Data may be collected from patients recruited at multiple institutions. Even within an institution, data may be collected and processed by different laboratories because of instrumentation and analysis requirements. Federated systems have been developed and employed [1,2,3,4] for these types of studies to support distributed data access and analysis requirements. An important component that is missing in most existing systems is a middleware framework for testing the data integrity and correct operation of a federated environment. Data sources in a federated environment change over time data management systems are modified, data models and ontologies are changed, new datasets are gathered, and existing datasets are updated. Federated databases are often managed by different groups; a group may modify their database without informing other groups, causing inconsistencies and breaking dependencies within the federated environment. Errors may arise from both human mistakes and faults in the software. For example, updates to data may introduce hard-to-detect errors. Indeed, such errors occurred in one of our studies, when a subset of the clinical database we accessed remotely was updated erroneously, replacing old diagnosis values with new values that did not match the known progression of the disease. Detecting and tracking this and other types of errors (Section 2 describes additional examples of errors) manually in a federated environment is impractical, and their presence can seriously compromise the results of a clinical research project. Some databases and ETL (Extract, Translate, Load) processes implement data quality and error checks. In practice, however, most implementations are done as one-off solutions via low-level scripts and programs, which can be difficult to extend or modify for new datasets and additional tests. Moreover, these implementations are targeted at a single instance of a resource and are not designed to test a federated environment. Our goal is to address these challenges by developing and evaluating a framework that can support systematic testing of the data integrity and correct operation of federated environments. Our framework has two main elements: a test model, representing constraints on and dependencies between datasets and data sources in the federated environment, and a family of testing techniques that leverage the model to test data integrity and accuracy of the environment. The test model is a set of rules derived from (1) data models of individual data sources and constraints expressed in the data models, (2) relationships among different data models and data sources, (3) business processes (e.g., study protocols), (4) user-defined rules and constraints, and (5) rules and constraints based on domain knowledge. The testing techniques are driven by the test model and assess the federated environment by (1) identifying relevant test scenarios for the environment, (2) creating test cases that realize such scenarios, (3) generating (when needed) suitably tagged synthetic data to enable the scenarios, and (4) executing the generated test cases. 2. Motivation and Objective Our effort is motivated mainly by translational research projects supported by the Atlanta Clinical and Translational Science Institute (ACTSI), a multiinstitutional partnership funded by the NIH Clinical and Translational Science Awards program. A common theme among a wide range of studies undertaken by ACTSI investigators is that biomedical data are captured at multiple locations and stored in different systems (e.g., multiple i2b2 [5] instances) hosted by 22
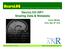
3 partnering institutions. It is worth noting that many of the research scenarios supported within ACTSI are common use cases in other clinical research efforts. To illustrate the issues we target, we consider a specific example: the study of brain tumors conducted in the In Silico Brain Tumor Research Center (ISBTRC) 1 through in silico experiments on data collected from a group of patients. Datasets for this study encompass high-throughput omics data, radiology and pathology image data, clinical data, such as diagnosis and survival, and tissue data. The datasets are obtained from public databases (e.g., Rembrandt 2 and The Cancer Genome Atlas 3 ), derived from primary datasets (e.g., image analysis results), and collected from subjects at the collaborating institutions. In the course of the study, the primary datasets from public resources are downloaded to local clinical, imaging, and genomic databases for further analysis and cyclically updated to include new data. These datasets are expected to have gene expression, microarray, mrna, and mirna data, radiology and high-power light microscopy image data, and clinical diagnosis and survival outcome for each patient. For subjects at the collaborating institutions, tissue samples are collected following the study protocol. Microscopy image data are obtained from the tissue samples; two modalities of microscopy image data are captured at Emory (brightfield microscopy images by a pathology imaging group for every subject and quantum dot immunohistochemistry images by a nanotechnology center for some subjects). Each subject s de-identified clinical information is maintained in a clinical datamanagement system. Gene expression datasets are stored in a molecular database, whereas imaging data are managed in radiology and microscopy imaging databases. The primary datasets are analyzed using a suite of analysis algorithms and by human experts (in the case of image data). The omics analysis results are stored in a molecular database, whereas the image analysis results are stored in image markup and annotation databases. The data gathering and management processes in this study are complex and error prone, as they involve multiple points of data acquisition/generation and multiple data management systems. There are multiple sources of error. For example, in one case, an image was accidentally deleted, leaving analysis results obtained from that image unlinked to any image data. Errors may also arise due to incorrect identifiers and foreign-key relationships across different data generation points and systems. Different institutions and systems maintain local identifiers (e.g., de identified specimen, patient, image identifiers), which need to be mapped to appropriate global identifiers and stored so that related data products can be linked and queried correctly. This is an error prone process. If a system in the environment does not support such identifiers correctly, or mapping scripts are implemented incorrectly, join queries involving that system will return incorrect results. As we mentioned in the Introduction, updates to data may introduce hard-to-detect errors. An erroneous update to some of the clinical data in our study, for instance, resulted in incorrect diagnosis values that conflicted with the expected progression of the disease. This is an example of the kind of domain knowledge that a testing framework should incorporate and check. As a final example, a time consuming inspection of the datasets from the public repositories revealed that some of the required data for some subjects were missing (e.g., image data). The TCGA study included a set of manual annotations generated by a set of neuropathologists; this process depended on the availability of digitized glass slides. In an early in silico experiment, we sought to replicate the results generated by the neuropathologists using a set of customdeveloped computer algorithms. We eventually discovered that not all images for slides used in the manual annotations were available for download on the TCGA website, which led to an extremely labor intensive process to track down additional slides. Our framework would be able to capture such a discrepancy early in the process, by checking for the presence of data based on the study protocol requirements, and notify the users of the issue. The goal of our testing framework is to be able to detect and report these types of errors using a suite of techniques that combine domain knowledge, modeling, and software testing methods. 3. Approach Figure 1 provides a depiction of our framework, which consists of a set of testing techniques and a test model that are tightly integrated. In addition, it contains a data-mining element, which can provide support for extracting rules from the federated environment. In this framework, test cases are run both offline, before deployment, and online, to monitor whether changes to the environment violate any of the dependencies or constraints encoded in the identified rules Test Model The test model is used to provide a specification of the expected correct state and function of a federated environment. It describes constraints, dependencies, and relationships imposed on data and resources. Our approach draws from the principles and practices 23
4 is the same as Attribute Y in Database B, encoded as havesamevalue(db_a,attrx,db_b,attry), could be created to express that the two attributes should have the same value. A third aspect of information semantics is the incorporation of domain knowledge. As our understanding of biological systems and diseases progresses, certain relationships, hierarchies, and axioms are deemed as domain facts and incorporated into ontologies representing domain knowledge. The test model makes use of domain ontologies to express rules Figure 1: Intuitive view of the proposed framework. that describe constraints and relationships derived from the domain knowledge. For instance, a domain ontology may define that Stage II always follows Stage I, and Stage I never follows Stage II for disease Z,. The test model then contains the rule t1>t2 DiseaseZ.Stage[t1]<DiseaseZ.Stage[t2], where t1 and t2 indicate timestamps. implemented in frameworks for data and systems interoperability [6]. The key components of these frameworks are system specifications (based on static, functional, and behavioral semantics), conformance statements, and conformance assertions. Conformance statements are derived from specifications, and conformance assertions are evaluated against the conformance statements to assess the level of interoperability between systems. The test model in our case becomes a key to system specifications and statements expressed as a set of properties and rules in a semantic language (OWL and SWRL [7,8]). Testing techniques evaluate conformance assertions against the specifications and statements. We employ the notions of information, functional, and process semantics to create the test model for a given federated environment Information Semantics Information semantics represent the set of properties and rules that can be derived from data models in individual databases and constraints associated with the data models. The first aspect of information semantics is the use of value domains and value sets to express permissible and non-permissible values for data elements. This aspect can be used to create rules to express data value constraints. For example, if a data attribute maintains values on a patient s height, it may have been assigned a value set of [1,8] feet. In that case, the test model could include the following corresponding set of rules: AttrX.domain=numerical; AttrX.unit=foot; AttrX.range =[1,8]. Another relevant aspect of information semantics is the description of relationships between data models within and across databases. In our framework, this aspect results in rules that describe how data elements in different databases are related, whether they are used to store semantically equivalent data, and so on. For example, a rule stating that Attribute X in Database A Functional Semantics Functional semantics describe the expected behavior of a system (e.g., error conditions, returns on successful invocation of the system) when it is interacted with. In our case, functional semantics are used to define rules on the behavior of the system for (1) data loads, updates, and deletes, (2) successful query executions, and (3) error conditions. For instance, if new object identifiers should be generated, when new data objects are loaded to a system, a rule expressing which identifiers should or should not be generated can be defined as part of the test model Process Semantics Process semantics describe business processes and relationships between databases and systems that are not part of information and functional semantics. For example, in the study described in Section 2, the study protocol corresponds to the business processes. The protocol describes which data should be obtained for each patient and how many instances of a given data type should be gathered. The process semantics of acquiring microscopy imaging data can lead to a rule stating that an image data item for the in silico brain tumor study must be of type InSilicoImageData, have two bright-field H&E whole-slide images, and have two quantum-dot images. (For clarity, we show these rules in natural language.) Another rule may combine the image data with other data types for the brain tumor study: A dataset for the in-silico brain tumor study must contain data items of type InSilicoImageData, patient survival data, sequence data, and mrna data. 24
5 This set of rules can be used to evaluate if the necessary data elements have been collected for a subject in the brain tumor study. In our framework, process semantics is also employed to derive userdefined rules on dependencies and relationships among two or more databases. For instance, a rule can be defined to specify that: if there are image-annotation results in the image-annotations database, there must be a corresponding image in the image database. We have identified these three components for test model creation based on our experience with several use cases. Our main goal with the test model is to provide a high-level mechanism for users and developers to represent constraints, dependencies, and relationships. Nevertheless, we are building the testing system such that application-specific test scripts can be executed for cases the test model is not sufficient. We plan to examine automated mechanisms and data mining to create rules from data models, datasets, and domain knowledge. Some rules in the test model can automatically be derived from the data models. For example, schema constraints on associations could be used to describe rules on such associations. In data models where data elements are semantically annotated, and associated with value sets for permissible or non-permissible values, rules could be created on such value sets to describe constraints on the data elements. Data mining may also be applied to identify strong correlations among the data that can indicate the presence of latent rules. Such inferred rules may be used to identify anomalies in the data -- data mining was successfully used to this end in previous work (e.g., [11,12]) Testing Techniques Our main goal is to assess the integrity and accuracy of the environment s data sources and datasets with respect to the rules in the test model. In this section, we describe how our approach accomplishes this goal and illustrate its application on some of the examples from Section Test Generation and Execution Tests are typically defined according to a given set of requirements (e.g., coverage of some code elements). In our case, the key aspects to be tested are the data elements and the rules defined over them. Thus, the starting point for our approach is the test model. More precisely, our approach analyzes the test model to identify relevant data elements, discover the rules involving such elements, and generate testing requirements (and ultimately tests) based on such rules. The specific kind of tests generated depends on the type of rule being analyzed. Consider, for instance, the rules presented in Section The analysis of rule havesamevalue(db_a,attrx,db_b,attry) would result in offline tests that check that for corresponding elements a and b in Databases A and B, respectively, a.x and b.y must have the same value. (The specific number of tests generated would depend on the amount of resources available for testing.) The framework can instantiate these tests and run them on the databases automatically, so as to identify and report violation of this rule. Another example is provided by rule t1>t2 DiseaseZ.Stage[t1]<DiseaseZ.Stage[t2]. In this case, the analysis of the rule would result in a set of online tests that would be implemented as runtime monitors deployed on the federated datasets. The monitors would be triggered by changes that affect the value of one or more DiseaseZ.Stage fields, would check that the new value of the fields is greater than the old values, and report a problem if this is not the case Data Generation In some cases, real datasets may be inadequate and prevent some of the tests from being run. For example, a test that checks whether n types of data collected from m different databases are aggregated correctly may need data of exactly the right types in the different databases involved, and this data may not be present. Moreover, for some more sophisticated tests, data with a specific distribution may be needed. Finally, in cases where an accurate test oracle is defined, differential testing may be needed to assess the outcome of a test. When generating synthetic data sets, the data must typically have characteristics representative of real data and cannot be simply randomly generated. We will leverage existing techniques for generating meaningful, valid synthetic data (e.g., [9,10]) and will extend them to support distributed data sources. Our framework will use information about relationships and dependencies among database schemas, generate synthetic datasets according to this information and on the generated tests, and populate data across the distributed databases. As an example of this scenario, consider another rule from Section 3.1: AttrX.domain=numerical; AttrX.unit=foot; AttrX.range =[1,8]. To execute a test that targets this rule, the framework may need to add to a database suitably tagged synthetic patients, whose AttrX s value violates the domain, unit, or range constraints. 4. Current State of Testing Framework We are in the process of implementing a prototype of our framework. Presently, we are building a brain tumor translational informatics test bed that contains a wide range of real data from the brain tumor study described in Section 2. The datasets come from the TCGA 3, Rembrandt 2, and NBIA 4 data repositories and 4 National Biomedical Imaging Archive 25
6 from Emory University, Thomas Jefferson University, and Henry Ford Hospital (three of the collaborating institutions in ISBTRC 1 ). Table 1 lists the datasets and data management systems for the test bed. The data management systems will be deployed on a set of virtual machines for easier portability and distributed deployment. The test bed will allow us to create scenarios for various types of errors, such as incorrect mappings from local identifiers to global identifiers (i.e., foreign key relationships the molecular, imaging, and clinical data stored in different systems should be linked through subject and/or specimen identifiers), missing data with respect to the study protocol (e.g., missing whole slide images for some patients), and updates to invariant data elements (e.g., disease diagnoses, dates of death). In parallel, we are incrementally building our test model by defining rules for properties, constraints, and relationships among the databases in the study. We have defined several rules using OWL and SWRL for immutable data elements, the study protocol, and foreign key relationships. Our test bed will eventually provide the end users with (1) a set of test cases that are automatically generated using the test model, (2) a method to automatically execute the test cases, and (3) a report of the test outcome for users. 5. Conclusion Testing is often overlooked in federated settings and accomplished via one-off implementations. An integrated middleware framework can provide a costeffective solution to this problem. Such a framework should enable researchers and database administrators to: (1) specify a description of the correct state and function of the system as a set of rules expressing dependencies, relationships, and constraints on data sources and datasets; and (2) create, based on the identified set of rules, relevant test scenarios for the federated environment, test cases that realize such scenarios, and suitably tagged synthetic data, when the existing data are not sufficient. Type of Dataset Radiology images in DICOM format, imaging metadata Manual annotations provided by neuroradiologists Neuroimaging Data Data Management System Virtual PACS 5, xnat 6 AIME 5,7 Molecular Data mrna, mirna, methylation data, copy number, sequence data in-house developed database with file system for data files extensible Neuroimaging Archive Toolkit, 7 Annotation and Image Markup, Clinical data (including days to death, diagnosis, year of initial pathologic diagnosis), specimen (e.g., sample type), etc., data Clinical Data Pathology Data Whole slide microscopy images as 20x and 40x magnification, image metadata Computer- and human-generated annotations of pathology images i2b2 8, in-house developed database camicroscope 9 PAIS 10 Table 1: Datasets and data management systems in the test bed. Acknowledgements. Partially funded by: Federal funds from the National Cancer Institute; National Institutes of Health Contracts HHSN E, 94995NBS23, and 85983CBS43; NIH PHS Grants (UL1 RR025008, KL2 RR or TL1 RR025010) from the CTSA program of NCRR; NHLBI R24 HL085343; NIH U54 CA113001; NLM R01LM A1, and BISTI P20 EB000591; NSF award CCF References 1. Grethe J.S. et al., Biomedical Informatics Research Network: Building a National Collaboratory to Hasten the Derivation of New Understanding and Treatment of Disease. Stud Health Technol Inform, 2005, 112: p Oster, S. et al., cagrid 1.0: An Enterprise Grid Infrastructure for Biomedical Research. Journal of American Medical Inf. Assoc. (JAMIA), 2008, 15(2): p von Eschenbach, A.C. and K. Buetow, Cancer Informatics Vision: cabig. Cancer Inform., 2006, 2: p CardioVascular Research Grid Mendis, M. et al., Integration of Hive and Cell Software in the i2b2 Architecture. AMIA Annual Symp., 2007: p Service-aware Interoperability Framework (SAIF) Web Ontology Language A Semantic Web Rule Language (SWRL) Chays, D., et al., A Framework for Testing Database Applications. ACM SIGSOFT International Symposium on Software Testing and Analysis, 2000: p Chays, D., et al., An AGENDA for Testing Relational Database Applications: Research Articles. Software Testing Verification, and Reliability, 2004, 14(1): p Orso, A. et al., Techniques for Classifying Executions of Deployed Software to Support Software Engineering Tasks. IEEE Trans. on Soft. Eng., 2007, 33(5): p Engler, D., et al., Bugs as Deviant Behavior: A General Approach to Inferring Errors in Systems Code. ACM Symposium on Operating Systems Principles, 2001: p Informatics for Integrating Biology & Bedside Pathology Analytical Imaging Standards ation 26
Detection of Conflicts and Inconsistencies in Taxonomy-based Authorization Policies
 Detection of Conflicts and Inconsistencies in Taxonomy-based Authorization Policies Apurva Mohan, Douglas M. Blough School of Electrical and Computer Engineering Georgia Institute of Technology Atlanta,
Detection of Conflicts and Inconsistencies in Taxonomy-based Authorization Policies Apurva Mohan, Douglas M. Blough School of Electrical and Computer Engineering Georgia Institute of Technology Atlanta,
TOOLS TO ANALYZE MORPHOLOGY AND SPATIALLY MAPPED MOLECULAR DATA. Tahsin Kurc Department of Biomedical Informatics Stony Brook University
 TOOLS TO ANALYZE MORPHOLOGY AND SPATIALLY MAPPED MOLECULAR DATA Tahsin Kurc Department of Biomedical Informatics Stony Brook University Research and Development Team Stony Brook University Joel Saltz Tahsin
TOOLS TO ANALYZE MORPHOLOGY AND SPATIALLY MAPPED MOLECULAR DATA Tahsin Kurc Department of Biomedical Informatics Stony Brook University Research and Development Team Stony Brook University Joel Saltz Tahsin
A Temporal Abstraction-based Extract, Transform and Load Process for Creating Registry Databases for Research.
 A Temporal Abstraction-based Extract, Transform and Load Process for Creating Registry Databases for Research. Andrew Post, Emory University Tahsin Kurc, Emory University Marc Overcash, Emory University
A Temporal Abstraction-based Extract, Transform and Load Process for Creating Registry Databases for Research. Andrew Post, Emory University Tahsin Kurc, Emory University Marc Overcash, Emory University
Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research
 Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research Ian Fore, D.Phil. Associate Director, Biorepository and Pathology Informatics Senior Program
Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research Ian Fore, D.Phil. Associate Director, Biorepository and Pathology Informatics Senior Program
A Vision for Bigger Biomedical Data: Integration of REDCap with Other Data Sources
 A Vision for Bigger Biomedical Data: Integration of REDCap with Other Data Sources Ram Gouripeddi Assistant Professor, Department of Biomedical Informatics, University of Utah Senior Biomedical Informatics
A Vision for Bigger Biomedical Data: Integration of REDCap with Other Data Sources Ram Gouripeddi Assistant Professor, Department of Biomedical Informatics, University of Utah Senior Biomedical Informatics
Full file at
 Chapter 2 Data Warehousing True-False Questions 1. A real-time, enterprise-level data warehouse combined with a strategy for its use in decision support can leverage data to provide massive financial benefits
Chapter 2 Data Warehousing True-False Questions 1. A real-time, enterprise-level data warehouse combined with a strategy for its use in decision support can leverage data to provide massive financial benefits
Acquiring Experience with Ontology and Vocabularies
 Acquiring Experience with Ontology and Vocabularies Walt Melo Risa Mayan Jean Stanford The author's affiliation with The MITRE Corporation is provided for identification purposes only, and is not intended
Acquiring Experience with Ontology and Vocabularies Walt Melo Risa Mayan Jean Stanford The author's affiliation with The MITRE Corporation is provided for identification purposes only, and is not intended
The NeuroLOG Platform Federating multi-centric neuroscience resources
 Software technologies for integration of process and data in medical imaging The Platform Federating multi-centric neuroscience resources Johan MONTAGNAT Franck MICHEL Vilnius, Apr. 13 th 2011 ANR-06-TLOG-024
Software technologies for integration of process and data in medical imaging The Platform Federating multi-centric neuroscience resources Johan MONTAGNAT Franck MICHEL Vilnius, Apr. 13 th 2011 ANR-06-TLOG-024
Ontology based Model and Procedure Creation for Topic Analysis in Chinese Language
 Ontology based Model and Procedure Creation for Topic Analysis in Chinese Language Dong Han and Kilian Stoffel Information Management Institute, University of Neuchâtel Pierre-à-Mazel 7, CH-2000 Neuchâtel,
Ontology based Model and Procedure Creation for Topic Analysis in Chinese Language Dong Han and Kilian Stoffel Information Management Institute, University of Neuchâtel Pierre-à-Mazel 7, CH-2000 Neuchâtel,
Semantic Web. Dr. Philip Cannata 1
 Semantic Web Dr. Philip Cannata 1 Dr. Philip Cannata 2 Dr. Philip Cannata 3 Dr. Philip Cannata 4 See data 14 Scientific American.sql on the class website calendar SELECT strreplace(x, 'sa:', '') "C" FROM
Semantic Web Dr. Philip Cannata 1 Dr. Philip Cannata 2 Dr. Philip Cannata 3 Dr. Philip Cannata 4 See data 14 Scientific American.sql on the class website calendar SELECT strreplace(x, 'sa:', '') "C" FROM
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR
 Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeaux 2, Guohui Xiao 3, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester,
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeaux 2, Guohui Xiao 3, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester,
PROJECT PERIODIC REPORT
 PROJECT PERIODIC REPORT Grant Agreement number: 257403 Project acronym: CUBIST Project title: Combining and Uniting Business Intelligence and Semantic Technologies Funding Scheme: STREP Date of latest
PROJECT PERIODIC REPORT Grant Agreement number: 257403 Project acronym: CUBIST Project title: Combining and Uniting Business Intelligence and Semantic Technologies Funding Scheme: STREP Date of latest
Rubicon: Scalable Bounded Verification of Web Applications
 Joseph P. Near Research Statement My research focuses on developing domain-specific static analyses to improve software security and reliability. In contrast to existing approaches, my techniques leverage
Joseph P. Near Research Statement My research focuses on developing domain-specific static analyses to improve software security and reliability. In contrast to existing approaches, my techniques leverage
ISO INTERNATIONAL STANDARD. Health informatics Genomic Sequence Variation Markup Language (GSVML)
 INTERNATIONAL STANDARD ISO 25720 First edition 2009-08-15 Health informatics Genomic Sequence Variation Markup Language (GSVML) Informatique de santé Langage de balisage de la variation de séquence génomique
INTERNATIONAL STANDARD ISO 25720 First edition 2009-08-15 Health informatics Genomic Sequence Variation Markup Language (GSVML) Informatique de santé Langage de balisage de la variation de séquence génomique
Healthcare IT A Monitoring Primer
 Healthcare IT A Monitoring Primer Published: February 2019 PAGE 1 OF 13 Contents Introduction... 3 The Healthcare IT Environment.... 4 Traditional IT... 4 Healthcare Systems.... 4 Healthcare Data Format
Healthcare IT A Monitoring Primer Published: February 2019 PAGE 1 OF 13 Contents Introduction... 3 The Healthcare IT Environment.... 4 Traditional IT... 4 Healthcare Systems.... 4 Healthcare Data Format
Integrating TCGA Clinical Data Using Metadata-driven Tools and NLP
 Integrating TCGA Clinical Data Using Metadata-driven Tools and NLP Guoqian Jiang, MD, PhD, Mayo Clinic Guergana Savova, PhD, HMS/Boston Children's Hospital Rebecca Jacobson, MD,MS, University of Pittsburgh
Integrating TCGA Clinical Data Using Metadata-driven Tools and NLP Guoqian Jiang, MD, PhD, Mayo Clinic Guergana Savova, PhD, HMS/Boston Children's Hospital Rebecca Jacobson, MD,MS, University of Pittsburgh
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR
 Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeax 2, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester, MN, 55905, USA 2
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeax 2, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester, MN, 55905, USA 2
Introduce Grid Service Authoring Toolkit
 Introduce Grid Service Authoring Toolkit Shannon Hastings hastings@bmi.osu.edu Multiscale Computing Laboratory Department of Biomedical Informatics The Ohio State University Outline Introduce Generated
Introduce Grid Service Authoring Toolkit Shannon Hastings hastings@bmi.osu.edu Multiscale Computing Laboratory Department of Biomedical Informatics The Ohio State University Outline Introduce Generated
Sharing Data and Analytical Resources Securely in a Biomedical Research Grid. Environment
 Sharing Data and Analytical Resources Securely in a Biomedical Research Grid Environment Stephen Langella, Shannon Hastings, Scott Oster, Tony Pan, Ashish Sharma, Justin Permar, David Ervin, B. Barla Cambazoglu,
Sharing Data and Analytical Resources Securely in a Biomedical Research Grid Environment Stephen Langella, Shannon Hastings, Scott Oster, Tony Pan, Ashish Sharma, Justin Permar, David Ervin, B. Barla Cambazoglu,
XML in the bipharmaceutical
 XML in the bipharmaceutical sector XML holds out the opportunity to integrate data across both the enterprise and the network of biopharmaceutical alliances - with little technological dislocation and
XML in the bipharmaceutical sector XML holds out the opportunity to integrate data across both the enterprise and the network of biopharmaceutical alliances - with little technological dislocation and
A Knowledge Model Driven Solution for Web-Based Telemedicine Applications
 Medical Informatics in a United and Healthy Europe K.-P. Adlassnig et al. (Eds.) IOS Press, 2009 2009 European Federation for Medical Informatics. All rights reserved. doi:10.3233/978-1-60750-044-5-443
Medical Informatics in a United and Healthy Europe K.-P. Adlassnig et al. (Eds.) IOS Press, 2009 2009 European Federation for Medical Informatics. All rights reserved. doi:10.3233/978-1-60750-044-5-443
Supporting Patient Screening to Identify Suitable Clinical Trials
 Supporting Patient Screening to Identify Suitable Clinical Trials Anca BUCUR a,1, Jasper VAN LEEUWEN a, Njin-Zu CHEN a, Brecht CLAERHOUT b Kristof DE SCHEPPER b, David PEREZ-REY c, Raul ALONSO-CALVO c,
Supporting Patient Screening to Identify Suitable Clinical Trials Anca BUCUR a,1, Jasper VAN LEEUWEN a, Njin-Zu CHEN a, Brecht CLAERHOUT b Kristof DE SCHEPPER b, David PEREZ-REY c, Raul ALONSO-CALVO c,
NeuroLOG WP1 Sharing Data & Metadata
 Software technologies for integration of process and data in medical imaging NeuroLOG WP1 Sharing Data & Metadata Franck MICHEL Paris, May 18 th 2010 NeuroLOG ANR-06-TLOG-024 http://neurolog.polytech.unice.fr
Software technologies for integration of process and data in medical imaging NeuroLOG WP1 Sharing Data & Metadata Franck MICHEL Paris, May 18 th 2010 NeuroLOG ANR-06-TLOG-024 http://neurolog.polytech.unice.fr
KEY BASED APPROACH FOR INTEGRATION OF HETEROGENEOUS DATA SOURCES
 KEY BASED APPROACH FOR INTEGRATION OF HETEROGENEOUS DATA SOURCES 1 KAMSURIAH AHMAD, 1 TENGKU SITI FATIMAH TENGKU WOOK, 2 REDUAN SAMAD 1 Strategic Management Research Group Faculty of Information Science
KEY BASED APPROACH FOR INTEGRATION OF HETEROGENEOUS DATA SOURCES 1 KAMSURIAH AHMAD, 1 TENGKU SITI FATIMAH TENGKU WOOK, 2 REDUAN SAMAD 1 Strategic Management Research Group Faculty of Information Science
Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies
 Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies Harold R. Solbrig 1, Guoqian Jiang 1 1 Mayo Clinic College of Medicine, Rochester, MN [solbrig.harold,
Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies Harold R. Solbrig 1, Guoqian Jiang 1 1 Mayo Clinic College of Medicine, Rochester, MN [solbrig.harold,
Populating the i2b2 Database with Heterogeneous EMR Data: a Semantic Network Approach
 Populating the i2b2 Database with Heterogeneous EMR Data: a Semantic Network Approach Sebastian Mate a,1, Thomas Bürkle a, Felix Köpcke a, Bernhard Breil b, Bernd Wullich c, Martin Dugas b, Hans-Ulrich
Populating the i2b2 Database with Heterogeneous EMR Data: a Semantic Network Approach Sebastian Mate a,1, Thomas Bürkle a, Felix Köpcke a, Bernhard Breil b, Bernd Wullich c, Martin Dugas b, Hans-Ulrich
Powering Knowledge Discovery. Insights from big data with Linguamatics I2E
 Powering Knowledge Discovery Insights from big data with Linguamatics I2E Gain actionable insights from unstructured data The world now generates an overwhelming amount of data, most of it written in natural
Powering Knowledge Discovery Insights from big data with Linguamatics I2E Gain actionable insights from unstructured data The world now generates an overwhelming amount of data, most of it written in natural
Data Cleansing. LIU Jingyuan, Vislab WANG Yilei, Theoretical group
 Data Cleansing LIU Jingyuan, Vislab WANG Yilei, Theoretical group What is Data Cleansing Data cleansing (data cleaning) is the process of detecting and correcting (or removing) errors or inconsistencies
Data Cleansing LIU Jingyuan, Vislab WANG Yilei, Theoretical group What is Data Cleansing Data cleansing (data cleaning) is the process of detecting and correcting (or removing) errors or inconsistencies
A System for Ontology-Based Annotation of Biomedical Data
 A System for Ontology-Based Annotation of Biomedical Data Clement Jonquet, Mark A. Musen, and Nigam Shah Stanford Center for Biomedical Informatics Research Stanford University School of Medicine Medical
A System for Ontology-Based Annotation of Biomedical Data Clement Jonquet, Mark A. Musen, and Nigam Shah Stanford Center for Biomedical Informatics Research Stanford University School of Medicine Medical
Federated queries of clinical data repositories: the sum of the parts does not equal the whole
 Federated queries of clinical data repositories: the sum of the parts does not equal the whole Griffin M Weber 1,2 Research and applications 1 Information Technology, Harvard Medical School, Boston, Massachusetts,
Federated queries of clinical data repositories: the sum of the parts does not equal the whole Griffin M Weber 1,2 Research and applications 1 Information Technology, Harvard Medical School, Boston, Massachusetts,
Integrative Informatics
 Early Vision Integrative Informatics Isaac S. Kohane 4.27.04 PIP s Integration Integrating Genomics and Pharmacology RNA expression in NCI 60 cell lines was determined using Affymetrix HU6000 arrays 5,223
Early Vision Integrative Informatics Isaac S. Kohane 4.27.04 PIP s Integration Integrating Genomics and Pharmacology RNA expression in NCI 60 cell lines was determined using Affymetrix HU6000 arrays 5,223
The NIH Collaboratory Distributed Research Network: A Privacy Protecting Method for Sharing Research Data Sets
 The NIH Collaboratory Distributed Research Network: A Privacy Protecting Method for Sharing Research Data Sets Jeffrey Brown, Lesley Curtis, and Rich Platt June 13, 2014 Previously The NIH Collaboratory:
The NIH Collaboratory Distributed Research Network: A Privacy Protecting Method for Sharing Research Data Sets Jeffrey Brown, Lesley Curtis, and Rich Platt June 13, 2014 Previously The NIH Collaboratory:
Cheshire 3 Framework White Paper: Implementing Support for Digital Repositories in a Data Grid Environment
 Cheshire 3 Framework White Paper: Implementing Support for Digital Repositories in a Data Grid Environment Paul Watry Univ. of Liverpool, NaCTeM pwatry@liverpool.ac.uk Ray Larson Univ. of California, Berkeley
Cheshire 3 Framework White Paper: Implementing Support for Digital Repositories in a Data Grid Environment Paul Watry Univ. of Liverpool, NaCTeM pwatry@liverpool.ac.uk Ray Larson Univ. of California, Berkeley
COINS Tools. MICIS DICOM Receiver Assessment Manager Self Assessment Tablet Query Builder Portals Data Exchange CAS My Security
 As neuroimaging research continues to grow, dynamic neuroinformatics systems are necessary to store, retrieve, mine and share the mass amounts of data. COINS has been created to facilitate communication
As neuroimaging research continues to grow, dynamic neuroinformatics systems are necessary to store, retrieve, mine and share the mass amounts of data. COINS has been created to facilitate communication
Anomaly Detection. You Chen
 Anomaly Detection You Chen 1 Two questions: (1) What is Anomaly Detection? (2) What are Anomalies? Anomaly detection refers to the problem of finding patterns in data that do not conform to expected behavior
Anomaly Detection You Chen 1 Two questions: (1) What is Anomaly Detection? (2) What are Anomalies? Anomaly detection refers to the problem of finding patterns in data that do not conform to expected behavior
Electronic Health Records with Cleveland Clinic and Oracle Semantic Technologies
 Electronic Health Records with Cleveland Clinic and Oracle Semantic Technologies David Booth, Ph.D., Cleveland Clinic (contractor) Oracle OpenWorld 20-Sep-2010 Latest version of these slides: http://dbooth.org/2010/oow/
Electronic Health Records with Cleveland Clinic and Oracle Semantic Technologies David Booth, Ph.D., Cleveland Clinic (contractor) Oracle OpenWorld 20-Sep-2010 Latest version of these slides: http://dbooth.org/2010/oow/
Measuring VDI Fitness and User Experience Technical White Paper
 Measuring VDI Fitness and User Experience Technical White Paper 3600 Mansell Road Suite 200 Alpharetta, GA 30022 866.914.9665 main 678.397.0339 fax info@liquidwarelabs.com www.liquidwarelabs.com Table
Measuring VDI Fitness and User Experience Technical White Paper 3600 Mansell Road Suite 200 Alpharetta, GA 30022 866.914.9665 main 678.397.0339 fax info@liquidwarelabs.com www.liquidwarelabs.com Table
Korea Institute of Oriental Medicine, South Korea 2 Biomedical Knowledge Engineering Laboratory,
 A Medical Treatment System based on Traditional Korean Medicine Ontology Sang-Kyun Kim 1, SeJin Nam 2, Dong-Hun Park 1, Yong-Taek Oh 1, Hyunchul Jang 1 1 Literature & Informatics Research Division, Korea
A Medical Treatment System based on Traditional Korean Medicine Ontology Sang-Kyun Kim 1, SeJin Nam 2, Dong-Hun Park 1, Yong-Taek Oh 1, Hyunchul Jang 1 1 Literature & Informatics Research Division, Korea
Reproducible Workflows Biomedical Research. P Berlin, Germany
 Reproducible Workflows Biomedical Research P11 2018 Berlin, Germany Contributors Leslie McIntosh Research Data Alliance, U.S., Executive Director Oya Beyan Aachen University, Germany Anthony Juehne RDA,
Reproducible Workflows Biomedical Research P11 2018 Berlin, Germany Contributors Leslie McIntosh Research Data Alliance, U.S., Executive Director Oya Beyan Aachen University, Germany Anthony Juehne RDA,
warwick.ac.uk/lib-publications
 Original citation: Zhao, Lei, Lim Choi Keung, Sarah Niukyun and Arvanitis, Theodoros N. (2016) A BioPortalbased terminology service for health data interoperability. In: Unifying the Applications and Foundations
Original citation: Zhao, Lei, Lim Choi Keung, Sarah Niukyun and Arvanitis, Theodoros N. (2016) A BioPortalbased terminology service for health data interoperability. In: Unifying the Applications and Foundations
Grid Platform for Medical Federated Queries Supporting Semantic and Visual Annotations
 Grid Platform for Medical Federated Queries Supporting Semantic and Visual Annotations, Juan Guillermo, Wilson Pérez, Lizandro Solano-Quinde, Washington Ramírez-Montalván, Alexandra La Cruz Content Introduction
Grid Platform for Medical Federated Queries Supporting Semantic and Visual Annotations, Juan Guillermo, Wilson Pérez, Lizandro Solano-Quinde, Washington Ramírez-Montalván, Alexandra La Cruz Content Introduction
Chapter 8 Software Testing. Chapter 8 Software testing
 Chapter 8 Software Testing 1 Topics covered Introduction to testing Stages for testing software system are: Development testing Release testing User testing Test-driven development as interleave approach.
Chapter 8 Software Testing 1 Topics covered Introduction to testing Stages for testing software system are: Development testing Release testing User testing Test-driven development as interleave approach.
and the bringing cabig cancer data to the.net Developer and Microsoft Office User Communities
 and the bringing cabig cancer data to the.net Developer and Microsoft Office User Communities http://xl-cabig-client.sourceforge.net/ Robert Macura Tom Macura escience Workshop October 2005 Science Paradigms
and the bringing cabig cancer data to the.net Developer and Microsoft Office User Communities http://xl-cabig-client.sourceforge.net/ Robert Macura Tom Macura escience Workshop October 2005 Science Paradigms
STANDARDISATION IN DIGITAL PATHOLOGY. Marcial García Rojo Hospital de Jerez. Cádiz. España Vice-President Spanish Society for Health Informatics
 STANDARDISATION IN DIGITAL PATHOLOGY Marcial García Rojo Hospital de Jerez. Cádiz. España Vice-President Spanish Society for Health Informatics Multiple image sources Introduction Digital imaging in pathology
STANDARDISATION IN DIGITAL PATHOLOGY Marcial García Rojo Hospital de Jerez. Cádiz. España Vice-President Spanish Society for Health Informatics Multiple image sources Introduction Digital imaging in pathology
AI Application and Development in ehealth Field. MIN Dong
 AI Application and Development in ehealth Field MIN Dong What s e-health? Defined by WHO ehealth is the cost-effective and secure use of information and communications technologies(icts) in support of
AI Application and Development in ehealth Field MIN Dong What s e-health? Defined by WHO ehealth is the cost-effective and secure use of information and communications technologies(icts) in support of
Ontrez Project Report National Center for Biomedical Ontology November, 2007
 Ontrez Project Report National Center for Biomedical Ontology November, 2007 Executive summary Currently, genomics data and data repositories in the public domain are expanding at an explosive pace. 1
Ontrez Project Report National Center for Biomedical Ontology November, 2007 Executive summary Currently, genomics data and data repositories in the public domain are expanding at an explosive pace. 1
Collaborative Framework for Testing Web Application Vulnerabilities Using STOWS
 Available Online at www.ijcsmc.com International Journal of Computer Science and Mobile Computing A Monthly Journal of Computer Science and Information Technology ISSN 2320 088X IMPACT FACTOR: 5.258 IJCSMC,
Available Online at www.ijcsmc.com International Journal of Computer Science and Mobile Computing A Monthly Journal of Computer Science and Information Technology ISSN 2320 088X IMPACT FACTOR: 5.258 IJCSMC,
A web interface for the quantification of microtubule dynamics
 A web interface for the quantification of microtubule dynamics Koon Yin Kong, Georgia Institute of Technology Adam Marcus, Emory University Paraskevi Giaanakakou, Emory University May D. Wang, Georgia
A web interface for the quantification of microtubule dynamics Koon Yin Kong, Georgia Institute of Technology Adam Marcus, Emory University Paraskevi Giaanakakou, Emory University May D. Wang, Georgia
Building Better Interfaces: HL7 Conformance Profiles
 Tutorials, W. Rishel Research Note 26 November 2002 Building Better Interfaces: HL7 Conformance Profiles The new Health Level Seven conformance technology allows individual healthcare organizations and
Tutorials, W. Rishel Research Note 26 November 2002 Building Better Interfaces: HL7 Conformance Profiles The new Health Level Seven conformance technology allows individual healthcare organizations and
UNIFYING SEMANTIC ANNOTATION AND QUERYING IN BIOMEDICAL IMAGE REPOSITORIES One Solution for Two Problems of Medical Knowledge Engineering
 UNIFYING SEMANTIC ANNOTATION AND QUERYING IN BIOMEDICAL IMAGE REPOSITORIES One Solution for Two Problems of Medical Knowledge Engineering Daniel Sonntag German Research Center for Artificial Intelligence,
UNIFYING SEMANTIC ANNOTATION AND QUERYING IN BIOMEDICAL IMAGE REPOSITORIES One Solution for Two Problems of Medical Knowledge Engineering Daniel Sonntag German Research Center for Artificial Intelligence,
SELF-SERVICE SEMANTIC DATA FEDERATION
 SELF-SERVICE SEMANTIC DATA FEDERATION WE LL MAKE YOU A DATA SCIENTIST Contact: IPSNP Computing Inc. Chris Baker, CEO Chris.Baker@ipsnp.com (506) 721 8241 BIG VISION: SELF-SERVICE DATA FEDERATION Biomedical
SELF-SERVICE SEMANTIC DATA FEDERATION WE LL MAKE YOU A DATA SCIENTIST Contact: IPSNP Computing Inc. Chris Baker, CEO Chris.Baker@ipsnp.com (506) 721 8241 BIG VISION: SELF-SERVICE DATA FEDERATION Biomedical
A Framework for Securing Databases from Intrusion Threats
 A Framework for Securing Databases from Intrusion Threats R. Prince Jeyaseelan James Department of Computer Applications, Valliammai Engineering College Affiliated to Anna University, Chennai, India Email:
A Framework for Securing Databases from Intrusion Threats R. Prince Jeyaseelan James Department of Computer Applications, Valliammai Engineering College Affiliated to Anna University, Chennai, India Email:
The Clinical Data Repository Provides CPR's Foundation
 Tutorials, T.Handler,M.D.,W.Rishel Research Note 6 November 2003 The Clinical Data Repository Provides CPR's Foundation The core of any computer-based patient record system is a permanent data store. The
Tutorials, T.Handler,M.D.,W.Rishel Research Note 6 November 2003 The Clinical Data Repository Provides CPR's Foundation The core of any computer-based patient record system is a permanent data store. The
Medical Imaging on the Semantic Web: Annotation and Image Markup
 Medical Imaging on the Semantic Web: Annotation and Image Markup Daniel L. Rubin, 1 Pattanasak Mongkolwat, 2 Vladimir Kleper, 2 Kaustubh Supekar, 1 and David S. Channin 2 1 Department of Radiology and
Medical Imaging on the Semantic Web: Annotation and Image Markup Daniel L. Rubin, 1 Pattanasak Mongkolwat, 2 Vladimir Kleper, 2 Kaustubh Supekar, 1 and David S. Channin 2 1 Department of Radiology and
Core Technology Development Team Meeting
 Core Technology Development Team Meeting To hear the meeting, you must call in Toll-free phone number: 1-866-740-1260 Access Code: 2201876 For international call in numbers, please visit: https://www.readytalk.com/account-administration/international-numbers
Core Technology Development Team Meeting To hear the meeting, you must call in Toll-free phone number: 1-866-740-1260 Access Code: 2201876 For international call in numbers, please visit: https://www.readytalk.com/account-administration/international-numbers
ICTR UW Institute of Clinical and Translational Research. i2b2 User Guide. Version 1.0 Updated 9/11/2017
 ICTR UW Institute of Clinical and Translational Research i2b2 User Guide Version 1.0 Updated 9/11/2017 Table of Contents Background/Search Criteria... 2 Accessing i2b2... 3 Navigating the Workbench...
ICTR UW Institute of Clinical and Translational Research i2b2 User Guide Version 1.0 Updated 9/11/2017 Table of Contents Background/Search Criteria... 2 Accessing i2b2... 3 Navigating the Workbench...
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview
 Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Supporting Discovery in Medicine by Association Rule Mining in Medline and UMLS
 Supporting Discovery in Medicine by Association Rule Mining in Medline and UMLS Dimitar Hristovski a, Janez Stare a, Borut Peterlin b, Saso Dzeroski c a IBMI, Medical Faculty, University of Ljubljana,
Supporting Discovery in Medicine by Association Rule Mining in Medline and UMLS Dimitar Hristovski a, Janez Stare a, Borut Peterlin b, Saso Dzeroski c a IBMI, Medical Faculty, University of Ljubljana,
Daniel L. Rubin and Kaustubh Supekar, Stanford University
 S e m a n t i c S c i e n t i f i c K n o w l e d g e I n t e g r a t i o n Annotation and Image Markup: Accessing and Interoperating with the Semantic Content in Medical Imaging The Annotation Daniel
S e m a n t i c S c i e n t i f i c K n o w l e d g e I n t e g r a t i o n Annotation and Image Markup: Accessing and Interoperating with the Semantic Content in Medical Imaging The Annotation Daniel
DIGITAL STEWARDSHIP SUPPLEMENTARY INFORMATION FORM
 OMB No. 3137 0071, Exp. Date: 09/30/2015 DIGITAL STEWARDSHIP SUPPLEMENTARY INFORMATION FORM Introduction: IMLS is committed to expanding public access to IMLS-funded research, data and other digital products:
OMB No. 3137 0071, Exp. Date: 09/30/2015 DIGITAL STEWARDSHIP SUPPLEMENTARY INFORMATION FORM Introduction: IMLS is committed to expanding public access to IMLS-funded research, data and other digital products:
i2b2 User Guide University of Minnesota Clinical and Translational Science Institute
 Clinical and Translational Science Institute i2b2 User Guide i2b2 is a tool for discovering research cohorts using existing, de-identified, clinical data This guide is provided by the Office of Biomedical
Clinical and Translational Science Institute i2b2 User Guide i2b2 is a tool for discovering research cohorts using existing, de-identified, clinical data This guide is provided by the Office of Biomedical
Feed the Future Innovation Lab for Peanut (Peanut Innovation Lab) Data Management Plan Version:
 Feed the Future Innovation Lab for Peanut (Peanut Innovation Lab) Data Management Plan Version: 20180316 Peanut Innovation Lab Management Entity The University of Georgia, Athens, Georgia Feed the Future
Feed the Future Innovation Lab for Peanut (Peanut Innovation Lab) Data Management Plan Version: 20180316 Peanut Innovation Lab Management Entity The University of Georgia, Athens, Georgia Feed the Future
IHE Radiation Oncology Technical Framework Supplement. Treatment Delivery Workflow (TDW) Draft for Public Comment
 Integrating the Healthcare Enterprise IHE Radiation Oncology Technical Framework Supplement Treatment Delivery Workflow (TDW) Draft for Public Comment Date: January 29, 2010 Author: David Murray Email:
Integrating the Healthcare Enterprise IHE Radiation Oncology Technical Framework Supplement Treatment Delivery Workflow (TDW) Draft for Public Comment Date: January 29, 2010 Author: David Murray Email:
Document Retrieval using Predication Similarity
 Document Retrieval using Predication Similarity Kalpa Gunaratna 1 Kno.e.sis Center, Wright State University, Dayton, OH 45435 USA kalpa@knoesis.org Abstract. Document retrieval has been an important research
Document Retrieval using Predication Similarity Kalpa Gunaratna 1 Kno.e.sis Center, Wright State University, Dayton, OH 45435 USA kalpa@knoesis.org Abstract. Document retrieval has been an important research
FIVE BEST PRACTICES FOR ENSURING A SUCCESSFUL SQL SERVER MIGRATION
 FIVE BEST PRACTICES FOR ENSURING A SUCCESSFUL SQL SERVER MIGRATION The process of planning and executing SQL Server migrations can be complex and risk-prone. This is a case where the right approach and
FIVE BEST PRACTICES FOR ENSURING A SUCCESSFUL SQL SERVER MIGRATION The process of planning and executing SQL Server migrations can be complex and risk-prone. This is a case where the right approach and
Developing a Workflow for the Integration of Patient Education Materials into EPIC
 Developing a Workflow for the Integration of Patient Education Materials into EPIC Muhammad-Sharif Moustafa Mentors: Kristine Petre and Linda Schwartz Library Services Abstract The purpose of this project
Developing a Workflow for the Integration of Patient Education Materials into EPIC Muhammad-Sharif Moustafa Mentors: Kristine Petre and Linda Schwartz Library Services Abstract The purpose of this project
The Data exacell DXC. J. Ray Scott DXC PI May 17, 2016
 The Data exacell DXC J. Ray Scott DXC PI May 17, 2016 DXC Leadership Mike Levine Co-Scientific Director Co-PI Nick Nystrom Senior Director of Research Co-PI Ralph Roskies Co-Scientific Director Co-PI Robin
The Data exacell DXC J. Ray Scott DXC PI May 17, 2016 DXC Leadership Mike Levine Co-Scientific Director Co-PI Nick Nystrom Senior Director of Research Co-PI Ralph Roskies Co-Scientific Director Co-PI Robin
Chevron Position Paper for W3C Workshop on Semantic Web in Oil & Gas Industry
 Enterprise Architecture Chevron Position Paper for W3C Workshop on Semantic Web in Oil & Gas Industry Frank Chum, ITC EA Mario Casetta, ETC IM Roger Cutler, ITC EA 9 December 2008 Houston, Texas 2008 Chevron
Enterprise Architecture Chevron Position Paper for W3C Workshop on Semantic Web in Oil & Gas Industry Frank Chum, ITC EA Mario Casetta, ETC IM Roger Cutler, ITC EA 9 December 2008 Houston, Texas 2008 Chevron
APPLYING HUMAN FACTORS ENGINEERING TO IMPROVE USABILITY AND WORKFLOW IN PATHOLOGY INFORMATICS
 Proceedings of the 2017 International Symposium on Human Factors and Ergonomics in Health Care 23 APPLYING HUMAN FACTORS ENGINEERING TO IMPROVE USABILITY AND WORKFLOW IN PATHOLOGY INFORMATICS Austin F.
Proceedings of the 2017 International Symposium on Human Factors and Ergonomics in Health Care 23 APPLYING HUMAN FACTORS ENGINEERING TO IMPROVE USABILITY AND WORKFLOW IN PATHOLOGY INFORMATICS Austin F.
Hybrid Approach for MRI Human Head Scans Classification using HTT based SFTA Texture Feature Extraction Technique
 Volume 118 No. 17 2018, 691-701 ISSN: 1311-8080 (printed version); ISSN: 1314-3395 (on-line version) url: http://www.ijpam.eu ijpam.eu Hybrid Approach for MRI Human Head Scans Classification using HTT
Volume 118 No. 17 2018, 691-701 ISSN: 1311-8080 (printed version); ISSN: 1314-3395 (on-line version) url: http://www.ijpam.eu ijpam.eu Hybrid Approach for MRI Human Head Scans Classification using HTT
Integrated Consortium of Laboratory Networks (ICLN) Brief to the NPDN National Meeting
 Integrated Consortium of Laboratory Networks (ICLN) Brief to the NPDN National Meeting January 30, 2007 1 Agenda ICLN Background Information Network Coordinating Group Accomplishments Responsible Federal
Integrated Consortium of Laboratory Networks (ICLN) Brief to the NPDN National Meeting January 30, 2007 1 Agenda ICLN Background Information Network Coordinating Group Accomplishments Responsible Federal
Federated XDMoD Requirements
 Federated XDMoD Requirements Date Version Person Change 2016-04-08 1.0 draft XMS Team Initial version Summary Definitions Assumptions Data Collection Local XDMoD Installation Module Support Data Federation
Federated XDMoD Requirements Date Version Person Change 2016-04-08 1.0 draft XMS Team Initial version Summary Definitions Assumptions Data Collection Local XDMoD Installation Module Support Data Federation
Part 5. Verification and Validation
 Software Engineering Part 5. Verification and Validation - Verification and Validation - Software Testing Ver. 1.7 This lecture note is based on materials from Ian Sommerville 2006. Anyone can use this
Software Engineering Part 5. Verification and Validation - Verification and Validation - Software Testing Ver. 1.7 This lecture note is based on materials from Ian Sommerville 2006. Anyone can use this
SCAN POINT IMAGE MANAGEMENT TECHNOLOGY CLINICAL USER'S MANUAL
 SCAN POINT IMAGE MANAGEMENT TECHNOLOGY CLINICAL USER'S MANUAL 0900 3076 03 60 SCAN POINT IMAGE MANAGEMENT TECHNOLOGY Clinical User's Manual Effective: May 18, 2017 CONTACT INFORMATION To obtain additional
SCAN POINT IMAGE MANAGEMENT TECHNOLOGY CLINICAL USER'S MANUAL 0900 3076 03 60 SCAN POINT IMAGE MANAGEMENT TECHNOLOGY Clinical User's Manual Effective: May 18, 2017 CONTACT INFORMATION To obtain additional
An Annotation Tool for Semantic Documents
 An Annotation Tool for Semantic Documents (System Description) Henrik Eriksson Dept. of Computer and Information Science Linköping University SE-581 83 Linköping, Sweden her@ida.liu.se Abstract. Document
An Annotation Tool for Semantic Documents (System Description) Henrik Eriksson Dept. of Computer and Information Science Linköping University SE-581 83 Linköping, Sweden her@ida.liu.se Abstract. Document
Goal-oriented Schema in Biological Database Design
 Goal-oriented Schema in Biological Database Design Ping Chen Department of Computer Science University of Helsinki Helsinki, Finland 00014 EMAIL: pchen@cs.helsinki.fi Abstract In this paper, I reviewed
Goal-oriented Schema in Biological Database Design Ping Chen Department of Computer Science University of Helsinki Helsinki, Finland 00014 EMAIL: pchen@cs.helsinki.fi Abstract In this paper, I reviewed
The Emerging Data Lake IT Strategy
 The Emerging Data Lake IT Strategy An Evolving Approach for Dealing with Big Data & Changing Environments bit.ly/datalake SPEAKERS: Thomas Kelly, Practice Director Cognizant Technology Solutions Sean Martin,
The Emerging Data Lake IT Strategy An Evolving Approach for Dealing with Big Data & Changing Environments bit.ly/datalake SPEAKERS: Thomas Kelly, Practice Director Cognizant Technology Solutions Sean Martin,
Supporting Bioinformatic Experiments with A Service Query Engine
 Supporting Bioinformatic Experiments with A Service Query Engine Xuan Zhou Shiping Chen Athman Bouguettaya Kai Xu CSIRO ICT Centre, Australia {xuan.zhou,shiping.chen,athman.bouguettaya,kai.xu}@csiro.au
Supporting Bioinformatic Experiments with A Service Query Engine Xuan Zhou Shiping Chen Athman Bouguettaya Kai Xu CSIRO ICT Centre, Australia {xuan.zhou,shiping.chen,athman.bouguettaya,kai.xu}@csiro.au
Data publication and discovery with Globus
 Data publication and discovery with Globus Questions and comments to outreach@globus.org The Globus data publication and discovery services make it easy for institutions and projects to establish collections,
Data publication and discovery with Globus Questions and comments to outreach@globus.org The Globus data publication and discovery services make it easy for institutions and projects to establish collections,
The Semantic Planetary Data System
 The Semantic Planetary Data System J. Steven Hughes 1, Daniel J. Crichton 1, Sean Kelly 1, and Chris Mattmann 1 1 Jet Propulsion Laboratory 4800 Oak Grove Drive Pasadena, CA 91109 USA {steve.hughes, dan.crichton,
The Semantic Planetary Data System J. Steven Hughes 1, Daniel J. Crichton 1, Sean Kelly 1, and Chris Mattmann 1 1 Jet Propulsion Laboratory 4800 Oak Grove Drive Pasadena, CA 91109 USA {steve.hughes, dan.crichton,
MarkLogic. A Modern Data Platform To Support Your Critical Path COPYRIGHT 2016 MARKLOGIC CORPORATION. ALL RIGHTS RESERVED.
 MarkLogic A Modern Data Platform To Support Your Critical Path SLIDE: 2 Inception Pre- Post- Distribution Archive Taxonomies Semantics Technical Descriptive Customers Usage SLIDE: 4 Inception Pre- Post-
MarkLogic A Modern Data Platform To Support Your Critical Path SLIDE: 2 Inception Pre- Post- Distribution Archive Taxonomies Semantics Technical Descriptive Customers Usage SLIDE: 4 Inception Pre- Post-
The Shared Health Research Information Network (SHRINE): A Prototype Federated Query Tool for Clinical Data Repositories
 624 Weber et al., Shared Health Research Information Network Application of Information Technology The Shared Health Research Information Network (SHRINE): A Prototype Federated Query Tool for Clinical
624 Weber et al., Shared Health Research Information Network Application of Information Technology The Shared Health Research Information Network (SHRINE): A Prototype Federated Query Tool for Clinical
Software Testing. Software Testing. in the textbook. Chapter 8. Verification and Validation. Verification and Validation: Goals
 Software Testing in the textbook Software Testing Chapter 8 Introduction (Verification and Validation) 8.1 Development testing 8.2 Test-driven development 8.3 Release testing 8.4 User testing 1 2 Verification
Software Testing in the textbook Software Testing Chapter 8 Introduction (Verification and Validation) 8.1 Development testing 8.2 Test-driven development 8.3 Release testing 8.4 User testing 1 2 Verification
Modeling Actuations in BCI-O: A Context-based Integration of SOSA and IoT-O
 Modeling Actuations in BCI-O: A Context-based Integration of SOSA and IoT-O https://w3id.org/bci-ontology# Semantic Models for BCI Data Analytics Sergio José Rodríguez Méndez Pervasive Embedded Technology
Modeling Actuations in BCI-O: A Context-based Integration of SOSA and IoT-O https://w3id.org/bci-ontology# Semantic Models for BCI Data Analytics Sergio José Rodríguez Méndez Pervasive Embedded Technology
A VO-friendly, Community-based Authorization Framework
 A VO-friendly, Community-based Authorization Framework Part 1: Use Cases, Requirements, and Approach Ray Plante and Bruce Loftis NCSA Version 0.1 (February 11, 2005) Abstract The era of massive surveys
A VO-friendly, Community-based Authorization Framework Part 1: Use Cases, Requirements, and Approach Ray Plante and Bruce Loftis NCSA Version 0.1 (February 11, 2005) Abstract The era of massive surveys
An Architecture for Semantic Enterprise Application Integration Standards
 An Architecture for Semantic Enterprise Application Integration Standards Nenad Anicic 1, 2, Nenad Ivezic 1, Albert Jones 1 1 National Institute of Standards and Technology, 100 Bureau Drive Gaithersburg,
An Architecture for Semantic Enterprise Application Integration Standards Nenad Anicic 1, 2, Nenad Ivezic 1, Albert Jones 1 1 National Institute of Standards and Technology, 100 Bureau Drive Gaithersburg,
Utilizing NCBO Tools to Develop & Use an ECG Ontology
 Utilizing NCBO Tools to Develop & Use an ECG Ontology Stephen J. Granite, MS, MBA The Johns Hopkins University Institute for Computational Medicine (sgranite at jhu dot edu) The CardioVascular Research
Utilizing NCBO Tools to Develop & Use an ECG Ontology Stephen J. Granite, MS, MBA The Johns Hopkins University Institute for Computational Medicine (sgranite at jhu dot edu) The CardioVascular Research
An Information Sharing Platform Prototype for Hadron Therapy
 An Information Sharing Platform Prototype for Hadron Therapy Faustin Laurentiu Roman*, Daniel Abler, Vassiliki Kanellopoulos CERN IFIC, Univ. of Oxford, Univ. of Surrey partner-grid@cern.ch *presently
An Information Sharing Platform Prototype for Hadron Therapy Faustin Laurentiu Roman*, Daniel Abler, Vassiliki Kanellopoulos CERN IFIC, Univ. of Oxford, Univ. of Surrey partner-grid@cern.ch *presently
ReGraph: Bridging Relational and Graph Databases
 ReGraph: Bridging Relational and Graph Databases Patrícia Cavoto 1, André Santanchè 1 1 Laboratory of Information Systems (LIS) Institute of Computing (IC) Univeristy of Campinas (UNICAMP) Campinas SP
ReGraph: Bridging Relational and Graph Databases Patrícia Cavoto 1, André Santanchè 1 1 Laboratory of Information Systems (LIS) Institute of Computing (IC) Univeristy of Campinas (UNICAMP) Campinas SP
How to Guide. For Personal Users
 How to Guide For Personal Users March 2016 Contents Introduction... 2 Features and Functions:... 2 Accessing UICollaboratory... 3 Home Page... 3 Homepage Key Features... 3 Collaboration Map... 4 Search
How to Guide For Personal Users March 2016 Contents Introduction... 2 Features and Functions:... 2 Accessing UICollaboratory... 3 Home Page... 3 Homepage Key Features... 3 Collaboration Map... 4 Search
DRAGEN Bio-IT Platform Enabling the Global Genomic Infrastructure
 TM DRAGEN Bio-IT Platform Enabling the Global Genomic Infrastructure About DRAGEN Edico Genome s DRAGEN TM (Dynamic Read Analysis for GENomics) Bio-IT Platform provides ultra-rapid secondary analysis of
TM DRAGEN Bio-IT Platform Enabling the Global Genomic Infrastructure About DRAGEN Edico Genome s DRAGEN TM (Dynamic Read Analysis for GENomics) Bio-IT Platform provides ultra-rapid secondary analysis of
Informatics Common Metric Pre-Pilot Landscape Assessment
 Informatics Common Metric Pre-Pilot Landscape Assessment University of Rochester Center for Leading Innovation and Collaboration (CLIC) Funded by the National Center for Advancing Translational Sciences
Informatics Common Metric Pre-Pilot Landscape Assessment University of Rochester Center for Leading Innovation and Collaboration (CLIC) Funded by the National Center for Advancing Translational Sciences
Medici for Digital Cultural Heritage Libraries. George Tsouloupas, PhD The LinkSCEEM Project
 Medici for Digital Cultural Heritage Libraries George Tsouloupas, PhD The LinkSCEEM Project Overview of Digital Libraries A Digital Library: "An informal definition of a digital library is a managed collection
Medici for Digital Cultural Heritage Libraries George Tsouloupas, PhD The LinkSCEEM Project Overview of Digital Libraries A Digital Library: "An informal definition of a digital library is a managed collection
Beyond PACS. From the Radiological Imaging Archive to the Medical Imaging Global Repository. Patricio Ledesma Project Manager
 Beyond PACS From the Radiological Imaging Archive to the Medical Imaging Global Repository Patricio Ledesma Project Manager pledesma@medting.com Medical Imaging Usually Medical Imaging and Radiological
Beyond PACS From the Radiological Imaging Archive to the Medical Imaging Global Repository Patricio Ledesma Project Manager pledesma@medting.com Medical Imaging Usually Medical Imaging and Radiological
A DICOM-based streaming service for the Digital Operating Room
 A DICOM-based streaming service for the Digital Operating Room Release 0.00 Adrian Vázquez, Rafael Mayoral and Oliver Burgert July 10, 2009 ICCAS, Universität Leipzig, Semmelweisstraße 14, D-04103 Leipzig
A DICOM-based streaming service for the Digital Operating Room Release 0.00 Adrian Vázquez, Rafael Mayoral and Oliver Burgert July 10, 2009 ICCAS, Universität Leipzig, Semmelweisstraße 14, D-04103 Leipzig
Dynamic Data Integration in Clinical Research
 Dynamic Data Integration in Clinical Research Judith R. Logan, MD, MS Department of Medical Informatics & Clinical Epidemiology Oregon Health & Science University Buenos Aires, June 16, 2011 in collaboration
Dynamic Data Integration in Clinical Research Judith R. Logan, MD, MS Department of Medical Informatics & Clinical Epidemiology Oregon Health & Science University Buenos Aires, June 16, 2011 in collaboration
Increasing Interoperability, what is the Impact on Reliability? Illustrated with Health care examples
 Illustrated with Health care examples by Gerrit Muller University of South-Eastern Norway-NISE e-mail: gaudisite@gmail.com www.gaudisite.nl Abstract In all domains the amount of interoperability between
Illustrated with Health care examples by Gerrit Muller University of South-Eastern Norway-NISE e-mail: gaudisite@gmail.com www.gaudisite.nl Abstract In all domains the amount of interoperability between
Healthcare mobility: selecting the right device for better patient care
 Healthcare mobility: selecting the right device for better patient care How Fujitsu Mobile Solutions help accelerate digital transformation with human-centric innovation* Fujitsu Thought Leadership Report
Healthcare mobility: selecting the right device for better patient care How Fujitsu Mobile Solutions help accelerate digital transformation with human-centric innovation* Fujitsu Thought Leadership Report
SEMANTIC SUPPORT FOR MEDICAL IMAGE SEARCH AND RETRIEVAL
 SEMANTIC SUPPORT FOR MEDICAL IMAGE SEARCH AND RETRIEVAL Wang Wei, Payam M. Barnaghi School of Computer Science and Information Technology The University of Nottingham Malaysia Campus {Kcy3ww, payam.barnaghi}@nottingham.edu.my
SEMANTIC SUPPORT FOR MEDICAL IMAGE SEARCH AND RETRIEVAL Wang Wei, Payam M. Barnaghi School of Computer Science and Information Technology The University of Nottingham Malaysia Campus {Kcy3ww, payam.barnaghi}@nottingham.edu.my
European Commission Initiatives in telemedicine Presentation endorsed by the European Commission
 European Commission Initiatives in telemedicine Presentation endorsed by the European Commission Nicole Denjoy COCIR Secretary General How does the EU contribute to the large-scale deployment of telemedicine?
European Commission Initiatives in telemedicine Presentation endorsed by the European Commission Nicole Denjoy COCIR Secretary General How does the EU contribute to the large-scale deployment of telemedicine?
