ectd Next Major Release Business Requirements Collation (11 NOV 10) TOPIC Requirement Comment ICH Req No.
|
|
|
- Maurice Rodgers
- 6 years ago
- Views:
Transcription
1 ICH Req No. ICH001 ICH002 ICH003 ICH004 ectd Next Major Release Business Requirements Collation (11 NOV 10) TOPIC Requirement Comment A regulated product application may have one or more regulatory activities associated with it It must be possible to define a 'Regulatory Activity' to which a group of sequences will belong A regulatory activity may have one or more sequences associated with it The message should support the ability to provide one sequence to multiple regulatory activities which may span more than one application. sequence number values would need to be unique to each application ICH005 Each sequence should have a unique identifier ICH006 Capability to identify which ectd sequences were used at which step of agency review. ICH007 The message should not restrict adoption/implementation of the standard at any point in a product's lifecycle. ICH008 Compatible both to US/EU product wise lifecycle and to Japan applicationwise lifecycle. ICH009 ARCHIVE It must be possible to review and to archive the sequence without need for transformation ICH010 AUTHOR REVIEW It should be possible to review the ectd ICH011 AUTHOR REVIEW The specification should not restrict the types of file formats which can be submitted for use with the standard (allowed file formats are defined by each implementation and are not defined by the exchange specification). ICH012 AUTHOR REVIEW It should be possible for the reviewer to have access to the entire application from any part of the application. ICH013 AUTHOR REVIEW Clear definition of views across multiple sequences (cumulative, current) viewer/implementation requirement ICH014 BACKWARD COMPATIBLE Elements and attributes defined by V3.x should be able to be mapped to v4.0 constructs (and not the reverse) ICH016 BACKWARD The message should support continued use of the information and COMPATIBLE documentation provided with previous regional implementations, e.g., Module 1, STF. ICH017 DATASETS It should be possible to provide data definitions for data sets. ICH018 DOCUMENT It should be possible to support the inclusion of scanned documents, FORMATTING primarily for legacy documents ICH019 DOCUMENT METADATA It must be possible to describe, in free text, the titles of the files being submitted Title can be different per usage (e.g., context)
2 ICH020 ICH021 ICH024 ICH025 DOCUMENT METADATA DOCUMENT METADATA Every submitted file will have a unique identifier It must be possible for a Submitter to provide user defined information or identifier for a file DOCUMENT REUSE It must be possible to include by reference, a file that physically resides in the same or another sequence within the same regulatory activity, different regulatory activity within the same application or in a different application (e.g.. cross product submission support) DOCUMENT REUSE It must be possible to include, by reference, a file that has been submitted in a previous sequence and to be able to identify that this is not new but is being used in a different context For each file submitted by the Sponsor, a data field is present in the message for the Sponsor to include Sponsor-specified meta data (e.g., Sponsor internal version number or tracking information or OID). This requirement is currently provided by the version attribute in the ICH ectd v3.2.2 leaf element and should continue as an option in the ectd NMV. As this free text field refers to the actual content file, it should be an attribute of the file element in the message. It is proposed the field be called something like owners-version-number or senders-identifier (note wording to account for two way communication). This field would be optional. A file has been submitted to a dossier as part of an application's supportive documentation. The file is assigned a unique identifier in an ectd submission. The information contained in the file is later required to be available for use in the review of a supplement/additional application. The applicant wishes to include the previously submitted file in the supplement/additional application by referring to the file by its unique identifier rather than submitting the file for a second time. A reviewer viewing the supplement/additional application would view the file as though it was submitted to the supplement/additional application along with the other supportive documentation that was actually submitted. implementation Reviewer Capability ICH026 ENVELOPE It must be possible to assign an identifier to a sequence to imply an order, to enable sorting (e.g., chronology or review order) or just to assign uniqueness within an application [unique identifier for the sequence within the application and a friendly identifier] ICH027 ENVELOPE It must be possible to identify the regulatory agency for which a specific sequence is intended ICH028 ENVELOPE It must be possible to identify the applicant submitting a sequence ICH029 ENVELOPE It must be possible to unambiguously associate a sequence to the application(s) to which it belongs ICH030 ENVELOPE It must be possible to assign a submission type being used for the sequence ICH031 ENVELOPE It must be possible to describe, in free text, the sequence (include a short description of the sequence in the administrative section) ICH032 ENVELOPE It must be possible to identify the Procedure type being used for the application ICH033 ENVELOPE It must be possible to assign an invented name (trade name) for the product covered by the application ICH034 ENVELOPE It must be possible to assign an international non proprietory name(s) (inn) for the drug substance(s) covered by the application Not just EU Regional Not just EU Regional
3 ICH035 EU REGIONAL It must be possible to identify to which specific country a file is relevant ICH036 EU REGIONAL It must be possible to identify that a file is relevant to all countries ICH037 EU REGIONAL It must be possible to identify for which country(ies) a specific sequence is intended ICH038 HARMONISATION The message should support ICH harmonized content (documentation and metadata) and ICH regional content ICH039 HARMONISATION Need to provide a structure that supports all terminologies for dossier (all controlled vocabularies / Implementation Guide regulatory activity related to a product) and regulatory activity (collection of sequences that lead to a decision by the regulatory agency (NDS, SNDS)) which can be mapped to individual ICH regional regulatory processes ICH040 HARMONISATION Files should only need to be submitted once to a Health Authority and can Clarify meaning of application be included by reference in multiple sequences to support multiple regulatory actions even across applications ICH041 HARMONISATION Ability to reuse of ectd submitted in other regions. e.g. reuse of leaf files, Industry building tool need XML instance by module, ecsr. To achieve this, it is critical to distinguish global part and regional part even in Module2 5, not only in Module1. ICH042 HARMONISATION When the same documentation is provided, it should be submitted in the same way across HAs. For example, when a study report is submitted in US it is submitted using the STF which is not acceptable in other HAs. This minimizes reuse capabilities and adds to Industry costs to prepare globally harmonized dossiers. ICH043 HYPERLINKING It should be possible for the applicant to include hyperlinks between information ICH044 HYPERLINKING It should be possible to utilise relative addressing for all links. ICH046 HYPERLINKING Need to support relative links across the product lifecycle ICH047 ICH PROCEDURE For validation purposes, It should be able to uniquely identify: where in CTD a leaf belongs the relationship between leafs the lifecycle relationship (append, delete, or replace) between files the relationship between sequences the relationship of a sequence to a regulatory activity the relationship between applications the type of relationship (parent child, reference, etc) ICH048 JP REGIONAL Message should support the ability to provide second and subsequent sequences which contain only additional information in XML Instance Principle Implementation Guide
4 ICH049 LANGUAGE It must be possible to assign a language to a document ICH050 LANGUAGE It must be possible to (incorporate unicode character sets) to deal with languages such as Bulgarian and Greek ICH051 LANGUAGE It must be possible to include files with 1 or 2 byte characters, or a mixture of both ICH052 LANGUAGE ectd viewer should recognize section titles defined in CTD, e.g. "2.5 Clinical Overview". It should have an interface capable to show CTD section titles in any languages by switching standard dictionary provided by regional agencies. ICH053 The message should provide the ability to assign new metadata to update information previously submitted e.g., related sequences, submission type, operation attribute, manufacturer name, etc. ICH054 The message should provide the ability to unassign metadata previously submitted, e.g., related sequences, submission type, operation attribute, manufacturer name, etc. ICH055 The message should provide the ability to revise metadata previously submitted, e.g., related sequences, submission type, operation attribute, manufacturer name, etc. ICH056 Information provided in the message ( i.e., metadata) used to categorize documentation (e.g., attributes of drug substance, manufacturer, etc) or supplied in the regional envelope (e.g., Company Name, Sponsor) can be modified (i.e., added, edited, removed) during the life cycle of the application. ICH057 Replacement of multiple leafs with single leaf and vice versa should be supported in ectd. ICH058 The process for concatenating individual sequences into a combined sequence view (i.e., the current view and the cumulative view) must be unambiguously defined ICH059 ICH060 LOGICAL GROUPINGS LOGICAL GROUPINGS Provide ability to group a collection (or set) of files that together represent a document or reviewable grouping (e.g., all files related to a study report, all files related to a labeling document, all files related to a manufacturer or manufacturing component (e.g., container closure)) Provide ability to treat a grouping of files as a single entity and to be treated as if it were a single file (complete with all descriptive attributes e.g., title) for all life cycle operations and relationship management and reuse needs ICH061 PHYSICAL FILE RULES Filenames can include underscores Deal with greek and cyrillic viewer requirement / controlled vocabulary Delete examples viewer requirement / implementation ICH062 PHYSICAL FILE RULES It should be possible to constrain the contents to ensure there are no security settings, such as passwords. Implementation Guide
5 ICH063 PHYSICAL FILE RULES The physical file structure. (file/folder structure) should be minimal ICH064 PHYSICAL FILE RULES The technical message should not restrict the types of files that may be transferred. However, implementation guides may restrict the types of files and versions of file formats to be transferred or may specify unique file formats for that region. ICH065 PHYSICAL FILE RULES It should be possible to support file systems of different operating systems ICH066 PHYSICAL FILE RULES It must be possible to constrain the maximum size of any file (reword to Implementation issue reflect ICH minimum standard) ICH067 SCOPE Allow the capacity to modify the ICH CTD organizational structure (ToC) without modifying or changing the ectd message structure ICH068 SCOPE It should be possible to compile an ectd equivalent to the CTD ICH069 STANDARDS The message should interoperate with other healthcare standards, e.g. use controlled vocabularies from established standard based vocabularies ICH070 STANDARDS It should be possible to restrict the technology utilized to use open (ISO, Principle W3C, IETF) standards when ever possible. ICH071 STRUCTURE It must be possible to constrain the inclusion of documents at inappropriate locations in the submission structure (e.g.. at highest levels of ectd) ICH072 STRUCTURE The message should allow for the control/enforcement of document/structural granularity. ICH073 STRUCTURE It must be possible to assign 'attributes' to the contents of specific sections to support the ICH CTD organizational structure (e.g., repeating section 3.2.S) ICH074 STRUCTURE It must be possible to ensure that all files submitted are defined and referenced ICH075 STRUCTURE It must be possible to validate the contents of a sequence against the CTD (e.g., module 6 is invalid) ICH076 STRUCTURE It should be possible to identify all of the files relevant to a specific section of the CTD ICH077 STRUCTURE It should be possible to review the sequence in its entirety or in sections. Method of transmission should support the consumption ICH078 TECHNOLOGY The standard must not be constrained by the need for delivery via a particular medium ICH079 TECHNOLOGY It should be possible to include colour and black & white images ICH080 TECHNOLOGY It should be possible to support the introduction of new technology to aid in the review process. ICH081 TERMINOLOGY The message should support the use of controlled vocabularies for harmonized metadata ICH082 TERMINOLOGY The message should support the use of controlled vocabularies for regional metadata.
6 ICH083 TERMINOLOGY It should be possible to specify date values in an unambiguous manner. ICH084 TRANSFER/SECURITY The message should support a means to enable the validation of the integrity of the electronic files within an instance ICH085 TRANSFER/SECURITY The message standard should not restrict the mechanism for transmitting the message (e.g., media type, network) ICH086 TRANSFER/SECURITY The message standard should not restrict or prevent regionally implemented secure electronic message delivery standards ICH087 TWO WAY The message should support submission of a sequence from a regulator to COMMUNICATION a regulated party. ICH090 TWO WAY COMMUNICATION It should be possible to define the security methods to be used for transmission to the agencies and acknowledgement from the agency. ICH091 US REGIONAL The message should support the identification of the role of the instance within the identified regulatory activity, e.g. presubmision, application, amendment, etc. ICH092 US REGIONAL The message should support the identification of the regulatory activity associated with the instance, e.g. original application, labelingsupplement, etc. ICH093 VALIDATION It must be possible to define unambiguously, the validation criteria for a sequence ICH094 VALIDATION Message should contain sufficient information to unambiguously identify which version(s) of the DTD/Schema and controlling vocabularies was used to create the instance ICH095 VALIDATION The message should not require the submission of the DTD/Schema and controlling vocabularies with each instance ICH097 COMPATIBILITY It should be possible for an applicant to build on an ectd lifecycle started using the ectd 3.2.x specification and continued using the ectd NMV specification ICH098 COMPATIBILITY No applicant should be required to resubmit data in the ectd NMV specification if it has previously been submitted using the ectd 3.2.x specification. (It is recognised that in the future, further major versions of the ectd specification may require data migration guidance to ensure the use of data over the life of a drug product). Implementation GuIde Principle ICH099 COMPATIBILITY Tools designed to view ectd NMV sequences must also be able to view a lifecycle started with the ectd 3.2.x specification. However, the reverse requirement is not needed (i.e. it is not needed that tools for the ectd 3.2.x specification should be able to view sequences created using the ectd NMV specification). ICH100 COMPATIBILITY It is expected that once an ectd lifecycle is transitioned to the ectd NMV specification, then no further submissions/sequences will be made in the ectd 3.2.x specification.
7 ICH101 COMPATIBILITY The implementation guide must state how lifecycle relationships can be maintained from ectd 3.2.x to ectd NMV. ICH102 COMPATIBILITY Ability to reuse content; files submitted in ectd sequences can be referenced in ectd NMV submission units ICH104 DESIGN CONCEPTS The file format of the message should be xml based. ICH105 DESIGN CONCEPTS The message standard should not prevent or restrict the ability to e sign the message. ICH106 DESIGN CONCEPTS The message standard should not prevent or restrict the ability to encrypt the message for secure transfer purposes ICH107 DESIGN CONCEPTS The message standard must not require encryption ICH108 INTEGRITY Integrity checks for all files included in the sequence are required. ICH109 DESIGN CONCEPTS The message should provide the ability to identify further specific usage of the file (e.g., SPL, SDTM, application format, packaging insert, CTN) beyond that defined by the CTD ICH110 INTEGRITY The ability to specify which algorithm is being used for file integrity checks is required. ICH112 TWO WAY COMMUNICATION It must be possible to relate any message to a particular message, regulated activity and/or application. Every ectd message must be uniquely identifiable. ICH113 TWO WAY COMMUNICATION ICH114 DESIGN CONCEPTS In, the number of xml files managing content should be kept to a minimum and use a consistent technical design approach even though the content models may differ regionally ICH115 ENVELOPE The message standard must provide the ability to include information required for the processing (e.g., message standard version) and integrity (e.g., checksum) of the message ICH116 ENVELOPE The message standard must provide a three level hierarchy of application, regulatory activity and submission unit. ICH117 ENVELOPE The message standard must provide information about the product. enumerate data points to be required regionally ICH118 ENVELOPE The message standard must provide enough information to identify the sender. ICH119 ENVELOPE The message standard must provide enough information to identify the recipient. enumerate data points to be required regionally enumerate data points to be required regionally
8 ICH121 The order/sequence of leaf elements within a CTD section must be able to be controlled ICH122 A file can be displayed in multiple sections of the CTD (preserving the leaf file concept in the current ectd specification) ICH123 Maintain a similar file leaf model as in the current ectd in the ectd NMV, with the following exception/changes: a. The operation attribute value append be removed from the list of allowed values (leaving only new, replace and delete) b. Allow a replace or delete leaf to modify more than one leaf in a previous sequence or sequences c. Allow a single leaf to be modified by more than one leaf in later sequences (supports changes in granularity) ICH124 A file can be replaced in one existing ectd section or context without impacting the use of the file in other ectd sections or contexts ICH125 Life cycle operations must occur within the same context as the existing (target) leaf ICH126 There should be no restrictions on the characters used in controlled vocabularies ICH127 There should be a basic ICH stylesheet for presentation purposes ICH128 STF construct should be integrated into the message standard ICH129 Cardinality rules of the current ectd Specification should be retained plus those cited in approved Change Requests (e.g., CR#1490/1500 Module 3.2.A.3 will be made a repeating attribute in Version 3.2 of the specification based on excipient). ICH130 DESIGN CONCEPTS The message standard should allow the ability to e sign the message.
ectd Next Major Release Business Requirements Collation (9-JUN-10) TOPIC Requirement Comment
 ICH Req No. ectd Next Major Release Business Requirements Collation (9-JUN-10) TOPIC Requirement Comment ICH001 ICH002 ICH003 ICH004 APPLICATION APPLICATION APPLICATION APPLICATION A regulated product
ICH Req No. ectd Next Major Release Business Requirements Collation (9-JUN-10) TOPIC Requirement Comment ICH001 ICH002 ICH003 ICH004 APPLICATION APPLICATION APPLICATION APPLICATION A regulated product
ectd Next Major Version Business Requirements (11-JUN-09)
 ICH Req No. ICH01 ICH02 ICH03 ICH04 TOPIC APPLICATION APPLICATION APPLICATION APPLICATION ectd Next Major Version Business Requirements (11-JUN-09) Requirement A regulated product application may have
ICH Req No. ICH01 ICH02 ICH03 ICH04 TOPIC APPLICATION APPLICATION APPLICATION APPLICATION ectd Next Major Version Business Requirements (11-JUN-09) Requirement A regulated product application may have
RPS/ICH Requirements. sequence number values would need to be unique to each application. principle. 1 of 14
 o. ICH01 ectd ext Major Release Business Requirements Collation (12 OV 08) APPLICATIO A regulated product application may have one or LIFECCLE more regulatory activities associated with it RPS 1 included
o. ICH01 ectd ext Major Release Business Requirements Collation (12 OV 08) APPLICATIO A regulated product application may have one or LIFECCLE more regulatory activities associated with it RPS 1 included
Orientation Material for M8: ectd EWG ectd v4.0 Implementation Package v1.3
 Orientation Material for M8: ectd EWG ectd v4.0 Implementation Package v1.3 International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal Notice This presentation
Orientation Material for M8: ectd EWG ectd v4.0 Implementation Package v1.3 International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal Notice This presentation
Get ready for ectd in South Africa. Current status at MCC
 Get ready for ectd in South Africa Current status at MCC E Taute Feb 2013 Overview Background Guidelines, Specifications, Forms ICH ectd Specification V 3.2.2 16-July-2008 2.21 South African Specification
Get ready for ectd in South Africa Current status at MCC E Taute Feb 2013 Overview Background Guidelines, Specifications, Forms ICH ectd Specification V 3.2.2 16-July-2008 2.21 South African Specification
ectd : Industry Experience
 ectd : Industry Experience SAPRAA - 26 March 2010 Anita Smal Agenda Introduction Experience with MCC pilot project Lessons learnt ectd structure Life cycle Further guidance required from MCC Submission-ready
ectd : Industry Experience SAPRAA - 26 March 2010 Anita Smal Agenda Introduction Experience with MCC pilot project Lessons learnt ectd structure Life cycle Further guidance required from MCC Submission-ready
1. Document Control 2. Change Record Version Date Author(s) Comments 3. Reviewers Version Name Organisation 4. Distribution Version Date Name Status
 for the paper submission of regulatory information in support of a marketing authorisation application when using the Electronic Common Technical Document ( ectd ) as the source submission. V1.0 February
for the paper submission of regulatory information in support of a marketing authorisation application when using the Electronic Common Technical Document ( ectd ) as the source submission. V1.0 February
1 2 December esubmissions? 1. How can baseline dossiers support global esubmissions? Disclaimer. What is a baseline submission?
 How can baseline dossiers support global Hans van Bruggen www.ectdconsultancy.com How can baseline dossiers support global 1 Disclaimer The views and opinions expressed in the following PowerPoint slides
How can baseline dossiers support global Hans van Bruggen www.ectdconsultancy.com How can baseline dossiers support global 1 Disclaimer The views and opinions expressed in the following PowerPoint slides
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL Questions & Answers Implementation of ectd in South Africa This document is intended to provide clarity on guidelines and specifications for applications for the registration
MEDICINES CONTROL COUNCIL Questions & Answers Implementation of ectd in South Africa This document is intended to provide clarity on guidelines and specifications for applications for the registration
INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE
 USER GUIDE Use of EDQM terminologies for Dose Forms and Routes of Administration for Individual Case Safety Reports in E2B(R3) message Version 1.0, 16 November 2017 Page 1 of 8 Document History Date of
USER GUIDE Use of EDQM terminologies for Dose Forms and Routes of Administration for Individual Case Safety Reports in E2B(R3) message Version 1.0, 16 November 2017 Page 1 of 8 Document History Date of
Work instructions for US-FDA ANDA Submissions Work Instructions for ectd US-FDA ANDA Submissions
 Work Instructions for ectd US-FDA ANDA Submissions 1. Logon to the PharmaReady ectd System Work instructions for US-FDA ANDA Submissions PharmaReady is a Web-based application. The logon screen and all
Work Instructions for ectd US-FDA ANDA Submissions 1. Logon to the PharmaReady ectd System Work instructions for US-FDA ANDA Submissions PharmaReady is a Web-based application. The logon screen and all
Variations in ectd format Q&A document
 February 2015 Q&A document This document uses a question and answer format to give some guidance when submitting variation applications in ectd format. For general guidance on variations, please refer
February 2015 Q&A document This document uses a question and answer format to give some guidance when submitting variation applications in ectd format. For general guidance on variations, please refer
GDUFA s Impact on ectds / Case Study: Implementing PDF Standards
 GDUFA s Impact on ectds / Case Study: Implementing PDF Standards GPhA 2015 Fall Technical Conference GDUFA S Impact on ectds GPhA 2015 Fall Technical Conference 1 Introductions Kevin Tompkins Director,
GDUFA s Impact on ectds / Case Study: Implementing PDF Standards GPhA 2015 Fall Technical Conference GDUFA S Impact on ectds GPhA 2015 Fall Technical Conference 1 Introductions Kevin Tompkins Director,
Release Notes for TIGes ectd Guidance Comparison of version 2.0 with 1.0
 General Changes throughout the document Previous NA Changes EMEA changed to EMA Cover Page NA Draft status of document removed. Changed the name of the document. 1. Introduction 1 Fully rewritten to align
General Changes throughout the document Previous NA Changes EMEA changed to EMA Cover Page NA Draft status of document removed. Changed the name of the document. 1. Introduction 1 Fully rewritten to align
US Food and Drug Administration. Revision History
 Specifications for ectd Validation Criteria US Food and Drug Administration Specifications for ectd Validation Criteria Revision History Date Description Version 2008-03-10 Initial Release of ectd Validation
Specifications for ectd Validation Criteria US Food and Drug Administration Specifications for ectd Validation Criteria Revision History Date Description Version 2008-03-10 Initial Release of ectd Validation
Steps to RPS Adoption
 Steps to RPS Adoption Joel Finkle Advisor, Emerging Practices CSC Life Sciences BPS Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
Steps to RPS Adoption Joel Finkle Advisor, Emerging Practices CSC Life Sciences BPS Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
THE OECD GLOBAL HARMONIZED SUBMISSION TRANSPORT STANDARD (GHSTS) PROJECT
 THE OECD GLOBAL HARMONIZED SUBMISSION TRANSPORT STANDARD (GHSTS) PROJECT May 2014 History Pesticide Regulatory Process and IT Requirements Duplicate efforts by industry to meet various authority needs
THE OECD GLOBAL HARMONIZED SUBMISSION TRANSPORT STANDARD (GHSTS) PROJECT May 2014 History Pesticide Regulatory Process and IT Requirements Duplicate efforts by industry to meet various authority needs
M2 Glossary of Terms and Abbreviations
 M2 Glossary of Terms and Abbreviations 11 June 2015 M2: Electronic Standards for the Transfer of Regulatory Information Updated at ICH Expert Working Group meeting, Fukuoka, June 2015 Definitions... 2
M2 Glossary of Terms and Abbreviations 11 June 2015 M2: Electronic Standards for the Transfer of Regulatory Information Updated at ICH Expert Working Group meeting, Fukuoka, June 2015 Definitions... 2
Guidance for electronic and paper submissions for Certificates of Suitability (CEP) applications
 FK/CB PUBLIC DOCUMENT (LEVEL 1) English only/nglais seulement Strasbourg, June 2013 Certification of suitability to Monographs of the European Pharmacopoeia Guidance for electronic and paper submissions
FK/CB PUBLIC DOCUMENT (LEVEL 1) English only/nglais seulement Strasbourg, June 2013 Certification of suitability to Monographs of the European Pharmacopoeia Guidance for electronic and paper submissions
ectd Next Major Version / Regulated Product Submission
 ectd Next Major Version / Regulated Product Submission Trusted ectd Solutions At GlobalSubmit, we re not only thought leaders, we re trusted advisors to the FDA, to our clients and to agencies worldwide.
ectd Next Major Version / Regulated Product Submission Trusted ectd Solutions At GlobalSubmit, we re not only thought leaders, we re trusted advisors to the FDA, to our clients and to agencies worldwide.
ICH Topic M 4 Common Technical Document for the Registration of Pharmaceuticals for Human Use Organisation CTD. Step 5
 European Medicines Agency February 2004 CPMP/ICH/2887/99 ICH Topic M 4 Common Technical Document for the Registration of Pharmaceuticals for Human Use Organisation CTD Step 5 COMMON TECHNICAL DOCUMENT
European Medicines Agency February 2004 CPMP/ICH/2887/99 ICH Topic M 4 Common Technical Document for the Registration of Pharmaceuticals for Human Use Organisation CTD Step 5 COMMON TECHNICAL DOCUMENT
Guidance for electronic submissions for Certificates of Suitability (CEP) applications
 CBW/CB PUBLIC DOCUMENT (Level 1) English only/nglais seulement Strasbourg, January 2018 Certification of Suitability to the Monographs of the European Pharmacopoeia Guidance for electronic submissions
CBW/CB PUBLIC DOCUMENT (Level 1) English only/nglais seulement Strasbourg, January 2018 Certification of Suitability to the Monographs of the European Pharmacopoeia Guidance for electronic submissions
Get ready for ectd in South Africa
 Get ready for ectd in South Africa Going from CTD to ectd Anita Smal, 14 & 15 February 2013 Agenda The goal Planning Prepare submission ready documents PDFs ectd content planning ectd structure planning
Get ready for ectd in South Africa Going from CTD to ectd Anita Smal, 14 & 15 February 2013 Agenda The goal Planning Prepare submission ready documents PDFs ectd content planning ectd structure planning
ectd in Canada Ginette Larocque, Purdue Pharma Canada August 23, 2016
 ectd in Canada Ginette Larocque, Purdue Pharma Canada August 23, 2016 AGENDA Eligibility for Filing in ectd Format ectd Mandatory? Structure and Content LCM Table and HC-SC 3011 Form Info Module 1 Administrative
ectd in Canada Ginette Larocque, Purdue Pharma Canada August 23, 2016 AGENDA Eligibility for Filing in ectd Format ectd Mandatory? Structure and Content LCM Table and HC-SC 3011 Form Info Module 1 Administrative
ICH M8 Expert Working Group. Specification for Submission Formats for ectd v1.1
 INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE ICH M8 Expert Working Group Specification for Submission Formats for ectd v1.1 November 10, 2016 DOCUMENT
INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE ICH M8 Expert Working Group Specification for Submission Formats for ectd v1.1 November 10, 2016 DOCUMENT
A CMC Reviewer s Perspective on the Quality Overall Summary. Arthur B. Shaw, Ph.D. FDA/CDER/ONDQA FDA DMF Expert June 15, 2010.
 A CMC Reviewer s Perspective on the Quality Overall Summary and Module 3 Arthur B. Shaw, Ph.D. FDA/CDER/ONDQA FDA DMF Expert June 15, 2010 Disclaimer The views and opinions expressed in the following PowerPoint
A CMC Reviewer s Perspective on the Quality Overall Summary and Module 3 Arthur B. Shaw, Ph.D. FDA/CDER/ONDQA FDA DMF Expert June 15, 2010 Disclaimer The views and opinions expressed in the following PowerPoint
ectd TECHNICAL CONFORMANCE GUIDE
 ectd TECHNICAL CONFORMANCE GUIDE Technical Specifications Document This Document is incorporated by reference into the following Guidance Document(s): Guidance for Industry Providing Regulatory Submissions
ectd TECHNICAL CONFORMANCE GUIDE Technical Specifications Document This Document is incorporated by reference into the following Guidance Document(s): Guidance for Industry Providing Regulatory Submissions
Update of ectd Module 1 specification for South Africa V2.0
 Update of ectd Module 1 specification for South Africa V2.0 Dr. Silke Nolkemper, Senior Business Consultant Christine Hirt, Managing Consultant Pretoria Copyright 2016 EXTEDO. All rights reserved. 2 New
Update of ectd Module 1 specification for South Africa V2.0 Dr. Silke Nolkemper, Senior Business Consultant Christine Hirt, Managing Consultant Pretoria Copyright 2016 EXTEDO. All rights reserved. 2 New
Administrative Guideline. SMPTE Metadata Registers Maintenance and Publication SMPTE AG 18:2017. Table of Contents
 SMPTE AG 18:2017 Administrative Guideline SMPTE Metadata Registers Maintenance and Publication Page 1 of 20 pages Table of Contents 1 Scope 3 2 Conformance Notation 3 3 Normative References 3 4 Definitions
SMPTE AG 18:2017 Administrative Guideline SMPTE Metadata Registers Maintenance and Publication Page 1 of 20 pages Table of Contents 1 Scope 3 2 Conformance Notation 3 3 Normative References 3 4 Definitions
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL GUIDANCE FOR THE SUBMISSION OF REGULATORY INFORMATION IN ectd FORMAT This guideline is intended to provide recommendations to applicants wishing to submit applications for the
MEDICINES CONTROL COUNCIL GUIDANCE FOR THE SUBMISSION OF REGULATORY INFORMATION IN ectd FORMAT This guideline is intended to provide recommendations to applicants wishing to submit applications for the
Criteria Webinar. 13 th July European Update on Validation of ectd & NeeS submissions, July 2012
 EU ectd and NeeS Validation Criteria Webinar TIGes Harmonisation Group 13 th July 2012 1 Presenters of the day from TIGes Harmonisation Group Alastair Nixon (EFPIA) Klaus Menges ges( (DE) Manuela Copier
EU ectd and NeeS Validation Criteria Webinar TIGes Harmonisation Group 13 th July 2012 1 Presenters of the day from TIGes Harmonisation Group Alastair Nixon (EFPIA) Klaus Menges ges( (DE) Manuela Copier
Guideline on the specifications for provision of an electronic submission (e-submission) for a veterinary medicinal product
 Guideline prepared by the TIGes-Vet Version 1.0 June 2009 Guideline on the specifications for provision of an electronic submission (e-submission) for a veterinary medicinal product 1. Introduction This
Guideline prepared by the TIGes-Vet Version 1.0 June 2009 Guideline on the specifications for provision of an electronic submission (e-submission) for a veterinary medicinal product 1. Introduction This
ectd Practical experiences of the ectd pilot project and way forward
 ectd Practical experiences of the ectd pilot project and way forward 26 August 2016 SAPRAA Estelle Taute 1 Overview Pilot Project Specifications & Guidelines Requirements vs Actual Validation issues Lifecycle
ectd Practical experiences of the ectd pilot project and way forward 26 August 2016 SAPRAA Estelle Taute 1 Overview Pilot Project Specifications & Guidelines Requirements vs Actual Validation issues Lifecycle
TH ectd Specification
 TH ectd Specification Module 1 and Regional Information Version 0.91, August 2014 About the Food and Drug Administration Thailand - Bureau of Drug Control The Bureau of Drug Control has set a vision as
TH ectd Specification Module 1 and Regional Information Version 0.91, August 2014 About the Food and Drug Administration Thailand - Bureau of Drug Control The Bureau of Drug Control has set a vision as
1 Supported Validation Sets
 EURS and EURSvalidator 5.9 Supported Validation Sets and Functionality 1 Supported Validation Sets The following table provides a summary of the available validation sets (x) in EURS and EURSvalidator.
EURS and EURSvalidator 5.9 Supported Validation Sets and Functionality 1 Supported Validation Sets The following table provides a summary of the available validation sets (x) in EURS and EURSvalidator.
EU Module 1 v1.4 Release Notes August 2009
 EU Module 1 v1.4 Release Notes August 2009 DOCUMENT CHANGE RECORD Version Date Description Sections 0.1 15/07/2009 First version All 1.0 06/08/2009 Final Inclusion of comments All REVIEW Version Date Person
EU Module 1 v1.4 Release Notes August 2009 DOCUMENT CHANGE RECORD Version Date Description Sections 0.1 15/07/2009 First version All 1.0 06/08/2009 Final Inclusion of comments All REVIEW Version Date Person
About Domestic Implementation of the Electronic File Specifications to Be Included in the ICH Electronic Common Technical Document (ectd) v1.1.
 Appendix 2 About Domestic Implementation of the Electronic File Specifications to Be Included in the ICH Electronic Common Technical Document (ectd) v1.1.0 between the Japanese original and the English
Appendix 2 About Domestic Implementation of the Electronic File Specifications to Be Included in the ICH Electronic Common Technical Document (ectd) v1.1.0 between the Japanese original and the English
The ectd Backbone Files Specification for Module 1. The ectd BACKBONE FILES SPECIFICATION FOR MODULE 1
 The ectd Backbone Files Specification for Module 1 The ectd BACKBONE FILES SPECIFICATION FOR MODULE 1 Revision History Date Version Summary of Changes 2003-08-13 1.0 Original version 2004-03-01 1.1 Clarifications
The ectd Backbone Files Specification for Module 1 The ectd BACKBONE FILES SPECIFICATION FOR MODULE 1 Revision History Date Version Summary of Changes 2003-08-13 1.0 Original version 2004-03-01 1.1 Clarifications
Revision of Technical Conformance Guide on Electronic Study Data Submissions
 Notification No. 0824001 August 24, 2016 To: Prefectural Health Department (Bureau) Director of the Advanced Review with Electronic Data Promotion Group, Pharmaceuticals and Medical Devices Agency Revision
Notification No. 0824001 August 24, 2016 To: Prefectural Health Department (Bureau) Director of the Advanced Review with Electronic Data Promotion Group, Pharmaceuticals and Medical Devices Agency Revision
ICH M2 REDACTION: POINTS TO CONSIDER
 1 PURPOSE OF THIS DOCUMENT ICH M2 REDACTION: POINTS TO CONSIDER This document provides points to consider on the topic of redaction of information from documents submitted to regulatory authorities and
1 PURPOSE OF THIS DOCUMENT ICH M2 REDACTION: POINTS TO CONSIDER This document provides points to consider on the topic of redaction of information from documents submitted to regulatory authorities and
CDASH Standards and EDC CRF Library. Guang-liang Wang September 18, Q3 DCDISC Meeting
 CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
EMEA IMPLEMENTATION OF ELECTRONIC ONLY SUBMISSION AND ectd SUBMISSION:
 European Medicines Agency Evaluation of Medicines for Human Use London, 20th July 2008 EMEA/596881/2007 v0.4 EMEA IMPLEMENTATION OF ELECTRONIC ONLY SUBMISSION AND ectd SUBMISSION: QUESTIONS AND ANSWERS
European Medicines Agency Evaluation of Medicines for Human Use London, 20th July 2008 EMEA/596881/2007 v0.4 EMEA IMPLEMENTATION OF ELECTRONIC ONLY SUBMISSION AND ectd SUBMISSION: QUESTIONS AND ANSWERS
ANSM 11/ Page 1 sur 6
 NOTICE TO ASMF HOLDERS FOR ELECTRONIC SUBMISSION OF ASMF TO ANSM VIA CESP... 2 1. ectd Format... 2 a. MR/DC Procedures... 2 b. National Procedures... 2 c. ASMF Guidance... 2 d. Recommendations... 2 2.
NOTICE TO ASMF HOLDERS FOR ELECTRONIC SUBMISSION OF ASMF TO ANSM VIA CESP... 2 1. ectd Format... 2 a. MR/DC Procedures... 2 b. National Procedures... 2 c. ASMF Guidance... 2 d. Recommendations... 2 2.
Guidance for Industry on Providing Regulatory Information in Electronic Format: ectd electronic Submissions
 DRAFT FOR TESTING Guidance for Industry on Providing Regulatory Information in Electronic Format: ectd electronic Submissions This document is published under the auspices of the EU Telematic Implementation
DRAFT FOR TESTING Guidance for Industry on Providing Regulatory Information in Electronic Format: ectd electronic Submissions This document is published under the auspices of the EU Telematic Implementation
ectd Tools Compatibility and Interoperability: Discussion and Practical Experience
 ectd Tools Compatibility and Interoperability: Discussion and Practical Experience Gabriel Bohl, Regulatory Affairs Manager, Quintiles, France DIA EDM 3-4 Dec 2009 Vienna Disclaimer The views and opinions
ectd Tools Compatibility and Interoperability: Discussion and Practical Experience Gabriel Bohl, Regulatory Affairs Manager, Quintiles, France DIA EDM 3-4 Dec 2009 Vienna Disclaimer The views and opinions
Biotechnology Industry Organization 1225 Eye Street NW, Suite 400 Washington, DC 20006
 Biotechnology Industry Organization 1225 Eye Street NW, Suite 400 Washington, DC 20006 December 22, 2003 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville,
Biotechnology Industry Organization 1225 Eye Street NW, Suite 400 Washington, DC 20006 December 22, 2003 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville,
Important Notes: The XML and there corresponding images should be added with the proper file names.
 ectd FAQ Contents ECTD HOW TO GUIDE... 3 SPL Naming Convention in ectd... 3 Procedure to Send SPC documents in WORD format... 4 File name exceeds maximum length (64 characters) per ectd specification...
ectd FAQ Contents ECTD HOW TO GUIDE... 3 SPL Naming Convention in ectd... 3 Procedure to Send SPC documents in WORD format... 4 File name exceeds maximum length (64 characters) per ectd specification...
Indexing Field Descriptions Recommended Practice
 Indexing Field Descriptions Recommended Practice Service Alberta Enterprise Information Management Developed: Last Updated: https://www.alberta.ca/enterprise-information-management.aspx Contents Indexing...
Indexing Field Descriptions Recommended Practice Service Alberta Enterprise Information Management Developed: Last Updated: https://www.alberta.ca/enterprise-information-management.aspx Contents Indexing...
Pharmaceuticals, Health Care, and Life Sciences. An Approach to CDISC SDTM Implementation for Clinical Trials Data
 An Approach to CDISC SDTM Implementation for Clinical Trials Data William T. Chen, Merck Research Laboratories, Rahway, NJ Margaret M. Coughlin, Merck Research Laboratories, Rahway, NJ ABSTRACT The Clinical
An Approach to CDISC SDTM Implementation for Clinical Trials Data William T. Chen, Merck Research Laboratories, Rahway, NJ Margaret M. Coughlin, Merck Research Laboratories, Rahway, NJ ABSTRACT The Clinical
esubmission Gateway and Web Client Training on the use of XML delivery files for Veterinary submissions
 esubmission Gateway and Web Client Training on the use of XML delivery files for Veterinary submissions Webinar training on v1.0 Presented by Kristiina Puusaari on 3 June 2016 An agency of the European
esubmission Gateway and Web Client Training on the use of XML delivery files for Veterinary submissions Webinar training on v1.0 Presented by Kristiina Puusaari on 3 June 2016 An agency of the European
Preparing for FDA Mandated ectd Submissions
 Preparing for FDA Mandated ectd Submissions Outsourcing in Clinical Trials West Coast 2016 11 Feb 2016 2 Agenda Introduction FDA Regulatory Background FDA Timetable International ectd What is an ectd Options
Preparing for FDA Mandated ectd Submissions Outsourcing in Clinical Trials West Coast 2016 11 Feb 2016 2 Agenda Introduction FDA Regulatory Background FDA Timetable International ectd What is an ectd Options
Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library
 PharmaSUG 2018 - Paper SS-12 Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library Veena Nataraj, Erica Davis, Shire ABSTRACT Establishing internal Data Standards helps companies
PharmaSUG 2018 - Paper SS-12 Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library Veena Nataraj, Erica Davis, Shire ABSTRACT Establishing internal Data Standards helps companies
Change Control Process for European ectd Standards Version 1.1. December 2006
 Change Control Process for European ectd Standards Version 1.1 December 2006 Document Control Change Record Version Date Author(s) Comments 0.1 10 September, 2003 Miguel Bley Draft 0.2 11 September, 2003
Change Control Process for European ectd Standards Version 1.1 December 2006 Document Control Change Record Version Date Author(s) Comments 0.1 10 September, 2003 Miguel Bley Draft 0.2 11 September, 2003
ICH M8 Expert Working Group. ICH Electronic Common Technical Document (ectd) v4.0 DRAFT ICH Implementation Guide v2.0
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
European Medicines Agency Standard Operating Procedure
 European Medicines Agency Standard Operating Procedure Title: Procedure to be followed by the EMEA in case of mechanical, programme, electronic or communication failure, which prevents a Sender from generating
European Medicines Agency Standard Operating Procedure Title: Procedure to be followed by the EMEA in case of mechanical, programme, electronic or communication failure, which prevents a Sender from generating
MedDRA Update. MedDRA Industry User Group Meeting. 28 September 2018
 MedDRA Update MedDRA Industry User Group Meeting 28 September 2018 Topics MedDRA Users Profile MedDRA Translations MSSO Email Distribution List Opt-In Retrieving MedDRA Unzip Passwords Device terms in
MedDRA Update MedDRA Industry User Group Meeting 28 September 2018 Topics MedDRA Users Profile MedDRA Translations MSSO Email Distribution List Opt-In Retrieving MedDRA Unzip Passwords Device terms in
ETSI TS V8.0.0 ( ) Technical Specification
 TS 102 224 V8.0.0 (2008-10) Technical Specification Smart Cards; Security mechanisms for UICC based Applications - Functional requirements (Release 8) 2 TS 102 224 V8.0.0 (2008-10) Reference RTS/SCP-R0282v800
TS 102 224 V8.0.0 (2008-10) Technical Specification Smart Cards; Security mechanisms for UICC based Applications - Functional requirements (Release 8) 2 TS 102 224 V8.0.0 (2008-10) Reference RTS/SCP-R0282v800
Memorandum. This memorandum requires Board action. EXECUTIVE SUMMARY
 California Independent System Operator Corporation Memorandum To: ISO Board of Governors From: Keith Casey, Vice President, Market and Infrastructure Development Date: January 30, 2019 Re: Decision on
California Independent System Operator Corporation Memorandum To: ISO Board of Governors From: Keith Casey, Vice President, Market and Infrastructure Development Date: January 30, 2019 Re: Decision on
Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai, India
 Paper CD15 PhUSE 2016 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences,
Paper CD15 PhUSE 2016 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences,
Helping The Define.xml User
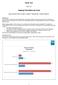 Paper TT01 Helping The Define.xml User Dave Iberson-Hurst, Assero Limited, Teignmouth, United Kingdom ABSTRACT The FDA often comment at industry gatherings on the quality of define.xml files received as
Paper TT01 Helping The Define.xml User Dave Iberson-Hurst, Assero Limited, Teignmouth, United Kingdom ABSTRACT The FDA often comment at industry gatherings on the quality of define.xml files received as
Information Technology Metadata registries (MDR) Part 6: Registration
 ISO/IEC 2013 All rights reserved ISO/IEC JTC 1/SC 32/WG 2 N1845 Date: 2013-11-08 ISO/IEC WD 11179-6 ISO/IEC JTC 1/SC 32/WG 2 Secretariat: ANSI Information Technology etadata registries (DR) Part 6: Registration
ISO/IEC 2013 All rights reserved ISO/IEC JTC 1/SC 32/WG 2 N1845 Date: 2013-11-08 ISO/IEC WD 11179-6 ISO/IEC JTC 1/SC 32/WG 2 Secretariat: ANSI Information Technology etadata registries (DR) Part 6: Registration
-Evolving ectd Technology Transforming Global Regulatory Submission Strategy. PART TWO 30-Mar ectd MASTERCLASS 1
 ectd MASTERCLASS -Evolving ectd Technology Transforming Global Regulatory Submission Strategy PART TWO 30-Mar-2016 ectd MASTERCLASS 1 Session Chair Handsome Ji Senior Publishing Team Manager, Worldwide
ectd MASTERCLASS -Evolving ectd Technology Transforming Global Regulatory Submission Strategy PART TWO 30-Mar-2016 ectd MASTERCLASS 1 Session Chair Handsome Ji Senior Publishing Team Manager, Worldwide
DATA ITEM DESCRIPTION
 helping projects succeed... 1.TITLE DATA ITEM DESCRIPTION SYSTEM/SUBSYSTEM DESIGN DESCRIPTION (SSDD) VERSION B 2. Identification Number PPA-003461-5 25 May 2012 3. DESCRIPTION/PURPOSE OF THE SSDD 3.1 The
helping projects succeed... 1.TITLE DATA ITEM DESCRIPTION SYSTEM/SUBSYSTEM DESIGN DESCRIPTION (SSDD) VERSION B 2. Identification Number PPA-003461-5 25 May 2012 3. DESCRIPTION/PURPOSE OF THE SSDD 3.1 The
Site User Guide. Oracle Health Sciences InForm CRF Submit Release Part Number:E
 Site User Guide Oracle Health Sciences InForm CRF Submit Release 4.0.2 Part Number:E79080-01 Copyright 2016, 2017, Oracle and/or its affiliates. All rights reserved. This software and related documentation
Site User Guide Oracle Health Sciences InForm CRF Submit Release 4.0.2 Part Number:E79080-01 Copyright 2016, 2017, Oracle and/or its affiliates. All rights reserved. This software and related documentation
FDA XML Data Format Requirements Specification
 FDA XML Data Format Barry Brown, Product Integration Manager, Mortara Instrument Mark Kohls, Engineering Director, GE Medical Systems-Information Technologies Norman Stockbridge, M.D., Ph. D., Medical
FDA XML Data Format Barry Brown, Product Integration Manager, Mortara Instrument Mark Kohls, Engineering Director, GE Medical Systems-Information Technologies Norman Stockbridge, M.D., Ph. D., Medical
ICH M8 Expert Working Group. ICH Electronic Common Technical Document (ectd) v4.0 Implementation Guide v1.2
 THE INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE (ICH) ICH M8 Expert Working Group ICH Electronic Common Technical Document () v4.0 Implementation
THE INTERNATIONAL COUNCIL FOR HARMONISATION OF TECHNICAL REQUIREMENTS FOR PHARMACEUTICALS FOR HUMAN USE (ICH) ICH M8 Expert Working Group ICH Electronic Common Technical Document () v4.0 Implementation
How to create a validated ectd submission based on the new guidelines and specification - Part 1
 How to create a validated ectd submission based on the new guidelines and specification - Part 1 2.22 ectd MCC Validation Criteria Michael Gessert Product Manager Global Review & Validation Dr. Gerhard
How to create a validated ectd submission based on the new guidelines and specification - Part 1 2.22 ectd MCC Validation Criteria Michael Gessert Product Manager Global Review & Validation Dr. Gerhard
Customer Guidance For Requesting Changes to SNOMED CT
 Customer Guidance For Requesting Changes to SNOMED CT Date 20161005 Version 2.0 Customer Guidance For Requesting Changes to SNOMED CT Page 1 of 12 Version 2.0 Amendment History Version Date Editor Comments
Customer Guidance For Requesting Changes to SNOMED CT Date 20161005 Version 2.0 Customer Guidance For Requesting Changes to SNOMED CT Page 1 of 12 Version 2.0 Amendment History Version Date Editor Comments
The Dublin Core Metadata Element Set
 ISSN: 1041-5635 The Dublin Core Metadata Element Set Abstract: Defines fifteen metadata elements for resource description in a crossdisciplinary information environment. A proposed American National Standard
ISSN: 1041-5635 The Dublin Core Metadata Element Set Abstract: Defines fifteen metadata elements for resource description in a crossdisciplinary information environment. A proposed American National Standard
Software Requirements Specification. <Project> for. Version 1.0 approved. Prepared by <author(s)> <Organization> <Date created>
 Software Requirements Specification for Version 1.0 approved Prepared by Software Requirements Specification for Page 2 Table of Contents Revision
Software Requirements Specification for Version 1.0 approved Prepared by Software Requirements Specification for Page 2 Table of Contents Revision
ISO/IEC INTERNATIONAL STANDARD. Information technology Software asset management Part 2: Software identification tag
 INTERNATIONAL STANDARD ISO/IEC 19770-2 First edition 2009-11-15 Information technology Software asset management Part 2: Software identification tag Technologies de l'information Gestion de biens de logiciel
INTERNATIONAL STANDARD ISO/IEC 19770-2 First edition 2009-11-15 Information technology Software asset management Part 2: Software identification tag Technologies de l'information Gestion de biens de logiciel
Introduction to ADaM and What s new in ADaM
 Introduction to ADaM and What s new in ADaM Italian CDISC UN Day - Milan 27 th October 2017 Silvia Faini Principal Statistical Programmer CROS NT - Verona ADaM Purpose Why are standards needed in analysis
Introduction to ADaM and What s new in ADaM Italian CDISC UN Day - Milan 27 th October 2017 Silvia Faini Principal Statistical Programmer CROS NT - Verona ADaM Purpose Why are standards needed in analysis
Records Management Metadata Standard
 Records Management Metadata Standard Standard No: RIM203 2008 City Clerk s Office Records and Information Management Records and Information Management Standard Subject: Records Management Metadata Standard
Records Management Metadata Standard Standard No: RIM203 2008 City Clerk s Office Records and Information Management Records and Information Management Standard Subject: Records Management Metadata Standard
Material covered in the Dec 2014 FDA Binding Guidances
 Accenture Accelerated R&D Services Rethink Reshape Restructure for better patient outcomes Sandra Minjoe Senior ADaM Consultant Preparing ADaM and Related Files for Submission Presentation Focus Material
Accenture Accelerated R&D Services Rethink Reshape Restructure for better patient outcomes Sandra Minjoe Senior ADaM Consultant Preparing ADaM and Related Files for Submission Presentation Focus Material
GEOFidelis SDSFIE Implementation Roles and Responsibilities Guide
 GEOFidelis SDSFIE Implementation Roles and Responsibilities Guide Version: 1.4 Prepared for: USMC Installation Geospatial Information and Services Program (GEOFidelis) November 19th, 2012 TABLE OF CONTENTS
GEOFidelis SDSFIE Implementation Roles and Responsibilities Guide Version: 1.4 Prepared for: USMC Installation Geospatial Information and Services Program (GEOFidelis) November 19th, 2012 TABLE OF CONTENTS
Pre-notification check for type IB Variations 1
 Pre-notification check for type IB Variations 1 This pre-notification checklist is aimed at facilitating submission of complete and correct Type IB variation notifications by Marketing Authorisation Holders
Pre-notification check for type IB Variations 1 This pre-notification checklist is aimed at facilitating submission of complete and correct Type IB variation notifications by Marketing Authorisation Holders
DATA Act Information Model Schema (DAIMS) Architecture. U.S. Department of the Treasury
 DATA Act Information Model Schema (DAIMS) Architecture U.S. Department of the Treasury September 22, 2017 Table of Contents 1. Introduction... 1 2. Conceptual Information Model... 2 3. Metadata... 4 4.
DATA Act Information Model Schema (DAIMS) Architecture U.S. Department of the Treasury September 22, 2017 Table of Contents 1. Introduction... 1 2. Conceptual Information Model... 2 3. Metadata... 4 4.
Standard CIP 005 2a Cyber Security Electronic Security Perimeter(s)
 A. Introduction 1. Title: Cyber Security Electronic Security Perimeter(s) 2. Number: CIP-005-2a 3. Purpose: Standard CIP-005-2 requires the identification and protection of the Electronic Security Perimeter(s)
A. Introduction 1. Title: Cyber Security Electronic Security Perimeter(s) 2. Number: CIP-005-2a 3. Purpose: Standard CIP-005-2 requires the identification and protection of the Electronic Security Perimeter(s)
Step: 9 Conduct Data Standardization
 Step: 9 Conduct Data Standardization Version 1.0, February 2005 1 Step Description/Objectives: Step 9, Conduct Data Standardization, is intended to reduce the life cycle cost of data through data integration,
Step: 9 Conduct Data Standardization Version 1.0, February 2005 1 Step Description/Objectives: Step 9, Conduct Data Standardization, is intended to reduce the life cycle cost of data through data integration,
April 28, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852
 701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
extended EudraVigilance Medicinal Product Report Message (XEVPRM) Step-by-Step Guide
 extended EudraVigilance Medicinal Product Report Message (XEVPRM) Step-by-Step Insert of a Development Medicinal Product (DMP) 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44
extended EudraVigilance Medicinal Product Report Message (XEVPRM) Step-by-Step Insert of a Development Medicinal Product (DMP) 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44
Medical devices Quality management Medical device nomenclature data structure
 INTERNATIONAL STANDARD ISO 15225 Third edition 2016-03-15 Medical devices Quality management Medical device nomenclature data structure Dispositifs médicaux Management de la qualité Structure des données
INTERNATIONAL STANDARD ISO 15225 Third edition 2016-03-15 Medical devices Quality management Medical device nomenclature data structure Dispositifs médicaux Management de la qualité Structure des données
Biocides Submission Manual How to use the SPC Editor
 1 How to use the SPC Editor May 2018 Biocides Submission Manual How to use the SPC Editor May 2018 2 Biocides Submission Manual Version 2.3 Disclaimer This document aims to assist users in complying with
1 How to use the SPC Editor May 2018 Biocides Submission Manual How to use the SPC Editor May 2018 2 Biocides Submission Manual Version 2.3 Disclaimer This document aims to assist users in complying with
PSUR Repository Interactive Q&A
 PSUR Repository Interactive Q&A Interactive Q&A session with MAHs on the new functionality provided in release 1.03.00 Presented by Kristiina Puusaari on 10 September 2015 An agency of the European Union
PSUR Repository Interactive Q&A Interactive Q&A session with MAHs on the new functionality provided in release 1.03.00 Presented by Kristiina Puusaari on 10 September 2015 An agency of the European Union
Agilent ICP-MS ChemStation Complying with 21 CFR Part 11. Application Note. Overview
 Agilent ICP-MS ChemStation Complying with 21 CFR Part 11 Application Note Overview Part 11 in Title 21 of the Code of Federal Regulations includes the US Federal guidelines for storing and protecting electronic
Agilent ICP-MS ChemStation Complying with 21 CFR Part 11 Application Note Overview Part 11 in Title 21 of the Code of Federal Regulations includes the US Federal guidelines for storing and protecting electronic
December EMA/668616/2014-Rev.2.4EMA/668616/2014-Rev.2.5
 Technical validation checklist 1 for veterinary electronic submission 2 - Version 2.45 Validation checklist for VNeeS submissions, approved by the Veterinary Harmonisation Group (VHG). There are two types
Technical validation checklist 1 for veterinary electronic submission 2 - Version 2.45 Validation checklist for VNeeS submissions, approved by the Veterinary Harmonisation Group (VHG). There are two types
How to handle different versions of SDTM & DEFINE generation in a Single Study?
 Paper CD15 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai,
Paper CD15 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai,
GMEI UTILITY BULK REGISTRATION AND RENEWAL USER'S GUIDE FOR TEMPLATE VERSION 5.4 JANUARY 29, 2018
 GMEI UTILITY BULK REGISTRATION AND RENEWAL USER'S GUIDE FOR TEMPLATE VERSION 5.4 JANUARY 29, 2018 Copyright 2018 DTCC. All rights reserved. This work (including, without limitation, all text, images, logos,
GMEI UTILITY BULK REGISTRATION AND RENEWAL USER'S GUIDE FOR TEMPLATE VERSION 5.4 JANUARY 29, 2018 Copyright 2018 DTCC. All rights reserved. This work (including, without limitation, all text, images, logos,
Biocides Submission Manual
 MANUAL Biocides Submission Manual Technical guide: using IUCLID 2 Biocides Submission Manual Version 4.0 BSM Technical guide: using IUCLID Reference: ECHA-14-B-21-EN Catalogue number: ISBN: DOI: Publ.
MANUAL Biocides Submission Manual Technical guide: using IUCLID 2 Biocides Submission Manual Version 4.0 BSM Technical guide: using IUCLID Reference: ECHA-14-B-21-EN Catalogue number: ISBN: DOI: Publ.
extended EudraVigilance Medicinal Product Dictionary (XEVMPD) e-learning
 extended EudraVigilance Medicinal Product Dictionary (XEVMPD) e-learning Session 3: Database Architecture Version 5.3 An agency of the European Union Roles of the XEVMPD in the EV System (1) The roles
extended EudraVigilance Medicinal Product Dictionary (XEVMPD) e-learning Session 3: Database Architecture Version 5.3 An agency of the European Union Roles of the XEVMPD in the EV System (1) The roles
Guidance for Industry on Providing Regulatory Information in ectd Format
 Guidance for Industry on Providing Regulatory Information in ectd Format Authors: Responsible: Lead: Ralph Maier, Swissmedic Review team Swissmedic Review team Interpharma Operational Support Services,
Guidance for Industry on Providing Regulatory Information in ectd Format Authors: Responsible: Lead: Ralph Maier, Swissmedic Review team Swissmedic Review team Interpharma Operational Support Services,
The Wonderful World of Define.xml.. Practical Uses Today. Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008
 The Wonderful World of Define.xml.. Practical Uses Today Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008 Agenda Introduction to Formedix What is Define.xml? Features and Benefits
The Wonderful World of Define.xml.. Practical Uses Today Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008 Agenda Introduction to Formedix What is Define.xml? Features and Benefits
PROPOSED DOCUMENT. International Medical Device Regulators Forum
 PROPOSED DOCUMENT International Medical Device Regulators Forum Title: Assembly and Technical Guide for IMDRF Table of Contents (ToC) Submissions (ToC-based submissions) Authoring Group: Regulated Product
PROPOSED DOCUMENT International Medical Device Regulators Forum Title: Assembly and Technical Guide for IMDRF Table of Contents (ToC) Submissions (ToC-based submissions) Authoring Group: Regulated Product
EUROPEAN MEDICINES AGENCY (EMA) CONSULTATION
 EUROPEAN MEDICINES AGENCY (EMA) CONSULTATION Guideline on GCP compliance in relation to trial master file (paper and/or electronic) for content, management, archiving, audit and inspection of clinical
EUROPEAN MEDICINES AGENCY (EMA) CONSULTATION Guideline on GCP compliance in relation to trial master file (paper and/or electronic) for content, management, archiving, audit and inspection of clinical
eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0
 eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Reviewer User Guide 2 Table of Contents 1. INTRODUCTION 3
eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Reviewer User Guide 2 Table of Contents 1. INTRODUCTION 3
Archivists Toolkit: Description Functional Area
 : Description Functional Area Outline D1: Overview D2: Resources D2.1: D2.2: D2.3: D2.4: D2.5: D2.6: D2.7: Description Business Rules Required and Optional Tasks Sequences User intentions / Application
: Description Functional Area Outline D1: Overview D2: Resources D2.1: D2.2: D2.3: D2.4: D2.5: D2.6: D2.7: Description Business Rules Required and Optional Tasks Sequences User intentions / Application
GEP Certificate Database System - User Manual Contents
 GEP Certificate Database System - User Manual Contents Introduction... 2 Purpose... 2 1) Storage... 2 2) Retrieval... 2 3) Reference... 2 Users... 2 1) Standard Users... 2 2) Administrators... 2 3) Viewers...
GEP Certificate Database System - User Manual Contents Introduction... 2 Purpose... 2 1) Storage... 2 2) Retrieval... 2 3) Reference... 2 Users... 2 1) Standard Users... 2 2) Administrators... 2 3) Viewers...
Submitting High Quality ectd Submissions to FDA/OGD
 Submitting High Quality ectd Submissions to FDA/OGD GPhA/FDA ANDA Labeling Workshop/ USP User Forum September 11, 2013 Constance Robinson Regulatory Information Specialist edata Management Solutions Team
Submitting High Quality ectd Submissions to FDA/OGD GPhA/FDA ANDA Labeling Workshop/ USP User Forum September 11, 2013 Constance Robinson Regulatory Information Specialist edata Management Solutions Team
PMS Iteration 1: analysis of the data efforts & value results and recommendation on the scope
 PMS Iteration 1: analysis of the data efforts & value results and recommendation on the scope EU ISO IDMP Task Force 19 February 2016 Presented by Ilaria Del Seppia Data Standardisation and Analytics,
PMS Iteration 1: analysis of the data efforts & value results and recommendation on the scope EU ISO IDMP Task Force 19 February 2016 Presented by Ilaria Del Seppia Data Standardisation and Analytics,
Comparison of FDA and PMDA Requirements for Electronic Submission of Study Data
 Comparison of FDA and PMDA Requirements for Electronic Submission of Study Data Monika Kawohl Statistical Programming Accovion CDISC GSUG Meeting 15-Sep-2015 1 References FDA Website: Study Data Standards
Comparison of FDA and PMDA Requirements for Electronic Submission of Study Data Monika Kawohl Statistical Programming Accovion CDISC GSUG Meeting 15-Sep-2015 1 References FDA Website: Study Data Standards
