USING BRAT ANALYSIS PIPELINE
|
|
|
- Rodger Briggs
- 6 years ago
- Views:
Transcription
1 USIN BR his new version has a new tool convert-to-sam that converts BR format to SM format. Please use this program as needed after remove-dupl in the pipeline below. 1 NLYSIS PIPELINE urrently BR includes the following tools: brat, brat-large, brat-large-build, acgt-count, trim, rev-compl, check-strands and remove-dupl. he flow of the analysis pipeline is given in the figure below. Reads in FSQ References in FS trim removedupl checkstrands brat or brat-large Mapped results Unmapped reads re reads sequenced only from 2 original genomic strands? No rev-compl acgt-count brat or brat-large Methylome First, tool trim takes reads in FSQ format, trims low-quality bases from both ends as well as Ns and outputs reads in raw reads format that are accepted by brat and brat-large. fter trim, brat or brat-large are used to map the reads to the reference genome. If the reads are sequenced from four PR-product strands, then rev-compl is used to take reverse-complements of the unmapped reads followed by mapping of the results with brat or brat-large. Next, mapped results are used as input for remove-dupl that removes copy-duplicates keeping only a randomly chosen one, where copy-duplicates are the reads that are mapped to the same start position in the reference. Finally acgt-count processes all the mapped results to produce methylome, a map with methylation status of each cytocine. ool check-strands is used for information on distribution in the reference and input reads.
2 2 SYSEM ND SPE REQUIREMENS BR stands for Bisulfite-treated Reads nalysis ool. here are two versions of BR: BR and BR-large. Both programs run on 64-bit architecture under Linux/Unix operating system. Both versions work with reads of lengths 24 bases and above. BR accepts up to 4.2 references with total size of references up to 4.2 base pairs. BR-large can work with up to 4.2 references with the size of the largest reference up to 4.2 base pairs. BR works best with relatively small genomes because it uses significantly more space than BR-large. BR is faster than BR-large that maps reads to the references sequentially: it maps all reads to the first reference, then all reads to the second reference and so on. he space requirement of BR-large depends on the size of the largest reference in the set of input references, whereas BR s space requirement depends on the total size of all input references (measured in base pairs). Let N be the total size of a genome in base pairs, be the size of the largest reference in base pairs and R be the total number of reads (each read is counted, i.e. a pair has 2 reads). hen the space required for both programs is bound as follows: Space BR = N + 24R + (3/8)N Space BR-large = R + (3/8) Space BR-large = R + (3/8) Bytes Bytes Bytes (when option S is provided) Whenever there is a space limitation, the user can choose to use BR-large. Here is an example of space usage with pairedend BS-mapping with non-bs-mismatches (mismatches other than -to- and -to-): BR uses 2.5 B on 1 million pairs and human chromosome 1, and BR-large on 10 million pairs and an entire human genome uses 1.7 B (or 2.7B with option S). In this version, BR allows for non-bs-mismatches with the restriction on the number of non-bs-mismatches in the first X bases (for BR) and X bases (for BR-large), where X is a threshold specified with the option f (X cannot be smaller than 24 or larger than 64). BR guarantees to map all reads that could be mapped to the genome with up to one non-bsmismatch in the first X bases of reads. user can specify any number of non-bs-mismatches with the option m, and as long as there is only one non-bs-mismatch in the first X bases of a read, BR will map this read. BR-large does not allow for non-bs-mismatches in the first X bases of reads. If a user specifies any number of non-bs-mismatches with the option m, BR-large will map all reads that could be mapped perfectly or with BS-mismatches within the first X bases, and any number of non-bs-mismatches in the other bases of reads. he package includes three additional tools: trim, brat-large-build and acgt-count. he latter aligns mapped reads to the genome and counts mapped nucleotides at each base for forward and reverse strands separately. he tool trim accepts FSQ files with reads/pairs as input, trims the ends of the reads whose base quality scores are lower than the user specified threshold and trims Ns at the ends of the reads. he tool brat-large-build is used with option P with BR-large. 3 OMMNDS ND INPU o uncompress run: tar zxvf brat-1.2.*.tar.gz o build: cd brat-1.2.* make his will create executable programs: brat-large, brat-large-build, acgt-count, trim, rev-compl, check-strands and removedupl.
3 Input format of the reads for BR and BR-large is raw reads: Read < string >: a read after using trim; Start <int>: the number of bases trimmed at the beginning of the original read; End <int>: the number of bases trimmed at the end of the original read. o convert reads from FSQ format to raw reads, one should run trim. If a user does not wish to trim reads low quality score bases, then he/she should omit the option for the base quality score threshold: the default threshold equals to zero, so all reads will be in the output without change in lengths (except for reads having Ns at the ends). If reads have Ns at the ends, trim trims Ns at the ends and outputs only those reads whose length after trimming is greater or equal to 24 bases. command to run trim his program trims low-quality bases (lower than a threshold given with option -q) and Ns from each end of a read: bases are trimmed one at a time from both ends of a read until a base with quality score greater or equal than q is encountered (similarly, all consecutive Ns from both ends of a read are trimmed). his tool outputs only those reads whose length is at least 24 after trimming and that have at most m internal Ns: the number of allowable internal Ns is set by option -m. o trim single-end reads in the file reads.fastq in FSQ format and output trimmed raw reads into a file with name prefix_reads1.txt, run the command:./trim -s reads.fastq -P prefix -q 20 -L 64 -m 2 his will trim bases whose base quality scores are lower than 20 from the ends of reads. he option L specifies the smallest value of the range of base quality scores in SII representation (please see ommands Options for details). o learn more about Phred scores, please visit Option -m allows each read having at most 2 internal Ns. Option -P provides prefix to the output file names (it might contain a path for an output file: -P /home/directory/prefix). If the user does not wish to trim ends with low base quality scores, the -q option is not specified. For single-end reads, there is a single output file with trimmed reads. o trim paired-end reads in the files reads1.fastq and reads2.fastq in FSQ format, run the command:./trim -1 reads1.fastq -2 reads2.fastq -P prefix -q 20 -L 64 -m 2 Here we assume, that reads1.fastq contains sequenced 5` mates, and reads2.fastq contains sequenced 3` mates. he output will be in four files with raw reads: prefix_reads1.txt, prefix_reads2.txt, prefix_mates1.txt and prefix_mates2.txt. o further map paired-end reads, use prefix_reads1.txt and prefix_reads2.txt as input files for paired-end mapping with brat or brat-large. he file prefix_mates1.txt contains reads from the file reads1.fastq whose mates have shorter length than 24 bases after trimming. Similarly, the file prefix_mates2.txt contains reads from the file reads2.fastq whose mates are shorter than 24 bases. he user can further map these files, prefix_mates1.txt and prefix_mates2.txt, as single-end reads: for BS-mapping of the reads in prefix_mates2.txt, the user must specify - option for mapping to work correctly (the same is true if a user wishes to map the reads in prefix_reads2.txt as single reads). dditional output files are prefix_pair1.fastq, prefix_pair2.fastq, prefix_mates1.fastq and prefix_mates2.fastq. hese files have the same reads as do files prefix_reads1.txt, prefix_reads2.txt, prefix_mates1.txt and prefix_mates2.txt respectively, except the files prefix_pair1.fastq, prefix_pair2.fastq, prefix_mates1.fastq and prefix_mates2.fastq are in FSQ format. NOE: current version of BR and BR-large do NO support FSQ format. hese additional files are for users to track original reads names and corresponding base quality scores. ommands to run brat and brat-large BR and BR-large map raw reads (output from trim) to references that have to be in FS format: one reference per FS file. BR accepts a single file that contains names of FS files with the references. o map bisulfite singleend reads, run either of the commands:./brat -r references_names.txt -s prefix_reads1.txt -bs -o output_results.txt./brat-large -r references_names.txt -s prefix_reads1.txt -bs -o output_results.txt./brat -r references_names.txt -s prefix_reads2.txt -bs -o output_results.txt -
4 ./brat-large -r references_names.txt -s prefix_reads2.txt -bs -o output_results.txt - he file references_names.txt contains the names of the FS files with the references. he file output_results.txt contains the results of the mapping: only uniquely mapped reads are in this file. he option -bs specifies that mapping is for bisulfite sequenced reads. o map normal (not bisulfite-treated) reads, run similar commands but without -bs option:./brat -r references_names.txt -s prefix_reads1.txt -o output_results.txt./brat-large -r references_names.txt -s prefix_reads1.txt -o output_results.txt o map bisulfite paired-end reads, run either of the following commands:./brat -r references_names.txt -1 prefix_reads1.txt -2 prefix_reads2.txt -pe -bs -o output_results.txt./brat-large -r references_names.txt -1 prefix_reads1.txt -2 prefix_reads2.txt -pe -bs -o output_results.txt he option -pe specifies paired-end mapping and as with single-end reads, -bs, specifies bisulfite mapping. he results of the mapping will be in output_results.txt. One can choose to pre-build genome index first and then use brat-large on this index to speed up mapping. Use option -P and tool brat-large-build:./brat-large-build -r references_names.txt -P some_directory [options: S, bs, f ]./brat-large -P some_directory -1 prefix_reads1.txt -2 prefix_reads2.txt -pe -o output_results.txt [options] o use this option that allows to separate hashing of the genome from mapping of reads, one has to create a directory, some_directory (the name of a directory in the example above), and to run the following commands (1) to build a hashing index and 2) to map reads. If the directory some_directory is the directory inside the directory in which you run the commands above, then providing the name, some_directory, is sufficient. However, if you run these commands in the directory that does not have some_directory in it, then please provide a full path to some_directory. he tool brat-large-build will output hashing index of the references into some_directory. For human genome, space on hard disk for hashing index is 15B (and 26B when option S is used). he command for tool brat-large with option P differs only by not specifying option r. If option bs was passed to brat-large-build, then this option must be passed to bratlarge. In other words, for mapping normal and bisulfite-treated reads, two different hashing indices must be built. he same true when option S is used: if a user wishes to use this option and P option, then S must be used with brat-large-build. When P is used, during mapping option f will have the same value that was set with brat-large-build. If a user does not specify this option, the default value, 24, will be used. If a user wishes to speed up mapping time, and to use option P, then the user could set option f to a higher value with brat-large-build. eneral rule for using option P: if a user wishes to change either of the parameters: f, S or bs; the user must apply the same parameters with brat-large-build. Default options for brat-large-build are f = 24, S is not set (i.e. preference is given to slower mapping, but also smaller space usage), and normal mapping (i.e. bs is not set). ommands Options: - specifies 3`mates (in our examples above, either of prefix_reads2.txt or prefix_mates2.txt files must be used with this option). If the user does not specify this option, and provides either of the files, prefix_mates2.txt or prefix_reads2.txt, as input reads for single-end mapping, the mapping will NO be correct; -u is used when a user wishes to output unmapped reads. he output will be in prefix_reads1.txt.unm file (assuming prefix_reads1.txt was provided in the command line with option -s) for single reads and with paired-end reads in prefix_reads1.txt.unm and prefix_reads2.txt.unm files (if prefix_reads1.txt and prefix_reads2.txt were used with the options -1 and -2 in the corresponding command line). Unmapped reads in the output files are in raw reads format: <read, X, Y>, where X and Y are the number of bases trimmed from the beginning and end of original read respectively (there are no ambiguous reads/pairs in the files with unmapped reads); -s <single-end reads file>: to specify the file with input reads for single-end reads mapping; -1 <paired-end reads file>: to specify the file with 5` mates for paired-end reads mapping (in our example, prefix_reads1.txt). his option is also used with acgt-count; -2 <paired-end reads file>: to specify the file with 3` mates for paired-end reads mapping (in our example above, prefix_reads2.txt); his option is also used with acgt-count; -pe to specify paired-end reads mapping (default is false, i.e. single-end mapping);
5 -bs to specify bisulfite sequenced reads mapping. Without this option, mapping will be done as for normal, not bisulfitetreated, sequenced reads; -i <positive integer>: to specify minimum insert size for paired-end mapping, the minimum distance allowed between the leftmost ends of the mapped mates on forward strand (default is 100); -a <positive integer>: to specify maximum insert size for paired-end mapping, the maximum distance allowed between the leftmost ends of the mapped mates on forward strand (default is 300); -o <string>: to specify the file with the results of mapping; -M is used when a user wishes to output ambiguous reads. he output will be in prefix_reads1.txt.amb for single reads and in prefix_reads1.txt.amb and prefix_reads2.txt.amb files with paired-end reads: a single read per line. his option does not output the mapping locations for ambiguous reads, but just the reads themselves; -m <integer>: the maximum number of non-bs-mismatches allowed by a user (default is 0). -f <integer>: the number of the first bases of a read, where the restriction on the number of non-bs-mismatches applies: for BR, only one non-bs-mismatch is allowed in the first <integer> bases, and for BR-large NO non-bs-mismatches are allowed in the first <integer> bases (for BR, default is 36, and for BR-large, default is 24). -S to specify speed mode for BR-large. Space usage with this option doubles, but running time is about three times faster. -P <directory name> o use this option that allows to separate hashing of the genome from mapping of reads. -L <integer>: the smallest value of the range of base quality scores in SII representation (default is 33). he table below gives examples of different quality scores and their range in SII representation (from Wikipedia). he option L uses the values in the Smallest Value in SII representation column. ype Smallest Score Largest Score Smallest Value in Largest Value in SII representation SII representation Phred quality score (Sanger format) Solexa/Illumina, Soloexa/Illumina, B: specifies the second option for output with acgt-count (please read Output format for acgt-count). command to run remove-dupl his program processes the mapping results and removes copy-duplicates: it outputs all reads that are mapped to a unique genomic location and only a randomly chosen one out of copy-duplicates (the reads mapped to the same location)../remove-dupl -r references_names.txt -p pairs_results.txt -1 single_results_mates1.txt -2 single_results_mates2.txt NOE: the file pairs_results.txt does not contain the actual results, it contains the names of the files with the results for paired-end mapping, and similarly, single_results.txt file contains the names of the files with the actual results for single-end mapping. For example, the content of pairs_results.txt is: output_pairs_lane1.txt output_pairs_lane2.txt and the content of single_results_mates1.txt is: output_singles_mates1_lane1.txt output_singles_mates1_lane2.txt and the content of single_results_mates2.txt is: output_singles_mates2_lane1.txt output_singles_mates2_lane2.txt he output of remove-dupl are the files with the same names as before with additional extension.nodupl : output_pairs_lane1.txt.nodupl output_pairs_lane2.txt.nodupl output_singles_mates1_lane1.txt.nodupl output_singles_mates1_lane2.txt.nodupl output_singles_mates2_lane1.txt.nodupl output_singles_mates2_lane2.txt.nodupl
6 For single-end mapping, run the following command:./remove-dupl -r references_names.txt -s single_results.txt he file single_results.txt contains the names of the files with mapping results. NOE: that the output files with extension.nodupl are opened with ++ app option (opens a file and appends output to the file s content). his means that once you have run remove-dupl, you will have.nodupl files, and if you want for some reason to run remove-dupl on the same files (and possibly some additional files), you need to remove.nodupl files for the corresponding files first and only then re-run remove-dupl. For example, as in the example above, you run remove-dupl and obtain.nodupl files: output_pairs_lane1.txt.nodupl output_pairs_lane2.txt.nodupl output_singles_mates1_lane1.txt.nodupl output_singles_mates1_lane2.txt.nodupl output_singles_mates2_lane1.txt.nodupl output_singles_mates2_lane2.txt.nodupl hen you wish to add output_pairs_lane3.txt to pairs_results.txt: output_pairs_lane1.txt output_pairs_lane2.txt output_pairs_lane3.txt and to re-run remove-dupl on all files. Make sure your delete existent files with extension.nodupl : rm output_pairs_lane1.txt.nodupl rm output_pairs_lane2.txt.nodupl rm output_singles_mates1_lane1.txt.nodupl rm output_singles_mates1_lane2.txt.nodupl rm output_singles_mates2_lane1.txt.nodupl rm output_singles_mates2_lane2.txt.nodupl and only then run remove-dupl. If you don t remove these files, you will have previous output plus new output (for example: if output_pairs_lane1.txt.nodupl had 100 lines, then re-running remove-dupl without removing this file will result in 200 lines). command to run convert-to-sam his program converts BR format to SM format../convert-to-sam -P prefix -p pairs_results.txt -1 single_results_mates1.txt -2 single_results_mates2.txt -s single_results.txt NOE: the file pairs_results.txt does not contain the actual results, it contains the names of the files with the results for paired-end mapping, and similarly, single_results*.txt files contain the names of the files with the actual results for single-end mapping (see examples in command to run remove-dupl ). NOE: Use options -1 and -2 only when mapping paired-end reads as singles (for 5 mates and 3 mates respectively), and option s if the original sequenced reads were single reads. he output will be in two files with names prefix_forw.sam and prefix_rev.sam if option P is provided, or mapped_to_forw.sam and mapped_to_rev.sam if not. he reason behind separating the mapped reads into two separate files is the following. o distinguish between methylated and unmethylated cytosines in aligned reads, one has to look at mapped to and mapped to respectively if reads come from forward strand, and one has to look at mapped to and mapped to respectively if reads come from reverse strand. If one has a mixture of reads from forward and reverse strands, it would be impossible to track methylated and unmethylated cytosines when visualizing aligned reads. Paired-end mapped mates mapped as pairs are both either in forward output file (prefix_forw.sam) if 5 mate (the first mate) is mapped to positive strand, or both in reverse output file (prefix_rev.sam). Reads from files with option 1 are in
7 forward output file if they are mapped to positive strand, and in reverse output file if they are mapped to negative strand. Reads from files with option 2 are in forward output file if they are mapped to negative strand, and in reverse output file if they are mapped to positive strand. Single reads from files with option s are in forward output file if they are mapped to positive strand, and in reverse output file if to negative strand. NOE: that the output files with extension.sam are opened with ++ app option. Please see details how to be careful with re-running convert-to-sam in command to run remove-dupl (since it is similar situation as with removedupl). command to run acgt-count o count mapped s, s, s and s at each base of forward and reverse strands of the references, use acgt-count../acgt-count -r references_names.txt -P prefix -p pairs_results.txt -s single_results.txt the file references_names.txt contains the names of FS files with the references, which are needed to calculate the sizes of the references. he output will be in two files per a reference: prefix_forw_areference_name and prefix_rev_areference_name. he option -p is to specify the results of paired-end mapping (if any), and -s is to specify the results of single-end mapping (if any). NOE: the file pairs_results.txt does not contain the actual results, it contains the names of the files with the results for paired-end mapping, and similarly, single_results.txt file contains the names of the files with the actual results for single-end mapping. t least one of these options must be provided. he files whose names are listed in the files pairs_results.txt and single_results.txt must be in BR s output format. o make this point clear, assume, a user ran brat on paired-end reads and had the output file with the results in output_pairs_results.txt; to run acgt-count, the user must store the name of this file in pairs_results.txt file and run acgt_count using pairs_results.txt (i.e. pairs_results.txt will have in this case one line, namely, output_pairs_results.txt). he command for this example is:./acgt-count -r references_names.txt -P prefix -p pairs_results.txt Please note that if a user has paired-end reads (files with mates 1 and mates 2) and wishes to map the mates as single-end reads, then the user must provide names of the files with results for mates 1 and mates 2 separately using options -1 and -2. his will ensure unbiased -counting when reads are sequenced from two original genomic strands:./acgt-count -r references_names.txt -P prefix -p pairs_results.txt -1 single_results_mates1.txt -2 single_results_mates2.txt o produce a more concise output, use option -B (choose an appropriate command from the commands below):./acgt-count -r references_names.txt -P prefix -p pairs_results.txt -s single_results.txt -B./acgt-count -r references_names.txt -P prefix -p pairs_results.txt -1 single_results_mates1.txt -2 single_results_mates2.txt -B his program takes care of overlapping mates: if two mates of a pair overlap, then -count is done only for one mate in the overlapped region. his program also takes care of producing un-biased -count from mates 2 (3` mates). Please see Details on -count section for details. command to run rev-compl Please read subsection How to use BR with reads sequenced from four strands. command to run check-strands Please read section Details on -count. 4 OUPU FORM
8 Output format for brat and brat-large for single-end mapping Read id < integer >: a consecutive number of a read in the reads input file that starts with 0; Read 1 < string >: the read given as in the input file (prefix_reads1.txt); Reference name < string >: a name of a reference to which the read is mapped (the first word following > in a FS file); Strand + if the read is mapped to forward strand, and - if the read is mapped to reverse strand; Position < integer >: position within the reference starting with 0, where the read is mapped (the leftmost position on forward strand). he number of non-bs-mismatches <int> Original position <integer>: position within the reference starting with 0, where the original read is mapped (the leftmost position on forward strand), where original read is the read before its ends have been trimmed. For example, if the number of trimmed bases at the beginning of a read is 2, and the read is mapped to positive strand at the position 10, then original position is 10 2 = 8. If the number of trimmed bases at the end of a read was 3 and the reverse-complement of the read is mapped to the position 10 on positive strand, then original position = position 3 = 10 3 = 7. he original positions are used to identify copy-duplicates. Output format for brat and brat-large for paired-end mapping Read id < integer >: a consecutive number of a read in the reads input file that starts with 0; Read 1 < string >: the first mate of a pair given as in the input file (prefix_reads1.txt); Read 2 < string >: the second mate of a pair given as in the input file (prefix_reads2.txt); Reference name < string >: a name of the reference to which the pair is mapped (the first word following > in a FS file); Strand + if 5` mate (from prefix_reads1.txt) is mapped to forward strand (consecutively, 3` mate, from prefix_reads2.txt, is mapped to reverse strand), and - if the 5` mate is mapped to reverse strand (and 3` mate to forward strand); Position 1 < integer >: position within the reference starting with 0, where 5` mate is mapped (the leftmost position on forward strand); Position 2 < integer >: position within the reference starting with 0, where 3` mate is mapped (the leftmost position on forward strand). he number of non-bs-mismatches < integer >: the number of mismatches in the alignment for 5` mate he number of non-bs-mismatches < integer >: the number of mismatches in the alignment for 3` mate Original position 1 <integer>: original position for 5` mate (see definition of original position above) Original position 2 <integer>: original position for 3` mate (see definition of original position above) Output format for acgt-count Starting with version brat , there are two choices for output format. he first choice: he number of output files will be double the number of input references: two for each reference listed in references_names.txt file (one file for forward strand and the other for reverse strand). In each file, there are M lines, where M is the size of a corresponding reference in base pairs. Each line corresponds to a base of a strand and contains counts for s, s, s and s at that base for all mapped reads (i.e. there are four integers per line: from left to right for s, s, s and s). For the reverse strand, the counts of s, s, s and s are given for the reads that are mapped to the reverse strand, but the counts are obtained by aligning the reverse-complements of these reads with the forward strand. Following is an example to illustrate this point. Let a read be mapped to a reverse strand at position i, then the corresponding forward strand starting at position i is, and the counts for the reverse strand at positions i i+5 from this read are incremented for the following nucleotides: i(), i+1(), i+2(), i+3(), i+4() and i+5(). he second choice: If a user provides option -B:./acgt-count -r references_names.txt -P prefix -p pairs_results.txt -s single_results.txt -B
9 then the output is in two files: one file for positive strand and another for negative strand (output files will contain words forw and rev to distinguish between strands). Each line in the output corresponds to a base in the genome that is either a cytocine on positive strand or cytocine on negative strand (given in separate files). Output format: chrom, start, end, total, methylation_level, strand where chrom is the reference name, start and end are positions in the genome (Note: base count in a reference starts with 0), total takes one of the values: HH:X, H:X or p:x, where X is the sum of counts of s and s mapped to this base, methylation level is calculated as the number of s over the total (methylation level = count_/(count_ + count_)). HH, H and p describe the sequence content following : if two consecutive bases that follow are not, then total = HH:X; if the first consecutive base following is non- and the second consecutive is, then total = H:X; and finally, if follows (we have di-nucleotide), then total = p:x. Output format for trim. he tool trim accepts FSQ files with reads/pairs as input, trims the ends of the reads whose base quality scores are lower than the user specified threshold or whose ends are Ns. he output for single reads is a single file with reads whose lengths might be different and whose lengths are greater than or equal to 24 bases. he output for pairs is four files: two for pairedend mapping, and two for single-end mapping. rimming of paired-end reads produces two files with single reads: if one mate is shorter than the minimum length allowed, and the other s length is correct, then the mate with the correct length will be output into a corresponding file with single reads. wo files for single reads are necessary because BS-mapping for 5` and 3` mates is different. Each file contains a single line (raw reads format) with the following fields: Read < string >: a read after using trim; Start <int>: the number of bases trimmed at the beginning of the original read; End <int>: the number of bases trimmed at the end of the original read.
10 5 DEILS ON -OUN Let us denote a mapping of a base in a read to a base in the reference as,,,. Initially, we thought that if a read maps to a positive strand, then the mappings and contribute to the count of methylation level of cytocines of the positive strand. Similarly, if the reverse-complement of a read maps to the positive strand (equivalently, a read maps to the negative strand), then the mappings and contribute to the count of methylation level of cytocines of the negative strand. Let us illustrate this idea in Figures 1-3 below. Figure 1. fter the special adapters with methylated cytokines are ligated to DN fragments, sodium bisulfite treatment (BS-treatment) is applied to DN fragments, after which unmethylated cytocines are converted to uracils and later to thymines during PR for library amplification. Note, that after BS-treatment, there are four distinct PR-product strands: PR1+ and PR2- (correspond to original genomic strands) and PR1- and PR2+ (the reverse-complements of PR1+ and PR2- respectively). Note, that PR1+ and PR2- are -rich and -depleted (since unmethylated cytocines converted to thymines), and PR1- and PR2+ are -rich and -depleted (as the reverse-complements of PR1+ and PR2- respectively).
11 + PR 1 5 ' 3 ' 3 ' 5 ' 3 ' PR 2-5 ' 5 ' 3 ' 3 ' PR 1-5 ' 5 ' 3 ' + PR 2 5 ' 3 ' 3 ' 5 ' sequenced pairs + PR 1 - PR 2 PR PR 2 Figure 2. Here we show the sequenced pairs resulted from sequencing all four PR-product strands, each of which serves as a template during sequencing. If PR1+ is attached to the flow cell, then in single-end sequencing, 5 -end of this strand is sequenced; in paired-end sequencing, 5 -end of PR1+ is sequenced, then PR1+ is copied into its reverse-complement PR1- and 5 -end of PR1- is sequenced as the second mate of a pair. Similarly, sequencing produces reads for the rest of strands. If procedure has some technical details that ensure that only original genomic strands serve as templates for sequencing, then even though we have four PR-product strands, we ll have reads sequenced only from PR1+ and PR2- with mates ordering shown above (in paired-end sequencing, the left column of reads are mates 1 will have reads IDs end with 1 and will be in one file, the right column of reads are mates 2 will have reads IDs end with 2 and will be in the other file of Illumina s sequenced reads).
12 Figure 3. Here, we show mapping of the pairs sequenced from all four strands. he bottom strand shows positive strand of the reference with methylated cytocine shown in blue and methylated cytocine on negative strand shown as in orange. his figure demonstrates that if we use simplified approach of counting methylated level of cytocines from positive and negative strands, then we introduce a bias in counting methylation level of unmethylated cytocines (either cytocines that are partially methylated across cells of a sample or completely unmethylated). For example, read 2 (mate 2) from PR1+ maps to negative strand (its reverse-complement maps to positive strand), and therefore, initially we contributed counts from this read toward negative strand. his was incorrect: in this Figure, bias is introduced by counting from this read (in red circle) toward unmethylated (i.e. unmethylated cytocine on negative strand). he count of another from this read (in green circle) toward methylated did not introduce a bias (since methylated has only s mapped to it), but the coverage of methylated s is also not what it would have been were we using the correct counting. Similar bias is introduced when counting contribution of from mates 2 from PR2+. From Figures 1-3, we can observe that mate 2 of PR1+ strand is reverse-complement 3 -end of PR1+; thus, mate 2 must reflect methylation on positive strand rather than on negative strand. For example, methylated on PR1+ will be on PR1-, and unmethylated (which is ) on PR1+ will be on PR1-. hus, and, where and respectively belong to PR1-, must contribute to the count of methylation level of cytocines on positive strand. Similarly,
13 and (with and belonging to PR2+) must contribute to the count of methylation level of cytocines on negative strand. hese changes have been made to acgt-count and now -counting from second mates of pairs sequenced from PR1+ and PR2- is done without bias, which is demonstrated in Figure 4. Figure 4. If mate 1 of a pair maps to positive strand (case of a pair sequenced from PR1+), then we increment count from both mates for positive strand, and methylation level of a cytocine on the positive strand can be measured as total s mapped to positive strand to the sum of total s and total s mapped to the positive strand. If mate 1 of a pair maps to negative strand (case of a pair sequenced from PR2-), then we increment -count from both mates for negative strand. Methylation level can be determined for each on positive strand of the reference ( on positive strand corresponds to on negative strand) by looking into acgt-count output for negative strand and taking the ratio of total s mapped to this position and the sum of total s and s mapped to this position. We received a couple of s concerned that the sequenced reads must be products of sequencing of all four strands. he best way to be sure how many strands have been sequenced is talking to Illumina tech support, but there is also a quick and dirty method to answer this question. his approach will work best with genomes whose -distribution is close to uniform, or with genomes that -rich. We cannot be positive that the way we will explain shortly can be successfully applied to -rich genomes. We added a new tool, check-strands, that calculates -distribution in the genome, and in mates 1 and mates 2 of paired-end reads. For the case, when pairs are sequenced only from two original genomic strands, you should notice significant increase of s in mates 1 (and decrease of s) compared to s and s in the genome, and significant increase of s in mates 2 (and consequently decrease of s) compared to s and s in the genome. If four strands are sequenced, then the file with mates 1 contains a mixture of reads sequenced from original genomic strands as well as their reverse-complements. hus, the increase of both s and s (and decrease of counts of s and s) should be observed in both files: with mates 1 and mates2. his conclusion is derived from the fact that after BS-treatment original
14 genomic strands are -rich and -depleted, and consequently their reverse-complements PR1- and PR2+ are -rich and -depleted. Below are the commands to run check-strands: Single-end reads: Paired-end reads:./check-strands -r references_names.txt -s reads1.fastq -o output_results.txt./check-strands -r references_names.txt -1 reads1.fastq -2 reads2.fastq -o output_results.txt Here, reference_names.txt as before: it is the file with names of files in FS with references; reads1.fastq and reads2.fastq are FSQ files from Illumina with sequenced reads (for paired-end reads, with mates 1 and mates 2). he output is in output_results.txt; it is self-explanatory with proportions of,, and (in that order) in references, in reads from reads1.fastq and in reads from reads2.fastq. How to use BR with reads sequenced from four strands. For single-end reads, if reads are sequenced from four PR-product strands, namely PR1+, PR2-, PR1- and PR2+, then run BR as explained before with option -u that will output unmapped reads into a separate file, say unmapped.txt. hen use our new tool rev-compl with unmapped.txt as follows:./rev-compl -s unmapped.txt -o rev_compl_of_unmapped.txt he output file rev_compl_of_unmapped.txt will contain reverse-complement of reads from unmapped.txt file. hen map reads from rev_compl_of_unmapped.txt as usual (see how to map single-end reads with BR). For paired-end reads, map reads as usual, but use -u option to output unmapped reads (use appropriate command of these two):./brat -r references_names.txt -1 prefix_reads1.txt -2 prefix_reads2.txt -pe -bs -o output_results.txt -u./brat-large -r references_names.txt -1 prefix_reads1.txt -2 prefix_reads2.txt -pe -bs -o output_results.txt -u he unmapped reads from prefix_reads1.txt will be in prefix_reads1.txt.unm and unmapped reads from prefix_reads2.txt will be in prefix_reads2.txt.unm hen take reverse-complements of unmapped reads (please provide output files with option -o):./rev-compl -s prefix_reads1.txt.unm -o rc_ prefix_reads1.txt.unm./rev-compl -s prefix_reads2.txt.unm -o rc_prefix_reads2.txt.unm hen run BR again on reverse-complements of unmapped reads and use option (use either of the commands below):./brat -r references_names.txt -1 rc_unm_prefix_reads1.txt -2 rc_unm_prefix_reads2.txt -o output_results2.txt -./brat-large -r references_names.txt -1 rc_unm_prefix_reads1.txt -2 rc_unm_prefix_reads2.txt -o output_results2.txt - he rest of the tools are used as before. Why does this work? BR allows only - BS-mismatches if mate 1 maps to positive strand, and only - BSmismatches if reverse-complement of mate 1 maps to positive strand. hus, when we first time map paired-end reads, only pairs sequenced from the original strands are mapped; after we take reverse-complement, then mapping is done for the pairs sequenced from PR1- and PR2+. However, if a read does not have BS-mismatches or the number of inappropriate BS-mismatches (- in case when mate 1 maps to positive strand and - when reverse-complemet of mate 1 maps to positive strand) is less than the total of allowed non-bs-mismatches, then some of the pairs from PR1- and PR2+ can be mapped together with the paired-end reads sequenced from the original strands inducing -count bias showed in Figure 5. BR does not have any counting bias for the case when only original strands are sequenced, however in case of four strands being sequenced, there might be a bias that users must account for by some other means (statistics, etc.) his is shown in Figure 5.
15 PR + 2, rc read 2 PR + 2, read 1 PR - 1, read 2 PR 1-, rc read 1 PR - 2, read 2 PR - 2, rc read 1 PR + 1, rc read 2 PR + 1, read 1 DS + 5 ' m 3 ' Figure 5. here is no acgt-count bias for the case when two original strands are sequenced, but there might be a bias from mapped paired-end reads sequenced from PR1- and PR2+ strands for unmethylated cytocines (or for partially methylated cytocines) if the mates have no BS-mismatches or the number of inappropriate (see above) BS-mismatches is less than or equal to the number of allowed non-bs mismatches.
USING BRAT UPDATES 2 SYSTEM AND SPACE REQUIREMENTS
 USIN BR-1.1.17 1 UPDES In version 1.1.17, we fixed a bug in acgt-count: in the previous versions it had only option -s to accept the file with names of the files with mapping results of single-end reads;
USIN BR-1.1.17 1 UPDES In version 1.1.17, we fixed a bug in acgt-count: in the previous versions it had only option -s to accept the file with names of the files with mapping results of single-end reads;
USING BRAT-BW Table 1. Feature comparison of BRAT-bw, BRAT-large, Bismark and BS Seeker (as of on March, 2012)
 USING BRAT-BW-2.0.1 BRAT-bw is a tool for BS-seq reads mapping, i.e. mapping of bisulfite-treated sequenced reads. BRAT-bw is a part of BRAT s suit. Therefore, input and output formats for BRAT-bw are
USING BRAT-BW-2.0.1 BRAT-bw is a tool for BS-seq reads mapping, i.e. mapping of bisulfite-treated sequenced reads. BRAT-bw is a part of BRAT s suit. Therefore, input and output formats for BRAT-bw are
BIOINFORMATICS APPLICATIONS NOTE
 BIOINFORMATICS APPLICATIONS NOTE Sequence analysis BRAT: Bisulfite-treated Reads Analysis Tool (Supplementary Methods) Elena Y. Harris 1,*, Nadia Ponts 2, Aleksandr Levchuk 3, Karine Le Roch 2 and Stefano
BIOINFORMATICS APPLICATIONS NOTE Sequence analysis BRAT: Bisulfite-treated Reads Analysis Tool (Supplementary Methods) Elena Y. Harris 1,*, Nadia Ponts 2, Aleksandr Levchuk 3, Karine Le Roch 2 and Stefano
BRAT-BW: Efficient and accurate mapping of bisulfite-treated reads [Supplemental Material]
![BRAT-BW: Efficient and accurate mapping of bisulfite-treated reads [Supplemental Material] BRAT-BW: Efficient and accurate mapping of bisulfite-treated reads [Supplemental Material]](/thumbs/72/66393769.jpg) BRAT-BW: Efficient and accurate mapping of bisulfite-treated reads [Supplemental Material] Elena Y. Harris 1, Nadia Ponts 2,3, Karine G. Le Roch 2 and Stefano Lonardi 1 1 Department of Computer Science
BRAT-BW: Efficient and accurate mapping of bisulfite-treated reads [Supplemental Material] Elena Y. Harris 1, Nadia Ponts 2,3, Karine G. Le Roch 2 and Stefano Lonardi 1 1 Department of Computer Science
EpiGnome Methyl Seq Bioinformatics User Guide Rev. 0.1
 EpiGnome Methyl Seq Bioinformatics User Guide Rev. 0.1 Introduction This guide contains data analysis recommendations for libraries prepared using Epicentre s EpiGnome Methyl Seq Kit, and sequenced on
EpiGnome Methyl Seq Bioinformatics User Guide Rev. 0.1 Introduction This guide contains data analysis recommendations for libraries prepared using Epicentre s EpiGnome Methyl Seq Kit, and sequenced on
adriaan van der graaf*
 B S S E Q D ATA A N A LY S I S adriaan van der graaf* contents 1 Introduction 1 2 Steps performed before the practical 2 2.1 Isolation of DNA.......................... 2 2.2 Sequencing library creation....................
B S S E Q D ATA A N A LY S I S adriaan van der graaf* contents 1 Introduction 1 2 Steps performed before the practical 2 2.1 Isolation of DNA.......................... 2 2.2 Sequencing library creation....................
ASAP - Allele-specific alignment pipeline
 ASAP - Allele-specific alignment pipeline Jan 09, 2012 (1) ASAP - Quick Reference ASAP needs a working version of Perl and is run from the command line. Furthermore, Bowtie needs to be installed on your
ASAP - Allele-specific alignment pipeline Jan 09, 2012 (1) ASAP - Quick Reference ASAP needs a working version of Perl and is run from the command line. Furthermore, Bowtie needs to be installed on your
HMPL User Manual. Shuying Sun or Texas State University
 HMPL User Manual Shuying Sun (ssun5211@yahoo.com or s_s355@txstate.edu), Texas State University Peng Li (pxl119@case.edu), Case Western Reserve University June 18, 2015 Contents 1. General Overview and
HMPL User Manual Shuying Sun (ssun5211@yahoo.com or s_s355@txstate.edu), Texas State University Peng Li (pxl119@case.edu), Case Western Reserve University June 18, 2015 Contents 1. General Overview and
The SAM Format Specification (v1.3 draft)
 The SAM Format Specification (v1.3 draft) The SAM Format Specification Working Group July 15, 2010 1 The SAM Format Specification SAM stands for Sequence Alignment/Map format. It is a TAB-delimited text
The SAM Format Specification (v1.3 draft) The SAM Format Specification Working Group July 15, 2010 1 The SAM Format Specification SAM stands for Sequence Alignment/Map format. It is a TAB-delimited text
The SAM Format Specification (v1.3-r837)
 The SAM Format Specification (v1.3-r837) The SAM Format Specification Working Group November 18, 2010 1 The SAM Format Specification SAM stands for Sequence Alignment/Map format. It is a TAB-delimited
The SAM Format Specification (v1.3-r837) The SAM Format Specification Working Group November 18, 2010 1 The SAM Format Specification SAM stands for Sequence Alignment/Map format. It is a TAB-delimited
m6aviewer Version Documentation
 m6aviewer Version 1.6.0 Documentation Contents 1. About 2. Requirements 3. Launching m6aviewer 4. Running Time Estimates 5. Basic Peak Calling 6. Running Modes 7. Multiple Samples/Sample Replicates 8.
m6aviewer Version 1.6.0 Documentation Contents 1. About 2. Requirements 3. Launching m6aviewer 4. Running Time Estimates 5. Basic Peak Calling 6. Running Modes 7. Multiple Samples/Sample Replicates 8.
When we search a nucleic acid databases, there is no need for you to carry out your own six frame translation. Mascot always performs a 6 frame
 1 When we search a nucleic acid databases, there is no need for you to carry out your own six frame translation. Mascot always performs a 6 frame translation on the fly. That is, 3 reading frames from
1 When we search a nucleic acid databases, there is no need for you to carry out your own six frame translation. Mascot always performs a 6 frame translation on the fly. That is, 3 reading frames from
The Smithlab DNA Methylation Data Analysis Pipeline (MethPipe)
 The Smithlab DNA Methylation Data Analysis Pipeline (MethPipe) Qiang Song Benjamin Decato Michael Kessler Fang Fang Jenny Qu Tyler Garvin Meng Zhou Andrew Smith October 4, 2013 The methpipe software package
The Smithlab DNA Methylation Data Analysis Pipeline (MethPipe) Qiang Song Benjamin Decato Michael Kessler Fang Fang Jenny Qu Tyler Garvin Meng Zhou Andrew Smith October 4, 2013 The methpipe software package
SAM : Sequence Alignment/Map format. A TAB-delimited text format storing the alignment information. A header section is optional.
 Alignment of NGS reads, samtools and visualization Hands-on Software used in this practical BWA MEM : Burrows-Wheeler Aligner. A software package for mapping low-divergent sequences against a large reference
Alignment of NGS reads, samtools and visualization Hands-on Software used in this practical BWA MEM : Burrows-Wheeler Aligner. A software package for mapping low-divergent sequences against a large reference
Sep. Guide. Edico Genome Corp North Torrey Pines Court, Plaza Level, La Jolla, CA 92037
 Sep 2017 DRAGEN TM Quick Start Guide www.edicogenome.com info@edicogenome.com Edico Genome Corp. 3344 North Torrey Pines Court, Plaza Level, La Jolla, CA 92037 Notice Contents of this document and associated
Sep 2017 DRAGEN TM Quick Start Guide www.edicogenome.com info@edicogenome.com Edico Genome Corp. 3344 North Torrey Pines Court, Plaza Level, La Jolla, CA 92037 Notice Contents of this document and associated
QIAseq DNA V3 Panel Analysis Plugin USER MANUAL
 QIAseq DNA V3 Panel Analysis Plugin USER MANUAL User manual for QIAseq DNA V3 Panel Analysis 1.0.1 Windows, Mac OS X and Linux January 25, 2018 This software is for research purposes only. QIAGEN Aarhus
QIAseq DNA V3 Panel Analysis Plugin USER MANUAL User manual for QIAseq DNA V3 Panel Analysis 1.0.1 Windows, Mac OS X and Linux January 25, 2018 This software is for research purposes only. QIAGEN Aarhus
Number representations
 Number representations Number bases Three number bases are of interest: Binary, Octal and Hexadecimal. We look briefly at conversions among them and between each of them and decimal. Binary Base-two, or
Number representations Number bases Three number bases are of interest: Binary, Octal and Hexadecimal. We look briefly at conversions among them and between each of them and decimal. Binary Base-two, or
Understanding and Pre-processing Raw Illumina Data
 Understanding and Pre-processing Raw Illumina Data Matt Johnson October 4, 2013 1 Understanding FASTQ files After an Illumina sequencing run, the data is stored in very large text files in a standard format
Understanding and Pre-processing Raw Illumina Data Matt Johnson October 4, 2013 1 Understanding FASTQ files After an Illumina sequencing run, the data is stored in very large text files in a standard format
Mar. Guide. Edico Genome Inc North Torrey Pines Court, Plaza Level, La Jolla, CA 92037
 Mar 2017 DRAGEN TM Quick Start Guide www.edicogenome.com info@edicogenome.com Edico Genome Inc. 3344 North Torrey Pines Court, Plaza Level, La Jolla, CA 92037 Notice Contents of this document and associated
Mar 2017 DRAGEN TM Quick Start Guide www.edicogenome.com info@edicogenome.com Edico Genome Inc. 3344 North Torrey Pines Court, Plaza Level, La Jolla, CA 92037 Notice Contents of this document and associated
SAM / BAM Tutorial. EMBL Heidelberg. Course Materials. Tobias Rausch September 2012
 SAM / BAM Tutorial EMBL Heidelberg Course Materials Tobias Rausch September 2012 Contents 1 SAM / BAM 3 1.1 Introduction................................... 3 1.2 Tasks.......................................
SAM / BAM Tutorial EMBL Heidelberg Course Materials Tobias Rausch September 2012 Contents 1 SAM / BAM 3 1.1 Introduction................................... 3 1.2 Tasks.......................................
Short Read Alignment. Mapping Reads to a Reference
 Short Read Alignment Mapping Reads to a Reference Brandi Cantarel, Ph.D. & Daehwan Kim, Ph.D. BICF 05/2018 Introduction to Mapping Short Read Aligners DNA vs RNA Alignment Quality Pitfalls and Improvements
Short Read Alignment Mapping Reads to a Reference Brandi Cantarel, Ph.D. & Daehwan Kim, Ph.D. BICF 05/2018 Introduction to Mapping Short Read Aligners DNA vs RNA Alignment Quality Pitfalls and Improvements
Resequencing Analysis. (Pseudomonas aeruginosa MAPO1 ) Sample to Insight
 Resequencing Analysis (Pseudomonas aeruginosa MAPO1 ) 1 Workflow Import NGS raw data Trim reads Import Reference Sequence Reference Mapping QC on reads Variant detection Case Study Pseudomonas aeruginosa
Resequencing Analysis (Pseudomonas aeruginosa MAPO1 ) 1 Workflow Import NGS raw data Trim reads Import Reference Sequence Reference Mapping QC on reads Variant detection Case Study Pseudomonas aeruginosa
merantk Version 1.1.1a
 DIVISION OF BIOINFORMATICS - INNSBRUCK MEDICAL UNIVERSITY merantk Version 1.1.1a User manual Dietmar Rieder 1/12/2016 Page 1 Contents 1. Introduction... 3 1.1. Purpose of this document... 3 1.2. System
DIVISION OF BIOINFORMATICS - INNSBRUCK MEDICAL UNIVERSITY merantk Version 1.1.1a User manual Dietmar Rieder 1/12/2016 Page 1 Contents 1. Introduction... 3 1.1. Purpose of this document... 3 1.2. System
Bismark Bisulfite Mapper User Guide - v0.7.3
 April 05, 2012 Bismark Bisulfite Mapper User Guide - v0.7.3 1) Quick Reference Bismark needs a working version of Perl and it is run from the command line. Furthermore, Bowtie (http://bowtie-bio.sourceforge.net/index.shtml)
April 05, 2012 Bismark Bisulfite Mapper User Guide - v0.7.3 1) Quick Reference Bismark needs a working version of Perl and it is run from the command line. Furthermore, Bowtie (http://bowtie-bio.sourceforge.net/index.shtml)
Description of a genome assembler: CABOG
 Theo Zimmermann Description of a genome assembler: CABOG CABOG (Celera Assembler with the Best Overlap Graph) is an assembler built upon the Celera Assembler, which, at first, was designed for Sanger sequencing,
Theo Zimmermann Description of a genome assembler: CABOG CABOG (Celera Assembler with the Best Overlap Graph) is an assembler built upon the Celera Assembler, which, at first, was designed for Sanger sequencing,
Benchmarking of RNA-seq aligners
 Lecture 17 RNA-seq Alignment STAR Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Based on this analysis the most reliable
Lecture 17 RNA-seq Alignment STAR Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Based on this analysis the most reliable
Running SNAP. The SNAP Team February 2012
 Running SNAP The SNAP Team February 2012 1 Introduction SNAP is a tool that is intended to serve as the read aligner in a gene sequencing pipeline. Its theory of operation is described in Faster and More
Running SNAP The SNAP Team February 2012 1 Introduction SNAP is a tool that is intended to serve as the read aligner in a gene sequencing pipeline. Its theory of operation is described in Faster and More
Pre-processing and quality control of sequence data. Barbera van Schaik KEBB - Bioinformatics Laboratory
 Pre-processing and quality control of sequence data Barbera van Schaik KEBB - Bioinformatics Laboratory b.d.vanschaik@amc.uva.nl Topic: quality control and prepare data for the interesting stuf Keep Throw
Pre-processing and quality control of sequence data Barbera van Schaik KEBB - Bioinformatics Laboratory b.d.vanschaik@amc.uva.nl Topic: quality control and prepare data for the interesting stuf Keep Throw
Mapping Reads to Reference Genome
 Mapping Reads to Reference Genome DNA carries genetic information DNA is a double helix of two complementary strands formed by four nucleotides (bases): Adenine, Cytosine, Guanine and Thymine 2 of 31 Gene
Mapping Reads to Reference Genome DNA carries genetic information DNA is a double helix of two complementary strands formed by four nucleotides (bases): Adenine, Cytosine, Guanine and Thymine 2 of 31 Gene
Omega: an Overlap-graph de novo Assembler for Metagenomics
 Omega: an Overlap-graph de novo Assembler for Metagenomics B a h l e l H a i d e r, Ta e - H y u k A h n, B r i a n B u s h n e l l, J u a n j u a n C h a i, A l e x C o p e l a n d, C h o n g l e Pa n
Omega: an Overlap-graph de novo Assembler for Metagenomics B a h l e l H a i d e r, Ta e - H y u k A h n, B r i a n B u s h n e l l, J u a n j u a n C h a i, A l e x C o p e l a n d, C h o n g l e Pa n
User's guide to ChIP-Seq applications: command-line usage and option summary
 User's guide to ChIP-Seq applications: command-line usage and option summary 1. Basics about the ChIP-Seq Tools The ChIP-Seq software provides a set of tools performing common genome-wide ChIPseq analysis
User's guide to ChIP-Seq applications: command-line usage and option summary 1. Basics about the ChIP-Seq Tools The ChIP-Seq software provides a set of tools performing common genome-wide ChIPseq analysis
Groups of two-state devices are used to represent data in a computer. In general, we say the states are either: high/low, on/off, 1/0,...
 Chapter 9 Computer Arithmetic Reading: Section 9.1 on pp. 290-296 Computer Representation of Data Groups of two-state devices are used to represent data in a computer. In general, we say the states are
Chapter 9 Computer Arithmetic Reading: Section 9.1 on pp. 290-296 Computer Representation of Data Groups of two-state devices are used to represent data in a computer. In general, we say the states are
arxiv: v2 [q-bio.gn] 13 May 2014
![arxiv: v2 [q-bio.gn] 13 May 2014 arxiv: v2 [q-bio.gn] 13 May 2014](/thumbs/81/83096958.jpg) BIOINFORMATICS Vol. 00 no. 00 2005 Pages 1 2 Fast and accurate alignment of long bisulfite-seq reads Brent S. Pedersen 1,, Kenneth Eyring 1, Subhajyoti De 1,2, Ivana V. Yang 1 and David A. Schwartz 1 1
BIOINFORMATICS Vol. 00 no. 00 2005 Pages 1 2 Fast and accurate alignment of long bisulfite-seq reads Brent S. Pedersen 1,, Kenneth Eyring 1, Subhajyoti De 1,2, Ivana V. Yang 1 and David A. Schwartz 1 1
SMALT Manual. December 9, 2010 Version 0.4.2
 SMALT Manual December 9, 2010 Version 0.4.2 Abstract SMALT is a pairwise sequence alignment program for the efficient mapping of DNA sequencing reads onto genomic reference sequences. It uses a combination
SMALT Manual December 9, 2010 Version 0.4.2 Abstract SMALT is a pairwise sequence alignment program for the efficient mapping of DNA sequencing reads onto genomic reference sequences. It uses a combination
Standard output. Some of the output files can be redirected into the standard output, which may facilitate in creating the pipelines:
 Lecture 18 RNA-seq Alignment Standard output Some of the output files can be redirected into the standard output, which may facilitate in creating the pipelines: Filtering of the alignments STAR performs
Lecture 18 RNA-seq Alignment Standard output Some of the output files can be redirected into the standard output, which may facilitate in creating the pipelines: Filtering of the alignments STAR performs
The Smithlab DNA Methylation Data Analysis Pipeline (MethPipe)
 The Smithlab DNA Methylation Data Analysis Pipeline (MethPipe) Qiang Song Benjamin Decato Michael Kessler Fang Fang Jenny Qu Tyler Garvin Meng Zhou Andrew Smith August 4, 2014 The methpipe software package
The Smithlab DNA Methylation Data Analysis Pipeline (MethPipe) Qiang Song Benjamin Decato Michael Kessler Fang Fang Jenny Qu Tyler Garvin Meng Zhou Andrew Smith August 4, 2014 The methpipe software package
IDBA - A Practical Iterative de Bruijn Graph De Novo Assembler
 IDBA - A Practical Iterative de Bruijn Graph De Novo Assembler Yu Peng, Henry Leung, S.M. Yiu, Francis Y.L. Chin Department of Computer Science, The University of Hong Kong Pokfulam Road, Hong Kong {ypeng,
IDBA - A Practical Iterative de Bruijn Graph De Novo Assembler Yu Peng, Henry Leung, S.M. Yiu, Francis Y.L. Chin Department of Computer Science, The University of Hong Kong Pokfulam Road, Hong Kong {ypeng,
HIPPIE User Manual. (v0.0.2-beta, 2015/4/26, Yih-Chii Hwang, yihhwang [at] mail.med.upenn.edu)
![HIPPIE User Manual. (v0.0.2-beta, 2015/4/26, Yih-Chii Hwang, yihhwang [at] mail.med.upenn.edu) HIPPIE User Manual. (v0.0.2-beta, 2015/4/26, Yih-Chii Hwang, yihhwang [at] mail.med.upenn.edu)](/thumbs/71/65752105.jpg) HIPPIE User Manual (v0.0.2-beta, 2015/4/26, Yih-Chii Hwang, yihhwang [at] mail.med.upenn.edu) OVERVIEW OF HIPPIE o Flowchart of HIPPIE o Requirements PREPARE DIRECTORY STRUCTURE FOR HIPPIE EXECUTION o
HIPPIE User Manual (v0.0.2-beta, 2015/4/26, Yih-Chii Hwang, yihhwang [at] mail.med.upenn.edu) OVERVIEW OF HIPPIE o Flowchart of HIPPIE o Requirements PREPARE DIRECTORY STRUCTURE FOR HIPPIE EXECUTION o
High-throughout sequencing and using short-read aligners. Simon Anders
 High-throughout sequencing and using short-read aligners Simon Anders High-throughput sequencing (HTS) Sequencing millions of short DNA fragments in parallel. a.k.a.: next-generation sequencing (NGS) massively-parallel
High-throughout sequencing and using short-read aligners Simon Anders High-throughput sequencing (HTS) Sequencing millions of short DNA fragments in parallel. a.k.a.: next-generation sequencing (NGS) massively-parallel
ITMO Ecole de Bioinformatique Hands-on session: smallrna-seq N. Servant 21 rd November 2013
 ITMO Ecole de Bioinformatique Hands-on session: smallrna-seq N. Servant 21 rd November 2013 1. Data and objectives We will use the data from GEO (GSE35368, Toedling, Servant et al. 2011). Two samples were
ITMO Ecole de Bioinformatique Hands-on session: smallrna-seq N. Servant 21 rd November 2013 1. Data and objectives We will use the data from GEO (GSE35368, Toedling, Servant et al. 2011). Two samples were
SOLiD GFF File Format
 SOLiD GFF File Format 1 Introduction The GFF file is a text based repository and contains data and analysis results; colorspace calls, quality values (QV) and variant annotations. The inputs to the GFF
SOLiD GFF File Format 1 Introduction The GFF file is a text based repository and contains data and analysis results; colorspace calls, quality values (QV) and variant annotations. The inputs to the GFF
Lecture 12. Short read aligners
 Lecture 12 Short read aligners Ebola reference genome We will align ebola sequencing data against the 1976 Mayinga reference genome. We will hold the reference gnome and all indices: mkdir -p ~/reference/ebola
Lecture 12 Short read aligners Ebola reference genome We will align ebola sequencing data against the 1976 Mayinga reference genome. We will hold the reference gnome and all indices: mkdir -p ~/reference/ebola
Shortest Path Algorithm
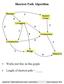 Shortest Path Algorithm C Works just fine on this graph. C Length of shortest path = Copyright 2005 DIMACS BioMath Connect Institute Robert Hochberg Dynamic Programming SP #1 Same Questions, Different
Shortest Path Algorithm C Works just fine on this graph. C Length of shortest path = Copyright 2005 DIMACS BioMath Connect Institute Robert Hochberg Dynamic Programming SP #1 Same Questions, Different
IDBA A Practical Iterative de Bruijn Graph De Novo Assembler
 IDBA A Practical Iterative de Bruijn Graph De Novo Assembler Yu Peng, Henry C.M. Leung, S.M. Yiu, and Francis Y.L. Chin Department of Computer Science, The University of Hong Kong Pokfulam Road, Hong Kong
IDBA A Practical Iterative de Bruijn Graph De Novo Assembler Yu Peng, Henry C.M. Leung, S.M. Yiu, and Francis Y.L. Chin Department of Computer Science, The University of Hong Kong Pokfulam Road, Hong Kong
Package Rbowtie. January 21, 2019
 Type Package Title R bowtie wrapper Version 1.23.1 Date 2019-01-17 Package Rbowtie January 21, 2019 Author Florian Hahne, Anita Lerch, Michael B Stadler Maintainer Michael Stadler
Type Package Title R bowtie wrapper Version 1.23.1 Date 2019-01-17 Package Rbowtie January 21, 2019 Author Florian Hahne, Anita Lerch, Michael B Stadler Maintainer Michael Stadler
Notes on Bloom filters
 Computer Science B63 Winter 2017 Scarborough Campus University of Toronto Notes on Bloom filters Vassos Hadzilacos A Bloom filter is an approximate or probabilistic dictionary. Let S be a dynamic set of
Computer Science B63 Winter 2017 Scarborough Campus University of Toronto Notes on Bloom filters Vassos Hadzilacos A Bloom filter is an approximate or probabilistic dictionary. Let S be a dynamic set of
Richard Feynman, Lectures on Computation
 Chapter 8 Sorting and Sequencing If you keep proving stuff that others have done, getting confidence, increasing the complexities of your solutions for the fun of it then one day you ll turn around and
Chapter 8 Sorting and Sequencing If you keep proving stuff that others have done, getting confidence, increasing the complexities of your solutions for the fun of it then one day you ll turn around and
High-throughput sequencing: Alignment and related topic. Simon Anders EMBL Heidelberg
 High-throughput sequencing: Alignment and related topic Simon Anders EMBL Heidelberg Established platforms HTS Platforms Illumina HiSeq, ABI SOLiD, Roche 454 Newcomers: Benchtop machines 454 GS Junior,
High-throughput sequencing: Alignment and related topic Simon Anders EMBL Heidelberg Established platforms HTS Platforms Illumina HiSeq, ABI SOLiD, Roche 454 Newcomers: Benchtop machines 454 GS Junior,
BLAST & Genome assembly
 BLAST & Genome assembly Solon P. Pissis Tomáš Flouri Heidelberg Institute for Theoretical Studies November 17, 2012 1 Introduction Introduction 2 BLAST What is BLAST? The algorithm 3 Genome assembly De
BLAST & Genome assembly Solon P. Pissis Tomáš Flouri Heidelberg Institute for Theoretical Studies November 17, 2012 1 Introduction Introduction 2 BLAST What is BLAST? The algorithm 3 Genome assembly De
Analysis of ChIP-seq data
 Before we start: 1. Log into tak (step 0 on the exercises) 2. Go to your lab space and create a folder for the class (see separate hand out) 3. Connect to your lab space through the wihtdata network and
Before we start: 1. Log into tak (step 0 on the exercises) 2. Go to your lab space and create a folder for the class (see separate hand out) 3. Connect to your lab space through the wihtdata network and
Welcome to MAPHiTS (Mapping Analysis Pipeline for High-Throughput Sequences) tutorial page.
 Welcome to MAPHiTS (Mapping Analysis Pipeline for High-Throughput Sequences) tutorial page. In this page you will learn to use the tools of the MAPHiTS suite. A little advice before starting : rename your
Welcome to MAPHiTS (Mapping Analysis Pipeline for High-Throughput Sequences) tutorial page. In this page you will learn to use the tools of the MAPHiTS suite. A little advice before starting : rename your
Copyright 2014 Regents of the University of Minnesota
 Quality Control of Illumina Data using Galaxy Contents September 16, 2014 1 Introduction 2 1.1 What is Galaxy?..................................... 2 1.2 Galaxy at MSI......................................
Quality Control of Illumina Data using Galaxy Contents September 16, 2014 1 Introduction 2 1.1 What is Galaxy?..................................... 2 1.2 Galaxy at MSI......................................
Sequence Alignment/Map Optional Fields Specification
 Sequence Alignment/Map Optional Fields Specification The SAM/BAM Format Specification Working Group 14 Jul 2017 The master version of this document can be found at https://github.com/samtools/hts-specs.
Sequence Alignment/Map Optional Fields Specification The SAM/BAM Format Specification Working Group 14 Jul 2017 The master version of this document can be found at https://github.com/samtools/hts-specs.
Demultiplexing Illumina sequencing data containing unique molecular indexes (UMIs)
 next generation sequencing analysis guidelines Demultiplexing Illumina sequencing data containing unique molecular indexes (UMIs) See what more we can do for you at www.idtdna.com. For Research Use Only
next generation sequencing analysis guidelines Demultiplexing Illumina sequencing data containing unique molecular indexes (UMIs) See what more we can do for you at www.idtdna.com. For Research Use Only
High-throughput sequencing: Alignment and related topic. Simon Anders EMBL Heidelberg
 High-throughput sequencing: Alignment and related topic Simon Anders EMBL Heidelberg Established platforms HTS Platforms Illumina HiSeq, ABI SOLiD, Roche 454 Newcomers: Benchtop machines: Illumina MiSeq,
High-throughput sequencing: Alignment and related topic Simon Anders EMBL Heidelberg Established platforms HTS Platforms Illumina HiSeq, ABI SOLiD, Roche 454 Newcomers: Benchtop machines: Illumina MiSeq,
Tutorial. Small RNA Analysis using Illumina Data. Sample to Insight. October 5, 2016
 Small RNA Analysis using Illumina Data October 5, 2016 Sample to Insight QIAGEN Aarhus Silkeborgvej 2 Prismet 8000 Aarhus C Denmark Telephone: +45 70 22 32 44 www.qiagenbioinformatics.com AdvancedGenomicsSupport@qiagen.com
Small RNA Analysis using Illumina Data October 5, 2016 Sample to Insight QIAGEN Aarhus Silkeborgvej 2 Prismet 8000 Aarhus C Denmark Telephone: +45 70 22 32 44 www.qiagenbioinformatics.com AdvancedGenomicsSupport@qiagen.com
Small RNA Analysis using Illumina Data
 Small RNA Analysis using Illumina Data September 7, 2016 Sample to Insight CLC bio, a QIAGEN Company Silkeborgvej 2 Prismet 8000 Aarhus C Denmark Telephone: +45 70 22 32 44 www.clcbio.com support-clcbio@qiagen.com
Small RNA Analysis using Illumina Data September 7, 2016 Sample to Insight CLC bio, a QIAGEN Company Silkeborgvej 2 Prismet 8000 Aarhus C Denmark Telephone: +45 70 22 32 44 www.clcbio.com support-clcbio@qiagen.com
Next Generation Sequence Alignment on the BRC Cluster. Steve Newhouse 22 July 2010
 Next Generation Sequence Alignment on the BRC Cluster Steve Newhouse 22 July 2010 Overview Practical guide to processing next generation sequencing data on the cluster No details on the inner workings
Next Generation Sequence Alignment on the BRC Cluster Steve Newhouse 22 July 2010 Overview Practical guide to processing next generation sequencing data on the cluster No details on the inner workings
SISTEMI EMBEDDED. Basic Concepts about Computers. Federico Baronti Last version:
 SISTEMI EMBEDDED Basic Concepts about Computers Federico Baronti Last version: 20170307 Embedded System Block Diagram Embedded Computer Embedded System Input Memory Output Sensor Sensor Sensor SENSOR CONDITIONING
SISTEMI EMBEDDED Basic Concepts about Computers Federico Baronti Last version: 20170307 Embedded System Block Diagram Embedded Computer Embedded System Input Memory Output Sensor Sensor Sensor SENSOR CONDITIONING
Tutorial: RNA-Seq Analysis Part II (Tracks): Non-Specific Matches, Mapping Modes and Expression measures
 : RNA-Seq Analysis Part II (Tracks): Non-Specific Matches, Mapping Modes and February 24, 2014 Sample to Insight : RNA-Seq Analysis Part II (Tracks): Non-Specific Matches, Mapping Modes and : RNA-Seq Analysis
: RNA-Seq Analysis Part II (Tracks): Non-Specific Matches, Mapping Modes and February 24, 2014 Sample to Insight : RNA-Seq Analysis Part II (Tracks): Non-Specific Matches, Mapping Modes and : RNA-Seq Analysis
World Inside a Computer is Binary
 C Programming 1 Representation of int data World Inside a Computer is Binary C Programming 2 Decimal Number System Basic symbols: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 Radix-10 positional number system. The radix
C Programming 1 Representation of int data World Inside a Computer is Binary C Programming 2 Decimal Number System Basic symbols: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 Radix-10 positional number system. The radix
Loops / Repetition Statements
 Loops / Repetition Statements Repetition statements allow us to execute a statement multiple times Often they are referred to as loops C has three kinds of repetition statements: the while loop the for
Loops / Repetition Statements Repetition statements allow us to execute a statement multiple times Often they are referred to as loops C has three kinds of repetition statements: the while loop the for
CLC Server. End User USER MANUAL
 CLC Server End User USER MANUAL Manual for CLC Server 10.0.1 Windows, macos and Linux March 8, 2018 This software is for research purposes only. QIAGEN Aarhus Silkeborgvej 2 Prismet DK-8000 Aarhus C Denmark
CLC Server End User USER MANUAL Manual for CLC Server 10.0.1 Windows, macos and Linux March 8, 2018 This software is for research purposes only. QIAGEN Aarhus Silkeborgvej 2 Prismet DK-8000 Aarhus C Denmark
15.4 Longest common subsequence
 15.4 Longest common subsequence Biological applications often need to compare the DNA of two (or more) different organisms A strand of DNA consists of a string of molecules called bases, where the possible
15.4 Longest common subsequence Biological applications often need to compare the DNA of two (or more) different organisms A strand of DNA consists of a string of molecules called bases, where the possible
RASER: Reads Aligner for SNPs and Editing sites of RNA (version 0.51) Manual
 RASER: Reads Aligner for SNPs and Editing sites of RNA (version 0.51) Manual July 02, 2015 1 Index 1. System requirement and how to download RASER source code...3 2. Installation...3 3. Making index files...3
RASER: Reads Aligner for SNPs and Editing sites of RNA (version 0.51) Manual July 02, 2015 1 Index 1. System requirement and how to download RASER source code...3 2. Installation...3 3. Making index files...3
Practical Bioinformatics for Life Scientists. Week 4, Lecture 8. István Albert Bioinformatics Consulting Center Penn State
 Practical Bioinformatics for Life Scientists Week 4, Lecture 8 István Albert Bioinformatics Consulting Center Penn State Reminder Before any serious work re-check the documentation for small but essential
Practical Bioinformatics for Life Scientists Week 4, Lecture 8 István Albert Bioinformatics Consulting Center Penn State Reminder Before any serious work re-check the documentation for small but essential
Biostrings/BSgenome Lab (BioC2009)
 Biostrings/BSgenome Lab (BioC2009) Hervé Pagès and Patrick Aboyoun Fred Hutchinson Cancer Research Center Seattle, WA July 26, 2009 1 Lab overview Learn the basics of Biostrings and the BSgenome data packages.
Biostrings/BSgenome Lab (BioC2009) Hervé Pagès and Patrick Aboyoun Fred Hutchinson Cancer Research Center Seattle, WA July 26, 2009 1 Lab overview Learn the basics of Biostrings and the BSgenome data packages.
Data Representation 1
 1 Data Representation Outline Binary Numbers Adding Binary Numbers Negative Integers Other Operations with Binary Numbers Floating Point Numbers Character Representation Image Representation Sound Representation
1 Data Representation Outline Binary Numbers Adding Binary Numbers Negative Integers Other Operations with Binary Numbers Floating Point Numbers Character Representation Image Representation Sound Representation
Dynamic Programming User Manual v1.0 Anton E. Weisstein, Truman State University Aug. 19, 2014
 Dynamic Programming User Manual v1.0 Anton E. Weisstein, Truman State University Aug. 19, 2014 Dynamic programming is a group of mathematical methods used to sequentially split a complicated problem into
Dynamic Programming User Manual v1.0 Anton E. Weisstein, Truman State University Aug. 19, 2014 Dynamic programming is a group of mathematical methods used to sequentially split a complicated problem into
Copyright 2014 Regents of the University of Minnesota
 Quality Control of Illumina Data using Galaxy August 18, 2014 Contents 1 Introduction 2 1.1 What is Galaxy?..................................... 2 1.2 Galaxy at MSI......................................
Quality Control of Illumina Data using Galaxy August 18, 2014 Contents 1 Introduction 2 1.1 What is Galaxy?..................................... 2 1.2 Galaxy at MSI......................................
ASSIGNMENT 4. COMP-202, Summer 2017, All Sections. Due: Friday, June 16 th, 11:59pm
 ASSIGNMENT 4 COMP-202, Summer 2017, All Sections Due: Friday, June 16 th, 11:59pm Please read the entire PDF before starting. You must do this assignment individually. Question 1: 100 points 100 points
ASSIGNMENT 4 COMP-202, Summer 2017, All Sections Due: Friday, June 16 th, 11:59pm Please read the entire PDF before starting. You must do this assignment individually. Question 1: 100 points 100 points
Sequencing. Short Read Alignment. Sequencing. Paired-End Sequencing 6/10/2010. Tobias Rausch 7 th June 2010 WGS. ChIP-Seq. Applied Biosystems.
 Sequencing Short Alignment Tobias Rausch 7 th June 2010 WGS RNA-Seq Exon Capture ChIP-Seq Sequencing Paired-End Sequencing Target genome Fragments Roche GS FLX Titanium Illumina Applied Biosystems SOLiD
Sequencing Short Alignment Tobias Rausch 7 th June 2010 WGS RNA-Seq Exon Capture ChIP-Seq Sequencing Paired-End Sequencing Target genome Fragments Roche GS FLX Titanium Illumina Applied Biosystems SOLiD
BLAST & Genome assembly
 BLAST & Genome assembly Solon P. Pissis Tomáš Flouri Heidelberg Institute for Theoretical Studies May 15, 2014 1 BLAST What is BLAST? The algorithm 2 Genome assembly De novo assembly Mapping assembly 3
BLAST & Genome assembly Solon P. Pissis Tomáš Flouri Heidelberg Institute for Theoretical Studies May 15, 2014 1 BLAST What is BLAST? The algorithm 2 Genome assembly De novo assembly Mapping assembly 3
UNIT - I: COMPUTER ARITHMETIC, REGISTER TRANSFER LANGUAGE & MICROOPERATIONS
 UNIT - I: COMPUTER ARITHMETIC, REGISTER TRANSFER LANGUAGE & MICROOPERATIONS (09 periods) Computer Arithmetic: Data Representation, Fixed Point Representation, Floating Point Representation, Addition and
UNIT - I: COMPUTER ARITHMETIC, REGISTER TRANSFER LANGUAGE & MICROOPERATIONS (09 periods) Computer Arithmetic: Data Representation, Fixed Point Representation, Floating Point Representation, Addition and
GSNAP: Fast and SNP-tolerant detection of complex variants and splicing in short reads by Thomas D. Wu and Serban Nacu
 GSNAP: Fast and SNP-tolerant detection of complex variants and splicing in short reads by Thomas D. Wu and Serban Nacu Matt Huska Freie Universität Berlin Computational Methods for High-Throughput Omics
GSNAP: Fast and SNP-tolerant detection of complex variants and splicing in short reads by Thomas D. Wu and Serban Nacu Matt Huska Freie Universität Berlin Computational Methods for High-Throughput Omics
Bits, Words, and Integers
 Computer Science 52 Bits, Words, and Integers Spring Semester, 2017 In this document, we look at how bits are organized into meaningful data. In particular, we will see the details of how integers are
Computer Science 52 Bits, Words, and Integers Spring Semester, 2017 In this document, we look at how bits are organized into meaningful data. In particular, we will see the details of how integers are
Sequence analysis Pairwise sequence alignment
 UMF11 Introduction to bioinformatics, 25 Sequence analysis Pairwise sequence alignment 1. Sequence alignment Lecturer: Marina lexandersson 12 September, 25 here are two types of sequence alignments, global
UMF11 Introduction to bioinformatics, 25 Sequence analysis Pairwise sequence alignment 1. Sequence alignment Lecturer: Marina lexandersson 12 September, 25 here are two types of sequence alignments, global
Sequence Preprocessing: A perspective
 Sequence Preprocessing: A perspective Dr. Matthew L. Settles Genome Center University of California, Davis settles@ucdavis.edu Why Preprocess reads We have found that aggressively cleaning and processing
Sequence Preprocessing: A perspective Dr. Matthew L. Settles Genome Center University of California, Davis settles@ucdavis.edu Why Preprocess reads We have found that aggressively cleaning and processing
15.4 Longest common subsequence
 15.4 Longest common subsequence Biological applications often need to compare the DNA of two (or more) different organisms A strand of DNA consists of a string of molecules called bases, where the possible
15.4 Longest common subsequence Biological applications often need to compare the DNA of two (or more) different organisms A strand of DNA consists of a string of molecules called bases, where the possible
CHAPTER 2 Data Representation in Computer Systems
 CHAPTER 2 Data Representation in Computer Systems 2.1 Introduction 37 2.2 Positional Numbering Systems 38 2.3 Decimal to Binary Conversions 38 2.3.1 Converting Unsigned Whole Numbers 39 2.3.2 Converting
CHAPTER 2 Data Representation in Computer Systems 2.1 Introduction 37 2.2 Positional Numbering Systems 38 2.3 Decimal to Binary Conversions 38 2.3.1 Converting Unsigned Whole Numbers 39 2.3.2 Converting
Preparation of alignments for variant calling with GATK: exercise instructions for BioHPC Lab computers
 Preparation of alignments for variant calling with GATK: exercise instructions for BioHPC Lab computers Data used in the exercise We will use D. melanogaster WGS paired-end Illumina data with NCBI accessions
Preparation of alignments for variant calling with GATK: exercise instructions for BioHPC Lab computers Data used in the exercise We will use D. melanogaster WGS paired-end Illumina data with NCBI accessions
γ 2 γ 3 γ 1 R 2 (b) a bounded Yin set (a) an unbounded Yin set
 γ 1 γ 3 γ γ 3 γ γ 1 R (a) an unbounded Yin set (b) a bounded Yin set Fig..1: Jordan curve representation of a connected Yin set M R. A shaded region represents M and the dashed curves its boundary M that
γ 1 γ 3 γ γ 3 γ γ 1 R (a) an unbounded Yin set (b) a bounded Yin set Fig..1: Jordan curve representation of a connected Yin set M R. A shaded region represents M and the dashed curves its boundary M that
File Formats: SAM, BAM, and CRAM. UCD Genome Center Bioinformatics Core Tuesday 15 September 2015
 File Formats: SAM, BAM, and CRAM UCD Genome Center Bioinformatics Core Tuesday 15 September 2015 / BAM / CRAM NEW! http://samtools.sourceforge.net/ - deprecated! http://www.htslib.org/ - SAMtools 1.0 and
File Formats: SAM, BAM, and CRAM UCD Genome Center Bioinformatics Core Tuesday 15 September 2015 / BAM / CRAM NEW! http://samtools.sourceforge.net/ - deprecated! http://www.htslib.org/ - SAMtools 1.0 and
CHAPTER 2 Data Representation in Computer Systems
 CHAPTER 2 Data Representation in Computer Systems 2.1 Introduction 37 2.2 Positional Numbering Systems 38 2.3 Decimal to Binary Conversions 38 2.3.1 Converting Unsigned Whole Numbers 39 2.3.2 Converting
CHAPTER 2 Data Representation in Computer Systems 2.1 Introduction 37 2.2 Positional Numbering Systems 38 2.3 Decimal to Binary Conversions 38 2.3.1 Converting Unsigned Whole Numbers 39 2.3.2 Converting
Next Generation Sequencing quality trimming (NGSQTRIM)
 Next Generation Sequencing quality trimming (NGSQTRIM) Danamma B.J 1, Naveen kumar 2, V.G Shanmuga priya 3 1 M.Tech, Bioinformatics, KLEMSSCET, Belagavi 2 Proprietor, GenEclat Technologies, Bengaluru 3
Next Generation Sequencing quality trimming (NGSQTRIM) Danamma B.J 1, Naveen kumar 2, V.G Shanmuga priya 3 1 M.Tech, Bioinformatics, KLEMSSCET, Belagavi 2 Proprietor, GenEclat Technologies, Bengaluru 3
Chapter 4. Operations on Data
 Chapter 4 Operations on Data 1 OBJECTIVES After reading this chapter, the reader should be able to: List the three categories of operations performed on data. Perform unary and binary logic operations
Chapter 4 Operations on Data 1 OBJECTIVES After reading this chapter, the reader should be able to: List the three categories of operations performed on data. Perform unary and binary logic operations
3.1 DATA REPRESENTATION (PART C)
 3.1 DATA REPRESENTATION (PART C) 3.1.3 REAL NUMBERS AND NORMALISED FLOATING-POINT REPRESENTATION In decimal notation, the number 23.456 can be written as 0.23456 x 10 2. This means that in decimal notation,
3.1 DATA REPRESENTATION (PART C) 3.1.3 REAL NUMBERS AND NORMALISED FLOATING-POINT REPRESENTATION In decimal notation, the number 23.456 can be written as 0.23456 x 10 2. This means that in decimal notation,
all M 2M_gt_15 2M_8_15 2M_1_7 gt_2m TopHat2
 Pairs processed per second 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 72,318 418 1,666 49,495 21,123 69,984 35,694 1,9 71,538 3,5 17,381 61,223 69,39 55 19,579 44,79 65,126 96 5,115 33,6 61,787
Pairs processed per second 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 72,318 418 1,666 49,495 21,123 69,984 35,694 1,9 71,538 3,5 17,381 61,223 69,39 55 19,579 44,79 65,126 96 5,115 33,6 61,787
Importing sequence assemblies from BAM and SAM files
 BioNumerics Tutorial: Importing sequence assemblies from BAM and SAM files 1 Aim With the BioNumerics BAM import routine, a sequence assembly in BAM or SAM format can be imported in BioNumerics. A BAM
BioNumerics Tutorial: Importing sequence assemblies from BAM and SAM files 1 Aim With the BioNumerics BAM import routine, a sequence assembly in BAM or SAM format can be imported in BioNumerics. A BAM
Number Systems. Binary Numbers. Appendix. Decimal notation represents numbers as powers of 10, for example
 Appendix F Number Systems Binary Numbers Decimal notation represents numbers as powers of 10, for example 1729 1 103 7 102 2 101 9 100 decimal = + + + There is no particular reason for the choice of 10,
Appendix F Number Systems Binary Numbers Decimal notation represents numbers as powers of 10, for example 1729 1 103 7 102 2 101 9 100 decimal = + + + There is no particular reason for the choice of 10,
NGS Data and Sequence Alignment
 Applications and Servers SERVER/REMOTE Compute DB WEB Data files NGS Data and Sequence Alignment SSH WEB SCP Manpreet S. Katari App Aug 11, 2016 Service Terminal IGV Data files Window Personal Computer/Local
Applications and Servers SERVER/REMOTE Compute DB WEB Data files NGS Data and Sequence Alignment SSH WEB SCP Manpreet S. Katari App Aug 11, 2016 Service Terminal IGV Data files Window Personal Computer/Local
STREAMING FRAGMENT ASSIGNMENT FOR REAL-TIME ANALYSIS OF SEQUENCING EXPERIMENTS. Supplementary Figure 1
 STREAMING FRAGMENT ASSIGNMENT FOR REAL-TIME ANALYSIS OF SEQUENCING EXPERIMENTS ADAM ROBERTS AND LIOR PACHTER Supplementary Figure 1 Frequency 0 1 1 10 100 1000 10000 1 10 20 30 40 50 60 70 13,950 Bundle
STREAMING FRAGMENT ASSIGNMENT FOR REAL-TIME ANALYSIS OF SEQUENCING EXPERIMENTS ADAM ROBERTS AND LIOR PACHTER Supplementary Figure 1 Frequency 0 1 1 10 100 1000 10000 1 10 20 30 40 50 60 70 13,950 Bundle
Performing a resequencing assembly
 BioNumerics Tutorial: Performing a resequencing assembly 1 Aim In this tutorial, we will discuss the different options to obtain statistics about the sequence read set data and assess the quality, and
BioNumerics Tutorial: Performing a resequencing assembly 1 Aim In this tutorial, we will discuss the different options to obtain statistics about the sequence read set data and assess the quality, and
Subset sum problem and dynamic programming
 Lecture Notes: Dynamic programming We will discuss the subset sum problem (introduced last time), and introduce the main idea of dynamic programming. We illustrate it further using a variant of the so-called
Lecture Notes: Dynamic programming We will discuss the subset sum problem (introduced last time), and introduce the main idea of dynamic programming. We illustrate it further using a variant of the so-called
QIAseq Targeted RNAscan Panel Analysis Plugin USER MANUAL
 QIAseq Targeted RNAscan Panel Analysis Plugin USER MANUAL User manual for QIAseq Targeted RNAscan Panel Analysis 0.5.2 beta 1 Windows, Mac OS X and Linux February 5, 2018 This software is for research
QIAseq Targeted RNAscan Panel Analysis Plugin USER MANUAL User manual for QIAseq Targeted RNAscan Panel Analysis 0.5.2 beta 1 Windows, Mac OS X and Linux February 5, 2018 This software is for research
Helpful Galaxy screencasts are available at:
 This user guide serves as a simplified, graphic version of the CloudMap paper for applicationoriented end-users. For more details, please see the CloudMap paper. Video versions of these user guides and
This user guide serves as a simplified, graphic version of the CloudMap paper for applicationoriented end-users. For more details, please see the CloudMap paper. Video versions of these user guides and
User Manual. This is the example for Oases: make color 'VELVET_DIR=/full_path_of_velvet_dir/' 'MAXKMERLENGTH=63' 'LONGSEQUENCES=1'
 SATRAP v0.1 - Solid Assembly TRAnslation Program User Manual Introduction A color space assembly must be translated into bases before applying bioinformatics analyses. SATRAP is designed to accomplish
SATRAP v0.1 - Solid Assembly TRAnslation Program User Manual Introduction A color space assembly must be translated into bases before applying bioinformatics analyses. SATRAP is designed to accomplish
A Nearest Neighbors Algorithm for Strings. R. Lederman Technical Report YALEU/DCS/TR-1453 April 5, 2012
 A randomized algorithm is presented for fast nearest neighbors search in libraries of strings. The algorithm is discussed in the context of one of the practical applications: aligning DNA reads to a reference
A randomized algorithm is presented for fast nearest neighbors search in libraries of strings. The algorithm is discussed in the context of one of the practical applications: aligning DNA reads to a reference
Binary Values. CSE 410 Lecture 02
 Binary Values CSE 410 Lecture 02 Lecture Outline Binary Decimal, Binary, and Hexadecimal Integers Why Place Value Representation Boolean Algebra 2 First: Why Binary? Electronic implementation Easy to store
Binary Values CSE 410 Lecture 02 Lecture Outline Binary Decimal, Binary, and Hexadecimal Integers Why Place Value Representation Boolean Algebra 2 First: Why Binary? Electronic implementation Easy to store
Tutorial: De Novo Assembly of Paired Data
 : De Novo Assembly of Paired Data September 20, 2013 CLC bio Silkeborgvej 2 Prismet 8000 Aarhus C Denmark Telephone: +45 70 22 32 44 Fax: +45 86 20 12 22 www.clcbio.com support@clcbio.com : De Novo Assembly
: De Novo Assembly of Paired Data September 20, 2013 CLC bio Silkeborgvej 2 Prismet 8000 Aarhus C Denmark Telephone: +45 70 22 32 44 Fax: +45 86 20 12 22 www.clcbio.com support@clcbio.com : De Novo Assembly
