Institutional Review Board. Application for Research Using Humans
|
|
|
- Victoria McKinney
- 6 years ago
- Views:
Transcription
1 Institutional Review Board 4500 Riverwalk Parkway Riverside, CA IRB Research Application Phone Fax Institutional Review Board Application for Research Using Humans Part 1: Administrative Information 1. Title of protocol 2. Contact information Applicant s Full Name ID# Dept. Address City State Zip Phone Applicant Status: _Undergraduate Student _Graduate Student _Faculty _Staff _ Others: specify Faculty Supervisor (if student project) Dept. Phone Dates of Entire Project Period from to 3. Funding Information Indicate if your project is funded by an external (non-la Sierra) sponsor, including a gift or a sponsored award. Federal Funding Agency Student projects funded fully or in part by Federal funds received by a Faculty Advisor Non Federal sponsor that requires compliance with Federal IRB regulations Other Funding source My research is not funded by any outside funding agency (this option should be available only if none of the above are selected) Name of the external funding agency: Provide the Sponsor s Project ID number La Sierra University Initial Approval Request 1
2 Part 2: Study Design, Methods and Procedures 1. Type of project/study: Please select ALL of the categories of work that apply to this proposed project. Active collection of data (not human biological materials or physiological data) Active collection and use of human biological materials or physiological data Use of physiological or biomedical devices, or drugs, biological, or chemical agents Use of existing data (not human biological materials) Use of existing human biological materials 2. Please provide a lay summary of the study, including the purpose, research questions and hypotheses to be evaluated. (If you need more space for your answer, please attach separately.) 3. Please describe briefly how this study will contribute to existing knowledge in the field. (If you need more space for your answer, please attach separately.) 4. Will your research involve a questionnaire, interview, or both? (If instruments will be used, please attach a copy of each to your protocol.) La Sierra University Initial Approval Request 2
3 Part 3: Participants, Recruitment and Compensation 1. Please provide the estimated number of participants you plan to recruit. 2. Please provide the age range of the participants. 3. Please select all the categories of participants that will be included in your study. Healthy adult volunteers Children or minor individuals under 18 La Sierra students La Sierra employees Cognitively impaired persons Pregnant or nursing women Prisoners or individuals under detention or on probation People in foreign countries People unable to read, speak or understand English People with limited literacy People with specific health conditions Other category of participants not listed above Please explain below: None of the above 4. Please select all of the tools that you plan to use to recruit your participants. Flyers Notices Mailers (U.S. Post) Online Advertisements Use of Internet social media or online networking sites TV, radio, print advertisements La Sierra participant pool recruiting methods (such as Sona Systems or other La Sierraaffiliated web-based participant pool management software) Face to face public intercept Presentations at meetings Other (Please describe below) 5. Please describe each recruitment method to be used. La Sierra University Initial Approval Request 3
4 6. Describe the inclusion or exclusion criteria for participants as applicable in this study. 7. Will participants be compensated for their participation? Yes No If yes, please describe the type and amount of compensation. 8. Please describe the tasks that the participants will be asked to perform for each phase of the study. 9. Please provide an estimate of the time commitment from each participant for each phase of the study. Part 4: Risks and Benefits 1. From the list below, please select ALL of the potential risks that are involved in your study. Use of deceptive techniques Use of private records (such as educational or medical records) Manipulation of psychological or social state such as sensory deprivation, social isolation, psychological stress Probing for personal or sensitive information in surveys or interviews (e.g.: private behaviors, sexual activities, employer assessments) Presentation of materials which some participants may consider sensitive, offensive, threatening or degrading Possible invasion of privacy of subject or subject s family Social or economic risk (reputational, cultural, employability, etc.) Identification of child, spousal, or elder abuse Identification of illegal activity (e.g. drug use) Risk of injury or bodily harm Other (Please specify) There are no risks of any kind to any participants enrolled in this study. This option is valid only if none of the risks above are selected. La Sierra University Initial Approval Request 4
5 2. Describe the nature and degree of the risks or harm selected above. All of the risks/harms must be disclosed in the consent form. 3. Describe the steps that will be taken to minimize risks or harms and to protect the welfare of participants. Include a description of how you will handle an adverse or unexpected outcome that could be potentially harmful (e.g., suicidal ideation). If the study will include protected populations, identify each group and provide an explanatory paragraph for each group. 4. Describe any benefits that individuals may reasonably expect from participation. If there are none, state None.. Describe the anticipated benefits of this study to society, academic knowledge or both. La Sierra University Initial Approval Request 5
6 Part 5: Privacy and Confidentiality 1. Will you or any member of your research team collect or have access to any of the personal identifiers listed below? Select all that apply. Name Date of birth Mailing or address Phone or fax numbers Social Security number Medical records License, certificate or Vehicle ID IP (internet protocol) address Biometric identifiers Photos/images/audio/video recording Signatures, handwriting samples Any unique identifier not mentioned above: No member of the research team will have access to any personal identifiers. This option is valid only if none of the other options in this question are selected. 1. Informed Consent: Part 6: Consent Process Will you use a written informed consent document? Yes No, I am requesting a waiver of written informed consent Not applicable Will you use a verbal informed consent? Yes No, I am requesting a waiver Not applicable 2. Written assent for individuals under 18: Will you obtain written assent for children and individuals under 18? Yes No, I am requesting a waiver of written assent Not applicable 3. Written parental permission: Will you obtain written parental or guardian permission for children and individuals under 18? Yes No, I am requesting a waiver of written parental or guardian permission Not applicable La Sierra University Initial Approval Request 6
7 Part 7: Triennial Review This project may be eligible for Triennial review, which means that this project will be reviewed every three years instead of annually. No changes to the study can be implemented however, before an amendment is submitted to the IRB and the PI receives written approval from the IRB office. The IRB policy on Triennial Review is on the IRB website at Indicate if you would like to proceed with Triennial review or request an Annual review (recommended if you are expecting to receive Federal funding for some part of this study). Options: Proceed with Triennial Review Request Annual Review Reminder Check List You have now completed this form. Please review it to ensure that it is filled out completely and accurately. Please save this form and proceed to the signature page for submission instructions. Please include (attach) informed consent form(s) with this application. If your research does not use a consent form, please indicate how you will obtain participant consent. In case of research involving children include both forms used to solicit the assent of the children and the permission of their parent(s) and/or guardian(s). Please include (attach) all the questionnaires, surveys, or any other data collection protocols with this application. Please include (attach) the certificate of completion of free online NIH Protecting Human Research Participants Training that can be found at (if you are a student of a class and need an IRB approval for your class project (i.e. PSYC 323, EXSC 364, etc.), ask your instructor about this training requirement.) If you have any questions or need assistance, please contact the IRB office. phone: (951) office: Ambs Hall irb@lasierra.edu La Sierra University Initial Approval Request 7
8 Signature This page is to be signed by the principal investigator. If the principal investigator is an undergraduate or graduate student, the faculty supervisor must also sign in the lower box. OPTIONAL: You may submit an electronic copy of this application by clicking on the attestation box below and entering your name and today s date. After clicking on the attestation box, please save a copy of the form before ing it to irb@lasierra.edu. If you are a student, send this file to your faculty supervisor (in addition to your sending this to the IRB office), and ask your faculty supervisor to send his/her approval to irb@lasierra.edu. The submission must come from your La Sierra account. (If you don t have a La Sierra account, contact the IRB office.) Principal Investigator I certify that the information I provide in this application is correct and complete. I also pledge that I will not change any of the procedures, forms, or protocols used in this study without first seeking review and approval from the Institutional Review Board for Human Participants. _ Attestation of Principal Investigator (for submissions) Applicant s Name Date Applicant s Signature Date Faculty Supervisor (if student project) Date Updated 01/17 La Sierra University Initial Approval Request 8
WITH MODIFICATIONS ), S, PHONE NUMBERS OR OTHER FORMS, PLEASE VISIT THE IRB S WEBSITE AT:
 Modification Request Dominican College Institutional Review Board (IRB) IRB #: Date Received: IRB Approval Name & Signature: FOR MORE IRB INSTRUCTIONS, APPLICATIONS (including the RENEWAL FORM that should
Modification Request Dominican College Institutional Review Board (IRB) IRB #: Date Received: IRB Approval Name & Signature: FOR MORE IRB INSTRUCTIONS, APPLICATIONS (including the RENEWAL FORM that should
University of North Florida
 Last edited by: Kayla Champaigne Last edited on: December 5, 2012 University of North Florida Institutional Review Board IRB Protocol Please note, this is a sample of the North Florida - IRB Protocol output.
Last edited by: Kayla Champaigne Last edited on: December 5, 2012 University of North Florida Institutional Review Board IRB Protocol Please note, this is a sample of the North Florida - IRB Protocol output.
Completing & Submitted the IRB Approval of Human Subjects Form
 Completing & Submitted the IRB Approval of Human Subjects Form All areas of the form should be completed. Once completed it must be submitted to the IRB by sending it to the EU IRB Chairperson. The following
Completing & Submitted the IRB Approval of Human Subjects Form All areas of the form should be completed. Once completed it must be submitted to the IRB by sending it to the EU IRB Chairperson. The following
Make sure you delete all red type explanations. NOTE: Anticipate no action on applications that do not meet the timeline as posted on the IRB web site
 APPLICATION FOR REVIEW OF HUMAN PARTICIPANTS RESEARCH The following is designed to briefly introduce you to important considerations when answering each item. There is no one correct answer for each item,
APPLICATION FOR REVIEW OF HUMAN PARTICIPANTS RESEARCH The following is designed to briefly introduce you to important considerations when answering each item. There is no one correct answer for each item,
HANDWRITTEN FORMS WILL NOT BE ACCEPTED APPLICATION MUST BE SINGLE SIDED DO NOT STAPLE. Directions for Completion of the IRB Application Form
 HANDWRITTEN FORMS WILL NOT BE ACCEPTED APPLICATION MUST BE SINGLE SIDED DO NOT STAPLE Directions for Completion of the IRB Application Form Handwritten forms will not be accepted. Check boxes by double
HANDWRITTEN FORMS WILL NOT BE ACCEPTED APPLICATION MUST BE SINGLE SIDED DO NOT STAPLE Directions for Completion of the IRB Application Form Handwritten forms will not be accepted. Check boxes by double
IRB APPLICATION INSTRUCTIONS & TEMPLATES
 1 Contents: Items Required for Every Application 2 Synopsis Template Instructions 3 Link to editable Synopsis Template (Word format) Other Types of Documentation to Submit 5 Checklist for Consent Forms
1 Contents: Items Required for Every Application 2 Synopsis Template Instructions 3 Link to editable Synopsis Template (Word format) Other Types of Documentation to Submit 5 Checklist for Consent Forms
Reviewers Guide on Clinical Trials
 Reviewers Guide on Clinical Trials Office of Research Integrity & Compliance Version 2 Updated: June 26, 2017 This document is meant to help board members conduct reviews for Full Board: Clinical Trial
Reviewers Guide on Clinical Trials Office of Research Integrity & Compliance Version 2 Updated: June 26, 2017 This document is meant to help board members conduct reviews for Full Board: Clinical Trial
COEUS LITE IRB COEUS 4.5.1_P3 USER GUIDE
 COEUS LITE IRB COEUS 4.5.1_P3 USER GUIDE Version: August 24, 2016 1 TABLE OF CONTENTS A note about this guide... 4 Getting access to Coeus Lite... 4 Preparing to enter a protocol... 4 Getting Started:
COEUS LITE IRB COEUS 4.5.1_P3 USER GUIDE Version: August 24, 2016 1 TABLE OF CONTENTS A note about this guide... 4 Getting access to Coeus Lite... 4 Preparing to enter a protocol... 4 Getting Started:
IRBManager Quick Start Guide INITIAL APPLICATION - OVERVIEW
 Page 1 of 16 GENERAL INFORMATION IRBManager Quick Start Guide INITIAL APPLICATION - OVERVIEW Initial Application Types: The IRBManager initial application form (xform) is available for specific types of
Page 1 of 16 GENERAL INFORMATION IRBManager Quick Start Guide INITIAL APPLICATION - OVERVIEW Initial Application Types: The IRBManager initial application form (xform) is available for specific types of
Contents. Researcher Guidance for Reportable Events & Notifying the IRB. Office of Research Integrity & Compliance Version 2 Updated: June 23, 2017
 Researcher Guidance for Reportable Events & Notifying the IRB Office of Research Integrity & Compliance Version 2 Updated: June 23, 2017 This document to help investigators determine what event(s) need
Researcher Guidance for Reportable Events & Notifying the IRB Office of Research Integrity & Compliance Version 2 Updated: June 23, 2017 This document to help investigators determine what event(s) need
Last revised: September 30, e-protocol User Guide 1
 e-protocol User Guide Last revised: September 30, 2015 e-protocol User Guide 1 e-protocol is an electronic system for submitting and monitoring the status of Institutional Review Board (IRB) submissions.
e-protocol User Guide Last revised: September 30, 2015 e-protocol User Guide 1 e-protocol is an electronic system for submitting and monitoring the status of Institutional Review Board (IRB) submissions.
Ontario Community College Multi-site REB Application Form. Application to Involve Human Participants in Research
 Ontario Community College Multi-site REB Application Form This form is for researchers who are planning to conduct research at St. Clair College or with multiple colleges in Ontario. Several colleges have
Ontario Community College Multi-site REB Application Form This form is for researchers who are planning to conduct research at St. Clair College or with multiple colleges in Ontario. Several colleges have
Informed Consent and the Consent Form
 Informed Consent and the Consent Form What is informed consent? What does the process look like? Who can obtain consent? Where can I find more information? Consent Form Informed Consent They are NOT the
Informed Consent and the Consent Form What is informed consent? What does the process look like? Who can obtain consent? Where can I find more information? Consent Form Informed Consent They are NOT the
***** ***** August
 SLU eirb Conversion Charts Saint Louis University ***** eirb Paper to Electronic Protocol Conversion Charts ***** Institutional Review Board August 2011 http://eirb.slu.edu Institutional Review Board Saint
SLU eirb Conversion Charts Saint Louis University ***** eirb Paper to Electronic Protocol Conversion Charts ***** Institutional Review Board August 2011 http://eirb.slu.edu Institutional Review Board Saint
BRANDMAN UNIVERSITY INSTITUTIONAL REVIEW BOARD Continuing Review Request/Closure Report
 BRANDMAN UNIVERSITY INSTITUTIONAL REVIEW BOARD Continuing Review Request/Closure Report Instructions: Please complete all sections of this form and submit with attached documents, forms, and/or explanations
BRANDMAN UNIVERSITY INSTITUTIONAL REVIEW BOARD Continuing Review Request/Closure Report Instructions: Please complete all sections of this form and submit with attached documents, forms, and/or explanations
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
APPLICATION DEADLINE:
 Application Directions for Applicants to The Ritchie Program for School Leaders An Innovative Partnership between the University of Denver and Denver Public Schools APPLICATION DEADLINE: The documents
Application Directions for Applicants to The Ritchie Program for School Leaders An Innovative Partnership between the University of Denver and Denver Public Schools APPLICATION DEADLINE: The documents
Kuali Research User Guide: Create an IRB Protocol
 Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
New Protocol - expedited & full board
 New Protocol - expedited & full board training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Expedited & Full Board 1 table of contents Table of Contents 2 table
New Protocol - expedited & full board training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Expedited & Full Board 1 table of contents Table of Contents 2 table
If this is your first time submitting a protocol for review, see FAQs for information to consider beforehand.
 IRB CHART REVIEW System Requirements: FORM If using Windows, use Internet Explorer (IE) or Firefox as your browser. If using Macintosh, use Safari or Firefox as your browser. Your browser must be configured
IRB CHART REVIEW System Requirements: FORM If using Windows, use Internet Explorer (IE) or Firefox as your browser. If using Macintosh, use Safari or Firefox as your browser. Your browser must be configured
Instructions to complete CITI Human Subjects Training - New User
 Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
UNIVERSITY OF NORTH FLORIDA. Institutional Review Board (IRB) Guide to IRBNet:
 UNIVERSITY OF NORTH FLORIDA Institutional Review Board (IRB) Guide to IRBNet: Version 2 11-15-2013 Table of Contents i Introduction 2 IRBNet User Registration 2 IRBNet ID Number 2 Training and Credentials
UNIVERSITY OF NORTH FLORIDA Institutional Review Board (IRB) Guide to IRBNet: Version 2 11-15-2013 Table of Contents i Introduction 2 IRBNet User Registration 2 IRBNet ID Number 2 Training and Credentials
Early Learning SF User Guide for Families
 Early Learning SF User Guide for Families Instructions Sherry Clark Contents 1 Home Page... 2 2 New Application... 2 2.1 Initial Assessment... 3 2.2 Ineligible Outcome... 3 2.3 Eligible Outcome... 4 2.4
Early Learning SF User Guide for Families Instructions Sherry Clark Contents 1 Home Page... 2 2 New Application... 2 2.1 Initial Assessment... 3 2.2 Ineligible Outcome... 3 2.3 Eligible Outcome... 4 2.4
Cayuse IRB. Submitting a New Protocol. Office of Research Compliance (657)
 Cayuse IRB Submitting a New Protocol Office of Research Compliance irb@fullerton.edu (657) 278-7719 How to use this tutorial This tutorial is written from the point of view of a Primary Investigator (PI),
Cayuse IRB Submitting a New Protocol Office of Research Compliance irb@fullerton.edu (657) 278-7719 How to use this tutorial This tutorial is written from the point of view of a Primary Investigator (PI),
ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL
 Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
University of Pennsylvania Institutional Review Board
 Reliance Agreement Guidance Creating Consent Templates This document provides step by step guidance on how to convert the Penn IRB approved consent form into a consent form template that can be shared
Reliance Agreement Guidance Creating Consent Templates This document provides step by step guidance on how to convert the Penn IRB approved consent form into a consent form template that can be shared
Office of Human Research
 Office of Human Research JeffTrial End-User Training Document Regulatory Coordinator Training for Non-Oncology personnel Office of Human Research 8/16/2013 Ver. 1.0 Contents The REG Role: Completing Basic
Office of Human Research JeffTrial End-User Training Document Regulatory Coordinator Training for Non-Oncology personnel Office of Human Research 8/16/2013 Ver. 1.0 Contents The REG Role: Completing Basic
era(infoed) Electronic Submission Instructions and Tips: Initial Application
 era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system.
era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system.
ADULT VOLUNTEER SERVICES APPLICATION
 ADULT VOLUNTEER SERVICES APPLICATION Adult - For Internal Use: Certifications: Community Service: PERSONAL INFORMATION First Middle Last Date of Birth Social Security # Driver s License # Photo Copy [
ADULT VOLUNTEER SERVICES APPLICATION Adult - For Internal Use: Certifications: Community Service: PERSONAL INFORMATION First Middle Last Date of Birth Social Security # Driver s License # Photo Copy [
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK. (Version date 03/03/2006)
 ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
EXAMPLE 2-JOINT PRIVACY AND SECURITY CHECKLIST
 Purpose: The purpose of this Checklist is to evaluate your proposal to use or disclose Protected Health Information ( PHI ) for the purpose indicated below and allow the University Privacy Office and Office
Purpose: The purpose of this Checklist is to evaluate your proposal to use or disclose Protected Health Information ( PHI ) for the purpose indicated below and allow the University Privacy Office and Office
Page 1/6 MEMORANDUM. DATE: 7 July Faculty & Graduate Students. John Allen, Melissa Soenke, & Jeff Greenberg
 MEMORANDUM Page 1/6 DATE: 7 July 2010 TO: FROM: RE: Faculty & Graduate Students John Allen, Melissa Soenke, & Jeff Greenberg INTRODUCTORY PSYCHOLOGY STUDENT SURVEY To all Mass Survey contributors: This
MEMORANDUM Page 1/6 DATE: 7 July 2010 TO: FROM: RE: Faculty & Graduate Students John Allen, Melissa Soenke, & Jeff Greenberg INTRODUCTORY PSYCHOLOGY STUDENT SURVEY To all Mass Survey contributors: This
New Protocol - emergency use training guide
 New Protocol - emergency use training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Emergency Use 1 table of contents Table of Contents 2 table of contents 3 about
New Protocol - emergency use training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Emergency Use 1 table of contents Table of Contents 2 table of contents 3 about
AGING STUDIES ADMISSIONS APPLICATION
 AGING STUDIES ADMISSIONS APPLICATION MASTER OF SCIENCE DEGREE IN GERONTOLOGY This application must be typed or printed legibly in black ink. If not legible, your application will be disqualified. TODAY'S
AGING STUDIES ADMISSIONS APPLICATION MASTER OF SCIENCE DEGREE IN GERONTOLOGY This application must be typed or printed legibly in black ink. If not legible, your application will be disqualified. TODAY'S
IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL
 Page 1 of 12 IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL NOTE: This Quick Start Guide provides instructions for removing and/or adding personnel, other than the Principal Investigator,
Page 1 of 12 IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL NOTE: This Quick Start Guide provides instructions for removing and/or adding personnel, other than the Principal Investigator,
OnCore Enterprise Research. Subject Administration Full Study
 OnCore Enterprise Research Subject Administration Full Study Principal Investigator Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Table of Contents What
OnCore Enterprise Research Subject Administration Full Study Principal Investigator Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Table of Contents What
Protocol Management System (PMS) Investigator User Guide. Harris Health System Administrative Approval
 Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
CCEI3001: The CDA Credentialing Process
 CCEI3001: The CDA Credentialing Process Welcome This course provides students with general information about the steps involved in obtaining a CDA Credential from the Council for Professional Recognition.
CCEI3001: The CDA Credentialing Process Welcome This course provides students with general information about the steps involved in obtaining a CDA Credential from the Council for Professional Recognition.
Effort Certification at KU. University of Kansas Summer 2016
 Effort Certification at KU University of Kansas Summer 2016 Agenda 1. Effort Reporting Overview 2. Effort Workflow and Basic Information 3. Certification Process 4. Resources 5. Questions Effort Reporting
Effort Certification at KU University of Kansas Summer 2016 Agenda 1. Effort Reporting Overview 2. Effort Workflow and Basic Information 3. Certification Process 4. Resources 5. Questions Effort Reporting
Certified Addiction Recovery Coach Application
 Certified Addiction Recovery Coach Application A Project of ASAP - Alcoholism & Substance Abuse Providers of New York State 11 North Pearl Street, Suite 801 Albany New York 12207 Phone: 518.426.0945 Fax:
Certified Addiction Recovery Coach Application A Project of ASAP - Alcoholism & Substance Abuse Providers of New York State 11 North Pearl Street, Suite 801 Albany New York 12207 Phone: 518.426.0945 Fax:
Drexel University. Page 1 of 48. Version November agf
 Drexel University Page 1 of 48 TABLE OF CONTENTS Getting Started. 3 Creating a New Protocol.. 4 General Information... 7 Organization Information... 9 Investigators/Study Personnel.. 10 Correspondents
Drexel University Page 1 of 48 TABLE OF CONTENTS Getting Started. 3 Creating a New Protocol.. 4 General Information... 7 Organization Information... 9 Investigators/Study Personnel.. 10 Correspondents
Information Privacy Statement
 Information Privacy Statement Commitment to Privacy The University of Florida values individuals' privacy and actively seeks to preserve the privacy rights of those who share information with us. Your
Information Privacy Statement Commitment to Privacy The University of Florida values individuals' privacy and actively seeks to preserve the privacy rights of those who share information with us. Your
Fill Out the Form to Name an Authorized Delegate
 Do you want us to share your health information with someone? Fill Out the Form to Name an Authorized Delegate For Blue Benefit Services Groups What Is the Purpose of This Form? Fill out this form to allow
Do you want us to share your health information with someone? Fill Out the Form to Name an Authorized Delegate For Blue Benefit Services Groups What Is the Purpose of This Form? Fill out this form to allow
Drexel University. Version April Page 1 of 23. Version April agf
 Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
LIFEWAY PREMARITAL INFORMATION FORM LIFEWAY REFERRAL INFORMATION
 LIFEWAY PREMARITAL INFORMATION FORM Date: / / Name: First MI Last Date of Birth: / / Gender: M F Marital Status: Single Engaged Divorced Address: City State: Zip: Primary Contact Phone: Secondary Contact
LIFEWAY PREMARITAL INFORMATION FORM Date: / / Name: First MI Last Date of Birth: / / Gender: M F Marital Status: Single Engaged Divorced Address: City State: Zip: Primary Contact Phone: Secondary Contact
AUTHORIZATION TO RELEASE HEALTH INFORMATION
 Request Completed Health Information Management AUTHORIZATION TO RELEASE HEALTH INFORMATION Completion of this form authorizes the use and/or disclosure (release) of individually identifiable health information,
Request Completed Health Information Management AUTHORIZATION TO RELEASE HEALTH INFORMATION Completion of this form authorizes the use and/or disclosure (release) of individually identifiable health information,
SECTION 2: PROGRAM IDENTIFICATION
 UTAH REGISTRY FOR PROFESSIONAL DEVELOPMENT PROFESSIONAL DEVELOPMENT INCENTIVE APPLICATION SECTION 1: CANDIDATE IDENTIFICATION (Use through 7/1/2017 5/31/2018) DATE OF BIRTH / / FILL OUT PAGE 1 OF THE ATTACHED
UTAH REGISTRY FOR PROFESSIONAL DEVELOPMENT PROFESSIONAL DEVELOPMENT INCENTIVE APPLICATION SECTION 1: CANDIDATE IDENTIFICATION (Use through 7/1/2017 5/31/2018) DATE OF BIRTH / / FILL OUT PAGE 1 OF THE ATTACHED
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
If you haven t already created a ParentVUE account, you ll need to do so by going to https://parentvue.beaverton.k12.or.
 If you are a parent or guardian of a new student, you can enroll your child using BSD's online registration system. If you are a parent/guardian of a current BSD student, you can also use BSD's online
If you are a parent or guardian of a new student, you can enroll your child using BSD's online registration system. If you are a parent/guardian of a current BSD student, you can also use BSD's online
Phase II CAQH CORE 202 Certification Policy version March 2011 CAQH 2011
 CAQH 2011 Phase II CAQH CORE 202 Certification Policy GUIDING PRINCIPLES Phase II CORE 202 Certification Policy After signing the CORE Pledge and/or Addendum, the entity has 180 days to complete CORE certification
CAQH 2011 Phase II CAQH CORE 202 Certification Policy GUIDING PRINCIPLES Phase II CORE 202 Certification Policy After signing the CORE Pledge and/or Addendum, the entity has 180 days to complete CORE certification
EXAMPLE 3-JOINT PRIVACY AND SECURITY CHECKLIST
 Purpose: The purpose of this Checklist is to evaluate your proposal to use or disclose Protected Health Information ( PHI ) for the purpose indicated below and allow the University Privacy Office and Office
Purpose: The purpose of this Checklist is to evaluate your proposal to use or disclose Protected Health Information ( PHI ) for the purpose indicated below and allow the University Privacy Office and Office
Introduction/Instructions
 Introduction/Instructions Registries (data banks) and repositories (tissue banks, usually with databases associated) all involve the collection and storage of information and/or biological specimens that
Introduction/Instructions Registries (data banks) and repositories (tissue banks, usually with databases associated) all involve the collection and storage of information and/or biological specimens that
CSL Data Subject Request Form Representative
 Please answer all of the following questions completely and truthfully. Enter the date you are making this request [Day/Month/Year] Enter your first name. Information about you Enter your surname. Enter
Please answer all of the following questions completely and truthfully. Enter the date you are making this request [Day/Month/Year] Enter your first name. Information about you Enter your surname. Enter
WEBSITE & SOCIAL MEDIA PRIVACY POLICY
 WEBSITE & SOCIAL MEDIA PRIVACY POLICY This website is the property of Girls on the Run of Northern Virginia ( Girls on the Run ), an Independent Council of Girls on the Run International, Inc. of Charlotte,
WEBSITE & SOCIAL MEDIA PRIVACY POLICY This website is the property of Girls on the Run of Northern Virginia ( Girls on the Run ), an Independent Council of Girls on the Run International, Inc. of Charlotte,
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
IEC-I Re-registration. ECR/229/lnst./MH/2013/RR-16,IEC-II registration. If additional collaborators attach details and letter of consent by the collab
 IEC-I Re-registration. ECR/229/lnst./MH/2013/RR-16,IEC-II registration. Please fill in the details in legible hand writing Annexure 1-B AX 1-B/SOP 05/V5 Project submission application form for initial
IEC-I Re-registration. ECR/229/lnst./MH/2013/RR-16,IEC-II registration. Please fill in the details in legible hand writing Annexure 1-B AX 1-B/SOP 05/V5 Project submission application form for initial
New Family Enrollment Guide August 2018 Revision
 New Family Enrollment Guide 2018-2019 August 2018 Revision Welcome to Pennsylvania 4-H! 2018-2019 Enrollment for 4-H Members and Adult Volunteers We are glad you are interested in joining Pennsylvania
New Family Enrollment Guide 2018-2019 August 2018 Revision Welcome to Pennsylvania 4-H! 2018-2019 Enrollment for 4-H Members and Adult Volunteers We are glad you are interested in joining Pennsylvania
Better Healthcare with My ehealth Record
 Better Healthcare with My ehealth Record What is My ehealth Record? My ehealth Record is a way of securely storing and sharing important healthcare information with your consent, so that it is easily and
Better Healthcare with My ehealth Record What is My ehealth Record? My ehealth Record is a way of securely storing and sharing important healthcare information with your consent, so that it is easily and
HIPAA FOR BROKERS. revised 10/17
 HIPAA FOR BROKERS revised 10/17 COURSE PURPOSE The purpose of this information is to help ensure that all Optima Health Brokers are prepared to protect the privacy and security of our members health information.
HIPAA FOR BROKERS revised 10/17 COURSE PURPOSE The purpose of this information is to help ensure that all Optima Health Brokers are prepared to protect the privacy and security of our members health information.
Admission, Discharge, Update Client Data and Associated Forms
 Admission, Discharge, Update Client Data and Associated Forms Table of Contents Introduction... 2 When to Update Client Data... 2 Admission Form... 2 Discharge Form...10 Update Client Data Form...11 CSI
Admission, Discharge, Update Client Data and Associated Forms Table of Contents Introduction... 2 When to Update Client Data... 2 Admission Form... 2 Discharge Form...10 Update Client Data Form...11 CSI
DATA PROTECTION PRIVACY NOTICE PROTECTING YOUR PERSONAL INFORMATION: YOUR RIGHTS, OUR RESPONSIBILITIES
 DATA PROTECTION PRIVACY NOTICE PROTECTING YOUR PERSONAL INFORMATION: YOUR RIGHTS, OUR RESPONSIBILITIES You have been given this Notice because Babington collects, uses and stores information about colleagues
DATA PROTECTION PRIVACY NOTICE PROTECTING YOUR PERSONAL INFORMATION: YOUR RIGHTS, OUR RESPONSIBILITIES You have been given this Notice because Babington collects, uses and stores information about colleagues
Official Student School Records Request
 Official Student School Records Request The Family Educational Rights and Privacy Act (FERPA) and the Illinois School Student Records Act afford parents/guardians and students over 18 years of age ( eligible
Official Student School Records Request The Family Educational Rights and Privacy Act (FERPA) and the Illinois School Student Records Act afford parents/guardians and students over 18 years of age ( eligible
2014 ISACA Academic Scholarship Competition DUE DATE EXTENDED TO MAY 1, 2014
 2014 ISACA Academic Scholarship Competition DUE DATE EXTENDED TO MAY 1, 2014 ISACA is a pace-setting global organization for IT professionals focusing on information governance, security and audit. IT
2014 ISACA Academic Scholarship Competition DUE DATE EXTENDED TO MAY 1, 2014 ISACA is a pace-setting global organization for IT professionals focusing on information governance, security and audit. IT
TEACHING Credential PROGRAM APPLICATION FALL 2005 Priority Filing Deadline: March 15, 2005 Final Filing Deadline: April 19, 2005
 PLEASE PRINT CLEARLY TEACHING Credential PROGRAM APPLICATION FALL 2005 Priority Filing Deadline: March 15, 2005 Final Filing Deadline: April 19, 2005 Name Last First Middle Maiden/Former Address Home Phone
PLEASE PRINT CLEARLY TEACHING Credential PROGRAM APPLICATION FALL 2005 Priority Filing Deadline: March 15, 2005 Final Filing Deadline: April 19, 2005 Name Last First Middle Maiden/Former Address Home Phone
HIPAA and Research Contracts JILL RAINES, ASSISTANT GENERAL COUNSEL AND UNIVERSITY PRIVACY OFFICIAL
 HIPAA and Research Contracts JILL RAINES, ASSISTANT GENERAL COUNSEL AND UNIVERSITY PRIVACY OFFICIAL Just a Few Reminders HIPAA applies to Covered Entities HIPAA is a federal law that governs the privacy
HIPAA and Research Contracts JILL RAINES, ASSISTANT GENERAL COUNSEL AND UNIVERSITY PRIVACY OFFICIAL Just a Few Reminders HIPAA applies to Covered Entities HIPAA is a federal law that governs the privacy
Certified Peer Recovery Mentor
 Michigan Certification Board for Addiction Professionals CERTIFICATION MANUAL For Certified Peer Recovery Mentor (Michigan Only, non-ic&rc reciprocal) CPRM-M Directions for Submitting Application Completion
Michigan Certification Board for Addiction Professionals CERTIFICATION MANUAL For Certified Peer Recovery Mentor (Michigan Only, non-ic&rc reciprocal) CPRM-M Directions for Submitting Application Completion
Investigator Submission Form for Multi-Center Protocols
 Western Institutional Review Board 3535 7th Avenue SW Olympia, WA 98502-5010 PO Box 12029 Olympia, WA 98508-2029 Office: (360) 252-2500 Toll Free: (800) 562-4789 www.wirb.com clientservices@wirb.com OHRP/FDA
Western Institutional Review Board 3535 7th Avenue SW Olympia, WA 98502-5010 PO Box 12029 Olympia, WA 98508-2029 Office: (360) 252-2500 Toll Free: (800) 562-4789 www.wirb.com clientservices@wirb.com OHRP/FDA
Phase I CAQH CORE 102: Eligibility and Benefits Certification Policy version March 2011
 Phase I CAQH CORE 102: Eligibility and Benefits Certification Policy GUIDING PRINCIPLES After signing the CORE Pledge, the entity has 180 days to complete CORE certification testing. CORE will not certify
Phase I CAQH CORE 102: Eligibility and Benefits Certification Policy GUIDING PRINCIPLES After signing the CORE Pledge, the entity has 180 days to complete CORE certification testing. CORE will not certify
Certified Recovery Peer Advocate-Provisional Application
 Certified Recovery Peer Advocate-Provisional Application Provisional A Project of Alcoholism & Substance Abuse Providers of New York State, Inc. 11 North Pearl Street, Suite 801 Albany, New York 12207
Certified Recovery Peer Advocate-Provisional Application Provisional A Project of Alcoholism & Substance Abuse Providers of New York State, Inc. 11 North Pearl Street, Suite 801 Albany, New York 12207
MI LAST NAME DATE OF BIRTH GENDER ADDRESS CITY STATE ZIP CODE MI LAST NAME DATE OF BIRTH GENDER
 PARTICIPANT FORM New member Update member information PRIMARY ACCOUNT HOLDER HOUSEHOLD #: FIRST NAME MI LAST NAME DATE OF BIRTH GENDER EMAIL ADDRESS CITY STATE ZIP CODE SECONDARY ACCOUNT HOLDER FIRST NAME
PARTICIPANT FORM New member Update member information PRIMARY ACCOUNT HOLDER HOUSEHOLD #: FIRST NAME MI LAST NAME DATE OF BIRTH GENDER EMAIL ADDRESS CITY STATE ZIP CODE SECONDARY ACCOUNT HOLDER FIRST NAME
General Guidance for Maintaining a Regulatory Binder
 General Guidance for Maintaining a Regulatory Binder Study documentation should be well organized, providing a complete and thorough history from protocol development to study completion. Maintaining a
General Guidance for Maintaining a Regulatory Binder Study documentation should be well organized, providing a complete and thorough history from protocol development to study completion. Maintaining a
Victim Assistance & Restorative Justice Program s Registrant/Victim Input at Offender Intake Form
 Victim Assistance & Restorative Justice Program s Registrant/Victim Input at Offender Intake Form The Minnesota Department of Corrections goal is to promote safety in the lives of victims and others who
Victim Assistance & Restorative Justice Program s Registrant/Victim Input at Offender Intake Form The Minnesota Department of Corrections goal is to promote safety in the lives of victims and others who
Governors State University
 MSW Advanced Field Application (2018-19) Last Name First Name Address Email Address Home phone Cell Phone The following questions are designed to help the Field Division faculty help you find the best
MSW Advanced Field Application (2018-19) Last Name First Name Address Email Address Home phone Cell Phone The following questions are designed to help the Field Division faculty help you find the best
Your mymeritain Personalized Member Website
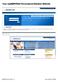 Your mymeritain Personalized Member Website 2008 Meritain Health, Inc. Last Updated 5.23.2008 Your mymeritain Member Website The mymeritain Member Website offers Members a user-friendly web experience,
Your mymeritain Personalized Member Website 2008 Meritain Health, Inc. Last Updated 5.23.2008 Your mymeritain Member Website The mymeritain Member Website offers Members a user-friendly web experience,
SLATER HIGH SCHOOL A+ STUDENT HANDBOOK SLATER HIGH SCHOOL HOME OF THE WILDCATS
 SLATER HIGH SCHOOL A+ STUDENT HANDBOOK SLATER HIGH SCHOOL HOME OF THE WILDCATS Purpose of the A+ Student Handbook The purpose of this manual is to provide a clear understanding of the various aspects of
SLATER HIGH SCHOOL A+ STUDENT HANDBOOK SLATER HIGH SCHOOL HOME OF THE WILDCATS Purpose of the A+ Student Handbook The purpose of this manual is to provide a clear understanding of the various aspects of
Bangor University Agent Portal
 Bangor University Agent Portal User Guide for Agents Last updated: 09 Mar 2017 Contents Introduction: Section I: Section II: Section III: Section IV: Section V: Section VI: What you can do on the Agent
Bangor University Agent Portal User Guide for Agents Last updated: 09 Mar 2017 Contents Introduction: Section I: Section II: Section III: Section IV: Section V: Section VI: What you can do on the Agent
Using the e Version of the Protocol Summary. University of Utah IRB Version: January 2012
 Using the e Version of the Protocol Summary University of Utah IRB Version: January 2012 What is the e Version of the Protocol Summary? Beginning January 2012, ERICA will create an up todate e version
Using the e Version of the Protocol Summary University of Utah IRB Version: January 2012 What is the e Version of the Protocol Summary? Beginning January 2012, ERICA will create an up todate e version
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
Human Subjects Development Guide
 Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Certification Guidelines: Credential Standards and Requirements Table
 Certification Guidelines: Credential Standards and Requirements Table Certified Behavioral Health Case Manager (CBHCM) Define Yourself as a Professional through Certification. 1715 S. Gadsden St. Tallahassee,
Certification Guidelines: Credential Standards and Requirements Table Certified Behavioral Health Case Manager (CBHCM) Define Yourself as a Professional through Certification. 1715 S. Gadsden St. Tallahassee,
EDENRED COMMUTER BENEFITS SOLUTIONS, LLC PRIVACY POLICY. Updated: April 2017
 This Privacy Policy (this Privacy Policy ) applies to Edenred Commuter Benefits Solutions, LLC, (the Company ) online interface (i.e., website or mobile application) and any Edenred Commuter Benefit Solutions,
This Privacy Policy (this Privacy Policy ) applies to Edenred Commuter Benefits Solutions, LLC, (the Company ) online interface (i.e., website or mobile application) and any Edenred Commuter Benefit Solutions,
Version GENESIS HEALTH SYSTEM. Institutional Review Board (IRB) IRBNet User s Guide
 Version 1 7-1-2012 GENESIS HEALTH SYSTEM Institutional Review Board (IRB) IRBNet User s Guide G E N E S I S H E A L T H S Y S T E M I N S T I T U T I O N A L R E V I E W B O A R D IRBNet User s Guide Genesis
Version 1 7-1-2012 GENESIS HEALTH SYSTEM Institutional Review Board (IRB) IRBNet User s Guide G E N E S I S H E A L T H S Y S T E M I N S T I T U T I O N A L R E V I E W B O A R D IRBNet User s Guide Genesis
Privacy Policy I. COOKEVILLE COMMUNICATIONS PRIVACY POLICY II. GENERAL PRIVACY GUIDELINES
 Privacy Policy I. COOKEVILLE COMMUNICATIONS PRIVACY POLICY Cookeville Communications Media is committed to maintaining robust privacy protections for its users. Our privacy policy is designed to help you
Privacy Policy I. COOKEVILLE COMMUNICATIONS PRIVACY POLICY Cookeville Communications Media is committed to maintaining robust privacy protections for its users. Our privacy policy is designed to help you
Parchment Guide to Ordering Official Transcripts
 Parchment Guide to Ordering Official Transcripts www. 2 Contents OVERVIEW 4 How it works 4 CREATE AN ACCOUNT AND ADD YOUR SCHOOL 5 ORDER YOUR TRANSCRIPT 7 TRACK YOUR TRANSCRIPT 12 TRANSCRIPT STATUSES 13
Parchment Guide to Ordering Official Transcripts www. 2 Contents OVERVIEW 4 How it works 4 CREATE AN ACCOUNT AND ADD YOUR SCHOOL 5 ORDER YOUR TRANSCRIPT 7 TRACK YOUR TRANSCRIPT 12 TRANSCRIPT STATUSES 13
Page 1 of 6 SURVEY: PROJECT TECH
 SURVEY: PROJECT TECH Case managers: Thank you for working with us to administer the survey. Please read each question as it is written. Instructions for you are written in italics. Information that you
SURVEY: PROJECT TECH Case managers: Thank you for working with us to administer the survey. Please read each question as it is written. Instructions for you are written in italics. Information that you
Certification. What: Who: Where:
 Certification What: Certification is a process by which the Wisconsin Technical College System Office evaluates the occupational, academic, and teaching experience of district employees to determine his/her
Certification What: Certification is a process by which the Wisconsin Technical College System Office evaluates the occupational, academic, and teaching experience of district employees to determine his/her
EDI ENROLLMENT AGREEMENT INSTRUCTIONS
 EDI ENROLLMENT AGREEMENT INSTRUCTIONS The Railroad EDI Enrollment Form (commonly referred to as the EDI Agreement) should be submitted when enrolling for electronic billing. It should be reviewed and signed
EDI ENROLLMENT AGREEMENT INSTRUCTIONS The Railroad EDI Enrollment Form (commonly referred to as the EDI Agreement) should be submitted when enrolling for electronic billing. It should be reviewed and signed
INDIANA DEPARTMENT OF CORRECTIONS Credit Recommendation Guide
 INDIANA DEPARTMENT OF CORRECTIONS 2009-2013 Credit Recommendation Guide The following courses have been evaluated by Corporate Articulation to potentially fulfill General Education or Elective credits
INDIANA DEPARTMENT OF CORRECTIONS 2009-2013 Credit Recommendation Guide The following courses have been evaluated by Corporate Articulation to potentially fulfill General Education or Elective credits
HOW TO APPLY. Access to the Internal Job Openings (click here)
 HOW TO APPLY Access to the Internal Job Openings (click here) Create a log in Enter your email address and create a password. Then, select and answer one security questions. This step will enable you to
HOW TO APPLY Access to the Internal Job Openings (click here) Create a log in Enter your email address and create a password. Then, select and answer one security questions. This step will enable you to
Red Flags/Identity Theft Prevention Policy: Purpose
 Red Flags/Identity Theft Prevention Policy: 200.3 Purpose Employees and students depend on Morehouse College ( Morehouse ) to properly protect their personal non-public information, which is gathered and
Red Flags/Identity Theft Prevention Policy: 200.3 Purpose Employees and students depend on Morehouse College ( Morehouse ) to properly protect their personal non-public information, which is gathered and
CPRC Renewal Changes
 CPRC Renewal Changes Please Note: All CPRC renewals must have six (6) hours of continuing education in Ethical Responsibility, out of the required twenty (20) hours. As of January 1 st, 2017 ethical responsibilities
CPRC Renewal Changes Please Note: All CPRC renewals must have six (6) hours of continuing education in Ethical Responsibility, out of the required twenty (20) hours. As of January 1 st, 2017 ethical responsibilities
Vision Services Application Overview
 The Georgia Lions Lighthouse is a 501(c)3 nonprofit. Our mission is to provide vision and hearing services through education, detection, prevention, and treatment. The services we provide are made possible
The Georgia Lions Lighthouse is a 501(c)3 nonprofit. Our mission is to provide vision and hearing services through education, detection, prevention, and treatment. The services we provide are made possible
Step-by-Step Guide. Electronic Proposal Routing in Cayuse 424. Office of Research & Sponsored Programs (ORSP)
 Step-by-Step Guide Electronic Proposal Routing in Cayuse 424 Office of Research & Sponsored Programs (ORSP) Step # 1 Look for an email from Cayuse entitled Cayuse424 System Notification: Proposal Awaiting
Step-by-Step Guide Electronic Proposal Routing in Cayuse 424 Office of Research & Sponsored Programs (ORSP) Step # 1 Look for an email from Cayuse entitled Cayuse424 System Notification: Proposal Awaiting
The next steps are numbered 1-7. These steps will collect information to register your account and place you in the correct course.
 CITI Instructions for First Time Users CITI Program New Learner Account Registration Go to www.citiprogram.org and click on the Register button located at the top right of the page. The next steps are
CITI Instructions for First Time Users CITI Program New Learner Account Registration Go to www.citiprogram.org and click on the Register button located at the top right of the page. The next steps are
NEW PROTOCOL - HUMANITARIAN USE DEVICE (HUD) TRAINING GUIDE
 NEW PROTOCOL - HUMANITARIAN USE DEVICE (HUD) TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol Humanitarian Use Device (HUD) 1 Table of Contents Table of Contents
NEW PROTOCOL - HUMANITARIAN USE DEVICE (HUD) TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol Humanitarian Use Device (HUD) 1 Table of Contents Table of Contents
SE Manager Users Guide
 SE Manager Users Guide Contents Forms... 4 Beginning a Form... 4 Individualized Education Program Meeting (IEP Meeting)... 5 I. Review of Evaluation Data... 7 Adding Evaluations... 8 Editing/Deleting Evaluations...
SE Manager Users Guide Contents Forms... 4 Beginning a Form... 4 Individualized Education Program Meeting (IEP Meeting)... 5 I. Review of Evaluation Data... 7 Adding Evaluations... 8 Editing/Deleting Evaluations...
BISHOP GROSSETESTE UNIVERSITY. Document Administration. This policy applies to staff, students, and relevant data subjects
 BISHOP GROSSETESTE UNIVERSITY Document Administration Document Title: Document Category: Privacy Policy Policy Version Number: 1.0 Status: Reason for development: Scope: Author / developer: Owner Approved
BISHOP GROSSETESTE UNIVERSITY Document Administration Document Title: Document Category: Privacy Policy Policy Version Number: 1.0 Status: Reason for development: Scope: Author / developer: Owner Approved
Certified Recovery Peer Advocate Application
 Certified Recovery Peer Advocate Application A Project of Alcoholism & Substance Abuse Providers of New York State, Inc. 11 North Pearl Street, Suite 801 Albany, NY 12207 Phone: 518.426.3122 x 101 Candidate
Certified Recovery Peer Advocate Application A Project of Alcoholism & Substance Abuse Providers of New York State, Inc. 11 North Pearl Street, Suite 801 Albany, NY 12207 Phone: 518.426.3122 x 101 Candidate
Connecticut Science & Engineering Fair Student/Project Registration
 Connecticut Science & Engineering Fair Student/Project Registration You may exit the site at any time (no Log Out required) and return later using your Login account. Save your work by clicking the "Save
Connecticut Science & Engineering Fair Student/Project Registration You may exit the site at any time (no Log Out required) and return later using your Login account. Save your work by clicking the "Save
FDA Audit Preparation
 Duke University Office of Audit, Risk and Compliance (OARC) FDA Audit Preparation Margaret M. Groves, JD, CRA, CCRP, CHRC Associate Compliance Officer for Human Subject Research Compliance (HSRC) External
Duke University Office of Audit, Risk and Compliance (OARC) FDA Audit Preparation Margaret M. Groves, JD, CRA, CCRP, CHRC Associate Compliance Officer for Human Subject Research Compliance (HSRC) External
