SDTM Automation with Standard CRF Pages Taylor Markway, SCRI Development Innovations, Carrboro, NC
|
|
|
- Peregrine Benson
- 6 years ago
- Views:
Transcription
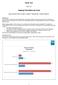
1 PharmaSUG Paper PO21 SDTM Automation with Standard CRF Pages Taylor Markway, SCRI Development Innovations, Carrboro, NC ABSTRACT Much has been written about automatically creating outputs for the Study Data Tabulation Model (SDTM). This paper provides brief descriptions of common approaches in practice and details an approach to automatically create SDTM outputs when case report form (CRF) pages are uniquely identifiable. The process uses a fixed SAS program structure that allows programs to be automatically constructed and deconstructed. This approach depends on three assumptions: 1) all CRF pages are identifiable, 2) all CRF pages that have the same set of identifiers are unique, and 3) all CRF pages are consistently represented in the electronic database. At SCRI, the statistical programming team and the data management team worked to meet these assumptions while designing the standard database. After meeting these three assumptions we can automatically create SAS SDTM programs for each domain. These programs that are easy to read due to their systematic structures. Considering that the programming team is still working directly with SAS, minimal training is needed to implement them. Deviations from the standard CRF or sponsor-specific SDTM mapping are implemented directly in SAS programs without complicated macro calls or outside mapping tools. INTRODUCTION This paper describes some SDTM automation techniques and how our approach fits into the existing paradigm. Our approach is detailed, and the results of the initial live cases are reported. This paper assumes the readers are familiar with SDTM. Our automatically generated SDTM programs take advantage of a metadata-driven approach. However, this paper will not detail the metadata-driven processes of these SDTM programs, as this topic has been covered in previous papers, including Using SAS Macros to simplify preparation of SDTM data, Annotated CRFs and Define.xml in a metadata driven environment by Niels Both (2009). SDTM AUTOMATION The creation of the SDTM data sets falls under a type of computing called Extract, Transform, Load or ETL. We first extract the clinical data from the case report forms, central labs and other vendors. Next, we transform it to the SDTM standard. Then we load it to a final location to be used for analysis and reporting. SDTM programs are an ETL tool. Every time we write an SDTM program that we have written before, we are rebuilding that tool much like a carpenter who builds a new hammer each time he builds a desk. This is wasting time and we need to automate. After surveying the landscape we found that there are many technologies and approaches available for SDTM automation. Black-box mappers usually have a graphical user interface (GUI) and do not require the user to input code. Metadata sources such as the CDISC ODM are typically used to automate the mapping in these systems. At the other end of the spectrum, we have whole SDTM programs that are recycled. On this side of the spectrum, all user inputs are in SAS code and each SDTM domain starts as the same version copied from the standards and is updated for each particular study. Many programmers informally practice this second type of automation when they copy SDTM programs from one study to the next. Figure 1 shows a graphic lay out of the landscape. This is, of course, an oversimplification, but it shows what our method is and is not supposed to be. The black-box method is typically automated the most and also creates restrictions and may not even use SAS. The domain level is the most flexible, yet it usually requires the most manual interventions. So our module approach falls between these two extremes. 1
2 Figure 1. SDTM Automation Landscape THE MODULE APPROACH We call our approach the module approach because at its core are SAS code fragments called modules. Each module is associated with a CRF page and an SDTM domain. Each SDTM domain also has a SAS program shell associated with it, and it is called a domain program. By putting the module and domain programs together, we can create SDTM programs for any combinations of the standard CRF pages. Think of this method as programming each CRF page to an SDTM domain in isolation. This is different than the usual approach where we think of programming an SDTM domain from all the CRF pages that map to that domain. This is a subtle distinction, but it allows us to separate the study-specific details from the CRF and the SDTM programs. All organization are going to face their own set of requirements when implementing new standards. To meet our goals for SDTM automation, we required minimum standards for our automation system: 1. Must be user friendly. 2. Must have a transparent output. 3. Must be easily maintained and updated. 4. Must work with any combination of standard CRF pages. At SCRI, the statistical programmers who create analyses and reporting outputs also create the SDTM data sets. Since our users would be professional SAS programmers, any tool that limits the functionality of SAS would be a restriction rather than a boon. Transparency means that no black-box solutions or complicated macros can be used to create SDTM output. In terms of maintenance, any solution that does not expand, grow, or adapt to changes is dead on arrival. In contrast, we require a solution that can be improved. It would also need to work for any combination of our standard CRF pages. No partial solutions are acceptable. We must have something that creates SDTM output without requiring user input when CRF pages are 100% standard. For this reason, the module approach creates SAS SDTM programs that can be fully edited in SAS and validated in the typical double programming fashion that many sponsors expect. However, the module approach provides greater efficiencies by incorporating alternatives to the traditional independent double programming that will be discussed later. MODULE METHOD DETAILS The Module method will be explained in three parts. First, I will go over the standard CRF structure. Second, I will cover the standard structure of the SDTM programs. Third, I will show how the standard programs are built and finalized when there are deviations from the standard CRF pages. 2
3 The module method of automation has two main parts; module programs associated with each CRF page and the domain programs that contain the code that will always be part of an SDTM domain. When the module and domain programs are combined, the final product is the SDTM program. STANDARD CRF For our module approach to work, we need to confirm three assumptions 1) all CRF pages are identifiable, 2) all CRF pages have unique identifiers, and 3) all CRF pages are consistently represented in the electronic database. At SCRI, we have used a template identifier and version date to uniquely identify CRF pages. Figure 2 is a simple example of an early clinical trial with the CRF template ENRL-01 and Version Date: The template and version date are stored as electronic metadata, and the information will be read and used to automatically combine the module programs into the domain programs to create the final SDTM programs. Figure 2. Sample CRF Page with Template and Version SDTM PROGRAMS To see how the module and domain programs work together, we will look at a SDTM program created using the Module method. All the programs are structured exactly the same way so that they can be programmatically constructed and deconstructed. Our approach creates an SDTM program that consists of five components: 1. Initialization of raw data 2. Mapping CRF pages to SDTM (the modules) 3. Combining all of the modules 4. Additional/Complex Derivations (the domain program) 5. Output All five pieces of the SDTM program are automatically brought together for each study that uses the standards. A standard macro is used to dynamically create components 1 and 3 by initializing the data and combining the modules. This ensures that the mapping of the CRF pages to the SDTM (component 2) will always have a consistent start and finish and allows the macro to copy any modules into the domain program. 3
4 Component 4 is the heart of the domain program as it contains the derivations that are required for the domain regardless of the CRF pages used. For example, derivations such as study day, baseline, and visit mapping take place in component 4. Component 5 is part of the domain program and outputs the final data set. It applies the final data set s metadata. All SDTM programs that use CRF data are organized this way. Initialize the Data After establishing a standard header, we initialize the data. This step is critical as it sets up the input for the modules in section 2. We use a fill-in-the-blank approach and create an empty SDTM data set shell that our module programs then fill. Other approaches could also be taken. In Figure 3, you can see the creation of the demographics shell via a macro (%mu_shell) and the use of a simple initialization macro (%ms_initial) to perform the repetitive tasks of ensuring that the data set assumptions for the module programs are correct. To ensure that all mappings are explicit in the final program, we renamed all of the raw data variables that have SDTM variable names with a leading underscore. Figure 3. Initializing the CRF Data Mapping CRF Pages to SDTM The modules, are the second part of any SDTM program and where the CRF variables are mapped to SDTM variables. All of the modules are included automatically and each module starts and ends with standard tags. In our case: *---- START: Map <module name> to <SDTM domain> ----*; <Module code> *---- END: Map <module name> to <SDTM domain> -----*; Though the modules are included automatically, they must still be written manually they are created. These tags make it easy to identify where the modules begin and end and make it possible to extract modules and save them in our standards library. This allows a module to be written only when it is first used and facilitates the creation of sponsor-specific standards if changes from our standard SDTM mapping are requested. 4
5 Figure 4. Death Details CRF Page Mapped to DM Domain Figure 4 shows the mapping from the Death CRF page to the demographics (DM) domain. In the full SDTM program, there is a module for each CRF page mapped to the DM domain. Note that the input data set dth matches the raw data set named for the initialization and the output name is the raw name, underscore, domain name. Combining the Modules As shown, all of the modules have programmatically determined output names. Using these standard names, all of the individual modules are combined into one data set. We have a metadata file called the module index that contains a list of all the modules for every CRF page and indicates how they relate to the core domain either as a set or merged. This is automatically generated for each program based on the CRF pages used. Figure 5. Combining All CRF Modules Figure 5 shows the merging of the module output to create a consistent domain output. We can set or merge any combination of our CRF pages. 5
6 Additional/Complex Derivations This fourth section is the heart of the domain program. Because the modules contain only the mapping of raw variables to SDTM variables this section contains any SDTM variables that are the result of derivations, for example, study day and the SDTM sequence number. In Figure 6 for the DM domain we have derivations for RFPENDTC, DTHFL, and AGE. Figure 6. Additional Derivation for DM Output The output section contains minimal coding and strictly creates the final output. In our case, this is both a SAS data set and a SAS version 5 transport file. Figure 7 displays this simple step. The macro variables (&domlabl and &keep) were created in the initialization step by the same macro that created the SDTM shell data set, %mu_shell. Figure 7. Final Output for DM Domain COMBINE MODULES AND DOMAINS The macro call to create our standard SDTM programs needs to specify the version number of the standard CRF used and the SDTM version. Figure 8 contains the actual macro call that ultimately creates over 25 SDTM domains. Figure 8. Macro to Automatically Create Standard SDTM 6
7 For transparency sake, we split the creation into two separate pieces. This first macro call created a program containing the macro calls that actually created the SDTM programs. This was so manual corrections could be made if a CRF page had incorrect metadata in the EDC system. Figure 9 contains the final macro calls used to create the SDTM programs. This is run once at the beginning of SDTM production. Based on the CRF and the SDTM versions the macro will go to a directory and copy the indicated modules and paste them into the domain program. The macro will also create the initialization and combination parts of the program from the module input. The input for the module parameter is <raw data set name>-<crf template>. So the DM domain in figure 9 has a raw data set of EXF that corresponds to the CRF template EX-01. Figure 9. Combing Modules to Domain Programs UPDATING NON-STANDARD PAGES When creating the study CRF we placed each page into one of three buckets: 1. EXACT: A match to the standard CRF page. 2. MODIFIED: Based on a standard CRF page modified in some way. 3. CUSTOM: A completely new CRF page that is not based on any existing standards. This category is determined by the template name and the version date (see Figure 2 as an example). The macro that generates the SDTM program treats the EXACT and MODIFIED the same. It simply copies the associated module program and pastes it into the domain program. If the standard CRF page was a good starting place for the CRF designer, it would be a good starting point for the SDTM programmer. Custom CRF pages will have to be programmed from scratch as these are not based off of any existing pages. When the domains and modules are combined into the SDTM programs reports are created that summarize the buckets for each page. These reports are reproduced in Tables 1 and 2. From this information, the programmer can now approximate the effort needed to create the SDTM programs for a study. RESULTS IN PRACTICE Our Module method was conceptualized in September and designed and built in October, followed by validation of all our standard CRF pages during November and December. Since December, we have had the opportunity to test the method on three live studies with great success. It is now being used on all new studies. Early phase oncology studies are SCRI s specialty, so the three test studies were all oncology and phase I or I/II. The percent of the CRF pages that followed the standards have been 49%, 73 and 75%. The second two studies were similar, so I will only provide details on the first two studies. 7
8 STUDY A 49% STANDARD The first study to use the standards had 47 CRF pages with 23 pages being an exact match to our standards. The programmer using the standards completed 20 SDTM domains in two days. This was the first time this programmer had used the standards after two one-hour training sessions. STUDY B 73% STANDARD The second study tested had 45 unique CRF pages. Based on feedback from the first test case, we implemented a standard report to help guide the programming effort. When we run the macros against the CRF metadata, it categorizes the pages into three groups: Standard, Modified and Custom. If a page falls into the standard category, then it is an exact match to the standard CRF page. The metadata for a modified page indicates the standard page it was created from, so we still use the standard module as a starting point, while still needing to update the SDTM program to account for the changes. Custom pages are so unique that they are not based on of any existing standard page, so these pages must be included in the SDTM programs from scratch. Tables 1 and 2 below are standard reports that are generated after running the macro that creates the SDTM programs. Table 2 has been slightly modified to show where the custom CRF page was mapped. Table 2 also tells us that there are 12 SDTM domains that are 100% standard and will not require manual intervention. Then there are eight domains that require a manual intervention to make updates for modified or custom CRF pages. Study B CRF Details Mapping Result Number of Pages Percentage Modified Standard CRF Page Standard CRF Page Custom Study B SDTM Mapping Details Domain Mapping Result Number of Pages Percentage of Domain for the Mapping Result AE Modified CE Modified CM Standard 2 66 CM Custom 1 33 DD Standard DM Standard 4 66 DM Modified 2 33 DS Standard 2 50 DS Modified 2 50 EC Standard EG Unknown EX Standard IE Standard LB Standard MH Standard PR Standard 3 60 PR Modified 2 40 QS Standard RS Standard SC Standard
9 Domain Mapping Result Number of Pages Percentage of Domain for the Mapping Result SS Modified TR Standard TU Standard VS Standard NEXT STEPS AND VALIDATION Some may dismiss the Module method as a clever trick at a CRO that specializes in early oncology studies. However, recall that three assumptions had to be met to make this approach work: 1) all CRF pages are identifiable, 2) all CRF pages that have the same set of identifiers are unique, and 3) all CRF pages are consistently represented in the electronic database. If you are working with multiple data collection systems, many different indications, or any other confounding factors, you only need to meet these three assumptions in four steps: 1. Identify the CRF page and the SDTM domain it maps to. 2. Grab the domain program from wherever it is stored. 3. Grab the module program associated with the CRF page. 4. Paste the module program into the domain program. That is really all we are doing here. Conceptually, it is a simple copy and paste. EXPANSION Since the programs are structured systematically, they can be taken apart. This allows us to keep adding new modules whenever they are encountered. If your custom CRF pages meet the criteria then they will be custom only once. The addition and storage of new CRF pages carries over to sponsors who have specific SDTM mapping needs. Again, after updating the SDTM programs to fit the sponsor s needs, we can then extract the pieces (module program and domain programs) and store them in a sponsor-specific directory. The next time we work with the sponsor, we can use the modules we created earlier. This process formalizes the copy-and-paste approach so many programmers are employing. By copying codes from a standard instead of from study to study we can easily copy the most appropriate code for a study and all it takes is our three assumptions. Hardly a herculean task. A NOTE ON VALIDATION Undoubtedly, we must validate outputs on every study, but what would be the appropriate level of validation when using the module method? To obtain the maximum benefit from the module method, we want to avoid re-validating something previously validated. We validated the Module method as a two-step process. First, all the modules and domain programs underwent a code review. Next, we tested every module in the domain program performing a form and content check of the final SDTM data set. We did not validate every possible combination of module within each domain. This would have been too time-consuming and some combinations are never likely to occur. This only made us certain that the method would work for any combination. Validation on individual studies still needs to occur. Presented here are two possible validation strategies to maximize the benefit of the Module method. Semi-Independent Validation Semi-Independent Validation would be similar to independent double programming. The key difference would be that both the production programmer and the validation programmer would be working from the combined module and domain programs. Other than having the same starting point, the programmers would remain independent. This validation technique allows for the validation side to benefit from the Module method and avoids the redundancy of having to recreate a validation set of modules and domain programs. 9
10 Tracking Module Combinations The tracking method would use full and independent double programming and track the validated module-domain combinations. Using this method, a combination can be validated and logged, and then the validation program can be stored in a central directory for reference and used on other studies. This method requires the validation programmers to be fully aware that their programs would be used on future studies and plan accordingly with judicious use of study specific programming. This method is still the gold-standard of independent double programming, but it adds complexity in that the validation programs would not be systematically structured like those in the module method. This may cause study-specific assumptions to be included that could make them worse starting points than starting from scratch. CONCLUSION The SDTM automation process presented in this paper can be implemented by anyone, even if you don t think you have time to devote to standards. Since the programs follow standard structures you can build up your standard library over time as you encounter new CRF pages. Nobody likes redoing an SDTM data set they ve done before and for good reason: it s a waste of resources. There are so many other things we can be doing with our time than mapping the latest exposure date to RFXENDTC for the umpteenth time. The module method in this paper can be used to free up time and create savings that eventually can be passed on to the patients who hope to one day receive the drugs we study. REFERENCES Ewing, D Automating SDTM File Creation: Metadata Files Speeding the Process. Proceedings of SAS Global Forum 2010 Conference. Malvern, PA: Available at Li, C., et. al. SDTM Domain Mapping with Structured Programming Methodology. Proceedings of PharmaSUG 2012 Conference. Ridgefield, CT. Available at Aerts, J., SDTM-ETL, website. March 18, Available at ACKNOWLEDGMENTS I would like to thank Jeffery Johnson, Rakesh Mucha and Zahir Karim for reaching out and creating the opportunity to be involved with SDTM automation at SCRI Development Innovations. CONTACT INFORMATION Your comments and questions are valued and encouraged. Contact the author at: Name: Taylor Markway Enterprise: SCRI Development Innovations Address: 3322 West End Avenue, Suite 900 City, State ZIP: Nashville, TN Work Phone: (615) Taylor.Markway@SCRI-Innovations.com SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. indicates USA registration. Other brand and product names are trademarks of their respective companies. 10
CDASH Standards and EDC CRF Library. Guang-liang Wang September 18, Q3 DCDISC Meeting
 CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
Submission-Ready Define.xml Files Using SAS Clinical Data Integration Melissa R. Martinez, SAS Institute, Cary, NC USA
 PharmaSUG 2016 - Paper SS12 Submission-Ready Define.xml Files Using SAS Clinical Data Integration Melissa R. Martinez, SAS Institute, Cary, NC USA ABSTRACT SAS Clinical Data Integration simplifies the
PharmaSUG 2016 - Paper SS12 Submission-Ready Define.xml Files Using SAS Clinical Data Integration Melissa R. Martinez, SAS Institute, Cary, NC USA ABSTRACT SAS Clinical Data Integration simplifies the
An Alternate Way to Create the Standard SDTM Domains
 PharmaSUG 2018 - Paper DS-12 ABSTRACT An Alternate Way to Create the Standard SDTM Domains Sunil Kumar Pusarla, Omeros Corporation Sponsors who initiate clinical trials after 2016-12-17 are required to
PharmaSUG 2018 - Paper DS-12 ABSTRACT An Alternate Way to Create the Standard SDTM Domains Sunil Kumar Pusarla, Omeros Corporation Sponsors who initiate clinical trials after 2016-12-17 are required to
PharmaSUG Paper PO21
 PharmaSUG 2015 - Paper PO21 Evaluating SDTM SUPP Domain For AdaM - Trash Can Or Buried Treasure Xiaopeng Li, Celerion, Lincoln, NE Yi Liu, Celerion, Lincoln, NE Chun Feng, Celerion, Lincoln, NE ABSTRACT
PharmaSUG 2015 - Paper PO21 Evaluating SDTM SUPP Domain For AdaM - Trash Can Or Buried Treasure Xiaopeng Li, Celerion, Lincoln, NE Yi Liu, Celerion, Lincoln, NE Chun Feng, Celerion, Lincoln, NE ABSTRACT
How to write ADaM specifications like a ninja.
 Poster PP06 How to write ADaM specifications like a ninja. Caroline Francis, Independent SAS & Standards Consultant, Torrevieja, Spain ABSTRACT To produce analysis datasets from CDISC Study Data Tabulation
Poster PP06 How to write ADaM specifications like a ninja. Caroline Francis, Independent SAS & Standards Consultant, Torrevieja, Spain ABSTRACT To produce analysis datasets from CDISC Study Data Tabulation
Why organizations need MDR system to manage clinical metadata?
 PharmaSUG 2018 - Paper SS-17 Why organizations need MDR system to manage clinical metadata? Abhinav Jain, Ephicacy Consulting Group Inc. ABSTRACT In the last decade, CDISC standards undoubtedly have transformed
PharmaSUG 2018 - Paper SS-17 Why organizations need MDR system to manage clinical metadata? Abhinav Jain, Ephicacy Consulting Group Inc. ABSTRACT In the last decade, CDISC standards undoubtedly have transformed
CDISC Variable Mapping and Control Terminology Implementation Made Easy
 PharmaSUG2011 - Paper CD11 CDISC Variable Mapping and Control Terminology Implementation Made Easy Balaji Ayyappan, Ockham Group, Cary, NC Manohar Sure, Ockham Group, Cary, NC ABSTRACT: CDISC SDTM (Study
PharmaSUG2011 - Paper CD11 CDISC Variable Mapping and Control Terminology Implementation Made Easy Balaji Ayyappan, Ockham Group, Cary, NC Manohar Sure, Ockham Group, Cary, NC ABSTRACT: CDISC SDTM (Study
An Efficient Solution to Efficacy ADaM Design and Implementation
 PharmaSUG 2017 - Paper AD05 An Efficient Solution to Efficacy ADaM Design and Implementation Chengxin Li, Pfizer Consumer Healthcare, Madison, NJ, USA Zhongwei Zhou, Pfizer Consumer Healthcare, Madison,
PharmaSUG 2017 - Paper AD05 An Efficient Solution to Efficacy ADaM Design and Implementation Chengxin Li, Pfizer Consumer Healthcare, Madison, NJ, USA Zhongwei Zhou, Pfizer Consumer Healthcare, Madison,
Harmonizing CDISC Data Standards across Companies: A Practical Overview with Examples
 PharmaSUG 2017 - Paper DS06 Harmonizing CDISC Data Standards across Companies: A Practical Overview with Examples Keith Shusterman, Chiltern; Prathima Surabhi, AstraZeneca; Binoy Varghese, Medimmune ABSTRACT
PharmaSUG 2017 - Paper DS06 Harmonizing CDISC Data Standards across Companies: A Practical Overview with Examples Keith Shusterman, Chiltern; Prathima Surabhi, AstraZeneca; Binoy Varghese, Medimmune ABSTRACT
Study Data Reviewer s Guide Completion Guideline
 Study Data Reviewer s Guide Completion Guideline 22-Feb-2013 Revision History Date Version Summary 02-Nov-2012 0.1 Draft 20-Nov-2012 0.2 Added Finalization Instructions 10-Jan-2013 0.3 Updated based on
Study Data Reviewer s Guide Completion Guideline 22-Feb-2013 Revision History Date Version Summary 02-Nov-2012 0.1 Draft 20-Nov-2012 0.2 Added Finalization Instructions 10-Jan-2013 0.3 Updated based on
Pharmaceuticals, Health Care, and Life Sciences. An Approach to CDISC SDTM Implementation for Clinical Trials Data
 An Approach to CDISC SDTM Implementation for Clinical Trials Data William T. Chen, Merck Research Laboratories, Rahway, NJ Margaret M. Coughlin, Merck Research Laboratories, Rahway, NJ ABSTRACT The Clinical
An Approach to CDISC SDTM Implementation for Clinical Trials Data William T. Chen, Merck Research Laboratories, Rahway, NJ Margaret M. Coughlin, Merck Research Laboratories, Rahway, NJ ABSTRACT The Clinical
The Wonderful World of Define.xml.. Practical Uses Today. Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008
 The Wonderful World of Define.xml.. Practical Uses Today Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008 Agenda Introduction to Formedix What is Define.xml? Features and Benefits
The Wonderful World of Define.xml.. Practical Uses Today Mark Wheeldon, CEO, Formedix DC User Group, Washington, 9 th December 2008 Agenda Introduction to Formedix What is Define.xml? Features and Benefits
The Benefits of Traceability Beyond Just From SDTM to ADaM in CDISC Standards Maggie Ci Jiang, Teva Pharmaceuticals, Great Valley, PA
 PharmaSUG 2017 - Paper DS23 The Benefits of Traceability Beyond Just From SDTM to ADaM in CDISC Standards Maggie Ci Jiang, Teva Pharmaceuticals, Great Valley, PA ABSTRACT Since FDA released the Analysis
PharmaSUG 2017 - Paper DS23 The Benefits of Traceability Beyond Just From SDTM to ADaM in CDISC Standards Maggie Ci Jiang, Teva Pharmaceuticals, Great Valley, PA ABSTRACT Since FDA released the Analysis
PharmaSUG Paper DS06 Designing and Tuning ADaM Datasets. Songhui ZHU, K&L Consulting Services, Fort Washington, PA
 PharmaSUG 2013 - Paper DS06 Designing and Tuning ADaM Datasets Songhui ZHU, K&L Consulting Services, Fort Washington, PA ABSTRACT The developers/authors of CDISC ADaM Model and ADaM IG made enormous effort
PharmaSUG 2013 - Paper DS06 Designing and Tuning ADaM Datasets Songhui ZHU, K&L Consulting Services, Fort Washington, PA ABSTRACT The developers/authors of CDISC ADaM Model and ADaM IG made enormous effort
One Project, Two Teams: The Unblind Leading the Blind
 ABSTRACT PharmaSUG 2017 - Paper BB01 One Project, Two Teams: The Unblind Leading the Blind Kristen Reece Harrington, Rho, Inc. In the pharmaceutical world, there are instances where multiple independent
ABSTRACT PharmaSUG 2017 - Paper BB01 One Project, Two Teams: The Unblind Leading the Blind Kristen Reece Harrington, Rho, Inc. In the pharmaceutical world, there are instances where multiple independent
How a Metadata Repository enables dynamism and automation in SDTM-like dataset generation
 Paper DH05 How a Metadata Repository enables dynamism and automation in SDTM-like dataset generation Judith Goud, Akana, Bennekom, The Netherlands Priya Shetty, Intelent, Princeton, USA ABSTRACT The traditional
Paper DH05 How a Metadata Repository enables dynamism and automation in SDTM-like dataset generation Judith Goud, Akana, Bennekom, The Netherlands Priya Shetty, Intelent, Princeton, USA ABSTRACT The traditional
DCDISC Users Group. Nate Freimark Omnicare Clinical Research Presented on
 DCDISC Users Group Nate Freimark Omnicare Clinical Research Presented on 2011-05-12 1 Disclaimer The opinions provided are solely those of the author and not those of the ADaM team or Omnicare Clinical
DCDISC Users Group Nate Freimark Omnicare Clinical Research Presented on 2011-05-12 1 Disclaimer The opinions provided are solely those of the author and not those of the ADaM team or Omnicare Clinical
PharmaSUG 2014 PO16. Category CDASH SDTM ADaM. Submission in standardized tabular form. Structure Flexible Rigid Flexible * No Yes Yes
 ABSTRACT PharmaSUG 2014 PO16 Automation of ADAM set Creation with a Retrospective, Prospective and Pragmatic Process Karin LaPann, MSIS, PRA International, USA Terek Peterson, MBA, PRA International, USA
ABSTRACT PharmaSUG 2014 PO16 Automation of ADAM set Creation with a Retrospective, Prospective and Pragmatic Process Karin LaPann, MSIS, PRA International, USA Terek Peterson, MBA, PRA International, USA
Creating a Patient Profile using CDISC SDTM Marc Desgrousilliers, Clinovo, Sunnyvale, CA Romain Miralles, Clinovo, Sunnyvale, CA
 Creating a Patient Profile using CDISC SDTM Marc Desgrousilliers, Clinovo, Sunnyvale, CA Romain Miralles, Clinovo, Sunnyvale, CA ABSTRACT CDISC SDTM data is the standard format requested by the FDA for
Creating a Patient Profile using CDISC SDTM Marc Desgrousilliers, Clinovo, Sunnyvale, CA Romain Miralles, Clinovo, Sunnyvale, CA ABSTRACT CDISC SDTM data is the standard format requested by the FDA for
Automated Creation of Submission-Ready Artifacts Silas McKee, Accenture, Pennsylvania, USA Lourdes Devenney, Accenture, Pennsylvania, USA
 Paper DH06 Automated Creation of Submission-Ready Artifacts Silas McKee, Accenture, Pennsylvania, USA Lourdes Devenney, Accenture, Pennsylvania, USA ABSTRACT Despite significant progress towards the standardization
Paper DH06 Automated Creation of Submission-Ready Artifacts Silas McKee, Accenture, Pennsylvania, USA Lourdes Devenney, Accenture, Pennsylvania, USA ABSTRACT Despite significant progress towards the standardization
Advantages of a real end-to-end approach with CDISC standards
 Advantages of a real end-to-end approach with CDISC standards Dr. Philippe Verplancke CEO XClinical GmbH 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna Austria Disclaimer The views and opinions expressed
Advantages of a real end-to-end approach with CDISC standards Dr. Philippe Verplancke CEO XClinical GmbH 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna Austria Disclaimer The views and opinions expressed
Main challenges for a SAS programmer stepping in SAS developer s shoes
 Paper AD15 Main challenges for a SAS programmer stepping in SAS developer s shoes Sebastien Jolivet, Novartis Pharma AG, Basel, Switzerland ABSTRACT Whether you work for a large pharma or a local CRO,
Paper AD15 Main challenges for a SAS programmer stepping in SAS developer s shoes Sebastien Jolivet, Novartis Pharma AG, Basel, Switzerland ABSTRACT Whether you work for a large pharma or a local CRO,
Automation of SDTM Programming in Oncology Disease Response Domain Yiwen Wang, Yu Cheng, Ju Chen Eli Lilly and Company, China
 ABSTRACT Study Data Tabulation Model (SDTM) is an evolving global standard which is widely used for regulatory submissions. The automation of SDTM programming is essential to maximize the programming efficiency
ABSTRACT Study Data Tabulation Model (SDTM) is an evolving global standard which is widely used for regulatory submissions. The automation of SDTM programming is essential to maximize the programming efficiency
The New SDTMIG What s Different from Previous Versions
 1 The New SDTMIG 3.1.4 What s Different from Previous Versions Presented by Dan Godoy (SDS Team Co-Lead) 2 Agenda Background Progress-to-Date SDTM 3.1.4 - What s Different? Key Features & Examples Q&A
1 The New SDTMIG 3.1.4 What s Different from Previous Versions Presented by Dan Godoy (SDS Team Co-Lead) 2 Agenda Background Progress-to-Date SDTM 3.1.4 - What s Different? Key Features & Examples Q&A
Introduction to ADaM and What s new in ADaM
 Introduction to ADaM and What s new in ADaM Italian CDISC UN Day - Milan 27 th October 2017 Silvia Faini Principal Statistical Programmer CROS NT - Verona ADaM Purpose Why are standards needed in analysis
Introduction to ADaM and What s new in ADaM Italian CDISC UN Day - Milan 27 th October 2017 Silvia Faini Principal Statistical Programmer CROS NT - Verona ADaM Purpose Why are standards needed in analysis
Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata
 Paper SD02 Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata Dr. Philippe Verplancke, XClinical GmbH, Munich, Germany ABSTRACT define.xml is often created at the end of
Paper SD02 Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata Dr. Philippe Verplancke, XClinical GmbH, Munich, Germany ABSTRACT define.xml is often created at the end of
SDTM-ETL 3.2 User Manual and Tutorial
 SDTM-ETL 3.2 User Manual and Tutorial Author: Jozef Aerts, XML4Pharma Last update: 2017-07-29 Adding mappings for the Vital Signs domain After loading and validating the ODM file with metadata, your load
SDTM-ETL 3.2 User Manual and Tutorial Author: Jozef Aerts, XML4Pharma Last update: 2017-07-29 Adding mappings for the Vital Signs domain After loading and validating the ODM file with metadata, your load
PharmaSUG. companies. This paper. will cover how. processes, a fairly linear. before moving. be carried out. Lifecycle. established.
 PharmaSUG 2016 - Paper PO17 Standards Implementationn & Governance: Carrot or Stick? Julie Smiley, Akana, San Antonio, Texas Judith Goud, Akana, Bennekom, Netherlands ABSTRACT With the looming FDA mandate
PharmaSUG 2016 - Paper PO17 Standards Implementationn & Governance: Carrot or Stick? Julie Smiley, Akana, San Antonio, Texas Judith Goud, Akana, Bennekom, Netherlands ABSTRACT With the looming FDA mandate
START CONVERTING FROM TEXT DATE/TIME VALUES
 A Macro Mapping Date and Time Variable to CDISC Date and Time Variable Song Liu, Biogen Idec, San Diego, California Brett Sellars, Biogen Idec, San Diego, California ABSTRACT The Clinical Data Interchange
A Macro Mapping Date and Time Variable to CDISC Date and Time Variable Song Liu, Biogen Idec, San Diego, California Brett Sellars, Biogen Idec, San Diego, California ABSTRACT The Clinical Data Interchange
Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library
 PharmaSUG 2018 - Paper SS-12 Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library Veena Nataraj, Erica Davis, Shire ABSTRACT Establishing internal Data Standards helps companies
PharmaSUG 2018 - Paper SS-12 Improving Metadata Compliance and Assessing Quality Metrics with a Standards Library Veena Nataraj, Erica Davis, Shire ABSTRACT Establishing internal Data Standards helps companies
Creating an ADaM Data Set for Correlation Analyses
 PharmaSUG 2018 - Paper DS-17 ABSTRACT Creating an ADaM Data Set for Correlation Analyses Chad Melson, Experis Clinical, Cincinnati, OH The purpose of a correlation analysis is to evaluate relationships
PharmaSUG 2018 - Paper DS-17 ABSTRACT Creating an ADaM Data Set for Correlation Analyses Chad Melson, Experis Clinical, Cincinnati, OH The purpose of a correlation analysis is to evaluate relationships
From Implementing CDISC Using SAS. Full book available for purchase here. About This Book... xi About The Authors... xvii Acknowledgments...
 From Implementing CDISC Using SAS. Full book available for purchase here. Contents About This Book... xi About The Authors... xvii Acknowledgments... xix Chapter 1: Implementation Strategies... 1 Why CDISC
From Implementing CDISC Using SAS. Full book available for purchase here. Contents About This Book... xi About The Authors... xvii Acknowledgments... xix Chapter 1: Implementation Strategies... 1 Why CDISC
From raw data to submission: A metadata-driven, repository-based process of data conversion to CDISC models
 Paper CD08 From raw data to submission: A metadata-driven, repository-based process of data conversion to CDISC models Dimitri Kutsenko, Entimo AG, Berlin, Germany ABSTRACT The paper presents a visionary
Paper CD08 From raw data to submission: A metadata-driven, repository-based process of data conversion to CDISC models Dimitri Kutsenko, Entimo AG, Berlin, Germany ABSTRACT The paper presents a visionary
PharmaSUG Paper PO22
 PharmaSUG 2015 - Paper PO22 Challenges in Developing ADSL with Baseline Data Hongyu Liu, Vertex Pharmaceuticals Incorporated, Boston, MA Hang Pang, Vertex Pharmaceuticals Incorporated, Boston, MA ABSTRACT
PharmaSUG 2015 - Paper PO22 Challenges in Developing ADSL with Baseline Data Hongyu Liu, Vertex Pharmaceuticals Incorporated, Boston, MA Hang Pang, Vertex Pharmaceuticals Incorporated, Boston, MA ABSTRACT
Automate Clinical Trial Data Issue Checking and Tracking
 PharmaSUG 2018 - Paper AD-31 ABSTRACT Automate Clinical Trial Data Issue Checking and Tracking Dale LeSueur and Krishna Avula, Regeneron Pharmaceuticals Inc. Well organized and properly cleaned data are
PharmaSUG 2018 - Paper AD-31 ABSTRACT Automate Clinical Trial Data Issue Checking and Tracking Dale LeSueur and Krishna Avula, Regeneron Pharmaceuticals Inc. Well organized and properly cleaned data are
Real Time Clinical Trial Oversight with SAS
 PharmaSUG 2017 - Paper DA01 Real Time Clinical Trial Oversight with SAS Ashok Gunuganti, Trevena ABSTRACT A clinical trial is an expensive and complex undertaking with multiple teams working together to
PharmaSUG 2017 - Paper DA01 Real Time Clinical Trial Oversight with SAS Ashok Gunuganti, Trevena ABSTRACT A clinical trial is an expensive and complex undertaking with multiple teams working together to
Dealing with changing versions of SDTM and Controlled Terminology (CT)
 CDISC UK Network Breakout session Notes 07/06/16 Afternoon Session 1: Dealing with changing versions of SDTM and Controlled Terminology (CT) How do people manage this? Is this managed via a sponsor Standards
CDISC UK Network Breakout session Notes 07/06/16 Afternoon Session 1: Dealing with changing versions of SDTM and Controlled Terminology (CT) How do people manage this? Is this managed via a sponsor Standards
Preparing the Office of Scientific Investigations (OSI) Requests for Submissions to FDA
 PharmaSUG 2018 - Paper EP15 Preparing the Office of Scientific Investigations (OSI) Requests for Submissions to FDA Ellen Lin, Wei Cui, Ran Li, and Yaling Teng Amgen Inc, Thousand Oaks, CA ABSTRACT The
PharmaSUG 2018 - Paper EP15 Preparing the Office of Scientific Investigations (OSI) Requests for Submissions to FDA Ellen Lin, Wei Cui, Ran Li, and Yaling Teng Amgen Inc, Thousand Oaks, CA ABSTRACT The
AUTOMATED CREATION OF SUBMISSION-READY ARTIFACTS SILAS MCKEE
 AUTOMATED CREATION OF SUBMISSION-READY ARTIFACTS SILAS MCKEE AGENDA 1. Motivation 2. Automation Overview 3. Architecture 4. Validating the System 5. Pilot Study Results 6. Future State Copyright 2012-2017
AUTOMATED CREATION OF SUBMISSION-READY ARTIFACTS SILAS MCKEE AGENDA 1. Motivation 2. Automation Overview 3. Architecture 4. Validating the System 5. Pilot Study Results 6. Future State Copyright 2012-2017
Now let s take a look
 1 2 3 4 Manage assets across the end to end life cycle of your studies This includes forms, datasets, terminologies, files, links and more, for example: - Studies may contain the protocol, a set of Forms,
1 2 3 4 Manage assets across the end to end life cycle of your studies This includes forms, datasets, terminologies, files, links and more, for example: - Studies may contain the protocol, a set of Forms,
Implementing CDISC Using SAS. Full book available for purchase here.
 Implementing CDISC Using SAS. Full book available for purchase here. Contents About the Book... ix About the Authors... xv Chapter 1: Implementation Strategies... 1 The Case for Standards... 1 Which Models
Implementing CDISC Using SAS. Full book available for purchase here. Contents About the Book... ix About the Authors... xv Chapter 1: Implementation Strategies... 1 The Case for Standards... 1 Which Models
PharmaSUG Paper TT11
 PharmaSUG 2014 - Paper TT11 What is the Definition of Global On-Demand Reporting within the Pharmaceutical Industry? Eric Kammer, Novartis Pharmaceuticals Corporation, East Hanover, NJ ABSTRACT It is not
PharmaSUG 2014 - Paper TT11 What is the Definition of Global On-Demand Reporting within the Pharmaceutical Industry? Eric Kammer, Novartis Pharmaceuticals Corporation, East Hanover, NJ ABSTRACT It is not
Lex Jansen Octagon Research Solutions, Inc.
 Converting the define.xml to a Relational Database to enable Printing and Validation Lex Jansen Octagon Research Solutions, Inc. Leading the Electronic Transformation of Clinical R&D PhUSE 2009, Basel,
Converting the define.xml to a Relational Database to enable Printing and Validation Lex Jansen Octagon Research Solutions, Inc. Leading the Electronic Transformation of Clinical R&D PhUSE 2009, Basel,
SAS Clinical Data Integration 2.4
 SAS Clinical Data Integration 2.4 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2013. SAS Clinical Data Integration 2.4: User's Guide.
SAS Clinical Data Integration 2.4 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2013. SAS Clinical Data Integration 2.4: User's Guide.
Programming checks: Reviewing the overall quality of the deliverables without parallel programming
 PharmaSUG 2016 Paper IB04 Programming checks: Reviewing the overall quality of the deliverables without parallel programming Shailendra Phadke, Baxalta US Inc., Cambridge MA Veronika Csom, Baxalta US Inc.,
PharmaSUG 2016 Paper IB04 Programming checks: Reviewing the overall quality of the deliverables without parallel programming Shailendra Phadke, Baxalta US Inc., Cambridge MA Veronika Csom, Baxalta US Inc.,
Legacy to SDTM Conversion Workshop: Tools and Techniques
 Legacy to SDTM Conversion Workshop: Tools and Techniques Mike Todd President Nth Analytics Legacy Data Old studies never die Legacy studies are often required for submissions or pharmacovigilence. Often
Legacy to SDTM Conversion Workshop: Tools and Techniques Mike Todd President Nth Analytics Legacy Data Old studies never die Legacy studies are often required for submissions or pharmacovigilence. Often
Managing CDISC version changes: how & when to implement? Presented by Lauren Shinaberry, Project Manager Business & Decision Life Sciences
 1 Managing CDISC version changes: how & when to implement? Presented by Lauren Shinaberry, Project Manager Business & Decision Life Sciences 2 Content Standards Technical Standards SDTM v1.1 SDTM IG v3.1.1
1 Managing CDISC version changes: how & when to implement? Presented by Lauren Shinaberry, Project Manager Business & Decision Life Sciences 2 Content Standards Technical Standards SDTM v1.1 SDTM IG v3.1.1
ADaM Compliance Starts with ADaM Specifications
 PharmaSUG 2017 - Paper DS16 ADaM Compliance Starts with ADaM Specifications Trevor Mankus, Kent Letourneau, PRA Health Sciences ABSTRACT As of December 17th, 2016, the FDA and PMDA require that all new
PharmaSUG 2017 - Paper DS16 ADaM Compliance Starts with ADaM Specifications Trevor Mankus, Kent Letourneau, PRA Health Sciences ABSTRACT As of December 17th, 2016, the FDA and PMDA require that all new
Applying ADaM Principles in Developing a Response Analysis Dataset
 PharmaSUG2010 Paper CD03 Applying ADaM Principles in Developing a Response Analysis Dataset Mei Dey, Merck & Co., Inc Lisa Pyle, Merck & Co., Inc ABSTRACT The Clinical Data Interchange Standards Consortium
PharmaSUG2010 Paper CD03 Applying ADaM Principles in Developing a Response Analysis Dataset Mei Dey, Merck & Co., Inc Lisa Pyle, Merck & Co., Inc ABSTRACT The Clinical Data Interchange Standards Consortium
ABSTRACT INTRODUCTION WHERE TO START? 1. DATA CHECK FOR CONSISTENCIES
 Developing Integrated Summary of Safety Database using CDISC Standards Rajkumar Sharma, Genentech Inc., A member of the Roche Group, South San Francisco, CA ABSTRACT Most individual trials are not powered
Developing Integrated Summary of Safety Database using CDISC Standards Rajkumar Sharma, Genentech Inc., A member of the Roche Group, South San Francisco, CA ABSTRACT Most individual trials are not powered
Codelists Here, Versions There, Controlled Terminology Everywhere Shelley Dunn, Regulus Therapeutics, San Diego, California
 ABSTRACT PharmaSUG 2016 - Paper DS16 lists Here, Versions There, Controlled Terminology Everywhere Shelley Dunn, Regulus Therapeutics, San Diego, California Programming SDTM and ADaM data sets for a single
ABSTRACT PharmaSUG 2016 - Paper DS16 lists Here, Versions There, Controlled Terminology Everywhere Shelley Dunn, Regulus Therapeutics, San Diego, California Programming SDTM and ADaM data sets for a single
SAS Application to Automate a Comprehensive Review of DEFINE and All of its Components
 PharmaSUG 2017 - Paper AD19 SAS Application to Automate a Comprehensive Review of DEFINE and All of its Components Walter Hufford, Vincent Guo, and Mijun Hu, Novartis Pharmaceuticals Corporation ABSTRACT
PharmaSUG 2017 - Paper AD19 SAS Application to Automate a Comprehensive Review of DEFINE and All of its Components Walter Hufford, Vincent Guo, and Mijun Hu, Novartis Pharmaceuticals Corporation ABSTRACT
Planning to Pool SDTM by Creating and Maintaining a Sponsor-Specific Controlled Terminology Database
 PharmaSUG 2017 - Paper DS13 Planning to Pool SDTM by Creating and Maintaining a Sponsor-Specific Controlled Terminology Database ABSTRACT Cori Kramer, Ragini Hari, Keith Shusterman, Chiltern When SDTM
PharmaSUG 2017 - Paper DS13 Planning to Pool SDTM by Creating and Maintaining a Sponsor-Specific Controlled Terminology Database ABSTRACT Cori Kramer, Ragini Hari, Keith Shusterman, Chiltern When SDTM
How Managers and Executives Can Leverage SAS Enterprise Guide
 Paper 8820-2016 How Managers and Executives Can Leverage SAS Enterprise Guide ABSTRACT Steven First and Jennifer First-Kluge, Systems Seminar Consultants, Inc. SAS Enterprise Guide is an extremely valuable
Paper 8820-2016 How Managers and Executives Can Leverage SAS Enterprise Guide ABSTRACT Steven First and Jennifer First-Kluge, Systems Seminar Consultants, Inc. SAS Enterprise Guide is an extremely valuable
Study Data Reviewer s Guide
 Revision History Date Study Data Reviewer s Guide Completion Guideline: Nonclinical (nnsdrg) Version Summary V1.1 03 March 2016 1.0 First Public Version: posted for Public Comment 1.1 Update from Public
Revision History Date Study Data Reviewer s Guide Completion Guideline: Nonclinical (nnsdrg) Version Summary V1.1 03 March 2016 1.0 First Public Version: posted for Public Comment 1.1 Update from Public
Automation of makefile For Use in Clinical Development Nalin Tikoo, BioMarin Pharmaceutical Inc., Novato, CA
 Automation of makefile For Use in Clinical Development Nalin Tikoo, BioMarin Pharmaceutical Inc., Novato, CA ABSTRACT The 'make' utility is a software engineering tool for managing and maintaining computer
Automation of makefile For Use in Clinical Development Nalin Tikoo, BioMarin Pharmaceutical Inc., Novato, CA ABSTRACT The 'make' utility is a software engineering tool for managing and maintaining computer
SAS Clinical Data Integration 2.6
 SAS Clinical Data Integration 2.6 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2015. SAS Clinical Data Integration 2.6: User's Guide.
SAS Clinical Data Integration 2.6 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2015. SAS Clinical Data Integration 2.6: User's Guide.
Material covered in the Dec 2014 FDA Binding Guidances
 Accenture Accelerated R&D Services Rethink Reshape Restructure for better patient outcomes Sandra Minjoe Senior ADaM Consultant Preparing ADaM and Related Files for Submission Presentation Focus Material
Accenture Accelerated R&D Services Rethink Reshape Restructure for better patient outcomes Sandra Minjoe Senior ADaM Consultant Preparing ADaM and Related Files for Submission Presentation Focus Material
PhUSE US Connect 2019
 PhUSE US Connect 2019 Paper SI04 Creation of ADaM Define.xml v2.0 Using SAS Program and Pinnacle 21 Yan Lei, Johnson & Johnson, Spring House, PA, USA Yongjiang Xu, Johnson & Johnson, Spring House, PA,
PhUSE US Connect 2019 Paper SI04 Creation of ADaM Define.xml v2.0 Using SAS Program and Pinnacle 21 Yan Lei, Johnson & Johnson, Spring House, PA, USA Yongjiang Xu, Johnson & Johnson, Spring House, PA,
Standardising The Standards The Benefits of Consistency
 Paper DH06 Standardising The Standards The Benefits of Consistency Nathan James, Roche Products Ltd., Welwyn Garden City, UK ABSTRACT The introduction of the Study Data Tabulation Model (SDTM) has had
Paper DH06 Standardising The Standards The Benefits of Consistency Nathan James, Roche Products Ltd., Welwyn Garden City, UK ABSTRACT The introduction of the Study Data Tabulation Model (SDTM) has had
PharmaSUG Paper CC22
 PharmaSUG 2011 - Paper CC22 Importing and Parsing Comments From a PDF Document With Help From Perl Regular Expressions Joel Campbell, PPD, Inc., Wilmington, NC Ryan Wilkins, PPD, Inc., Wilmington, NC ABSTRACT
PharmaSUG 2011 - Paper CC22 Importing and Parsing Comments From a PDF Document With Help From Perl Regular Expressions Joel Campbell, PPD, Inc., Wilmington, NC Ryan Wilkins, PPD, Inc., Wilmington, NC ABSTRACT
Doctor's Prescription to Re-engineer Process of Pinnacle 21 Community Version Friendly ADaM Development
 PharmaSUG 2018 - Paper DS-15 Doctor's Prescription to Re-engineer Process of Pinnacle 21 Community Version Friendly ADaM Development Aakar Shah, Pfizer Inc; Tracy Sherman, Ephicacy Consulting Group, Inc.
PharmaSUG 2018 - Paper DS-15 Doctor's Prescription to Re-engineer Process of Pinnacle 21 Community Version Friendly ADaM Development Aakar Shah, Pfizer Inc; Tracy Sherman, Ephicacy Consulting Group, Inc.
The Implementation of Display Auto-Generation with Analysis Results Metadata Driven Method
 PharmaSUG 2015 - Paper AD01 The Implementation of Display Auto-Generation with Analysis Results Metadata Driven Method Chengxin Li, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA ABSTRACT
PharmaSUG 2015 - Paper AD01 The Implementation of Display Auto-Generation with Analysis Results Metadata Driven Method Chengxin Li, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA ABSTRACT
Helping The Define.xml User
 Paper TT01 Helping The Define.xml User Dave Iberson-Hurst, Assero Limited, Teignmouth, United Kingdom ABSTRACT The FDA often comment at industry gatherings on the quality of define.xml files received as
Paper TT01 Helping The Define.xml User Dave Iberson-Hurst, Assero Limited, Teignmouth, United Kingdom ABSTRACT The FDA often comment at industry gatherings on the quality of define.xml files received as
The Submission Data File System Automating the Creation of CDISC SDTM and ADaM Datasets
 Paper AD-08 The Submission Data File System Automating the Creation of CDISC SDTM and ADaM Datasets Marcus Bloom, Amgen Inc, Thousand Oaks, CA David Edwards, Amgen Inc, Thousand Oaks, CA ABSTRACT From
Paper AD-08 The Submission Data File System Automating the Creation of CDISC SDTM and ADaM Datasets Marcus Bloom, Amgen Inc, Thousand Oaks, CA David Edwards, Amgen Inc, Thousand Oaks, CA ABSTRACT From
Advanced Visualization using TIBCO Spotfire and SAS
 PharmaSUG 2018 - Paper DV-04 ABSTRACT Advanced Visualization using TIBCO Spotfire and SAS Ajay Gupta, PPD, Morrisville, USA In Pharmaceuticals/CRO industries, you may receive requests from stakeholders
PharmaSUG 2018 - Paper DV-04 ABSTRACT Advanced Visualization using TIBCO Spotfire and SAS Ajay Gupta, PPD, Morrisville, USA In Pharmaceuticals/CRO industries, you may receive requests from stakeholders
PhUSE EU Connect Paper PP15. Stop Copying CDISC Standards. Craig Parry, SyneQuaNon, Diss, England
 Paper PP15 Abstract Stop Copying CDISC Standards Craig Parry, SyneQuaNon, Diss, England We repeatedly see repositories which require a large amount of front loading, a lot of duplicating of the Clinical
Paper PP15 Abstract Stop Copying CDISC Standards Craig Parry, SyneQuaNon, Diss, England We repeatedly see repositories which require a large amount of front loading, a lot of duplicating of the Clinical
Use of Traceability Chains in Study Data and Metadata for Regulatory Electronic Submission
 PharmaSUG 2017 - Paper SS03 Use of Traceability Chains in Study Data and Metadata for Regulatory Electronic Submission ABSTRACT Tianshu Li, Celldex Therapeutics, Hampton, NJ Traceability is one of the
PharmaSUG 2017 - Paper SS03 Use of Traceability Chains in Study Data and Metadata for Regulatory Electronic Submission ABSTRACT Tianshu Li, Celldex Therapeutics, Hampton, NJ Traceability is one of the
It s All About Getting the Source and Codelist Implementation Right for ADaM Define.xml v2.0
 PharmaSUG 2018 - Paper SS-15 It s All About Getting the Source and Codelist Implementation Right for ADaM Define.xml v2.0 ABSTRACT Supriya Davuluri, PPD, LLC, Morrisville, NC There are some obvious challenges
PharmaSUG 2018 - Paper SS-15 It s All About Getting the Source and Codelist Implementation Right for ADaM Define.xml v2.0 ABSTRACT Supriya Davuluri, PPD, LLC, Morrisville, NC There are some obvious challenges
Hanming Tu, Accenture, Berwyn, USA
 Hanming Tu, Accenture, Berwyn, USA Agenda Issue Statement Create Mapping Build Reusable Codes Define Repeatable Workflow Check compliance Conclusion Copyright 2016 Accenture. All rights reserved. 2 Issue
Hanming Tu, Accenture, Berwyn, USA Agenda Issue Statement Create Mapping Build Reusable Codes Define Repeatable Workflow Check compliance Conclusion Copyright 2016 Accenture. All rights reserved. 2 Issue
CDISC Standards and the Semantic Web
 CDISC Standards and the Semantic Web Dave Iberson-Hurst 12 th October 2015 PhUSE Annual Conference, Vienna 1 Abstract With the arrival of the FDA guidance on electronic submissions, CDISC SHARE and the
CDISC Standards and the Semantic Web Dave Iberson-Hurst 12 th October 2015 PhUSE Annual Conference, Vienna 1 Abstract With the arrival of the FDA guidance on electronic submissions, CDISC SHARE and the
SDTM Implementation Guide Clear as Mud: Strategies for Developing Consistent Company Standards
 Paper CD02 SDTM Implementation Guide Clear as Mud: Strategies for Developing Consistent Company Standards Brian Mabe, UCB Biosciences, Raleigh, USA ABSTRACT Many pharmaceutical companies are now entrenched
Paper CD02 SDTM Implementation Guide Clear as Mud: Strategies for Developing Consistent Company Standards Brian Mabe, UCB Biosciences, Raleigh, USA ABSTRACT Many pharmaceutical companies are now entrenched
PharmaSUG2014 Paper DS09
 PharmaSUG2014 Paper DS09 An ADaM Interim Dataset for Time-to-Event Analysis Needs Tom Santopoli, Accenture, Berwyn, PA Kim Minkalis, Accenture, Berwyn, PA Sandra Minjoe, Accenture, Berwyn, PA ABSTRACT
PharmaSUG2014 Paper DS09 An ADaM Interim Dataset for Time-to-Event Analysis Needs Tom Santopoli, Accenture, Berwyn, PA Kim Minkalis, Accenture, Berwyn, PA Sandra Minjoe, Accenture, Berwyn, PA ABSTRACT
A Taste of SDTM in Real Time
 A Taste of SDTM in Real Time Changhong Shi, Merck & Co., Inc., Rahway, NJ Beilei Xu, Merck & Co., Inc., Rahway, NJ ABSTRACT The Study Data Tabulation Model (SDTM) is a Clinical Data Interchange Standards
A Taste of SDTM in Real Time Changhong Shi, Merck & Co., Inc., Rahway, NJ Beilei Xu, Merck & Co., Inc., Rahway, NJ ABSTRACT The Study Data Tabulation Model (SDTM) is a Clinical Data Interchange Standards
The application of SDTM in a disease (oncology)-oriented organization
 Paper CD01 The application of SDTM in a disease (oncology)-oriented organization Angelo Tinazzi, Alessandro Cattaneo, Enrica Paschetto, Sonia Colombini SENDO-Tech S.r.l., Milan, Italy ABSTRACT Applying
Paper CD01 The application of SDTM in a disease (oncology)-oriented organization Angelo Tinazzi, Alessandro Cattaneo, Enrica Paschetto, Sonia Colombini SENDO-Tech S.r.l., Milan, Italy ABSTRACT Applying
SAS. IT Service Level Management 2.1: Migration Documentation
 SAS IT Service Level Management 2.1: Migration Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2005. SAS IT Service Level Management 2.1: Migration Documentation.
SAS IT Service Level Management 2.1: Migration Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2005. SAS IT Service Level Management 2.1: Migration Documentation.
CDASH MODEL 1.0 AND CDASHIG 2.0. Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams
 CDASH MODEL 1.0 AND CDASHIG 2.0 Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams 1 What is CDASH? Clinical Data Acquisition Standards Harmonization (CDASH) Standards for the collection
CDASH MODEL 1.0 AND CDASHIG 2.0 Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams 1 What is CDASH? Clinical Data Acquisition Standards Harmonization (CDASH) Standards for the collection
CDISC SDTM and ADaM Real World Issues
 CDISC SDTM and ADaM Real World Issues Washington DC CDISC Data Standards User Group Meeting Sy Truong President MXI, Meta-Xceed, Inc. http://www.meta-x.com Agenda CDISC SDTM and ADaM Fundamentals CDISC
CDISC SDTM and ADaM Real World Issues Washington DC CDISC Data Standards User Group Meeting Sy Truong President MXI, Meta-Xceed, Inc. http://www.meta-x.com Agenda CDISC SDTM and ADaM Fundamentals CDISC
Leveraging Study Data Reviewer s Guide (SDRG) in Building FDA s Confidence in Sponsor s Submitted Datasets
 PharmaSUG 2017 - Paper SS09 Leveraging Study Data Reviewer s Guide (SDRG) in Building FDA s Confidence in Sponsor s Submitted Datasets Xiangchen (Bob) Cui, Min Chen, and Letan (Cleo) Lin, Alkermes Inc.,
PharmaSUG 2017 - Paper SS09 Leveraging Study Data Reviewer s Guide (SDRG) in Building FDA s Confidence in Sponsor s Submitted Datasets Xiangchen (Bob) Cui, Min Chen, and Letan (Cleo) Lin, Alkermes Inc.,
Customizing SAS Data Integration Studio to Generate CDISC Compliant SDTM 3.1 Domains
 Paper AD17 Customizing SAS Data Integration Studio to Generate CDISC Compliant SDTM 3.1 Domains ABSTRACT Tatyana Kovtun, Bayer HealthCare Pharmaceuticals, Montville, NJ John Markle, Bayer HealthCare Pharmaceuticals,
Paper AD17 Customizing SAS Data Integration Studio to Generate CDISC Compliant SDTM 3.1 Domains ABSTRACT Tatyana Kovtun, Bayer HealthCare Pharmaceuticals, Montville, NJ John Markle, Bayer HealthCare Pharmaceuticals,
How to handle different versions of SDTM & DEFINE generation in a Single Study?
 Paper CD15 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai,
Paper CD15 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai,
Paper FC02. SDTM, Plus or Minus. Barry R. Cohen, Octagon Research Solutions, Wayne, PA
 Paper FC02 SDTM, Plus or Minus Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT The CDISC Study Data Tabulation Model (SDTM) has become the industry standard for the regulatory submission
Paper FC02 SDTM, Plus or Minus Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT The CDISC Study Data Tabulation Model (SDTM) has become the industry standard for the regulatory submission
XML in the DATA Step Michael Palmer, Zurich Biostatistics, Inc., Morristown, New Jersey
 Paper 25-28 XML in the DATA Step Michael Palmer, Zurich Biostatistics, Inc., Morristown, New Jersey ABSTRACT This paper discusses a DATA-step method to import, export, and transform user-defined XML vocabularies.
Paper 25-28 XML in the DATA Step Michael Palmer, Zurich Biostatistics, Inc., Morristown, New Jersey ABSTRACT This paper discusses a DATA-step method to import, export, and transform user-defined XML vocabularies.
How to review a CRF - A statistical programmer perspective
 Paper DH07 How to review a CRF - A statistical programmer perspective Elsa Lozachmeur, Novartis Pharma AG, Basel, Switzerland ABSTRACT The design of the Case Report Form (CRF) is critical for the capture
Paper DH07 How to review a CRF - A statistical programmer perspective Elsa Lozachmeur, Novartis Pharma AG, Basel, Switzerland ABSTRACT The design of the Case Report Form (CRF) is critical for the capture
Power Data Explorer (PDE) - Data Exploration in an All-In-One Dynamic Report Using SAS & EXCEL
 Power Data Explorer (PDE) - Data Exploration in an All-In-One Dynamic Report Using SAS & EXCEL ABSTRACT Harry Chen, Qian Zhao, Janssen R&D China Lisa Lyons, Janssen R&D US Getting to know your data is
Power Data Explorer (PDE) - Data Exploration in an All-In-One Dynamic Report Using SAS & EXCEL ABSTRACT Harry Chen, Qian Zhao, Janssen R&D China Lisa Lyons, Janssen R&D US Getting to know your data is
Taming Rave: How to control data collection standards?
 Paper DH08 Taming Rave: How to control data collection standards? Dimitri Kutsenko, Entimo AG, Berlin, Germany Table of Contents Introduction... 1 How to organize metadata... 2 How to structure metadata...
Paper DH08 Taming Rave: How to control data collection standards? Dimitri Kutsenko, Entimo AG, Berlin, Germany Table of Contents Introduction... 1 How to organize metadata... 2 How to structure metadata...
Managing your metadata efficiently - a structured way to organise and frontload your analysis and submission data
 Paper TS06 Managing your metadata efficiently - a structured way to organise and frontload your analysis and submission data Kirsten Walther Langendorf, Novo Nordisk A/S, Copenhagen, Denmark Mikkel Traun,
Paper TS06 Managing your metadata efficiently - a structured way to organise and frontload your analysis and submission data Kirsten Walther Langendorf, Novo Nordisk A/S, Copenhagen, Denmark Mikkel Traun,
Streamline SDTM Development and QC
 Paper SI09 Streamline SDTM Development and QC Stephen Gormley, Amgen, United Kingdom ABSTRACT Amgen s Global Statistical Programming ( GSP ) function have one centralised team (The CDISC Consultancy and
Paper SI09 Streamline SDTM Development and QC Stephen Gormley, Amgen, United Kingdom ABSTRACT Amgen s Global Statistical Programming ( GSP ) function have one centralised team (The CDISC Consultancy and
SDTM Attribute Checking Tool Ellen Xiao, Merck & Co., Inc., Rahway, NJ
 PharmaSUG2010 - Paper CC20 SDTM Attribute Checking Tool Ellen Xiao, Merck & Co., Inc., Rahway, NJ ABSTRACT Converting clinical data into CDISC SDTM format is a high priority of many pharmaceutical/biotech
PharmaSUG2010 - Paper CC20 SDTM Attribute Checking Tool Ellen Xiao, Merck & Co., Inc., Rahway, NJ ABSTRACT Converting clinical data into CDISC SDTM format is a high priority of many pharmaceutical/biotech
Best Practice for Creation and Maintenance of a SAS Infrastructure
 Paper 2501-2015 Best Practice for Creation and Maintenance of a SAS Infrastructure Paul Thomas, ASUP Ltd. ABSTRACT The advantage of using metadata to control and maintain data and access to data on databases,
Paper 2501-2015 Best Practice for Creation and Maintenance of a SAS Infrastructure Paul Thomas, ASUP Ltd. ABSTRACT The advantage of using metadata to control and maintain data and access to data on databases,
Once the data warehouse is assembled, its customers will likely
 Clinical Data Warehouse Development with Base SAS Software and Common Desktop Tools Patricia L. Gerend, Genentech, Inc., South San Francisco, California ABSTRACT By focusing on the information needed by
Clinical Data Warehouse Development with Base SAS Software and Common Desktop Tools Patricia L. Gerend, Genentech, Inc., South San Francisco, California ABSTRACT By focusing on the information needed by
Creating a Departmental Standard SAS Enterprise Guide Template
 Paper 1288-2017 Creating a Departmental Standard SAS Enterprise Guide Template ABSTRACT Amanda Pasch and Chris Koppenhafer, Kaiser Permanente This paper describes an ongoing effort to standardize and simplify
Paper 1288-2017 Creating a Departmental Standard SAS Enterprise Guide Template ABSTRACT Amanda Pasch and Chris Koppenhafer, Kaiser Permanente This paper describes an ongoing effort to standardize and simplify
PharmaSUG Paper PO10
 PharmaSUG 2013 - Paper PO10 How to make SAS Drug Development more efficient Xiaopeng Li, Celerion Inc., Lincoln, NE Chun Feng, Celerion Inc., Lincoln, NE Peng Chai, Celerion Inc., Lincoln, NE ABSTRACT
PharmaSUG 2013 - Paper PO10 How to make SAS Drug Development more efficient Xiaopeng Li, Celerion Inc., Lincoln, NE Chun Feng, Celerion Inc., Lincoln, NE Peng Chai, Celerion Inc., Lincoln, NE ABSTRACT
Data Integrity through DEFINE.PDF and DEFINE.XML
 Data Integrity through DEFINE.PDF and DEFINE.XML Sy Truong, Meta Xceed, Inc, Fremont, CA ABSTRACT One of the key questions asked in determining if an analysis dataset is valid is simply, what did you do
Data Integrity through DEFINE.PDF and DEFINE.XML Sy Truong, Meta Xceed, Inc, Fremont, CA ABSTRACT One of the key questions asked in determining if an analysis dataset is valid is simply, what did you do
Paper ###-YYYY. SAS Enterprise Guide: A Revolutionary Tool! Jennifer First, Systems Seminar Consultants, Madison, WI
 Paper ###-YYYY SAS Enterprise Guide: A Revolutionary Tool! Jennifer First, Systems Seminar Consultants, Madison, WI ABSTRACT Whether you are a novice or a pro with SAS, Enterprise Guide has something for
Paper ###-YYYY SAS Enterprise Guide: A Revolutionary Tool! Jennifer First, Systems Seminar Consultants, Madison, WI ABSTRACT Whether you are a novice or a pro with SAS, Enterprise Guide has something for
esubmission - Are you really Compliant?
 ABSTRACT PharmaSUG 2018 - Paper SS21 esubmission - Are you really Compliant? Majdoub Haloui, Merck & Co., Inc., Upper Gwynedd, PA, USA Suhas R. Sanjee, Merck & Co., Inc., Upper Gwynedd, PA, USA Pinnacle
ABSTRACT PharmaSUG 2018 - Paper SS21 esubmission - Are you really Compliant? Majdoub Haloui, Merck & Co., Inc., Upper Gwynedd, PA, USA Suhas R. Sanjee, Merck & Co., Inc., Upper Gwynedd, PA, USA Pinnacle
Journey to the center of the earth Deep understanding of SAS language processing mechanism Di Chen, SAS Beijing R&D, Beijing, China
 Journey to the center of the earth Deep understanding of SAS language processing Di Chen, SAS Beijing R&D, Beijing, China ABSTRACT SAS is a highly flexible and extensible programming language, and a rich
Journey to the center of the earth Deep understanding of SAS language processing Di Chen, SAS Beijing R&D, Beijing, China ABSTRACT SAS is a highly flexible and extensible programming language, and a rich
PharmaSUG Paper AD03
 PharmaSUG 2017 - Paper AD03 Three Issues and Corresponding Work-Around Solution for Generating Define.xml 2.0 Using Pinnacle 21 Enterprise Jeff Xia, Merck & Co., Inc., Rahway, NJ, USA Lugang (Larry) Xie,
PharmaSUG 2017 - Paper AD03 Three Issues and Corresponding Work-Around Solution for Generating Define.xml 2.0 Using Pinnacle 21 Enterprise Jeff Xia, Merck & Co., Inc., Rahway, NJ, USA Lugang (Larry) Xie,
Applications Development. Paper 38-28
 Paper 38-28 The California Template or How to keep from reinventing the wheel using SAS/IntrNet, JavaScript and process reengineering Blake R. Sanders, U.S. Census Bureau, Washington, DC ABSTRACT Creating
Paper 38-28 The California Template or How to keep from reinventing the wheel using SAS/IntrNet, JavaScript and process reengineering Blake R. Sanders, U.S. Census Bureau, Washington, DC ABSTRACT Creating
Creating Define-XML v2 with the SAS Clinical Standards Toolkit 1.6 Lex Jansen, SAS
 Creating Define-XML v2 with the SAS Clinical Standards Toolkit 1.6 Lex Jansen, SAS Agenda Introduction to the SAS Clinical Standards Toolkit (CST) Define-XML History and Background What is Define-XML?
Creating Define-XML v2 with the SAS Clinical Standards Toolkit 1.6 Lex Jansen, SAS Agenda Introduction to the SAS Clinical Standards Toolkit (CST) Define-XML History and Background What is Define-XML?
