Electronic Supplementary Material for PCCP This journal is The Owner Societies 2005 EXPERIMENTAL SECTION
|
|
|
- Carmella Peters
- 5 years ago
- Views:
Transcription
1 This journal is The wner Societies 00 EXPERIMETAL SECTI General Techniques: All reactions were carried out with dry, freshly distilled solvents under anhydrous conditions, unless otherwise stated. Tetrahydrofuran (TF) was distilled from sodium benzophenone, and methylene chloride (C) was distilled from calcium hydride. Acetonitrile was distilled over Ca and stored over Å molecular sieves. Yields refer to chromatographically and spectroscopically ( MR) homogeneous materials, unless otherwise stated. All reactions were monitored by thin layer chromatography (TLC) carried out on 0. mm E. Merck silica gel plates (0F ) using UV light ( nm). Merck silica gel (0 00 mesh) was used for flash chromatography. MR spectra were recorded on JEL JM-EX0 (0 Mz) or JEL Eclipse FT (00 Mz) instruments and calibrated using a solvent peak as an internal reference. The following abbreviations are used to indicate the multiplicities; s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. Mass spectra were obtained using Agilent 00 via Electron Spray Ionisation geometry.ir spectra were recorded on a Perkin Elmer Spectrum ne spectrophotometer and only typical absorptions were cited. GEERAL PRCEDURE FR TE IMIE-FRMATI: To a stirred solution of indole--carbaldehyde ( equiv.) in C was added the respective amine ( equiv.) and MgS (0. equiv.). The reaction mixture was refluxed. After filtration of the MgS and evaporation of the solvent, the imine was obtained with good yield (9-9 %). If necessary, crystallisation in ethanol is possible. Compounds a, b have been described before in : P. Kumar, C. ath, K.P. Bhargava, K. Shanker, Indian Journal of Chem., 98, B, 8. A. Alemany, E. Fernandez Alvarez, J.M. Martinez-Lopez, Bull. Soc. Chim. France, 9, -,. Y. Shimoji, T. ashimoto, Y. Furukawa,. Yanagisawa, eterocycles, 99,,. a a -MR (0 Mz, CD, ppm): δ =.8 (, s, C (Ph)),.-. (0, m, C, C, C, C, C, x C(Ph)), 8. (, ~d, C=), 8. (, s, ); C-MR (8 Mz, CD, ppm): δ =. (C (Ph)),.8 (C ),.99 (C ),.9 (C ),. (C ),.0 (C a ),.0 (C ), 8.0 (x C(Ph)), 8.9 (C(Ph)), 8. (x C(Ph)), 9. (C quat (Ph)),.9 (C ), 0. (C a ),. (C=); IR (KBr, cm - ): (ν ); MS (0eV): m/z = (M + ); Mp.:. C. b a a -MR (00 Mz, CD, ppm): δ =.9 (, d, J =. z, x C (ipr)),.0 (, sept, J =. z, C(iPr)),.8-. (, m, C, C ),. (, ~d, J = 8. z, C or C ),. (, s, C ), 8.0 (, d, J =. z, C or C ), 8. (, s, (Ind)C=), not visible; C-MR (, Mz, CD, ppm):.8 (x C (ipr)),.90 (C(iPr)),. (C or C ),.9 (C ), 0.98 (C or C or C or C ),.0 (C or C or C or C ),.98 (C or C ),.90 (C a ),.8 (C ),.0 (C a ),.8 ((Ind)C=); IR (KBr, cm - ): 9 (ν C= ),,, 9, 8; MS (ESI): m/z = 8 (M + +); Mp.:. C.
2 This journal is The wner Societies 00 c (Et) P.0 C. a -MR (0 Mz, CD, ppm): δ =. (, t, J =. z, P()C C ),.0 (, d, J P =. z, C P),.8 (, m, P()C C ),.0 (, s, C ),.0 (, m, C, C ),. (, dd, J P =.8 z J =. z, C ), 8.09 (, d, J =. z, C ), 8. (, d, J =. z, C=), 0.00 (, s, ); C-MR (8 Mz, CD, ppm): δ =. (C, J CP =. z, P()C C ), 8. (J CP =.8 z, C P),.8 (C, J CP =. z, P()C C ),.8 (C ),.0 (d, J CP =. z C ),.0 (C ),.9 (C ),.8 (C ),.9 (C a ),.9 (C ),.8 (C a ), 0.9 (J CP =. z, C=); P-MR (09 Mz, CD, ppm): δ =.8; IR (KBr, cm - ): 0 (ν ), 9 (ν C= ); MS (0eV): m/z = 9 (M + ); Mp.: Preparation of d-g. To a stirred solution of indole--carbaldehyde (0.8 g, equiv.) in acetonitrile (0 ml) was added diëthyl amino(phenyl)methylphosphonate (0.98 g, equiv.) and 0. g (0. equiv.) MgS. The reaction mixture was refluxed for days. After filtration of the MgS and evaporation of the solvent, diëthyl (phenyl){[(e)--indole--ylmethylideen]amino} methylphosphonate was obtained with a yield of 98 %. Crystallisation in ethanol afforded yellow crystals with 8% yield. d a (Et) P -MR (0 Mz, CD, ppm): δ =.9 (, t, J =. z, P()C C ),. (, t, J =.9 z, P()C C ),.09 (, m, P()C C ),.8 (, d, J P =.8 z, CP),.09 (, s, C ),.-. (, m, C, C, C, C (Ph), C (Ph), C (Ph)),.8 (, d, J =.9 z, C (Ph), C (Ph)), 8. (, d, J =. z, C=), 8. (, d, J =. z, C ), 0.0 (, s, ); C-MR (8 Mz, CD, ppm): δ =. (C, J CP =.9 z, P()C C ),. (J CP =. z, P()C C ),. (J CP =. z, P()C C ),.8 (J CP =. z, CP),.9 (C ),. (C ),. (C ),.9 (C ),.8 (C ),.8 (C a ),. (J CP =. z, C(Ph)), 8. (C, J CP =.9 z, C(Ph)), 8. (C, J CP =.9 z, C(Ph)),.8 (C ),. (d, J CP =. z, C quat (Ph)),. (C a ), 9. (J CP =.8 z, C=); P-MR (09 Mz, CD, ppm): δ =.9; IR (KBr, cm - ): 0 (ν ), 9 (ν C= ); MS (0eV): m/z = (M + ); Mp.: 0. C. e a (Et) P F -MR (0 Mz, CD, ppm): δ =. (, t, J =.9 z, P()C C ),. (, t, J =. z, P()C C ),. (, m, P()C C ),.8 (, d, J P =. z, CP),.0 (, m, C, x C(Ph)),. (, m, C, C ),. (, dd, J =. z, J =.8 z, C ),. (, m, x C(Ph)), 8. (, d, J P =. z, C=), 8. (, dd, J =. z, J =.8 z, C ), 0. (, s, ); C-MR (8 Mz, CD, ppm): δ =.8 (C, J CP =. z, P()C C ),.09 (J CP =. z, P()C C ),.0 (J CP =. z, P()C C ),.0 (J CP =. z, CP),.88 (C ),. (J CP =. z, C ),. (C, d, J CF =. z, C(Ph)),.0 (C ),.90 (C ),.98 (C ),.8 (C a ), 9.88 (C, t, J CF =. z, C(Ph)),. (C ),. (dd, J =. z, J =. z, C quat (Ph)),. (C a ), 9. (J CP =.9 z, C=),. (dd, J CF =. z, J CP =. z, CF), P-MR (09 Mz, CD, ppm): δ = 0.; IR (KBr, cm - ): 8 (ν ), 9 (ν C= ); MS (0eV): m/z = 88 (M + ); Mp.:.8 C.
3 This journal is The wner Societies 00 f -MR (0 Mz, CD, ppm): δ =.8 (, t, J =. z, P()C C ),. (, t, J =. z, P()C C ),. (, m, P()C C ),. (, d, J P = 8. z, CP),.0 (, s, C ),. (, m, C, C ),.9 (, m, C, x C(Ph)), 8. (, d, J =. z, C=), 8. (, m, C ), 0.0 (, s, ); C- MR (8 Mz, CD, ppm): δ =.8 (C, t, J CP =. z, P()C C ),. (J CP = 8. z, P()C C ),.08 (J CP =. z, P()C C ),.8 (J CP =.8 z, CP),.88 (C ),. (J CP =. z, C ),. (C ),.0 (d, J CP =.9 z, CBr),.9 (C ),.0 (C ),. (C a ), 0.00 (C, J CP =.9 z, C(Ph)),. (C, J CP =. z, C(Ph)),. (C ),. (d, J = 8. z, C quat (Ph)),. (C a ), 9. (J CP =.9 z, C=); P-MR (09 Mz, CD, ppm): δ = 0.; IR (KBr, cm - ):. C. g (ν ), (ν C= ); MS (0eV): m/z = /9 (M + ); Mp.: -MR (0 Mz, CD, ppm): δ =.9 (, t, J =.8 z, P()C C ),. (, t, J =. z, P()C C ),.80 (, s, C ),.9 (, m, P()C C ),.8 (, d, J P =. z, CP),.9 (, d, J = 8. z, C(Ph)),.0 (, s, C ),. (, m, C, C ),. (, C ),. (, m, C(Ph)), 8. (, t, J =. z, C=), 8. (, m, C ), 0. (, s, ); C-MR (8 Mz, CD, ppm): δ =. (C, P()C C ),. (C ). (J CP =. z, P()C C ),.9 (J CP =.8 z, P()C C ),.8 (J CP =.0 z, CP),.00 (C ),.8 (C, C(Ph)),.8 (C ),. (C ),.0 (C ),.0 (C ),. (C a ), 9. (C a ), 9. (CMe), 9. (J CP =. z, C=); P-MR (09 Mz, CD, ppm): δ =.90; IR (KBr, cm - ): 8 (ν ), 9 (ν P= ); MS (0eV): m/z = 0. (M + ); Mp.:. C. Summary a (Et) P a (Et) P Br Me Compound Reaction Yield (%) Compound Reaction Yield (%) Time (hours) Time(days) a 0 9 d 8 b 8 88 e 8 c f g 88
4 This journal is The wner Societies 00 GEERAL PRCEDURE FR TE AMIE-FRMATI: The imine from the previous section was dissolved in absolute ethanol and equiv. ab was added. After stirring at room temperature h, the mixture was quenched by the addition of 0. M a and stirred for 0 minutes. ext, the mixture was poured in dichloromethane and washed three times with 0. M a. After filtration of the MgS and evaporation of the solvent, the amine was obtained in good yields. a a -MR (0 Mz, CD, ppm): δ =.80 (, bs, ),.8 (, s, (C (Ph)),.00 (, s, (Ind)C ),.0 (, s, C ),.-. (8, m, C, C, C, x C(Ph)),. (, d, J =. z, C ), 8. (, bs, (ind)); C-MR (8 Mz, CD, ppm): δ =. (C (Ph)),. ((Ind)C ),. (C ),.9 (C ), 8.9 (C ), 9. (C ),. (C ),. (C ),. (C a ), 8. (C(Ph)), 8. (C, x C(Ph)), 8. (C, x C(Ph)),. (C a ), 0. (C quat (Ph)); IR (KBr, cm - ): (ν ); MS (0eV): m/z = (M + ); Mp.:. C. b a a -MR (00 Mz, CD, ppm):. (, d, J =, z, x C (ipr)),.8 (, bs, ),.9 (, sept, J =. z, C(iPr)),.98 (, s, (Ind)C ),.9 (, s, C ),. (, dxd, J = J =. z, C or C ),. z (, ~t, J = J =. z, C or C ),. (, d, J = 8. z, C or C ),. (, d, J =. z, C or C ), 8.98 (, bs, (Ind)); C-MR (. Mz, CD, ppm):.8 (x C (ipr)),. ((Ind)C ), 8.8 (C(iPr)),. (C or C ),.0 (C ), 8. (C or C ), 9.9 (C or C ),.8 (C or C ),.0 (C ),.9 (C a or C a ),. (C a or C a ); IR (a, cm-): (ν ),, 0; MS (ESI): m/z = 90 (M++), (Ind-C + +). c (Et) P a -MR (0 Mz, CD, ppm): δ =.9 (, t, J =.9 z, P()C C ),. (, s, ),.0 (, d, J P =. z, C P),. (, m, P()C C, (Ind)C ),.0 (, d, J =.0 z, C ),. (, m, C, C ),. (, d,.9 z, C ),. (, d, J =.9 z, C ), 8.9 (, s, (Ind)); C-MR (8 Mz, CD, ppm): δ =. (C, J CP =.9 z, P()C C ),.8 (J CP =.8 z, C P),.8 (J CP =.9 z, (Ind)C ),. (C, J CP =. z, P()C C ),. (C ),.9 (C ), 8. (C ), 9. (C ),.9 (C ),.0 (C ),.9 (C a ),. (C a ), P-MR (09 Mz, CD, ppm): δ =.8; IR (a, cm - ): (ν P= ), (ν ) ; MS (0eV): m/z = (M + ). Preparation of d-g. To a stirred solution of diëthyl (phenyl){[(e)--indole--ylmethylideen] amino} methylphosphonate d in ethanol (0 ml) was added at 0 C 0. g (. equiv.) ab. After days at room temperature the reaction mixture was quenched with 0 ml of 0. M a. The reaction mixture was poured in C (0 ml).the organic phase was washed three times with 0 ml 0. M a and dried over anhydrous MgS. After filtration and evaporation of the solvent the crude residue was further purified by flash chromatography (EtAc/PE/triethylamine: 89/0/, Rf= 0.0, yield= %).
5 This journal is The wner Societies 00 d a (Et) P -MR (00 Mz, CD, ppm): δ =. (, t, J =.0 z, P()C C ),. (, t, J =.0 z, P()C C ),. (, s, ),. (, d, J AB =. z, (Ind)C ),.98 (, m, P()C C ),.0 (, d, J AB =. z, (Ind)C ),. (, d, J P = 9.8 z, CP),.9 (, d, J =. z, C ),.0 (, dxdxd, J = J =. z, J =.0 z, C ),. (, dxdxd, J = J =. z, J =. z, C ),.8 (, m, C, x C(Ph)),. (, m, x C(Ph)),. (, d,. z, C ), 9.9 (, s, (Ind)); C-MR (. Mz, CD, ppm): δ =. (J CP =. z, P()C C ),. (J CP =. z, P()C C ),. (J CP = 8. z, (Ind)C ), 9. (J CP =. z, CP),.98 (J CP =. z, P()C C ),.0 (J CP =.9 z, P()C C ),. (C ),. (C ), 9.00 (C ), 9. (C ),.8 (C ),. (C ),. (C a ), 8.08 (C (Ph)), 8. (C (Ph), C (Ph)), 8.9 (C, J CP =. z, C (Ph), C (Ph)),.9 (C a ),.9 (C (Ph)); P-MR (. Mz, CD, ppm): δ =.; IR (a, cm - ): 09 (ν ), 9 (ν ), 0 (ν P= ); MS (0eV): m/z = (M + ). e a (Et) P F -MR (0 Mz, CD, ppm): δ =. (, t, J =.0 z, P()C C ),. (, t, J =.0 z, P()C C ),. (, s, ),. (, d, J AB =. z, (Ind)C ),.0 (, m, P()C C ),.0 (, d, J AB =. z, (Ind)C ),. (, d, J P = 9. z, CP),.9 (, d, J =. z, C ),.0 (, m, x C(Ph), C ),. (, dxd, J = J =.0 z, C ),. (, d, J = 8, z, C ),. (, m, C(Ph)),.8 (, d, J =. z, C ), 8.9 (, s, (Ind)); C-MR (. Mz, CD, ppm): δ =. (C, t, J CP =.8 z, P()C C ),. (J CP =.9 z, (Ind)C ), 8.9 (J CP =.0 z, CP),.9 (J CP =. z, P()C C ),. (J CP =. z, P()C C ),.0 (C ),. (C ),.0 (C, d, J CF = 0.8 z, C(Ph)), 0. (x C(Ph)),. (C a ),.9 (C q (Ph)),. (d, J CP =. z, CF); P-MR (09 Mz, CD, ppm): δ =.; IR (KBr, cm - ): (ν P= ), (ν ); MS (0eV): m/z = 9 (M + ), Rf (ethylacetate) = 0.0. f a (Et) P Br -MR (0 Mz, CD, ppm): δ =. (, t, J =. z, P()C C ),. (, t, J =. z, P()C C ),. (, s, ),. (, d, J AB =. z, (Ind)C ),.9 (, m, P()C C ),.99 (, d, J AB =. z, (Ind)C ),. (, d, J P = 9.8 z, CP),.9 (, d, J =.9 z, C ),.09 (, dxd, J = J =. z, C ),.8 (, dxd, J = J =. z, C ),.0 (, m, C x C(Ph)),.8 (, d, J =. z, C ), 8. (, s, (Ind)); C-MR (8 Mz, CD, ppm): δ =. (C, t, J CP =. z, P()C C ),. (J CP =.9 z, (Ind)C ), 9. (J CP =.9 z, CP),.00 (J CP =.9 z, P()C C ),.8 (J CP =. z, P()C C ),. (C ),. (C ), 9.0 (C ), 9. (C ),.9 (CBr),. (C ),. (C ),.0 (C a ), 0. (d, J CP =. z, x C(Ph)),.8 (x C(Ph)),. (C a ),. (C q (Ph)); P-MR (09 Mz, CD, ppm): δ =.; IR (KBr, cm - ): (ν P= ), (ν ); MS (0eV): m/z = (M + ), Rf (ethylacetate/petrolether 9/) = 0.0.
6 This journal is The wner Societies 00 g -MR (0 Mz, CD, ppm): δ =. (, t, J =. z, P()C C ),. (, t, J =. z, P()C C ),. (, s, ),. (, d, J AB =. z, (Ind)C ),.8 (, s, C ),.00 (, d, J AB =. z, (Ind)C ),.0 (, m, P()C C ),.0 (, d, J P = 9. z, CP),.9 (, d, J = 8. z, C(Ph)),.9 (, d, J =. z, C ),.08 (, dxdxd, J = J =. z, J =. z, C ),.8 (, dxdxd, J = J =. z, J =. z C ),. (, d, J = 8.0 z, C ),.9 (, dd, J P = 8.8 z, J =. z, x C(Ph)),.9 (, d, J = 8.0 z, C ), 8.80 (, s, (Ind)); C-MR (8 Mz, CD, ppm): δ =.8 (d, J CP =.8 z, P()C C ),. (d, J CP =.8 z, P()C C ),. (J CP = 8. z, (Ind)C ),. (C ), 8.99 (J CP =. z, CP),.8 (J CP =.9 z, P()C C ),.0 (J CP =. z, P()C C ),. (C ),. (C ),.00 (x C(Ph)), 9.08 (C ), 9. (C ),.98 (C ),. (C ),. (C a ),. (C q (Ph)), 9.9 (C, J CP =.8 z, x C(Ph)),. (C a ), 9.0 (C(Ph)Me); P-MR (09 Mz, CD, ppm): δ =.9; IR (a, cm - ): 8 (ν P= ), (ν ); MS (0eV): m/z = 0 (M + ), Rf (ethyl acetate/petrolether /) = 0.. Summary a (Et) P Me Compound Yield (%) Compound Yield (%) a 9 d b 9 e c 80 f g
7 This journal is The wner Societies 00 GEERAL PRCEDURE FR TE ACYLATI: To a stirred solution of the respective amine and equiv. of pyridine, equiv. trichloroacetyl chloride was added dropwise at 0 C and under nitrogen atmosphere. The reaction mixture was carefully protected from the light and stirred for to 8 h at room temperature under a nitrogen atmosphere. The reaction mixture was poured in M a and extracted three times with C. The combined organic fractions were dried over MgS. The crude mixture was further purified through filtration over a small ( cm) silica column using petroleumether/ ethyl acetate. Because of the photo lability of the compound, it is important to protect the compound as much as possible from the light. The spectrum shows the existance of rotamers. The mixture consist of % rotamer A and % rotamer B. -MR (00 Mz, CD, ppm): δ =. (, s, C (Ph), rot B),. (, s, (Ind)C, rot A),.8 (, s, C (Ph), rot A),.08 (, s, (Ind)C, rot B),.0 (, s, C, rot A),.0 (, s, C, rot B), a.-.0 (8 (rot A), 8 (rot B), m, C, C, C or C, x C(Ph), rot A, rot B),.0 (, ~d, J =. z, C or C, rot A, rot B), 8. (, bs,, rot A), 8.8 (, bs,, rot B); a C-MR (. Mz, CD, ppm): δ =. ((Ind)C, rot A),. ((Ind)C, rot B), 0.0 (C (Ph)), rot B),. (C (Ph)), rot A), 9.8 (C, rot A, rot B), 0.0 (C, rot B), 0.0 (C, rot A),. (C or C, rot A),.8 (C or C, rot B), 9.09 (C or C, rot B), 9.0 (C or C, rot A), 0. (C or C, rot A, rot B),.9 (C or C, rot A, rot B),. (C, rot B),.0 (C, rot A),.0 (C a ),. (C, x C(Ph)),.98 (C, x C(Ph)), 9.0 (C(Ph)),. (C quat(ph), rot A, rot B),. (C a, rot A),. (C a, rot B),. (C=, rot A, rot B); IR (KBr, cm - ): 0(ν ), (ν C= ), 9,, ; MS (ESI): m/z = 0 (Ind-C + ); Mp.:. C. b a a C -MR (00 Mz, CD, ppm):. (, d, J =. z, x C (ipr)),. (, s, (Ind)C ), (, m, C(iPr)),.-. (, m, C, C, C ),. (, d, J =. z, C or C ),. (, d, J = 8. z, C or C ), 8.09 (, bs, (Ind)); C-MR (. Mz, CD, ppm): 0. (x C (ipr)), 8.0 ((Ind)C ), 0.8 (C(iPr)), 9.99 (C ),.8 (C or C ),. (C ), 8.8 (C or C ), 9.,.99,. (C, C, C ),.8 (C a or C a ),. (C a or C a ), 0. (C=); IR (KBr, cm-): 9 (ν ), (ν C= ); MS (ESI): m/z = (Ind-C + +); Mp.:.8 C. c a (Et) P C -MR (0 Mz, CD, ppm): δ =. (, t, J =. z, P()C C ),. (, t, J =.9 z, P()C C ),.8 (, d, J P =. z, C P),. (, m, P()C C ),. (, s, (Ind)C ),. (, dxd, J = J =. z, C ),. (, m, C, C ),. (, d, J =.9 z, C ),. (, d, J =.9 z, C ), 9.0 (, s, (Ind)); C-MR (8 Mz, CD, ppm): δ =. (C, J CP =. z, P()C C ),.8 (J CP =.0 z, C P),.90 ((Ind)C ),. (C, J CP =. z, P()C C ), 9.0 (C ), 08.8 (C ),.8 (C ), 8.8 (C ), 0. (C ),. (C ),. (C ),. (C a ),. (C a ), 0. (C=); P-MR (09 Mz, CD, ppm): δ =.00; IR (a, cm - ): (ν P= ), (ν ), (ν C= ), ; MS (0eV): m/z = 0. (Ind-C + ).
8 This journal is The wner Societies 00 Preparation of d. To a stirred solution of 0. g diëthyl (phenyl)[(-indole--ylmethyl)amino]methylphosphonate d and pyridine (0.8 g, equiv.) in 0 ml TF, was added trichloroacetyl chloride (0. g, equiv.) dropwise at 0 C and under a nitrogen atmosphere. The reaction mixture was carefully protected from the light and stirred for exactly hours at room temperature under a nitrogen atmosphere. An excess of Et was added to the reaction mixture and stirred for 0 minutes at 0 C. The salts were filtered over celite and washed with Et. The organic layer was washed three times with water (0 ml), dried over MgS, filtered, and concentrated under reduced pressure to afford ( %) as a bright yellow oil. The residue has a purity > 9 %. Because of the photo lability it is important to protect the reaction mixture as much as possible from the light. -MR (00 Mz, CD, ppm):.08 (, t, J =.0 z, (Et) P P()C C ),.0 (, t, J =. z, P()C C ),.-.90 (, m, P()C C ),. (, q, J =. z, P()C C ),. (, d, J AB =. z, (Ind)C ),.89 (, d, J -P =. z, CP),.8 C a (, dxd, J AB =. z, J =. z, (Ind)C ),.0 (, dxd, J = J =. z, C or C ),.0 (, dxd, J = J =. z, C or C ),.-.9 (, m, x C(Ph)),. (, ~ t, C, C ),.9 (, s, d C ), 9. (, bs, (ind)); C-MR (. Mz, CD, ppm): δ =. (J CP =.9 z, P()C C ),.8 (J CP =.8 z, P()C C ),.8 ((Ind)C ),. (J CP =. z, P()C C ),),.8 (J CP = 9. z, CP),. (J CP =. z, P()C C ), 9.0 (C ), 08.9 (C ),.9 (C or C ), 8.0 (C or C ), 9.0 (C or C ),. (C or C ),. (C ),.8 (C a ), 8.9 (C(Ph)), 8. (C(Ph)), 8. (C(Ph)), 8.8 (C, x C(Ph)),. (C quat (Ph)),. (C a ),. (C=); P-MR (. Mz, CD, ppm): δ = 0.; IR (a, cm - ): (ν ), 90, 8(ν C= ), (ν P= ), 09 (ν P- ); MS (ESI): m/z = 0 (Ind-C + ). Summary Compound Reaction Yield (%) Time (hours) a 8-9 b c 8 d 8
9 This journal is The wner Societies 00 GEERAL PRCEDURE FR TE ALGE TRASFER RADICAL CYCLIZATI: A mixture of the trichloroacetyl amino-derivative ( equiv.) and TMEDA (0.8 equiv.) was dissolved in C and flushed with argon for minutes. Cu(I) (0. equiv.) was added and the reaction mixture was stirred for hours at room temperature under argon atmosphere and finally quenched with water and stirred for 0 minutes. The organic phase was washed with water until the blue colour of the wash water disappeared. After drying over MgS, filtration and evaporation of the solvent, the spiro compound was obtained. By adding chloroform/diethylether or ethanol it was sometimes possible to obtain the compount as an amorf (more pure) precipitate. -MR (00 Mz, CD, ppm): δ =. (, d, J AB = 0. z, (Ind)C ),.9 (, d, J AB = 0. z, (Ind)C ),.9 (, d, J AB =. z, C (Ph)),. (, d, J AB =. z, C (Ph)),.0-. (8, m, C, C, C or C, x C Ph)),. (, dxd, J = 8. z, J =. z, C or C ),.98 (, s, C ); C-MR (. Mz, CD, ppm): δ = 8.09 a ((Ind)C ), 8.8 (C (Ph)),. (C ), 8. (C ),. (C or C ), {.,., 8., 0. (C, C, C(Ph), C or C )}, 0a 8. (C, x C(Ph)), 9. (C, x C(Ph)),. (C a ),. (C quat, (Ph)),. (C a ),.8 (C=), 9.0 (C ); IR (a, cm - ): 9(ν =C(Ind) ), (ν C= ), 8,, 8; MS (ESI): m/z = //9 (M+ + ). -MR (00 Mz, CD, ppm):. (, d, J =. z, C (ipr)),. (, d, J =. z, C (ipr)),. (, d, J AB = 9.9 z, (Ind)C )),. (, d, J AB = 0. z, (Ind)C )),. (, sept, J =. z, C(iPr)),. (, dxdxd, J = J =. z, J = 0.8 z, C or C ),.0 (, dxdxd, J = J =. z, J =. z, C or a C ),. (, d, J =. z, C or C ),.0 (, d, J =. z, C or C ), 8. (, s, C ); C-MR (. Mz, CD, ppm): 9. (C (ipr)), 9. (C (ipr)),.0 ((Ind)C ),.9 (C(iPr)),.8 (C ), 8.9 (C ),.8 0b (C or C ),.9 (C or C ),. (C or C ), 0. (C or C ),.0 (C a ),. (C a ),.8 (C=), 9.0 (C ); IR (a, cm - ): (ν =C(Ind) ), (ν C= ); MS (ESI): m/z = 9/99/0 (M + +). -MR (00 Mz, CD, ppm):. (, t, J =.9 z, P()C C ), (Et) P. (, t, J =.0 z, P()C C ),.8 (, s, (Ind)C ),.8 (, dd, J P =.8 z, J =. z, C P),.9 (, dd, J P =.8 z, J =. z, C P),. (, m, P()C C ),. (, dxd, J =J =. z, a C ),.8 (, dxdxd, J = J =. z, J =. z, C ),.8 (, d, J =. z, C ),.8 (, d, J =. z, C ), 8. (, s, C ); C-MR (8 0c Mz, CD, ppm): δ =. (J CP =. z, P()C C ),.8 (J CP =. z, P()C C ), 9.8 (J CP =.8 z, C P), 9. ((Ind)C ),.0 (J CP =. z, P()C C ),. (J CP =. z, P()C C ), 8.0 (C ), 8. (C ),. (C ),.8 (C ),. (C ), 0. (C ),. (C a ),.89 (C a ),. (C=), 8.8 (C ); P-MR (09 Mz, CD, ppm): δ = 9.8; IR (a, cm - ): (ν P= ), (ν C= ), (ν ); MS (0eV): m/z = 09. 9
10 This journal is The wner Societies 00 -MR (00 Mz, CD, ppm):.-. (, m, P()C C ),.9 (, ~t, (Ind)C ),.9-. (, m, (Ind)C, P()C C ),.9 (, d, J P = 0. z, CP, m),.80 (, d, J -P = 0.9 z, CP, M), (Et) P.-. (9, C, C, C, C, x C(Ph)), 8. (, s, C, M), 8.8 (, ~dxd, C, m); C-MR (. Mz, CD, ppm):.8 (J CP =.8 z, P()C C ),.9 (J CP =.9 z, P()C C ),. ((Ind)C, a m),.90 ((Ind)C, M),.9 (J CP = 8. z, CP),.98 (J CP =.9 z, P()C C, M ),.0 (J CP =.9 z, P()C C, m ),.9 (J CP =.9 0d z, P()C C, M),.0 (J CP =.9 z, P()C C, m),.9 (C ), 8. (C ), {.99,.0,.9, 0.9 (C, C, C and C )}, 9. (C, x C(Ph)), 9.8 (C, x C(Ph)), 9.89 (C(Ph)),.8 (C a or C quat (Ph)),.9 (C a or C quat (Ph)),.80 (C a ),. (C=), 8.8 (C, m), 8.9 (C, M); P-MR (. Mz, CD, ppm): δ = 8. (M), 8.0 (m); IR (a, cm - ): 80(ν ), 98, (ν C= ), (ν P= ), 09 (ν P- ) ; MS (ESI): m/z = 80/8/8 (M+ + ),. Compound Yield (%) Purity (%) 0a 9 8 0b c 9 8 0d 8 GEERAL PRCEDURE FR TE REDUCTI F TE SPIR-IMIE: To a mixture of the previous spiro-imine 0 and. equiv. of glacial acetic acid in methanol was added equiv. of acb. The reaction mixture was stirred for h at room temperature, poured in 0. M a and extracted three times with dichloromethane. After drying over MgS the compound was obtained with moderate purity. a a -MR (00 Mz, CD, ppm): δ =. (, d, J AB = 9.8 z, C ),.0 (, d, J AB = 9.8 z, C ),. (, d, J AB = 0. z, (Ind)C ),.80 (, bs, ),.09 (, d, J AB = 0. z, (Ind)C ),.9 (, d, J AB =. z, C (Ph)),.8 (, d, J AB =. z, C (Ph)),.-.9 (, m, C, C ),.9 (, dxd, J =.8 z, J =. z, C ),. (, dxdxd, J = J =. z, J =.z, C ),.-. (, m, x C(Ph)); C-MR (. Mz, CD, ppm): δ = 8. (C (Ph)),. ((Ind)C ),.0 (C ), 9. (C ), (C ), 0.8 (C ), 9.0 (C ),.80 (C ), 8.9 (C a ), 8. (C(Ph)), 8.8 (C, x C(Ph)), 9. (C, x C(Ph)), 0.0 (C ),.8 (C quat, (Ph)),.9 (C a ),. (C=); IR (a, cm - ): 8(ν ), 0 (ν C= ), 8,, 8; MS (ESI): m/z = /9/ (M+ + ). 0
11 This journal is The wner Societies 00 c (Et) P a -MR (0 Mz, CD, ppm):. (, t, J =.0 z, P()C C ),. (, t, J =. z, P()C C ),.8 (, d, J = 0. z, (Ind)C ),.8 (, dd, J P =. z, J = 0.9 z, C P),.9 (, d, J = 0.0 z, C ),.8 (, d, J = 0. z, C ),.9 (, dd, J P =.0 z, J =. z, C P),.0 (,dt, J = 0. z, (Ind)C ),. (, m, P()C C ),.8 (, m, C, C ),.98 (, dxd, J = 8.0 z, J =. z, C ),. (, dxdxd, J = J =. z, J =. z, C ); C-MR (8 Mz, CD, ppm): δ =. (J CP =.8 z, P()C C ),. (J CP =.8 z, P()C C ), 9.0 (J CP =. z, C P),. ((Ind)C ),. (C ), 9.8 (C ),.8 (J CP =. z, P()C C ),.0 (J CP =.8 z, P()C C ), 8.90 (C ), 0. (C ), 9.0 (C ),.99 (C ), 8. (C a ), 0.0 (C ),. (C a ),.8(C=); P-MR (09 Mz, CD, ppm): δ = 0.; IR (a, cm - ): 0 (ν C= ), (ν ); MS (0eV): m/z =. Compound Yield (%) Purity (%) a 88 c 90 Procedure for the synthesis of the Boc-derivate g (. mmol) of a was dissolved together with. g (.9 mmol,. equiv.) Boc and 0.0 g (0. mmol, 0. equiv.) DMAP in acetonitrile. The reaction was stirred for h at room temperature. The solvent was then concentrated in vacuo and the mixture was again dissolved in C. The organic layer was washed times with M a. After drying over MgS and evaporating the solvent, the crude mixture was further purified through filtration over a small silica column ( cm) with ethylacetate/petroleum ether (/) as solvent. The compound was obtained as a yellow powder (9 %). The spectrum shows the existance of rotamers. -MR (00 Mz, CD, ppm): δ =. (9, bs, C(C ) ),. (, bs, (Ind)C ),.9 (,~ d, C (Ph)),.-. (9, m, C, C or C, C, C, x C(Ph)), 8. (, d, J =.9 z, (C or a C ); C-MR (. Mz, CD, ppm): δ = 8.08 (C, C(C ) ), C.8 ((Ind)C ),. (C (Ph)), 8. (CC ), 9. (C ), xx. (C ),. (C or C ), 9.9 (C or C ), {.8,.,.,.0,.8, 8.8,.99,. (C, C, C, C a, C a, x C(Ph), x C(Ph), C(Ph))}, 9.8 (C=()), 0.8 (C=(C )) The signal of C quat (Ph) coincide with an other peak; IR (KBr, cm - ):, (ν C=() ), (ν C=(C) ),,,, 088; MS (0eV): m/z =00, 0 (Ind-C + ), 0 (Boc-Ind-C + ), 0; Mp.:. C. Procedure for the synthesis of -[(-methylphenyl)sulfonyl]--indole--carbaldehyde To a solution of 0 g (8.8 mmol) indole--carbaldehyde in TF was added at 0 C.8 g (. mmol,. equiv) a. The reaction mixture was stirred for h at room temperature. ext. g (8.8 mmol, equiv.) tosylchloride was added and the mixture was stirred for h at room temperature. The mixture was quenched with an excess of water. Carefully stirring for minutes afforded rose crystals. These crystals werd filtrated and washed three times with 0.M cold a. After drying at high vacuum the compound was obtained with a yield of 8 %. If necessary recrystallisation is possible in TF/water.
12 This journal is The wner Societies 00 xx -MR (00 Mz, CD, ppm):.8 (, s, C ),.-.8 (, m, C (Ts), C (Ts), C, C ),.8-.8 (, m, C (Ts), C (Ts)),.9-.9 (, m, C or C ), 8. (, s, C ), (, m, C or C ), 0.0 (, s, (C=)); C-MR (. Mz, CD, ppm): δ =. (C ),. (C or C ),. (C or C a or C a ),.9 (C or C ),. (C or C ),. (C or C ),. (C, C (Ts), C (Ts)), 0. (C, C (Ts), C (Ts)),. (C or C a or C a ),. (C or C a or C a ),.(C ),.8 (C quat (Ts)), 8.9 ((C=)), the signal C quat (Ts) coincide with an other peak; IR (KBr, cm - ): (ν C=() ), 9,,, (ν S ); MS (ESI): m/z = 00(M+ + ); Mp.: 0. C. Procedure for the synthesis of -benzyl-{-[(-methylfenyl)sulfonyl]--indole-yl} metanamine via reductive amination. The imine obtained according the general procedure was immediately reduced according the general procudere for reduction. Crystallisation in ethanol/0. M a afforded yellow crystals in % yield. -MR (00 Mz, CD, ppm):. (, bs, ),. (, s, C ),.9 (, s, C (Ph)),.8 (, s, (Ind)C ),. (, d, J = 8.0 z, C (Ts), C (Ts)),.8-. (, m, C, C, x C(Ph)),.9 (, s, C ),. (, d, J =. z, C or C ),. (, d, J = xx. z, C (Ts), C (Ts)),.99 (, d, J = 8. z, C or C ); C-MR (. Mz, CD, ppm): δ =. (C ),.9 ((Ind)C ),.9 (C (Ph)),.88 (C or C ), 9.99 (C or C ),. (C ),.8 (C or C ),.89 (C ),.9 (C or C ),.9 (C, C (Ts), C (Ts)),. (C(Ph)), 8. (C, x C(Ph)), 8.0 (C, x C(Ph)), 9.9 (C, C (Ts), C (Ts)), 0. (C a or C a or C quat(ts)),.8 C a or C a or C quat(ts)),. (C a or C a or C quat(ts)), 0. (C quat(ph)),.98 (C quat(ts)); IR (KBr, cm - ): (ν ), 9, 9,, (ν S ); MS (ESI): m/z = 8 (Ts-Ind-C + ); Mp.: 9. C. The acylation was performed according the previous general procedure. Reaction time h, yield: %. The spectrum shows the existance of rotamers. The mixture consist of % rotamer A and % rotamer B. -MR (00 Mz, CD, ppm): δ =. (, s, C, rot A, rot B),. (, s, C (Ph), rot B),.9 (, s, (Ind)C, rot a a a a S a a S C S a A),.8 (, s, C (Ph), rot A),.9 (, s, (Ind)C, rot B),.08-. (, m, C, C or C, C, C, x C(Ph), {C (Ts), C (Ts) or C (Ts), C (Ts)}, rot A, rot B),. (, d, J = 8. z, C (Ts), C (Ts) or C (Ts), C (Ts), rot A, rot B), 8.0 (, d, J = 8.0 z, C or C, rot A, rot B); C-MR (. Mz, CD, ppm): δ =. (C, rot A, rot B),. ((Ind)C, rot A),. ((Ind)C, rot B), 0. (C (Ph)), rot B),.8 (C (Ph)), rot A), 9. (C, rot A, rot B),.8 (C or C, rot A),.9 (C or C, rot B),.0 (C, rot A, rot B), 9.0 (C or C, rot B), 9.8 (C or C, rot A),. (C ), {.,.9,.08, 8.09, 8.9, 9. (C, C, x C(Ph), x C(Ph), C(Ph), C quat (Ph), rot A, rot B)},. (C (Ts), C (Ts) or C (Ts), C (Ts), rot A, rot B), 9.89 (C (Ts), C (Ts) or C (Ts), C (Ts), rot A, rot B), {.,.9,. (C a, C a, C quat (Ts), rot A, rot B)},. (C quat (Ts), rot A, rot B),.00 (C=, rot A, rot B); IR (KBr, cm - ):, 8 (ν C= ), 8,, (ν S ); MS (ESI): m/z = 8 (Ts-Ind-C + ); Mp.:. C.
13 This journal is The wner Societies 00 Reaction time: h, yield: %. -MR (00 Mz, CD, ppm):.8 (, d, J=. z, x C (ipr)),. (, s, C (Ts)),.9 (, s, (Ind)C ),.9-.9 (, m, C(iPr)),.8 (, d, J = 8. z, C (Ts), C (Ts)),. (, dxdxd, J = J =. z, J =. z, a C C or C ),. (, dxd, J = J =. z, C or C ),. (, s, C ),. (, d, J = 8.0 z, C or C ),.9 (, d, J = 8. z, C (Ts), C (Ts)),.98 (, d, J = 8.0 z, C or C ); C-MR (. Mz, CD, ppm): 0. (x S C (ipr)),.0 (C (Ts)), 8. ((Ind)C ), 0.9 (C(iPr)), 9. (C ),.9 and 9. (C and C ), 9.8 (C ),.8 (C or C ),.08 (C ),.9 (C or C ),. (C, C (Ts), C (Ts)), 9. (C a or C a ), b 9.8 (C, C (Ts), C (Ts)),.9 (C (Ts)),. (C a or C a ),.9 (C (Ts)), 0. (C=); IR (KBr, cm - ):, (ν C= ),, 9 (ν S= ); MS (ESI): m/z = 9// ([M +] + +); Mp.: 8. C. Procedure for the preparation of a A mixture of 0. g (0. mmol, equiv.) a and. mg (0.mmol, 0.8 equiv.) TMEDA was dissolved in refluxing C and was flushed with argon for minutes.. mg (0. mmol, 0. equiv.) Cu(I) was added to the reaction mixture. The reaction mixture was refluxed for h under argon atmosphere and finally quenched with water and stirred for 0 minutes. The organic phase was washed three times with water until the blue colour of the wash water disappeared. After drying over MgS, filtration and evaporation of the solvent the spiro compound was obtained. Crystallisation in chloroform/diethylether afforded the compound in % yield. -MR (00 Mz, CD, ppm): δ =.9 (, s, C ),.8 (, d, J AB = 0.9 z, (Ind)C ),.8 (, d, J AB = 0.9 z, (Ind)C ),.8 (, d, J AB =. z, C (Ph)),.0 (, d, J AB =. z, C (Ph)),. (, d, J =. z, C or C ), a S.9 (, dxd, J = J =. z, C or C ),.99 (, s, C ), a.-. (8, m, C or C, x C(Ph), x C(Ts)),.9 (, d, J = 8.0 z, C or C ),.88 (, d, J = 8.0 z, x C(Ts)); a C-MR (. Mz, CD, ppm): δ =. (C ), 8.0 (C (Ph)), 0.8 ((Ind)C ),. (C ), 80.9 (C ), 8. (C ),. (C or C ),. (C or C ),.8 (C or C ),.88 (C, x C(Ts)), 8.80 (C(Ph)), 8.90 (C, x C(Ph)), 9. (C, x C(Ph)), 9.8 (C a or C a or C quat (Ts)), 0.0 (C or C ),. (C, x C(Ts)),. (C quat (Ph)),. (C a or C a or C quat (Ts)), 9. (C a or C a or C quat (Ts)),.8 (C quat (Ts)),.8 (C=); IR (KBr, cm - ):, (ν C= ), 98,, (ν S ); MS (ESI): ): m/z = /9/ (M+ + ); Mp./decomposition temperature: 0. C.
14 This journal is The wner Societies 00 Crystallisation in methanol; yield: %. -MR (00 Mz, CD, ppm):. (, d, J =.9 z, C (ipr)), (, d, J =.9 z, C (ipr)),.9 (, s, C (Ts)),.0 (, d, J AB = 0. z, (Ind)C ),.88 (, d, J AB = 0. z, (Ind)C ),,9 (, sept, J =.9 z, C(iPr)),.0 (, s, C ),.0-. (, a m, C or C and C or C ),.0 (, d, J = 8.0 z, C (Ts), C (Ts)),.8 (, dxdxd, J = J =.9 z, J =.9 z, C or C ),. (, d, J = 8, z, C or C ),.90 (, d, J = 8. z, C (Ts), C (Ts)); S C-MR (, Mz, CD, ppm): 9. (C (ipr)), 9.0 (C (ipr)),. (C (Ts)),.0 (C(iPr)),. ((Ind)C ),. (C ), 8. (C ), 8. b (C ),.9 (C or C ),. and.8 (C or C and C or C ),.8 (C, C (Ts), C (Ts)), 9. (C a or C a ), 9.99 (C, C (Ts), C (Ts)),.0 (C or C ),.8, 9. and.0 (C quat (Ts), C a and C a ),.0 (C=); IR (KBr, cm - ): (ν C= ),, 9 (ν S= ); MS (ESI): m/z = 9// ([M +] + +); Mp./decomposition temperature:. C. GEERAL PRCEDURE FR TE SYTESIS F TE EMI-AMIAL 0. g of was dissolved in C together with 0. g silica. The mixture was stirred for h at room temperature. The silica was filtrated over celite and the solvent was concentrated in vacuo. The compound was obtained in 80 % yield. -MR (00 Mz, CD, ppm): δ =. (, s, C ),. a S a a (, d, J AB = 0. z, (Ind)C ),.0 (, d, J =.0 z, C, D exchangeable),.99 (, d, J AB = 0. z, (Ind)C ),. (, d, J AB =. z, C (Ph)),. (, d, J AB =. z, C (Ph)),.0 (, d, J =.0 z, C ),.8 (, dxd, J =. z, J = 0.8 z, C or C ),.89 (, dxdxd, J = J =. z, J = 0.8 z, C or C ),.-.0 (8, m, C or C, x C(Ph), x C(Ts)),.9 (, d, J = 8.0 z, C or C ),. (, d, J = 8. z, x C(Ts)); C-MR (. Mz, CD, ppm): δ =. (C ), 8. (C (Ph)), 9.9 ((Ind)C ),.8 (C ), 8.0 (C ), 8. (C ),.09 (C or C ),.8 (C or C ),. (C or C ),. (C, x C(Ph) or x C(Ts)), 8. (C(Ph)), 8.8 (C, x C(Ph) or x C(Ts)), 9. (C, x C(Ph) or x C(Ts)), 9.8 (C quat (Ts)), 0.0 (C, x C(Ph) or x C(Ts)), 0.8 (C or C ), {.,., 9. (C a, C a, C quat(ph))},.0 (C quat (Ts)),. (C=); IR (KBr, cm - ): (ν ), (ν C= ), 99, 8,, (ν S ); MS (ESI): ): m/z = /9/ (M+ + ); Mp./decomposition temperature: 0. C. yield: %. -MR (00 Mz, CD, ppm):. (, d, J =. z, C (ipr)),. (, d, J =. z, C (ipr)),. (, s, C (Ts)),. (, d, J AB = 0. z, (Ind)C ),. (, d, J =.0 z, ),.0 (, d, J AB = 0. z, (Ind)C ),. (, sept, a J =.9 z, C(iPr)),.0 (, d, J =.0 z, C ),.0 (, d, J = 8.0 z, C or C ),.0 (, dxdxd, J = J = 8.0 z, J =. z, C or C ),. (, d, J = 8. z, C (Ts), C (Ts)),. (, dxd, J = J =. z, C or C ),. (, d, J = 8.0 z, C or C ),.8 (, d, J = 8. z, C (Ts), C (Ts)); C-MR (, S Mz, CD, ppm): 9. (C (ipr)), 9. (C (ipr)),. (C (Ts)),. (C, (Ind)C, C(iPr)), 0.9 (C ), 8.8 (C ), 8.9 (C ),.0 (C or b C ),.9 (C or C ),. (C or C ),.8 (C, C (Ts), C (Ts)), 9. (C a ), 9.99 (C, C (Ts), C (Ts)), 0.80 (C or C ),.8 (C (Ts)), 0. (C a ),.0 (C (Ts)),. (C=); IR (KBr, cm - ): 0 (ν ), 9 (ν C= ), 8, 9 (ν S= ); MS (ESI): m/z = 9// (M + +); Mp.: 9. C.
Synthesis and their spectroscopic distinction of. benzonaphthonaphthyridines and its isomer
 Synthesis and their spectroscopic distinction of benzonaphthonaphthyridines and its isomer Kolandaivel Prabha and Karnam Jayaramapillai Rajendra Prasad* Department of Chemistry, Bharathiar University Coimbatore,
Synthesis and their spectroscopic distinction of benzonaphthonaphthyridines and its isomer Kolandaivel Prabha and Karnam Jayaramapillai Rajendra Prasad* Department of Chemistry, Bharathiar University Coimbatore,
Synthesis of derivatives of potent antitumor bistramide D and A leading to the first crystal structure of natural bistramide D
 SUPPLEMETARY MATERIAL Synthesis of derivatives of potent antitumor bistramide D and A leading to the first crystal structure of natural bistramide D Claude Bauder,* a,c Jean-François Biard b and Guy Solladié
SUPPLEMETARY MATERIAL Synthesis of derivatives of potent antitumor bistramide D and A leading to the first crystal structure of natural bistramide D Claude Bauder,* a,c Jean-François Biard b and Guy Solladié
Rapid consecutive three-component coupling-fiesselmann synthesis of luminescent 2,4-disubstituted thiophenes and oligothiophenes
 Rapid consecutive three-component coupling-fiesselmann synthesis of luminescent 2,4-disubstituted thiophenes and oligothiophenes Marco Teiber, and Thomas J. J. Müller* Institut für Organische Chemie und
Rapid consecutive three-component coupling-fiesselmann synthesis of luminescent 2,4-disubstituted thiophenes and oligothiophenes Marco Teiber, and Thomas J. J. Müller* Institut für Organische Chemie und
Novel Enzymatic Synthesis of 4-O-Cinnamoyl Quinic and Shikimic Acid Derivatives
 Revised: May 003 Novel Enzymatic Synthesis of --Cinnamoyl Quinic and Shikimic Acid Derivatives Nuria Armesto, Miguel Ferrero, Susana Fernández, and Vicente Gotor* Departamento de Química rgánica e Inorgánica,
Revised: May 003 Novel Enzymatic Synthesis of --Cinnamoyl Quinic and Shikimic Acid Derivatives Nuria Armesto, Miguel Ferrero, Susana Fernández, and Vicente Gotor* Departamento de Química rgánica e Inorgánica,
Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry Electronic Supplementary Information
 Electronic Supplementary Information Synthesis of the bis potassium salts of 5-hydroxy-3-oxo-pent-4-enoic acids and their use for the efficient preparation of 4-hydroxy-2H-pyran-2-ones and other heterocycles
Electronic Supplementary Information Synthesis of the bis potassium salts of 5-hydroxy-3-oxo-pent-4-enoic acids and their use for the efficient preparation of 4-hydroxy-2H-pyran-2-ones and other heterocycles
Supporting Information for: 1: Kansas State University, Department of Chemistry, 213 CB Building, 1212 Mid-Campus
 Supporting Information for: Insights from Theory and Experiment on the Photochromic spiro- Dihydropyrrolo-Pyridazine/Betaine System Amendra Fernando, Tej B. Shrestha,,2 Yao Liu, 3 Aruni P. Malalasekera,
Supporting Information for: Insights from Theory and Experiment on the Photochromic spiro- Dihydropyrrolo-Pyridazine/Betaine System Amendra Fernando, Tej B. Shrestha,,2 Yao Liu, 3 Aruni P. Malalasekera,
Modular Synthesis of Naphtho-Thiophenes by Pd- Catalyzed Tandem Direct-Arylation/Suzuki- Coupling
 Modular Synthesis of Naphtho-Thiophenes by Pd- Catalyzed Tandem Direct-Arylation/Suzuki- Coupling Norman Nicolaus, Patrick T. Franke and Mark Lautens* [*]Davenport Chemical Laboratories Department of Chemistry,
Modular Synthesis of Naphtho-Thiophenes by Pd- Catalyzed Tandem Direct-Arylation/Suzuki- Coupling Norman Nicolaus, Patrick T. Franke and Mark Lautens* [*]Davenport Chemical Laboratories Department of Chemistry,
Supporting Information. One-pot four-component synthesis of pyrimidyl and. pyrazolyl substituted azulenes by glyoxylation
 Supporting Information for One-pot four-component synthesis of pyrimidyl and pyrazolyl substituted azulenes by glyoxylation decarbonylative alkynylation cyclocondensation sequences Charlotte F. Gers, Julia
Supporting Information for One-pot four-component synthesis of pyrimidyl and pyrazolyl substituted azulenes by glyoxylation decarbonylative alkynylation cyclocondensation sequences Charlotte F. Gers, Julia
Supporting Information. for. TEMPO-derived spin labels linked to the nucleobases. adenine and cytosine for probing local structural
 Supporting Information for TEMPO-derived spin labels linked to the nucleobases adenine and cytosine for probing local structural perturbations in DNA by EPR spectroscopy Dnyaneshwar B. Gophane and Snorri
Supporting Information for TEMPO-derived spin labels linked to the nucleobases adenine and cytosine for probing local structural perturbations in DNA by EPR spectroscopy Dnyaneshwar B. Gophane and Snorri
Unprecedented In Water Imidazole- Carbonylation: Paradigm shift for Preparation of Urea and Carbamate
 Unprecedented In Water Imidazole- Carbonylation: Paradigm shift for Preparation of Urea and Carbamate Kamlesh J. Padiya* Sandip Gavade, Bhavana Kardile, Manojkumar Tiwari, Swapnil Bajare, Madhav Mane,
Unprecedented In Water Imidazole- Carbonylation: Paradigm shift for Preparation of Urea and Carbamate Kamlesh J. Padiya* Sandip Gavade, Bhavana Kardile, Manojkumar Tiwari, Swapnil Bajare, Madhav Mane,
SUPPORTING INFORMATION
 Dual-Cavity Baskets Promote Encapsulation in Water in an Allosteric Fashion Shigui Chen, Makoto Yamasaki, Shane Polen, Judith Gallucci, Christopher M. Hadad and Jovica D. Badjić* Department of Chemistry
Dual-Cavity Baskets Promote Encapsulation in Water in an Allosteric Fashion Shigui Chen, Makoto Yamasaki, Shane Polen, Judith Gallucci, Christopher M. Hadad and Jovica D. Badjić* Department of Chemistry
Supporting Information. for the manuscript entitled
 upporting Information for the manuscript entitled ynthesis of -ubstituted ulfamate Esters from ulfamic Acid alts by Activation with Triphenylphosphine Ditriflate J. Miles Blackburn, lanie. A. hort, Thomas
upporting Information for the manuscript entitled ynthesis of -ubstituted ulfamate Esters from ulfamic Acid alts by Activation with Triphenylphosphine Ditriflate J. Miles Blackburn, lanie. A. hort, Thomas
Supplementary Figures
 Supplementary Information Bottom-up synthesis of finite models of helical (n,m)-single-wall carbon nanotubes Shunpei Hitosugi, Waka Nakanishi, Takashi Yamasaki and Hiroyuki Isobe* Department of Chemistry,
Supplementary Information Bottom-up synthesis of finite models of helical (n,m)-single-wall carbon nanotubes Shunpei Hitosugi, Waka Nakanishi, Takashi Yamasaki and Hiroyuki Isobe* Department of Chemistry,
Halogen-free water-stable aluminates as replacement for persistent fluorinated weakly-coordinating anions
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2014 Halogen-free water-stable aluminates as replacement for persistent fluorinated weakly-coordinating
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2014 Halogen-free water-stable aluminates as replacement for persistent fluorinated weakly-coordinating
Supporting Information for Efficient Two-Step Synthesis of Biodiesel from Greases
 Supporting Information for Efficient Two-Step Synthesis of Biodiesel from Greases Helen L. Ngo,a,*, Nicholas A. Zafiropoulos,a, Thomas A. Foglia,*, Edward T. Samulski, and Wenbin Lin * U.S. Department
Supporting Information for Efficient Two-Step Synthesis of Biodiesel from Greases Helen L. Ngo,a,*, Nicholas A. Zafiropoulos,a, Thomas A. Foglia,*, Edward T. Samulski, and Wenbin Lin * U.S. Department
Three-component synthesis of polysubstituted pyrrole core containing heterocyclic scaffolds over magnetically separable nanocrystalline copper ferrite
 Three-component synthesis of polysubstituted pyrrole core containing heterocyclic scaffolds over magnetically separable nanocrystalline copper ferrite Sanjay Paul, Gargi Pal, Asish R. Das* Department of
Three-component synthesis of polysubstituted pyrrole core containing heterocyclic scaffolds over magnetically separable nanocrystalline copper ferrite Sanjay Paul, Gargi Pal, Asish R. Das* Department of
Electronic Supplementary Information
 Electronic Supplementary Information Si-H activation of Hydrosilanes leading to Hydrido Silyl and Bis(silyl) Nickel Complexes Thomas Zell, a Thomas Schaub, a Krzysztof Radacki, a Udo Radius* a a Institut
Electronic Supplementary Information Si-H activation of Hydrosilanes leading to Hydrido Silyl and Bis(silyl) Nickel Complexes Thomas Zell, a Thomas Schaub, a Krzysztof Radacki, a Udo Radius* a a Institut
Pd-Catalyzed Intramolecular Oxyalkynylation of Alkenes with Hypervalent Iodine
 Supporting information Pd-Catalyzed Intramolecular xyalkynylation of Alkenes with Hypervalent Iodine Stefano Nicolai, Stéphane Erard, Davinia Fernández González and Jérôme Waser Laboratory of Catalysis
Supporting information Pd-Catalyzed Intramolecular xyalkynylation of Alkenes with Hypervalent Iodine Stefano Nicolai, Stéphane Erard, Davinia Fernández González and Jérôme Waser Laboratory of Catalysis
Supporting Information. Organocatalytic Stereoselective Synthesis of Passifloricin A
 Supporting Information Organocatalytic Stereoselective Synthesis of Passifloricin A Pradeep Kumar,* a Menaka Pandey a, Priti Gupta a, Dilip D. Dhavale b a Division of Organic Chemistry, National Chemical
Supporting Information Organocatalytic Stereoselective Synthesis of Passifloricin A Pradeep Kumar,* a Menaka Pandey a, Priti Gupta a, Dilip D. Dhavale b a Division of Organic Chemistry, National Chemical
molecules ISSN
 Molecules 2006, 11, 183-187 Full Paper molecules ISSN 1420-3049 http://www.mdpi.org Synthesis of 3,4,7,8-Tetrahydronaphthalene-1,5(2,6)-dione Garrett B. Minne and Pierre J. De Clercq* Ghent University,
Molecules 2006, 11, 183-187 Full Paper molecules ISSN 1420-3049 http://www.mdpi.org Synthesis of 3,4,7,8-Tetrahydronaphthalene-1,5(2,6)-dione Garrett B. Minne and Pierre J. De Clercq* Ghent University,
Total Synthesis of the Neoclerodane Diterpene Salvinorin A via an Intramolecular Diels Alder Strategy
 Total Synthesis of the Neoclerodane Diterpene Salvinorin A via an Intramolecular Diels Alder Strategy Yuzhou Wang and Peter Metz* Fakultät Chemie und Lebensmittelchemie, Organische Chemie I, Technische
Total Synthesis of the Neoclerodane Diterpene Salvinorin A via an Intramolecular Diels Alder Strategy Yuzhou Wang and Peter Metz* Fakultät Chemie und Lebensmittelchemie, Organische Chemie I, Technische
Supporting Information. for
 Supporting Information for Copper Complexes for Fluorescence-Based NO Detection in Aqueous Solution Mi Hee Lim and Stephen J. Lippard Department of Chemistry, Massachusetts Institute of Technology, Cambridge,
Supporting Information for Copper Complexes for Fluorescence-Based NO Detection in Aqueous Solution Mi Hee Lim and Stephen J. Lippard Department of Chemistry, Massachusetts Institute of Technology, Cambridge,
Hydrogen-bonding controlled rigidity of an isoindoline-derived nitroxide spin label for nucleic acids
 Page S1 Hydrogen-bonding controlled rigidity of an isoindoline-derived nitroxide spin label for nucleic acids Dnyaneshwar B. Gophane and Snorri Th. Sigurdsson* Department of Chemistry, Science Institute,
Page S1 Hydrogen-bonding controlled rigidity of an isoindoline-derived nitroxide spin label for nucleic acids Dnyaneshwar B. Gophane and Snorri Th. Sigurdsson* Department of Chemistry, Science Institute,
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Construction of flexible metal-organic framework (MOF) papers
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Construction of flexible metal-organic framework (MOF) papers
Elucidation of the Teixobactin Pharmacophore
 Supporting Information for Elucidation of the Teixobactin Pharmacophore Authors: yunjun Yang a, Kevin. Chen a, and James S. owick*,a a Department of Chemistry, University of California, Irvine, Irvine,
Supporting Information for Elucidation of the Teixobactin Pharmacophore Authors: yunjun Yang a, Kevin. Chen a, and James S. owick*,a a Department of Chemistry, University of California, Irvine, Irvine,
Supporting Information for. Selective Double-Carbomagnesiation of Internal Alkynes Catalyzed by Iron-N-Heterocyclic Carbene
 Supporting Information for Selective Double-Carbomagnesiation of Internal Alkynes Catalyzed by Iron-N-Heterocyclic Carbene Complexes: A Convenient Method to Highly Substituted 1,3-Dienyl Magnesium Reagents
Supporting Information for Selective Double-Carbomagnesiation of Internal Alkynes Catalyzed by Iron-N-Heterocyclic Carbene Complexes: A Convenient Method to Highly Substituted 1,3-Dienyl Magnesium Reagents
FRET in Orthogonally Arranged Chromophores. Supporting Information
 FRET in rthogonally Arranged Chromophores Heinz Langhals* a, Andreas Walter and Andreas J. Esterbauer a Eberhard Riedle* b and Igor Pugliesi b a Department of Chemistry, LMU University of Munich, Butenandtstraße
FRET in rthogonally Arranged Chromophores Heinz Langhals* a, Andreas Walter and Andreas J. Esterbauer a Eberhard Riedle* b and Igor Pugliesi b a Department of Chemistry, LMU University of Munich, Butenandtstraße
Regioselective Heck Arylation of Unsaturated Alcohols by
 Supporting Information for: Regioselective Heck Arylation of Unsaturated Alcohols by Palladium Catalysis in Ionic Liquid Jun Mo, Lijin Xu, Jiwu Ruan, Shifang Liu and Jianliang Xiao* Liverpool Centre for
Supporting Information for: Regioselective Heck Arylation of Unsaturated Alcohols by Palladium Catalysis in Ionic Liquid Jun Mo, Lijin Xu, Jiwu Ruan, Shifang Liu and Jianliang Xiao* Liverpool Centre for
Stereoselective Palladium Catalyzed Synthesis of Indolines via Intramolecular Trapping of N-Ylides with Alkenes
 Supporting Information Stereoselective Palladium Catalyzed Synthesis of Indolines via Intramolecular Trapping of N-Ylides with Alkenes Angula Chandra Shekar Reddy, Venkata Surya Kumar Choutipalli, Jayanta
Supporting Information Stereoselective Palladium Catalyzed Synthesis of Indolines via Intramolecular Trapping of N-Ylides with Alkenes Angula Chandra Shekar Reddy, Venkata Surya Kumar Choutipalli, Jayanta
SUPPORTING INFORMATION
 S1 SUPPORTING INFORMATION Cyclization of 1,6-enynes catalyzed by gold nanoparticles supported on TiO 2 : Significant changes in selectivity and mechanism, as compared to homogeneous Au-catalysis Charis
S1 SUPPORTING INFORMATION Cyclization of 1,6-enynes catalyzed by gold nanoparticles supported on TiO 2 : Significant changes in selectivity and mechanism, as compared to homogeneous Au-catalysis Charis
Rhodium- and Non-Metal-Catalyzed Approaches for the Conversion of Isoxazol-5-ones to 2,3-Dihydro-6H-1,3- Oxazin-6-ones
 Supporting Information Rhodium- and Non-Metal-Catalyzed Approaches for the Conversion of Isoxazol-5-ones to 2,3-Dihydro-6H-1,3- Oxazin-6-ones Igor D. Jurberg a,b,* and Huw M. L. Davies a,* a Department
Supporting Information Rhodium- and Non-Metal-Catalyzed Approaches for the Conversion of Isoxazol-5-ones to 2,3-Dihydro-6H-1,3- Oxazin-6-ones Igor D. Jurberg a,b,* and Huw M. L. Davies a,* a Department
Supporting Information. Rapid access to unsymmetrical tolanes and alkynones by sequentially palladium catalyzed one pot processes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Rapid access to unsymmetrical tolanes and alkynones
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Rapid access to unsymmetrical tolanes and alkynones
Sustainable Radical Cascades to Synthesize Difluoroalkylated Pyrrolo[1,2-a]indoles
![Sustainable Radical Cascades to Synthesize Difluoroalkylated Pyrrolo[1,2-a]indoles Sustainable Radical Cascades to Synthesize Difluoroalkylated Pyrrolo[1,2-a]indoles](/thumbs/88/116200608.jpg) Sustainable Radical Cascades to Synthesize Difluoroalkylated Pyrrolo[1,2-a]indoles Honggui Huang a, Menglin Yu a, Xiaolong Su a, Peng Guo a, Jia Zhao a, Jiabing Zhou a and Yi Li a, b * a Department of
Sustainable Radical Cascades to Synthesize Difluoroalkylated Pyrrolo[1,2-a]indoles Honggui Huang a, Menglin Yu a, Xiaolong Su a, Peng Guo a, Jia Zhao a, Jiabing Zhou a and Yi Li a, b * a Department of
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Iron(II) Bromide-Catalyzed Oxidative Coupling of Benzylamines with ortho-substituted
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Iron(II) Bromide-Catalyzed Oxidative Coupling of Benzylamines with ortho-substituted
Cross-Coupling of Aromatic Bromides with Allylic Silanolate Salts
 S1 Cross-Coupling of Aromatic Bromides with Allylic Silanolate Salts Scott E. Denmark* and Nathan S. Werner Roger Adams Laboratory, University of Illinois, 600 S. Mathews Avenue, Urbana, Illinois 6101
S1 Cross-Coupling of Aromatic Bromides with Allylic Silanolate Salts Scott E. Denmark* and Nathan S. Werner Roger Adams Laboratory, University of Illinois, 600 S. Mathews Avenue, Urbana, Illinois 6101
One-Pot Acid-Catalyzed Ring-Opening/Cyclization/Oxidation of Aziridines with N-Tosylhydrazones: Access to 1,2,4- Triazines
 One-Pot Acid-Catalyzed Ring-Opening/Cyclization/Oxidation of Aziridines with N-Tosylhydrazones: Access to 1,2,4- Triazines Lorène Crespin,*, Lorenzo Biancalana, Tobias Morack, David C. Blakemore, Steven
One-Pot Acid-Catalyzed Ring-Opening/Cyclization/Oxidation of Aziridines with N-Tosylhydrazones: Access to 1,2,4- Triazines Lorène Crespin,*, Lorenzo Biancalana, Tobias Morack, David C. Blakemore, Steven
Supporting Information
 Electronic upplementary Material (EI) for RC dvances. This journal is The Royal ociety of Chemistry 204 upporting Information for ynthesis of thiazolidines via regioselective addition of unsymmetric thioureas
Electronic upplementary Material (EI) for RC dvances. This journal is The Royal ociety of Chemistry 204 upporting Information for ynthesis of thiazolidines via regioselective addition of unsymmetric thioureas
Pd(0)-Catalyzed Oxy- and Amino- Alkynylation of Olefins for the Synthesis of Tetrahydrofurans and Pyrrolidines.
 Supporting information Pd(0)-Catalyzed Oxy- and Amino- Alkynylation of Olefins for the Synthesis of Tetrahydrofurans and Pyrrolidines. Stefano Nicolai and Jérôme Waser Laboratory of Catalysis and Organic
Supporting information Pd(0)-Catalyzed Oxy- and Amino- Alkynylation of Olefins for the Synthesis of Tetrahydrofurans and Pyrrolidines. Stefano Nicolai and Jérôme Waser Laboratory of Catalysis and Organic
One-pot selective synthesis of a fullerene bisadduct for
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Electronic Supporting Information: One-pot selective synthesis of a fullerene bisadduct
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Electronic Supporting Information: One-pot selective synthesis of a fullerene bisadduct
Agilent GC/MSD Instructions
 Agilent GC/MSD Instructions GC/MS SAMPLE PREPARATION: Sample Components to Avoid Completely: The following should never be injected: metals, strong acids or bases, salts, oligomeric and polymeric material.
Agilent GC/MSD Instructions GC/MS SAMPLE PREPARATION: Sample Components to Avoid Completely: The following should never be injected: metals, strong acids or bases, salts, oligomeric and polymeric material.
Supporting Information
 Supporting Information for The application of a monolithic triphenylphosphine reagent for conducting Appel reactions in flow microreactors Kimberley A. Roper 1, Heiko Lange 1, Anastasios Polyzos 1, Malcolm
Supporting Information for The application of a monolithic triphenylphosphine reagent for conducting Appel reactions in flow microreactors Kimberley A. Roper 1, Heiko Lange 1, Anastasios Polyzos 1, Malcolm
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2141 Catalytic, Stereospecific Syn-Dichlorination of Alkenes Alexander J. Cresswell, Stanley T.-C. Eey and Scott E. Denmark* Department of Chemistry, University of Illinois Urbana-Champaign,
DOI: 10.1038/NCHEM.2141 Catalytic, Stereospecific Syn-Dichlorination of Alkenes Alexander J. Cresswell, Stanley T.-C. Eey and Scott E. Denmark* Department of Chemistry, University of Illinois Urbana-Champaign,
Conductivity and TDS Meter Pen Style Water Quality Meter
 User's Guide 99 Washington Street Melrose, MA 02176 Phone 781-665-1400 Toll Free 1-800-517-8431 Visit us at www.testequipmentdepot.com Conductivity and TDS Meter Pen Style Water Quality Meter Model EC150
User's Guide 99 Washington Street Melrose, MA 02176 Phone 781-665-1400 Toll Free 1-800-517-8431 Visit us at www.testequipmentdepot.com Conductivity and TDS Meter Pen Style Water Quality Meter Model EC150
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2015. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201503969 Generalizable Synthesis of Metal-Sulfides/Carbon Hybrids with
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2015. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201503969 Generalizable Synthesis of Metal-Sulfides/Carbon Hybrids with
SUPPORTING INFORMATION
 Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2016 SUPPORTING INFORMATION Investigation of triazole linked indole and oxindole glycoconjugates
Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2016 SUPPORTING INFORMATION Investigation of triazole linked indole and oxindole glycoconjugates
Infrared Laser Writing of MOFs
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information Infrared Laser Writing of MOFs Kenji Hirai* and Kazuki Sada* Department
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information Infrared Laser Writing of MOFs Kenji Hirai* and Kazuki Sada* Department
DDS-12DW Benchtop Conductivity Meter. Instruction Manual BANTE INSTRUMENTS CO., LTD
 DDS-12DW Benchtop Conductivity Meter Instruction Manual BANTE INSTRUMENTS CO., LTD DDS-12DW Benchtop Conductivity Meter 1 Introduction Thank you for selecting the DDS-12DW benchtop conductivity meter.
DDS-12DW Benchtop Conductivity Meter Instruction Manual BANTE INSTRUMENTS CO., LTD DDS-12DW Benchtop Conductivity Meter 1 Introduction Thank you for selecting the DDS-12DW benchtop conductivity meter.
Supporting information for the manuscript
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2019 Supporting information for the manuscript Facile N-functionalization and strong magnetic
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2019 Supporting information for the manuscript Facile N-functionalization and strong magnetic
NMR Users Guide Organic Chemistry Laboratory
 NMR Users Guide Organic Chemistry Laboratory Introduction The chemistry department is fortunate to have a high field (400 MHz) Nuclear Magnetic Resonance (NMR) spectrometer. You will be using this instrument
NMR Users Guide Organic Chemistry Laboratory Introduction The chemistry department is fortunate to have a high field (400 MHz) Nuclear Magnetic Resonance (NMR) spectrometer. You will be using this instrument
CHROMABOND Flash cartridges
 Flash chromatography outperforming the standard CHROMABOND Flash cartridges from the silica experts www.mn-net.com MN products for Flash chromatography Contents CHROMABOND Flash solutions separations from
Flash chromatography outperforming the standard CHROMABOND Flash cartridges from the silica experts www.mn-net.com MN products for Flash chromatography Contents CHROMABOND Flash solutions separations from
Aqueous GPC Instructions - Detailed
 Aqueous GPC Instructions - Detailed Waste Bottle Columns PL Datastream Injection Port GPC Computer UV Detector GPC Pump Eluent Bottle The Aqueous GPC Set Up RI Detector Important The points below must
Aqueous GPC Instructions - Detailed Waste Bottle Columns PL Datastream Injection Port GPC Computer UV Detector GPC Pump Eluent Bottle The Aqueous GPC Set Up RI Detector Important The points below must
Supporting Information
 Enantioselective Synthesis of (+) Estrone exploiting a ydrogen Bond Promoted Diels Alder Reaction Marko Weimar, Gerd Dürner, Jan W. Bats, Michael W. Göbel* Institut für rganische Chemie und Chemische Biologie
Enantioselective Synthesis of (+) Estrone exploiting a ydrogen Bond Promoted Diels Alder Reaction Marko Weimar, Gerd Dürner, Jan W. Bats, Michael W. Göbel* Institut für rganische Chemie und Chemische Biologie
FACULTY OF SCIENCE MID-TERM II CHEMISTRY 222 / 234
 Version 1 Seat Number FACULTY F SCIENCE MID-TERM II CEMISTRY 222 / 234 LAST NAME FIRST NAME STUDENT NUMBER *LAST TREE DIGITS NLY* EXAMINERS: Prof. D. F. Perepichka, Prof. D. N. arpp DATE: March 9, 2009
Version 1 Seat Number FACULTY F SCIENCE MID-TERM II CEMISTRY 222 / 234 LAST NAME FIRST NAME STUDENT NUMBER *LAST TREE DIGITS NLY* EXAMINERS: Prof. D. F. Perepichka, Prof. D. N. arpp DATE: March 9, 2009
Novel bi- and tridentate phosphane and thioether ligands derived from chiral α-hydroxy acids
 Pergamon Tetrahedron: Asymmetry 10 (1999) 1207 1215 TETRAHEDRON: ASYMMETRY Novel bi- and tridentate phosphane and thioether ligands derived from chiral α-hydroxy acids Jens Christoffers and Ulrich Rößler
Pergamon Tetrahedron: Asymmetry 10 (1999) 1207 1215 TETRAHEDRON: ASYMMETRY Novel bi- and tridentate phosphane and thioether ligands derived from chiral α-hydroxy acids Jens Christoffers and Ulrich Rößler
Sepacore X10 / X50 system Technical data sheet
 Sepacore X10 / X50 system Technical data sheet Sepacore X10 and X50 flash chromatography systems address most requirements for the purification of organic compounds. Whether a crude synthesis mixture or
Sepacore X10 / X50 system Technical data sheet Sepacore X10 and X50 flash chromatography systems address most requirements for the purification of organic compounds. Whether a crude synthesis mixture or
More Chemistries, More Choices For Your Toughest Separation Challenges
 Ordering Guide More Chemistries, More Choices For Your Toughest Separation Challenges Agilent InfinityLab Poroshell 120 Columns Efficiently separate the widest variety of columns Based on superficially
Ordering Guide More Chemistries, More Choices For Your Toughest Separation Challenges Agilent InfinityLab Poroshell 120 Columns Efficiently separate the widest variety of columns Based on superficially
Supporting Information
 Supporting Information Photocatalytic color switching of transition metal hexacyanometalate nanoparticles for high-performance light-printable rewritable paper Wenshou Wang,, * Ji Feng, Yifan Ye, Fenglei
Supporting Information Photocatalytic color switching of transition metal hexacyanometalate nanoparticles for high-performance light-printable rewritable paper Wenshou Wang,, * Ji Feng, Yifan Ye, Fenglei
HiQ sil TM HPLC Columns
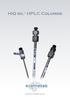 www.kromatek.co.uk KYA Technologies of Japan KYA Technologies Corporation, Tokyo, Japan established in 1998 to develop a range of HPLC products. The latest product HiQ sil HS is a high performance silica
www.kromatek.co.uk KYA Technologies of Japan KYA Technologies Corporation, Tokyo, Japan established in 1998 to develop a range of HPLC products. The latest product HiQ sil HS is a high performance silica
Acetylation of Alcohols and Molecular Modeling
 Exp t 202 Acetylation of Alcohols and Molecular Modeling Adapted by R. Minard and M. Alibhai (PSU) from procedures by S.E. Branz, J. Chem,. Ed., 62, 899 (1985) and K.L. Lipkowitz, J. Chem. Ed., 66, 275
Exp t 202 Acetylation of Alcohols and Molecular Modeling Adapted by R. Minard and M. Alibhai (PSU) from procedures by S.E. Branz, J. Chem,. Ed., 62, 899 (1985) and K.L. Lipkowitz, J. Chem. Ed., 66, 275
Electronic Supporting Information Available. Efficient and weak efficiency-roll-off near-infrared (NIR) polymer lightemitting
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information Available Efficient and weak efficiency-roll-off
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information Available Efficient and weak efficiency-roll-off
Isolera. User Manual
 Isolera User Manual Safety and Document Conventions Isolera system should only be operated and maintained by trained individuals. Please read this manual carefully before working with the system. To guarantee
Isolera User Manual Safety and Document Conventions Isolera system should only be operated and maintained by trained individuals. Please read this manual carefully before working with the system. To guarantee
Agilent G6855AA MassHunter Personal
 Agilent G6855AA MassHunter Personal Forensic Toxicology Database Kit Quick Start Guide What is the MassHunter Personal Forensic Toxicology Database Kit? 1 Kit Content 2 Where to find more information 4
Agilent G6855AA MassHunter Personal Forensic Toxicology Database Kit Quick Start Guide What is the MassHunter Personal Forensic Toxicology Database Kit? 1 Kit Content 2 Where to find more information 4
Chemical Polarimeter (Order Code CHEM-POL)
 Chemical Polarimeter (Order Code CHEM-POL) The Chemical Polarimeter is a device used for measuring the rotation of plane-polarized light caused by an optically active substance such as an organic, inorganic,
Chemical Polarimeter (Order Code CHEM-POL) The Chemical Polarimeter is a device used for measuring the rotation of plane-polarized light caused by an optically active substance such as an organic, inorganic,
MassHunter Pesticides PCD or PCDL Quick Start Guide
 MassHunter Pesticides PCD or PCDL Quick Start Guide What is the MassHunter Pesticides PCD or PCDL? 3 Kit Contents 4 Where to find more information Before You Begin 7 Installation 7 Required reagents and
MassHunter Pesticides PCD or PCDL Quick Start Guide What is the MassHunter Pesticides PCD or PCDL? 3 Kit Contents 4 Where to find more information Before You Begin 7 Installation 7 Required reagents and
SUPPORTING INFORMATION. Sequential metal-free thermal 1,3-dipolar cycloaddition of unactivated azomethine ylides
 SUPPORTING INFORMATION Sequential metal-free thermal 1,3-dipolar cycloaddition of unactivated azomethine ylides Verónica Selva, a,b,c Elisabet Selva, a,b,c,d Pedro Merino, e Carmen Nájera, a,b José M.
SUPPORTING INFORMATION Sequential metal-free thermal 1,3-dipolar cycloaddition of unactivated azomethine ylides Verónica Selva, a,b,c Elisabet Selva, a,b,c,d Pedro Merino, e Carmen Nájera, a,b José M.
Getting started with BatchColumn
 Getting started with BatchColumn Example: Simulation of solvents mixture separation Introduction 2 This document presents the different steps to follow in order to simulate a batch distillation using BatchColumn
Getting started with BatchColumn Example: Simulation of solvents mixture separation Introduction 2 This document presents the different steps to follow in order to simulate a batch distillation using BatchColumn
icolumn 12 Operation Manual
 icolumn 12 Operation Manual AccuBioMed Co., Ltd. 8F.-8, No.5, Wuquan 1st Rd., Xinzhuang Dist., New Taipei City 24892, Taiwan (R.O.C.) Tel: +886-2-2299-5989 Fax: +886-2-2299-2678 www.accubiomed.com AccuBioMed
icolumn 12 Operation Manual AccuBioMed Co., Ltd. 8F.-8, No.5, Wuquan 1st Rd., Xinzhuang Dist., New Taipei City 24892, Taiwan (R.O.C.) Tel: +886-2-2299-5989 Fax: +886-2-2299-2678 www.accubiomed.com AccuBioMed
Nicolet FT-IR Procedure
 Nicolet FT-IR Procedure Getting Started Jerry Hu (Lab Manager), ext. 7914 or 7940 jghu@mrl.ucsb.edu Jaya Nolt (Lab Tech), ext. 4997 jaya@mrl.ucsb.edu Materials Research Laboratory University of California,
Nicolet FT-IR Procedure Getting Started Jerry Hu (Lab Manager), ext. 7914 or 7940 jghu@mrl.ucsb.edu Jaya Nolt (Lab Tech), ext. 4997 jaya@mrl.ucsb.edu Materials Research Laboratory University of California,
Supporting Information. The application of a monolithic triphenylphosphine. reagent for conducting Ramirez gemdibromoolifination
 Supporting Information for The application of a monolithic triphenylphosphine reagent for conducting Ramirez gemdibromoolifination reactions in flow Kimberley A. Roper 1, Malcolm B. Berry 2 and Steven
Supporting Information for The application of a monolithic triphenylphosphine reagent for conducting Ramirez gemdibromoolifination reactions in flow Kimberley A. Roper 1, Malcolm B. Berry 2 and Steven
Supramolecular Approach to Enzyme Sensing on Paper Discs Using Lanthanide Photoluminescence
 Supporting Information for Supramolecular Approach to Enzyme Sensing on Paper Discs Using Lanthanide Photoluminescence Tumpa Gorai and Uday Maitra* Department of Organic Chemistry, Indian Institute of
Supporting Information for Supramolecular Approach to Enzyme Sensing on Paper Discs Using Lanthanide Photoluminescence Tumpa Gorai and Uday Maitra* Department of Organic Chemistry, Indian Institute of
Keywords: Bacillus anthracis, anthrax, binary toxins, aminoquinoline, aminoquinolinium salts
 PostDoc Journal Vol. 2, No. 11, November 2014 Journal of Postdoctoral Research www.postdocjournal.com Design and Synthesis of Small Molecule Inhibitors against the Protective Antigen of Bacillus anthracis
PostDoc Journal Vol. 2, No. 11, November 2014 Journal of Postdoctoral Research www.postdocjournal.com Design and Synthesis of Small Molecule Inhibitors against the Protective Antigen of Bacillus anthracis
Reverse-direction synthesis of oligonucleotides containing a 3 -S-phosphorothiolate linkage and 3 - terminal 3 -thionucleosides
 S1 [Electronic Supplementary Information] Reverse-direction synthesis of oligonucleotides containing a 3 -S-phosphorothiolate linkage and 3 - terminal 3 -thionucleosides James W Gaynor, Michael Piperakis,
S1 [Electronic Supplementary Information] Reverse-direction synthesis of oligonucleotides containing a 3 -S-phosphorothiolate linkage and 3 - terminal 3 -thionucleosides James W Gaynor, Michael Piperakis,
Synthesis and Structural Characterization of Novel Monoesters of Succinic Anhydride with Aryl Alcohols
 Research Paper Synthesis and Structural Characterization of Novel Monoesters of Succinic Anhydride with Aryl Alcohols Abstract Muhammad Iqbal, Imam Bakhsh Baloch and Musa Kaleem Baloch* *Department of
Research Paper Synthesis and Structural Characterization of Novel Monoesters of Succinic Anhydride with Aryl Alcohols Abstract Muhammad Iqbal, Imam Bakhsh Baloch and Musa Kaleem Baloch* *Department of
Chapter 2: Wave Optics
 Chapter : Wave Optics P-1. We can write a plane wave with the z axis taken in the direction of the wave vector k as u(,) r t Acos tkzarg( A) As c /, T 1/ and k / we can rewrite the plane wave as t z u(,)
Chapter : Wave Optics P-1. We can write a plane wave with the z axis taken in the direction of the wave vector k as u(,) r t Acos tkzarg( A) As c /, T 1/ and k / we can rewrite the plane wave as t z u(,)
USER GUIDE. Salinity Meter Pen Style Water Quality Meter. Model EC170
 USER GUIDE Salinity Meter Pen Style Water Quality Meter Model EC170 Introduction Congratulations on your purchase of the Extech Pen Style Water Quality instrument; the Model EC170 measures Salinity and
USER GUIDE Salinity Meter Pen Style Water Quality Meter Model EC170 Introduction Congratulations on your purchase of the Extech Pen Style Water Quality instrument; the Model EC170 measures Salinity and
11.4 Synthesis of Alcohols From Alkenes
 RGANIC CEMISTRY 11.4 Synthesis of Alcohols From Alkenes RGANIC CEMISTRY C C C C Acid-Catalyzed hydration of Alkenes xymercuration-demeruration ydroboration-xidation RGANIC CEMISTRY 11.5 Alcohols From Alkenes
RGANIC CEMISTRY 11.4 Synthesis of Alcohols From Alkenes RGANIC CEMISTRY C C C C Acid-Catalyzed hydration of Alkenes xymercuration-demeruration ydroboration-xidation RGANIC CEMISTRY 11.5 Alcohols From Alkenes
Introduction to the HPLC ChemStation and Acquisition
 Introduction to the HPLC ChemStation and Acquisition In This Section, We Will Discuss: How to work in the Microsoft Windows Environment The structure of the ChemStation Software. How to set up an acquisition
Introduction to the HPLC ChemStation and Acquisition In This Section, We Will Discuss: How to work in the Microsoft Windows Environment The structure of the ChemStation Software. How to set up an acquisition
Agilent G6854AA MassHunter Personal Pesticide Database Kit Quick Start Guide
 Agilent G6854AA MassHunter Personal Pesticide Database Kit Quick Start Guide What is the MassHunter Personal Pesticide Database Kit? 1 Kit Content 2 Where to find more information 3 Before You Begin 4
Agilent G6854AA MassHunter Personal Pesticide Database Kit Quick Start Guide What is the MassHunter Personal Pesticide Database Kit? 1 Kit Content 2 Where to find more information 3 Before You Begin 4
Replacing Pen and Paper in the Analytical Laboratory
 Replacing Pen and Paper in the Analytical Laboratory A Database for analytical chemistry Jürgen Bienert Max-Planck-Institute for Biophysical Chemistry; Facility for Synthetic Organic Chemisty Göttingen,
Replacing Pen and Paper in the Analytical Laboratory A Database for analytical chemistry Jürgen Bienert Max-Planck-Institute for Biophysical Chemistry; Facility for Synthetic Organic Chemisty Göttingen,
Determination of suppressor with CVS using the calibration technique smartdt with dynamic addition volumes
 The Application Bulletin describes the determination of suppressor in acid copper baths by smartdt. The determination of suppressor with dilution titration (DT) involves numerous additions with standard
The Application Bulletin describes the determination of suppressor in acid copper baths by smartdt. The determination of suppressor with dilution titration (DT) involves numerous additions with standard
Rh-Catalyzed Stereospecific Synthesis of Allenes from Propargylic Benzoates and Arylboronic Acids. Supporting Information
 Rh-Catalyzed Stereospecific Synthesis of Allenes from Propargylic Benzoates and Arylboronic Acids Jonathan Ruchti and Erick M. Carreira* Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich, Switzerland
Rh-Catalyzed Stereospecific Synthesis of Allenes from Propargylic Benzoates and Arylboronic Acids Jonathan Ruchti and Erick M. Carreira* Laboratorium für Organische Chemie, ETH Zürich, 8093 Zürich, Switzerland
Facile and eco-friendly synthesis of bis(2- tetrahydrobenzofuranyl)alkanes catalyzed by H 2 SO 4 SiO 2 under solvent-free conditions
 Facile and eco-friendly synthesis of bis(2- tetrahydrobenzofuranyl)alkanes catalyzed by H 2 SO 4 SiO 2 under solvent-free conditions Lei Jin, Wenbin Wang, Chengqiao Cao, Nianyu Huang*, Junzhi Wang, Kun
Facile and eco-friendly synthesis of bis(2- tetrahydrobenzofuranyl)alkanes catalyzed by H 2 SO 4 SiO 2 under solvent-free conditions Lei Jin, Wenbin Wang, Chengqiao Cao, Nianyu Huang*, Junzhi Wang, Kun
BABA B F C FINE CHEMICALS. Product List. Baba Fine Chemicals
 BABA B F C FINE CHEMICALS Product List Baba Fine Chemicals (IS 9001 : 2008 Certified) D-119, EXPRT PRMTIN INDUSTRIAL PARK (EPIP), SITE-5, INDUSTRIAL AREA, KASNA, GREATER NIDA, GAUTAM BUDH NAGAR-201306
BABA B F C FINE CHEMICALS Product List Baba Fine Chemicals (IS 9001 : 2008 Certified) D-119, EXPRT PRMTIN INDUSTRIAL PARK (EPIP), SITE-5, INDUSTRIAL AREA, KASNA, GREATER NIDA, GAUTAM BUDH NAGAR-201306
Automated Fraction Re-Analysis Does it really make sense?
 Automated Fraction Re-Analysis Does it really make sense? Presented by: Udo Huber 1995 PHD in organic chemistry from the University Karlsruhe/Germany 1996-97 Postdoctoral fellow at the University of Hawai
Automated Fraction Re-Analysis Does it really make sense? Presented by: Udo Huber 1995 PHD in organic chemistry from the University Karlsruhe/Germany 1996-97 Postdoctoral fellow at the University of Hawai
NEW NEW. Diaphragm agm Pumps MPC for Chemical Applications MPC 301 E. MPR 060 E with power pack
 Diaphragm agm Pumps MPC for Chemical Applications The diaphragm pumps for chemical applications -Type MPC- are resistant to aggressive solvents and acidic vapors typically used in laboratory processes.
Diaphragm agm Pumps MPC for Chemical Applications The diaphragm pumps for chemical applications -Type MPC- are resistant to aggressive solvents and acidic vapors typically used in laboratory processes.
GC-MS ChemStation Reference Guide. Last Updated July 2014
 GC-MS ChemStation Reference Guide Last Updated July 2014 Overview GC-MS Instrumentation Description Sequence Programming Data Analysis Tips and Tricks Method programming reference is for your enrichment
GC-MS ChemStation Reference Guide Last Updated July 2014 Overview GC-MS Instrumentation Description Sequence Programming Data Analysis Tips and Tricks Method programming reference is for your enrichment
Minisart Syringe Filters Removal of Particles and Microorganisms from Liquids and Gases
 58 Filtration Devices Minisart Syringe Filters Minisart Syringe Filters Removal of Particles and Microorganisms from Liquids and Gases Sample Preparation HPLC UHPLC Analytics Elimination of particles from
58 Filtration Devices Minisart Syringe Filters Minisart Syringe Filters Removal of Particles and Microorganisms from Liquids and Gases Sample Preparation HPLC UHPLC Analytics Elimination of particles from
Jasco 4700 FTIR. Laser Spectroscopy Labs. Jasco 4700 FTIR. Operation instructions. Laser Spectroscopy Labs, UCI
 Laser Spectroscopy Labs Jasco 4700 FTIR Operation instructions Turn the system on 1. Turn on the computer in case it has been switched off. Normally computer stays on. 2. The instrument should be always
Laser Spectroscopy Labs Jasco 4700 FTIR Operation instructions Turn the system on 1. Turn on the computer in case it has been switched off. Normally computer stays on. 2. The instrument should be always
Technical Procedure for Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for Gunshot Residue Analysis
 Technical Procedure for Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for Gunshot Residue Analysis 1.0 Purpose This technical procedure shall be followed for the operation of the Inductively Coupled
Technical Procedure for Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for Gunshot Residue Analysis 1.0 Purpose This technical procedure shall be followed for the operation of the Inductively Coupled
Allylic and benzylic sp 3 C-H oxidation in water
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information Allylic and benzylic sp 3 C-H oxidation in water
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information Allylic and benzylic sp 3 C-H oxidation in water
Bante902 Benchtop ph/conductivity Meter. Instruction Manual BANTE INSTRUMENTS CO., LTD
 Bante902 Benchtop ph/conductivity Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante902 Benchtop ph/conductivity Meter 1 Introduction Thank you for selecting the Bante902 benchtop ph/conductivity
Bante902 Benchtop ph/conductivity Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante902 Benchtop ph/conductivity Meter 1 Introduction Thank you for selecting the Bante902 benchtop ph/conductivity
BIDHAN CHANDRA KRISHI VISWAVIDYALAYA FACULTY OF AGRICULTURE Department of Agricultural Chemicals Mohanpur , West Bengal, INDIA
 From: Dr. S. Roy Assistant Professor & PI, BIDHAN CHANDRA KRISHI VISWAVIDYALAYA FACULTY OF AGRICULTURE Department of Agricultural Chemicals Mohanpur-741 252, West Bengal, INDIA Ref. No. SR/54/2015-16 Date:
From: Dr. S. Roy Assistant Professor & PI, BIDHAN CHANDRA KRISHI VISWAVIDYALAYA FACULTY OF AGRICULTURE Department of Agricultural Chemicals Mohanpur-741 252, West Bengal, INDIA Ref. No. SR/54/2015-16 Date:
How to get acceptance of CEP revisions quickly
 How to get acceptance of CEP revisions quickly EDQM WEBINAR 13 November 2017 Florence SCHULIAR - Certification of Substances Department 1 How to get acceptance of CEP revisions quickly Aim of this presentation
How to get acceptance of CEP revisions quickly EDQM WEBINAR 13 November 2017 Florence SCHULIAR - Certification of Substances Department 1 How to get acceptance of CEP revisions quickly Aim of this presentation
Bante902P Portable ph/conductivity Meter Instruction Manual
 Bante902P Portable ph/conductivity Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante902P Portable ph/conductivity Meter 1 Introduction Thank you for selecting the Bante902P portable ph/conductivity
Bante902P Portable ph/conductivity Meter Instruction Manual BANTE INSTRUMENTS CO., LTD Bante902P Portable ph/conductivity Meter 1 Introduction Thank you for selecting the Bante902P portable ph/conductivity
Keyboard Unit Sigmatek
 KEYBOARD UNIT SIGMATEK Keyboard Unit Sigmatek 10.10.2005 Page 1 KEYBOARD UNIT SIGMATEK Technical Data Performance data Interfacing Control panel Signal generator Left or right: chip card reader Back panel:
KEYBOARD UNIT SIGMATEK Keyboard Unit Sigmatek 10.10.2005 Page 1 KEYBOARD UNIT SIGMATEK Technical Data Performance data Interfacing Control panel Signal generator Left or right: chip card reader Back panel:
Platinum-Catalyzed Domino Reaction with Benziodoxole Reagents for Accessing Benzene-Alkynylated Indoles
 COMMUNICATION Platinum-Catalyzed Domino Reaction with Benziodoxole Reagents for Accessing Benzene-Alkynylated Indoles Yifan Li and Jerome Waser* [a] Dedication ((optional)) Abstract: Indoles are omnipresent
COMMUNICATION Platinum-Catalyzed Domino Reaction with Benziodoxole Reagents for Accessing Benzene-Alkynylated Indoles Yifan Li and Jerome Waser* [a] Dedication ((optional)) Abstract: Indoles are omnipresent
Supplementary Information for. A self-cleaning underwater superoleophobic mesh for oil-water separation
 Supplementary Information for A self-cleaning underwater superoleophobic mesh for oil-water separation Lianbin Zhang 1, Yujiang Zhong 1, Dongkyu Cha 2 & Peng Wang 1 1 Biological and Environmental Sciences
Supplementary Information for A self-cleaning underwater superoleophobic mesh for oil-water separation Lianbin Zhang 1, Yujiang Zhong 1, Dongkyu Cha 2 & Peng Wang 1 1 Biological and Environmental Sciences
CH142: Instructions for the Vernier LabPro
 1 CH142: Instructions for the Vernier LabPro INTRODUCTION: The Vernier LabPro is a versatile data collection interface that can be used in combination with more than 45 sensors, and with a computer, TI
1 CH142: Instructions for the Vernier LabPro INTRODUCTION: The Vernier LabPro is a versatile data collection interface that can be used in combination with more than 45 sensors, and with a computer, TI
Equipment Overview: Safety:
 PerkinElmer Series 200 HPLC: Operating Procedure 12/2/11 R.C Equipment Overview: This document outlines the basic procedure for using the Sedex light scattering detector and the PE series 200 HPLC system
PerkinElmer Series 200 HPLC: Operating Procedure 12/2/11 R.C Equipment Overview: This document outlines the basic procedure for using the Sedex light scattering detector and the PE series 200 HPLC system
SUPPLEMENTARY INFORMATION
 Photoinduced Synthesis of Dibenzofurans. Intramolecular and Intermolecular Comparative Methodologies Patricia Camargo Solórzano, Federico Brigante, Adriana B. Pierini, Liliana B. Jimenez* INFIQC, Departamento
Photoinduced Synthesis of Dibenzofurans. Intramolecular and Intermolecular Comparative Methodologies Patricia Camargo Solórzano, Federico Brigante, Adriana B. Pierini, Liliana B. Jimenez* INFIQC, Departamento
