POSTER ABSTRACTS ARE CLASSIFIED AS ONE THE FOLLOWING: June 8-12, 2019 Boston, MA
|
|
|
- Charleen Stephany Garrett
- 5 years ago
- Views:
Transcription
1 Thank you for your interest in presenting a poster. This document contains detailed instructions on formatting your abstract, developing content for your abstract, and other pertinent information. Read this document in its entirety. Failure to follow the instructions provided in this document could result in your abstract not being selected for presentation. SUBMISSION DEADLINE March 15, 2019 at 11:59 p.m. (Pacific) Abstracts must be complete and submitted by this date; no new submission or edits will be accepted after this deadline. ASHP will not edit abstracts. Incomplete abstracts will be deleted from the system after this deadline. POSTER ABSTRACTS ARE CLASSIFIED AS ONE THE FOLLOWING: D = Descriptive Reports: Describes new, improved or innovative roles or services in pharmacy practice, or unusual clinical cases in one or a few patients that have not been formally evaluated but are of such importance that they must be brought to the attention of practitioners. Descriptive reports must contain detailed rationale of the project or case, and the importance of the report to pharmacy practice. E = Evaluative Study Reports: Describes original research, including clinical research on drug effects in humans, drug-use evaluations, and evaluations of innovative pharmacy services. Evaluative study reports must include scientific results and/or data to support the conclusions, and indicate that all clinical research represented in the abstract was approved by the appropriate ethics committee or institutional review board and, if appropriate, informed consent was obtained for all subjects. C = Case Reports: Describes an unusual patient-specific case that was not part of a study but the findings are of interest to clinical pharmacists. Case Reports do not need the headings Purpose, Methods, Results, or Conclusions. 1
2 PRACTICE AREA CATEGORIES Administrative Practice/ Management /Financial Management / Human Resources Ambulatory Care Cardiology/ Anticoagulation Chronic/Managed Care Clinical Services Management Clinical Topics/Therapeutics Complementary Alternative Medicine (Herbals, etc.) Critical Care Drug Information/ Drug-Use Evaluation Emergency Medicine Emergency Preparedness Geriatrics Home Care Infectious Diseases/HIV Informatics/Technology/Automation Investigational Drugs I.V. Therapy/ Infusion Devices Leadership Development Nuclear Pharmacy Nutrition Support Oncology/Hematology Operating Room Pharmacy Pain Management/ Palliative Care Pediatrics Pharmacokinetics Pharmacy Law/ Regulatory/ Accreditation Pharmacy Technicians: Competencies Development/Other Precepting/Preceptor Skills/Education and Training Professionalism and Career Development Psychiatry/Neurology Safety/Quality Small and/or Rural Practice Toxicology Transplant/Immunology Women s Health GETTING STARTED LOGIN ADDRESS & ACCESS KEY To submit an abstract, you must create an account profile which includes your contact information, mailing address, and your access key. Do not delete or alter the address that is shown on your profile. It is imperative that this address is a working address that is not spamprotected. If you have spam protection, you may not receive our s. Your address and the access key you create will be used as your login information for the poster submission site. The that is used for logging into the poster abstract submission site must belong to the primary author not an assistant or colleague. PRIMARY AUTHOR The person entering the information online is considered the primary author as well as the primary presenter. The primary author s name will automatically appear first on the citation and the abstract. Only the primary author s contact information will be printed on the 2
3 published version of the abstract. The primary author is responsible for verifying that all co-authors are aware of the content of the abstract and support the data. CREATE A POSTER ABSTRACT TITLE Create a title for your abstract that accurately and concisely reflects the abstract content. Only put the title of the abstract in the title field. DO NOT put the title in any of the abstract fields. Format your title as follows: Sentence case format only. NO proprietary (brand) names in the title. Use capitalized letters only for acronyms or proper nouns (e.g. countries, etc.). Do not use A, An, or The as the first word in the title. Title Format Examples Incorrect: IMPLEMENTATION OF COMPUTERIZED PRESCRIBER ORDER ENTRY (CPOE) IN A SURGICAL UNIT: ONE YEAR LATER Incorrect: Implementation of Computerized Prescriber Order Entry In A Surgical Unit: One Year Later CORRECT: Implementation of computerized prescriber order entry (CPOE) in a surgical unit: One year later SELECT A PRACTICE AREA CATEGORY Select one category from the dropdown that appropriately reflects the content of the abstract. The category will be used to match your submission with the reviewer for the peer review process. (see page 2 for a complete list of categories) ONLINE SUBMISSION PROCESS The online submission process consists of six (6) tasks. All six (6) tasks must be completed by the primary author to submit a poster abstract. TASK 1: POSTER ABSTRACT CONTENT Enter your poster abstract content details. ABSTRACT CONTENT MUST: Be complete at the time of submission. Planned projects or descriptions of projects still being implemented will not be accepted. Contain Purpose, Methods, Results and Conclusions. NOT contain the statement details/results will be discussed. Abstracts with this statement will not be accepted. 3
4 Be supported by scientific merit. Methodology is consistent with sound research design; study designed in a manner likely to answer the research questions; research questions aligned with proposed data collection and conclusion. Exhibit a balanced presentation. Abstracts must be non-promotional in nature and free of commercial bias. Abstracts written in a manner that promotes a company, service or product will not be accepted. Support a topic of relevance and importance to our attendees. ABSTRACT FORMAT: Correctly format your title. (See page 3 for details on correct title format.) Word Limits your entire abstract should be approximately words Do not use special functions such as tabs, underlines, Word Limits trademarks, superscript, subscript, bold, or italics. Spell out special symbols - Greek letters, degrees, plus and/or Purpose ~ 100 words minus signs, greater than or less signs, percentage, etc. Use Methods ~ 225 words standard abbreviations. Results ~ 200 words Do not include graphs, tables, or illustrations in your abstract. Conclusion ~ 100 words Spell out all pharmaceutical acronyms. Total ~ 625 words max Do not include the title or authors in the body of the abstract. Abstracts in outline form will be rejected. Submission Type Your abstract must be a Descriptive Report, Evaluative Study Report, or a Case Report. TASK 2: PRIMARY AUTHOR Review the primary author s information and make necessary edits. Click the Continue button to save your changes. Click the Save Primary Author button to move to the next task. Remember: Do not use ALL CAPS Include a period after your middle initial Do not place degrees in the Last Name field Add degrees in the credentials field TASK 3: AFFIRMATION OF CONTENT The primary author must affirm the content of the submission on behalf of all authors listed on the abstract. Affirmation indicates that all co-authors are aware of the content and the primary author or one of the co-authors will present the poster during the time assigned if the abstract submission is accepted. 4
5 TASK 4: CO-AUTHORS Each submission may have to up to five (5) authors, the primary author and four (4) additional authors. It is the responsibility of the primary author to ensure all authors are included and in the order they will appear on the abstract, citation, and on the poster display. ASHP will not add forgotten authors or make changes to the order of the authors. TASK 5: FINANCIAL RELATIONSHIP DISCLOSURE Only the financial relationships of the primary author must be disclosed. An individual has a relevant financial relationship if he or she (or spouse/domestic partner) has a financial relationship in any amount occurring in the last 12 months with a commercial interest whose products or services are discussed in the activity content over which the individual has control. TASK 6: CONFLICT OF INTEREST AGREEMENT The primary author must complete and sign the conflict of interest agreement terms for their submission. This includes agreeing to display the disclosures on the poster display. CONFIRMATION & PROPOSAL ID NUMBER After all the submission tasks are completed (shown with a green check mark) you must save your submission before you can submit it. Click the Submit button to submit the abstract. You will automatically get a confirmation with your submission details. Please save it for your records. Proposal ID Number: Your Proposal ID will appear on the screen with the list of tasks you completed as well as in your confirmation. Save this number for your records. INCOMPLETE SUBMISSIONS Incomplete submissions will be deleted from our online system (i.e. missing required elements, etc.). 5
6 NOTIFICATIONS After April 15, you will receive an notification indicating that your poster abstract has been accepted or rejected. All correspondence including confirmations, reminders, and accept/reject notifications will be sent to the primary author's address only. It is the primary author s responsibility to notify the coauthors of the status of the submission. Notification s will come from posters@ashp.org. WITHDRAWALS & CANCELLATIONS Written notification is required for all submission withdrawals. Only the primary author may withdraw a submission. Send your withdrawal request to: posters@ashp.org. Please include your full name, presentation title, and proposal ID number in all correspondence. Due to early publication deadlines, if you withdraw after receiving your acceptance notice we cannot guarantee that your presentation citation and/or abstract will not appear in print, on the ASHP Website, or in other print or electronic media. CONTACT INFORMATION If you have any questions regarding your submission, please send an to posters@ashp.org. Please include your name, title of submission, and your abstract submission number. ASHP will provide information to the primary author only. Thank you for your interest in presenting a poster at the ASHP Summer Meeting American Society of Health-System Pharmacists, Inc. ASHP is a service mark of the American Society of Health-System Pharmacists, Inc.; registered in the U.S. Patent and Trademark Office. 6
Training Guide for Arkansas Practitioners and Pharmacists. Arkansas Department of Health Prescription Monitoring Program
 Training Guide for Arkansas Practitioners and Pharmacists Arkansas Department of Health Prescription Monitoring Program May 2013 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 2 System
Training Guide for Arkansas Practitioners and Pharmacists Arkansas Department of Health Prescription Monitoring Program May 2013 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 2 System
Arkansas Prescription Drug Monitoring Program. User Support Manual
 Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
Canadian Neurological Sciences Federation Detailed Instructions for Submitting Abstracts
 Instructions for Online Abstract Submissions Authors are invited to submit an abstract, on or before January 15, 2014. Prepare your abstract in word processing or text editing software. Do not use Powerpoint
Instructions for Online Abstract Submissions Authors are invited to submit an abstract, on or before January 15, 2014. Prepare your abstract in word processing or text editing software. Do not use Powerpoint
BOARD OF PHARMACY SPECIALTIES POLICIES AND PROCEDURES
 BOARD OF PHARMACY SPECIALTIES POLICIES AND PROCEDURES XIII. Recertification Process A. General Information BPS mandates periodic recertification of an individual who is certified in any of its specialties
BOARD OF PHARMACY SPECIALTIES POLICIES AND PROCEDURES XIII. Recertification Process A. General Information BPS mandates periodic recertification of an individual who is certified in any of its specialties
Updated: 10/2016. Regional Meeting/ Specialty Conference. Abstract Submitter. MAPS User Guide
 Updated: 10/2016 Regional Meeting/ Specialty Conference Abstract Submitter MAPS User Guide Table of Contents GETTING STARTED...2 MAPS..2 Creating an ACS ID..2 CREATING A NEW ABSTRACT..3 Step1: Title/Body.4
Updated: 10/2016 Regional Meeting/ Specialty Conference Abstract Submitter MAPS User Guide Table of Contents GETTING STARTED...2 MAPS..2 Creating an ACS ID..2 CREATING A NEW ABSTRACT..3 Step1: Title/Body.4
Instructions for Authors Canadian Journal of Pathology (CJP)
 Instructions for Authors Canadian Journal of Pathology (CJP) Canadian Journal of Pathology is a quarterly, peer-reviewed publication. It is published by Clockwork Communications as the agent for Canadian
Instructions for Authors Canadian Journal of Pathology (CJP) Canadian Journal of Pathology is a quarterly, peer-reviewed publication. It is published by Clockwork Communications as the agent for Canadian
PDMP User s Guide. Oregon Health Authority Prescription Drug Monitoring Program
 Oregon Health Authority Prescription Drug Monitoring Program March 2014 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 System Overview... 3 About the RxSentry
Oregon Health Authority Prescription Drug Monitoring Program March 2014 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 System Overview... 3 About the RxSentry
Training Guide for Wisconsin Practitioners and Pharmacists. Pharmacy Examining Board Wisconsin Department of Safety and Professional Services
 Training Guide for Wisconsin Practitioners and Pharmacists Pharmacy Examining Board Wisconsin Department of Safety and Professional Services October 2013 Contents Contents 1 Document Overview... 1 Purpose
Training Guide for Wisconsin Practitioners and Pharmacists Pharmacy Examining Board Wisconsin Department of Safety and Professional Services October 2013 Contents Contents 1 Document Overview... 1 Purpose
Alabama PDMP AWA E. User Support Manual
 Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
ABSTRACT SUBMISSION GUIDELINES & INSTRUCTIONS
 ABSTRACT SUBMISSION GUIDELINES & INSTRUCTIONS Please read the following instructions on how to use the Oxford Abstracts Submission System to register your details, submit, edit or withdraw an abstract
ABSTRACT SUBMISSION GUIDELINES & INSTRUCTIONS Please read the following instructions on how to use the Oxford Abstracts Submission System to register your details, submit, edit or withdraw an abstract
New Mexico PDMP AWA E. User Support Manual
 New Mexico PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 10 2.3 Email Verification... 10 2.4 Validation
New Mexico PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 10 2.3 Email Verification... 10 2.4 Validation
IMPORTANT DATE: THE SUBMISSION DEADLINE FOR ALL ABSTRACTS IS EXTENDED TO Friday, March 9, 5:00 PM PT
 Thank you for your interest in submitting an Abstract for the 2018 Florida Society of Anesthesiologists Annual Meeting. This document is intended to be your guide in using the online submission software
Thank you for your interest in submitting an Abstract for the 2018 Florida Society of Anesthesiologists Annual Meeting. This document is intended to be your guide in using the online submission software
Training Guide for Practitioners. Washington State Department of Health Washington State Prescription Monitoring Program
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
ScholarOne Abstracts. Author User Guide
 ScholarOne Abstracts Author User Guide 26-November-2018 Clarivate Analytics ScholarOne Abstracts Author User Guide Page i TABLE OF CONTENTS Select an item in the table of contents to go to that topic in
ScholarOne Abstracts Author User Guide 26-November-2018 Clarivate Analytics ScholarOne Abstracts Author User Guide Page i TABLE OF CONTENTS Select an item in the table of contents to go to that topic in
Training Guide for Practitioners
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Ten Steps for Completing Your Submission
 Ten Steps for Completing Your Submission The ACC is currently accepting submissions for the Young Investigator Awards. All submitting authors must file up to date disclosures on the ACC's Disclosure Website
Ten Steps for Completing Your Submission The ACC is currently accepting submissions for the Young Investigator Awards. All submitting authors must file up to date disclosures on the ACC's Disclosure Website
THE SUBMISSION DEADLINE FOR ALL ABSTRACTS IS FRIDAY, SEPTEMBER 7, 2018, 5:00 PM PDT
 Thank you for your interest in submitting an abstract for the upcoming North American Skull Based Society Annual Meeting. This document is intended to be your guide in using the online submission software
Thank you for your interest in submitting an abstract for the upcoming North American Skull Based Society Annual Meeting. This document is intended to be your guide in using the online submission software
* Video abstract submissions must include a written abstract in addition to the video file.
 ABSTRACT SUBMISSION GUIDELINES FOR VIDEO SESSIONS On-line Abstract Submission Process Begins: August 1, 2017 Abstract Deadline Date for Video Sessions: November 6, 2017@ 11:59 PM EST * Video abstract submissions
ABSTRACT SUBMISSION GUIDELINES FOR VIDEO SESSIONS On-line Abstract Submission Process Begins: August 1, 2017 Abstract Deadline Date for Video Sessions: November 6, 2017@ 11:59 PM EST * Video abstract submissions
Orientation. Certification, Licensure, Registration. Pharmacy Technician Training Systems PassAssured, LLC
 Orientation Certification, Licensure, Registration Pharmacy Technician Training Systems PassAssured, LLC PTCB & ExCPT PassAssured's Pharmacy Technician Training Program Orientation Exams and Re Certification
Orientation Certification, Licensure, Registration Pharmacy Technician Training Systems PassAssured, LLC PTCB & ExCPT PassAssured's Pharmacy Technician Training Program Orientation Exams and Re Certification
IMPORTANT DATE: THE SUBMISSION DEADLINE FOR ALL ABSTRACTS IS OCTOBER 26, :00 PM PDT
 Thank you for your interest in submitting an abstract for the 2019 AHNS Annual Meeting, held during the Combined Otolaryngology Spring Meetings (COSM). This document is intended to be your guide in using
Thank you for your interest in submitting an abstract for the 2019 AHNS Annual Meeting, held during the Combined Otolaryngology Spring Meetings (COSM). This document is intended to be your guide in using
POLICY AND PROCEDURES OFFICE OF SURVEILLANCE AND EPIDEMIOLOGY. Procedures for Handling Requests for Proprietary Name Review.
 POLICY AND PROCEDURES OFFICE OF SURVEILLANCE AND EPIDEMIOLOGY Procedures for Handling Requests for Proprietary Name Review Table of Contents PURPOSE...1 BACKGROUND...1 POLICY...2 RESPONSIBILITIES AND PROCEDURES...3
POLICY AND PROCEDURES OFFICE OF SURVEILLANCE AND EPIDEMIOLOGY Procedures for Handling Requests for Proprietary Name Review Table of Contents PURPOSE...1 BACKGROUND...1 POLICY...2 RESPONSIBILITIES AND PROCEDURES...3
Thank you, and enjoy the webinar.
 Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws. This should not be used as legal advice. Itentive recognizes that
Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws. This should not be used as legal advice. Itentive recognizes that
ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL
 Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
All abstracts should be reviewed by meeting organisers prior to submission to BioMed Central to ensure suitability for publication.
 Abstract supplements - Guidelines for Organisers General requirements Abstracts submitted to the journal must be original and must not have been previously published elsewhere. Abstracts published on a
Abstract supplements - Guidelines for Organisers General requirements Abstracts submitted to the journal must be original and must not have been previously published elsewhere. Abstracts published on a
CHAP LinQ User Guide. CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC Version 1.
 2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
Updated: 10/2016. National Meeting. Abstract Submitter. MAPS User Guide
 Updated: 10/2016 National Meeting Abstract Submitter MAPS User Guide Table of Contents GETTING STARTED...2 MAPS 2 Creating an ACS ID.2 CREATING A NEW ABSTRACT.3 Step 1: Program Area (Division/Committee)
Updated: 10/2016 National Meeting Abstract Submitter MAPS User Guide Table of Contents GETTING STARTED...2 MAPS 2 Creating an ACS ID.2 CREATING A NEW ABSTRACT.3 Step 1: Program Area (Division/Committee)
Investigator Activities Quick Reference Guide. Sanofi/Genzyme October 2013
 Investigator Activities Quick Reference Guide Sanofi/Genzyme October 2013 Contents INVESTIGATOR ACTIVITIES QUICK REFERENCE GUIDE... 1 I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG IN... 6 III. HOW TO
Investigator Activities Quick Reference Guide Sanofi/Genzyme October 2013 Contents INVESTIGATOR ACTIVITIES QUICK REFERENCE GUIDE... 1 I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG IN... 6 III. HOW TO
IMPORTANT DATE: THE SUBMISSION DEADLINE FOR ALL ABSTRACTS IS JANUARY 5, 11:59 PM PST
 Thank you for your interest in submitting an abstract for the upcoming Emerging Technology Session. This document is intended to be your guide in using the online submission software and we strongly suggest
Thank you for your interest in submitting an abstract for the upcoming Emerging Technology Session. This document is intended to be your guide in using the online submission software and we strongly suggest
EPHAR Certification. EUROPEAN CERTIFIED PHARMACOLOGIST (EuCP) Guidelines for Certification
 EPHAR Certification EUROPEAN CERTIFIED PHARMACOLOGIST (EuCP) Guidelines for Certification current as of: 28.03.2014 Introduction The European Certification of Pharmacologists is a system of The Federation
EPHAR Certification EUROPEAN CERTIFIED PHARMACOLOGIST (EuCP) Guidelines for Certification current as of: 28.03.2014 Introduction The European Certification of Pharmacologists is a system of The Federation
Odyssey File & Serve. Firm Administrator User Guide Release 3.10
 Odyssey File & Serve Firm Administrator User Guide Release 3.10 OFS-FS-220-3324 v.1 April 2015 Copyright and Confidentiality Copyright 2015 Tyler Technologies, Inc. All rights reserved. All documentation,
Odyssey File & Serve Firm Administrator User Guide Release 3.10 OFS-FS-220-3324 v.1 April 2015 Copyright and Confidentiality Copyright 2015 Tyler Technologies, Inc. All rights reserved. All documentation,
Implementation of the Minor Ailment Service Produced by NES Pharmacy
 Implementation of the Minor Ailment Service Produced by NES Pharmacy Using emas Software SECTION 3 Using emas Software NHS pharmacists in Scotland can apply for a printed copy of this pack, please contact:
Implementation of the Minor Ailment Service Produced by NES Pharmacy Using emas Software SECTION 3 Using emas Software NHS pharmacists in Scotland can apply for a printed copy of this pack, please contact:
MPRC Guidelines & Requirements
 MPRC Guidelines & Requirements MPRC Website Registration for the Midwest Pharmacy Residents Conference (MPRC) must be submitted online. To register for the conference and obtain further information and
MPRC Guidelines & Requirements MPRC Website Registration for the Midwest Pharmacy Residents Conference (MPRC) must be submitted online. To register for the conference and obtain further information and
Abstract Submission Instructions
 Abstract Submission Instructions Entering Abstract Information A. Title 1. Do not bold, italicize, underline, superscript or subscript any items in the title. 2. Do not include authors in the title. If
Abstract Submission Instructions Entering Abstract Information A. Title 1. Do not bold, italicize, underline, superscript or subscript any items in the title. 2. Do not include authors in the title. If
Guide to Use of the CFP Marks by Education Partners
 Guide to Use of the CFP Marks by Education Partners CFP CM, CERTIFIED FINANCIAL PLANNER CM and are certification marks owned outside the U.S. by Financial Planning Standards Board Ltd. (FPSB). Financial
Guide to Use of the CFP Marks by Education Partners CFP CM, CERTIFIED FINANCIAL PLANNER CM and are certification marks owned outside the U.S. by Financial Planning Standards Board Ltd. (FPSB). Financial
Personal Online Banking Quick Start Guide
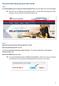 Personal Online Banking Quick Start Guide Step 1 Visit AmericanBank.com and locate the Personal Login ID box in the top right corner of the homepage. TIP: There are now two different online banking systems
Personal Online Banking Quick Start Guide Step 1 Visit AmericanBank.com and locate the Personal Login ID box in the top right corner of the homepage. TIP: There are now two different online banking systems
European Congress on Thrombosis and Haemostasis Abstract submission guidelines
 European Congress on Thrombosis and Haemostasis Abstract submission guidelines Table of contents Introduction The abstract submission guidelines of the ECTH Congress are intended to provide clear instructions
European Congress on Thrombosis and Haemostasis Abstract submission guidelines Table of contents Introduction The abstract submission guidelines of the ECTH Congress are intended to provide clear instructions
IMPORTANT DATE: THE SUBMISSION DEADLINE FOR ALL ABSTRACTS AND VIDEOS IS SEPTEMBER 21, :59 PM PDT
 Thank you for your interest in submitting an abstract for the upcoming Scientific Session. This document is intended to be your guide in using the online submission software and we strongly suggest you
Thank you for your interest in submitting an abstract for the upcoming Scientific Session. This document is intended to be your guide in using the online submission software and we strongly suggest you
Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives
 Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives Arkansas Department of Health Prescription Monitoring Program March 2016 Contents Contents 1 Document Overview...
Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives Arkansas Department of Health Prescription Monitoring Program March 2016 Contents Contents 1 Document Overview...
ACC.19 FIT Clinical Decision Making Submission Instructions
 ACC.19 FIT Clinical Decision Making Submission Instructions Eligibility for Submission Eligibility for FIT Clinical Decision Making: Anyone who is currently a medical student or in a fellowship or residency
ACC.19 FIT Clinical Decision Making Submission Instructions Eligibility for Submission Eligibility for FIT Clinical Decision Making: Anyone who is currently a medical student or in a fellowship or residency
Pre-registration Exam workshop 2017
 Pre-registration Exam workshop 2017 Programme TIME ITEM PRESENTER 09:00 09:30 Welcome and SAPC presentation SAPC (30 min) 09:30 10:30 Examination Paper 1 Facilitator (60 min) 10:30-10:45 SHORT BREAK 15
Pre-registration Exam workshop 2017 Programme TIME ITEM PRESENTER 09:00 09:30 Welcome and SAPC presentation SAPC (30 min) 09:30 10:30 Examination Paper 1 Facilitator (60 min) 10:30-10:45 SHORT BREAK 15
Reminder: Please refer to the 2018 Convening & Collaborating (C 2 ) competition page for more details.
 Reminder: Please refer to the 2018 Convening & Collaborating (C 2 ) competition page for more details. Starting the Process Login to MSFHR ApplyNet. IMPORTANT: If you have forgotten your login ID for your
Reminder: Please refer to the 2018 Convening & Collaborating (C 2 ) competition page for more details. Starting the Process Login to MSFHR ApplyNet. IMPORTANT: If you have forgotten your login ID for your
PCORI Online: Awardee User Guide Research Awards
 PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
American Diabetes Association Scientific Sessions Online Disclosure Collection System
 American Diabetes Association Scientific Sessions Online Disclosure Collection System To simplify the disclosure collection process, ADA has implemented a new online disclosure collection system for the
American Diabetes Association Scientific Sessions Online Disclosure Collection System To simplify the disclosure collection process, ADA has implemented a new online disclosure collection system for the
Frequently Asked Questions Electronic Filing
 SECOND JUDICIAL DISTRICT COURT WASHOE COUNTY STATE OF NEVADA Frequently Asked Questions Electronic Filing Revised February 17, 2017 Who can efile? Anyone can efile, if they have an account. If you are
SECOND JUDICIAL DISTRICT COURT WASHOE COUNTY STATE OF NEVADA Frequently Asked Questions Electronic Filing Revised February 17, 2017 Who can efile? Anyone can efile, if they have an account. If you are
DO NOT SEND DUPLICATE COPIES OF YOUR LOG AND DO NOT SEND A PRINTED COPY.
 AMERICAN BOARD OF UROLOGY 2018 LIFE LONG LEARNING (LLL) LEVEL 2 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions
AMERICAN BOARD OF UROLOGY 2018 LIFE LONG LEARNING (LLL) LEVEL 2 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions
National Standards of Continued Competence In Acupuncture and Oriental Medicine NCCAOM
 NCCAOM PDA Application: National Standards of Continued Competence In Acupuncture and Oriental Medicine NCCAOM PDA Department 76 S. Laura Street, Suite 1290 Jacksonville, FL 32202 904-598-1005 www.nccaom.org
NCCAOM PDA Application: National Standards of Continued Competence In Acupuncture and Oriental Medicine NCCAOM PDA Department 76 S. Laura Street, Suite 1290 Jacksonville, FL 32202 904-598-1005 www.nccaom.org
EBSCO Publishing Health Library Editorial Policy
 EBSCO Publishing Health Library Editorial Policy Introduction EBSCO Publishing is a leader in publishing health and medical information on the Internet. While we make every effort to ensure that our content
EBSCO Publishing Health Library Editorial Policy Introduction EBSCO Publishing is a leader in publishing health and medical information on the Internet. While we make every effort to ensure that our content
LIFE LONG LEARNING LEVEL INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS
 AMERICAN BOARD OF UROLOGY LIFE LONG LEARNING LEVEL 2 2018 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions carefully before preparing your log. It is imperative that you carefully
AMERICAN BOARD OF UROLOGY LIFE LONG LEARNING LEVEL 2 2018 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions carefully before preparing your log. It is imperative that you carefully
ICIS 2019 Review Guide Tips and Tricks
 ICIS 2019 Review Guide Tips and Tricks Table of Contents Table of Contents... 1 Getting started... 1 Logging In... 1 Reviewing Submissions... 6 Submitting your review... 7 Getting started Logging In 1.
ICIS 2019 Review Guide Tips and Tricks Table of Contents Table of Contents... 1 Getting started... 1 Logging In... 1 Reviewing Submissions... 6 Submitting your review... 7 Getting started Logging In 1.
Reference Guide for Students
 Ministry of Training, Colleges and Universities PARIS Program Approval and Registration Information System Reference Guide for Students February 2019 Table of Contents Table of Contents Introduction...
Ministry of Training, Colleges and Universities PARIS Program Approval and Registration Information System Reference Guide for Students February 2019 Table of Contents Table of Contents Introduction...
Step By Step Registration Process
 The Florida Prescription Drug Monitoring Program, known as E FORCSE (Electronic Florida Online Reporting of Controlled Substance Evaluation Program), was created by the 2009 Florida Legislature in an initiative
The Florida Prescription Drug Monitoring Program, known as E FORCSE (Electronic Florida Online Reporting of Controlled Substance Evaluation Program), was created by the 2009 Florida Legislature in an initiative
erequest Frequently Asked Questions
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what we can support or sponsor. Online requests will go through an internal review and approval
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what we can support or sponsor. Online requests will go through an internal review and approval
Long-Term and Cross-Disciplinary Fellowships Instructions for host supervisors
 Long-Term and Cross-Disciplinary Fellowships 2019 Instructions for host supervisors The host supervisor is defined as the senior scientist who will mentor the applicant in the proposed host institution
Long-Term and Cross-Disciplinary Fellowships 2019 Instructions for host supervisors The host supervisor is defined as the senior scientist who will mentor the applicant in the proposed host institution
Candidate Handbook. Application and Registration Instructions for the Certified Pain Educator (CPE) Examination
 Candidate Handbook Candidate Handbook Application and Registration Instructions for the Certified Pain Educator (CPE) Examination American Society Of Pain Educators (ASPE) 6 Erie Street, Montclair, NJ
Candidate Handbook Candidate Handbook Application and Registration Instructions for the Certified Pain Educator (CPE) Examination American Society Of Pain Educators (ASPE) 6 Erie Street, Montclair, NJ
NHS Education for Scotland Portal https://www.portal.scot.nhs.uk Dental Audit: A user guide from application to completion
 Dental Audit: A user guide from application to completion 1. Audit Guidance 2. New Application: Getting Started 3. New Application: The Audit Application Form 4. New Application: Submitting Your Application
Dental Audit: A user guide from application to completion 1. Audit Guidance 2. New Application: Getting Started 3. New Application: The Audit Application Form 4. New Application: Submitting Your Application
Odyssey File & Serve. Firm Administrator User Guide Release 3.14
 Odyssey File & Serve Firm Administrator User Guide Release 3.14 OFS-FS-220-3680 v.1 April 2016 COPYRIGHT AND CONFIDENTIALITY Copyright 2016 Tyler Technologies, Inc. All rights reserved. Use of these materials
Odyssey File & Serve Firm Administrator User Guide Release 3.14 OFS-FS-220-3680 v.1 April 2016 COPYRIGHT AND CONFIDENTIALITY Copyright 2016 Tyler Technologies, Inc. All rights reserved. Use of these materials
DISCLOSER GUIDE. Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure. Submitting Disclosures Post-Submission Actions
 DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
VISIONTRACKER FREQUENTLY ASKED QUESTIONS FAQ
 VISIONTRACKER FREQUENTLY ASKED QUESTIONS FAQ FREQUENTLY ASKED QUESTIONS 1.1. TABLE OF CONTENTS 1. Frequently Asked Questions... 1 1.1. Table of Contents... 1 1.2. How to View or Open a Saved Application...
VISIONTRACKER FREQUENTLY ASKED QUESTIONS FAQ FREQUENTLY ASKED QUESTIONS 1.1. TABLE OF CONTENTS 1. Frequently Asked Questions... 1 1.1. Table of Contents... 1 1.2. How to View or Open a Saved Application...
Registration and Award Application Full Text
 Registration and Award Application Full Text The following document is a text preview of our online application tool. All website logic has been moved for readability. All registrations and award applications
Registration and Award Application Full Text The following document is a text preview of our online application tool. All website logic has been moved for readability. All registrations and award applications
AUSTRALIAN INTERN WRITTEN EXAM
 AUSTRALIAN INTERN WRITTEN EXAM Graduates of approved Australian and New Zealand pharmacy programs who hold provisional registration with the Pharmacy Board of Australia (PBA) or equivalent registration
AUSTRALIAN INTERN WRITTEN EXAM Graduates of approved Australian and New Zealand pharmacy programs who hold provisional registration with the Pharmacy Board of Australia (PBA) or equivalent registration
Training Guide for Alabama Practitioners and Pharmacists. Alabama Department of Public Health Prescription Drug Monitoring Program
 Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program August 2015 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program August 2015 Contents Contents 1 Document Overview... 3 Purpose and Contents...
HCPIR Job Aid Massage Therapy Submissions. Purpose. Overview
 Purpose As of April 1, 2019, the Health Care Provider Invoicing and Reporting (HCPIR) application is available to support Massage Therapy practitioners. The HCPIR application is designed to streamline
Purpose As of April 1, 2019, the Health Care Provider Invoicing and Reporting (HCPIR) application is available to support Massage Therapy practitioners. The HCPIR application is designed to streamline
JITs Portal. Quick Start Guide
 JITs Portal Quick Start Guide December 2017 Table of Contents 1. INTRODUCTION... 2 2. GETTING STARTED... 2 2.1. JITs Portal Home Page (Public site)... 2 2.2. JITs Portal Home Page (Authenticated area)...
JITs Portal Quick Start Guide December 2017 Table of Contents 1. INTRODUCTION... 2 2. GETTING STARTED... 2 2.1. JITs Portal Home Page (Public site)... 2 2.2. JITs Portal Home Page (Authenticated area)...
HCPIR Job Aid Counselling Submissions. Purpose. Overview
 Purpose As of April 1, 2019, the Health Care Provider Invoicing and Reporting (HCPIR) application is available to support Counselling practitioners. The HCPIR application is designed to streamline the
Purpose As of April 1, 2019, the Health Care Provider Invoicing and Reporting (HCPIR) application is available to support Counselling practitioners. The HCPIR application is designed to streamline the
INVESTIGATION REPORT , , ,
 INVESTIGATION REPORT 206-2018, 207-2018, 208-2018, 214-2018 ehealth Saskatchewan and University of Saskatchewan January 29, 2019 Summary: ehealth Saskatchewan (ehealth) detected that two medical residents
INVESTIGATION REPORT 206-2018, 207-2018, 208-2018, 214-2018 ehealth Saskatchewan and University of Saskatchewan January 29, 2019 Summary: ehealth Saskatchewan (ehealth) detected that two medical residents
IRMA Researcher User Guide v2 DRAFT. IRMA Researcher User Guide
 IRMA Researcher User Guide v2 IRMA Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets
IRMA Researcher User Guide v2 IRMA Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets
INITIATING A WORKDAY SECURITY ACCESS REQUEST
 INITIATING A PURPOSE This user guide provides an in-depth look at the functionality and characteristics of the Workday Security Access Request form and how to initiate a request for access to various LSU
INITIATING A PURPOSE This user guide provides an in-depth look at the functionality and characteristics of the Workday Security Access Request form and how to initiate a request for access to various LSU
Guidelines for Oral/Poster Abstract Submission
 Guidelines for Oral/Poster Abstract Submission Contents General Abstract Submission Guidelines... 2 Oral/Poster Abstract Submission Guidelines... 2 1. Select Major Section:... 2 2. Select the Most Appropriate
Guidelines for Oral/Poster Abstract Submission Contents General Abstract Submission Guidelines... 2 Oral/Poster Abstract Submission Guidelines... 2 1. Select Major Section:... 2 2. Select the Most Appropriate
Patient Portal Guide
 Patient Portal Guide Please navigate to www.coendo.com. In the upper right hand portion of the screen, you will notice a link to the patient portal: From there, you will be brought to the Portal login
Patient Portal Guide Please navigate to www.coendo.com. In the upper right hand portion of the screen, you will notice a link to the patient portal: From there, you will be brought to the Portal login
HRA Open User Guide for Awardees
 HRA Open User Guide for Awardees HRA Open is an web-based service sponsored by the Health Research Alliance (HRA) that promotes open access by linking your award information with PubMed Central so you
HRA Open User Guide for Awardees HRA Open is an web-based service sponsored by the Health Research Alliance (HRA) that promotes open access by linking your award information with PubMed Central so you
Activity Director Portal Online Application 2017 Reference Guide
 Activity Director Portal Online Application 2017 Reference Guide Table of Contents Reference Notes... 6 Status:... 6 Start new application... 7 Step 1 of 21: Basic Application Information... 7 Application
Activity Director Portal Online Application 2017 Reference Guide Table of Contents Reference Notes... 6 Status:... 6 Start new application... 7 Step 1 of 21: Basic Application Information... 7 Application
ACC.19 Late Breaking Clinical Trial Submission Instructions
 ACC.19 Late Breaking Clinical Trial Submission Instructions Entering LBCT Information A. Title 1. Do not bold, italicize, underline, superscript or subscript any items in the title. 2. Do not include authors
ACC.19 Late Breaking Clinical Trial Submission Instructions Entering LBCT Information A. Title 1. Do not bold, italicize, underline, superscript or subscript any items in the title. 2. Do not include authors
Patient Portal: Policies and Procedures & User Reference Guide Patient Portal Version 5.8.1
 Patient Portal: Policies and Procedures & User Reference Guide Patient Portal Version 5.8.1 1 Welcome to the Patient Portal We would like to welcome you to the Patient Portal. The Patient Portal is a secure
Patient Portal: Policies and Procedures & User Reference Guide Patient Portal Version 5.8.1 1 Welcome to the Patient Portal We would like to welcome you to the Patient Portal. The Patient Portal is a secure
AMERICAN BOARD OF UROLOGY 2017 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS
 AMERICAN BOARD OF UROLOGY 2017 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions carefully before preparing your log. It is imperative that you carefully review the data contained
AMERICAN BOARD OF UROLOGY 2017 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions carefully before preparing your log. It is imperative that you carefully review the data contained
2017 MOC PEDIATRIC PRACTICE LOG TEMPLATE:
 AMERICAN BOARD OF UROLOGY 2017 MAINTENANCE OF CERTIFICATION (MOC) Level 4 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all
AMERICAN BOARD OF UROLOGY 2017 MAINTENANCE OF CERTIFICATION (MOC) Level 4 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all
Act CXII of 2011 on the right to information self-determination and freedom of information. Act ;
 PRIVACY POLICY THE COMPANY'S DATA MANAGEMENT PRINCIPLES M2M Rendszerház Kft. and WM Systems LLC. (hereinafter referred to as the Company as a joint Data Administrator) provide detailed information management
PRIVACY POLICY THE COMPANY'S DATA MANAGEMENT PRINCIPLES M2M Rendszerház Kft. and WM Systems LLC. (hereinafter referred to as the Company as a joint Data Administrator) provide detailed information management
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
Welcome to the. Patient Portal!
 Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider and take control of your medical information. Using this quick reference guide,
Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider and take control of your medical information. Using this quick reference guide,
2017 PTCOG NA Conference Abstract Submission Form
 2017 PTCOG NA Conference Abstract Submission Form Deadline is June 14, 2017 Submitter s Name Submitter s Email Abstract Title Please follow instructions carefully. Do not use TRADE NAMES OR INSTITUTIONS
2017 PTCOG NA Conference Abstract Submission Form Deadline is June 14, 2017 Submitter s Name Submitter s Email Abstract Title Please follow instructions carefully. Do not use TRADE NAMES OR INSTITUTIONS
Provider Portal. User Manual. Therapists and Health Practitioners
 Provider Portal User Manual Therapists and Health Practitioners Table of Contents 1. Introduction... 3 2. Registering for the Provider Portal... 4 i. Changing Your Password...6 ii. Accepting Terms and
Provider Portal User Manual Therapists and Health Practitioners Table of Contents 1. Introduction... 3 2. Registering for the Provider Portal... 4 i. Changing Your Password...6 ii. Accepting Terms and
Odyssey File & Serve. Review Queue User Guide Release 3.11
 Odyssey File & Serve Review Queue User Guide Release 3.11 OFS-FS 200 3375 v.1 June 2015 COPYRIGHT AND CONFIDENTIALITY Copyright 2015 Tyler Technologies, Inc. All rights reserved. All documentation, source
Odyssey File & Serve Review Queue User Guide Release 3.11 OFS-FS 200 3375 v.1 June 2015 COPYRIGHT AND CONFIDENTIALITY Copyright 2015 Tyler Technologies, Inc. All rights reserved. All documentation, source
How to access My.QuestForHealth.com
 How to access My.QuestForHealth.com Go to HertzCareCoordinators.com and log in to your account. Click My Plan on the top of the page and then click Rally Health. After you log in to your Rally account,
How to access My.QuestForHealth.com Go to HertzCareCoordinators.com and log in to your account. Click My Plan on the top of the page and then click Rally Health. After you log in to your Rally account,
Certification and Re-Certification Policies and Procedures
 National Wellness Institute Certified Worksite Wellness Specialist (CWWS) and Program Manager (CWWPM) expiring between 3/1/2016 and 12/31/2018 Certification and Re-Certification Policies and Procedures
National Wellness Institute Certified Worksite Wellness Specialist (CWWS) and Program Manager (CWWPM) expiring between 3/1/2016 and 12/31/2018 Certification and Re-Certification Policies and Procedures
IRMA Human Ethics Researcher User Guide
 IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
The Lilly Safety Mailing Process
 The Lilly Safety Mailing Process 1 After this presentation you will be able to: Define Safety Mailings and the type of adverse events that trigger safety mailings. Define Principal Investigator (PI) and
The Lilly Safety Mailing Process 1 After this presentation you will be able to: Define Safety Mailings and the type of adverse events that trigger safety mailings. Define Principal Investigator (PI) and
Frequently Asked Questions (FAQs) Relating to the SNIA s New IP Policy v4
 Frequently Asked Questions (FAQs) Relating to the SNIA s New IP Policy v4 In September of 2015, the SNIA Board of Directors voted to approve an updated version of the SNIA Intellectual Property Policy
Frequently Asked Questions (FAQs) Relating to the SNIA s New IP Policy v4 In September of 2015, the SNIA Board of Directors voted to approve an updated version of the SNIA Intellectual Property Policy
Sanofi Investigator Sponsored Studies (ISS) External Reference Guide. 1 November 2017
 Sanofi Investigator Sponsored Studies (ISS) External Reference Guide 1 November 2017 Sanofi ISS Overview Sanofi is committed to supporting medically and scientifically sound research aimed at the advancement
Sanofi Investigator Sponsored Studies (ISS) External Reference Guide 1 November 2017 Sanofi ISS Overview Sanofi is committed to supporting medically and scientifically sound research aimed at the advancement
PHARM 309 Secondary Resources Terry Ann Jankowski, MLS, AHIP 9 Oct 2006
 PHARM 309 Secondary Resources Terry Ann Jankowski, MLS, AHIP 9 Oct 2006 Lecture objective: By the end of this lecture (and with a little practice outside of class), you will be able to: Define secondary
PHARM 309 Secondary Resources Terry Ann Jankowski, MLS, AHIP 9 Oct 2006 Lecture objective: By the end of this lecture (and with a little practice outside of class), you will be able to: Define secondary
Patient Portal Guide
 Patient Portal Guide Please navigate to www.tnpeds.com. In the upper right hand portion of the screen, you will notice a link to the patient portal: From there, you will be brought to the Portal login
Patient Portal Guide Please navigate to www.tnpeds.com. In the upper right hand portion of the screen, you will notice a link to the patient portal: From there, you will be brought to the Portal login
PORTAL TRANSITION GUIDE. 1 February 2019
 PORTAL TRANSITION GUIDE 1 February 2019 PORTAL TRANSITION GUIDE Portal Transition Guide - 1 February 2019 1 WELCOME The Pharmacy Programs Administrator welcomes you to the new Registration and Claiming
PORTAL TRANSITION GUIDE 1 February 2019 PORTAL TRANSITION GUIDE Portal Transition Guide - 1 February 2019 1 WELCOME The Pharmacy Programs Administrator welcomes you to the new Registration and Claiming
efiletexas.gov Review Queue User Guide Release
 efiletexas.gov Review Queue User Guide Release 2017.1 EFS-TF-200-4075 v.1 October 2017 Copyright and Confidentiality Copyright 2017 Tyler Technologies, Inc. All rights reserved Use of these materials is
efiletexas.gov Review Queue User Guide Release 2017.1 EFS-TF-200-4075 v.1 October 2017 Copyright and Confidentiality Copyright 2017 Tyler Technologies, Inc. All rights reserved Use of these materials is
RelayHealth Legal Notices
 Page 1 of 7 RelayHealth Legal Notices PRIVACY POLICY Revised August 2010 This policy only applies to those RelayHealth services for which you also must accept RelayHealth s Terms of Use. RelayHealth respects
Page 1 of 7 RelayHealth Legal Notices PRIVACY POLICY Revised August 2010 This policy only applies to those RelayHealth services for which you also must accept RelayHealth s Terms of Use. RelayHealth respects
ADEA PASS CUSTOMER SERVICE
 May 2014 April 2015 -2- TABLE OF CONTENTS Customer Service... 2 Overview... 3 ETS PPI for ADEA: Steps at a Glance... 3 Terms and Conditions... 3 ETS E-Records Disclosure... 3 ETS Data Transfer... 4 Federal
May 2014 April 2015 -2- TABLE OF CONTENTS Customer Service... 2 Overview... 3 ETS PPI for ADEA: Steps at a Glance... 3 Terms and Conditions... 3 ETS E-Records Disclosure... 3 ETS Data Transfer... 4 Federal
Evaluating and Reviewing Proposals in PAPERS
 Evaluating and Reviewing Proposals in PAPERS Contents Deadlines... 2 Logging In... 2 Welcome Page... 4 Rating and Commenting on Proposals... 5 Reviewing Papers Sessions.... 7 Anonymity and Ratings/Comments
Evaluating and Reviewing Proposals in PAPERS Contents Deadlines... 2 Logging In... 2 Welcome Page... 4 Rating and Commenting on Proposals... 5 Reviewing Papers Sessions.... 7 Anonymity and Ratings/Comments
Research Poster Forum Guidelines
 Research Poster Forum Guidelines Eligibility All osteopathic medical students, residents, and faculty in Arizona. General One entry per person Do not need to register for the AOMA Convention in order to
Research Poster Forum Guidelines Eligibility All osteopathic medical students, residents, and faculty in Arizona. General One entry per person Do not need to register for the AOMA Convention in order to
SCHOLARONE ABSTRACTS GLOSSARY
 SCHOLARONE ABSTRACTS GLOSSARY Abstract Book Compilation of submitted abstracts sorted by client specifications; an export type. Abstract Details Submission step which can include client-specific custom
SCHOLARONE ABSTRACTS GLOSSARY Abstract Book Compilation of submitted abstracts sorted by client specifications; an export type. Abstract Details Submission step which can include client-specific custom
COEUS IRB MODULE Written & Designed by: Maria Martinez Weill Medical College of Cornell
 COEUS IRB MODULE Table of Contents Short Cut Identification Key 3 Searching How To s 6 How to Run Reports 11 Protocol Screen Description 13 How to Enter a New Protocol 18 How to Submit a Protocol for Review
COEUS IRB MODULE Table of Contents Short Cut Identification Key 3 Searching How To s 6 How to Run Reports 11 Protocol Screen Description 13 How to Enter a New Protocol 18 How to Submit a Protocol for Review
Graduate Research Fellowship Program. Applicant User Guide. October 21, Version 2.3
 H E L P D O C U M E N T A T I O N Graduate Research Fellowship Program Applicant User Guide October 21, 2006 Version 2.3 TABLE OF CONTENTS 1 CONVENTIONS USED IN THIS GUIDE... 1 2 GETTING ACCESS... 2 2.1
H E L P D O C U M E N T A T I O N Graduate Research Fellowship Program Applicant User Guide October 21, 2006 Version 2.3 TABLE OF CONTENTS 1 CONVENTIONS USED IN THIS GUIDE... 1 2 GETTING ACCESS... 2 2.1
ATS 2016 Call for Abstracts. Instructions for Title, Type, Category and Presentation Preference
 ATS 2016 Call for Abstracts Instructions for Title, Type, Category and Presentation Preference Add Title Although there are limitations to the text formatting of the abstract title, submitters can still
ATS 2016 Call for Abstracts Instructions for Title, Type, Category and Presentation Preference Add Title Although there are limitations to the text formatting of the abstract title, submitters can still
In Basket Folder Overview Epic Ambulatory Training Document
 In Basket Folder Overview Epic Ambulatory Training Document Purpose This document should be used as a guide for faculty and staff to use when working tasks within the Epic In This reference tool provides
In Basket Folder Overview Epic Ambulatory Training Document Purpose This document should be used as a guide for faculty and staff to use when working tasks within the Epic In This reference tool provides
