Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0)
|
|
|
- Randolf Leonard
- 5 years ago
- Views:
Transcription
1 Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0)
2 Internal Investigators These are BU/BMC faculty and staff Boston Public Health Commission and Boston Healthnet Community Health Center investigators are also internal investigators for BUMC IRB protocols Internal investigators must be listed in Section 3 of the IRB application In order to be listed, all internal investigators and study staff MUST have a BU username and Kerberos password Investigators from other locations and other institutions are not listed under Internal investigators (they are added in Section 4 External Investigators)
3 Internal Investigators An Internal Study Personnel Change Form can be made to add/remove internal investigators from Section 3 at any time. An Internal Study Personnel Change Form can be made While another Internal Study Personnel Change Form is pending While another standard amendment is pending While a Progress Report (continuing review) is pending An Internal Study Personnel Change Form cannot be used to remove study staff from Section 5 (migrated from INSPIR I) - a standard amendment is necessary An Internal Study Personnel Change Form cannot be used to add External Investigators a standard amendment must be submitted An Internal Study Personnel Change Form should be used whenever new staff is added this should not be done on a standard amendment or as part of a progress report
4 Login into INSPIR and from your Home page, click on My Studies to list all the studies that you have access to
5 This is my personal My Studies page You can use the filter or the Find by feature to locate the study or draft. Or you can just scroll down. Once you locate the study or draft, click on Open.
6 Click on Internal Study Personnel Changes)- blue arrow
7 This will bring you to the Internal Study Personnel Changes page. (blue arrow) Here you will see any previous personnel changes (yellow arrow) To open a new Internal Study Personnel Change form click on the Add New Form button (red arrow)
8 This will bring you to the Amendment information page. In section 1.1 describe the changes requested (e.g. Add Danielle Littee to protocol) Then scroll down to complete the form. (yellow arrow)
9 Adding New Internal Investigators to a Study
10 Locate the appropriate section/role; and using the Add buttons (yellow arrow), add the personnel to the application. Clicking on the Add button will bring you to the Directories where you can search for the investigators by name.
11 When you get to the Search User Directory page (blue arrow) 1- Enter the last name (green arrow) 2- Click on the Find button (yellow arrow) to search for the person. 1 Banks 2 Tip If you can t find this person in INSPIR, check the radio button for LDAP Directory (purple arrow), and try again. If you still can t find him/her, then they should submit the following form to IT:
12 A list of people in INSPIR with that last name will be displayed. Locate the person you re looking to add and click on the check symbol ( ) (red arrow) to add this person to the application. This will take you back to the Internal Study personnel changes form. Note: Here, you can check the training status of the person you are adding by clicking on the head icon ( green arrow)
13 The person that you just added should show up now (yellow arrow). Some sections, requires that you select the user s role in the study from a drop-down menu- (green arrow). Select the user s role when required. To add more staff, repeat the instructions in the previous three screens.
14 Notes for adding New Investigators If the new investigator(s) that you add will need to receive notifications about the protocol and be assigned tasks in their Incomplete Tasks, you will also have to add their name(s) under Add a Study Contact. Study Contact is not a study role. So anyone that is listed as a Study Contact will also need to be listed under another study role such as A) Additional Investigators or B) Research Staff, if they are not already listed there.
15 Requesting the Removal of Existing Internal Investigators from the Study
16 To request for someone to be removed from the study, scroll down to the section titled Please select any existing Personnel you wish to remove:, and click on Select. This will take you to the next screen with the list of all personnel that are currently listed on the study.
17 Select the one(s) that you want to remove by clicking on the check box(es) next to their name(s). Then click on Save Selections
18 The list of people that you want removed from the study should appear in this section. Note: If you make a mistake, you can unselect someone by clicking on the check box next to the name and then on De-select to remove that person from this list. If you de-select someone, that person will no longer be included in the list of people that you want removed from the study, and thus, will remain on the study.
19 Submitting the Form Once you have added all the new investigators/study staff, or/and selected all the existing investigators/study staff that you wish to remove, scroll to the last section Training and COI verification. You will need to agree to both statements (even though they don t apply if you re only removing personnel from the study), before the system will allow you to proceed. When done, click on the Save and Continue to the Next Section button in the upper right hand corner This will bring you to the Form Has Been Completed page. From here you will send the Internal Study Personnel Change Form to the PI for sign-off (even if you are the PI completing the form, you will need to go to the sign-off page to authenticate).
20 Select the notify PI to signoff button to send this amendment to the PI for signoff before it goes to the IRB (yellow arrow) The PI must sign off on these amendments even if the PI completed the this form. After you click the notify PI button, you will get a popup message saying that the PI has been notified about this signoff
21 PI Signoff The PI will receive an notice that there is a protocol (in this case an Internal Study Personnel Change Form ) awaiting his/her signoff The PI will also be able to locate this task by going to his/her homepage and looking under Incomplete Tasks Go to the IRB website ( and under Instructions for Investigator click on How to sign off on a protocol PI for further instructions.
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II
 How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
How to Create and Submit an Amendment in INSPIR II
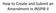 How to Create and Submit an Amendment in INSPIR II Login into INSPIR and from your Home page, click on My Studies to list all the studies that you have access to Locate the study and click on the Open
How to Create and Submit an Amendment in INSPIR II Login into INSPIR and from your Home page, click on My Studies to list all the studies that you have access to Locate the study and click on the Open
Review Response Submission Form for Continuing Review Form
 Review Response Submission Form for Continuing Review Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it,
Review Response Submission Form for Continuing Review Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it,
Review Response Submission Form
 Review Response Submission Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it, returns it as Incomplete
Review Response Submission Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it, returns it as Incomplete
University of the Sciences. IRBNet User s Guide
 University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
Create a new Human Research Ethics Application in InfoEd
 Create a new Human Research Ethics Application in InfoEd IMPORTANT! A Co-Investigator or Student can create, complete and submit new human research ethics applications in InfoEd on behalf of the Chief
Create a new Human Research Ethics Application in InfoEd IMPORTANT! A Co-Investigator or Student can create, complete and submit new human research ethics applications in InfoEd on behalf of the Chief
SUBMITTING A NEW IBC PROJECT APPLICATION (INITIAL)
 SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
STUDY ASSISTANT. Adding a New Study & Submitting to the Review Board. Version 10.03
 STUDY ASSISTANT Adding a New Study & Submitting to the Review Board Version 10.03 Contents Introduction... 3 Add a Study... 3 Selecting an Application... 3 1.0 General Information... 3 2.0 Add Department(s)...
STUDY ASSISTANT Adding a New Study & Submitting to the Review Board Version 10.03 Contents Introduction... 3 Add a Study... 3 Selecting an Application... 3 1.0 General Information... 3 2.0 Add Department(s)...
6. To access the online IRB protocol module, click the "My IRB Protocol" link found in the top navigation of any CoeusLite screen
 IPFW CoeusLite Online IRB Process CoeusLite IRB Login 1. Open a web browser page and go to: https://coeus.itap.purdue.edu/coeus/userauthaction.do 2. Enter your Career Account User Name in the USERNAME
IPFW CoeusLite Online IRB Process CoeusLite IRB Login 1. Open a web browser page and go to: https://coeus.itap.purdue.edu/coeus/userauthaction.do 2. Enter your Career Account User Name in the USERNAME
Guide for Researchers: Online Human Ethics Application Form
 Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
RWANDA DEVELOPMENT BOARD TOURISM LICENSING SYSTEM
 RWANDA DEVELOPMENT BOARD TOURISM LICENSING SYSTEM FRONTEND USER MANUAL 1 P a g e Contents 1.1 ACCESSING THE SYSTEM... 3 1.2 REGISTRATION... 3 1.3 LOGGING IN... 4 1. 4 DASHBOARD... 5 1.5 LOGGING OUT...
RWANDA DEVELOPMENT BOARD TOURISM LICENSING SYSTEM FRONTEND USER MANUAL 1 P a g e Contents 1.1 ACCESSING THE SYSTEM... 3 1.2 REGISTRATION... 3 1.3 LOGGING IN... 4 1. 4 DASHBOARD... 5 1.5 LOGGING OUT...
Logging into the system
 Logging into the system Access to the booking system is via a web portal. Point any of the major browsers to: http://bsys.lsi.nus.edu.sg/cmpr or http://bsys.lsi.nus.edu.sg/imaging depending on which set
Logging into the system Access to the booking system is via a web portal. Point any of the major browsers to: http://bsys.lsi.nus.edu.sg/cmpr or http://bsys.lsi.nus.edu.sg/imaging depending on which set
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
CHAP LinQ User Guide. CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC Version 1.
 2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
Scheduling Module Client Booking Quick Guide Online-Scheduling
 Scheduling Module Last Updated: November 26, 2009 System Administration Contact: Colin Bryant Phone: 604-822-7374 E-mail: PsychIT@exchange.ubc.ca Logon to Book King Enter the following URL into your address
Scheduling Module Last Updated: November 26, 2009 System Administration Contact: Colin Bryant Phone: 604-822-7374 E-mail: PsychIT@exchange.ubc.ca Logon to Book King Enter the following URL into your address
Advanced Training Manual: Approvals
 Advanced Training Manual: Approvals Last Updated: March 2012 Advanced Training Manual: Approvals Page 1 of 16 Table of Contents Introduction Training Objective Approvals Settings Approval Options Configure
Advanced Training Manual: Approvals Last Updated: March 2012 Advanced Training Manual: Approvals Page 1 of 16 Table of Contents Introduction Training Objective Approvals Settings Approval Options Configure
Edit an Existing Submission in InfoEd
 Edit an Existing Submission in InfoEd 1. Locate the project in the InfoEd Chief Investigator on the project: Log into InfoEd, In the Home screen, go to My Items tab and find the record. Once the record
Edit an Existing Submission in InfoEd 1. Locate the project in the InfoEd Chief Investigator on the project: Log into InfoEd, In the Home screen, go to My Items tab and find the record. Once the record
eibc Program User Guide
 eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
Instructions to complete CITI Human Subjects Training - New User
 Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
Buck-IRB Amendment User Guide
 Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
Training Manual for Researchers. How to Create an Online Human Ethics Application
 Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
Animal Protocol Development Instructions for Researchers
 Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
BOULDER IRB era InfoEd Amendments
 BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
The Ethic Management System (EMS) User guide
 The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
Easy Survey Creator: User s Guide
 Easy Survey Creator: User s Guide The Easy Survey Creator software is designed to enable faculty, staff, and students at the University of Iowa Psychology Department to quickly and easily create surveys
Easy Survey Creator: User s Guide The Easy Survey Creator software is designed to enable faculty, staff, and students at the University of Iowa Psychology Department to quickly and easily create surveys
JDRF Operations External FAQs Preaward and Postaward
 JDRF Operations External FAQs Preaward and Postaward Page 1 RMS360 Preaward FAQ Application Content 1. How do I apply for a grant? 2. How do I add my RO (Research Office contact) and FO (Finance Officer)
JDRF Operations External FAQs Preaward and Postaward Page 1 RMS360 Preaward FAQ Application Content 1. How do I apply for a grant? 2. How do I add my RO (Research Office contact) and FO (Finance Officer)
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
INSTRUCTIONS FOR THE eiaf COMPLETING THE FORM AND REQUIRED APPROVALS AND CERTIFICATIONS
 INSTRUCTIONS FOR THE eiaf COMPLETING THE FORM AND REQUIRED APPROVALS AND CERTIFICATIONS ACCESSING A NEW BLANK FORM From the UK website, click on link blue. (Click refers to pressing the mouse button.)
INSTRUCTIONS FOR THE eiaf COMPLETING THE FORM AND REQUIRED APPROVALS AND CERTIFICATIONS ACCESSING A NEW BLANK FORM From the UK website, click on link blue. (Click refers to pressing the mouse button.)
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
What is A web-based protocol submission system for IACUC and IRB protocols
 What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
DISCOVR-e USER MANUAL. Vanderbilt University Human Research Protection Program
 DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
PAGE 1. IRBManager. Instruction Manual For IRB members
 PAGE 1 IRBManager Instruction Manual For IRB members IRBManager PAGE 2 What is IRBManager? IRBManager is a web-based system designed specifically for the IRB review process, all the way from submission
PAGE 1 IRBManager Instruction Manual For IRB members IRBManager PAGE 2 What is IRBManager? IRBManager is a web-based system designed specifically for the IRB review process, all the way from submission
Nova Scotia Health Authority Research Ethics Board. Researcher s (PI) User Manual
 Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Cayuse IRB. Submitting a New Protocol. Office of Research Compliance (657)
 Cayuse IRB Submitting a New Protocol Office of Research Compliance irb@fullerton.edu (657) 278-7719 How to use this tutorial This tutorial is written from the point of view of a Primary Investigator (PI),
Cayuse IRB Submitting a New Protocol Office of Research Compliance irb@fullerton.edu (657) 278-7719 How to use this tutorial This tutorial is written from the point of view of a Primary Investigator (PI),
ORE Researcher Guide for using Kuali. October 2018
 ORE Researcher Guide for using Kuali October 2018 Navigating the system...3 Manage protocols page... 3 Viewing the full application... 4 Applications...6 Beginning an application... 6 General Information
ORE Researcher Guide for using Kuali October 2018 Navigating the system...3 Manage protocols page... 3 Viewing the full application... 4 Applications...6 Beginning an application... 6 General Information
Conflict of Interest Electronic Document Quick Reference Guide
 Conflict of Interest Electronic Document Quick Reference Guide The following quick reference provides guidance for using the Electronic Document Signature (EDS) system for the Conflict of Interest (COI)
Conflict of Interest Electronic Document Quick Reference Guide The following quick reference provides guidance for using the Electronic Document Signature (EDS) system for the Conflict of Interest (COI)
Nova Southeastern University IRBManager User Manual IRBMANAGER USER MANUAL
 IRBMANAGER USER MANUAL Contents 1. What is IRBManager?...1 2. IRBManager User Accounts/Login...1 a. Creating an IRBManager Account...1 b. Logging into IRBManager...2 c. Forgot Password...2 d. Locked Out
IRBMANAGER USER MANUAL Contents 1. What is IRBManager?...1 2. IRBManager User Accounts/Login...1 a. Creating an IRBManager Account...1 b. Logging into IRBManager...2 c. Forgot Password...2 d. Locked Out
Reference Card for ORSP
 Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Administrative and Research Staff Profile Instruction Guide
 Administrative and Research Staff Profile Instruction Guide In this guide you will learn how to: Log into your staff profile Find your staff profile Update primary contact information Update Bio (if applicable)
Administrative and Research Staff Profile Instruction Guide In this guide you will learn how to: Log into your staff profile Find your staff profile Update primary contact information Update Bio (if applicable)
Requesting Meetings with Mentors
 Requesting Meetings with Mentors WEBSITE 1. Login to the website: http://alinnovationconf.zerista.com 2. Click on the Mentors tab in the main menu 3. To view a mentor s profile click directly on his/her
Requesting Meetings with Mentors WEBSITE 1. Login to the website: http://alinnovationconf.zerista.com 2. Click on the Mentors tab in the main menu 3. To view a mentor s profile click directly on his/her
International Trade Online
 International Trade Online USER GUIDE For informational purposes only, not considered an advertisement. INTERNATIONAL TRADE ONLINE International Trade Online is the web-based offering from M&T Bank to
International Trade Online USER GUIDE For informational purposes only, not considered an advertisement. INTERNATIONAL TRADE ONLINE International Trade Online is the web-based offering from M&T Bank to
PRESS, PHOTOGRAPHER AND NON RIGHT HOLDER USER GUIDE
 PRESS, PHOTOGRAPHER AND NON RIGHT HOLDER USER GUIDE ACCESSING AND REGISTERING TO THE ISU The accreditation application procedure begins by creating a login to the ISU Online Media Accreditation System.
PRESS, PHOTOGRAPHER AND NON RIGHT HOLDER USER GUIDE ACCESSING AND REGISTERING TO THE ISU The accreditation application procedure begins by creating a login to the ISU Online Media Accreditation System.
WebDFS Creating Personnel Document
 WebDFS Creating Personnel Document 1. Start Internet Explorer 2. In the browser address bar type www.webdfs.uga.edu. 3. On the logon page type your TSO/IMS userid and password and click on the Logon button.
WebDFS Creating Personnel Document 1. Start Internet Explorer 2. In the browser address bar type www.webdfs.uga.edu. 3. On the logon page type your TSO/IMS userid and password and click on the Logon button.
User Guide. Welcome to FREEfax. Our online fax filing service that s Fast, Easy and FREE! PLEASE NOTE the following, before accessing our User Guide:
 User Guide Welcome to FREEfax Our online fax filing service that s Fast, Easy and FREE! PLEASE NOTE the following, before accessing our User Guide: FREEfax is available for both Public and Government Entities
User Guide Welcome to FREEfax Our online fax filing service that s Fast, Easy and FREE! PLEASE NOTE the following, before accessing our User Guide: FREEfax is available for both Public and Government Entities
Macquarie Research Centres (MQRC) Online Annual Progress Report
 Macquarie Research Centres (MQRC) Online Annual Progress Report USER GUIDE This guide is provided for Researchers and admin assistant submitting MQRC Annual Progress Report Online Version 1.0 Prepared
Macquarie Research Centres (MQRC) Online Annual Progress Report USER GUIDE This guide is provided for Researchers and admin assistant submitting MQRC Annual Progress Report Online Version 1.0 Prepared
OC RDC HTML User Guide
 CRA - Monitor OC RDC 4.5.3 HTML User Guide Page 1 of 46 TABLE OF CONTENTS Accessing OC RDC Steps for Access Logging On Change Password Computer and System Security Study and Site 3 4 5 5 6 Navigating OC
CRA - Monitor OC RDC 4.5.3 HTML User Guide Page 1 of 46 TABLE OF CONTENTS Accessing OC RDC Steps for Access Logging On Change Password Computer and System Security Study and Site 3 4 5 5 6 Navigating OC
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol, contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol, contact:
IRBNet Picture Guide
 IRBNet Picture Guide Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org For any questions or concerns regarding your submission, visit CSUSM
IRBNet Picture Guide Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org For any questions or concerns regarding your submission, visit CSUSM
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Audits of Surgical Mortality ONLINE USER GUIDE
 Audits of Surgical Mortality ONLINE USER GUIDE Contacts If you have any questions regarding the online audits of surgical mortality, please call or email your audit office. ACT Audit of Surgical Mortality
Audits of Surgical Mortality ONLINE USER GUIDE Contacts If you have any questions regarding the online audits of surgical mortality, please call or email your audit office. ACT Audit of Surgical Mortality
Personal Online Banking Quick Start Guide
 Personal Online Banking Quick Start Guide Step 1 Visit AmericanBank.com and locate the Personal Login ID box in the top right corner of the homepage. TIP: There are now two different online banking systems
Personal Online Banking Quick Start Guide Step 1 Visit AmericanBank.com and locate the Personal Login ID box in the top right corner of the homepage. TIP: There are now two different online banking systems
The IACUC is charged with the responsibility of ensuring that the Animal Welfare Act policies and procedures are followed.
 Protis is an online application system in support of research activities that require approval by the Institutional Animal Care and Use Committee (IACUC) or the Institutional Review Board (IRB). The IACUC
Protis is an online application system in support of research activities that require approval by the Institutional Animal Care and Use Committee (IACUC) or the Institutional Review Board (IRB). The IACUC
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Human Subjects Development Guide
 Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
IRBManager Faculty Adviser Role
 IRBManager Faculty Adviser Role Human Subjects Research Institutional Review Board irb@okstate.edu 405-744-3377 Page 1 Table of Contents ABOUT... 3 NOTIFICATION OF REVIEW... 3 LOGGING INTO YOUR IRBMANAGER
IRBManager Faculty Adviser Role Human Subjects Research Institutional Review Board irb@okstate.edu 405-744-3377 Page 1 Table of Contents ABOUT... 3 NOTIFICATION OF REVIEW... 3 LOGGING INTO YOUR IRBMANAGER
EHS360 IT SYSTEM AIMS USER GUIDE
 EHS360 IT SYSTEM AIMS USER GUIDE FOR INVESTIGATORS Copyright National University of Singapore. All Rights Reserved. Version 1.1 CONTENTS 1. Objective 2. Before logging in 3. Logging in to EHS360 4. User
EHS360 IT SYSTEM AIMS USER GUIDE FOR INVESTIGATORS Copyright National University of Singapore. All Rights Reserved. Version 1.1 CONTENTS 1. Objective 2. Before logging in 3. Logging in to EHS360 4. User
Quick Start. for Users. Online Banking
 Quick Start for Users Online Banking Table of Contents Getting Started... 1 Multifactor Authentication.... 2 Log In.... 3 Reset Your Password.... 4 Reset Your Security Question... 6 Change Your Phone Number....
Quick Start for Users Online Banking Table of Contents Getting Started... 1 Multifactor Authentication.... 2 Log In.... 3 Reset Your Password.... 4 Reset Your Security Question... 6 Change Your Phone Number....
In this guide you will learn how to:
 Faculty Profile Instruction Guide In this guide you will learn how to: Log into your faculty profile Find your faculty profile Update primary contact information Update Bio Update Expertise Update Education
Faculty Profile Instruction Guide In this guide you will learn how to: Log into your faculty profile Find your faculty profile Update primary contact information Update Bio Update Expertise Update Education
renew & Amend A Protocol training guide
 renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
esd Portal: Parent View User Guide v
 esd Portal: Parent View User Guide v. 3.7.0 Copyright 2002-2013 eschooldata, LLC All rights reserved. TABLE OF CONTENTS Overview... 3 Account Registration... 3 Logging In... 5 Getting Help... 7 Navigating
esd Portal: Parent View User Guide v. 3.7.0 Copyright 2002-2013 eschooldata, LLC All rights reserved. TABLE OF CONTENTS Overview... 3 Account Registration... 3 Logging In... 5 Getting Help... 7 Navigating
NZ Online Forms for Research Software Manual
 NZ Online Forms for Research Software Manual Version 1.5 Released May 2016 2 P a g e N Z O n l i n e F o r m s f o r R e s e a r c h 1 INTRODUCTION... 6 2 GETTING STARTED... 6 2.1 Creating an Account...
NZ Online Forms for Research Software Manual Version 1.5 Released May 2016 2 P a g e N Z O n l i n e F o r m s f o r R e s e a r c h 1 INTRODUCTION... 6 2 GETTING STARTED... 6 2.1 Creating an Account...
Digital StoreFront TRAINING
 Florida Agricultural and Mechanical University Digital StoreFront TRAINING Faculty and Staff January 2017 What is Digital StoreFront (DSF)? Digital StoreFront is a web-to-print e-commerce site that allows
Florida Agricultural and Mechanical University Digital StoreFront TRAINING Faculty and Staff January 2017 What is Digital StoreFront (DSF)? Digital StoreFront is a web-to-print e-commerce site that allows
External Funds Transfer Part I Adding an External Account
 External Funds Transfer Part I Adding an External Account 1. Log in to your Online Banking profile. In the column on the left, scroll down to Services and select Other Services. 2. From the list, select
External Funds Transfer Part I Adding an External Account 1. Log in to your Online Banking profile. In the column on the left, scroll down to Services and select Other Services. 2. From the list, select
Employee Online. Logging in to Employee Online LOAD INTERNET EXPLORER
 Logging in to Employee Online LOAD INTERNET EXPLORER EOL can be accessed via the staff intranet: www.southernhealth.nhs.uk/staff Click on the book Leave icon. Scroll down and click on Purple Access EOL
Logging in to Employee Online LOAD INTERNET EXPLORER EOL can be accessed via the staff intranet: www.southernhealth.nhs.uk/staff Click on the book Leave icon. Scroll down and click on Purple Access EOL
Report HQ. Quick Start Guide. Report HQ Quick Start Guide - Version 1.2
 Report HQ Quick Start Guide STEP 1 Requesting An Account 1) Request an account via the Report HQ Request Form 2) Nasdaq will verify your account 3) Once your account is verified and provisioned, you will
Report HQ Quick Start Guide STEP 1 Requesting An Account 1) Request an account via the Report HQ Request Form 2) Nasdaq will verify your account 3) Once your account is verified and provisioned, you will
Darwin Manual for FACC Coordinators
 Darwin Manual for FACC Coordinators McGill University Updated: October 21, 2013 Table of Contents Table of Contents...2 Logging into Darwin...4 Home Page...5 Introduction...8 Glossary of terms... 8 Process...
Darwin Manual for FACC Coordinators McGill University Updated: October 21, 2013 Table of Contents Table of Contents...2 Logging into Darwin...4 Home Page...5 Introduction...8 Glossary of terms... 8 Process...
The most current protocol information should be entered. This includes incorporation of all past amendments and modifications.
 Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Cayuse 424 Training VT PI Update Professional Profile
 This handout is step by step instructions for a Principal Investigator (PI) to update their Professional Profile permissions in Cayuse 424 so that a staff person/business manager can be designated as a
This handout is step by step instructions for a Principal Investigator (PI) to update their Professional Profile permissions in Cayuse 424 so that a staff person/business manager can be designated as a
You may also scroll through the Table of Contents and click on the topic or question of interest.
 We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
College Deans, Department Chairs & Supervising Faculty
 Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
Nexonia Time Off. Getting Started: A User Guide to Nexonia Time off. Delighted Customers. Unbeatable Integrations.
 Nexonia Time Off Getting Started: A User Guide to Nexonia Time off 1 Using Nexonia Time Off Nexonia s Time Off application is accessible through any major web browser: Google Chrome Mozilla Firefox Safari
Nexonia Time Off Getting Started: A User Guide to Nexonia Time off 1 Using Nexonia Time Off Nexonia s Time Off application is accessible through any major web browser: Google Chrome Mozilla Firefox Safari
Course Approval Proposal System (CAPS) User Guide
 Page 1 of 11 Course Approval Proposal System (CAPS) User Guide Creating a Resource and Planning (R&P) / Training and Assessment Strategy (TAS) Proposal Vocational Education Training (VET) About this guide
Page 1 of 11 Course Approval Proposal System (CAPS) User Guide Creating a Resource and Planning (R&P) / Training and Assessment Strategy (TAS) Proposal Vocational Education Training (VET) About this guide
Topaz Electronic Protocol Reviews and Electronic Meeting Instructions
 Topaz Electronic Protocol Reviews and Electronic Meeting Instructions Topaz Electronic Protocol Reviews and Meetings Page 1 Topaz Electronic Protocol Reviews & Electronic Meetings Instructions **********************************
Topaz Electronic Protocol Reviews and Electronic Meeting Instructions Topaz Electronic Protocol Reviews and Meetings Page 1 Topaz Electronic Protocol Reviews & Electronic Meetings Instructions **********************************
The e-marks System: Instructions for Faculty of Arts and Science Users
 The e-marks System: Instructions for Faculty of Arts and Science Users Contents Section A: Logging in... 2 Section B: Submitting Your Marks... 3 Section C: Marks Amendments... 8 Section D: How to Approve
The e-marks System: Instructions for Faculty of Arts and Science Users Contents Section A: Logging in... 2 Section B: Submitting Your Marks... 3 Section C: Marks Amendments... 8 Section D: How to Approve
5/13/2016. Punching in. Punching out for a Meal. Punching in from a Meal Punch back in from a meal to resume working.
 Punching Punching in Punch in to start your shift. After punching in you can begin working. Punching Punching out for a Meal Punch out to start your meal. Note: You do not have to punch out for 15 minute
Punching Punching in Punch in to start your shift. After punching in you can begin working. Punching Punching out for a Meal Punch out to start your meal. Note: You do not have to punch out for 15 minute
Amend A Protocol training guide
 Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
NEW PROTOCOL - NON-HUMAN SUBJECTS RESEARCH TRAINING GUIDE
 NEW PROTOCOL - NON-HUMAN SUBJECTS RESEARCH TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 Table of Contents Table of Contents
NEW PROTOCOL - NON-HUMAN SUBJECTS RESEARCH TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 Table of Contents Table of Contents
Approving the Outside Interests Disclosure Form Without Conflicts. Supervisor Level. IRIS Mobile through the Web
 Approving the Outside Interests Disclosure Form Without Conflicts Supervisor Level IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff
Approving the Outside Interests Disclosure Form Without Conflicts Supervisor Level IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff
Applicant Tutorial Foundant Grant Lifecycle Manager
 Foundant Grant Lifecycle Manager Overview This document is designed to provide grant applicants instructions for use of the Foundant Grant Lifecycle Manager application on The Kinsman Foundation website.
Foundant Grant Lifecycle Manager Overview This document is designed to provide grant applicants instructions for use of the Foundant Grant Lifecycle Manager application on The Kinsman Foundation website.
Legal Kiosk TM v3.0. Internal User Guide
 Legal Kiosk TM v3.0 Internal User Guide Table of Contents Overview... 2 Legal Kiosk Admin Management... 3 User Access Section... 3 Adding A New User... 3 Adding a New Contact... 6 Granting Access to Files
Legal Kiosk TM v3.0 Internal User Guide Table of Contents Overview... 2 Legal Kiosk Admin Management... 3 User Access Section... 3 Adding A New User... 3 Adding a New Contact... 6 Granting Access to Files
Production Assistance for Cellular Therapies (PACT) PACT Application System User s Guide
 Production Assistance for Cellular Therapies (PACT) PACT Application System User s Guide Version 1.0 February 9, 2017 Version 1.0 TABLE OF CONTENTS 1.0 Getting Started... 1 1.1 Access to the Internet...
Production Assistance for Cellular Therapies (PACT) PACT Application System User s Guide Version 1.0 February 9, 2017 Version 1.0 TABLE OF CONTENTS 1.0 Getting Started... 1 1.1 Access to the Internet...
IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL
 Page 1 of 12 IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL NOTE: This Quick Start Guide provides instructions for removing and/or adding personnel, other than the Principal Investigator,
Page 1 of 12 IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL NOTE: This Quick Start Guide provides instructions for removing and/or adding personnel, other than the Principal Investigator,
Grower Priority Database User Guide
 Have a Question? Select Questions? in the upper right corner or email inquiry@mrlpriority.com. All Users: Request an Account Select the Request an Account link at the top right corner of the site. Proceed
Have a Question? Select Questions? in the upper right corner or email inquiry@mrlpriority.com. All Users: Request an Account Select the Request an Account link at the top right corner of the site. Proceed
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE
 Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
ecampus 9.2 Faculty Homepage
 1 I. ecampus Features In ecampus 9.2, the ecampus Faculty Homepage features three (3) tiles. The tiles have all the functionalities found on the previous ecampus Faculty Homepage. ecampus 9.2 Faculty Homepage
1 I. ecampus Features In ecampus 9.2, the ecampus Faculty Homepage features three (3) tiles. The tiles have all the functionalities found on the previous ecampus Faculty Homepage. ecampus 9.2 Faculty Homepage
Concur Expense Management System
 GETTING STARTED IN CONCUR Concur Expense Management System User Guide: Getting Started in Concur Printed copies of this User Guide should not be regarded as the current version. For the latest User Guides:
GETTING STARTED IN CONCUR Concur Expense Management System User Guide: Getting Started in Concur Printed copies of this User Guide should not be regarded as the current version. For the latest User Guides:
Revised: February 28, Investigator/Coordinator Guide
 2014 Revised: February 28, 2014 Table of Contents Getting Started... 3 Navigational Tips... 4 Adding a New Application (Form 1)... 6 Routing Form... 11 Routing for Signatures... 15 Workflow Tracking and
2014 Revised: February 28, 2014 Table of Contents Getting Started... 3 Navigational Tips... 4 Adding a New Application (Form 1)... 6 Routing Form... 11 Routing for Signatures... 15 Workflow Tracking and
New Online Process for Updating TN BME Committee on Physician Assistants Attachment 4 & 5 Authorization for Prescribing for PAs
 New Online Process for Updating TN BME Committee on Physician Assistants Attachment 4 & 5 Authorization for Prescribing for PAs As of May 2017, the Authorization for Prescribing for Physician Assistants
New Online Process for Updating TN BME Committee on Physician Assistants Attachment 4 & 5 Authorization for Prescribing for PAs As of May 2017, the Authorization for Prescribing for Physician Assistants
Welcome to the QParents Portal... 2
 Table of contents Welcome to the QParents Portal... 2 Introduction: about the QParents Portal... 2 Online security... 2 About this guide... 3 How to provide feedback in QParents... 4 Help and support...
Table of contents Welcome to the QParents Portal... 2 Introduction: about the QParents Portal... 2 Online security... 2 About this guide... 3 How to provide feedback in QParents... 4 Help and support...
PI Certification Quick Guide
 PI Certification Quick Guide 1 P age Table of Contents Certifications... 3 PI Self-Certification... 3 PI is Certifying via Link in Email Notification... 3 PI Goes Directly to the Proposal... 6 Certification
PI Certification Quick Guide 1 P age Table of Contents Certifications... 3 PI Self-Certification... 3 PI is Certifying via Link in Email Notification... 3 PI Goes Directly to the Proposal... 6 Certification
IRMA Human Ethics Researcher User Guide
 IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
FAQs. Q What is the status of my study application?
 FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
Western Water Online User Guide
 Western Water Online User Guide Western Water December 2017 About Western Water Online Western Water Online is a self-service portal for plumbers, builders and developers. With the portal, you can apply
Western Water Online User Guide Western Water December 2017 About Western Water Online Western Water Online is a self-service portal for plumbers, builders and developers. With the portal, you can apply
How to Use My NetTeller
 How to Use My NetTeller Whether you re someone who prefers taking advantage of the latest technology or you re committed to traditional, face-to-face banking, First National Bank of Michigan is here to
How to Use My NetTeller Whether you re someone who prefers taking advantage of the latest technology or you re committed to traditional, face-to-face banking, First National Bank of Michigan is here to
VNB Connect Plus Automated Clearing House (ACH) Pass-Thru Reference Guide
 VNB Connect Plus Automated Clearing House (ACH) Pass-Thru Reference Guide 2015 Valley National Bank. Member FDIC. Equal Opportunity Lender. All Rights Reserved. About ACH Pass-Thru The ACH Pass-Thru module
VNB Connect Plus Automated Clearing House (ACH) Pass-Thru Reference Guide 2015 Valley National Bank. Member FDIC. Equal Opportunity Lender. All Rights Reserved. About ACH Pass-Thru The ACH Pass-Thru module
