Discrepancy Management
|
|
|
- Preston Shelton
- 6 years ago
- Views:
Transcription
1 Discrepancy Management Oracle Clinical Discrepancy Statuses IN OC RDC Discrepancies tab: While working in OC RDC Classic, you will encounter discrepancies with various assigned statuses on the Discrepancies task tab. The statuses you will see and a brief explanation are provided below. Note that the screen shots provided are from the perspective of a CRA. The List of Discrepancies tab is the central location for working with the discrepancies associated with one CRF. It provides various features: provides you with an overview of discrepancy information about that CRF allows you to initiate an action on a discrepancy allows you to enter informative text about a discrepancy allows you to generate a new discrepancy, either for: o the entire CRF, or o a single datapoint view the history associated with a discrepancy. Discrepancy not reviewed by user: This is the Unreviewed status of a discrepancy that requires site personnel review i.e. coordinator or Investigator. When logged in as a CRA or other study team member, you will see these discrepancies in yellow as Other Discrepancy. They will appear Red to the site as Open Discrepancy. INV Review: This is the status of a manually assigned discrepancy that requires site personnel review i.e. coordinator or Investigator. When logged in as a CRA or other study team member, you will see these discrepancies in yellow as Other Discrepancy. They will appear Red to the site as Open Discrepancy. CRA Review This is the status of a query that requires PPD review when you are logged in as a CRA. You will see these in red as Open Discrepancy. They will appear Yellow to the site as Other Discrepancy. Note that if the discrepancy was raised as a manual discrepancy by another department e.g. by Data Management or PVG and the site has responded to it and sent it back to PPD the status bar will still read CRA review (there is no option for the site to send this query to DM for review or to PVG for review). You should work with DM and PVG to determine whether the query can be closed and by whom. CLOSED These are discrepancies which have been manually closed by the CRA, i.e. manual discrepancies or automatic discrepancies which were first resolved by the investigator and for which the CRA has accepted the resolution text. Version 1.1 Page 1 of 16
2 Discrepancy Statuses IN OC RDC Classic Discrepancies task tab: TMS IN PROGRESS (relates to PPDs Data Management coding system) These discrepancies are reviewed by data management, who often works with Pharmacovigilance/Safety, or other coding resources to resolve the discrepancies. These discrepancies do appear on the ecrf but should be addressed ONLY by Data Management. The ecrf cell icon and patient icon on the Activity List will display the appropriate discrepancy color relative to a users role, i.e. these will be YELLOW to the CRA and site but RED to Data Management. If the terms cannot be resolved or coded against the dictionaries, data management can set the query status to INV Review. If this occurs, the discrepancy would then be an active discrepancy, appearing to the site as RED for their action. DM REVIEW or DM LAB REVIEW These are discrepancies that are set for data management to review. Most commonly, TMS queries would be set to DM REVIEW after someone from coding has reviewed the queries and the terms are not able to be coded to any medical dictionary. Site users and CRAs will see these discrepancies as YELLOW since they do not require their action. Sites and CRAs do not have the option to change a discrepancy status to DM Review. ADDITIONAL DISCREPANCY STATUSES IN DETAILED RDC QUERY TRACKING REPORT: When running the Detailed RDC Query Tracking Report, one of the standard reports available in OC RDC to all users, some additional query statuses can appear. The statuses and a brief explanation are provided below. OBSOLETE These are discrepancies that have been closed automatically by OC RDC. These discrepancies were automatically closed during batch validation, etc., due to a change in the data that made the discrepancy obsolete. Version 1.1 Page 2 of 16
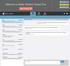
3 Access By default, the List of Discrepancies tab is displayed whenever you click the Discrepancies task tab. If focus is elsewhere within the Discrepancy task tab, you can access the List of discrepancies by selecting its tab. Components The List of Discrepancies tab provides a substantial amount of information about each discrepancy associated with a CRF. Components of the List of Discrepancies tab Seq Text Label Type Description Field Field Sequence number a number that is assigned to each discrepancy that is based on its current status, its timestamp, and the CRF datapoint with which it is associated. An editable field that describes the discrepancy. You can double-click the field to display the Field Editor window, if you wish to draft text in it; otherwise, you can simply type the description directly in the field. Status Field A descriptive field that displays the current status of the discrepancy. Comments Action Resolution Resolution Comment Field Dropdown list Field Field An editable text box that displays the current comment text associated with a given discrepancy. You can double-click the field to display the Fiedl Editor window, if you wish to draft text in it; otherwise, you can simply type the description directly in the field. A drop-down list that gives you access to the set of Discrepancy actions available. An informative field that displays the action that was taken for a discrepancy that resulted in its resolution. An editable field that contains text that describes the resolution of the discrepancy. You can double-click the field to display the Field Editor window, if you wish to draft text in it; otherwise, you can simply type the description directly in the field. New Button Initiates the process of creating a new discrepancy. History Button Opens the Discrepancy History window, which lists the changes to the currently selected discrepancy. Ordering of discrepancies in the list A set of criteria determines the sequence number of each discrepancy in the List of Discrepancies tab. It is based on the current discrepancy status and the location of the datapoint with which each discrepancy associated. As the status of a discrepancy changes, as well as the status of other discrepancies, and other discrepancies are added to the CRF, the sequence number associated with a discrepancy changes. Version 1.1 Page 3 of 16
4 Example of a selected discrepancy list List tab Shows all discrepancies for an ecrf page Text Status Seq New History View resolution and resolution coments in the history window. Comments Action Individual Discrepancy tab For each discrepancy listed in the List of Discrepancies tab, there is a corresponding individual tab, which displays the complete set of information related to a single discrepancy. Access To access an individual discrepancy tab, click the appropriate tab. When you do this, the tab you select is displayed "on top," and you can view or work with that discrepancy. Components of an individual discrepancy tab The components of an individual discrepancy tab are identical for all statuses. The table below lists and describes each of the components that are present on each tab. Version 1.1 Page 4 of 16
5 Components of an Individual Discrepancy Tab Tab Title Label Type Description Info Text The label that is displayed on the tab, it includes the current sequence number and the discrepancy status. Text Info Text The error message that describes the discrepancy. Status Info Text A descriptive field that displays the current status of the discrepancy. Id Action History Resolution Comments and Reason Comments Resolution Comments Variable Value Info Text Drop-down list Button Info Text and Text Field Text Field Info Text Data field Data field An internal identification number that is assigned to the discrepancy by Oracle Clinical. A drop-down list that gives you access to the set of Discrepancy actions that are designated for the study. Opens the Discrepancy History window, which lists the changes in status that have occurred to the current discrepancy. The Resoultion Reason text field displays the reason that was selected when the discrepancy was resolved. The Resolution Comments text box displays the comment that was included when the discrepancy was resolved. An editable text box that displays the current discrepancy comment. You can type directly in the field or double-click in the text box to open the Field Editor window. A field that displays the comment that was included with the resolution reason. A field that displays one variable that is associated with the discrepancy. (Multivariate discrepancies only) A field that displays the current value of the corresponding variable. (Multivariate discrepancies only) Version 1.1 Page 5 of 16
6 Select a discrepancy In order to work with a discrepancy from the List of discrepancies tab, you must first select it. To select a discrepancy, click on the appropriate sub-tab for that discrepancy. Example of a selected discrepancy Tab Title Text Action History Status Id Comments Variable Value Resolution Comments Resolution Comments and Reason Version 1.1 Page 6 of 16
7 Step by Step Instructions for Discrepancy Resolution in Classic Mode Recall that discrepancies requiring your attention will be red. You will have two options when reviewing discrepancies: Route the discrepancy back to the site for further clarification Close the discrepancy if appropriate (Manual discrepancies that have been generated by another user group such as Data Management or PVG should not be closed by you. Follow your study specific guidelines on who should close them.) Routing a discrepancy back to the site: Step Procedure Step 1 To open the ecrf with open discrepancies, click on the red ecrf icon in the main screen. Click on the Discrepancies tab to view the list of discrepancies. Select the sub-tab to view the individual discrepancy. Step 2 Review the discrepancy and determine what action needs to be taken. In this example, we are going to route the discrepancy back to the site for further clarification of the patient s race. Version 1.1 Page 7 of 16
8 Step Step 3 Procedure Click on the Action drop down list and select Send to Investigator. Step 4 The Internal comments for Discrepancy window appears. Delete the current comment, note that all deleted comments will be viewable in the window. Step 5 Enter the appropriate comment requesting the clarification needed to correct the data and close the discrepancy. Step 6 Click the Save button to close the Internal comments for Discrepancy window. Note that the discrepancy status and sequence number has changed. Version 1.1 Page 8 of 16
9 Closing a discrepancy: Step Step 1 Procedure Open the ecrf. Click on the Discrepancies tab and open the discrepancy sub-tab that will be reviewed and closed. Step 2 Double click in the comments field to open the Editor window to view the entire comment. In this view, you will be able to view the site comments, manual discrepancy comments. If you scroll to the data field questioned in the discrepancy, you will be able to view all of the information needed to determine if you can close the discrepancy. Site Correction Site Response Manual Discrepancy Request Ensure that the site has made the corrections on the ecrf as noted in the Editor window. Version 1.1 Page 9 of 16
10 Step Procedure Step 3 Click on the Action drop down list. To close the discrepancy, select Close resolved. Step 4 The Resolution Reason window appears. The data had been changed by the site therefore the correct resolution is Data value changed. Disc no longer applicable. 7. From the Resolution drop down list, select the Data value changed. Disc no longer applicable. Note: Comments may be added if appropriate. Step 5 Click Save Discrepancy has been closed, fields are greyed and status updated. Reminder: Discrepancies that have been generated by another user group should not be closed unless indicated by your project specific guidelines for discrepancy management. Version 1.1 Page 10 of 16
11 Generating a manual discrepancy: Assmune that during SDV, the medical record indicated that a rash was noted on the Physical Exam (PE). The ecrf data recorded indicates that the PE for Skin was normal. A discrepancy will need to be created to reflect the source documents. Step Procedure Step 1 Click in the Physical Assessment data field for Skin (blue highlight) Step 2 To create a manual discrepancy, you will use the Discrepancy (manual) icon in the tool bar. Click on the Discrepancy (manual) icon Discrepancy (manual) Version 1.1 Page 11 of 16
12 Step Procedure The manual discrepancy window will open. The title bar indicates the data field that the discrepancy will be related to (Comment for Question Normal/Abnormal/Not Done). Message text field Comment Reason field Comments field Do not enter text in this field. Action field Step 3 In the Comment Reason field, click on the drop down list and select DE Comment from the list. Version 1.1 Page 12 of 16
13 Step Step 4 Procedure In the Message field, type the clarification that will needed to made by the site. In this example, the following error message was added: MANUAL CRA...As noted in the source documents, the Physical Exam performed on 28 Feb 2006 indicated a rash. Consider adding this to the Physical Assessment for Skin, indicating that the assessment was abnormal and adding Rash to the Specify if Abnormal field. Do not enter comments in the Comments (optional) field. Refer to your project specific guidelines for naming conventions when generating a manual discrepancy. In this example, the naming convention to start all manual discrepancies created by the CRA is Manual CRA Type your discrepancy in the Message field or you can click on this icon to open the Comment message window to view entire text or type your discrepancy in this window. Step 5 In the Action: drop down list select Send to Investigator to route the discrepancy to the site. Version 1.1 Page 13 of 16
14 Step Step 6 Procedure The Internal Comment window will appear. DO NOT enter comments in this field. Click OK or Cancel to close this window. Step 7 Click OK to close the discrepancy window. Version 1.1 Page 14 of 16
15 After completing the above steps for generating a manual discrepancy, the view below indicates that you have successfully generated a manual discrepancy. The discrepancy appears in the Discrepancies tab list. The yellow discrepancy icon appears in the DEW tab indicating an active discrepancy that needs to be addressed by the site. The data field is now highlighted in yellow with blue text indicating there is an outstanding discrepancy. Reminder: Yellow fields with blue text indicate a discrepancy that has been generated on data entry or manually. Version 1.1 Page 15 of 16
16 Process Flows Oracle Clinical Data Entry, Monitoring and Discrepancy Flow The following diagram highlights the RDC process for data entry, monitoring and discrepancy flow. Investigator Site Performs Data Entry into ecrf System Generated Discrepancies (during data entry and upon save of ecrf) Discrepancies Generated Investigator site makes corrections on ecrf Validation Checks (run automatically every night, or when initiated by site users or CRAs) Discrepancies Generated Manual Discrepancies (appear immediately after they have been saved) Discrepancies Generated Investigator site makes corrections on ecrf or comments on data entered Changes Rejected CRA reviews changes or comments on data Changes Accepted or No Change Required CRA creates discrepancies ecrf Corrections /Clarifications Discrepancy Closed CRA Performs SDV ecrf Data Correct CRA Validates ecrf (Verification) Investigator Approves ecrf Note: Additional discrepancies may be raised by PVG or DM during review of data. These discrepancies would require site response, and a reverification and reapproval of any changed ecrfs, following the process outlined in the flow diagram. Version 1.1 Page 16 of 16
Discrepancy Statuses In OC RDC PDF Discrepancy Management Window:
 Discrepancy Statuses In OC RDC PDF Discrepancy Management Window: While working in OC RDC, you will encounter discrepancies with various assigned statuses on the Discrepancy Management window. The statuses
Discrepancy Statuses In OC RDC PDF Discrepancy Management Window: While working in OC RDC, you will encounter discrepancies with various assigned statuses on the Discrepancy Management window. The statuses
Discrepancy Management
 Discrepancy Management While working in OC RDC, you will encounter discrepancies with various assigned statuses. The description of each type of status is listed below. Unreviewed This is the Unreviewed
Discrepancy Management While working in OC RDC, you will encounter discrepancies with various assigned statuses. The description of each type of status is listed below. Unreviewed This is the Unreviewed
OC RDC HTML User Guide
 CRA - Monitor OC RDC 4.5.3 HTML User Guide Page 1 of 46 TABLE OF CONTENTS Accessing OC RDC Steps for Access Logging On Change Password Computer and System Security Study and Site 3 4 5 5 6 Navigating OC
CRA - Monitor OC RDC 4.5.3 HTML User Guide Page 1 of 46 TABLE OF CONTENTS Accessing OC RDC Steps for Access Logging On Change Password Computer and System Security Study and Site 3 4 5 5 6 Navigating OC
CRA OC RDC Classic User Guide
 CRA OC RDC Classic User Guide Version 1.0 Page 1 of 37 TABLE OF CONTENTS Accessing OC RDC Steps for Access 3 Logging On 3 Change Password 5 Change Study 5 Laptop and System Security 6 Navigating OC RDC
CRA OC RDC Classic User Guide Version 1.0 Page 1 of 37 TABLE OF CONTENTS Accessing OC RDC Steps for Access 3 Logging On 3 Change Password 5 Change Study 5 Laptop and System Security 6 Navigating OC RDC
Investigator Site OC RDC PDF User Guide
 Investigator Site OC RDC PDF User Guide Version 1.0 Page 1 of 40 TABLE OF CONTENTS Accessing OC RDC Steps for Access 3 Logging On 4 Change Password 4 Laptop and System Security 5 Change Study 5 Navigating
Investigator Site OC RDC PDF User Guide Version 1.0 Page 1 of 40 TABLE OF CONTENTS Accessing OC RDC Steps for Access 3 Logging On 4 Change Password 4 Laptop and System Security 5 Change Study 5 Navigating
OC RDC 4.6. User Guide
 OC RDC 4.6 Read-Only User Guide Version 1.0 Page 1 of 25 TABLE OF CONTENTS ACCESSING OC RDC...3 Steps for Obtaining Access...3 Logging On...4 Password Changes...5 Computer System and Security...5 VIEWING
OC RDC 4.6 Read-Only User Guide Version 1.0 Page 1 of 25 TABLE OF CONTENTS ACCESSING OC RDC...3 Steps for Obtaining Access...3 Logging On...4 Password Changes...5 Computer System and Security...5 VIEWING
 RDC Roles and Responsibilities RDC has several basic roles that are available to users that enter, update, review and approve data. Site User Data Entry Responds to queries Principal Investigator Reviews
RDC Roles and Responsibilities RDC has several basic roles that are available to users that enter, update, review and approve data. Site User Data Entry Responds to queries Principal Investigator Reviews
InForm Functionality Reference Manual for Sites. Version 1.0
 InForm Functionality Reference Manual for Sites Version 1.0 1-Mar-2012 2012 by Merck & Co., Inc., Whitehouse Station, New Jersey, USA All Rights Reserved No part of this book may be reproduced in any form
InForm Functionality Reference Manual for Sites Version 1.0 1-Mar-2012 2012 by Merck & Co., Inc., Whitehouse Station, New Jersey, USA All Rights Reserved No part of this book may be reproduced in any form
Site Coordinator User Guide
 Site Coordinator User Guide for Oracle Clinical RDC (Remote Data Capture) Onsite Data Entry and Discrepancy Management Purpose: The purpose of this user guide is to document the Oracle Clinical RDC (RDC)
Site Coordinator User Guide for Oracle Clinical RDC (Remote Data Capture) Onsite Data Entry and Discrepancy Management Purpose: The purpose of this user guide is to document the Oracle Clinical RDC (RDC)
Frequently Asked Questions
 Frequently Asked Questions ACCESS AND NAVIGATION 1. Can I change my password? 2. What are the guidelines for a new password? 3. What types of information will I get in RDC news? 4. I closed RDC Onsite
Frequently Asked Questions ACCESS AND NAVIGATION 1. Can I change my password? 2. What are the guidelines for a new password? 3. What types of information will I get in RDC news? 4. I closed RDC Onsite
ORACLE RDC ONSITE RESEARCH COORDINATOR TRAINING
 ORACLE RDC ONSITE RESEARCH COORDINATOR TRAINING TRAINING REQUIREMENTS RDC system training is designed and conducted for access to OnSite. Additional RDC training will be provided on a per study basis by
ORACLE RDC ONSITE RESEARCH COORDINATOR TRAINING TRAINING REQUIREMENTS RDC system training is designed and conducted for access to OnSite. Additional RDC training will be provided on a per study basis by
InForm for Primary Investigators Performing esignature Only (v4.6) Narration
 InForm for Primary Investigators Performing esignature Only (v4.6) Narration Page 1: Title Slide No narration Page 2: Welcome Welcome to the InForm for Primary Investigators Performing esignature Only
InForm for Primary Investigators Performing esignature Only (v4.6) Narration Page 1: Title Slide No narration Page 2: Welcome Welcome to the InForm for Primary Investigators Performing esignature Only
ORACLE RDC ONSITE RESEARCH COORDINATOR TRAINING
 ORACLE RDC ONSITE RESEARCH COORDINATOR TRAINING TRAINING REQUIREMENTS RDC system training is designed and conducted for access to OnSite. Additional RDC training will be provided on a per study basis by
ORACLE RDC ONSITE RESEARCH COORDINATOR TRAINING TRAINING REQUIREMENTS RDC system training is designed and conducted for access to OnSite. Additional RDC training will be provided on a per study basis by
Manual data entry in OpenClinica
 Manual data entry in OpenClinica Table of contents Page 1. User Support Information... 2 2. Getting started in OpenClinica... 2 2a. Account Procedure... 2 2b. User Roles applicable for site personnel...
Manual data entry in OpenClinica Table of contents Page 1. User Support Information... 2 2. Getting started in OpenClinica... 2 2a. Account Procedure... 2 2b. User Roles applicable for site personnel...
MTN-036 Medidata Rave Training SCHARP. Tanya Harrell & Jen Berthiaume
 MTN-036 Medidata Rave Training SCHARP Tanya Harrell & Jen Berthiaume Presentation Overview Medidata Navigation, Functionality, and Features Query management SCHARP Data Reviews and PPD monitoring in Rave
MTN-036 Medidata Rave Training SCHARP Tanya Harrell & Jen Berthiaume Presentation Overview Medidata Navigation, Functionality, and Features Query management SCHARP Data Reviews and PPD monitoring in Rave
Verifying CRFs 03-Mar-2016
 Verifying CRFs 03-Mar-2016 What is verifying CRFs? The verification process means Checking the entered data against the source data. 2 Studing Purpose After studying these slides, you may know: 1. Find
Verifying CRFs 03-Mar-2016 What is verifying CRFs? The verification process means Checking the entered data against the source data. 2 Studing Purpose After studying these slides, you may know: 1. Find
ALEA instructions for Local Investigators
 ALEA instructions for Local Investigators This document provides instructions, guidelines and background information for Local Investigators (LI) regarding the Electronic Data Capture (EDC) system of ALEA,
ALEA instructions for Local Investigators This document provides instructions, guidelines and background information for Local Investigators (LI) regarding the Electronic Data Capture (EDC) system of ALEA,
ICON Laboratory Services, Inc. isite User Guide
 ICON Laboratory Services, Inc. isite User Guide TABLE OF CONTENTS Section 1 Introduction and Creating an Account in isite... 2 Section 2 Log In... 3 2.1 Selecting a Study... 3 Section 3 Viewing Lab Reports...
ICON Laboratory Services, Inc. isite User Guide TABLE OF CONTENTS Section 1 Introduction and Creating an Account in isite... 2 Section 2 Log In... 3 2.1 Selecting a Study... 3 Section 3 Viewing Lab Reports...
OpenClinica Site Data Entry Guide
 Contents Accessing OpenClinica... 2 Entering Data... 2 Subject Matrix Familiarisation... 2 Scheduling an Event... 4 Accessing and Navigating CRFs... 5 CRF General Familiarisation... 6 CRF Header Info...
Contents Accessing OpenClinica... 2 Entering Data... 2 Subject Matrix Familiarisation... 2 Scheduling an Event... 4 Accessing and Navigating CRFs... 5 CRF General Familiarisation... 6 CRF Header Info...
Training Booking System User Guide Contents:
 Training Booking System User Guide Contents: Register to Use the System... 2 Password Reminder... 4 Log In and Page Overview... 6 Book a Course for Yourself... 7 Book Yourself and Another Staff Member
Training Booking System User Guide Contents: Register to Use the System... 2 Password Reminder... 4 Log In and Page Overview... 6 Book a Course for Yourself... 7 Book Yourself and Another Staff Member
eclinical ecrf Guideline
 eclinical ecrf Guideline 1 Table of Contents The Basics... 3 Getting Started... 3 Logging In... 3 Accessing Folders... 4 Navigating through Data Review/Capture... 7 Creating a Patient... 8 Entering Data...
eclinical ecrf Guideline 1 Table of Contents The Basics... 3 Getting Started... 3 Logging In... 3 Accessing Folders... 4 Navigating through Data Review/Capture... 7 Creating a Patient... 8 Entering Data...
December CTMS Site Management. User Reference Guide
 December 2016 CTMS Site Management User Reference Guide Trademarks DCRI is a registered trademark of Duke University. Cognos is a registered trademark of Cognos, Incorporated. Chrome is a trademark of
December 2016 CTMS Site Management User Reference Guide Trademarks DCRI is a registered trademark of Duke University. Cognos is a registered trademark of Cognos, Incorporated. Chrome is a trademark of
A white icon denotes there are no queries at the present time
 RDC Icons RDC Color Scheme There is a color scheme in RDC which denotes the query status of a subject, a CRF page and the queries (discrepancies) within the CRF. A red icon denotes the presence of a query
RDC Icons RDC Color Scheme There is a color scheme in RDC which denotes the query status of a subject, a CRF page and the queries (discrepancies) within the CRF. A red icon denotes the presence of a query
ALEA instructions for Local Data Managers
 ALEA instructions for Local Data Managers This document provides instructions, guidelines and background information for Local Data Management (LDM) regarding the Electronic Data Capture (EDC) system of
ALEA instructions for Local Data Managers This document provides instructions, guidelines and background information for Local Data Management (LDM) regarding the Electronic Data Capture (EDC) system of
PPD s Guide for External Users Requesting OC RDC Access
 Welcome to PPD s guide to obtaining Oracle Clinical Remote Data Capture 4.6.x access. This guide has been written to walk you through the steps you will need to take in order to obtain OC RDC access for
Welcome to PPD s guide to obtaining Oracle Clinical Remote Data Capture 4.6.x access. This guide has been written to walk you through the steps you will need to take in order to obtain OC RDC access for
How to Void or Correct CPA Service Claims DD Agency Providers (updated 9/25/2018)
 How to Void or Correct CPA Service Claims DD Agency Providers (updated 9/25/2018) As an Agency provider of services managed and paid via exprs, it is your responsibility to review your claims for payment
How to Void or Correct CPA Service Claims DD Agency Providers (updated 9/25/2018) As an Agency provider of services managed and paid via exprs, it is your responsibility to review your claims for payment
Annual Performance Assessment Process Additional Comments Guide
 Annual Performance Assessment Process Introduction The Reviewer can send the draft version of the online assessment out to someone else (a Third Party) in Halogen for further input - for review and additional
Annual Performance Assessment Process Introduction The Reviewer can send the draft version of the online assessment out to someone else (a Third Party) in Halogen for further input - for review and additional
... User Manual v CECM Clinical Trials
 ... User Manual v. 2.7.0... CECM Clinical Trials 2014-12-15 T a b l e o f C o n t e n t s i Table of Contents... 1. Table of Contents........................................................... i 2. About........................................................................
... User Manual v. 2.7.0... CECM Clinical Trials 2014-12-15 T a b l e o f C o n t e n t s i Table of Contents... 1. Table of Contents........................................................... i 2. About........................................................................
MAR Tab Overview. Search Criteria bar
 MAR Tab Overview The MAR (Medication Administration Record) displays a patient s medication orders and administration tasks. Medications will display following order entry by physicians, nurses, or pharmacy
MAR Tab Overview The MAR (Medication Administration Record) displays a patient s medication orders and administration tasks. Medications will display following order entry by physicians, nurses, or pharmacy
Welcome! A few things to go over before we get started
 Welcome! A few things to go over before we get started 1 Medidata Rave Training SCHARP March 19, 2017 Karen Patterson Jen Berthiaume Melissa Peda Medidata Rave Overview Medidata Rave and EDC User roles
Welcome! A few things to go over before we get started 1 Medidata Rave Training SCHARP March 19, 2017 Karen Patterson Jen Berthiaume Melissa Peda Medidata Rave Overview Medidata Rave and EDC User roles
INFORMATION SEARCH POWERING SUCCESSFUL PRACTICES TM VETERINARY SOLUTIONS
 INFORMATION SEARCH 855-478-7920 www.avimark.net support@avimark.net POWERING SUCCESSFUL PRACTICES TM VETERINARY SOLUTIONS Table of Contents Getting Started...3 Creating a New Search...4 Edit an Existing
INFORMATION SEARCH 855-478-7920 www.avimark.net support@avimark.net POWERING SUCCESSFUL PRACTICES TM VETERINARY SOLUTIONS Table of Contents Getting Started...3 Creating a New Search...4 Edit an Existing
EDC Training: Rave Architect Lite. Participant Guide 2.0 [30 Mar 11]
![EDC Training: Rave Architect Lite. Participant Guide 2.0 [30 Mar 11] EDC Training: Rave Architect Lite. Participant Guide 2.0 [30 Mar 11]](/thumbs/80/80801732.jpg) EDC Training: Rave Architect Lite Participant Guide 2.0 [30 Mar 11] ACKNOWLEDGMENTS This version of this guide owes its development to Process and Training Management (PTM). Version 2.0 Document Date 30
EDC Training: Rave Architect Lite Participant Guide 2.0 [30 Mar 11] ACKNOWLEDGMENTS This version of this guide owes its development to Process and Training Management (PTM). Version 2.0 Document Date 30
Recording Vital Signs and Test Results in Med-Center
 Recording Vital Signs and Test Results in Med-Center Introduction This technical note discusses Med-Center s ability to record and plot lab test results or patient vital signs. This capability is a new
Recording Vital Signs and Test Results in Med-Center Introduction This technical note discusses Med-Center s ability to record and plot lab test results or patient vital signs. This capability is a new
PROST USER GUIDE FOR VENDORS
 PROST USER GUIDE FOR VENDORS Revised 06/18/2018 Introduction Setup Welcome Email Account Verification Vendor Login Preferences Services Provided Voucher Voucher Inbox Invoice Inbox Security My Profile
PROST USER GUIDE FOR VENDORS Revised 06/18/2018 Introduction Setup Welcome Email Account Verification Vendor Login Preferences Services Provided Voucher Voucher Inbox Invoice Inbox Security My Profile
Smart Measurement System for Late Phase
 Smart Measurement System for Late Phase Electronic Data Capture (EDC) User Guide - Site Staff Version 6.6 Contents Contents 2 Section 1: Signing into Smart Measurement System (SMS) for Late Phase 4 1.1
Smart Measurement System for Late Phase Electronic Data Capture (EDC) User Guide - Site Staff Version 6.6 Contents Contents 2 Section 1: Signing into Smart Measurement System (SMS) for Late Phase 4 1.1
Vanderbilt Outpatient Order Management
 Vanderbilt Outpatient Order Management Ordering Application VOOM provides users with an application to manage their outpatient orders by electronically communicating the orders to the appropriate staff.
Vanderbilt Outpatient Order Management Ordering Application VOOM provides users with an application to manage their outpatient orders by electronically communicating the orders to the appropriate staff.
Maplewood connected version 5.8 a guide for non-markbook users. Contents
 Maplewood connected version 5.8 a guide for non-markbook users In the 5.8 release, the Maplewood connected Achievement and Markbook pages are being consolidated. The Class Achievement and Individual Achievement
Maplewood connected version 5.8 a guide for non-markbook users In the 5.8 release, the Maplewood connected Achievement and Markbook pages are being consolidated. The Class Achievement and Individual Achievement
INAB CAB Portal User Guide
 INAB CAB Portal User Guide CRM 2 INAB Cab Portal User Guide CRM Documentation Issue 2 June 2018 Contents 1. Login...4 1.1. Set up Portal password... 5 1.2. Login... 6 1.3. Forgot my password... 7 1.4.
INAB CAB Portal User Guide CRM 2 INAB Cab Portal User Guide CRM Documentation Issue 2 June 2018 Contents 1. Login...4 1.1. Set up Portal password... 5 1.2. Login... 6 1.3. Forgot my password... 7 1.4.
Messages tour Welcome to Medic Mobile s Guided Tour. Reports tour. Analytics tour. Begin the tour
 Welcome to Medic Mobile s Guided Tour Begin the tour This is where you receive and reply to text messages from the field. Message tab Here you can communicate with patients, community health workers, and
Welcome to Medic Mobile s Guided Tour Begin the tour This is where you receive and reply to text messages from the field. Message tab Here you can communicate with patients, community health workers, and
Family Medical Group NE Follow My Health How To s
 1 Family Medical Group NE Follow My Health How To s IF you want to Request an appointment: THEN 1. From your Home screen, click Schedule an appointment or Request (See example below.) NOTE: If you are
1 Family Medical Group NE Follow My Health How To s IF you want to Request an appointment: THEN 1. From your Home screen, click Schedule an appointment or Request (See example below.) NOTE: If you are
Compliance Document Manager User Guide
 Compliance Document Manager User Guide Contents OVERVIEW... 3 SYSTEM REQUIREMENTS... 3 VENDORMATE PASSWORD REQUIREMENTS... 3 LOGIN... 4 THE HOME SCREEN... 5 BA Screening... 5 BA Oversight... 5 My Screening
Compliance Document Manager User Guide Contents OVERVIEW... 3 SYSTEM REQUIREMENTS... 3 VENDORMATE PASSWORD REQUIREMENTS... 3 LOGIN... 4 THE HOME SCREEN... 5 BA Screening... 5 BA Oversight... 5 My Screening
PLATA Data Management User Manual. Version: 1.0
 1. Table of Contents 1. Access to the PLATA Data Management environment 3 1.1 Web address and login procedure. 3 2. Features of the ALEA Data Management module 6 2.1 Patient Grid 6 2.1.1. Layouts 9 2.2
1. Table of Contents 1. Access to the PLATA Data Management environment 3 1.1 Web address and login procedure. 3 2. Features of the ALEA Data Management module 6 2.1 Patient Grid 6 2.1.1. Layouts 9 2.2
QUALITY MEDICAL SOLUTIONS USER GUIDE PRACTICE FOCUS REFRESH (EMIS WEB) AND EXPORT PROCESS
 QUALITY MEDICAL SOLUTIONS USER GUIDE PRACTICE FOCUS REFRESH (EMIS WEB) AND EXPORT PROCESS User Guide January 2013 COMMERCIAL IN CONFIDENCE Quality Medical Solutions Ltd, 2012 INDEX 1.Introduction... 3
QUALITY MEDICAL SOLUTIONS USER GUIDE PRACTICE FOCUS REFRESH (EMIS WEB) AND EXPORT PROCESS User Guide January 2013 COMMERCIAL IN CONFIDENCE Quality Medical Solutions Ltd, 2012 INDEX 1.Introduction... 3
Design of Case Report Forms. Case Report Form. Purpose. ..CRF Official clinical data-recording document or tool used in a clinical study
 Design of Case Report Forms David W. Mailhot February 23, 2010 Case Report Form..CRF Official clinical data-recording document or tool used in a clinical study PAPER RDC/RDE (Remote Data Capture, Remote
Design of Case Report Forms David W. Mailhot February 23, 2010 Case Report Form..CRF Official clinical data-recording document or tool used in a clinical study PAPER RDC/RDE (Remote Data Capture, Remote
Augusta University Health: Physician Portal User Guide. Improved Access to Patient Information from Augusta University Medical Center
 Augusta University Health: Physician Portal User Guide Improved Access to Patient Information from Augusta University Medical Center Rev. 7/06 User Guide Index. Accessing the AU Health Physician Portal.
Augusta University Health: Physician Portal User Guide Improved Access to Patient Information from Augusta University Medical Center Rev. 7/06 User Guide Index. Accessing the AU Health Physician Portal.
ehepqual- HCV Quality of Care Performance Measure Program
 NEW YORK STATE DEPARTMENT OF HEALTH AIDS INSTITUTE ehepqual- HCV Quality of Care Performance Measure Program USERS GUIDE A GUIDE FOR PRIMARY CARE AND HEPATITIS C CARE PROVIDERS * * For use with ehepqual,
NEW YORK STATE DEPARTMENT OF HEALTH AIDS INSTITUTE ehepqual- HCV Quality of Care Performance Measure Program USERS GUIDE A GUIDE FOR PRIMARY CARE AND HEPATITIS C CARE PROVIDERS * * For use with ehepqual,
How to Find & Void Foster Care Service Claims Foster Care (FC) Providers (updated 9/24/2018)
 How to Find & Void Foster Care Service Claims Foster Care (FC) Providers (updated 9/24/2018) As a DD Foster Care provider of services managed and paid via exprs, it is your responsibility to review your
How to Find & Void Foster Care Service Claims Foster Care (FC) Providers (updated 9/24/2018) As a DD Foster Care provider of services managed and paid via exprs, it is your responsibility to review your
Managing Your Requests in ilab
 Managing Your Requests in ilab The Vanderbilt s ilab Site is: https://vanderbilt.corefacilities.org/landing/2191 Introduction: Managing Your Requests Section 1: Request Status Section 2: Icons Section
Managing Your Requests in ilab The Vanderbilt s ilab Site is: https://vanderbilt.corefacilities.org/landing/2191 Introduction: Managing Your Requests Section 1: Request Status Section 2: Icons Section
Standard Operating Procedure. SOP effective: 06 February 2017 Review date: 06 February 2019
 Standard Operating Procedure SOP number: SOP full title: SOP-JRO-26-001 Data Management SOP effective: 06 February 2017 Review date: 06 February 2019 SOP author signature: SIGNED COPY HELD WITHIN THE NJRO
Standard Operating Procedure SOP number: SOP full title: SOP-JRO-26-001 Data Management SOP effective: 06 February 2017 Review date: 06 February 2019 SOP author signature: SIGNED COPY HELD WITHIN THE NJRO
OpenClinica Training Exercises for Data Managers
 OpenClinica 3.1 for CTC Studies OpenClinica Training Exercises for Data Managers Version 1.1 15 May 2013 Page 1 of 41 Contents EXERCISE 1.... 3 LOGGING ON... 3 EXERCISE 2.... 5 THE SUBJECT MATRIX BROWSING
OpenClinica 3.1 for CTC Studies OpenClinica Training Exercises for Data Managers Version 1.1 15 May 2013 Page 1 of 41 Contents EXERCISE 1.... 3 LOGGING ON... 3 EXERCISE 2.... 5 THE SUBJECT MATRIX BROWSING
Expense Approvals on Nexonia s Web Application
 Expense Approvals on Nexonia s Web Application Expense Approvals on Web Nexonia Expenses on the web gives approvers the ability to review expenses submitted to them for approval. You can review submitted
Expense Approvals on Nexonia s Web Application Expense Approvals on Web Nexonia Expenses on the web gives approvers the ability to review expenses submitted to them for approval. You can review submitted
Standard Operating Procedure Clinical Data Management
 P-CTU-010 Standard Operating Procedure Topic area: Data Management and Statistics Based on SCTO Matrix: Not applicable Identification number: P-CTU-010 Version: /- Valid from: 01.06.2014 Review and Approval
P-CTU-010 Standard Operating Procedure Topic area: Data Management and Statistics Based on SCTO Matrix: Not applicable Identification number: P-CTU-010 Version: /- Valid from: 01.06.2014 Review and Approval
CONSOLIDATED LABORATORY SERVICES
 TABLE OF CONTENTS 2 INTRODUCTION 3 LOGIN 4 DESKTOP 5 TEST RESULTS 6 Basic Features 12 Advanced Features 16 TEST ORDERS Coming Soon 17 ACTIVITY REPORTS 17 Trace Accession 18 Activity Report 19 ADMINISTRATOR
TABLE OF CONTENTS 2 INTRODUCTION 3 LOGIN 4 DESKTOP 5 TEST RESULTS 6 Basic Features 12 Advanced Features 16 TEST ORDERS Coming Soon 17 ACTIVITY REPORTS 17 Trace Accession 18 Activity Report 19 ADMINISTRATOR
Managing Company Policy
 Managing Company Policy End users with Manage Company Policy rights can manage the Company Policy on behalf of the company. The Company Policy can be edited, but never deleted. Company administrators with
Managing Company Policy End users with Manage Company Policy rights can manage the Company Policy on behalf of the company. The Company Policy can be edited, but never deleted. Company administrators with
Notes: The course is suitable for CTC or external staff in the following roles: Clinical Research Coordinators, e.g. Site Data Manager/Study Nurse
 This course provides an introduction to OpenClinica version 3.1, including key terminology and concepts, as well as basic skills such as navigation. The course is suitable for CTC or external staff in
This course provides an introduction to OpenClinica version 3.1, including key terminology and concepts, as well as basic skills such as navigation. The course is suitable for CTC or external staff in
CTEP, NCI AE Reporting Guidelines and AdEERS
 CTEP, NCI AE Reporting Guidelines and AdEERS Ann Setser, BSN, Med, Nurse Consultant Cancer Therapy Evaluation Program, National Cancer Institute setsera@ctep.nci.nih.gov CALGB CRA Orientation, October
CTEP, NCI AE Reporting Guidelines and AdEERS Ann Setser, BSN, Med, Nurse Consultant Cancer Therapy Evaluation Program, National Cancer Institute setsera@ctep.nci.nih.gov CALGB CRA Orientation, October
CPOE Order Management (Basics)
 Orders Workspace Layout The Orders Workspace layout is broken into 4 sections. Customizing Orders Workspace It is necessary to ensure that the appropriate icons are visible in the Type column and that
Orders Workspace Layout The Orders Workspace layout is broken into 4 sections. Customizing Orders Workspace It is necessary to ensure that the appropriate icons are visible in the Type column and that
Marketo ON24 Adapter. User Guide Version 4.1. Updated May 3, 2013
 Marketo ON24 Adapter User Guide Version 4.1 Updated May 3, 2013 CONTENTS EVENT INTEGRATION OVERVIEW... 2 BEFORE YOU BEGIN... 3 REQUIREMENTS... 3 HOW TO CREATE AN EVENT IN MARKETO WITH AN ON24 INTEGRATION...
Marketo ON24 Adapter User Guide Version 4.1 Updated May 3, 2013 CONTENTS EVENT INTEGRATION OVERVIEW... 2 BEFORE YOU BEGIN... 3 REQUIREMENTS... 3 HOW TO CREATE AN EVENT IN MARKETO WITH AN ON24 INTEGRATION...
Quick Start Guide to Dynamic Templates
 Quick Start Guide to Dynamic Templates CS Version 2.7.7 (EMIS) Getting Started CHECKLIST: You must be logged into your Clinical System (EMIS) with a patient selected to access Clinical Support (CS) o Double
Quick Start Guide to Dynamic Templates CS Version 2.7.7 (EMIS) Getting Started CHECKLIST: You must be logged into your Clinical System (EMIS) with a patient selected to access Clinical Support (CS) o Double
Providers Approval Queue (PAQ) Quick Reference Guide
 PAQ The Providers Approval Queue (PAQ) is used to review and sign-off on medical information such as; Office visit notes, HIE Continuity of Care documents, Lab Results, Document Management images, and
PAQ The Providers Approval Queue (PAQ) is used to review and sign-off on medical information such as; Office visit notes, HIE Continuity of Care documents, Lab Results, Document Management images, and
QUICK TIPS FOR FULL-ACCESS ACCOUNTS. Florida SHOTS. Contact Information.
 Florida SHOTS FOR FULL-ACCESS ACCOUNTS Contact Information www.flshots.com Free help desk: 877-888-SHOT (7468) Monday Friday, 8 A.M. to 5 P.M. Eastern Quick Content Finder LOGGING IN 1 FORGOTTEN PASSWORD
Florida SHOTS FOR FULL-ACCESS ACCOUNTS Contact Information www.flshots.com Free help desk: 877-888-SHOT (7468) Monday Friday, 8 A.M. to 5 P.M. Eastern Quick Content Finder LOGGING IN 1 FORGOTTEN PASSWORD
LOGIN INTO OPENCLINICA...
 TABLE OF CONTENTS 1. INTRODUCTION... 3 2. LOGIN INTO OPENCLINICA... 3 2.1 Login... 3 2.2 Reset password... 3 2.3 Homepage... 4 2.4 Change Study or Site... 4 3. MODULES AND TASKS AVAILABLE TO A MONITOR...
TABLE OF CONTENTS 1. INTRODUCTION... 3 2. LOGIN INTO OPENCLINICA... 3 2.1 Login... 3 2.2 Reset password... 3 2.3 Homepage... 4 2.4 Change Study or Site... 4 3. MODULES AND TASKS AVAILABLE TO A MONITOR...
Reviewing Radiology Results
 AED14 Version 3.1 Emergency Department Operational Areas Included ED Doctors ED Consultants Roles Responsible for Carrying out this Process Operational Areas Excluded All areas other than the Emergency
AED14 Version 3.1 Emergency Department Operational Areas Included ED Doctors ED Consultants Roles Responsible for Carrying out this Process Operational Areas Excluded All areas other than the Emergency
Data Entry in InForm 4.6 Course version 1.0 [27 February 2013]
![Data Entry in InForm 4.6 Course version 1.0 [27 February 2013] Data Entry in InForm 4.6 Course version 1.0 [27 February 2013]](/thumbs/78/77110032.jpg) This course will show you how to enter data in InForm version 4.6 The course is suitable for CTC or external staff in the following roles: Clinical Trials Assistant Data Manager Site Data Manager/Study
This course will show you how to enter data in InForm version 4.6 The course is suitable for CTC or external staff in the following roles: Clinical Trials Assistant Data Manager Site Data Manager/Study
Creating an Online Course
 Definitions Online Course: a course that is completed entirely online and may be comprised of all or some of the following components: PowerPoint, PDF, Word Doc, Video, SCORM modules, Surveys, and Assessment.
Definitions Online Course: a course that is completed entirely online and may be comprised of all or some of the following components: PowerPoint, PDF, Word Doc, Video, SCORM modules, Surveys, and Assessment.
Researcher User Manual
 Researcher User Manual Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: November 24, 2017 Checkpoint *PLEASE NOTE* Prior to leveraging
Researcher User Manual Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: November 24, 2017 Checkpoint *PLEASE NOTE* Prior to leveraging
Audits of Surgical Mortality ONLINE USER GUIDE
 Audits of Surgical Mortality ONLINE USER GUIDE Contacts If you have any questions regarding the online audits of surgical mortality, please call or email your audit office. ACT Audit of Surgical Mortality
Audits of Surgical Mortality ONLINE USER GUIDE Contacts If you have any questions regarding the online audits of surgical mortality, please call or email your audit office. ACT Audit of Surgical Mortality
Transcribe a New Document in MTM
 Get Started 1. From your AppBar, select the Transcription Entry icon. 2. From the Startup dialog box, select Open Document and click the button. 3. From the Open Assistant dialog box, select Document Explorer
Get Started 1. From your AppBar, select the Transcription Entry icon. 2. From the Startup dialog box, select Open Document and click the button. 3. From the Open Assistant dialog box, select Document Explorer
Admission Process: Module 1.3
 Definitions StarPanel: Vanderbilt s electronic medical record (EMR) system used to collect and save medical information from both inpatient and outpatient visits for all Vanderbilt patients. Actions Menu:
Definitions StarPanel: Vanderbilt s electronic medical record (EMR) system used to collect and save medical information from both inpatient and outpatient visits for all Vanderbilt patients. Actions Menu:
National Louis University Faculty Training Guide
 Student Success Collaborative TM National Louis University Faculty Training Guide EAB Campus 2016 EAB All Rights Reserved 1 eab.com Table of Contents Table of Contents... 2 Searching for a Student... 3
Student Success Collaborative TM National Louis University Faculty Training Guide EAB Campus 2016 EAB All Rights Reserved 1 eab.com Table of Contents Table of Contents... 2 Searching for a Student... 3
Oracle Clinical Remote Data Capture Onsite
 Oracle Clinical Remote Data Capture Onsite User's Guide Release 4.6.2 E18822-01 April 2011 Oracle Clinical Remote Data Capture Onsite User's Guide, Release 4.6.2 E18822-01 Copyright 2007, 2011, Oracle
Oracle Clinical Remote Data Capture Onsite User's Guide Release 4.6.2 E18822-01 April 2011 Oracle Clinical Remote Data Capture Onsite User's Guide, Release 4.6.2 E18822-01 Copyright 2007, 2011, Oracle
Researcher User Manual
 Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
OpenClinica 3.4.x for Investigators
 OpenClinica 3.4.x for Investigators http://www.trialdatasolutions.com 2015 TrialDataSolutions 2015 OpenClinica 3.4.x for Investigators Page 1 of 20 Preface This manual describes the main activities of
OpenClinica 3.4.x for Investigators http://www.trialdatasolutions.com 2015 TrialDataSolutions 2015 OpenClinica 3.4.x for Investigators Page 1 of 20 Preface This manual describes the main activities of
THE TENANTS DASHBOARD USER MANUAL
 THE TENANTS DASHBOARD USER MANUAL Elogbooks Facilities Management Ltd, 2013 The Tenants Dashboard Manual The Tenants Dashboard offers a limited amount of Elogbooks functionality. It allows tenants to carry
THE TENANTS DASHBOARD USER MANUAL Elogbooks Facilities Management Ltd, 2013 The Tenants Dashboard Manual The Tenants Dashboard offers a limited amount of Elogbooks functionality. It allows tenants to carry
RouteOne / Groupe PPP System Integration
 Importing Deals from Groupe PPP System to RouteOne Before importing deals from Groupe PPP System to RouteOne, the solution must be first installed in the F&I software. If you have not done so yet, please
Importing Deals from Groupe PPP System to RouteOne Before importing deals from Groupe PPP System to RouteOne, the solution must be first installed in the F&I software. If you have not done so yet, please
ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL
 Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
Implementing targeted Source Data Verification (SDV) Strategy in idatafax
 Implementing targeted Source Data Verification (SDV) Strategy in idatafax Sadia Yousuf Research Coordinator, Population Health Research Institute DFUG 2017, Orlando, Florida Source Data Verification (SDV)
Implementing targeted Source Data Verification (SDV) Strategy in idatafax Sadia Yousuf Research Coordinator, Population Health Research Institute DFUG 2017, Orlando, Florida Source Data Verification (SDV)
SERVICE PROVIDER S ELOGBOOK USER MANUAL
 SERVICE PROVIDER S ELOGBOOK USER MANUAL Elogbooks Facilities Management Ltd, 2012 Service Provider s Elogbook Manual Welcome to the user manual for the service provider s electronic logbook. If you are
SERVICE PROVIDER S ELOGBOOK USER MANUAL Elogbooks Facilities Management Ltd, 2012 Service Provider s Elogbook Manual Welcome to the user manual for the service provider s electronic logbook. If you are
file:///c:/nhardy%20documents/5-systems/oracle/oracle_clinical/oc/466/readme_oc4...
 Page 1 of 48 Oracle Clinical Release Notes Release 4.6.6 E27598-01 Oracle Clinical Release Notes Release 4.6.6 My Oracle Support Article ID 1459178.1 August 2012 Patch Number: 14198756 Contents Section
Page 1 of 48 Oracle Clinical Release Notes Release 4.6.6 E27598-01 Oracle Clinical Release Notes Release 4.6.6 My Oracle Support Article ID 1459178.1 August 2012 Patch Number: 14198756 Contents Section
Intensive Care Unit and Paediatric Intensive Care Unit Essential Toolbar Buttons
 Intensive Care Unit and Paediatric Intensive Care Unit Essential Toolbar Buttons The following buttons are situated on the PowerChart Toolbar which are commonly used throughout the Digital Release. Care
Intensive Care Unit and Paediatric Intensive Care Unit Essential Toolbar Buttons The following buttons are situated on the PowerChart Toolbar which are commonly used throughout the Digital Release. Care
Electronic Submission System User procedures document MAY 2018
 Electronic Submission System User procedures document MAY 2018 Electronic Submission System User Guide 1 What s new? This user guide was updated in May 2018 to include recent changes to the Electronic
Electronic Submission System User procedures document MAY 2018 Electronic Submission System User Guide 1 What s new? This user guide was updated in May 2018 to include recent changes to the Electronic
Bar Code Medication Administration and MAR Resource Manual
 Bar Code Medication Administration and MAR Resource Manual Basic Information Glossary of Terms... 2 MAR Summary... 5 MAR Summary Frequently Asked Questions... 8 Changing MAR Summary Defaults... 9 MAR Tab...
Bar Code Medication Administration and MAR Resource Manual Basic Information Glossary of Terms... 2 MAR Summary... 5 MAR Summary Frequently Asked Questions... 8 Changing MAR Summary Defaults... 9 MAR Tab...
REDCap User Guide Version: September 30, 2010
 REDCap User Guide Version: September 30, 2010 Introduction This REDCap Manual functions as a resource for successful completion of electronic case report forms (ecrfs) for the PEP up Study. The manual
REDCap User Guide Version: September 30, 2010 Introduction This REDCap Manual functions as a resource for successful completion of electronic case report forms (ecrfs) for the PEP up Study. The manual
Shopping Cart: Queries, Personalizations, Filters, and Settings
 Shopping Cart: Queries, Personalizations, Filters, and Settings on the Shopping Cart Home Page Use this Job Aid to: Learn how to organize the Shopping Cart home page so that it is easier to use. BEFORE
Shopping Cart: Queries, Personalizations, Filters, and Settings on the Shopping Cart Home Page Use this Job Aid to: Learn how to organize the Shopping Cart home page so that it is easier to use. BEFORE
Using the Ramsar Sites Information Service
 Using the Ramsar Sites Information Service These instructions are intended to help Contracting Parties designate a new Wetland of International importance, update the information on an existing Ramsar
Using the Ramsar Sites Information Service These instructions are intended to help Contracting Parties designate a new Wetland of International importance, update the information on an existing Ramsar
USCCB/MRS Migration & Refugee Information System Resource Guide
 USCCB/MRS Migration & Refugee Information System Resource Guide R&P Remote Placement Program (RPP) Guide Pre-Arrival Case Management This MRIS Instructional Guide highlights the various requirements in
USCCB/MRS Migration & Refugee Information System Resource Guide R&P Remote Placement Program (RPP) Guide Pre-Arrival Case Management This MRIS Instructional Guide highlights the various requirements in
Scheduling Book Set-Up & Check-In Preferences
 Clinical Informatics Scheduling Book Set-Up & Check-In Preferences 10/23/2018 Clinical Informatics 1 Scheduling Book Settings It is important that ALL settings in the Scheduling Book are set up correctly,
Clinical Informatics Scheduling Book Set-Up & Check-In Preferences 10/23/2018 Clinical Informatics 1 Scheduling Book Settings It is important that ALL settings in the Scheduling Book are set up correctly,
My Practice Profile Attestation QUICK REFERENCE
 My Practice Profile Attestation QUICK REFERENCE My Practice Profile allows you to view, update and attest (update and attest capability is only available for the Optum ID administrator) group and provider
My Practice Profile Attestation QUICK REFERENCE My Practice Profile allows you to view, update and attest (update and attest capability is only available for the Optum ID administrator) group and provider
Supervision Guide For software version September 2013 and later OCR 2014
 4713521716 Supervision Guide For software version September 2013 and later OCR 2014 Introduction Table of contents Introduction 3 Marking quality control 5 Viewing your team Screen shortcuts Set up team
4713521716 Supervision Guide For software version September 2013 and later OCR 2014 Introduction Table of contents Introduction 3 Marking quality control 5 Viewing your team Screen shortcuts Set up team
Absence Management. 1. From SharePoint hover your cursor over My HR and click Associate Self Service.
 Oracle s Absence Management System is designed to make monitoring your accrued paid time off and requesting paid leave as quick and simple as possible. We can access the absence Management System in just
Oracle s Absence Management System is designed to make monitoring your accrued paid time off and requesting paid leave as quick and simple as possible. We can access the absence Management System in just
Centricity Cardio Workflow (CCW)
 Centricity Cardio Workflow (CCW) Staff Application Training 1. Open/log into, Verify that Worklist and Filters are set appropriately. Examination Echo Staff Current User Your organization You should see
Centricity Cardio Workflow (CCW) Staff Application Training 1. Open/log into, Verify that Worklist and Filters are set appropriately. Examination Echo Staff Current User Your organization You should see
Insurer User Manual Chapter 5: Common Functionality
 Insurer User Manual Chapter 5: Common Functionality 2018 HCAI Communications Table of Contents Search... 2 Advanced Search... 3 Search Results... 4 Navigating in an OCF plan or invoice... 5 Description
Insurer User Manual Chapter 5: Common Functionality 2018 HCAI Communications Table of Contents Search... 2 Advanced Search... 3 Search Results... 4 Navigating in an OCF plan or invoice... 5 Description
Check Positive Pay. User Guide
 Bu Check Positive Pay User Guide Table of Contents Overview... 2 Issues... 2 Issue Entry... 2 Import Issues... 5 Issue Activity... 17 Decisions... 20 Decision Items... 20 Decision Activity... 25 Subscriptions...
Bu Check Positive Pay User Guide Table of Contents Overview... 2 Issues... 2 Issue Entry... 2 Import Issues... 5 Issue Activity... 17 Decisions... 20 Decision Items... 20 Decision Activity... 25 Subscriptions...
Nova Scotia Health Authority Research Ethics Board. Researcher s (PI) User Manual
 Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
UCC ESS Online Leave Requests Manager Dashboard
 University College Cork UCC ESS Online Leave Requests Manager Dashboard Version 2.1 Contents How to Access UCC Employee Self Service (ESS)... 1 Manager Dashboard... 2 My Team... 2 My Approvals... 4 Additional
University College Cork UCC ESS Online Leave Requests Manager Dashboard Version 2.1 Contents How to Access UCC Employee Self Service (ESS)... 1 Manager Dashboard... 2 My Team... 2 My Approvals... 4 Additional
Creating and Viewing Curriculum
 Creating and Viewing Click the button on the META home screen. A dropdown menu will appear with the following options: Course, Program and Assessment. Click the desired option to proceed. options are explained
Creating and Viewing Click the button on the META home screen. A dropdown menu will appear with the following options: Course, Program and Assessment. Click the desired option to proceed. options are explained
HousingBrixx Top Tips Tutorial. March 2018
 HousingBrixx Top Tips Tutorial March 2018 Author: Achie Gyedu Version: v1.1 Created on 12/03/2018 Last Updated: 12/03/2018 Contents Introduction... 2 Selecting similar objects... 2 Populating multiple
HousingBrixx Top Tips Tutorial March 2018 Author: Achie Gyedu Version: v1.1 Created on 12/03/2018 Last Updated: 12/03/2018 Contents Introduction... 2 Selecting similar objects... 2 Populating multiple
Action Items Definitions of Activity Status How to Resubmit an Incomplete Activity How to Find Incomplete Activities...
 Contents Quick Reference Guide... 3 Accepting Terms and Conditions... 3 Fund Balances... 5 Setting a Proxy... 6 Email Notifications... 9 Banking Details and Partner Payments... 9 Claim Updates to Paid
Contents Quick Reference Guide... 3 Accepting Terms and Conditions... 3 Fund Balances... 5 Setting a Proxy... 6 Email Notifications... 9 Banking Details and Partner Payments... 9 Claim Updates to Paid
Open Referrals Manual
 Open Referrals Manual Centricity Business 4.3 MSU HealthTeam Training and Education (M-F 8a 5p) Melody Frye 517-432-0898 melody.frye@ht.msu.edu Page 2 of 31 Table of Contents Open Referrals Objectives
Open Referrals Manual Centricity Business 4.3 MSU HealthTeam Training and Education (M-F 8a 5p) Melody Frye 517-432-0898 melody.frye@ht.msu.edu Page 2 of 31 Table of Contents Open Referrals Objectives
Australia Online Forms for Research Software User Manual
 Australia Online Forms for Research Software User Manual Version 1.3 Released 21 August 2010 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting
Australia Online Forms for Research Software User Manual Version 1.3 Released 21 August 2010 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting
