Frequently Asked Questions (FAQs)
|
|
|
- Rosalind Green
- 5 years ago
- Views:
Transcription
1 Frequently Asked Questions (FAQs) This document contains answers of the most frequently asked questions about SDR system and drug file submissions. The questions are grouped by category to make search easier and the answers are cross-referenced with other information on the topic available on this document or linked websites. The categories are: Where to start? Application form in SDR Submitting Drug Application Data Requirements General Questions If you have any comments, or if you have a question about SDR that was not answered here, please send it to: sdr.drug@sfda.gov.sa This FAQ is created and maintained by Regulatory Affairs Department Drug Sector SFDA. Saudi Food & Drug Authority 1
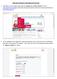
2 Where to start? 1. How to register a product? The applicant should be familiar with the product type by comparing the product with the published drug lists. If the product is not available in the drug lists, then can choose to either classify it or apply directly. Please follow the figure below for more information. Product No Needs classification? Yes No Step No. 1 Company is registered in DENR? Yes Human drug Classification result: Go to step no 1 Register in DENR Go to SDR Herbal & Health Go to step no 1 Veterinary drug Go to step no 1 Prohibited Not allowed in KSA Not subject for registrations Depends on the product Cosmetics Not allowed in KSA Figure 1: schematic diagram for how to register a product 2. How to register my Pharmaceutical Establishment in Drug Sector? Visit the DENR website at the link: Saudi Food & Drug Authority 2
3 3. I have created a user ID and a password from DENR system, can I start applying in SDR? No, you cannot. You need to activate the account by following the instructions in the and the DENR guidance. 4. How long it will take to register my Pharmaceutical Establishment in DENR? Usually around 7 days. 5. How I can create a subaccount? By choosing the Add sub account menu in the main account page. 6. When I can log in to SDR? You can when you receive an from DENR containing the approval of the account and the DENR number. Moreover, the DENR user ID and password can be used for the Drug Sector e-services, such as SDR, IBRCS and others. 7. Who should apply in SDR, the agent (MAH) or the scientific office manager? This is done by an agreement between the two. Taking into consideration that once one of them started, the other cannot continue or step in the middle of the registration process (per application). Saudi Food & Drug Authority 3
4 Application form in SDR 8. What types of applications are available in SDR? There are three types: Marketing Authorization Application of Medicinal Product for Human Use Marketing Authorization Application of Medicinal Product for Veterinary Use Marketing Authorization Application of Herbal & Health Product Type of Application 9. What does the following means? a. Is Saudi Arabia the Country of Origin (COO)? It means, is the manufacturer a local establishment located in Saudi Arabia? b. Marketing Authorization holder? It means the MAH holder in the country of origin. Names and ATC Code 10. What is the Pharmacotherapeutic group (ATC code)? The Anatomical Therapeutic Chemical (ATC) Classification System is used for the classification of drugs. It is controlled by the WHO Collaborating Centre for Drug Statistics Methodology (WHOCC). Please, visit the website: How to choose the right ATC code? The active substances are divided into different groups according to the organ or system on which they act and their therapeutic, pharmacological and chemical properties. Drugs are classified in groups at five different levels. The drugs are divided into fourteen main groups (1st level), with pharmacological/therapeutic subgroups (2nd level). The 3rd and 4th levels are chemical/pharmacological/therapeutic subgroups and the 5th level is the chemical substance. It is available in the WHO website at the previous website. For example, vancomycin has 2 codes. One is (J01XA01) as glycopeptide antibacterials under the pharmacological/therapeutic subgroups of Saudi Food & Drug Authority 4
5 Antiinfectives for systemic use. The other one is (A07AA09) as antibiotics under the pharmacological/therapeutic subgroups of antidiarrheals, intestinal anti-inflammatory / antiinfective. Product Information 12. How to fill the package size, volume and unit fields? It depends on your product, such as tablet or a liquid dosage form. Below table shows some examples: Dosage Form Package Size Volume Unit Ampoule or Vial 5 3 ml Cartridge ml Cream or ointment 1 10 g Enema ml Eye/ear drops 1 10 ml Inhaler DF 1 Powder g Syrup ml Tablet or Capsule 30 Leave it blank Leave it blank Contact Information 13. What does Proposed marketing authorization holder/person legally responsible for placing the product on the market in KSA mean? This is the MAH in KSA, i.e. the agent. Manufacturers 14. What does the field Activity mean? The activity can be anything from manufacturing, primary packaging, secondary packaging, batch release, or other activities. 15. If a product has two manufacturing sites, where to include the 2nd site in the application form for new registration? Both the two manufactures should be included in the Finished Product Manufacturer section, and the activity can be to one of them as secondary source. 1 DF: Dosage Form Saudi Food & Drug Authority 5
6 Fees 16. How to pay the required amount for registering a drug? Under Fees page, choose the proper application type then generate a SADAD bill. Please make sure you have chosen the right type in order to generate a bill with the right fee amount. Scheduling 17. How to schedule and reschedule an appointment? SDR Guidance explains the procedure in details. 18. Do I have to notify for cancelation? What the minimum time for notification? Yes, it is recommended to notify SFDA by at least two days prior to the scheduled day. 19. If I am (as applicant) late for the appointment, what is the procedure? You will have the remaining time for checking, then by the end of meeting time the unrevised parts of your application will be considered missing, and another appointment is needed. Saudi Food & Drug Authority 6
7 Submitting Drug Application 20. What is a Drug Application? A drug application is a term used to describe the combination of: the application form in SDR, the product file, and drug samples. 21. How to present the product file to SFDA? Simply, follow the Guidance for Submission (latest published version). 22. Can non-saudi pharmacist make the delivery of the product file? According to the government law, the person should be Saudi. 23. In module 1, if I missed a signature or a stamp or a copy of a certificate, is it acceptable? No it is not. The file will be returned with an acknowledgement letter with deficiency. 24. If any information or documentation is missing/incorrect, would SFDA receive it until I complete it? The Drug Sector will not accept the Drug Application and will be returned back to the applicant. 25. Would providing a valid legalized CPP copy issued to any other country a possibility, or should the CPP be issued to Saudi Arabia by the related health authorities? The CPP should be issued to Saudi Arabia by the related health authorities. 26. How would I receive an inquiry? What to do then? An auto- will be sent from SDR notifying you of an inquiry. For full details, please read the Guidance for Submission (latest published version). However, Drug Sector does not recommend that the applicant sending a further clarifications or questions regarding the query in SDR system. If the applicant have any question or need clarifications, he/she should send an to sdr.drug@sfda.gov.sa. 27. When the samples (batch number) is different, what to do? This is not acceptable. All samples, its letter (1.3.5), and the certificate of Analysis (1.7.3) must have the same batch number and expiry date. Saudi Food & Drug Authority 7
8 28. In case the Drug Samples are different than the Artwork, e.g. no Arabic trade name, different design or different pack size etc, what is the procedure? SFDA will accept the provided samples as long as they meet the conditions in module 1 (1.3.5) and the certificate of Analysis (1.7.3). 29. What is NeeS? What are the differences between NeeS and ectd? NeeS is defined as Non-eCTD electronic Submission. The difference from an ectd is that the two relevant XML files, the index.xml and gc-regional.xml for the backbone of Modules 2 to 5 and Module 1 for the GCC, respectively and the util folder are not present, so navigation through a NeeS is based on electronic Tables of Content, bookmarks and hypertext links. The XML backbone (recognizable as index.xml at the root level of the submission folder) of the ectd provides two useful functions: It provides a hyperlinked table of contents to the entire submission when viewed in web browser with a suitable stylesheet It provides descriptive information ( meta-data ) on the files that make up the actual content of the ectd 30. Is the NeeS accepted for Herbal and health products? Veterinary products? For the time being, they are excluded. 31. How to prepare a NeeS? Simply, follow the Guidance for Submission (latest published version). 32. Does a NeeS application cover all dosage forms, strengths, and pack sizes of a product? Yes it does. 33. Are the folders and the folders naming mandatory? For Modules 1-5: the folder structure and names must follow the GCC or ICH defined structure and names. 34. In case of empty folder, should we put not applicable or not available? No, these folders should be deleted. Saudi Food & Drug Authority 8
9 35. If we have a pdf file size above 100MB, what to do? Files larger than 100 MB should be avoided. If there is a need to exceed this limit, please contact the Drug Sector. 36. When I can submit a NeeS or ectd? A timeline has been published in the Drug Sector website. Saudi Food & Drug Authority 9
10 Data requirements 37. How to prepare the drug file? The drug file should be prepared according to the Data Requirements for Human Drugs Submission - Content of the Dossier published in the website. 38. Where should I include the certificate of suitability (COS)? In the folder name 32r-reg-info. 39. What do we have to submit in (2.3.R) & (3.2.R) Section? Refer to the Data Requirements for Human Drugs Submission - Content of the Dossier guideline. 40. Following are recommendations for the presentation of the information in the Quality Module for different scenarios that may be encountered: For a drug product containing more than one drug substance: one complete 3.2.S section should be provided for one drug substance, followed by other complete 3.2.S sections for each drug substance. For a drug substance from multiple manufacturers: one complete 3.2.S section should be provided for the drug substance from one manufacturer, followed by other complete 3.2.S sections for each drug substance manufacturer. For a drug product with multiple strengths: one complete 3.2.P section should be provided with the information for the different strengths provided within the subsections. One complete copy of the dossier should be provided for each strength. For a drug product with multiple container closure systems (e.g. bottles and unit dose blisters): one complete 3.2.P section should be provided with the information for the different presentations provided within the subsections. For multiple drug products (e.g. tablets and a parenteral product): a separate dossier is required for each drug product. For a drug product supplied with reconstitution diluent(s), the information on the diluent should be provided in a separate part 3.2.P if the diluent is co-packaged with the drug product. However, if the diluent is not copackaged with the drug product, the compatibility of the diluent with the drug product should be discussed in 3.2.P.2.6. Saudi Food & Drug Authority 10
11 41. Where should the information on bioequivalence studies for a generic application be included? Bioavailability study reports should be included in Module 5, under section Reports of Biopharmaceutical Studies. More specifically, reports of comparative Bioavailability/Bioequivalence studies should go under section For a registered product, how to register the following: a. A new pack size. Follow the Guidelines for Variation Requirements, specifically the section Change in pack size of the finished product. b. A new package type (for example from aluminum blister to tablet container, or from plastic bottle to a glass bottle). Apply for a new registration. c. A new strength. Apply for a new registration. Saudi Food & Drug Authority 11
12 General Questions 43. How can we submit the Herbal & Health products? Veterinary drugs? As mentioned in the Guidance for Submission (latest published version). 44. Is SFDA offering pre-submission meetings? In the time being, no. 45. After registering a product, who is the MAH? The MAH in KSA is then the agent. However, the MAH for the product worldwide is the license holder. 46. How to contact the Drug Sector regarding registration? There are several ways: Phone: Extensions: 5171, 5169 Fax: sdr.drug@sfda.gov.sa Mail: 3292 Northern Ring Road An nafal District Riyadh Kingdom of Saudi Arabia Website: Saudi Food & Drug Authority 12
Pre-notification check for type IB Variations 1
 Pre-notification check for type IB Variations 1 This pre-notification checklist is aimed at facilitating submission of complete and correct Type IB variation notifications by Marketing Authorisation Holders
Pre-notification check for type IB Variations 1 This pre-notification checklist is aimed at facilitating submission of complete and correct Type IB variation notifications by Marketing Authorisation Holders
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL Questions & Answers Implementation of ectd in South Africa This document is intended to provide clarity on guidelines and specifications for applications for the registration
MEDICINES CONTROL COUNCIL Questions & Answers Implementation of ectd in South Africa This document is intended to provide clarity on guidelines and specifications for applications for the registration
Guidance for electronic submissions for Certificates of Suitability (CEP) applications
 CBW/CB PUBLIC DOCUMENT (Level 1) English only/nglais seulement Strasbourg, January 2018 Certification of Suitability to the Monographs of the European Pharmacopoeia Guidance for electronic submissions
CBW/CB PUBLIC DOCUMENT (Level 1) English only/nglais seulement Strasbourg, January 2018 Certification of Suitability to the Monographs of the European Pharmacopoeia Guidance for electronic submissions
LiiV Handbook. Version 2.1. Supplier information in the VARA register. This handbook describes pharmaceutical companies work in LiiV
 LiiV Handbook Supplier information in the VARA register Version 2.1 This handbook describes pharmaceutical companies work in LiiV Contents 1. Introduction... 4 1.1 Information owner... 4 2. How does LiiV
LiiV Handbook Supplier information in the VARA register Version 2.1 This handbook describes pharmaceutical companies work in LiiV Contents 1. Introduction... 4 1.1 Information owner... 4 2. How does LiiV
Guidance for electronic and paper submissions for Certificates of Suitability (CEP) applications
 FK/CB PUBLIC DOCUMENT (LEVEL 1) English only/nglais seulement Strasbourg, June 2013 Certification of suitability to Monographs of the European Pharmacopoeia Guidance for electronic and paper submissions
FK/CB PUBLIC DOCUMENT (LEVEL 1) English only/nglais seulement Strasbourg, June 2013 Certification of suitability to Monographs of the European Pharmacopoeia Guidance for electronic and paper submissions
Get ready for ectd in South Africa. Current status at MCC
 Get ready for ectd in South Africa Current status at MCC E Taute Feb 2013 Overview Background Guidelines, Specifications, Forms ICH ectd Specification V 3.2.2 16-July-2008 2.21 South African Specification
Get ready for ectd in South Africa Current status at MCC E Taute Feb 2013 Overview Background Guidelines, Specifications, Forms ICH ectd Specification V 3.2.2 16-July-2008 2.21 South African Specification
Work instructions for US-FDA ANDA Submissions Work Instructions for ectd US-FDA ANDA Submissions
 Work Instructions for ectd US-FDA ANDA Submissions 1. Logon to the PharmaReady ectd System Work instructions for US-FDA ANDA Submissions PharmaReady is a Web-based application. The logon screen and all
Work Instructions for ectd US-FDA ANDA Submissions 1. Logon to the PharmaReady ectd System Work instructions for US-FDA ANDA Submissions PharmaReady is a Web-based application. The logon screen and all
GDUFA s Impact on ectds / Case Study: Implementing PDF Standards
 GDUFA s Impact on ectds / Case Study: Implementing PDF Standards GPhA 2015 Fall Technical Conference GDUFA S Impact on ectds GPhA 2015 Fall Technical Conference 1 Introductions Kevin Tompkins Director,
GDUFA s Impact on ectds / Case Study: Implementing PDF Standards GPhA 2015 Fall Technical Conference GDUFA S Impact on ectds GPhA 2015 Fall Technical Conference 1 Introductions Kevin Tompkins Director,
Q&A QP Declaration. Table of contents
 CMDh/340/2015/Rev.5 December 2018 Table of contents 1. Why can an audit performed by a European National Health Authority not be used in order to support a QP Declaration? /Why is an on-site audit performed
CMDh/340/2015/Rev.5 December 2018 Table of contents 1. Why can an audit performed by a European National Health Authority not be used in order to support a QP Declaration? /Why is an on-site audit performed
Submitting High Quality ectd Submissions to FDA/OGD
 Submitting High Quality ectd Submissions to FDA/OGD GPhA/FDA ANDA Labeling Workshop/ USP User Forum September 11, 2013 Constance Robinson Regulatory Information Specialist edata Management Solutions Team
Submitting High Quality ectd Submissions to FDA/OGD GPhA/FDA ANDA Labeling Workshop/ USP User Forum September 11, 2013 Constance Robinson Regulatory Information Specialist edata Management Solutions Team
US Food and Drug Administration. Revision History
 Specifications for ectd Validation Criteria US Food and Drug Administration Specifications for ectd Validation Criteria Revision History Date Description Version 2008-03-10 Initial Release of ectd Validation
Specifications for ectd Validation Criteria US Food and Drug Administration Specifications for ectd Validation Criteria Revision History Date Description Version 2008-03-10 Initial Release of ectd Validation
GCC Module 1 Specifications Version 1.4 Sep 2015
 GCC Module 1 Specifications Version 1.4 GCC Module 1 Specifications Version 1.4 Drug Sector Saudi Food & Drug Authority Please visit SFDA s website at http://www.sfda.gov.sa/en/drug for the latest update
GCC Module 1 Specifications Version 1.4 GCC Module 1 Specifications Version 1.4 Drug Sector Saudi Food & Drug Authority Please visit SFDA s website at http://www.sfda.gov.sa/en/drug for the latest update
esubmission Gateway and Web Client Training on the use of XML delivery files for Veterinary submissions
 esubmission Gateway and Web Client Training on the use of XML delivery files for Veterinary submissions Webinar training on v1.0 Presented by Kristiina Puusaari on 3 June 2016 An agency of the European
esubmission Gateway and Web Client Training on the use of XML delivery files for Veterinary submissions Webinar training on v1.0 Presented by Kristiina Puusaari on 3 June 2016 An agency of the European
EXEMPTIONS: PROCEDURE TO BE FOLLOWED FOR MEDICINES FOR HUMAN USE
 EXEMPTIONS: PROCEDURE TO BE FOLLOWED FOR MEDICINES FOR HUMAN USE Table OF CONTENTS Introduction... 2 Category 1. Deviations from the primary and secondary (harmonised) packaging for which an exemption
EXEMPTIONS: PROCEDURE TO BE FOLLOWED FOR MEDICINES FOR HUMAN USE Table OF CONTENTS Introduction... 2 Category 1. Deviations from the primary and secondary (harmonised) packaging for which an exemption
MEDICINES CONTROL COUNCIL
 CMs ZACTD MEDICINES CONTROL COUNCIL COMPLEMENTARY MEDICINES - USE OF THE ZA-CTD FORMAT IN THE PREPARATION OF A REGISTRATION APPLICATION This guideline is intended to provide recommendations to applicants
CMs ZACTD MEDICINES CONTROL COUNCIL COMPLEMENTARY MEDICINES - USE OF THE ZA-CTD FORMAT IN THE PREPARATION OF A REGISTRATION APPLICATION This guideline is intended to provide recommendations to applicants
TH ectd Specification
 TH ectd Specification Module 1 and Regional Information Version 0.91, August 2014 About the Food and Drug Administration Thailand - Bureau of Drug Control The Bureau of Drug Control has set a vision as
TH ectd Specification Module 1 and Regional Information Version 0.91, August 2014 About the Food and Drug Administration Thailand - Bureau of Drug Control The Bureau of Drug Control has set a vision as
QUALITY OVERALL SUMMARY - CHEMICAL ENTITIES (Applications for Drug Identification Number Submissions) (QOS-CE (DINA))
 QUALITY OVERALL SUMMARY - CHEMICAL ENTITIES (Applications for Drug Identification Number Submissions) (QOS-CE (DINA)) (version: 2004-04-01) FOREWORD The Quality Overall Summary (QOS) is a summary of the
QUALITY OVERALL SUMMARY - CHEMICAL ENTITIES (Applications for Drug Identification Number Submissions) (QOS-CE (DINA)) (version: 2004-04-01) FOREWORD The Quality Overall Summary (QOS) is a summary of the
JORDAN Module 1 ectd Specification Version 1.0.1
 المو سسة العامة للغذاء والدواء Jordan Food & Drug Administration JORDAN Module 1 ectd Specification Version 1.0.1 Drug Directorate Jordan Food & Drug Administration Vision: To excel regionally & globally
المو سسة العامة للغذاء والدواء Jordan Food & Drug Administration JORDAN Module 1 ectd Specification Version 1.0.1 Drug Directorate Jordan Food & Drug Administration Vision: To excel regionally & globally
ectd in Canada Ginette Larocque, Purdue Pharma Canada August 23, 2016
 ectd in Canada Ginette Larocque, Purdue Pharma Canada August 23, 2016 AGENDA Eligibility for Filing in ectd Format ectd Mandatory? Structure and Content LCM Table and HC-SC 3011 Form Info Module 1 Administrative
ectd in Canada Ginette Larocque, Purdue Pharma Canada August 23, 2016 AGENDA Eligibility for Filing in ectd Format ectd Mandatory? Structure and Content LCM Table and HC-SC 3011 Form Info Module 1 Administrative
ectd : Industry Experience
 ectd : Industry Experience SAPRAA - 26 March 2010 Anita Smal Agenda Introduction Experience with MCC pilot project Lessons learnt ectd structure Life cycle Further guidance required from MCC Submission-ready
ectd : Industry Experience SAPRAA - 26 March 2010 Anita Smal Agenda Introduction Experience with MCC pilot project Lessons learnt ectd structure Life cycle Further guidance required from MCC Submission-ready
CBT Appointment Registration Guide
 CBT Appointment Registration Guide This IBLCE CBT Appointment Registration Guide is for those who: need to make an appointment for their Computer Based Testing examination As an International Organisation,
CBT Appointment Registration Guide This IBLCE CBT Appointment Registration Guide is for those who: need to make an appointment for their Computer Based Testing examination As an International Organisation,
MANUAL OF SUBMISSION OF APPLICATIONS FOR CERTIFICATES OF A PHARMACEUTICAL PRODUCT WHO MODEL AND STATEMENTS OF PHARMACEUTICAL PRODUCTS
 MANUAL OF SUBMISSION OF APPLICATIONS FOR CERTIFICATES OF A PHARMACEUTICAL PRODUCT WHO MODEL AND STATEMENTS OF PHARMACEUTICAL PRODUCTS GLOSSARY... 2 INTRDUCTION... 3 Objective... 3 DEFINITIONS... 3 Certificate
MANUAL OF SUBMISSION OF APPLICATIONS FOR CERTIFICATES OF A PHARMACEUTICAL PRODUCT WHO MODEL AND STATEMENTS OF PHARMACEUTICAL PRODUCTS GLOSSARY... 2 INTRDUCTION... 3 Objective... 3 DEFINITIONS... 3 Certificate
Ontario Labour Relations Board
 Ontario Labour Relations Board Frequently Asked Questions E-Filing 1. What is E-Filing? Electronic filing or E-filing is the ability to file forms and submissions online with the Ontario Labour Relations
Ontario Labour Relations Board Frequently Asked Questions E-Filing 1. What is E-Filing? Electronic filing or E-filing is the ability to file forms and submissions online with the Ontario Labour Relations
Quality Overall Summary Chemical Entities Clinical Trial Application Phase III QOS - CTA GRP(PQ)-01-1(v1): Date 2008/11/12
 Therapeutic Products Directorate To: [Name], Director, Office of Clinical Trials Security Classification: HC Protected From: [Name], Manager, Clinical Trials Quality Division, Office of Clinical Trials
Therapeutic Products Directorate To: [Name], Director, Office of Clinical Trials Security Classification: HC Protected From: [Name], Manager, Clinical Trials Quality Division, Office of Clinical Trials
User Manual NDS-WEB. Version: 3.0 status Published by: < Authors: Swissmedic Narcotics Division
 User Manual NDS-WEB Published by: Authors: Swissmedic Narcotics Division 1 / 29 Contents User Manual NDS-WEB 1 Basic principles of NDS-WEB 3 2 Registration and applications 3 2.1 Self-registration
User Manual NDS-WEB Published by: Authors: Swissmedic Narcotics Division 1 / 29 Contents User Manual NDS-WEB 1 Basic principles of NDS-WEB 3 2 Registration and applications 3 2.1 Self-registration
ectd TECHNICAL CONFORMANCE GUIDE
 ectd TECHNICAL CONFORMANCE GUIDE Technical Specifications Document This Document is incorporated by reference into the following Guidance Document(s): Guidance for Industry Providing Regulatory Submissions
ectd TECHNICAL CONFORMANCE GUIDE Technical Specifications Document This Document is incorporated by reference into the following Guidance Document(s): Guidance for Industry Providing Regulatory Submissions
NSTIP Portal Registration Manual NSTIP Portal Registration Manual
 NSTIP Portal Registration Manual Jan 2013 1 Contents 1. Introduction to NSTIP Portal... 3 2. Steps... 4 2.1. Registration... 4 2.2. Login... 6 2.3. Update Profile... 8 2 1. Introduction to NSTIP Portal
NSTIP Portal Registration Manual Jan 2013 1 Contents 1. Introduction to NSTIP Portal... 3 2. Steps... 4 2.1. Registration... 4 2.2. Login... 6 2.3. Update Profile... 8 2 1. Introduction to NSTIP Portal
VISUAL ARTS & NEW MEDIA INDIVIDUAL PROJECTS
 VISUAL ARTS & NEW MEDIA INDIVIDUAL PROJECTS INSTRUCTIONS FOR GATE ONLINE APPLICATIONS If you are a FIRST-TIME user, please read through the guide in full BEFORE starting your application. If you a RETURNING
VISUAL ARTS & NEW MEDIA INDIVIDUAL PROJECTS INSTRUCTIONS FOR GATE ONLINE APPLICATIONS If you are a FIRST-TIME user, please read through the guide in full BEFORE starting your application. If you a RETURNING
The ectd Backbone Files Specification for Module 1. The ectd BACKBONE FILES SPECIFICATION FOR MODULE 1
 The ectd Backbone Files Specification for Module 1 The ectd BACKBONE FILES SPECIFICATION FOR MODULE 1 Revision History Date Version Summary of Changes 2003-08-13 1.0 Original version 2004-03-01 1.1 Clarifications
The ectd Backbone Files Specification for Module 1 The ectd BACKBONE FILES SPECIFICATION FOR MODULE 1 Revision History Date Version Summary of Changes 2003-08-13 1.0 Original version 2004-03-01 1.1 Clarifications
March Canadian Public Tenders Buyer Guide
 March 2010 Canadian Public Tenders Buyer Guide Table of Contents WELCOME TO MERX... 1 ABOUT THIS REFERENCE GUIDE... 1 SECTION 1 GETTING STARTED... 2 SYSTEM REQUIREMENTS... 2 NAVIGATING IN MERX... 2 SECTION
March 2010 Canadian Public Tenders Buyer Guide Table of Contents WELCOME TO MERX... 1 ABOUT THIS REFERENCE GUIDE... 1 SECTION 1 GETTING STARTED... 2 SYSTEM REQUIREMENTS... 2 NAVIGATING IN MERX... 2 SECTION
Get ready for ectd in South Africa
 Get ready for ectd in South Africa Going from CTD to ectd Anita Smal, 14 & 15 February 2013 Agenda The goal Planning Prepare submission ready documents PDFs ectd content planning ectd structure planning
Get ready for ectd in South Africa Going from CTD to ectd Anita Smal, 14 & 15 February 2013 Agenda The goal Planning Prepare submission ready documents PDFs ectd content planning ectd structure planning
1 2 December esubmissions? 1. How can baseline dossiers support global esubmissions? Disclaimer. What is a baseline submission?
 How can baseline dossiers support global Hans van Bruggen www.ectdconsultancy.com How can baseline dossiers support global 1 Disclaimer The views and opinions expressed in the following PowerPoint slides
How can baseline dossiers support global Hans van Bruggen www.ectdconsultancy.com How can baseline dossiers support global 1 Disclaimer The views and opinions expressed in the following PowerPoint slides
Pay Equity Hearings Tribunal
 Pay Equity Hearings Tribunal Frequently Asked Questions E-Filing 1. WHAT IS E-FILING? Electronic filing or E-filing is the ability to file forms and submissions online with the Pay Equity Hearings Tribunal
Pay Equity Hearings Tribunal Frequently Asked Questions E-Filing 1. WHAT IS E-FILING? Electronic filing or E-filing is the ability to file forms and submissions online with the Pay Equity Hearings Tribunal
NAPAMS HELP DOCUMENT
 NAPAMS HELP DOCUMENT What you should know All documents to be uploaded must be PDF documents and not more than 250KB All images should be in JPEG and not more than 250KB There are two payment options,
NAPAMS HELP DOCUMENT What you should know All documents to be uploaded must be PDF documents and not more than 250KB All images should be in JPEG and not more than 250KB There are two payment options,
Criteria Webinar. 13 th July European Update on Validation of ectd & NeeS submissions, July 2012
 EU ectd and NeeS Validation Criteria Webinar TIGes Harmonisation Group 13 th July 2012 1 Presenters of the day from TIGes Harmonisation Group Alastair Nixon (EFPIA) Klaus Menges ges( (DE) Manuela Copier
EU ectd and NeeS Validation Criteria Webinar TIGes Harmonisation Group 13 th July 2012 1 Presenters of the day from TIGes Harmonisation Group Alastair Nixon (EFPIA) Klaus Menges ges( (DE) Manuela Copier
How to Interact with the Natural and Non-prescription Health Products Directorate Electronically. Guidance Document
 How to Interact with the Natural and Non-prescription Health Products Directorate Electronically Guidance Document Table of Contents 1. INTRODUCTION... 3 1.1 System Requirements... 3 2. EPOST CONNECT...
How to Interact with the Natural and Non-prescription Health Products Directorate Electronically Guidance Document Table of Contents 1. INTRODUCTION... 3 1.1 System Requirements... 3 2. EPOST CONNECT...
How to get acceptance of CEP revisions quickly
 How to get acceptance of CEP revisions quickly EDQM WEBINAR 13 November 2017 Florence SCHULIAR - Certification of Substances Department 1 How to get acceptance of CEP revisions quickly Aim of this presentation
How to get acceptance of CEP revisions quickly EDQM WEBINAR 13 November 2017 Florence SCHULIAR - Certification of Substances Department 1 How to get acceptance of CEP revisions quickly Aim of this presentation
[ USER REGISTRATION MANUAL ]
![[ USER REGISTRATION MANUAL ] [ USER REGISTRATION MANUAL ]](/thumbs/83/87097543.jpg) [ USER REGISTRATION MANUAL ] [New Account Creation ] ABSTRACT [To use the on-line submission system (e-jdws) you should have an account at JFDA, this manual will help you to create an account] Author:
[ USER REGISTRATION MANUAL ] [New Account Creation ] ABSTRACT [To use the on-line submission system (e-jdws) you should have an account at JFDA, this manual will help you to create an account] Author:
Variations in ectd format Q&A document
 February 2015 Q&A document This document uses a question and answer format to give some guidance when submitting variation applications in ectd format. For general guidance on variations, please refer
February 2015 Q&A document This document uses a question and answer format to give some guidance when submitting variation applications in ectd format. For general guidance on variations, please refer
December EMA/668616/2014-Rev.2.4EMA/668616/2014-Rev.2.5
 Technical validation checklist 1 for veterinary electronic submission 2 - Version 2.45 Validation checklist for VNeeS submissions, approved by the Veterinary Harmonisation Group (VHG). There are two types
Technical validation checklist 1 for veterinary electronic submission 2 - Version 2.45 Validation checklist for VNeeS submissions, approved by the Veterinary Harmonisation Group (VHG). There are two types
Important Notes: The XML and there corresponding images should be added with the proper file names.
 ectd FAQ Contents ECTD HOW TO GUIDE... 3 SPL Naming Convention in ectd... 3 Procedure to Send SPC documents in WORD format... 4 File name exceeds maximum length (64 characters) per ectd specification...
ectd FAQ Contents ECTD HOW TO GUIDE... 3 SPL Naming Convention in ectd... 3 Procedure to Send SPC documents in WORD format... 4 File name exceeds maximum length (64 characters) per ectd specification...
ICH Topic M 4 Common Technical Document for the Registration of Pharmaceuticals for Human Use Organisation CTD. Step 5
 European Medicines Agency February 2004 CPMP/ICH/2887/99 ICH Topic M 4 Common Technical Document for the Registration of Pharmaceuticals for Human Use Organisation CTD Step 5 COMMON TECHNICAL DOCUMENT
European Medicines Agency February 2004 CPMP/ICH/2887/99 ICH Topic M 4 Common Technical Document for the Registration of Pharmaceuticals for Human Use Organisation CTD Step 5 COMMON TECHNICAL DOCUMENT
Guideline on the specifications for provision of an electronic submission (e-submission) for a veterinary medicinal product
 Guideline prepared by the TIGes-Vet Version 1.0 June 2009 Guideline on the specifications for provision of an electronic submission (e-submission) for a veterinary medicinal product 1. Introduction This
Guideline prepared by the TIGes-Vet Version 1.0 June 2009 Guideline on the specifications for provision of an electronic submission (e-submission) for a veterinary medicinal product 1. Introduction This
Harmonised Technical Guidance for Using of Electronic Application Forms (eaf) for human and veterinary medicinal products in the EU. Version 1.
 Harmonised Technical Guidance for Using of Electronic Application Forms (eaf) for human and veterinary medicinal products in the EU Version 1.1 September 2015 Remark to the reader This document reflects
Harmonised Technical Guidance for Using of Electronic Application Forms (eaf) for human and veterinary medicinal products in the EU Version 1.1 September 2015 Remark to the reader This document reflects
Guidance for Industry on Providing Regulatory Information in Electronic Format: ectd electronic Submissions
 DRAFT FOR TESTING Guidance for Industry on Providing Regulatory Information in Electronic Format: ectd electronic Submissions This document is published under the auspices of the EU Telematic Implementation
DRAFT FOR TESTING Guidance for Industry on Providing Regulatory Information in Electronic Format: ectd electronic Submissions This document is published under the auspices of the EU Telematic Implementation
Release Notes for TIGes ectd Guidance Comparison of version 2.0 with 1.0
 General Changes throughout the document Previous NA Changes EMEA changed to EMA Cover Page NA Draft status of document removed. Changed the name of the document. 1. Introduction 1 Fully rewritten to align
General Changes throughout the document Previous NA Changes EMEA changed to EMA Cover Page NA Draft status of document removed. Changed the name of the document. 1. Introduction 1 Fully rewritten to align
Orientation Material for M8: ectd EWG ectd v4.0 Implementation Package v1.3
 Orientation Material for M8: ectd EWG ectd v4.0 Implementation Package v1.3 International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal Notice This presentation
Orientation Material for M8: ectd EWG ectd v4.0 Implementation Package v1.3 International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal Notice This presentation
WCB Online A User Guide for Tiered Service Providers
 WCB Online User Guide for Tiered Service Providers WCB Online A User Guide for Tiered Service Providers A Nova Scotians safe and secure from workplace injury Table of Contents WCB Online 1 Profile Creation
WCB Online User Guide for Tiered Service Providers WCB Online A User Guide for Tiered Service Providers A Nova Scotians safe and secure from workplace injury Table of Contents WCB Online 1 Profile Creation
1. Document Control 2. Change Record Version Date Author(s) Comments 3. Reviewers Version Name Organisation 4. Distribution Version Date Name Status
 for the paper submission of regulatory information in support of a marketing authorisation application when using the Electronic Common Technical Document ( ectd ) as the source submission. V1.0 February
for the paper submission of regulatory information in support of a marketing authorisation application when using the Electronic Common Technical Document ( ectd ) as the source submission. V1.0 February
Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) for human medicinal products
 Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) for human medicinal products This document is published under the auspices of the
Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) for human medicinal products This document is published under the auspices of the
Update of ectd Module 1 specification for South Africa V2.0
 Update of ectd Module 1 specification for South Africa V2.0 Dr. Silke Nolkemper, Senior Business Consultant Christine Hirt, Managing Consultant Pretoria Copyright 2016 EXTEDO. All rights reserved. 2 New
Update of ectd Module 1 specification for South Africa V2.0 Dr. Silke Nolkemper, Senior Business Consultant Christine Hirt, Managing Consultant Pretoria Copyright 2016 EXTEDO. All rights reserved. 2 New
DUBAI CUSTOMS New Registration User s Manual
 1 1 DUBAI CUSTOMS New Registration User s Manual Copyright Information Copyright 2009 by Dubai Trade. All rights reserved This document and all associated attachments mentioned therein are the intellectual
1 1 DUBAI CUSTOMS New Registration User s Manual Copyright Information Copyright 2009 by Dubai Trade. All rights reserved This document and all associated attachments mentioned therein are the intellectual
API documentation from the perspective of WHO-PQP
 API documentation from the perspective of WHO-PQP Antony Fake PhD WHO Medicines Prequalification Programme 1 API documentation 3.2.S.3.2 from Impurities, the perspective of WHO PQP Malaysia, Mumbai, 29
API documentation from the perspective of WHO-PQP Antony Fake PhD WHO Medicines Prequalification Programme 1 API documentation 3.2.S.3.2 from Impurities, the perspective of WHO PQP Malaysia, Mumbai, 29
CJA evoucher. Attorney User Manual Release 5.2 October 2018
 CJA evoucher Attorney User Manual Release 5. October 08 CJA evoucher for Attorneys i Contents Nota Bene: Edit before using!... Introduction... Panel Management... Voucher & Authorization Request Submission...
CJA evoucher Attorney User Manual Release 5. October 08 CJA evoucher for Attorneys i Contents Nota Bene: Edit before using!... Introduction... Panel Management... Voucher & Authorization Request Submission...
Training Guide for Practitioners. Washington State Department of Health Washington State Prescription Monitoring Program
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
EMC Documentum Quality and Manufacturing
 EMC Documentum Quality and Manufacturing Version 3.1 User Guide EMC Corporation Corporate Headquarters Hopkinton, MA 01748-9103 1-508-435-1000 www.emc.com Legal Notice Copyright 2012-2016 EMC Corporation.
EMC Documentum Quality and Manufacturing Version 3.1 User Guide EMC Corporation Corporate Headquarters Hopkinton, MA 01748-9103 1-508-435-1000 www.emc.com Legal Notice Copyright 2012-2016 EMC Corporation.
Submissions to the PSUR Repository using EMA Gateway/Web Client
 Submissions to the PSUR Repository using EMA Gateway/Web Client Webinar training to existing Gateway users Presented by Kristiina Puusaari on 10 February 2015 An agency of the European Union Presenters
Submissions to the PSUR Repository using EMA Gateway/Web Client Webinar training to existing Gateway users Presented by Kristiina Puusaari on 10 February 2015 An agency of the European Union Presenters
STEPS FOR CANDIDATE FOR SCHEDULING ITB EXAM
 STEPS FOR CANDIDATE FOR SCHEDULING ITB EXAM 1. Candidates can start registering through the Register for an ITB Certification link on www.pearsonvue.com/itb or directly by clicking on Enrollment link of
STEPS FOR CANDIDATE FOR SCHEDULING ITB EXAM 1. Candidates can start registering through the Register for an ITB Certification link on www.pearsonvue.com/itb or directly by clicking on Enrollment link of
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL South African Specification for ectd Regional - Module1 This document is intended to provide requirements to applicants wishing to submit applications for the registration of
MEDICINES CONTROL COUNCIL South African Specification for ectd Regional - Module1 This document is intended to provide requirements to applicants wishing to submit applications for the registration of
Guidance for registration with EudraVigilance Veterinary
 11 July 2014 Veterinary Medicines and Product Data Management Table of Contents 1. Summary.2 2. Overview of the registration process 3 3. General information you should familiarise yourself with before
11 July 2014 Veterinary Medicines and Product Data Management Table of Contents 1. Summary.2 2. Overview of the registration process 3 3. General information you should familiarise yourself with before
Eforms Full Application Guide New Contractor
 Eforms Full Application Guide New Contractor 1 Completing a Full application form (Pages 2-6) 1.1 Initial application screen (Page 2) 1.2 Assessment scope (Page 3) 1.3 Declaration and fees (Page 3) 1.4
Eforms Full Application Guide New Contractor 1 Completing a Full application form (Pages 2-6) 1.1 Initial application screen (Page 2) 1.2 Assessment scope (Page 3) 1.3 Declaration and fees (Page 3) 1.4
extended EudraVigilance Medicinal Product Dictionary (XEVMPD) e-learning
 extended EudraVigilance Medicinal Product Dictionary (XEVMPD) e-learning Session 3: Database Architecture Version 5.3 An agency of the European Union Roles of the XEVMPD in the EV System (1) The roles
extended EudraVigilance Medicinal Product Dictionary (XEVMPD) e-learning Session 3: Database Architecture Version 5.3 An agency of the European Union Roles of the XEVMPD in the EV System (1) The roles
User Guide to the e-recruitment System
 User Guide to the e-recruitment System Last Updated in August 2018 Public Service Commission and Disciplined Forces Service Commission Contents Page 1. General Information 2 2. Before you apply 3 3. Searching
User Guide to the e-recruitment System Last Updated in August 2018 Public Service Commission and Disciplined Forces Service Commission Contents Page 1. General Information 2 2. Before you apply 3 3. Searching
User Guidance for National Competent Authorities for PSUR Repository
 20 February 2017 EMA/52448/2015 v9.0 User Guidance for National Competent Authorities for PSUR Repository 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0)20 3660 8427 Facsimile
20 February 2017 EMA/52448/2015 v9.0 User Guidance for National Competent Authorities for PSUR Repository 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44 (0)20 3660 8427 Facsimile
ANSM 11/ Page 1 sur 6
 NOTICE TO ASMF HOLDERS FOR ELECTRONIC SUBMISSION OF ASMF TO ANSM VIA CESP... 2 1. ectd Format... 2 a. MR/DC Procedures... 2 b. National Procedures... 2 c. ASMF Guidance... 2 d. Recommendations... 2 2.
NOTICE TO ASMF HOLDERS FOR ELECTRONIC SUBMISSION OF ASMF TO ANSM VIA CESP... 2 1. ectd Format... 2 a. MR/DC Procedures... 2 b. National Procedures... 2 c. ASMF Guidance... 2 d. Recommendations... 2 2.
Document Image System
 Document Image System Document Image System Overview/Benefits The Document Image System (DIS) facilitates automated submission of documents and specific data by participating Trade Partners to CBP. The
Document Image System Document Image System Overview/Benefits The Document Image System (DIS) facilitates automated submission of documents and specific data by participating Trade Partners to CBP. The
ProviderLink. Healthcare Providers eclaim Application. User Guide - Release 1.0 MAY in partnership with
 Healthcare Providers eclaim Application User Guide - Release 1.0 MAY 2012 in partnership with www.eclaimlink.ae 1 Table of Contents Getting Started 3 Registration 4 Logging In & Setup 5 ProviderLink System
Healthcare Providers eclaim Application User Guide - Release 1.0 MAY 2012 in partnership with www.eclaimlink.ae 1 Table of Contents Getting Started 3 Registration 4 Logging In & Setup 5 ProviderLink System
All Outreach Training Card Requests and payments will be submitted via the NEW ONLINE OUTREACH TRAINER PORTAL. https://outreach.msosha.
 All Outreach Training Card Requests and payments will be submitted via the NEW ONLINE OUTREACH TRAINER PORTAL https://outreach.msosha.com Register Yourself As An OSHA Authorized Outreach Trainer Register
All Outreach Training Card Requests and payments will be submitted via the NEW ONLINE OUTREACH TRAINER PORTAL https://outreach.msosha.com Register Yourself As An OSHA Authorized Outreach Trainer Register
Practical User Guide for Electronic Application Forms (eaf) for human and veterinary medicinal products in the EU
 ` 10 August 2018 Practical User Guide for Electronic Application Forms (eaf) for human and veterinary medicinal products in the EU Version 1.7.1 Page 1 of 80 Note to readers This guidance reflects the
` 10 August 2018 Practical User Guide for Electronic Application Forms (eaf) for human and veterinary medicinal products in the EU Version 1.7.1 Page 1 of 80 Note to readers This guidance reflects the
for Missouri Performance Assessments
 ETS Submission System User Guide for Missouri Performance Assessments Version 4.0 7/26/17 Copyright 2017 by Educational Testing Service. All rights reserved. ETS and the ETS logo are registered trademarks
ETS Submission System User Guide for Missouri Performance Assessments Version 4.0 7/26/17 Copyright 2017 by Educational Testing Service. All rights reserved. ETS and the ETS logo are registered trademarks
Quest 3 Refresher & Issue
 Quest 3 Refresher & Issue Hotel Singgahsana, PJ ( 10 September 2013,Tuesday) Prepared by: Eva Senior Assistant Director, NPCB WHO Collaborating Centre for Regulatory Control of Pharmaceuticals Member of
Quest 3 Refresher & Issue Hotel Singgahsana, PJ ( 10 September 2013,Tuesday) Prepared by: Eva Senior Assistant Director, NPCB WHO Collaborating Centre for Regulatory Control of Pharmaceuticals Member of
A guide to filling out Health Canada Exemption Applications
 Page 1 of 16 A guide to filling out Health Canada Exemption Applications A controlled substance exemption is a document issued by Health Canada authorising you to possess a specific quantity of a controlled
Page 1 of 16 A guide to filling out Health Canada Exemption Applications A controlled substance exemption is a document issued by Health Canada authorising you to possess a specific quantity of a controlled
Takeda Pharmaceuticals North America, Inc. Takeda Supplier Registration User Guide. v1.0
 Takeda Pharmaceuticals North America, Inc. Takeda Supplier Registration User Guide v1.0 1.0 OVERVIEW Takeda utilizes an automated supplier registration solution. A supplier will receive an e-mail detailing
Takeda Pharmaceuticals North America, Inc. Takeda Supplier Registration User Guide v1.0 1.0 OVERVIEW Takeda utilizes an automated supplier registration solution. A supplier will receive an e-mail detailing
Standard operating procedure
 Standard operating procedure Title: of medicinal products Status: PUBLIC Document no.: SOP/INSP/2000 Lead author Approver Effective date: 03-APR-12 Name: Camelia Manta Name: Fergus Sweeney Review date:
Standard operating procedure Title: of medicinal products Status: PUBLIC Document no.: SOP/INSP/2000 Lead author Approver Effective date: 03-APR-12 Name: Camelia Manta Name: Fergus Sweeney Review date:
extended EudraVigilance Medicinal Product Report Message (XEVPRM) Step-by-Step Guide
 extended EudraVigilance Medicinal Product Report Message (XEVPRM) Step-by-Step Insert of a Development Medicinal Product (DMP) 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44
extended EudraVigilance Medicinal Product Report Message (XEVPRM) Step-by-Step Insert of a Development Medicinal Product (DMP) 30 Churchill Place Canary Wharf London E14 5EU United Kingdom Telephone +44
Creating a New Application. A ROMEO Hasty Handbook Presented by the CHEO Research Institute
 Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Applies to: SA Administrative Assistant, SAWP Scientific Secretary and SA secretarial team in Scientific Advice Section
 Work instructions Title: Use of scientific advice and protocol assistance database Applies to: SA Administrative Assistant, SAWP Scientific Secretary and SA secretarial team in Scientific Advice Section
Work instructions Title: Use of scientific advice and protocol assistance database Applies to: SA Administrative Assistant, SAWP Scientific Secretary and SA secretarial team in Scientific Advice Section
Questions & Answers on Swissmedic ectd Implementation
 Questions & Answers on Swissmedic ectd Implementation Authors: Lead: Christiane Hofstetter, Swissmedic Madeleine Meusburger, Swissmedic Janine Weix, Swissmedic Ralph Maier, Swissmedic Céline Jurt Kuster,
Questions & Answers on Swissmedic ectd Implementation Authors: Lead: Christiane Hofstetter, Swissmedic Madeleine Meusburger, Swissmedic Janine Weix, Swissmedic Ralph Maier, Swissmedic Céline Jurt Kuster,
Frequently Asked Questions (FAQs) about Japan Display Inc. (JDI) Green Procurement Guideline
 Frequently Asked Questions (FAQs) about Japan Display Inc. (JDI) Green Procurement Guideline Last updated on April 1, 2017 (1) Green Procurement Guideline in general: Q: We are a trading company. Should
Frequently Asked Questions (FAQs) about Japan Display Inc. (JDI) Green Procurement Guideline Last updated on April 1, 2017 (1) Green Procurement Guideline in general: Q: We are a trading company. Should
Ferring Pharmaceuticals Inc. Educational Grant Applicant Working Guide
 Ferring Pharmaceuticals Inc. Educational Grant Applicant Working Guide 2014 Contents FERRING I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 4 III. HOW TO SUBMIT AN APPLICATION... 6 IV. HOW TO PROVIDE
Ferring Pharmaceuticals Inc. Educational Grant Applicant Working Guide 2014 Contents FERRING I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 4 III. HOW TO SUBMIT AN APPLICATION... 6 IV. HOW TO PROVIDE
How to register to MF in Japan Points to Consider
 How to register to MF in Japan Points to Consider Master File Management Group Division of Pharmacopoeia and Standards for Drugs Office of Standards and Guidelines Development KENTARO HASHIMOTO CPhI Korea
How to register to MF in Japan Points to Consider Master File Management Group Division of Pharmacopoeia and Standards for Drugs Office of Standards and Guidelines Development KENTARO HASHIMOTO CPhI Korea
USER GUIDE: NMLS Course Provider Application Process (Initial)
 USER GUIDE: NMLS Course Provider Application Process (Initial) Version 2.0 May 1, 2011 Nationwide Mortgage Licensing System & Registry State Regulatory Registry, LLC 1129 20 th St, N.W., 9 th Floor Washington,
USER GUIDE: NMLS Course Provider Application Process (Initial) Version 2.0 May 1, 2011 Nationwide Mortgage Licensing System & Registry State Regulatory Registry, LLC 1129 20 th St, N.W., 9 th Floor Washington,
Meritain Connect User Manual. for Employees. 1 Meritain Connect User Guide for Employees
 Meritain Connect User Manual for Employees 1 Meritain Connect User Guide for Employees Contents Introduction... 4 Accessing Meritain Connect... 5 Logging In... 5 Forgot Password... 6 Registration Process...
Meritain Connect User Manual for Employees 1 Meritain Connect User Guide for Employees Contents Introduction... 4 Accessing Meritain Connect... 5 Logging In... 5 Forgot Password... 6 Registration Process...
Electronic Appraisal Delivery (EAD) Portal. FHA EAD General User Guide
 Electronic Appraisal Delivery (EAD) Portal FHA EAD General User Guide Last Updated: October 2015 FHA EAD General User Guide Page 2 of 87 Version 1.3.1 TABLE OF CONTENTS INTRODUCTION... 6 WHAT IS THE ELECTRONIC
Electronic Appraisal Delivery (EAD) Portal FHA EAD General User Guide Last Updated: October 2015 FHA EAD General User Guide Page 2 of 87 Version 1.3.1 TABLE OF CONTENTS INTRODUCTION... 6 WHAT IS THE ELECTRONIC
Daman isupplier Portal User Guide. Procurement
 Procurement Table of Content 1. Introduction... 4 Benefits associated with using isupplier include:... 4 2. System Requirements... 4 2.1 Recommended Operating System:... 4 2.2 Browser Requirements:...
Procurement Table of Content 1. Introduction... 4 Benefits associated with using isupplier include:... 4 2. System Requirements... 4 2.1 Recommended Operating System:... 4 2.2 Browser Requirements:...
PSUR Repository Interactive Q&A
 PSUR Repository Interactive Q&A Interactive Q&A session with MAHs on the new functionality provided in release 1.03.00 Presented by Kristiina Puusaari on 10 September 2015 An agency of the European Union
PSUR Repository Interactive Q&A Interactive Q&A session with MAHs on the new functionality provided in release 1.03.00 Presented by Kristiina Puusaari on 10 September 2015 An agency of the European Union
Guidance for Industry on Providing Regulatory Information in ectd Format
 Guidance for Industry on Providing Regulatory Information in ectd Format Authors: Responsible: Lead: Ralph Maier, Swissmedic Review team Swissmedic Review team Interpharma Operational Support Services,
Guidance for Industry on Providing Regulatory Information in ectd Format Authors: Responsible: Lead: Ralph Maier, Swissmedic Review team Swissmedic Review team Interpharma Operational Support Services,
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
PIMS Getting Started. Click Here to open the PIMS website. Step 1 Click Create Account
 PIMS Getting Started Click Here to open the PIMS website Step 1 Click Create Account Business New to PIMS? Business/Agency name not in the drop down? Select New Account Select Account Type Non Profit or
PIMS Getting Started Click Here to open the PIMS website Step 1 Click Create Account Business New to PIMS? Business/Agency name not in the drop down? Select New Account Select Account Type Non Profit or
FAQ MyCoID SERVICES GENERAL. 1. What is MyCoID 2016?
 1 FAQ MyCoID 2016 FAQ MyCoID SERVICES GENERAL 1. What is MyCoID 2016? MyCoID is an online company incorporation and registration services launched by SSM that allows company incorporation services to be
1 FAQ MyCoID 2016 FAQ MyCoID SERVICES GENERAL 1. What is MyCoID 2016? MyCoID is an online company incorporation and registration services launched by SSM that allows company incorporation services to be
Zambia Medicines Regulatory Authority APPLICATION FOR MARKETING AUTHORISATION OF A MEDICINE FOR HUMAN USE
 Zambia Medicines Regulatory Authority APPLICATION FOR MARKETING AUTHORISATION OF A MEDICINE FOR HUMAN USE COMMON TECHNICAL DOCUMENT FORMAT ZAMBIA Module 1 CTD-Modules 2-5 Version 03, April 2015 ZAMBIA
Zambia Medicines Regulatory Authority APPLICATION FOR MARKETING AUTHORISATION OF A MEDICINE FOR HUMAN USE COMMON TECHNICAL DOCUMENT FORMAT ZAMBIA Module 1 CTD-Modules 2-5 Version 03, April 2015 ZAMBIA
EMC Documentum Quality and Manufacturing
 EMC Documentum Quality and Manufacturing Version 4.0 User Guide EMC Corporation Corporate Headquarters Hopkinton, MA 01748-9103 1-508-435-1000 www.emc.com Legal Notice Copyright 2012-2016 EMC Corporation.
EMC Documentum Quality and Manufacturing Version 4.0 User Guide EMC Corporation Corporate Headquarters Hopkinton, MA 01748-9103 1-508-435-1000 www.emc.com Legal Notice Copyright 2012-2016 EMC Corporation.
Digital Intelligence Systems, LLC PeopleSoft Guide Vendors
 Digital Intelligence Systems, LLC PeopleSoft Guide Vendors Version 1.0 July 2016 CONTENTS INTRODUCTION... 3 1.1 Change Password... 3 PROFILE INFORMATION... 5 2.1 Identifying Information... 6 2.2 Address...
Digital Intelligence Systems, LLC PeopleSoft Guide Vendors Version 1.0 July 2016 CONTENTS INTRODUCTION... 3 1.1 Change Password... 3 PROFILE INFORMATION... 5 2.1 Identifying Information... 6 2.2 Address...
UNDP etendering: User Guide for Bidders. January 2018
 UNDP etendering: User Guide for Bidders January 2018 Quick References to the Guide The UNDP etendering Guide for Bidders is a manual for individuals or companies who wish to participate in a UNDP tender
UNDP etendering: User Guide for Bidders January 2018 Quick References to the Guide The UNDP etendering Guide for Bidders is a manual for individuals or companies who wish to participate in a UNDP tender
ON-LINE INCIDENT REPORT FORM: GUIDANCE FOR INDUSTRY
 ON-LINE INCIDENT REPORT FORM: GUIDANCE FOR INDUSTRY Industry Guidance August 2007 1 Food Standards Agency On-line incident report form: Guidance for industry Contents Title Section Page No(s). Introduction
ON-LINE INCIDENT REPORT FORM: GUIDANCE FOR INDUSTRY Industry Guidance August 2007 1 Food Standards Agency On-line incident report form: Guidance for industry Contents Title Section Page No(s). Introduction
Sanofi Investigator Sponsored Studies (ISS) External Reference Guide. 1 November 2017
 Sanofi Investigator Sponsored Studies (ISS) External Reference Guide 1 November 2017 Sanofi ISS Overview Sanofi is committed to supporting medically and scientifically sound research aimed at the advancement
Sanofi Investigator Sponsored Studies (ISS) External Reference Guide 1 November 2017 Sanofi ISS Overview Sanofi is committed to supporting medically and scientifically sound research aimed at the advancement
ectd Next Major Release Business Requirements Collation (9-JUN-10) TOPIC Requirement Comment
 ICH Req No. ectd Next Major Release Business Requirements Collation (9-JUN-10) TOPIC Requirement Comment ICH001 ICH002 ICH003 ICH004 APPLICATION APPLICATION APPLICATION APPLICATION A regulated product
ICH Req No. ectd Next Major Release Business Requirements Collation (9-JUN-10) TOPIC Requirement Comment ICH001 ICH002 ICH003 ICH004 APPLICATION APPLICATION APPLICATION APPLICATION A regulated product
Managing Product Master Data
 Managing Product Master Data Table of Contents Managing Product Master Data 3 Product Information 4 Adding Product Master Data 5 Updating Products 27 Accessing Product Master Data 28 Exporting Product
Managing Product Master Data Table of Contents Managing Product Master Data 3 Product Information 4 Adding Product Master Data 5 Updating Products 27 Accessing Product Master Data 28 Exporting Product
Swiss Module 1 Specification for ectd
 Project SIMES Swiss Module 1 Specification for ectd Authors: Lead: Christiane Hofstetter, Swissmedic Madeleine Meusburger, Swissmedic Ralph Maier, Swissmedic Céline Jurt Kuster, Swissmedic Susanne Kienberger,
Project SIMES Swiss Module 1 Specification for ectd Authors: Lead: Christiane Hofstetter, Swissmedic Madeleine Meusburger, Swissmedic Ralph Maier, Swissmedic Céline Jurt Kuster, Swissmedic Susanne Kienberger,
ectd Next Major Release Business Requirements Collation (11 NOV 10) TOPIC Requirement Comment ICH Req No.
 ICH Req No. ICH001 ICH002 ICH003 ICH004 ectd Next Major Release Business Requirements Collation (11 NOV 10) TOPIC Requirement Comment A regulated product application may have one or more regulatory activities
ICH Req No. ICH001 ICH002 ICH003 ICH004 ectd Next Major Release Business Requirements Collation (11 NOV 10) TOPIC Requirement Comment A regulated product application may have one or more regulatory activities
