ARC IRB Chair s Manual
|
|
|
- Colin Joseph
- 5 years ago
- Views:
Transcription
1 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application.
2 ARC IRB Chairs Manual Page 2 Table of Contents Welcome to ARC... 2 Accounts... 3 New Account Registration...3 Account Changes...3 Passwords...4 Navigation... 4 Login...4 Forgot Your User Name or Password?...4 My Home Folder...5 Navigation Tabs...5 My Inbox Tab... 6 My Reviews Tab... 6 Application Workspace...6 Working with Smart Forms... 8 IRB Committee Chair... 9 My Roles...9 Review: a Two-Part Process...10 Part 1 - Perform the Review...10 Part 2 - Submit the Review...11 Submit Expedited Review Submit Exempt Review Submit Full Board Review Meeting Workspace...13 Review and Sign Approval Letter...14 Expedited Study Exempt Study Full Board Study View Approval Letter...16 Welcome to ARC Our on-line ARC system streamlines the process of submitting, approving, tracking, and managing IRB applications. ARC is available via Internet connection 24 hours a day, 7 days a week. The ARC HelpDesk is available during regular business hours at (813) and by at RSCH-arc@usf.edu.
3 ARC IRB Chairs Manual Page 3 Accounts New Account Registration To open your new ARC account: 1. Go to the ARC Web Site: 2. Click Register Here on the right hand side of the page. 3. Complete the required fields ( * ) and your USF Net ID or Employee ID. 4. Select all relevant roles, such as Chair, Committee Member, Study Staff, PI. 5. Click Register. 6. Within two business days your new account will be activated and you will receive an e- mail containing your account information (i.e., User Name & Password). Account Changes It is important to keep your account information current. To make changes to your account, click your name in the upper right hand corner of your screen to open your account properties. Then make the necessary changes and click Apply. Note For changes to your department affiliation or assigned roles, you will need to contact the helpdesk.
4 ARC IRB Chairs Manual Page 4 Passwords To change your password, click on your name (as described above). On your Account page, click the Account tab. Type in your old password, your new password, and your new password again in their respective boxes. Click Apply. Navigation Login 1. Type your User Name in the login section on the right side of the ARC screen. 2. Type in your Password. 3. Click Log in. Forgot Your User Name or Password? 1. The Forgot Password and Forgot User Name options are available under Need Help?.
5 ARC IRB Chairs Manual Page 5 2. If you select Forgot user name?, you will be prompted to confirm your address. Once confirmed, your user name will be ed to you. 3. If you select Forgot password?, you will be prompted to confirm your user name and address. Once confirmed, a new temporary password will be ed to you. Upon log in, you will be required to change your password. My Home Folder After logging in, the screen displays your Folder which is like a personal home page. Here you will be able to view and manage those studies relevant to your Role. When you are in other sections of the ARC system, you can easily get back to your personal Folder by clicking the link to My Home. Navigation Tabs The central area of your Folder (My Home) provides a row of navigation tabs.
6 ARC IRB Chairs Manual Page 6 My Inbox Tab My Inbox lists all applications (studies, amendments, continuing reviews, and reportable events) that require your attention. Whenever you login or return to your Folder (My Home), My Inbox is displayed. To find out where the application is in the IRB process, look in the State column. You can access applications in My Inbox by clicking the application Name. Once you have completed the required activities, the application is moved electronically from one respective Inbox to the next according to who needs to work on it next. If an application is not in your Inbox, it is someone else's turn to work on it. If an item is still in your Inbox, it still requires your attention. My Reviews Tab The My Reviews tab lists all applications which you have reviewed or been assigned to review regardless of the State they are in. In the My Reviews tab, you can monitor the State of any application you have reviewed or been assigned to review. Application Workspace Review of an application begins in the application Workspace which is like a home page for the application. Open an application Workspace by clicking on its Name in My Inbox. The application Workspace provides: information about the application links to specific sections and documents related to the application buttons to initiate Activities and move the application to the next step in the IRB Process (these buttons are only available to you when the application is in your Inbox)
7 ARC IRB Chairs Manual Page 7 history of all activities performed on the application Below is an example of a Study Workspace screen: Key to the study Workspace screen: 1. The summary panel displays information about this study, including the study title. The information changes when a study becomes active. 2. IRB Study Number (also referred to as the Pro#). 3. Current State indicates the stage in the IRB review process for this study. This changes as Activities are completed. 4. View Study button opens the application SmartForm for viewing. Use this to view the entire IRB application. 5. Printer Version button opens all of the relevant SmartForm screens in one easy-toprint window. You may find that this is an easier way to review an application. 6. View Differences button allows you to view differences between versions. 7. Use the Edit Reviewer Checklist to perform the IRB Review. This opens the application SmartForm with the reviewer checklist in a column on the right. Completion of the Reviewer Checklist does not move the application to the next State. 8. The Pending Ancillary Approvals section lists ancillary reviews that are required but that have not been submitted yet. 9. The My Activities section lists those Activities that can be performed on the application under its Current State. Performing one of these Activities will move the application to the next state or will send an (as noted on the button). The legend on each button indicates which role can perform this activity. Click the button/link to open the Activity screen.
8 ARC IRB Chairs Manual Page Use Submit Full/Expedited/Exempt Board Review to submit your review and move the application to the next State. This Activity requires you to submit your Checklist and Reviewer Notes and to recommend a motion and approval period. 11. The History tab lists chronologically all actions that have been performed on the study. Click the Activity name in the listed History to view details. 12. The Attachments tab lists all documents that have been uploaded for this study. Successive versions are archived automatically so that you have access to the most currently approved versions, i.e., protocol, informed consent, advertisement, etc. 13. Reviewer Notes lists the Reviewer Notes, Change Requests, and study team responses. Jump To links are included that take you directly to the item in the application SmartForm. 14. Reviewer Checklists lists each Reviewer Checklist that has been saved for this application. Click the Link to SmartForm to open the respective Reviewer Checklist. Working with Smart Forms All applications in ARC use SmartForms which present only those questions that are relevant to your study. Navigation controls are located in the navigation bar at the top or bottom of each page. Use the Continue and Back buttons to move to the next or last-viewed screen. Use the SmartForm navigation controls instead of the controls in the browser bar (e.g., Internet Explorer, Firefox, Chrome, Safari, Opera). Save your work by clicking Save or Continue. WARNING: The Back button does not save changes. After you enter or edit data on a screen click Save before going Back! Use Exit to close the application and return to that application s Workspace. WARNING: Always Save before exiting!
9 ARC IRB Chairs Manual Page 9 Each section and question is numbered for easy navigation and reference. Numbering is consistent through all SmartForm applications; however, remember that only the relevant questions for each specific study are displayed. Once new or revised data on a page has been saved, you can navigate directly to other sections and questions by using the Jump To drop-down menu. The title of the displayed page will be red. Items not relevant to this study (based upon PI s answers) will appear gray in the Jump To menu. IRB Committee Chair My Roles A person can have multiple Roles in ARC (PI, Co-Investigator, Study Coordinator, Committee Chair, IRB Committee member, etc.). Different Roles provide access to different applications, information, and activities. When you log in to ARC, the system opens the Folder in one of your Roles. Each role has specific views and Activities associated with it, so be sure to select the correct Role. Your current Role will be displayed in the red banner at the top of the column on the left side of your Folder and will be Bold in the listing of your available Roles. If you have more than one role, make sure that the correct role is selected. Click on a role to make it your current role. Committee Chair - use this Role to manage applications that require Chair approval. You cannot conduct expedited or exempt reviews in the role of Chair.
10 ARC IRB Chairs Manual Page 10 Committee Member use this Role to manage applications that require expedited and exempt review by the Chair and those that require full board review by Primary and Secondary Reviewers. Study Staff use this Role to manage applications involving your own study as the PI or other member of a study team. Review: a Two-Part Process 1. Perform the Review: The Reviewer reviews the entire application, completes the Reviewer Checklist Questions, and makes notes on the SmartForm as needed. The application remains in your Inbox. 2. Submit the Review: The Reviewer submits the completed Checklist, Reviewer Notes, and motion with an approval period. The specifics of this step vary depending on the type of review required (full board, expedited, or exempt). Submission moves the application out of your Inbox to the next State. Part 1 - Perform the Review 1. Begin in your Folder (My Inbox). 2. Select Committee Member (1) in My Roles to display the applications that need attention. 3. Click on the application Name (2) to open the application Workspace. 4. In the application Workspace click View Study (left column) to view the entire application. 5. After you have reviewed the entire application, click Exit. 6. In the application Workspace click Edit Reviewer Checklist. 7. Review all relevant questions in the application. Answer the Reviewer Checklist Questions in the right column of each screen. The screens vary in vertical length so be
11 ARC IRB Chairs Manual Page 11 sure to scroll down the page to view all questions. Make notes as you go using the Review Notes bar at the top of each section. WARNING: The Reviewer Checklist does not include all sections of an application because it displays only those sections that are matched to checklist questions. 8. Before exiting the Reviewer Checklist, click Save and then Exit. WARNING: Completing the review and saving reviewer notes does not submit your review. It will remain in My Inbox until you have submitted it. Part 2 - Submit the Review To submit your completed review, follow the steps below for the type of review conducted. Submit Expedited Review 1. In the left column of the application workspace click Submit Expedited Review: 2. Choose the appropriate Expedited Review Motion: 3. If needed, add comments and/or attachments. 4. Click OK. WARNING: Once the motion (with review) has been submitted, you will not be able to make changes to the review. 5. The application will move back to the IRB Staff Inbox for the next activity.
12 ARC IRB Chairs Manual Page 12 Submit Exempt Review 1. In the left column of the application workspace click Submit Exempt Review: 2. Choose the appropriate Exempt Review Motion: 3. If needed, add comments and/or attachments. 4. Click OK. WARNING: Once the motion has been submitted, you will not be able to make changes to the review. 5. The application will move back to the IRB Staff Inbox for the next activity. Submit Full Board Review 1. In the left column of the study workspace click Submit Full Board Review. 2. Choose the appropriate Recommended Motion and Recommended Approval Period.
13 ARC IRB Chairs Manual Page If needed, add comments and/or attachments. 4. Click OK. WARNING: Once the motion has been submitted, you will not be able to make changes to the review. 5. The application will move back to the IRB Staff Inbox for the next activity. Meeting Workspace Management of meeting activities is completed in a meeting s Workspace. Typical activities include viewing the agenda and confirming or declining your attendance. 1. In your folder (My Home), click the Upcoming Meetings tab to view the list of scheduled meetings. 2. To open the meeting Workspace, click the name/date of the meeting. 3. The meeting Workspace will be displayed.
14 ARC IRB Chairs Manual Page 14 Key to Meeting Workspace: 1. The Agenda tab displays the main panel, with links to each item Workspace. 2. Click Meeting Agenda to display the formal version of the Agenda. 3. Click respective buttons to confirm or decline your attendance. Review and Sign Approval Letter Expedited Study 1. Begin in your Folder (My Inbox). 2. Select Committee Chair in My Roles to display the applications that need attention. 3. Click on the application Name to open the application Workspace. 4. In the left column of the Workspace, click Send Final IRB Letter To Study Team. 5. To review the approval letter click View. 6. Choose the meeting date. 7. Click OK. 8. The IRB approval letter will be ed to the study team.
15 ARC IRB Chairs Manual Page 15 Exempt Study 1. Begin in your Folder (My Inbox). 2. Select Committee Chair in My Roles to display the applications that need attention. 3. Click on the application Name to open the application Workspace. 4. In the left column of the study Workspace, click Send Final IRB Letter To Study Team. 5. To review the approval letter click View. 6. Click OK. 7. The approval letter will be sent to the Study Team and the study will be filed as certified exempt. Full Board Study 1. Begin in your Folder (My Inbox). 2. Select Committee Chair in My Roles to display the applications that need attention. 3. Click on the application Name to open the application Workspace. 4. In the left column of the study Workspace, click Send Final IRB Letter To Study Team. 5. To review the approval letter click View. 6. Choose the meeting date. 7. Click OK. 8. The IRB approval letter will be ed to the study team.
16 ARC IRB Chairs Manual Page 16 View Approval Letter When the IRB has approved an application, the approval letter is ed to the PI. The approval letter will also be available in the study Workspace. To view the approval letter: 1. In your Folder (My Home), click the Approved Studies tab. 2. In the Approved Studies folder click the study name. 3. In the study Workspace, the summary panel will now display a link to view the Letter of Approval. Click on the file next to IRB Letter.
The most current protocol information should be entered. This includes incorporation of all past amendments and modifications.
 Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Core Staff Review Approve with Contingencies
 Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
How Does the PI make Pre Review ICF Changes?
 How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
Nova Southeastern University IRBManager User Manual IRBMANAGER USER MANUAL
 IRBMANAGER USER MANUAL Contents 1. What is IRBManager?...1 2. IRBManager User Accounts/Login...1 a. Creating an IRBManager Account...1 b. Logging into IRBManager...2 c. Forgot Password...2 d. Locked Out
IRBMANAGER USER MANUAL Contents 1. What is IRBManager?...1 2. IRBManager User Accounts/Login...1 a. Creating an IRBManager Account...1 b. Logging into IRBManager...2 c. Forgot Password...2 d. Locked Out
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
eiacuc Reference for Veterinarians
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
FAQs. Q What is the status of my study application?
 FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
Submitting a Renewal Application in WebCAMP
 Submitting a Renewal Application in WebCAMP (NOTE: This is for existing studies with the CTSI.) 1. Click on the following link: http://cfqa01.med.nyu.edu/webcamp IMPORTANT: For proper functioning of WebCAMP,
Submitting a Renewal Application in WebCAMP (NOTE: This is for existing studies with the CTSI.) 1. Click on the following link: http://cfqa01.med.nyu.edu/webcamp IMPORTANT: For proper functioning of WebCAMP,
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment)
 Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Module 1: Core Committee Staff Basics
 Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
You may also scroll through the Table of Contents and click on the topic or question of interest.
 We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eresearch IBC Staff Review Step-by-Step Procedures Core Committee Staff Home Page
 1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
PAGE 1. IRBManager. Instruction Manual For IRB members
 PAGE 1 IRBManager Instruction Manual For IRB members IRBManager PAGE 2 What is IRBManager? IRBManager is a web-based system designed specifically for the IRB review process, all the way from submission
PAGE 1 IRBManager Instruction Manual For IRB members IRBManager PAGE 2 What is IRBManager? IRBManager is a web-based system designed specifically for the IRB review process, all the way from submission
DISCLOSER GUIDE. Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure. Submitting Disclosures Post-Submission Actions
 DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Click. IRB Study Submission Guide
 Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
How to Submit a New Application
 ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
era(infoed) Electronic Submission Instructions and Tips: Initial Application
 era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system.
era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system.
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK. (Version date 03/03/2006)
 ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
IRB Committee Member Adverse Event (AE) Single Member Review
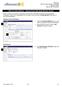 IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
Revised: February 28, Investigator/Coordinator Guide
 2014 Revised: February 28, 2014 Table of Contents Getting Started... 3 Navigational Tips... 4 Adding a New Application (Form 1)... 6 Routing Form... 11 Routing for Signatures... 15 Workflow Tracking and
2014 Revised: February 28, 2014 Table of Contents Getting Started... 3 Navigational Tips... 4 Adding a New Application (Form 1)... 6 Routing Form... 11 Routing for Signatures... 15 Workflow Tracking and
Electronic MTA Training Manual & User Guide for Administrative Staff & Principal Investigators
 Electronic MTA Training Manual & User Guide for Administrative Staff & Principal Investigators 1 P a g e Table of Contents Getting Started... 3 Introduction... 3 Helpful Hints... 3 Login Process... 6 Profile...
Electronic MTA Training Manual & User Guide for Administrative Staff & Principal Investigators 1 P a g e Table of Contents Getting Started... 3 Introduction... 3 Helpful Hints... 3 Login Process... 6 Profile...
Kuali Research User Guide: Create an IRB Protocol
 Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
retrieving study documents training guide
 retrieving study documents training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this document 4 retrieving study documents 5 current
retrieving study documents training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this document 4 retrieving study documents 5 current
Applying for EMSWCD Small Project and Community Events (SPACE) Grants
 ZOOMGRANTS TUTORIAL Applying for EMSWCD Small Project and Community Events (SPACE) Grants Instructions for ZoomGrants ZoomGrants is an online tool that helps facilitate grant applications, committee review,
ZOOMGRANTS TUTORIAL Applying for EMSWCD Small Project and Community Events (SPACE) Grants Instructions for ZoomGrants ZoomGrants is an online tool that helps facilitate grant applications, committee review,
Information Sheet for Scientific Approvers
 1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
IBC Reviewer User Guide
 IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
Talking Point: Today s topic will help you answers these questions in the future without having to send an or pick up the telephone!
 1 Today s topic will help you answers these questions in the future without having to send an email or pick up the telephone! 2 3 And sites did not have direct access to what activities were occurring
1 Today s topic will help you answers these questions in the future without having to send an email or pick up the telephone! 2 3 And sites did not have direct access to what activities were occurring
BOULDER IRB era InfoEd Continuing Review
 BOULDER IRB era InfoEd Continuing Review Last Update: 2017/11/30 Preface: This guide explains how to submit a continuing review for a previously approved expedited or full board study that is nearing expiration.
BOULDER IRB era InfoEd Continuing Review Last Update: 2017/11/30 Preface: This guide explains how to submit a continuing review for a previously approved expedited or full board study that is nearing expiration.
Adding (Amending) SSNs to an I-9
 Adding (Amending) SSNs to an I-9 When an employee does not have an SSN, you may still complete an I-9 form. Once they apply for their SSN, SSA will mail them their new card (this usually takes 2-3 weeks).
Adding (Amending) SSNs to an I-9 When an employee does not have an SSN, you may still complete an I-9 form. Once they apply for their SSN, SSA will mail them their new card (this usually takes 2-3 weeks).
Amend A Protocol training guide
 Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Click IRB 7.3 IRB Ancillary Reviewer s Guide
 Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Questions and Answers. Converting Existing Protocols into CHeRP IRB
 Questions and Answers Converting Existing Protocols into CHeRP IRB Questions: (hold ctrl and click on the question to jump directly to that answer) 1. Do I need to convert my existing protocol applications
Questions and Answers Converting Existing Protocols into CHeRP IRB Questions: (hold ctrl and click on the question to jump directly to that answer) 1. Do I need to convert my existing protocol applications
College Deans, Department Chairs & Supervising Faculty
 Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event
 Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Logging into myclinicalexchange
 Logging into myclinicalexchange 1. Navigate to https://myclinicalexchange.com 2. Click on Students Login Here. 3. You will be redirected to Students login page. 4. Under the Login area, click on the Forgot
Logging into myclinicalexchange 1. Navigate to https://myclinicalexchange.com 2. Click on Students Login Here. 3. You will be redirected to Students login page. 4. Under the Login area, click on the Forgot
Manual: Create a Staff Posting Initiator
 Manual: Create a Staff Posting Initiator Revised: 11-27-17 Introduction The University of Georgia has implemented a new version of its applicant tracking system, ipaws. The objective of ipaws is to streamline
Manual: Create a Staff Posting Initiator Revised: 11-27-17 Introduction The University of Georgia has implemented a new version of its applicant tracking system, ipaws. The objective of ipaws is to streamline
Version 2.1 June 12, 2018
 Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
IACUC Module. March 2018
 z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
Top Ten Questions About IRBNet.org
 Top Ten Questions About IRBNet.org 1. How do I register? Registration is easy. To register, simply navigate your web browser www.irbnet.org. The login area for IRBNet is in the upper right corner of the
Top Ten Questions About IRBNet.org 1. How do I register? Registration is easy. To register, simply navigate your web browser www.irbnet.org. The login area for IRBNet is in the upper right corner of the
Memorial Hermann ecredentialing Portal & Application Frequently Asked Questions
 Memorial Hermann ecredentialing Portal & Application Frequently Asked Questions Implementation & Go-Live What platform does Memorial Hermann s electronic application use? Memorial Hermann Health System
Memorial Hermann ecredentialing Portal & Application Frequently Asked Questions Implementation & Go-Live What platform does Memorial Hermann s electronic application use? Memorial Hermann Health System
Drexel University. Version April Page 1 of 23. Version April agf
 Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
BOULDER IRB era InfoEd Amendments
 BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
Animal Protocol Development Instructions for Researchers
 Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Buck-IRB Amendment User Guide
 Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
A User s Guide to EHF s Online Application Process
 A User s Guide to EHF s Online Application Process Thank you so much for applying to the Episcopal Health Foundation. Below you will find tips and instructions that we hope will be helpful as you move
A User s Guide to EHF s Online Application Process Thank you so much for applying to the Episcopal Health Foundation. Below you will find tips and instructions that we hope will be helpful as you move
What is A web-based protocol submission system for IACUC and IRB protocols
 What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
Insight 3.4: Release Date September 27, Help Desk/ Service Center # (If Available) Internal TFS # Description
 Insight 3.4: Release Date September 27, 2013 Internal TFS # Help Desk/ Service Center # (If Available) Description Agreements- Patient Care Implement Patient Care Details for BWH Patient Care Details are
Insight 3.4: Release Date September 27, 2013 Internal TFS # Help Desk/ Service Center # (If Available) Description Agreements- Patient Care Implement Patient Care Details for BWH Patient Care Details are
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
IBC Committee Manager User Guide
 IBC Committee Manager User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Version 1.2 Copyright 2018 Key Solutions 2002-2018 Key Solutions, Inc. 2803 Lakeview Court Fremont,
IBC Committee Manager User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Version 1.2 Copyright 2018 Key Solutions 2002-2018 Key Solutions, Inc. 2803 Lakeview Court Fremont,
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Training Booking System User Guide Contents:
 Training Booking System User Guide Contents: Register to Use the System... 2 Password Reminder... 4 Log In and Page Overview... 6 Book a Course for Yourself... 7 Book Yourself and Another Staff Member
Training Booking System User Guide Contents: Register to Use the System... 2 Password Reminder... 4 Log In and Page Overview... 6 Book a Course for Yourself... 7 Book Yourself and Another Staff Member
Module 3: Changes and Correspondence
 Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
IACUC Researcher s Quick Reference
 IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
IRB Staff Approve Repository Application
 IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
Acuity 504. User Guide. Administrators 504 Coordinators Teachers. MSB Customer Care msb-services.
 TM Acuity 504 User Guide Administrators 504 Coordinators Teachers MSB Customer Care 800.810.4220 support@ Copyright 2014 MSB All rights reserved 1 Copyright MSB 2014 Table of Contents MSB Mission Statement...
TM Acuity 504 User Guide Administrators 504 Coordinators Teachers MSB Customer Care 800.810.4220 support@ Copyright 2014 MSB All rights reserved 1 Copyright MSB 2014 Table of Contents MSB Mission Statement...
inspira 9.2 e-performance User Guide March 2018
 inspira 9.2 e-performance User Guide March 2018 1 Contents GETTING STARTED... 3 PROCESS OVERVIEW... 4 PHASE 1: WORKPLAN... 5 PHASE 2: MID-POINT REVIEW... 13 PHASE 3: END-OF-CYCLE EVALUATION... 16 2 Getting
inspira 9.2 e-performance User Guide March 2018 1 Contents GETTING STARTED... 3 PROCESS OVERVIEW... 4 PHASE 1: WORKPLAN... 5 PHASE 2: MID-POINT REVIEW... 13 PHASE 3: END-OF-CYCLE EVALUATION... 16 2 Getting
PowerUp provides Referees with tools and features to support and manage their game assignments, review schedules and view payment history.
 PowerUp provides with tools and features to support and manage their game assignments, review schedules and view payment history. Major features include: 1) Game assignment confirmation 2) Calendar Overview
PowerUp provides with tools and features to support and manage their game assignments, review schedules and view payment history. Major features include: 1) Game assignment confirmation 2) Calendar Overview
renew & Amend A Protocol training guide
 renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
SUBMITTING A NEW IBC PROJECT APPLICATION (INITIAL)
 SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
FIELDS REQUIRED TO SAVE A PROTOCOL
 Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
The Ethic Management System (EMS) User guide
 The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
Web Portal Manual. For Self-Managing Clients. manawanui INDIVIDUALISED FUNDING SUPPORT
 Web Portal Manual For Self-Managing Clients manawanui INDIVIDUALISED FUNDING SUPPORT Contents Welcome 3 Overview 4 Getting Started 5 Web Portal Basics 6 Help and Support 8 Statement Screen 9 Detailed Transaction
Web Portal Manual For Self-Managing Clients manawanui INDIVIDUALISED FUNDING SUPPORT Contents Welcome 3 Overview 4 Getting Started 5 Web Portal Basics 6 Help and Support 8 Statement Screen 9 Detailed Transaction
1. Log in Forgot password? Change language Dashboard... 7
 Table of contents 1. Log in... 2 2. Forgot password?... 3 3. Change language... 6 4. Dashboard... 7 Page 1 of 8 IndFak has been updated to make it possible to use the following recommended browsers: Internet
Table of contents 1. Log in... 2 2. Forgot password?... 3 3. Change language... 6 4. Dashboard... 7 Page 1 of 8 IndFak has been updated to make it possible to use the following recommended browsers: Internet
Navigating Your Profile
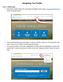 Part 1: Profile Login Navigating Your Profile 1. Start at the Greater Kansas City Community Foundation s home page, www.growyourgiving.org. Select Nonprofit Search. 2. This is the Nonprofit Search home
Part 1: Profile Login Navigating Your Profile 1. Start at the Greater Kansas City Community Foundation s home page, www.growyourgiving.org. Select Nonprofit Search. 2. This is the Nonprofit Search home
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
eprotocol IRB Reviewer Role Manual
 eprotocol IRB Reviewer Role Manual MARCH 2018 Table of Contents 1 Overview... 4 1.1 Things to Remember... 4 2 Review Process... 5 2.1 Reviewer Role Overview...5 2.2 Protocol Event Types...6 Assigned as
eprotocol IRB Reviewer Role Manual MARCH 2018 Table of Contents 1 Overview... 4 1.1 Things to Remember... 4 2 Review Process... 5 2.1 Reviewer Role Overview...5 2.2 Protocol Event Types...6 Assigned as
eprmc User Manual For Investigators and Research Staff
 eprmc User Manual For Investigators and Research Staff Table of Contents How to Get an Account... 3 Logging In... 3 Overview of the eprmc Database... 4 Searching for a Protocol... 5 Adding a New Protocol...
eprmc User Manual For Investigators and Research Staff Table of Contents How to Get an Account... 3 Logging In... 3 Overview of the eprmc Database... 4 Searching for a Protocol... 5 Adding a New Protocol...
***** ***** June
 SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
1. More about the Online Record Book (ORB)
 1. More about the Online Record Book (ORB) The Online Record Book (ORB) is a secure web platform that allow participants to record their activities and submit their Awards, and also allow Award Leaders
1. More about the Online Record Book (ORB) The Online Record Book (ORB) is a secure web platform that allow participants to record their activities and submit their Awards, and also allow Award Leaders
UNIVERSITY OF NORTH FLORIDA. Institutional Review Board (IRB) Guide to IRBNet:
 UNIVERSITY OF NORTH FLORIDA Institutional Review Board (IRB) Guide to IRBNet: Version 2 11-15-2013 Table of Contents i Introduction 2 IRBNet User Registration 2 IRBNet ID Number 2 Training and Credentials
UNIVERSITY OF NORTH FLORIDA Institutional Review Board (IRB) Guide to IRBNet: Version 2 11-15-2013 Table of Contents i Introduction 2 IRBNet User Registration 2 IRBNet ID Number 2 Training and Credentials
PORTAL USER GUIDE DOSE ADMINISTRATION AIDS
 PORTAL USER GUIDE DOSE ADMINISTRATION AIDS 1 February 2019 Portal User Guide Dose Administration Aids / February 2019 i PHARMACY PROGRAMS ADMINISTRATOR PORTAL USER GUIDE DOSE ADMINISTRATION AIDS INTRODUCTION...
PORTAL USER GUIDE DOSE ADMINISTRATION AIDS 1 February 2019 Portal User Guide Dose Administration Aids / February 2019 i PHARMACY PROGRAMS ADMINISTRATOR PORTAL USER GUIDE DOSE ADMINISTRATION AIDS INTRODUCTION...
Contents. TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs
 TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
Employee Access Manual
 Is there a Help Manual for Employee Self-Service Users? Welcome to BambooHR! We are excited that you will be using BambooHR to keep track of and manage your employee information. Depending on the customized
Is there a Help Manual for Employee Self-Service Users? Welcome to BambooHR! We are excited that you will be using BambooHR to keep track of and manage your employee information. Depending on the customized
Oakland University Obtaining Your 1098-T Electronically
 Accessing a student 1098-T is easy - simply go to tra.vangent.com, click on First Time Student and follow the instructions. 1. Open a web browser (such as Internet Explorer, Safari, Chrome, Firefox, etc.
Accessing a student 1098-T is easy - simply go to tra.vangent.com, click on First Time Student and follow the instructions. 1. Open a web browser (such as Internet Explorer, Safari, Chrome, Firefox, etc.
eirb Training Investigators/Research Coordinators Topics Covered:
 eirb Training Investigators/Research Coordinators Topics Covered: 1. Home Page 2. My IRB Protocols 3. Completing New Protocols 4. Amendments & Continuing Reviews 5. Submitting a Protocol 6. Certifying
eirb Training Investigators/Research Coordinators Topics Covered: 1. Home Page 2. My IRB Protocols 3. Completing New Protocols 4. Amendments & Continuing Reviews 5. Submitting a Protocol 6. Certifying
Registering for and Signing Into myclinicalexchange
 Registering for and Signing Into myclinicalexchange Dear myclinicalexchange Student, Welcome to the myclinicalexchange program (mce). We are making your clinical rotations more organized, efficient, and
Registering for and Signing Into myclinicalexchange Dear myclinicalexchange Student, Welcome to the myclinicalexchange program (mce). We are making your clinical rotations more organized, efficient, and
PDA Workspace User Guide
 PDA Workspace User Guide An Introduction to PDA s Online Task Force/Technical Report Team Management and Collaboration Platform Table of Topic Page # Account sign up 3 Finding your group 4-5 Accessing
PDA Workspace User Guide An Introduction to PDA s Online Task Force/Technical Report Team Management and Collaboration Platform Table of Topic Page # Account sign up 3 Finding your group 4-5 Accessing
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
BM Solutions Mortgage Portal. Document upload - Case tracking - Secure messaging Mobile technology. User Guide 15 January 2018 V3.
 BM Solutions Mortgage Portal Document upload - Case tracking - Secure messaging Mobile technology User Guide 15 January 2018 V3.0 KEY BENEFITS Moving forward together with secure online document upload
BM Solutions Mortgage Portal Document upload - Case tracking - Secure messaging Mobile technology User Guide 15 January 2018 V3.0 KEY BENEFITS Moving forward together with secure online document upload
Manual: Create a Faculty Search Posting Manager/Supervisor
 Manual: Create a Faculty Search Posting Manager/Supervisor Revised: 7-11-2017 Introduction The University of Georgia has implemented a new online faculty applicant tracking system, FacultyJobs@UGA. The
Manual: Create a Faculty Search Posting Manager/Supervisor Revised: 7-11-2017 Introduction The University of Georgia has implemented a new online faculty applicant tracking system, FacultyJobs@UGA. The
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
CERTIFIED HIRING MANAGER USER GUIDE
 CERTIFIED HIRING MANAGER USER GUIDE University of California Santa Cruz Recruitment Management System 12/21/12 TABLE OF CONTENTS INTRODUCTION... 3 BEFORE YOU GET STARTED... 4 Your Web Browser... 4 Web
CERTIFIED HIRING MANAGER USER GUIDE University of California Santa Cruz Recruitment Management System 12/21/12 TABLE OF CONTENTS INTRODUCTION... 3 BEFORE YOU GET STARTED... 4 Your Web Browser... 4 Web
IT Access Portal User Guide (Employees)
 IT Access Portal User Guide (Employees) Introduction The University of Salford IT Access Portal provides University employees with secure, off-campus access to core IT applications and resources; for example:
IT Access Portal User Guide (Employees) Introduction The University of Salford IT Access Portal provides University employees with secure, off-campus access to core IT applications and resources; for example:
Applying for Funding in Fluxx. Quick Start Instructions
 Applying for Funding in Fluxx Quick Start Instructions GETTING STARTED The Hogg Foundation Fluxx Grant Portal is optimized for use with Chrome or Safari browsers and using another browser may cause technical
Applying for Funding in Fluxx Quick Start Instructions GETTING STARTED The Hogg Foundation Fluxx Grant Portal is optimized for use with Chrome or Safari browsers and using another browser may cause technical
ivisions Employee Guide Portal Employee User Guide Town of Needham Terry Wolfson Created: 1/27/2014 Updated: 10/5/2016
 ivisions Employee Guide Portal Employee User Guide Town of Needham Terry Wolfson Created: 1/27/2014 Updated: 10/5/2016 Contents What is ivisions?... 2 Registering to ivisions Portal... 3 Logging into the
ivisions Employee Guide Portal Employee User Guide Town of Needham Terry Wolfson Created: 1/27/2014 Updated: 10/5/2016 Contents What is ivisions?... 2 Registering to ivisions Portal... 3 Logging into the
Portal User Guide. Best practice tips and shortcuts Icon Legend Informational notes about functions. Important warnings about a function
 Portal User Guide Tips Best practice tips and shortcuts Icon Legend Notes Warning Informational notes about functions Important warnings about a function Your Portal https://www.clientaxcess.com Your Portal
Portal User Guide Tips Best practice tips and shortcuts Icon Legend Notes Warning Informational notes about functions Important warnings about a function Your Portal https://www.clientaxcess.com Your Portal
Nova Scotia Health Authority Research Ethics Board. Researcher s (PI) User Manual
 Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0
 eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Reviewer User Guide 2 Table of Contents 1. INTRODUCTION 3
eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Reviewer User Guide 2 Table of Contents 1. INTRODUCTION 3
Parent Portal User Guide
 Parent Portal User Guide Table of Contents LOGIN TO THE PARENT PORTAL... 2 RETRIEVE LOST LOGIN DETAILS... 3 CHANGE YOUR PASSWORD... 5 CHANGE OR CONFIRM YOUR DETAILS & MEDICAL INFORMATION... 6 NAVIGATING
Parent Portal User Guide Table of Contents LOGIN TO THE PARENT PORTAL... 2 RETRIEVE LOST LOGIN DETAILS... 3 CHANGE YOUR PASSWORD... 5 CHANGE OR CONFIRM YOUR DETAILS & MEDICAL INFORMATION... 6 NAVIGATING
JDRF Operations External FAQs Preaward and Postaward
 JDRF Operations External FAQs Preaward and Postaward Page 1 RMS360 Preaward FAQ Application Content 1. How do I apply for a grant? 2. How do I add my RO (Research Office contact) and FO (Finance Officer)
JDRF Operations External FAQs Preaward and Postaward Page 1 RMS360 Preaward FAQ Application Content 1. How do I apply for a grant? 2. How do I add my RO (Research Office contact) and FO (Finance Officer)
erequest Frequently Asked Questions
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what we can support or sponsor. Online requests will go through an internal review and approval
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what we can support or sponsor. Online requests will go through an internal review and approval
