Module 1: Core Committee Staff Basics
|
|
|
- Herbert McKinney
- 5 years ago
- Views:
Transcription
1 Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in eresearch. This document is divided into several modules that will help you manage submissions for human subject research in eresearch. Module 1 Objective: Covers basic information that Core Committee Staff need to understand before working in eresearch to manage human subject research submissions. Refer to the table of contents below for the topics covered in Module 1. Table of Contents Module 1: Core Committee Staff Basics... 1 Approval and Expiration Dates... 2 Automatic Notifications... 2 Logging into eresearch... 3 Using the eresearch Home Workspace Last updated: 9/29/10 1 of 7 v.2.1
2 Approval and Expiration Dates Approval and Expiration Dates The following table details how approval and expiration dates are set in eresearch. Review Type System Action Approval Date Expiration Date Full Committee Expedited Administrative or Expedited - Exempt Administrative or Expedited - Not Regulated Record Committee Decision Submit Reviewer Checklist Submit Reviewer Checklist Submit Reviewer Checklist Edit Approval Period Date of Meeting Date reviewer submits review with a review determination of Approved or Approved with Contingencies Date reviewer submits review with a review determination of Exempt Date reviewer submits review with a review determination of Not Regulated Specific Date entered by Core Staff Approval Date + Approval Period 1 day Approval Date + Approval Period 1 day or Other Date specifically entered by the reviewer No Expiration Date Set No Expiration Date Set Specific Date entered by Core Staff Automatic Notifications eresearch automatically notifies Study Teams of: Upcoming expiration at 90, 60, and 30 days prior to expiration. When the study expires. Last updated: 9/29/10 2 of 7 v.2.1
3 eresearch is accessed at Important Information Logging into eresearch For more information on how to obtain a uniqname and a UMICH Kerberos password, go to: eresearch supports the following system configurations: o Windows 2000 or Windows XP and Internet Explorer 6.0 o Macintosh OSX and the Safari For optimal viewing, the recommended screen resolution size is 1024 x 768 or higher. 1 2 eresearch Home Page 1. Go to 2. Click. Cosign Login 3. Enter your Login ID and your Password. 4. Click. 3 4 Last updated: 9/29/10 3 of 7 v.2.1
4 eresearch Home Workspace 5 5. eresearch opens and displays your Home Workspace. Last updated: 9/29/10 4 of 7 v.2.1
5 Using the eresearch Home Workspace eresearch Home Workspace Verify that is displayed on the home workspace. Note: If Core Committee Staff is not displayed, click the Core Committee Staff link under My Roles. 2. Your Home Workspace displays all the eresearch submissions associated with you, organized by status (Inbox, Unassigned, In Progress, Completed, and All Reviews). Submissions can be accessed by clicking the appropriate tab for each category. Submissions that require action appear under. Note: New submissions will not appear in the you are required to take some action. unless you are assigned as the owner and 2. Individuals with multiple roles in eresearch, such as Core Committee Staff and Study Team Member, can select the appropriate role under My Roles. 3. Click to display submissions that require action by you. 4. Click from any page in eresearch to return to your Home Workspace. 5. Click to exit eresearch. Last updated: 9/29/10 5 of 7 v.2.1
6 Tip: The easiest way to add a new user is to have the person requesting access log into eresearch. o If you need to add a new user, you will need the user s uniqname, first and last name, and address. eresearch Home Workspace 1. Select the Study Team Member role. 1 2 Note: appears. 2. Click If the new user is affiliated with UM, select the Uniqname radio button. If not, skip to step Type the user s uniqname in the Uniqname field. Note: You can also search by the user s last name by selecting the Last Name radio button and typing his/her last name in the field. 5. Click Click the new user s uniqname in the Uniqname field Confirm the information listed is correct for the new user. 8. Click and you are finished. Last updated: 9/29/10 6 of 7 v.2.1
7 9. If the user is not affiliated with UM, turn on the Not Currently Associated with UM check box Enter the new user s contact information. 11. Click Last updated: 9/29/10 7 of 7 v.2.1
Module 3: Changes and Correspondence
 Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Core Staff Review Approve with Contingencies
 Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
IRB Staff Approve Repository Application
 IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
IRB Committee Member Adverse Event (AE) Single Member Review
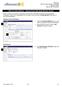 IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
Create an eresearch Account
 If you cannot find a user in eresearch you can create an eresearch account. If a person has logged into eresearch before he/she will have the PI/Project Team role. If you need to add a person who has never
If you cannot find a user in eresearch you can create an eresearch account. If a person has logged into eresearch before he/she will have the PI/Project Team role. If you need to add a person who has never
eresearch IBC Staff Review Step-by-Step Procedures Core Committee Staff Home Page
 1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
Update Your Company Profile in M-Inform
 in M-Inform As a U-M employee, student, or trainee, you are expected to disclose your ownership of, or relationship with, any company you start in order to option, license, distribute, or commercialize
in M-Inform As a U-M employee, student, or trainee, you are expected to disclose your ownership of, or relationship with, any company you start in order to option, license, distribute, or commercialize
ARC IRB Chair s Manual
 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
eiacuc Reference for Veterinarians
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
Reference Card for ORSP
 Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Click IRB 7.3 IRB Ancillary Reviewer s Guide
 Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Welcome to the. Patient Portal!
 Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider, schedule and request appointments, take control of your medical information,
Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider, schedule and request appointments, take control of your medical information,
PI & Project Team. Proposal Management. Create a PAF: Basics. Step-By-Step Procedure
 Create a PAF: Basics This procedure details how to: Create a PAF: the fields that must be completed in order to create a PAF. Hide/Show Errors: the function that allows you to view a list of fields that
Create a PAF: Basics This procedure details how to: Create a PAF: the fields that must be completed in order to create a PAF. Hide/Show Errors: the function that allows you to view a list of fields that
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II
 How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
Welcome to the. Patient Portal!
 Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider and take control of your medical information. Using this quick reference guide,
Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider and take control of your medical information. Using this quick reference guide,
Create an Animal Use Form
 Overview Principal Investigators (PIs) and Lab Personnel with the role of Protocol Editor or Authorized Signer can request the purchase or transfer of animals and report animal acquisitions or deliveries
Overview Principal Investigators (PIs) and Lab Personnel with the role of Protocol Editor or Authorized Signer can request the purchase or transfer of animals and report animal acquisitions or deliveries
IACUC Researcher s Quick Reference
 IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
The most current protocol information should be entered. This includes incorporation of all past amendments and modifications.
 Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
Affinity Provider Portal Training Manual
 Training Manual Login This page enables a user to either login and/or register if he/she is not already a regstered user (ie. Providers and Staff users). The following are the functionalities which can
Training Manual Login This page enables a user to either login and/or register if he/she is not already a regstered user (ie. Providers and Staff users). The following are the functionalities which can
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK. (Version date 03/03/2006)
 ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
Create an Action Item
 Overview Action Items are created within animal use forms, amendments and protocols to notify a person or group of a related task. If the Action Item requires an amendment, the Action Item and amendment
Overview Action Items are created within animal use forms, amendments and protocols to notify a person or group of a related task. If the Action Item requires an amendment, the Action Item and amendment
Click. IRB Study Submission Guide
 Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Version 2.1 June 12, 2018
 Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
NJDEP. Bureau of Water System Engineering. January 2015
 Physical Connection E-Permitting Renewal Service Instructions NJDEP Bureau of Water System Engineering January 2015 Instructions on how to use the Regulatory Service Portal (RSP) for the renewal of backflow
Physical Connection E-Permitting Renewal Service Instructions NJDEP Bureau of Water System Engineering January 2015 Instructions on how to use the Regulatory Service Portal (RSP) for the renewal of backflow
Submitting an Order Request Using LSA s Online Purchasing System
 Submitting an Order Request Using LSA s Online Purchasing System Overview LSA s Online Purchasing System is a web based system that allows departments to submit order requests, route through appropriate
Submitting an Order Request Using LSA s Online Purchasing System Overview LSA s Online Purchasing System is a web based system that allows departments to submit order requests, route through appropriate
Drexel University. Version April Page 1 of 23. Version April agf
 Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Kuali Research User Guide: Create an IRB Protocol
 Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
Prior Authorization and Notification Prior Authorization/Notification Submission QUICK REFERENCE
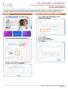 Prior Authorization and Notification Prior Authorization/Notification Submission QUICK REFERENCE You may submit Prior Authorizations/Notifications and attach documents in the Prior Authorization and Notification
Prior Authorization and Notification Prior Authorization/Notification Submission QUICK REFERENCE You may submit Prior Authorizations/Notifications and attach documents in the Prior Authorization and Notification
TAM Recruitment & Induction system guide for Approvers
 TAM Recruitment & Induction system guide for Approvers Table of Contents Table of Contents... 2 Recruitment Centre overview... 3 Accessing the TAM system... 4 Approving a job... 5 Approving an offer...
TAM Recruitment & Induction system guide for Approvers Table of Contents Table of Contents... 2 Recruitment Centre overview... 3 Accessing the TAM system... 4 Approving a job... 5 Approving an offer...
How Does the PI make Pre Review ICF Changes?
 How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
EasyChair instructions for Authors
 EasyChair instructions for Authors The submission and review of papers for NICFD 2016 will be managed through an online conference paper management system called EasyChair. This system gives you, the author,
EasyChair instructions for Authors The submission and review of papers for NICFD 2016 will be managed through an online conference paper management system called EasyChair. This system gives you, the author,
The Ethic Management System (EMS) User guide
 The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
CTools QuickGuide. A W h a t i s C T o o l s? O p e n i n g C T o o l s
 CTools QuickGuide This QuickGuide is a very condensed version of the CTools Online Help Guide. It includes information about the following: A What is CTools? E Joining a site B Opening CTools F Using the
CTools QuickGuide This QuickGuide is a very condensed version of the CTools Online Help Guide. It includes information about the following: A What is CTools? E Joining a site B Opening CTools F Using the
eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0
 eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Reviewer User Guide 2 Table of Contents 1. INTRODUCTION 3
eprotocol - Protocol Management System (PMS) Reviewer User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Reviewer User Guide 2 Table of Contents 1. INTRODUCTION 3
***** ***** June
 SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment)
 Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Conflict of Interest Web System
 Conflict of Interest Web System Process Overview All Users Contact information Logging in with ONID Security Navigation menu Help Faculty My profile My draft declaration My submitted declaration History
Conflict of Interest Web System Process Overview All Users Contact information Logging in with ONID Security Navigation menu Help Faculty My profile My draft declaration My submitted declaration History
ITU/TIES USER ACCOUNT: REQUEST TIES ACCESS. SAP AG. All rights reserved. 1
 ITU/TIES USER ACCOUNT: REQUEST TIES ACCESS SAP AG. All rights reserved. 1 Login to ITU/TIES User Management Page To request TIES access, you need to first create and activate your ITU user account. To
ITU/TIES USER ACCOUNT: REQUEST TIES ACCESS SAP AG. All rights reserved. 1 Login to ITU/TIES User Management Page To request TIES access, you need to first create and activate your ITU user account. To
College Deans, Department Chairs & Supervising Faculty
 Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
ESS Approver Training Leave Management
 ESS Approver Training Leave Management Table of Contents 1. LEAVE MANAGEMENT... 1 2. EXAMPLE OF AN ESS LEAVE REQUEST WORKFLOW... 1 3. LEAVE REQUEST NOTIFICATION E-MAIL... 2 4. INBOX... 4 4.1 VIEWING THE
ESS Approver Training Leave Management Table of Contents 1. LEAVE MANAGEMENT... 1 2. EXAMPLE OF AN ESS LEAVE REQUEST WORKFLOW... 1 3. LEAVE REQUEST NOTIFICATION E-MAIL... 2 4. INBOX... 4 4.1 VIEWING THE
Voic Instructions
 NUMBER: PIN: TELEPHONE VOICEMAIL ACCESS To log into your voicemail box from your greeting: 1. Dial your phone number and let it ring to voicemail. 2. Press * to interrupt your greeting. 3. Enter your PIN
NUMBER: PIN: TELEPHONE VOICEMAIL ACCESS To log into your voicemail box from your greeting: 1. Dial your phone number and let it ring to voicemail. 2. Press * to interrupt your greeting. 3. Enter your PIN
FAQs. Q What is the status of my study application?
 FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
eiacuc Reference for PIs and Research Team
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
Group Admin Guide. NetBrain Consultant Edition 6.2
 NetBrain Consultant Edition 6.2 Group Admin Guide Version 6.2 Last Updated 2017-08-16 Copyright 2004-2017 NetBrain Technologies, Inc. All rights reserved. Contents 1. Overview... 3 2. Logging in to Admin
NetBrain Consultant Edition 6.2 Group Admin Guide Version 6.2 Last Updated 2017-08-16 Copyright 2004-2017 NetBrain Technologies, Inc. All rights reserved. Contents 1. Overview... 3 2. Logging in to Admin
Questions and Answers. Converting Existing Protocols into CHeRP IRB
 Questions and Answers Converting Existing Protocols into CHeRP IRB Questions: (hold ctrl and click on the question to jump directly to that answer) 1. Do I need to convert my existing protocol applications
Questions and Answers Converting Existing Protocols into CHeRP IRB Questions: (hold ctrl and click on the question to jump directly to that answer) 1. Do I need to convert my existing protocol applications
JITs Portal. Quick Start Guide
 JITs Portal Quick Start Guide December 2017 Table of Contents 1. INTRODUCTION... 2 2. GETTING STARTED... 2 2.1. JITs Portal Home Page (Public site)... 2 2.2. JITs Portal Home Page (Authenticated area)...
JITs Portal Quick Start Guide December 2017 Table of Contents 1. INTRODUCTION... 2 2. GETTING STARTED... 2 2.1. JITs Portal Home Page (Public site)... 2 2.2. JITs Portal Home Page (Authenticated area)...
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review
 Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
You may also scroll through the Table of Contents and click on the topic or question of interest.
 We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
eprost System Policies & Procedures
 eprost System Policies & Procedures Initial Approval Date: 12/07/2010 Revision Date: 02/25/2011 Introduction eprost [ Electronic Protocol Submission and Tracking ] is the Human Subject Research Office's
eprost System Policies & Procedures Initial Approval Date: 12/07/2010 Revision Date: 02/25/2011 Introduction eprost [ Electronic Protocol Submission and Tracking ] is the Human Subject Research Office's
As ESS allows for customisation of certain screens, the client will be able to extract other information from the ESS system too.
 Reporting on ESS Three different reports can be run from ESS Leave Balance Report Leave Transactions Report Manager Leave Calendar As ESS allows for customisation of certain screens, the client will be
Reporting on ESS Three different reports can be run from ESS Leave Balance Report Leave Transactions Report Manager Leave Calendar As ESS allows for customisation of certain screens, the client will be
Outside Interests Disclosure Form for Staff. IRIS Mobile through the Web
 Outside Interests Disclosure Form for Staff IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff to disclose outside interests as required
Outside Interests Disclosure Form for Staff IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff to disclose outside interests as required
Managing Short Codes
 Managing Short Codes Managing Short Codes Overview Short codes are used for making purchases at U-M, including purchasing animals, equipment and/or other services related to Animal Management protocols,
Managing Short Codes Managing Short Codes Overview Short codes are used for making purchases at U-M, including purchasing animals, equipment and/or other services related to Animal Management protocols,
Click IACUC Researcher s Quick Reference
 Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond
Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond
Instructions for the Hearst Foundations Online Grant Application
 Instructions for the Hearst Foundations Online Grant Application NOTE: All applicants should read this document thoroughly BEFORE starting the application process or seeking assistance from the Hearst
Instructions for the Hearst Foundations Online Grant Application NOTE: All applicants should read this document thoroughly BEFORE starting the application process or seeking assistance from the Hearst
Ohio Child Licensing and Quality System (OCLQS) Create User Account Login Procedure Request Program Access
 Ohio Child Licensing and Quality System (OCLQS) Create User Account Login Procedure Request Program Access Create Web Portal Create Web Portal User Account When you first enter the Ohio Child Licensing
Ohio Child Licensing and Quality System (OCLQS) Create User Account Login Procedure Request Program Access Create Web Portal Create Web Portal User Account When you first enter the Ohio Child Licensing
Workspace Desktop Edition User's Guide. Transfer An SMS or MMS Interaction
 Workspace Desktop Edition User's Guide Transfer An SMS or MMS Interaction 11/30/2017 Transfer An SMS or MMS Interaction [Modified: 8.5.110.13] In this lesson, you will learn how to transfer SMS or MMS
Workspace Desktop Edition User's Guide Transfer An SMS or MMS Interaction 11/30/2017 Transfer An SMS or MMS Interaction [Modified: 8.5.110.13] In this lesson, you will learn how to transfer SMS or MMS
BOULDER IRB era InfoEd Continuing Review
 BOULDER IRB era InfoEd Continuing Review Last Update: 2017/11/30 Preface: This guide explains how to submit a continuing review for a previously approved expedited or full board study that is nearing expiration.
BOULDER IRB era InfoEd Continuing Review Last Update: 2017/11/30 Preface: This guide explains how to submit a continuing review for a previously approved expedited or full board study that is nearing expiration.
How to Submit a New Application
 ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
Automated Background Check System (ABCS)- Approving Access Guide. April 2018
 Automated Background Check System ()- Approving Access Guide April 2018 How do I approve access to? Complete Background Check HHS Enterprise Portal Add User to There are four main steps as an approver
Automated Background Check System ()- Approving Access Guide April 2018 How do I approve access to? Complete Background Check HHS Enterprise Portal Add User to There are four main steps as an approver
- Direct your browser to This is the homepage for the IRB application.
 I. Logging on/setting up an account. - Direct your browser to http://irb.kean.edu. This is the homepage for the IRB application. - First time applicants will have to create a new account. Click on the
I. Logging on/setting up an account. - Direct your browser to http://irb.kean.edu. This is the homepage for the IRB application. - First time applicants will have to create a new account. Click on the
Grants emanagement System (GeMS) Affiliate Grants Reviewer Manual. Version 2.1
 Grants emanagement System (GeMS) Affiliate Grants Reviewer Manual Version 2.1 Table of Contents GeMS System Requirements... 3 Accessing GeMS... 4 Application Review Checklist... 4 New Users GeMS Registration...
Grants emanagement System (GeMS) Affiliate Grants Reviewer Manual Version 2.1 Table of Contents GeMS System Requirements... 3 Accessing GeMS... 4 Application Review Checklist... 4 New Users GeMS Registration...
irb.unm.edu
 IRBNet Submission Instructions The purpose of this document is to provide IRBNet submission instructions for UNM researchers using IRBNet. Please contact the Office of the IRB for assistance: irb.unm.edu
IRBNet Submission Instructions The purpose of this document is to provide IRBNet submission instructions for UNM researchers using IRBNet. Please contact the Office of the IRB for assistance: irb.unm.edu
How to Sign Up for a Volunteer Activity
 How to Sign Up for a Volunteer Activity Visit www.catholiccharitiesdc.org/volunteer Click the One-Time volunteer button to see the upcoming volunteer activities On the Calendar, click the activity where
How to Sign Up for a Volunteer Activity Visit www.catholiccharitiesdc.org/volunteer Click the One-Time volunteer button to see the upcoming volunteer activities On the Calendar, click the activity where
IEEE Standards Association. Sponsor Balloting Process Using myproject
 IEEE Standards Association Sponsor Balloting Process Using myproject What is the standards process? There are 6 steps to the IEEE Standards Process 1. Initiating the project An idea for a standard is proposed
IEEE Standards Association Sponsor Balloting Process Using myproject What is the standards process? There are 6 steps to the IEEE Standards Process 1. Initiating the project An idea for a standard is proposed
ADMIN GUIDE. Easily manage your staff s access to Snap, reset passwords and update user profiles.
 ADMIN GUIDE Easily manage your staff s access to Snap, reset passwords and update user profiles. Welcome to Snap As an Agency Administrator, you can easily manage the Agency staff s access to Snap. Using
ADMIN GUIDE Easily manage your staff s access to Snap, reset passwords and update user profiles. Welcome to Snap As an Agency Administrator, you can easily manage the Agency staff s access to Snap. Using
retrieving study documents training guide
 retrieving study documents training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this document 4 retrieving study documents 5 current
retrieving study documents training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this document 4 retrieving study documents 5 current
How to apply for the e-tip using the ZIMRA e-tip Portal. 1. Sign Up on a Mobile app. Select the e-tip app on your phone
 How to apply for the e-tip using the ZIMRA e-tip Portal 1. Sign Up on a Mobile app Select the e-tip app on your phone Select Sign Up if you don t have an account Capture your Sign Up details Select SUBMIT
How to apply for the e-tip using the ZIMRA e-tip Portal 1. Sign Up on a Mobile app Select the e-tip app on your phone Select Sign Up if you don t have an account Capture your Sign Up details Select SUBMIT
Financial Center Administration Console USER GUIDE
 Financial Center Administration Console USER GUIDE For Client Use Only Effective April 2018 Table of contents Introduction 3 Communicating securely with Union Bank 3 Change Security Settings 4 Manage
Financial Center Administration Console USER GUIDE For Client Use Only Effective April 2018 Table of contents Introduction 3 Communicating securely with Union Bank 3 Change Security Settings 4 Manage
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event
 Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Logging into myclinicalexchange
 Logging into myclinicalexchange 1. Navigate to https://myclinicalexchange.com 2. Click on Students Login Here. 3. You will be redirected to Students login page. 4. Under the Login area, click on the Forgot
Logging into myclinicalexchange 1. Navigate to https://myclinicalexchange.com 2. Click on Students Login Here. 3. You will be redirected to Students login page. 4. Under the Login area, click on the Forgot
Honeywell Source Inspection Request Instructions
 Honeywell Source Inspection Request Instructions [Suppliers] 1) Access the website by going to https://hia.honeywell.com Frequently Asked Questions Q: I do not have an account, how do I request for one?
Honeywell Source Inspection Request Instructions [Suppliers] 1) Access the website by going to https://hia.honeywell.com Frequently Asked Questions Q: I do not have an account, how do I request for one?
Easy Chair Online Conference Submission, Tracking and Distribution Process: Getting Started
 Easy Chair Online Conference Submission, Tracking and Distribution Process: Getting Started AMS WMC 2014 Click on play to begin show AMS Conference Information You can always access information about the
Easy Chair Online Conference Submission, Tracking and Distribution Process: Getting Started AMS WMC 2014 Click on play to begin show AMS Conference Information You can always access information about the
Approving the Outside Interests Disclosure Form Without Conflicts. Supervisor Level. IRIS Mobile through the Web
 Approving the Outside Interests Disclosure Form Without Conflicts Supervisor Level IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff
Approving the Outside Interests Disclosure Form Without Conflicts Supervisor Level IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff
SmartSolutions Portal User Guide
 SmartSolutions Portal User Guide Managing group permissions Updated 28/04/17 v1 In this guide we will show you how to manage and edit the permissions of other users within your organisation. 1. First,
SmartSolutions Portal User Guide Managing group permissions Updated 28/04/17 v1 In this guide we will show you how to manage and edit the permissions of other users within your organisation. 1. First,
USER MANUAL [QUICK REFERENCE]
![USER MANUAL [QUICK REFERENCE] USER MANUAL [QUICK REFERENCE]](/thumbs/74/71390282.jpg) USER MANUAL [QUICK REFERENCE] PROJECT : STUDENT MANAGEMENT SYSTEM MODULE : E PROGRESS REPORT DATE : FEBRUARY VERSION : 1.0 Contents No Description Page No 1. SECTION A : LOGIN... 4 2. SECTION B : EVALUATION
USER MANUAL [QUICK REFERENCE] PROJECT : STUDENT MANAGEMENT SYSTEM MODULE : E PROGRESS REPORT DATE : FEBRUARY VERSION : 1.0 Contents No Description Page No 1. SECTION A : LOGIN... 4 2. SECTION B : EVALUATION
How to Use the Comprehensive Ancillary Forms Engine (CAFÉ) for DSMB/ISPRC Initial Submission
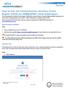 How to Use the Comprehensive Ancillary Forms Engine (CAFÉ) for DSMB/ISPRC Initial Submission The Comprehensive Ancillary Forms Engine (CAFÉ) allows s to submit DSMB and ISPRC submissions to the Jonsson
How to Use the Comprehensive Ancillary Forms Engine (CAFÉ) for DSMB/ISPRC Initial Submission The Comprehensive Ancillary Forms Engine (CAFÉ) allows s to submit DSMB and ISPRC submissions to the Jonsson
ASME Conformity Assessment: Statistics, Process, and CA Connect. Dan Sharp Manager, Process Management Dept. August 10, 2016
 ASME Conformity Assessment: Statistics, Process, and CA Connect Dan Sharp Manager, Process Management Dept. August 10, 2016 Topics ASME Conformity Assessment Statistics Overview of the Certification Process
ASME Conformity Assessment: Statistics, Process, and CA Connect Dan Sharp Manager, Process Management Dept. August 10, 2016 Topics ASME Conformity Assessment Statistics Overview of the Certification Process
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
NHPNet Provider Enrollment Portal User Guide
 NHPNet Provider Enrollment Portal User Guide Updated February 2017 NHP s Provider Enrollment Portal allows you direct control over how NHP configures your provider data. Key features of the tool include:
NHPNet Provider Enrollment Portal User Guide Updated February 2017 NHP s Provider Enrollment Portal allows you direct control over how NHP configures your provider data. Key features of the tool include:
Using the Study Abroad online application (Portico)
 Using the Study Abroad online application (Portico) Completing the full online application 1. Once you have received notification that your application to study abroad has been approved by your tutor,
Using the Study Abroad online application (Portico) Completing the full online application 1. Once you have received notification that your application to study abroad has been approved by your tutor,
Office of Research Integrity. ARIES Research Management System. Human Ethics User Guide
 Office of Research Integrity ARIES Research Management System Human Ethics User Guide Version: 5.0 Date: June 2014 Using the Human Ethics module of ARIES Aries is accessed on the web using Mozilla Firefox,
Office of Research Integrity ARIES Research Management System Human Ethics User Guide Version: 5.0 Date: June 2014 Using the Human Ethics module of ARIES Aries is accessed on the web using Mozilla Firefox,
Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0)
 Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0) Internal Investigators These are BU/BMC faculty and staff Boston Public Health Commission and Boston Healthnet Community
Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0) Internal Investigators These are BU/BMC faculty and staff Boston Public Health Commission and Boston Healthnet Community
Bell County. E-Discovery Portal. Training Guide. 1/8/2014 Version 1.0
 Bell County E-Discovery Portal Training Guide 1/8/2014 Version 1.0 The E-Discovery portal has been developed to provide the District Attorney s Office with the ability to electronically upload discoverable
Bell County E-Discovery Portal Training Guide 1/8/2014 Version 1.0 The E-Discovery portal has been developed to provide the District Attorney s Office with the ability to electronically upload discoverable
CHaRMS Primary Sponsor User Guide. CHaRMS Primary Sponsor User Guide Page 1
 CHaRMS Primary Sponsor User Guide CHaRMS Primary Sponsor User Guide Page 1 Table of Contents Overview... 3 Request an Account... 4 Account Access... 10 The Dashboard... 12 View Course Status... 14 My Organization
CHaRMS Primary Sponsor User Guide CHaRMS Primary Sponsor User Guide Page 1 Table of Contents Overview... 3 Request an Account... 4 Account Access... 10 The Dashboard... 12 View Course Status... 14 My Organization
My Learning Plan Professional Development Management System SY User s Guide
 My Learning Plan Professional Development Management System SY 2009-10 User s Guide Logging In...2 Update Your User Profile...3 Changing Your Password...3 Enrolling in a Class...3 Dropping a Class...6
My Learning Plan Professional Development Management System SY 2009-10 User s Guide Logging In...2 Update Your User Profile...3 Changing Your Password...3 Enrolling in a Class...3 Dropping a Class...6
Accessing the SIM PCMH Dashboard
 Accessing the SIM PCMH Dashboard Setting up Duo, Creating Your Level-2 Password, and Setting up Citrix Receiver to Log in to the Dashboard P R O C EDURAL GUID E Document File Name Accessing_the_SIM_Dashboard.docx
Accessing the SIM PCMH Dashboard Setting up Duo, Creating Your Level-2 Password, and Setting up Citrix Receiver to Log in to the Dashboard P R O C EDURAL GUID E Document File Name Accessing_the_SIM_Dashboard.docx
UCSC Visa Document Request Tool Quick Guide **Please make sure you are able to receive s from
 UCSC Visa Document Request Tool Quick Guide **Please make sure you are able to receive emails from istudent@ucsc.edu** Part 1: Accessing the Document Request Tool 1. All communication will be sent to your
UCSC Visa Document Request Tool Quick Guide **Please make sure you are able to receive emails from istudent@ucsc.edu** Part 1: Accessing the Document Request Tool 1. All communication will be sent to your
HEI User Authorization
 HEI User Authorization ODHE Administrator User Manual Version 1.1 - January 2018 System Access HEI Authorization Login HEI Institution Administrator / Liaison and Financial Aid Institution Administrator
HEI User Authorization ODHE Administrator User Manual Version 1.1 - January 2018 System Access HEI Authorization Login HEI Institution Administrator / Liaison and Financial Aid Institution Administrator
USER MANUAL FOR AUTHOR.
 USER MANUAL FOR AUTHOR http://conference.binus.ac.id/ocs/index.php/icimtech/icimtech2017 Version : 1.0 May 2017 Table of Contents Create an Account... 2 Forgot Password... 6 Submit Paper... 9 Check Submitted
USER MANUAL FOR AUTHOR http://conference.binus.ac.id/ocs/index.php/icimtech/icimtech2017 Version : 1.0 May 2017 Table of Contents Create an Account... 2 Forgot Password... 6 Submit Paper... 9 Check Submitted
Talent Connect User Guide
 Talent Connect User Guide Table of Contents Register As A New User Search For Jobs/Save Jobs Basic Search Advanced Search Save Search Queries Job Cart Apply For A Job Resume Upload Candidate Personal Info
Talent Connect User Guide Table of Contents Register As A New User Search For Jobs/Save Jobs Basic Search Advanced Search Save Search Queries Job Cart Apply For A Job Resume Upload Candidate Personal Info
Coaches and Team Managers Getting Started Guide. For coaches and parent volunteers who use SportsEngine to manage teams and schedules.
 Coaches and Team Managers Getting Started Guide For coaches and parent volunteers who use SportsEngine to manage teams and schedules. Table of Contents Introduction... 3 Verifying Your Access... 3 Editing
Coaches and Team Managers Getting Started Guide For coaches and parent volunteers who use SportsEngine to manage teams and schedules. Table of Contents Introduction... 3 Verifying Your Access... 3 Editing
