Create a new Human Research Ethics Application in InfoEd
|
|
|
- Roland Armstrong
- 5 years ago
- Views:
Transcription
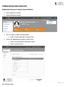
1 Create a new Human Research Ethics Application in InfoEd IMPORTANT! A Co-Investigator or Student can create, complete and submit new human research ethics applications in InfoEd on behalf of the Chief Investigator (CI). The Research Ethics Office will not receive the application until after the Chief Investigator and all Co-Investigators or Students have reviewed and signed off on the ethics application in InfoEd. A Student who is also a Curtin University staff member must be listed as a student investigator if they are applying for ethics approval for their (student) project. They must select their student personnel profile when adding themselves as an investigator in the ethics application form in InfoEd. The student s supervisor must be added and selected as the Chief Investigator of the ethics application. 1. Create a new record Log in to InfoEd => In the Home page, hover your cursor over Human Ethics and click on Create New Record. A separate window will appear on your screen with the following message New Human Protocol in Human Ethics Development. Click Continue. Page 1 of 12
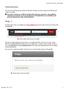
2 A Protocol Creation window will appear on your screen. Type or copy/paste your project title in the text box, and click Continue. A Select CI window will appear on your screen. Please note that the Chief Investigator (CI) must be a Curtin University staff member! Click Continue if you are the Chief Investigator (CI). Otherwise, delete your name and start typing in the last name of the Chief Investigator (CI). A list should appear once you start typing, hence select the correct CI name and then click Continue. Page 2 of 12
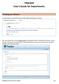
3 You should now be in the Initial Application window. Your record number is on the top left-hand side of the Initial Application window 2. Add a blank human ethics application form to the submission Click on the Add button to open the upload window: In the upload window, tick the box against the Human Research Ethics Application form name and click Add above the tick box: Page 3 of 12
4 The upload window will disappear and you will be taken back to the Initial Application submission window. A blank human ethics application form has been added to the Initial Application window. The status of this blank form will display as Incomplete until you answer all the questions in the form. 3. Fill in the ethics application form To start filling in your ethics form, click on the Human Research Ethics Application link in the Initial Application screen. Click Save to save your answers. Once you have answered all the questions, tick the Complete box and close the form. Important! InfoEd will not allow you to progress successfully to Complete status if you have not answered all the mandatory questions on the ethics form in InfoEd. After successful completion of the form, click Close to close the window. Page 4 of 12
5 4. Add investigators to your application form Click on Investigators on the left side of the form window. In the Investigators page, click on the + icon to add your co-investigator to the application. If you have more than one co-investigator, you will have to repeat this action for each coinvestigator you need to add. Once you have clicked on the + icon, a separate window titled Personnel will appear on your screen. In the Personnel window, you can search by clicking on the alphabet link matching the first letter of the co-investigator s last name, to bring up the list of surnames matching your selection. Once you have done this, type in the LAST NAME of the co-investigator in the Search for a particular entry text box. This will narrow down your search results, and you can click on the drop-down arrow next to the Select button to display the results. Page 5 of 12
6 Once you have located the name of the co-investigator, click on the name and it will display in the Personnel window. Press Select. The separate Personnel window will disappear, and the name of your selected coninvestigator will appear in the Investigators section. Repeat the same steps as above in this section for each Co-Investigator to be added to your human research ethics application. Page 6 of 12
7 If you are a Co-Investigator who is creating a new human research ethics application on behalf of the Chief Investigator, you must check the Chief Investigator box under the name of the Chief Investigator. 5. Attach supporting documents to your submission Click on the Add button to open the upload window: Page 7 of 12
8 Click Browse to locate the document you would like to upload from your computer drive. You can only upload one document at a time. Select a category from the Category drop-down menu, and click Upload. Page 8 of 12
9 To complete the upload of the documents, close the upload window. The document/s you uploaded will appear in the submission window: Page 9 of 12
10 6. Submit your human ethics application When you are ready to submit your ethics application, click the Submit button in the Initial Application window. If a Chief Investigator submits the ethics application: A new window will appear, displaying the investigator declaration. Select Accepted, and then press Continue to proceed with the submission. Page 10 of 12
11 If the application has been successfully submitted, the status of your application should change from Draft to Faculty Ethics Support Officer review in the Initial Application window. Once all listed investigators have signed off on the application, you will receive an automated from InfoEd in your Outlook inbox, notifying you that the application has been submitted. The same message will also appear in the Messages page on your InfoEd Home screen. If a Co-Investigator/Student submits the ethics application: If the application has been successfully submitted, the status of your application should change from Draft to Submitted in the Initial Application window. Page 11 of 12
12 The application will now be assigned to the Chief Investigator for their electronic sign-off. When the Chief Investigator has signed off on the application, all listed Co-Investigators/Students will receive an automated notification , requesting for them to provide electronic sign-off for the application. When all listed investigators in the human ethics application have completed their sign-off, the application will be assigned to the Research Ethics Office for processing. IMPORTANT! Your human ethics application will not proceed in InfoEd until all listed investigators have signed off on it. If you need to make changes to your ethics application after you have submitted it, please contact the Ethics Officer or Ethics Support Officer for your faculty at first instance for assistance. Please DO NOT create and submit another ethics application or submission to do so, e.g. please do not submit additional supporting documents as an Amendment Request! Support Available Faculty CBS Health Science Humanities Science & Engineering Non Faculty Non-low risk & reciprocals Technical Support Page 12 of 12
Edit an Existing Submission in InfoEd
 Edit an Existing Submission in InfoEd 1. Locate the project in the InfoEd Chief Investigator on the project: Log into InfoEd, In the Home screen, go to My Items tab and find the record. Once the record
Edit an Existing Submission in InfoEd 1. Locate the project in the InfoEd Chief Investigator on the project: Log into InfoEd, In the Home screen, go to My Items tab and find the record. Once the record
Guide for Researchers: Online Human Ethics Application Form
 Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
IRMA Human Ethics Researcher User Guide
 IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
IRMA Researcher User Guide v2 DRAFT. IRMA Researcher User Guide
 IRMA Researcher User Guide v2 IRMA Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets
IRMA Researcher User Guide v2 IRMA Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets
Research Management Information System (RIMS) Human Ethics - User Guide
 Research Management Information System (RIMS) Human Ethics - User Guide Contents How to create an Initial Application to the HREC... 2 Step 1: Create a new protocol... 2 Step 2: Title... 3 Step 3: Chief
Research Management Information System (RIMS) Human Ethics - User Guide Contents How to create an Initial Application to the HREC... 2 Step 1: Create a new protocol... 2 Step 2: Title... 3 Step 3: Chief
Guide for Researchers: Online Human Ethics Application Form
 Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Training Manual for Researchers. How to Create an Online Human Ethics Application
 Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
JOURNAL RELATED PUBLICATION TYPES. Adding New Research Output (Journal Article) Click Add New Content Select Research Output
 JOURNAL RELATED PUBLICATION TYPES Adding New Research Output (Journal Article) Click Add New Content Select Research Output Do you want to import existing data? o Select Create Manually or Import from
JOURNAL RELATED PUBLICATION TYPES Adding New Research Output (Journal Article) Click Add New Content Select Research Output Do you want to import existing data? o Select Create Manually or Import from
Student Guide. Click here to log in. A: Log in
 Middlesex Online Research Ethics (MORE) Applicant Guidance Notes (Student & Staff) Note: PhD ethics applications must be submitted as Student Applications. Creating, completing and submitting a Research
Middlesex Online Research Ethics (MORE) Applicant Guidance Notes (Student & Staff) Note: PhD ethics applications must be submitted as Student Applications. Creating, completing and submitting a Research
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0)
 Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0) Internal Investigators These are BU/BMC faculty and staff Boston Public Health Commission and Boston Healthnet Community
Adding/Removing Internal Investigators from an Already Approved Study (Section 3.0) Internal Investigators These are BU/BMC faculty and staff Boston Public Health Commission and Boston Healthnet Community
Ethical Review Manager. Applicant User Guide
 Ethical Review Manager Applicant User Guide Last Updated: June 2017 Introduction The Ethical Review Manager (ERM) System has been designed to enable applications for ethical approval to conduct research
Ethical Review Manager Applicant User Guide Last Updated: June 2017 Introduction The Ethical Review Manager (ERM) System has been designed to enable applications for ethical approval to conduct research
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
Add new qualification to scope of registration
 Add new qualification to scope of registration Purpose This guide provides information to allow users to add a new qualification to scope of registration. Gain access The school s Organisation Administrator,
Add new qualification to scope of registration Purpose This guide provides information to allow users to add a new qualification to scope of registration. Gain access The school s Organisation Administrator,
Guide for Candidates: Online Progress Reports
 Guide for Candidates: Online Progress Reports What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University. All Progress Reports
Guide for Candidates: Online Progress Reports What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University. All Progress Reports
NZ Online Forms for Research Software Manual
 NZ Online Forms for Research Software Manual Version 1.5 Released May 2016 2 P a g e N Z O n l i n e F o r m s f o r R e s e a r c h 1 INTRODUCTION... 6 2 GETTING STARTED... 6 2.1 Creating an Account...
NZ Online Forms for Research Software Manual Version 1.5 Released May 2016 2 P a g e N Z O n l i n e F o r m s f o r R e s e a r c h 1 INTRODUCTION... 6 2 GETTING STARTED... 6 2.1 Creating an Account...
TURNITIN STUDENT S MANUAL
 TURNITIN STUDENT S MANUAL Account Creation 1 2 Fig 1 After being enrolled into Turnitin a student will receive an email that looks like the diagram in Fig 1. Use the login credentials given in that email
TURNITIN STUDENT S MANUAL Account Creation 1 2 Fig 1 After being enrolled into Turnitin a student will receive an email that looks like the diagram in Fig 1. Use the login credentials given in that email
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
The Ethic Management System (EMS) User guide
 The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
The Ethic Management System (EMS) User guide On the web browser, type the URL link: https://www.witsethics.co.za Click on Login (on right corner of top menu bar) to access the Ethics Management System
My Research, Human Protocols (MRHP) PI User Guide October 2017
 My Research, Human Protocols (MRHP) PI User Guide October 2017 October, 2017 Page 1 of 22 Contents System Requirements... 3 Browsers... 3 Pop-ups... 3 Chrome... 3 Internet Explorer... 4 Firefox... 4 Safari...
My Research, Human Protocols (MRHP) PI User Guide October 2017 October, 2017 Page 1 of 22 Contents System Requirements... 3 Browsers... 3 Pop-ups... 3 Chrome... 3 Internet Explorer... 4 Firefox... 4 Safari...
R I M S. ETHICS APPLICATIONS TRAINING MANUAL How to complete an Online Ethics Application
 R I M S (R e s e a r c h I n f o r m a t io n M a n a g e m e n t S y s t e m) ETHICS APPLICATIONS TRAINING MANUAL How to complete an Online Ethics Application By The Directorate Research Development 2016
R I M S (R e s e a r c h I n f o r m a t io n M a n a g e m e n t S y s t e m) ETHICS APPLICATIONS TRAINING MANUAL How to complete an Online Ethics Application By The Directorate Research Development 2016
DISCOVR-e USER MANUAL. Vanderbilt University Human Research Protection Program
 DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
Instructions to complete CITI Human Subjects Training - New User
 Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
SUBMITTING A NEW IBC PROJECT APPLICATION (INITIAL)
 SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
Ethical Review Online Application System: User Guide
 Ethical Review Online Application System: User Guide 1 Contents Purpose of this document... 3 Accessing the system... 3 Setting internet browsers to check spelling... 4 Creating a new Application... 5
Ethical Review Online Application System: User Guide 1 Contents Purpose of this document... 3 Accessing the system... 3 Setting internet browsers to check spelling... 4 Creating a new Application... 5
INSTRUCTIONS FOR THE eiaf COMPLETING THE FORM AND REQUIRED APPROVALS AND CERTIFICATIONS
 INSTRUCTIONS FOR THE eiaf COMPLETING THE FORM AND REQUIRED APPROVALS AND CERTIFICATIONS ACCESSING A NEW BLANK FORM From the UK website, click on link blue. (Click refers to pressing the mouse button.)
INSTRUCTIONS FOR THE eiaf COMPLETING THE FORM AND REQUIRED APPROVALS AND CERTIFICATIONS ACCESSING A NEW BLANK FORM From the UK website, click on link blue. (Click refers to pressing the mouse button.)
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
New Protocol - NoN-humaN subjects research training guide
 New Protocol - NoN-humaN subjects research training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 table of contents Table of Contents
New Protocol - NoN-humaN subjects research training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 table of contents Table of Contents
BOULDER IRB era InfoEd Amendments
 BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
LaserFiche Appointments Manual
 LaserFiche Appointments Manual Guide to Academic Appointments Using Web Forms and LaserFiche8 Human Resources Version Discovery Commons September 2013 Table of Contents Appointments Processed in the LaserFiche
LaserFiche Appointments Manual Guide to Academic Appointments Using Web Forms and LaserFiche8 Human Resources Version Discovery Commons September 2013 Table of Contents Appointments Processed in the LaserFiche
THE UNIVERSITY OF EDINBURGH FINANCE PROCESS MANAGER USER GUIDE. Student/Visitor efinancials Payment Record Creation
 THE UNIVERSITY OF EDINBURGH FINANCE PROCESS MANAGER USER GUIDE Student/Visitor efinancials Payment Record Creation CONTENTS Notes Page 2 Submitting a Student/Visitor efinancials Payment Record Creation
THE UNIVERSITY OF EDINBURGH FINANCE PROCESS MANAGER USER GUIDE Student/Visitor efinancials Payment Record Creation CONTENTS Notes Page 2 Submitting a Student/Visitor efinancials Payment Record Creation
Australia Online Forms for Research Software User Manual
 Australia Online Forms for Research Software User Manual Version 1.3 Released 21 August 2010 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting
Australia Online Forms for Research Software User Manual Version 1.3 Released 21 August 2010 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting
Part-Time Faculty Guide View and Print Roster, Online Grading, and Notify Button
 Faculty may access the system from the CLC Home page located at: http://www.clcillinois.edu On the CLC Home page use the Faculty/Staff link in the top navigation bar.. Under Resources for Faculty & Staff
Faculty may access the system from the CLC Home page located at: http://www.clcillinois.edu On the CLC Home page use the Faculty/Staff link in the top navigation bar.. Under Resources for Faculty & Staff
Creating a grant submission record in Themis
 Creating a grant submission record in Themis Creating a submission record for a grant requires completion of a number of screens. You will be able to monitor your progress on the progress train that displays
Creating a grant submission record in Themis Creating a submission record for a grant requires completion of a number of screens. You will be able to monitor your progress on the progress train that displays
The e-marks System: Instructions for Faculty of Arts and Science Users
 The e-marks System: Instructions for Faculty of Arts and Science Users Contents Section A: Logging in... 2 Section B: Submitting Your Marks... 3 Section C: Marks Amendments... 8 Section D: How to Approve
The e-marks System: Instructions for Faculty of Arts and Science Users Contents Section A: Logging in... 2 Section B: Submitting Your Marks... 3 Section C: Marks Amendments... 8 Section D: How to Approve
R I M S. (R e s e a r c h I n f o r m a t i o n M a n a g e m e n t S y s t e m) ETHICS APPLICATIONS TRAINING MANUAL
 R I M S (R e s e a r c h I n f o r m a t i o n M a n a g e m e n t S y s t e m) ETHICS APPLICATIONS TRAINING MANUAL How to apply for ethical clearance on RIMS By The Directorate Research Development 2018
R I M S (R e s e a r c h I n f o r m a t i o n M a n a g e m e n t S y s t e m) ETHICS APPLICATIONS TRAINING MANUAL How to apply for ethical clearance on RIMS By The Directorate Research Development 2018
Eforms Full Application Guide New Contractor
 Eforms Full Application Guide New Contractor 1 Completing a Full application form (Pages 2-6) 1.1 Initial application screen (Page 2) 1.2 Assessment scope (Page 3) 1.3 Declaration and fees (Page 3) 1.4
Eforms Full Application Guide New Contractor 1 Completing a Full application form (Pages 2-6) 1.1 Initial application screen (Page 2) 1.2 Assessment scope (Page 3) 1.3 Declaration and fees (Page 3) 1.4
User Guide for Staff and Postgraduate Research Students using the Ethics Online Approval System
 User Guide for Staff and Postgraduate Research Students using the Ethics Online Approval System To access Ethics Online go to Ethics and Governance webpage https://www.northumbria.ac.uk/research/ethics-andgovernance/
User Guide for Staff and Postgraduate Research Students using the Ethics Online Approval System To access Ethics Online go to Ethics and Governance webpage https://www.northumbria.ac.uk/research/ethics-andgovernance/
Review Response Submission Form
 Review Response Submission Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it, returns it as Incomplete
Review Response Submission Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it, returns it as Incomplete
Create and Complete IFS eform
 Research Services Create and Complete IFS eform Research Fellowships Introduction The purpose of this Quick Reference Guide is to provide guidance to create and complete the Internal Fund Scheme eform
Research Services Create and Complete IFS eform Research Fellowships Introduction The purpose of this Quick Reference Guide is to provide guidance to create and complete the Internal Fund Scheme eform
Web CMS Sub Administrator Training
 Web CMS Sub Administrator Training - Introduction... 2 User Administration... 2 User Roles... 2 Administrator... 2 Sub Administrator... 2 Content Contributor... 2 Site User... 3 Overview of User Management...
Web CMS Sub Administrator Training - Introduction... 2 User Administration... 2 User Roles... 2 Administrator... 2 Sub Administrator... 2 Content Contributor... 2 Site User... 3 Overview of User Management...
eibc Program User Guide
 eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
NYU Cayuse IRB Manual
 IRB NYU Cayuse IRB Manual prepared by the NYU UCAIHS (University Committee on Activities Involving Human Subjects) What is Cayuse? The Cayuse Research Suite is NYU s system to support the submission of
IRB NYU Cayuse IRB Manual prepared by the NYU UCAIHS (University Committee on Activities Involving Human Subjects) What is Cayuse? The Cayuse Research Suite is NYU s system to support the submission of
New Protocol - emergency use training guide
 New Protocol - emergency use training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Emergency Use 1 table of contents Table of Contents 2 table of contents 3 about
New Protocol - emergency use training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Emergency Use 1 table of contents Table of Contents 2 table of contents 3 about
Outside Interests Disclosure Form for Staff. IRIS Mobile through the Web
 Outside Interests Disclosure Form for Staff IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff to disclose outside interests as required
Outside Interests Disclosure Form for Staff IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff to disclose outside interests as required
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
STELLENBOSCH UNIVERSITY
 STELLENBOSCH UNIVERSITY Research Information Management System INFONETICA Training Manual Compliance/Ethics New Application Contents 1. Login 2. Create your Project 3. Complete the questions online 4.
STELLENBOSCH UNIVERSITY Research Information Management System INFONETICA Training Manual Compliance/Ethics New Application Contents 1. Login 2. Create your Project 3. Complete the questions online 4.
You may have to enter your university login details the first time you access the system.
 To access Ethics Online go to Ethics and Governance webpage https://www.northumbria.ac.uk/research/ethics-andgovernance/ or the Staff or Student portals. You may have to enter your university login details
To access Ethics Online go to Ethics and Governance webpage https://www.northumbria.ac.uk/research/ethics-andgovernance/ or the Staff or Student portals. You may have to enter your university login details
Creating a New Application. A ROMEO Hasty Handbook Presented by the CHEO Research Institute
 Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
NEW PROTOCOL - NON-HUMAN SUBJECTS RESEARCH TRAINING GUIDE
 NEW PROTOCOL - NON-HUMAN SUBJECTS RESEARCH TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 Table of Contents Table of Contents
NEW PROTOCOL - NON-HUMAN SUBJECTS RESEARCH TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 Table of Contents Table of Contents
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
JITs Portal. User Manual
 JITs Portal User Manual November 2017 JITs Portal 1 Table of Contents Chapter 1 Introduction... 2 1.1 What is the JITs Portal?...3 1.2 User Guide structure...4 Chapter 2 Working with the JITs Portal...
JITs Portal User Manual November 2017 JITs Portal 1 Table of Contents Chapter 1 Introduction... 2 1.1 What is the JITs Portal?...3 1.2 User Guide structure...4 Chapter 2 Working with the JITs Portal...
REPORTING A HAZARD. MySAFETY Training Guide CONTACT
 MySAFETY Training Guide REPORTING A HAZARD CONTACT Phone: +61 3 6226 6298 Location: Level 3, Corporate Services Building, TT Flynn Street, Sandy Bay Tasmania 7000 Email: Website: health.safety@utas.edu.au
MySAFETY Training Guide REPORTING A HAZARD CONTACT Phone: +61 3 6226 6298 Location: Level 3, Corporate Services Building, TT Flynn Street, Sandy Bay Tasmania 7000 Email: Website: health.safety@utas.edu.au
Eforms Full Application Guide Returning Contractor
 Eforms Full Application Guide Returning Contractor 1 Completing a Full application form (Pages 2-6) 1.1 Login to Eforms system (Page 2) 1.2 Assessment scope (Page 3) 1.3 Declaration and fees (Page 3) 1.4
Eforms Full Application Guide Returning Contractor 1 Completing a Full application form (Pages 2-6) 1.1 Login to Eforms system (Page 2) 1.2 Assessment scope (Page 3) 1.3 Declaration and fees (Page 3) 1.4
User Manual. Declaration Creation, Submission and Approvals for ERDF For Beneficiary, Approver Beneficiary and Intermediary Body users. Version 0.
 User Manual Declaration Creation, Submission and Approvals for ERDF For Beneficiary, Approver Beneficiary and Intermediary Body users. Version 0.3 Document Version Control Version Amendment Author Date
User Manual Declaration Creation, Submission and Approvals for ERDF For Beneficiary, Approver Beneficiary and Intermediary Body users. Version 0.3 Document Version Control Version Amendment Author Date
This quickstart will help you get started with Turnitin. To begin, you need to register with Turnitin and create a user profile.
 Introduction This quickstart will help you get started with Turnitin. To begin, you need to register with Turnitin and create a user profile.! If you have received an e-mail from Turnitin with a temporary
Introduction This quickstart will help you get started with Turnitin. To begin, you need to register with Turnitin and create a user profile.! If you have received an e-mail from Turnitin with a temporary
New Protocol - expedited & full board
 New Protocol - expedited & full board training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Expedited & Full Board 1 table of contents Table of Contents 2 table
New Protocol - expedited & full board training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Expedited & Full Board 1 table of contents Table of Contents 2 table
Unit Outline Builder. Training Guide
 Unit Outline Builder Training Guide Curtin Learning & Teaching December 2017 CONTENTS 1. Purpose... 3 2. Log in and Select a Unit... 3 3. Select an Availability... 5 4. View Availability... 5 5. Preview
Unit Outline Builder Training Guide Curtin Learning & Teaching December 2017 CONTENTS 1. Purpose... 3 2. Log in and Select a Unit... 3 3. Select an Availability... 5 4. View Availability... 5 5. Preview
Human Research Ethics Application (HREA) How to Guide 6 February 2017 Version 2.0
 Human Research Ethics Application (HREA) How to Guide 6 February 2017 Version 2.0 Page 1 Contents 1 Introduction... 3 2 How-to guide... 4 2.1 Creating and managing your HREA account... 4 2.1.1 How to create
Human Research Ethics Application (HREA) How to Guide 6 February 2017 Version 2.0 Page 1 Contents 1 Introduction... 3 2 How-to guide... 4 2.1 Creating and managing your HREA account... 4 2.1.1 How to create
Researcher User Manual
 Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
TRACKER User s Guide for Departments
 TRACKER User s Guide for Departments Creating your Account You will receive an email from one of the ISFS staff welcoming you to Tracker. You must activate your account within 3 days of receiving this
TRACKER User s Guide for Departments Creating your Account You will receive an email from one of the ISFS staff welcoming you to Tracker. You must activate your account within 3 days of receiving this
EPA Research Programme EPA s Online Grant Application & Project Management Portal
 EPA Research Programme 2014-2020 EPA s Online Grant Application & Project Management Portal QUICK GUIDE TO MAKING AN APPLICATION April 2018 Version 3 Powered by SmartSimple The EPA Research Programme is
EPA Research Programme 2014-2020 EPA s Online Grant Application & Project Management Portal QUICK GUIDE TO MAKING AN APPLICATION April 2018 Version 3 Powered by SmartSimple The EPA Research Programme is
Romeo. How to Apply for a Faculty Conference Travel Grant
 Romeo How to Apply for a Faculty Conference Travel Grant Please note: Romeo is compatible with Internet Explorer, Firefox, Edge, Google Chrome and Safari. If you have any problems or questions, please
Romeo How to Apply for a Faculty Conference Travel Grant Please note: Romeo is compatible with Internet Explorer, Firefox, Edge, Google Chrome and Safari. If you have any problems or questions, please
Australia Online Forms for Research Software User Manual
 Australia Online Forms for Research Software User Manual Version 1.6 Released August 2014 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting Started
Australia Online Forms for Research Software User Manual Version 1.6 Released August 2014 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting Started
ecampus 9.2 Faculty Homepage
 1 I. ecampus Features In ecampus 9.2, the ecampus Faculty Homepage features three (3) tiles. The tiles have all the functionalities found on the previous ecampus Faculty Homepage. ecampus 9.2 Faculty Homepage
1 I. ecampus Features In ecampus 9.2, the ecampus Faculty Homepage features three (3) tiles. The tiles have all the functionalities found on the previous ecampus Faculty Homepage. ecampus 9.2 Faculty Homepage
Buck-IRB Amendment User Guide
 Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
E-OAR Mentor Guide Date Written 30 May 2017 Date Updated 20 Dec 2017
 E-OAR Mentor Guide Date Written 30 May 2017 Date Updated 20 Dec 2017 Electronic Student Assessment e-oar... 2 TO LOGIN TO E-OAR... 2 SEARCHING FOR A STUDENT... 3 VIEWING, VERIFYING OR REJECTING A DEVELOPMENT
E-OAR Mentor Guide Date Written 30 May 2017 Date Updated 20 Dec 2017 Electronic Student Assessment e-oar... 2 TO LOGIN TO E-OAR... 2 SEARCHING FOR A STUDENT... 3 VIEWING, VERIFYING OR REJECTING A DEVELOPMENT
FUNDING FORM NAVIGATION INITIATOR S GUIDE
 FUNDING FORM NAVIGATION INITIATOR S GUIDE TABLE OF CONTENTS INTRODUCTION... 1 ACCESS FUNDING FORM IN PEOPLESOFT HR... 2 INITIATE A FUNDING FORM... 2 ENTERING SEARCH CRITERIA TO START FORM... 3 FUNDING
FUNDING FORM NAVIGATION INITIATOR S GUIDE TABLE OF CONTENTS INTRODUCTION... 1 ACCESS FUNDING FORM IN PEOPLESOFT HR... 2 INITIATE A FUNDING FORM... 2 ENTERING SEARCH CRITERIA TO START FORM... 3 FUNDING
renew & Amend A Protocol training guide
 renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
SGSonSITE User Guide. User Guide to SGSonSITE ecertification. ecertificate OPERATION. Version 3.0
 Issue date: 07.06.2012 Author: Eleonor Tolete Page n : 1 of 45 SGSonSITE User Guide ecertificate Version 3.0 Back Office User s Manual ecertificate E.Tolete Page n : 2 of 47 What is SGSonSITE? SGSonSITE
Issue date: 07.06.2012 Author: Eleonor Tolete Page n : 1 of 45 SGSonSITE User Guide ecertificate Version 3.0 Back Office User s Manual ecertificate E.Tolete Page n : 2 of 47 What is SGSonSITE? SGSonSITE
How to Process a Work List Transaction
 Business Process Guide How to Process a Work List Transaction A guide for approving, returning and canceling a workflow transaction HR Data Management Contacts Please refer to the Data Management staff
Business Process Guide How to Process a Work List Transaction A guide for approving, returning and canceling a workflow transaction HR Data Management Contacts Please refer to the Data Management staff
Amend A Protocol training guide
 Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
ORE Researcher Guide for using Kuali. October 2018
 ORE Researcher Guide for using Kuali October 2018 Navigating the system...3 Manage protocols page... 3 Viewing the full application... 4 Applications...6 Beginning an application... 6 General Information
ORE Researcher Guide for using Kuali October 2018 Navigating the system...3 Manage protocols page... 3 Viewing the full application... 4 Applications...6 Beginning an application... 6 General Information
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
RTO / TRAINER USER MANUAL
 Dear RTO / Trainer System User, Welcome to profile21 system, This user guide describes the basic functions of the profile21 software application. Login Helpdesk Email Go to the profiling website: www.profile21.com.au
Dear RTO / Trainer System User, Welcome to profile21 system, This user guide describes the basic functions of the profile21 software application. Login Helpdesk Email Go to the profiling website: www.profile21.com.au
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II
 How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
Human Subjects Development Guide
 Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Main Toolbar. Toolbars Icon Cheat Sheet. The Coeus Main toolbar, located below the menu bar, is consistent in every module of Coeus.
 Toolbars Icon Cheat Sheet Main Toolbar The Coeus Main toolbar, located below the menu bar, is consistent in every module of Coeus. Maintain Inbox - Inbox contains messages regarding routing and approval
Toolbars Icon Cheat Sheet Main Toolbar The Coeus Main toolbar, located below the menu bar, is consistent in every module of Coeus. Maintain Inbox - Inbox contains messages regarding routing and approval
HOW TO ACCESS AND SUBMIT THE ONLINE APPLICATION TO AMEND ENROLMENT FORM
 Student Business Systems, Student Operations STUDENT GUIDE HOW TO ACCESS AND SUBMIT THE ONLINE APPLICATION TO AMEND ENROLMENT FORM 1) To access the Application to Amend Enrolment Form, (i) Log in to Blackboard,
Student Business Systems, Student Operations STUDENT GUIDE HOW TO ACCESS AND SUBMIT THE ONLINE APPLICATION TO AMEND ENROLMENT FORM 1) To access the Application to Amend Enrolment Form, (i) Log in to Blackboard,
Kuali Research User Guide: Create an IRB Protocol
 Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
User guide: Submitting programme and location approval requests via GMC Connect
 May 2018 User guide: Submitting programme and location approval requests via GMC Connect This guide is for colleagues at local offices and deaneries who are responsible for making submissions to the GMC
May 2018 User guide: Submitting programme and location approval requests via GMC Connect This guide is for colleagues at local offices and deaneries who are responsible for making submissions to the GMC
Attach Documents to an Order
 Attach Documents to an Order Who: Why: When: Any User who can view the Order Files (documents, spreadsheets etc.) can be attached to an Order or Project View using the Attach tab. The attachments are then
Attach Documents to an Order Who: Why: When: Any User who can view the Order Files (documents, spreadsheets etc.) can be attached to an Order or Project View using the Attach tab. The attachments are then
How Does the PI make Pre Review ICF Changes?
 How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
Module 3: Changes and Correspondence
 Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Monash University Policy Management. User Guide
 Monash University Policy Management User Guide 1 Table of Contents 1. GENERAL NAVIGATION... 4 1.1. Logging In to Compliance 360 - Single Sign On... 4 1.2. Help... 4 1.2.1. The University Policy Bank...
Monash University Policy Management User Guide 1 Table of Contents 1. GENERAL NAVIGATION... 4 1.1. Logging In to Compliance 360 - Single Sign On... 4 1.2. Help... 4 1.2.1. The University Policy Bank...
Protocol Management System (PMS) Investigator User Guide. Harris Health System Administrative Approval
 Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Unit Outline Builder. Training Guide
 Unit Outline Builder Training Guide Sonia Ferns and Eric artini Curtin Teaching & Learning ay 2013 CONTENTS 1. Purpose... 3 2. Log in and select a unit... 3 3. Select an availability... 4 4. View availability...
Unit Outline Builder Training Guide Sonia Ferns and Eric artini Curtin Teaching & Learning ay 2013 CONTENTS 1. Purpose... 3 2. Log in and select a unit... 3 3. Select an availability... 4 4. View availability...
University of the Sciences. IRBNet User s Guide
 University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
Nova Scotia Health Authority Research Ethics Board. Researcher s (PI) User Manual
 Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Quick guide to the SmartSimple on-line portal (making an application)
 EPA Research Programme 2014-2020 Quick guide to the SmartSimple on-line portal (making an application) POWERED BY SMARTSIMPLE Disclaimer Please read this document carefully prior to using the on-line portal.
EPA Research Programme 2014-2020 Quick guide to the SmartSimple on-line portal (making an application) POWERED BY SMARTSIMPLE Disclaimer Please read this document carefully prior to using the on-line portal.
IDF Appraisee Guide to the IDF Online Appraisal Form. Contents
 IDF Appraisee Guide to the IDF Online Appraisal Form The IDF Appraisee Guide is a step by step handbook on how to complete the IDF online appraisal form. This document contains detailed guidance on how
IDF Appraisee Guide to the IDF Online Appraisal Form The IDF Appraisee Guide is a step by step handbook on how to complete the IDF online appraisal form. This document contains detailed guidance on how
Research Funding Module InfoEd User Guide. Version 3.3
 Research Funding Module InfoEd User Guide Version 3.3 Table of Contents Login... 3 Create New Proposal or Expression of Interest (EOI)... 3 Primary Application Details... 4 Principal Investigator Information...
Research Funding Module InfoEd User Guide Version 3.3 Table of Contents Login... 3 Create New Proposal or Expression of Interest (EOI)... 3 Primary Application Details... 4 Principal Investigator Information...
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
1st Reviewer. Last Updated: March 31, 2015
 1st Reviewer Last Updated: March 31, 2015 Table of Contents Introduction... 3 Roles... 4 Login... 5 Main Screen... 6 Home menu... 7 Search for an Equivalency Request... 8 Search for an Equivalency Request
1st Reviewer Last Updated: March 31, 2015 Table of Contents Introduction... 3 Roles... 4 Login... 5 Main Screen... 6 Home menu... 7 Search for an Equivalency Request... 8 Search for an Equivalency Request
Special Consideration Online application
 Special Consideration Online application Student User Guide Copyright Deakin University All rights reserved. No part of this work covered by Deakin University's copyright may be reproduced or copied in
Special Consideration Online application Student User Guide Copyright Deakin University All rights reserved. No part of this work covered by Deakin University's copyright may be reproduced or copied in
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE
 Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
