Wave correction for arrays
|
|
|
- Edward Campbell
- 5 years ago
- Views:
Transcription
1 Wave correction for arrays Eitan Halper-Stromberg and Robert B. Scharpf April 30, 2018 Abstract ArrayTV is a GC correction algorithm for microarray data designed to mitigate the appearance of waves. This work is inspired by a method for mitigating waves in sequencing data [1]. We find the genomic window for computing GC that best captures the dependence of signal intensity on GC content for each array using a score similar to the total variance (TV) statistic defined in [1]. The correction each probe receives is the mean signal intensity of all probes sharing the same local GC. We center windows at the starting position of each probe. 1 Adjusting copy number estimates for GC waves Importing data. The wave correction methods in ArrayTV handle simple matrices with rows indexing markers and columns indexing samples, as well as more complex data structures commonly used to process Affymetrix and Illumina platforms made available in the oligoclasses package. In this vignette, we illustrate our approach with a single female lymphoblastoid cell line sample assayed on the Agilent 1M, Affymetrix 2.7M, and NimbleGen 2.1M platforms (available in full from spike-in tube 2)[2]. For each platform, we provide a matrix. The rows of the matrix correspond to markers on chromosome 15 and the columns correspond to the physical position of the first nucleotide of the marker using UCSC build hg18 (note: for genotyping arrays, the position should be adjusted to the start of the marker and not the location of the SNP) and preprocessed signal intensities that we expect to be proportional to the copy number as well as sequence artifacts such as GC content. Agilent and NimbleGen signal intensities were preprocessed by taking the log ratio of loess normalized raw signal. Affymetrix performed their own preprocessing to obtain log R ratios. Hereafter, we refer to the preprocessed signal as M values. To keep the size of this package small, we provide the M values as integers (M 1000). In the following code, we load the data for each platform, inspect the first few rows, and transform the M values for each platform to the original scale. > library(arraytv) > ### parallelization will be discussed later but for now we provide the following > ### command to avoid warnings > if(require(doparallel)){ + cl <- makecluster(1) + registerdoparallel(cl) > path <- system.file("extdata", package="arraytv") > load(file.path(path, "array_logratios.rda")) > head(agilent) position M [1,] [2,] [3,] [4,] [5,] [6,]
2 > head(affymetrix) position M [1,] [2,] [3,] [4,] [5,] [6,] > head(nimblegen) position M [1,] [2,] [3,] [4,] [5,] [6,] > agilent[, "M"] <- agilent[, "M"]/1000 > affymetrix[, "M"] <- affymetrix[, "M"]/1000 > nimblegen[, "M"] <- nimblegen[, "M"]/1000 To determine the optimal window(s) for GC correction, we generate TV scores for several window sizes. Again, we assume that the positions denote the start location of each probe. While our analysis in this vignette is limited to chromosome 15 for computational reasons, in practice we recommend implementing the wave correction on all autosomes simultaneously. We specify the windows to search by providing two vectors that indicate the maximum window size and the increment: > max.window <- c(100,10e3,1e6) > increms <- c(20, 2000, 200e3) The above specification indicates that we will compute TV scores for three sequential window sizes as follows. The first series of windows has a maximum window extension of 100 from center, (max.window[1]) (effective maximum window size is therefore 200 since we extend in both directions) and increment extension value 20 (increms[1]) again, extending from the center of the window, indicating that windows extending 20, 40,..., and 100 from the probe starts will be evaluated. For the second series, the maximum window extension is 10e3 (max.window[2]) and window extensions 2000, 4000,..., 10e3 will be evaluated since the increment value is 2000 (increms[2]). For each of the three series, the number of windows to be evaluated is 5. The sequence for each series must have the same length. Evaluating TV scores as a function of window size for computing GC. The class of the first argument to the gccorrect method determines the dispatch to the low-level function gccorrectmain. Any of the arguments to gccorrectmain can be passed through the... operator and we refer the user to the documentation for gccorrectmain for additional arguments and gccorrect for the classes of objects handled by the gccorrect method. Computing the GC content of the sequence for each marker requires that the appropriate BSgenome package is loaded. For example, here the package BSgenome.Hsapiens.UCSC.hg18 is loaded internally. The following codechunk performs GC correction on the M values for the NimbleGen, Agilent, and Affymetrix platforms, respectively, for the windows specified previously. > nimcm1list <- gccorrect(object=nimblegen[, "M", drop=false], + chr=rep("chr15", nrow(nimblegen)), + starts=nimblegen[, "position"], + increms=increms, + maxwins=max.window, 2
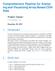
3 + build='hg18') > afcm1list <- gccorrect(affymetrix[, "M", drop=false], + chr=rep("chr15", nrow(affymetrix)), + starts=affymetrix[, "position"], + increms=increms, + maxwins=max.window, + build="hg18") > ## We load the corrected NimbleGen values computed during unit-testing and Affy > ## values, pre-computed, to save time > load(file=file.path(path,"nimcm1list.rda")) > load(file=file.path(path,"afcm1list.rda")) > agcm1list <- gccorrect(agilent[, "M", drop=false], + chr=rep("chr15", nrow(agilent)), + starts=agilent[, "position"], + increms=increms, + maxwins=max.window, + build="hg18") > if(require(doparallel)) + stopcluster(cl) To plot the TV score for each of the windows evaluated, we concatenate the results from each platform into a list and then convert the elements representing the TVscores into a data.frame. Plotting is based on the size of the genomic windows used to compute GC (small, medium, and large). > results <- list(nimcm1list,agcm1list,afcm1list) > tvscores <- do.call("rbind", lapply(results, "[[", "tvscore"))[, 1] > tv.df <- data.frame(tv=tvscores, platform=rep(c("nimblegen", "Agilent", "Affy 2.7M"), each=15), + window=c(seq(20, 100, 20), + seq(2000, 10e3, 2e3), seq(2e5, 1e6, 2e5))*2) > op <- par(no.readonly = TRUE) > par(las=1, mar=c(6, 4, 3, 2)+0.1) > with(tv.df, plot(log10(window), tv, pch=20, col=tv.df$platform, + xaxt="n", xlab="window (bp)", ylab="tv score", cex=1.2)) > invisible(lapply(split(tv.df, tv.df$platform), function(x) + points(log10(x$window),x$tv, type="l",col=x$platform, lwd=1.5))) > axis(1, at=log10(tv.df$window), labels=false) #labels=false, tick=true)a > text(x=log10(tv.df$window), par("usr")[3]-0.005, + labels=tv.df$window, + srt=45, xpd=true, cex=0.7, adj=1) > legend("bottomright", legend=unique(tv.df$platform), + pch=20, col=unique(tv.df$platform), lwd=1.2) > par(op) View corrected and uncorrected M values Our GC-adjusted signal was computed when we called gccorrect and is now stored in objects nimcm1list, agcm1list, and afcm1list. Here we create a data.frame to store corrected and uncorrected signal, ahead of plotting. > wave.df <- data.frame(position=c(rep(nimblegen[, "position"]/1e6, 2), + rep(agilent[, "position"]/1e6, 2), + rep(affymetrix[, "position"]/1e6,2)), + r=c(nimblegen[, "M"], + nimcm1list[['correctedvals']], 3
4 0.08 TV score NimbleGen Agilent Affy 2.7M e+05 8e e+06 window (bp) Figure 1: TV scores for three array platforms indicated by color. For the Agilent and NimbleGen platforms, the window selected for wave correction is relatively large ( 1.2Mb). For the Affymetrix platform, the optimal window is 8kb. Often, the optimal window is closer to the size of the probe or the PCR fragment. A correction for both large and small windows could be acheived by a second iteration of the gccorrect function. + agilent[,"m"], + agcm1list[['correctedvals']], + affymetrix[,"m"], afcm1list[['correctedvals']]), + platform=rep(c(rep("nimblegen", 2), + rep("agilent", 2), + rep("affymetrix", 2)), c(rep(length(nimblegen[, "M"]),2), + rep(length(agilent[,"m"]),2), + rep(length(affymetrix[,"m"]),2))), + wave.corr=c(rep("raw", length(nimblegen[, "M"])), + rep("arraytv", length(nimblegen[, "M"])), + rep("raw", length(agilent[,"m"])), + rep("arraytv", length(agilent[,"m"])), + rep("raw", length(affymetrix[,"m"])), + rep("arraytv",length(affymetrix[,"m"])))) > wave.df$platform <- factor(wave.df$platform, levels=c("nimblegen", + "Agilent", "Affymetrix")) > wave.df$wave.corr <- factor(wave.df$wave.corr, levels=c("raw", "ArrayTV")) > ## remove some points to make subsequent figure smaller > wave.df <- wave.df[seq(1,nrow(wave.df),4),] We use the R package lattice to visualize the M values before and after correction for the three platforms (Figure 2) > ## For each of the 3 example platforms, the uncorrected signal appears immediately above the > ## corrected signal, across chromosome 15. A smoothed loess line may be drawn through each > ## by uncommenting the commented line in the xyplot function > require("lattice") && require("latticeextra") [1] TRUE 4
5 Nimblegen Agilent Affymetrix raw M ArrayTV position (Mb) on chr Figure 2: Comparison of M values before and after ArrayTV wave correction (rows) for three array platforms (columns). > p <- xyplot(r~position platform+wave.corr, wave.df, pch=".", col="gray", + ylim=c(-2,1.5), xlim=range(nimblegen[, "position"]/1e6), + ylab="m", xlab="position (Mb) on chr15", + scales=list(y=list(tick.number=8)), + panel=function(x,y,subscripts,...){ + panel.grid(lty=2) + panel.xyplot(x, y,...) + panel.abline(h=0) + ## panel.loess(x, y, span=1/20, lwd=2, col="black"), par.strip.text=list(cex=0.9), + layout=c(2,3), + as.table=true) > p2 <- useouterstrips(p) > print(p2) 5
6 2 Spike-In Example To demonstrate that the GC-correction does not remove true CNV signals, we illustrate our approach on a spike-in datasets containing a small amplification. While the signal for the spike-in is subtle, the main point here is that the small inflection is not effected by the GC correction. The amplification was spiked in to each of the three array platforms as described in Halper-Stromberg et al. [2]. As the segmentation of the data using circular binary segmentation (CBS; [3]) is only for visualization and is not critical to the functionality of this package, the following code chunk is static (not evaluated) to reduce computation: > if(require("dnacopy")){ + cbs.segs <- list() + platforms <- c("nimblegen", "Agilent", "Affymetrix") + correction <- c("raw", "ArrayTV") + Ms <- list(nimblegen[, "M"], nimcm1list[['correctedvals']], + agilent[, "M"], agcm1list[['correctedvals']], + affymetrix[, "M"], afcm1list[['correctedvals']]) + starts <- list(nimblegen[, "position"], + agilent[, "position"], + affymetrix[, "position"]) + k <- 0 + for(i in 1:3){ + for(j in 1:2){ + k <- k+1 + MsUse <- Ms[[k]] + chruse <- rep("chr15", length(msuse)) + startsuse <- starts[[i]] + touse <-!(duplicated(startsuse)) + cna1 <- CNA(as.matrix(MsUse[toUse]), + chruse[touse], + startsuse[touse], + data.type="logratio", + paste(platforms[i], correction[j], sep="_")) + smooth.cna1 <- smooth.cna(cna1) + cbs.segs[[k]] <- segment(smooth.cna1, verbose=1,min.width = 5) + cbs.out <- do.call("rbind", lapply(cbs.segs, "[[", "output")) We load the results from the above computation into our workspace as follows: > load(file.path(path, "cbs_out.rda")) To facilitate downstream analyses such as visualization of the results, we coerce the results from the CBS algorithm to an object of class GRanges. > ids <- cbs.out$id > ## create vectors indicating corrected/uncorrected and platform for each cbs segment > platforms <- gsub("_raw", "", ids) > platforms <- gsub("_arraytv", "", platforms) > correction <- gsub("nimblegen_", "", ids) > correction <- gsub("affymetrix_", "", correction) > correction <- gsub("agilent_", "", correction) > ## store cbs segments in a GRanges object with designations for corrected/uncorrected > ## and platform 6
7 > cbs.segs <- GRanges(rep("chr14", nrow(cbs.out)), + IRanges(cbs.out$loc.start, cbs.out$loc.end), + nummarkers=cbs.out$num.mark, + seg.mean = cbs.out$seg.mean, + platform = platforms, + correction=correction) > ## separate corrected and uncorrected cbs segments int separate objects > cbs.segs.raw <- cbs.segs[cbs.segs$correction=="raw", ] > cbs.segs.arraytv <- cbs.segs[cbs.segs$correction=="arraytv", ] > ## create GRanges objects with M values, platform, and > ## corrected/uncorrected designation > marker.data <- GRanges(rep("chr14", nrow(wave.df)), + IRanges(wave.df$position*1e6, width=1), + nummarkers=1, + seg.mean = wave.df$r, + platform=wave.df$platform, + correction=wave.df$wave.corr) > marker.raw <- marker.data[marker.data$correction=="raw",] > marker.arraytv <- marker.data[marker.data$correction=="arraytv",] > ## populate cbs.seg metadata column by joining M values with CBS segments by location > olaps1 <- findoverlaps(marker.raw, cbs.segs.raw, select="first") > marker.raw$cbs.seg <- cbs.segs.raw$seg.mean[olaps1] > olaps2 <- findoverlaps(marker.arraytv, cbs.segs.arraytv, select="first") > marker.arraytv$cbs.seg <- cbs.segs.arraytv$seg.mean[olaps2] > ## populate SpikeIn metadata column if a marker falls within the Spiked-in region > spikein <- IRanges(start= ,end= ) > spikeinstart < ; spikeinend < > marker.raw$spikein <- marker.raw$cbs.seg > marker.arraytv$spikein <- marker.arraytv$cbs.seg > index1 <- which(!overlapsany(unlist(as(ranges(marker.raw), "IntegerList")), + spikein)) > index2 <- which(!overlapsany(unlist(as(ranges(marker.arraytv), "IntegerList")), + spikein)) > marker.raw$spikein[index1] <- NA > marker.arraytv$spikein[index2] <- NA > ## put GRanges objects into a dataframe ahead of plotting > rawd <- as.data.frame(marker.raw) > atvd <- as.data.frame(marker.arraytv) > rawd <- rbind(rawd, atvd) > rawd$platform <- factor(rawd$platform, levels=c("nimblegen", + "Agilent", "Affymetrix")) > rawd$correction <- factor(rawd$correction, levels=c("raw", "ArrayTV")) > ## remove some points to make subsequent figure smaller > rawd <- rawd[seq(1,nrow(rawd),2),] Black points in Figure 3 represent signal intensities for each probe, and red line segments correspond to the segmentation of the intensities from CBS. The red line segments demarcate the spike-in. As in the previous figure, raw signal intensities appear immediately above the corrected intensities for each platform > p2 <- xyplot(seg.mean+cbs.seg+spikein~start/1e6 platform+correction, rawd, + ylim=c(-1.5, 1.5), + pch=".", + xlim=c(spikeinstart/1e6-1.5,spikeinend/1e6+1.5), + ylab="m", + xlab="position (Mb)",##pch=c(".", NA,NA), 7
8 + col=c('gray','black','red'), + distribute.type=true, + type=c('p','l','l'),lwd=c(na,2,3), + scales=list(y=list(tick.number=8)), + par.strip.text=list(cex=0.9), layout=c(2,3), as.table=true) > p3 <- useouterstrips(p2) > print(p3) Nimblegen Agilent Affymetrix raw M 1.0 ArrayTV position (Mb) Figure 3: Comparison of CBS segments (lines) and M values (points) before and after ArrayTV wave correction (rows) for three array platforms (columns) in the vicinity of a known amplification of copy number 2.5 (red). 3 Parallelization When multiple cores are available, computation can proceed in parallel for computing the GC content for the various window sizes specified. To enable parallelization across 8 cores using the doparallel package, one could execute the following (unevaluated) code chunk prior to calling the gccorrect function: > if(require(doparallel)){ + cl <- makecluster(8) + registerdoparallel(cl) > ##... execute gccorrect > if(require(doparallel)) + stopcluster(cl) 8
9 4 Session Information ˆ R version ( ), x86_64-pc-linux-gnu ˆ Locale: LC_CTYPE=en_US.UTF-8, LC_NUMERIC=C, LC_TIME=en_US.UTF-8, LC_COLLATE=C, LC_MONETARY=en_US.UTF-8, LC_MESSAGES=en_US.UTF-8, LC_PAPER=en_US.UTF-8, LC_NAME=C, LC_ADDRESS=C, LC_TELEPHONE=C, LC_MEASUREMENT=en_US.UTF-8, LC_IDENTIFICATION=C ˆ Running under: Ubuntu LTS ˆ Matrix products: default ˆ BLAS: /home/biocbuild/bbs-3.7-bioc/r/lib/librblas.so ˆ LAPACK: /home/biocbuild/bbs-3.7-bioc/r/lib/librlapack.so ˆ Base packages: base, datasets, grdevices, graphics, methods, parallel, stats, stats4, utils ˆ Other packages: ArrayTV , BSgenome , BSgenome.Hsapiens.UCSC.hg , BiocGenerics , Biostrings , GenomeInfoDb , GenomicRanges , IRanges , RColorBrewer 1.1-2, S4Vectors , XVector , doparallel , foreach 1.4.4, iterators 1.0.9, lattice , latticeextra , rtracklayer ˆ Loaded via a namespace (and not attached): Biobase , BiocInstaller , BiocParallel , DBI 0.8, DNAcopy , DelayedArray 0.6.0, GenomeInfoDbData 1.1.0, GenomicAlignments , Matrix , RCurl , Rsamtools , SummarizedExperiment , XML , affyio , bit , bitops 1.0-6, codetools , compiler 3.5.0, ff , grid 3.5.0, matrixstats , oligoclasses , tools 3.5.0, zlibbioc References [1] Yuval Benjamini and Terence P. Speed. Summarizing and correcting the GC content bias in highthroughput sequencing. Nucleic Acids Res, 40(10):e72, May [2] Eitan Halper-Stromberg, Laurence Frelin, Ingo Ruczinski, Robert Scharpf, Chunfa Jie, Benilton Carvalho, Haiping Hao, Kurt Hetrick, Anne Jedlicka, Amanda Dziedzic, Kim Doheny, Alan F. Scott, Steve Baylin, Jonathan Pevsner, Forrest Spencer, and Rafael A. Irizarry. Performance assessment of copy number microarray platforms using a spike-in experiment. Bioinformatics, 27(8): , [3] Adam B Olshen, E. S. Venkatraman, Robert Lucito, and Michael Wigler. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics, 5(4):557 72, Oct
segmentseq: methods for detecting methylation loci and differential methylation
 segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 30, 2018 1 Introduction This vignette introduces analysis methods for data from high-throughput
segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 30, 2018 1 Introduction This vignette introduces analysis methods for data from high-throughput
segmentseq: methods for detecting methylation loci and differential methylation
 segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 13, 2015 1 Introduction This vignette introduces analysis methods for data from high-throughput
segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 13, 2015 1 Introduction This vignette introduces analysis methods for data from high-throughput
The bumphunter user s guide
 The bumphunter user s guide Kasper Daniel Hansen khansen@jhsph.edu Martin Aryee aryee.martin@mgh.harvard.edu Rafael A. Irizarry rafa@jhu.edu Modified: November 23, 2012. Compiled: April 30, 2018 Introduction
The bumphunter user s guide Kasper Daniel Hansen khansen@jhsph.edu Martin Aryee aryee.martin@mgh.harvard.edu Rafael A. Irizarry rafa@jhu.edu Modified: November 23, 2012. Compiled: April 30, 2018 Introduction
Working with aligned nucleotides (WORK- IN-PROGRESS!)
 Working with aligned nucleotides (WORK- IN-PROGRESS!) Hervé Pagès Last modified: January 2014; Compiled: November 17, 2017 Contents 1 Introduction.............................. 1 2 Load the aligned reads
Working with aligned nucleotides (WORK- IN-PROGRESS!) Hervé Pagès Last modified: January 2014; Compiled: November 17, 2017 Contents 1 Introduction.............................. 1 2 Load the aligned reads
Visualisation, transformations and arithmetic operations for grouped genomic intervals
 ## Warning: replacing previous import ggplot2::position by BiocGenerics::Position when loading soggi Visualisation, transformations and arithmetic operations for grouped genomic intervals Thomas Carroll
## Warning: replacing previous import ggplot2::position by BiocGenerics::Position when loading soggi Visualisation, transformations and arithmetic operations for grouped genomic intervals Thomas Carroll
An Introduction to ShortRead
 Martin Morgan Modified: 21 October, 2013. Compiled: April 30, 2018 > library("shortread") The ShortRead package provides functionality for working with FASTQ files from high throughput sequence analysis.
Martin Morgan Modified: 21 October, 2013. Compiled: April 30, 2018 > library("shortread") The ShortRead package provides functionality for working with FASTQ files from high throughput sequence analysis.
An Overview of the S4Vectors package
 Patrick Aboyoun, Michael Lawrence, Hervé Pagès Edited: February 2018; Compiled: June 7, 2018 Contents 1 Introduction.............................. 1 2 Vector-like and list-like objects...................
Patrick Aboyoun, Michael Lawrence, Hervé Pagès Edited: February 2018; Compiled: June 7, 2018 Contents 1 Introduction.............................. 1 2 Vector-like and list-like objects...................
Preprocessing and Genotyping Illumina Arrays for Copy Number Analysis
 Preprocessing and Genotyping Illumina Arrays for Copy Number Analysis Rob Scharpf September 18, 2012 Abstract This vignette illustrates the steps required prior to copy number analysis for Infinium platforms.
Preprocessing and Genotyping Illumina Arrays for Copy Number Analysis Rob Scharpf September 18, 2012 Abstract This vignette illustrates the steps required prior to copy number analysis for Infinium platforms.
Analysis of two-way cell-based assays
 Analysis of two-way cell-based assays Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Analysis of two-way cell-based assays Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Basic4Cseq: an R/Bioconductor package for the analysis of 4C-seq data
 Basic4Cseq: an R/Bioconductor package for the analysis of 4C-seq data Carolin Walter October 30, 2017 Contents 1 Introduction 1 1.1 Loading the package...................................... 2 1.2 Provided
Basic4Cseq: an R/Bioconductor package for the analysis of 4C-seq data Carolin Walter October 30, 2017 Contents 1 Introduction 1 1.1 Loading the package...................................... 2 1.2 Provided
riboseqr Introduction Getting Data Workflow Example Thomas J. Hardcastle, Betty Y.W. Chung October 30, 2017
 riboseqr Thomas J. Hardcastle, Betty Y.W. Chung October 30, 2017 Introduction Ribosome profiling extracts those parts of a coding sequence currently bound by a ribosome (and thus, are likely to be undergoing
riboseqr Thomas J. Hardcastle, Betty Y.W. Chung October 30, 2017 Introduction Ribosome profiling extracts those parts of a coding sequence currently bound by a ribosome (and thus, are likely to be undergoing
Image Analysis with beadarray
 Mike Smith October 30, 2017 Introduction From version 2.0 beadarray provides more flexibility in the processing of array images and the extraction of bead intensities than its predecessor. In the past
Mike Smith October 30, 2017 Introduction From version 2.0 beadarray provides more flexibility in the processing of array images and the extraction of bead intensities than its predecessor. In the past
How to use CNTools. Overview. Algorithms. Jianhua Zhang. April 14, 2011
 How to use CNTools Jianhua Zhang April 14, 2011 Overview Studies have shown that genomic alterations measured as DNA copy number variations invariably occur across chromosomal regions that span over several
How to use CNTools Jianhua Zhang April 14, 2011 Overview Studies have shown that genomic alterations measured as DNA copy number variations invariably occur across chromosomal regions that span over several
How to Use pkgdeptools
 How to Use pkgdeptools Seth Falcon February 10, 2018 1 Introduction The pkgdeptools package provides tools for computing and analyzing dependency relationships among R packages. With it, you can build
How to Use pkgdeptools Seth Falcon February 10, 2018 1 Introduction The pkgdeptools package provides tools for computing and analyzing dependency relationships among R packages. With it, you can build
How to use cghmcr. October 30, 2017
 How to use cghmcr Jianhua Zhang Bin Feng October 30, 2017 1 Overview Copy number data (arraycgh or SNP) can be used to identify genomic regions (Regions Of Interest or ROI) showing gains or losses that
How to use cghmcr Jianhua Zhang Bin Feng October 30, 2017 1 Overview Copy number data (arraycgh or SNP) can be used to identify genomic regions (Regions Of Interest or ROI) showing gains or losses that
Advanced analysis using bayseq; generic distribution definitions
 Advanced analysis using bayseq; generic distribution definitions Thomas J Hardcastle October 30, 2017 1 Generic Prior Distributions bayseq now offers complete user-specification of underlying distributions
Advanced analysis using bayseq; generic distribution definitions Thomas J Hardcastle October 30, 2017 1 Generic Prior Distributions bayseq now offers complete user-specification of underlying distributions
RAPIDR. Kitty Lo. November 20, Intended use of RAPIDR 1. 2 Create binned counts file from BAMs Masking... 1
 RAPIDR Kitty Lo November 20, 2014 Contents 1 Intended use of RAPIDR 1 2 Create binned counts file from BAMs 1 2.1 Masking.................................................... 1 3 Build the reference 2 3.1
RAPIDR Kitty Lo November 20, 2014 Contents 1 Intended use of RAPIDR 1 2 Create binned counts file from BAMs 1 2.1 Masking.................................................... 1 3 Build the reference 2 3.1
Basic4Cseq: an R/Bioconductor package for the analysis of 4C-seq data
 Basic4Cseq: an R/Bioconductor package for the analysis of 4C-seq data Carolin Walter April 16, 2015 Contents 1 Introduction 1 1.1 Loading the package...................................... 2 1.2 Provided
Basic4Cseq: an R/Bioconductor package for the analysis of 4C-seq data Carolin Walter April 16, 2015 Contents 1 Introduction 1 1.1 Loading the package...................................... 2 1.2 Provided
survsnp: Power and Sample Size Calculations for SNP Association Studies with Censored Time to Event Outcomes
 survsnp: Power and Sample Size Calculations for SNP Association Studies with Censored Time to Event Outcomes Kouros Owzar Zhiguo Li Nancy Cox Sin-Ho Jung Chanhee Yi June 29, 2016 1 Introduction This vignette
survsnp: Power and Sample Size Calculations for SNP Association Studies with Censored Time to Event Outcomes Kouros Owzar Zhiguo Li Nancy Cox Sin-Ho Jung Chanhee Yi June 29, 2016 1 Introduction This vignette
Creating a New Annotation Package using SQLForge
 Creating a New Annotation Package using SQLForge Marc Carlson, HervÃľ PagÃĺs, Nianhua Li April 30, 2018 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
Creating a New Annotation Package using SQLForge Marc Carlson, HervÃľ PagÃĺs, Nianhua Li April 30, 2018 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
MMDiff2: Statistical Testing for ChIP-Seq Data Sets
 MMDiff2: Statistical Testing for ChIP-Seq Data Sets Gabriele Schwikert 1 and David Kuo 2 [1em] 1 The Informatics Forum, University of Edinburgh and The Wellcome
MMDiff2: Statistical Testing for ChIP-Seq Data Sets Gabriele Schwikert 1 and David Kuo 2 [1em] 1 The Informatics Forum, University of Edinburgh and The Wellcome
A complete analysis of peptide microarray binding data using the pepstat framework
 A complete analysis of peptide microarray binding data using the pepstat framework Greg Imholte, Renan Sauteraud, Mike Jiang and Raphael Gottardo April 30, 2018 This document present a full analysis, from
A complete analysis of peptide microarray binding data using the pepstat framework Greg Imholte, Renan Sauteraud, Mike Jiang and Raphael Gottardo April 30, 2018 This document present a full analysis, from
VanillaICE: Hidden Markov Models for the Assessment of Chromosomal Alterations using High-throughput SNP Arrays
 VanillaICE: Hidden Markov Models for the Assessment of Chromosomal Alterations using High-throughput SNP Arrays Robert Scharpf March 16, 2016 Abstract This package provides an implementation of a hidden
VanillaICE: Hidden Markov Models for the Assessment of Chromosomal Alterations using High-throughput SNP Arrays Robert Scharpf March 16, 2016 Abstract This package provides an implementation of a hidden
Overview of ensemblvep
 Overview of ensemblvep Lori Shepherd and Valerie Obenchain October 30, 2017 Contents 1 Introduction 1 2 Results as R objects 1 3 Write results to a file 4 4 Configuring runtime options 5 5 sessioninfo()
Overview of ensemblvep Lori Shepherd and Valerie Obenchain October 30, 2017 Contents 1 Introduction 1 2 Results as R objects 1 3 Write results to a file 4 4 Configuring runtime options 5 5 sessioninfo()
The rtracklayer package
 The rtracklayer package Michael Lawrence January 22, 2018 Contents 1 Introduction 2 2 Gene expression and microrna target sites 2 2.1 Creating a target site track..................... 2 2.1.1 Constructing
The rtracklayer package Michael Lawrence January 22, 2018 Contents 1 Introduction 2 2 Gene expression and microrna target sites 2 2.1 Creating a target site track..................... 2 2.1.1 Constructing
Overview of ensemblvep
 Overview of ensemblvep Lori Shepherd and Valerie Obenchain October 30, 2018 Contents 1 Introduction 1 2 Results as R objects 1 3 Write results to a file 4 4 Configuring runtime options 5 5 sessioninfo()
Overview of ensemblvep Lori Shepherd and Valerie Obenchain October 30, 2018 Contents 1 Introduction 1 2 Results as R objects 1 3 Write results to a file 4 4 Configuring runtime options 5 5 sessioninfo()
Introduction to GenomicFiles
 Valerie Obenchain, Michael Love, Martin Morgan Last modified: October 2014; Compiled: October 30, 2018 Contents 1 Introduction.............................. 1 2 Quick Start..............................
Valerie Obenchain, Michael Love, Martin Morgan Last modified: October 2014; Compiled: October 30, 2018 Contents 1 Introduction.............................. 1 2 Quick Start..............................
Triform: peak finding in ChIP-Seq enrichment profiles for transcription factors
 Triform: peak finding in ChIP-Seq enrichment profiles for transcription factors Karl Kornacker * and Tony Håndstad October 30, 2018 A guide for using the Triform algorithm to predict transcription factor
Triform: peak finding in ChIP-Seq enrichment profiles for transcription factors Karl Kornacker * and Tony Håndstad October 30, 2018 A guide for using the Triform algorithm to predict transcription factor
Introduction: microarray quality assessment with arrayqualitymetrics
 Introduction: microarray quality assessment with arrayqualitymetrics Audrey Kauffmann, Wolfgang Huber April 4, 2013 Contents 1 Basic use 3 1.1 Affymetrix data - before preprocessing.......................
Introduction: microarray quality assessment with arrayqualitymetrics Audrey Kauffmann, Wolfgang Huber April 4, 2013 Contents 1 Basic use 3 1.1 Affymetrix data - before preprocessing.......................
Introduction to QDNAseq
 Ilari Scheinin October 30, 2017 Contents 1 Running QDNAseq.......................... 1 1.1 Bin annotations.......................... 1 1.2 Processing bam files....................... 2 1.3 Downstream analyses......................
Ilari Scheinin October 30, 2017 Contents 1 Running QDNAseq.......................... 1 1.1 Bin annotations.......................... 1 1.2 Processing bam files....................... 2 1.3 Downstream analyses......................
An Introduction to GenomeInfoDb
 Martin Morgan, Hervé Pagès, Marc Carlson, Sonali Arora Modified: 17 January, 2014. Compiled: January 21, 2018 Contents 1 Introduction.............................. 2 2 Functionality for all existing organisms...............
Martin Morgan, Hervé Pagès, Marc Carlson, Sonali Arora Modified: 17 January, 2014. Compiled: January 21, 2018 Contents 1 Introduction.............................. 2 2 Functionality for all existing organisms...............
RMassBank for XCMS. Erik Müller. January 4, Introduction 2. 2 Input files LC/MS data Additional Workflow-Methods 2
 RMassBank for XCMS Erik Müller January 4, 2019 Contents 1 Introduction 2 2 Input files 2 2.1 LC/MS data........................... 2 3 Additional Workflow-Methods 2 3.1 Options..............................
RMassBank for XCMS Erik Müller January 4, 2019 Contents 1 Introduction 2 2 Input files 2 2.1 LC/MS data........................... 2 3 Additional Workflow-Methods 2 3.1 Options..............................
Shrinkage of logarithmic fold changes
 Shrinkage of logarithmic fold changes Michael Love August 9, 2014 1 Comparing the posterior distribution for two genes First, we run a DE analysis on the Bottomly et al. dataset, once with shrunken LFCs
Shrinkage of logarithmic fold changes Michael Love August 9, 2014 1 Comparing the posterior distribution for two genes First, we run a DE analysis on the Bottomly et al. dataset, once with shrunken LFCs
Count outlier detection using Cook s distance
 Count outlier detection using Cook s distance Michael Love August 9, 2014 1 Run DE analysis with and without outlier removal The following vignette produces the Supplemental Figure of the effect of replacing
Count outlier detection using Cook s distance Michael Love August 9, 2014 1 Run DE analysis with and without outlier removal The following vignette produces the Supplemental Figure of the effect of replacing
SimBindProfiles: Similar Binding Profiles, identifies common and unique regions in array genome tiling array data
 SimBindProfiles: Similar Binding Profiles, identifies common and unique regions in array genome tiling array data Bettina Fischer October 30, 2018 Contents 1 Introduction 1 2 Reading data and normalisation
SimBindProfiles: Similar Binding Profiles, identifies common and unique regions in array genome tiling array data Bettina Fischer October 30, 2018 Contents 1 Introduction 1 2 Reading data and normalisation
Creating a New Annotation Package using SQLForge
 Creating a New Annotation Package using SQLForge Marc Carlson, Herve Pages, Nianhua Li November 19, 2013 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
Creating a New Annotation Package using SQLForge Marc Carlson, Herve Pages, Nianhua Li November 19, 2013 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
Package SNPchip. May 3, 2018
 Version 2.26.0 Title Visualizations for copy number alterations Package SNPchip May 3, 2018 Author Robert Scharpf and Ingo Ruczinski Maintainer Robert Scharpf Depends
Version 2.26.0 Title Visualizations for copy number alterations Package SNPchip May 3, 2018 Author Robert Scharpf and Ingo Ruczinski Maintainer Robert Scharpf Depends
Creating IGV HTML reports with tracktables
 Thomas Carroll 1 [1em] 1 Bioinformatics Core, MRC Clinical Sciences Centre; thomas.carroll (at)imperial.ac.uk June 13, 2018 Contents 1 The tracktables package.................... 1 2 Creating IGV sessions
Thomas Carroll 1 [1em] 1 Bioinformatics Core, MRC Clinical Sciences Centre; thomas.carroll (at)imperial.ac.uk June 13, 2018 Contents 1 The tracktables package.................... 1 2 Creating IGV sessions
Bioconductor: Annotation Package Overview
 Bioconductor: Annotation Package Overview April 30, 2018 1 Overview In its current state the basic purpose of annotate is to supply interface routines that support user actions that rely on the different
Bioconductor: Annotation Package Overview April 30, 2018 1 Overview In its current state the basic purpose of annotate is to supply interface routines that support user actions that rely on the different
The analysis of rtpcr data
 The analysis of rtpcr data Jitao David Zhang, Markus Ruschhaupt October 30, 2017 With the help of this document, an analysis of rtpcr data can be performed. For this, the user has to specify several parameters
The analysis of rtpcr data Jitao David Zhang, Markus Ruschhaupt October 30, 2017 With the help of this document, an analysis of rtpcr data can be performed. For this, the user has to specify several parameters
An Introduction to the methylumi package
 An Introduction to the methylumi package Sean Davis and Sven Bilke April 30, 2018 1 Introduction Gene expression patterns are very important in understanding any biologic system. The regulation of gene
An Introduction to the methylumi package Sean Davis and Sven Bilke April 30, 2018 1 Introduction Gene expression patterns are very important in understanding any biologic system. The regulation of gene
HowTo: Querying online Data
 HowTo: Querying online Data Jeff Gentry and Robert Gentleman November 12, 2017 1 Overview This article demonstrates how you can make use of the tools that have been provided for on-line querying of data
HowTo: Querying online Data Jeff Gentry and Robert Gentleman November 12, 2017 1 Overview This article demonstrates how you can make use of the tools that have been provided for on-line querying of data
MultipleAlignment Objects
 MultipleAlignment Objects Marc Carlson Bioconductor Core Team Fred Hutchinson Cancer Research Center Seattle, WA April 30, 2018 Contents 1 Introduction 1 2 Creation and masking 1 3 Analytic utilities 7
MultipleAlignment Objects Marc Carlson Bioconductor Core Team Fred Hutchinson Cancer Research Center Seattle, WA April 30, 2018 Contents 1 Introduction 1 2 Creation and masking 1 3 Analytic utilities 7
crlmm to downstream data analysis
 crlmm to downstream data analysis VJ Carey, B Carvalho March, 2012 1 Running CRLMM on a nontrivial set of CEL files To use the crlmm algorithm, the user must load the crlmm package, as described below:
crlmm to downstream data analysis VJ Carey, B Carvalho March, 2012 1 Running CRLMM on a nontrivial set of CEL files To use the crlmm algorithm, the user must load the crlmm package, as described below:
CQN (Conditional Quantile Normalization)
 CQN (Conditional Quantile Normalization) Kasper Daniel Hansen khansen@jhsph.edu Zhijin Wu zhijin_wu@brown.edu Modified: August 8, 2012. Compiled: April 30, 2018 Introduction This package contains the CQN
CQN (Conditional Quantile Normalization) Kasper Daniel Hansen khansen@jhsph.edu Zhijin Wu zhijin_wu@brown.edu Modified: August 8, 2012. Compiled: April 30, 2018 Introduction This package contains the CQN
INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis
 INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis Stefano de Pretis April 7, 20 Contents 1 Introduction 1 2 Quantification of Exon and Intron features 3 3 Time-course
INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis Stefano de Pretis April 7, 20 Contents 1 Introduction 1 2 Quantification of Exon and Intron features 3 3 Time-course
Djork-Arné Clevert and Andreas Mitterecker. Institute of Bioinformatics, Johannes Kepler University Linz
 Software Manual Institute of Bioinformatics, Johannes Kepler University Linz cn.farms: a latent variable model to detect copy number variations in microarray data with a low false discovery rate Manual
Software Manual Institute of Bioinformatics, Johannes Kepler University Linz cn.farms: a latent variable model to detect copy number variations in microarray data with a low false discovery rate Manual
How To Use GOstats and Category to do Hypergeometric testing with unsupported model organisms
 How To Use GOstats and Category to do Hypergeometric testing with unsupported model organisms M. Carlson October 30, 2017 1 Introduction This vignette is meant as an extension of what already exists in
How To Use GOstats and Category to do Hypergeometric testing with unsupported model organisms M. Carlson October 30, 2017 1 Introduction This vignette is meant as an extension of what already exists in
Analysis of screens with enhancer and suppressor controls
 Analysis of screens with enhancer and suppressor controls Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Analysis of screens with enhancer and suppressor controls Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
An Introduction to the methylumi package
 An Introduction to the methylumi package Sean Davis and Sven Bilke October 13, 2014 1 Introduction Gene expression patterns are very important in understanding any biologic system. The regulation of gene
An Introduction to the methylumi package Sean Davis and Sven Bilke October 13, 2014 1 Introduction Gene expression patterns are very important in understanding any biologic system. The regulation of gene
Introduction: microarray quality assessment with arrayqualitymetrics
 : microarray quality assessment with arrayqualitymetrics Audrey Kauffmann, Wolfgang Huber October 30, 2017 Contents 1 Basic use............................... 3 1.1 Affymetrix data - before preprocessing.............
: microarray quality assessment with arrayqualitymetrics Audrey Kauffmann, Wolfgang Huber October 30, 2017 Contents 1 Basic use............................... 3 1.1 Affymetrix data - before preprocessing.............
Introduction to the Codelink package
 Introduction to the Codelink package Diego Diez October 30, 2018 1 Introduction This package implements methods to facilitate the preprocessing and analysis of Codelink microarrays. Codelink is a microarray
Introduction to the Codelink package Diego Diez October 30, 2018 1 Introduction This package implements methods to facilitate the preprocessing and analysis of Codelink microarrays. Codelink is a microarray
Using crlmm for copy number estimation and genotype calling with Illumina platforms
 Using crlmm for copy number estimation and genotype calling with Illumina platforms Rob Scharpf November, Abstract This vignette illustrates the steps necessary for obtaining marker-level estimates of
Using crlmm for copy number estimation and genotype calling with Illumina platforms Rob Scharpf November, Abstract This vignette illustrates the steps necessary for obtaining marker-level estimates of
INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis
 INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis Stefano de Pretis October 30, 2017 Contents 1 Introduction.............................. 1 2 Quantification of
INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis Stefano de Pretis October 30, 2017 Contents 1 Introduction.............................. 1 2 Quantification of
SCnorm: robust normalization of single-cell RNA-seq data
 SCnorm: robust normalization of single-cell RNA-seq data Rhonda Bacher and Christina Kendziorski February 18, 2019 Contents 1 Introduction.............................. 1 2 Run SCnorm.............................
SCnorm: robust normalization of single-cell RNA-seq data Rhonda Bacher and Christina Kendziorski February 18, 2019 Contents 1 Introduction.............................. 1 2 Run SCnorm.............................
ExomeDepth. Vincent Plagnol. May 15, What is new? 1. 4 Load an example dataset 4. 6 CNV calling 5. 9 Visual display 9
 ExomeDepth Vincent Plagnol May 15, 2016 Contents 1 What is new? 1 2 What ExomeDepth does and tips for QC 2 2.1 What ExomeDepth does and does not do................................. 2 2.2 Useful quality
ExomeDepth Vincent Plagnol May 15, 2016 Contents 1 What is new? 1 2 What ExomeDepth does and tips for QC 2 2.1 What ExomeDepth does and does not do................................. 2 2.2 Useful quality
The REDseq user s guide
 The REDseq user s guide Lihua Julie Zhu * October 30, 2017 Contents 1 Introduction 1 2 Examples of using REDseq 2 2.1 Task 1: Build a RE map for a genome..................... 2 2.2 Task 2: Assign mapped
The REDseq user s guide Lihua Julie Zhu * October 30, 2017 Contents 1 Introduction 1 2 Examples of using REDseq 2 2.1 Task 1: Build a RE map for a genome..................... 2 2.2 Task 2: Assign mapped
NacoStringQCPro. Dorothee Nickles, Thomas Sandmann, Robert Ziman, Richard Bourgon. Modified: April 10, Compiled: April 24, 2017.
 NacoStringQCPro Dorothee Nickles, Thomas Sandmann, Robert Ziman, Richard Bourgon Modified: April 10, 2017. Compiled: April 24, 2017 Contents 1 Scope 1 2 Quick start 1 3 RccSet loaded with NanoString data
NacoStringQCPro Dorothee Nickles, Thomas Sandmann, Robert Ziman, Richard Bourgon Modified: April 10, 2017. Compiled: April 24, 2017 Contents 1 Scope 1 2 Quick start 1 3 RccSet loaded with NanoString data
Comprehensive Pipeline for Analyzing and Visualizing Array-Based CGH Data
 Comprehensive Pipeline for Analyzing and Visualizing Array-Based CGH Data Frederic Commo fredcommo@gmail.com November 30, 2017 1 Introduction Genomic profiling using array-based comparative genomic hybridization
Comprehensive Pipeline for Analyzing and Visualizing Array-Based CGH Data Frederic Commo fredcommo@gmail.com November 30, 2017 1 Introduction Genomic profiling using array-based comparative genomic hybridization
Making and Utilizing TxDb Objects
 Marc Carlson, Patrick Aboyoun, HervÃľ PagÃĺs, Seth Falcon, Martin Morgan October 30, 2017 1 Introduction The GenomicFeatures package retrieves and manages transcript-related features from the UCSC Genome
Marc Carlson, Patrick Aboyoun, HervÃľ PagÃĺs, Seth Falcon, Martin Morgan October 30, 2017 1 Introduction The GenomicFeatures package retrieves and manages transcript-related features from the UCSC Genome
Djork-Arné Clevert and Andreas Mitterecker. Institute of Bioinformatics, Johannes Kepler University Linz
 Software Manual Institute of Bioinformatics, Johannes Kepler University Linz cn.farms: a latent variable model to detect copy number variations in microarray data with a low false discovery rate Manual
Software Manual Institute of Bioinformatics, Johannes Kepler University Linz cn.farms: a latent variable model to detect copy number variations in microarray data with a low false discovery rate Manual
bayseq: Empirical Bayesian analysis of patterns of differential expression in count data
 bayseq: Empirical Bayesian analysis of patterns of differential expression in count data Thomas J. Hardcastle October 30, 2017 1 Introduction This vignette is intended to give a rapid introduction to the
bayseq: Empirical Bayesian analysis of patterns of differential expression in count data Thomas J. Hardcastle October 30, 2017 1 Introduction This vignette is intended to give a rapid introduction to the
Analyse RT PCR data with ddct
 Analyse RT PCR data with ddct Jitao David Zhang, Rudolf Biczok and Markus Ruschhaupt October 30, 2017 Abstract Quantitative real time PCR (qrt PCR or RT PCR for short) is a laboratory technique based on
Analyse RT PCR data with ddct Jitao David Zhang, Rudolf Biczok and Markus Ruschhaupt October 30, 2017 Abstract Quantitative real time PCR (qrt PCR or RT PCR for short) is a laboratory technique based on
MIGSA: Getting pbcmc datasets
 MIGSA: Getting pbcmc datasets Juan C Rodriguez Universidad Católica de Córdoba Universidad Nacional de Córdoba Cristóbal Fresno Instituto Nacional de Medicina Genómica Andrea S Llera Fundación Instituto
MIGSA: Getting pbcmc datasets Juan C Rodriguez Universidad Católica de Córdoba Universidad Nacional de Córdoba Cristóbal Fresno Instituto Nacional de Medicina Genómica Andrea S Llera Fundación Instituto
ssviz: A small RNA-seq visualizer and analysis toolkit
 ssviz: A small RNA-seq visualizer and analysis toolkit Diana HP Low Institute of Molecular and Cell Biology Agency for Science, Technology and Research (A*STAR), Singapore dlow@imcb.a-star.edu.sg August
ssviz: A small RNA-seq visualizer and analysis toolkit Diana HP Low Institute of Molecular and Cell Biology Agency for Science, Technology and Research (A*STAR), Singapore dlow@imcb.a-star.edu.sg August
M 3 D: Statistical Testing for RRBS Data Sets
 Tom Mayo Modified: 5 September, 2016. Compiled: October 30, 2018 Contents 1 Introduction 1 2 Analysis Pipeline 2 2.1 Reading in Data. 2 2.2 Computing the MMD, and coverage-mmd. 5 2.3 Generating p-values..
Tom Mayo Modified: 5 September, 2016. Compiled: October 30, 2018 Contents 1 Introduction 1 2 Analysis Pipeline 2 2.1 Reading in Data. 2 2.2 Computing the MMD, and coverage-mmd. 5 2.3 Generating p-values..
SIBER User Manual. Pan Tong and Kevin R Coombes. May 27, Introduction 1
 SIBER User Manual Pan Tong and Kevin R Coombes May 27, 2015 Contents 1 Introduction 1 2 Using SIBER 1 2.1 A Quick Example........................................... 1 2.2 Dealing With RNAseq Normalization................................
SIBER User Manual Pan Tong and Kevin R Coombes May 27, 2015 Contents 1 Introduction 1 2 Using SIBER 1 2.1 A Quick Example........................................... 1 2.2 Dealing With RNAseq Normalization................................
poplite vignette Daniel Bottomly and Beth Wilmot October 12, 2017
 poplite vignette Daniel Bottomly and Beth Wilmot October 12, 2017 1 Introduction Prior to utilizing a given database in a research context, it has to be designed, scripts written to format existing data
poplite vignette Daniel Bottomly and Beth Wilmot October 12, 2017 1 Introduction Prior to utilizing a given database in a research context, it has to be designed, scripts written to format existing data
Package dmrseq. September 14, 2018
 Type Package Package dmrseq September 14, 2018 Title Detection and inference of differentially methylated regions from Whole Genome Bisulfite Sequencing Version 1.1.15 Author Keegan Korthauer ,
Type Package Package dmrseq September 14, 2018 Title Detection and inference of differentially methylated regions from Whole Genome Bisulfite Sequencing Version 1.1.15 Author Keegan Korthauer ,
Using branchpointer for annotation of intronic human splicing branchpoints
 Using branchpointer for annotation of intronic human splicing branchpoints Beth Signal October 30, 2018 Contents 1 Introduction.............................. 1 2 Preparation..............................
Using branchpointer for annotation of intronic human splicing branchpoints Beth Signal October 30, 2018 Contents 1 Introduction.............................. 1 2 Preparation..............................
DupChecker: a bioconductor package for checking high-throughput genomic data redundancy in meta-analysis
 DupChecker: a bioconductor package for checking high-throughput genomic data redundancy in meta-analysis Quanhu Sheng, Yu Shyr, Xi Chen Center for Quantitative Sciences, Vanderbilt University, Nashville,
DupChecker: a bioconductor package for checking high-throughput genomic data redundancy in meta-analysis Quanhu Sheng, Yu Shyr, Xi Chen Center for Quantitative Sciences, Vanderbilt University, Nashville,
Package GOTHiC. November 22, 2017
 Title Binomial test for Hi-C data analysis Package GOTHiC November 22, 2017 This is a Hi-C analysis package using a cumulative binomial test to detect interactions between distal genomic loci that have
Title Binomial test for Hi-C data analysis Package GOTHiC November 22, 2017 This is a Hi-C analysis package using a cumulative binomial test to detect interactions between distal genomic loci that have
Cardinal design and development
 Kylie A. Bemis October 30, 2017 Contents 1 Introduction.............................. 2 2 Design overview........................... 2 3 iset: high-throughput imaging experiments............ 3 3.1 SImageSet:
Kylie A. Bemis October 30, 2017 Contents 1 Introduction.............................. 2 2 Design overview........................... 2 3 iset: high-throughput imaging experiments............ 3 3.1 SImageSet:
Analyze Illumina Infinium methylation microarray data
 Analyze Illumina Infinium methylation microarray data Pan Du, Gang Feng, Spencer Huang, Warren A. Kibbe, Simon Lin May 3, 2016 Genentech Inc., South San Francisco, CA, 94080, USA Biomedical Informatics
Analyze Illumina Infinium methylation microarray data Pan Du, Gang Feng, Spencer Huang, Warren A. Kibbe, Simon Lin May 3, 2016 Genentech Inc., South San Francisco, CA, 94080, USA Biomedical Informatics
Package CopywriteR. April 20, 2018
 Type Package Package CopywriteR April 20, 2018 Title Copy number information from targeted sequencing using off-target reads Version 2.11.0 Date 2016-02-23 Author Thomas Kuilman Maintainer Thomas Kuilman
Type Package Package CopywriteR April 20, 2018 Title Copy number information from targeted sequencing using off-target reads Version 2.11.0 Date 2016-02-23 Author Thomas Kuilman Maintainer Thomas Kuilman
Using the qrqc package to gather information about sequence qualities
 Using the qrqc package to gather information about sequence qualities Vince Buffalo Bioinformatics Core UC Davis Genome Center vsbuffalo@ucdavis.edu 2012-02-19 Abstract Many projects in bioinformatics
Using the qrqc package to gather information about sequence qualities Vince Buffalo Bioinformatics Core UC Davis Genome Center vsbuffalo@ucdavis.edu 2012-02-19 Abstract Many projects in bioinformatics
The DMRcate package user s guide
 The DMRcate package user s guide Peters TJ, Buckley MJ, Statham A, Pidsley R, Clark SJ, Molloy PL March 31, 2017 Summary DMRcate extracts the most differentially methylated regions (DMRs) and variably
The DMRcate package user s guide Peters TJ, Buckley MJ, Statham A, Pidsley R, Clark SJ, Molloy PL March 31, 2017 Summary DMRcate extracts the most differentially methylated regions (DMRs) and variably
qplexanalyzer Matthew Eldridge, Kamal Kishore, Ashley Sawle January 4, Overview 1 2 Import quantitative dataset 2 3 Quality control 2
 qplexanalyzer Matthew Eldridge, Kamal Kishore, Ashley Sawle January 4, 2019 Contents 1 Overview 1 2 Import quantitative dataset 2 3 Quality control 2 4 Data normalization 8 5 Aggregation of peptide intensities
qplexanalyzer Matthew Eldridge, Kamal Kishore, Ashley Sawle January 4, 2019 Contents 1 Overview 1 2 Import quantitative dataset 2 3 Quality control 2 4 Data normalization 8 5 Aggregation of peptide intensities
An Introduction to Bioconductor s ExpressionSet Class
 An Introduction to Bioconductor s ExpressionSet Class Seth Falcon, Martin Morgan, and Robert Gentleman 6 October, 2006; revised 9 February, 2007 1 Introduction Biobase is part of the Bioconductor project,
An Introduction to Bioconductor s ExpressionSet Class Seth Falcon, Martin Morgan, and Robert Gentleman 6 October, 2006; revised 9 February, 2007 1 Introduction Biobase is part of the Bioconductor project,
fastseg An R Package for fast segmentation Günter Klambauer and Andreas Mitterecker Institute of Bioinformatics, Johannes Kepler University Linz
 Software Manual Institute of Bioinformatics, Johannes Kepler University Linz fastseg An R Package for fast segmentation Günter Klambauer and Andreas Mitterecker Institute of Bioinformatics, Johannes Kepler
Software Manual Institute of Bioinformatics, Johannes Kepler University Linz fastseg An R Package for fast segmentation Günter Klambauer and Andreas Mitterecker Institute of Bioinformatics, Johannes Kepler
Getting started with flowstats
 Getting started with flowstats F. Hahne, N. Gopalakrishnan October, 7 Abstract flowstats is a collection of algorithms for the statistical analysis of flow cytometry data. So far, the focus is on automated
Getting started with flowstats F. Hahne, N. Gopalakrishnan October, 7 Abstract flowstats is a collection of algorithms for the statistical analysis of flow cytometry data. So far, the focus is on automated
Analysis of Pairwise Interaction Screens
 Analysis of Pairwise Interaction Screens Bernd Fischer April 11, 2014 Contents 1 Introduction 1 2 Installation of the RNAinteract package 1 3 Creating an RNAinteract object 2 4 Single Perturbation effects
Analysis of Pairwise Interaction Screens Bernd Fischer April 11, 2014 Contents 1 Introduction 1 2 Installation of the RNAinteract package 1 3 Creating an RNAinteract object 2 4 Single Perturbation effects
Prepare input data for CINdex
 1 Introduction Prepare input data for CINdex Genomic instability is known to be a fundamental trait in the development of tumors; and most human tumors exhibit this instability in structural and numerical
1 Introduction Prepare input data for CINdex Genomic instability is known to be a fundamental trait in the development of tumors; and most human tumors exhibit this instability in structural and numerical
PROPER: PROspective Power Evaluation for RNAseq
 PROPER: PROspective Power Evaluation for RNAseq Hao Wu [1em]Department of Biostatistics and Bioinformatics Emory University Atlanta, GA 303022 [1em] hao.wu@emory.edu October 30, 2017 Contents 1 Introduction..............................
PROPER: PROspective Power Evaluation for RNAseq Hao Wu [1em]Department of Biostatistics and Bioinformatics Emory University Atlanta, GA 303022 [1em] hao.wu@emory.edu October 30, 2017 Contents 1 Introduction..............................
Simulation of molecular regulatory networks with graphical models
 Simulation of molecular regulatory networks with graphical models Inma Tur 1 inma.tur@upf.edu Alberto Roverato 2 alberto.roverato@unibo.it Robert Castelo 1 robert.castelo@upf.edu 1 Universitat Pompeu Fabra,
Simulation of molecular regulatory networks with graphical models Inma Tur 1 inma.tur@upf.edu Alberto Roverato 2 alberto.roverato@unibo.it Robert Castelo 1 robert.castelo@upf.edu 1 Universitat Pompeu Fabra,
Preliminary Figures for Renormalizing Illumina SNP Cell Line Data
 Preliminary Figures for Renormalizing Illumina SNP Cell Line Data Kevin R. Coombes 17 March 2011 Contents 1 Executive Summary 1 1.1 Introduction......................................... 1 1.1.1 Aims/Objectives..................................
Preliminary Figures for Renormalizing Illumina SNP Cell Line Data Kevin R. Coombes 17 March 2011 Contents 1 Executive Summary 1 1.1 Introduction......................................... 1 1.1.1 Aims/Objectives..................................
mocluster: Integrative clustering using multiple
 mocluster: Integrative clustering using multiple omics data Chen Meng Modified: June 03, 2015. Compiled: October 30, 2018. Contents 1 mocluster overview......................... 1 2 Run mocluster............................
mocluster: Integrative clustering using multiple omics data Chen Meng Modified: June 03, 2015. Compiled: October 30, 2018. Contents 1 mocluster overview......................... 1 2 Run mocluster............................
Evaluation of VST algorithm in lumi package
 Evaluation of VST algorithm in lumi package Pan Du 1, Simon Lin 1, Wolfgang Huber 2, Warrren A. Kibbe 1 December 22, 2010 1 Robert H. Lurie Comprehensive Cancer Center Northwestern University, Chicago,
Evaluation of VST algorithm in lumi package Pan Du 1, Simon Lin 1, Wolfgang Huber 2, Warrren A. Kibbe 1 December 22, 2010 1 Robert H. Lurie Comprehensive Cancer Center Northwestern University, Chicago,
GxE.scan. October 30, 2018
 GxE.scan October 30, 2018 Overview GxE.scan can process a GWAS scan using the snp.logistic, additive.test, snp.score or snp.matched functions, whereas snp.scan.logistic only calls snp.logistic. GxE.scan
GxE.scan October 30, 2018 Overview GxE.scan can process a GWAS scan using the snp.logistic, additive.test, snp.score or snp.matched functions, whereas snp.scan.logistic only calls snp.logistic. GxE.scan
GenoGAM: Genome-wide generalized additive
 GenoGAM: Genome-wide generalized additive models Georg Stricker 1, Julien Gagneur 1 [1em] 1 Technische Universität München, Department of Informatics, Garching, Germany Abstract April 30, 2018 Many genomic
GenoGAM: Genome-wide generalized additive models Georg Stricker 1, Julien Gagneur 1 [1em] 1 Technische Universität München, Department of Informatics, Garching, Germany Abstract April 30, 2018 Many genomic
Raman Spectra of Chondrocytes in Cartilage: hyperspec s chondro data set
 Raman Spectra of Chondrocytes in Cartilage: hyperspec s chondro data set Claudia Beleites CENMAT and DI3, University of Trieste Spectroscopy Imaging, IPHT Jena e.v. February 13,
Raman Spectra of Chondrocytes in Cartilage: hyperspec s chondro data set Claudia Beleites CENMAT and DI3, University of Trieste Spectroscopy Imaging, IPHT Jena e.v. February 13,
rhdf5 - HDF5 interface for R
 Bernd Fischer October 30, 2017 Contents 1 Introduction 1 2 Installation of the HDF5 package 2 3 High level R -HDF5 functions 2 31 Creating an HDF5 file and group hierarchy 2 32 Writing and reading objects
Bernd Fischer October 30, 2017 Contents 1 Introduction 1 2 Installation of the HDF5 package 2 3 High level R -HDF5 functions 2 31 Creating an HDF5 file and group hierarchy 2 32 Writing and reading objects
An Introduction to the GenomicRanges Package
 Marc Carlson, Patrick Aboyoun, Hervé Pagès, and Martin Morgan October 30, 2017; updated 16 November, 2016 Contents 1 Introduction.............................. 1 2 GRanges: Genomic Ranges....................
Marc Carlson, Patrick Aboyoun, Hervé Pagès, and Martin Morgan October 30, 2017; updated 16 November, 2016 Contents 1 Introduction.............................. 1 2 GRanges: Genomic Ranges....................
Managing and analyzing multiple NGS samples with Bioconductor bamviews objects: application to RNA-seq
 Managing and analyzing multiple NGS samples with Bioconductor bamviews objects: application to RNA-seq VJ Carey April 4, 13 Contents 1 Introduction 2 2 Basic design 2 3 Illustration 2 4 Comparative counts
Managing and analyzing multiple NGS samples with Bioconductor bamviews objects: application to RNA-seq VJ Carey April 4, 13 Contents 1 Introduction 2 2 Basic design 2 3 Illustration 2 4 Comparative counts
genefu: a package for breast cancer gene expression analysis
 genefu: a package for breast cancer gene expression analysis Benjamin Haibe-Kains 1, Markus Schröder 2, Christos Sotiriou 3, Gianluca Bontempi 4, and John Quackenbush 5,6 1 Bioinformatics and Computational
genefu: a package for breast cancer gene expression analysis Benjamin Haibe-Kains 1, Markus Schröder 2, Christos Sotiriou 3, Gianluca Bontempi 4, and John Quackenbush 5,6 1 Bioinformatics and Computational
Sparse Model Matrices
 Sparse Model Matrices Martin Maechler R Core Development Team maechler@r-project.org July 2007, 2008 (typeset on April 6, 2018) Introduction Model matrices in the very widely used (generalized) linear
Sparse Model Matrices Martin Maechler R Core Development Team maechler@r-project.org July 2007, 2008 (typeset on April 6, 2018) Introduction Model matrices in the very widely used (generalized) linear
Package GreyListChIP
 Type Package Version 1.14.0 Package GreyListChIP November 2, 2018 Title Grey Lists -- Mask Artefact Regions Based on ChIP Inputs Date 2018-01-21 Author Gord Brown Maintainer Gordon
Type Package Version 1.14.0 Package GreyListChIP November 2, 2018 Title Grey Lists -- Mask Artefact Regions Based on ChIP Inputs Date 2018-01-21 Author Gord Brown Maintainer Gordon
Package RAPIDR. R topics documented: February 19, 2015
 Package RAPIDR February 19, 2015 Title Reliable Accurate Prenatal non-invasive Diagnosis R package Package to perform non-invasive fetal testing for aneuploidies using sequencing count data from cell-free
Package RAPIDR February 19, 2015 Title Reliable Accurate Prenatal non-invasive Diagnosis R package Package to perform non-invasive fetal testing for aneuploidies using sequencing count data from cell-free
An Introduction to the genoset Package
 An Introduction to the genoset Package Peter M. Haverty April 4, 2013 Contents 1 Introduction 2 1.1 Creating Objects........................................... 2 1.2 Accessing Genome Information...................................
An Introduction to the genoset Package Peter M. Haverty April 4, 2013 Contents 1 Introduction 2 1.1 Creating Objects........................................... 2 1.2 Accessing Genome Information...................................
Fitting Baselines to Spectra
 Fitting Baselines to Spectra Claudia Beleites DIA Raman Spectroscopy Group, University of Trieste/Italy (2005 2008) Spectroscopy Imaging, IPHT, Jena/Germany (2008 2016) ÖPV, JKI,
Fitting Baselines to Spectra Claudia Beleites DIA Raman Spectroscopy Group, University of Trieste/Italy (2005 2008) Spectroscopy Imaging, IPHT, Jena/Germany (2008 2016) ÖPV, JKI,
