Quality Data Model (QDM)
|
|
|
- Dwayne Welch
- 6 years ago
- Views:
Transcription
1 Quality Data Model (QDM) Overview Document National Quality Forum 4/20/2011
2 QUALITY DATA MODEL (QDM): OVERVIEW TABLE OF CONTENTS Quality Data Model (QDM): Overview... 2 National Quality Forum: Overview and Goals... 3 Performance Measurement: Information Needs and the Quality Data Model... 3 Electronic Measures (emeasures) and QDM s Role... 4 Quality Data Model: Status and the Public Comment Process... 5 Quality Data Model: The Full Description, Specificity, and Technical Detail... 6 Figure 1. QDM Composition Diagram... 7 Figure 2. QDM Element Structure... 8 Figure 3. QDM Use of Value Sets Table 1. QDM Concepts Table 2. QDM States Figure 4: Timing Attribute Figure 5: Data Flow Attribute Figure 6. Actor Attribute Figure 7. Concept Specific Attribute Table 3. QDM Concepts and Specific Attributes April 20, 2011 Quality Data Model Overview pg. 2
3 National Quality Forum: Overview and Goals The National Quality Forum (NQF) is a nonprofit organization that operates under a three-part mission to improve the quality of American healthcare by: building consensus on national priorities and goals for performance improvement and working in partnership to achieve them; endorsing national consensus standards for measuring and publicly reporting on performance; and promoting the attainment of national goals through education and outreach programs. NQF drives improvements in care by rigorously endorsing evidence-based measures of performance focusing on measurement for accountability and quality improvement. Measurement has the greatest impact on quality when it supports transparency and public reporting, but it also provides information to help clinicians make improvements in care delivery. To date, quality measurement and public reporting have been thought of as secondary data uses rather than as drivers of care. By setting standardized performance measures and properly designing and building health IT, however, it will now be possible to capture performance data as part of the care process and provide immediate information feedback and clinical decision support to clinicians to improve care. Designing and building health IT to support performance improvement requires close collaboration between the quality and health IT communities. NQF plays a key role in the quality community as the national standard-endorsing body for performance measures and as a neutral convener of multiple stakeholders to set National Priorities for improvement and drive quality improvement. Performance Measurement: Information Needs and the Quality Data Model Collecting and reporting accurate, comparative healthcare performance data is a complex and largely time-consuming and manual process. Much of the information required to measure performance is collected in the process of routine clinical care and is available in electronic health records (EHRs) and other clinical data sources. It has not, however, been routinely available for export and use for reporting or performance measurement. Performance measures are most frequently developed based on routinely available sources of data and therefore are often based on claims and clinically enriched administrative data. Taking advantage of comprehensive clinical data contained in EHRs and other clinical applications, including personal health records (PHRs) requires that measures are specified to account for the way data are expressed in such products. April 20, 2011 Quality Data Model Overview pg. 3
4 NQF, through the Health Information Technology Expert Panel (HITEP), a committee of health IT industry experts, established the Quality Data Model (QDM) to enable such expression of data criteria for measurement to address data that are available in EHRs and other clinical information sources. QDM s development was based on a request by the American Health Information Community (AHIC) and the Office of the National Coordinator for Health Information Technology (ONC), with funding from the Agency for Healthcare Research and Quality (AHRQ). The QDM (formerly referred to as the Quality Data Set, or QDS) is an information model that clearly defines concepts used in quality measures and clinical care and is intended to enable automation of structured data capture in EHRs, PHRs, and other clinical applications. It provides a way to describe clinical concepts in a standardized format so individuals (i.e., providers, researchers, or measure developers) monitoring clinical performance and outcomes can clearly and concisely communicate necessary information. The QDM also describes information so EHR and other clinical electronic system vendors can consistently interpret and easily locate the data required. The QDM provides the potential for more precisely defined, universally adopted electronic quality measures to automate measurement and compare and improve quality using electronic health information. Use of the QDM will enable more standardized, less-burdensome quality measurement and reporting and more consistent use and communication of EHRs for direct patient care. The QDM is described in detail in the Health Information Technology Expert Panel Report: Recommended Common Data Types and Prioritized Performance Measures for Electronic Healthcare Information Systems. The model was further clarified and described in a second report, Health Information Technology Automation of Quality Measurement: Quality Data Set and Data Flow. Electronic Measures (emeasures) and QDM s Role During the last 18 months, NQF and many measure stewards have been involved in efforts to rapidly convert, or retool, existing measures for use on an electronic platform. As part of the Department of Health and Human Services (HHS) contract, NQF has worked with measure stewards to retool an initial set of more than 100 performance measures.many of these are being used for the Health Information Technology for Economic and Clinical Health Act (HITECH) incentive payments linked to meaningful use of EHRs. In a recent child health quality measures project, NQF received, for the first time, measures submitted with EHR specifications. NQF has been laying the groundwork for the endorsement of emeasures for some time. The Testing Task Force Report released in 2011 specifies requirements for testing new and retooled e-measures. The QDM is an essential building block of both performance measures and EHRs. April 20, 2011 Quality Data Model Overview pg. 4
5 With support from HHS, NQF subcontracted with the IFMC to develop a Measure Authoring Tool that will help developers generate specifications for emeasures in a consistent fashion. The tool is expected to be publicly available to measure developers starting in fall Quality Data Model: Status and the Public Comment Process To ensure QDM s continued value and use, NQF will enhance and update it as needed in response to evolving quality measurement needs. The QDM Version 2.1 was published for public comment in September The current version, QDM 3.0, contains updates based on feedback from the comment period as well as from information learned in applying the QDM to retooling more than 100 measures in This new version has a number of modifications, including: 1. A more hierarchical structure has been implemented. 2. A more detailed set of information about each QDM element has been included to allow a more complete description of information required. 3. A method for describing data relationships enabling more expressive measure criteria has been employed. 4. Standard categories are now referred to as concepts. 5. States are subdivided into states of action or states of being. (States of action are present-tense verbs, and states of being are nouns.) 6. The terminology and definitions of some concepts, states, and attributes has shifted in some cases to make these components more applicable and reusable. For example, allergy is now a concept to allow clearer description of information requirements rather than its prior use as a context of medications or substances. A new concept has also been added to enable measurement of the use of health IT as part of care coordination, the health record component. This new concept is derived from the Health IT Assessment Framework published in Data type previously was used to combine a concept (referred to as standard category ) and its context of use. For example, the data type Diagnostic study order now is referenced by the concept Diagnostic study and the state in which it is expected, for example, order. This change allows states to be more interchangeable. 8. Data types context components are now referred to as states. 9. Each concept has a defined list of states (states of action and/or states of being) that can be used to describe how it is used within a measure statement. To see the full list of 1 NQF, Expert Panel Report: Driving Quality: A Health IT Assessment Framework for Measurement, 2010, Washington, DC: NQF; Available at: _A_Health_IT_Assessment_Framework_for_Measurement.aspx. Last accessed April April 20, 2011 Quality Data Model Overview pg. 5
6 concepts and states, refer to the list below and more complete detail in the Appendix under QDM Concepts and States. Individuals can submit public comments on the most current QDM version on a continual basis. NQF will use relevant elements of its Consensus Development Process to receive and review comments on the most current version of the QDM. NQF s Health Information Technology Advisory Committee (HITAC), in collaboration with NQF staff, will regularly review public comments on the QDM. Based on the findings and HITAC recommendations, the QDM will be updated and new versions released as needed. Each new version of the QDM will be posted to the NQF website and will then be available for public comment. The next version of the QDM will be posted for public comment at the end of April 2011 and released in early summer. Quality Data Model: The Full Description, Specificity, and Technical Detail The QDM is a model of information used to express patient, clinical, and community characteristics as well as the basic logic required to express quality measure criteria. Measure specifications include population, denominator, numerator, exclusion, and optionally risk adjustment criteria. The QDM describes the data elements and the states, or contexts in which the data elements are expected to exist in clinical information systems (see Figure 1, below). As such, coordination with standards is required to ensure the information is clear, unambiguous, consistent, and accurate. Readers interested in understanding more of QDM s technical details and specifications (e.g., expression language, relative timing) should refer to the companion document, QDM Technical Specification. April 20, 2011 Quality Data Model Overview pg. 6
7 Quality Data Model: QDM Element Structure Example of a Performance Measure Phrase Diagnosis active: Asthma Starts before or during Encounter Office & Outpatient Consult QDM Element Comparator QDM Element Consists of a Concept Has many Has an Has an State Has many Attribute Relevant Attributes Relevant Attributes Instance Is defined by a Is defined by a OR Which contains each of the following Type(s) of Attributes OR State of Action Timing Codesets Codesets Code List Code List Grouping State of Being Actor Codesets Codesets Is defined by an Is defined by many Data Flow Codesets Codesets Allowable Taxonomy Code Lists Relevant Attributes Relevant Attributes Each of which Is defined by an Concept Specific Codesets (OPTIONAL) Codesets Allowable Taxonomy Relevant Attributes Relevant Attributes Figure 1. QDM Composition Diagram The diagram depicts the direction and interrelationship between the various QDM element components (i.e., concepts, states, and attributes.) The left side of the diagram shows the definition of a QDM element. This begins with defining a concept for which each use April 20, 2011 Quality Data Model Overview pg. 7
8 has an instance (or specific use), which in turn is defined by a value set. Value sets may be individual or comprised of child value sets. An example of child value sets is provided in Figure 3. Each value set is defined by a taxonomy that is based on established standards for clinical system use and interoperability. The middle section of the diagram shows the application of a state to the defined instance of a concept. States may be actions (states of action) or indicate the existence of a specific concept instance (states of being). The concept-state pair comprises the QDM element, which can be further described using the elements on the right of the diagram the attributes. Attributes include four basic categories: timing, actor, data flow, and concept-specific. Greater detail is provided in Figures 4, 5, 6, and 7. Enhancements are incorporated into the QDM to enable expanding concepts of measurement. This helps to ensure the QDM covers data required to evaluate care coordination across venues of care, patient, and family engagement in care and longitudinal outcomes. These QDM elements are used in different contexts, depending on the measure (see Figure 2, below). For example, one measure may assess if a lab test was ordered, while another may assess if it was performed, and a third may compare the actual lab result to a guideline threshold or the amount of change in the result over time. Figure 2. QDM Element Structure The components of a QDM element are shown in the figure. The figure on the left identifies the terms used for each component of the QDM element. The figure on the right uses each of these components to describe a QDM element indicating Medication, administer aspirin. Each QDM element is composed of a concept, the state in which that concept is expected to be used and a value set of codes in a defined taxonomy to specify which instance of the concept is expected. The boxes in the lower section of the QDM element specify individual attributes, or additional data to April 20, 2011 Quality Data Model Overview pg. 8
9 describe the QDM element. Attributes include: timing, actor, data flow, and concept-specific. More detail about each of these QDM components is provided in the text. Figure 3. QDM Use of Value Sets This figure shows three QDM elements, each of which uses value sets. The figure on the left shows a value set for medication (aspirin) that includes a single set of codes using a single taxonomy. The middle figure shows the instance diabetes of the concept diagnosis. In the middle figure the value set provided is a parent value set composed of three child value sets, one each using the taxonomies SNOMED-CT, ICD-9-CM, and ICD-10-CM, respectively. In this case the parent value set indicates the same concept instance but expressed in different taxonomies. The figure on the right provides a parent value set titled ACEI/ARB* comprised of two child value sets, each in the same taxonomy (RxNorm). This example uses a parent value set for convenience, expressing a combination of two different concept instances (both ACEI and ARB medications). *ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker. April 20, 2011 Quality Data Model Overview pg. 9
10 1. QDM Concepts and States Table: The following table lists all the QDM concepts and states. More details on the possibilities of each concept and state and the relationships among all concepts and all states are included in the companion document, QDM Technical Specifications. The QDM element (concept, state, value set, and attributes) comprise the atomic data expression that is used to specify data criteria required for the quality measure. Table 1. QDM Concepts QDM Concepts 1. Allergy 2. Characteristics 3. Communication 4. Condition/Diagnosis/Problem 5. Device 6. Diagnostic Study 7. Encounter 8. Experience 9. Family History 10. Functional Status 11. Health Record Component 12. Intervention 13. Intolerance 14. Laboratory Test 15. Medication 16. Physical Exam 17. Preference 18. Procedure 19. Risk Evaluation 20. Substance 21. Symptom 22. System Resources 23. Transfer April 20, 2011 Quality Data Model Overview pg. 10
11 Table 2. QDM States QDM States QDM State of QDM State of Action Being Access 1. Active 2. Acknowledge 2. Inactive 3. Administer 3. Resolved 4. Alert 5. Apply 6. Assess 7. Calculate 8. Create 9. Decline 10. Discontinue 11. Dispense 12. Document 13. Implement 14. Notify 15. Order 16. Perform 17. Plan 18. Receive 19. Recommend 20. Reconcile 21. Record 22. Remind 23. Report 24. Request 25. Review 26. Stratify 27. Transmit 28. Update April 20, 2011 Quality Data Model Overview pg. 11
12 Attributes QDM Element attributes include four basic categories: timing, actor, data flow, and conceptspecific. Greater detail is provided in Figures 4, 5, 6, and 7. Figure 4. Timing Attribute: The timing attribute indicates the time of occurrence, including whether the beginning or end of a process is the time of interest. Timing provides the process context for the QDM element. Figure 5. Data Flow Attribute: The data flow attribute allows specification of a specific sender or receiver of a transaction, enabling expression of criteria that a specific health care component is shared by a clinician (sender) with a patient (receiver). April 20, 2011 Quality Data Model Overview pg. 12
13 Figure 6. Actor Attribute: The actor attribute allows the measure developer to define the expected source, recorder, and subject for each QDM element; thus, it is possible to specify data only derived and recorded by devices, patients, or clinicians. Figure 7. Concept-Specific Attribute: Concept-specific attributes are listed separately in Table 3 of this document. April 20, 2011 Quality Data Model Overview pg. 13
14 Table 3. QDM Concepts and Specific Attributes: This table provides detail on the specific relationships between the attributes and concepts. The individual concept-specific attributes are provided as headers for each column. The concepts define the rows. Concept-specific attributes that apply to each concept are depicted with an x in the applicable columns. Full descriptions of the concepts and attributes are provided in the QDM Technical Specification (glossary section) companion document. Concept (From Anatomical Environmental Facility Health Record Version X.X) structure Dosage location location Field Laterality Ordinality Reason Result Route Severity Status 1. Allergy x x x 2. Characteristics x x 3. Communication x x x 4. Condition/ x Diagnosis/ Problem x X X x x 5. Device x x X X x 6. Diagnostic x study X x X x x 7. Encounter x X x x 8. Experience x 9. Family History x x 10. Functional x Status 11. Health record component x x X x x x x X 12. Intervention x x x x X X x x 13. Intolerance x x x 14. Laboratory test x x x x x x x 15. Medication x x x 16. Physical Exam x x x x x x x 17. Preference x x 18. Procedure x x x x x X x x x 19. Risk evaluation x x x x 20. Substance x x x 21. Symptom x x x x x X x x April 20, 2011 Quality Data Model Overview pg. 14
15 Concept (From Anatomical Environmental Facility Health Record Version X.X) structure Dosage location location Field Laterality Ordinality Reason Result Route Severity Status 22. System resources x x x x 23. Transfer x x x x April 20, 2011 Quality Data Model Overview pg. 15
16 The companion document, QDM Technical Specification, provides greater detail on the technical issues and specifications (e.g., expression language, relative timing) associated with the QDM. This document includes the following: QDM Glossary: Provides detailed definitions with relevant examples for all terms and components of the QDM. QDM Relative Timings Functions and Operators: Describes the interactions of QDM components related to the: 1) relative timing, 2) operator, or 3) function. Relative timings allow a measure developer to describe timing relationships among individual QDM elements. Combining the QDM elements in this way allows the measure developer to create phrases that can add meaning to the individual elements. Operators allow measure developers to compare two or more QDM elements logically or mathematically. Functions use a QDM element as an input and return a calculation based on that input. QDM Mapping of Concepts to States: Provides detail on the possibilities of each concept and state and describes the relationships among all concepts and all states (e.g., every state that corresponds with diagnosis). April 20, 2011 Quality Data Model Overview pg. 16
HITSP/IS06. January 25, 2010 Version 2.0. Healthcare Information Technology Standards Panel. Population Perspective Technical Committee.
 January 25, 2010 Version 2.0 HITSP/IS06 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Technical Committee DOCUMENT CHANGE HISTORY Version Number Description of Change Name
January 25, 2010 Version 2.0 HITSP/IS06 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Technical Committee DOCUMENT CHANGE HISTORY Version Number Description of Change Name
Terminology Harmonization
 Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
HEALTH INITIATIVES CONSULTING, INC. Maintaining Data Mapping in i2itracks
 HEALTH INITIATIVES CONSULTING, INC. Healthcare Transformation, Redesigned! Your Partner in Data Stewardship Maintaining Data Mapping in i2itracks Data Stewardship is a process of establishing and maintaining
HEALTH INITIATIVES CONSULTING, INC. Healthcare Transformation, Redesigned! Your Partner in Data Stewardship Maintaining Data Mapping in i2itracks Data Stewardship is a process of establishing and maintaining
User Manual. phr.mtbc.com
 User Manual Table of Contents Introduction Appointments Appointment History Claims History CCDA Report Demographics Health History Lab Reports Online Payment Secure Messages Health Recommendation Patient
User Manual Table of Contents Introduction Appointments Appointment History Claims History CCDA Report Demographics Health History Lab Reports Online Payment Secure Messages Health Recommendation Patient
Data Quality Attributes
 Data Quality Improvement Unit 11.2b: Data Quality Attributes 1 Data Quality Attributes 11 2 Consistency of Data Value of the data should be reliable and the same across applications 3 1 Consistency of
Data Quality Improvement Unit 11.2b: Data Quality Attributes 1 Data Quality Attributes 11 2 Consistency of Data Value of the data should be reliable and the same across applications 3 1 Consistency of
April 25, Dear Secretary Sebelius,
 April 25, 2014 Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2015 Edition EHR Standards and Certification Criteria Proposed Rule
April 25, 2014 Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2015 Edition EHR Standards and Certification Criteria Proposed Rule
HITSP/T16. October 15, 2007 Version 1.1. Healthcare Information Technology Standards Panel. Security and Privacy Technical Committee.
 October 15, 2007 Version 1.1 HITSP/T16 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Security and Privacy Technical Committee 20071015 V1.1 D O C U M E N T C H A N G E H
October 15, 2007 Version 1.1 HITSP/T16 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Security and Privacy Technical Committee 20071015 V1.1 D O C U M E N T C H A N G E H
NIST Normative Test Process Document: e-prescribing (erx) Test Tool
 NIST Normative Test Process Document: e-prescribing (erx) Test Tool Test Tool and Test Descriptions to Conduct ONC 2015 Edition Certification Version 1.7 Date: December 3, 2015 Developed by the National
NIST Normative Test Process Document: e-prescribing (erx) Test Tool Test Tool and Test Descriptions to Conduct ONC 2015 Edition Certification Version 1.7 Date: December 3, 2015 Developed by the National
Certification Commission for Healthcare Information Technology. CCHIT A Catalyst for EHR Adoption
 Certification Commission for Healthcare Information Technology CCHIT A Catalyst for EHR Adoption Alisa Ray, Executive Director, CCHIT Sarah Corley, MD, Chief Medical Officer, NextGen Healthcare Systems;
Certification Commission for Healthcare Information Technology CCHIT A Catalyst for EHR Adoption Alisa Ray, Executive Director, CCHIT Sarah Corley, MD, Chief Medical Officer, NextGen Healthcare Systems;
Information Technology (CCHIT): Report on Activities and Progress
 Certification Commission for Healthcare Information Technology Certification Commission for Healthcare Information Technology (CCHIT): Report on Activities and Progress Mark Leavitt, MD, PhD Chair, CCHIT
Certification Commission for Healthcare Information Technology Certification Commission for Healthcare Information Technology (CCHIT): Report on Activities and Progress Mark Leavitt, MD, PhD Chair, CCHIT
Diabetes Data Strategy Project ( Diabe-DS ) Overview and Status Update May 2010
 Diabetes Data Strategy Project ( Diabe-DS ) Overview and Status Update May 2010 Uses of Data Have Significant Overlap Premise of project: Develop a process to identify a common set of data elements in
Diabetes Data Strategy Project ( Diabe-DS ) Overview and Status Update May 2010 Uses of Data Have Significant Overlap Premise of project: Develop a process to identify a common set of data elements in
Overview of the Multi-Payer Claims Database (MPCD)
 Overview of the Multi-Payer Claims Database (MPCD) Genesis of the MPCD The MPCD project is one of a number of initiatives related to comparative effectiveness research (CER) funded by the American Recovery
Overview of the Multi-Payer Claims Database (MPCD) Genesis of the MPCD The MPCD project is one of a number of initiatives related to comparative effectiveness research (CER) funded by the American Recovery
Meaningful Use and the 3M Healthcare Data Dictionary
 Meaningful Use and the 3M Healthcare Data Dictionary Now, a few years into the HITECH Act, healthcare organizations and providers are discovering that EHRs and standard terminologies may not fully meet
Meaningful Use and the 3M Healthcare Data Dictionary Now, a few years into the HITECH Act, healthcare organizations and providers are discovering that EHRs and standard terminologies may not fully meet
Meaningful Use Setup Guide
 Meaningful Use Setup Guide Table of Contents ChiroWrite Certified Settings... 3 Exports... 3 Dr. First... 4 Imports... 4 Microsoft HealthVault... 5 Additional Settings... 7 CPOE... 7 Orders... 8 Order
Meaningful Use Setup Guide Table of Contents ChiroWrite Certified Settings... 3 Exports... 3 Dr. First... 4 Imports... 4 Microsoft HealthVault... 5 Additional Settings... 7 CPOE... 7 Orders... 8 Order
EZ-Suite Interfacing Applications. Jeremy Powell (Chief Growth Officer, Citra Health Solutions) Rini Jose (Product Manager, Citra Health Solutions)
 EZ-Suite Interfacing Applications Jeremy Powell (Chief Growth Officer, Citra Health Solutions) Rini Jose (Product Manager, Citra Health Solutions) Agenda Items CITRA suite Current External Integrations
EZ-Suite Interfacing Applications Jeremy Powell (Chief Growth Officer, Citra Health Solutions) Rini Jose (Product Manager, Citra Health Solutions) Agenda Items CITRA suite Current External Integrations
Pennsylvania PROMISe Companion Guide
 Pennsylvania Companion Guide Unsolicited 277 Claim Response Version 5010 September 2010 Version 1 Pennsylvania PROMISe Unsolicited 277 Claim Companion Guide This page intentionally left blank. September
Pennsylvania Companion Guide Unsolicited 277 Claim Response Version 5010 September 2010 Version 1 Pennsylvania PROMISe Unsolicited 277 Claim Companion Guide This page intentionally left blank. September
Document Number: HITSP 08 N 378 Date: December 17, 2008 Report from the HITSP Education, Communication and Outreach (HITSP-ECO) Committee
 0 Document Number: HITSP 08 N 378 Date: December 17, 2008 Report from the HITSP Education, Communication and Outreach (HITSP-ECO) Committee Co-Chairs: Walter G. Suarez, MD, Institute for HIPAA/HIT Education
0 Document Number: HITSP 08 N 378 Date: December 17, 2008 Report from the HITSP Education, Communication and Outreach (HITSP-ECO) Committee Co-Chairs: Walter G. Suarez, MD, Institute for HIPAA/HIT Education
HPH SCC CYBERSECURITY WORKING GROUP
 HPH SCC A PRIMER 1 What Is It? The cross sector coordinating body representing one of 16 critical infrastructure sectors identified in Presidential Executive Order (PPD 21) A trust community partnership
HPH SCC A PRIMER 1 What Is It? The cross sector coordinating body representing one of 16 critical infrastructure sectors identified in Presidential Executive Order (PPD 21) A trust community partnership
Certification for Meaningful Use Experiences and Observations from the Field June 2011
 Certification for Meaningful Use Experiences and Observations from the Field June 2011 Principles for Certification to Support Meaningful Use Certification should promote EHR adoption by giving providers
Certification for Meaningful Use Experiences and Observations from the Field June 2011 Principles for Certification to Support Meaningful Use Certification should promote EHR adoption by giving providers
MAQ DASHBOARD USERS GUIDE
 USERS GUIDE V10 - July 2014 eclinicalworks, 2014. All rights reserved CONTENTS ABOUT THIS GUIDE 4 Product Documentation 4 Webinars 4 eclinicalworks Newsletter 4 Getting Support 5 Conventions 5 MAQ DASHBOARD
USERS GUIDE V10 - July 2014 eclinicalworks, 2014. All rights reserved CONTENTS ABOUT THIS GUIDE 4 Product Documentation 4 Webinars 4 eclinicalworks Newsletter 4 Getting Support 5 Conventions 5 MAQ DASHBOARD
What is Usability? What is the Current State? Role and Activities of NIST in Usability Reactions from Stakeholders What s Next?
 What is Usability? What is the Current State? Role and Activities of NIST in Usability Reactions from Stakeholders What s Next? Usability is "the extent to which a product can be used by specified users
What is Usability? What is the Current State? Role and Activities of NIST in Usability Reactions from Stakeholders What s Next? Usability is "the extent to which a product can be used by specified users
ONC Health IT Certification Program
 ONC Health IT Certification Program Certification Requirements Update March 17, 2016 ICSA Labs Health IT Program Agenda Introduction Mandatory Product Disclosures and Transparency Requirements Certified
ONC Health IT Certification Program Certification Requirements Update March 17, 2016 ICSA Labs Health IT Program Agenda Introduction Mandatory Product Disclosures and Transparency Requirements Certified
Healthcare FHIR API TECHNICAL SPECIFICATION 7.4
 Healthcare FHIR API TECHNICAL SPECIFICATION 7.4 2018 Pegasystems Inc., Cambridge, MA All rights reserved. Trademarks For Pegasystems Inc. trademarks and registered trademarks, all rights reserved. All
Healthcare FHIR API TECHNICAL SPECIFICATION 7.4 2018 Pegasystems Inc., Cambridge, MA All rights reserved. Trademarks For Pegasystems Inc. trademarks and registered trademarks, all rights reserved. All
ProviderConnect Registered Services Autism Service Provider User Manual ASD Behavioral Assessment, Treatment Plan and Program Book Development
 ProviderConnect Registered Services Autism Service Provider User Manual ASD Behavioral Assessment, Treatment Plan and Program Book Development Created 9/1/17 Table of Contents Introduction... 3 Accessing
ProviderConnect Registered Services Autism Service Provider User Manual ASD Behavioral Assessment, Treatment Plan and Program Book Development Created 9/1/17 Table of Contents Introduction... 3 Accessing
IHE Patient Care Coordination (PCC) Technical Framework Supplement. CDA Document Summary Sections (CDA-DSS) Revision 1.1 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Sections (CDA-DSS) 15 Revision 1.1 Trial Implementation 20 Date: September
Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Sections (CDA-DSS) 15 Revision 1.1 Trial Implementation 20 Date: September
Chapter Two: Conformance Clause
 HL7 EHR TC Electronic Health Record - System Functional Model, Release 1 February 2007 Chapter Two: Conformance Clause EHR Technical Committee Co-chairs: Linda Fischetti, RN, MS Veterans Health Administration
HL7 EHR TC Electronic Health Record - System Functional Model, Release 1 February 2007 Chapter Two: Conformance Clause EHR Technical Committee Co-chairs: Linda Fischetti, RN, MS Veterans Health Administration
CMS and ehealth. Robert Tagalicod Director, Office of ehealth Standards and Services (OESS)
 CMS and ehealth Robert Tagalicod Director, Office of ehealth Standards and Services (OESS) Robert Anthony Deputy Director, Health IT Initiatives Group, OESS September 16, 2013 www.cms.gov/ehealth 2 ehealth
CMS and ehealth Robert Tagalicod Director, Office of ehealth Standards and Services (OESS) Robert Anthony Deputy Director, Health IT Initiatives Group, OESS September 16, 2013 www.cms.gov/ehealth 2 ehealth
PANEL 5: IHE CONFORMITY ASSESSMENT TESTING IN A GLOBAL CONTEXT
 PANEL 5: IHE CONFORMITY ASSESSMENT TESTING IN A GLOBAL CONTEXT Panel Chair: Chris Carr, RSNA (United States) Lapo Bertini, IHE Europe (Italy) Joyce Sensmeier, HIMSS (United States) Alexander Berler, IHE
PANEL 5: IHE CONFORMITY ASSESSMENT TESTING IN A GLOBAL CONTEXT Panel Chair: Chris Carr, RSNA (United States) Lapo Bertini, IHE Europe (Italy) Joyce Sensmeier, HIMSS (United States) Alexander Berler, IHE
Report of the Working Group on mhealth Assessment Guidelines February 2016 March 2017
 Report of the Working Group on mhealth Assessment Guidelines February 2016 March 2017 1 1 INTRODUCTION 3 2 SUMMARY OF THE PROCESS 3 2.1 WORKING GROUP ACTIVITIES 3 2.2 STAKEHOLDER CONSULTATIONS 5 3 STAKEHOLDERS'
Report of the Working Group on mhealth Assessment Guidelines February 2016 March 2017 1 1 INTRODUCTION 3 2 SUMMARY OF THE PROCESS 3 2.1 WORKING GROUP ACTIVITIES 3 2.2 STAKEHOLDER CONSULTATIONS 5 3 STAKEHOLDERS'
Slide 1 Welcome to Networking and Health Information Exchange, Health Data Interchange Standards. This is lecture b.
 HEALTH DATA EXCHANGE AND PRIVACY AND SECURITY Audio Transcript Component 9 Unit 5 Lecture B Networking and Health Information Exchange Slide 1 Welcome to Networking and Health Information Exchange, Health
HEALTH DATA EXCHANGE AND PRIVACY AND SECURITY Audio Transcript Component 9 Unit 5 Lecture B Networking and Health Information Exchange Slide 1 Welcome to Networking and Health Information Exchange, Health
Health Information Exchange Content Model Architecture Building Block HISO
 Health Information Exchange Content Model Architecture Building Block HISO 10040.2 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary HISO 10040.1 Health Information
Health Information Exchange Content Model Architecture Building Block HISO 10040.2 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary HISO 10040.1 Health Information
Prior Authorization and Clinician Burden: Updates from ONC
 Prior Authorization and Clinician Burden: Updates from ONC Thomas A. Mason, MD, FACP Chief Medical Officer Office of the National Coordinator for Health Information Technology (ONC) U.S. Department of
Prior Authorization and Clinician Burden: Updates from ONC Thomas A. Mason, MD, FACP Chief Medical Officer Office of the National Coordinator for Health Information Technology (ONC) U.S. Department of
Ch 4: Requirements Engineering. What are requirements?
 Ch 4: Engineering What are? Functional and non-functional The software document specification engineering processes elicitation and analysis validation management The descriptions of what the system should
Ch 4: Engineering What are? Functional and non-functional The software document specification engineering processes elicitation and analysis validation management The descriptions of what the system should
IHE Patient Care Coordination (PCC) Technical Framework Supplement. CDA Document Summary Section (CDA-DSS) Revision 1.0 Draft for Public Comment
 Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Section (CDA-DSS) 15 Revision 1.0 Draft for Public Comment 20 Date: May
Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Section (CDA-DSS) 15 Revision 1.0 Draft for Public Comment 20 Date: May
ConCert FAQ s Last revised December 2017
 ConCert FAQ s Last revised December 2017 What is ConCert by HIMSS? ConCert by HIMSS is a comprehensive interoperability testing and certification program governed by HIMSS and built on the work of the
ConCert FAQ s Last revised December 2017 What is ConCert by HIMSS? ConCert by HIMSS is a comprehensive interoperability testing and certification program governed by HIMSS and built on the work of the
pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Provincial Advisory Group [PAG])
![pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Provincial Advisory Group [PAG]) pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Provincial Advisory Group [PAG])](/thumbs/90/101387747.jpg) pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Provincial Advisory Group [PAG]) Nivolumab-Ipilimumab for Renal Cell Carcinoma November
pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Provincial Advisory Group [PAG]) Nivolumab-Ipilimumab for Renal Cell Carcinoma November
Primary Health Care (PHC) Indicator Inventory: Instructions for Use
 Primary Health Care (PHC) Indicator Inventory: Instructions for Use This document contains instructions for using the PHC Indicator Inventory, developed by the Joint Learning Network for Universal Health
Primary Health Care (PHC) Indicator Inventory: Instructions for Use This document contains instructions for using the PHC Indicator Inventory, developed by the Joint Learning Network for Universal Health
EBSCO Publishing Health Library Editorial Policy
 EBSCO Publishing Health Library Editorial Policy Introduction EBSCO Publishing is a leader in publishing health and medical information on the Internet. While we make every effort to ensure that our content
EBSCO Publishing Health Library Editorial Policy Introduction EBSCO Publishing is a leader in publishing health and medical information on the Internet. While we make every effort to ensure that our content
The HITECH Act. 5 things you can do Right Now to pave the road to compliance. 1. Secure PHI in motion.
 The HITECH Act 5 things you can do Right Now to pave the road to compliance Beginning in 2011, HITECH Act financial incentives will create a $5,800,000 opportunity over four years for mid-size hospital
The HITECH Act 5 things you can do Right Now to pave the road to compliance Beginning in 2011, HITECH Act financial incentives will create a $5,800,000 opportunity over four years for mid-size hospital
HIT Policy Committee. Recommendations by the Certification and Adoption Workgroup. Paul Egerman Marc Probst, Intermountain Healthcare.
 HIT Policy Committee Recommendations by the Certification and Adoption Workgroup Paul Egerman Marc Probst, Intermountain Healthcare July 16, 2009 Agenda The Workgroup The Workgroup s Charge Workgroup Process
HIT Policy Committee Recommendations by the Certification and Adoption Workgroup Paul Egerman Marc Probst, Intermountain Healthcare July 16, 2009 Agenda The Workgroup The Workgroup s Charge Workgroup Process
Update from HIMSS National Privacy & Security. Lisa Gallagher, VP Technology Solutions November 14, 2013
 Update from HIMSS National Privacy & Security Lisa Gallagher, VP Technology Solutions November 14, 2013 Agenda Update on HIMSS new Technology Solutions Department HIPAA Omnibus Rules Meaningful Use 2 P&S
Update from HIMSS National Privacy & Security Lisa Gallagher, VP Technology Solutions November 14, 2013 Agenda Update on HIMSS new Technology Solutions Department HIPAA Omnibus Rules Meaningful Use 2 P&S
The SHARPn phenotyping funnel. Phenotype specific patient cohorts
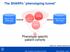 The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
10-CM/PCS Barriers and Opportunities
 HIPAA Summit West VI ICD-10 10-CM/PCS Barriers and Opportunities Dan Rode, MBA, CHPS, FHFMA Vice President, Advocacy and Policy AHIMA ICD-10 10-CM/PCS What we ll cover: AHIMA What is now known about ICD-10
HIPAA Summit West VI ICD-10 10-CM/PCS Barriers and Opportunities Dan Rode, MBA, CHPS, FHFMA Vice President, Advocacy and Policy AHIMA ICD-10 10-CM/PCS What we ll cover: AHIMA What is now known about ICD-10
Edition. MONTEREY COUNTY BEHAVIORAL HEALTH MD User Guide
 Edition 1 MONTEREY COUNTY BEHAVIORAL HEALTH MD User Guide i Table of Content OderConnect/InfoScriber Registration CH1 Pg.2 Sign In to MyAvatar CH2..Pg.10 Sync OrderConnect Password CH3.Pg.14 Client Look
Edition 1 MONTEREY COUNTY BEHAVIORAL HEALTH MD User Guide i Table of Content OderConnect/InfoScriber Registration CH1 Pg.2 Sign In to MyAvatar CH2..Pg.10 Sync OrderConnect Password CH3.Pg.14 Client Look
Discuss and finalize recommendations on Entity-Level Provider Directories (ELPDs):
 Agenda Discuss and finalize recommendations on Entity-Level Provider Directories (ELPDs): Users Uses/Functionality Directory Content Operating Requirements/Business Models Terminology Two TF calls to complete
Agenda Discuss and finalize recommendations on Entity-Level Provider Directories (ELPDs): Users Uses/Functionality Directory Content Operating Requirements/Business Models Terminology Two TF calls to complete
Vision for PACE Data Environment, PACE Quantum and the Common Data Set. October 21, 2015
 Vision for PACE Data Environment, PACE Quantum and the Common Data Set October 21, 2015 Vision for PACE Data Environment Processes and systems created to collect standardized data which can be used to
Vision for PACE Data Environment, PACE Quantum and the Common Data Set October 21, 2015 Vision for PACE Data Environment Processes and systems created to collect standardized data which can be used to
Architecture and Standards Development Lifecycle
 Architecture and Standards Development Lifecycle Architecture and Standards Branch Author: Architecture and Standards Branch Date Created: April 2, 2008 Last Update: July 22, 2008 Version: 1.0 ~ This Page
Architecture and Standards Development Lifecycle Architecture and Standards Branch Author: Architecture and Standards Branch Date Created: April 2, 2008 Last Update: July 22, 2008 Version: 1.0 ~ This Page
NextGen UD2 Upgrade Enhancements
 NextGen UD2 Upgrade Enhancements Summary NextGen EHR Enhancements May 23, 2016: Workflow Module Patient Information Bar Alerts Medication Module Allergy Module Encounter/Category View Filters NG Share
NextGen UD2 Upgrade Enhancements Summary NextGen EHR Enhancements May 23, 2016: Workflow Module Patient Information Bar Alerts Medication Module Allergy Module Encounter/Category View Filters NG Share
Direct / Secure Messaging
 Direct / Secure Messaging What is Direct Messaging? Direct or Secure messaging is any electronic communication between either a provider and a patient OR a provider to another provider that ensures only
Direct / Secure Messaging What is Direct Messaging? Direct or Secure messaging is any electronic communication between either a provider and a patient OR a provider to another provider that ensures only
Patient Portal- Instructions Overview
 Patient Portal- Instructions Overview Your Healthcare Provider will now be able to send you results and other health information via our secure Patient Portal. When your Provider has sent something to
Patient Portal- Instructions Overview Your Healthcare Provider will now be able to send you results and other health information via our secure Patient Portal. When your Provider has sent something to
CERECONS. Provider Training
 CERECONS Provider Training February 2012 Table of Contents 1. Physician Dashboard 2 Eligibility Highlights 2 Clinical Alerts 3 Referral Alerts and Stats 4 Group Information 5 2. Edit Profile 6 3. Eligibility
CERECONS Provider Training February 2012 Table of Contents 1. Physician Dashboard 2 Eligibility Highlights 2 Clinical Alerts 3 Referral Alerts and Stats 4 Group Information 5 2. Edit Profile 6 3. Eligibility
Regulating Telemedicine: the
 Regulating Telemedicine: the EU perspective ETSI ehealth workshop On telemedicine 6-7 May 2014 Céline Deswarte, Policy officer Unit Health and Well-Being European Commission i Table of Contents t 1) Legal
Regulating Telemedicine: the EU perspective ETSI ehealth workshop On telemedicine 6-7 May 2014 Céline Deswarte, Policy officer Unit Health and Well-Being European Commission i Table of Contents t 1) Legal
ICD-10 Customer Testing Guidelines and Scenarios
 ICD-10 Customer Testing Guidelines and Scenarios Revision 2.0 Updated July 21, 2015 This document includes pre-release information and user interface designs that are in development for upcoming enhancements
ICD-10 Customer Testing Guidelines and Scenarios Revision 2.0 Updated July 21, 2015 This document includes pre-release information and user interface designs that are in development for upcoming enhancements
CPRD Aurum Frequently asked questions (FAQs)
 CPRD Aurum Frequently asked questions (FAQs) Version 1.0 Date: 6 December 2017 Author: Helen Booth and Daniel Dedman, CPRD, UK Documentation Control Sheet During the course of the project it may be necessary
CPRD Aurum Frequently asked questions (FAQs) Version 1.0 Date: 6 December 2017 Author: Helen Booth and Daniel Dedman, CPRD, UK Documentation Control Sheet During the course of the project it may be necessary
National Antimicrobial Resistance Surveillance
 Discover Discover Kalamazoo Strategic Profile 017-00 APRIL 017 INTRODUCTION Strategic Profile: 017 00 Surveillance for antimicrobial resistance provides vital data on the emergence and spread of resistant
Discover Discover Kalamazoo Strategic Profile 017-00 APRIL 017 INTRODUCTION Strategic Profile: 017 00 Surveillance for antimicrobial resistance provides vital data on the emergence and spread of resistant
HL7 Development Framework
 HL7 Development Framework Version 3.0 Model Driven Standards Development Abdul-Malik Shakir Principal Consultant, Shakir Consulting October 2005 Introduction to Health Level Seven Health Level Seven (HL7)
HL7 Development Framework Version 3.0 Model Driven Standards Development Abdul-Malik Shakir Principal Consultant, Shakir Consulting October 2005 Introduction to Health Level Seven Health Level Seven (HL7)
Thank you, and enjoy the webinar.
 Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws. This should not be used as legal advice. Itentive recognizes that
Disclaimer This webinar may be recorded. This webinar presents a sampling of best practices and overviews, generalities, and some laws. This should not be used as legal advice. Itentive recognizes that
Diabetes Data Strategy Project ( Diabe-DS ) Overview and Status Update January 2010
 Diabetes Data Strategy Project ( Diabe-DS ) Overview and Status Update January 2010 Objectives Understand the purpose and scope of the Diabe-DS proof-of-concept project Review the status of project work
Diabetes Data Strategy Project ( Diabe-DS ) Overview and Status Update January 2010 Objectives Understand the purpose and scope of the Diabe-DS proof-of-concept project Review the status of project work
QRDA Category I: Ballot Development
 QRDA Category I: Ballot Development HL7 Structured Documents Sub Workgroup for Developing the QRDA I Ballot for May Telecom: +1 770-657-9270 Participant Code: 310940 Web: https://www3.gotomeeting.com/join/412175430
QRDA Category I: Ballot Development HL7 Structured Documents Sub Workgroup for Developing the QRDA I Ballot for May Telecom: +1 770-657-9270 Participant Code: 310940 Web: https://www3.gotomeeting.com/join/412175430
Can We Reliably Benchmark HTA Organizations? Michael Drummond Centre for Health Economics University of York
 Can We Reliably Benchmark HTA Organizations? Michael Drummond Centre for Health Economics University of York Outline of Presentation Some background Methods Results Discussion Some Background In recent
Can We Reliably Benchmark HTA Organizations? Michael Drummond Centre for Health Economics University of York Outline of Presentation Some background Methods Results Discussion Some Background In recent
Testing for Reliable and Dependable Health Information Exchange
 Testing for Reliable and Dependable Health Information Exchange Presented by Didi Davis, Testing Programs Director 1 Copyright 2016 The Sequoia Project. All rights reserved. Discussion Topics 1. ehealth
Testing for Reliable and Dependable Health Information Exchange Presented by Didi Davis, Testing Programs Director 1 Copyright 2016 The Sequoia Project. All rights reserved. Discussion Topics 1. ehealth
MAPIR User Guide for Eligible Hospitals. Medical Assistance Provider Incentive Repository (MAPIR): User Guide for Eligible Hospitals
 Medical Assistance Provider Incentive Repository (MAPIR): User Guide for Eligible Hospitals Version: 1.0 Original Version Date: 02/23/2018 Last Revision Date: 02/23/2018 Table of Contents Table of Contents
Medical Assistance Provider Incentive Repository (MAPIR): User Guide for Eligible Hospitals Version: 1.0 Original Version Date: 02/23/2018 Last Revision Date: 02/23/2018 Table of Contents Table of Contents
Health Information Exchange - A Critical Assessment: How Does it Work in the US and What Has Been Achieved?
 Health Information Exchange - A Critical Assessment: How Does it Work in the US and What Has Been Achieved? Use cases, best practice and examples for successful implementations 1 Agenda Overview of The
Health Information Exchange - A Critical Assessment: How Does it Work in the US and What Has Been Achieved? Use cases, best practice and examples for successful implementations 1 Agenda Overview of The
IHE Endoscopy Technical Framework Supplement. Endoscopy Image Archiving (EIA) Rev. 1.1 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Endoscopy Technical Framework Supplement 10 Endoscopy Image Archiving (EIA) 15 Rev. 1.1 Trial Implementation 20 Date: November 28, 2018 Author: IHE Endoscopy
Integrating the Healthcare Enterprise 5 IHE Endoscopy Technical Framework Supplement 10 Endoscopy Image Archiving (EIA) 15 Rev. 1.1 Trial Implementation 20 Date: November 28, 2018 Author: IHE Endoscopy
NATIONAL COMMISSION ON FORENSIC SCIENCE
 NATIONAL COMMISSION ON FORENSIC SCIENCE Recommendation for the Accreditation of Digital and Multimedia Forensic Science Service Providers 1 Subcommittee Date of Current Version 25/02/16 Accreditation and
NATIONAL COMMISSION ON FORENSIC SCIENCE Recommendation for the Accreditation of Digital and Multimedia Forensic Science Service Providers 1 Subcommittee Date of Current Version 25/02/16 Accreditation and
A Pilot Implementation of DIRECT Messaging and Provider Directory Services in the Palomar Health District
 A Pilot Implementation of DIRECT Messaging and Provider Directory Services in the Palomar Health District Project Overview and Plan Sujansky & Associates, LLC 1. Project Objectives Figure 1. High-level
A Pilot Implementation of DIRECT Messaging and Provider Directory Services in the Palomar Health District Project Overview and Plan Sujansky & Associates, LLC 1. Project Objectives Figure 1. High-level
Table of Contents [Revised: February 6, 2015] PEI-OMA User Manual MAP Supplemental
![Table of Contents [Revised: February 6, 2015] PEI-OMA User Manual MAP Supplemental Table of Contents [Revised: February 6, 2015] PEI-OMA User Manual MAP Supplemental](/thumbs/95/124379762.jpg) Table of Contents Overview Managing and Adapting Practice (MAP)... 1 Section 1 Sign In... 2 Section 2 Select a Provider... 3 Section 3 Select MAP as the EBP... 4 Section 4 Select a Client... 6 Section
Table of Contents Overview Managing and Adapting Practice (MAP)... 1 Section 1 Sign In... 2 Section 2 Select a Provider... 3 Section 3 Select MAP as the EBP... 4 Section 4 Select a Client... 6 Section
Clinical Decision Support
 Clinical Decision Support W. Ed Hammond, Ph.D. Director, Duke Center for Health Informatics Clinical Decision Support Clinical decision support (CDS) systems apply information technology to address, in
Clinical Decision Support W. Ed Hammond, Ph.D. Director, Duke Center for Health Informatics Clinical Decision Support Clinical decision support (CDS) systems apply information technology to address, in
United States Government Cloud Standards Perspectives
 United States Government Cloud Standards Perspectives in the context of the NIST initiative to collaboratively build a USG Cloud Computing Technology Roadmap NIST Mission: To promote U.S. innovation and
United States Government Cloud Standards Perspectives in the context of the NIST initiative to collaboratively build a USG Cloud Computing Technology Roadmap NIST Mission: To promote U.S. innovation and
Prevention and Early Intervention Outcome Measures Application (PEI-OMA)
 Chief Information Office Bureau Solutions Delivery Division Solutions Development Section Prevention and Early Intervention Outcome Measures Application (PEI-OMA) User Manual v1.5 February 5, 2015 Table
Chief Information Office Bureau Solutions Delivery Division Solutions Development Section Prevention and Early Intervention Outcome Measures Application (PEI-OMA) User Manual v1.5 February 5, 2015 Table
Chapter Two: Conformance Clause
 HL7 EHR Work Group Electronic Health Record - System Functional Model, Release 2.0 December 2011 Chapter Two: Conformance Clause Gary Dickinson Co-Chair, Hl7 EHR Work Group CentriHealth Don Mon PhD Co-Chair,
HL7 EHR Work Group Electronic Health Record - System Functional Model, Release 2.0 December 2011 Chapter Two: Conformance Clause Gary Dickinson Co-Chair, Hl7 EHR Work Group CentriHealth Don Mon PhD Co-Chair,
Compass Practice Transformation Network (PTN) User Guide
 Compass Practice Transformation Network (PTN) User Guide Page 1 Table of Contents 1 Preface... 9 2 Introduction... 11 2.1 Operational Model... 11 2.2 How to Use this User Guide... 12 3 System Availability
Compass Practice Transformation Network (PTN) User Guide Page 1 Table of Contents 1 Preface... 9 2 Introduction... 11 2.1 Operational Model... 11 2.2 How to Use this User Guide... 12 3 System Availability
Vaccine data collection tool Oct Functions, Indicators & Sub-Indicators
 data collection tool Oct. 2011 A. National Regulatory System RS01: Legal framework for establishment of a regulatory system, mandate and enforcement power for each function RS01.01: Legislation or and
data collection tool Oct. 2011 A. National Regulatory System RS01: Legal framework for establishment of a regulatory system, mandate and enforcement power for each function RS01.01: Legislation or and
Overview Functionality Maintenance Future
 LEXICON Overview Functionality Maintenance Future OVERVIEW What is Lexicon Standard reference for terminology across VA Provides a basis for a common language of terminology so that all members of a healthcare
LEXICON Overview Functionality Maintenance Future OVERVIEW What is Lexicon Standard reference for terminology across VA Provides a basis for a common language of terminology so that all members of a healthcare
Qualifying Alternative Payment Model Participants (QPs) Methodology Fact Sheet
 Qualifying Alternative Payment Model Participants (QPs) Methodology Fact Sheet Overview This methodology fact sheet describes the process and methodology that the Centers for Medicare & Medicaid Services
Qualifying Alternative Payment Model Participants (QPs) Methodology Fact Sheet Overview This methodology fact sheet describes the process and methodology that the Centers for Medicare & Medicaid Services
Infinedi, LLC. Frequently Asked Questions
 Infinedi, LLC Frequently Asked Questions Who are we? Infinedi has been helping medical providers better manage their practices since 1986 by providing the finest EDI services available. Infinedi is a privately
Infinedi, LLC Frequently Asked Questions Who are we? Infinedi has been helping medical providers better manage their practices since 1986 by providing the finest EDI services available. Infinedi is a privately
Response to CMS. WEDI Attachment Forum Questions. August 9th Attachment Standard
 Response to CMS WEDI Attachment Forum Questions August 9th 2016 Attachment Standard August 25, 2016 Cooperative Exchange National Association of Clearinghouses 28 Clearinghouse member companies Represent
Response to CMS WEDI Attachment Forum Questions August 9th 2016 Attachment Standard August 25, 2016 Cooperative Exchange National Association of Clearinghouses 28 Clearinghouse member companies Represent
Workflow. Workflow Tabs
 Workflow Workflow provides a consolidated view of information contained throughout the electronic medical record. It also integrates with the ios app PowerChart Touch. Clinicians' efficiency and satisfaction
Workflow Workflow provides a consolidated view of information contained throughout the electronic medical record. It also integrates with the ios app PowerChart Touch. Clinicians' efficiency and satisfaction
SNOMED CT (Systematized Nomenclature of Medicine Clinical Terms)
 SNOMED CT (Systematized Nomenclature of Medicine Clinical Terms) February 2019 Agenda What is SNOMED CT and it s benefits Differences and changes between Read and SNOMED CT terms The new SNOMED CT code
SNOMED CT (Systematized Nomenclature of Medicine Clinical Terms) February 2019 Agenda What is SNOMED CT and it s benefits Differences and changes between Read and SNOMED CT terms The new SNOMED CT code
Preparing for v11.4. From the PM and UC perspective
 Preparing for v11.4 From the PM and UC perspective Introduction Matt Leyva Consultant with Galen Healthcare Solutions Over 10 years of Project Management experience, with past two years focused on EHR
Preparing for v11.4 From the PM and UC perspective Introduction Matt Leyva Consultant with Galen Healthcare Solutions Over 10 years of Project Management experience, with past two years focused on EHR
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview
 Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Illinois Medicaid EHR Incentive Program for EPs
 The Chicago HIT Regional Extension Center Bringing Chicago together through health IT Illinois Medicaid EHR Incentive Program for EPs A Guide to Attesting for the 2017 Program Year in the emipp System
The Chicago HIT Regional Extension Center Bringing Chicago together through health IT Illinois Medicaid EHR Incentive Program for EPs A Guide to Attesting for the 2017 Program Year in the emipp System
Matt Quinn.
 Matt Quinn matt.quinn@nist.gov Roles of AHRQ and NIST What s at Stake Current State of Usability in Certified EHRs Projects to Support Improved Usability Moving Forward June 7 NIST Workshop Questions NIST's
Matt Quinn matt.quinn@nist.gov Roles of AHRQ and NIST What s at Stake Current State of Usability in Certified EHRs Projects to Support Improved Usability Moving Forward June 7 NIST Workshop Questions NIST's
ICD-10 Testing: Testing Your EHR, Practice Management System and Internal Processes for ICD-10 Readiness
 : Testing Your EHR, Practice Management System and Internal Processes for ICD-10 Readiness Learning Objectives: Understand testing variables and procedures for addressing applications that store and use
: Testing Your EHR, Practice Management System and Internal Processes for ICD-10 Readiness Learning Objectives: Understand testing variables and procedures for addressing applications that store and use
Identity Theft: Enterprise-Wide Strategies for Prevention, Detection and Remediation
 Booz Allen Hamilton Proprietary 1 Conference Presentation Identity Theft: Enterprise-Wide Strategies for Prevention, Detection and Remediation Kris O Neal Dan Steinberg Harvard Privacy Symposium August
Booz Allen Hamilton Proprietary 1 Conference Presentation Identity Theft: Enterprise-Wide Strategies for Prevention, Detection and Remediation Kris O Neal Dan Steinberg Harvard Privacy Symposium August
Outcomes and Lessons Learned From 5010 Testing Project Collaboration
 Outcomes and Lessons Learned From 5010 Testing Project Collaboration Elliot Sloane, PhD, CCE Villanova University Co-Chair, IHE International Bob Bowman CORE Manager CAQH Paul Decrosta Manager, IPP Regulatory
Outcomes and Lessons Learned From 5010 Testing Project Collaboration Elliot Sloane, PhD, CCE Villanova University Co-Chair, IHE International Bob Bowman CORE Manager CAQH Paul Decrosta Manager, IPP Regulatory
Putting It All Together:
 Putting It All Together: The Interplay of Privacy & Security Regina Verde, MS, MBA, CHC Chief Corporate Compliance & Privacy Officer University of Virginia Health System 2017 ISPRO Conference October 24,
Putting It All Together: The Interplay of Privacy & Security Regina Verde, MS, MBA, CHC Chief Corporate Compliance & Privacy Officer University of Virginia Health System 2017 ISPRO Conference October 24,
pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Manufacturer)
 pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Manufacturer) Blinatumomab (Blincyto) for Philadelphia chromosome positive Acute Lymphoblastic
pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation (Manufacturer) Blinatumomab (Blincyto) for Philadelphia chromosome positive Acute Lymphoblastic
HL7 s Common Terminology Services Standard (CTS)
 HL7 s Common Terminology Services Standard (CTS) HIMSS06 Annual Conference and Exhibition February 15, 2006 San Diego, CA Russell Hamm Objectives Describe the HL7 Common Terminology Services Specification
HL7 s Common Terminology Services Standard (CTS) HIMSS06 Annual Conference and Exhibition February 15, 2006 San Diego, CA Russell Hamm Objectives Describe the HL7 Common Terminology Services Specification
July 13, Via to RE: International Internet Policy Priorities [Docket No ]
![July 13, Via to RE: International Internet Policy Priorities [Docket No ] July 13, Via to RE: International Internet Policy Priorities [Docket No ]](/thumbs/91/104515045.jpg) July 13, 2018 Honorable David J. Redl Assistant Secretary for Communications and Information and Administrator, National Telecommunications and Information Administration U.S. Department of Commerce Washington,
July 13, 2018 Honorable David J. Redl Assistant Secretary for Communications and Information and Administrator, National Telecommunications and Information Administration U.S. Department of Commerce Washington,
IPC Integrated Food Security Phase Classification. Lesson: IPC Quality Assurance
 IPC Integrated Food Security Phase Classification Version 2.0 Lesson: Text-only version In partnership with: In this lesson LEARNING OBJECTIVES... 2 INTRODUCTION... 2 WHERE YOU ARE IN THE IPC PACKAGE...
IPC Integrated Food Security Phase Classification Version 2.0 Lesson: Text-only version In partnership with: In this lesson LEARNING OBJECTIVES... 2 INTRODUCTION... 2 WHERE YOU ARE IN THE IPC PACKAGE...
2017 Partners in Excellence Executive Overview, Targets, and Methodology
 2017 Partners in Excellence Executive Overview, s, and Methodology Overview The Partners in Excellence program forms the basis for HealthPartners financial and public recognition for medical or specialty
2017 Partners in Excellence Executive Overview, s, and Methodology Overview The Partners in Excellence program forms the basis for HealthPartners financial and public recognition for medical or specialty
SUSTAINABLE ELECTRIFICATION OF HEALTH FACILITIES: UGANDA
 Project Information Sheet SUSTAINABLE ELECTRIFICATION OF HEALTH FACILITIES: UGANDA This project information sheet provides an overview of the activities planned under the Sustainable Electrification of
Project Information Sheet SUSTAINABLE ELECTRIFICATION OF HEALTH FACILITIES: UGANDA This project information sheet provides an overview of the activities planned under the Sustainable Electrification of
Near-port Community Capacity Building Toolkit for Effective EJ Stakeholder Engagement
 Near-port Community Capacity Building Toolkit for Effective EJ Stakeholder Engagement Sabrina Johnson, Project Lead EPA Office of Transportation & Air Quality presented at 2016 Southeast Brownfields Conference
Near-port Community Capacity Building Toolkit for Effective EJ Stakeholder Engagement Sabrina Johnson, Project Lead EPA Office of Transportation & Air Quality presented at 2016 Southeast Brownfields Conference
Inpatient Quality Reporting (IQR) Program
 PSVA Demonstration and ecqm Q&A Session Questions & Answers Moderator: Debra Price, PhD Manager, Continuing Education Hospital Inpatient Value, Incentives, and Quality Reporting (VIQR) Outreach and Education
PSVA Demonstration and ecqm Q&A Session Questions & Answers Moderator: Debra Price, PhD Manager, Continuing Education Hospital Inpatient Value, Incentives, and Quality Reporting (VIQR) Outreach and Education
pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation
 pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation Avelumab (Bavencio) for metastatic Merkel Cell Carcinoma March 21, 2018 3 Feedback on perc
pan-canadian Oncology Drug Review Stakeholder Feedback on a pcodr Expert Review Committee Initial Recommendation Avelumab (Bavencio) for metastatic Merkel Cell Carcinoma March 21, 2018 3 Feedback on perc
European Commission Initiatives in telemedicine Presentation endorsed by the European Commission
 European Commission Initiatives in telemedicine Presentation endorsed by the European Commission Nicole Denjoy COCIR Secretary General How does the EU contribute to the large-scale deployment of telemedicine?
European Commission Initiatives in telemedicine Presentation endorsed by the European Commission Nicole Denjoy COCIR Secretary General How does the EU contribute to the large-scale deployment of telemedicine?
Open Source Software Quality Certification
 Open Source Software Quality Certification The Emerging ANSI Standard Wes Turner Director, Open Source Operations OSEHRA Mike Henderson Director, Open Source Product Management OSEHRA Wednesday, September
Open Source Software Quality Certification The Emerging ANSI Standard Wes Turner Director, Open Source Operations OSEHRA Mike Henderson Director, Open Source Product Management OSEHRA Wednesday, September
ACCREDITATION COMMISSION FOR CONFORMITY ASSESSMENT BODIES
 ACCREDITATION COMMISSION FOR CONFORMITY ASSESSMENT BODIES ACCREDITATION SCHEME MANUAL Document Title: Document Number: Various Accreditation Schemes ACCAB-ASM-7.0 CONTROLLED COPY Revision Number Revision
ACCREDITATION COMMISSION FOR CONFORMITY ASSESSMENT BODIES ACCREDITATION SCHEME MANUAL Document Title: Document Number: Various Accreditation Schemes ACCAB-ASM-7.0 CONTROLLED COPY Revision Number Revision
DATA VALIDATION RESOURCES RCHC Data Group Webinar By Ben Fouts MPH November 14, 2017
 DATA VALIDATION RESOURCES RCHC Data Group Webinar By Ben Fouts MPH November 14, 2017 Agenda 1. Documents on new RCHC Peer Collaboration web page 2. Data Validation Resources (Introduction to the new version
DATA VALIDATION RESOURCES RCHC Data Group Webinar By Ben Fouts MPH November 14, 2017 Agenda 1. Documents on new RCHC Peer Collaboration web page 2. Data Validation Resources (Introduction to the new version
