Dispenser s Implementation Guide
|
|
|
- Stephen Perry
- 6 years ago
- Views:
Transcription
1 Dispenser s Implementation Guide Colorado Board of Pharmacy Prescription Drug Monitoring Program June 2014 Note This document is periodically updated. Please refer to the Colorado PDMP website, for the most current version. This document is formatted for duplex printing.
2 Dispenser s Implementation Guide This page intentionally left blank. Copyright 2012 Health Information Designs, LLC
3 Contents Contents 1 Document Overview... 1 Purpose and Content Program Overview... 3 Purpose Data Collection and Tracking... 5 Data Collection equirements... 5 eporting equirements... 5 Exemptions... 6 equired Prescription Information... 6 eporting Noncompliance... 7 Zero eports Data ubmission... 9 About This Chapter... 9 Timeline and equirements... 9 Upload pecifications... 9 Creating Your Account Modifying Your Upload Account eporting Zero Dispensing eport Zero Activity xentry eport Zero Activity File Upload Data Delivery Methods About This Chapter ecure FTP over H Encrypted File with OpenPGP Via FTP L Website Universal Claim Form (UCF) ubmission eporting equirements for UCF ubmissions Notes about NDC Numbers Online UCF ubmission Upload eports and Edit Definitions Upload eports View Upload eports View Zero eports Error Correction ubmit a New ecord evise a ecord Void a ecord Copyright Health Information Designs, LLC i
4 Contents Edit Definitions Assistance and upport Technical Assistance Administrative Assistance Glossary Document Information Copyright Notice and Trademarks Disclaimer Formatting Conventions Version History Change Log Appendix A: AAP 4.2 pecifications... A-1 Appendix B: Zero eport pecifications... B-1 Copyright Health Information Designs, LLC ii
5 Document Overview 1 Document Overview Purpose and Content The xentry Dispenser s Implementation Guide serves as a step-by-step implementation and training guide for pharmacies registered by the tate of Colorado as either prescription drug outlets or nonresident prescription drug outlets. It includes such topics as: eporting requirements for Colorado-registered pharmacies Data file submission guidelines and methods Creating your upload account Creating a data file Uploading or reporting your data Understanding upload error codes and definitions This guide has been customized to target the specific training needs of Coloradoregistered pharmacies. It is intended for use by Colorado-registered pharmacies required to report dispensing of controlled substances. Copyright Health Information Designs, LLC 1
6 Document Overview This page intentionally left blank. Copyright Health Information Designs, LLC 2
7 Program Overview 2 Program Overview Purpose Colorado s Electronic Prescription Drug Monitoring Program (PDMP) was originally authorized in 2005 by Colorado evised tatutes (C...) and was reauthorized in This program was created to improve patient care and foster the goal of reducing misuse, abuse, and diversion of controlled substances; and to encourage cooperation and coordination among state, local, and federal agencies and other states to reduce the misuse, abuse, and diversion of controlled substances. The Colorado tate Board of Pharmacy oversees the operation of the PDMP. The Colorado tate Board of Pharmacy (Board) has selected Health Information Designs (HID) to develop a database that will collect and store prescribing and dispensing data for controlled substances in chedules II, III, IV, and V and any other drugs specified by Colorado law as amended. HID s xentry is a Web-based program that facilitates the collection, analysis, and reporting of information on the prescribing, dispensing, and use of controlled substance prescription drugs. xentry leads the industry in flexibility, functionality, and ease of use. Colorado law requires that each dispenser shall submit, by electronic means, information regarding each prescription dispensed for a controlled substance. Each dispenser shall submit the information required by Colorado law to the central repository twice each month at time periods specified by Board rule unless the Board waives this requirement for good cause shown by the dispenser. The Board shall establish and maintain procedures to ensure that the privacy, confidentiality, and security of patient information collected, recorded, transmitted, and maintained is not disclosed except as provided in the C... Copyright Health Information Designs, LLC 3
8 Program Overview This page intentionally left blank. Copyright Health Information Designs, LLC 4
9 Data Collection and Tracking 3 Data Collection and Tracking Data Collection equirements This guide provides information regarding the Colorado Prescription Drug Monitoring Program (CO PDMP). The program was established to collect data on all chedules II, III, IV, and V controlled substances dispensed to an individual in the tate of Colorado by Colorado-registered pharmacies (prescription drug outlets and nonresident prescription drug outlets). Each time a controlled substance is dispensed to an individual, the controlled substance shall be reported to the CO PDMP, using a format approved by the Board, twice per month. All dispensers of controlled substances must meet the reporting requirements set forth by Colorado law in a secure methodology and format. uch approved formats may include secure FTP over H, FTP of a PGP-encrypted file, L website, or online universal claim form (UCF). Note: A dispenser is identified as a pharmacy registered with the Colorado tate Board of Pharmacy as a prescription drug outlet or nonresident prescription drug outlet. eporting equirements All dispensers of chedules II, III, IV, and V controlled substance prescriptions are required to collect and report their dispensing information. If you are a chain pharmacy, your data will likely be submitted from your home office. Please verify this with your home office. If you are an independent pharmacy or other entity, please forward the reporting requirements to your software vendor. They will need to create the data file, and they may be able to submit the data on your behalf. If not, follow the instructions provided in the Data ubmission chapter to submit the data. Note: Additions or deletions of other drugs specified by Colorado law may happen periodically. These changes must go through the regulatory process in order to be added or deleted. You will be notified of any changes that are made in the future. For detailed information for each of the fields required by the tate of Colorado and the fields required by the American ociety for Automation in Pharmacy (AAP), please see Appendix A: AAP 4.2 pecifications. Copyright Health Information Designs, LLC 5
10 Data Collection and Tracking Exemptions The following entities are exempt from reporting data: A prescription drug outlet not using electronic automation of the dispenser s business may apply for a waiver and, if granted by the Board, is exempt from reporting requirements. Please see the Board s website ( for waiver request forms. A hospital licensed or certified pursuant to section , C... or a prescription drug outlet located within the hospital that is dispensing a controlled substance for a chart order or dispensing less than or equal to a twenty-four (24) hour supply of a controlled substance. Emergency medical services personnel certified pursuant to section , C... equired Prescription Information The following information must be reported for each controlled substance dispensed to a patient: Field Name Field ID Pharmacy Header DEA Number Pharmacy Name Address Information 1 City Address tate Address ZIP Code Pharmacy DEA Number PHA03 PHA04 PHA05 PHA07 PHA08 PHA09 PHA03 Patient Information Last Name First Name Address Information 1 City Address tate Address ZIP Code Address Date of Birth Gender Code PAT07 PAT08 PAT12 PAT14 PAT15 PAT16 PAT18 PAT19 Dispensing ecord Prescription Number DP02 Copyright Health Information Designs, LLC 6
11 Data Collection and Tracking Field Name Date Written efills Authorized Date Filled efill Number Product ID *Note: NDC is required Compound Code Quantity Dispensed Days upply Classification Code for Payment Type (if available) Field ID DP03 DP04 DP05 DP06 DP08 DP07 DP09 DP10 DP16 Prescriber Information DEA Number DEA Number uffix, if applicable Last Name First Name PE02 PE03 PE05 PE06 The Data ubmission chapter provides all the instructions necessary to submit the required information. eporting Noncompliance Any dispenser who fails to submit prescription monitoring information to the Board as required by Colorado law and Board rule or submits incorrect prescription information may be disciplined by the Board. Zero eports If a pharmacy has no dispensing transactions to report for the preceding reporting period, the pharmacy must report this information to the CO PDMP by filing a zero report, as described in the eporting Zero Dispensing topic in this guide. Copyright Health Information Designs, LLC 7
12 Data Collection and Tracking This page intentionally left blank. Copyright Health Information Designs, LLC 8
13 Data ubmission 4 Data ubmission About This Chapter This chapter provides information and instructions for submitting data to the CO PDMP. Timeline and equirements Pharmacies or software vendors can establish submission accounts upon receipt of this guide and the date listed below. Instructions for setting up an account are provided in the Creating Your Account topic in this chapter. You can begin submitting data as soon as your account has been established. Mandatory reporting began on August 15, Dispensers are required to report data regarding their dispensing of controlled substances twice per month at the following schedule: o For dispensing transactions from the first through the 15 th day of each month, data shall be transmitted to the PDMP between the 16 th and 25 th day of that month. o For dispensing transactions from the 16 th though the last day of the month, data shall be transmitted to the PDMP between the first and 10 th day of the subsequent month. o If the pharmacy does not dispense any controlled substances for the reporting period, it must file a zero report during the above listed time frames or it will be considered noncompliant. Upload pecifications Files must be in the AAP 4.2 format, as defined in Appendix A: AAP 4.2 pecifications. Files for upload should be named in a unique fashion, with a prefix constructed from the date (YYYYMMDD) and a suffix of.dat. An example file name would be dat. All of your upload files will be kept separate from the files of others. eports for multiple pharmacies can be in the same upload file in any order. After a controlled substance has been dispensed, controlled substance prescription information must be reported twice per month at the schedule detailed in the Timeline and equirements topic, unless a waiver has been obtained from the Board. Copyright Health Information Designs, LLC 9
14 Data ubmission Creating Your Account Prior to submitting data, you must either create an upload account or verify your existing account information. If you have already created your account, proceed to the appropriate section of this document that outlines the steps you must follow to upload your data. Note: Multiple pharmacies can be uploaded in the same file. For example, chain pharmacies may send in one file containing controlled substance dispensing information for all of their pharmacies licensed in the tate of Colorado. Therefore, chains with multiple stores only have to set up one account to upload a file. Perform the following steps to create an account: 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Data Uploader. A window similar to the following is displayed: 3 Click Uploader ite. A window similar to the following is displayed: 4 Type newacct in the User Name field. Note: Existing users must also enter newacct in the User Name field. Do not use your current user name; you will be prompted to enter it in a subsequent step. 5 Type welcome in the Password field, and then click OK. Note: Existing users must also enter welcome in the Password field. Do not use your current password. Copyright Health Information Designs, LLC 10
15 Data ubmission A window similar to the following is displayed: 6 Click etup Upload Account. 7 elect one of the following options: Existing CO PMP data submitter New CO PMP data submitter Note: If you selected New CO PMP data submitter, please proceed to step 9. 8 If you selected Existing CO PMP data submitter, a window similar to the following is displayed: Enter your existing user ID, and then proceed to step 12 to verify your account information. 9 If you selected New CO PMP data submitter, a window similar to the following is displayed: 10 Enter your DEA number in the Physician or Pharmacy DEA number field. 11 Type your ZIP code in the ZIP Code field. 12 Click Next. Copyright Health Information Designs, LLC 11
16 Data ubmission A window similar to the following is displayed: 13 Complete all required fields (indicated by an asterisk) on the New Account etup for Upload Access window, using the information in the following table as a guideline: Field Description/Usage Account selection Choose Keep <account number> as my account for a single Pharmacy if you wish to use the suggested account name. Choose Create an account using <suggested account name> as my ID for uploading more than one Pharmacy s Data if you wish to enter an account name of your choosing. If this option is selected, type the desired account name in this field. Contact Information Note: Information in this section is used for contact purposes in the event a problem occurs with a data upload. Contact Name Contact Address Contact Contact Phone (equired) Type the first and last name of the contact person. (equired) Type the contact s street address, city, state, and ZIP code in the appropriate fields. (equired) Type the contact s address. Click the down arrow in the field to the right of the Contact field to select Edit eports for All Uploads. (equired) Type the contact s phone number, using the format Copyright Health Information Designs, LLC 12
17 Data ubmission Field Contact Fax Anticipated Upload Method Pharmacies I will be reporting Description/Usage (equired) Type the contact s fax number, using the format Click the down arrow in the field to the right of the Contact Fax field and select Fax Edit eports for All Uploads. elect the method of data upload you plan to use to report your data: ecure FTP over H FTP of file Encrypted with OpenPGP Upload with Internet browser using L UCF ubmission (equired) A list of all pharmacies with names similar to your store name/pharmacy name is displayed in this field. To select additional pharmacies for which you will be reporting, press the [CTL] key and then click the name of each pharmacy you wish to select. The pharmacies you select will be tied to your user name. Note: Failure to select the pharmacy or pharmacies for which you will be reporting will cause fatal errors and file rejections. 14 After completing all required fields, click Next. A window similar to the following is displayed: A randomly-assigned password for the FTP and FTP upload process is provided to you. oftware vendors setting up multiple accounts may choose from the following options: Create each account separately by using the method listed above. After you finish one pharmacy s account, click etup Upload Account on the home page, and repeat the process; O Create multiple accounts using one pharmacy s DEA number and ZIP code. If you choose this method, select et up user name as a group. Note: Data error reports are submitted to the address(es) supplied for the account(s). Copyright Health Information Designs, LLC 13
18 Data ubmission Modifying Your Upload Account Use this function if you need to modify the information supplied when you originally created your account. Important Note: The Colorado Prescription Drug Monitoring Program has implemented a change that will affect your reporting beginning in the reporting window running from 06/01/13 to 06/10/13 (for controlled substance prescriptions dispensed from 05/16/13 to 05/31/13). Each data uploader must modify his/her upload account and describe each pharmacy that he/she reports for on that account. Each data uploader will be prompted to do so when logging in to the Colorado PDMP during the reporting window from 06/01/13 to 06/10/13. Each data uploader that submits controlled substance dispensing reports to the Colorado PDMP via ecure FTP must also modify his/her upload account and indicate each pharmacy that the data uploader submits ecure FTP files for. Any failure to clearly identify the pharmacies on each data uploader account will cause fatal errors and file rejections for all non-identified pharmacies during the reporting window from 06/01/13 to 06/10/13. Each data uploader should utilize the View Upload eports option to ensure that it has correctly identified all of the pharmacies on his/her account, as any non-identified pharmacies will result in fatal errors and file rejections. If a data uploader has pharmacies for which no controlled substances were dispensed during the dispensing time frame, the data uploader must file a zero dispensing report for that particular pharmacy, either by submitting an AAP version 4.2 file for a zero report or by logging into his/her data uploader account in the Colorado PDMP and manually filing a zero report. Any questions or concerns may be addressed to the Help Desk at Open an Internet browser window and type the following UL in the address bar: 2 Click Data Uploader, and then click Uploader ite. A logon window is displayed. 3 Type your user name in the User Name field. 4 Type your password in the Password field. 5 Click OK. 6 From the xentry home page, click Modify Upload Account. 7 Update the information as necessary, using the field descriptions provided in the Creating Your Account topic as a guideline. 8 Click Next. A message displays that your account information was successfully updated. Copyright Health Information Designs, LLC 14
19 Data ubmission eporting Zero Dispensing If you have no dispensing transactions to report for the preceding reporting period, you must report this information to the CO PDMP. You may report zero dispensing by using the functionality provided within xentry via the eport Zero Activity menu item, or by creating and uploading a zero report data file. The steps you must perform for each method are provided in the following sections. eport Zero Activity xentry The information in the following topics explains the processes single dispensers and dispensers reporting for a group of pharmacies should use to report zero activity using xentry s eport Zero Activity menu item ingle Dispensers If you are a single dispenser, perform the following steps to report zero activity using xentry: 1 If you do not have an account, perform the steps in Creating Your Account. 2 Open an Internet browser window and type the following UL in the address bar: 3 Click Data Uploader, and then click Uploader ite. A logon window is displayed. 4 Type your user name in the User Name field. 5 Type your password in the Password field. 6 Click OK. 7 From the xentry home page, click eport Zero Activity. A window similar to the following is displayed: Copyright Health Information Designs, LLC 15
20 Data ubmission 8 Type the start date for this report in the Period tart Date field, using the dd/mm/yy format. Notes: The Period End Date field is populated with the current date. You may adjust this date to reflect the dates during which no dispensing occurred. All other pharmacy information is populated with the information provided when you created your account. 9 Click Continue. A message similar to the following is displayed: Group Pharmacies If you are responsible for reporting for a group of pharmacies, perform the following steps to report zero activity using xentry. Note: You are required to repeat this process for every pharmacy for which you are responsible for reporting. 1 If you do not have an account, perform the steps in Creating Your Account. 2 Open an Internet browser window and type the following UL in the address bar: 3 Click Data Uploader, and then click Uploader ite. A logon window is displayed. 4 Type your user name in the User Name field. 5 Type your password in the Password field. 6 Click OK. 7 From the xentry home page, click eport Zero Activity. Copyright Health Information Designs, LLC 16
21 Data ubmission A window similar to the following is displayed: 8 Type the start date for this report in the Period tart Date field, using the dd/mm/yy format. Notes: The Period End Date field is populated with the current date. You may adjust this date to reflect the dates during which no dispensing occurred. All other pharmacy information is populated with the information provided when you created your account. 9 elect the Use ID/Name listed above option to manually enter the pharmacy ID whose information you are reporting. If you choose to enter the pharmacy ID manually, type the pharmacy ID in the Pharmacy ID/Name field. Or elect the Choose from list option to select the pharmacy ID whose information you are reporting from a list of pharmacies with a name similar to your pharmacy. 10 Click Continue. If you selected the Use ID/Name listed above option, a message similar to the following is displayed: Or Copyright Health Information Designs, LLC 17
22 Data ubmission If you selected the Choose from list option, a window similar to the following is displayed: 11 Click the radio button next to the correct pharmacy ID. 12 Click Continue. A window similar to the following is displayed: eport Zero Activity File Upload 1 If you have not created an account, perform the steps in Creating Your Account. 2 Prepare the zero report data file for submission, using the specifications described in Appendix B: Zero eport pecifications. Important Notes: The file name should be constructed using the date of submission to HID as the file name and should have a.dat extension. For example, name the file dat if you submit it on July 1, Do not include spaces in the file name. If you submit more than one file within the same day, you must uniquely name each file so the system does not overwrite existing uploaded files. For example, if uploading three files within the same day, you could use the following file names: a.dat, b.dat, and c.dat. Copyright Health Information Designs, LLC 18
23 Data ubmission The system will accept zipped files and you should name them using the date of submission to HID. For example, name the file zip if you submit it on July 1, Before transmitting your file, rename it to include the suffix.up (e.g., dat.up). This will ensure that we do not try to load the file while you are transmitting it. Once transmission is complete, rename the file back to the original name (e.g., dat). 3 Upload the file using the steps provided in one of the following data delivery topics: ecure FTP over H Encrypted File with OpenPGP Via FTP L Website HID tracks the use of the Web-based tool, date stamps incoming files, and notifies you of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. Copyright Health Information Designs, LLC 19
24 Data ubmission This page intentionally left blank. Copyright Health Information Designs, LLC 20
25 Data Delivery Methods 5 Data Delivery Methods About This Chapter This chapter provides information about data delivery methods you can use to upload your controlled substance reporting data file(s). For quick reference, click the desired hyperlink in the following table to view the stepby-step instructions for your chosen data delivery method: Delivery Method Page ecure FTP over H 21 Encrypted File with OpenPGP Via FTP 22 L Website 23 Universal Claim Form (UCF) ubmission eporting equirements for UCF ubmissions 24 Notes about NDC Numbers 24 Online UCF ubmission 25 ecure FTP over H There are many free software products that support ecure FTP. Neither the Board nor HID is in a position to direct or support your installation of operating system software for ecure FTP; however, we have information that WinCP ( has been used successfully by other pharmacies. 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important Notes: The file name should be constructed using the date of submission to the CO PDMP as the file name and should have a.dat extension. For example, name the file dat if it is submitted on July 1, Do not include spaces in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.dat, b.dat, and c.dat. Zipped files can be accepted and should be named using the date of submission. For example, name the file zip if it is submitted on July 1, Copyright Health Information Designs, LLC 21
26 Data Delivery Methods Before transmitting your file, rename it to include the suffix.up (e.g., dat.up). This will ensure that we do not try to load the file while you are transmitting it. Once transmission is complete, rename the file back to the original name (e.g., dat). 3 FTP the file to sftp://copdmreporting.hidinc.com. 4 When prompted, type copdm (lower case) in front of your DEA number (or Generic ID) as your user ID and enter the password supplied when you created your account. 5 Place the file in the new directory. 6 Once the transmission is complete, rename the file without the.up extension (e.g., dat). 7 If desired, view the results of the transfer/upload in your user directory. The file name is YYYYMMDD.rpt. 8 Log off when the file transfer/upload is complete. HID tracks the use of the Web-based tool, and incoming files are date stamped. You are notified of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. Encrypted File with OpenPGP Via FTP There are many free software products which support file encryption using the PGP standard. Neither the Board nor HID is in a position to direct or support your installation of PGP compatible software utilities; however, our usage indicates that software from the GnuPG Project ( ) should be compatible with many operating systems. 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Import the PGP public key, supplied during the account creation, into your PGP key ring. 3 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important notes: The file name should be constructed using the date of submission as the file name and should have a.pgp extension. For example, name the file pgp if it is submitted on July 1, Do not include spaces in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.pgp, b.pgp, and c.pgp. Copyright Health Information Designs, LLC 22
27 Data Delivery Methods Before transmitting your file, rename it to include the suffix.up (e.g., pgp.up). This will ensure that we do not try to load the file while you are transmitting it. Once transmission is complete, rename the file back to the original name (e.g., pgp). 4 Encrypt the file with the PGP software, using the public key supplied during account creation. Note: PGP encryption performs a single compression as it encrypts, so there is no need to zip the file. 5 FTP the file to ftp://copdmreporting.hidinc.com. 6 When prompted, type copdm (lower case) in front of your DEA number (or Generic ID) as your user ID and enter the password you supplied when creating your account. 7 Place the file in the new directory. 8 Once the transmission is complete, rename the file without the.up extension (e.g., pgp). 9 If desired, view the results of the transfer/upload in your user directory. The file name is YYYYMMDD.rpt. 10 Log off when the file transfer/upload is complete. HID tracks the use of the Web-based tool, and incoming files are date stamped. You are notified of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. L Website 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important notes: The file name should be constructed using the date of submission to the CO PDMP as the file name and should have a.dat extension. For example, name the file dat if it is submitted on July 1, Do not include spaces or parentheses in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.dat, b.dat, and c.dat. Zipped files can be accepted and should be named using the date of submission. For example, name the file zip if it is submitted on July 1, Copyright Health Information Designs, LLC 23
28 Data Delivery Methods 3 Open a Web browser and enter the following UL: 4 When prompted, type the user ID and password supplied when the account was created. 5 Click Upload a File. 6 Click Browse to navigate to the location where you saved the file created in step 2. 7 If not previously named according to upload requirements, rename the file using the format YYYYMMDD.dat, for example, dat. 8 Click to select the file, and then click Open. 9 Click end File. HID tracks the use of the Web-based tool, and incoming files are date stamped. You are notified of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. Universal Claim Form (UCF) ubmission If you have Internet access, but are unable to submit your data in a batch upload, you may submit prescription information using xentry s online universal claim form (UCF). When submitting information using the online UCF, the information provided must be complete and accurate. Only complete and accurate submissions are entered into the CO PDMP database. Please use the information in the Notes about NDC Numbers section below as a guideline for providing accurate NDC numbers. eporting equirements for UCF ubmissions ee the equired Prescription Information topic for details for reporting requirements. Notes about NDC Numbers Use the following information when entering NDC numbers on the UCF: NDCs are 11 digits and use the format When adding an NDC, do not include the dashes, for example, NDCs are typically located on the original medication bottle on the top right corner of the label, prefaced with NDC- and followed by the number. Manufacturers often leave off a zero in the NDC. In these instances, you should add the 0 where appropriate, using the following examples as a guideline: If the NDC appears this way Enter it this way (missing 0 in first segment) (missing 0 in 2nd segment) Copyright Health Information Designs, LLC 24
29 Data Delivery Methods Online UCF ubmission If you do not have an automated record-keeping system capable of producing an electronic report using the AAP 4.2 format, you may submit prescription information using xentry s online UCF. The following new terms are introduced in this topic: ecord the patient, pharmacy, and prescription information that you enter for one patient on the UCF Batch a single record, or group of records, that you upload using the ubmit Batch function Note: ecords can be continually added to a batch a convenient feature that allows you to enter records at your convenience and not all at one time. We recommend that you add as many records as possible to a batch before submitting it; however, you must submit and close batches in accordance with the required reporting time frame. Perform the following steps to use the online UCF to submit prescription information: 1 If you do not have an account, perform the steps in Creating Your Account. 2 Open an Internet browser window and type the following UL in the address bar: 3 Click Data Uploader, and then click Uploader ite. A logon window is displayed. 4 Type your user name in the User Name field. 5 Type your password in the Password field. 6 Click OK. 7 From the xentry home page, click UCF Form Entry. A window similar to the following is displayed: Enter Next Form allows you to prepare one or more records for submission. how Batch Counts displays the number of records in the batch currently being prepared for submission and the number of records that have been previously been submitted. 8 Click Enter Next Form. Copyright Health Information Designs, LLC 25
30 Data Delivery Methods A window similar to the following is displayed: The UCF contains three sections Patient Information, Dispenser Information, and Prescription Information. efer to the following information to complete these sections on the UCF: Patient Information Complete all fields in this section. Dispenser Information In this section, supply your DEA number in the DEA field. Once this information is provided, all associated pharmacy information available within the xentry database is auto-populated in the appropriate fields. Prescription Information Information for up to three prescriptions may be entered in this section, and all fields for each prescription must be completed. If entering more than one prescription for the same prescriber, you may select the Use Prescriber Information From Above check box to auto-populate each prescription with the previously-used prescriber information. Copyright Health Information Designs, LLC 26
31 Data Delivery Methods 9 Once all information has been entered, click ubmit. Notes: If information is missing from any required fields on the UCF, the UCF window will display again with the required fields indicated. Click Modify to add the missing information, and then click ubmit. If the system indicates that the DEA number or the NDC number you have provided is invalid, and you are certain you have provided the correct number, contact HID using the information supplied in Assistance and upport. 10 The UCF is displayed for your review. If all information is correct, click ubmit. If you need to modify any information, click Modify. Once you click ubmit, a window similar to the following is displayed: 11 Perform one of the following functions: Click Enter Next Form to add additional records to this batch. Click how Batch Counts to display the number of records in the current batch. Click ubmit/close Batch to upload this batch of records. Copyright Health Information Designs, LLC 27
32 Data Delivery Methods This page intentionally left blank. Copyright Health Information Designs, LLC 28
33 Upload eports and Edit Definitions 6 Upload eports and Edit Definitions Upload eports xentry provides all submitters of data with an upload report. When creating an account, you are required to submit an address and a fax number. You must also specify the method by which you wish to receive your upload report. If you FTP/FTP the data, a report will be placed in your home directory on the FTP server. Below is an example of an error report: A single record may be rejected, or if a certain percentage of records are rejected in an individual file, the entire file may be rejected. We track three types of errors: Minor Incorrect data in non-vital field erious ecord can be loaded with missing or inappropriate data Fatal ecord cannot be loaded A single record will be rejected if it contains a fatal error. An entire batch will be rejected if: ALL records have Fatal or erious errors More than 10% of the records have Fatal errors More than 20% of the records have erious errors Pharmacies are required to correct fatal errors and resubmit the records within five (5) days of the initial record submission. Copyright Health Information Designs, LLC 29
34 Upload eports and Edit Definitions View Upload eports This function provides uploaders access to upload reports that were previously delivered via or fax following a data submission. By default, the reports that display for reviewing are provided for a 31-day period. However, uploaders can view reports outside of the 31-day default period by entering start and end dates for the desired date range. Perform the following steps to view upload reports: 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Data Uploader, and then click Uploader ite. A logon window is displayed. 3 Type your user name in the User Name field. 4 Type your password in the Password field. 5 Click OK. 6 From the xentry home page, click View Upload eports. A window similar to the following is displayed: 7 Click a hyperlink in the eport Name field to open an upload report for viewing. To view reports for a different time frame, type a start and end date in the eport Timeframe fields, and then click ubmit. View Zero eports This function provides uploaders the ability to view previously submitted zero reports. By default, the reports that display for reviewing are provided for a 31-day period. However, uploaders can view reports outside of the 31-day default period by entering start and end dates for the desired date range. Perform the following steps to view zero reports: 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Data Uploader, and then click Uploader ite. Copyright Health Information Designs, LLC 30
35 Upload eports and Edit Definitions A login window is displayed. 3 Type your user name in the User Name field. 4 Type your password in the Password field. 5 Click OK. 6 From the xentry home page, click View Zero eports. A window similar to the following is displayed: Error Correction Fatal errors will cause a record NOT to be loaded. If this occurs, correct the data that caused the error and resubmit the entire record. Fatal error corrections must be resubmitted within five (5) days of the initial record submission. If a record with a serious or minor error is loaded and a correction is required, records can be corrected using the DP01 values as explained below. Note: Edit Number V1 as shown in the Edit Definitions table should not be resubmitted. All other records with errors that are not fatal will be loaded unless the batch thresholds are reached. Error thresholds are defined in the Upload eports section. The AAP 4.2 standard requires a dispenser to select an indicator in the DP01 (eporting tatus) field. Dispensers may submit new records, revise and resubmit records, and void (delete) erroneous records. These actions are indicated by supplying one of the following values in the DP01 field: 00 New ecord indicates a new record 01 evise indicates that one or more data elements in a previously-submitted record has been revised 02 Void indicates that the original record should be voided Use the information in the following topics to create, revise/resubmit, or void an erroneous record. Copyright Health Information Designs, LLC 31
36 Upload eports and Edit Definitions ubmit a New ecord Perform the following steps to submit a new record: 1 Create a record with the value 00 in the DP01 field. 2 Populate all other required fields and submit the record. Note: These steps are used to submit new records or to submit records that were previously submitted but received a fatal status on your error report. ecords with fatal errors are not loaded to the system. The errors in these records must be corrected in your system and resubmitted using the 00 status in the DP01 field. evise a ecord Perform the following steps to revise a record: 1 Create a record with the value 01 in the DP01 field. 2 Populate the following fields with the same information originally submitted in the erroneous record: PHA03 (DEA Provider ID) DP02 (Prescription Number) DP05 (Date Filled) 3 Fill in all other data fields with the correct information. This information will override the original data linked to the fields referenced in step 2. 4 ubmit the record. Important note: If any of the fields referenced in step 2 are part of the correction, the record must first be voided using the steps provided in the Void a ecord section, and then you must re-submit the record using the value 00 in the DP01 field. Void a ecord Perform the following steps to void (delete) a record: 1 end a record with the value 02 in the DP01 field. 2 Fill in all other data identical to the original record. This will void the original record submission. Copyright Health Information Designs, LLC 32
37 Upload eports and Edit Definitions Edit Definitions The following table describes the current list of edits: Edit Number Message everity Edit 01 Format of File Error Fatal Edit 02 Pharmacy DEA is blank Fatal Edit 05 Pharmacy ID not found Fatal Edit 09 Invalid DOB erious Edit 10 Gender must be valid erious Edit 14 eporting status is invalid Fatal Edit 15 Date Dispensed is invalid or irrational erious Edit 17 efill Code must be a valid number Minor Edit 18 Quantity is invalid erious Edit 20 Days upply > 360 erious Edit 21 NDC not found Fatal NDC not found (used when CDI segment is used) Fatal Edit 22 Product ID Qualifier is invalid Fatal Edit 25 Prescriber ID not found Minor Prescriber ID cannot be blank Fatal Edit 26 Prescriber Last Name is blank Minor Edit 27 Prescriber First Name is blank Minor Edit 28 Date X Written is invalid Minor Edit 31 Classification Code for Payment Type invalid erious Edit 50 Customer Last Name blank Fatal Edit 51 Customer First Name blank Fatal Edit 52 Customer Address blank erious Edit 53 Customer ZIP Code is blank erious Edit 54 Customer ZIP and tate Code conflict erious Edit 56 Customer City is blank Minor Edit 60 Customer tate Code is blank erious Edit 61 Customer tate Code is invalid erious Edit 200 Prescription Number is blank Fatal Edit 354 Patient ID Qualifier is invalid erious Edit 355 Pharmacy ID is not registered to the uploader Fatal Edit 360 Date dispensed prior to December 1, 2010 Fatal Copyright Health Information Designs, LLC 33
38 Upload eports and Edit Definitions Edit Number Message everity Edit V1 ecord already exists Note: Duplicate records are not loaded. The number of duplicate records, if any, is displayed on the upload report produced after data file transmission has completed. Minor Copyright Health Information Designs, LLC 34
39 Assistance and upport 7 Assistance and upport Technical Assistance If you need additional help with any of the procedures outlined in this guide, you can: Contact HID by at copdmp-info@hidinc.com O Call the HID Help Desk at Technical assistance is available Monday through Friday (except for holidays) from 9:00 a.m. to 5:00 p.m. Mountain Time. Administrative Assistance If you have non-technical questions regarding the Colorado PDMP, please contact: Colorado tate Board of Pharmacy Prescription Drug Monitoring Program 1560 Broadway, uite 1350 Denver, CO Phone: (303) Fax: (303) pdmpinqr@dora.state.co.us Copyright Health Information Designs, LLC 35
40 Assistance and upport This page intentionally left blank. Copyright Health Information Designs, LLC 36
41 Glossary 8 Glossary AAP American ociety for Automation in Pharmacy Batch Group of files (report or query requests) that are processed in the background while other work is continued Board Colorado tate Board of Pharmacy Dispense To interpret, evaluate, and implement a prescription drug order or chart order, including the preparation of a drug or device for a patient or a patient s agent in a suitable container appropriately labeled for subsequent administration to or use by a patient. Dispenser A pharmacy registered with the Colorado tate Board of Pharmacy as a prescription drug outlet or nonresident prescription drug outlet FTP HID NDC File Transfer Protocol; commonly-used protocol for exchanging files over any network Health Information Designs, LLC National Drug Code; describes specific drugs by drug manufacturer and package size PDMP Prescription Drug Monitoring Program PMP Prescription Monitoring Program; term used by AAP Prescriber An individual licensed, registered, or otherwise authorized by the jurisdiction in which the individual is practicing to prescribe drugs in the course of professional practice. Copyright Health Information Designs, LLC 37
42 Glossary xentry Prescription drug monitoring system developed by Health Information Designs, LLC FTP L ecure File Transfer Protocol (also referred to as H File Transfer Protocol ); provides file transfer and manipulation functionality over any reliable data stream ecure ockets Layer; cryptographic protocol that provides secure communications for data transfers Universal Claim Form (UCF) Electronic form used by a pharmacy that has Internet access, but is unable to submit its data in a batch upload Uploader A dispenser that uploads a data file containing controlled substance dispensing information Copyright Health Information Designs, LLC 38
43 Document Information 9 Document Information Copyright Notice and Trademarks Copyright Health Information Designs, LLC. All rights reserved. Health Information Designs, LLC 391 Industry Drive Auburn, AL xentry is a registered trademark of Health Information Designs, LLC (HID). Microsoft and Internet Explorer are registered trademarks or trademarks of Microsoft Corporation in the United tates and/or other countries. All other product names may be trademarks or registered trademarks of their respective companies. Disclaimer HID has made every effort to ensure the accuracy of the information in this document at the time of printing. However, information may change without notice. Please refer to the Colorado PDMP website, for the most current version of this document. Formatting Conventions The following formatting conventions are used throughout this document. Format Bold Arial Italic Blue underline Table 1 Text Formats Used to Designate eferences to execution buttons, windows, file names, menus, icons, or options Text you must type in a field or window, for example, \\server_name\printer_name for a network printer Hyperlinks to other sections of this document or external websites Copyright Health Information Designs, LLC 39
44 Document Information Version History The Version History records the publication history of this document. ee the Change Log for more details regarding the changes and enhancements included in each version. Publication Date Version Number Comments 08/03/ Initial publication 09/21/ Updated publication 10/02/ Updated publication 10/20/ Updated publication 04/19/ Updated publication 05/29/ Updated publication 06/20/ Updated publication Table 2 Document Version History Change Log The Change Log records the changes and enhancements included in each version. Version Number Chapter/ection Change 1.0 N/A N/A 1.1 Chapter 6/Edit Definitions 1.2 Chapter 3/equired Prescription Information Chapter 6/Edit Definitions Appendix A/AAP pecifications Table 1.3 Chapter 6/Edit Definitions 1.4 Chapter 4/eporting Zero Dispensing Chapter 6/View Zero eports Added edits 07 and 354 emoved fields PAT02 and PAT03 emoved edit 07 Changed field usage for fields PAT02 and PAT03 from to Added edits 26, 27, and 31 eparated topic into two sub-topics, ingle Dispensers and Group Pharmacies, to clarify the way zero reports must be submitted for each group Added new topic Copyright Health Information Designs, LLC 40
45 Document Information Version Number Chapter/ection Change 1.5 Chapter 4: Creating Your Account Modifying Your Upload Account Chapter 6/Edit Definitions Added a note specifying that selecting the pharmacies for which you are reporting is now required, and that failure to indicate the pharmacy for which you are reporting will result in fatal errors and file rejections Added Edit Cover page emoved state seal Table 3 Document Change Log Copyright Health Information Designs, LLC 41
46 Document Information This page intentionally left blank. Copyright Health Information Designs, LLC 42
Dispenser s Implementation Guide
 AAP 4.2 Florida Department of Health Prescription Drug Monitoring Program July 2015 This project was supported by Grant No. 2009-PM-BX-4004 awarded by the Bureau of Justice Assistance, Office of Justice
AAP 4.2 Florida Department of Health Prescription Drug Monitoring Program July 2015 This project was supported by Grant No. 2009-PM-BX-4004 awarded by the Bureau of Justice Assistance, Office of Justice
Dispenser s Implementation Guide
 Dispenser s Implementation Guide Arkansas Department of Health Prescription Monitoring Program March 2013 Note: This document is periodically updated. Please refer to the A PMP website, www.arkansaspmp.com,
Dispenser s Implementation Guide Arkansas Department of Health Prescription Monitoring Program March 2013 Note: This document is periodically updated. Please refer to the A PMP website, www.arkansaspmp.com,
Dispenser s Implementation Guide
 Dispenser s Implementation Guide Prescription Drug Monitoring Program July 2011 Note This document may be updated prior to the implementation of E-FORCE on eptember 1, 2011. Please refer to the Florida
Dispenser s Implementation Guide Prescription Drug Monitoring Program July 2011 Note This document may be updated prior to the implementation of E-FORCE on eptember 1, 2011. Please refer to the Florida
Dispenser s Implementation Guide
 Minnesota Board of Pharmacy Prescription Monitoring Program July 2016 This document is formatted for duplex printing. This page intentionally left blank. Do not copy or distribute without the express written
Minnesota Board of Pharmacy Prescription Monitoring Program July 2016 This document is formatted for duplex printing. This page intentionally left blank. Do not copy or distribute without the express written
Data Submitter s Implementation Guide
 Office of Substance Abuse and Mental Health Services Maine Department of Health and Human Services Prescription Monitoring Program December 2014 This document is formatted for duplex printing. This page
Office of Substance Abuse and Mental Health Services Maine Department of Health and Human Services Prescription Monitoring Program December 2014 This document is formatted for duplex printing. This page
Dispenser s Implementation Guide
 Dispenser s Implementation Guide Oregon Health Authority Prescription Drug Monitoring Program February 2011 This document is formatted for duplex printing. Dispenser s Implementation Guide This page intentionally
Dispenser s Implementation Guide Oregon Health Authority Prescription Drug Monitoring Program February 2011 This document is formatted for duplex printing. Dispenser s Implementation Guide This page intentionally
Dispenser s Implementation Guide
 Minnesota Board of Pharmacy Prescription Monitoring Program April 2017 This document is formatted for duplex printing. This page intentionally left blank. Contents Contents 1 Document Overview... A-1 2
Minnesota Board of Pharmacy Prescription Monitoring Program April 2017 This document is formatted for duplex printing. This page intentionally left blank. Contents Contents 1 Document Overview... A-1 2
Alabama Department of Public Health Prescription Drug Monitoring Program
 Prescription Drug Monitoring Program Dispenser s Implementation Guide v1.3 May 2009 391 Industry Drive Auburn, AL 36832 Phone: (334) 502-3262 Fax: (334) 466-6947 Auburn, Alabama Jackson, Mississippi Little
Prescription Drug Monitoring Program Dispenser s Implementation Guide v1.3 May 2009 391 Industry Drive Auburn, AL 36832 Phone: (334) 502-3262 Fax: (334) 466-6947 Auburn, Alabama Jackson, Mississippi Little
Implementation Guide for Dispensing Practitioners
 Arizona Controlled Substances Prescription Monitoring Program Implementation Guide for Dispensing Practitioners v 2.2 September 2010 This document is formatted for duplex printing. 391 Industry Drive Auburn,
Arizona Controlled Substances Prescription Monitoring Program Implementation Guide for Dispensing Practitioners v 2.2 September 2010 This document is formatted for duplex printing. 391 Industry Drive Auburn,
Dispenser Reporting Manual. October 1, 2007
 South Carolina Department of Health and Environmental Control Prescription Monitoring Program Dispenser Reporting Manual October 1, 2007 Prepared by Health Information Designs, Inc. 391 Industry Dr Auburn,
South Carolina Department of Health and Environmental Control Prescription Monitoring Program Dispenser Reporting Manual October 1, 2007 Prepared by Health Information Designs, Inc. 391 Industry Dr Auburn,
Controlled Substances Reporting System. Dispenser Reporting Manual
 North Carolina Department of Health and Human Services Division of Mental Health, Developmental Disabilities and Substance Abuse Services Controlled Substances Reporting System Dispenser Reporting Manual
North Carolina Department of Health and Human Services Division of Mental Health, Developmental Disabilities and Substance Abuse Services Controlled Substances Reporting System Dispenser Reporting Manual
Dispenser s Implementation Guide ASAP 4.2 Washington State Department of Health Washington State Prescription Monitoring Program
 ASAP 4.2 Washington State Department of Health Washington State Monitoring Program April 2017 ote This document is periodically updated. Please refer to the WA PMP website, www.wapmp.org, for the most
ASAP 4.2 Washington State Department of Health Washington State Monitoring Program April 2017 ote This document is periodically updated. Please refer to the WA PMP website, www.wapmp.org, for the most
Frequently Asked Questions
 This document provides answers to some common questions regarding the reporting of controlled substances to the Arkansas (PMP) database. A list of the questions answered in this document is provided below:
This document provides answers to some common questions regarding the reporting of controlled substances to the Arkansas (PMP) database. A list of the questions answered in this document is provided below:
Data Submission Dispenser Guide Florida Department of Health Prescription Drug Monitoring Program
 Data ubmission Dispenser Guide Florida Department of Health Prescription Drug Monitoring Program March 2018 Version 1.0 Effective April 18, 2018 Table of Contents 1 E-FOCE Overview...4 1.1 Florida s PDMP
Data ubmission Dispenser Guide Florida Department of Health Prescription Drug Monitoring Program March 2018 Version 1.0 Effective April 18, 2018 Table of Contents 1 E-FOCE Overview...4 1.1 Florida s PDMP
Training Guide for Arkansas Practitioners and Pharmacists. Arkansas Department of Health Prescription Monitoring Program
 Training Guide for Arkansas Practitioners and Pharmacists Arkansas Department of Health Prescription Monitoring Program May 2013 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 2 System
Training Guide for Arkansas Practitioners and Pharmacists Arkansas Department of Health Prescription Monitoring Program May 2013 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 2 System
Data Submission Guide for Dispensers Minnesota Prescription Monitoring Program
 Minnesota Prescription Monitoring Program ovember 2018 Version 1.0, Effective December 4, 2018 9901 Linn Station oad Louisville, KY 40223 apprisshealth.com Table of Contents Table of Contents 1 Document
Minnesota Prescription Monitoring Program ovember 2018 Version 1.0, Effective December 4, 2018 9901 Linn Station oad Louisville, KY 40223 apprisshealth.com Table of Contents Table of Contents 1 Document
PRESCRIPTION MONITORING PROGRAM (PMP)
 PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives
 Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives Arkansas Department of Health Prescription Monitoring Program March 2016 Contents Contents 1 Document Overview...
Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives Arkansas Department of Health Prescription Monitoring Program March 2016 Contents Contents 1 Document Overview...
Training Guide for Practitioners
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Training Guide for Wisconsin Practitioners and Pharmacists. Pharmacy Examining Board Wisconsin Department of Safety and Professional Services
 Training Guide for Wisconsin Practitioners and Pharmacists Pharmacy Examining Board Wisconsin Department of Safety and Professional Services October 2013 Contents Contents 1 Document Overview... 1 Purpose
Training Guide for Wisconsin Practitioners and Pharmacists Pharmacy Examining Board Wisconsin Department of Safety and Professional Services October 2013 Contents Contents 1 Document Overview... 1 Purpose
Training Guide for Colorado Practitioners and Pharmacists. Colorado State Board of Pharmacy Prescription Drug Monitoring Program
 Training Guide for Colorado Practitioners and Pharmacists Colorado State Board of Pharmacy Prescription Drug Monitoring Program April 2016 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Colorado Practitioners and Pharmacists Colorado State Board of Pharmacy Prescription Drug Monitoring Program April 2016 Contents Contents 1 Document Overview... 3 Purpose and Contents...
PDMP User s Guide. Oregon Health Authority Prescription Drug Monitoring Program
 Oregon Health Authority Prescription Drug Monitoring Program March 2014 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 System Overview... 3 About the RxSentry
Oregon Health Authority Prescription Drug Monitoring Program March 2014 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 System Overview... 3 About the RxSentry
1 Data Collection and Tracking Data Collection Overview Data Collection Requirements Reporting Requirements Exemptions...
 Data ubmission Dispenser Guide Arkansas Department of Health Prescription Monitoring Program November 2017 Version 1.2 Effective November 13, 2017 Table of Contents 1 Data Collection and Tracking... 5
Data ubmission Dispenser Guide Arkansas Department of Health Prescription Monitoring Program November 2017 Version 1.2 Effective November 13, 2017 Table of Contents 1 Data Collection and Tracking... 5
Training Guide for Practitioners. Washington State Department of Health Washington State Prescription Monitoring Program
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
NEW JERSEY PRESCRIPTION MONITORING AND REPORTING SYSTEM (NJPMRS) DATA COLLECTION MANUAL Effective: September 2011
 NEW JERSEY PRESCRIPTION MONITORING AND REPORTING SYSTEM (NJPMRS) DATA COLLECTION MANUAL Effective: September 2011 Optimum Technology, Inc. Contact Information 866-683-2476 njrxreport@otech.com 1 P a g
NEW JERSEY PRESCRIPTION MONITORING AND REPORTING SYSTEM (NJPMRS) DATA COLLECTION MANUAL Effective: September 2011 Optimum Technology, Inc. Contact Information 866-683-2476 njrxreport@otech.com 1 P a g
Training Guide for Alabama Practitioners and Pharmacists. Alabama Department of Public Health Prescription Drug Monitoring Program
 Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program August 2015 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program August 2015 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Data Submission Dispenser Guide Rhode Island Prescription Drug Monitoring Program (RI PDMP) February 2016 Version 1.0
 Data Submission Dispenser Guide hode Island Prescription Drug Monitoring Program (I PDMP) February 2016 Version 1.0 1 Table of Contents 1 Data Collection and Tracking...4 Data Collection equirements...
Data Submission Dispenser Guide hode Island Prescription Drug Monitoring Program (I PDMP) February 2016 Version 1.0 1 Table of Contents 1 Data Collection and Tracking...4 Data Collection equirements...
Ohio Automated Reporting System Handbook
 Ohio Automated Reporting System Handbook Ohio State Board of Pharmacy Prescription Monitoring Program 77 South High St., Room 1702 Columbus, OH 43215-6126 Phone: 614-466-4143 (Option 1) OARRS Fax: 614-644-8556
Ohio Automated Reporting System Handbook Ohio State Board of Pharmacy Prescription Monitoring Program 77 South High St., Room 1702 Columbus, OH 43215-6126 Phone: 614-466-4143 (Option 1) OARRS Fax: 614-644-8556
Training Guide for Florida Practitioners and Pharmacists. Florida Department of Health Prescription Drug Monitoring Program
 Training Guide for Florida Practitioners and Pharmacists Florida Department of Health Prescription Drug Monitoring Program February 2017 Contents Contents 1 Program Overview... 1 2 Document Overview...
Training Guide for Florida Practitioners and Pharmacists Florida Department of Health Prescription Drug Monitoring Program February 2017 Contents Contents 1 Program Overview... 1 2 Document Overview...
Training Guide for Alabama Practitioners and Pharmacists. Alabama Department of Public Health Prescription Drug Monitoring Program
 Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program March 2015 Contents Contents 1 Document Overview... 2 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program March 2015 Contents Contents 1 Document Overview... 2 Purpose and Contents...
Data Submission Dispenser Guide
 Data ubmission Dispenser Guide Delaware Division of Professional Regulation Prescription Monitoring Program October 2017 Version 1.3 Effective November 7, 2017 Table of Contents 1 Data Collection and Tracking...4
Data ubmission Dispenser Guide Delaware Division of Professional Regulation Prescription Monitoring Program October 2017 Version 1.3 Effective November 7, 2017 Table of Contents 1 Data Collection and Tracking...4
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program March 2016 Version 1.0
 Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program March 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program March 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program May 2016 Version 1.0
 Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program May 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program May 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
3 Accessing RxSentry. About This Chapter. Request Access to RxSentry
 Accessing RxSentry 3 Accessing RxSentry About This Chapter This chapter provides the steps you must follow to request an RxSentry account, log in to the system, retrieve system messages, retrieve a forgotten
Accessing RxSentry 3 Accessing RxSentry About This Chapter This chapter provides the steps you must follow to request an RxSentry account, log in to the system, retrieve system messages, retrieve a forgotten
Companion Guide Institutional Billing 837I
 Companion Guide Institutional Billing 837I Release 3 X12N 837 (Version 5010A2) Healthcare Claims Submission Implementation Guide Published December 2016 Revision History Date Release Appendix name/ loop
Companion Guide Institutional Billing 837I Release 3 X12N 837 (Version 5010A2) Healthcare Claims Submission Implementation Guide Published December 2016 Revision History Date Release Appendix name/ loop
Dispenser s Implementation Guide (ASAP 4.2) Nebraska Prescription Drug Monitoring Program
 Dispenser s Implementation Guide (ASAP 4.2) Nebraska Prescription Drug Monitoring Program Version: 3.6 DrFirst 9420 Key West Ave, Suite 101 Rockville, MD 20850 Office: 888-271-9898 Contents Document Change
Dispenser s Implementation Guide (ASAP 4.2) Nebraska Prescription Drug Monitoring Program Version: 3.6 DrFirst 9420 Key West Ave, Suite 101 Rockville, MD 20850 Office: 888-271-9898 Contents Document Change
Arkansas Prescription Drug Monitoring Program. User Support Manual
 Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
AK PDMP Data Submission Dispenser Guide
 AK PDMP Data Submission Dispenser Guide V1rE February 23, 2012 Additional information at http://pmp.relayhealth.com/ak 2011 RelayHealth All Rights Reserved No part of this document may be copied or reproduced
AK PDMP Data Submission Dispenser Guide V1rE February 23, 2012 Additional information at http://pmp.relayhealth.com/ak 2011 RelayHealth All Rights Reserved No part of this document may be copied or reproduced
HealthInfoNet CLINICAL PORTAL USER REFERENCE GUIDE. Revised: Page 1 of 24
 HealthInfoNet CLINICAL PORTAL USER REFERENCE GUIDE Revised: 6.3.2015 Page 1 of 24 HealthInfoNet User Reference Guide INSIDE: Accessing HealthInfoNet (HIN) 3-5 Clinical Portal 6-11 Notifications and Worklists
HealthInfoNet CLINICAL PORTAL USER REFERENCE GUIDE Revised: 6.3.2015 Page 1 of 24 HealthInfoNet User Reference Guide INSIDE: Accessing HealthInfoNet (HIN) 3-5 Clinical Portal 6-11 Notifications and Worklists
Health Services provider user guide
 Health Services provider user guide online claims submission... convenient service, delivered through an easy-to-use secure web site http://provider.ab.bluecross.ca/health... convenient service, delivered
Health Services provider user guide online claims submission... convenient service, delivered through an easy-to-use secure web site http://provider.ab.bluecross.ca/health... convenient service, delivered
Data Submission Dispenser Guide. Oregon Health Authority Prescription Drug Monitoring Program
 Data Submission Dispenser Guide Oregon Health Authority Prescription Drug Monitoring Program August 2017 Version 1.1 Effective October 23, 2017 Table of Contents 1 Data Collection and Tracking...4 Data
Data Submission Dispenser Guide Oregon Health Authority Prescription Drug Monitoring Program August 2017 Version 1.1 Effective October 23, 2017 Table of Contents 1 Data Collection and Tracking...4 Data
Meritain Connect User Manual. for Employees. 1 Meritain Connect User Guide for Employees
 Meritain Connect User Manual for Employees 1 Meritain Connect User Guide for Employees Contents Introduction... 4 Accessing Meritain Connect... 5 Logging In... 5 Forgot Password... 6 Registration Process...
Meritain Connect User Manual for Employees 1 Meritain Connect User Guide for Employees Contents Introduction... 4 Accessing Meritain Connect... 5 Logging In... 5 Forgot Password... 6 Registration Process...
Provider Secure Portal User Manual
 Provider Secure Portal User Manual Copyright 2011 Centene Corporation. All rights reserved. Operational Training 2 August 2011 Table of Contents Provider Secure Portal... 5 Registration... 6 Provider -
Provider Secure Portal User Manual Copyright 2011 Centene Corporation. All rights reserved. Operational Training 2 August 2011 Table of Contents Provider Secure Portal... 5 Registration... 6 Provider -
Alabama PDMP AWA E. User Support Manual
 Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
Table of Contents Data Collection and Tracking...4 Data Submission...8 Creating Your Account...9 Data Delivery Methods Data Compliance...
 Data Submission Dispenser Guide Arizona Controlled Substances Prescription Monitoring Program (AZ CSPMP) December 2016 Version 1.2 Effective January 12, 2017 Table of Contents 1 Data Collection and Tracking...4
Data Submission Dispenser Guide Arizona Controlled Substances Prescription Monitoring Program (AZ CSPMP) December 2016 Version 1.2 Effective January 12, 2017 Table of Contents 1 Data Collection and Tracking...4
BULLETIN BOARD SCREENS for HIPAA (BBS) UPDATED JULY 22, Once connected, the first screen displays the node number that you are connected to.
 BULLETIN BOARD SCREENS for HIPAA (BBS) UPDATED JULY 22, 2010 The following provides information and screen instructions on: 1. Transmitting 837 Transactions 2. TA1 Acknowledgement Retrievals 3. 997 Acknowledgement
BULLETIN BOARD SCREENS for HIPAA (BBS) UPDATED JULY 22, 2010 The following provides information and screen instructions on: 1. Transmitting 837 Transactions 2. TA1 Acknowledgement Retrievals 3. 997 Acknowledgement
Revision History. Document Version. Date Name Comments /26/2017 Training and Development Initial Creation
 Pharmaceutical Assistance Contract for the Elderly (PACE)/ Pharmaceutical Assistance Contract for the Elderly Needs Enhancement Tier (PACENET)Web Provider Enrollment/Provider Management Corporate User
Pharmaceutical Assistance Contract for the Elderly (PACE)/ Pharmaceutical Assistance Contract for the Elderly Needs Enhancement Tier (PACENET)Web Provider Enrollment/Provider Management Corporate User
Loan Closing Advisor SM. User Guide. December 2017
 Loan Closing Advisor SM User Guide December 2017 Notice This User Guide is Freddie Mac s CONFIDENTIAL INFORMATION as defined in and subject to the provisions of the Freddie Mac Single Family Seller/Servicer
Loan Closing Advisor SM User Guide December 2017 Notice This User Guide is Freddie Mac s CONFIDENTIAL INFORMATION as defined in and subject to the provisions of the Freddie Mac Single Family Seller/Servicer
Louisiana Medicaid Management Information System (LMMIS)
 Louisiana Medicaid Management Information System (LMMIS) Batch Eligibility Verification System Pilot User Manual Date Created: 03/22/2017 Date Modified: 08/02/2018 Prepared By Technical Communications
Louisiana Medicaid Management Information System (LMMIS) Batch Eligibility Verification System Pilot User Manual Date Created: 03/22/2017 Date Modified: 08/02/2018 Prepared By Technical Communications
March Canadian Public Tenders Buyer Guide
 March 2010 Canadian Public Tenders Buyer Guide Table of Contents WELCOME TO MERX... 1 ABOUT THIS REFERENCE GUIDE... 1 SECTION 1 GETTING STARTED... 2 SYSTEM REQUIREMENTS... 2 NAVIGATING IN MERX... 2 SECTION
March 2010 Canadian Public Tenders Buyer Guide Table of Contents WELCOME TO MERX... 1 ABOUT THIS REFERENCE GUIDE... 1 SECTION 1 GETTING STARTED... 2 SYSTEM REQUIREMENTS... 2 NAVIGATING IN MERX... 2 SECTION
(EHR) Incentive Program
 REGISTRATION USER GUIDE For Eligible Professionals Medicare Electronic Health Record (EHR) Incentive Program JULY 2012 (07.02.12 ver8) CONTENTS Step 1...Getting started 3 Step 2... Login 5 Step 3...Welcome
REGISTRATION USER GUIDE For Eligible Professionals Medicare Electronic Health Record (EHR) Incentive Program JULY 2012 (07.02.12 ver8) CONTENTS Step 1...Getting started 3 Step 2... Login 5 Step 3...Welcome
HPHConnect for Employers User s Guide
 HPHConnect for Employers User s Guide Copyright 2017 Harvard Pilgrim Health Care, Inc. All rights reserved. Harvard Pilgrim Health Care and the Harvard Pilgrim Health Care logo are trademarks of Harvard
HPHConnect for Employers User s Guide Copyright 2017 Harvard Pilgrim Health Care, Inc. All rights reserved. Harvard Pilgrim Health Care and the Harvard Pilgrim Health Care logo are trademarks of Harvard
efiletexas.gov Individual Filer User Guide Release
 efiletexas.gov Individual Filer User Guide Release 2017.1 EFS-TF-200-4071 v.1 October 2017 Copyright and Confidentiality Copyright 2017 Tyler Technologies, Inc. All rights reserved Use of these materials
efiletexas.gov Individual Filer User Guide Release 2017.1 EFS-TF-200-4071 v.1 October 2017 Copyright and Confidentiality Copyright 2017 Tyler Technologies, Inc. All rights reserved Use of these materials
Beam Software User Policies
 Beam Software User Policies Table of Contents 1 Overview... 3 2 Technical Support... 3 2.1 What Do I Do When I Have a Question or Encounter a Problem?... 4 2.1.1 Telephone Submissions... 4 2.1.2 Critical
Beam Software User Policies Table of Contents 1 Overview... 3 2 Technical Support... 3 2.1 What Do I Do When I Have a Question or Encounter a Problem?... 4 2.1.1 Telephone Submissions... 4 2.1.2 Critical
School Data Submission: Educator
 Ministry of Education Information Management Branch School Data Submission: Educator For Publicly-Funded and Inspected Private Schools User Guide February 2007 This page is left intentionally blank. OnSIS
Ministry of Education Information Management Branch School Data Submission: Educator For Publicly-Funded and Inspected Private Schools User Guide February 2007 This page is left intentionally blank. OnSIS
Version 1/Revision 4 Page 1 of 23. epaces PA/DVS Request REFERENCE GUIDE. Table of Contents
 Version 1/Revision 4 Page 1 of 23 Table of Contents PA/DVS Request... 2 Provider Service Address... 3 Contact Information... 4 Referring Provider... 4 Ordering Provider... 5 Event Information... 6 Pattern
Version 1/Revision 4 Page 1 of 23 Table of Contents PA/DVS Request... 2 Provider Service Address... 3 Contact Information... 4 Referring Provider... 4 Ordering Provider... 5 Event Information... 6 Pattern
CAQH ProView. Practice Manager and Provider Frequently Asked Questions
 CAQH ProView Practice Manager and Provider Frequently Asked s *s related to the CAQH ProView Practice Manager Module begin on page 2. *s on the CAQH ProView system for providers begin on page 6. 1 P a
CAQH ProView Practice Manager and Provider Frequently Asked s *s related to the CAQH ProView Practice Manager Module begin on page 2. *s on the CAQH ProView system for providers begin on page 6. 1 P a
Front End Risk Adjustment System [ FERAS ] User Guide
![Front End Risk Adjustment System [ FERAS ] User Guide Front End Risk Adjustment System [ FERAS ] User Guide](/thumbs/77/74723317.jpg) Front End Risk Adjustment System [ FERAS ] User Guide Centers for Medicare & Medicaid Services Table of Contents Table of Contents TRADEMARK INFORMATION... 1 INTRODUCTION... 2 FERAS OVERVIEW... 2 WHO TO
Front End Risk Adjustment System [ FERAS ] User Guide Centers for Medicare & Medicaid Services Table of Contents Table of Contents TRADEMARK INFORMATION... 1 INTRODUCTION... 2 FERAS OVERVIEW... 2 WHO TO
e-frr SYSTEM USER GUIDE
 e-frr SYSTEM USER GUIDE for Electronic Submission of Financial Return Version 1.5 Jun 2015 Table of Contents 1. Introduction... 4 2. Background... 4 3. System Purpose... 4 4. Baseline Specification of
e-frr SYSTEM USER GUIDE for Electronic Submission of Financial Return Version 1.5 Jun 2015 Table of Contents 1. Introduction... 4 2. Background... 4 3. System Purpose... 4 4. Baseline Specification of
Quick reference guide. Creating a Controlled Substance Export File with the ASAP 4.2 Format. Completion requirements
 Quick reference guide Creating a Controlled Substance Export File with the ASAP 4.2 Format If you have Cornerstone* 8.6 NEXT or later software, you can create a controlled substance export file that uses
Quick reference guide Creating a Controlled Substance Export File with the ASAP 4.2 Format If you have Cornerstone* 8.6 NEXT or later software, you can create a controlled substance export file that uses
Welcome to the. Patient Portal!
 Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider, schedule and request appointments, take control of your medical information,
Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider, schedule and request appointments, take control of your medical information,
Louisiana Medicaid Management Information System (LMMIS)
 Louisiana Medicaid Management Information System (LMMIS) EFT Authorization Application User Guide Date Created: 1/23/2014 Date Revised: 8/03/2018 Prepared By Technical Communications Group Molina Medicaid
Louisiana Medicaid Management Information System (LMMIS) EFT Authorization Application User Guide Date Created: 1/23/2014 Date Revised: 8/03/2018 Prepared By Technical Communications Group Molina Medicaid
SCRIPT: MA Enrollment Long Form
 SCRIPT: MA Enrollment Long Form (Purpose: This script is to be used for Medicare Advantage plan telephone enrollment for new enrollments only. Telephone enrollment may be offered: 1. If the telephone call
SCRIPT: MA Enrollment Long Form (Purpose: This script is to be used for Medicare Advantage plan telephone enrollment for new enrollments only. Telephone enrollment may be offered: 1. If the telephone call
Electronic Prescribing of Controlled Substances (EPCS)
 Electronic Prescribing of Controlled Substances (EPCS) This document, as well as the software described in it, is provided under a software license agreement with STI Computer Services, Inc. Use of this
Electronic Prescribing of Controlled Substances (EPCS) This document, as well as the software described in it, is provided under a software license agreement with STI Computer Services, Inc. Use of this
NANP Administration System (NAS) User Registration Guide 2.6v
 NANP Administration System (NAS) User Registration Guide 2.6v November 8, 2017 TABLE OF CONTENTS 1.0 Introduction... 4 1.1 Purpose... 4 1.2 NAS Overview... 4 1.3 Content Summary... 4 1.4 Problem Solving...
NANP Administration System (NAS) User Registration Guide 2.6v November 8, 2017 TABLE OF CONTENTS 1.0 Introduction... 4 1.1 Purpose... 4 1.2 NAS Overview... 4 1.3 Content Summary... 4 1.4 Problem Solving...
General Companion Guide 837 Professional and Institutional Healthcare Claims Submission Version Version Date: June 2017
 General Companion Guide 837 Professional and Institutional Healthcare Claims Submission Version 5010 Version Date: June 2017 1 Introduction ************************************************************************
General Companion Guide 837 Professional and Institutional Healthcare Claims Submission Version 5010 Version Date: June 2017 1 Introduction ************************************************************************
CAQH ProView. Participating Organization User Guide
 CAQH ProView Participating Organization User Guide Table of Contents CHAPTER 1: Introduction... 4 CAQH ProView Overview...4 Becoming a CAQH ProView Participating Organization...5 System Security...5 CHAPTER
CAQH ProView Participating Organization User Guide Table of Contents CHAPTER 1: Introduction... 4 CAQH ProView Overview...4 Becoming a CAQH ProView Participating Organization...5 System Security...5 CHAPTER
Supplement. Medicare. eapplication Quick Reference Guide. Coverage where Medicare leaves off. Americo
 Americo Medicare Supplement Coverage where Medicare leaves off eapplication Quick Reference Guide For agent use only. Not for public use. 15-138-15 (03/16) Americo This guide provides information on how
Americo Medicare Supplement Coverage where Medicare leaves off eapplication Quick Reference Guide For agent use only. Not for public use. 15-138-15 (03/16) Americo This guide provides information on how
Financial Center Administration Console USER GUIDE
 Financial Center Administration Console USER GUIDE For Client Use Only Effective April 2018 Table of contents Introduction 3 Communicating securely with Union Bank 3 Change Security Settings 4 Manage
Financial Center Administration Console USER GUIDE For Client Use Only Effective April 2018 Table of contents Introduction 3 Communicating securely with Union Bank 3 Change Security Settings 4 Manage
PearsonAccess User Guide PARCC
 PearsonAccess User Guide PARCC Copyright 2013, Pearson Education, Inc. Published December 16, 2013 1.0 Document Revisions... 5 2.0 Getting Started... 6 2.1 Getting Started - Introduction... 7 2.2 Getting
PearsonAccess User Guide PARCC Copyright 2013, Pearson Education, Inc. Published December 16, 2013 1.0 Document Revisions... 5 2.0 Getting Started... 6 2.1 Getting Started - Introduction... 7 2.2 Getting
First Data Global Gateway SM Virtual Terminal User Manual
 First Data Global Gateway SM Virtual Terminal User Manual Version 1.0 2015 First Data Corporation. All Rights Reserved. All trademarks, service marks, and trade names referenced in this material are the
First Data Global Gateway SM Virtual Terminal User Manual Version 1.0 2015 First Data Corporation. All Rights Reserved. All trademarks, service marks, and trade names referenced in this material are the
MEDICAID MARYLAND PRE-ENROLLMENT INSTRUCTIONS MCDMD
 MEDICAID MARYLAND PRE-ENROLLMENT INSTRUCTIONS MCDMD HOW LONG DOES PRE-ENROLLMENT TAKE? Standard processing time is 2 weeks. WHAT FORM(S) SHOULD I COMPLETE? Maryland Medical Care Programs Submitter Identification
MEDICAID MARYLAND PRE-ENROLLMENT INSTRUCTIONS MCDMD HOW LONG DOES PRE-ENROLLMENT TAKE? Standard processing time is 2 weeks. WHAT FORM(S) SHOULD I COMPLETE? Maryland Medical Care Programs Submitter Identification
e-submission System User Manual Authorised Person, Administrator, DI User and Security Officer
 e-submission System User Manual For Authorised Person, Administrator, DI User and Security Officer June 2017 Version 1.14 Table of Contents 1 GENERAL OVERVIEW... 2 1.1 INTRODUCTION... 2 1.2 REGISTRATION...
e-submission System User Manual For Authorised Person, Administrator, DI User and Security Officer June 2017 Version 1.14 Table of Contents 1 GENERAL OVERVIEW... 2 1.1 INTRODUCTION... 2 1.2 REGISTRATION...
QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting
 QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting Updated February 2017 Release Notes November 2016 Initial posting of this resource February 2017 Updated posting of this resource NOTE:
QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting Updated February 2017 Release Notes November 2016 Initial posting of this resource February 2017 Updated posting of this resource NOTE:
What is a Prescription Drug Monitoring Program?
 What is a Prescription Drug Monitoring Program? A prescription drug monitoring program (PDMP) is a state program that collects controlled substance prescription records from dispensers (e.g., pharmacies)
What is a Prescription Drug Monitoring Program? A prescription drug monitoring program (PDMP) is a state program that collects controlled substance prescription records from dispensers (e.g., pharmacies)
MEDICARE Texas (TRAILBLAZERS) PRE-ENROLLMENT INSTRUCTIONS 00900
 MEDICARE Texas (TRAILBLAZERS) PRE-ENROLLMENT INSTRUCTIONS 00900 HOW LONG DOES PRE-ENROLLMENT TAKE? Standard processing time is 5 business days after receipt. WHAT FORM(S) SHOULD I COMPLETE? EDI Provider
MEDICARE Texas (TRAILBLAZERS) PRE-ENROLLMENT INSTRUCTIONS 00900 HOW LONG DOES PRE-ENROLLMENT TAKE? Standard processing time is 5 business days after receipt. WHAT FORM(S) SHOULD I COMPLETE? EDI Provider
Therapy Provider Portal. User Guide
 Therapy Provider Portal User Guide Page 2 of 16 UCare User Guide V1.7 Table of Contents I. Introduction...3 About HSM Therapy Management... 4 Terms of Use... 4 Contact Information... 6 II. Using the Therapy
Therapy Provider Portal User Guide Page 2 of 16 UCare User Guide V1.7 Table of Contents I. Introduction...3 About HSM Therapy Management... 4 Terms of Use... 4 Contact Information... 6 II. Using the Therapy
Agent Online Application User Guide
 Agent Online Application User Guide Contact Phone Numbers: Agent Licensing & Supplies: 1-800-321-0102 Marketing Support: 1-866-644-3988 Claims, Underwriting, Cust. Svc., & Commissions: 1-855-664-5517 02/20/2015
Agent Online Application User Guide Contact Phone Numbers: Agent Licensing & Supplies: 1-800-321-0102 Marketing Support: 1-866-644-3988 Claims, Underwriting, Cust. Svc., & Commissions: 1-855-664-5517 02/20/2015
Aerial iexchange Users Guide
 Aerial iexchange Users Guide 2014.1 How to Run the Util\\\ \user Disclaimer How to reach us Copyright Information contained in this document is subject to change without notice and does not present a commitment
Aerial iexchange Users Guide 2014.1 How to Run the Util\\\ \user Disclaimer How to reach us Copyright Information contained in this document is subject to change without notice and does not present a commitment
ONTARIO CHIROPRACTIC ASSOCIATION PATIENT MANAGEMENT PROGRAM PUTTING EXPERIENCE INTO PRACTICE. PMP HCAI & OCF Guide
 ONTARIO CHIROPRACTIC ASSOCIATION PATIENT MANAGEMENT PROGRAM PUTTING EXPERIENCE INTO PRACTICE PMP HCAI & OCF Guide December 2010 HCAI - Patient Management Program Contents Contact Information... 3 PMP HCAI
ONTARIO CHIROPRACTIC ASSOCIATION PATIENT MANAGEMENT PROGRAM PUTTING EXPERIENCE INTO PRACTICE PMP HCAI & OCF Guide December 2010 HCAI - Patient Management Program Contents Contact Information... 3 PMP HCAI
Benefit Tracker. User Manual
 Benefit Tracker User Manual 2017 www.modahealth.com Revised 10/20/2017 Table of Contents Introduction Page 3 Benefit Tracker Overview Page 3 Security and Password Protection Page 3 Passwords Page 4 Getting
Benefit Tracker User Manual 2017 www.modahealth.com Revised 10/20/2017 Table of Contents Introduction Page 3 Benefit Tracker Overview Page 3 Security and Password Protection Page 3 Passwords Page 4 Getting
PCORI Online: Awardee User Guide Research Awards
 PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
RelayHealth Legal Notices
 Page 1 of 7 RelayHealth Legal Notices PRIVACY POLICY Revised August 2010 This policy only applies to those RelayHealth services for which you also must accept RelayHealth s Terms of Use. RelayHealth respects
Page 1 of 7 RelayHealth Legal Notices PRIVACY POLICY Revised August 2010 This policy only applies to those RelayHealth services for which you also must accept RelayHealth s Terms of Use. RelayHealth respects
User Guidance Manual
 User Guidance Manual Table of Contents TABLE OF CONTENTS... 1 PROGRAM OVERVIEW... 3 PORTAL OVERVIEW... 3 GETTING TO THE LOGIN PAGE... 4 LOGGING INTO THE PORTAL... 5 IF YOU HAVE AN ACCOUNT... 5 YOU DO NOT
User Guidance Manual Table of Contents TABLE OF CONTENTS... 1 PROGRAM OVERVIEW... 3 PORTAL OVERVIEW... 3 GETTING TO THE LOGIN PAGE... 4 LOGGING INTO THE PORTAL... 5 IF YOU HAVE AN ACCOUNT... 5 YOU DO NOT
CAQH ProView. Dentist Practice Manager Module User Guide
 CAQH ProView Dentist Practice Manager Module User Guide Table of Contents Chapter 1: Introduction... 1 CAQH ProView Overview... 1 System Security... 2 Chapter 2: Registration... 3 Existing Practice Managers...
CAQH ProView Dentist Practice Manager Module User Guide Table of Contents Chapter 1: Introduction... 1 CAQH ProView Overview... 1 System Security... 2 Chapter 2: Registration... 3 Existing Practice Managers...
EDI Billing Guide for L.A. County. Clinitrak. for Behavioral Health Providers
 EDI Billing Guide for L.A. County Clinitrak for Behavioral Health Providers Clinitrak EDI Billing Guide Document Number CLEG-100923 First edition printed April 2007 2010 Clinivate, LLC All Rights Reserved
EDI Billing Guide for L.A. County Clinitrak for Behavioral Health Providers Clinitrak EDI Billing Guide Document Number CLEG-100923 First edition printed April 2007 2010 Clinivate, LLC All Rights Reserved
Ascribe Pharmacy System User Guide
 Ascribe Pharmacy System User Guide i Ascribe Pharmacy System User Guide Contents Introduction... 1 Terminology... 1 Navigation... 1 Hot keys... 2 Required fields... 2 Resetting a forgotten password...
Ascribe Pharmacy System User Guide i Ascribe Pharmacy System User Guide Contents Introduction... 1 Terminology... 1 Navigation... 1 Hot keys... 2 Required fields... 2 Resetting a forgotten password...
Both of these paths will eventually lead you to the Welcome page starting on page 5.
 1] When you click on the Sponsorship link on the www.ti.com/giving page, you are taken to the login screen shown below. Here you have two choices: A] If you are a first time user, follow the directions
1] When you click on the Sponsorship link on the www.ti.com/giving page, you are taken to the login screen shown below. Here you have two choices: A] If you are a first time user, follow the directions
Oregon registration will open next Monday, the 26th. First register with CMS, then with your state.
 Sent: Tuesday, September 20, 2011 2:53 PM Subject: Meaningful Use Registration I want to make sure that the Portland Area I/T/U's are on track to receive the EHR Incentive payments. So far, who has registered
Sent: Tuesday, September 20, 2011 2:53 PM Subject: Meaningful Use Registration I want to make sure that the Portland Area I/T/U's are on track to receive the EHR Incentive payments. So far, who has registered
(EHR) Incentive Program
 REGISTRATION USER GUIDE For Eligible Professionals Medicaid Electronic Health Record (EHR) Incentive Program DECEMBER 2010 (12.28.10 ver2) CONTENTS Step 1... Getting started 3 Step 2... Login instruction
REGISTRATION USER GUIDE For Eligible Professionals Medicaid Electronic Health Record (EHR) Incentive Program DECEMBER 2010 (12.28.10 ver2) CONTENTS Step 1... Getting started 3 Step 2... Login instruction
Arkansas All-Payer Claims Database (APCD) Onboarding Packet
 Arkansas All-Payer Claims Database (APCD) Onboarding Packet October 2018 Introduction The onboarding packet contains information for submitting entities (SEs) to establish connectivity for secure data
Arkansas All-Payer Claims Database (APCD) Onboarding Packet October 2018 Introduction The onboarding packet contains information for submitting entities (SEs) to establish connectivity for secure data
National Drug Treatment Monitoring System (NDTMS) NDTMS Drug and Alcohol Monitoring System (DAMS) Agency User Guide
 National Drug Treatment Monitoring System (NDTMS) NDTMS Drug and Alcohol Monitoring System (DAMS) Agency User Guide Updated: 10/01/2011 Author: Approver: P Mantrala M Hinchcliffe NDTMS DAMS - Agency User
National Drug Treatment Monitoring System (NDTMS) NDTMS Drug and Alcohol Monitoring System (DAMS) Agency User Guide Updated: 10/01/2011 Author: Approver: P Mantrala M Hinchcliffe NDTMS DAMS - Agency User
Mississippi Medicaid. Mississippi Medicaid Program Provider Enrollment P.O. Box Jackson, Mississippi Complete form and mail original to:
 Mississippi Medicaid Complete form and mail original to: Blank forms may by copied. Call LTC at 888-941-8967 if you have questions. Please complete the following Mississippi Medicaid Provider EDI Enrollment
Mississippi Medicaid Complete form and mail original to: Blank forms may by copied. Call LTC at 888-941-8967 if you have questions. Please complete the following Mississippi Medicaid Provider EDI Enrollment
Quick guide to the SmartSimple on-line portal (making an application)
 EPA Research Programme 2014-2020 Quick guide to the SmartSimple on-line portal (making an application) POWERED BY SMARTSIMPLE Disclaimer Please read this document carefully prior to using the on-line portal.
EPA Research Programme 2014-2020 Quick guide to the SmartSimple on-line portal (making an application) POWERED BY SMARTSIMPLE Disclaimer Please read this document carefully prior to using the on-line portal.
Step By Step Registration Process
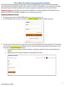 The Florida Prescription Drug Monitoring Program, known as E FORCSE (Electronic Florida Online Reporting of Controlled Substance Evaluation Program), was created by the 2009 Florida Legislature in an initiative
The Florida Prescription Drug Monitoring Program, known as E FORCSE (Electronic Florida Online Reporting of Controlled Substance Evaluation Program), was created by the 2009 Florida Legislature in an initiative
YOUR GUIDE TO I-STOP COMPLIANCE AND EPCS
 YOUR GUIDE TO I-STOP COMPLIANCE AND EPCS Q: I prescribe medication in New York. Why does EPCS matter to me? A: Beginning March 2015, paper prescriptions will no longer be accepted. Beginning March 27,
YOUR GUIDE TO I-STOP COMPLIANCE AND EPCS Q: I prescribe medication in New York. Why does EPCS matter to me? A: Beginning March 2015, paper prescriptions will no longer be accepted. Beginning March 27,
HIPAA 837 Claims EDI Agreement. Agreement. HIPAA 837 Claims EDI Agreement
 HIPAA 837 Claims EDI Agreement Agreement All New Jersey Medicaid and Charity Care Providers desiring to submit HIPAA formatted electronic claims must complete a HIPAA 837 Claims EDI Agreement as required
HIPAA 837 Claims EDI Agreement Agreement All New Jersey Medicaid and Charity Care Providers desiring to submit HIPAA formatted electronic claims must complete a HIPAA 837 Claims EDI Agreement as required
Vendor Registration and Training
 Vendor Registration and Training Bid Express Registration Guide Bid Express Vendor Guide February 2015 Prepared By Address: 5700 SW 34th Street, Suite 1235, Gainesville, Florida 32608-5371 Web: www.infotechfl.com
Vendor Registration and Training Bid Express Registration Guide Bid Express Vendor Guide February 2015 Prepared By Address: 5700 SW 34th Street, Suite 1235, Gainesville, Florida 32608-5371 Web: www.infotechfl.com
IDRP Portal User Guide for Providers and Plans
 IDRP Portal User Guide for Providers and Plans Version 1.0, September 2017 Controlled electronic version prevails over printed copy of this document. Provided by MAXIMUS Federal Services, Folsom, CA. Work
IDRP Portal User Guide for Providers and Plans Version 1.0, September 2017 Controlled electronic version prevails over printed copy of this document. Provided by MAXIMUS Federal Services, Folsom, CA. Work
Your mymeritain Personalized Member Website
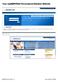 Your mymeritain Personalized Member Website 2008 Meritain Health, Inc. Last Updated 5.23.2008 Your mymeritain Member Website The mymeritain Member Website offers Members a user-friendly web experience,
Your mymeritain Personalized Member Website 2008 Meritain Health, Inc. Last Updated 5.23.2008 Your mymeritain Member Website The mymeritain Member Website offers Members a user-friendly web experience,
