Dispenser s Implementation Guide
|
|
|
- Augustus Barber
- 6 years ago
- Views:
Transcription
1 Dispenser s Implementation Guide Arkansas Department of Health Prescription Monitoring Program March 2013 Note: This document is periodically updated. Please refer to the A PMP website, for the most current version. This document is formatted for duplex printing.
2 Dispenser s Implementation Guide This page intentionally left blank. Copyright Health Information Designs, LLC
3 Contents Contents 1 Document Overview... 1 Purpose and Content Program Overview... 3 Purpose Data Collection and Tracking... 5 Data Collection equirements... 5 eporting equirements... 5 Exemptions... 6 equired Prescription Information... 6 eporting Noncompliance... 7 Zero eports... 7 tate of Emergency Data ubmission... 9 About This Chapter... 9 Timeline and equirements... 9 Upload pecifications... 9 Creating Your Account Modifying Your Upload Account eporting Zero Dispensing eport Zero Activity xentry eport Zero Activity File Upload Data Delivery Methods About This Chapter ecure FTP over H Encrypted File with OpenPGP Via FTP L Website Physical Media (CD or DVD) Universal Claim Form (UCF) ubmission eporting equirements for UCF ubmissions Notes about NDC Numbers Online UCF ubmission Upload eports and Edit Definitions Upload eports View Upload eports View Zero eports Error Correction ubmit a New ecord Copyright Health Information Designs, LLC i
4 Contents evise a ecord Void a ecord Edit Definitions Assistance and upport Technical Assistance Administrative Assistance Glossary Document Information Copyright Notice and Trademarks Disclaimer Formatting Conventions Version History Change Log Appendix A: AAP 4.2 pecifications... A-1 Appendix B: Zero eport pecifications... B-1 Copyright Health Information Designs, LLC ii
5 Document Overview 1 Document Overview Purpose and Content The xentry Dispenser s Implementation Guide serves as a step-by-step implementation and training guide for dispensers in the tate of Arkansas who use xentry as a repository for the reporting of their chedule II, III, IV, and V controlled substances. It includes such topics as: eporting requirements for dispensers in the tate of Arkansas Data file submission guidelines and methods Creating your upload account Creating a data file Uploading or reporting your data Understanding upload error codes and definitions This guide has been customized to target the specific training needs of Arkansas dispensers. It is intended for use by all dispensers in the tate of Arkansas required to report their dispensing of controlled substances. Copyright Health Information Designs, LLC 1
6 Document Overview This page intentionally left blank. Copyright Health Information Designs, LLC 2
7 Program Overview 2 Program Overview Purpose Arkansas s Electronic Prescription Monitoring Program (PMP) was authorized in 2011 by Arkansas tate Legislature Act 304. This program was created for the following purposes: To enhance patient care by providing prescription monitoring information that will ensure legitimate use of controlled substances in health care To help curtail the misuse and abuse of controlled substances To assist in combating illegal trade in and diversion of controlled substances To enable access to prescription information by practitioners, law enforcement agents, and other authorized individuals and agencies The Arkansas Department of Health (ADH) oversees the operation of the PMP and has selected Health Information Designs (HID) to develop a database that will collect and store prescribing and dispensing data for controlled substances in chedules II, III, IV, and V. HID s xentry is a Web-based program that facilitates the collection, analysis, and reporting of information on the prescribing, dispensing, and use of controlled substance prescription drugs. xentry leads the industry in flexibility, functionality, and ease of use. Arkansas law requires that each dispenser shall submit, by electronic means, information regarding each prescription dispensed for a controlled substance. Each time a controlled substance is dispensed to an individual, the dispenser shall submit the information required by Arkansas law to the central repository as soon thereafter as possible, but not more than one week after the controlled substance was dispensed. The ADH shall establish and maintain procedures to ensure that the privacy, confidentiality, and security of patient information collected, recorded, transmitted, and maintained is not disclosed except as provided in Act 304. Copyright Health Information Designs, LLC 3
8 Program Overview This page intentionally left blank. Copyright Health Information Designs, LLC 4
9 Data Collection and Tracking 3 Data Collection and Tracking Data Collection equirements This guide provides information regarding the Arkansas Prescription Monitoring Program (A PMP). The program was established to collect data on all chedules II, III, IV, and V controlled substances dispensed to an individual in the tate of Arkansas. This includes state-controlled chedule IV substances tramadol and nalbuphine, and chedule V solid dosage forms of ephedrine, pseudoephedrine, and phenylpropanolamine. Each time a controlled substance is dispensed to an individual, the controlled substance shall be reported to the A PMP, using a format approved by the ADH, as soon thereafter as possible, but not more than one week after the controlled substance was dispensed. All dispensers of controlled substances must meet the reporting requirements set forth by Arkansas law in a secure methodology and format. uch approved formats may include secure FTP over H, L website, CD OM, or other agreed-upon method. Note: A dispenser is identified as a practitioner who is authorized by the state to deliver a controlled substance to an ultimate user or research subject by or pursuant to the lawful order of a practitioner, including without limitation, the prescribing, administering, packaging, labeling, or compounding necessary to prepare the controlled substance for that delivery. eporting equirements All dispensers of chedules II, III, IV, and V controlled substance prescriptions are required to collect and report their dispensing information. If you are a chain pharmacy, your data will likely be submitted from your home office. Please verify this with your home office. If you are an independent pharmacy or other entity, please forward the reporting requirements to your software vendor. They will need to create the data file, and they may be able to submit the data on your behalf. If not, follow the instructions provided in the Data ubmission chapter to submit the data. Note: Additions or deletions of other drugs specified by Arkansas law may happen periodically. These changes must go through the regulatory process in order to be added or deleted. You will be notified of any changes that are made in the future. For detailed information for each of the fields required by the state of Arkansas and the fields required by the American ociety for Automation in Pharmacy (AAP), please see Appendix A: AAP 4.2 pecifications. Copyright Health Information Designs, LLC 5
10 Data Collection and Tracking Exemptions The following entities are exempt from reporting data: A licensed hospital pharmacy that is dispensing controlled substances for the purpose of outpatient services, inpatient hospital care, or at the time of discharge from a hospital, except for a pharmacy owned by a hospital that has a retail pharmacy permit and is distributing controlled substances directly to the public A wholesale distributor of chedule II chedule V controlled substances A practitioner or other authorized person who administers a controlled substance equired Prescription Information All dispensers of chedule II, III, IV, and V controlled substances are required to collect and report the information in the following table. For detailed information for each of these fields, please see Appendix A: AAP 4.2 pecifications. Field Name Field ID Pharmacy Header NPI Number DEA Number Pharmacy/Dispensing Practitioner Name PHA01 PHA03 PHA04 Patient Information Last Name First Name Address Information 1 City Address tate Address ZIP Code Address Date of Birth Gender Code PAT07 PAT08 PAT12 PAT14 PAT15 PAT16 PAT18 PAT19 Dispensing ecord Prescription Number Date Written efills Authorized Date Filled efill Number Product ID *Note: NDC is required DP02 DP03 DP04 DP05 DP06 DP08 Copyright Health Information Designs, LLC 6
11 Data Collection and Tracking Field Name Quantity Dispensed Days upply Classification Code for Payment Type (if available) Field ID DP09 DP10 DP16 Prescriber Information DEA Number Last Name First Name PE02 PE05 PE06 The Data ubmission chapter provides all the instructions necessary to submit the required information. eporting Noncompliance Any dispenser who knowingly fails to submit prescription monitoring information as required by Arkansas law, intentionally submits incorrect prescription information, or discloses confidential information obtained from the PMP database may be subject to criminal charges or may be disciplined by the dispenser s licensing board. Zero eports If a pharmacy usually dispenses controlled substances in Arkansas, but has no dispensing transactions to report for the preceding week, the pharmacy must report this information to the A PMP by filing a zero report, as described in the eporting Zero Dispensing topic in this guide. tate of Emergency In the event that a state of emergency is declared in Arkansas, and a dispenser is unable to report to xentry due to the declared state of emergency, reporting will not be required until the state of emergency is lifted. Once lifted the dispenser must report the backlog of data as soon as possible in order to bring reporting current. Copyright Health Information Designs, LLC 7
12 Data Collection and Tracking This page intentionally left blank. Copyright Health Information Designs, LLC 8
13 Data ubmission 4 Data ubmission About This Chapter This chapter provides information and instructions for submitting data to the A PMP. Timeline and equirements Dispensers or software vendors can establish submission accounts upon receipt of this guide and the date listed below. Instructions for setting up an account are provided in the Creating Your Account topic in this chapter. You may create your account and begin submitting test data files on or after February 14, Beginning March 1, 2013, dispensers are required to report their data weekly for the previous week, unday through aturday. However, dispensers are encouraged to report more frequently if they would like. The ADH requests that dispensers report retroactive data for the period eptember 1, 2012 through February 28, Dispensers will have until May 1, 2013 to report their retroactive data. If a dispenser does not dispense any controlled substances for the reporting period, it must file a zero report for that reporting period or it will be considered noncompliant. Upload pecifications Files must be in the AAP 4.2 format, as defined in Appendix A: AAP 4.2 pecifications. Files for upload should be named in a unique fashion, with a prefix constructed from the date (YYYYMMDD) and a suffix of.dat. An example file name would be dat. All of your upload files will be kept separate from the files of others. eports for multiple dispensers can be in the same upload file in any order. Controlled substance prescription information must be reported weekly for the previous week, unday through aturday. Copyright Health Information Designs, LLC 9
14 Data ubmission Creating Your Account Prior to submitting data, you must create an upload account. If you have already created your account, proceed to the appropriate section of this document that outlines the steps you must follow to upload your data. Note: Multiple pharmacies can be uploaded in the same file. For example, chain pharmacies may send in one file containing controlled substance dispensing information for all of their pharmacies licensed in the state of Arkansas. Therefore, chains with multiple stores only have to set up one account to upload a file. Perform the following steps to create an account: 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Dispenser. A window similar to the following is displayed: 3 Click xentry Data Upload. A window similar to the following is displayed: 4 Type newacct in the User Name field. 5 Type welcome in the Password field. 6 Click OK. Copyright Health Information Designs, LLC 10
15 Data ubmission A window similar to the following is displayed: 7 Click etup Upload Account. A window similar to the following is displayed: 8 Enter your DEA number in the Physician or Pharmacy DEA number field. 9 Enter your ZIP code in the ZIP Code field. 10 Click Next. The New Account etup for A PDMP Upload Access window is displayed as shown on the following page. Copyright Health Information Designs, LLC 11
16 Data ubmission 11 Complete all required fields (indicated by an asterisk) on the New Account etup for A PDMP Upload Access window, using the information in the following table as a guideline: Field Description/Usage Account selection Choose Keep <account number> as my account for a single Pharmacy if you wish to use the suggested account name. Choose Create an account using <suggested account name> as my ID for uploading more than one Pharmacy s Data if you wish to enter an account name of your choosing. If this option is selected, type the desired account name in this field. Contact Information Note: Information in this section is used for contact purposes in the event a problem occurs with a data upload. Contact Name (equired) Type the first and last name of the contact person. Copyright Health Information Designs, LLC 12
17 Data ubmission Field Contact Address Contact Contact Phone Contact Fax Anticipated Upload Method Pharmacies I will be reporting Or Manually Enter List of Ids eporting for: Comma eparated List Dispenser Type Description/Usage (equired) Type the contact s street address, city, state, and ZIP code in the appropriate fields. (equired) Type the contact s address. Click the down arrow in the field to the right of the Contact field to select Edit eports for All Uploads. (equired) Type the contact s phone number, using the format (equired) Type the contact s fax number, using the format Click the down arrow in the field to the right of the Contact Fax field and select Fax Edit eports for All Uploads. elect the method of data upload you plan to use to report your data: ecure FTP using H FTP of file Encrypted with OpenPGP Upload with Internet browser using L Mail a CD Mail a DVD UCF ubmission A list of all pharmacies with names similar to your store name/pharmacy name is displayed in this field. To select additional pharmacies for which you will be reporting, press the [CTL] key and then click the name of each pharmacy you wish to select. The pharmacies you select will be tied to your user name. You may use this field to manually enter a list of pharmacy IDs you will be reporting for, rather than selecting from the list in the field above. When manually entering multiple pharmacy IDs, separate each ID with a comma. (equired) elect whether you are a Pharmacy or a Dispensing Practitioner. Note: If you select the wrong dispenser type, you will be required to re-enter all of your account setup information once the dispenser type has been corrected. Copyright Health Information Designs, LLC 13
18 Data ubmission Field Dispenser ub-type Dispenser Location Description/Usage (equired) elect the appropriate dispenser sub-type. Note: The options that display in this field are dependent on whether you answered Pharmacy or Dispensing Practitioner in the Dispenser Type field. Pharmacy ub-types Pharmacy (Chain) Pharmacy (Community) Pharmacy (Hospital) Non-esident Pharmacy Mail Order Pharmacy Mfg/Wholesalers/ Distributors Mfg/Whse/Dist. Oxygen Pharmacy Nuclear Pharmacy Parenteral Pharmacy Precursor Pharmacy Veterinary Pharmacy Dispensing Practitioner ub-types Dentist/Oral urgeon Medical Doctor Optometrist Podiatrist Veterinarian (equired) elect whether you are an In-tate or Outof-tate dispenser. 12 After completing all required fields, click Next. A window similar to the following is displayed: A randomly-assigned password for the FTP and FTP upload process is provided to you. oftware vendors setting up multiple accounts may choose from the following options: Create each account separately by using the method listed above. After you finish one pharmacy s account, click etup Upload Account on the home page, and repeat the process; Or Create multiple accounts using one pharmacy s DEA number and ZIP code. If you choose this method, select et up user name as a group. Note: Data error reports are submitted to the address(es) supplied for the account(s). Copyright Health Information Designs, LLC 14
19 Data ubmission Modifying Your Upload Account Use this function if you need to modify the information supplied when you originally created your account. 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Dispenser, and then click xentry Data Upload. A login window is displayed. 3 Type your user name in the User Name field. 4 Type your password in the Password field. 5 Click OK. 6 From the xentry home page, click Modify Upload Account. 7 Update the information as necessary, using the field descriptions provided in the Creating Your Account topic as a guideline. 8 Click Next. A message displays that your account information was successfully updated. eporting Zero Dispensing If you have no dispensing transactions to report for the preceding reporting period, you must report this information to the A PMP. You may report zero dispensing by using the functionality provided within xentry via the eport Zero Activity menu item, or by creating and uploading a zero report data file. The steps you must perform for each method are provided in the following sections. eport Zero Activity xentry 1 If you do not have an account, perform the steps in Creating Your Account. 2 Open an Internet browser window and type the following UL in the address bar: 3 Click Dispenser, and then click xentry Data Upload. A login window is displayed. 4 Type your user name in the User Name field. 5 Type your password in the Password field. 6 Click OK. 7 From the xentry home page, click eport Zero Activity. Copyright Health Information Designs, LLC 15
20 Data ubmission A window similar to the following is displayed: 8 Type the start date for this report in the Period tart Date field, using the dd/mm/yy format. Notes: The Period End Date field is populated with the current date. You may adjust this date, if necessary. All other pharmacy information is populated with the information provided when you created your account. 9 Click Continue. A message similar to the following is displayed: eport Zero Activity File Upload 1 If you have not created an account, perform the steps in Creating Your Account. 2 Prepare the zero report data file for submission, using the specifications described in Appendix B: Zero eport pecifications. Important Notes: The file name should be constructed using the date of submission to HID as the file name, and should have a.dat extension. For example, name the file dat if you submit it on March 1, Copyright Health Information Designs, LLC 16
21 Data ubmission Do not include spaces in the file name. If you submit more than one file within the same day, you must uniquely name each file so the system does not overwrite existing uploaded files. For example, if uploading three files within the same day, you could use the following file names: a.dat, b.dat, and c.dat. The system will accept zipped files and you should name them using the date of submission to HID. For example, name the file zip if you submit it on March 1, Before transmitting your file, rename it to include the suffix.up (e.g., dat.up). This will ensure that we do not try to load the file while you are transmitting it. Once transmission is complete, rename the file back to the original name (e.g., dat). 3 Upload the file using the steps provided in one of the following data delivery topics: ecure FTP over H Encrypted File with OpenPGP Via FTP L Website HID tracks the use of the Web-based tool, date stamps incoming files, and notifies you of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. Copyright Health Information Designs, LLC 17
22 Data ubmission This page intentionally left blank. Copyright Health Information Designs, LLC 18
23 Data Delivery Methods 5 Data Delivery Methods About This Chapter This chapter provides information about data delivery methods you can use to upload your controlled substance reporting data file(s). For quick reference, click the desired hyperlink in the following table to view the stepby-step instructions for your chosen data delivery method: Delivery Method Page ecure FTP over H 19 Encrypted File with OpenPGP Via FTP 20 L Website 21 Physical Media 22 Universal Claim Form (UCF) ubmission eporting equirements for UCF ubmissions 23 Notes about NDC Numbers 23 Online UCF ubmission 23 ecure FTP over H There are many free software products that support ecure FTP. Neither the ADH nor HID is in a position to direct or support your installation of operating system software for ecure FTP; however, we have information that WinCP ( has been used successfully by other pharmacies. 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important Notes: The file name should be constructed using the date of submission to the A PMP as the file name and should have a.dat extension. For example, name the file dat if it is submitted on March 1, Do not include spaces in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.dat, b.dat, and c.dat. Zipped files can be accepted and should be named using the date of submission. For example, name the file zip if it is submitted on March 1, Copyright Health Information Designs, LLC 19
24 Data Delivery Methods Before transmitting your file, rename it to include the suffix.up (e.g., dat.up). This will ensure that we do not try to load the file while you are transmitting it. Once transmission is complete, rename the file back to the original name (e.g., dat). 3 FTP the file to sftp://arpmpreporting.hidinc.com. 4 When prompted, type arpdm (lower case) in front of your DEA number (or Generic ID) as your user ID and enter the password you supplied when creating your account. 5 Place the file in the new directory. 6 Once the transmission is complete, rename the file without the.up extension (e.g., dat). 7 If desired, view the results of the transfer/upload in your user directory. The file name is YYYYMMDD.rpt. 8 Log off when the file transfer/upload is complete. HID tracks the use of the Web-based tool, and incoming files are date stamped. You are notified of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. Encrypted File with OpenPGP Via FTP There are many free software products which support file encryption using the PGP standard. Neither the ADH nor HID is in a position to direct or support your installation of PGP compatible software utilities; however, our usage indicates that software from the GnuPG Project ( ) should be compatible with many operating systems. 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Import the PGP public key, supplied during the account creation, into your PGP key ring. 3 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important notes: The file name should be constructed using the date of submission as the file name and should have a.pgp extension. For example, name the file pgp if it is submitted on March 1, Do not include spaces in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.pgp, b.pgp, and c.pgp. Copyright Health Information Designs, LLC 20
25 Data Delivery Methods Before transmitting your file, rename it to include the suffix.up (e.g., pgp.up). This will ensure that we do not try to load the file while you are transmitting it. Once transmission is complete, rename the file back to the original name (e.g., pgp). 4 Encrypt the file with the PGP software, using the public key supplied during account creation. Note: PGP encryption performs a single compression as it encrypts, so there is no need to zip the file. 5 FTP the file to ftp://arpmpreporting.hidinc.com. 6 When prompted, type arpdm (lower case) in front of your DEA number (or Generic ID) as your user ID and enter the password you supplied when creating your account. 7 Place the file in the new directory. 8 Once the transmission is complete, rename the file without the.up extension (e.g., pgp). 9 If desired, view the results of the transfer/upload in your user directory. The file name is YYYYMMDD.rpt. 10 Log off when the file transfer/upload is complete. HID tracks the use of the Web-based tool, and incoming files are date stamped. You are notified of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. L Website 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important notes: The file name should be constructed using the date of submission to the A PMP as the file name and should have a.dat extension. For example, name the file dat if it is submitted on March 1, Do not include spaces or parentheses in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.dat, b.dat, and c.dat. Zipped files can be accepted and should be named using the date of submission. For example, name the file zip if it is submitted on March 1, Copyright Health Information Designs, LLC 21
26 Data Delivery Methods 3 Open a Web browser and enter the following UL: 4 Click Dispenser, and then click xentry Data Upload. 5 When prompted, enter the user ID and password supplied when the account was created. 6 Click Upload a File. 7 Click Browse to navigate to the location where you saved the file created in step 2. 8 If not previously named according to upload requirements, rename the file using the format YYYYMMDD.dat, for example, dat. 9 Click to select the file, and then click Open. 10 Click end File. HID tracks the use of the Web-based tool, and incoming files are date stamped. You are notified of a successful file transmission. After the file is reviewed for accuracy, you are notified of the status of the submitted file. Physical Media (CD or DVD) 1 If an account has not yet been created, perform the steps in Creating Your Account. 2 Prepare the data file for submission, using the AAP specifications described in Appendix A: AAP 4.2 pecifications. Important notes: The file name should be constructed using the date of submission to HID as the file name, and should have a.dat extension. For example, name the file dat if it is submitted on March 1, Do not include spaces in the file name. If more than one file is submitted within the same day, each file must be uniquely named so that existing uploaded files are not overwritten. For example, if uploading three files within the same day, the following file names could be used: a.dat, b.dat, and c.dat. Zipped files can be accepted and should be named using the date of submission to HID. For example, name the file zip if it is submitted on March 1, Write the file to the preferred media (CD or DVD). 4 Add a label to the outside of the media that contains the following information: DEA Number Date of ubmission Contact Person Copyright Health Information Designs, LLC 22
27 Data Delivery Methods 5 end the media by certified mail or courier to: Health Information Designs, LLC A PMP Program 391 Industry Drive Auburn, AL Universal Claim Form (UCF) ubmission If you have Internet access, but are unable to submit your data in a batch upload, you may submit prescription information using xentry s online universal claim form (UCF). When submitting information using the online UCF, the information provided must be complete and accurate. Only complete and accurate submissions are entered into the A PMP database. Please use the information in the Notes about NDC Numbers section below as a guideline for providing accurate NDC numbers. eporting equirements for UCF ubmissions ee the equired Prescription Information topic for details for reporting requirements. Notes about NDC Numbers Use the following information when entering NDC numbers on the UCF: NDCs are 11 digits and use the format When adding an NDC, do not include the dashes, for example, NDCs are typically located on the original medication bottle on the top right corner of the label, prefaced with NDC- and followed by the number. Manufacturers often leave off a zero in the NDC. In these instances, you should add the 0 where appropriate, using the following examples as a guideline: If the NDC appears this way Enter it this way (missing 0 in first segment) (missing 0 in 2nd segment) Online UCF ubmission If you do not have an automated record-keeping system capable of producing an electronic report using the AAP 4.2 format, you may submit prescription information using xentry s online UCF. The following new terms are introduced in this topic: ecord the patient, pharmacy, and prescription information that you enter for one patient on the UCF Batch a single record, or group of records, that you upload using the ubmit Batch function Copyright Health Information Designs, LLC 23
28 Data Delivery Methods Note: ecords can be continually added to a batch a convenient feature that allows you to enter records at your convenience and not all at one time. We recommend that you add as many records as possible to a batch before submitting it; however, you must submit and close batches in accordance with the required reporting time frame. Perform the following steps to use the online UCF to submit prescription information: 1 If you do not have an account, perform the steps in Creating Your Account. 2 Open an Internet browser window and type the following UL in the address bar: 3 Click Dispenser, and then click xentry Data Upload. A login window is displayed. 4 Type your user name in the User Name field. 5 Type your password in the Password field. 6 Click OK. 7 From the xentry home page, click UCF Form Entry. A window similar to the following is displayed: Enter Next Form allows you to prepare one or more records for submission. how Batch Counts displays the number of records in the batch currently being prepared for submission and the number of records that have been previously been submitted. 8 Click Enter Next Form. Copyright Health Information Designs, LLC 24
29 Data Delivery Methods A window similar to the following is displayed: The UCF contains three sections Patient Information, Dispenser Information, and Prescription Information. efer to the following information to complete these sections on the UCF: Patient Information Complete all fields in this section. Dispenser Information In this section, supply your DEA number in the DEA field. Once this information is provided, all associated pharmacy information available within the xentry database is auto-populated in the appropriate fields. Prescription Information Information for up to three prescriptions may be entered in this section, and all fields for each prescription must be completed. If entering more than one prescription for the same prescriber, you may select the Use Prescriber Information From Above check box to auto-populate each prescription with the previously-used prescriber information. Copyright Health Information Designs, LLC 25
30 Data Delivery Methods 9 Once all information has been entered, click ubmit. Notes: If information is missing from any required fields on the UCF, the UCF window will display again with the required fields indicated. Click Modify to add the missing information, and then click ubmit. If the system indicates that the DEA number or the NDC number you have provided is invalid, and you are certain you have provided the correct number, contact HID using the information supplied in Assistance and upport. 10 The UCF is displayed for your review. If all information is correct, click ubmit. If you need to modify any information, click Modify. Once you click ubmit, a window similar to the following is displayed: 11 Perform one of the following functions: Click Enter Next Form to add additional records to this batch. Click how Batch Counts to display the number of records in the current batch. Click ubmit/close Batch to upload this batch of records. Copyright Health Information Designs, LLC 26
31 Upload eports and Edit Definitions 6 Upload eports and Edit Definitions Upload eports xentry provides all data submitters with an upload report. When creating an account, you are required to submit an address and a fax number. You must also specify the method by which you wish to receive your upload report. If you FTP/FTP the data, a report will be placed in your home directory on the FTP server. Below is an example of an error report: A single record may be rejected, or if a certain percentage of records are rejected in an individual file, the entire file may be rejected. We track three types of errors: Minor Incorrect data in non-vital field erious ecord can be loaded with missing or inappropriate data Fatal ecord cannot be loaded A single record will be rejected if it contains a fatal error. An entire batch will be rejected if: ALL records have Fatal or erious errors More than 10% of the records have Fatal errors More than 20% of the records have erious errors Pharmacies are required to correct fatal errors and resubmit the records within seven (7) days of the initial record submission. Copyright Health Information Designs, LLC 27
32 Upload eports and Edit Definitions View Upload eports This function provides uploaders access to upload reports that were previously delivered via or fax following a data submission. By default, the reports that display for reviewing are provided for a 31-day period. However, uploaders can view reports outside of the 31-day default period by entering start and end dates for the desired date range. Perform the following steps to view upload reports: 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Dispenser, and then click xentry Data Upload. A login window is displayed. 3 Type your user name in the User Name field. 4 Type your password in the Password field. 5 Click OK. 6 From the xentry home page, click View Upload eports. A window similar to the following is displayed: 7 Click a hyperlink in the eport Name field to open an upload report for viewing. To view reports for a different time frame, type a start and end date in the eport Timeframe fields, and then click ubmit. View Zero eports This function provides uploaders the ability to view previously submitted zero reports. By default, the reports that display for reviewing are provided for a 31-day period. However, uploaders can view reports outside of the 31-day default period by entering start and end dates for the desired date range. Perform the following steps to view zero reports: 1 Open an Internet browser window and type the following UL in the address bar: 2 Click Dispenser, and then click xentry Data Upload. A login window is displayed. Copyright Health Information Designs, LLC 28
33 Upload eports and Edit Definitions 3 Type your user name in the User Name field. 4 Type your password in the Password field. 5 Click OK. 6 From the xentry home page, click View Zero eports. A window similar to the following is displayed: Error Correction Fatal errors will cause a record NOT to be loaded. If this occurs, correct the data that caused the error and resubmit the entire record. Fatal error corrections must be resubmitted within seven (7) days of the initial record submission. If a record with a serious or minor error is loaded and a correction is required, records can be corrected using the DP01 values as explained below. Note: Edit Number V1 as shown in the Edit Definitions table should not be resubmitted. All other records with errors that are not fatal will be loaded unless the batch thresholds are reached. Error thresholds are defined in the Upload eports section. The AAP 4.2 standard requires a dispenser to select an indicator in the DP01 (eporting tatus) field. Dispensers may submit new records, revise and resubmit records, and void (delete) erroneous records. These actions are indicated by supplying one of the following values in the DP01 field: 00 New ecord indicates a new record 01 evise indicates that one or more data elements in a previously-submitted record has been revised 02 Void indicates that the original record should be voided Use the information in the following topics to create, revise/resubmit, or void an erroneous record. Copyright Health Information Designs, LLC 29
34 Upload eports and Edit Definitions ubmit a New ecord Perform the following steps to submit a new record: 1 Create a record with the value 00 in the DP01 field. 2 Populate all other required fields and submit the record. Note: These steps are used to submit new records or to submit records that were previously submitted but received a fatal status on your error report. ecords with fatal errors are not loaded to the system. The errors in these records must be corrected in your system and resubmitted using the 00 status in the DP01 field. evise a ecord Perform the following steps to revise a record: 1 Create a record with the value 01 in the DP01 field. 2 Populate the following fields with the same information originally submitted in the erroneous record: PHA03 (DEA Provider ID) DP02 (Prescription Number) DP05 (Date Filled) 3 Fill in all other data fields with the correct information. This information will override the original data linked to the fields referenced in step 2. 4 ubmit the record. Important note: If any of the fields referenced in step 2 are part of the correction, the record must first be voided using the steps provided in the Void a ecord section, and then you must re-submit the record using the value 00 in the DP01 field. Void a ecord Perform the following steps to void (delete) a record: 1 end a record with the value 02 in the DP01 field. 2 Fill in all other data identical to the original record. This will void the original record submission. Copyright Health Information Designs, LLC 30
35 Upload eports and Edit Definitions Edit Definitions The following table describes the current list of edits: Edit Number Message everity Edit 01 Format of File Error Fatal Edit 02 Pharmacy DEA is blank Fatal Edit 05 Pharmacy ID not found Fatal Edit 09 Invalid DOB erious Edit 10 Gender is invalid Minor Edit 14 eporting status is invalid Fatal Edit 15 Date Dispensed is invalid or irrational erious Edit 18 Quantity is invalid erious Edit 20 Days upply > 360 erious Edit 21 NDC not found NDC not found (used when CDI segment is used) erious erious Edit 22 Product ID Qualifier is invalid Fatal Edit 25 Prescriber ID not found Prescriber ID cannot be blank Minor Fatal Edit 28 Date X Written is invalid Minor Edit 50 Customer Last Name blank Fatal Edit 51 Customer First Name blank Fatal Edit 52 Customer Address blank erious Edit 53 Customer ZIP Code is blank erious Edit 54 Customer ZIP and tate Code conflict erious Edit 56 Customer City is blank Minor Edit 60 Customer tate Code is blank erious Edit 61 Customer tate Code is invalid erious Edit 200 Prescription Number is blank Fatal Edit V1 ecord already exists Note: Duplicate records are not loaded. The number of duplicate records, if any, is displayed on the upload report produced after data file transmission has completed. Minor Copyright Health Information Designs, LLC 31
36 Upload eports and Edit Definitions This page intentionally left blank. Copyright Health Information Designs, LLC 32
37 Assistance and upport 7 Assistance and upport Technical Assistance If you need additional help with any of the procedures outlined in this guide, you can: Contact HID by at arpmp-info@hidinc.com O Call the HID Help Desk at Technical assistance is available Monday through Friday (except for holidays) from 8:00 a.m. to 5:00 p.m. Central tandard Time. Administrative Assistance If you have non-technical questions regarding the Arkansas PMP, please contact: Denise obertson, P.D. PMP Administrator Arkansas Department of Health 4815 West Markham, lot 25 Little ock, A Phone: (501) Fax: (501) denise.robertson@arkansas.gov Copyright Health Information Designs, LLC 33
38 Assistance and upport This page intentionally left blank. Copyright Health Information Designs, LLC 34
39 Glossary 8 Glossary ADH AAP Arkansas Department of Health American ociety for Automation in Pharmacy Batch Group of files (report or query requests) that are processed in the background while other work is continued Dispense To deliver a controlled substance to an ultimate user or research subject by or pursuant to the lawful order of a practitioner, including without limitation, the prescribing, administering, packaging, labeling, or compounding necessary to prepare the controlled substance for that delivery Dispenser A practitioner who is authorized to dispense controlled substances in the state of Arkansas FTP HID NDC PMP File Transfer Protocol; commonly-used protocol for exchanging files over any network Health Information Designs, LLC National Drug Code; describes specific drugs by drug manufacturer and package size Prescription Monitoring Program; term used by AAP Prescriber An individual licensed, registered, or otherwise authorized by the jurisdiction in which the individual is practicing to prescribe drugs in the course of professional practice. xentry Prescription drug monitoring system developed by Health Information Designs, LLC Copyright Health Information Designs, LLC 35
40 Glossary FTP L ecure File Transfer Protocol (also referred to as H File Transfer Protocol ); provides file transfer and manipulation functionality over any reliable data stream ecure ockets Layer; cryptographic protocol that provides secure communications for data transfers Universal Claim Form (UCF) Electronic form used by a pharmacy that has Internet access, but is unable to submit its data in a batch upload Uploader A dispenser that uploads a data file containing controlled substance dispensing information Copyright Health Information Designs, LLC 36
41 Document Information 9 Document Information Copyright Notice and Trademarks Copyright Health Information Designs, LLC. All rights reserved. Health Information Designs, LLC 391 Industry Drive Auburn, AL xentry is a registered trademark of Health Information Designs, LLC (HID). Microsoft and Internet Explorer are registered trademarks or trademarks of Microsoft Corporation in the United tates and/or other countries. All other product names may be trademarks or registered trademarks of their respective companies. Disclaimer HID has made every effort to ensure the accuracy of the information in this document at the time of printing. However, information may change without notice. Please refer to the Arkansas PMP website, for the most current version of this document. Formatting Conventions The following formatting conventions are used throughout this document. Format Bold Arial Italic Blue underline Table 1 Text Formats Used to Designate eferences to execution buttons, windows, file names, menus, icons, or options Text you must type in a field or window, for example, \\server_name\printer_name for a network printer Hyperlinks to other sections of this document or external websites Copyright Health Information Designs, LLC 37
42 Document Information Version History The Version History records the publication history of this document. ee the Change Log for more details regarding the changes and enhancements included in each version. Publication Date Version Number Comments 01/14/ Initial publication 01/22/ Updated publication 02/28/ Updated publication 03/08/ Updated publication 03/12/ Updated publication Table 2 Document Version History Change Log The Change Log records the changes and enhancements included in each version. Version Number Chapter/ection Change 1.0 N/A N/A 1.1 Chapter 3/equired Prescription Information Chapter 6/Edit Definitions Appendix A/AAP pecifications Table 1.2 Appendix A/AAP pecifications Table emoved field PAT21, as it is not required emoved Edit 151, as field PAT21 is not required Changed Field Usage for PAT 21 from to Changed Field Usage for DP 04 and DP 06 from s to 1.3 Global Updated screen shots 1.4 Chapter 6/View Zero eports Added new topic Table 3 Document Change Log Copyright Health Information Designs, LLC 38
43 Appendix A: AAP 4.2 pecifications Appendix A: AAP 4.2 pecifications The information on the following pages contains the definitions for the specific contents required of uploaded records in the American ociety for Automation in Pharmacy (AAP) version 4, release 2 format to comply with the Arkansas Prescription Monitoring Program s requirements. The following elements are used in each upload file: egment Identifier indicates the beginning of a new segment, for example PHA. Data Delimiter character used to separate segments and the data elements within a segment, for example, an asterisk (*). Each completed field should be followed by an asterisk, and each blank field should contain a single asterisk. If the last field in the segment is blank, it should contain an asterisk and a tilde (~). egment Terminator character used to mark the end of a segment, for example, the tilde (~). Note: Field TH09 in the Transaction Header segment contains a built-in segment terminator. ince TH09 also signifies the end of the segment, it should contain two tildes (~~). Field Usage o = equired by AAP o = equired by the A PMP o = ituational (not required; however, supply if available) Both and fields must be reported. Note: For more information regarding AAP 4.2 specifications, contact the American ociety for Automation in Pharmacy at for the full Implementation Guide for the AAP tandard for Prescription-Monitoring Programs. This guide includes field lengths, acceptable attributes, and examples. Copyright Health Information Designs, LLC A-1
44 Appendix A: AAP 4.2 pecifications egment Field ID Field Name Field Usage TH: Transaction Header equired segment; used to indicate the start of a transaction. It also assigns the data element separator, segment terminator, and control number. TH01 TH02 TH03 TH04 TH05 TH06 TH07 TH08 TH09 Version/elease Number Code uniquely identifying the transaction. Format = xx.x Transaction Control Number ender assigned code uniquely identifying a transaction. Transaction Type Identifies the purpose of initiating the transaction. 01 end/equest Transaction 02 Acknowledgement (used in esponse only) 03 Error eceiving (used in esponse only) 04 Void (used to void a specific x in a real-time transmission or an entire batch that has been transmitted) esponse ID Contains the Transaction Control Number of a transaction that initiated the transaction. equired in response transaction only. Creation Date Date the transaction was created. Format: CCYYMMDD. Creation Time Time the transaction was created. Format: HHMM or HHMM. File Type P = Production T = Test outing Number eserved for real-time transmissions that go through a network switch to indicate, if necessary, the specific state PMP the transaction should be routed to. egment Terminator Character This terminates the TH segment and sets the actual value of the data segment terminator for the entire transaction. I: Information ource equired segment; used to convey the name and identification numbers of the entity supplying the information. I01 I02 I03 Unique Information ource ID eference number or identification number. (Example: phone number) Information ource Entity Name Entity name of the Information ource. Message Free-form text message. Copyright Health Information Designs, LLC A-2
Dispenser s Implementation Guide
 Dispenser s Implementation Guide Colorado Board of Pharmacy Prescription Drug Monitoring Program June 2014 Note This document is periodically updated. Please refer to the Colorado PDMP website, http://www.hidinc.com/copdmp,
Dispenser s Implementation Guide Colorado Board of Pharmacy Prescription Drug Monitoring Program June 2014 Note This document is periodically updated. Please refer to the Colorado PDMP website, http://www.hidinc.com/copdmp,
Dispenser s Implementation Guide
 AAP 4.2 Florida Department of Health Prescription Drug Monitoring Program July 2015 This project was supported by Grant No. 2009-PM-BX-4004 awarded by the Bureau of Justice Assistance, Office of Justice
AAP 4.2 Florida Department of Health Prescription Drug Monitoring Program July 2015 This project was supported by Grant No. 2009-PM-BX-4004 awarded by the Bureau of Justice Assistance, Office of Justice
Dispenser s Implementation Guide
 Dispenser s Implementation Guide Prescription Drug Monitoring Program July 2011 Note This document may be updated prior to the implementation of E-FORCE on eptember 1, 2011. Please refer to the Florida
Dispenser s Implementation Guide Prescription Drug Monitoring Program July 2011 Note This document may be updated prior to the implementation of E-FORCE on eptember 1, 2011. Please refer to the Florida
Frequently Asked Questions
 This document provides answers to some common questions regarding the reporting of controlled substances to the Arkansas (PMP) database. A list of the questions answered in this document is provided below:
This document provides answers to some common questions regarding the reporting of controlled substances to the Arkansas (PMP) database. A list of the questions answered in this document is provided below:
Data Submitter s Implementation Guide
 Office of Substance Abuse and Mental Health Services Maine Department of Health and Human Services Prescription Monitoring Program December 2014 This document is formatted for duplex printing. This page
Office of Substance Abuse and Mental Health Services Maine Department of Health and Human Services Prescription Monitoring Program December 2014 This document is formatted for duplex printing. This page
Dispenser s Implementation Guide
 Minnesota Board of Pharmacy Prescription Monitoring Program July 2016 This document is formatted for duplex printing. This page intentionally left blank. Do not copy or distribute without the express written
Minnesota Board of Pharmacy Prescription Monitoring Program July 2016 This document is formatted for duplex printing. This page intentionally left blank. Do not copy or distribute without the express written
Dispenser s Implementation Guide
 Dispenser s Implementation Guide Oregon Health Authority Prescription Drug Monitoring Program February 2011 This document is formatted for duplex printing. Dispenser s Implementation Guide This page intentionally
Dispenser s Implementation Guide Oregon Health Authority Prescription Drug Monitoring Program February 2011 This document is formatted for duplex printing. Dispenser s Implementation Guide This page intentionally
Dispenser s Implementation Guide
 Minnesota Board of Pharmacy Prescription Monitoring Program April 2017 This document is formatted for duplex printing. This page intentionally left blank. Contents Contents 1 Document Overview... A-1 2
Minnesota Board of Pharmacy Prescription Monitoring Program April 2017 This document is formatted for duplex printing. This page intentionally left blank. Contents Contents 1 Document Overview... A-1 2
Dispenser s Implementation Guide ASAP 4.2 Washington State Department of Health Washington State Prescription Monitoring Program
 ASAP 4.2 Washington State Department of Health Washington State Monitoring Program April 2017 ote This document is periodically updated. Please refer to the WA PMP website, www.wapmp.org, for the most
ASAP 4.2 Washington State Department of Health Washington State Monitoring Program April 2017 ote This document is periodically updated. Please refer to the WA PMP website, www.wapmp.org, for the most
Alabama Department of Public Health Prescription Drug Monitoring Program
 Prescription Drug Monitoring Program Dispenser s Implementation Guide v1.3 May 2009 391 Industry Drive Auburn, AL 36832 Phone: (334) 502-3262 Fax: (334) 466-6947 Auburn, Alabama Jackson, Mississippi Little
Prescription Drug Monitoring Program Dispenser s Implementation Guide v1.3 May 2009 391 Industry Drive Auburn, AL 36832 Phone: (334) 502-3262 Fax: (334) 466-6947 Auburn, Alabama Jackson, Mississippi Little
Dispenser Reporting Manual. October 1, 2007
 South Carolina Department of Health and Environmental Control Prescription Monitoring Program Dispenser Reporting Manual October 1, 2007 Prepared by Health Information Designs, Inc. 391 Industry Dr Auburn,
South Carolina Department of Health and Environmental Control Prescription Monitoring Program Dispenser Reporting Manual October 1, 2007 Prepared by Health Information Designs, Inc. 391 Industry Dr Auburn,
Data Submission Dispenser Guide Florida Department of Health Prescription Drug Monitoring Program
 Data ubmission Dispenser Guide Florida Department of Health Prescription Drug Monitoring Program March 2018 Version 1.0 Effective April 18, 2018 Table of Contents 1 E-FOCE Overview...4 1.1 Florida s PDMP
Data ubmission Dispenser Guide Florida Department of Health Prescription Drug Monitoring Program March 2018 Version 1.0 Effective April 18, 2018 Table of Contents 1 E-FOCE Overview...4 1.1 Florida s PDMP
Implementation Guide for Dispensing Practitioners
 Arizona Controlled Substances Prescription Monitoring Program Implementation Guide for Dispensing Practitioners v 2.2 September 2010 This document is formatted for duplex printing. 391 Industry Drive Auburn,
Arizona Controlled Substances Prescription Monitoring Program Implementation Guide for Dispensing Practitioners v 2.2 September 2010 This document is formatted for duplex printing. 391 Industry Drive Auburn,
Training Guide for Arkansas Practitioners and Pharmacists. Arkansas Department of Health Prescription Monitoring Program
 Training Guide for Arkansas Practitioners and Pharmacists Arkansas Department of Health Prescription Monitoring Program May 2013 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 2 System
Training Guide for Arkansas Practitioners and Pharmacists Arkansas Department of Health Prescription Monitoring Program May 2013 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 2 System
Controlled Substances Reporting System. Dispenser Reporting Manual
 North Carolina Department of Health and Human Services Division of Mental Health, Developmental Disabilities and Substance Abuse Services Controlled Substances Reporting System Dispenser Reporting Manual
North Carolina Department of Health and Human Services Division of Mental Health, Developmental Disabilities and Substance Abuse Services Controlled Substances Reporting System Dispenser Reporting Manual
Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives
 Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives Arkansas Department of Health Prescription Monitoring Program March 2016 Contents Contents 1 Document Overview...
Training Guide for Arkansas Law Enforcement Officers and Licensing Board Representatives Arkansas Department of Health Prescription Monitoring Program March 2016 Contents Contents 1 Document Overview...
1 Data Collection and Tracking Data Collection Overview Data Collection Requirements Reporting Requirements Exemptions...
 Data ubmission Dispenser Guide Arkansas Department of Health Prescription Monitoring Program November 2017 Version 1.2 Effective November 13, 2017 Table of Contents 1 Data Collection and Tracking... 5
Data ubmission Dispenser Guide Arkansas Department of Health Prescription Monitoring Program November 2017 Version 1.2 Effective November 13, 2017 Table of Contents 1 Data Collection and Tracking... 5
Data Submission Guide for Dispensers Minnesota Prescription Monitoring Program
 Minnesota Prescription Monitoring Program ovember 2018 Version 1.0, Effective December 4, 2018 9901 Linn Station oad Louisville, KY 40223 apprisshealth.com Table of Contents Table of Contents 1 Document
Minnesota Prescription Monitoring Program ovember 2018 Version 1.0, Effective December 4, 2018 9901 Linn Station oad Louisville, KY 40223 apprisshealth.com Table of Contents Table of Contents 1 Document
PRESCRIPTION MONITORING PROGRAM (PMP)
 PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
Training Guide for Wisconsin Practitioners and Pharmacists. Pharmacy Examining Board Wisconsin Department of Safety and Professional Services
 Training Guide for Wisconsin Practitioners and Pharmacists Pharmacy Examining Board Wisconsin Department of Safety and Professional Services October 2013 Contents Contents 1 Document Overview... 1 Purpose
Training Guide for Wisconsin Practitioners and Pharmacists Pharmacy Examining Board Wisconsin Department of Safety and Professional Services October 2013 Contents Contents 1 Document Overview... 1 Purpose
Training Guide for Practitioners
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Data Submission Dispenser Guide Rhode Island Prescription Drug Monitoring Program (RI PDMP) February 2016 Version 1.0
 Data Submission Dispenser Guide hode Island Prescription Drug Monitoring Program (I PDMP) February 2016 Version 1.0 1 Table of Contents 1 Data Collection and Tracking...4 Data Collection equirements...
Data Submission Dispenser Guide hode Island Prescription Drug Monitoring Program (I PDMP) February 2016 Version 1.0 1 Table of Contents 1 Data Collection and Tracking...4 Data Collection equirements...
Ohio Automated Reporting System Handbook
 Ohio Automated Reporting System Handbook Ohio State Board of Pharmacy Prescription Monitoring Program 77 South High St., Room 1702 Columbus, OH 43215-6126 Phone: 614-466-4143 (Option 1) OARRS Fax: 614-644-8556
Ohio Automated Reporting System Handbook Ohio State Board of Pharmacy Prescription Monitoring Program 77 South High St., Room 1702 Columbus, OH 43215-6126 Phone: 614-466-4143 (Option 1) OARRS Fax: 614-644-8556
Data Submission Dispenser Guide
 Data ubmission Dispenser Guide Delaware Division of Professional Regulation Prescription Monitoring Program October 2017 Version 1.3 Effective November 7, 2017 Table of Contents 1 Data Collection and Tracking...4
Data ubmission Dispenser Guide Delaware Division of Professional Regulation Prescription Monitoring Program October 2017 Version 1.3 Effective November 7, 2017 Table of Contents 1 Data Collection and Tracking...4
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program March 2016 Version 1.0
 Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program March 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program March 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
PDMP User s Guide. Oregon Health Authority Prescription Drug Monitoring Program
 Oregon Health Authority Prescription Drug Monitoring Program March 2014 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 System Overview... 3 About the RxSentry
Oregon Health Authority Prescription Drug Monitoring Program March 2014 Contents Contents 1 Document Overview... 1 Purpose and Contents... 1 RxSentry Update... 1 2 System Overview... 3 About the RxSentry
NEW JERSEY PRESCRIPTION MONITORING AND REPORTING SYSTEM (NJPMRS) DATA COLLECTION MANUAL Effective: September 2011
 NEW JERSEY PRESCRIPTION MONITORING AND REPORTING SYSTEM (NJPMRS) DATA COLLECTION MANUAL Effective: September 2011 Optimum Technology, Inc. Contact Information 866-683-2476 njrxreport@otech.com 1 P a g
NEW JERSEY PRESCRIPTION MONITORING AND REPORTING SYSTEM (NJPMRS) DATA COLLECTION MANUAL Effective: September 2011 Optimum Technology, Inc. Contact Information 866-683-2476 njrxreport@otech.com 1 P a g
Training Guide for Practitioners. Washington State Department of Health Washington State Prescription Monitoring Program
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program May 2016 Version 1.0
 Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program May 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
Data Submission Dispenser Guide Pennsylvania Prescription Drug Monitoring Program May 2016 Version 1.0 Table of Contents 1 Assistance and Support...4 Technical Assistance... 4 Administrative Assistance...
Training Guide for Colorado Practitioners and Pharmacists. Colorado State Board of Pharmacy Prescription Drug Monitoring Program
 Training Guide for Colorado Practitioners and Pharmacists Colorado State Board of Pharmacy Prescription Drug Monitoring Program April 2016 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Colorado Practitioners and Pharmacists Colorado State Board of Pharmacy Prescription Drug Monitoring Program April 2016 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists. Alabama Department of Public Health Prescription Drug Monitoring Program
 Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program August 2015 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program August 2015 Contents Contents 1 Document Overview... 3 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists. Alabama Department of Public Health Prescription Drug Monitoring Program
 Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program March 2015 Contents Contents 1 Document Overview... 2 Purpose and Contents...
Training Guide for Alabama Practitioners and Pharmacists Alabama Department of Public Health Prescription Drug Monitoring Program March 2015 Contents Contents 1 Document Overview... 2 Purpose and Contents...
Training Guide for Florida Practitioners and Pharmacists. Florida Department of Health Prescription Drug Monitoring Program
 Training Guide for Florida Practitioners and Pharmacists Florida Department of Health Prescription Drug Monitoring Program February 2017 Contents Contents 1 Program Overview... 1 2 Document Overview...
Training Guide for Florida Practitioners and Pharmacists Florida Department of Health Prescription Drug Monitoring Program February 2017 Contents Contents 1 Program Overview... 1 2 Document Overview...
Dispenser s Implementation Guide (ASAP 4.2) Nebraska Prescription Drug Monitoring Program
 Dispenser s Implementation Guide (ASAP 4.2) Nebraska Prescription Drug Monitoring Program Version: 3.6 DrFirst 9420 Key West Ave, Suite 101 Rockville, MD 20850 Office: 888-271-9898 Contents Document Change
Dispenser s Implementation Guide (ASAP 4.2) Nebraska Prescription Drug Monitoring Program Version: 3.6 DrFirst 9420 Key West Ave, Suite 101 Rockville, MD 20850 Office: 888-271-9898 Contents Document Change
Table of Contents Data Collection and Tracking...4 Data Submission...8 Creating Your Account...9 Data Delivery Methods Data Compliance...
 Data Submission Dispenser Guide Arizona Controlled Substances Prescription Monitoring Program (AZ CSPMP) December 2016 Version 1.2 Effective January 12, 2017 Table of Contents 1 Data Collection and Tracking...4
Data Submission Dispenser Guide Arizona Controlled Substances Prescription Monitoring Program (AZ CSPMP) December 2016 Version 1.2 Effective January 12, 2017 Table of Contents 1 Data Collection and Tracking...4
AK PDMP Data Submission Dispenser Guide
 AK PDMP Data Submission Dispenser Guide V1rE February 23, 2012 Additional information at http://pmp.relayhealth.com/ak 2011 RelayHealth All Rights Reserved No part of this document may be copied or reproduced
AK PDMP Data Submission Dispenser Guide V1rE February 23, 2012 Additional information at http://pmp.relayhealth.com/ak 2011 RelayHealth All Rights Reserved No part of this document may be copied or reproduced
Arkansas Prescription Drug Monitoring Program. User Support Manual
 Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
Companion Guide Institutional Billing 837I
 Companion Guide Institutional Billing 837I Release 3 X12N 837 (Version 5010A2) Healthcare Claims Submission Implementation Guide Published December 2016 Revision History Date Release Appendix name/ loop
Companion Guide Institutional Billing 837I Release 3 X12N 837 (Version 5010A2) Healthcare Claims Submission Implementation Guide Published December 2016 Revision History Date Release Appendix name/ loop
3 Accessing RxSentry. About This Chapter. Request Access to RxSentry
 Accessing RxSentry 3 Accessing RxSentry About This Chapter This chapter provides the steps you must follow to request an RxSentry account, log in to the system, retrieve system messages, retrieve a forgotten
Accessing RxSentry 3 Accessing RxSentry About This Chapter This chapter provides the steps you must follow to request an RxSentry account, log in to the system, retrieve system messages, retrieve a forgotten
Data Submission Dispenser Guide. Oregon Health Authority Prescription Drug Monitoring Program
 Data Submission Dispenser Guide Oregon Health Authority Prescription Drug Monitoring Program August 2017 Version 1.1 Effective October 23, 2017 Table of Contents 1 Data Collection and Tracking...4 Data
Data Submission Dispenser Guide Oregon Health Authority Prescription Drug Monitoring Program August 2017 Version 1.1 Effective October 23, 2017 Table of Contents 1 Data Collection and Tracking...4 Data
Quick reference guide. Creating a Controlled Substance Export File with the ASAP 4.2 Format. Completion requirements
 Quick reference guide Creating a Controlled Substance Export File with the ASAP 4.2 Format If you have Cornerstone* 8.6 NEXT or later software, you can create a controlled substance export file that uses
Quick reference guide Creating a Controlled Substance Export File with the ASAP 4.2 Format If you have Cornerstone* 8.6 NEXT or later software, you can create a controlled substance export file that uses
IDEXX Cornerstone * Practice Management System
 IDEXX Cornerstone * Practice Management System Creating a Controlled Substance Export File with the ASAP 4.1 Format Guide Overview If you have Service Pack 4 or higher of the Cornerstone* 8.3 Practice
IDEXX Cornerstone * Practice Management System Creating a Controlled Substance Export File with the ASAP 4.1 Format Guide Overview If you have Service Pack 4 or higher of the Cornerstone* 8.3 Practice
Provider Secure Portal User Manual
 Provider Secure Portal User Manual Copyright 2011 Centene Corporation. All rights reserved. Operational Training 2 August 2011 Table of Contents Provider Secure Portal... 5 Registration... 6 Provider -
Provider Secure Portal User Manual Copyright 2011 Centene Corporation. All rights reserved. Operational Training 2 August 2011 Table of Contents Provider Secure Portal... 5 Registration... 6 Provider -
Alabama PDMP AWA E. User Support Manual
 Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
BULLETIN BOARD SCREENS for HIPAA (BBS) UPDATED JULY 22, Once connected, the first screen displays the node number that you are connected to.
 BULLETIN BOARD SCREENS for HIPAA (BBS) UPDATED JULY 22, 2010 The following provides information and screen instructions on: 1. Transmitting 837 Transactions 2. TA1 Acknowledgement Retrievals 3. 997 Acknowledgement
BULLETIN BOARD SCREENS for HIPAA (BBS) UPDATED JULY 22, 2010 The following provides information and screen instructions on: 1. Transmitting 837 Transactions 2. TA1 Acknowledgement Retrievals 3. 997 Acknowledgement
Version 1/Revision 4 Page 1 of 23. epaces PA/DVS Request REFERENCE GUIDE. Table of Contents
 Version 1/Revision 4 Page 1 of 23 Table of Contents PA/DVS Request... 2 Provider Service Address... 3 Contact Information... 4 Referring Provider... 4 Ordering Provider... 5 Event Information... 6 Pattern
Version 1/Revision 4 Page 1 of 23 Table of Contents PA/DVS Request... 2 Provider Service Address... 3 Contact Information... 4 Referring Provider... 4 Ordering Provider... 5 Event Information... 6 Pattern
Health Services provider user guide
 Health Services provider user guide online claims submission... convenient service, delivered through an easy-to-use secure web site http://provider.ab.bluecross.ca/health... convenient service, delivered
Health Services provider user guide online claims submission... convenient service, delivered through an easy-to-use secure web site http://provider.ab.bluecross.ca/health... convenient service, delivered
Revision History. Document Version. Date Name Comments /26/2017 Training and Development Initial Creation
 Pharmaceutical Assistance Contract for the Elderly (PACE)/ Pharmaceutical Assistance Contract for the Elderly Needs Enhancement Tier (PACENET)Web Provider Enrollment/Provider Management Corporate User
Pharmaceutical Assistance Contract for the Elderly (PACE)/ Pharmaceutical Assistance Contract for the Elderly Needs Enhancement Tier (PACENET)Web Provider Enrollment/Provider Management Corporate User
HealthInfoNet CLINICAL PORTAL USER REFERENCE GUIDE. Revised: Page 1 of 24
 HealthInfoNet CLINICAL PORTAL USER REFERENCE GUIDE Revised: 6.3.2015 Page 1 of 24 HealthInfoNet User Reference Guide INSIDE: Accessing HealthInfoNet (HIN) 3-5 Clinical Portal 6-11 Notifications and Worklists
HealthInfoNet CLINICAL PORTAL USER REFERENCE GUIDE Revised: 6.3.2015 Page 1 of 24 HealthInfoNet User Reference Guide INSIDE: Accessing HealthInfoNet (HIN) 3-5 Clinical Portal 6-11 Notifications and Worklists
ONTARIO CHIROPRACTIC ASSOCIATION PATIENT MANAGEMENT PROGRAM PUTTING EXPERIENCE INTO PRACTICE. PMP HCAI & OCF Guide
 ONTARIO CHIROPRACTIC ASSOCIATION PATIENT MANAGEMENT PROGRAM PUTTING EXPERIENCE INTO PRACTICE PMP HCAI & OCF Guide December 2010 HCAI - Patient Management Program Contents Contact Information... 3 PMP HCAI
ONTARIO CHIROPRACTIC ASSOCIATION PATIENT MANAGEMENT PROGRAM PUTTING EXPERIENCE INTO PRACTICE PMP HCAI & OCF Guide December 2010 HCAI - Patient Management Program Contents Contact Information... 3 PMP HCAI
(EHR) Incentive Program
 REGISTRATION USER GUIDE For Eligible Professionals Medicare Electronic Health Record (EHR) Incentive Program JULY 2012 (07.02.12 ver8) CONTENTS Step 1...Getting started 3 Step 2... Login 5 Step 3...Welcome
REGISTRATION USER GUIDE For Eligible Professionals Medicare Electronic Health Record (EHR) Incentive Program JULY 2012 (07.02.12 ver8) CONTENTS Step 1...Getting started 3 Step 2... Login 5 Step 3...Welcome
Meritain Connect User Manual. for Employees. 1 Meritain Connect User Guide for Employees
 Meritain Connect User Manual for Employees 1 Meritain Connect User Guide for Employees Contents Introduction... 4 Accessing Meritain Connect... 5 Logging In... 5 Forgot Password... 6 Registration Process...
Meritain Connect User Manual for Employees 1 Meritain Connect User Guide for Employees Contents Introduction... 4 Accessing Meritain Connect... 5 Logging In... 5 Forgot Password... 6 Registration Process...
March Canadian Public Tenders Buyer Guide
 March 2010 Canadian Public Tenders Buyer Guide Table of Contents WELCOME TO MERX... 1 ABOUT THIS REFERENCE GUIDE... 1 SECTION 1 GETTING STARTED... 2 SYSTEM REQUIREMENTS... 2 NAVIGATING IN MERX... 2 SECTION
March 2010 Canadian Public Tenders Buyer Guide Table of Contents WELCOME TO MERX... 1 ABOUT THIS REFERENCE GUIDE... 1 SECTION 1 GETTING STARTED... 2 SYSTEM REQUIREMENTS... 2 NAVIGATING IN MERX... 2 SECTION
efiletexas.gov Individual Filer User Guide Release
 efiletexas.gov Individual Filer User Guide Release 2017.1 EFS-TF-200-4071 v.1 October 2017 Copyright and Confidentiality Copyright 2017 Tyler Technologies, Inc. All rights reserved Use of these materials
efiletexas.gov Individual Filer User Guide Release 2017.1 EFS-TF-200-4071 v.1 October 2017 Copyright and Confidentiality Copyright 2017 Tyler Technologies, Inc. All rights reserved Use of these materials
837 Health Care Claim Professional, Institutional & Dental Companion Guide
 837 Health Care Claim Professional, Institutional & Dental Companion Guide 005010X222A1 & 005010X223A1 V. 1.2 Created 07/18/14 Disclaimer Blue Cross of Idaho created this companion guide for 837 healthcare
837 Health Care Claim Professional, Institutional & Dental Companion Guide 005010X222A1 & 005010X223A1 V. 1.2 Created 07/18/14 Disclaimer Blue Cross of Idaho created this companion guide for 837 healthcare
Louisiana Medicaid Management Information System (LMMIS)
 Louisiana Medicaid Management Information System (LMMIS) Batch Eligibility Verification System Pilot User Manual Date Created: 03/22/2017 Date Modified: 08/02/2018 Prepared By Technical Communications
Louisiana Medicaid Management Information System (LMMIS) Batch Eligibility Verification System Pilot User Manual Date Created: 03/22/2017 Date Modified: 08/02/2018 Prepared By Technical Communications
Provider File Management Guide
 Provider File Management Guide March 2018 Independence Blue Cross offers products through its subsidiaries Independence Hospital Indemnity Plan, Keystone Health Plan East, and QCC Insurance Company, and
Provider File Management Guide March 2018 Independence Blue Cross offers products through its subsidiaries Independence Hospital Indemnity Plan, Keystone Health Plan East, and QCC Insurance Company, and
Loan Closing Advisor SM. User Guide. December 2017
 Loan Closing Advisor SM User Guide December 2017 Notice This User Guide is Freddie Mac s CONFIDENTIAL INFORMATION as defined in and subject to the provisions of the Freddie Mac Single Family Seller/Servicer
Loan Closing Advisor SM User Guide December 2017 Notice This User Guide is Freddie Mac s CONFIDENTIAL INFORMATION as defined in and subject to the provisions of the Freddie Mac Single Family Seller/Servicer
Louisiana Medicaid Management Information System (LMMIS)
 Louisiana Medicaid Management Information System (LMMIS) EFT Authorization Application User Guide Date Created: 1/23/2014 Date Revised: 8/03/2018 Prepared By Technical Communications Group Molina Medicaid
Louisiana Medicaid Management Information System (LMMIS) EFT Authorization Application User Guide Date Created: 1/23/2014 Date Revised: 8/03/2018 Prepared By Technical Communications Group Molina Medicaid
Front End Risk Adjustment System [ FERAS ] User Guide
![Front End Risk Adjustment System [ FERAS ] User Guide Front End Risk Adjustment System [ FERAS ] User Guide](/thumbs/77/74723317.jpg) Front End Risk Adjustment System [ FERAS ] User Guide Centers for Medicare & Medicaid Services Table of Contents Table of Contents TRADEMARK INFORMATION... 1 INTRODUCTION... 2 FERAS OVERVIEW... 2 WHO TO
Front End Risk Adjustment System [ FERAS ] User Guide Centers for Medicare & Medicaid Services Table of Contents Table of Contents TRADEMARK INFORMATION... 1 INTRODUCTION... 2 FERAS OVERVIEW... 2 WHO TO
Provider Portal Claim Features Training MHO
 Provider Portal Claim Features Training MHO-2585 0119 MOLINA HEALTHCARE S PROVIDER PORTAL The Provider Portal is secure and available 24 hours a day, seven days a week. Register for access to our Provider
Provider Portal Claim Features Training MHO-2585 0119 MOLINA HEALTHCARE S PROVIDER PORTAL The Provider Portal is secure and available 24 hours a day, seven days a week. Register for access to our Provider
Your mymeritain Personalized Member Website
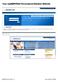 Your mymeritain Personalized Member Website 2008 Meritain Health, Inc. Last Updated 5.23.2008 Your mymeritain Member Website The mymeritain Member Website offers Members a user-friendly web experience,
Your mymeritain Personalized Member Website 2008 Meritain Health, Inc. Last Updated 5.23.2008 Your mymeritain Member Website The mymeritain Member Website offers Members a user-friendly web experience,
Change Healthcare Provider Portal
 MED3000, a wholly owned subsidiary of Change Healthcare Change Healthcare Provider Portal Overview The provider portal provides secure, web-enabled, role-based access. You will be able to perform the following
MED3000, a wholly owned subsidiary of Change Healthcare Change Healthcare Provider Portal Overview The provider portal provides secure, web-enabled, role-based access. You will be able to perform the following
CAQH ProView. Participating Organization User Guide
 CAQH ProView Participating Organization User Guide Table of Contents CHAPTER 1: Introduction... 4 CAQH ProView Overview...4 Becoming a CAQH ProView Participating Organization...5 System Security...5 CHAPTER
CAQH ProView Participating Organization User Guide Table of Contents CHAPTER 1: Introduction... 4 CAQH ProView Overview...4 Becoming a CAQH ProView Participating Organization...5 System Security...5 CHAPTER
Mississippi Medicaid. Mississippi Medicaid Program Provider Enrollment P.O. Box Jackson, Mississippi Complete form and mail original to:
 Mississippi Medicaid Complete form and mail original to: Blank forms may by copied. Call LTC at 888-941-8967 if you have questions. Please complete the following Mississippi Medicaid Provider EDI Enrollment
Mississippi Medicaid Complete form and mail original to: Blank forms may by copied. Call LTC at 888-941-8967 if you have questions. Please complete the following Mississippi Medicaid Provider EDI Enrollment
Authorization Submission and Inquiry Guide
 Authorization Submission and Inquiry Guide January 2018 Independence Blue Cross offers products through its subsidiaries Independence Hospital Indemnity Plan, Keystone Health Plan East, and QCC Insurance
Authorization Submission and Inquiry Guide January 2018 Independence Blue Cross offers products through its subsidiaries Independence Hospital Indemnity Plan, Keystone Health Plan East, and QCC Insurance
Alternate Data Submission Users Guide
 Alternate Data Submission Users Guide AHRQ Common Formats for Event Reporting Community Pharmacy Version 1.0 Change Log Version Author Date Revision 1.0 PSOPPC 12/2016 Initial Version 1.1 PSOPPC 04/2018
Alternate Data Submission Users Guide AHRQ Common Formats for Event Reporting Community Pharmacy Version 1.0 Change Log Version Author Date Revision 1.0 PSOPPC 12/2016 Initial Version 1.1 PSOPPC 04/2018
Electronic Prescribing of Controlled Substances (EPCS)
 Electronic Prescribing of Controlled Substances (EPCS) This document, as well as the software described in it, is provided under a software license agreement with STI Computer Services, Inc. Use of this
Electronic Prescribing of Controlled Substances (EPCS) This document, as well as the software described in it, is provided under a software license agreement with STI Computer Services, Inc. Use of this
MEDICAID MARYLAND PRE-ENROLLMENT INSTRUCTIONS MCDMD
 MEDICAID MARYLAND PRE-ENROLLMENT INSTRUCTIONS MCDMD HOW LONG DOES PRE-ENROLLMENT TAKE? Standard processing time is 2 weeks. WHAT FORM(S) SHOULD I COMPLETE? Maryland Medical Care Programs Submitter Identification
MEDICAID MARYLAND PRE-ENROLLMENT INSTRUCTIONS MCDMD HOW LONG DOES PRE-ENROLLMENT TAKE? Standard processing time is 2 weeks. WHAT FORM(S) SHOULD I COMPLETE? Maryland Medical Care Programs Submitter Identification
MEDICAID MARYLAND PART A (MCDMD) PRE-ENROLLMENT INSTRUCTIONS
 MEDICAID MARYLAND PART A (MCDMD) PRE-ENROLLMENT INSTRUCTIONS WHAT FORM(S) SHOULD I DO? Maryland Medical Care Programs Submitter Identification Form Trading Partner Agreement o Both Forms must have original
MEDICAID MARYLAND PART A (MCDMD) PRE-ENROLLMENT INSTRUCTIONS WHAT FORM(S) SHOULD I DO? Maryland Medical Care Programs Submitter Identification Form Trading Partner Agreement o Both Forms must have original
CAQH Solutions TM EnrollHub TM Provider User Guide Chapter 3 - Create & Manage Enrollments. Table of Contents
 CAQH Solutions TM EnrollHub TM Provider User Guide Chapter 3 - Create & Manage Enrollments Table of Contents 3 CREATE & MANAGE EFT ENROLLMENTS 2 3.1 OVERVIEW OF THE EFT ENROLLMENT PROCESS 3 3.2 ADD PROVIDER
CAQH Solutions TM EnrollHub TM Provider User Guide Chapter 3 - Create & Manage Enrollments Table of Contents 3 CREATE & MANAGE EFT ENROLLMENTS 2 3.1 OVERVIEW OF THE EFT ENROLLMENT PROCESS 3 3.2 ADD PROVIDER
Data Requestor Support Manual Minnesota Prescription Monitoring Program
 Minnesota Prescription Monitoring Program November 2018 Version 1.0 9901 Linn Station Road Louisville, KY 40223 apprisshealth.com Table of Contents Table of Contents 1 Document Overview...1 1.1 What is
Minnesota Prescription Monitoring Program November 2018 Version 1.0 9901 Linn Station Road Louisville, KY 40223 apprisshealth.com Table of Contents Table of Contents 1 Document Overview...1 1.1 What is
Louisiana Medicaid Management Information System (LMMIS)
 Louisiana Medicaid Management Information System (LMMIS) Prescriber Practices and Diabetes Management User Manual Date Created: 03/15/2017 Date Modified: 09/04/2018 Prepared By Technical Communications
Louisiana Medicaid Management Information System (LMMIS) Prescriber Practices and Diabetes Management User Manual Date Created: 03/15/2017 Date Modified: 09/04/2018 Prepared By Technical Communications
(EHR) Incentive Program
 REGISTRATION USER GUIDE For Eligible Professionals Medicaid Electronic Health Record (EHR) Incentive Program DECEMBER 2010 (12.28.10 ver2) CONTENTS Step 1... Getting started 3 Step 2... Login instruction
REGISTRATION USER GUIDE For Eligible Professionals Medicaid Electronic Health Record (EHR) Incentive Program DECEMBER 2010 (12.28.10 ver2) CONTENTS Step 1... Getting started 3 Step 2... Login instruction
CAQH ProView. Dentist Practice Manager Module User Guide
 CAQH ProView Dentist Practice Manager Module User Guide Table of Contents Chapter 1: Introduction... 1 CAQH ProView Overview... 1 System Security... 2 Chapter 2: Registration... 3 Existing Practice Managers...
CAQH ProView Dentist Practice Manager Module User Guide Table of Contents Chapter 1: Introduction... 1 CAQH ProView Overview... 1 System Security... 2 Chapter 2: Registration... 3 Existing Practice Managers...
1500 Claim Submission Guide
 1500 Claim Submission Guide February 2016 Independence Blue Cross offers products through its subsidiaries Independence Hospital Indemnity Plan, Keystone Health Plan East and QCC Insurance Company, and
1500 Claim Submission Guide February 2016 Independence Blue Cross offers products through its subsidiaries Independence Hospital Indemnity Plan, Keystone Health Plan East and QCC Insurance Company, and
Office - Claims EMDEON OFFICE USER GUIDE - CLAIMS
 Office - Claims EMDEON OFFICE USER GUIDE - CLAIMS September, 2014 CONTENTS 1 INTRODUCTION... 9 1.1 OVERVIEW... 9 1.2 IMPORT... 9 1.3 CREATE... 9 1.4 LIST... 9 1.5 SUPPLEMENT... 10 1.6 REPORTING & ANALYTICS...
Office - Claims EMDEON OFFICE USER GUIDE - CLAIMS September, 2014 CONTENTS 1 INTRODUCTION... 9 1.1 OVERVIEW... 9 1.2 IMPORT... 9 1.3 CREATE... 9 1.4 LIST... 9 1.5 SUPPLEMENT... 10 1.6 REPORTING & ANALYTICS...
NANP Administration System (NAS) User Registration Guide 2.6v
 NANP Administration System (NAS) User Registration Guide 2.6v November 8, 2017 TABLE OF CONTENTS 1.0 Introduction... 4 1.1 Purpose... 4 1.2 NAS Overview... 4 1.3 Content Summary... 4 1.4 Problem Solving...
NANP Administration System (NAS) User Registration Guide 2.6v November 8, 2017 TABLE OF CONTENTS 1.0 Introduction... 4 1.1 Purpose... 4 1.2 NAS Overview... 4 1.3 Content Summary... 4 1.4 Problem Solving...
Louisiana Medicaid Management Information System (LMMIS)
 Louisiana Medicaid Management Information System (LMMIS) SMO Applications User Manual Date Created: 04/06/2017 Date Modified: 08/08/2018 Prepared By Technical Communications Group Molina Medicaid Solutions
Louisiana Medicaid Management Information System (LMMIS) SMO Applications User Manual Date Created: 04/06/2017 Date Modified: 08/08/2018 Prepared By Technical Communications Group Molina Medicaid Solutions
Maine ASO Provider Portal Atrezzo End User Guide
 Maine ASO Provider Portal Atrezzo End User Guide October 2018 CONTENTS INTRODUCTION... 4 The KEPRO/Maine s Atrezzo Portal Guide... 4 SETUP AND ACCESS ATREZZO... 5 A. New Provider Registration/ Register
Maine ASO Provider Portal Atrezzo End User Guide October 2018 CONTENTS INTRODUCTION... 4 The KEPRO/Maine s Atrezzo Portal Guide... 4 SETUP AND ACCESS ATREZZO... 5 A. New Provider Registration/ Register
Aerial iexchange Users Guide
 Aerial iexchange Users Guide 2014.1 How to Run the Util\\\ \user Disclaimer How to reach us Copyright Information contained in this document is subject to change without notice and does not present a commitment
Aerial iexchange Users Guide 2014.1 How to Run the Util\\\ \user Disclaimer How to reach us Copyright Information contained in this document is subject to change without notice and does not present a commitment
Trading Partner Account (TPA) User Guide. for. State of Idaho MMIS
 Trading Partner Account (TPA) User Guide for State of Idaho MMIS Date of Publication: 3/8/2018 Document Number: RF019 Version: 4.0 This document and information contains proprietary information and copyrighted
Trading Partner Account (TPA) User Guide for State of Idaho MMIS Date of Publication: 3/8/2018 Document Number: RF019 Version: 4.0 This document and information contains proprietary information and copyrighted
MEDICAID MARYLAND PRE ENROLLMENT INSTRUCTIONS MCDMD
 MEDICAID MARYLAND PRE ENROLLMENT INSTRUCTIONS MCDMD HOW LONG DOES PRE ENROLLMENT TAKE? Standard processing time is 2 weeks. WHAT FORM(S) SHOULD I COMPLETE? Maryland Medical Care Programs Submitter Identification
MEDICAID MARYLAND PRE ENROLLMENT INSTRUCTIONS MCDMD HOW LONG DOES PRE ENROLLMENT TAKE? Standard processing time is 2 weeks. WHAT FORM(S) SHOULD I COMPLETE? Maryland Medical Care Programs Submitter Identification
Oregon registration will open next Monday, the 26th. First register with CMS, then with your state.
 Sent: Tuesday, September 20, 2011 2:53 PM Subject: Meaningful Use Registration I want to make sure that the Portland Area I/T/U's are on track to receive the EHR Incentive payments. So far, who has registered
Sent: Tuesday, September 20, 2011 2:53 PM Subject: Meaningful Use Registration I want to make sure that the Portland Area I/T/U's are on track to receive the EHR Incentive payments. So far, who has registered
Therapy Provider Portal. User Guide
 Therapy Provider Portal User Guide Page 2 of 16 UCare User Guide V1.7 Table of Contents I. Introduction...3 About HSM Therapy Management... 4 Terms of Use... 4 Contact Information... 6 II. Using the Therapy
Therapy Provider Portal User Guide Page 2 of 16 UCare User Guide V1.7 Table of Contents I. Introduction...3 About HSM Therapy Management... 4 Terms of Use... 4 Contact Information... 6 II. Using the Therapy
Secure Provider Website. Instructional Guide
 Secure Provider Website Instructional Guide Operational Training 1 March 2017 Introduction The Secure Provider Web is a secure website developed to allow Providers across Centene health plans to perform
Secure Provider Website Instructional Guide Operational Training 1 March 2017 Introduction The Secure Provider Web is a secure website developed to allow Providers across Centene health plans to perform
Health Link Frequently Asked Questions
 Health Link Frequently Asked Questions We hope that you find our Health Link patient portal easy to use. If you have any questions or comments, please contact Health Link Support by email at healthlink@hvhs.org
Health Link Frequently Asked Questions We hope that you find our Health Link patient portal easy to use. If you have any questions or comments, please contact Health Link Support by email at healthlink@hvhs.org
Provider File Management Guide
 Provider File Management Guide June 2018 AmeriHealth HMO, Inc. AmeriHealth Insurance Company of New Page Jersey 1 of 29 The Provider File Management transaction allows professional providers to view and
Provider File Management Guide June 2018 AmeriHealth HMO, Inc. AmeriHealth Insurance Company of New Page Jersey 1 of 29 The Provider File Management transaction allows professional providers to view and
Provider Portal User Guide. For the Provider Portal External Use
 Provider Portal User Guide For the Provider Portal External Use IT Department Issued January 2017 mynexus 2017. All rights reserved. Version 1.4 Revised 07122017 Contents Getting Started with the Portal...
Provider Portal User Guide For the Provider Portal External Use IT Department Issued January 2017 mynexus 2017. All rights reserved. Version 1.4 Revised 07122017 Contents Getting Started with the Portal...
837 Health Care Claim Companion Guide. Professional and Institutional
 837 Health Care Claim Companion Guide Professional and Institutional Revised December 2011 Table of Contents Introduction... 3 Purpose... 3 References... 3 Additional information... 4 Delimiters Supported...
837 Health Care Claim Companion Guide Professional and Institutional Revised December 2011 Table of Contents Introduction... 3 Purpose... 3 References... 3 Additional information... 4 Delimiters Supported...
Ordering New & Refill Prescriptions Online With Costco Mail Order
 Ordering New & Refill Prescriptions Online With Costco Mail Order Last updated: 09/2018 Register an Account Visit: pharmacy.costco.com Click Sign In/Register and then Create Account to get started on your
Ordering New & Refill Prescriptions Online With Costco Mail Order Last updated: 09/2018 Register an Account Visit: pharmacy.costco.com Click Sign In/Register and then Create Account to get started on your
HPHConnect for Employers User s Guide
 HPHConnect for Employers User s Guide Copyright 2017 Harvard Pilgrim Health Care, Inc. All rights reserved. Harvard Pilgrim Health Care and the Harvard Pilgrim Health Care logo are trademarks of Harvard
HPHConnect for Employers User s Guide Copyright 2017 Harvard Pilgrim Health Care, Inc. All rights reserved. Harvard Pilgrim Health Care and the Harvard Pilgrim Health Care logo are trademarks of Harvard
Beam Software User Policies
 Beam Software User Policies Table of Contents 1 Overview... 3 2 Technical Support... 3 2.1 What Do I Do When I Have a Question or Encounter a Problem?... 4 2.1.1 Telephone Submissions... 4 2.1.2 Critical
Beam Software User Policies Table of Contents 1 Overview... 3 2 Technical Support... 3 2.1 What Do I Do When I Have a Question or Encounter a Problem?... 4 2.1.1 Telephone Submissions... 4 2.1.2 Critical
THCIC 837 Electronic File Processing Tests and Production
 Purpose: The purpose of testing the electronic data files is to ensure the file format is compatible with the THCIC System and includes all required data fields. Before beginning the testing of data files,
Purpose: The purpose of testing the electronic data files is to ensure the file format is compatible with the THCIC System and includes all required data fields. Before beginning the testing of data files,
Step By Step Registration Process
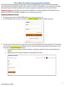 The Florida Prescription Drug Monitoring Program, known as E FORCSE (Electronic Florida Online Reporting of Controlled Substance Evaluation Program), was created by the 2009 Florida Legislature in an initiative
The Florida Prescription Drug Monitoring Program, known as E FORCSE (Electronic Florida Online Reporting of Controlled Substance Evaluation Program), was created by the 2009 Florida Legislature in an initiative
Provider Portal. User Manual. Therapists and Health Practitioners
 Provider Portal User Manual Therapists and Health Practitioners Table of Contents 1. Introduction... 3 2. Registering for the Provider Portal... 4 i. Changing Your Password...6 ii. Accepting Terms and
Provider Portal User Manual Therapists and Health Practitioners Table of Contents 1. Introduction... 3 2. Registering for the Provider Portal... 4 i. Changing Your Password...6 ii. Accepting Terms and
PROVIDER PORTAL. Inpatient Authorization Module
 PROVIDER PORTAL Inpatient Authorization Module AUTHORIZATIONS A Dean Health Plan (DHP) authorization should be completed in full by a Primary Care Practitioner (PCP) or a DHP Specialty Provider. The authorization
PROVIDER PORTAL Inpatient Authorization Module AUTHORIZATIONS A Dean Health Plan (DHP) authorization should be completed in full by a Primary Care Practitioner (PCP) or a DHP Specialty Provider. The authorization
WASHINGTON UNIVERSITY HIPAA Privacy Policy # 7. Appropriate Methods of Communicating Protected Health Information
 WASHINGTON UNIVERSITY HIPAA Privacy Policy # 7 Appropriate Methods of Communicating Protected Health Information Statement of Policy Washington University and its member organizations (collectively, Washington
WASHINGTON UNIVERSITY HIPAA Privacy Policy # 7 Appropriate Methods of Communicating Protected Health Information Statement of Policy Washington University and its member organizations (collectively, Washington
Refers to the Technical Reports Type 3 Based on ASC X12 version X /277 Health Care Claim Status Inquiry and Response
 HIPAA Transaction Standard Companion Guide For Availity Health Information Network Users Refers to the Technical Reports Type 3 Based on ASC X12 version 005010X212 276/277 Health Care Claim Status Inquiry
HIPAA Transaction Standard Companion Guide For Availity Health Information Network Users Refers to the Technical Reports Type 3 Based on ASC X12 version 005010X212 276/277 Health Care Claim Status Inquiry
BLUE CROSS AND BLUE SHIELD OF LOUISIANA PROFESSIONAL CLAIMS COMPANION GUIDE
 BLUE CROSS AND BLUE SHIELD OF LOUISIANA Table of Contents I. Introduction...3 II. General Specifications...4 III. Enveloping Specifications...5 IV. Loop and Data Element Specifications...7 V. Transaction
BLUE CROSS AND BLUE SHIELD OF LOUISIANA Table of Contents I. Introduction...3 II. General Specifications...4 III. Enveloping Specifications...5 IV. Loop and Data Element Specifications...7 V. Transaction
