Synoptic Reporting in Tumor Pathology Advantages of a Web-Based System
|
|
|
- Randolph Morton
- 5 years ago
- Views:
Transcription
1 Informatics / SYNOPTIC REPORTING IN TUMOR PATHOLOGY Synoptic Reporting in Tumor Pathology Advantages of a Web-Based System Zhenhong Qu, MD, PhD, 1 Shibu Ninan, MS, 1 Ahmed Almosa, MS, 1 K.G. Chang, MD, 2 Supriya Kuruvilla, MD, 1 and Nghia Nguyen, MD 1 Key Words: Cancer registry; Tumor reporting; Cancer report template DOI: /6VKCQDC69595KYVE Abstract The American College of Surgeons Commission on Cancer (ACS-CoC) mandates that pathology reports at ACS-CoC approved cancer programs include all scientifically validated data elements for each site and tumor specimen. The College of American Pathologists (CAP) has produced cancer checklists in static text formats to assist reporting. To be inclusive, the CAP checklists are pages long, requiring extensive text editing and multiple intermediate steps. We created a set of dynamic tumor-reporting templates, using Microsoft Active Server Page (ASP.NET), with dropdown list and data-compile features, and added a reminder function to indicate missing information. Users can access this system on the Internet, prepare the tumor report by selecting relevant data from dropdown lists with an embedded tumor staging scheme, and directly transfer the final report into a laboratory information system by using the copy-and-paste function. By minimizing extensive text editing and eliminating intermediate steps, this system can reduce reporting errors, improve work efficiency, and increase compliance. Historically, surgical pathologists had confined their reporting of malignant neoplasms to the identification of tumor type (diagnosis) and nodal involvement. However, recognizing that a plethora of morphologic attributes have significant predictive value for biologic behavior and therapeutic response of the malignant tumors, the Association of Directors of Anatomic and Surgical Pathology (ADASP) issued its official recommendation in 1992 also to include important morphologic attributes in surgical pathology reports. 1 Because of the large number of tumors encountered in surgical pathology practice and because the amount of information required in pathology reports is often extensive, pathologists usually cannot consistently remember all of the information required for each tumor and each tumor site. 2,3 As a result, important information is often omitted in pathology tumor reports. Based on a landmark study of 15,940 pathology reports of colorectal cancer from 332 laboratories, Zarbo et al 4 reported in 1991 that essential elements such as gross tumor size, depth of tumor invasion, status of resection margins, and tumor grades were omitted in a significant portion of surgical pathology reports. Recently, Gomez and Tamboli, 5 in an abstract, reported that 27% of referral and internal pathology reports in a highly specialized cancer treatment center failed to include at least 1 element with great clinical significance, such as the status of vascular lymphatic invasion. In addition, diversity in terms, individual styles, and amount of information obtained constitutes another layer of confusion and obstacle for communication. 2,6 The overwhelming diagnostic information and diverse styles, formats, and terminology have been the major sources of inconsistency and lack of uniformity in pathology tumor reporting. To address this issue, in 1993, Rosai, 7 in a proposal to standardize 898 Am J Clin Pathol 2007;127: DOI: /6VKCQDC69595KYVE
2 Informatics / ORIGINAL ARTICLE reporting of surgical pathology diagnoses of major tumor types, formulated the checklist format in an attempt to make the reporting process more efficient, uniform, and complete. In 1992, Zarbo 8 also reported that the single practice that was significantly associated with increased likelihood of providing information on gross and microscopic features surveyed was the use of standardized report forms or checklists. In the ensuing years, the College of American Pathologists (CAP) and the American Society for Clinical Pathology have made persistent efforts to establish guidelines for reporting the most commonly encountered human malignant neoplasms Effective implementation of these recommendations gained momentum with the mandate by the American College of Surgeons Commission on Cancer (ACS-CoC), which accredits more than 1,400 cancer treatment centers in the United States, that pathologists at its approved cancer programs include all scientifically validated or regularly used data elements in their reports for each site and specimen. 18 In accordance, the CAP has developed more than 40 site-specific cancer protocols and checklists as a resource for pathologists for effectively providing this information. 19 To be inclusive, the CAP checklists are pages long, often containing too detailed information. Despite being checklists, extensive text editing is inevitable. In practice, extensive deletion and typing (eg, measurements) are usually necessary to ensure that only pertinent text or code data are retained for the cancer registry. Because the checklists or templates are in static text formats (ie, Microsoft Word [Microsoft, Redmond, WA] and portable document format [PDF]), multiple intermediate steps usually have to take place before the reporting information can be incorporated into the final pathology report in the laboratory information system (LIS). Because the collected data must be incorporated into the main LIS, the reporting process comprises at least 2 major segments: data collection from specimen examination by pathologists and data transfer into the main LIS, most often now by transcriptionists. Currently, the workflow of tumor reporting using CAP checklists or other institutional static templates usually takes a tortuous route that includes downloading and printing the checklist or template, list checking and filling out during data collection by a pathologist, extensive text editing and transferring into the LIS by a transcriptionist, and final review and additional editing by the pathologist before the report is released. There are variations in the order of the process, but extensive editing and multiple steps inevitably take place. Extensive text editing and multiple intermediate steps involving at least 2 entities (pathologists and transcriptionists) have several weak links for potential reporting errors. Typographic errors are unavoidable. Because the generic CAP checklist or an institutional static template is not an integral part of the pathology report and is without patient demographic information, the resulting typographic separation of the tumor report, albeit transiently, from the rest of the pathology report creates another vulnerable link. The most dangerous is mismatch of the tumor report to the pathology report of the wrong patient. Conventional designation of data collection and data entry into the LIS to different entities (ie, pathologists and office staff) has always been a third weak link unless the final report is transferred into the LIS by electronic copying and pasting by pathologists. Although uniformity in the content is desired, inclusive information in a rigid format has been viewed by many of our users as a major drawback of CAP checklists. In this report, we introduce a simple template-based tumor reporting system that minimizes exhaustive list checking and extensive text editing, thereby markedly simplifying the process of routine reporting of tumor pathology. Materials and Methods A set of tumor-reporting templates on commonly encountered tumors was created with reference to samples collected from several academic institutions. These templates were converted to hypertext markup language (HTML), and tool controls such as drop-down menus, text boxes, and function buttons were created for dynamic presentation (ie, page display in response to inputs by users via a Web browser) on the World Wide Web. The static text was converted into Microsoft Active Server Page (ASP.NET) files. A Microsoft Access database table was created to store and organize the specific morphologic attributes from the templates by the file name and the name of specific organ sites. The stored information could be retrieved as the content of a dropdown list on the Web page for users to select. Specific command source codes were programmed in ASP.NET to implement the dynamic presentation. The completed files with the dynamic functionality were then uploaded and implemented on our institutional server and were accessible to all Internet users. The specific organ sites are first presented in a drop-down list on the initial Web page. The programmed ASP.NET codes, on selection of an organ site on the drop-down list by the user on the Internet, bring the corresponding HTML rendering of the ASP.NET page of the report form with multiple dropdown lists in categories, each containing specific attributes to be selected. When the final selections are submitted, the ASP.NET codes collate the selections and other text entries and format them into HTML text output on the Web page. This HTML output can be copied and pasted to text-editing buffers on any LIS system. The resultant Web page content (collated tumor report data) is cleared when the client browser is closed. Am J Clin Pathol 2007;127: DOI: /6VKCQDC69595KYVE 899
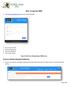
3 Qu et al / SYNOPTIC REPORTING IN TUMOR PATHOLOGY Results The synoptic reporting system can be accessed on the World Wide Web by all Internet users at tmc.edu/cap/webform1.aspx or edu/path/zqu/cap/webforml.aspx. Along with brief background information and specific instructions on how to use the synoptic reporting system, the entry page provides a dropdown list of specific organ sites for which synoptic report forms are available Image 1. Users can select an organ to bring up the complete synoptic report form. In each form, in addition to general information (eg, surgical accession number, specimen type, and surgical procedure), specific histopathologic attributes are provided in drop-down lists to choose or marked as a blank field to be filled out. A pathology staging scheme for each tumor is also embedded in each form for reference. At the end of each form, a freetext comment field is provided for adding additional information or unique attributes not provided in the standard forms. On completion of the form, users can compile all selections and entries into a short, concise tumor synoptic report containing only the selected attributes. The system also simultaneously generates and appends to the final report a list of the attributes that have not been selected or included in the final pathology report Image 2, serving as a reminder of the information missing from the report. If changes to the tumor synoptic report should be made, users can navigate in the browser back to the form and make any changes before compiling the final report. The final tumor synoptic report in the Internet browser window can then be copied and pasted into text-editing buffers in various LISs that accept plain text data. After the final pathology report is transferred into the LIS, all information in the tumor synoptic report on the Internet browser will be permanently purged when the browser is closed. Although the final synoptic pathology report can also be printed on regular paper and submitted for transcription, most users understandably prefer the direct copy-and-paste mode of information transfer to the LIS system. The tumor synoptic reporting system is compatible with all common Internet browsers such as Microsoft Internet Explorer (Microsoft), FireFox (Mozilla, Mountain View, CA), and Netscape (Netscape Communications, Mountain View, Image 1 Front page and site-specific form. The front page provides a drop-down list of specific organ sites to select (left). When a specific site or organ is selected, a corresponding form appears that contains site-specific reporting information in a drop-down list and staging scheme as a reference (right). In this image, the liver is selected as an example Am J Clin Pathol 2007;127: DOI: /6VKCQDC69595KYVE
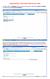
4 Informatics / ORIGINAL ARTICLE CA). The plain text transfer function with these browsers is compatible with all LISs, allowing final report transfer into the LIS by using the copy-and-paste function. The synoptic reports, of course, can be transferred electronically via or saved in a secured server for future retrieval. Discussion In this report, we introduced a simple Web-based tumor synoptic reporting system with several practical features: drop-down list, reminder function, and convenient Web interface. Because users can directly access this reporting system on the Web, enter reporting data by selecting from drop-down lists, eliminate irrelevant information, and directly transfer the final report into an LIS by simple copying and pasting, this system offers several practical benefits. First and foremost, it reduces the typographic efforts and errors by minimizing extensive text editing. Second, it greatly simplifies the reporting process. The direct copy-and-paste mode of data transfer into LIS by pathologists eliminates multiple error-prone intermediate steps by bypassing the tortuous route of the paper copy of the CAP checklist from the pathologist to the transcriptionist and, after extensive editing, to the LIS, and then back to the pathologist. The pathologist, by this time, may not have the original paper copy of the checklist and, thus, may be unable to ascertain accurate transcription. In addition, the added reminder function helps avoid a common reporting defect, ie, missing required information. 5 These features effectively counteract the inherent drawbacks of static checklists and templates considered to be the most serious potential risk in standardizing pathology reports by using the checklist model. 7 The drop-down list feature not only provides a reporting advantage but is also consistent with specific recommendation by Centers for Disease Control and Prevention National Program of Cancer Registries. 18 Moreover, the system is made available on the Web. It adopts the modes of navigation and data input commonly encountered Image 2 Data entry and final report page. The user can enter collected information by selection of relevant attributes from the drop-down list or by typing (left). When completed, the collected information can be compiled as the final report by clicking the submit button at the end of the form (not shown). The final report contains only the collected (relevant) information (right). At the end of the final report, a note is present to remind of any missing information (right, lower portion). The final report can then be copied and pasted into the main laboratory information system (not shown). Am J Clin Pathol 2007;127: DOI: /6VKCQDC69595KYVE 901
5 Qu et al / SYNOPTIC REPORTING IN TUMOR PATHOLOGY on the Web and, thus, is familiar to most Internet users. Users do not need training to use this tumor synoptic reporting system. The simplified process and its user-friendliness can help increase work efficiency and compliance. This system helps collect and summarize diagnostic information independent of any LIS, yet it is compatible with most, if not all, LISs in terms of reported data transfer (entry into the standard LIS system). The independence of this reporting system from existing LISs has 2 key advantages. First, it can be used in conjunction with a wide variety of existing LISs. This is particularly useful for laboratories or institutions that use more than one LIS for different clinical clients. Second, implementation of this system requires no modification of most existing LISs. Incorporation of a complex tumor reporting system in any existing LIS is usually technically demanding because it may affect the underlying software architecture of the existing LIS. The incorporation and subsequent maintenance can also be costly. In contrast, update or modification of this independent tumor synoptic reporting system is easily amendable because the changes are not limited by the complexity of the main LIS and will not affect the underlying architecture of the main systems. This proposed system, however, has its drawbacks. The data generated by this reporting system are input en bloc into existing LISs, technically in a single field of the underlying database. This will make future data retrieval by single attribute for a retrospective study difficult. However, if the format of the form is consistent, specific data can still be retrieved by more sophisticated search modalities, although certain computer programming (such as a parsing application) will be needed. Ideally, the tumor reporting function should be embedded in the LIS as an integral component and should have features such as drop-down lists, error reminders, and other relevant functions. The task is expected largely, however, to be that of main commercial LIS vendors. Because the need exists, it is reasonable to believe that LISs in the future will incorporate this capability. An existing alternative is a hybrid of these two, a reporting system with drop-down lists and other features that can collect report data and disperse the data into specific fields in the main LIS, using some sort of middleware. Generally, such smooth data transfer into LIS is at the expense of independence of the reporting system because this functionality depends on mutual compatibility. Implementation of the system with an individual LIS requires technical expertise and specific modification of either or both systems. As a result, any changes or updates of either system will potentially create an impediment to the functionality of this tumor reporting system. Most third-party reporting systems are also commercial products, and the cost is a significant consideration not in its favor. Although ACS-CoC accredited cancer treatment centers are required to include all scientifically validated data elements for each site and tumor specimen, the ACS-CoC imposes no restriction on the format of the pathology report or requirement of variables for which the clinical importance and prognostic value have not been well established. To most users (practicing pathologists), concise tumor-reporting templates with scientifically validated data elements and clinically important information are highly desirable and more manageable than exhaustive, long checklists. Although it is not our intention to judge the adequacy of existing tumor report formats or contents or to take a stance on which reporting system or method should be used, we thought it would greatly increase compliance and reduce clerical error if a user-friendly, simple, and flexible reporting system or method could be made available. Ideally, the tumor reporting system should be standard and uniform and also institution-specific. In practice, this balance is difficult to achieve, if even possible, at the multi-institutional level. However, this is a real need and should be addressed. The best way, we believe, is to allow users to modify the proposed system to meet their needs. We, therefore, have kept the HTML code an open source for users to copy and modify, although some programming (mainly HTML) knowledge, though quite rudimentary, is needed for this task. As described in the Materials and Methods section, a close collaboration between the pathologists and LIS staff is necessary to create or modify such a Web-based reporting system. At the time of this report, at least 1 major LIS vendor provides an added module with this capability. 20 However, a significant lag time is expected before it is widely adopted owing to the additional cost and compatibility with existing LISs. Although a new generation of LISs with tumor reporting functions in compliance with ACS-CoC requirements will be widely available eventually, the length of the lag time is difficult to predict. Given the facts that the ACS-CoC accredits more than 1,400 cancer treatment centers in the United States 18 and that there are approximately 450,000 new cancer cases each year in the United States, 21 development of simple and easily accessible systems to efficiently and accurately provide the scientifically validated data, such as the one described in this article, will have significant impacts on cancer registries, at least during this transition period. It is reasonable to believe that a simple and user-friendly reporting system will also increase voluntary compliance with the ACS-CoC mandate by many small non ACS-CoC accredited hospitals and improve the overall standard of tumor reporting. From the Departments of 1 Pathology and Laboratory Medicine, The University of Texas Health Science Center in Houston; and 2 Pathology, Lahey Clinic, Burlington, MA. Address reprint requests to Dr Qu: Dept of Pathology & Laboratory Medicine, University of Rochester Medical Center, 601Elmwood Ave, Rochester, NY Am J Clin Pathol 2007;127: DOI: /6VKCQDC69595KYVE
6 Informatics / ORIGINAL ARTICLE Acknowledgments: We thank Cheryl Breitenbuecher for assistance in Web implementation of this reporting system at the University of Rochester Medical Center, and Bob Brown, MD, University of Texas Health Science Center in Houston, for critical review of the manuscript. References 1. Association of Directors of Anatomic and Surgical Pathology. Standardization of the surgical pathology report. Am J Surg Pathol. 1992;16: Kempson RL. The time is now: checklists for surgical pathology reports [editorial]. Arch Pathol Lab Med. 1992;116: Kempson RL. Checklists for surgical pathology reports: an important step forward [editorial]. Am J Clin Pathol. 1993;100: Zarbo RJ, Hoffman GG, Howanitz PJ. Interinstitutional comparison of frozen-section consultation: a College of American Pathologists Q-Probes study of 79,647 consultations in 297 North American institutions. Arch Pathol Lab Med. 1991;115: Gomez JA, Tamboli P. Reporting of testicular germ cell tumors: are the clinically relevant data in the pathology report [abstract]. Mod Pathol. 2005;18:142A. 6. Foucar E. Individuality in the specialty of surgical pathology: self-expression or just another source of diagnostic error? Am J Surg Pathol. 2000;24: Rosai J. Standardized reporting of surgical pathology diagnoses for the major tumor types: a proposal. The Department of Pathology, Memorial Sloan-Kettering Cancer Center. Am J Clin Pathol. 1993;100: Zarbo RJ. Interinstitutional assessment of colorectal carcinoma surgical pathology report adequacy: a College of American Pathologists Q-Probes study of practice patterns from 532 laboratories and 15,940 reports. Arch Pathol Lab Med. 1992;116: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of breast carcinoma. Am J Clin Pathol. 1995;104: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of urinary bladder specimens containing bladder neoplasms. Am J Clin Pathol. 1996;106: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of urinary bladder specimens containing bladder neoplasms. Hum Pathol. 1996;27: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of resected large intestinal carcinomas. Am J Clin Pathol. 1996;106: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of urinary bladder specimens containing bladder neoplasms. Virchows Arch. 1996;428: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of resected large intestinal carcinomas. Hum Pathol. 1996;27: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of larynx specimens containing laryngeal neoplasms. Virchows Arch. 1997;431: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of tissues removed as part of the surgical treatment of cutaneous melanoma. Virchows Arch. 1997;431: Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of tissues removed as part of the surgical treatment of cutaneous melanoma. Pathol Int. 1998;48: Centers for Disease Control and Prevention. Report on the Reporting Pathology Protocols for Colon and Rectum Cancers Project. Available at rpp_report_ pdf. Accessed July 20, College of American Pathologists. Cancer protocols and checklists. Available at protocols/protocols_index.html. Accessed May 30, Cerner. Cerner launches Millennium PathNet synoptic reporting. Available at NewsReleases.asp?id=257&cid=229. Accessed April 10, American Cancer Society. Cancer statistics 2006 presentation. Available at content/pro_1_1_cancer_statistics_2006_presentation.asp. Accessed August 2, Am J Clin Pathol 2007;127: DOI: /6VKCQDC69595KYVE 903
Building an Ancillary System for Cancer Registries from SDC CAP Templates
 Building an Ancillary System for Cancer Registries from SDC CAP Templates Jennifer Seiffert, MLIS, CTR, Northrop Grumman, under contract to CDC s NPCR Sanjeev Baral, Northrop Grumman, under contract to
Building an Ancillary System for Cancer Registries from SDC CAP Templates Jennifer Seiffert, MLIS, CTR, Northrop Grumman, under contract to CDC s NPCR Sanjeev Baral, Northrop Grumman, under contract to
Jovanka N. Harrison, Ph.D., Chair For the NAACCR Pathology Data Work Group, and the NAACCR Supplement WG/Task Force
 Jovanka N. Harrison, Ph.D., Chair For the NAACCR Pathology Data Work Group, and the NAACCR Supplement WG/Task Force North American Association of Central Cancer Registries Annual Conference Austin, Texas
Jovanka N. Harrison, Ph.D., Chair For the NAACCR Pathology Data Work Group, and the NAACCR Supplement WG/Task Force North American Association of Central Cancer Registries Annual Conference Austin, Texas
The first approach to accessing pathology diagnostic information is the use of synoptic worksheets produced by the
 ABSTRACT INTRODUCTION Expediting Access to Critical Pathology Data Leanne Goldstein, City of Hope, Duarte, CA Rebecca Ottesen, City of Hope, Duarte, CA Julie Kilburn, City of Hope, Duarte, CA Joyce Niland,
ABSTRACT INTRODUCTION Expediting Access to Critical Pathology Data Leanne Goldstein, City of Hope, Duarte, CA Rebecca Ottesen, City of Hope, Duarte, CA Julie Kilburn, City of Hope, Duarte, CA Joyce Niland,
CAP Electronic Cancer Checklists (CAP ecc) Overview. An International Implementation of SNOMED CT
 CAP Electronic Cancer Checklists (CAP ecc) Overview An International Implementation of SNOMED CT 1 Outline Structured Reporting The CAP Cancer checklists Moving from paper form to application XML & the
CAP Electronic Cancer Checklists (CAP ecc) Overview An International Implementation of SNOMED CT 1 Outline Structured Reporting The CAP Cancer checklists Moving from paper form to application XML & the
pathx pathx Laboratory Information System
 A LETTER FROM THE PRESIDENT. At PathX, we fully understand that your lab is focused on more than just patient reports and information processing. For most pathology labs, primary concerns include responsible
A LETTER FROM THE PRESIDENT. At PathX, we fully understand that your lab is focused on more than just patient reports and information processing. For most pathology labs, primary concerns include responsible
Electronic skin cancer reporting tool User guide
 Electronic skin cancer reporting tool User guide Dr Michael Eden m.eden@nhs.net PUBS 280514 1 V2 Final Contents Introduction... 3 Overview... 3 How it works... 3 Benefits... 3 Getting started... 4 Starting
Electronic skin cancer reporting tool User guide Dr Michael Eden m.eden@nhs.net PUBS 280514 1 V2 Final Contents Introduction... 3 Overview... 3 How it works... 3 Benefits... 3 Getting started... 4 Starting
Pathology Informatics Training and Education Workshop Lab InfoTech Summit April 9, 2008
 Pathology Informatics Training and Education Workshop Lab InfoTech Summit April 9, 2008 Walter H. Henricks, M.D. Cleveland Clinic Pathology Informatics Mission of pathology is to provide information necessary
Pathology Informatics Training and Education Workshop Lab InfoTech Summit April 9, 2008 Walter H. Henricks, M.D. Cleveland Clinic Pathology Informatics Mission of pathology is to provide information necessary
7/31/2012. NCI SEER CDC NPCR ACOS COC Other States Florida-Specific Collaborative Stage NAACCR EDITS Working Group
 Blank Field Checks Single Item Edit Valid Code Checks Single Item Edit Valid Date Checks Single Item Edit Inter-Field Edits Relationships Between Items Inter-Record Edits Relationships Between Cases CS
Blank Field Checks Single Item Edit Valid Code Checks Single Item Edit Valid Date Checks Single Item Edit Inter-Field Edits Relationships Between Items Inter-Record Edits Relationships Between Cases CS
A fast breast nonlinear elastography reconstruction technique using the Veronda-Westman model
 A fast breast nonlinear elastography reconstruction technique using the Veronda-Westman model Mohammadhosein Amooshahi a and Abbas Samani abc a Department of Electrical & Computer Engineering, University
A fast breast nonlinear elastography reconstruction technique using the Veronda-Westman model Mohammadhosein Amooshahi a and Abbas Samani abc a Department of Electrical & Computer Engineering, University
How To Log Into MDX. How To Print Your Memberships MDX Forms
 How To Log Into MDX 1. Type https://axis.mdxnet.com into your Internet Browser 2. Enter account ID 119 3. Enter you User Name 4. Enter your Password 5. Click Login Button AV Form or Member Information
How To Log Into MDX 1. Type https://axis.mdxnet.com into your Internet Browser 2. Enter account ID 119 3. Enter you User Name 4. Enter your Password 5. Click Login Button AV Form or Member Information
OnCore Enterprise Research. Subject Administration Full Study
 OnCore Enterprise Research Subject Administration Full Study Principal Investigator Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Table of Contents What
OnCore Enterprise Research Subject Administration Full Study Principal Investigator Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Table of Contents What
Site Specific Data Items (SSDI)
 Jennifer Ruhl Site Specific Data Items (SSDI) Developed by Jennifer Ruhl and the SSDI Taskforce What is an SSDI Site-Specific Data Item Based on primary site AJCC Chapter Summary Stage chapter Previously
Jennifer Ruhl Site Specific Data Items (SSDI) Developed by Jennifer Ruhl and the SSDI Taskforce What is an SSDI Site-Specific Data Item Based on primary site AJCC Chapter Summary Stage chapter Previously
Med-Info. Council Directive 93/42/EEC on medical devices. TÜV SÜD Product Service GmbH
 Med-Info International expert information for the medical device industry Council Directive 93/42/E on medical devices Practice-oriented summary of the most important aspects and requirements contained
Med-Info International expert information for the medical device industry Council Directive 93/42/E on medical devices Practice-oriented summary of the most important aspects and requirements contained
Med-Info. Council Directive 93/42/EEC on Medical Devices. TÜV SÜD Product Service GmbH
 Med-Info International expert information for the Medical Device industry Council Directive 93/42/E on Medical Devices Practice-oriented summary of the most important aspects and requirements contained
Med-Info International expert information for the Medical Device industry Council Directive 93/42/E on Medical Devices Practice-oriented summary of the most important aspects and requirements contained
Med-Info. Council Directive 93/42/EEC on medical devices. TÜV SÜD Product Service GmbH
 Med-Info International expert information for the medical device industry Council Directive 93/42/E on medical devices Practice-oriented summary of the most important aspects and requirements contained
Med-Info International expert information for the medical device industry Council Directive 93/42/E on medical devices Practice-oriented summary of the most important aspects and requirements contained
P A T I E N T C E N T E R E D M E D I C A L H O M E ( P C M H ) A T T E S T A T I O N O F F A C I L I T Y C O M P L I A N C E
 P A T I E N T C E N T E R E D M E D I C A L H O M E ( P C M H ) A T T E S T A T I O N O F F A C I L I T Y C O M P L I A N C E State of Wyoming, Department of Health, Division of Healthcare Financing 2015
P A T I E N T C E N T E R E D M E D I C A L H O M E ( P C M H ) A T T E S T A T I O N O F F A C I L I T Y C O M P L I A N C E State of Wyoming, Department of Health, Division of Healthcare Financing 2015
AAPM Standard of Practice: CT Protocol Review Physicist
 AAPM Standard of Practice: CT Protocol Review Physicist Dianna Cody, Ph.D., DABR, FAAPM U.T.M.D. Anderson Cancer Center September 11, 2014 2014 Texas Radiation Regulatory Conference Goals Understand purpose
AAPM Standard of Practice: CT Protocol Review Physicist Dianna Cody, Ph.D., DABR, FAAPM U.T.M.D. Anderson Cancer Center September 11, 2014 2014 Texas Radiation Regulatory Conference Goals Understand purpose
UCare Therapy Authorization Web Application. User Guide
 UCare Therapy Authorization Web Application User Guide 1 Table of Contents I. Introduction...3 Contact Information... 3 II. Using the Therapy Authorization System...3 Log On... 3-7 Member Identification...
UCare Therapy Authorization Web Application User Guide 1 Table of Contents I. Introduction...3 Contact Information... 3 II. Using the Therapy Authorization System...3 Log On... 3-7 Member Identification...
CERTIFICATION GUIDE. Detailed Instructions for Health Care Homes Certification & Recertification: Letter of Intent Application Assessment
 CERTIFICATION GUIDE Detailed Instructions for Health Care Homes Certification & Recertification: Letter of Intent Application Assessment Version 5: reviewed March 2018 For more information, please go to
CERTIFICATION GUIDE Detailed Instructions for Health Care Homes Certification & Recertification: Letter of Intent Application Assessment Version 5: reviewed March 2018 For more information, please go to
Anthem East (Connecticut, Maine, New Hampshire) HIPAA Supplemental Billing Guidelines Professional
 Objectives The purpose of these guidelines is to provide billing offices with information about several significant changes and features of the HIPAA-compliant professional claims transaction (837P). These
Objectives The purpose of these guidelines is to provide billing offices with information about several significant changes and features of the HIPAA-compliant professional claims transaction (837P). These
CliniSync Community Health Record. Version Release Notes Live 05/20/17
 CliniSync Community Health Record Version 7.5.4.4 Release Notes Live 05/20/17 1 Contents Appearance and Performance... 3 Browser Support Validation... 3 Advanced Search Option... 4 Patient Age Display...
CliniSync Community Health Record Version 7.5.4.4 Release Notes Live 05/20/17 1 Contents Appearance and Performance... 3 Browser Support Validation... 3 Advanced Search Option... 4 Patient Age Display...
Scottish Care Information. SCI Gateway v10.3. Sending Referrals & Receiving Discharges User Guide
 Scottish Care Information SCI Gateway v10.3 Sending Referrals & Receiving Discharges User Guide Contents 1 Introduction... 1-1 2 Accessing SCI Gateway... 2-1 Accessing SCI Gateway Through GPASS... 2-2
Scottish Care Information SCI Gateway v10.3 Sending Referrals & Receiving Discharges User Guide Contents 1 Introduction... 1-1 2 Accessing SCI Gateway... 2-1 Accessing SCI Gateway Through GPASS... 2-2
Summary of FERC Order No. 791
 Summary of FERC Order No. 791 On November 22, 2013, the Federal Energy Regulatory Commission ( FERC or Commission ) issued Order No. 791 adopting a rule that approved Version 5 of the Critical Infrastructure
Summary of FERC Order No. 791 On November 22, 2013, the Federal Energy Regulatory Commission ( FERC or Commission ) issued Order No. 791 adopting a rule that approved Version 5 of the Critical Infrastructure
Working with Health IT Systems is available under a Creative Commons Attribution-NonCommercial- ShareAlike 3.0 Unported license.
 Working with Health IT Systems is available under a Creative Commons Attribution-NonCommercial- ShareAlike 3.0 Unported license. Johns Hopkins University. Welcome to Quality Improvement: Data Quality Improvement.
Working with Health IT Systems is available under a Creative Commons Attribution-NonCommercial- ShareAlike 3.0 Unported license. Johns Hopkins University. Welcome to Quality Improvement: Data Quality Improvement.
Data Consistency and Quality Issues in SEND Datasets
 Data Consistency and Quality Issues in SEND Datasets PointCross has reviewed numerous SEND datasets prepared for test submissions to the FDA and has worked with the FDA on their KickStart program to ensure
Data Consistency and Quality Issues in SEND Datasets PointCross has reviewed numerous SEND datasets prepared for test submissions to the FDA and has worked with the FDA on their KickStart program to ensure
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
Annotating PDFs for ereturn version 1.0; August 28, 2007; full release
 Annotating PDFs for ereturn version 1.0; August 28, 2007; full release 1. Introduction eproof files are self-contained PDF documents for viewing on-screen and for printing. They contain all appropriate
Annotating PDFs for ereturn version 1.0; August 28, 2007; full release 1. Introduction eproof files are self-contained PDF documents for viewing on-screen and for printing. They contain all appropriate
NIST Risk Assessment for Part 11 Compliance: Evaluation of a GXP Case Study
 NIST Risk Assessment for Part 11 Compliance: Evaluation of a GXP Case Study Monica Fanjoy* 109 Fairground Road, Holly Springs, NC 27540, USA Summary Current guidance for compliance with 21 Code of Federal
NIST Risk Assessment for Part 11 Compliance: Evaluation of a GXP Case Study Monica Fanjoy* 109 Fairground Road, Holly Springs, NC 27540, USA Summary Current guidance for compliance with 21 Code of Federal
Departmental Reports: Posted 48 Hours After the Report Reaches a Signed Status
 What is My Noyes HEALTH? My Noyes Health offers patients personalized and secure online access to portions of your Noyes HEALTH record. My Noyes HEALTH enables you to securely use the Internet to help
What is My Noyes HEALTH? My Noyes Health offers patients personalized and secure online access to portions of your Noyes HEALTH record. My Noyes HEALTH enables you to securely use the Internet to help
Consultation document: Summary of Clinical Trial Results for Laypersons
 SANTE-B4-GL-results-laypersons@ec.europa.eu Consultation document: Summary of Clinical Trial Results for Laypersons Professor DK Theo Raynor, University of Leeds d.k.raynor@leeds.ac.uk This is my response
SANTE-B4-GL-results-laypersons@ec.europa.eu Consultation document: Summary of Clinical Trial Results for Laypersons Professor DK Theo Raynor, University of Leeds d.k.raynor@leeds.ac.uk This is my response
CT Protocol Review: Practical Tips for the Imaging Physicist Physicist
 CT Protocol Review: Practical Tips for the Imaging Physicist Physicist Dianna Cody, Ph.D., DABR, FAAPM U.T.M.D. Anderson Cancer Center August 8, 2013 AAPM Annual Meeting Goals Understand purpose and importance
CT Protocol Review: Practical Tips for the Imaging Physicist Physicist Dianna Cody, Ph.D., DABR, FAAPM U.T.M.D. Anderson Cancer Center August 8, 2013 AAPM Annual Meeting Goals Understand purpose and importance
Regulatory Aspects of Digital Healthcare Solutions
 Regulatory Aspects of Digital Healthcare Solutions TÜV SÜD Product Service GmbH Dr. Markus Siebert Rev. 02 / 2017 02.05.2017 TÜV SÜD Product Service GmbH Slide 1 Contents Digital solutions as Medical Device
Regulatory Aspects of Digital Healthcare Solutions TÜV SÜD Product Service GmbH Dr. Markus Siebert Rev. 02 / 2017 02.05.2017 TÜV SÜD Product Service GmbH Slide 1 Contents Digital solutions as Medical Device
Office of Human Research
 Office of Human Research JeffTrial End-User Training Document Regulatory Coordinator Training for Non-Oncology personnel Office of Human Research 8/16/2013 Ver. 1.0 Contents The REG Role: Completing Basic
Office of Human Research JeffTrial End-User Training Document Regulatory Coordinator Training for Non-Oncology personnel Office of Human Research 8/16/2013 Ver. 1.0 Contents The REG Role: Completing Basic
Academic Program Review at Illinois State University PROGRAM REVIEW OVERVIEW
 Academic Program Review at Illinois State University PROGRAM REVIEW OVERVIEW For Research and Service Centers Submitting Self-Study Reports Fall 2017 INTRODUCTION Primary responsibility for maintaining
Academic Program Review at Illinois State University PROGRAM REVIEW OVERVIEW For Research and Service Centers Submitting Self-Study Reports Fall 2017 INTRODUCTION Primary responsibility for maintaining
i2b2 User Guide University of Minnesota Clinical and Translational Science Institute
 Clinical and Translational Science Institute i2b2 User Guide i2b2 is a tool for discovering research cohorts using existing, de-identified, clinical data This guide is provided by the Office of Biomedical
Clinical and Translational Science Institute i2b2 User Guide i2b2 is a tool for discovering research cohorts using existing, de-identified, clinical data This guide is provided by the Office of Biomedical
By choosing to view this document, you agree to all provisions of the copyright laws protecting it.
 Copyright 2009 IEEE. Reprinted from 31 st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009. EMBC 2009. Sept. 2009. This material is posted here with permission
Copyright 2009 IEEE. Reprinted from 31 st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009. EMBC 2009. Sept. 2009. This material is posted here with permission
Views on the Framework for Improving Critical Infrastructure Cybersecurity
 This document is scheduled to be published in the Federal Register on 12/11/2015 and available online at http://federalregister.gov/a/2015-31217, and on FDsys.gov Billing Code: 3510-13 DEPARTMENT OF COMMERCE
This document is scheduled to be published in the Federal Register on 12/11/2015 and available online at http://federalregister.gov/a/2015-31217, and on FDsys.gov Billing Code: 3510-13 DEPARTMENT OF COMMERCE
Title of Manual: Specimen Collection Document Number: GPA.SPC.52.0
 Page 1 of 17 I._PURPOSE This document is a guideline of procedures for Outreach 2.0 ordering and results application. It is intended to outline the features and functions of Outreach 2.0. All major functionality
Page 1 of 17 I._PURPOSE This document is a guideline of procedures for Outreach 2.0 ordering and results application. It is intended to outline the features and functions of Outreach 2.0. All major functionality
Yes No Comments. SHQ Form Compliance Checklist. Sponsor Name: Anticipated Launch Date: Url (s) used for site audit: Audit performed by: Audit Date:
 SHQ Form Compliance Checklist Sponsor Name: Anticipated Launch Date: Url (s) used for site audit: Audit performed by: Audit Date: Documentation Received: Design/Functional Specification Yes No Comments
SHQ Form Compliance Checklist Sponsor Name: Anticipated Launch Date: Url (s) used for site audit: Audit performed by: Audit Date: Documentation Received: Design/Functional Specification Yes No Comments
ehepqual- HCV Quality of Care Performance Measure Program
 NEW YORK STATE DEPARTMENT OF HEALTH AIDS INSTITUTE ehepqual- HCV Quality of Care Performance Measure Program USERS GUIDE A GUIDE FOR PRIMARY CARE AND HEPATITIS C CARE PROVIDERS * * For use with ehepqual,
NEW YORK STATE DEPARTMENT OF HEALTH AIDS INSTITUTE ehepqual- HCV Quality of Care Performance Measure Program USERS GUIDE A GUIDE FOR PRIMARY CARE AND HEPATITIS C CARE PROVIDERS * * For use with ehepqual,
Experience fast on-demand or batch cassette printing in any color
 Experience fast on-demand or batch cassette printing in any color Tissue-Tek SmartWrite Cassette Printers The SMART system solution for cassette printing Increase efficiency for your laboratory while reducing
Experience fast on-demand or batch cassette printing in any color Tissue-Tek SmartWrite Cassette Printers The SMART system solution for cassette printing Increase efficiency for your laboratory while reducing
NCDB Vendor Webinar: NCDB Call for Data April 2019 and RQRS Updates
 NCDB Vendor Webinar: NCDB Call for Data April 2019 and RQRS Updates Topics to be Covered Today NCDB: 2019 Call for Data Submission Specifications Record layout and edits Mapping of NPI Data Items Facilitating
NCDB Vendor Webinar: NCDB Call for Data April 2019 and RQRS Updates Topics to be Covered Today NCDB: 2019 Call for Data Submission Specifications Record layout and edits Mapping of NPI Data Items Facilitating
DO NOT SEND DUPLICATE COPIES OF YOUR LOG AND DO NOT SEND A PRINTED COPY.
 AMERICAN BOARD OF UROLOGY 2018 LIFE LONG LEARNING (LLL) LEVEL 2 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions
AMERICAN BOARD OF UROLOGY 2018 LIFE LONG LEARNING (LLL) LEVEL 2 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions
STANDARDS FOR AUTOMATED REPORTING
 North Carolina Central Cancer Registry STANDARDS FOR AUTOMATED REPORTING In accordance with the North American Association of Central Cancer Registries Record Layout Version 16.0 Effective 1/1/2016 January
North Carolina Central Cancer Registry STANDARDS FOR AUTOMATED REPORTING In accordance with the North American Association of Central Cancer Registries Record Layout Version 16.0 Effective 1/1/2016 January
OncoEMR Certified Workflows Meaningful Use Core Measure 15: Summary of Care
 In an effort to support oncology practices striving to achieve CMS s Meaningful Use Stage 2, Altos would like to share the following support information with you. The CMS website is the ultimate source
In an effort to support oncology practices striving to achieve CMS s Meaningful Use Stage 2, Altos would like to share the following support information with you. The CMS website is the ultimate source
RF-PTCAS CONFIGURATION MANAGER HELP GUIDE
 RF-PTCAS CONFIGURATION MANAGER HELP GUIDE The Configuration Manager Help Guide is designed to help you navigate through the RF-PTCAS Configuration Portal, which is the tool you will use to set up your
RF-PTCAS CONFIGURATION MANAGER HELP GUIDE The Configuration Manager Help Guide is designed to help you navigate through the RF-PTCAS Configuration Portal, which is the tool you will use to set up your
IVDR Breakout. Copyright 2017 BSI. All rights reserved.
 IVDR Breakout 1 IVDR Classification and conformity assessment 2 Classification- IVDR 3 Classification of IVDs Re-classification of IVDs will mean 80-90 % will no longer be able to self certify conformity
IVDR Breakout 1 IVDR Classification and conformity assessment 2 Classification- IVDR 3 Classification of IVDs Re-classification of IVDs will mean 80-90 % will no longer be able to self certify conformity
WISER. Protocol Creation, Activation, and Management TRAINING MANUAL. Wake Integrated Solution for Enterprise Research. For Oncology Studies
 WISER Wake Integrated Solution for Enterprise Research Protocol Creation, Activation, and Management For Oncology Studies TRAINING MANUAL Version June 11, 2018 WELCOME to WISER! Navigation and Home Page
WISER Wake Integrated Solution for Enterprise Research Protocol Creation, Activation, and Management For Oncology Studies TRAINING MANUAL Version June 11, 2018 WELCOME to WISER! Navigation and Home Page
Certification Commission for Healthcare Information Technology. CCHIT A Catalyst for EHR Adoption
 Certification Commission for Healthcare Information Technology CCHIT A Catalyst for EHR Adoption Alisa Ray, Executive Director, CCHIT Sarah Corley, MD, Chief Medical Officer, NextGen Healthcare Systems;
Certification Commission for Healthcare Information Technology CCHIT A Catalyst for EHR Adoption Alisa Ray, Executive Director, CCHIT Sarah Corley, MD, Chief Medical Officer, NextGen Healthcare Systems;
 1 von 5 13/10/2005 17:44 high graphics home search browse about whatsnew submit sitemap Factors affecting the quality of an information source The purpose of this document is to explain the factors affecting
1 von 5 13/10/2005 17:44 high graphics home search browse about whatsnew submit sitemap Factors affecting the quality of an information source The purpose of this document is to explain the factors affecting
APPLYING HUMAN FACTORS ENGINEERING TO IMPROVE USABILITY AND WORKFLOW IN PATHOLOGY INFORMATICS
 Proceedings of the 2017 International Symposium on Human Factors and Ergonomics in Health Care 23 APPLYING HUMAN FACTORS ENGINEERING TO IMPROVE USABILITY AND WORKFLOW IN PATHOLOGY INFORMATICS Austin F.
Proceedings of the 2017 International Symposium on Human Factors and Ergonomics in Health Care 23 APPLYING HUMAN FACTORS ENGINEERING TO IMPROVE USABILITY AND WORKFLOW IN PATHOLOGY INFORMATICS Austin F.
2017 MOC PEDIATRIC PRACTICE LOG TEMPLATE:
 AMERICAN BOARD OF UROLOGY 2017 MAINTENANCE OF CERTIFICATION (MOC) Level 4 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all
AMERICAN BOARD OF UROLOGY 2017 MAINTENANCE OF CERTIFICATION (MOC) Level 4 PEDIATRIC UROLOGY SUBSPECIALTY CERTIFICATION EXAMINATION PROCESS INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all
Monash University Policy Management. User Guide
 Monash University Policy Management User Guide 1 Table of Contents 1. GENERAL NAVIGATION... 4 1.1. Logging In to Compliance 360 - Single Sign On... 4 1.2. Help... 4 1.2.1. The University Policy Bank...
Monash University Policy Management User Guide 1 Table of Contents 1. GENERAL NAVIGATION... 4 1.1. Logging In to Compliance 360 - Single Sign On... 4 1.2. Help... 4 1.2.1. The University Policy Bank...
Research Data Browser UCSF ITS Academic Research Systems ACADEMIC RESEARCH SYSTEMS
 Research Data Browser Research Data Browser UCSF ITS Academic Research Systems Research Data Browser (RDB) Overview The Research Data Browser allows you to view Epic Patient Data through the Cogito Data
Research Data Browser Research Data Browser UCSF ITS Academic Research Systems Research Data Browser (RDB) Overview The Research Data Browser allows you to view Epic Patient Data through the Cogito Data
Guide to Completing the Online Application
 Guide to Completing the Online Application Table of Contents 1. Before You Begin.......2 a. Invitation to Apply....2 b. Please Sign In.....2 c. Application Time Out...........3 d. Navigating the Online
Guide to Completing the Online Application Table of Contents 1. Before You Begin.......2 a. Invitation to Apply....2 b. Please Sign In.....2 c. Application Time Out...........3 d. Navigating the Online
... user-friendly explanations for HL7, SNOMED, DICOM, XML, BDT
 ... user-friendly explanations for HL7, SNOMED, DICOM, XML, BDT HL 7 (Health Level 7) The quality of modern software can be measured by its ability to integrate with other systems. For this reason, an
... user-friendly explanations for HL7, SNOMED, DICOM, XML, BDT HL 7 (Health Level 7) The quality of modern software can be measured by its ability to integrate with other systems. For this reason, an
Maximizing Statistical Interactions Part II: Database Issues Provided by: The Biostatistics Collaboration Center (BCC) at Northwestern University
 Maximizing Statistical Interactions Part II: Database Issues Provided by: The Biostatistics Collaboration Center (BCC) at Northwestern University While your data tables or spreadsheets may look good to
Maximizing Statistical Interactions Part II: Database Issues Provided by: The Biostatistics Collaboration Center (BCC) at Northwestern University While your data tables or spreadsheets may look good to
From Lexicon To Mammographic Ontology: Experiences and Lessons
 From Lexicon To Mammographic : Experiences and Lessons Bo Hu, Srinandan Dasmahapatra and Nigel Shadbolt Department of Electronics and Computer Science University of Southampton United Kingdom Email: {bh,
From Lexicon To Mammographic : Experiences and Lessons Bo Hu, Srinandan Dasmahapatra and Nigel Shadbolt Department of Electronics and Computer Science University of Southampton United Kingdom Email: {bh,
Healthcare FHIR API TECHNICAL SPECIFICATION 7.4
 Healthcare FHIR API TECHNICAL SPECIFICATION 7.4 2018 Pegasystems Inc., Cambridge, MA All rights reserved. Trademarks For Pegasystems Inc. trademarks and registered trademarks, all rights reserved. All
Healthcare FHIR API TECHNICAL SPECIFICATION 7.4 2018 Pegasystems Inc., Cambridge, MA All rights reserved. Trademarks For Pegasystems Inc. trademarks and registered trademarks, all rights reserved. All
HealthlinkOnline Lung Cancer Referral User Guide
 HealthlinkOnline Lung Cancer Referral User Guide To begin, click the Referrals tab from across the top menu. Select St. James s Hospital and referral type Lung Cancer Referral. Next you will be presented
HealthlinkOnline Lung Cancer Referral User Guide To begin, click the Referrals tab from across the top menu. Select St. James s Hospital and referral type Lung Cancer Referral. Next you will be presented
Trainer Outline: Provider: Documenting a Visit with Note Capture
 Trainer Outline: Provider: Documenting a Visit with Note Capture Prerequisites Please reference the OP15 Resource List ILTs: All Roles: Navigating the Electronic Chart All Roles: Messaging Who Needs to
Trainer Outline: Provider: Documenting a Visit with Note Capture Prerequisites Please reference the OP15 Resource List ILTs: All Roles: Navigating the Electronic Chart All Roles: Messaging Who Needs to
INSPIRE. User Screen Guide: MST, Administrative
 INSPIRE User Screen Guide: MST, Administrative The EPISCenter is a project of the Prevention Research Center, College of Health and Human Development, Penn State University, and is funded by the Pennsylvania
INSPIRE User Screen Guide: MST, Administrative The EPISCenter is a project of the Prevention Research Center, College of Health and Human Development, Penn State University, and is funded by the Pennsylvania
University of Wisconsin-Madison Policy and Procedure
 Page 1 of 10 I. Policy The Health Information Technology for Economic and Clinical Health Act regulations ( HITECH ) amended the Health Information Portability and Accountability Act ( HIPAA ) to establish
Page 1 of 10 I. Policy The Health Information Technology for Economic and Clinical Health Act regulations ( HITECH ) amended the Health Information Portability and Accountability Act ( HIPAA ) to establish
Using Probability Maps for Multi organ Automatic Segmentation
 Using Probability Maps for Multi organ Automatic Segmentation Ranveer Joyseeree 1,2, Óscar Jiménez del Toro1, and Henning Müller 1,3 1 University of Applied Sciences Western Switzerland (HES SO), Sierre,
Using Probability Maps for Multi organ Automatic Segmentation Ranveer Joyseeree 1,2, Óscar Jiménez del Toro1, and Henning Müller 1,3 1 University of Applied Sciences Western Switzerland (HES SO), Sierre,
CERECONS. Provider Training
 CERECONS Provider Training February 2012 Table of Contents 1. Physician Dashboard 2 Eligibility Highlights 2 Clinical Alerts 3 Referral Alerts and Stats 4 Group Information 5 2. Edit Profile 6 3. Eligibility
CERECONS Provider Training February 2012 Table of Contents 1. Physician Dashboard 2 Eligibility Highlights 2 Clinical Alerts 3 Referral Alerts and Stats 4 Group Information 5 2. Edit Profile 6 3. Eligibility
VetConnect* PLUS. Online Services. User s Guide
 VetConnect* PLUS Online Services User s Guide Proprietary rights notice Information in this document is subject to change without notice. Companies, names and data used in examples are fictitious unless
VetConnect* PLUS Online Services User s Guide Proprietary rights notice Information in this document is subject to change without notice. Companies, names and data used in examples are fictitious unless
This guide is designed to be used in conjunction with the UNF APC Workflow System.
 Requests Involving Existing Degree Programs in the APC Workflow System (WfS) This guide is designed to be used in conjunction with the UNF APC Workflow System. Prepared by: Lisa Jamba & Arturo Sánchez-Ruíz
Requests Involving Existing Degree Programs in the APC Workflow System (WfS) This guide is designed to be used in conjunction with the UNF APC Workflow System. Prepared by: Lisa Jamba & Arturo Sánchez-Ruíz
RADIOMICS: potential role in the clinics and challenges
 27 giugno 2018 Dipartimento di Fisica Università degli Studi di Milano RADIOMICS: potential role in the clinics and challenges Dr. Francesca Botta Medical Physicist Istituto Europeo di Oncologia (Milano)
27 giugno 2018 Dipartimento di Fisica Università degli Studi di Milano RADIOMICS: potential role in the clinics and challenges Dr. Francesca Botta Medical Physicist Istituto Europeo di Oncologia (Milano)
RULES OF TENNESSEE BOARD OF MEDICAL EXAMINERS DIVISION OF HEALTH RELATED BOARDS
 RULES OF TENNESSEE BOARD OF MEDICAL EXAMINERS DIVISION OF HEALTH RELATED BOARDS CHAPTER 0880-9 GENERAL RULES AND REGULATIONS GOVERNING TABLE OF CONTENTS 0880-9-.01 Definitions 0880-9-.06 Certification
RULES OF TENNESSEE BOARD OF MEDICAL EXAMINERS DIVISION OF HEALTH RELATED BOARDS CHAPTER 0880-9 GENERAL RULES AND REGULATIONS GOVERNING TABLE OF CONTENTS 0880-9-.01 Definitions 0880-9-.06 Certification
2011 Clinical. Breast Imaging. Superior Image Quality. FREE SYLLABUS with purchase of entire set 16 AMA PRA Category 1 Credit(s) tm
 A DVD Teaching Program 2011 Clinical Breast Imaging Digital Mammography 1.5 AMA PRA Category 1 Credit(s) TM Breast Ultrasound 1.0 AMA PRA Category 1 Credit(s) TM Breast MR 1.75 AMA PRA Category 1 Credit(s)
A DVD Teaching Program 2011 Clinical Breast Imaging Digital Mammography 1.5 AMA PRA Category 1 Credit(s) TM Breast Ultrasound 1.0 AMA PRA Category 1 Credit(s) TM Breast MR 1.75 AMA PRA Category 1 Credit(s)
PULSE TAKING THE PHYSICIAN S
 TAKING THE PHYSICIAN S PULSE TACKLING CYBER THREATS IN HEALTHCARE Accenture and the American Medical Association (AMA) surveyed U.S. physicians regarding their experiences and attitudes toward cybersecurity.
TAKING THE PHYSICIAN S PULSE TACKLING CYBER THREATS IN HEALTHCARE Accenture and the American Medical Association (AMA) surveyed U.S. physicians regarding their experiences and attitudes toward cybersecurity.
September 26, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852
 701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
Accuracy made easy. Improving patient safety through lean workflow. Thermo Scientific Labeling and Tracking Solutions
 Thermo Scientific Labeling and Tracking Solutions Accuracy made easy Improving patient safety through lean workflow PrintMate AS Cassette Printer SlideMate AS Slide Printer LabWriter Software Primary labeling
Thermo Scientific Labeling and Tracking Solutions Accuracy made easy Improving patient safety through lean workflow PrintMate AS Cassette Printer SlideMate AS Slide Printer LabWriter Software Primary labeling
Monday, Tuesday, Wednesday, and Thursday, 1 pm to 3 or 4 pm. (See Course Schedule for details)
 Anatomy 6201 Course Director: Dr. Ernesto Phone: (303) 724-3430 Office: RC1 South Rm 11124 Office Hours: by appointment Email: ernesto.salcedo@ucdenver Location ED 2 South Room 2206.! Course Hours Monday,
Anatomy 6201 Course Director: Dr. Ernesto Phone: (303) 724-3430 Office: RC1 South Rm 11124 Office Hours: by appointment Email: ernesto.salcedo@ucdenver Location ED 2 South Room 2206.! Course Hours Monday,
Physician Online Data System
 Physician Online Data System Medical Network One, PC 4986 Adams Rd., Suite D Rochester, MI 48306 Physician Online Data System (PODS) Register for PODS... 4 User name and password... 7 Login... 8 Member
Physician Online Data System Medical Network One, PC 4986 Adams Rd., Suite D Rochester, MI 48306 Physician Online Data System (PODS) Register for PODS... 4 User name and password... 7 Login... 8 Member
The IBMS: 2016 and beyond
 The IBMS: 2016 and beyond Ian Sturdgess IBMS President Pathology Services Manager West Hertfordshire Hospitals NHS Trust Institute of Biomedical Science The professional body for the biomedical science
The IBMS: 2016 and beyond Ian Sturdgess IBMS President Pathology Services Manager West Hertfordshire Hospitals NHS Trust Institute of Biomedical Science The professional body for the biomedical science
LIFE LONG LEARNING LEVEL INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS
 AMERICAN BOARD OF UROLOGY LIFE LONG LEARNING LEVEL 2 2018 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions carefully before preparing your log. It is imperative that you carefully
AMERICAN BOARD OF UROLOGY LIFE LONG LEARNING LEVEL 2 2018 INSTRUCTIONS FOR SUBMISSION OF ELECTRONIC LOGS Please read all instructions carefully before preparing your log. It is imperative that you carefully
NCDB Special Study: Post-Active Treatment Surveillance in Prostate Cancer. Webinar #2 Eileen Tonner, MS
 NCDB Special Study: Post-Active Treatment Surveillance in Prostate Cancer Webinar #2 Eileen Tonner, MS Veterans Affairs If a patient was diagnosed at a VA institution and was referred to your facility
NCDB Special Study: Post-Active Treatment Surveillance in Prostate Cancer Webinar #2 Eileen Tonner, MS Veterans Affairs If a patient was diagnosed at a VA institution and was referred to your facility
CAP Quick Guide for Proficiency Testing
 CAP Quick Guide for Proficiency Testing Definitions e-lab Solutions Suite (ELSS): The CAP s online portal to manage your laboratory improvement programs, including proficiency testing (PT), accreditation,
CAP Quick Guide for Proficiency Testing Definitions e-lab Solutions Suite (ELSS): The CAP s online portal to manage your laboratory improvement programs, including proficiency testing (PT), accreditation,
Florida Hospital Electronic Documentation PowerNote
 Florida Hospital Electronic Documentation PowerNote January 2014 Version 2.0 POWERNOTE PowerNote is electronic provider documentation within PowerChart. Storing information electronically rather than
Florida Hospital Electronic Documentation PowerNote January 2014 Version 2.0 POWERNOTE PowerNote is electronic provider documentation within PowerChart. Storing information electronically rather than
Augusta University Health: Physician Portal User Guide. Improved Access to Patient Information from Augusta University Medical Center
 Augusta University Health: Physician Portal User Guide Improved Access to Patient Information from Augusta University Medical Center Rev. 7/06 User Guide Index. Accessing the AU Health Physician Portal.
Augusta University Health: Physician Portal User Guide Improved Access to Patient Information from Augusta University Medical Center Rev. 7/06 User Guide Index. Accessing the AU Health Physician Portal.
COLLECTION & HOW THE INFORMATION WILL BE USED
 Privacy Policy INTRODUCTION As our esteemed client, your privacy is essential to us. Here, at www.indushealthplus.com, we believe that privacy is a top priority. We know that you care how information about
Privacy Policy INTRODUCTION As our esteemed client, your privacy is essential to us. Here, at www.indushealthplus.com, we believe that privacy is a top priority. We know that you care how information about
SERIOUS ADVERSE EVENTS SERIOUS ADVERSE EVENT REPORTING SERIOUS ADVERSE EVENT. Clinical Trials Training Course Serious Adverse Event Reporting
 SERIOUS ADVERSE EVENT REPORTING SERIOUS ADVERSE EVENT SAE s are a sub-set of all adverse events collected The reporting of SAE s is in addition to, and does not supplant, the necessity of adequately reporting
SERIOUS ADVERSE EVENT REPORTING SERIOUS ADVERSE EVENT SAE s are a sub-set of all adverse events collected The reporting of SAE s is in addition to, and does not supplant, the necessity of adequately reporting
IRB RESEARCH REPOSITORY COMPLIANCE PROGRAM. FAQs: Designing and Managing Repositories. Compliance Deadline: August 31, 2011
 IRB RESEARCH REPOSITORY COMPLIANCE PROGRAM FAQs: Designing and Managing Repositories Compliance Deadline: August 31, 2011 Susan Bankowski, MS, JD IRB Chair Kathryn Schuff, MD, MCR IRB Co-Chair Agenda Review
IRB RESEARCH REPOSITORY COMPLIANCE PROGRAM FAQs: Designing and Managing Repositories Compliance Deadline: August 31, 2011 Susan Bankowski, MS, JD IRB Chair Kathryn Schuff, MD, MCR IRB Co-Chair Agenda Review
Pacific Knowledge Systems. RippleDown Deployment Guide: v8.0.5
 Pacific Knowledge Systems RippleDown Deployment Guide: v8.0.5 Copyright Notice The information provided in this User's Guide is subject to change without notice and is not a commitment by Pacific Knowledge
Pacific Knowledge Systems RippleDown Deployment Guide: v8.0.5 Copyright Notice The information provided in this User's Guide is subject to change without notice and is not a commitment by Pacific Knowledge
Children s Surgery Verification Program - Center Portal Instructions
 Children s Surgery Verification Program - Center Portal Instructions Thank you for your interest in joining the Children s Surgery Verification Quality Improvement Program. This portal guide is designed
Children s Surgery Verification Program - Center Portal Instructions Thank you for your interest in joining the Children s Surgery Verification Quality Improvement Program. This portal guide is designed
Cascade Health Alliance. User Guide. Authorization Management Provider Portal
 Cascade Health Alliance User Guide Authorization Management Provider Portal 8-16-2017 Table of Contents USING THIS GUIDE... 2 SIGNING IN... 2 PROVIDER PORTAL NAVIGATION... 3 Portal... 3 Core... 3 Resources...
Cascade Health Alliance User Guide Authorization Management Provider Portal 8-16-2017 Table of Contents USING THIS GUIDE... 2 SIGNING IN... 2 PROVIDER PORTAL NAVIGATION... 3 Portal... 3 Core... 3 Resources...
2016 e-mds, Inc.
 Capability and Certification Criteria Electronic Prescribing of Medications Certification Criteria: 170.314(a)(1) Computerized provider order entry (CPOE) of Medications Description of capability Electronic
Capability and Certification Criteria Electronic Prescribing of Medications Certification Criteria: 170.314(a)(1) Computerized provider order entry (CPOE) of Medications Description of capability Electronic
Building Better Interfaces: HL7 Conformance Profiles
 Tutorials, W. Rishel Research Note 26 November 2002 Building Better Interfaces: HL7 Conformance Profiles The new Health Level Seven conformance technology allows individual healthcare organizations and
Tutorials, W. Rishel Research Note 26 November 2002 Building Better Interfaces: HL7 Conformance Profiles The new Health Level Seven conformance technology allows individual healthcare organizations and
Cancer Waiting Times. User Manual. Version 7.0 Published 4 August 2016
 Cancer Waiting Times User Manual Version 7.0 Published 4 August 2016 Copyright 2016 Health and Social Care Information Centre. NHS Digital is the trading name of the Health and Social Care Information
Cancer Waiting Times User Manual Version 7.0 Published 4 August 2016 Copyright 2016 Health and Social Care Information Centre. NHS Digital is the trading name of the Health and Social Care Information
2017 Essentials Brief: Cloud
 2017 Essentials Brief: Cloud www.himssanalytics.com Enabling better health through information technology. Healthcare Information and Management Systems Society (HIMSS) HIMSS is a global, cause-based,
2017 Essentials Brief: Cloud www.himssanalytics.com Enabling better health through information technology. Healthcare Information and Management Systems Society (HIMSS) HIMSS is a global, cause-based,
GMO Register User Guide
 GMO Register User Guide A. Rana and F. Foscarini Institute for Health and Consumer Protection 2007 EUR 22697 EN The mission of the Institute for Health and Consumer Protection is to provide scientific
GMO Register User Guide A. Rana and F. Foscarini Institute for Health and Consumer Protection 2007 EUR 22697 EN The mission of the Institute for Health and Consumer Protection is to provide scientific
ScholarOne Manuscripts. Editor User Guide
 ScholarOne Manuscripts Editor User Guide 28-November-2017 Clarivate Analytics ScholarOne Manuscripts Editor User Guide Page i TABLE OF CONTENTS INTRODUCTION... 1 Use Get Help Now and FAQs... 1 Site Configuration
ScholarOne Manuscripts Editor User Guide 28-November-2017 Clarivate Analytics ScholarOne Manuscripts Editor User Guide Page i TABLE OF CONTENTS INTRODUCTION... 1 Use Get Help Now and FAQs... 1 Site Configuration
The IDN Variant TLD Program: Updated Program Plan 23 August 2012
 The IDN Variant TLD Program: Updated Program Plan 23 August 2012 Table of Contents Project Background... 2 The IDN Variant TLD Program... 2 Revised Program Plan, Projects and Timeline:... 3 Communication
The IDN Variant TLD Program: Updated Program Plan 23 August 2012 Table of Contents Project Background... 2 The IDN Variant TLD Program... 2 Revised Program Plan, Projects and Timeline:... 3 Communication
APPENDIX 1 7 APPENDIX 2 8 APPENDIX 3 10 APPENDIX 4 11
 Trust Policy and Procedure Document ref. no: PP(16)276 Form Creation Policy For use in: For use by: For use for: Document owner: Status: Trust wide All staff Management of Form Creation Health Records
Trust Policy and Procedure Document ref. no: PP(16)276 Form Creation Policy For use in: For use by: For use for: Document owner: Status: Trust wide All staff Management of Form Creation Health Records
BUILDING A TOWN WEBSITE Teacher s Guide
 A Basic Dreamweaver MX Project from Macromedia BUILDING A TOWN WEBSITE Teacher s Guide Table of Contents Project Description...3 ISTE National Educational Technology Standards for Students...3 Timing...4
A Basic Dreamweaver MX Project from Macromedia BUILDING A TOWN WEBSITE Teacher s Guide Table of Contents Project Description...3 ISTE National Educational Technology Standards for Students...3 Timing...4
UMDNJ VISUAL IDENTITY GUIDELINES
 UMDNJ VISUAL IDENTITY GUIDELINES One University The University of Medicine and Dentistry of New Jersey is a diverse academic community, and this diversity adds value and strength to the institution. UMDNJ
UMDNJ VISUAL IDENTITY GUIDELINES One University The University of Medicine and Dentistry of New Jersey is a diverse academic community, and this diversity adds value and strength to the institution. UMDNJ
Collaborative Stage Data Collection System. Version Implementation Guide for. Registries and Vendors. Elaine N. Collins, RHIA, CTR
 Collaborative Stage Data Collection System Version 02.05 Implementation Guide for Registries and Vendors Elaine N. Collins, RHIA, CTR Collaborative Stage Informatics Team Revised January 14, 2014 Informatics
Collaborative Stage Data Collection System Version 02.05 Implementation Guide for Registries and Vendors Elaine N. Collins, RHIA, CTR Collaborative Stage Informatics Team Revised January 14, 2014 Informatics
Presenter(s): Dave Venier. Topic Rosetta Interoperability (C-CDA and IHE) Level 200
 Presenter(s): Dave Venier Topic Rosetta Interoperability (C-CDA and IHE) Level 200 Safe Harbor Provisions/Legal Disclaimer This presentation may contain forward-looking statements within the meaning of
Presenter(s): Dave Venier Topic Rosetta Interoperability (C-CDA and IHE) Level 200 Safe Harbor Provisions/Legal Disclaimer This presentation may contain forward-looking statements within the meaning of
A TEXT MINER ANALYSIS TO COMPARE INTERNET AND MEDLINE INFORMATION ABOUT ALLERGY MEDICATIONS Chakib Battioui, University of Louisville, Louisville, KY
 Paper # DM08 A TEXT MINER ANALYSIS TO COMPARE INTERNET AND MEDLINE INFORMATION ABOUT ALLERGY MEDICATIONS Chakib Battioui, University of Louisville, Louisville, KY ABSTRACT Recently, the internet has become
Paper # DM08 A TEXT MINER ANALYSIS TO COMPARE INTERNET AND MEDLINE INFORMATION ABOUT ALLERGY MEDICATIONS Chakib Battioui, University of Louisville, Louisville, KY ABSTRACT Recently, the internet has become
