AtmoCONTROL FDA EDITION IQ DOCUMENTATION OQ DOCUMENTATION 100% ATMOSAFE. MADE IN GERMANY.
|
|
|
- Suzanna Neal
- 5 years ago
- Views:
Transcription
1 AtmoCONTROL FDA EDITION IQ DOCUMENTATION OQ DOCUMENTATION 100% ATMOSAFE. MADE IN GERMANY.
2 Manufacturer and customer service Memmert GmbH + Co. KG Willi-Memmert-Straße D Büchenbach Deutschland/Germany Phone: +49 (0) Fax: +49 (0) sales@memmert.com Internet: Customer service: Service hotline: +49 (0) Service fax: +49 (0) service@memmert.com 2018 MEMMERT GmbH + Co. KG D39051 Date 05/2018 v2.6.x We reserve the right to make changes
3 Contents AtmoCONTROL FDA Edition IQ-OQ-Documentation 1. Introduction Purpose of Qualification Installation Qualification Packing List Verification Software Installation (to be completed by user) Assessment of Installation Qualification Operational Qualification Login Admin and User Rights... 9 User Rights Audit Trail Connect database Signatures Security Editor Signatures Export Backup Help Assessment of Operational Qualification D39051 Date 05/2018 v2.6.x 3
4 Please follow the instructions below and fill out each gap in order to perform the installation qualification as well as the operational qualification. Examination object (to be completed by user) Manufacturer: Designation: MEMMERT GmbH + Co.KG, Schwabach, Germany Memmert AtmoCONTROL FDA Version: Site: Occasion: Inspection Personnel (to be completed by user) Name Position Signature Date Performed by: Approved by: Memmert recommends that the IQ/OQ protocols are performed in total when AtmoCONTROL FDA is initially installed. Installation Qualification should be performed as well when the software is upgraded and when the computer that runs AtmoCONTROL FDA is changed or modified. Operational qualification should also be run on a regular basis to confirm that the system is performing to specification and also when there is any doubt that the instrument is not performing to specifications. 4 D39051 Date 05/2018 v2.6.x
5 1. Introduction The task of the FDA is the protection of public health in the US and the approval of pharmaceutical products. The FDA controls the safety and efficacy of medical products for human and veterinary medicine, biological products, medical devices, food and radiation - emitting devices, that arrive in the American market. This applies to products manufactured in the US as well as imported products. FDA plays a significant role in addressing the Nation s counterterrorism capability and ensuring the security of the food supply. The Electronic Records and Signatures Rule, known as 21 CFR Part 11, were introduced by the U.S. Food and Drug Administration (FDA) in order to define requirements for the use of electronic documents instead of paper records. The law, published in the Federal Register on March 20, 1997 and in effect since August 20, 1997, specifies the system elements, controls, and procedures that are necessary to make sure the trustworthiness of electronically stored records. Conformity requires both procedural controls and administrative controls, such as Standard Operating Procedures (SOPs), training and administration to be put in place by the user, in addition to the technical controls and elements that the system can offer. Therefore, no product alone can fully meet the regulatory requirements. However, products with unified functions that support 21 CFR Part 11 requirements can significantly ease the task of achieving and maintaining full conformity with the law. 1.1 Purpose of Qualification Qualification of instruments or software is a formal process of documenting that an instrument or software is fit for its intended use and that is kept maintained and calibrated. IQ: The purpose of this qualification is to examine that the product designated as examination object is complete and is essentially ready for operation. It also documents that all important specifications and parameters described by the manufacturer, as well as its safety requirements, are fulfilled. OQ: The purpose of this qualification is to ensure the function of the product designated as examination objects and that it is ready for operation. The following functions are tested: Security functions (according to FDA Titel 21 CFR Part 11) Control functions relevant for quality and/or process 2. Installation Qualification Installation Qualification (IQ) demonstrates that the AtmoCONTROL FDA software is properly installed. Installation Qualification tests should be performed at the following times: When the system is installed Every time the software is upgraded Any time the associated computer is changed or modified Prior to running OQ tests D39051 Date 05/2018 v2.6.x 5
6 2.1 Packing List Verification Operating software AtmoCONTROL FDA Version on USB stick included? Operating instructions for AtmoCONTROL FDA Version on USB stick included? Is the AtmoCONTROL FDA Version Software USB stick undamaged? No externally visible damage to the USB stick or packaging material? Delivery complete according to order and order confirmation (e.g. Software and Installation Manual) The answer to all questions must be yes in order to move on to the next qualification stage, otherwise rectify the fault! 2.2 Software Installation (to be completed by user) Are the following requirements fulfilled? Computer is running operating system Windows 7, Windows 8, Windows XP or Windows Vista? Enough internal memory is available for a successful installation (at least 1GB)? Installer has administrator rights? For the installation process please refer to the AtmoCONTROL User Manual and follow the instructions under Section Installing AtmoCONTROL. The manual was delivered along with the AtmoCONTROL FDA Version Software USB stick. Installation During installation, are you prompted to create an Admin account with a password? 6 D39051 Date 05/2018 v2.6.x
7 Was the software installed successfully? AtmoCONTROL FDA Edition IQ-OQ-Documentation 2.3 Assessment of Installation Qualification The installation qualification has been passed successfully if all previous questions have been answered with yes. If not, the errors have to be fixed and the installation qualification needs to be repeated. I hereby confirm that all previous questions have been answered with YES. Name Position Date Signature D39051 Date 05/2018 v2.6.x 7
8 3. Operational Qualification Operational Qualification (OQ) demonstrates that the AtmoCONTROL FDA software is functioning to specification. Operational Qualification tests should be performed: Once upon receipt of the instrument When the computer or software that runs the instrument is changed or modified Software IQ tests should be performed prior to the OQ tests in order to verify that the software is installed properly. 3.1 Login Start the AtmoCONTROL FDA Version by double-clicking the appropriate icon or in the start menu. Does the Login window appear after starting AtmoCONTROL FDA? If the username and/or password are incorrect, does the login fail? Can you login with your user name which was created during Setup? 8 D39051 Date 05/2018 v2.6.x
9 After login, does the program start with the AtmoCONTROL window? Is Admin displayed in the title bar? 3.2 Admin and User Rights Is the menu item Security available? Does the following window appear after clicking Edit users in the Security menu? D39051 Date 05/2018 v2.6.x 9
10 Does the Admin appear in the list? Does the Admin have all user rights, is locked and can t be edited? Does the button Add user exist? Create a new user account by clicking the Add user button and name the new user TestUser1. Does the new user have no user rights? Can the user rights be edited? 10 D39051 Date 05/2018 v2.6.x
11 Create a new user account with the same name as before. Does the following message appear? Can the admin reset the password of TestUser1? Close the User Management window. Click the Switch user button in the Security menu. Can you login as TestUser1? TestUser1 ******** Is TestUser1 displayed in the title bar? User Rights Login as TestUser1. Check if it is not possible to change the system settings (Options options or Edit backup folder) Is it impossible? D39051 Date 05/2018 v2.6.x 11
12 Check if it is not possible to open the User management (Security Edit user) Is it impossible? Audit Trail Login as Admin. To confirm the functionality of the Audit Trail function, select the Security tab on top of the tool bar and click on Audit Trail. Is the Audit Trail window below displayed? Does the Audit Trail window appear without any error messages? Activate Select time span. Is it possible to select the current day as time span? 12 D39051 Date 05/2018 v2.6.x
13 Click the Show audit trail button. Does it open up the following browser window? AtmoCONTROL FDA Edition IQ-OQ-Documentation 3.3 Connect database To confirm the functionality of the Connect database function, click Device Connect offline from Database. Does the following window appear? Connect demo device Y atdbx. D39051 Date 05/2018 v2.6.x 13
14 3.4 Signatures Click the Security button. Can the Sign document button be selected? Does the following window appear? Can the starting and ending point for signatures be selected? Does the following window appear after clicking the Sign button? Can all five roles be selected? 14 D39051 Date 05/2018 v2.6.x
15 Does the following window appear after clicking the Sign button? AtmoCONTROL FDA Edition IQ-OQ-Documentation Enter the admin data. Does the Signature window close without any errors after clicking the Sign button? Does the new signature appear in the signature bar? Do all informations appear in the signature bar after moving the cursor over the bar? D39051 Date 05/2018 v2.6.x 15
16 Select the Security tab from the top tool bar and click Sign document. Does the window Sign protocol range show the newly created signature? Can the newly created signature be selected? 16 D39051 Date 05/2018 v2.6.x
17 Does the Select roles to sign window appear after clicking the Sign button and is the admin shown in every role? After clicking on an info symbol, is the comment redisplayed? Close the dialog by clicking the Cancel button. Security Login with the newly created user account, without user rights. Is it possible? Check if it is possible to sign the protocol as TestUser1. Is it impossible? Close the dialog by clicking the Cancel button. D39051 Date 05/2018 v2.6.x 17
18 3.5 Editor Login as Admin. Switch to the Editor view. Does AtmoCONTROL FDA show the following window? Can you create a new profile by dragging and dropping the program symbols? 18 D39051 Date 05/2018 v2.6.x
19 Save the new profile. Can it be saved? Does the selected filename appear in the program view? Click New program. Are all program elements removed? Click Program. Open the previously saved program. Is it possible? D39051 Date 05/2018 v2.6.x 19
20 Signatures Can you select the menu item Sign document? Does the window Select roles to sign appear after clicking the Sign button? Can all five roles be selected? Does the following window appear after clicking the Sign button? Enter the admin data. Does the Signature window close without any errors after clicking the Sign button? 20 D39051 Date 05/2018 v2.6.x
21 Does the Select roles to sign window appear after clicking the Sign button and show the admin in every role? Close the dialog by clicking the Cancel button. Does the editor show a lock symbol in the top left corner? Try to move the editor symbols around. Are they blocked resp. is the program write-protected? 3.6 Export Switch to Protocol view. D39051 Date 05/2018 v2.6.x 21
22 Does the Export of Measurements Data window appear after clicking Protocol Export? Can the desired time span be selected? 22 D39051 Date 05/2018 v2.6.x
23 Can you select the following items: Excel PDF Text File/CSV Does the window show an input mask for the GLP data when selecting Excel or PDF? Can the protocol be exported to all three files types? Excel PDF Text File/CSV D39051 Date 05/2018 v2.6.x 23
24 Does the following window appear after clicking the Print document button? Please fill the gaps and print the protocol. Does the printout contain the signatures? Does AtmoCONTROL print the document without any errors? 3.7 Backup Does the following window appear after clicking Edit backup folder in the Options menu? If Default backup folder is activated, is it impossible to deactivate it? Is the path write-protected? Activate User specific backup folder. Is it possible to edit the folder? 24 D39051 Date 05/2018 v2.6.x
25 Activate User specific backup folder and click Save. Close the dialog by clicking the Cancel button. Reopen the dialog and be sure that the changes are present. AtmoCONTROL FDA Edition IQ-OQ-Documentation Deactivate User specific backup folder and click Save. Does the window close without any errors? 3.8 Help Does the following window appear after clicking the About button in the Help menu and show the activated version? Does the following PDF document appear after clicking User manual in the Help menu? D39051 Date 05/2018 v2.6.x 25
26 3.9 Assessment of Operational Qualification The operational qualification has been passed successfully if all previous questions have been answered with Yes. If not, the errors have to be fixed and the operational qualification needs to be repeated. I hereby confirm that all previous questions have been answered with YES. Name Position Date Signature 26 D39051 Date 05/2018 v2.6.x
27
28 AtmoCONTROL FDA Edition IQ Documentation OQ Documentation D39051 Date 05/2018 v2.6.x englisch Memmert GmbH + Co. KG Willi-Memmert-Straße D Büchenbach Tel Fax sales@memmert.com facebook.com/memmert.family Die Experten-Plattform:
WBCalib WATERBATH CALIBRATION TOOL VERSION OPERATING MANUAL 100% ATMOSAFE. MADE IN GERMANY.
 WBCalib WATERBATH CALIBRATION TOOL VERSION 1.0.1 OPERATING MANUAL 100% ATMOSAFE. MADE IN GERMANY. www.memmert.com www.atmosafe.net Manufacturer and customer service Memmert GmbH + Co. KG Willi-Memmert-Straße
WBCalib WATERBATH CALIBRATION TOOL VERSION 1.0.1 OPERATING MANUAL 100% ATMOSAFE. MADE IN GERMANY. www.memmert.com www.atmosafe.net Manufacturer and customer service Memmert GmbH + Co. KG Willi-Memmert-Straße
AtmoCONTROL FDA Edition according to 21CFR Part 11 U. S. Food and Drug Administration Version 2.6 SOFTWARE MANUAL
 AtmoCONTROL FDA Edition according to 21CFR Part 11 U. S. Food and Drug Administration Version 2.6 SOFTWARE MANUAL 100% ATMOSAFE. MADE IN GERMANY. www.memmert.com www.atmosafe.net Manufacturer and customer
AtmoCONTROL FDA Edition according to 21CFR Part 11 U. S. Food and Drug Administration Version 2.6 SOFTWARE MANUAL 100% ATMOSAFE. MADE IN GERMANY. www.memmert.com www.atmosafe.net Manufacturer and customer
Introduction. So what is 21 CFR Part 11? Who Should Comply with 21CFR Part 11?
 Introduction The following guide is an explanation of the term 21 CFR Part 11, and gives some background into the tools/features that Comark includes in its 21 CFR Part 11 products to aid compliance with
Introduction The following guide is an explanation of the term 21 CFR Part 11, and gives some background into the tools/features that Comark includes in its 21 CFR Part 11 products to aid compliance with
testo Comfort Software CFR 4 Instruction manual
 testo Comfort Software CFR 4 Instruction manual 2 1 Contents 1 Contents 1 Contents... 3 2 Specifications... 4 2.1. Intended purpose... 4 2.2. 21 CFR Part 11 and terminology used... 5 3 First steps... 9
testo Comfort Software CFR 4 Instruction manual 2 1 Contents 1 Contents 1 Contents... 3 2 Specifications... 4 2.1. Intended purpose... 4 2.2. 21 CFR Part 11 and terminology used... 5 3 First steps... 9
System Assessment Report Relating to Electronic Records and Electronic Signatures; 21 CFR Part 11. System: tiamo (Software Version 2.
 Page 1 /15 System Assessment Report Relating to Electronic Records and Electronic Signatures; 21 CFR Part 11 System: tiamo (Software Version 2.5) Page 2 /15 1 Procedures and Controls for Closed Systems
Page 1 /15 System Assessment Report Relating to Electronic Records and Electronic Signatures; 21 CFR Part 11 System: tiamo (Software Version 2.5) Page 2 /15 1 Procedures and Controls for Closed Systems
System Assessment Report Relating to Electronic Records and Electronic Signatures; 21 CFR Part 11. System: StabNet (Software Version 1.
 Page 1 /16 System Assessment Report Relating to Electronic Records and Electronic Signatures; 21 CFR Part 11 System: StabNet (Software Version 1.1) Page 2 /16 1 Procedures and Controls for Closed Systems
Page 1 /16 System Assessment Report Relating to Electronic Records and Electronic Signatures; 21 CFR Part 11 System: StabNet (Software Version 1.1) Page 2 /16 1 Procedures and Controls for Closed Systems
SDA COMPLIANCE SOFTWARE For Agilent ICP-MS MassHunter Software
 SDA COMPLIANCE SOFTWARE For Agilent ICP-MS MassHunter Software Part 11 in Title 21 of the US Code of Federal Regulations (commonly referred to as 21 CFR Part 11) governs food and drugs in the US, and includes
SDA COMPLIANCE SOFTWARE For Agilent ICP-MS MassHunter Software Part 11 in Title 21 of the US Code of Federal Regulations (commonly referred to as 21 CFR Part 11) governs food and drugs in the US, and includes
System Assessment Report Relating to Electronic Records and Electronic Signatures; Final Rule, 21 CFR Part 11
 Page 1 /16 System Assessment Report Relating to Electronic Records and Electronic Signatures; Final Rule, 21 CFR Part 11 System: Touch Control for Titrando (Software version 5.840.0150) Page 2 /16 1 Procedures
Page 1 /16 System Assessment Report Relating to Electronic Records and Electronic Signatures; Final Rule, 21 CFR Part 11 System: Touch Control for Titrando (Software version 5.840.0150) Page 2 /16 1 Procedures
21 CFR Part 11 LIMS Requirements Electronic signatures and records
 21 CFR Part 11 LIMS Requirements Electronic signatures and records Compiled by Perry W. Burton Version 1.0, 16 August 2014 Table of contents 1. Purpose of this document... 1 1.1 Notes to version 1.0...
21 CFR Part 11 LIMS Requirements Electronic signatures and records Compiled by Perry W. Burton Version 1.0, 16 August 2014 Table of contents 1. Purpose of this document... 1 1.1 Notes to version 1.0...
Guide for 21 CFR part 11 on NucleoView NC-200
 Guide for 21 CFR part 11 on NucleoView NC-200 Contents Introduction... 2 Background... 2 Implementation... 2 Defining NucleoView NC-200 TM user groups using Windows Active Directory... 3 Enabling 21 CFR
Guide for 21 CFR part 11 on NucleoView NC-200 Contents Introduction... 2 Background... 2 Implementation... 2 Defining NucleoView NC-200 TM user groups using Windows Active Directory... 3 Enabling 21 CFR
Rotronic Monitoring System Technical paper Version 1.0. Rotronic Monitoring System - Technical paper - Understanding RMS from an IT perspective
 Version 1.0 Rotronic Monitoring System - Technical paper - Understanding RMS from an IT perspective 2 Abbreviations CFR CL CS FAT FDA FRS FS Code of Federal Regulations Change Log Configuration Specification
Version 1.0 Rotronic Monitoring System - Technical paper - Understanding RMS from an IT perspective 2 Abbreviations CFR CL CS FAT FDA FRS FS Code of Federal Regulations Change Log Configuration Specification
Mastersizer CFR Part 11 User Guide
 Mastersizer 3000 21 CFR Part 11 User Guide Abstract This document provides details on how to use the 21 CFR Part 11 features provided for the Malvern Mastersizer 3000 software. Assumptions This document
Mastersizer 3000 21 CFR Part 11 User Guide Abstract This document provides details on how to use the 21 CFR Part 11 features provided for the Malvern Mastersizer 3000 software. Assumptions This document
MicroLab FTIR Software 21 CFR Part 11 Compliance
 MicroLab FTIR Software 21 CFR Part 11 Compliance Technical Overview Introduction Electronic data submitted to the United States FDA must comply with specifications set forth in the Code of Federal Regulations,
MicroLab FTIR Software 21 CFR Part 11 Compliance Technical Overview Introduction Electronic data submitted to the United States FDA must comply with specifications set forth in the Code of Federal Regulations,
Operating Instructions. LMI 5000 LaVA Suite Quick Start Manual. Rev:
 LMI 5000 LaVA Suite Quick Start Manual Operating Instructions -1- Notice This manual contains descriptions, drawings and specifications for an Alpha Technologies Product. Equipment or products made prior
LMI 5000 LaVA Suite Quick Start Manual Operating Instructions -1- Notice This manual contains descriptions, drawings and specifications for an Alpha Technologies Product. Equipment or products made prior
Getting Started With the Cisco PAM Desktop Software
 CHAPTER 3 Getting Started With the Cisco PAM Desktop Software This chapter describes how to install the Cisco PAM desktop client software, log on to Cisco PAM, and begin configuring access control features
CHAPTER 3 Getting Started With the Cisco PAM Desktop Software This chapter describes how to install the Cisco PAM desktop client software, log on to Cisco PAM, and begin configuring access control features
Software.
 Software ebro offers exactly the software you need: Evaluation software for any applications: basic, light and pro Evaluation software for EBI 25 data loggers: web and wave Evaluation software for pharmaceutical
Software ebro offers exactly the software you need: Evaluation software for any applications: basic, light and pro Evaluation software for EBI 25 data loggers: web and wave Evaluation software for pharmaceutical
Using "IC Net 2.2 " software to comply with 21 CFR Part 11
 CH-9101 Herisau/Switzerland E-Mail info@metrohm.com Internet www.metrohm.com Using "IC Net 2.2 " software to comply with 21 CFR Part 11 Compliance white paper 8.110.8273 CH-9101 Herisau/Switzerland E-Mail
CH-9101 Herisau/Switzerland E-Mail info@metrohm.com Internet www.metrohm.com Using "IC Net 2.2 " software to comply with 21 CFR Part 11 Compliance white paper 8.110.8273 CH-9101 Herisau/Switzerland E-Mail
Good Laboratory Practice GUIDELINES FOR THE ARCHIVING OF ELECTRONIC RAW DATA IN A GLP ENVIRONMENT. Release Date:
 AGIT: Swiss Working Group on Information Technology in a GLP Environment Good Laboratory Practice GUIDELINES FOR THE ARCHIVING OF ELECTRONIC RAW DATA IN A GLP ENVIRONMENT Release Date: 31.01.2018 Version:
AGIT: Swiss Working Group on Information Technology in a GLP Environment Good Laboratory Practice GUIDELINES FOR THE ARCHIVING OF ELECTRONIC RAW DATA IN A GLP ENVIRONMENT Release Date: 31.01.2018 Version:
Benutzerhandbuch "CELSIUS 2007 FDA-Edition" USER MANUAL
 Benutzerhandbuch "CELSIUS 2007 FDA-Edition" USER MANUAL CELSIUS 10 Control software for MEMMERT appliances FDA Edition Validatable in according to 21 CFR Part 11 U. S. Food and Drug Administration Manufacturer
Benutzerhandbuch "CELSIUS 2007 FDA-Edition" USER MANUAL CELSIUS 10 Control software for MEMMERT appliances FDA Edition Validatable in according to 21 CFR Part 11 U. S. Food and Drug Administration Manufacturer
Avigilon Control Center Server User Guide
 Avigilon Control Center Server User Guide Version 4.12 PDF-SERVER-E-Rev1 Copyright 2012 Avigilon. All rights reserved. The information presented is subject to change without notice. No copying, distribution,
Avigilon Control Center Server User Guide Version 4.12 PDF-SERVER-E-Rev1 Copyright 2012 Avigilon. All rights reserved. The information presented is subject to change without notice. No copying, distribution,
Sparta Systems TrackWise Solution
 Systems Solution 21 CFR Part 11 and Annex 11 Assessment October 2017 Systems Solution Introduction The purpose of this document is to outline the roles and responsibilities for compliance with the FDA
Systems Solution 21 CFR Part 11 and Annex 11 Assessment October 2017 Systems Solution Introduction The purpose of this document is to outline the roles and responsibilities for compliance with the FDA
Compliance of Shimadzu Total Organic Carbon (TOC) Analyzer with FDA 21 CFR Part 11 Regulations on Electronic Records and Electronic Signatures
 NT1D-1275 Compliance of Shimadzu Total Organic Carbon (TOC) Analyzer with FDA 21 CFR Part 11 Regulations on Electronic Records and Electronic Signatures TOC-Control L Ver.1 / LabSolutions DB/CS Ver.6 Part
NT1D-1275 Compliance of Shimadzu Total Organic Carbon (TOC) Analyzer with FDA 21 CFR Part 11 Regulations on Electronic Records and Electronic Signatures TOC-Control L Ver.1 / LabSolutions DB/CS Ver.6 Part
Kaye Validator AVS Version Z3007, Rev D June Advanced Validation System Console SW Enhancements
 Kaye Validator AVS Version 1.2.3 Z3007, Rev D June 2017 Kaye Advanced Validation System (AVS) version 1.2.3 is an upgrade for the Advanced Validation System version 1.2.1, including the enhancements and
Kaye Validator AVS Version 1.2.3 Z3007, Rev D June 2017 Kaye Advanced Validation System (AVS) version 1.2.3 is an upgrade for the Advanced Validation System version 1.2.1, including the enhancements and
21 CFR PART 11 COMPLIANCE
 21 CFR PART 11 COMPLIANCE PRODUCT OVERVIEW ADD-ONS & INDIVIDUAL SOLUTIONS PLA SUPPORT CONTRACT TRAINING CONSULTING 21 CFR PART 11 COMPLIANCE PLA 3.0 Software For Biostatistical Analysis PLA 3.0 21 CFR
21 CFR PART 11 COMPLIANCE PRODUCT OVERVIEW ADD-ONS & INDIVIDUAL SOLUTIONS PLA SUPPORT CONTRACT TRAINING CONSULTING 21 CFR PART 11 COMPLIANCE PLA 3.0 Software For Biostatistical Analysis PLA 3.0 21 CFR
Sparta Systems TrackWise Digital Solution
 Systems TrackWise Digital Solution 21 CFR Part 11 and Annex 11 Assessment February 2018 Systems TrackWise Digital Solution Introduction The purpose of this document is to outline the roles and responsibilities
Systems TrackWise Digital Solution 21 CFR Part 11 and Annex 11 Assessment February 2018 Systems TrackWise Digital Solution Introduction The purpose of this document is to outline the roles and responsibilities
Lab Assistant GLP Software for AcqKnowledge
 Lab Assistant GLP Software for AcqKnowledge Lab Assistant GLP Features include Network or Stand-alone Solutions Security Management Data Archiving Customizable Software Solutions Advanced Audit Trails
Lab Assistant GLP Software for AcqKnowledge Lab Assistant GLP Features include Network or Stand-alone Solutions Security Management Data Archiving Customizable Software Solutions Advanced Audit Trails
CLIQ Web Manager. User Manual. The global leader in door opening solutions V 6.1
 CLIQ Web Manager User Manual V 6.1 The global leader in door opening solutions Program version: 6.1 Document number: ST-003478 Date published: 2016-03-31 Language: en-gb Table of contents 1 Overview...9
CLIQ Web Manager User Manual V 6.1 The global leader in door opening solutions Program version: 6.1 Document number: ST-003478 Date published: 2016-03-31 Language: en-gb Table of contents 1 Overview...9
Smooth and secure integration of ChemStation Plus with LIMS systems using Labtronics LimsLink CDS. Application. Steve Bolton Ute Bober.
 Smooth and secure integration of ChemStation Plus with LIMS systems using Labtronics LimsLink CDS Application Steve Bolton Ute Bober Abstract Laboratories today are facing an urgent requirement to make
Smooth and secure integration of ChemStation Plus with LIMS systems using Labtronics LimsLink CDS Application Steve Bolton Ute Bober Abstract Laboratories today are facing an urgent requirement to make
ChromQuest 5.0. Tools to Aid in 21 CFR Part 11 Compliance. Introduction. General Overview. General Considerations
 ChromQuest 5.0 Tools to Aid in 21 CFR Part 11 Compliance Introduction Thermo Scientific, Inc. is pleased to offer the ChromQuest chromatography data system (CDS) as a solution for chromatography labs seeking
ChromQuest 5.0 Tools to Aid in 21 CFR Part 11 Compliance Introduction Thermo Scientific, Inc. is pleased to offer the ChromQuest chromatography data system (CDS) as a solution for chromatography labs seeking
3M Molecular Detection System Software Upgrade/Installation Instructions
 User Manual Supplement Number: TB.342837.03 Effective Date: March 2018 Supersedes: TB.342837.02 Technology Platform: 3M Molecular Detection System Originating Location: St. Paul, MN 3M Molecular Detection
User Manual Supplement Number: TB.342837.03 Effective Date: March 2018 Supersedes: TB.342837.02 Technology Platform: 3M Molecular Detection System Originating Location: St. Paul, MN 3M Molecular Detection
SIMATIC Automation License Manager Manual 02/2008 A5E
 s Contents SIMATIC Automation License Manager Product Overview 1 Installation 2 Working with the Automation License Manager 3 Glossar Index Manual 02/2008 A5E02128430-01 Safety Guidelines This manual contains
s Contents SIMATIC Automation License Manager Product Overview 1 Installation 2 Working with the Automation License Manager 3 Glossar Index Manual 02/2008 A5E02128430-01 Safety Guidelines This manual contains
Internet Key Administration
 Internet Key Administration An Internet Key allows multiple users access to a common Earthwork 4D software license. Users are created by the Internet Key s administrator. As an Internet Key administrator
Internet Key Administration An Internet Key allows multiple users access to a common Earthwork 4D software license. Users are created by the Internet Key s administrator. As an Internet Key administrator
User Guide. Oracle Health Sciences Central Coding Release 3.1. Part Number: E
 User Guide Oracle Health Sciences Central Coding Release 3.1 Part Number: E69156-01 Copyright 2006, 2016, Oracle and/or its affiliates. All rights reserved. This software and related documentation are
User Guide Oracle Health Sciences Central Coding Release 3.1 Part Number: E69156-01 Copyright 2006, 2016, Oracle and/or its affiliates. All rights reserved. This software and related documentation are
Adobe Sign and 21 CFR Part 11
 Adobe Sign and 21 CFR Part 11 Today, organizations of all sizes are transforming manual paper-based processes into end-to-end digital experiences speeding signature processes by 500% with legal, trusted
Adobe Sign and 21 CFR Part 11 Today, organizations of all sizes are transforming manual paper-based processes into end-to-end digital experiences speeding signature processes by 500% with legal, trusted
X-Series System Manual Part 5: Information for administrators
 X-Series System Manual Part 5: Information for administrators Version check: Version No. Date Version 3.0 April 2011 Version 2.0 October 2007 Mettler-Toledo Garvens GmbH Kampstrasse 7 31180 Giesen OT Hasede
X-Series System Manual Part 5: Information for administrators Version check: Version No. Date Version 3.0 April 2011 Version 2.0 October 2007 Mettler-Toledo Garvens GmbH Kampstrasse 7 31180 Giesen OT Hasede
Integration of Agilent UV-Visible ChemStation with OpenLAB ECM
 Integration of Agilent UV-Visible ChemStation with OpenLAB ECM Compliance with Introduction in Title 21 of the Code of Federal Regulations includes the US Federal guidelines for storing and protecting
Integration of Agilent UV-Visible ChemStation with OpenLAB ECM Compliance with Introduction in Title 21 of the Code of Federal Regulations includes the US Federal guidelines for storing and protecting
Metrohm White paper. FDA 21 CFR Part 11 Requirements for NIR Spectroscopy. Dr. N. Rühl
 FDA 21 CFR Part 11 Requirements for NIR Spectroscopy Dr. N. Rühl The prosperity of a society can be evaluated based on many criteria, and the focus is certainly different for each individual. However,
FDA 21 CFR Part 11 Requirements for NIR Spectroscopy Dr. N. Rühl The prosperity of a society can be evaluated based on many criteria, and the focus is certainly different for each individual. However,
System Assessment Report Relating to Electronic Records and Electronic Signatures; Final Rule, 21 CFR Part 11. System: tiamo 2.3
 Page 1 /14 System Assessment Report Relating to Electronic Records and Electronic Signatures; Final le, 21 CFR Part 11 System: tiamo 23 052011 / doe Page 2 /14 1 Procedures and Controls for Closed Systems
Page 1 /14 System Assessment Report Relating to Electronic Records and Electronic Signatures; Final le, 21 CFR Part 11 System: tiamo 23 052011 / doe Page 2 /14 1 Procedures and Controls for Closed Systems
AtmoCONTROL SOFTWARE MANUAL 100% ATMOSAFE. MADE IN GERMANY.
 SOFTWARE MANUAL 100% ATMOSAFE. MADE IN GERMANY. www.memmert.com www.atmosafe.net Manufacturer and customer service Memmert GmbH + Co. KG Willi-Memmert-Straße 90 96 D-91186 Büchenbach Deutschland/Germany
SOFTWARE MANUAL 100% ATMOSAFE. MADE IN GERMANY. www.memmert.com www.atmosafe.net Manufacturer and customer service Memmert GmbH + Co. KG Willi-Memmert-Straße 90 96 D-91186 Büchenbach Deutschland/Germany
Installing Authoring Manager
 Installing Authoring Manager Installing Authoring Manager v5.2 (PC only) System Requirements: Before you install Authoring Manager, you should ensure that your system meets the minimum software and hardware
Installing Authoring Manager Installing Authoring Manager v5.2 (PC only) System Requirements: Before you install Authoring Manager, you should ensure that your system meets the minimum software and hardware
Agilent ICP-MS ChemStation Complying with 21 CFR Part 11. Application Note. Overview
 Agilent ICP-MS ChemStation Complying with 21 CFR Part 11 Application Note Overview Part 11 in Title 21 of the Code of Federal Regulations includes the US Federal guidelines for storing and protecting electronic
Agilent ICP-MS ChemStation Complying with 21 CFR Part 11 Application Note Overview Part 11 in Title 21 of the Code of Federal Regulations includes the US Federal guidelines for storing and protecting electronic
Bp Premier Quick Start to Applying a Data Update
 Bp Premier Quick Start to Applying a Data Update Updating the drug database Best Practice Software releases data (drug) updates at the beginning of each month. Updates include Pharmaceutical Benefits Scheme
Bp Premier Quick Start to Applying a Data Update Updating the drug database Best Practice Software releases data (drug) updates at the beginning of each month. Updates include Pharmaceutical Benefits Scheme
Electronic Data Capture (EDC) Systems and Part 11 Compliance
 Electronic Data Capture (EDC) Systems and Part 11 Compliance Office of New Animal Drug Evaluation Center for Veterinary Medicine Society of Quality Assurance Gaylord Hotel, Washington DC March 28, 2017
Electronic Data Capture (EDC) Systems and Part 11 Compliance Office of New Animal Drug Evaluation Center for Veterinary Medicine Society of Quality Assurance Gaylord Hotel, Washington DC March 28, 2017
21 CFR PART 11 FREQUENTLY ASKED QUESTIONS (FAQS)
 21 CFR PART 11 FREQUENTLY ASKED QUESTIONS (S) The United States Food and Drug Administration (FDA) defines the criteria under which electronic records and electronic signatures are considered trustworthy,
21 CFR PART 11 FREQUENTLY ASKED QUESTIONS (S) The United States Food and Drug Administration (FDA) defines the criteria under which electronic records and electronic signatures are considered trustworthy,
Compliance Matrix for 21 CFR Part 11: Electronic Records
 Compliance Matrix for 21 CFR Part 11: Electronic Records Philip E. Plantz, PhD, Applications Manager David Kremer, Senior Software Engineer Application Note SL-AN-27 Revision B Provided By: Microtrac,
Compliance Matrix for 21 CFR Part 11: Electronic Records Philip E. Plantz, PhD, Applications Manager David Kremer, Senior Software Engineer Application Note SL-AN-27 Revision B Provided By: Microtrac,
GLP Administrator. AcqKnowledge GLP
 GLP Administrator AcqKnowledge GLP Introduction When AcqKnowledge is used with the GLP System installed, each user is assigned permissions for the program that indicate what operations that user is and
GLP Administrator AcqKnowledge GLP Introduction When AcqKnowledge is used with the GLP System installed, each user is assigned permissions for the program that indicate what operations that user is and
UNIVERSAL SOFTWARE. Universal Software. Data Sheet
 Universal Software Data Sheet System Requirements: The minimum requirements for using the Software are: 1). Windows XP/Vista/7 2). A minimum of 512 MB RAM 3). 1 GB of hard disk space 4). Microsoft Office
Universal Software Data Sheet System Requirements: The minimum requirements for using the Software are: 1). Windows XP/Vista/7 2). A minimum of 512 MB RAM 3). 1 GB of hard disk space 4). Microsoft Office
N2KExtractor. Maretron Data Extraction Software User s Manual
 N2KExtractor Maretron Data Extraction Software User s Manual Revision 3.1.6 Copyright 2017 Maretron, LLP All Rights Reserved Maretron, LLP 9014 N. 23rd Ave #10 Phoenix, AZ 85021-7850 http://www.maretron.com
N2KExtractor Maretron Data Extraction Software User s Manual Revision 3.1.6 Copyright 2017 Maretron, LLP All Rights Reserved Maretron, LLP 9014 N. 23rd Ave #10 Phoenix, AZ 85021-7850 http://www.maretron.com
JUNG Visu Pro Server
 Content: JUNG Visu Pro Server 1 TECHNICAL REQUIREMENTS...2 1.1 HARDWARE...2 1.2 OPERATING SYSTEMS...2 1.3 SOFTWARE...2 1.4 CLIENT/BROWSER SETTINGS...2 2 FIRST RUN...3 3 ADMINISTRATION SURFACE...3 3.1 PROJECT
Content: JUNG Visu Pro Server 1 TECHNICAL REQUIREMENTS...2 1.1 HARDWARE...2 1.2 OPERATING SYSTEMS...2 1.3 SOFTWARE...2 1.4 CLIENT/BROWSER SETTINGS...2 2 FIRST RUN...3 3 ADMINISTRATION SURFACE...3 3.1 PROJECT
How to Comply with FDA 21 CFR Part11. Sample Screen Manual. Mitsubishi Electric Corporation
 How to Comply with FDA 21 CFR Part11 Sample Screen Manual Mitsubishi Electric Corporation Using the Samples The sample screen data and files such as the instruction manual can be used upon agreement to
How to Comply with FDA 21 CFR Part11 Sample Screen Manual Mitsubishi Electric Corporation Using the Samples The sample screen data and files such as the instruction manual can be used upon agreement to
Using the Titrando system to comply with 21 CFR Part 11
 01.2003/sn Using the Titrando system to comply with 21 CFR Part 11 The Electronic Records and Signatures Rule, known as 21 CFR Part 11, was established by the U.S. Food and Drug Administration (FDA) to
01.2003/sn Using the Titrando system to comply with 21 CFR Part 11 The Electronic Records and Signatures Rule, known as 21 CFR Part 11, was established by the U.S. Food and Drug Administration (FDA) to
Integration of Agilent OpenLAB CDS EZChrom Edition with OpenLAB ECM Compliance with 21 CFR Part 11
 OpenLAB CDS Integration of Agilent OpenLAB CDS EZChrom Edition with OpenLAB ECM Compliance with 21 CFR Part 11 Technical Note Introduction Part 11 in Title 21 of the Code of Federal Regulations includes
OpenLAB CDS Integration of Agilent OpenLAB CDS EZChrom Edition with OpenLAB ECM Compliance with 21 CFR Part 11 Technical Note Introduction Part 11 in Title 21 of the Code of Federal Regulations includes
21 CFR Part 11 FAQ (Frequently Asked Questions)
 21 CFR Part 11 FAQ (Frequently Asked Questions) and Roles and Responsibilities for Assessment of METTLER TOLEDO STAR e Software Version 16.00, including: - 21 CFR 11 Compliance software option for Compliance
21 CFR Part 11 FAQ (Frequently Asked Questions) and Roles and Responsibilities for Assessment of METTLER TOLEDO STAR e Software Version 16.00, including: - 21 CFR 11 Compliance software option for Compliance
ISF Watchkeeper 3 Getting Started Users Guide
 ISF Watchkeeper 3 Getting Started Users Guide ISF Watchkeeper is a computer software program, for use on board ships. The software is designed for maintaining records of seafarers hours of work and rest,
ISF Watchkeeper 3 Getting Started Users Guide ISF Watchkeeper is a computer software program, for use on board ships. The software is designed for maintaining records of seafarers hours of work and rest,
DIGIOP ELEMENTS V8.1 Software-only Installation Guide
 DIGIOP ELEMENTS V8.1 Software-only Installation Guide About this installation guide Use this document as a guide to install your DIGIOP ELEMENTS software system. It includes procedures to install your
DIGIOP ELEMENTS V8.1 Software-only Installation Guide About this installation guide Use this document as a guide to install your DIGIOP ELEMENTS software system. It includes procedures to install your
MASTERSIZER 2000 SOFTWARE: v6.00 (PSS ) SOFTWARE UPDATE NOTIFICATION
 MASTERSIZER 2000 SOFTWARE: v6.00 (PSS0002-35) SOFTWARE UPDATE NOTIFICATION Introduction This document details the release of software PSS0002-35. This is version 6.00 of software for the Mastersizer 2000
MASTERSIZER 2000 SOFTWARE: v6.00 (PSS0002-35) SOFTWARE UPDATE NOTIFICATION Introduction This document details the release of software PSS0002-35. This is version 6.00 of software for the Mastersizer 2000
QUICK START GUIDE FIRMWARE UPDATE VIA MIRA SOFTWARE APPLICABLE TO ALL PATH MEDICAL DEVICES
 Before beginning a Firmware update for your Sentiero device through MIRA, please make sure that your MIRA software version is up to date. To get the latest version of MIRA software on your PC, you can
Before beginning a Firmware update for your Sentiero device through MIRA, please make sure that your MIRA software version is up to date. To get the latest version of MIRA software on your PC, you can
Agilent ChemStation OpenLAB Option
 Agilent ChemStation OpenLAB Option Concepts Guide ChemStation OpenLAB Option Concepts Guide Agilent Technologies Notices Agilent Technologies, Inc. 2008-2009, 2010 No part of this manual may be reproduced
Agilent ChemStation OpenLAB Option Concepts Guide ChemStation OpenLAB Option Concepts Guide Agilent Technologies Notices Agilent Technologies, Inc. 2008-2009, 2010 No part of this manual may be reproduced
SOFTWARE UPDATE NOTIFICATION SPRAYTEC SOFTWARE v3.30: PSS
 SOFTWARE UPDATE NOTIFICATION SPRAYTEC SOFTWARE v3.30: PSS0024-11 Introduction This document details the release of software PSS0024-11: version 3.30 of the software for the Spraytec laser diffraction system.
SOFTWARE UPDATE NOTIFICATION SPRAYTEC SOFTWARE v3.30: PSS0024-11 Introduction This document details the release of software PSS0024-11: version 3.30 of the software for the Spraytec laser diffraction system.
Getting Started. SpotOn! Flexo 2.6. All you need to know to get started, every step of the way.
 2013 Starter Guide Getting Started SpotOn! Flexo 2.6 All you need to know to get started, every step of the way. How to install the software How to activate the software How to contact us Languages SpotOn!
2013 Starter Guide Getting Started SpotOn! Flexo 2.6 All you need to know to get started, every step of the way. How to install the software How to activate the software How to contact us Languages SpotOn!
Frequently Asked Questions UNIFI v1.9 Service Release 4 Upgrade
 Frequently Asked Questions UNIFI v1.9 Service Release 4 Upgrade Q. What are the features and benefits of upgrading to UNIFI v1.9 SR4? A. UNIFI v1.9 SR4 incorporates improvements across all application
Frequently Asked Questions UNIFI v1.9 Service Release 4 Upgrade Q. What are the features and benefits of upgrading to UNIFI v1.9 SR4? A. UNIFI v1.9 SR4 incorporates improvements across all application
User Manual. Version 1 1/10/ of 26
 User Manual Version 1 1/10/2006 1 of 26 Software Disclosure This Software is Compliant with all 21 CFR 11 regulations pertaining to Closed Systems. We currently do not comply with Open System requirements.
User Manual Version 1 1/10/2006 1 of 26 Software Disclosure This Software is Compliant with all 21 CFR 11 regulations pertaining to Closed Systems. We currently do not comply with Open System requirements.
EZChrom Elite Chromatography Data System. Regulatory Compliance with FDA Rule of Electronic Records and Electronic Signatures (21 CFR Part 11)
 EZChrom Elite Chromatography Data System Regulatory Compliance with FDA Rule of Electronic Records and Electronic Signatures (21 CFR Part 11) Scope On August 20, 1997 the final rule of the United States
EZChrom Elite Chromatography Data System Regulatory Compliance with FDA Rule of Electronic Records and Electronic Signatures (21 CFR Part 11) Scope On August 20, 1997 the final rule of the United States
Sparta Systems Stratas Solution
 Systems Solution 21 CFR Part 11 and Annex 11 Assessment October 2017 Systems Solution Introduction The purpose of this document is to outline the roles and responsibilities for compliance with the FDA
Systems Solution 21 CFR Part 11 and Annex 11 Assessment October 2017 Systems Solution Introduction The purpose of this document is to outline the roles and responsibilities for compliance with the FDA
This information applies to versions 2015 or greater of CODESOFT Enterprise Network Licenses.
 Healthcare and Pharmaceutical Label Printing Tools for 21 CFR Part 11 Compliance This information applies to versions 2015 or greater of CODESOFT Enterprise Network Licenses. CODESOFT Enterprise label
Healthcare and Pharmaceutical Label Printing Tools for 21 CFR Part 11 Compliance This information applies to versions 2015 or greater of CODESOFT Enterprise Network Licenses. CODESOFT Enterprise label
Offline Audit Tool (OAT) User Guide Version 3.0
 Offline Audit Tool (OAT) User Guide Version 3.0 Contents Downloading Java Development Kit... 2 Downloading the Offline Client... 7 Completing Your Audit & Utilizing the Offline Audit Tool... 14 Importing
Offline Audit Tool (OAT) User Guide Version 3.0 Contents Downloading Java Development Kit... 2 Downloading the Offline Client... 7 Completing Your Audit & Utilizing the Offline Audit Tool... 14 Importing
White Paper Assessment of Veriteq viewlinc Environmental Monitoring System Compliance to 21 CFR Part 11Requirements
 White Paper Assessment of Veriteq viewlinc Environmental Monitoring System Compliance to 21 CFR Part 11Requirements Introduction The 21 CFR Part 11 rule states that the FDA view is that the risks of falsification,
White Paper Assessment of Veriteq viewlinc Environmental Monitoring System Compliance to 21 CFR Part 11Requirements Introduction The 21 CFR Part 11 rule states that the FDA view is that the risks of falsification,
Using "TiNet 2.5 Compliant SR1" software to comply with 21 CFR Part 11
 2003-08-08/dö Using "TiNet 2.5 Compliant SR1" software to comply with 21 CFR Part 11 The Title 21 Code of Federal Regulations Electronic Records; Electronic Signatures of the U.S. Food and Drug Administration,
2003-08-08/dö Using "TiNet 2.5 Compliant SR1" software to comply with 21 CFR Part 11 The Title 21 Code of Federal Regulations Electronic Records; Electronic Signatures of the U.S. Food and Drug Administration,
Downloading Java Development Kit (JDK) & Offline Client For Use of Reliance Offline Audit Tool
 Downloading Java Development Kit (JDK) & Offline Client For Use of Reliance Offline Audit Tool Contents Downloading Java Development Kit... 1 Downloading the Offline Client... 6 Downloading Java Development
Downloading Java Development Kit (JDK) & Offline Client For Use of Reliance Offline Audit Tool Contents Downloading Java Development Kit... 1 Downloading the Offline Client... 6 Downloading Java Development
CODESOFT uses NT security. The network administrator will need to set up the users as needed per the requirements of 21 CFR Part 11.
 Healthcare and Pharmaceutical Label Printing Tools for 21 CFR Part 11 Compliance This applies to versions 7.x or greater of CODESOFT Enterprise CODESOFT Enterprise label design software provides features
Healthcare and Pharmaceutical Label Printing Tools for 21 CFR Part 11 Compliance This applies to versions 7.x or greater of CODESOFT Enterprise CODESOFT Enterprise label design software provides features
Australia Online Forms for Research Software User Manual
 Australia Online Forms for Research Software User Manual Version 1.3 Released 21 August 2010 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting
Australia Online Forms for Research Software User Manual Version 1.3 Released 21 August 2010 2 P a g e A u s t r a l i a O n l i n e F o r m s f o r R e s e a r c h Contents 1. Introduction 5 2. Getting
Aligned Elements The professional Product Suite built to keep the Design History Files complete and consistent at all times, using minimal effort and
 Aligned Elements The professional Product Suite built to keep the Design History Files complete and consistent at all times, using minimal effort and tying up a minimum of resources Aligned Elements will
Aligned Elements The professional Product Suite built to keep the Design History Files complete and consistent at all times, using minimal effort and tying up a minimum of resources Aligned Elements will
Managing GSS Devices from the GUI
 CHAPTER 1 This chapter describes how to configure and manage your Global Site Selector Manager (GSSM) and Global Site Selector (GSS) devices from the primary GSSM graphical user interface. It includes
CHAPTER 1 This chapter describes how to configure and manage your Global Site Selector Manager (GSSM) and Global Site Selector (GSS) devices from the primary GSSM graphical user interface. It includes
EU Annex 11 Compliance Regulatory Conformity of eve
 White Paper EU Annex 11 Compliance Regulatory Conformity of eve Franco Berz, Head of Quality Management INFORS HT Dr. Britta Abellan, Computer System Validation Manager INFORS HT 1. Introduction More and
White Paper EU Annex 11 Compliance Regulatory Conformity of eve Franco Berz, Head of Quality Management INFORS HT Dr. Britta Abellan, Computer System Validation Manager INFORS HT 1. Introduction More and
Assessment of Vaisala Veriteq viewlinc Continuous Monitoring System Compliance to 21 CFR Part 11 Requirements
 / White PAPer Assessment of Vaisala Veriteq viewlinc Continuous Monitoring System Compliance to 21 CFR Part 11 Requirements The 21 CFR Part 11 rule states that the FDA view is that the risks of falsification,
/ White PAPer Assessment of Vaisala Veriteq viewlinc Continuous Monitoring System Compliance to 21 CFR Part 11 Requirements The 21 CFR Part 11 rule states that the FDA view is that the risks of falsification,
Flow Computer. Manual Configuration of Device Software. FC1-CDS-EN b i From ensuite version 3.4
 Flow Computer encore FC1 Manual Configuration of Device Software FC1-CDS-EN b 2015-11-18 i 2015-11-18 From ensuite version 3.4 Elster GmbH Schloßstraße 95a D - 44357 Dortmund/Germany Tel.: +49 231 937110-0
Flow Computer encore FC1 Manual Configuration of Device Software FC1-CDS-EN b 2015-11-18 i 2015-11-18 From ensuite version 3.4 Elster GmbH Schloßstraße 95a D - 44357 Dortmund/Germany Tel.: +49 231 937110-0
V-STATS 4.01 HB g Copyright: SenTec AG. Installation Manual. for V-STATS with. V-CareNeT Package. Copyright:
 Installation Manual for V-STATS 4.01 with V-CareNeT Package Copyright: SenTec AG Ringstrasse 39 4106 Therwil Switzerland Tel.: +41 61 726 97 60 Fax : +41 61 726 97 61 info@sentec.com www.sentec.com HB-006144-g
Installation Manual for V-STATS 4.01 with V-CareNeT Package Copyright: SenTec AG Ringstrasse 39 4106 Therwil Switzerland Tel.: +41 61 726 97 60 Fax : +41 61 726 97 61 info@sentec.com www.sentec.com HB-006144-g
MPX Server Software User Manual
 MPX Server Software User Manual Contents 1 Server Software Installation... - 3 - Initial Setup... - 6-2 Software Interface... - 10 - Login Page:... - 10-2.1 Homepage... - 12-2.2 Resources... - 13-2.3 Composer...
MPX Server Software User Manual Contents 1 Server Software Installation... - 3 - Initial Setup... - 6-2 Software Interface... - 10 - Login Page:... - 10-2.1 Homepage... - 12-2.2 Resources... - 13-2.3 Composer...
Getting Started With DO Analyser Software Version 4
 Getting Started With DO Analyser Software Version 4 Getting Started With The DO Analyser Sofware Version 4 Jochen Arndt SiS Sensoren Instrumente Systeme GmbH Schwentinental The author and publisher have
Getting Started With DO Analyser Software Version 4 Getting Started With The DO Analyser Sofware Version 4 Jochen Arndt SiS Sensoren Instrumente Systeme GmbH Schwentinental The author and publisher have
POLKADOTS SOFTWARE PrePage-it 09 Technical Primer GETTING STARTED WITH PREPAGE-IT 09
 POLKADOTS SOFTWARE PrePage-it 09 Technical Primer GETTING STARTED WITH PREPAGE-IT 09 Table of Contents INTRODUCTION...3 PREPAGE-IT 09 OVERVIEW...4 PREPAGE-IT 09: MAIN MODULES...4 Optional modules...4 INSTALLATION...4
POLKADOTS SOFTWARE PrePage-it 09 Technical Primer GETTING STARTED WITH PREPAGE-IT 09 Table of Contents INTRODUCTION...3 PREPAGE-IT 09 OVERVIEW...4 PREPAGE-IT 09: MAIN MODULES...4 Optional modules...4 INSTALLATION...4
SureClose Product Line
 SureClose Product Line Release Notes 3.7 June 21, 2013 SureClose 3.7 Release Notes June 2013 1 Table of Contents Overview... 4 Post-Installation Considerations... 4 Features and Functionality... 6 New
SureClose Product Line Release Notes 3.7 June 21, 2013 SureClose 3.7 Release Notes June 2013 1 Table of Contents Overview... 4 Post-Installation Considerations... 4 Features and Functionality... 6 New
New Dropbox Users (don t have a Dropbox account set up with your Exeter account)
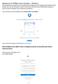 The setup process will determine if you already have a Dropbox account associated with an Exeter email address, and if so, you'll be given a choice to move those contents to your Phillips Exeter Dropbox
The setup process will determine if you already have a Dropbox account associated with an Exeter email address, and if so, you'll be given a choice to move those contents to your Phillips Exeter Dropbox
Log on to the server. The user name and password for this will be issued for each user by Pellcomp.
 USING PICS HOSTED BY PELLCOMP This note is for PICS users whose database is hosted by Pellcomp on our servers. It is not relevant if PICS is kept on your own company network, other hosted servers or just
USING PICS HOSTED BY PELLCOMP This note is for PICS users whose database is hosted by Pellcomp on our servers. It is not relevant if PICS is kept on your own company network, other hosted servers or just
ComplianceQuest Support of Compliance to FDA 21 CFR Part 11Requirements WHITE PAPER. ComplianceQuest In-Depth Analysis and Review
 ComplianceQuest Support of Compliance to FDA 21 CFR Part 11 WHITE PAPER ComplianceQuest In-Depth Analysis and Review ComplianceQuest Support of Compliance to FDA is the FDA guideline that defines the criteria
ComplianceQuest Support of Compliance to FDA 21 CFR Part 11 WHITE PAPER ComplianceQuest In-Depth Analysis and Review ComplianceQuest Support of Compliance to FDA is the FDA guideline that defines the criteria
Work instructions for US-FDA ANDA Submissions Work Instructions for ectd US-FDA ANDA Submissions
 Work Instructions for ectd US-FDA ANDA Submissions 1. Logon to the PharmaReady ectd System Work instructions for US-FDA ANDA Submissions PharmaReady is a Web-based application. The logon screen and all
Work Instructions for ectd US-FDA ANDA Submissions 1. Logon to the PharmaReady ectd System Work instructions for US-FDA ANDA Submissions PharmaReady is a Web-based application. The logon screen and all
PORTAL USER. Jayme Pina Version GUIDE
 PORTAL USER Jayme Pina Version 2.6.2018 GUIDE Contents User Access Roles... 3 How to Log-in... 3 Patient Look Up... 5 My Patients... 5 All Patients... 6 Patient Notification... 8 Opt-out / Opt-Back-In...
PORTAL USER Jayme Pina Version 2.6.2018 GUIDE Contents User Access Roles... 3 How to Log-in... 3 Patient Look Up... 5 My Patients... 5 All Patients... 6 Patient Notification... 8 Opt-out / Opt-Back-In...
First steps digivod. Version 3.4 Trial version
 First steps digivod Version 3.4 Trial version Content 1. Introduction 3 1.1 Copyright / License agreements 3 2. Installation and first steps 4 2.1 System requirements 4 2.2 Installation of digivod 5 2.3
First steps digivod Version 3.4 Trial version Content 1. Introduction 3 1.1 Copyright / License agreements 3 2. Installation and first steps 4 2.1 System requirements 4 2.2 Installation of digivod 5 2.3
MindView Online - Quick Start Guide
 MindView Online - Quick Start Guide Overview MindView Online is an online concept mapping program that allows users to organize their thoughts visually to create, share, and export mind maps to Microsoft
MindView Online - Quick Start Guide Overview MindView Online is an online concept mapping program that allows users to organize their thoughts visually to create, share, and export mind maps to Microsoft
Policy Manager in Compliance 360 Version 2018
 Policy Manager in Compliance 360 Version 2018 Policy Manager Overview 3 Create a Policy 4 Relate a Policy to Other Policies, Departments, and Incidents 8 Edit a Policy 10 Edit a Policy by Using the Edit
Policy Manager in Compliance 360 Version 2018 Policy Manager Overview 3 Create a Policy 4 Relate a Policy to Other Policies, Departments, and Incidents 8 Edit a Policy 10 Edit a Policy by Using the Edit
INFORMATION. Guidance on the use of the SM1000 and SM2000 Videographic Recorders for Electronic Record Keeping in FDA Approved Processes
 INFORMATION No. INF02/70 Issue 3 Date: October 2007 Product SM1000 and SM2000 Videographic Recorders Manuals IM/SM1000 and IM/SM2000 Guidance on the use of the SM1000 and SM2000 Videographic Recorders
INFORMATION No. INF02/70 Issue 3 Date: October 2007 Product SM1000 and SM2000 Videographic Recorders Manuals IM/SM1000 and IM/SM2000 Guidance on the use of the SM1000 and SM2000 Videographic Recorders
Employee-User Guide. The Answers to Frequently Asked Questions. Support
 Employee-User Guide The Answers to Frequently Asked Questions Support 1.844.402.6557 Support@purelyhr.com How to login to To log into the system, go to http://www.purelyhr.com, and click on the Login button
Employee-User Guide The Answers to Frequently Asked Questions Support 1.844.402.6557 Support@purelyhr.com How to login to To log into the system, go to http://www.purelyhr.com, and click on the Login button
Widgets for SAP BusinessObjects Business Intelligence Platform User Guide SAP BusinessObjects Business Intelligence platform 4.1 Support Package 2
 Widgets for SAP BusinessObjects Business Intelligence Platform User Guide SAP BusinessObjects Business Intelligence platform 4.1 Support Package 2 Copyright 2013 SAP AG or an SAP affiliate company. All
Widgets for SAP BusinessObjects Business Intelligence Platform User Guide SAP BusinessObjects Business Intelligence platform 4.1 Support Package 2 Copyright 2013 SAP AG or an SAP affiliate company. All
Operation Manual. for the. Data Logging Software. Version 7.1. (Isoft.xls)
 for the Data Logging Software Version 7.1 (Isoft.xls) TetraTec Instruments GmbH 1 GENERAL HINTS 1.1 Typographical Conventions Displayment Means marks a work procedure, which you must implement references
for the Data Logging Software Version 7.1 (Isoft.xls) TetraTec Instruments GmbH 1 GENERAL HINTS 1.1 Typographical Conventions Displayment Means marks a work procedure, which you must implement references
Aventail Connect Client with Smart Tunneling
 Aventail Connect Client with Smart Tunneling User s Guide Windows v8.9.0 1996-2007 Aventail Corporation. All rights reserved. Aventail, Aventail Cache Control, Aventail Connect, Aventail Connect Mobile,
Aventail Connect Client with Smart Tunneling User s Guide Windows v8.9.0 1996-2007 Aventail Corporation. All rights reserved. Aventail, Aventail Cache Control, Aventail Connect, Aventail Connect Mobile,
Table of Contents.
 Table of Contents 1. Items Included with the BioAxxis ThumbLock Audit Trail Software: 2 2. IMPORTANT NOTE:... 2 3. Supported Operating Systems:... 3 Section 1 Installing the BioAxxis ThumbLock AT Software...
Table of Contents 1. Items Included with the BioAxxis ThumbLock Audit Trail Software: 2 2. IMPORTANT NOTE:... 2 3. Supported Operating Systems:... 3 Section 1 Installing the BioAxxis ThumbLock AT Software...
OM-MICROLITE-8 AND OM- MICROLITE-16 DATA LOGGERS AND OM-MICROLAB
 User s Guide Shop online at omega.com e-mail: info@omega.com For latest product manuals: omegamanual.info OM-MICROLITE-8 AND OM- MICROLITE-16 DATA LOGGERS AND OM-MICROLAB And DatPass Administration Software
User s Guide Shop online at omega.com e-mail: info@omega.com For latest product manuals: omegamanual.info OM-MICROLITE-8 AND OM- MICROLITE-16 DATA LOGGERS AND OM-MICROLAB And DatPass Administration Software
August August 21, FADV.com. First Advantage 2013
 August 2014 August 21, 2014 First Advantage 2013 FADV.com Emails sent to Candidate Username (Log in ID) & Password Once an order is placed, the Candidate will be sent two emails (with Username and subsequently
August 2014 August 21, 2014 First Advantage 2013 FADV.com Emails sent to Candidate Username (Log in ID) & Password Once an order is placed, the Candidate will be sent two emails (with Username and subsequently
Password Memory 7 User s Guide
 C O D E : A E R O T E C H N O L O G I E S Password Memory 7 User s Guide 2007-2018 by code:aero technologies Phone: +1 (321) 285.7447 E-mail: info@codeaero.com Table of Contents How secure is Password
C O D E : A E R O T E C H N O L O G I E S Password Memory 7 User s Guide 2007-2018 by code:aero technologies Phone: +1 (321) 285.7447 E-mail: info@codeaero.com Table of Contents How secure is Password
Understanding Word Lesson 1
 Understanding Word Lesson 1 Objectives Software Orientation Before you begin working in Microsoft Word, you need to acquaint yourself with the primary user interface (UI). When you first launch Microsoft
Understanding Word Lesson 1 Objectives Software Orientation Before you begin working in Microsoft Word, you need to acquaint yourself with the primary user interface (UI). When you first launch Microsoft
SOFTWARE UPDATE NOTIFICATION MASTERSIZER 2000 SOFTWARE v5.61: PSS
 SOFTWARE UPDATE NOTIFICATION MASTERSIZER 2000 SOFTWARE v5.61: PSS0002-34 Introduction This document details the release of software PSS0002-34: version 5.61 of the software for the Mastersizer 2000 laser
SOFTWARE UPDATE NOTIFICATION MASTERSIZER 2000 SOFTWARE v5.61: PSS0002-34 Introduction This document details the release of software PSS0002-34: version 5.61 of the software for the Mastersizer 2000 laser
