ISA internals. October 18, Speeding up the ISA iteration 2
|
|
|
- Caitlin Turner
- 5 years ago
- Views:
Transcription
1 ISA internals Gábor Csárdi October 18, 2011 Contents 1 Introduction 1 2 Why two packages? 1 3 Speeding up the ISA iteration 2 4 Running time analysis The hardware and software Getting the data Measuring running time Number of rows and columns Number of seeds Running ISA in parallel 11 6 Session information 12 1 Introduction The Iterative Signature Algorithm (ISA) is a biclustering method, it finds consistent blocks (modules, biclusters) in tabular data, e.g. gene expression measurements across a number of samples. Please see the introductory tutorials at the ISA homepage, and also [Bergmann et al., 2003] for details. In this document we specifically deal with the implementation of the ISA in the isa2 and eisa R packages. 2 Why two packages? We implemented the ISA in two R packages, isa2 and eisa. ISA is a very general algorithm, that can be used for any tabular data to find correlated blocks. Examples for such tabular data are gene expression [Ihmels and Bergmann, 2004], 1
2 response of different cell lines to a number of drugs [Kutalik et al., 2008]. It can also be used as a general classifier, for biological or other data. It is also true, however, that the ISA is frequently used for gene expression analysis. Thus, we decided to provide two different user interfaces to the ISA. One interface, provided by isa2 package, is very general, the input of the isa2 functions is a numeric matrix, and the output contains two matrices, defining the ISA modules. The isa2 package can be used for the modular analysis of tabular data of any kind, from any source. The eisa package (the leading e stands for expression) provides a second interface, for people that specifically deal with gene expression data. This package builds on the infrastructure created by the BioConductor project [Gentleman et al., 2004]. The input of the eisa functions is an Expression- Set object, the standard BioConductor data structure for storing gene expression data. BioConductor provides functions to create such an ExpressionSet object from raw data and to download data from public repositories, such as the Gene Expression Omnibus [Davis and Meltzer, 2007, Barrett et al., 2009] and transform it into an ExpressionSet. The output of the eisa functions is an object that contains the ISA modules, the annotation of the genes and samples in the data set and possibly also further experimental meta data. The eisa package provides functions for calculating enrichment statistics against various databases for the ISA modules. The eisa package uses already existing BioConductor annotation packages, so it works for any organism that is supported by BioConductor. Having two ISA packages, however, does not mean two implementations of the ISA. The eisa package is fully built on top of the services of the isa2 package, only the latter one contains the implementation of the ISA iteration. The two packages allow ease of installation and use: users dealing with gene expression data install the eisa package, and this automatically installs the isa2 package as well. Users analyzing other data install the isa2 package only, this does not need any BioConductor packages. The isa2 package is part of the standard R package repository (CRAN), the eisa package has been accepted as an official BioConductor package and is included in BioConductor from the 2.6 release, due in April, Speeding up the ISA iteration ISA is an unsupervised, iterative, randomized algorithm. It starts with a seed vector. r 0. This vector is an initial guess for the rows of the input matrix that form a single module. This guess is then refined, by iterating itself and another vector, c i, that defines the columns of the module. During the iteration, the ISA uses two matrices, E r and E c, derived from the input matrix, by standardizing it row-wise and column-wise, respectively. One step of the ISA iteration involves (1) multiplying E r by r n and then (2) thresholding the result to keep elements that are further away from its mean 2
3 than a prescribed value, Θ c, in standard deviation units. This gives c n+1. Next, (3) E c is multiplied by c n+1 and (4) the result is thresholded with Θ r. This gives r n+1. The iteration is finished if r n+1 is close to r n and c n+1 is close to c n. Considerable speedup can be achived, if the ISA iteration is performed in batches of seed vectors, instead of handling them individually. The reason for this is the availability of the highly optimized linear algebra libraries that perform matrix-matrix multiplication much faster than all the corresponding matrix-vector multiplications individually. The seed vectors can be merged into a seed matrix. This can be done, even if the ISA thresholds are different for the different seeds, the isa2 and eisa packages support this. We considered using sparse matrices during the ISA iteration, because the matrix of seed vectors is sparse, but according to our measurements, sparse matrices are only marginally faster, and only in some cases. The reason for this is, that the product of the two matrices is always dense, and gets sparse only after the thresholding; the dense-sparse matrix multiplication, plus the conversion to a sparse matrix again, takes about the same time as the densedense matrix multiplication. Different input seeds converge in different number of steps, in parctice many seeds tend to converge quickly, and very few seeds need a lot of steps. Because of this, it is essential to remove the seeds that have already converged, from the seed matrix, so that only a smaller seed matrix needs to be iterated for many steps. As loops are typically slow in R, we implemented the thresholding operations in C. 4 Running time analysis In this section we show an experimental running time analysis for our ISA implementation. 4.1 The hardware and software The code in the following sections were run under Linux operating system, release el5, version #1 SMP Tue May 31 13:22:04 EDT 2011, on an x86_64 machine with 12 processors of type Intel(R) Xeon(R) CPU 2.67GHz and GiB memory. 4.2 Getting the data We use subsets of the same data set for the test, this is a data set that is publicly available in the Gene Expression Omnibus (GEO) repository, its id is GSE Note, that for the analysis of gene expression data, the eisa package is a better choice. It makes sense, however, if this tutorial can be run without any BioConductor packages, so we will use the isa2 package. 3
4 The non-normalized data set of the experiment is available from GEO as a numeric matrix. To spare time and bandwidth, we only download it once, even if the code of this tutorial is run multiple times. We store the data file in the current local directory. If the file is already there, then there is nothing to do. > GEO <- "GSE18858" > GEOfile <- paste(sep = "", GEO, "_series_matrix.txt.gz") > GEOurl <- paste(sep = "", "ftp://ftp.ncbi.nih.gov/pub/geo/data/seriesmatrix/", GEO, "/", GEOfile) > if (!file.exists(geofile)) { download.file(geourl, GEOfile) Next, we are read in the data. Lines that are part of the file header start with an exclamation mark in GEO files, so we skip them by setting the comment.char argument of read.table(). The table has a header, the first lines are the sample names; it also has the probeset names in the first column, we use these as row names. > data <- read.table(gzfile(geofile), comment.char = "!", header = TRUE, row.names = 1) > data <- as.matrix(data) Let s check the size of the data matrix. > dim(data) [1] It has rows (=probesets) and 242 columns. We load the isa2 package, to perform the ISA. No BioConductor packages are needed. > library(isa2) 4.3 Measuring running time We define a simple function first, that runs the various steps of the ISA toolchain and measures the running time of each step. We use the system.time() function for this. The results are returned in table. > mesisa <- function(e, thr.row, thr.col, no.seeds) { t1 <- system.time({ NE <- isa.normalize(e) ) t2 <- system.time({ seeds <- generate.seeds(length = nrow(e), count = no.seeds) 4
5 ) t3 <- system.time({ mods <- isa.iterate(ne, row.seeds = seeds, thr.row = thr.row, thr.col = thr.col) ) t4 <- system.time({ mods2 <- isa.unique(ne, mods) ) cbind(normalization = t1, seeds.generation = t2, isa.iteration = t3, module.merge = t4, full = t1 + t2 + t3 + t4) We quickly test this function, on a small subset of our data, with 1000 rows and 30 columns, chosen randomly. > mydata <- data[sample(nrow(data), 1000), sample(ncol(data), 30)] > mesisa(mydata, 3, 3, 100) normalization seeds.generation isa.iteration user.self sys.self elapsed user.child sys.child module.merge full user.self sys.self elapsed user.child sys.child The table has one column for each step of the ISA toolchain: normalization, seed generation, performing the ISA iteration, merging the modules, and there is a column for the total time of these operations as well. The first row shows the processor time used by process itself, the second row the time spent in system calls. In the following we will use the total of both of them to measure the speed of the implementation. 4.4 Number of rows and columns First, we increase the number of rows in the data matrix gradually, and measure the running time of ISA. We do this for variuos (row) thresholds, to see if the trend is threshold-dependent. The column threshold will be fixed now. > row.thresholds <- seq(1, 3, by = 0.5) > col.thresholds <- 2 5
6 We create a function, do.row.thr(), that runs the ISA for fixed threshold parameters, and different number of rows in the data matrix. The function also does replications, 5 by default. > no.rows <- seq(5000, min(40000, nrow(data)), by = 5000) > do.row.thr <- function(thr, rep = 5) { res <- lapply(no.rows, function(x) { lapply(1:rep, function(xxx) { mydata <- data[sample(nrow(data), x), ] mesisa(mydata, thr, col.thresholds, 100) ) ) res We are ready to do the running time measurement now; separately for each row threshold parameter. This takes about three hours to run, on the platform mentioned above. > by.rows <- lapply(row.thresholds, do.row.thr) Next, we define a function to plot the results, with error bars. Error bars are not supported by the builtin R plotting functions, so we put them together from line segments. > myplot <- function(x, y, sd, xlim = range(x), ylim = c(min(y - sd), max(y + sd)), xlab = "", ylab = "",...) { plot(na, type = "n", xlim = xlim, ylim = ylim, xlab = xlab, ylab = ylab) xmin <- par("usr")[1] xr <- par("usr")[2] - xmin bw <- xr/200 segments(x, y - sd, x, y + sd) segments(x - bw, y - sd, x + bw, y - sd) segments(x - bw, y + sd, x + bw, y + sd) points(x, y,...) The following two functions calculate the mean and standard deviation of the running times in the result lists, we will use them later. > get.mean <- function(xx) { sapply(xx, function(x) mean(sapply(x, function(y) sum(y[1:2, 5])))) 6
7 > get.sd <- function(xx) { sapply(xx, function(x) sd(sapply(x, function(y) sum(y[1:2, 5])))) We are ready to create a plot, the running times in the function of the number of rows in the input matrix. The results can be seen in Fig. 1. > layout(rbind(1:2, 3:4, 5:6)) > for (i in 1:length(row.thresholds)) { par(mar = c(5, 4, 1, 1) + 0.1) y <- get.mean(by.rows[[i]]) s <- get.sd(by.rows[[i]]) myplot(no.rows, y, s, type = "b", pch = 20, xlab = "# of rows", ylab = "") rt <- row.thresholds[i] text(min(no.rows), max(y + s), substitute(theta[r] == rt, list(rt = rt)), adj = c(0, 1), cex = 1.3) In the following, we perform a similar analysis for the number of columns in the input matrix. ISA is a symmetric algorithm, rows are treated the same way as columns. The reason for discussing them separately here, is that gene expression matrices have usually much more rows than columns and this difference might affect the running time. For these runs, the row threshold is fixed and the column threshold changes from 1 to 3. > row.thresholds2 <- 2 > col.thresholds2 <- seq(1, 3, by = 0.5) We create a function to perform all the runs for a given column threshold, with replications five by default. > no.cols <- seq(30, ncol(data), by = 30) > do.col.thr <- function(thr, rep = 5) { res <- lapply(no.cols, function(x) { lapply(1:rep, function(xxx) { mydata <- data[, sample(ncol(data), x)] mesisa(mydata, row.thresholds2, thr, 100) ) ) res We are ready to do the measurements. On the above mentioned hardware configuration, this takes about 4 hours to run, depending on the load of the system. 7
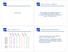
8 Θ r = Θ r = # of rows # of rows Θ r = Θ r = # of rows # of rows Θ r = # of rows Figure 1: Running time of the ISA, in the function of the number of rows in the input matrix, for various row thresholds. Crearly, the running time increases linearly with the number of rows, for all row thresholds. It is also true, that for higher thresholds the running times tend to be smaller, this is because in these cases more seeds converge quickly to the null vector, and these don t need to be iterated further. Each data point is the mean of five runs. 8
9 > by.cols <- lapply(col.thresholds2, do.col.thr) The mean running times are plotted, the results are shown in Fig. 2. > layout(rbind(1:2, 3:4, 5:6)) > for (i in 1:length(col.thresholds2)) { par(mar = c(5, 4, 1, 1) + 0.1) y <- get.mean(by.cols[[i]]) s <- get.sd(by.cols[[i]]) myplot(no.cols, y, s, type = "b", pch = 20, xlab = "# of cols", ylab = "") rt <- col.thresholds2[i] text(min(no.cols), max(y + s), substitute(theta[c] == rt, list(rt = rt)), adj = c(0, 1), cex = 1.3) 4.5 Number of seeds Finally, we also check the running time in the function of the number of starting seeds. We define three threshold configurations to test. The first has intermediate thresholds for both the rows and the columns, the second has a low threshold for the rows and a high threshold for the columns, the third is the opposite of the second. > thr.comb <- list(c(2, 2), c(1, 3), c(3, 1)) > no.seeds <- seq(50, 400, by = 50) The following function does all the runs for a given threshold combination. The size of the data matrix is fixed here, times 100. > do.no.seeds <- function(thr, rep = 5) { nr <- min(20000, nrow(data)) nc <- min(100, ncol(data)) res <- lapply(no.seeds, function(x) { lapply(1:rep, function(xxx) { mydata <- data[sample(nrow(data), nr), sample(ncol(data), nc)] mesisa(mydata, thr[1], thr[2], x) ) ) res We are ready to do the measurement now. > by.no.seeds <- lapply(thr.comb, do.no.seeds) The results are plotted in Fig. 3. 9
10 Θ c = Θ c = # of cols # of cols Θ c = Θ c = # of cols # of cols Θ c = # of cols Figure 2: Running times, in the function of the number of columns in the input matrix, for various column thresholds. Interestingly, the running time can be considered as independent of the number of columns. This is simply because the row seed matrix is two orders of magnitude larger than the column seed matrix, and the former dominates the running time. Each data point is the mean of five runs. 10
11 Θ r = 2, Θ c = Θ r = 1, Θ c = # of seeds # of seeds Θ r = 3, Θ c = # of seeds Figure 3: The running time, in the function of the number of starting seeds, for various row and column threshold combinations. Each point is the average of five ISA runs. The running time is increasing linearly with the number of seeds. > layout(rbind(1:2, 3:4)) > for (i in 1:length(thr.comb)) { par(mar = c(5, 4, 1, 1) + 0.1) y <- get.mean(by.no.seeds[[i]]) s <- get.sd(by.no.seeds[[i]]) myplot(no.seeds, y, s, type = "b", pch = 20, xlab = "# of seeds", ylab = "") th <- thr.comb[[i]] text(min(no.seeds), max(y + s), substitute(paste(theta[r] == r1, ", ", Theta[c] == r2), list(r1 = th[1], r2 = th[2])), adj = c(0, 1), cex = 1.3) 5 Running ISA in parallel It is trivial to run an ISA analysis in parallel, on a multi-processor machine, or a computer cluster: one just runs different threshold-combinations and/or 11
12 different seeds on the different processors. The achived speedup is close to linear, since the ISA iteration step dominates in the toolchain. Please see more about this on the ISA homepage at 6 Session information The version number of R and packages loaded for generating this vignette were: ˆ R version ( ), x86_64-unknown-linux-gnu ˆ Locale: LC_CTYPE=en_US.UTF-8, LC_NUMERIC=C, LC_TIME=en_US.UTF-8, LC_COLLATE=en_US.UTF-8, LC_MONETARY=C, LC_MESSAGES=en_US.UTF-8, LC_PAPER=en_US.UTF-8, LC_NAME=C, LC_ADDRESS=C, LC_TELEPHONE=C, LC_MEASUREMENT=en_US.UTF-8, LC_IDENTIFICATION=C ˆ Base packages: base, datasets, graphics, grdevices, methods, stats, utils ˆ Other packages: isa References [Barrett et al., 2009] Barrett, T., Troup, D., Wilhite, S., Ledoux, P., Rudnev, D., Evangelista, C., Kim, I., Soboleva, A., Tomashevsky, M., Marshall, K., Phillippy, K., Sherman, P., Muertter, R., and R, E. (2009). NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Research, 37:D [Bergmann et al., 2003] Bergmann, S., Ihmels, J., and Barkai, N. (2003). Iterative signature algorithm for the analysis of large-scale gene expression data. Physical Review E, 67: [Davis and Meltzer, 2007] Davis, S. and Meltzer, P. (2007). GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics, 14: [Gentleman et al., 2004] Gentleman, R. C., Carey, V. J., Bates, D. M., et al. (2004). BioConductor: Open software development for computational biology and bioinformatics. Genome Biology, 5:R80. [Ihmels and Bergmann, 2004] Ihmels, J. H. and Bergmann, S. (2004). Challenges and prospects in the analysis of large-scale gene expression data. Brief Bioinform, 5(4): [Kutalik et al., 2008] Kutalik, Z., Beckmann, J. S., and Bergmann, S. (2008). A modular approach for integrative analysis of large-scale gene-expression and drug-response data. Nat Biotechnol, 26(5):
Using metama for differential gene expression analysis from multiple studies
 Using metama for differential gene expression analysis from multiple studies Guillemette Marot and Rémi Bruyère Modified: January 28, 2015. Compiled: January 28, 2015 Abstract This vignette illustrates
Using metama for differential gene expression analysis from multiple studies Guillemette Marot and Rémi Bruyère Modified: January 28, 2015. Compiled: January 28, 2015 Abstract This vignette illustrates
CQN (Conditional Quantile Normalization)
 CQN (Conditional Quantile Normalization) Kasper Daniel Hansen khansen@jhsph.edu Zhijin Wu zhijin_wu@brown.edu Modified: August 8, 2012. Compiled: April 30, 2018 Introduction This package contains the CQN
CQN (Conditional Quantile Normalization) Kasper Daniel Hansen khansen@jhsph.edu Zhijin Wu zhijin_wu@brown.edu Modified: August 8, 2012. Compiled: April 30, 2018 Introduction This package contains the CQN
Shrinkage of logarithmic fold changes
 Shrinkage of logarithmic fold changes Michael Love August 9, 2014 1 Comparing the posterior distribution for two genes First, we run a DE analysis on the Bottomly et al. dataset, once with shrunken LFCs
Shrinkage of logarithmic fold changes Michael Love August 9, 2014 1 Comparing the posterior distribution for two genes First, we run a DE analysis on the Bottomly et al. dataset, once with shrunken LFCs
How to use CNTools. Overview. Algorithms. Jianhua Zhang. April 14, 2011
 How to use CNTools Jianhua Zhang April 14, 2011 Overview Studies have shown that genomic alterations measured as DNA copy number variations invariably occur across chromosomal regions that span over several
How to use CNTools Jianhua Zhang April 14, 2011 Overview Studies have shown that genomic alterations measured as DNA copy number variations invariably occur across chromosomal regions that span over several
The analysis of rtpcr data
 The analysis of rtpcr data Jitao David Zhang, Markus Ruschhaupt October 30, 2017 With the help of this document, an analysis of rtpcr data can be performed. For this, the user has to specify several parameters
The analysis of rtpcr data Jitao David Zhang, Markus Ruschhaupt October 30, 2017 With the help of this document, an analysis of rtpcr data can be performed. For this, the user has to specify several parameters
Seminar III: R/Bioconductor
 Leonardo Collado Torres lcollado@lcg.unam.mx Bachelor in Genomic Sciences www.lcg.unam.mx/~lcollado/ August - December, 2009 1 / 25 Class outline Working with HTS data: a simulated case study Intro R for
Leonardo Collado Torres lcollado@lcg.unam.mx Bachelor in Genomic Sciences www.lcg.unam.mx/~lcollado/ August - December, 2009 1 / 25 Class outline Working with HTS data: a simulated case study Intro R for
survsnp: Power and Sample Size Calculations for SNP Association Studies with Censored Time to Event Outcomes
 survsnp: Power and Sample Size Calculations for SNP Association Studies with Censored Time to Event Outcomes Kouros Owzar Zhiguo Li Nancy Cox Sin-Ho Jung Chanhee Yi June 29, 2016 1 Introduction This vignette
survsnp: Power and Sample Size Calculations for SNP Association Studies with Censored Time to Event Outcomes Kouros Owzar Zhiguo Li Nancy Cox Sin-Ho Jung Chanhee Yi June 29, 2016 1 Introduction This vignette
Count outlier detection using Cook s distance
 Count outlier detection using Cook s distance Michael Love August 9, 2014 1 Run DE analysis with and without outlier removal The following vignette produces the Supplemental Figure of the effect of replacing
Count outlier detection using Cook s distance Michael Love August 9, 2014 1 Run DE analysis with and without outlier removal The following vignette produces the Supplemental Figure of the effect of replacing
DupChecker: a bioconductor package for checking high-throughput genomic data redundancy in meta-analysis
 DupChecker: a bioconductor package for checking high-throughput genomic data redundancy in meta-analysis Quanhu Sheng, Yu Shyr, Xi Chen Center for Quantitative Sciences, Vanderbilt University, Nashville,
DupChecker: a bioconductor package for checking high-throughput genomic data redundancy in meta-analysis Quanhu Sheng, Yu Shyr, Xi Chen Center for Quantitative Sciences, Vanderbilt University, Nashville,
Analysis of two-way cell-based assays
 Analysis of two-way cell-based assays Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Analysis of two-way cell-based assays Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Drug versus Disease (DrugVsDisease) package
 1 Introduction Drug versus Disease (DrugVsDisease) package The Drug versus Disease (DrugVsDisease) package provides a pipeline for the comparison of drug and disease gene expression profiles where negatively
1 Introduction Drug versus Disease (DrugVsDisease) package The Drug versus Disease (DrugVsDisease) package provides a pipeline for the comparison of drug and disease gene expression profiles where negatively
segmentseq: methods for detecting methylation loci and differential methylation
 segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 30, 2018 1 Introduction This vignette introduces analysis methods for data from high-throughput
segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 30, 2018 1 Introduction This vignette introduces analysis methods for data from high-throughput
Triform: peak finding in ChIP-Seq enrichment profiles for transcription factors
 Triform: peak finding in ChIP-Seq enrichment profiles for transcription factors Karl Kornacker * and Tony Håndstad October 30, 2018 A guide for using the Triform algorithm to predict transcription factor
Triform: peak finding in ChIP-Seq enrichment profiles for transcription factors Karl Kornacker * and Tony Håndstad October 30, 2018 A guide for using the Triform algorithm to predict transcription factor
segmentseq: methods for detecting methylation loci and differential methylation
 segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 13, 2015 1 Introduction This vignette introduces analysis methods for data from high-throughput
segmentseq: methods for detecting methylation loci and differential methylation Thomas J. Hardcastle October 13, 2015 1 Introduction This vignette introduces analysis methods for data from high-throughput
Advanced analysis using bayseq; generic distribution definitions
 Advanced analysis using bayseq; generic distribution definitions Thomas J Hardcastle October 30, 2017 1 Generic Prior Distributions bayseq now offers complete user-specification of underlying distributions
Advanced analysis using bayseq; generic distribution definitions Thomas J Hardcastle October 30, 2017 1 Generic Prior Distributions bayseq now offers complete user-specification of underlying distributions
Bioconductor: Annotation Package Overview
 Bioconductor: Annotation Package Overview April 30, 2018 1 Overview In its current state the basic purpose of annotate is to supply interface routines that support user actions that rely on the different
Bioconductor: Annotation Package Overview April 30, 2018 1 Overview In its current state the basic purpose of annotate is to supply interface routines that support user actions that rely on the different
Iterative Signature Algorithm for the Analysis of Large-Scale Gene Expression Data. By S. Bergmann, J. Ihmels, N. Barkai
 Iterative Signature Algorithm for the Analysis of Large-Scale Gene Expression Data By S. Bergmann, J. Ihmels, N. Barkai Reasoning Both clustering and Singular Value Decomposition(SVD) are useful tools
Iterative Signature Algorithm for the Analysis of Large-Scale Gene Expression Data By S. Bergmann, J. Ihmels, N. Barkai Reasoning Both clustering and Singular Value Decomposition(SVD) are useful tools
Analyse RT PCR data with ddct
 Analyse RT PCR data with ddct Jitao David Zhang, Rudolf Biczok and Markus Ruschhaupt October 30, 2017 Abstract Quantitative real time PCR (qrt PCR or RT PCR for short) is a laboratory technique based on
Analyse RT PCR data with ddct Jitao David Zhang, Rudolf Biczok and Markus Ruschhaupt October 30, 2017 Abstract Quantitative real time PCR (qrt PCR or RT PCR for short) is a laboratory technique based on
Image Analysis with beadarray
 Mike Smith October 30, 2017 Introduction From version 2.0 beadarray provides more flexibility in the processing of array images and the extraction of bead intensities than its predecessor. In the past
Mike Smith October 30, 2017 Introduction From version 2.0 beadarray provides more flexibility in the processing of array images and the extraction of bead intensities than its predecessor. In the past
SIBER User Manual. Pan Tong and Kevin R Coombes. May 27, Introduction 1
 SIBER User Manual Pan Tong and Kevin R Coombes May 27, 2015 Contents 1 Introduction 1 2 Using SIBER 1 2.1 A Quick Example........................................... 1 2.2 Dealing With RNAseq Normalization................................
SIBER User Manual Pan Tong and Kevin R Coombes May 27, 2015 Contents 1 Introduction 1 2 Using SIBER 1 2.1 A Quick Example........................................... 1 2.2 Dealing With RNAseq Normalization................................
highscreen: High-Throughput Screening for Plate Based Assays
 highscreen: High-Throughput Screening for Plate Based Assays Ivo D. Shterev Cliburn Chan Gregory D. Sempowski August 22, 2018 1 Introduction This vignette describes the use of the R extension package highscreen
highscreen: High-Throughput Screening for Plate Based Assays Ivo D. Shterev Cliburn Chan Gregory D. Sempowski August 22, 2018 1 Introduction This vignette describes the use of the R extension package highscreen
How to Use pkgdeptools
 How to Use pkgdeptools Seth Falcon February 10, 2018 1 Introduction The pkgdeptools package provides tools for computing and analyzing dependency relationships among R packages. With it, you can build
How to Use pkgdeptools Seth Falcon February 10, 2018 1 Introduction The pkgdeptools package provides tools for computing and analyzing dependency relationships among R packages. With it, you can build
Introduction to R (BaRC Hot Topics)
 Introduction to R (BaRC Hot Topics) George Bell September 30, 2011 This document accompanies the slides from BaRC s Introduction to R and shows the use of some simple commands. See the accompanying slides
Introduction to R (BaRC Hot Topics) George Bell September 30, 2011 This document accompanies the slides from BaRC s Introduction to R and shows the use of some simple commands. See the accompanying slides
Creating a New Annotation Package using SQLForge
 Creating a New Annotation Package using SQLForge Marc Carlson, Herve Pages, Nianhua Li November 19, 2013 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
Creating a New Annotation Package using SQLForge Marc Carlson, Herve Pages, Nianhua Li November 19, 2013 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
PROPER: PROspective Power Evaluation for RNAseq
 PROPER: PROspective Power Evaluation for RNAseq Hao Wu [1em]Department of Biostatistics and Bioinformatics Emory University Atlanta, GA 303022 [1em] hao.wu@emory.edu October 30, 2017 Contents 1 Introduction..............................
PROPER: PROspective Power Evaluation for RNAseq Hao Wu [1em]Department of Biostatistics and Bioinformatics Emory University Atlanta, GA 303022 [1em] hao.wu@emory.edu October 30, 2017 Contents 1 Introduction..............................
Simulation of molecular regulatory networks with graphical models
 Simulation of molecular regulatory networks with graphical models Inma Tur 1 inma.tur@upf.edu Alberto Roverato 2 alberto.roverato@unibo.it Robert Castelo 1 robert.castelo@upf.edu 1 Universitat Pompeu Fabra,
Simulation of molecular regulatory networks with graphical models Inma Tur 1 inma.tur@upf.edu Alberto Roverato 2 alberto.roverato@unibo.it Robert Castelo 1 robert.castelo@upf.edu 1 Universitat Pompeu Fabra,
HowTo: Querying online Data
 HowTo: Querying online Data Jeff Gentry and Robert Gentleman November 12, 2017 1 Overview This article demonstrates how you can make use of the tools that have been provided for on-line querying of data
HowTo: Querying online Data Jeff Gentry and Robert Gentleman November 12, 2017 1 Overview This article demonstrates how you can make use of the tools that have been provided for on-line querying of data
Comparing Least Squares Calculations
 Comparing Least Squares Calculations Douglas Bates R Development Core Team Douglas.Bates@R-project.org November 16, 2017 Abstract Many statistics methods require one or more least squares problems to be
Comparing Least Squares Calculations Douglas Bates R Development Core Team Douglas.Bates@R-project.org November 16, 2017 Abstract Many statistics methods require one or more least squares problems to be
RDAVIDWebService: a versatile R interface to DAVID
 RDAVIDWebService: a versatile R interface to DAVID Cristóbal Fresno 1,2 and Elmer A. Fernández 1,2 1 Bio-science Data Mining Group, Catholic University of Córdoba, Córdoba, Argentina. 2 CONICET, Argentina
RDAVIDWebService: a versatile R interface to DAVID Cristóbal Fresno 1,2 and Elmer A. Fernández 1,2 1 Bio-science Data Mining Group, Catholic University of Córdoba, Córdoba, Argentina. 2 CONICET, Argentina
SEEK User Manual. Introduction
 SEEK User Manual Introduction SEEK is a computational gene co-expression search engine. It utilizes a vast human gene expression compendium to deliver fast, integrative, cross-platform co-expression analyses.
SEEK User Manual Introduction SEEK is a computational gene co-expression search engine. It utilizes a vast human gene expression compendium to deliver fast, integrative, cross-platform co-expression analyses.
Using the dorng package
 Using the dorng package dorng package Version 1.6 Renaud Gaujoux March 5, 2014 Contents Introduction............. 1 1 The %dorng% operator...... 3 1.1 How it works......... 3 1.2 Seeding computations.....
Using the dorng package dorng package Version 1.6 Renaud Gaujoux March 5, 2014 Contents Introduction............. 1 1 The %dorng% operator...... 3 1.1 How it works......... 3 1.2 Seeding computations.....
Visualisation, transformations and arithmetic operations for grouped genomic intervals
 ## Warning: replacing previous import ggplot2::position by BiocGenerics::Position when loading soggi Visualisation, transformations and arithmetic operations for grouped genomic intervals Thomas Carroll
## Warning: replacing previous import ggplot2::position by BiocGenerics::Position when loading soggi Visualisation, transformations and arithmetic operations for grouped genomic intervals Thomas Carroll
Raman Spectra of Chondrocytes in Cartilage: hyperspec s chondro data set
 Raman Spectra of Chondrocytes in Cartilage: hyperspec s chondro data set Claudia Beleites CENMAT and DI3, University of Trieste Spectroscopy Imaging, IPHT Jena e.v. February 13,
Raman Spectra of Chondrocytes in Cartilage: hyperspec s chondro data set Claudia Beleites CENMAT and DI3, University of Trieste Spectroscopy Imaging, IPHT Jena e.v. February 13,
How To Use GOstats and Category to do Hypergeometric testing with unsupported model organisms
 How To Use GOstats and Category to do Hypergeometric testing with unsupported model organisms M. Carlson October 30, 2017 1 Introduction This vignette is meant as an extension of what already exists in
How To Use GOstats and Category to do Hypergeometric testing with unsupported model organisms M. Carlson October 30, 2017 1 Introduction This vignette is meant as an extension of what already exists in
MultipleAlignment Objects
 MultipleAlignment Objects Marc Carlson Bioconductor Core Team Fred Hutchinson Cancer Research Center Seattle, WA April 30, 2018 Contents 1 Introduction 1 2 Creation and masking 1 3 Analytic utilities 7
MultipleAlignment Objects Marc Carlson Bioconductor Core Team Fred Hutchinson Cancer Research Center Seattle, WA April 30, 2018 Contents 1 Introduction 1 2 Creation and masking 1 3 Analytic utilities 7
Package hbm. February 20, 2015
 Type Package Title Hierarchical Block Matrix Analysis Version 1.0 Date 2015-01-25 Author Maintainer Package hbm February 20, 2015 A package for building hierarchical block matrices from
Type Package Title Hierarchical Block Matrix Analysis Version 1.0 Date 2015-01-25 Author Maintainer Package hbm February 20, 2015 A package for building hierarchical block matrices from
Classification of Breast Cancer Clinical Stage with Gene Expression Data
 Classification of Breast Cancer Clinical Stage with Gene Expression Data Zhu Wang Connecticut Children s Medical Center University of Connecticut School of Medicine zwang@connecticutchildrens.org July
Classification of Breast Cancer Clinical Stage with Gene Expression Data Zhu Wang Connecticut Children s Medical Center University of Connecticut School of Medicine zwang@connecticutchildrens.org July
Analysis of screens with enhancer and suppressor controls
 Analysis of screens with enhancer and suppressor controls Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Analysis of screens with enhancer and suppressor controls Lígia Brás, Michael Boutros and Wolfgang Huber April 16, 2015 Contents 1 Introduction 1 2 Assembling the data 2 2.1 Reading the raw intensity files..................
Introduction to the Codelink package
 Introduction to the Codelink package Diego Diez October 30, 2018 1 Introduction This package implements methods to facilitate the preprocessing and analysis of Codelink microarrays. Codelink is a microarray
Introduction to the Codelink package Diego Diez October 30, 2018 1 Introduction This package implements methods to facilitate the preprocessing and analysis of Codelink microarrays. Codelink is a microarray
An Overview of the S4Vectors package
 Patrick Aboyoun, Michael Lawrence, Hervé Pagès Edited: February 2018; Compiled: June 7, 2018 Contents 1 Introduction.............................. 1 2 Vector-like and list-like objects...................
Patrick Aboyoun, Michael Lawrence, Hervé Pagès Edited: February 2018; Compiled: June 7, 2018 Contents 1 Introduction.............................. 1 2 Vector-like and list-like objects...................
Calibration of Quinine Fluorescence Emission Vignette for the Data Set flu of the R package hyperspec
 Calibration of Quinine Fluorescence Emission Vignette for the Data Set flu of the R package hyperspec Claudia Beleites CENMAT and DI3, University of Trieste Spectroscopy Imaging,
Calibration of Quinine Fluorescence Emission Vignette for the Data Set flu of the R package hyperspec Claudia Beleites CENMAT and DI3, University of Trieste Spectroscopy Imaging,
Fitting Baselines to Spectra
 Fitting Baselines to Spectra Claudia Beleites DIA Raman Spectroscopy Group, University of Trieste/Italy (2005 2008) Spectroscopy Imaging, IPHT, Jena/Germany (2008 2016) ÖPV, JKI,
Fitting Baselines to Spectra Claudia Beleites DIA Raman Spectroscopy Group, University of Trieste/Italy (2005 2008) Spectroscopy Imaging, IPHT, Jena/Germany (2008 2016) ÖPV, JKI,
How to use cghmcr. October 30, 2017
 How to use cghmcr Jianhua Zhang Bin Feng October 30, 2017 1 Overview Copy number data (arraycgh or SNP) can be used to identify genomic regions (Regions Of Interest or ROI) showing gains or losses that
How to use cghmcr Jianhua Zhang Bin Feng October 30, 2017 1 Overview Copy number data (arraycgh or SNP) can be used to identify genomic regions (Regions Of Interest or ROI) showing gains or losses that
Introduction: microarray quality assessment with arrayqualitymetrics
 Introduction: microarray quality assessment with arrayqualitymetrics Audrey Kauffmann, Wolfgang Huber April 4, 2013 Contents 1 Basic use 3 1.1 Affymetrix data - before preprocessing.......................
Introduction: microarray quality assessment with arrayqualitymetrics Audrey Kauffmann, Wolfgang Huber April 4, 2013 Contents 1 Basic use 3 1.1 Affymetrix data - before preprocessing.......................
Seminar III: R/Bioconductor
 Leonardo Collado Torres lcollado@lcg.unam.mx Bachelor in Genomic Sciences www.lcg.unam.mx/~lcollado/ August - December, 2009 1 / 50 Class outline Public Data Intro biomart GEOquery ArrayExpress annotate
Leonardo Collado Torres lcollado@lcg.unam.mx Bachelor in Genomic Sciences www.lcg.unam.mx/~lcollado/ August - December, 2009 1 / 50 Class outline Public Data Intro biomart GEOquery ArrayExpress annotate
Package sigqc. June 13, 2018
 Title Quality Control Metrics for Gene Signatures Version 0.1.20 Package sigqc June 13, 2018 Description Provides gene signature quality control metrics in publication ready plots. Namely, enables the
Title Quality Control Metrics for Gene Signatures Version 0.1.20 Package sigqc June 13, 2018 Description Provides gene signature quality control metrics in publication ready plots. Namely, enables the
riboseqr Introduction Getting Data Workflow Example Thomas J. Hardcastle, Betty Y.W. Chung October 30, 2017
 riboseqr Thomas J. Hardcastle, Betty Y.W. Chung October 30, 2017 Introduction Ribosome profiling extracts those parts of a coding sequence currently bound by a ribosome (and thus, are likely to be undergoing
riboseqr Thomas J. Hardcastle, Betty Y.W. Chung October 30, 2017 Introduction Ribosome profiling extracts those parts of a coding sequence currently bound by a ribosome (and thus, are likely to be undergoing
GS Analysis of Microarray Data
 GS01 0163 Analysis of Microarray Data Keith Baggerly and Kevin Coombes Section of Bioinformatics Department of Biostatistics and Applied Mathematics UT M. D. Anderson Cancer Center kabagg@mdanderson.org
GS01 0163 Analysis of Microarray Data Keith Baggerly and Kevin Coombes Section of Bioinformatics Department of Biostatistics and Applied Mathematics UT M. D. Anderson Cancer Center kabagg@mdanderson.org
matter: Rapid prototyping with data on disk
 matter: Rapid prototyping with data on disk Kylie A. Bemis March 17, 2017 Contents 1 Introduction 1 2 Installation 1 3 Basic use and data manipulation 1 4 Linear regression for on-disk datasets 5 5 Principal
matter: Rapid prototyping with data on disk Kylie A. Bemis March 17, 2017 Contents 1 Introduction 1 2 Installation 1 3 Basic use and data manipulation 1 4 Linear regression for on-disk datasets 5 5 Principal
Biobase development and the new eset
 Biobase development and the new eset Martin T. Morgan * 7 August, 2006 Revised 4 September, 2006 featuredata slot. Revised 20 April 2007 minor wording changes; verbose and other arguments passed through
Biobase development and the new eset Martin T. Morgan * 7 August, 2006 Revised 4 September, 2006 featuredata slot. Revised 20 April 2007 minor wording changes; verbose and other arguments passed through
Evaluation of VST algorithm in lumi package
 Evaluation of VST algorithm in lumi package Pan Du 1, Simon Lin 1, Wolfgang Huber 2, Warrren A. Kibbe 1 December 22, 2010 1 Robert H. Lurie Comprehensive Cancer Center Northwestern University, Chicago,
Evaluation of VST algorithm in lumi package Pan Du 1, Simon Lin 1, Wolfgang Huber 2, Warrren A. Kibbe 1 December 22, 2010 1 Robert H. Lurie Comprehensive Cancer Center Northwestern University, Chicago,
An Introduction to Bioconductor s ExpressionSet Class
 An Introduction to Bioconductor s ExpressionSet Class Seth Falcon, Martin Morgan, and Robert Gentleman 6 October, 2006; revised 9 February, 2007 1 Introduction Biobase is part of the Bioconductor project,
An Introduction to Bioconductor s ExpressionSet Class Seth Falcon, Martin Morgan, and Robert Gentleman 6 October, 2006; revised 9 February, 2007 1 Introduction Biobase is part of the Bioconductor project,
The bumphunter user s guide
 The bumphunter user s guide Kasper Daniel Hansen khansen@jhsph.edu Martin Aryee aryee.martin@mgh.harvard.edu Rafael A. Irizarry rafa@jhu.edu Modified: November 23, 2012. Compiled: April 30, 2018 Introduction
The bumphunter user s guide Kasper Daniel Hansen khansen@jhsph.edu Martin Aryee aryee.martin@mgh.harvard.edu Rafael A. Irizarry rafa@jhu.edu Modified: November 23, 2012. Compiled: April 30, 2018 Introduction
BRB-ArrayTools Data Archive for Human Cancer Gene Expression: A Unique and Efficient Data Sharing Resource
 SHORT REPORT BRB-ArrayTools Data Archive for Human Cancer Gene Expression: A Unique and Efficient Data Sharing Resource Yingdong Zhao and Richard Simon Biometric Research Branch, National Cancer Institute,
SHORT REPORT BRB-ArrayTools Data Archive for Human Cancer Gene Expression: A Unique and Efficient Data Sharing Resource Yingdong Zhao and Richard Simon Biometric Research Branch, National Cancer Institute,
How to store and visualize RNA-seq data
 How to store and visualize RNA-seq data Gabriella Rustici Functional Genomics Group gabry@ebi.ac.uk EBI is an Outstation of the European Molecular Biology Laboratory. Talk summary How do we archive RNA-seq
How to store and visualize RNA-seq data Gabriella Rustici Functional Genomics Group gabry@ebi.ac.uk EBI is an Outstation of the European Molecular Biology Laboratory. Talk summary How do we archive RNA-seq
MIGSA: Getting pbcmc datasets
 MIGSA: Getting pbcmc datasets Juan C Rodriguez Universidad Católica de Córdoba Universidad Nacional de Córdoba Cristóbal Fresno Instituto Nacional de Medicina Genómica Andrea S Llera Fundación Instituto
MIGSA: Getting pbcmc datasets Juan C Rodriguez Universidad Católica de Córdoba Universidad Nacional de Córdoba Cristóbal Fresno Instituto Nacional de Medicina Genómica Andrea S Llera Fundación Instituto
An introduction to gaucho
 An introduction to gaucho Alex Murison Alexander.Murison@icr.ac.uk Christopher P Wardell Christopher.Wardell@icr.ac.uk October 30, 2017 This vignette serves as an introduction to the R package gaucho.
An introduction to gaucho Alex Murison Alexander.Murison@icr.ac.uk Christopher P Wardell Christopher.Wardell@icr.ac.uk October 30, 2017 This vignette serves as an introduction to the R package gaucho.
Cardinal design and development
 Kylie A. Bemis October 30, 2017 Contents 1 Introduction.............................. 2 2 Design overview........................... 2 3 iset: high-throughput imaging experiments............ 3 3.1 SImageSet:
Kylie A. Bemis October 30, 2017 Contents 1 Introduction.............................. 2 2 Design overview........................... 2 3 iset: high-throughput imaging experiments............ 3 3.1 SImageSet:
genefu: a package for breast cancer gene expression analysis
 genefu: a package for breast cancer gene expression analysis Benjamin Haibe-Kains 1, Markus Schröder 2, Christos Sotiriou 3, Gianluca Bontempi 4, and John Quackenbush 5,6 1 Bioinformatics and Computational
genefu: a package for breast cancer gene expression analysis Benjamin Haibe-Kains 1, Markus Schröder 2, Christos Sotiriou 3, Gianluca Bontempi 4, and John Quackenbush 5,6 1 Bioinformatics and Computational
Controlling and visualizing the precision-recall tradeoff for external performance indices
 Controlling and visualizing the precision-recall tradeoff for external performance indices Blaise Hanczar 1 and Mohamed Nadif 2 1 IBISC, University of Paris-Saclay, Univ. Evry, Evry, France blaise.hanczar@ibisc.univ-evry.fr
Controlling and visualizing the precision-recall tradeoff for external performance indices Blaise Hanczar 1 and Mohamed Nadif 2 1 IBISC, University of Paris-Saclay, Univ. Evry, Evry, France blaise.hanczar@ibisc.univ-evry.fr
ChIP-Seq Tutorial on Galaxy
 1 Introduction ChIP-Seq Tutorial on Galaxy 2 December 2010 (modified April 6, 2017) Rory Stark The aim of this practical is to give you some experience handling ChIP-Seq data. We will be working with data
1 Introduction ChIP-Seq Tutorial on Galaxy 2 December 2010 (modified April 6, 2017) Rory Stark The aim of this practical is to give you some experience handling ChIP-Seq data. We will be working with data
bayseq: Empirical Bayesian analysis of patterns of differential expression in count data
 bayseq: Empirical Bayesian analysis of patterns of differential expression in count data Thomas J. Hardcastle October 30, 2017 1 Introduction This vignette is intended to give a rapid introduction to the
bayseq: Empirical Bayesian analysis of patterns of differential expression in count data Thomas J. Hardcastle October 30, 2017 1 Introduction This vignette is intended to give a rapid introduction to the
The Command Pattern in R
 The Command Pattern in R Michael Lawrence September 2, 2012 Contents 1 Introduction 1 2 Example pipelines 2 3 sessioninfo 8 1 Introduction Command pattern is a design pattern used in object-oriented programming,
The Command Pattern in R Michael Lawrence September 2, 2012 Contents 1 Introduction 1 2 Example pipelines 2 3 sessioninfo 8 1 Introduction Command pattern is a design pattern used in object-oriented programming,
Contents. Introduction 2. PerturbationClustering 2. SubtypingOmicsData 5. References 8
 PINSPlus: Clustering Algorithm for Data Integration and Disease Subtyping Hung Nguyen, Sangam Shrestha, and Tin Nguyen Department of Computer Science and Engineering University of Nevada, Reno, NV 89557
PINSPlus: Clustering Algorithm for Data Integration and Disease Subtyping Hung Nguyen, Sangam Shrestha, and Tin Nguyen Department of Computer Science and Engineering University of Nevada, Reno, NV 89557
Biclustering Algorithms for Gene Expression Analysis
 Biclustering Algorithms for Gene Expression Analysis T. M. Murali August 19, 2008 Problems with Hierarchical Clustering It is a global clustering algorithm. Considers all genes to be equally important
Biclustering Algorithms for Gene Expression Analysis T. M. Murali August 19, 2008 Problems with Hierarchical Clustering It is a global clustering algorithm. Considers all genes to be equally important
Bioconductor tutorial
 Bioconductor tutorial Adapted by Alex Sanchez from tutorials by (1) Steffen Durinck, Robert Gentleman and Sandrine Dudoit (2) Laurent Gautier (3) Matt Ritchie (4) Jean Yang Outline The Bioconductor Project
Bioconductor tutorial Adapted by Alex Sanchez from tutorials by (1) Steffen Durinck, Robert Gentleman and Sandrine Dudoit (2) Laurent Gautier (3) Matt Ritchie (4) Jean Yang Outline The Bioconductor Project
R/BioC Exercises & Answers: Unsupervised methods
 R/BioC Exercises & Answers: Unsupervised methods Perry Moerland April 20, 2010 Z Information on how to log on to a PC in the exercise room and the UNIX server can be found here: http://bioinformaticslaboratory.nl/twiki/bin/view/biolab/educationbioinformaticsii.
R/BioC Exercises & Answers: Unsupervised methods Perry Moerland April 20, 2010 Z Information on how to log on to a PC in the exercise room and the UNIX server can be found here: http://bioinformaticslaboratory.nl/twiki/bin/view/biolab/educationbioinformaticsii.
Introduction to R. UCLA Statistical Consulting Center R Bootcamp. Irina Kukuyeva September 20, 2010
 UCLA Statistical Consulting Center R Bootcamp Irina Kukuyeva ikukuyeva@stat.ucla.edu September 20, 2010 Outline 1 Introduction 2 Preliminaries 3 Working with Vectors and Matrices 4 Data Sets in R 5 Overview
UCLA Statistical Consulting Center R Bootcamp Irina Kukuyeva ikukuyeva@stat.ucla.edu September 20, 2010 Outline 1 Introduction 2 Preliminaries 3 Working with Vectors and Matrices 4 Data Sets in R 5 Overview
rhdf5 - HDF5 interface for R
 Bernd Fischer October 30, 2017 Contents 1 Introduction 1 2 Installation of the HDF5 package 2 3 High level R -HDF5 functions 2 31 Creating an HDF5 file and group hierarchy 2 32 Writing and reading objects
Bernd Fischer October 30, 2017 Contents 1 Introduction 1 2 Installation of the HDF5 package 2 3 High level R -HDF5 functions 2 31 Creating an HDF5 file and group hierarchy 2 32 Writing and reading objects
Creating a New Annotation Package using SQLForge
 Creating a New Annotation Package using SQLForge Marc Carlson, HervÃľ PagÃĺs, Nianhua Li April 30, 2018 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
Creating a New Annotation Package using SQLForge Marc Carlson, HervÃľ PagÃĺs, Nianhua Li April 30, 2018 1 Introduction The AnnotationForge package provides a series of functions that can be used to build
jackstraw: Statistical Inference using Latent Variables
 jackstraw: Statistical Inference using Latent Variables Neo Christopher Chung August 7, 2018 1 Introduction This is a vignette for the jackstraw package, which performs association tests between variables
jackstraw: Statistical Inference using Latent Variables Neo Christopher Chung August 7, 2018 1 Introduction This is a vignette for the jackstraw package, which performs association tests between variables
xmapbridge: Graphically Displaying Numeric Data in the X:Map Genome Browser
 xmapbridge: Graphically Displaying Numeric Data in the X:Map Genome Browser Tim Yates, Crispin J Miller April 30, 2018 Contents 1 Introduction 1 2 Quickstart 2 3 Plotting Graphs, the simple way 4 4 Multiple
xmapbridge: Graphically Displaying Numeric Data in the X:Map Genome Browser Tim Yates, Crispin J Miller April 30, 2018 Contents 1 Introduction 1 2 Quickstart 2 3 Plotting Graphs, the simple way 4 4 Multiple
ROTS: Reproducibility Optimized Test Statistic
 ROTS: Reproducibility Optimized Test Statistic Fatemeh Seyednasrollah, Tomi Suomi, Laura L. Elo fatsey (at) utu.fi March 3, 2016 Contents 1 Introduction 2 2 Algorithm overview 3 3 Input data 3 4 Preprocessing
ROTS: Reproducibility Optimized Test Statistic Fatemeh Seyednasrollah, Tomi Suomi, Laura L. Elo fatsey (at) utu.fi March 3, 2016 Contents 1 Introduction 2 2 Algorithm overview 3 3 Input data 3 4 Preprocessing
BGGN 213 Working with R packages Barry Grant
 BGGN 213 Working with R packages Barry Grant http://thegrantlab.org/bggn213 Recap From Last Time: Why it is important to visualize data during exploratory data analysis. Discussed data visualization best
BGGN 213 Working with R packages Barry Grant http://thegrantlab.org/bggn213 Recap From Last Time: Why it is important to visualize data during exploratory data analysis. Discussed data visualization best
INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis
 INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis Stefano de Pretis April 7, 20 Contents 1 Introduction 1 2 Quantification of Exon and Intron features 3 3 Time-course
INSPEcT - INference of Synthesis, Processing and degradation rates in Time-course analysis Stefano de Pretis April 7, 20 Contents 1 Introduction 1 2 Quantification of Exon and Intron features 3 3 Time-course
GxE.scan. October 30, 2018
 GxE.scan October 30, 2018 Overview GxE.scan can process a GWAS scan using the snp.logistic, additive.test, snp.score or snp.matched functions, whereas snp.scan.logistic only calls snp.logistic. GxE.scan
GxE.scan October 30, 2018 Overview GxE.scan can process a GWAS scan using the snp.logistic, additive.test, snp.score or snp.matched functions, whereas snp.scan.logistic only calls snp.logistic. GxE.scan
limma: A brief introduction to R
 limma: A brief introduction to R Natalie P. Thorne September 5, 2006 R basics i R is a command line driven environment. This means you have to type in commands (line-by-line) for it to compute or calculate
limma: A brief introduction to R Natalie P. Thorne September 5, 2006 R basics i R is a command line driven environment. This means you have to type in commands (line-by-line) for it to compute or calculate
Release Notes. JMP Genomics. Version 4.0
 JMP Genomics Version 4.0 Release Notes Creativity involves breaking out of established patterns in order to look at things in a different way. Edward de Bono JMP. A Business Unit of SAS SAS Campus Drive
JMP Genomics Version 4.0 Release Notes Creativity involves breaking out of established patterns in order to look at things in a different way. Edward de Bono JMP. A Business Unit of SAS SAS Campus Drive
Sparse Model Matrices
 Sparse Model Matrices Martin Maechler R Core Development Team maechler@r-project.org July 2007, 2008 (typeset on April 6, 2018) Introduction Model matrices in the very widely used (generalized) linear
Sparse Model Matrices Martin Maechler R Core Development Team maechler@r-project.org July 2007, 2008 (typeset on April 6, 2018) Introduction Model matrices in the very widely used (generalized) linear
The supclust Package
 The supclust Package May 18, 2005 Title Supervised Clustering of Genes Version 1.0-5 Date 2005-05-18 Methodology for Supervised Grouping of Predictor Variables Author Marcel Dettling and Martin Maechler
The supclust Package May 18, 2005 Title Supervised Clustering of Genes Version 1.0-5 Date 2005-05-18 Methodology for Supervised Grouping of Predictor Variables Author Marcel Dettling and Martin Maechler
Package LSDinterface
 Type Package Title Reading LSD Results (.res) Files Version 0.3.1 Date 2017-11-24 Author Package LSDinterface November 27, 2017 Maintainer Interfaces R with LSD. Reads object-oriented
Type Package Title Reading LSD Results (.res) Files Version 0.3.1 Date 2017-11-24 Author Package LSDinterface November 27, 2017 Maintainer Interfaces R with LSD. Reads object-oriented
Introduction to the TSSi package: Identification of Transcription Start Sites
 Introduction to the TSSi package: Identification of Transcription Start Sites Julian Gehring, Clemens Kreutz October 30, 2017 Along with the advances in high-throughput sequencing, the detection of transcription
Introduction to the TSSi package: Identification of Transcription Start Sites Julian Gehring, Clemens Kreutz October 30, 2017 Along with the advances in high-throughput sequencing, the detection of transcription
Design Issues in Matrix package Development
 Design Issues in Matrix package Development Martin Maechler and Douglas Bates R Core Development Team maechler@stat.math.ethz.ch, bates@r-project.org Spring 2008 (typeset on October 26, 2018) Abstract
Design Issues in Matrix package Development Martin Maechler and Douglas Bates R Core Development Team maechler@stat.math.ethz.ch, bates@r-project.org Spring 2008 (typeset on October 26, 2018) Abstract
Introduction to Mfuzz package and its graphical user interface
 Introduction to Mfuzz package and its graphical user interface Matthias E. Futschik SysBioLab, Universidade do Algarve URL: http://mfuzz.sysbiolab.eu and Lokesh Kumar Institute for Advanced Biosciences,
Introduction to Mfuzz package and its graphical user interface Matthias E. Futschik SysBioLab, Universidade do Algarve URL: http://mfuzz.sysbiolab.eu and Lokesh Kumar Institute for Advanced Biosciences,
Package cgh. R topics documented: February 19, 2015
 Package cgh February 19, 2015 Version 1.0-7.1 Date 2009-11-20 Title Microarray CGH analysis using the Smith-Waterman algorithm Author Tom Price Maintainer Tom Price
Package cgh February 19, 2015 Version 1.0-7.1 Date 2009-11-20 Title Microarray CGH analysis using the Smith-Waterman algorithm Author Tom Price Maintainer Tom Price
Package ChIPXpress. October 4, 2013
 Package ChIPXpress October 4, 2013 Type Package Title ChIPXpress: enhanced transcription factor target gene identification from ChIP-seq and ChIP-chip data using publicly available gene expression profiles
Package ChIPXpress October 4, 2013 Type Package Title ChIPXpress: enhanced transcription factor target gene identification from ChIP-seq and ChIP-chip data using publicly available gene expression profiles
Clustering. RNA-seq: What is it good for? Finding Similarly Expressed Genes. Data... And Lots of It!
 RNA-seq: What is it good for? Clustering High-throughput RNA sequencing experiments (RNA-seq) offer the ability to measure simultaneously the expression level of thousands of genes in a single experiment!
RNA-seq: What is it good for? Clustering High-throughput RNA sequencing experiments (RNA-seq) offer the ability to measure simultaneously the expression level of thousands of genes in a single experiment!
Package ibbig. R topics documented: December 24, 2018
 Type Package Title Iterative Binary Biclustering of Genesets Version 1.26.0 Date 2011-11-23 Author Daniel Gusenleitner, Aedin Culhane Package ibbig December 24, 2018 Maintainer Aedin Culhane
Type Package Title Iterative Binary Biclustering of Genesets Version 1.26.0 Date 2011-11-23 Author Daniel Gusenleitner, Aedin Culhane Package ibbig December 24, 2018 Maintainer Aedin Culhane
Design Issues in Matrix package Development
 Design Issues in Matrix package Development Martin Maechler and Douglas Bates R Core Development Team maechler@stat.math.ethz.ch, bates@r-project.org Spring 2008 (typeset on November 16, 2017) Abstract
Design Issues in Matrix package Development Martin Maechler and Douglas Bates R Core Development Team maechler@stat.math.ethz.ch, bates@r-project.org Spring 2008 (typeset on November 16, 2017) Abstract
SimBindProfiles: Similar Binding Profiles, identifies common and unique regions in array genome tiling array data
 SimBindProfiles: Similar Binding Profiles, identifies common and unique regions in array genome tiling array data Bettina Fischer October 30, 2018 Contents 1 Introduction 1 2 Reading data and normalisation
SimBindProfiles: Similar Binding Profiles, identifies common and unique regions in array genome tiling array data Bettina Fischer October 30, 2018 Contents 1 Introduction 1 2 Reading data and normalisation
ddhazard Diagnostics Benjamin Christoffersen
 ddhazard Diagnostics Benjamin Christoffersen 2017-11-25 Introduction This vignette will show examples of how the residuals and hatvalues functions can be used for an object returned by ddhazard. See vignette("ddhazard",
ddhazard Diagnostics Benjamin Christoffersen 2017-11-25 Introduction This vignette will show examples of how the residuals and hatvalues functions can be used for an object returned by ddhazard. See vignette("ddhazard",
Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice
 Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice 2.b Step-by-step network construction and module detection Peter Langfelder and Steve
Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice 2.b Step-by-step network construction and module detection Peter Langfelder and Steve
Package rain. March 4, 2018
 Package rain March 4, 2018 Type Package Title Rhythmicity Analysis Incorporating Non-parametric Methods Version 1.13.0 Date 2015-09-01 Author Paul F. Thaben, Pål O. Westermark Maintainer Paul F. Thaben
Package rain March 4, 2018 Type Package Title Rhythmicity Analysis Incorporating Non-parametric Methods Version 1.13.0 Date 2015-09-01 Author Paul F. Thaben, Pål O. Westermark Maintainer Paul F. Thaben
Creating an annotation package with a new database schema
 Creating an annotation package with a new database schema Gábor Csárdi April 7, 2011 Contents 1 Introduction 2 2 Creating the template of your package 2 2.1 Update DESCRIPTION file.....................
Creating an annotation package with a new database schema Gábor Csárdi April 7, 2011 Contents 1 Introduction 2 2 Creating the template of your package 2 2.1 Update DESCRIPTION file.....................
Package BEARscc. May 2, 2018
 Type Package Package BEARscc May 2, 2018 Title BEARscc (Bayesian ERCC Assesstment of Robustness of Single Cell Clusters) Version 1.0.0 Author David T. Severson Maintainer
Type Package Package BEARscc May 2, 2018 Title BEARscc (Bayesian ERCC Assesstment of Robustness of Single Cell Clusters) Version 1.0.0 Author David T. Severson Maintainer
Package munfold. R topics documented: February 8, Type Package. Title Metric Unfolding. Version Date Author Martin Elff
 Package munfold February 8, 2016 Type Package Title Metric Unfolding Version 0.3.5 Date 2016-02-08 Author Martin Elff Maintainer Martin Elff Description Multidimensional unfolding using
Package munfold February 8, 2016 Type Package Title Metric Unfolding Version 0.3.5 Date 2016-02-08 Author Martin Elff Maintainer Martin Elff Description Multidimensional unfolding using
ECS 234: Data Analysis: Clustering ECS 234
 : Data Analysis: Clustering What is Clustering? Given n objects, assign them to groups (clusters) based on their similarity Unsupervised Machine Learning Class Discovery Difficult, and maybe ill-posed
: Data Analysis: Clustering What is Clustering? Given n objects, assign them to groups (clusters) based on their similarity Unsupervised Machine Learning Class Discovery Difficult, and maybe ill-posed
Description/History Objects/Language Description Commonly Used Basic Functions. More Specific Functionality Further Resources
 R Outline Description/History Objects/Language Description Commonly Used Basic Functions Basic Stats and distributions I/O Plotting Programming More Specific Functionality Further Resources www.r-project.org
R Outline Description/History Objects/Language Description Commonly Used Basic Functions Basic Stats and distributions I/O Plotting Programming More Specific Functionality Further Resources www.r-project.org
Package SCAN.UPC. July 17, 2018
 Type Package Package SCAN.UPC July 17, 2018 Title Single-channel array normalization (SCAN) and Universal expression Codes (UPC) Version 2.22.0 Author and Andrea H. Bild and W. Evan Johnson Maintainer
Type Package Package SCAN.UPC July 17, 2018 Title Single-channel array normalization (SCAN) and Universal expression Codes (UPC) Version 2.22.0 Author and Andrea H. Bild and W. Evan Johnson Maintainer
Package loomr. April 23, 2018
 Type Package Title An R interface for loom files Version 0.2.0 Date 2018-23-18 Package loomr April 23, 2018 Description An interface for the single-cell RNAseq-oriented loom format. Loom files are an HDF5-
Type Package Title An R interface for loom files Version 0.2.0 Date 2018-23-18 Package loomr April 23, 2018 Description An interface for the single-cell RNAseq-oriented loom format. Loom files are an HDF5-
