Non FDA approved:investigational device
|
|
|
- Charles Cooper
- 6 years ago
- Views:
Transcription
1 Carotid Stenting with the Fibernet Protection Device: A New Generation in EPD Gary M Ansel MD FACC Clinical Director of Peripheral Vascular Intervention MidOhio Cardiology and Vascular Consultants Riverside Methodist Hospital Columbus, Ohio Non FDA approved:investigational device
2 Relevant Disclosures Cordis JJ Bard Medtronic Abbott/Guidant Lumen biomedical Cook ev3 Advisory, education Advisory Advisory Education, research Advisory Royalties, advisory Advisory
3 This Carotid Looks Like a Ripe One
4 SAPPHIRE Randomized Patients: Kaplan Meier Analysis CEA CEA26.7% 26.7% % MAE 20.1% 20.1% Stent 19.2% Stent 19.2% 12.2% 12.2% 9.8% 9.8% 4.8% 4.8% 30 Days After Initial Procedure Days: N at Risk (CEA): N at Risk (Stent):
5 Filters: Newer Devices FilterWire EZ Interceptor Rubicon SPIDER
6 Carotid Stent Trials: 30-Day MACE* Patients (%) 10 8 * MACE=Death, CVA, MI SAPPHIRE ARCHER SECURITY BEACH CABERNET
7
8 Population The 30 day clinical outcomes in a pop. of patients recruited in to the LEAD-IN Phase of the CREST study.
9 Patient Population Inclusion Criteria Symptomatic - >50% diameter stenosis Asymptomatic - >70% diameter stenosis High and Standard Risk CEA Pts. Initially no age exclusions. Later recruitment only < 80 yrs.
10 Total Population data at 30 days N=1303 Event rate (%) P=NS 4.6 % 4.6 % 5.4 % 5.4 % 10 0 Death/Stroke Death/Stroke/MI
11 Symptomatic population at 30 days N = 343 Event rates (%) % 6.8 % 10 0 Death/Stroke Death/Stroke/MI
12 CREST: 30 day death/stroke rate by age group n= % P= % 30 day stroke and death 12% 10% 8% 6.5% 6% 4% 2.6% 2% 1.2% 0% <60 years n= years n= years n=594 >80 years n=145
13 Primary Events <30 days: CAPTURE 2500ARCHeR vs ARCHeR CAPTURE DIFFERENCE EVENT (N=2500) (n= 581) 95% CI 5.7% 8.3% -2.54% [ -4.96%, -0.13%] Death 1.6% 2.1% -0.47% [ -1.72%, 0.79%] Stroke-Related Death 0.8% 0.5% 0.24% [-0.43%, 0.92%] All Stroke 4.2% 5.5% -1.27% [ -3.28%, 0.75%] Major Stroke 1.7% 1.5% 0.13% [ -0.99%, 1.25%] Minor Stroke 2.6% 4.0% -1.32% [ -3.02%, 0.39%] MI 0.9% 2.4% All Stroke and Death* 5.1% 6.9% -1.49% [ -2.79%, -0.19%] -1.80% [-4.04%, 0.43%] Major Stroke and Death* 2.5% 2.9% -0.41% [-1.91%, 1.10%] Death, Stroke and MI* *Hierarchical- Includes only the most serious event for each patient and includes only each patient first occurrence of each event. Denotes statistically significant difference at the 0.05 level
14 CAPTURE 2500: DSMI by Combined Octogenarian/Symptomatic Status % of Patients DSMI* 18% 16% 14% 12% 10% 8% 6% 4% 2% 0% 80 < % 14.2% 12.7% 7.0% 5.7% All Patients (N=2500) 4.9% Symptomatic (N=233) Asymptomatic (N=2267) Asymptomatic patients <80 year: Stroke and death: 3.6% (63/1741) [ 2.8%, 4.6%] 4.2%
15 Controversial Studies
16 30 day death and stroke (%) EVA-3S: Randomized CEA vs. CAS 20 CEA CAS 15 RR 2.5 (95% CI, ) P= Primary Endpoint Mas JL et al. New Engl J Med 2006;355:
17 EVA-3S critique Slow enrollment resulted in limited investigator experience 1.7 CAS patients/year/site Early and/or non-standard technique resulted in unnecessary morbidity Use of EPD not widespread or familiar Lack of use in the early phase of the trial likely responsible for 4-5 excess strokes (~20% of all strokes in the CAS arm) 5% stent procedure failure requiring emergency surgery in this trial resulting in 2 strokes in the CAS group Major pivotal trials in this country (e.g., SAPPHIRE, ARCHeR) have not reported any emergent surgical conversions No pre-dilation in >80% of procedures (standard in US) Significant (beyond local) anesthesia was employed in ~30% of procedures (estimated <5% in US) William Gray
18 EVA-3S critique Limited investigator experience and number of trained sites/operators Experienced operators defined by 12 lifetime CAS procedures or 5 CAS procedure if 35 supra-aortic procedure These operators were deemed experienced and allowed to tutor the non-experienced No centralized training qualification process (local proctors pronounced the operators qualified) Approximately 2/3 of sites were under tutelage at the beginning of their randomized participation. William Gray
19
20 Silent Cerebral Embolization Animal Data Microspheres 15 microns no effect Microspheres 50 microns neuro effect Heistaded DD et al Measurement of cerebral blood flown in animals Williams and Wilkins;1980:202-11
21 Silent Cerebral Embolization MRI data N = 20 DW MRI Carotid Stent placement Protection successful in 16 Abnormal study in 3/20 (15%) No permanent deficits Jaeger et al. Cardiovasc Intervent Radiol 2001:24:249-56
22 Silent Cerebral Embolization MRI data N = 42 DW MRI Carotid Stent placement Protection in all (multiple types) Abnormal study in 10/42 (22.7%) 1 major stroke 9 no deficit Jaeger et al. Cardiovasc Intervent Radiol 2001:24:249-56
23 Silent Cerebral Embolization MRI data N = 88 DW MRI Carotid Surgery Abnormal study in 17/88 (17%) 2 major stroke Eur J Vasc Endovasc Surg 2004;27:167-71
24 Possible Technology answers?
25 FiberNet Embolic Protection System Lumen Biomedical, Inc. m
26 FiberNet EPS Device Description The FiberNet system consists of - Expandable filter mounted on a high performance guidewire - RX focal-suction retrieval catheter. The filter - numerous strands of polymer fibers. - seek the vessel wall, forming a 3-D depth filter. Focal-suction catheter
27
28 FiberNet EPS Features Effective Particulate Capture/Retrieval: - completely fills (even eccentric) vessels - 3-D depth filter efficiently traps particles 40 microns Greater Access: - flexible high performance wire; low crossing profile; no delivery catheter - very small landing zone requirement Maintains blood flow during the intervention:
29 FiberNet: Fills (even eccentric) Vessels FiberNet FilterWire
30 FiberNet: Efficient 3D Depth Filter Bench Tests Confirm Filtration Efficacy: 99% particulate 100 µm 93% particulate 40 µm data on file at Lumen Biomedical
31 FiberNet: Low Crossing Profile Other Device Specifications FiberNet Model Vessel Size Crossing Profile mm 1.7F mm 2.1F mm 2.4F mm 2.7F mm 3.2F Device Vessel Size Crossing Profile EmboShield mm F AccuNet RX mm F FilterWire EZ mm 3.2F SpideRX mm 3.2F GuardWire mm 2.7F
32 High Capture Efficiency: Clinical Cases Greece, Germany, US Visible debris has been removed in 100% of the cases to date.
33 Clinical Studies Epic US Carotid Study: High surgical risk, multicenter, single arm registry, 30 day follow-up Symptomatic with 50% stenosis, Asymptomatic with 70% stenosis Enrollment Ongoing Epic European Carotid Study: Multicenter, single arm registry, 30 day follow-up Symptomatic with 50% stenosis, Asymptomatic with 70% stenosis Enrollment Ongoing Retrieve European Saphenous Vein Graft Study: Multicenter, single arm registry, 30 day follow-up Enrollment Pending
34 Evaluating the Use of the FiberNet Emboli Protection Device in Carotid Artery Stenting: The EPIC US Feasibility Study CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
35 EPIC Study Objective Multicenter, prospective, feasibility study designed to demonstrate the performance and safety of the Lumen Biomedical, Inc. FiberNet Embolic Protection System as an adjunctive device during carotid artery percutaneous intervention in high risk patients. CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
36 EPIC Study Design, Scope, and Duration High Surgical Risk Patient Carotid Registry study. Up to 10 US Centers enrolling up to 30 subjects. All subjects who meet criteria will be treated with the FiberNet device and Acculink stent. Patients will be followed through 30 days. Pivotal study to support commercial clearance to begin first quarter 2007 Principle Investigator: Michael Bacharach MD CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
37 EPIC Study Endpoints Primary Endpoint: the rate of all death, stroke, and MI within 30 days of the procedure. Secondary Endpoints: All death and stroke rates Non-stroke neurological event rates Technical success rates Procedural success rates Access site complication rates CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
38 FiberNet Procedures Done in Greece with Dr. Henry and Dr. Polydorou CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B Non FDA approved:investigational device
39 Greece Baseline Characteristics N = 46 Carotid Patients and 4 Renal Patients Mean Age at Procedure Percent Male 69 years 68% Mean Percent Stenosis 84.6% CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
40 Greece FiberNet Results Patients N = 50 subjects (51 lesions) Procedure Success % Cases Visible Debris Caught 96% 100% Two Instances of TIMI 1 flow and one instance of TIMI 0 flow. TIMI 3 flow restored after suction in all cases. Three vessel spasm treated with nitro (not at the location of distal protection device) No Changes noted in 30 day follow-up CT/MRI CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
41 Debris Analysis Area of Debris Captured (mm2) FiberNet vs. other Distal Filter devices Areas in square mm Average - FiberNet 60 Avg (other Distal Filter devices) CAUTION Not commercially available: INVESTIGATIONAL DEVICE ,B
PASSION for Peripheral and Endovascular. Kathleen Hewitt American College of Cardiology
 PASSION for Peripheral and Endovascular Kathleen Hewitt American College of Cardiology PVI Registry an expansion beyond our CARE Registry CARE PVI Carotid stenting and endarterectomy NCDR s first multi-specialist
PASSION for Peripheral and Endovascular Kathleen Hewitt American College of Cardiology PVI Registry an expansion beyond our CARE Registry CARE PVI Carotid stenting and endarterectomy NCDR s first multi-specialist
Urgent: Important Safety Update Medical Device Correction for the AFX Endovascular AAA System
 July 20, 2018 Urgent: Important Safety Update Medical Device Correction for the AFX Endovascular AAA System Dear Physician, This letter provides important information related to the AFX Endovascular AAA
July 20, 2018 Urgent: Important Safety Update Medical Device Correction for the AFX Endovascular AAA System Dear Physician, This letter provides important information related to the AFX Endovascular AAA
PTA-Balloons. PTA Balloons
 General Information...32.035 Guidewire Compatibility POWERFLEX Pro...34 POWERFLEX EXTREME...35 MAXI LD...36.018 Guidewire Compatibility SABER...37 SABERX...39 SAVVY...40 SAVVY Long...40 SLALOM...41.014
General Information...32.035 Guidewire Compatibility POWERFLEX Pro...34 POWERFLEX EXTREME...35 MAXI LD...36.018 Guidewire Compatibility SABER...37 SABERX...39 SAVVY...40 SAVVY Long...40 SLALOM...41.014
Endovascular aortic repair Thoracic
 Endovascular aortic repair Thoracic Zenith ENDOVASCULAR GRAFTS MEDICAL www.cookmedical.com/web/aortic-intervention Average number of pieces used per procedure Zenith TX2 Trial 1 1.59 TAG Trial 2 Talent
Endovascular aortic repair Thoracic Zenith ENDOVASCULAR GRAFTS MEDICAL www.cookmedical.com/web/aortic-intervention Average number of pieces used per procedure Zenith TX2 Trial 1 1.59 TAG Trial 2 Talent
Optimization of Flow Diverter Treatment for a Patient-specific Giant Aneurysm Using STAR-CCM+ László Daróczy, Philipp Berg, Gábor Janiga
 Optimization of Flow Diverter Treatment for a Patient-specific Giant Aneurysm Using STAR-CCM+ László Daróczy, Philipp Berg, Gábor Janiga Introduction Definition of aneurysm: permanent and locally limited
Optimization of Flow Diverter Treatment for a Patient-specific Giant Aneurysm Using STAR-CCM+ László Daróczy, Philipp Berg, Gábor Janiga Introduction Definition of aneurysm: permanent and locally limited
B. Braun Interventional Systems Inc. Balloon Dilatation Catheter Family
 B. Braun Interventional Systems Inc. Balloon Dilatation Catheter Family Rapid Inflation/Deflation High Burst Pressure Balloon Diameters from 2mm to 40mm Mini Ghost Catheter Z-MED-X Catheter Z-MED and Z-MED
B. Braun Interventional Systems Inc. Balloon Dilatation Catheter Family Rapid Inflation/Deflation High Burst Pressure Balloon Diameters from 2mm to 40mm Mini Ghost Catheter Z-MED-X Catheter Z-MED and Z-MED
7/7/2015. Disclosures. ARS question #1. Michael Allon, M.D. University of Alabama at Birmingham, USA. CorMedix consultant Gore - consultant
 7/7/25 Michael Allon, M.D. University of Alabama at Birmingham, USA Disclosures CorMedix consultant Gore - consultant Forearm AVG before upper arm AVF: Pros and Cons Tradeoffs of upper arm AVF vs AVG Retrospectively
7/7/25 Michael Allon, M.D. University of Alabama at Birmingham, USA Disclosures CorMedix consultant Gore - consultant Forearm AVG before upper arm AVF: Pros and Cons Tradeoffs of upper arm AVF vs AVG Retrospectively
TREO is Engineered for Confidence. MODULAR DESIGN Three-piece system available in a wide range of sizes, lengths, and tapers.
 MODULAR DESIGN Three-piece system available in a wide range of sizes, lengths, and tapers. A D V A N C E D D E S I G N Every aspect of the TREO Abdominal Stent-Graft system is engineered for performance.
MODULAR DESIGN Three-piece system available in a wide range of sizes, lengths, and tapers. A D V A N C E D D E S I G N Every aspect of the TREO Abdominal Stent-Graft system is engineered for performance.
Reduced Oder Modeling techniques to predict the risk of fatigue fracture of peripheral stents. Michel Rochette
 Reduced Oder Modeling techniques to predict the risk of fatigue fracture of peripheral stents Michel Rochette 1 RT3S: Real Time Simulation for Safer vascular Stenting 2 The vascular disease in peripheral
Reduced Oder Modeling techniques to predict the risk of fatigue fracture of peripheral stents Michel Rochette 1 RT3S: Real Time Simulation for Safer vascular Stenting 2 The vascular disease in peripheral
Working with Composite Endpoints: Constructing Analysis Data Pushpa Saranadasa, Merck & Co., Inc., Upper Gwynedd, PA
 PharmaSug2016- Paper HA03 Working with Composite Endpoints: Constructing Analysis Data Pushpa Saranadasa, Merck & Co., Inc., Upper Gwynedd, PA ABSTRACT A composite endpoint in a Randomized Clinical Trial
PharmaSug2016- Paper HA03 Working with Composite Endpoints: Constructing Analysis Data Pushpa Saranadasa, Merck & Co., Inc., Upper Gwynedd, PA ABSTRACT A composite endpoint in a Randomized Clinical Trial
OnCore Enterprise Research. Subject Administration Full Study
 OnCore Enterprise Research Subject Administration Full Study Principal Investigator Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Table of Contents What
OnCore Enterprise Research Subject Administration Full Study Principal Investigator Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Table of Contents What
! " # $%! &% '()*+ *, % '% + & -(
 ! " # $%! &% '()*+ *, % '% + & -( * '*. /. 01 ' 2.'* ' 3 *4.* 1 15' *63 1 7' *2# -' 8*' * * 9! ' &+:! ( '& *( &;:"+ +, +( 1 %! 5/, &%(
! " # $%! &% '()*+ *, % '% + & -( * '*. /. 01 ' 2.'* ' 3 *4.* 1 15' *63 1 7' *2# -' 8*' * * 9! ' &+:! ( '& *( &;:"+ +, +( 1 %! 5/, &%(
PDA closure: device selection
 PDA & ASD CLOSURE: DEVICE SELECTION Dr. Alejandro Peirone, FSCAI Hospital Privado y Hospital de Niños de Córdoba Argentina. October 5, 2012. Buenos Aires. Conflicts of interest: AGA St Jude pfm Medical
PDA & ASD CLOSURE: DEVICE SELECTION Dr. Alejandro Peirone, FSCAI Hospital Privado y Hospital de Niños de Córdoba Argentina. October 5, 2012. Buenos Aires. Conflicts of interest: AGA St Jude pfm Medical
Non-rigid 2D-3D image registration for use in Endovascular repair of Abdominal Aortic Aneurysms.
 RAHEEM ET AL.: IMAGE REGISTRATION FOR EVAR IN AAA. 1 Non-rigid 2D-3D image registration for use in Endovascular repair of Abdominal Aortic Aneurysms. Ali Raheem 1 ali.raheem@kcl.ac.uk Tom Carrell 2 tom.carrell@gstt.nhs.uk
RAHEEM ET AL.: IMAGE REGISTRATION FOR EVAR IN AAA. 1 Non-rigid 2D-3D image registration for use in Endovascular repair of Abdominal Aortic Aneurysms. Ali Raheem 1 ali.raheem@kcl.ac.uk Tom Carrell 2 tom.carrell@gstt.nhs.uk
Family of Diagnostic and Therapeutic Catheters
 Family of Diagnostic and Therapeutic Catheters Full line of Percutaneous Transluminal Angioplasty (PTA), Percutaneous Transluminal Valvuloplasty (PTV) and Aortic Valvuloplastly (BAV) Catheters PTS and
Family of Diagnostic and Therapeutic Catheters Full line of Percutaneous Transluminal Angioplasty (PTA), Percutaneous Transluminal Valvuloplasty (PTV) and Aortic Valvuloplastly (BAV) Catheters PTS and
Psoriasis Registry Graz Austria. User Manual. Version 1.0 Page 1 of 19
 Version 1.0 Page 1 of 19 Table of content Table of content... 2 1. Introduction... 3 1.1. Contact person... 3 2. Login... 4 2.1. Edit user details and change password... 4 2.1.1. Edit User Details:...
Version 1.0 Page 1 of 19 Table of content Table of content... 2 1. Introduction... 3 1.1. Contact person... 3 2. Login... 4 2.1. Edit user details and change password... 4 2.1.1. Edit User Details:...
BRANDMAN UNIVERSITY INSTITUTIONAL REVIEW BOARD Continuing Review Request/Closure Report
 BRANDMAN UNIVERSITY INSTITUTIONAL REVIEW BOARD Continuing Review Request/Closure Report Instructions: Please complete all sections of this form and submit with attached documents, forms, and/or explanations
BRANDMAN UNIVERSITY INSTITUTIONAL REVIEW BOARD Continuing Review Request/Closure Report Instructions: Please complete all sections of this form and submit with attached documents, forms, and/or explanations
Topics Raised by EFPIA. Webinar on Policy Jun 2017, FINAL. Presentation
 Topics Raised by EFPIA Webinar on Policy 70 29 Jun 2017, FINAL Presentation Definition of listings out of scope of Phase 1 Examples Topics to discuss Previously submitted studies in scope Reiterating EFPIA
Topics Raised by EFPIA Webinar on Policy 70 29 Jun 2017, FINAL Presentation Definition of listings out of scope of Phase 1 Examples Topics to discuss Previously submitted studies in scope Reiterating EFPIA
Creating an ADaM Data Set for Correlation Analyses
 PharmaSUG 2018 - Paper DS-17 ABSTRACT Creating an ADaM Data Set for Correlation Analyses Chad Melson, Experis Clinical, Cincinnati, OH The purpose of a correlation analysis is to evaluate relationships
PharmaSUG 2018 - Paper DS-17 ABSTRACT Creating an ADaM Data Set for Correlation Analyses Chad Melson, Experis Clinical, Cincinnati, OH The purpose of a correlation analysis is to evaluate relationships
Specification of a Cardiovascular Metadata Model: A Consensus Standard
 Specification of a Cardiovascular Metadata Model: A Consensus Standard Rebecca Wilgus, RN MSN 1 ; Dana Pinchotti 2 ; Salvatore Mungal 3 ; David F Kong, MD AM 1 ; James E Tcheng, MD 1 ; Brian McCourt 1
Specification of a Cardiovascular Metadata Model: A Consensus Standard Rebecca Wilgus, RN MSN 1 ; Dana Pinchotti 2 ; Salvatore Mungal 3 ; David F Kong, MD AM 1 ; James E Tcheng, MD 1 ; Brian McCourt 1
The Latest Technology. Digi-Lite TM Key Features & Benefits
 Digi-Lite TM The Latest Technology Digi-Lite TM is a Digital Transcranial Doppler (TCD) System with an advanced and proprietary M-Mode display. The product is based on the latest technology in digital
Digi-Lite TM The Latest Technology Digi-Lite TM is a Digital Transcranial Doppler (TCD) System with an advanced and proprietary M-Mode display. The product is based on the latest technology in digital
4 Channel 4~20mA/0~10VDC Analog Data Fiber Link System
 USER GUIDE RLH Industries, Inc. The leader in rugged fiber optic technology. U-022 2017A-0330 4 Channel 4~20mA/0~10VDC Analog Data Fiber Link System SYSTEM INSTALLATION INFORMATION Description The 4 Channel
USER GUIDE RLH Industries, Inc. The leader in rugged fiber optic technology. U-022 2017A-0330 4 Channel 4~20mA/0~10VDC Analog Data Fiber Link System SYSTEM INSTALLATION INFORMATION Description The 4 Channel
UDI TAC Meeting January 15, 2014
 UDI TAC Meeting January 15, 2014 UDI TAC Meeting Call in Information DIAL-IN: (605) 475-6767 ACCESS CODE: 7786079 Please do NOT put this call on hold as we cannot mute your hold music Kindly MUTE your
UDI TAC Meeting January 15, 2014 UDI TAC Meeting Call in Information DIAL-IN: (605) 475-6767 ACCESS CODE: 7786079 Please do NOT put this call on hold as we cannot mute your hold music Kindly MUTE your
The results section of a clinicaltrials.gov file is divided into discrete parts, each of which includes nested series of data entry screens.
 OVERVIEW The ClinicalTrials.gov Protocol Registration System (PRS) is a web-based tool developed for submitting clinical trials information to ClinicalTrials.gov. This document provides step-by-step instructions
OVERVIEW The ClinicalTrials.gov Protocol Registration System (PRS) is a web-based tool developed for submitting clinical trials information to ClinicalTrials.gov. This document provides step-by-step instructions
Hospital Compare Downloadable Database
 Hospital Compare Downloadable Database Generally, health policy researchers and the media download the Hospital Compare database as an easy way to obtain a large set of data. The data in the Downloadable
Hospital Compare Downloadable Database Generally, health policy researchers and the media download the Hospital Compare database as an easy way to obtain a large set of data. The data in the Downloadable
CDRH s Expedited Access PMA (EAP) Program. What is Adjudication s Role?
 CDRH s Expedited Access PMA (EAP) Program. What is Adjudication s Role? March 11, 2016 Wendel Smith, M.D. Director Global Medical Safety Trans-catheter Heart Valve Can we speed approval of devices with
CDRH s Expedited Access PMA (EAP) Program. What is Adjudication s Role? March 11, 2016 Wendel Smith, M.D. Director Global Medical Safety Trans-catheter Heart Valve Can we speed approval of devices with
Zenith Flex AAA Endovascular Graft
 Zenith Flex AAA Endovascular Graft Ancillary components with Z-Trak Introduction System Zenith Flex AAA Endovascular Graft Ancillary Components Converter Main body extension 2 internal stents Main body
Zenith Flex AAA Endovascular Graft Ancillary components with Z-Trak Introduction System Zenith Flex AAA Endovascular Graft Ancillary Components Converter Main body extension 2 internal stents Main body
Requirements on clinical data in Europe
 Requirements on clinical data in Europe Dr. Bassil Akra Director Global Clinical Affairs TÜV SÜD Product Service Current Medical Device Directives Applicable Directives Active Implantable Medical Devices
Requirements on clinical data in Europe Dr. Bassil Akra Director Global Clinical Affairs TÜV SÜD Product Service Current Medical Device Directives Applicable Directives Active Implantable Medical Devices
CURRICULUM COMMITTEE MEETING Friday, March 18, :00 p.m. Student Life Center, Faculty Dining Room (Building 23, First Floor) AGENDA
 CURRICULUM COMMITTEE MEETING Friday, March 18, 2016-2:00 p.m. Student Life Center, Faculty Dining Room (Building 23, First Floor) I. Call to Order AGENDA II. Roll Call III. Minutes of meeting of January
CURRICULUM COMMITTEE MEETING Friday, March 18, 2016-2:00 p.m. Student Life Center, Faculty Dining Room (Building 23, First Floor) I. Call to Order AGENDA II. Roll Call III. Minutes of meeting of January
Online Reports. ACS NSQIP National Conference Salt Lake City, Utah Pre-Conference Session July 21, 2012
 Online Reports ACS NSQIP National Conference Salt Lake City, Utah Pre-Conference Session July 21, 2012 Accessing Online Reports Data Main Page Right Hand Side Menu o Quick link that jumps you to an individual
Online Reports ACS NSQIP National Conference Salt Lake City, Utah Pre-Conference Session July 21, 2012 Accessing Online Reports Data Main Page Right Hand Side Menu o Quick link that jumps you to an individual
Preparing the Office of Scientific Investigations (OSI) Requests for Submissions to FDA
 PharmaSUG 2018 - Paper EP15 Preparing the Office of Scientific Investigations (OSI) Requests for Submissions to FDA Ellen Lin, Wei Cui, Ran Li, and Yaling Teng Amgen Inc, Thousand Oaks, CA ABSTRACT The
PharmaSUG 2018 - Paper EP15 Preparing the Office of Scientific Investigations (OSI) Requests for Submissions to FDA Ellen Lin, Wei Cui, Ran Li, and Yaling Teng Amgen Inc, Thousand Oaks, CA ABSTRACT The
CorPath GRX System Operator s Manual
 CorPath GRX System Operator s Manual Important Safety Notice to Users of the Corindus CorPath GRX System This manual and the equipment it describes are for use only by qualified medical professionals with
CorPath GRX System Operator s Manual Important Safety Notice to Users of the Corindus CorPath GRX System This manual and the equipment it describes are for use only by qualified medical professionals with
Robot Control for Medical Applications and Hair Transplantation
 Dr. John Tenney Director of Research Restoration Robotics, Inc. Robot Control for Medical Applications and Hair Transplantation Presented to the IEEE Control Systems Society, Santa Clara Valley 18 November
Dr. John Tenney Director of Research Restoration Robotics, Inc. Robot Control for Medical Applications and Hair Transplantation Presented to the IEEE Control Systems Society, Santa Clara Valley 18 November
Issues that Matter Notification and Escalation
 Issues that Matter and and Clinical Development Enterprise Occurrences/Situations that require a simple correction, with no effect on clinical development activities, are managed through normal business
Issues that Matter and and Clinical Development Enterprise Occurrences/Situations that require a simple correction, with no effect on clinical development activities, are managed through normal business
INTRODUCTION TO THE QUALIFIED COURSE PROFESSIONAL GUIDELINES
 INTRODUCTION TO THE QUALIFIED COURSE PROFESSIONAL GUIDELINES As the challenge course industry matures, it becomes more important to designate those who are qualified to deliver services. For this reason,
INTRODUCTION TO THE QUALIFIED COURSE PROFESSIONAL GUIDELINES As the challenge course industry matures, it becomes more important to designate those who are qualified to deliver services. For this reason,
Standards: Implementation, Certification and Testing Work group Friday, May 8, :00 Pm-1:30 Pm ET.
 Standards: Implementation, Certification and Testing Work group Friday, May 8, 2015. 12:00 Pm-1:30 Pm ET. Agenda Complete Work group Comments- Group 1 Review Group 2 Comments. 2015 Edition Certification
Standards: Implementation, Certification and Testing Work group Friday, May 8, 2015. 12:00 Pm-1:30 Pm ET. Agenda Complete Work group Comments- Group 1 Review Group 2 Comments. 2015 Edition Certification
Programming checks: Reviewing the overall quality of the deliverables without parallel programming
 PharmaSUG 2016 Paper IB04 Programming checks: Reviewing the overall quality of the deliverables without parallel programming Shailendra Phadke, Baxalta US Inc., Cambridge MA Veronika Csom, Baxalta US Inc.,
PharmaSUG 2016 Paper IB04 Programming checks: Reviewing the overall quality of the deliverables without parallel programming Shailendra Phadke, Baxalta US Inc., Cambridge MA Veronika Csom, Baxalta US Inc.,
Michael V. McConnell, MD, MSEE Professor of CV Medicine and Electrical Engineering (courtesy) Director, Cardiovascular Health
 Michael V. McConnell, MD, MSEE Professor of CV Medicine and Electrical Engineering (courtesy) Director, Cardiovascular Health Innovation @StopHeartDz Disclosures Apple, Inc. In-kind software development
Michael V. McConnell, MD, MSEE Professor of CV Medicine and Electrical Engineering (courtesy) Director, Cardiovascular Health Innovation @StopHeartDz Disclosures Apple, Inc. In-kind software development
Validation System of MR Image Overlay and Other Needle Insertion Techniques
 Submitted to the Proceedings fro Medicine Meets Virtual Reality 15 February 2007, Long Beach, California To appear in Stud. Health Inform Validation System of MR Image Overlay and Other Needle Insertion
Submitted to the Proceedings fro Medicine Meets Virtual Reality 15 February 2007, Long Beach, California To appear in Stud. Health Inform Validation System of MR Image Overlay and Other Needle Insertion
Reviewers Guide on Clinical Trials
 Reviewers Guide on Clinical Trials Office of Research Integrity & Compliance Version 2 Updated: June 26, 2017 This document is meant to help board members conduct reviews for Full Board: Clinical Trial
Reviewers Guide on Clinical Trials Office of Research Integrity & Compliance Version 2 Updated: June 26, 2017 This document is meant to help board members conduct reviews for Full Board: Clinical Trial
How to write ADaM specifications like a ninja.
 Poster PP06 How to write ADaM specifications like a ninja. Caroline Francis, Independent SAS & Standards Consultant, Torrevieja, Spain ABSTRACT To produce analysis datasets from CDISC Study Data Tabulation
Poster PP06 How to write ADaM specifications like a ninja. Caroline Francis, Independent SAS & Standards Consultant, Torrevieja, Spain ABSTRACT To produce analysis datasets from CDISC Study Data Tabulation
Computer Aided Diagnosis Based on Medical Image Processing and Artificial Intelligence Methods
 International Journal of Information and Computation Technology. ISSN 0974-2239 Volume 3, Number 9 (2013), pp. 887-892 International Research Publications House http://www. irphouse.com /ijict.htm Computer
International Journal of Information and Computation Technology. ISSN 0974-2239 Volume 3, Number 9 (2013), pp. 887-892 International Research Publications House http://www. irphouse.com /ijict.htm Computer
Abstract Submission Instructions
 Abstract Submission Instructions Entering Abstract Information A. Title 1. Do not bold, italicize, underline, superscript or subscript any items in the title. 2. Do not include authors in the title. If
Abstract Submission Instructions Entering Abstract Information A. Title 1. Do not bold, italicize, underline, superscript or subscript any items in the title. 2. Do not include authors in the title. If
3 piece system. Treat necks. 75 degrees of angulation* mm. 17 mm. Overlapping stents provide multiple sealing points for every apex
 3 piece system with multiple options for patient customization. Broad range of sizes in the standard offerings Overlapping stents provide multiple sealing points for every apex Multiple treatment options
3 piece system with multiple options for patient customization. Broad range of sizes in the standard offerings Overlapping stents provide multiple sealing points for every apex Multiple treatment options
INTERVENTIONAL PRODUCTS. (866)
 INTERVENTIONAL PRODUCTS Nitinol Tracheal Stent Placement: Trachea, self-expanding Configuration: Braided stent loaded on delivery system, sterile Flexibility: Highly elastic and strong radial forces Visibility:
INTERVENTIONAL PRODUCTS Nitinol Tracheal Stent Placement: Trachea, self-expanding Configuration: Braided stent loaded on delivery system, sterile Flexibility: Highly elastic and strong radial forces Visibility:
Submission Guidelines
 Submission Guidelines Clinical Trial Results invites the submission of phase I, II, and III clinical trials for publication in a brief print format, with full trials results online. We encourage the submission
Submission Guidelines Clinical Trial Results invites the submission of phase I, II, and III clinical trials for publication in a brief print format, with full trials results online. We encourage the submission
Practical Elements of Database Design
 Practical Elements of Database Design Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to: identify principles of database design
Practical Elements of Database Design Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to: identify principles of database design
The Evolving Role of Primary Care and Technology in Cardiology
 The Evolving Role of Primary Care and Technology in Cardiology Peter Tilkemeier, MD, MMM, FACC Chair, Department of Medicine Greenville Health System Professor, University of South Carolina School of Medicine
The Evolving Role of Primary Care and Technology in Cardiology Peter Tilkemeier, MD, MMM, FACC Chair, Department of Medicine Greenville Health System Professor, University of South Carolina School of Medicine
Ports Designed to Change. Your Point of View
 Ports Designed to Change Your Point of View Features That Allow You to Focus on Your Patient s Care Caring for patients is a dynamic process. Most, if not all, of your patients are simultaneously receiving
Ports Designed to Change Your Point of View Features That Allow You to Focus on Your Patient s Care Caring for patients is a dynamic process. Most, if not all, of your patients are simultaneously receiving
THE GLOBAL MARKET FOR INTRAVASCULAR ULTRASOUND TOOLS AND ANCILLARY EQUIPMENT. HLC145A July Himani Singhi Wadhwa Project Analyst
 THE GLOBAL MARKET FOR INTRAVASCULAR ULTRASOUND TOOLS AND ANCILLARY EQUIPMENT HLC145A July 2013 Himani Singhi Wadhwa Project Analyst ISBN: 1-56965-445-X BCC Research 49 Walnut Park, Building 2 Wellesley,
THE GLOBAL MARKET FOR INTRAVASCULAR ULTRASOUND TOOLS AND ANCILLARY EQUIPMENT HLC145A July 2013 Himani Singhi Wadhwa Project Analyst ISBN: 1-56965-445-X BCC Research 49 Walnut Park, Building 2 Wellesley,
Patient Safety Initiatives Implementation (WP5): Presentation of Main Outcomes
 Patient Safety Initiatives Implementation (WP5): Presentation of Main Outcomes Lena Mehrmann, Tugce Aksoy, Christian Thomeczek German Agency for Quality in Medicine (AQuMed / ÄZQ) PaSQ 5 th Coordination
Patient Safety Initiatives Implementation (WP5): Presentation of Main Outcomes Lena Mehrmann, Tugce Aksoy, Christian Thomeczek German Agency for Quality in Medicine (AQuMed / ÄZQ) PaSQ 5 th Coordination
The Next Frontier in Medical Device Security
 The Next Frontier in Medical Device Security Session #76, February 21, 2017 Denise Anderson, President, NH-ISAC Dr. Dale Nordenberg, Executive Director, MDISS 1 Speaker Introduction Denise Anderson, MBA
The Next Frontier in Medical Device Security Session #76, February 21, 2017 Denise Anderson, President, NH-ISAC Dr. Dale Nordenberg, Executive Director, MDISS 1 Speaker Introduction Denise Anderson, MBA
Evolution of Massive Transfusion Strategies
 Evolution of Massive Transfusion Strategies Kerry Gunn Department of Anaesthesia and Perioperative Medicine Auckland City Hospital Conflict of Interest Honorarium for Novo Nordisk to Chair Meetings Summary
Evolution of Massive Transfusion Strategies Kerry Gunn Department of Anaesthesia and Perioperative Medicine Auckland City Hospital Conflict of Interest Honorarium for Novo Nordisk to Chair Meetings Summary
Medical Device Vulnerability Management
 Medical Device Vulnerability Management MDISS / NH-ISAC Process Draft Dale Nordenberg, MD June 2015 Market-based public health: collaborative acceleration Objectives Define a trusted and repeatable process
Medical Device Vulnerability Management MDISS / NH-ISAC Process Draft Dale Nordenberg, MD June 2015 Market-based public health: collaborative acceleration Objectives Define a trusted and repeatable process
New Approaches to Computer-based Interventional Neuroradiology Training
 New Approaches to Computer-based Interventional Neuroradiology Training Xunlei WU, Vincent PEGORARO, Vincent LUBOZ, Paul F. NEUMANN, Ryan BARDSLEY, Steven DAWSON, Stephane COTIN The Simulation Group, CIMIT,
New Approaches to Computer-based Interventional Neuroradiology Training Xunlei WU, Vincent PEGORARO, Vincent LUBOZ, Paul F. NEUMANN, Ryan BARDSLEY, Steven DAWSON, Stephane COTIN The Simulation Group, CIMIT,
Protocol Deviations and Protocol Violations Made Simple. Donna Brassil, MA, RN, CCRC Rhonda G. Kost, MD
 Protocol Deviations and Protocol Violations Made Simple Donna Brassil, MA, RN, CCRC Rhonda G. Kost, MD Objectives Upon completion of this class, the learner will be able to: verbalize the difference between
Protocol Deviations and Protocol Violations Made Simple Donna Brassil, MA, RN, CCRC Rhonda G. Kost, MD Objectives Upon completion of this class, the learner will be able to: verbalize the difference between
Office of Human Research
 Office of Human Research JeffTrial End-User Training Document Regulatory Coordinator Training for Non-Oncology personnel Office of Human Research 8/16/2013 Ver. 1.0 Contents The REG Role: Completing Basic
Office of Human Research JeffTrial End-User Training Document Regulatory Coordinator Training for Non-Oncology personnel Office of Human Research 8/16/2013 Ver. 1.0 Contents The REG Role: Completing Basic
AAPM Standard of Practice: CT Protocol Review Physicist
 AAPM Standard of Practice: CT Protocol Review Physicist Dianna Cody, Ph.D., DABR, FAAPM U.T.M.D. Anderson Cancer Center September 11, 2014 2014 Texas Radiation Regulatory Conference Goals Understand purpose
AAPM Standard of Practice: CT Protocol Review Physicist Dianna Cody, Ph.D., DABR, FAAPM U.T.M.D. Anderson Cancer Center September 11, 2014 2014 Texas Radiation Regulatory Conference Goals Understand purpose
QAngio XA 7.3. Quick Start Manual. July 31, v6.0
 QAngio XA 7.3 Quick Start Manual July 31, 2018 9.04.250.73.6 v6.0 Medis medical imaging systems bv Schuttersveld 9, 2316 XG Leiden, the Netherlands http://www.medis.nl Medis medical imaging systems bv
QAngio XA 7.3 Quick Start Manual July 31, 2018 9.04.250.73.6 v6.0 Medis medical imaging systems bv Schuttersveld 9, 2316 XG Leiden, the Netherlands http://www.medis.nl Medis medical imaging systems bv
Pooling strategy of clinical data
 Pooling strategy of clinical data Abraham Yeh, Xiaohong (Grace) Zhang, Shin-Ru Wang, Novartis Pharmaceuticals Corporation, East Hanover, NJ ABSTRACT Pooling of clinical data is used by all pharmaceutical
Pooling strategy of clinical data Abraham Yeh, Xiaohong (Grace) Zhang, Shin-Ru Wang, Novartis Pharmaceuticals Corporation, East Hanover, NJ ABSTRACT Pooling of clinical data is used by all pharmaceutical
CorPath GRX System Operator s Manual
 CorPath GRX System Operator s Manual Important Safety Notice to Users of the Corindus CorPath GRX System This manual and the equipment it describes are for use only by qualified medical professionals with
CorPath GRX System Operator s Manual Important Safety Notice to Users of the Corindus CorPath GRX System This manual and the equipment it describes are for use only by qualified medical professionals with
MINIMALLY invasive vascular interventions have become
 1998 IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 61, NO. 7, JULY 2014 Which Spring is the Best? Comparison of Methods for Virtual Stenting Katerina Spranger and Yiannis Ventikos Abstract This paper
1998 IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 61, NO. 7, JULY 2014 Which Spring is the Best? Comparison of Methods for Virtual Stenting Katerina Spranger and Yiannis Ventikos Abstract This paper
CPM Specifications Document Healthy Vertebral:
 CPM Specifications Document Healthy Vertebral: OSMSC 0078_0000, 0079_0000, 0166_000, 0167_0000 May 1, 2013 Version 1 Open Source Medical Software Corporation 2013 Open Source Medical Software Corporation.
CPM Specifications Document Healthy Vertebral: OSMSC 0078_0000, 0079_0000, 0166_000, 0167_0000 May 1, 2013 Version 1 Open Source Medical Software Corporation 2013 Open Source Medical Software Corporation.
Optical Flow Method for Blood Flow Velocimetry Based on Digital X-Ray Subtraction Angiography: A Brief Review
 Optical Flow Method for Blood Flow Velocimetry Based on Digital X-Ray Subtraction Angiography: A Brief Review Zifeng Yang* Department of Mechanical and Materials Engineering, Wright State University, Dayton,
Optical Flow Method for Blood Flow Velocimetry Based on Digital X-Ray Subtraction Angiography: A Brief Review Zifeng Yang* Department of Mechanical and Materials Engineering, Wright State University, Dayton,
***** ***** August
 SLU eirb Conversion Charts Saint Louis University ***** eirb Paper to Electronic Protocol Conversion Charts ***** Institutional Review Board August 2011 http://eirb.slu.edu Institutional Review Board Saint
SLU eirb Conversion Charts Saint Louis University ***** eirb Paper to Electronic Protocol Conversion Charts ***** Institutional Review Board August 2011 http://eirb.slu.edu Institutional Review Board Saint
The National Medical Device Information Sharing & Analysis Organization (MD-ISAO) Initiative Session 2, February 19, 2017 Moderator: Suzanne
 The National Medical Device Information Sharing & Analysis Organization (MD-ISAO) Initiative Session 2, February 19, 2017 Moderator: Suzanne Schwartz, Assoc. Dir., CDRH, FDA Denise Anderson, MBA, President,
The National Medical Device Information Sharing & Analysis Organization (MD-ISAO) Initiative Session 2, February 19, 2017 Moderator: Suzanne Schwartz, Assoc. Dir., CDRH, FDA Denise Anderson, MBA, President,
Allobone Graft Behavior after Calcaneal Lengthening
 Allobone Graft Behavior after Calcaneal Lengthening 2014. 9. 20. In Hyeok Lee, MD Department of Orthopedic Surgery Seoul National University Bundang Hospital Planovalgus SEOUL NATIONAL UNIVERSITY BUNDANG
Allobone Graft Behavior after Calcaneal Lengthening 2014. 9. 20. In Hyeok Lee, MD Department of Orthopedic Surgery Seoul National University Bundang Hospital Planovalgus SEOUL NATIONAL UNIVERSITY BUNDANG
Life Sciences Applications: Modeling and Simulation for Biomedical Device Design SGC 2013
 Life Sciences Applications: Modeling and Simulation for Biomedical Device Design Kristian.Debus@cd-adapco.com SGC 2013 Modeling and Simulation for Biomedical Device Design Biomedical device design and
Life Sciences Applications: Modeling and Simulation for Biomedical Device Design Kristian.Debus@cd-adapco.com SGC 2013 Modeling and Simulation for Biomedical Device Design Biomedical device design and
registries can download relevant information about all medical devices used to treat peripheral arterial
 Registry Assessment of Peripheral Interventional Devices (RAPID) project: A report from the GUDID (Global Unique Device Identification) Integration Working Group Introduction The Registry Assessment of
Registry Assessment of Peripheral Interventional Devices (RAPID) project: A report from the GUDID (Global Unique Device Identification) Integration Working Group Introduction The Registry Assessment of
Secondary Surge Arrester Instruction Manual Page 1 of 5
 Secondary Surge Arrester Instruction Manual Page 1 of 5 GENERAL SAFETY INSTRUCTIONS WARNING: ARRESTER CAN FAIL VIOLENTLY AND CAUSE INJURY AND PROPERTY DAMAGE WHEN APPLIED AT VOLTAGES GREATER THAN 120V.
Secondary Surge Arrester Instruction Manual Page 1 of 5 GENERAL SAFETY INSTRUCTIONS WARNING: ARRESTER CAN FAIL VIOLENTLY AND CAUSE INJURY AND PROPERTY DAMAGE WHEN APPLIED AT VOLTAGES GREATER THAN 120V.
GPU Based Region Growth and Vessel Tracking. Supratik Moulik M.D. Jason Walsh
 GPU Based Region Growth and Vessel Tracking Supratik Moulik M.D. (supratik@moulik.com) Jason Walsh Conflict of Interest Dr. Supratik Moulik does not have a significant financial stake in any company, nor
GPU Based Region Growth and Vessel Tracking Supratik Moulik M.D. (supratik@moulik.com) Jason Walsh Conflict of Interest Dr. Supratik Moulik does not have a significant financial stake in any company, nor
Zenith Alpha Thoracic case planning guide
 Zenith Alpha Thoracic case planning guide Zenith Alpha T HORACIC ENDOVASCULAR GRAFT MEDICAL www.cookmedical.com Zenith Alpha Thoracic case planning guide Zenith Alpha T HORACIC ENDOVASCULAR GRAFT Zenith
Zenith Alpha Thoracic case planning guide Zenith Alpha T HORACIC ENDOVASCULAR GRAFT MEDICAL www.cookmedical.com Zenith Alpha Thoracic case planning guide Zenith Alpha T HORACIC ENDOVASCULAR GRAFT Zenith
Three-dimensional (3D) printed endovascular simulation models: a feasibility study
 Original Article Page 1 of 8 Three-dimensional (3D) printed endovascular simulation models: a feasibility study Sebastian Mafeld 1, Craig Nesbitt 2, James McCaslin 2, Alan Bagnall 3, Philip Davey 4, Pentop
Original Article Page 1 of 8 Three-dimensional (3D) printed endovascular simulation models: a feasibility study Sebastian Mafeld 1, Craig Nesbitt 2, James McCaslin 2, Alan Bagnall 3, Philip Davey 4, Pentop
Gas Infrastructure Europe. Security Risk Assessment Methodology
 Gas Infrastructure Europe Security Risk Assessment Methodology May 2015 Introduction Gas Infrastructure Europe (GIE) is an association representing the interests of European natural gas infrastructure
Gas Infrastructure Europe Security Risk Assessment Methodology May 2015 Introduction Gas Infrastructure Europe (GIE) is an association representing the interests of European natural gas infrastructure
better images mean better results
 better images mean better results A better way for YOU and YOUR patient brought to you by Advanced Neuro analysis with access to studies wherever you need it Advanced Neuro from Invivo Advancements in
better images mean better results A better way for YOU and YOUR patient brought to you by Advanced Neuro analysis with access to studies wherever you need it Advanced Neuro from Invivo Advancements in
NRS STATE DATA QUALITY CHECKLIST
 A Project of the U.S. Department of Education NRS STATE DATA QUALITY CHECKLIST State: Date: Completed by (name and title): A. Data Foundation and Structure Acceptable Quality 1. State has written assessment
A Project of the U.S. Department of Education NRS STATE DATA QUALITY CHECKLIST State: Date: Completed by (name and title): A. Data Foundation and Structure Acceptable Quality 1. State has written assessment
OnCore Enterprise Research. Exercises: Subject Administration
 OnCore Enterprise Research Exercises: Subject Administration Clinical Research Coordinator August 2018 P a g e 1 This page is intentionally blank. P a g e 2 Exercises: Subject Administration Contents OnCore
OnCore Enterprise Research Exercises: Subject Administration Clinical Research Coordinator August 2018 P a g e 1 This page is intentionally blank. P a g e 2 Exercises: Subject Administration Contents OnCore
Guidance for the format and content of the final study report of non-interventional post-authorisation safety studies
 23 January 2013 EMA/48663/2013 Patient Health Protection Guidance for the format and content of the final study report of non-interventional post-authorisation safety studies Introduction From 10 January
23 January 2013 EMA/48663/2013 Patient Health Protection Guidance for the format and content of the final study report of non-interventional post-authorisation safety studies Introduction From 10 January
FDA & Medical Device Cybersecurity
 FDA & Medical Device Cybersecurity Closing Keynote, February 19, 2017 Suzanne B. Schwartz, M.D., MBA Associate Director for Science & Strategic Partnerships Center for Devices and Radiological Health US
FDA & Medical Device Cybersecurity Closing Keynote, February 19, 2017 Suzanne B. Schwartz, M.D., MBA Associate Director for Science & Strategic Partnerships Center for Devices and Radiological Health US
Medical Device Innovations: Welcome to the Future
 Medical Device Innovations: Welcome to the Future Sonali Gunawardhana, Of Counsel, Shook, Hardy & Bacon LLP Bakul Patel, Associate Director for Digital Health, CDRH, FDA Zachary Rothstein, Associate Vice
Medical Device Innovations: Welcome to the Future Sonali Gunawardhana, Of Counsel, Shook, Hardy & Bacon LLP Bakul Patel, Associate Director for Digital Health, CDRH, FDA Zachary Rothstein, Associate Vice
Impact of the Rethinking Personal Choice Program On Inmate Behavior and Facility Operations At Florida State Prison (FSP)
 Impact of the Rethinking Personal Choice Program On Inmate Behavior and Facility Operations At Florida State Prison (FSP) September 2002 Michael W. Moore, Secretary Richard L. Dugger, Deputy Secretary
Impact of the Rethinking Personal Choice Program On Inmate Behavior and Facility Operations At Florida State Prison (FSP) September 2002 Michael W. Moore, Secretary Richard L. Dugger, Deputy Secretary
4 Channel Contact Closure DIN Fiber Link System
 USER GUIDE RLH Industries, Inc. The leader in rugged fiber optic technology. U-027 2017A-0420 4 Channel Contact Closure DIN Fiber Link System SYSTEM INSTALLATI INFORMATI Description The 4 Channel Contact
USER GUIDE RLH Industries, Inc. The leader in rugged fiber optic technology. U-027 2017A-0420 4 Channel Contact Closure DIN Fiber Link System SYSTEM INSTALLATI INFORMATI Description The 4 Channel Contact
TIVATO 700 from ZEISS Seize The Digital Future
 TIVATO 700 from ZEISS Seize The Digital Future Transforming possibilities into realities. ZEISS TIVATO 700 Enhance precision and efficiency in the OR Today s microsurgical landscapes require advanced technology
TIVATO 700 from ZEISS Seize The Digital Future Transforming possibilities into realities. ZEISS TIVATO 700 Enhance precision and efficiency in the OR Today s microsurgical landscapes require advanced technology
Planning Image-Guided Endovascular Interventions: Guidewire Simulation using Shortest Path Algorithms
 Planning Image-Guided Endovascular Interventions: Guidewire Simulation using Shortest Path Algorithms Sebastian Schafer 1,2, Vikas Singh 2,3, Kenneth R. Hoffmann 1,2, Peter B. Noël 2,3, Jinhui Xu 3 1 Department
Planning Image-Guided Endovascular Interventions: Guidewire Simulation using Shortest Path Algorithms Sebastian Schafer 1,2, Vikas Singh 2,3, Kenneth R. Hoffmann 1,2, Peter B. Noël 2,3, Jinhui Xu 3 1 Department
ICTR UW Institute of Clinical and Translational Research. i2b2 User Guide. Version 1.0 Updated 9/11/2017
 ICTR UW Institute of Clinical and Translational Research i2b2 User Guide Version 1.0 Updated 9/11/2017 Table of Contents Background/Search Criteria... 2 Accessing i2b2... 3 Navigating the Workbench...
ICTR UW Institute of Clinical and Translational Research i2b2 User Guide Version 1.0 Updated 9/11/2017 Table of Contents Background/Search Criteria... 2 Accessing i2b2... 3 Navigating the Workbench...
Computer-Aided Detection system for Hemorrhage contained region
 Computer-Aided Detection system for Hemorrhage contained region Myat Mon Kyaw Faculty of Information and Communication Technology University of Technology (Yatanarpon Cybercity), Pyin Oo Lwin, Myanmar
Computer-Aided Detection system for Hemorrhage contained region Myat Mon Kyaw Faculty of Information and Communication Technology University of Technology (Yatanarpon Cybercity), Pyin Oo Lwin, Myanmar
Essential Guide. Chapter 1: The Physiotherapy Competency Exam (PCE)
 Essential Guide Chapter 1: The Physiotherapy Competency Exam (PCE) 1 After reading this chapter, you will know: what the purpose of the exam is; how the exam is structured; how to apply for the exam; what
Essential Guide Chapter 1: The Physiotherapy Competency Exam (PCE) 1 After reading this chapter, you will know: what the purpose of the exam is; how the exam is structured; how to apply for the exam; what
SERIOUS ADVERSE EVENTS SERIOUS ADVERSE EVENT REPORTING SERIOUS ADVERSE EVENT. Clinical Trials Training Course Serious Adverse Event Reporting
 SERIOUS ADVERSE EVENT REPORTING SERIOUS ADVERSE EVENT SAE s are a sub-set of all adverse events collected The reporting of SAE s is in addition to, and does not supplant, the necessity of adequately reporting
SERIOUS ADVERSE EVENT REPORTING SERIOUS ADVERSE EVENT SAE s are a sub-set of all adverse events collected The reporting of SAE s is in addition to, and does not supplant, the necessity of adequately reporting
MISSING DATA REPORT Survey Data
 MISSING DATA REPORT Survey Data 2012-2016 Abstract The rates of non response for ANZDATA survey items over the last 5 years anzdata@anzdata.org.au www.anzdata.org.au The tables below show the rates of
MISSING DATA REPORT Survey Data 2012-2016 Abstract The rates of non response for ANZDATA survey items over the last 5 years anzdata@anzdata.org.au www.anzdata.org.au The tables below show the rates of
1. Study Registration. 2. Confirm Registration
 USER MANUAL 1. Study Registration Diabetic patients are more susceptible to experiencing cardiovascular events, but this can be minimized with control of blood glucose levels and other risk factors (blood
USER MANUAL 1. Study Registration Diabetic patients are more susceptible to experiencing cardiovascular events, but this can be minimized with control of blood glucose levels and other risk factors (blood
Unique Device Identification (UDI) Updates on US Activities
 1 Unique Device Identification (UDI) Updates on US Activities U.S. - China Healthcare Cooperation Series China FDA Medical Device Executive Development Delegation Visit Symposium and Reception 2017-07-25
1 Unique Device Identification (UDI) Updates on US Activities U.S. - China Healthcare Cooperation Series China FDA Medical Device Executive Development Delegation Visit Symposium and Reception 2017-07-25
A Picture is worth 3000 words!! 3D Visualization using SAS Suhas R. Sanjee, Novartis Institutes for Biomedical Research, INC.
 DG04 A Picture is worth 3000 words!! 3D Visualization using SAS Suhas R. Sanjee, Novartis Institutes for Biomedical Research, INC., Cambridge, USA ABSTRACT Data visualization is an important aspect in
DG04 A Picture is worth 3000 words!! 3D Visualization using SAS Suhas R. Sanjee, Novartis Institutes for Biomedical Research, INC., Cambridge, USA ABSTRACT Data visualization is an important aspect in
Image-Based Device Tracking for the Co-registration of Angiography and Intravascular Ultrasound Images
 Image-Based Device Tracking for the Co-registration of Angiography and Intravascular Ultrasound Images Peng Wang 1, Terrence Chen 1, Olivier Ecabert 2, Simone Prummer 2, Martin Ostermeier 2, and Dorin
Image-Based Device Tracking for the Co-registration of Angiography and Intravascular Ultrasound Images Peng Wang 1, Terrence Chen 1, Olivier Ecabert 2, Simone Prummer 2, Martin Ostermeier 2, and Dorin
ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL
 Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
Version 1.0 24May16 ELECTRONIC SITE DELEGATION LOG (esdl) USER MANUAL - Table of Contents - 1. INTRODUCTION... 3 Background... 3 Purpose of the Site Delegation Log... 3 TNCC Contacts... 3 2. SYSTEM REQUIREMENTS...
Functional Requirements For the California Joint Replacement Registry I.T. Infrastructure
 Functional Requirements For the California Joint Replacement Registry I.T. Infrastructure Prepared by Sujansky & Associates, LLC On behalf of the Pacific Business Group on Health August 13, 2010 Outline
Functional Requirements For the California Joint Replacement Registry I.T. Infrastructure Prepared by Sujansky & Associates, LLC On behalf of the Pacific Business Group on Health August 13, 2010 Outline
Leading Innovation in Health Care Delivery
 Leading Innovation in Health Care Delivery Presented by: Chris Trimble Adjunct Professor Dartmouth College 2015: Chris Trimble. These slides may be freely distributed, with this copyright notice, so long
Leading Innovation in Health Care Delivery Presented by: Chris Trimble Adjunct Professor Dartmouth College 2015: Chris Trimble. These slides may be freely distributed, with this copyright notice, so long
OnCore Enterprise Research. Exercises: Subject Administration
 OnCore Enterprise Research Exercises: Subject Administration Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Exercises: Subject Administration Contents OnCore
OnCore Enterprise Research Exercises: Subject Administration Clinical Research Coordinator June 2017 P a g e 1 This page is intentionally blank. P a g e 2 Exercises: Subject Administration Contents OnCore
Magnetic Resonance Imaging Velocity. Information. Joe Lee. April 4, 2000
 Locating Arteriovenous Malformations using Magnetic Resonance Imaging Velocity Information Joe Lee April 4, 2000 1 Introduction An arteriovenous malformation (AVM) is a congenital vascular defect where
Locating Arteriovenous Malformations using Magnetic Resonance Imaging Velocity Information Joe Lee April 4, 2000 1 Introduction An arteriovenous malformation (AVM) is a congenital vascular defect where
A c c e s s o r i e s. r s & t o P Y R A. s C o n n e c T H E I V. s e d N e e d l e l e s. C l o. bionector Product Range
 I V T H E R A s e d N e e d l e l e s P Y s C o n n e c t o r s & A c c e s s o r i e s C l o Product Range is a needleless connector that allows medication and infusate to be administered via the patient
I V T H E R A s e d N e e d l e l e s P Y s C o n n e c t o r s & A c c e s s o r i e s C l o Product Range is a needleless connector that allows medication and infusate to be administered via the patient
Quantitative IntraVascular UltraSound (QCU)
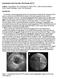 Quantitative IntraVascular UltraSound (QCU) Authors: Jouke Dijkstra, Ph.D. and Johan H.C. Reiber, Ph.D., Leiden University Medical Center, Dept of Radiology, Leiden, The Netherlands Introduction: For decades,
Quantitative IntraVascular UltraSound (QCU) Authors: Jouke Dijkstra, Ph.D. and Johan H.C. Reiber, Ph.D., Leiden University Medical Center, Dept of Radiology, Leiden, The Netherlands Introduction: For decades,
