Click IACUC Researcher s Quick Reference
|
|
|
- Natalie McBride
- 5 years ago
- Views:
Transcription
1 Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond to Reviewer Requests... 6 Create and Submit a Follow-On Submission... 7 Navigation and Basic Tasks... 8 Search the System Using Key Words... 0
2 Before You Create a Protocol Plan out your protocol: Summarize the research (science), reasons for performing the research, and its benefits. Determine the experiments and the number of animals required. Determine the procedures you will perform and the substances required for your experiments. Identify any supporting documents to include in your protocol, such as flowcharts, explanations of the science, grant applications, and other information explaining or justifying your research. Set up building blocks: The diagram below shows the concept of building blocks. Once they are set up, you can create your protocol. Create a Research Team for the substances, procedures, and protocols you create. Check the IACUC standard library for the procedures and substances required for your protocol experiments (see Check for Building Blocks). If not in the library: Create the missing substances Create the missing procedures s & Procedures Library Research Team s Procedures s Procedures Protocols Create substances Create procedures* *Team procedures can have one or more team or standard substances Create protocols with experiments* *Experiments can have one or more team or standard procedures Protocol Experiment Procedure Procedure Procedure March 06
3 Create a Research Team As a member of a research team, you can create substances, procedures, and protocols for your research team. When you create a protocol, your research team members appear on the protocol by default. Create Research Team. From My Inbox, click Create Research Team.. Type a name for the research team and select the team s PI. Note: If you are not the PI, add yourself as a team member if you will create team procedures and substances.. Complete the rest of the page and click Finish. You are taken to the research team workspace page. Check for Existing Building Blocks Check the system to see if there are IACUC standard building blocks that you can use in your protocols. Check for Building Blocks a. From My Inbox, click your research team on the left.. In the research team workspace, click the Procedures tab and check for the procedures you need. Do the same for substances (on the s tab). If missing, see Create Building Blocks.. If the building block exists, review its details: a. On the Procedures or s tab, click the item name. b. In the item s workspace, click View Procedure or View. b March 06
4 Create Building Blocks After you have checked the system for IACUC standard building blocks, create any that are required for your protocols. Create a or Procedure. From the research team workspace, click Create or Create Procedure. Note: Create the substances you will use in your procedures before creating the procedures.. Complete the pages. If more than one page, click Continue to move to the next page.. When done, click Finish. You are taken to the research team s substance or procedure workspace page. March 06 4
5 Create and Submit a Protocol Once all the building blocks are set up, you are ready to create your protocol. Create a Protocol. From the research team workspace or My Inbox page, click Create Protocol. a b. Complete the pages. Click Continue to move to the next page.. Pay attention to the following pages: a. Protocol Team Members: Add each person who will edit the protocol. Do not add the PI here. b. Experiments: When you add an experiment, select the procedures that apply to all animals (common procedures) and those that apply to some animals or are used differently across animals (variable procedures). 4. On the final page, click Finish. 4 You are taken to the protocol workspace page. You can continue to edit the protocol (Edit Protocol button) until you submit it for review. Submit a Protocol for Review 5. From the protocol workspace, click Submit Click OK to agree to the statement and submit the protocol for review. You can log off the system (top right). 5 6 March 06 5
6 Respond to Reviewer Requests If a reviewer has questions or requires you to change your submission, you will receive an indicating this. Review the request details and then respond to the request. Review the Request Details. Click the link to open the submission. If you no longer have the , see Open a Submission and then View History to see reviewer comments.. On the History tab, find the Clarification Requested activity and read the comments.. If the reviewer added reviewer notes, click the Reviewer Notes tab and go to Respond to Reviewer Notes. If not, go to Submit Response. Respond to Reviewer Notes For each reviewer note: 4. To edit the protocol in response to the reviewer note, click the Jump To link. 5. From the protocol page or the Reviewer Notes tab, click the Click here to respond link Select a response from the list and explain your response in the box. 7. Click OK. If on a protocol page, exit the protocol when done. Submit Response 9 8. On the protocol workspace, click Submit Response In the Comments box, explain your response to the reviewer. 0. Click OK. You can log off the system (top right). March 06 6
7 Create and Submit a Follow-On Submission If you need to make changes to an approved protocol (amendment) or submit an annual or triennial review, follow these steps. Note: For annual reviews, make sure you review all protocol information (e.g., team members, funding sources, activities, and approved and used animal numbers) and submit any needed amendments immediately. If an amendment is already in review, wait until the first amendment is processed. Create a Follow-On Submission. From My Inbox, click the research team on the left.. Select the name of the approved protocol.. On the left, click the Create button. 4. Complete the pages. Click Continue to move to the next page. 5. When done, click Exit and save changes or click Finish on the final page. You are taken to the submission s workspace page. You can continue to edit the submission ( Edit button) until you submit it for review. Submit Follow-On Submission for Review From the follow-on submission s workspace, click Submit. 7. Click OK to agree to the statement and submit it for review. You can log off the system (top right). 6 7 March 06 7
8 Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where do I find? From My Inbox, you can find:. Submissions that require you to take action.. Your research teams.. Actions you can perform such as create a protocol. What do I do? Review the state of submissions in My Inbox. The state gives a clue as to what to do next. For example, Pre-Submission means you haven t submitted the protocol. You can finish and submit it for review. Open a Submission 6 5. From My Inbox, click the submission name. 6. The submission workspace opens. View History 7. From the submission workspace, click the History tab The history lists the activity taken on a submission including any comments, attachments, or correspondence added. 8 March 06 8
9 9 0 Find Previous Submissions 9. From My Inbox, click Submissions. 0. Click the appropriate tab to see your submissions: In-Review: All submissions undergoing IACUC review Active: All approved submissions Archived: All discarded and closed submissions All Submissions: All submissions, in any state See also Search for Submissions Using Key Words. To find specific data in a table, see Filter Data. Filter Data Many pages contain tables that you can filter to show specific data.. Select the column to filter by.. Type the beginning characters for the items you want to find. You can also type a % symbol as a wildcard before the characters. Examples: 7 shows all items beginning with 7 %7 shows all items containing For operators you can type in the text box, click the Help icon. 4. Click Go to apply the filter. 5. To combine multiple filter criteria, click Add Filter. March 06 9
10 Search the System Using Key Words You can also search for submissions using key words. For example: Submissions involving a specific PI Submissions that involve a certain substance or procedure Note: The system will only return those items you have permission to view. Search for Submissions. From My Inbox, click Submissions.. In the Search field, type your search criteria. You can use the following operators: And: Finds all of the specified words. Or: Finds at least one of the specified words. Not: Excludes the specified word. Asterisk (*): Wildcard character. Finds part of a word. Quotation marks: Finds the exact phrase.. Press ENTER or click the magnifying glass to perform the search. March 06 0
11 06 Huron Technologies Inc. All rights reserved. Use and distribution prohibited except through written agreement with Huron. Click is a registered service mark of Huron Consulting Group, Inc. All other trademarks, registered trademarks, service marks, and trade names are the property of their respective owners. Information in this document is subject to change without notice. Published by Huron Technologies Inc. 95 NW AmberGlen Pkwy Suite 400 Beaverton, OR March 06
IACUC Researcher s Quick Reference
 IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
eiacuc Reference for PIs and Research Team
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
eiacuc Reference for Veterinarians
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
Click. IRB Study Submission Guide
 Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB 7.3 IRB Ancillary Reviewer s Guide
 Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
IACUC Module. March 2018
 z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
Procedural Amendment
 Creating an Amendment in the eacuc system differs depending on what type of amendment you are submitting. Procedural Amendment Adding New Procedures to an Approved Protocol: If you are adding a brand new
Creating an Amendment in the eacuc system differs depending on what type of amendment you are submitting. Procedural Amendment Adding New Procedures to an Approved Protocol: If you are adding a brand new
eresearch IBC Staff Review Step-by-Step Procedures Core Committee Staff Home Page
 1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
How Does the PI make Pre Review ICF Changes?
 How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
Submitting an Protocol for De Novo Review
 Submitting an Protocol for De Novo Review Important: A protocol can be created, edited and submitted by the Principal Investigator (PI), Laboratory Contact or Alternate Lab Contact. However, ONLY the Principal
Submitting an Protocol for De Novo Review Important: A protocol can be created, edited and submitted by the Principal Investigator (PI), Laboratory Contact or Alternate Lab Contact. However, ONLY the Principal
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
Introduction to ARFMS. (Animal Research Facility Management Software)
 Introduction to ARFMS (Animal Research Facility Management Software) Agenda 1. Overview Existing active protocols Course Access Users & Teams IACUC Viewing of AUPs assigned 2. Creation of New AUP Filling
Introduction to ARFMS (Animal Research Facility Management Software) Agenda 1. Overview Existing active protocols Course Access Users & Teams IACUC Viewing of AUPs assigned 2. Creation of New AUP Filling
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment)
 Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
Responding to REB Feedback
 Responding to REB Feedback A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: What is REB Feedback? The REB Feedback Letter The Investigator Response Letter Finding the
Responding to REB Feedback A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: What is REB Feedback? The REB Feedback Letter The Investigator Response Letter Finding the
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Research Inventory System (RIS) Outgoing Subaward Request Process Guide STACCEE RAMEY
 2016 Research Inventory System (RIS) Outgoing Subaward Request Process Guide STACCEE RAMEY Table of Contents Introduction... 2 System overview... 2 Mandatory Entries... 2 Tabs... 2 Tab colors... 2 Warning
2016 Research Inventory System (RIS) Outgoing Subaward Request Process Guide STACCEE RAMEY Table of Contents Introduction... 2 System overview... 2 Mandatory Entries... 2 Tabs... 2 Tab colors... 2 Warning
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Module 3: Changes and Correspondence
 Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
Creating a New Application. A ROMEO Hasty Handbook Presented by the CHEO Research Institute
 Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Contents. TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs
 TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
ARC IRB Chair s Manual
 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
How to Submit a New Application
 ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
Banner Security Access Request
 is a Web Form designed for Supervisors to submit Banner Access Requests for their employees. This online form replaces the previous paper form in a secure environment. This helps the Banner team respond
is a Web Form designed for Supervisors to submit Banner Access Requests for their employees. This online form replaces the previous paper form in a secure environment. This helps the Banner team respond
Training Manual for Researchers. How to Create an Online Human Ethics Application
 Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
Create an Action Item
 Overview Action Items are created within animal use forms, amendments and protocols to notify a person or group of a related task. If the Action Item requires an amendment, the Action Item and amendment
Overview Action Items are created within animal use forms, amendments and protocols to notify a person or group of a related task. If the Action Item requires an amendment, the Action Item and amendment
6. To access the online IRB protocol module, click the "My IRB Protocol" link found in the top navigation of any CoeusLite screen
 IPFW CoeusLite Online IRB Process CoeusLite IRB Login 1. Open a web browser page and go to: https://coeus.itap.purdue.edu/coeus/userauthaction.do 2. Enter your Career Account User Name in the USERNAME
IPFW CoeusLite Online IRB Process CoeusLite IRB Login 1. Open a web browser page and go to: https://coeus.itap.purdue.edu/coeus/userauthaction.do 2. Enter your Career Account User Name in the USERNAME
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
DISCLOSER GUIDE. Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure. Submitting Disclosures Post-Submission Actions
 DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
Maximo Self Service Center
 Maximo Self Service Center Once you have received an email regarding your registration approval, go to the following web address: https://maximo.mysodexo.com Log in to the Self Service Center: Your User
Maximo Self Service Center Once you have received an email regarding your registration approval, go to the following web address: https://maximo.mysodexo.com Log in to the Self Service Center: Your User
Animal Protocol Development Instructions for Researchers
 Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Secure Source to Pay (SS2P) Sourcing Administration December 2017
 Secure Source to Pay (SS2P) Sourcing Administration December 2017 1 Contents 1 Overview... 3 Access the Admin Center... 3 Side Navigation... 3 To search for a Supplier... 3 Supplier Account Maintenance...
Secure Source to Pay (SS2P) Sourcing Administration December 2017 1 Contents 1 Overview... 3 Access the Admin Center... 3 Side Navigation... 3 To search for a Supplier... 3 Supplier Account Maintenance...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
Reference Card for ORSP
 Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Core Staff Review Approve with Contingencies
 Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Payment Calculator Data Pages
 Payment Calculator Data Pages The Data Pages are a feature housed within the Strategic Sourcing module. They contain information about project parameters taken directly from the application and are used
Payment Calculator Data Pages The Data Pages are a feature housed within the Strategic Sourcing module. They contain information about project parameters taken directly from the application and are used
IRBNet for IACUC The Electronic Platform for Submission and Processing of Animals Study Documents
 IRBNet for IACUC The Electronic Platform for Submission and Processing of Animals Study Documents Please see the attached information regarding the submission of your protocol via IRBNet for IACUC. NOTE:
IRBNet for IACUC The Electronic Platform for Submission and Processing of Animals Study Documents Please see the attached information regarding the submission of your protocol via IRBNet for IACUC. NOTE:
eprotocol Investigator Role Manual
 eprotocol Investigator Role Manual Table of Contents Table of Contents Auto-Population of Stored User Data...17 5.4 Module Specific Work Flow Sections...18 1 Overview...4 1.1 Things to Remember...4 2 Creating
eprotocol Investigator Role Manual Table of Contents Table of Contents Auto-Population of Stored User Data...17 5.4 Module Specific Work Flow Sections...18 1 Overview...4 1.1 Things to Remember...4 2 Creating
Introduction to a-tune Jen Walker, Assistant to the Attending Veterinarian Cara Fink, IACUC Post-Approval Monitor
 Introduction to a-tune tick@lab Jen Walker, Assistant to the Attending Veterinarian Cara Fink, IACUC Post-Approval Monitor What we will cover General overview Logging-in and orientation of software Creating
Introduction to a-tune tick@lab Jen Walker, Assistant to the Attending Veterinarian Cara Fink, IACUC Post-Approval Monitor What we will cover General overview Logging-in and orientation of software Creating
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Customs & Remarks tab... 7 Review & Complete tab 8 Submit Booking. 8 Booking Received tab.. 9 Disclaimer... 9
 Quick Start Guide Introduction 1 New Booking Request.. 2 Contact and Reference tab 2 Contract & Quotation tab. 3 Routing & Schedule tab (page1) 4 Routing & Schedule tab (page2) 5 Cargo & Equipment tab
Quick Start Guide Introduction 1 New Booking Request.. 2 Contact and Reference tab 2 Contract & Quotation tab. 3 Routing & Schedule tab (page1) 4 Routing & Schedule tab (page2) 5 Cargo & Equipment tab
IRBNet Instructions for IBC, SCRO, IACUC Committees v
 Stony Brook University IRBNet Instructions for IBC, SCRO, IACUC Committees v. 04.23.14 Introduction: This document will explain, step by step, how to upload the required forms and documents you need to
Stony Brook University IRBNet Instructions for IBC, SCRO, IACUC Committees v. 04.23.14 Introduction: This document will explain, step by step, how to upload the required forms and documents you need to
Guide for Researchers: Online Human Ethics Application Form
 Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Researcher User Manual
 Researcher User Manual Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: November 24, 2017 Checkpoint *PLEASE NOTE* Prior to leveraging
Researcher User Manual Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: November 24, 2017 Checkpoint *PLEASE NOTE* Prior to leveraging
eprotocol - Protocol Management System (PMS) Investigator User Guide Version 2.0
 eprotocol - Protocol Management System (PMS) Investigator User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Investigator User Guide 2 Table of Contents 1. INTRODUCTION
eprotocol - Protocol Management System (PMS) Investigator User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Investigator User Guide 2 Table of Contents 1. INTRODUCTION
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
Guide for Researchers: Online Human Ethics Application Form
 Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event
 Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
IACUC PROTOCOL REVIEW
 IACUC PROTOCOL REVIEW Last Updated: 1/28/2016 Contents Accessing a Protocol for Review... 2 1) Log In To Topaz Elements... 2 2) Open Protocol... 3 Reviewing and Commenting... 6 1) Review Each Section Of
IACUC PROTOCOL REVIEW Last Updated: 1/28/2016 Contents Accessing a Protocol for Review... 2 1) Log In To Topaz Elements... 2 2) Open Protocol... 3 Reviewing and Commenting... 6 1) Review Each Section Of
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE
 Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
eirb Training Investigators/Research Coordinators Topics Covered:
 eirb Training Investigators/Research Coordinators Topics Covered: 1. Home Page 2. My IRB Protocols 3. Completing New Protocols 4. Amendments & Continuing Reviews 5. Submitting a Protocol 6. Certifying
eirb Training Investigators/Research Coordinators Topics Covered: 1. Home Page 2. My IRB Protocols 3. Completing New Protocols 4. Amendments & Continuing Reviews 5. Submitting a Protocol 6. Certifying
Researcher User Manual
 Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
Logging in, Navigating, & Finding your study
 Logging in, Navigating, & Finding your study A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Accessing ROMEO Logging in to ROMEO Home Page: Profile Contact Us Apply New
Logging in, Navigating, & Finding your study A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Accessing ROMEO Logging in to ROMEO Home Page: Profile Contact Us Apply New
Resource Account Instructions
 Resource Account Instructions Contents: click to skip to a section Managing a Resource Email Account in Outlook Overview of Resource Accounts for Owners... 2 Email Retention and Archives... 2 Accessing
Resource Account Instructions Contents: click to skip to a section Managing a Resource Email Account in Outlook Overview of Resource Accounts for Owners... 2 Email Retention and Archives... 2 Accessing
Module 1: Core Committee Staff Basics
 Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
Forms Management Walkthrough
 Forms Management Walkthrough Creating a paperless organization is easy in OrgSync - take all your forms online using the Form builder! The Forms tool can be used for a variety of purposes and the easy-to-use
Forms Management Walkthrough Creating a paperless organization is easy in OrgSync - take all your forms online using the Form builder! The Forms tool can be used for a variety of purposes and the easy-to-use
IBC Reviewer User Guide
 IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
PI & Project Team. Proposal Management. Create a PAF: Basics. Step-By-Step Procedure
 Create a PAF: Basics This procedure details how to: Create a PAF: the fields that must be completed in order to create a PAF. Hide/Show Errors: the function that allows you to view a list of fields that
Create a PAF: Basics This procedure details how to: Create a PAF: the fields that must be completed in order to create a PAF. Hide/Show Errors: the function that allows you to view a list of fields that
ADP Vantage HCM: Navigation for Time Managers
 ADP Vantage HCM: General Navigation 2 Login Page 2 Basic User Interface 3 Privacy, Legal, Feedback, Copyright 3 Preferences, Support, Logout 4 Menus and Activity Options 8 Messages, Calendar, My Profile
ADP Vantage HCM: General Navigation 2 Login Page 2 Basic User Interface 3 Privacy, Legal, Feedback, Copyright 3 Preferences, Support, Logout 4 Menus and Activity Options 8 Messages, Calendar, My Profile
MCES Industrial Online Reporting System Annual Statement Submittal Instructions for Dental Offices
 MCES Industrial Online Reporting System Annual Statement Submittal Instructions for Dental Offices MCES Industrial Online Reporting System (IORS) Annual Statement Log into the MCES Industrial Online Reporting
MCES Industrial Online Reporting System Annual Statement Submittal Instructions for Dental Offices MCES Industrial Online Reporting System (IORS) Annual Statement Log into the MCES Industrial Online Reporting
Supplier User Guide for Responding to Atlantic Lottery Tender Opportunities
 Supplier User Guide for Responding to Atlantic Lottery Tender Opportunities Version 4 Released November 2017 TABLE OF CONTENTS OVERVIEW... 3 Help & Support... 3 Definitions... 3 GENERAL FLOW OF SUPPLIER
Supplier User Guide for Responding to Atlantic Lottery Tender Opportunities Version 4 Released November 2017 TABLE OF CONTENTS OVERVIEW... 3 Help & Support... 3 Definitions... 3 GENERAL FLOW OF SUPPLIER
Oracle. Service Cloud Knowledge Advanced User Guide
 Oracle Service Cloud Release November 2016 Oracle Service Cloud Part Number: E80589-02 Copyright 2015, 2016, Oracle and/or its affiliates. All rights reserved Authors: The Knowledge Information Development
Oracle Service Cloud Release November 2016 Oracle Service Cloud Part Number: E80589-02 Copyright 2015, 2016, Oracle and/or its affiliates. All rights reserved Authors: The Knowledge Information Development
IRBNet User Manual IACUC
 IRBNet User Manual IACUC Version 5/29/2012 1 User Manual This user manual is designed to assist in the use of IRBNet. You will find assistance in registration, submission, continuing review, amendments
IRBNet User Manual IACUC Version 5/29/2012 1 User Manual This user manual is designed to assist in the use of IRBNet. You will find assistance in registration, submission, continuing review, amendments
Quick guide to the SmartSimple on-line portal (making an application)
 EPA Research Programme 2014-2020 Quick guide to the SmartSimple on-line portal (making an application) POWERED BY SMARTSIMPLE Disclaimer Please read this document carefully prior to using the on-line portal.
EPA Research Programme 2014-2020 Quick guide to the SmartSimple on-line portal (making an application) POWERED BY SMARTSIMPLE Disclaimer Please read this document carefully prior to using the on-line portal.
University of the Sciences. IRBNet User s Guide
 University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
What is A web-based protocol submission system for IACUC and IRB protocols
 What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
Travel Activities Creating a Cash Advance
 Travel Activities Creating a Cash Advance Overview: Understanding How to Create a Cash Advance In this topic, you will learn the steps to create a cash advance. OMNI Security Role Required: FSU_TE_TRAVEL_REP
Travel Activities Creating a Cash Advance Overview: Understanding How to Create a Cash Advance In this topic, you will learn the steps to create a cash advance. OMNI Security Role Required: FSU_TE_TRAVEL_REP
Coeus IACUC Administrative Process Deactivate Protocols
 Coeus IACUC Administrative Process Deactivate Protocols This action can be initiated by the PI or anyone who obtains the Aggregator Role on the protocol record. There are also 2 ways this action can be
Coeus IACUC Administrative Process Deactivate Protocols This action can be initiated by the PI or anyone who obtains the Aggregator Role on the protocol record. There are also 2 ways this action can be
PROST USER GUIDE FOR VENDORS
 PROST USER GUIDE FOR VENDORS Revised 06/18/2018 Introduction Setup Welcome Email Account Verification Vendor Login Preferences Services Provided Voucher Voucher Inbox Invoice Inbox Security My Profile
PROST USER GUIDE FOR VENDORS Revised 06/18/2018 Introduction Setup Welcome Email Account Verification Vendor Login Preferences Services Provided Voucher Voucher Inbox Invoice Inbox Security My Profile
and Systems Process April 2009
 NAVAIR's Process Asset Library (PAL) for Software and Systems Process Assets Prepared for 20-24 April 2009 Approved for Public Release by NAVAIR Public Affairs Office 16 MAR 2009 Topics Purpose Design
NAVAIR's Process Asset Library (PAL) for Software and Systems Process Assets Prepared for 20-24 April 2009 Approved for Public Release by NAVAIR Public Affairs Office 16 MAR 2009 Topics Purpose Design
Macquarie Research Centres (MQRC) Online Annual Progress Report
 Macquarie Research Centres (MQRC) Online Annual Progress Report USER GUIDE This guide is provided for Researchers and admin assistant submitting MQRC Annual Progress Report Online Version 1.0 Prepared
Macquarie Research Centres (MQRC) Online Annual Progress Report USER GUIDE This guide is provided for Researchers and admin assistant submitting MQRC Annual Progress Report Online Version 1.0 Prepared
Delegating Access & Managing Another Person s Mail/Calendar with Outlook. Information Technology
 Delegating Access & Managing Another Person s Mail/Calendar with Outlook Information Technology 1. Click the File tab 2. Click Account Settings, and then click Delegate Access 3. Click Add 4. Type the
Delegating Access & Managing Another Person s Mail/Calendar with Outlook Information Technology 1. Click the File tab 2. Click Account Settings, and then click Delegate Access 3. Click Add 4. Type the
Quality Notes QNO Role Guide March 2018
 Quality Notes QNO Role Guide March 2018 Copyright 2018 Exostar, LLC All rights reserved. 1 Contents Introduction... 3 About the QN Originator (QNO) Role... 3 Search... 3 Using Search to Locate Quality
Quality Notes QNO Role Guide March 2018 Copyright 2018 Exostar, LLC All rights reserved. 1 Contents Introduction... 3 About the QN Originator (QNO) Role... 3 Search... 3 Using Search to Locate Quality
ActivePay CARDHOLDER GUIDE
 ActivePay CARDHOLDER GUIDE PNC - ACTIVEPAY CARDHOLDER TRAINING MANUAL TABLE OF CONTENTS Accessing the PNC ActivePay Web Application 2 Self Registration for Cardholders.. 3 Forgotten Username or Password
ActivePay CARDHOLDER GUIDE PNC - ACTIVEPAY CARDHOLDER TRAINING MANUAL TABLE OF CONTENTS Accessing the PNC ActivePay Web Application 2 Self Registration for Cardholders.. 3 Forgotten Username or Password
2 Requests for Changes. About Requests for Changes
 2 Requests for Changes About Requests for Changes Requests for changes are made when you want to change the details of a project (research expenses or project members) after the project has been adopted.
2 Requests for Changes About Requests for Changes Requests for changes are made when you want to change the details of a project (research expenses or project members) after the project has been adopted.
IRIS Quick guide to the portal for Orphan Industry users
 28 June 2018 EMA/444925/2018 Information Management Division IRIS Quick guide to the portal for Orphan Industry users Version 1.3 1. Purpose and context... 2 1.1. Purpose of this Quick Guide... 2 1.2.
28 June 2018 EMA/444925/2018 Information Management Division IRIS Quick guide to the portal for Orphan Industry users Version 1.3 1. Purpose and context... 2 1.1. Purpose of this Quick Guide... 2 1.2.
ScholarOne Manuscripts. Editor User Guide
 ScholarOne Manuscripts Editor User Guide 18-June-2018 Clarivate Analytics ScholarOne Manuscripts Editor User Guide Page i TABLE OF CONTENTS INTRODUCTION... 1 Use Get Help Now and FAQs... 1 Site Configuration
ScholarOne Manuscripts Editor User Guide 18-June-2018 Clarivate Analytics ScholarOne Manuscripts Editor User Guide Page i TABLE OF CONTENTS INTRODUCTION... 1 Use Get Help Now and FAQs... 1 Site Configuration
Citizen Self Service Portal Guide to Online Permits
 Citizen Self Service Portal Guide to Online Permits The City has transitioned to a new online permit system. Citizen Self Service (CSS) is a web portal offering contractors a convenient way of conducting
Citizen Self Service Portal Guide to Online Permits The City has transitioned to a new online permit system. Citizen Self Service (CSS) is a web portal offering contractors a convenient way of conducting
istar User Reference Guide
 istar User Reference Guide Last Updated: 12/21/17 Table of Contents Questions and Issues 1 Getting Started 2 Dashboard 2 New Applications 4 Main Committee Page 5 Main Workspace page 6 Edit/View Application
istar User Reference Guide Last Updated: 12/21/17 Table of Contents Questions and Issues 1 Getting Started 2 Dashboard 2 New Applications 4 Main Committee Page 5 Main Workspace page 6 Edit/View Application
UNIVERSITY OF PENNSYLVANIA
 UNIVERSITY OF PENNSYLVANIA Research Inventory System- Subaward Generator Module (RIS-SGM) August 2013 This document contains a user instruction guide for the creation and submission of subaward requests.
UNIVERSITY OF PENNSYLVANIA Research Inventory System- Subaward Generator Module (RIS-SGM) August 2013 This document contains a user instruction guide for the creation and submission of subaward requests.
FIELDS REQUIRED TO SAVE A PROTOCOL
 Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
NQF ONLINE MEASURE SUBMISSION FORM USERS GUIDE
 NQF ONLINE MEASURE SUBMISSION FORM USERS GUIDE VERSION 1.1 Guide Version 1.0 01/11 TABLE OF CONTENTS PART 1: TECHNICAL SUPPORT FOR SUBMISSION FORM TABLE OF CONTENTS... CREATING AN INDIVIDUAL ACCOUNT...
NQF ONLINE MEASURE SUBMISSION FORM USERS GUIDE VERSION 1.1 Guide Version 1.0 01/11 TABLE OF CONTENTS PART 1: TECHNICAL SUPPORT FOR SUBMISSION FORM TABLE OF CONTENTS... CREATING AN INDIVIDUAL ACCOUNT...
What s New in FootPrints11.5
 What s New in FootPrints11.5 Administrator Guide & User Manual University of New Brunswick UNB FootPrints Acceptable Use and Best Practices Table of Contents Note: items in italics indicate administrator
What s New in FootPrints11.5 Administrator Guide & User Manual University of New Brunswick UNB FootPrints Acceptable Use and Best Practices Table of Contents Note: items in italics indicate administrator
Protocol Management System (PMS) Investigator User Guide. Harris Health System Administrative Approval
 Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Steps on How to Create Cash Advances
 FAST Travel System Guide: How to Create Cash Advances Purpose: Definition: Navigation: Notes: To provide instructions on how to create Cash Advances (CAs) in the FAST Travel module. Cash Advance - In some
FAST Travel System Guide: How to Create Cash Advances Purpose: Definition: Navigation: Notes: To provide instructions on how to create Cash Advances (CAs) in the FAST Travel module. Cash Advance - In some
Information Sheet for Scientific Approvers
 1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
REUTERS/Tim Wimborne SCHOLARONE MANUSCRIPTS TM EDITOR USER GUIDE
 REUTERS/Tim Wimborne SCHOLARONE MANUSCRIPTS TM EDITOR USER GUIDE 21-JUNE-2016 TABLE OF CONTENTS Select an item in the table of contents to go to that topic in the document. INTRODUCTION... 1 USE GET HELP
REUTERS/Tim Wimborne SCHOLARONE MANUSCRIPTS TM EDITOR USER GUIDE 21-JUNE-2016 TABLE OF CONTENTS Select an item in the table of contents to go to that topic in the document. INTRODUCTION... 1 USE GET HELP
HCM Instructions. Tip: Use the Magnifying Glass to search for values. Click the Add button to start a new request. 2/5/16 Page 1 of 8
 Creating Security Request Form Log in to the My BGSU portal and select Security Request under MISC SERVICES within the Employees tab. You will be redirected to the Security Request launch page within the
Creating Security Request Form Log in to the My BGSU portal and select Security Request under MISC SERVICES within the Employees tab. You will be redirected to the Security Request launch page within the
About the To-Do Bar in Outlook 2007
 Tasks in the Microsoft Office system are similar to a to-do list. Tasks make it easy to use Microsoft Office Outlook 007 to organize your time and your work. Tasks are integrated across Outlook 007, Microsoft
Tasks in the Microsoft Office system are similar to a to-do list. Tasks make it easy to use Microsoft Office Outlook 007 to organize your time and your work. Tasks are integrated across Outlook 007, Microsoft
The Center for Translational Medicine at UT Southwestern Medical Center. The Research Launchpad: Your One-Stop Shop to CTSA Resources and Services
 The Center for Translational Medicine at UT Southwestern Medical Center The Research Launchpad: Your One-Stop Shop to CTSA Resources and Services Users Guide Table of Contents a. Introduction b. Where
The Center for Translational Medicine at UT Southwestern Medical Center The Research Launchpad: Your One-Stop Shop to CTSA Resources and Services Users Guide Table of Contents a. Introduction b. Where
Employment Ontario Information System (EOIS) Case Management System Service Provider User Guide
 Employment Ontario Information System (EOIS) Case Management System Service Provider User Guide Chapter 8C: Service Plan Management for the Canada- Ontario Job Grant Version 2.1July 2018 Table of Contents
Employment Ontario Information System (EOIS) Case Management System Service Provider User Guide Chapter 8C: Service Plan Management for the Canada- Ontario Job Grant Version 2.1July 2018 Table of Contents
elp (Blackboard) Blackboard Spaces
 elp (Blackboard) Blackboard Spaces O Overview Blackboard spaces is a social area on Blackboard. It can be used to share content for collaborative working. This help guide will show you how to setup a profile,
elp (Blackboard) Blackboard Spaces O Overview Blackboard spaces is a social area on Blackboard. It can be used to share content for collaborative working. This help guide will show you how to setup a profile,
19_Amend Supplier Contract
 19_Amend Supplier Contract Purpose: How to Access: Audience: The purpose of this task is to create an amendment to an approved Supplier Contract. Enter Create Supplier Contract Amendment in the search
19_Amend Supplier Contract Purpose: How to Access: Audience: The purpose of this task is to create an amendment to an approved Supplier Contract. Enter Create Supplier Contract Amendment in the search
Click For Industry Professionals From the toolbar click Regulatory Filing & Reporting Under Related Links, click Entitlement Program
 Table of Contents Overview of the Advertising Regulation Electronic Files (AREF) System... 1 Getting Started Entitlement... 1 AREF Users... 2 Adding Submit an Advertisement to My Quicklinks... 2 The AREF
Table of Contents Overview of the Advertising Regulation Electronic Files (AREF) System... 1 Getting Started Entitlement... 1 AREF Users... 2 Adding Submit an Advertisement to My Quicklinks... 2 The AREF
E-RATE PRODUCTIVITY CENTER (EPC)
 Last Modified: June 2015 E-RATE PRODUCTIVITY CENTER (EPC) Filing FCC Forms 470 Before you Begin Before you start creating an FCC Form 470 in EPC, you should be familiar with the eligibility rules and filing
Last Modified: June 2015 E-RATE PRODUCTIVITY CENTER (EPC) Filing FCC Forms 470 Before you Begin Before you start creating an FCC Form 470 in EPC, you should be familiar with the eligibility rules and filing
ARIBA Supplier Information Management (SIM) Supplier User Guide
 ARIBA Supplier Information Management (SIM) Supplier User Guide Thomson Reuters are unable to review and process your company as a supplier until the registration and subsequent questionnaire are completed.
ARIBA Supplier Information Management (SIM) Supplier User Guide Thomson Reuters are unable to review and process your company as a supplier until the registration and subsequent questionnaire are completed.
Tools and Navigation. Navigating the Main Landing Page
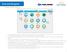 Navigating the Main Landing Page 3 4 6 2 5 1 7 1. On the Workday landing page, you will find worklets that provide access to tasks and reports. Click on any icon to view your available options. 2. Your
Navigating the Main Landing Page 3 4 6 2 5 1 7 1. On the Workday landing page, you will find worklets that provide access to tasks and reports. Click on any icon to view your available options. 2. Your
