IACUC Researcher s Quick Reference
|
|
|
- Caroline Glenn
- 5 years ago
- Views:
Transcription
1 IACUC Researcher s Quick Reference
2 Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where do I find? From My Inbox, you can find:. Submissions that require you to take action. Your research teams 3. Actions you can perform such as create a protocol What do I do? 3. Review the state of submissions in your inbox. The state gives a clue as to what to do next. For example, Pre-Submission means you haven t submitted the protocol. You can finish and submit it for review. 4 Open a Submission 5. From your inbox, click the submission name The submission workspace opens. View History 4. From the submission workspace, click the History tab. 5. The history tab lists the activity taken on a submission including any comments, attachments, or correspondence added. 7 8
3 9 0 Find Previous Submissions 6. On the left, click Submissions. 7. Click the appropriate tab to see your submissions: In-Review: All submissions undergoing IACUC review Active: All approved submissions Archived: All discarded and closed submissions All Submissions: All submissions, in any state To find specific data, see Filter Data. Filter Data Many pages contain tables that you can filter to show specific data. 8. Select the column to filter. 9. Type the beginning characters for the items you want to find. You can also type a % symbol as a wildcard before the characters. Examples: 7 shows all items beginning with 7 %7% shows all items containing 7 0. Click the Help icon to see explanation about search capabilities.. Click Go to apply the filter To combine multiple filter criteria, click Add Filter.
4 Before You Create a Protocol Plan out your protocol: Summarize the research (science), reasons for performing the research, and benefits from it. Determine the experiments and the number of animals needed. Determine the procedures you will perform and the substances needed for your experiments. Identify any supporting documents to include in your protocol, such as flowcharts, explanations of the science, grant applications, and other information explaining or justifying your research. Set up building blocks: The diagram below shows the concept of building blocks. Once they are set up, you can create your protocol. Create a research team for the substances, procedures, and protocols you create. Check the IACUC standard library for the procedures and substances needed for your protocol experiments (see Check for Building Blocks). If not in the library: Create the missing substances Create the missing procedures s & Procedures Library Research Team s Procedures s Procedures Protocols Create substances Create procedures* *Team procedures can have one or more team or standard substances Create protocols with experiments* *Experiments can have one or more team or standard procedures Protocol Experiment Procedure Procedure Procedure
5 Create a Research Team As a member of a research team, you can create substances, procedures, and protocols for your research team. When you create a protocol, your research team members appear on the protocol by default. Create Research Team 3. From My Inbox, click Create Research Team. 4. Type a name for the research team and select the team s PI. Note: If you are not the PI, add yourself as a team member if you will create team procedures and substances Complete the rest of the page and click Finish. You are taken to the research team workspace page. Check for Existing Building Blocks Check the system to see if there are IACUC standard building blocks that you can use in your protocols. Check for Building Blocks. From My Inbox, click your research team on the left. 3. In the research team workspace, click the Procedures tab and check for the procedures you need. Do the same for substances (on s tab). If missing, see Create Building Blocks. 3a 3b 3. If the building block exists, review its details: a. On the Procedures or s tab, click the item name. b. In the item s workspace, click View Procedure or View. *If minor changes are needed, you can copy the selected procedure and edit it. Refer to the Create a Protocol Procedure for guidance on the IACUC website.
6 Create Building Blocks After you have checked the system for IACUC standard building blocks, create any that are required for your protocols. Create a or Procedure. From the research team workspace, click Create or Create Procedure. Note: Create the substances you will use in your procedures before creating the procedures. 3. Complete the pages. If more than one page, click Continue to move to the next page. 3. When done, click Finish. You are taken to the research team s substance or procedure workspace page.
7 Create and Submit a Protocol Once all the building blocks are set up, you are ready to create your protocol. Create a Protocol. From the research team workspace or My Inbox page, click Create Protocol.. Complete the pages. Click Continue to move to the next page. 3a 3b 3. Pay attention to the following pages: a. Protocol Team Members: Add each person who will edit the protocol. Do not add the PI here. b. Experiments: When you add an experiment, select the procedures that apply to all animals (common procedures) and those that apply to some animals or are used differently across animals (variable procedures) On the final page, click Finish. You are taken to the protocol workspace page. You can continue to edit the protocol (Edit Protocol button) until you submit it for review. Submit a Protocol for Review 5. From the protocol workspace, click Submit Click OK to agree to the statement and submit the protocol for review. You can log off the system (top right).
8 Respond to Reviewer Requests If a reviewer has questions or requests you to revise/change your submission, you will receive an indicating this. Review the request details and then respond to the request. Review the Request Details. Click the link to open the submission. If you no longer have the , see Open a Submission and then View History to see reviewer comments. 3. On the History tab, find the Clarification by Requested activity and read the comments. 3. If the reviewer added reviewer notes, click the Reviewer Notes tab and go to Respond to Reviewer Notes. If not, go to Submit Response. Respond to Reviewer Notes For each reviewer note that requires a response: 4. To edit the protocol in response to the reviewer note, click the Jump To link. 5. From the protocol page or the Reviewer Notes tab, click the Click here to respond link Select a response and explain your response in the text box Click OK. If on a protocol page, exit the protocol when done. Go to Submit Response. Submit Response 8. On the protocol workspace, click Submit Response. 9. In the Comments box, explain your response to the reviewer. 0. Click OK. You can log off the system (top right).
9 Create and Submit a Follow-On Submission (Amendment/Annual Review) If you need to make changes to an approved protocol or submit an annual review, follow these steps. Create a Follow-On Submission. From My Inbox, click the research team on the left.. Select the name of the approved protocol. 3. On the left, click the Create button. 4. Complete the pages. Click Continue to move to the next page. 5. When done, click Exit and save changes or Finish on the final page. You are taken to the submission s workspace page. You can continue to edit the submission ( Edit button) until you submit it for review Submit Follow-On Submission for Review 6. From the follow-on submission s workspace, click Submit. 7. Click OK to agree to the statement and submit it for review. You can log off the system (top right). 6 7
10
Click IACUC Researcher s Quick Reference
 Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond
Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond
eiacuc Reference for PIs and Research Team
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
eiacuc Reference for Veterinarians
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
Procedural Amendment
 Creating an Amendment in the eacuc system differs depending on what type of amendment you are submitting. Procedural Amendment Adding New Procedures to an Approved Protocol: If you are adding a brand new
Creating an Amendment in the eacuc system differs depending on what type of amendment you are submitting. Procedural Amendment Adding New Procedures to an Approved Protocol: If you are adding a brand new
IACUC Module. March 2018
 z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
How Does the PI make Pre Review ICF Changes?
 How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
eresearch IBC Staff Review Step-by-Step Procedures Core Committee Staff Home Page
 1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
Submitting an Protocol for De Novo Review
 Submitting an Protocol for De Novo Review Important: A protocol can be created, edited and submitted by the Principal Investigator (PI), Laboratory Contact or Alternate Lab Contact. However, ONLY the Principal
Submitting an Protocol for De Novo Review Important: A protocol can be created, edited and submitted by the Principal Investigator (PI), Laboratory Contact or Alternate Lab Contact. However, ONLY the Principal
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Introduction to ARFMS. (Animal Research Facility Management Software)
 Introduction to ARFMS (Animal Research Facility Management Software) Agenda 1. Overview Existing active protocols Course Access Users & Teams IACUC Viewing of AUPs assigned 2. Creation of New AUP Filling
Introduction to ARFMS (Animal Research Facility Management Software) Agenda 1. Overview Existing active protocols Course Access Users & Teams IACUC Viewing of AUPs assigned 2. Creation of New AUP Filling
Click. IRB Study Submission Guide
 Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
and Systems Process April 2009
 NAVAIR's Process Asset Library (PAL) for Software and Systems Process Assets Prepared for 20-24 April 2009 Approved for Public Release by NAVAIR Public Affairs Office 16 MAR 2009 Topics Purpose Design
NAVAIR's Process Asset Library (PAL) for Software and Systems Process Assets Prepared for 20-24 April 2009 Approved for Public Release by NAVAIR Public Affairs Office 16 MAR 2009 Topics Purpose Design
Module 3: Changes and Correspondence
 Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Contents. TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs
 TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
ARC IRB Chair s Manual
 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
EPS Electronic Paper System CIMAC Congress 2019
 Manual Author ABSTRACT PHASE EPS Electronic Paper System CIMAC Congress 2019 IMPORTANT: For best experience please work with the following browsers in using this software: Latest Versions of Mozilla Firefox
Manual Author ABSTRACT PHASE EPS Electronic Paper System CIMAC Congress 2019 IMPORTANT: For best experience please work with the following browsers in using this software: Latest Versions of Mozilla Firefox
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
How to Submit a New Application
 ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment)
 Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Introduction to a-tune Jen Walker, Assistant to the Attending Veterinarian Cara Fink, IACUC Post-Approval Monitor
 Introduction to a-tune tick@lab Jen Walker, Assistant to the Attending Veterinarian Cara Fink, IACUC Post-Approval Monitor What we will cover General overview Logging-in and orientation of software Creating
Introduction to a-tune tick@lab Jen Walker, Assistant to the Attending Veterinarian Cara Fink, IACUC Post-Approval Monitor What we will cover General overview Logging-in and orientation of software Creating
DISCLOSER GUIDE. Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure. Submitting Disclosures Post-Submission Actions
 DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
DISCLOSER GUIDE Getting Started Creating an FCOE Disclosure Creating an Outside Activity Disclosure Submitting Disclosures Post-Submission Actions Contents Welcome!...3 Section One: Let s Get Started...3
Creating a New Application. A ROMEO Hasty Handbook Presented by the CHEO Research Institute
 Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Creating a New Application A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Home Page Apply New New Applications List Navigation Notes Project Info Project Team Info Application
Click IRB 7.3 IRB Ancillary Reviewer s Guide
 Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Core Staff Review Approve with Contingencies
 Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
istar User Reference Guide
 istar User Reference Guide Last Updated: 12/21/17 Table of Contents Questions and Issues 1 Getting Started 2 Dashboard 2 New Applications 4 Main Committee Page 5 Main Workspace page 6 Edit/View Application
istar User Reference Guide Last Updated: 12/21/17 Table of Contents Questions and Issues 1 Getting Started 2 Dashboard 2 New Applications 4 Main Committee Page 5 Main Workspace page 6 Edit/View Application
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Information Sheet for Scientific Approvers
 1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
Animal Protocol Development Instructions for Researchers
 Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
About the To-Do Bar in Outlook 2007
 Tasks in the Microsoft Office system are similar to a to-do list. Tasks make it easy to use Microsoft Office Outlook 007 to organize your time and your work. Tasks are integrated across Outlook 007, Microsoft
Tasks in the Microsoft Office system are similar to a to-do list. Tasks make it easy to use Microsoft Office Outlook 007 to organize your time and your work. Tasks are integrated across Outlook 007, Microsoft
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
Tools and Navigation. Navigating the Main Landing Page
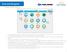 Navigating the Main Landing Page 3 4 6 2 5 1 7 1. On the Workday landing page, you will find worklets that provide access to tasks and reports. Click on any icon to view your available options. 2. Your
Navigating the Main Landing Page 3 4 6 2 5 1 7 1. On the Workday landing page, you will find worklets that provide access to tasks and reports. Click on any icon to view your available options. 2. Your
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
IACUC PROTOCOL REVIEW
 IACUC PROTOCOL REVIEW Last Updated: 1/28/2016 Contents Accessing a Protocol for Review... 2 1) Log In To Topaz Elements... 2 2) Open Protocol... 3 Reviewing and Commenting... 6 1) Review Each Section Of
IACUC PROTOCOL REVIEW Last Updated: 1/28/2016 Contents Accessing a Protocol for Review... 2 1) Log In To Topaz Elements... 2 2) Open Protocol... 3 Reviewing and Commenting... 6 1) Review Each Section Of
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
Forms Management Walkthrough
 Forms Management Walkthrough Creating a paperless organization is easy in OrgSync - take all your forms online using the Form builder! The Forms tool can be used for a variety of purposes and the easy-to-use
Forms Management Walkthrough Creating a paperless organization is easy in OrgSync - take all your forms online using the Form builder! The Forms tool can be used for a variety of purposes and the easy-to-use
eprotocol - Protocol Management System (PMS) Investigator User Guide Version 2.0
 eprotocol - Protocol Management System (PMS) Investigator User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Investigator User Guide 2 Table of Contents 1. INTRODUCTION
eprotocol - Protocol Management System (PMS) Investigator User Guide Version 2.0 Last Updated: 05/18/2011 Product Version: 2.0.16 eprotocol - PMS - Investigator User Guide 2 Table of Contents 1. INTRODUCTION
Module 1: Core Committee Staff Basics
 Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
Blackboard Help Topic
 Blackboard Help Topic Walden Blackboard Help Topic: How to Submit an Assignment Walden 2012 Welcome to the How to Submit an Assignment demonstration. After you have completed this demonstration, you will
Blackboard Help Topic Walden Blackboard Help Topic: How to Submit an Assignment Walden 2012 Welcome to the How to Submit an Assignment demonstration. After you have completed this demonstration, you will
Ohio Child Licensing and Quality System (OCLQS) SUTQ Change Rating Registration Process
 Ohio Child Licensing and Quality System (OCLQS) SUTQ Change Rating Registration Process My Program Activities Screen 1.Navigate to this screen by clicking: My Programs on the top of the page and select
Ohio Child Licensing and Quality System (OCLQS) SUTQ Change Rating Registration Process My Program Activities Screen 1.Navigate to this screen by clicking: My Programs on the top of the page and select
IRB Committee Member Adverse Event (AE) Single Member Review
 IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
Viveport Beta Testing Guide
 Viveport Beta Testing Guide Beta Testing: Test your titles before unveiling to consumers! [Note* - Red lines are optional description that might apply to first release. Green lines are description that
Viveport Beta Testing Guide Beta Testing: Test your titles before unveiling to consumers! [Note* - Red lines are optional description that might apply to first release. Green lines are description that
eprotocol Reviewer Role Manual
 eprotocol Reviewer Role Manual Table of Contents Table of Contents 1 Overview...4 1.1 Things to Remember... 4 2 Review Process...5 2.1 Reviewer Role Overview...5 2.2 Protocol Event Types...6 Assigned as
eprotocol Reviewer Role Manual Table of Contents Table of Contents 1 Overview...4 1.1 Things to Remember... 4 2 Review Process...5 2.1 Reviewer Role Overview...5 2.2 Protocol Event Types...6 Assigned as
IBC Reviewer User Guide
 IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Table of Contents. Manual for Principal Investigators + Lab Financial Administrators - Core Facilities Management System
 Table of Contents 1. Overview 2. My Homepage 3. How do I Manage My Lab Settings? a. How do I Set Projected Cost Amounts that Require My Approval? b. How do I Create Budgets and Monitor Spending? 4. Can
Table of Contents 1. Overview 2. My Homepage 3. How do I Manage My Lab Settings? a. How do I Set Projected Cost Amounts that Require My Approval? b. How do I Create Budgets and Monitor Spending? 4. Can
PI & Project Team. Proposal Management. Create a PAF: Basics. Step-By-Step Procedure
 Create a PAF: Basics This procedure details how to: Create a PAF: the fields that must be completed in order to create a PAF. Hide/Show Errors: the function that allows you to view a list of fields that
Create a PAF: Basics This procedure details how to: Create a PAF: the fields that must be completed in order to create a PAF. Hide/Show Errors: the function that allows you to view a list of fields that
IRBNet Instructions for IBC, SCRO, IACUC Committees v
 Stony Brook University IRBNet Instructions for IBC, SCRO, IACUC Committees v. 04.23.14 Introduction: This document will explain, step by step, how to upload the required forms and documents you need to
Stony Brook University IRBNet Instructions for IBC, SCRO, IACUC Committees v. 04.23.14 Introduction: This document will explain, step by step, how to upload the required forms and documents you need to
Travel Activities Creating a Cash Advance
 Travel Activities Creating a Cash Advance Overview: Understanding How to Create a Cash Advance In this topic, you will learn the steps to create a cash advance. OMNI Security Role Required: FSU_TE_TRAVEL_REP
Travel Activities Creating a Cash Advance Overview: Understanding How to Create a Cash Advance In this topic, you will learn the steps to create a cash advance. OMNI Security Role Required: FSU_TE_TRAVEL_REP
The IACUC is charged with the responsibility of ensuring that the Animal Welfare Act policies and procedures are followed.
 Protis is an online application system in support of research activities that require approval by the Institutional Animal Care and Use Committee (IACUC) or the Institutional Review Board (IRB). The IACUC
Protis is an online application system in support of research activities that require approval by the Institutional Animal Care and Use Committee (IACUC) or the Institutional Review Board (IRB). The IACUC
ecoi Department Reviewer User Guide
 ecoi Department Reviewer User Guide Click/Huron IRB XPress Using the Department Reviewer Workspace In This Section: Learn about the workspace Learning about the workspace Use the following picture to identify
ecoi Department Reviewer User Guide Click/Huron IRB XPress Using the Department Reviewer Workspace In This Section: Learn about the workspace Learning about the workspace Use the following picture to identify
Reference Card for ORSP
 Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
IACUC Proposal Entry Instructions
 IACUC Proposal Entry Instructions Version 1.0 October 10, 2016 Notes to Primary Investigator The following instructions are based on the IACUC Proposal form as currently found in iris. This instruction
IACUC Proposal Entry Instructions Version 1.0 October 10, 2016 Notes to Primary Investigator The following instructions are based on the IACUC Proposal form as currently found in iris. This instruction
How to Access If Rubrics does not appear on your course navbar, click Edit Course, Tools, Rubrics to activate..
 KODIAK QUICK GUIDE Rubrics Overview Rubrics allow you to establish set criteria for grading assignments; you can attach Rubrics to Dropbox folders or Discussion topics so that the criteria are available
KODIAK QUICK GUIDE Rubrics Overview Rubrics allow you to establish set criteria for grading assignments; you can attach Rubrics to Dropbox folders or Discussion topics so that the criteria are available
Workflow. Overview. Workflow Screen
 Workflow Overview The Workflow screen allows users to track content that has been sent for review through the approval process. The Workflow list view shows content that the current user has submitted
Workflow Overview The Workflow screen allows users to track content that has been sent for review through the approval process. The Workflow list view shows content that the current user has submitted
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event
 Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Kuali Research User Guide: Create a Protocol Amendment, Renewal or Event Version.0: November 06 Purpose: To create an amendment, renewal or event on an existing IRB Protocol document. Trigger / Timing
Maximo Self Service Center
 Maximo Self Service Center Once you have received an email regarding your registration approval, go to the following web address: https://maximo.mysodexo.com Log in to the Self Service Center: Your User
Maximo Self Service Center Once you have received an email regarding your registration approval, go to the following web address: https://maximo.mysodexo.com Log in to the Self Service Center: Your User
Create an Action Item
 Overview Action Items are created within animal use forms, amendments and protocols to notify a person or group of a related task. If the Action Item requires an amendment, the Action Item and amendment
Overview Action Items are created within animal use forms, amendments and protocols to notify a person or group of a related task. If the Action Item requires an amendment, the Action Item and amendment
Banner Security Access Request
 is a Web Form designed for Supervisors to submit Banner Access Requests for their employees. This online form replaces the previous paper form in a secure environment. This helps the Banner team respond
is a Web Form designed for Supervisors to submit Banner Access Requests for their employees. This online form replaces the previous paper form in a secure environment. This helps the Banner team respond
What s New in FootPrints11.5
 What s New in FootPrints11.5 Administrator Guide & User Manual University of New Brunswick UNB FootPrints Acceptable Use and Best Practices Table of Contents Note: items in italics indicate administrator
What s New in FootPrints11.5 Administrator Guide & User Manual University of New Brunswick UNB FootPrints Acceptable Use and Best Practices Table of Contents Note: items in italics indicate administrator
eprotocol Investigator Role Manual
 eprotocol Investigator Role Manual Table of Contents Table of Contents Auto-Population of Stored User Data...17 5.4 Module Specific Work Flow Sections...18 1 Overview...4 1.1 Things to Remember...4 2 Creating
eprotocol Investigator Role Manual Table of Contents Table of Contents Auto-Population of Stored User Data...17 5.4 Module Specific Work Flow Sections...18 1 Overview...4 1.1 Things to Remember...4 2 Creating
Arts & Humanities Faculty and Graduate Student Grants
 Arts & Humanities Faculty and Graduate Student Grants Instructions on how to work TITLE SLIDE GOES HERE with A&H grants using Optional subhead would go here DocuSign Power Forms 1 General Steps for submission:
Arts & Humanities Faculty and Graduate Student Grants Instructions on how to work TITLE SLIDE GOES HERE with A&H grants using Optional subhead would go here DocuSign Power Forms 1 General Steps for submission:
Online Labor Redistributions Entry Reference Guide
 Online Labor Redistributions Entry Reference Guide Contents: Submitting a Labor Redistribution... 2 Changing Multiple Pay Periods... 13 Additional Search Criteria... 17 Overview: The on-line labor redistribution
Online Labor Redistributions Entry Reference Guide Contents: Submitting a Labor Redistribution... 2 Changing Multiple Pay Periods... 13 Additional Search Criteria... 17 Overview: The on-line labor redistribution
ORGANIZATION OF AMERICAN STATES
 ORGANIZATION OF AMERICAN STATES OASES DESKTOP PROCEDURE PURCHASING MODULE CREATING PURCHASE ORDERS FOR THE SECRETARIAT FOR CONFERENCES AND MEETINGS ( SCM ) JANUARY 2004 Secretariat for Management Department
ORGANIZATION OF AMERICAN STATES OASES DESKTOP PROCEDURE PURCHASING MODULE CREATING PURCHASE ORDERS FOR THE SECRETARIAT FOR CONFERENCES AND MEETINGS ( SCM ) JANUARY 2004 Secretariat for Management Department
Instructor Feedback Location and Printing. Locating Instructor Feedback When Available within Canvas
 Instructor Feedback Location and Printing This document will identify the locations in Canvas where students may find instructor comments, feedback, inline editing, and rubric scores and comments. Also
Instructor Feedback Location and Printing This document will identify the locations in Canvas where students may find instructor comments, feedback, inline editing, and rubric scores and comments. Also
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
University of the Sciences. IRBNet User s Guide
 University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
6. To access the online IRB protocol module, click the "My IRB Protocol" link found in the top navigation of any CoeusLite screen
 IPFW CoeusLite Online IRB Process CoeusLite IRB Login 1. Open a web browser page and go to: https://coeus.itap.purdue.edu/coeus/userauthaction.do 2. Enter your Career Account User Name in the USERNAME
IPFW CoeusLite Online IRB Process CoeusLite IRB Login 1. Open a web browser page and go to: https://coeus.itap.purdue.edu/coeus/userauthaction.do 2. Enter your Career Account User Name in the USERNAME
What is A web-based protocol submission system for IACUC and IRB protocols
 What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
What is Tick@Lab? A web-based protocol submission system for IACUC and IRB protocols The system offers: o Available in one place 24/7 from anywhere o Smart forms available to complete your desired request
Logging in, Navigating, & Finding your study
 Logging in, Navigating, & Finding your study A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Accessing ROMEO Logging in to ROMEO Home Page: Profile Contact Us Apply New
Logging in, Navigating, & Finding your study A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: Accessing ROMEO Logging in to ROMEO Home Page: Profile Contact Us Apply New
Researcher User Manual
 Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
Researcher User Manual Post-Review Application Management Cloning Applications, Creating and Managing Events Audience: Principal Investigators & Project Team Members Updated: December 1, 2016 Checkpoint
Review Response Submission Form for Continuing Review Form
 Review Response Submission Form for Continuing Review Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it,
Review Response Submission Form for Continuing Review Form This form is used to reply to the IRB and address the issues/stipulations raised when the IRB conditionally approves the submission, defers it,
Responding to REB Feedback
 Responding to REB Feedback A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: What is REB Feedback? The REB Feedback Letter The Investigator Response Letter Finding the
Responding to REB Feedback A ROMEO Hasty Handbook Presented by the CHEO Research Institute Table of Contents: What is REB Feedback? The REB Feedback Letter The Investigator Response Letter Finding the
Payment Calculator Data Pages
 Payment Calculator Data Pages The Data Pages are a feature housed within the Strategic Sourcing module. They contain information about project parameters taken directly from the application and are used
Payment Calculator Data Pages The Data Pages are a feature housed within the Strategic Sourcing module. They contain information about project parameters taken directly from the application and are used
Using WebNow to Process the Fund Establishment Form
 Using WebNow to Process the Fund Establishment Form Fund Est in WebNow 1 Last Updated 3/28/14 Table of Contents TOPIC PAGE 1. General WebNow Information a. Introduction 3 b. Log in 3 c. Adobe Reader 3
Using WebNow to Process the Fund Establishment Form Fund Est in WebNow 1 Last Updated 3/28/14 Table of Contents TOPIC PAGE 1. General WebNow Information a. Introduction 3 b. Log in 3 c. Adobe Reader 3
Effort Certification. User s Guide
 Effort Certification User s Guide 1 Contents The Effort Certification Process.. 3 Sample Effort Reports Sample Summary Effort Report.5 Sample Detailed Effort Report..5 Workers/Employees How to Certify
Effort Certification User s Guide 1 Contents The Effort Certification Process.. 3 Sample Effort Reports Sample Summary Effort Report.5 Sample Detailed Effort Report..5 Workers/Employees How to Certify
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
IRBNet User Manual IACUC
 IRBNet User Manual IACUC Version 5/29/2012 1 User Manual This user manual is designed to assist in the use of IRBNet. You will find assistance in registration, submission, continuing review, amendments
IRBNet User Manual IACUC Version 5/29/2012 1 User Manual This user manual is designed to assist in the use of IRBNet. You will find assistance in registration, submission, continuing review, amendments
Virginia Henderson Global Nursing e-repository ( Henderson repository or the repository ) Revision and Re-Submission Instructions
 Virginia Henderson Global Nursing e-repository ( Henderson repository or the repository ) Revision and Re-Submission Instructions If your submission has been rejected by a reviewer for any reason, you
Virginia Henderson Global Nursing e-repository ( Henderson repository or the repository ) Revision and Re-Submission Instructions If your submission has been rejected by a reviewer for any reason, you
AIMS Applicant user guide to the online application system July 2016, v3.0
 AIMS Applicant user guide to the online application system July 2016, v3.0 This user guide is for applicants applying to join Q using AIMS, our new online application system. We welcome any feedback that
AIMS Applicant user guide to the online application system July 2016, v3.0 This user guide is for applicants applying to join Q using AIMS, our new online application system. We welcome any feedback that
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
Institutional Animal Care & Use Committee Protocol Review
 Institutional Animal Care & Use Committee Protocol Review I. Purpose To describe MSU s IACUC procedures for reviewing new Animal Care and Use Protocols, changes to existing protocols (amendments) and annual
Institutional Animal Care & Use Committee Protocol Review I. Purpose To describe MSU s IACUC procedures for reviewing new Animal Care and Use Protocols, changes to existing protocols (amendments) and annual
Electronic Submission System User procedures document MAY 2018
 Electronic Submission System User procedures document MAY 2018 Electronic Submission System User Guide 1 What s new? This user guide was updated in May 2018 to include recent changes to the Electronic
Electronic Submission System User procedures document MAY 2018 Electronic Submission System User Guide 1 What s new? This user guide was updated in May 2018 to include recent changes to the Electronic
ecampus Submission Process
 ecampus Submission Process Progress Report Submission, and Installment Submission & Feedback 1 All Progress Reports and Installment Submissions are found on the Assignments Page. 2 Individual assignments
ecampus Submission Process Progress Report Submission, and Installment Submission & Feedback 1 All Progress Reports and Installment Submissions are found on the Assignments Page. 2 Individual assignments
Steps on How to Create Cash Advances
 FAST Travel System Guide: How to Create Cash Advances Purpose: Definition: Navigation: Notes: To provide instructions on how to create Cash Advances (CAs) in the FAST Travel module. Cash Advance - In some
FAST Travel System Guide: How to Create Cash Advances Purpose: Definition: Navigation: Notes: To provide instructions on how to create Cash Advances (CAs) in the FAST Travel module. Cash Advance - In some
Workforce Central Navigating My Timecard- Student
 Navigating My Timecard- Student With Workforce Central, you can use the My Timecard widget to view your time entry data, make edits, and address or view exceptions within your timecard. Accessing the My
Navigating My Timecard- Student With Workforce Central, you can use the My Timecard widget to view your time entry data, make edits, and address or view exceptions within your timecard. Accessing the My
eprotocol Committee Manager & RCA Role Manual
 eprotocol Committee Manager & Manual Table of Contents Table of Contents 1 Overview...6 1.1 Things to Remember... 6 2 Committee Manager Role...7 2.1 Committee Manager Role Overview... 7 2.2 Assigning a
eprotocol Committee Manager & Manual Table of Contents Table of Contents 1 Overview...6 1.1 Things to Remember... 6 2 Committee Manager Role...7 2.1 Committee Manager Role Overview... 7 2.2 Assigning a
eprotocol Committee Manager & RCA Role Manual
 eprotocol Committee Manager & RCA Role Manual Table of Contents 1 Overview... 6 1.1 Things to Remember...6 2 Committee Manager Role... 7 2.1 Committee Manager Role Overview...7 2.2 Assigning a Protocol
eprotocol Committee Manager & RCA Role Manual Table of Contents 1 Overview... 6 1.1 Things to Remember...6 2 Committee Manager Role... 7 2.1 Committee Manager Role Overview...7 2.2 Assigning a Protocol
Ohio Child Licensing and Quality System (OCLQS) SUTQ Initial Rating Registration Process
 Ohio Child Licensing and Quality System (OCLQS) SUTQ Initial Rating Registration Process If your program is not currently rated, you will be registering for an Initial Rating. 1. After logging into the
Ohio Child Licensing and Quality System (OCLQS) SUTQ Initial Rating Registration Process If your program is not currently rated, you will be registering for an Initial Rating. 1. After logging into the
v. 01/13/ of 6
 TCSPP Online Submission System User Guide for IRB Members: Application Review Topics A. How to locate an application... 2 B. How to start and complete a review... 3 C. How to review a response... 6 v.
TCSPP Online Submission System User Guide for IRB Members: Application Review Topics A. How to locate an application... 2 B. How to start and complete a review... 3 C. How to review a response... 6 v.
Coeus IACUC Administrative Process Deactivate Protocols
 Coeus IACUC Administrative Process Deactivate Protocols This action can be initiated by the PI or anyone who obtains the Aggregator Role on the protocol record. There are also 2 ways this action can be
Coeus IACUC Administrative Process Deactivate Protocols This action can be initiated by the PI or anyone who obtains the Aggregator Role on the protocol record. There are also 2 ways this action can be
Creating a Quote. Topics covered in this guide: 1. Full Quotes 2. esignature with DocuSign 3. Duplicate a Quote 4. Quick Quotes
 Creating a Quote Creating a Quote Topics covered in this guide: 1. Full Quotes 2. esignature with DocuSign 3. Duplicate a Quote 4. Quick Quotes 2 Full Quotes 3 Full Quote 1. Click on the First InSite Enhanced
Creating a Quote Creating a Quote Topics covered in this guide: 1. Full Quotes 2. esignature with DocuSign 3. Duplicate a Quote 4. Quick Quotes 2 Full Quotes 3 Full Quote 1. Click on the First InSite Enhanced
RFQ Response Submission
 You will learn: How to complete and submit a Request for Qualifications (RFQ) response in ED Grants How to open and continue working on a previously saved RFQ response in ED Grants How to enter your organization
You will learn: How to complete and submit a Request for Qualifications (RFQ) response in ED Grants How to open and continue working on a previously saved RFQ response in ED Grants How to enter your organization
Introducing eram Phase 2 August 2012
 Introducing eram Phase 2 August 2012 Phase 1 2011 o o eram Phases esirius Animal Application/Protocol system was retired eram Application/Protocol system implemented to support UCUCA business processes
Introducing eram Phase 2 August 2012 Phase 1 2011 o o eram Phases esirius Animal Application/Protocol system was retired eram Application/Protocol system implemented to support UCUCA business processes
Getting Started with My Groups Author: Kevin Urasaki Revised by: Deanna Pasternak
 INET1002 November 2006 Getting Started with My Groups Author: Kevin Urasaki Revised by: Deanna Pasternak Introduction...1 What are My Groups?...2 How do I access My Groups?...2 How do I join a My Group?...5
INET1002 November 2006 Getting Started with My Groups Author: Kevin Urasaki Revised by: Deanna Pasternak Introduction...1 What are My Groups?...2 How do I access My Groups?...2 How do I join a My Group?...5
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE
 Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Training Manual for Researchers. How to Create an Online Human Ethics Application
 Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
Training Manual for Researchers How to Create an Online Human Ethics Application What is in this document This manual is intended to provide general tips on using functionality specific to QUEST online
Electronics Manufacturer e-submission Manual
 MAIL CODE 401-02C CHRIS CHRISTIE SOLID AND HAZARDOUS WASTE MANAGEMENT PROGRAM BOB MARTIN Governor ENVIRONMENTAL MANAGEMENT Commissioner NEW JERSEY DEPARTMENT OF ENVIRONMENTAL PROTECTION KIM GUADAGNO P.O.
MAIL CODE 401-02C CHRIS CHRISTIE SOLID AND HAZARDOUS WASTE MANAGEMENT PROGRAM BOB MARTIN Governor ENVIRONMENTAL MANAGEMENT Commissioner NEW JERSEY DEPARTMENT OF ENVIRONMENTAL PROTECTION KIM GUADAGNO P.O.
Requesting Testing Accommodations for the SSAT
 Requesting Testing Accommodations for the SSAT This document provides the step-by-step process for submitting an application for accommodations on the SSAT. Additional information for students regarding
Requesting Testing Accommodations for the SSAT This document provides the step-by-step process for submitting an application for accommodations on the SSAT. Additional information for students regarding
