Click IRB 7.3 IRB Ancillary Reviewer s Guide
|
|
|
- Beverly Quinn
- 5 years ago
- Views:
Transcription
1 Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013
2 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing Changes to a Study... 7 Submitting a Review Decision... 8 Accessing a Study... 9 Add Comments Generating Reports Finding More Information Contacting Support October 2013 page 2
3 Logging In The IRB system is secure, which means only authorized individuals have access to it. When you log in to the system, you get a personalized view of the information and possible actions pertinent to you. To log in: 1. Click the Login link located at the top right corner of your screen. 2. Enter your CAS (CaneID) user name and password. Tips: Press the Tab key after typing your user name to move to the Password box. If you do not know your CAS/CaneID user name or password, contact the Cane ID Help Desk for assistance. (See Contacting Support on page 12) 3. Click Login (or press Enter). Login issues If you receive this error message......contact... Sorry, you entered an invalid CaneID or password CaneID Help Desk ( ) for help resetting your password or re-enabling your CaneID account. We were unable to match your CaneID to an active eprost account and you already have an eprost account We were unable to match your CaneID to an active eprost account and you do not yet have an eprost account Research IT Help Desk ( or ) for assistance with re-enabling your eprost account. Research IT Help Desk ( or ) for assistance with requesting an eprost account. October 2013 page 3
4 Locating Your To-Do List IRB studies that are assigned to you for action generally appear in My Inbox with a link to the study. You may also receive an with a link to the study. An indicates that you must take action or informs you of important changes, such as an IRB decision about your study. Note: A continuing review, modification, or RNI (reportable new information) submission can be handled similarly to a study. To access a study that does not appear in My Inbox, see Accessing a Study on page 9. To access studies or other submissions assigned to you: 1. Click the My Inbox link in the top right navigation header. 2. Identify the reason the study appears in My Inbox by looking at the State column. 3. Open the study by clicking the link in the Name column. The study workspace opens. To view the details of the study, click View Study on the left. For instructions, see Viewing the Study Details on page 6. October 2013 page 4
5 Understanding My Inbox The list called My Inbox consists of two separate inboxes: your Study Staff Inbox and your Reviewer Inbox. The Study Staff Inbox contains studies or other submissions that require you (or your study team members) to take action. The Reviewer Inbox contains studies or other submissions that require your review. See the examples below to understand what you should and should not expect to appear in My Inbox. Your role Ancillary reviewer or Department reviewer State One of several In My Inbox Explanation You have been selected as a reviewer (either by name or representing a specific organization). The IRB can begin its review before you submit your review. The IRB may or may not wait for your input before completing its review of the study. Not in My Inbox Studies not yet submitted for review Important note for JHS and UMH ancillary committee members only: Since you may sometimes review submissions after they have already received IRB approval, the items requiring your review may not always be in your Inbox. To view a complete list of the submissions pending your review, go to the Custom Reports tab and run the report named REPORT_Items_with_Ancillary_Reviews _Pending. See Generating Reports on page 10. October 2013 page 5
6 Viewing the Study Details As a reviewer, you often need to view all the information submitted as part of the study. To view the details of a study: 1. From My Inbox, click the name of the study to open it. Note: If the study does not appear in your inbox, see Accessing a Study on page Click View Study on the left. Tips: For a continuing review or modification, click View Modification / CR instead. For a new information report, click View RNI instead. 3. Use the Continue and Back buttons to view all of the forms. Tip: Clicking Continue from the Supporting Documents page (the last page of the forms) exits the study. To view the documents submitted as part of the study, you have these options: While viewing the details of the study (as instructed above), click the name of each document when you encounter it on the various forms. Documents are listed in tables throughout the forms. When you have opened the study workspace (as in step 1 above), you can view a list of all the attached documents in one place by clicking the Documents tab. Important! Supplemental forms required by your ancillary committee can be found in the Documents tab, unless the study is going to an External IRB (such as WIRB). For External IRB studies, please go to the History tab, and look for the Study Information Updated activity. Click on the activity name and the documents will be listed at the top of the Activity Details page. (This page may take a few moments to load). Tip: If the study team updated the documents, they may contain tracked changes. You can use the review features in Word to toggle between showing the original and final versions of the document. When the IRB approves the documents, all tracked changes will be accepted and comments removed in the final versions. October 2013 page 6
7 Viewing Changes to a Study When a study changes based on reviewer requests, you may want to: View the changes submitted for a particular request View the differences between two versions of a study To view changes submitted for a clarification request: 1. From My Inbox, click the name of the study to open it. 2. Click the History tab. 3. Click the Changes Submitted activity link to see any notes or documents added to the study. To view the differences between two versions of a study: 1. From My Inbox, click the name of the study to open it. 2. Click View Differences on the left. 3. Next to Show Changes, select a version to compare the current study to. 4. Look for red and green changes in the current form. Click the arrow to show the details. The changes since the version you selected appear as follows: Additions to text since that version are shown with green highlighting. Deletions to text show in a light red box below the current text. Additions and deletions of selectable items show the changes (such as old values) in a light red box after the current values that appear normally. October 2013 page 7
8 5. Next to Changed Steps, click the arrow (or use the drop-down list) to view each of the other forms that have changed. 6. Exit the View Differences screen by clicking Close on the right. Tip: If the study team updated the documents, they may contain tracked changes. You can use the review features in Word to toggle between showing the original and final versions of the document. When the IRB approves the documents, all tracked changes will be accepted and comments removed in the final versions. Submitting a Review Decision After reviewing a study or other submission, you must record the decision in the system. Recording the decision completes the review and moves the study forward in the IRB process. Tip: You can include comments when you record your decision. To avoid regulatory issues, it is best to phrase your comments as questions or requests for information. If you need the study team to answer a question before you can complete the review, request clarifications using the Add Comments activity or the Submit Ancillary Review activity. The IRB Staff will send the submission back to the study team for revisions/clarifications. Note: The procedures below assume that the study team has completed any requested clarifications. To open the study: 1. From My Inbox, click the name of the study to open it. 2. Choose the appropriate procedure below. To complete an ancillary (or department/division) review: 1. Click Submit Ancillary Review on the left. 2. Select the review you are completing from the list. 3. Indicate whether you think the submission is acceptable as it is currently written. 4. (Optional) Add comments and attach documents related to the review. 5. Click OK. The review comments are shown in the study. October 2013 page 8
9 Accessing a Study You may want to open a specific study to view or update its contents, submit it for review, review it, or take other actions on the study. Note: Your access to a study is personalized based on your role in the system and the role you play in relation to the particular study. In addition, the actions you can take on a study are personalized. To open a study, click its name when you find it in a list of studies. To find a list that includes the study, try these suggestions: Check this list... My Inbox IRB In- Review tab For... Studies assigned to you for action, such as a study you are: Preparing to submit Assigned to review Studies the IRB has not reviewed or for which it has not communicated a decision How to find this list Click the My Inbox link in the top right navigation header. Click IRB in the top left navigation area and select the In- Review tab. IRB Active tab IRB All Submissions tab IRB New Information Reports tab Studies approved by the IRB and currently in progress All studies, continuing reviews, modifications, and reportable new information (RNI) entered into the system that you have permissions to view Reportable new information (RNI) submissions, possibly related to one or more studies Click IRB in the top left navigation area and select the Active tab. Click IRB in the top left navigation area and select the All Submissions tab. Tip: Try filtering this list by the study name or principal investigator. Next to Filter by, select Name or Investigator. Then type the beginning of the name and click Go. Click IRB in the top left navigation area and select the New Information Reports tab. October 2013 page 9
10 Add Comments Anyone who has read access to a study may add comments to the study and read comments posted by others. These comments will be visible only in the History Log of the study/submission. The system will NOT send an notification to notify others that you have posted a comment. To add comments: 1. Open the study by clicking the study's name. (For instructions about finding the study, see Accessing a Study on page 9) 2. Click Add Comment from the My Current Actions list on the left. A new window opens. 3. Enter your comments. You may also upload supporting documents. 4. Click OK. Tip: Comments directly related to your ancillary review decision and requests for clarification/changes should be posted via the Submit Ancillary Review activity, rather than through the Add Comments activity. This helps ensure that your comments will be seen by the IRB staff as the submission moves through the review process. Generating Reports The IRB system includes many standard reports regarding studies and reportable new information (RNI) to help you find relevant submissions and understand the overall operation of the IRB. In addition, there are a number of custom reports available to UM/JHS ancillary committee members and department/division reviewers. The reports provide links to the individual submissions, as well as sorting and filtering options. Any user has access to reports, but the data in the reports is limited to the studies visible to the individual. For example, a Studies Involving Children report generated by a PI will include only the studies that person is allowed to see elsewhere in the system--studies for which the person is included on the study team or guest list. IRB coordinators, directors, ancillary reviewers, and IRB members have a higher level of access to report data. To generate a report: 1. Click IRB in the top navigator. 2. Click IRB Reports on the left. The list of standard study and RNI reports appears. Tip: To find a custom report, click the Custom Reports tab. 3. Identify the report to generate and click the link. The report appears, listing the relevant submissions. Tip: Try filtering the list by status. Next to Filter by, select Status. Then type the state to view, such as Approved for a study report or Acknowledged for an RNI report and click Go. October 2013 page 10
11 Finding More Information To find this......look for this......and click... More information about a question or form. The full online help system, with search and table of contents. The online help contains additional procedures and information for all users. Click the question mark icon next to the question or form title. Click the Help link in the Shortcuts area on the left. Instructions for submitting a study for review. Click the Study Submission Guide link in the Shortcuts area on the left. Document templates, checklists, and IRB procedures. Click IRB and then IRB library in the upper left corner. October 2013 page 11
12 Contacting Support For additional answers to your questions, feel free to use the following resources: Resource How to access it Documentation See Finding More Information on page 11 Training materials in the IRB Library See Finding More Information on page 11 For regulatory questions, contact the HSRO Phone: For technical issues, contact Research IT Phone: or For login problems, contact the Cane ID help desk Phone: , option 2 October 2013 page 12
13 Huron Technologies Inc. All rights reserved. Use and distribution prohibited except through written agreement with Huron. Click is a registered service mark of Huron Consulting Group, Inc. All other trademarks, registered trademarks, service marks, and trade names are the property of their respective owners. Information in this document is subject to change without notice. Published by Huron Technologies Inc NW AmberGlen Pkwy Suite 400 Beaverton, OR October 2013 page 13
Click. IRB Study Submission Guide
 Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IACUC Researcher s Quick Reference
 Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond
Click IACUC Researcher s Quick Reference Before You Create a Protocol... Create a Research Team... Check for Existing Building Blocks... Create Building Blocks... 4 Create and Submit a Protocol... 5 Respond
Module 3: Changes and Correspondence
 Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
Table of Contents Module 3: Changes and Correspondence Module 3 Objective: Covers how to request application changes and communicate with study team, core staff and reviewers. Important Points: eresearch
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
eiacuc Reference for Veterinarians
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
Core Staff Review Approve with Contingencies
 Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
IRB Committee Member Adverse Event (AE) Single Member Review
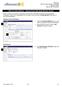 IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
eresearch IBC Staff Review Step-by-Step Procedures Core Committee Staff Home Page
 1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
IRB Staff Approve Repository Application
 IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
ARC IRB Chair s Manual
 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
eprost System Policies & Procedures
 eprost System Policies & Procedures Initial Approval Date: 12/07/2010 Revision Date: 02/25/2011 Introduction eprost [ Electronic Protocol Submission and Tracking ] is the Human Subject Research Office's
eprost System Policies & Procedures Initial Approval Date: 12/07/2010 Revision Date: 02/25/2011 Introduction eprost [ Electronic Protocol Submission and Tracking ] is the Human Subject Research Office's
How Does the PI make Pre Review ICF Changes?
 How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
How Does the PI make Pre Review ICF Changes? 1 You, as PI of a study, have received notification from the IRB that Changes [are] required by IRB Staff. These changes include a request for a revision of
Module 1: Core Committee Staff Basics
 Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
Table of Contents Module 1: Core Committee Staff Basics Guide Introduction This guide provides procedures that Core Committee Staff members will use to manage submissions for human subject research in
SUBMITTING A NEW IBC PROJECT APPLICATION (INITIAL)
 SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
IACUC Module. March 2018
 z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
z IACUC Module March 2018 1 Page left intentionally blank 2 Contents Logins Required for Exercises... 4 Navigation Exercises... 6 Log into the Click IACUC Module... 6 Explore the Inbox... 6 Explore Submissions...
The most current protocol information should be entered. This includes incorporation of all past amendments and modifications.
 Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Contents. TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs
 TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
TCSPP Online Submission System User Guide for Thesis and Dissertation Chairs Contents A. Login and Select the Application... 2 B. Review the Q&A form... 3 C. Adding Comments to the Q&A form... 4 D. Reviewing
JeffTrial Training Document Introduction to JeffTrial. Jefferson Coordinating Center for Clinical Research 9/26/2013 Ver. 1.1
 JeffTrial Training Document Introduction to JeffTrial Jefferson Coordinating Center for Clinical Research 9/26/2013 Ver. 1.1 Contents Navigation... 2 Getting Started... 2 User Roles... 3 My Profile...
JeffTrial Training Document Introduction to JeffTrial Jefferson Coordinating Center for Clinical Research 9/26/2013 Ver. 1.1 Contents Navigation... 2 Getting Started... 2 User Roles... 3 My Profile...
Electronic MTA Training Manual & User Guide for Administrative Staff & Principal Investigators
 Electronic MTA Training Manual & User Guide for Administrative Staff & Principal Investigators 1 P a g e Table of Contents Getting Started... 3 Introduction... 3 Helpful Hints... 3 Login Process... 6 Profile...
Electronic MTA Training Manual & User Guide for Administrative Staff & Principal Investigators 1 P a g e Table of Contents Getting Started... 3 Introduction... 3 Helpful Hints... 3 Login Process... 6 Profile...
Reference Card for ORSP
 Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Reference Card for ORSP https://eresearch.umich.edu Log in from eresearch Homepage 1. Go to https://eresearch.umich.edu. 2. Click Login in the Proposal Management box. 3. Enter your Login ID (uniqname)
Version 2.1 June 12, 2018
 Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
IACUC Researcher s Quick Reference
 IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
IACUC Researcher s Quick Reference Navigation and Basic Tasks When you first log in, you will be on the My Inbox page. This topic lists where to find submissions and the basic tasks you will perform. Where
How to Use the Comprehensive Ancillary Forms Engine (CAFÉ) for DSMB/ISPRC Initial Submission
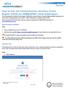 How to Use the Comprehensive Ancillary Forms Engine (CAFÉ) for DSMB/ISPRC Initial Submission The Comprehensive Ancillary Forms Engine (CAFÉ) allows s to submit DSMB and ISPRC submissions to the Jonsson
How to Use the Comprehensive Ancillary Forms Engine (CAFÉ) for DSMB/ISPRC Initial Submission The Comprehensive Ancillary Forms Engine (CAFÉ) allows s to submit DSMB and ISPRC submissions to the Jonsson
Buck-IRB Amendment User Guide
 Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
ROCHE/GENENTECH PRACTICAL WORKING GUIDE FOR REQUESTORS
 ROCHE/GENENTECH PRACTICAL WORKING GUIDE FOR REQUESTORS Contents I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 3 III. HOW TO SUBMIT AN APPLICATION... 7 IV. HOW TO PROVIDE ADDITIONAL STUDY INFORMATION...
ROCHE/GENENTECH PRACTICAL WORKING GUIDE FOR REQUESTORS Contents I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 3 III. HOW TO SUBMIT AN APPLICATION... 7 IV. HOW TO PROVIDE ADDITIONAL STUDY INFORMATION...
Office of Human Resources. Hiring Adjuncts CREATING THE POSTING (NO POSITION DESCRIPTION REQUIRED!)
 Office of Human Resources Hiring Adjuncts CREATING THE POSTING (NO POSITION DESCRIPTION REQUIRED!) Adjunct Initiator Dept Chair Employment Team Confirm that you are in the Applicant Tracking module (blue).
Office of Human Resources Hiring Adjuncts CREATING THE POSTING (NO POSITION DESCRIPTION REQUIRED!) Adjunct Initiator Dept Chair Employment Team Confirm that you are in the Applicant Tracking module (blue).
How to Submit a New Application
 ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
ehirb User Guide: How to Submit a New Application Last Update February 7, 2014 Intended Audience Purpose Principal Investigator/Researcher To provide the user with step- by- step instructions on how to
Approving the Outside Interests Disclosure Form Without Conflicts. Supervisor Level. IRIS Mobile through the Web
 Approving the Outside Interests Disclosure Form Without Conflicts Supervisor Level IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff
Approving the Outside Interests Disclosure Form Without Conflicts Supervisor Level IRIS Mobile through the Web The Outside Interests Disclosure Form is for the University of Tennessee faculty and staff
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
CAHIIM HELP for Program Users
 CAHIIM HELP for Program Users 2018 CAHIIM Table of Contents GETTING STARTED... 1 Performing Basic Functions... 1 Logging In... 1 Logging Out... 1 Resetting Password... 1 Viewing or Changing User Information...
CAHIIM HELP for Program Users 2018 CAHIIM Table of Contents GETTING STARTED... 1 Performing Basic Functions... 1 Logging In... 1 Logging Out... 1 Resetting Password... 1 Viewing or Changing User Information...
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK. (Version date 03/03/2006)
 ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
STUDY ASSISTANT. Adding a New Study & Submitting to the Review Board. Version 10.03
 STUDY ASSISTANT Adding a New Study & Submitting to the Review Board Version 10.03 Contents Introduction... 3 Add a Study... 3 Selecting an Application... 3 1.0 General Information... 3 2.0 Add Department(s)...
STUDY ASSISTANT Adding a New Study & Submitting to the Review Board Version 10.03 Contents Introduction... 3 Add a Study... 3 Selecting an Application... 3 1.0 General Information... 3 2.0 Add Department(s)...
IBC Reviewer User Guide
 IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
IBC Reviewer User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software version 2.5.43.0 Document Version 1.0 Copyright 2016 Key Solutions 2002-2016 Key Solutions, Inc.
Laserfiche Agenda Workflow Training. Submitting an Agenda Item Through Laserfiche Web Access Version 10.2
 Laserfiche Agenda Workflow Training Submitting an Agenda Item Through Laserfiche Web Access Version 10.2 May 2017 Contents LASERFICHE LASERFICHE AGENDA REVIEW WORKFLOW... 3 IMPORTING DOCUMENTS INTO WEB
Laserfiche Agenda Workflow Training Submitting an Agenda Item Through Laserfiche Web Access Version 10.2 May 2017 Contents LASERFICHE LASERFICHE AGENDA REVIEW WORKFLOW... 3 IMPORTING DOCUMENTS INTO WEB
Guide for Candidates: Online Progress Reports
 Guide for Candidates: Online Progress Reports What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University. All Progress Reports
Guide for Candidates: Online Progress Reports What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University. All Progress Reports
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment)
 Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Coeus Extension IRB Administrative Process Grant Exemption/Expedited Approval (Initial Protocol or Amendment) Complete the following steps only when ALL Reviewers have indicated Grant Exemption AND there
Guide for Researchers: Online Human Ethics Application Form
 Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Guide for Researchers: Online Human Ethics Application Form
 Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
PAGE 1. IRBManager. Instruction Manual For IRB members
 PAGE 1 IRBManager Instruction Manual For IRB members IRBManager PAGE 2 What is IRBManager? IRBManager is a web-based system designed specifically for the IRB review process, all the way from submission
PAGE 1 IRBManager Instruction Manual For IRB members IRBManager PAGE 2 What is IRBManager? IRBManager is a web-based system designed specifically for the IRB review process, all the way from submission
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
FIELDS REQUIRED TO SAVE A PROTOCOL
 Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
IACUC Proposal Entry Instructions
 IACUC Proposal Entry Instructions Version 1.0 October 10, 2016 Notes to Primary Investigator The following instructions are based on the IACUC Proposal form as currently found in iris. This instruction
IACUC Proposal Entry Instructions Version 1.0 October 10, 2016 Notes to Primary Investigator The following instructions are based on the IACUC Proposal form as currently found in iris. This instruction
WebEOC. User Guide. Version 8.1. County Emergency Managers Edition 6/5/17
 WebEOC User Guide Version 8.1 County Emergency Managers Edition 6/5/17 2016 Intermedix Corporation. All rights reserved. This document contains confidential or proprietary information of Intermedix Corporation
WebEOC User Guide Version 8.1 County Emergency Managers Edition 6/5/17 2016 Intermedix Corporation. All rights reserved. This document contains confidential or proprietary information of Intermedix Corporation
v. 01/13/ of 6
 TCSPP Online Submission System User Guide for IRB Members: Application Review Topics A. How to locate an application... 2 B. How to start and complete a review... 3 C. How to review a response... 6 v.
TCSPP Online Submission System User Guide for IRB Members: Application Review Topics A. How to locate an application... 2 B. How to start and complete a review... 3 C. How to review a response... 6 v.
irb.unm.edu
 IRBNet Submission Instructions The purpose of this document is to provide IRBNet submission instructions for UNM researchers using IRBNet. Please contact the Office of the IRB for assistance: irb.unm.edu
IRBNet Submission Instructions The purpose of this document is to provide IRBNet submission instructions for UNM researchers using IRBNet. Please contact the Office of the IRB for assistance: irb.unm.edu
PCORI Online: Awardee User Guide Research Awards
 PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol, contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol, contact:
How to Submit a New UIW IRB Application
 How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
How to Submit a New UIW IRB Application Step Log In Visit https://uiw.forms.ethicalreviewmanager.com/ click on at the top right corner of the page. New Users: Click on New User Fill in the applicable information
IBC Committee Manager User Guide
 IBC Committee Manager User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Version 1.2 Copyright 2018 Key Solutions 2002-2018 Key Solutions, Inc. 2803 Lakeview Court Fremont,
IBC Committee Manager User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Version 1.2 Copyright 2018 Key Solutions 2002-2018 Key Solutions, Inc. 2803 Lakeview Court Fremont,
CHAP LinQ User Guide. CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC Version 1.
 2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
eiacuc Reference for PIs and Research Team
 1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
1. Go to https://eiacuc.rutgers.edu 2. Enter your Rutgers NetID and Password. 3. Click Login to enter the site. Logging In My Inbox My Inbox displays all eiacuc submissions associated with you. Identify
Office 365 Groups. Creating, Managing, and Joining an Office 365 Group
 Office 365 Groups Creating, Managing, and Joining an Office 365 Group Table of Contents Creating, Managing, and Joining an Office 365 Group... 3 Creating a Group... 3 Add Group Members... 5 Remove Group
Office 365 Groups Creating, Managing, and Joining an Office 365 Group Table of Contents Creating, Managing, and Joining an Office 365 Group... 3 Creating a Group... 3 Add Group Members... 5 Remove Group
TABLE OF CONTENTS CHAPTER 1. PROTOCOL APPLICATION PROCESS OVERVIEW
 IBC Creating Protocol Applications TABLE OF CONTENTS CHAPTER 1. PROTOCOL APPLICATION PROCESS OVERVIEW... 1.1 CHAPTER 2. GENERAL INFORMATION... 2.1 2.1. STARTING AND LOGGING IN... 2.1 2.2. USING NAVIGATION
IBC Creating Protocol Applications TABLE OF CONTENTS CHAPTER 1. PROTOCOL APPLICATION PROCESS OVERVIEW... 1.1 CHAPTER 2. GENERAL INFORMATION... 2.1 2.1. STARTING AND LOGGING IN... 2.1 2.2. USING NAVIGATION
Blackboard Student Quick Reference Guide
 Blackboard Student Quick Reference Guide Welcome to Blackboard, UTT s E-Learning System! This Quick Reference Guide is designed to help get you started using Blackboard Release 9.1.120113.0. Page 1 of
Blackboard Student Quick Reference Guide Welcome to Blackboard, UTT s E-Learning System! This Quick Reference Guide is designed to help get you started using Blackboard Release 9.1.120113.0. Page 1 of
University of the Sciences. IRBNet User s Guide
 University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
Child and Adult Care Food Program User Manual for Institution Users
 COLYAR TECHNOLOGY SOLUTIONS, INC. NC Cares Child and Adult Care Food Program User Manual for Institution Users 1 TABLE OF CONTENTS USER MANUAL FOR INSTITUTION USERS... 1... 5 INTRODUCTION... 5 WEBSITE
COLYAR TECHNOLOGY SOLUTIONS, INC. NC Cares Child and Adult Care Food Program User Manual for Institution Users 1 TABLE OF CONTENTS USER MANUAL FOR INSTITUTION USERS... 1... 5 INTRODUCTION... 5 WEBSITE
LaserFiche Appointments Manual
 LaserFiche Appointments Manual Guide to Academic Appointments Using Web Forms and LaserFiche8 Human Resources Version Discovery Commons September 2013 Table of Contents Appointments Processed in the LaserFiche
LaserFiche Appointments Manual Guide to Academic Appointments Using Web Forms and LaserFiche8 Human Resources Version Discovery Commons September 2013 Table of Contents Appointments Processed in the LaserFiche
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
Odyssey File & Serve. Review Queue User Guide Release 3.11
 Odyssey File & Serve Review Queue User Guide Release 3.11 OFS-FS 200 3375 v.1 June 2015 COPYRIGHT AND CONFIDENTIALITY Copyright 2015 Tyler Technologies, Inc. All rights reserved. All documentation, source
Odyssey File & Serve Review Queue User Guide Release 3.11 OFS-FS 200 3375 v.1 June 2015 COPYRIGHT AND CONFIDENTIALITY Copyright 2015 Tyler Technologies, Inc. All rights reserved. All documentation, source
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
RHS INFORMATION SERVICES TECHNOLOGY GUIDE. PeopleAdmin User Guide
 RHS INFORMATION SERVICES TECHNOLOGY GUIDE PeopleAdmin User Guide Overview The RHS People Admin system offers a paperless, centralized method whereby applicants can apply for RHS jobs online and RHS hiring
RHS INFORMATION SERVICES TECHNOLOGY GUIDE PeopleAdmin User Guide Overview The RHS People Admin system offers a paperless, centralized method whereby applicants can apply for RHS jobs online and RHS hiring
Drexel University. Page 1 of 48. Version November agf
 Drexel University Page 1 of 48 TABLE OF CONTENTS Getting Started. 3 Creating a New Protocol.. 4 General Information... 7 Organization Information... 9 Investigators/Study Personnel.. 10 Correspondents
Drexel University Page 1 of 48 TABLE OF CONTENTS Getting Started. 3 Creating a New Protocol.. 4 General Information... 7 Organization Information... 9 Investigators/Study Personnel.. 10 Correspondents
SFU Connect Calendar. Guide. Sharing Calendars
 SFU Connect Calendar How-To Guide Sharing Calendars Last updated: January 2009 Table of Contents Creating a Share... 3 Share Properties Menu... 3 Sharing with Internal Users or Groups... 4 Sharing with
SFU Connect Calendar How-To Guide Sharing Calendars Last updated: January 2009 Table of Contents Creating a Share... 3 Share Properties Menu... 3 Sharing with Internal Users or Groups... 4 Sharing with
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
Children s Fund Referral Request Submission Guide. Revised 9/28/15
 Children s Fund Referral Request Submission Guide Revised 9/28/15 Table of Contents Section Page Logging in 3 Resetting Your Password 5 Dashboard 10 Account and Password 11 Submitting a New Request 12
Children s Fund Referral Request Submission Guide Revised 9/28/15 Table of Contents Section Page Logging in 3 Resetting Your Password 5 Dashboard 10 Account and Password 11 Submitting a New Request 12
Human Subjects Development Guide
 Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
eibc Program User Guide
 eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
You may also scroll through the Table of Contents and click on the topic or question of interest.
 We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
Production Assistance for Cellular Therapies (PACT) PACT Application System User s Guide
 Production Assistance for Cellular Therapies (PACT) PACT Application System User s Guide Version 1.0 February 9, 2017 Version 1.0 TABLE OF CONTENTS 1.0 Getting Started... 1 1.1 Access to the Internet...
Production Assistance for Cellular Therapies (PACT) PACT Application System User s Guide Version 1.0 February 9, 2017 Version 1.0 TABLE OF CONTENTS 1.0 Getting Started... 1 1.1 Access to the Internet...
EDI Web Portal Quick Start Guide
 Including ALLIANCE OF NONPROFITS FOR INSURANCE (ANI) & NONPROFITS INSURANCE ALLIANCE OF CALIFORNIA (NIAC) www.insurancefornonprofits.org EDI Web Portal Quick Start Guide Table of Contents Welcome!... 2
Including ALLIANCE OF NONPROFITS FOR INSURANCE (ANI) & NONPROFITS INSURANCE ALLIANCE OF CALIFORNIA (NIAC) www.insurancefornonprofits.org EDI Web Portal Quick Start Guide Table of Contents Welcome!... 2
***** ***** June
 SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
Rabo Supplier Finance User Manual - Suppliers -
 Rabo Supplier Finance User Manual - Suppliers - Page 2 of 33 Table of Contents 1 About This Document... 3 1.1 Objectives of This Document... 3 1.2 Inside This Document... 3 2 Rabo Supplier Finance platform...
Rabo Supplier Finance User Manual - Suppliers - Page 2 of 33 Table of Contents 1 About This Document... 3 1.1 Objectives of This Document... 3 1.2 Inside This Document... 3 2 Rabo Supplier Finance platform...
Updating your Profile in Concur for Non-Travelers who Have Procurement Cards
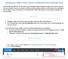 Updating your Profile in Concur for Non-Travelers who Have Procurement Cards Some faculty and staff who do not travel or their delegate handles all aspects of their travel will not need to use Concur often.
Updating your Profile in Concur for Non-Travelers who Have Procurement Cards Some faculty and staff who do not travel or their delegate handles all aspects of their travel will not need to use Concur often.
Reminder: Please refer to the 2018 Convening & Collaborating (C 2 ) competition page for more details.
 Reminder: Please refer to the 2018 Convening & Collaborating (C 2 ) competition page for more details. The MSFHR ApplyNet system identifies the Researcher and Research User Co-Leads as Third Party Personnel.
Reminder: Please refer to the 2018 Convening & Collaborating (C 2 ) competition page for more details. The MSFHR ApplyNet system identifies the Researcher and Research User Co-Leads as Third Party Personnel.
Research Funding Module InfoEd User Guide. Version 3.3
 Research Funding Module InfoEd User Guide Version 3.3 Table of Contents Login... 3 Create New Proposal or Expression of Interest (EOI)... 3 Primary Application Details... 4 Principal Investigator Information...
Research Funding Module InfoEd User Guide Version 3.3 Table of Contents Login... 3 Create New Proposal or Expression of Interest (EOI)... 3 Primary Application Details... 4 Principal Investigator Information...
eprotocol IRB Reviewer Role Manual
 eprotocol IRB Reviewer Role Manual MARCH 2018 Table of Contents 1 Overview... 4 1.1 Things to Remember... 4 2 Review Process... 5 2.1 Reviewer Role Overview...5 2.2 Protocol Event Types...6 Assigned as
eprotocol IRB Reviewer Role Manual MARCH 2018 Table of Contents 1 Overview... 4 1.1 Things to Remember... 4 2 Review Process... 5 2.1 Reviewer Role Overview...5 2.2 Protocol Event Types...6 Assigned as
DISCOVR-e USER MANUAL. Vanderbilt University Human Research Protection Program
 DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
REGULATORY DIVISION BOURSE DE MONTRÉAL INC.
 REGULATORY DIVISION BOURSE DE MONTRÉAL INC. Date of Issue: January 7, 2019 Table of contents Introduction... 1 Overview... 1 Background... 1 Definitions... 1 Getting Started... 1 Account Enabling... 1
REGULATORY DIVISION BOURSE DE MONTRÉAL INC. Date of Issue: January 7, 2019 Table of contents Introduction... 1 Overview... 1 Background... 1 Definitions... 1 Getting Started... 1 Account Enabling... 1
Drexel University. Version April Page 1 of 23. Version April agf
 Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
SharePoint User Manual
 SharePoint User Manual Developed By The CCAP SharePoint Team Revision: 10/2009 TABLE OF CONTENTS SECTION 1... 5 ABOUT SHAREPOINT... 5 1. WHAT IS MICROSOFT OFFICE SHAREPOINT SERVER (MOSS OR SHAREPOINT)?...
SharePoint User Manual Developed By The CCAP SharePoint Team Revision: 10/2009 TABLE OF CONTENTS SECTION 1... 5 ABOUT SHAREPOINT... 5 1. WHAT IS MICROSOFT OFFICE SHAREPOINT SERVER (MOSS OR SHAREPOINT)?...
IRBNet User Manual IACUC
 IRBNet User Manual IACUC Version 5/29/2012 1 User Manual This user manual is designed to assist in the use of IRBNet. You will find assistance in registration, submission, continuing review, amendments
IRBNet User Manual IACUC Version 5/29/2012 1 User Manual This user manual is designed to assist in the use of IRBNet. You will find assistance in registration, submission, continuing review, amendments
era(infoed) Electronic Submission Instructions and Tips: Initial Application
 era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system.
era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system.
NZ Online Forms for Research Software Manual
 NZ Online Forms for Research Software Manual Version 1.5 Released May 2016 2 P a g e N Z O n l i n e F o r m s f o r R e s e a r c h 1 INTRODUCTION... 6 2 GETTING STARTED... 6 2.1 Creating an Account...
NZ Online Forms for Research Software Manual Version 1.5 Released May 2016 2 P a g e N Z O n l i n e F o r m s f o r R e s e a r c h 1 INTRODUCTION... 6 2 GETTING STARTED... 6 2.1 Creating an Account...
Raptor University. Raptor System Entry Admin Training. Instructor: RAPTOR TECHNOLOGIES, LLC
 Raptor University Raptor System Entry Admin Training Instructor: RAPTOR TECHNOLOGIES, LLC This training will provide you with the skills necessary to perform the following functions within the Raptor System:
Raptor University Raptor System Entry Admin Training Instructor: RAPTOR TECHNOLOGIES, LLC This training will provide you with the skills necessary to perform the following functions within the Raptor System:
LifeStructures Link. P a g e 1 12
 LIFESTRUCTURESLINK: A web-based PROJECT MANAGEMENT AND COLLABORATION SERVICE that addresses the needs of the design and construction fields. LifeStructuresLink offers clear and efficient communication
LIFESTRUCTURESLINK: A web-based PROJECT MANAGEMENT AND COLLABORATION SERVICE that addresses the needs of the design and construction fields. LifeStructuresLink offers clear and efficient communication
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II
 How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
How to Create and Submit a Continuing Review Form (Progress Report) in INSPIR II Creating and Submitting a Continuing Review Form If you are listed as the Study Contact on the study, the system will send
KeyNavigator Book Transfer
 Created (10/2018) KeyNavigator Book Transfer User Guide Table of Contents 1. Introduction to Book Transfer... 3 Overview... 3 Features... 3 Navigation... 3 Transfer Processing Timing... 4 2. User-Level
Created (10/2018) KeyNavigator Book Transfer User Guide Table of Contents 1. Introduction to Book Transfer... 3 Overview... 3 Features... 3 Navigation... 3 Transfer Processing Timing... 4 2. User-Level
Use the table below to learn about the various user roles currently available in the Educator Dashboard. User Role For Who?
 How to Create Educator Accounts in Partner Districts Educators with a Site Admin or District Admin user role in the Educator Dashboard on CaliforniaColleges.edu have the ability to create accounts for
How to Create Educator Accounts in Partner Districts Educators with a Site Admin or District Admin user role in the Educator Dashboard on CaliforniaColleges.edu have the ability to create accounts for
Processing Submittals via Prolog Converge
 Processing Submittals via Prolog Converge Submittal Review Procedure Receiving Notification An automatic notification will be sent to your email when the team creates a new Submittal Package and assigns
Processing Submittals via Prolog Converge Submittal Review Procedure Receiving Notification An automatic notification will be sent to your email when the team creates a new Submittal Package and assigns
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review
 Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
Data Management Unit, V3.1 University of Pennsylvania Treatment Research Center, 2010 Page 2
 Welcome to the Data Entry System User s Manual. This manual will cover all of the steps necessary for you to successfully navigate and operate the Data Management Unit s Web based data entry system. We
Welcome to the Data Entry System User s Manual. This manual will cover all of the steps necessary for you to successfully navigate and operate the Data Management Unit s Web based data entry system. We
