era(infoed) Electronic Submission Instructions and Tips: Initial Application
|
|
|
- Byron Marshall
- 6 years ago
- Views:
Transcription
1 era(infoed) Electronic Submission Instructions and Tips: Initial Application This instructional document introduces users to submitting NEW research protocols electronically through the era(infoed) system. Make sure you know whether your project requires EXEMPT, EXPEDITED, or FULL BOARD REVIEW. Visit our step-by-step guide for more information. Before you can submit documents electronically, you should first review the following required elements to gain era(infoed) access. The era(infoed) website is where the COMIRB database and electronic submission website is housed. If you are an Employee of the University of Colorado Denver, you have the ability to log into this website using your CU Denver employee login information. If, however, you are a researcher or staff member at a CU Denver Affiliate Site, user access to the era/infoed) website requires Person of Interest (POI) Number. Follow the instructions for obtaining access to the COMIRB era(infoed) data system and completing other tasks necessary to start or join a COMIRB study. Important Note On Study Access: Access to studies on era(infoed) that are In Development is limited to the individuals listed on the study until its approval. This means only study personnel listed on the PERSONNEL FORM can edit or access the study prior to its submission to COMIRB. If you are a coordinator working on a submission on behalf of your PI, make sure to complete the Personnel Form (Step 3.E. below) before exiting the submission to ensure that everyone has the necessary access. STEP 1: LOGIN INTO ERA(INFOED) A. Navigate to in your preferred Internet browser. B. Enter your POI or CU Denver username and password. Select DENVER as the Campus. C. Click the SIGN IN button. STEP 2: Create A NEW PROTOCOL A. Select MY HUMAN SUBJECTS TAB from the menu located on the lefthand side of your browser window. B. Click the CREATE NEW and a new window will open. C. Click the CONTINUE BUTTON. 1
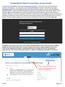
2 D. Enter the protocol title and click the CONTINUE BUTTON. E. Select a PRINCIPAL INVESTIGATOR. Your name should appear in the field. To select someone else as the PI, delete your name from the field, then begin typing their name in the field in the format Last Name, First Name (e.g. Smith, John). There may be multiple listings for the same person. Please select the one with the relevant department after the name for the research being conducted. F. Click the CONTINUE BUTTON, refreshing the window to the INITIAL APPLICATION screen. Please note: once this screen refreshes, era(infoed) assigns your protocol number automatically. The number can be viewed in the upper left-hand corner of the screen. This is what the INITIAL APPLICATION screen looks like: 2
3 STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS Last Updated: December 15, 14 The INITIAL APPLICATION SCREEN serves as the workspace for uploading not only the required elements that must be completed with each study submission, but also all relevant supporting documentation depending on the type of review. Please make sure to maintain electronic copies of all submitted documents. Below are links to the 6 steps required to submit a study for COMIRB review: you will find the links included again after each step in the process: A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB 3
4 A. ROUTING FORM To Edit the Routing Form: i. Click on the linked name of the ROUTING (EFORM) to open the routing form in a new browser window. ii. Select if you are submitting to CIRB, then choose the type of REVIEW being requested (Exempt/Expedited/Full Board) from the drop-down menu under heading labeled TYPE OF SUBMISSION. The Routing Form generates questions based on answers; if FULL BOARD is selected, then you will be prompted to answer a series of questions directing you to the appropriate panel. iii. If you find that you are unable to edit the Routing Form, click the CHECK OUT button in the top left hand corner of the form and ensure the form is checked out to you. iv. Click the button once all of the questions have been answered, and then check the COMPLETE box located in the upper right corner of the browser window. The Routing Form window will close and return to the COMPONENTS FOR INITIAL APPLICATION screen. 4
5 STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB 5
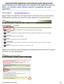
6 B. THE APPLICATION FOR PROTOCOL REVIEW DOWNLOAD, EDIT, AND UPLOAD THE APPLICATION FOR PROTOCOL REVIEW: i. Download the Application for Protocol Review. *To save the Application for Protocol Review, or if it says Please Wait, press the download icon in your internet browser to download the Application Form to your desktop, then open the document from there using Adobe Reader (Always use an Adobe product to edit the Application for Protocol Review). Download Icons - Firefox: icon in the top right-hand corner; Chrome: icon in the bottom righthand corner; Internet Explorer: icon in the top-left hand corner. ii. Open the Application for Protocol Review (subsequently referred to as APPLICATION FORM) and answer the questions as they pertain to the research protocol. Be sure to SAVE the application form in a specified location, either on a shared drive or on a local hard drive. iii. To upload the Application Form into the era(infoed) system, click linked word UPLOAD. iv. On the screen that appears, click on the button next to the subheading labeled LOCATION, and then navigate to the folder or location where you have saved the completed Application Form and select the file. (At this point you should be prepared to upload your completed form to the era(infoed) Website.) Please ignore the other fields on the upload page besides Location. v. Click the UPLOAD button to add the document to the INITIAL APPLICATION screen. Once the browser window has refreshed and uploaded the Application Form, the UPLOAD link changes to REPLACE and the status changes to COMPLETED. vi. Click the REPLACE link if you find that you need to replace the uploaded document with another version. 6
7 STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB 7
8 C. THE PROTOCOL Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) i. Download the PROTOCOL TEMPLATE from the COMIRB Forms Library and save it to your computer: Protocol Template Save the Protocol to either your computer or a simple drive location for uploading (the longer the path name, the more likely you will encounter trouble uploading documents). Download how to complete the COMIRB s protocol templates Biomedical and Social if you need additional help completing the protocol template. ii. iii. Open the Protocol Template (subsequently referred to as the PROTOCOL) and answer the questions as they pertain to the research project. Be sure to SAVE the protocol form in a specified location, either on a shared drive or on a local hard drive. Click the ADD link located on the INITIAL APPLICATION screen. iv. NAME the document to be uploaded in the provided field in the format: Protocol-v-Version Date (i.e. Protocol-v ) v. Click on the button next to the subheading labeled LOCATION, and then navigate to the folder or location where you have saved the completed application form, and select the file. (At this point you should be prepared to 8
9 upload your completed PROTOCOL to the era/(infoed) system.) vi. Choose the appropriate category: PROTOCOL. Disregard the other provided fields marked out with red x s in the screenshot above. Then, click the UPLOAD BUTTON. vii. Click the CLOSE BUTTON to complete the upload of the PROTOCOL. STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB 9
10 D. SUPPORTING DOCUMENTS FOR PROTOCOL REVIEW i. Click the ADD link located on the INITIAL APPLICATION screen. ii. NAME the supporting document to be uploaded in the provided field. *Please name the document according to our naming convention. COMIRB electronically stamps documents in the top righthand corner which covers any content in that area. Please do not place content in the top right-hand corner of documents. The table below includes a list of supporting documents that may apply to a review submission: Consent forms, assent forms, verbal consent scripts; templates can be found here. HIPAA Authorization B (if not using combined consent and HIPAA); templates can be found here Grant application (only when funded by a federal grant) Surveys, diaries, questionnaires, interview guides, scripts used with subjects (if applicable) Other committee approvals (if applicable, including SARC, PRMC, RDRC, IBC, CIR) Investigator drug brochure or package inserts (if applicable) Recruitment materials, advertisements, invitations to participate, informational materials subjects (if applicable); click here for advertisement guidance. If you are submitting an advertisement, please use our Advertisement Components Submission Form that can be found by clicking here. VA or Denver Health clearance letter (if research is conducted at or involves employees of these sites) Documentation of IND number or exempt (if applicable) FDA 1572 Form (if applicable) Background information for food supplements (if applicable) Device manual (if applicable) Documentation of IDE number, Non-significant risk device determination or IDE exemption (if applicable) Conflict of Interest management plan (if applicable) HIPAA Authorization A (if applicable) for recruiting subjects; templates can be found here 10
11 iii. Click on the button next to the subheading labeled LOCATION, and then navigate to the folder or location where you have saved the supporting document and select the file. Please submit supporting documents as separate computer files and do not combine them as a single file. Please do not upload Microsoft Office Documents in read-only format and please do not submit Microsoft Outlook.MSG files. iv. Choose the CATEGORY that most appropriately coincides with the supporting document. Disregard the other provided fields marked out with red x s in the screenshot above. v. Click the UPLOAD BUTTON to add the document to the INITIAL APPLICATION. Once the browser window has refreshed, click the CLOSE BUTTON to complete the upload of the document. vi. Click the linked document name to confirm that the supporting document can be opened and is readable. Click the REMOVE if you find that you need to completely remove the document from the submission. vii. Please always upload a COVER LETTER that generally outlines your request for review, includes any information you feel is important for the reviewer to be aware of, and includes an itemized list of all the documents included in the submission. STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB 11
12 E. Personnel Form Last Updated: December 15, 14 All individuals involved with the study should be included on the PERSONNEL FORM. It is the investigator s responsibility to ensure that the list of individuals included on the PERSONNEL FORM is accurate. Our Application Form only includes room to list 3 individuals. These 3 individuals, along with everyone else involved in the study, should be listed on the PERSONNEL FORM (EFORM). i. Click the linked document name to edit the PERSONNEL FORM (EFORM). ii. Click the button to add all of the personnel involved with your study, and click the button to remove personnel. When you click the button, the listed individuals are organized alphabetically according to LAST NAME. To select someone to add to the study, first select the first letter of the individuals last name from the list of letters, and then begin typing their last name into the Search for a particular entry field. The individual s name should then appear in the dropdown menu. Be careful when selecting a name; there may be more than one person with same name in the system. You may have to click on DROP-DOWN MENU to view other names. There may be multiple listings for the same person. Please select the one with the relevant department after the name for the research being conducted. iii. If an investigator has never been a part of a COMIRB study click here for information on obtaining access to the era(infoed) system and completing new investigator tasks. iv. Select an appropriate ROLE from the dropdown menu for each individual. Then, please make sure that the individuals you add have current Certifications that they completed the two required (starred) instructional courses: the COMIRB HS (Human Subjects Protection) course and HIPAA Course. The HIPAA Course needs to be completed only once, so as long as it is listed, it is valid; however, the COMIRB HS Course needs to be updated every 3 years, so it is important to pay attention to the end date for this Certification. 12
13 v. Please click here for information on how to update or complete Certifications for the courses, if necessary. Also, Conflict of Interest Disclosures need to be completed once per academic year. The system is reset each year September 1 st at which time a new disclosure needs to be made. Instructions on how to refresh CITI Courses and submit a Conflict of Interest Disclosure can be found by clicking here. vi. Please ignore the START DATE and END DATE marked out with red X s above. vii. If you find that you are unable to edit the Personnel Form, click the CHECK OUT button in the top left hand corner of the form and ensure the form is checked out to you. viii. Click the button after everyone has been added, and then check COMPLETE box located in the upper right corner of the browser window. The PERSONNEL FORM will close and return to the COMPONENTS FOR INITIAL APPLICATION screen. 13
14 STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB 14
15 F. PI ATTESTATION FORM The PI Attestation Form gives the ability for the Principal Investigator to have another individual listed on the study submit on behalf of the PI. i. This requirement can be fulfilled in several different ways depending on whether or not the PI plans to submit themselves or plans to have another member of the study team submit on behalf of the PI. Click the linked name of the PI ATTESTATION form for instructions on the different options to meet this requirement. Once you have decided which option is appropriate for the PI, click the UPLOAD link to upload the appropriate document. ii. Select the file location then click the Upload button. --CONTINUE TO NEXT PAGE-- 15
16 STEP 3: COMPLETE THE INITIAL APPLICATION SUBMISSION COMPONENTS A. Edit the required Routing (eform) B. Download, edit, and upload the Application for Protocol Review C. Download, edit, and upload the Protocol (not needed for Exempt/Non-human Subjects Submissions) D. Determine which supporting documents are needed for the review submission E. Complete the Personnel Form (eform) F. PI Attestation Form G. Submit to COMIRB Your submission should look approximately like this before you Submit to COMIRB (depending on the supporting documents you upload); the STATUS for the Forms should be COMPLETED: 16
17 G. SUBMIT AND CONFIRM COMIRB HAS RECEIVED THE SUBMISSION When the NECESSARY documents have been uploaded into the submission, you can SUBMIT to COMIRB. Please note that this can only be done by the Principal Investigator for the study unless the appropriate form has been completed and uploaded for the PI Attestation Form. i. Click the button located in upper right corner of the INITIAL APPLICATION screen. If no button is present, please call COMIRB at ii. Select ACCEPTED on the CERTIFICATION window that appears. Click the CONTINUE button once you have finished. iii. Be aware that the next screen you see ROUTE PATH SCREEN only shows you the route your protocol is going to take. Your protocol has not yet been submitted until you click submit. Please do not click the link Add New Person to Review Path marked out with a red X below. If the submit it greyed out, please contact COMIRB at : 17
18 iv. To confirm that COMIRB has received your submission. Check the STATUS in the top right-hand corner of the screen. If the status says IN DEVELOPMENT then the submission was not completed and you should click the submit button again or contact COMIRB at The PI should receive a submission confirmation , regardless of who submits. v. When you have completed your submission, make sure to click the button in the top left hand corner of the screen to close the submission, otherwise, no one else will be able to edit the study later. vi. Mostly, COMIRB correspondence will be made through your UCDenver.edu account at If you have forgotten your password, please visit If you are not CU Denver faculty or staff, you were assigned an account when you applied for a POI Number. 18
Mentor eirb Researcher User Manual
 Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
Ascension Wisconsin IRB Mentor eirb Researcher User Manual Contents 1 Introduction 1.1 Purpose of this Manual 1.2 User Support 2 Mentor eirb & User Accounts 2.1 About Mentor 2.2 User Roles 2.3 Requesting
SUBMITTING A NEW IBC PROJECT APPLICATION (INITIAL)
 SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
You may also scroll through the Table of Contents and click on the topic or question of interest.
 We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
Revised: February 28, Investigator/Coordinator Guide
 2014 Revised: February 28, 2014 Table of Contents Getting Started... 3 Navigational Tips... 4 Adding a New Application (Form 1)... 6 Routing Form... 11 Routing for Signatures... 15 Workflow Tracking and
2014 Revised: February 28, 2014 Table of Contents Getting Started... 3 Navigational Tips... 4 Adding a New Application (Form 1)... 6 Routing Form... 11 Routing for Signatures... 15 Workflow Tracking and
Route IRB Submission to Faculty Advisor
 Route IRB Submission to Faculty Advisor Updated: 7/25/2016 Contents Purpose... 2 Data Needed to Complete this Process... 2 Special Cases... 3 1) Respond to an Incomplete Determination... 3 PART 1: Login
Route IRB Submission to Faculty Advisor Updated: 7/25/2016 Contents Purpose... 2 Data Needed to Complete this Process... 2 Special Cases... 3 1) Respond to an Incomplete Determination... 3 PART 1: Login
NAU IRBNet Application
 NAU IRBNet Application Guidance for NAU Research Application Revised 17-0202 Go to IRBNet Website address: irbnet.org/release/index.html Login to your account - Enter your Username and Password and select
NAU IRBNet Application Guidance for NAU Research Application Revised 17-0202 Go to IRBNet Website address: irbnet.org/release/index.html Login to your account - Enter your Username and Password and select
NAU New Project Submission. Guidance for creating a new project
 NAU New Project Submission Guidance for creating a new project Go to IRBNet Website address: https://www.irbnet.org/release/index.html Login to your account Enter your Username and Password and select
NAU New Project Submission Guidance for creating a new project Go to IRBNet Website address: https://www.irbnet.org/release/index.html Login to your account Enter your Username and Password and select
ARC IRB Chair s Manual
 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
UVMClick IRB Study Submission Guide
 UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
UVMClick IRB Study Submission Guide September 2018 Table of Contents How to Login 3 How to Create a New Study 4 Find More Information 5 How to Edit a Study 6 Check the Study for Errors 7 Submit the Study
BOULDER IRB era InfoEd Amendments
 BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
BOULDER IRB era InfoEd Amendments Last Update: 2017/12/06 Preface: This guide explains how to submit an Amendment to modify an approved study. If you already have a submission pending review do not submit
Last revised: September 30, e-protocol User Guide 1
 e-protocol User Guide Last revised: September 30, 2015 e-protocol User Guide 1 e-protocol is an electronic system for submitting and monitoring the status of Institutional Review Board (IRB) submissions.
e-protocol User Guide Last revised: September 30, 2015 e-protocol User Guide 1 e-protocol is an electronic system for submitting and monitoring the status of Institutional Review Board (IRB) submissions.
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
Click. IRB Study Submission Guide
 Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
Click IRB Study Submission Guide October 2016 Table of Contents Logging In... 3 Creating a New Study... 4 Finding More Information... 5 Editing a Study... 5 Checking the Study for Errors... 6 Submitting
New Protocol - expedited & full board
 New Protocol - expedited & full board training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Expedited & Full Board 1 table of contents Table of Contents 2 table
New Protocol - expedited & full board training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Expedited & Full Board 1 table of contents Table of Contents 2 table
BOULDER IRB era InfoEd Continuing Review
 BOULDER IRB era InfoEd Continuing Review Last Update: 2017/11/30 Preface: This guide explains how to submit a continuing review for a previously approved expedited or full board study that is nearing expiration.
BOULDER IRB era InfoEd Continuing Review Last Update: 2017/11/30 Preface: This guide explains how to submit a continuing review for a previously approved expedited or full board study that is nearing expiration.
COEUS LITE IRB COEUS 4.5.1_P3 USER GUIDE
 COEUS LITE IRB COEUS 4.5.1_P3 USER GUIDE Version: August 24, 2016 1 TABLE OF CONTENTS A note about this guide... 4 Getting access to Coeus Lite... 4 Preparing to enter a protocol... 4 Getting Started:
COEUS LITE IRB COEUS 4.5.1_P3 USER GUIDE Version: August 24, 2016 1 TABLE OF CONTENTS A note about this guide... 4 Getting access to Coeus Lite... 4 Preparing to enter a protocol... 4 Getting Started:
retrieving study documents training guide
 retrieving study documents training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this document 4 retrieving study documents 5 current
retrieving study documents training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this document 4 retrieving study documents 5 current
Kuali Research User Guide: Create an IRB Protocol
 Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
Kuali Research User Guide: Create an IRB Protocol Version.0: November 06 Purpose: To create an IRB Protocol document to be used for tracking new Human Subjects Research requests. Trigger / Timing / Frequency:
ANNUAL PROGRESS REPORT SUBMISSIONS
 SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
SUBMISSIONS The submission of an annual report of an open study requires the creation of a subsequent PACKAGE in a project. As part of the DU IACUC post-approval monitoring program, all IACUC-approved
IRBManager Quick Start Guide INITIAL APPLICATION - OVERVIEW
 Page 1 of 16 GENERAL INFORMATION IRBManager Quick Start Guide INITIAL APPLICATION - OVERVIEW Initial Application Types: The IRBManager initial application form (xform) is available for specific types of
Page 1 of 16 GENERAL INFORMATION IRBManager Quick Start Guide INITIAL APPLICATION - OVERVIEW Initial Application Types: The IRBManager initial application form (xform) is available for specific types of
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol contact:
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
Institutional Review Board (IRB)
 IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol, contact:
IRBNet User s Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org If you have any questions regarding the submission of an IRB protocol, contact:
Drexel University. Version April Page 1 of 23. Version April agf
 Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Drexel University Page 1 of 23 TABLE OF CONTENTS Getting Started 3 IRB Protocol Committee Member Reviewer..4 Review IRB Protocol Submission..6 Enter Reviewer Comments... 7 Upload Reviewer Attachments......9
Reviewers Guide on Clinical Trials
 Reviewers Guide on Clinical Trials Office of Research Integrity & Compliance Version 2 Updated: June 26, 2017 This document is meant to help board members conduct reviews for Full Board: Clinical Trial
Reviewers Guide on Clinical Trials Office of Research Integrity & Compliance Version 2 Updated: June 26, 2017 This document is meant to help board members conduct reviews for Full Board: Clinical Trial
***** ***** June
 SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
SLU eirb Investigator Guide Saint Louis University ***** eirb Investigator Submitter Guide ***** Institutional Review Board June 2011 http://eirb.slu.edu Institutional Review Board Saint Louis University
UNIVERSITY OF NORTH FLORIDA. Institutional Review Board (IRB) Guide to IRBNet:
 UNIVERSITY OF NORTH FLORIDA Institutional Review Board (IRB) Guide to IRBNet: Version 2 11-15-2013 Table of Contents i Introduction 2 IRBNet User Registration 2 IRBNet ID Number 2 Training and Credentials
UNIVERSITY OF NORTH FLORIDA Institutional Review Board (IRB) Guide to IRBNet: Version 2 11-15-2013 Table of Contents i Introduction 2 IRBNet User Registration 2 IRBNet ID Number 2 Training and Credentials
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Human Subjects Development Guide
 Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
Research Integrity & Compliance Institutional Review Board Human Subjects Development Guide ERA Version 15, Nov. 2016 Table of Contents Logging In... 3 Adding a Delegate... 4 Viewing Items as a Delegate...
eprmc User Manual For Investigators and Research Staff
 eprmc User Manual For Investigators and Research Staff Table of Contents How to Get an Account... 3 Logging In... 3 Overview of the eprmc Database... 4 Searching for a Protocol... 5 Adding a New Protocol...
eprmc User Manual For Investigators and Research Staff Table of Contents How to Get an Account... 3 Logging In... 3 Overview of the eprmc Database... 4 Searching for a Protocol... 5 Adding a New Protocol...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
University of North Florida
 Last edited by: Kayla Champaigne Last edited on: December 5, 2012 University of North Florida Institutional Review Board IRB Protocol Please note, this is a sample of the North Florida - IRB Protocol output.
Last edited by: Kayla Champaigne Last edited on: December 5, 2012 University of North Florida Institutional Review Board IRB Protocol Please note, this is a sample of the North Florida - IRB Protocol output.
The most current protocol information should be entered. This includes incorporation of all past amendments and modifications.
 Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL
 Page 1 of 12 IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL NOTE: This Quick Start Guide provides instructions for removing and/or adding personnel, other than the Principal Investigator,
Page 1 of 12 IRBManager Quick Start Guide AMENDMENT SUBMISSION - CHANGE IN PERSONNEL NOTE: This Quick Start Guide provides instructions for removing and/or adding personnel, other than the Principal Investigator,
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS
 STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
STREAMLYNE GUIDE FOR STUDENTS/PRINCIPAL INVESTIGATORS Rev: 01/2017 In This Document Logging In... 1 Creating a New Protocol... 2 Revising a Returned Protocol... 7 Submitting an Amendment or Renewal Application...
Nova Southeastern University IRBManager User Manual IRBMANAGER USER MANUAL
 IRBMANAGER USER MANUAL Contents 1. What is IRBManager?...1 2. IRBManager User Accounts/Login...1 a. Creating an IRBManager Account...1 b. Logging into IRBManager...2 c. Forgot Password...2 d. Locked Out
IRBMANAGER USER MANUAL Contents 1. What is IRBManager?...1 2. IRBManager User Accounts/Login...1 a. Creating an IRBManager Account...1 b. Logging into IRBManager...2 c. Forgot Password...2 d. Locked Out
Drexel University. Page 1 of 48. Version November agf
 Drexel University Page 1 of 48 TABLE OF CONTENTS Getting Started. 3 Creating a New Protocol.. 4 General Information... 7 Organization Information... 9 Investigators/Study Personnel.. 10 Correspondents
Drexel University Page 1 of 48 TABLE OF CONTENTS Getting Started. 3 Creating a New Protocol.. 4 General Information... 7 Organization Information... 9 Investigators/Study Personnel.. 10 Correspondents
Top Ten Questions About IRBNet.org
 Top Ten Questions About IRBNet.org 1. How do I register? Registration is easy. To register, simply navigate your web browser www.irbnet.org. The login area for IRBNet is in the upper right corner of the
Top Ten Questions About IRBNet.org 1. How do I register? Registration is easy. To register, simply navigate your web browser www.irbnet.org. The login area for IRBNet is in the upper right corner of the
Weill Research Gateway
 Table of Contents The - What is the? - Logging In to WRG - The WRG Homepage Conflicts of Interest - What are Conflicts of Interest? - Submitting your Conflicts Survey - Submitting a Travel Disclosure -
Table of Contents The - What is the? - Logging In to WRG - The WRG Homepage Conflicts of Interest - What are Conflicts of Interest? - Submitting your Conflicts Survey - Submitting a Travel Disclosure -
IRMA Human Ethics Researcher User Guide
 IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
IRMA Human Ethics Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets 2.02 Templates
FAQs. Q What is the status of my study application?
 FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
FQs Q What is the status of my study application? One of the best things about the eirb system is the transparency regarding the status of your study at any point through the application process. You can
Romeo. How to Apply for a Faculty Conference Travel Grant
 Romeo How to Apply for a Faculty Conference Travel Grant Please note: Romeo is compatible with Internet Explorer, Firefox, Edge, Google Chrome and Safari. If you have any problems or questions, please
Romeo How to Apply for a Faculty Conference Travel Grant Please note: Romeo is compatible with Internet Explorer, Firefox, Edge, Google Chrome and Safari. If you have any problems or questions, please
IRMA Researcher User Guide v2 DRAFT. IRMA Researcher User Guide
 IRMA Researcher User Guide v2 IRMA Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets
IRMA Researcher User Guide v2 IRMA Researcher User Guide IRMA Researcher User Guide 1. Overview 1.01 What is IRMA? 1.02 What are the Benefits? 1.03 ISLHD Research and IRMA 2. Key Terms in IRMA 2.01 Coversheets
Heart Foundation Walking Website user guide for Local Coordinators
 Heart Foundation Walking Website user guide for Local Coordinators Proudly supported by Website User Guide for Local Coordinator Contents 1. Introduction... 2 1.1 Finding the HFW website... 2 1.2 Log in
Heart Foundation Walking Website user guide for Local Coordinators Proudly supported by Website User Guide for Local Coordinator Contents 1. Introduction... 2 1.1 Finding the HFW website... 2 1.2 Log in
Buck-IRB Amendment User Guide
 Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
Office of Research Buck-IRB Amendment User Guide Office of Responsible Research Practices 1960 Kenny Road, Columbus, OH 43210-1016 Institutional Review Board General Guidance for Amendments: The Start
eibc Program User Guide
 eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
Portal/Extranet User Guide for Clients
 Portal/Extranet User Guide for Clients Welcome to the ichannel Portal/Extranet. This guide will walk you through logging into your personalized, secure portal/extranet site. It will also show you how to
Portal/Extranet User Guide for Clients Welcome to the ichannel Portal/Extranet. This guide will walk you through logging into your personalized, secure portal/extranet site. It will also show you how to
Version GENESIS HEALTH SYSTEM. Institutional Review Board (IRB) IRBNet User s Guide
 Version 1 7-1-2012 GENESIS HEALTH SYSTEM Institutional Review Board (IRB) IRBNet User s Guide G E N E S I S H E A L T H S Y S T E M I N S T I T U T I O N A L R E V I E W B O A R D IRBNet User s Guide Genesis
Version 1 7-1-2012 GENESIS HEALTH SYSTEM Institutional Review Board (IRB) IRBNet User s Guide G E N E S I S H E A L T H S Y S T E M I N S T I T U T I O N A L R E V I E W B O A R D IRBNet User s Guide Genesis
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK. (Version date 03/03/2006)
 ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
ELECTRONIC RESEARCH STUDY APPLICATION (ERSA) INITIAL APPLICATION GUIDEBOOK (Version date 03/03/2006) Table of Contents ERSA Home Page and Login 3 CRC Desktop 4 Creating a: New Study Application 5 Amendment
renew & Amend A Protocol training guide
 renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
renew & Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before You Begin Hyperlinks The Table
University of the Sciences. IRBNet User s Guide
 University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
University of the Sciences IRBNet User s Guide Instructions for On-line Submission of Research Protocols to the Research Committees - IRB, IACUC or IBC. www.irbnet.org If you have any questions regarding
WITH MODIFICATIONS ), S, PHONE NUMBERS OR OTHER FORMS, PLEASE VISIT THE IRB S WEBSITE AT:
 Modification Request Dominican College Institutional Review Board (IRB) IRB #: Date Received: IRB Approval Name & Signature: FOR MORE IRB INSTRUCTIONS, APPLICATIONS (including the RENEWAL FORM that should
Modification Request Dominican College Institutional Review Board (IRB) IRB #: Date Received: IRB Approval Name & Signature: FOR MORE IRB INSTRUCTIONS, APPLICATIONS (including the RENEWAL FORM that should
PEOPLEADMIN SELECTSUITE USER MANUAL
 PEOPLEADMIN SELECTSUITE USER MANUAL TABLE OF CONTENTS OVERVIEW INTRODUCTION 1 CHAPTER 1 GETTING STARTED 2 1.1 LOGIN 2 1.2 ACCESS 2 1.3 USER GROUPS 4 1.4 NAVIGATION 4 1.5 MODULES 5 1.6 PAGE ORGANIZATION
PEOPLEADMIN SELECTSUITE USER MANUAL TABLE OF CONTENTS OVERVIEW INTRODUCTION 1 CHAPTER 1 GETTING STARTED 2 1.1 LOGIN 2 1.2 ACCESS 2 1.3 USER GROUPS 4 1.4 NAVIGATION 4 1.5 MODULES 5 1.6 PAGE ORGANIZATION
Submitting a Response to Modifications Required by the IRB to Secure Approval
 Submitting a Response to Modifications Required by the IRB to Secure Approval If you have submitted an item to the IRB, and the IRB has responded with a Modifications Required to Secure Approval memorandum,
Submitting a Response to Modifications Required by the IRB to Secure Approval If you have submitted an item to the IRB, and the IRB has responded with a Modifications Required to Secure Approval memorandum,
Once you have created a Project, you must submit the Project s first Package to the ORA.
 CREATING, SIGNING, AND SUBMITTING PACKAGE #1 Once you have created a Project, you must submit the Project s first Package to the ORA. CREATE PACKAGE #1 1. Go to the Designer page. This is where an IRB
CREATING, SIGNING, AND SUBMITTING PACKAGE #1 Once you have created a Project, you must submit the Project s first Package to the ORA. CREATE PACKAGE #1 1. Go to the Designer page. This is where an IRB
DISCOVR-e USER MANUAL. Vanderbilt University Human Research Protection Program
 DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
DISCOVR-e USER MANUAL Vanderbilt University Human Research Protection Program Table of Contents Introduction and Overview... 3 Log into the System... 4 Investigator Dashboard... 5 Submitting a New Study...
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review
 Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
CITY UNIVERSITY OF NEW YORK. i. Visit:
 CITY UNIVERSITY OF NEW YORK I. ACCESSING IRB NET (New Registration) i. Visit: https://www.irbnet.org/release/index.html ii. New users: Click on New Registration in the top right corner iii. Fill-out the
CITY UNIVERSITY OF NEW YORK I. ACCESSING IRB NET (New Registration) i. Visit: https://www.irbnet.org/release/index.html ii. New users: Click on New Registration in the top right corner iii. Fill-out the
College Deans, Department Chairs & Supervising Faculty
 Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
STUDY ASSISTANT. Adding a New Study & Submitting to the Review Board. Version 10.03
 STUDY ASSISTANT Adding a New Study & Submitting to the Review Board Version 10.03 Contents Introduction... 3 Add a Study... 3 Selecting an Application... 3 1.0 General Information... 3 2.0 Add Department(s)...
STUDY ASSISTANT Adding a New Study & Submitting to the Review Board Version 10.03 Contents Introduction... 3 Add a Study... 3 Selecting an Application... 3 1.0 General Information... 3 2.0 Add Department(s)...
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE
 Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Pennsylvania. Special Olympics Pennsylvania - Vsys Software User Manual
 Pennsylvania Special Olympics Pennsylvania - Vsys Software User Manual Applicable to all Vsys software programs including Terminal Service, Vsys One, Vsys Anywhere, and the Online Volunteer Portal Created
Pennsylvania Special Olympics Pennsylvania - Vsys Software User Manual Applicable to all Vsys software programs including Terminal Service, Vsys One, Vsys Anywhere, and the Online Volunteer Portal Created
JDRF Operations External FAQs Preaward and Postaward
 JDRF Operations External FAQs Preaward and Postaward Page 1 RMS360 Preaward FAQ Application Content 1. How do I apply for a grant? 2. How do I add my RO (Research Office contact) and FO (Finance Officer)
JDRF Operations External FAQs Preaward and Postaward Page 1 RMS360 Preaward FAQ Application Content 1. How do I apply for a grant? 2. How do I add my RO (Research Office contact) and FO (Finance Officer)
Institutional Planning Process Software Navigation Guide Annual Program Plan and Program Review Plan
 Institutional Planning Process Software Navigation Guide Annual Program Plan and Program Review Plan 2018-2019 Contents INTRODUCTION...3 Accessing Taskstream...3 Rio Hondo College s Taskstream Home Page...4
Institutional Planning Process Software Navigation Guide Annual Program Plan and Program Review Plan 2018-2019 Contents INTRODUCTION...3 Accessing Taskstream...3 Rio Hondo College s Taskstream Home Page...4
How to use People Admin Creating Staff Postings. How to Create Staff Postings How to Select Applicants for interview 25 26
 How to use People Admin Creating Staff Postings Index Page How to Create Staff Postings 2 24 How to Select Applicants for interview 25 26 How to Recommend a Candidate for Hire 27 How to Complete Hiring
How to use People Admin Creating Staff Postings Index Page How to Create Staff Postings 2 24 How to Select Applicants for interview 25 26 How to Recommend a Candidate for Hire 27 How to Complete Hiring
Applying for Funding in Fluxx. Quick Start Instructions
 Applying for Funding in Fluxx Quick Start Instructions GETTING STARTED The Hogg Foundation Fluxx Grant Portal is optimized for use with Chrome or Safari browsers and using another browser may cause technical
Applying for Funding in Fluxx Quick Start Instructions GETTING STARTED The Hogg Foundation Fluxx Grant Portal is optimized for use with Chrome or Safari browsers and using another browser may cause technical
PCORI Online: Awardee User Guide Research Awards
 PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
Nova Scotia Health Authority Research Ethics Board. Researcher s (PI) User Manual
 Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Filing Forms Electronically COGCC Denver, CO
 Filing Forms Electronically COGCC Denver, CO 303-894-2100 First Time Users: Set-up and Create Users First-time users will need to install Silverlight. Go to https://cogcc.state.co.us/eform/, the site will
Filing Forms Electronically COGCC Denver, CO 303-894-2100 First Time Users: Set-up and Create Users First-time users will need to install Silverlight. Go to https://cogcc.state.co.us/eform/, the site will
New Protocol - emergency use training guide
 New Protocol - emergency use training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Emergency Use 1 table of contents Table of Contents 2 table of contents 3 about
New Protocol - emergency use training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Emergency Use 1 table of contents Table of Contents 2 table of contents 3 about
New Protocol - NoN-humaN subjects research training guide
 New Protocol - NoN-humaN subjects research training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 table of contents Table of Contents
New Protocol - NoN-humaN subjects research training guide 2015, The Trustees of Indiana University KC IRB Training Guide: New Protocol - Non-Human Subjects Research 1 table of contents Table of Contents
VISUAL ARTS & NEW MEDIA INDIVIDUAL PROJECTS
 VISUAL ARTS & NEW MEDIA INDIVIDUAL PROJECTS INSTRUCTIONS FOR GATE ONLINE APPLICATIONS If you are a FIRST-TIME user, please read through the guide in full BEFORE starting your application. If you a RETURNING
VISUAL ARTS & NEW MEDIA INDIVIDUAL PROJECTS INSTRUCTIONS FOR GATE ONLINE APPLICATIONS If you are a FIRST-TIME user, please read through the guide in full BEFORE starting your application. If you a RETURNING
PeopleAdmin Search Committee Access Guide
 PeopleAdmin Search Committee Access Guide To log into your PeopleAdmin account go to https://jobs.ncat.edu/hr. At the log in screen you will need your username and password. Your username is your email
PeopleAdmin Search Committee Access Guide To log into your PeopleAdmin account go to https://jobs.ncat.edu/hr. At the log in screen you will need your username and password. Your username is your email
Amend A Protocol training guide
 Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Amend A Protocol training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 About this guide 4 login 5 Before you Begin Hyperlinks The Table of
Guide for Researchers: Online Human Ethics Application Form
 Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
Guide for Researchers: Online Human Ethics Application Form What is Quest Quest is our comprehensive research management system used to administer and support research activity at Victoria University.
Guide for Researchers: Online Human Ethics Application Form
 Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
FIELDS REQUIRED TO SAVE A PROTOCOL
 Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
Human Subject Research Protocols If conducting human subject research, a protocol must be submitted in WVU+kc to the WVU Institutional Review Board for review. Click on the Create IRB Protocol link located
University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Update a Pre-Proposal for Full Submission
 University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Update a Pre-Proposal for Full Submission In order to obtain external funding for their research programs, sometimes investigators
University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Update a Pre-Proposal for Full Submission In order to obtain external funding for their research programs, sometimes investigators
HIRING MANAGER S JOB SITE USER S GUIDE. Fitchburg State University Hiring System
 HIRING MANAGER S JOB SITE USER S GUIDE Fitchburg State University Hiring System TABLE OF CONTENTS INTRODUCTION... 3 GETTING STARTED... 5 CREATING A POSTING.7 Creating Posting from Position Type... 7 Posting
HIRING MANAGER S JOB SITE USER S GUIDE Fitchburg State University Hiring System TABLE OF CONTENTS INTRODUCTION... 3 GETTING STARTED... 5 CREATING A POSTING.7 Creating Posting from Position Type... 7 Posting
Publisher Onboarding Kit
 Publisher Onboarding Kit Smart content. Smart business. Publishing, Supporting & Selling HotDocs Market Templates A HotDocs Market publisher s guide for loading templates, answering customer questions
Publisher Onboarding Kit Smart content. Smart business. Publishing, Supporting & Selling HotDocs Market Templates A HotDocs Market publisher s guide for loading templates, answering customer questions
BHSF Physician User Guide
 PHYSICIAN GUIDE BHSF Physician User Guide The only requirement to use Ambra is a computer with Internet access. When using the web uploader, a JAVA plug- in (already installed on most computers) is required
PHYSICIAN GUIDE BHSF Physician User Guide The only requirement to use Ambra is a computer with Internet access. When using the web uploader, a JAVA plug- in (already installed on most computers) is required
IRB APPLICATION INSTRUCTIONS & TEMPLATES
 1 Contents: Items Required for Every Application 2 Synopsis Template Instructions 3 Link to editable Synopsis Template (Word format) Other Types of Documentation to Submit 5 Checklist for Consent Forms
1 Contents: Items Required for Every Application 2 Synopsis Template Instructions 3 Link to editable Synopsis Template (Word format) Other Types of Documentation to Submit 5 Checklist for Consent Forms
PISA 2018 COMPUTER-BASED SCHOOL QUESTIONNAIRE: PRINCIPAL S MANUAL
 P 2 I S A 0 1 8 PISA 2018 COMPUTER-BASED SCHOOL QUESTIONNAIRE: PRINCIPAL S MANUAL Doc.: CY7_CBA_ScQPrincipalManual.docx Produced by ETS, Core A Contractor TABLE OF CONTENTS Part 1 Introduction Introduction
P 2 I S A 0 1 8 PISA 2018 COMPUTER-BASED SCHOOL QUESTIONNAIRE: PRINCIPAL S MANUAL Doc.: CY7_CBA_ScQPrincipalManual.docx Produced by ETS, Core A Contractor TABLE OF CONTENTS Part 1 Introduction Introduction
Research Management Information System (RIMS) Human Ethics - User Guide
 Research Management Information System (RIMS) Human Ethics - User Guide Contents How to create an Initial Application to the HREC... 2 Step 1: Create a new protocol... 2 Step 2: Title... 3 Step 3: Chief
Research Management Information System (RIMS) Human Ethics - User Guide Contents How to create an Initial Application to the HREC... 2 Step 1: Create a new protocol... 2 Step 2: Title... 3 Step 3: Chief
Student Guide to ebear powered by Symplicity
 Student Guide to ebear powered by Symplicity Center for Career Development C-224 Montebello Complex 443.885.3110 careers@morgan.edu www.morgan.edu/careerdevelopment TABLE OF CONTENTS LOGGING IN... 2 HOME
Student Guide to ebear powered by Symplicity Center for Career Development C-224 Montebello Complex 443.885.3110 careers@morgan.edu www.morgan.edu/careerdevelopment TABLE OF CONTENTS LOGGING IN... 2 HOME
Quick-Start Guide: Applying for a Grant Using the Grants Ontario System
 Grants Ontario has recently made some changes to the application process. Below you will find a quick-reference guide to completing your grant application. Please navigate to: http://www.grants.gov.on.ca
Grants Ontario has recently made some changes to the application process. Below you will find a quick-reference guide to completing your grant application. Please navigate to: http://www.grants.gov.on.ca
NEW PROTOCOL - HUMANITARIAN USE DEVICE (HUD) TRAINING GUIDE
 NEW PROTOCOL - HUMANITARIAN USE DEVICE (HUD) TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol Humanitarian Use Device (HUD) 1 Table of Contents Table of Contents
NEW PROTOCOL - HUMANITARIAN USE DEVICE (HUD) TRAINING GUIDE 2014, The Trustees of Indiana University KC IRB Training Guide: New Protocol Humanitarian Use Device (HUD) 1 Table of Contents Table of Contents
copying a protocol in Kc irb training guide
 copying a protocol in Kc irb training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this guide 4 copying a protocol 5 protocol actions
copying a protocol in Kc irb training guide 2015, The Trustees of Indiana University 1 table of contents Table of Contents 2 table of contents 3 about this guide 4 copying a protocol 5 protocol actions
ROCHE/GENENTECH PRACTICAL WORKING GUIDE FOR REQUESTORS
 ROCHE/GENENTECH PRACTICAL WORKING GUIDE FOR REQUESTORS Contents I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 3 III. HOW TO SUBMIT AN APPLICATION... 7 IV. HOW TO PROVIDE ADDITIONAL STUDY INFORMATION...
ROCHE/GENENTECH PRACTICAL WORKING GUIDE FOR REQUESTORS Contents I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 3 III. HOW TO SUBMIT AN APPLICATION... 7 IV. HOW TO PROVIDE ADDITIONAL STUDY INFORMATION...
Protocol Management System (PMS) Investigator User Guide. Harris Health System Administrative Approval
 Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Protocol Management System (PMS) Investigator User Guide Harris Health System Administrative Approval November 2013 eprotocol - Investigator User Guide 2 Table of Contents 1. INTRODUCTION 3 2. STARTING
Easy Survey Creator: User s Guide
 Easy Survey Creator: User s Guide The Easy Survey Creator software is designed to enable faculty, staff, and students at the University of Iowa Psychology Department to quickly and easily create surveys
Easy Survey Creator: User s Guide The Easy Survey Creator software is designed to enable faculty, staff, and students at the University of Iowa Psychology Department to quickly and easily create surveys
Special Purpose User Guide
 Special Purpose User Guide Table of Contents 1 Instructions for Logging in... 3 1.1 Link to the EYCC Funds Management System... 3 1.2 Logging In... 3 2 Transformation Application... 4 2.1 Project Details...
Special Purpose User Guide Table of Contents 1 Instructions for Logging in... 3 1.1 Link to the EYCC Funds Management System... 3 1.2 Logging In... 3 2 Transformation Application... 4 2.1 Project Details...
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
IRBNet Picture Guide
 IRBNet Picture Guide Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org For any questions or concerns regarding your submission, visit CSUSM
IRBNet Picture Guide Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org For any questions or concerns regarding your submission, visit CSUSM
From the Online Tools list, scroll down to SBS Connect, and click on the Register for SBS Connect link. The SBS Connect login screen loads.
 SBS EXTERNAL HEALTHCARE REVIEW USER GUIDE Create New Account Register an Entity View Attachment Upload Attachment SBS CONNECT CREATE NEW ACCOUNT Before using SBS Connect for the first time, 1) create an
SBS EXTERNAL HEALTHCARE REVIEW USER GUIDE Create New Account Register an Entity View Attachment Upload Attachment SBS CONNECT CREATE NEW ACCOUNT Before using SBS Connect for the first time, 1) create an
Velos ecompliance. Researcher User Manual
 Velos ecompliance Researcher User Manual Table of Contents Disclaimer... 4 ecompliance Overview... 5 Contacts... 6 Administrator... 6 Super Users... 6 IT/Technical Support... 6 Quick Help... 7 Getting
Velos ecompliance Researcher User Manual Table of Contents Disclaimer... 4 ecompliance Overview... 5 Contacts... 6 Administrator... 6 Super Users... 6 IT/Technical Support... 6 Quick Help... 7 Getting
irb.unm.edu
 IRBNet Submission Instructions The purpose of this document is to provide IRBNet submission instructions for UNM researchers using IRBNet. Please contact the Office of the IRB for assistance: irb.unm.edu
IRBNet Submission Instructions The purpose of this document is to provide IRBNet submission instructions for UNM researchers using IRBNet. Please contact the Office of the IRB for assistance: irb.unm.edu
