Haemodynamics and the Site Specificity of Aneurysmal Disease in the Iliac Arteries
|
|
|
- Caitlin Banks
- 6 years ago
- Views:
Transcription
1 Haemodynamics and the Site Specificity of Aneurysmal Disease in the Iliac Arteries Lachlan J. Kelsey School of Mechanical and Chemical Engineering, The University of Western Australia Supervisor: Dr. Barry J. Doyle Intelligent Systems for Medicine Laboratory, School of Mechanical and Chemical Engineering, The University of Western Australia Final Year Project School of Mechanical and Chemical Engineering, The University of Western Australia Submitted: 26 th May 2014
2 Summary An aneurysm is a localised dilation of an artery wall that is life threatening when ruptured. Iliac artery aneurysms (IAA) may occur in isolation, but often accompany other large artery aneurysms, such as abdominal aortic aneurysms (AAA). For people over 65 years, the prevalence of AAA is high, at approximately 5 6% for men and 1 2% for women. Of the iliac arteries, aneurysms often occur in the common and internal iliac arteries, but are rarely found in the external iliac arteries. These three arteries are in close proximity to each another, and there is no obvious explanation as to why aneurysms form in certain arteries and not others. The aim of this investigation is to examine the site specificity of aneurysmal disease in the iliac arteries using computational fluid dynamic (CFD) models. The commercial CFD solver, STAR-CCM+ was used to calculate the three-dimensional, unsteady numerical solutions to the Navier-Stokes equations for laminar, pulsatile blood flow through both a healthy and a diseased patient arterial geometry. The modelling methodology focused on commonly-used and recommended practices associated with large artery CFD. The Time- Averaged Wall Shear Stress (TAWSS), maximal wall shear stress and Oscillatory Shear Index (OSI) were investigated and compared with the physical stresses and flow phenomena commonly associated with aneurysmal disease. The results support the low-flow, or low wall shear stress theory for the site specificity of aneurysmal progression. Only aneurysmal-prone regions are afflicted with areas of TAWSS low enough for the onset of monocyte adhesion; associated with a local inflammatory response and the degradation of the extracellular matrix. Also, the aneurysmal-prone regions show no focal increases in wall shear stress values (that is, there is no indication of high-flow aneurysmal progression). While there are some improvements that should be made to the models, the results provide a strong foundation for future CFD investigations into aneurysmal disease within the iliac arteries. i
3 Letter of Transmittal Lachlan J. Kelsey 112 Eric Street Cottesloe, WA, th May, 2014 Winthrop Professor John Dell Dean Faculty of Engineering, Computing and Mathematics University of Western Australia 35 Stirling Highway Crawley, WA, 6009 Dear Professor Dell I am pleased to submit this thesis, entitled Haemodynamics and the Site Specificity of Aneurysmal Disease in the Iliac Arteries, as part of the requirement for the degree of Bachelor of Engineering. Yours Sincerely Lachlan J. Kelsey ii
4 Acknowledgements I would like to acknowledge my supervisor Dr. Barry Doyle for providing the arterial geometries used in this study, and thank him for his readily available support and the enthusiasm that accompanied it. I would also like to extend my gratitude to Peter Ewing of CD-adapco Australia for providing the use of the commercial CFD package STAR-CCM+, a machine to run it on and his time spent discussing CFD concepts in detail. Kynan Maley of CD-adapco Australia must also be thanked for his support. Finally, I offer special thanks to my family, and especially Bonnie Kelsey for their on-going support. iii
5 Table of Contents Summary... i Letter of Transmittal... ii Acknowledgements... iii Table of Contents... iv Nomenclature... vi List of Abbreviations... vi List of Medical Definitions... vi 1 Introduction Literature Review Haeomodynamics and Arterial Biology Arterial Branching Structure Methods for Measuring Wall Shear Stress Computational Fluid Dynamics and Haemodynamics Initial Work Process Geometry and Boundary Conditions Physical Approximations Initial Conditions Meshing Monitoring Steady State Convergence Results and Discussion Arterial Modelling Process Geometries Meshing Physical Approximations and Boundary Conditions Wall Shear Stress and the Oscillatory Shear Index Results and Discussion Analysis of CFD Practices Used Maximal WSS and Time-Averaged Wall Shear Stress Fields Oscillatory Shear Index and Time-Averaged Wall Shear Stress Limitations iv
6 4 Conclusions and Future Work References Appendices Appendix A Appendix B v
7 Nomenclature List of Abbreviations AAA CC CFD GCI IA IAA ILT NO OSI PL SMC STL TAWSS WSS Abdominal Aortic Aneurysm Circumferential Cells Computational Fluid Dynamics Grid Convergence Index Iliac Artery Iliac Artery Aneurysm Intraluminal Thrombus Nitric Oxide Oscillatory Shear Index Prism Layer Smooth Muscle Cells Stereo-Lithography Time-Averaged Wall Shear Stress (Magnitude) Wall Shear Stress List of Medical Definitions Aneurysm Atherogenesis Atherogenic Atheroprotective Atherosclerosis Diastole A local dilation of an artery wall The formation of sub-intimal plaques (lipid deposits) in the lining of arteries A phenotype that promotes atherogenesis A phenotype that promotes protection against atherosclerosis/atherogenesis A progressive disease characterised by the accumulation of lipids and fibrous elements in the large arteries The phase of the cardiac cycle where the heart relaxes and blood fills its chambers vi
8 Hypoplastic Leukocyte Lumen Macrophage Monocyte Phenotype Systole Thrombus Underdevelopment or incomplete development of a tissue or organ Another name for a white blood cell The inside space of an artery Cells produced by the differentiation of monocytes in tissues The largest of all leukocytes An expression of specific traits pertaining to an organisms genetic make-up and surrounding environment The phase of the cardiac cycle where blood is pumped from the heart to the arteries A blood clot vii
9 1 Introduction An aneurysm is a localised dilation of an artery wall which is life threatening when ruptured. Aneurysms in the iliac arteries can occur in isolation, or in association with other large vessel aneurysms, such as abdominal aorta or femoral artery aneurysms (Kirkwood 2013). Large-scale population screening studies of abdominal aortic aneurysms (AAA) show that, for people older than 65 years, the prevalence of an AAA is approximately 5 6% in men and 1 2% in women. In 25% of these cases, sufferers also have aneurysms in one or both common iliac arteries; and in 7% of these cases the sufferers also have aneurysms in the internal iliac arteries (Norman & Powell 2010). Aneurysms are often found in the common and internal iliac arteries, but are rarely found in the external iliac arteries (Figure 1-1) (Norman & Powell 2010). Figure 1-1: Diagram showing healthy branches of the iliac arteries (a. = artery). (Fitzgerald 2014). 1
10 The underlying causes of most aneurysms in medium-to-large arteries are unknown. Specific pathological causes are generally only identified in a small number of cases. Interestingly, the distribution of iliac artery aneurysms (IAA) coincides with those arteries involved in placental circulation (Norman & Powell 2010). Regarding this, both the common and internal iliac arteries are subjected to accelerated structural adaption during early life, which encourages the formation of calcified incrustations in these vessels. This may impair the normal remodelling of these arteries during growth (Meyer & Lind 1972). However, other than this association, the site specificity of aneurysmal disease in the iliac arteries has not been given much attention. The aim of this investigation is to examine the site specificity of aneurysmal disease in the iliac arteries using computational fluid dynamics (CFD) models. Specifically, the low- and high-flow theories for aneurysmal progression are studied. This includes several important sub-steps: 1) The development of a three-dimensional mesh that accurately represents the abdominal aorta iliac artery domain is developed. The mesh must accurately capture the viscous boundary layer and accurately represent the wall shear stress (WSS) throughout the domain. The chosen mesh should be efficient and minimise the spatial discretisation error. To assess the influence that different mesh configurations may have on CFD solutions to arterial flow models, the impacts of different mesh configurations on CFD-modelled pipe-flow are examined. To achieve this, the numerical solutions for different mesh configurations are compared with the analytical, developed flow solution (Section 2). 2) The development and application of meshes for both healthy and diseased patient geometries are created and modelled. 3) The application of boundary conditions and physical approximations that sufficiently represent the expected pulsatile flow field through the patient geometries are applied (as patient-specific waveforms are not available). The CFD modelling procedures are validated against the CFD best practices discussed in Section
11 1.1 Literature Review Arteries consist of several sub-structures or layers: an endothelium, intimal sub-layer, media and adventitia (Figure 1-2). The endothelium is composed of endothelial cells, which line the inside of the artery and are in direct contact with blood flow. The endothelium rests upon a basement membrane, and serves a number of unique biological functions. The intimal sub-layer in adults generally contains a small number of smooth muscle cells scattered within an extracellular matrix, and is separated from the media by the internal elastic lamella (not shown in Figure 1-2) (Libby 2002). The media provides most of an arteries structural support and elasticity as it contains tightly-packed smooth muscle cells (SMC), connective tissue and elastin (elastic fibre). The media is separated from the adventitia by an elastic membrane. The adventitia is composed of connective tissue, nutrient vessels and autonomic nerves (D Souza 2013). Figure 1-2: Artery diagram (artery cross-section) (Libby 2002). The site specificity of aneurysmal disease is thought to depend on developmental biology, vascular cell lineage, haemodynamics and anatomic factors (Norman & Powell 2010). The interaction between haemodynamic forces and the vessel wall biology is integral to any hypothesis about aneurysm growth and rupture, as haemodynamic studies have not found evidence to explain the wall failure on a purely mechanical basis (Sforza et al. 2009). There must be an alteration that results in the mechanical weakening of the artery wall over time. For example, the degeneration of endothelial cells and the internal elastic lamella, and the thinning of the medial layer have been observed at aneurysm sites (Sforza et al. 2009). 3
12 In adults, the thickness of the elastic lamellae gradually decreases with age (Norman & Powell 2010). When compared with other aortic regions the abdominal aorta has relatively few lamellar units, a smaller cross-section, and a stiffer wall. This increases its exposure to the reflected pressure wave from the periphery, while also subjecting it to higher pulse and systolic pressures (Latham et al. 1985). Furthermore, the abdominal aorta experiences periods of zero and reversed flow direction during diastole, this leads to increased oscillatory WSS and peripheral resistance (Taylor et al. 2002). The peripheral resistance increases the responsiveness of inflammatory and proteolytic pathways, predisposing this segment of aorta to both atherosclerotic and aneurysmal disease (VanderLaan et al. 2004), such that intraluminal thrombus (ILT) is found in over 70% of clinically-relevant AAA (Doyle et al. 2013). When considering the aforementioned, the relationships between physical-flow phenomena and aneurysmal disease may provide useful insight into the site specificity of IAA Haeomodynamics and Arterial Biology Plaque Formation and Structural Remodelling The propensity for plaque formation at artery bifurcations and curvatures has led to conjectures that local mechanical factors such as WSS and tensile stress potentiate atherogenesis (Glagov et al. 1988). Quantitative evidence suggests that plaques tend to occur where WSS and flow velocities are low, and the flow has departed from a laminar unidirectional pattern. These characteristics increase the residence time of circulating particles in susceptible regions, while, particles are cleared rapidly from regions of relatively high WSS and laminar unidirectional flow (Glagov et al. 1988). The enlargement of arteries occurs as plaques build up; however, the plaque and wall configuration tends to preserve an adequate and regular lumen cross-section, so as to restore baseline levels of WSS. The wall thickness architecture and composition are closely related to the tensile stress (Glagov et al. 1988). Ultimately, the capacity of the endothelial cells to sense WSS is an important determinant of lumen diameter, and the arteries ability to undergo appropriate structural remodelling. This may be retarded by abnormal or oscillatory haemodynamic conditions. In the presence 4
13 of a uniform WSS field, the endothelial cells tend to elongate and align in the direction of blood flow, while, a low, oscillatory WSS field causes irregular endothelial cell shape, and a loss of any particular orientation (Sforza et al. 2009). Aneurysmal Progression: High-Flow and Low-Flow Theories It is thought that if WSS is increased focally, it may potentially cause a focal enlargement of, and damage to the arterial wall (destructive remodelling and possible aneurysm formation) (Sforza et al. 2009). The high-flow theory for aneurysm growth and rupture then suggests that a vascular endothelium malfunction and/or an abnormal WSS field may cause an overexpression of endothelium-dependent nitric oxide (NO) production. This leads to lower arterial tone, owing to the scarcity and apoptosis (programmed cell death) of SMC (Sforza et al. 2009). This theory is often applied in studies of intracranial aneurysms. The other main school of thought is the low-flow theory, which suggests that low WSS causes a dysfunction of flow-induced NO. This results in the aggregation of red blood cells and the accumulation and adhesion of platelets (thrombocytes) and leukocytes (white blood cells), causing intimal damage and inflammation, leading to localised degeneration of the aneurysm wall (Sforza et al. 2009). Once initiated, the mechanical growth of aneurysms could be understood as a passive yield to blood pressure and reactive healing and thickening of the wall with increasing aneurysm diameter (Sforza et al. 2009). The following images (Figure 1-3) show the growth of an AAA (affected by ILT) at locations of low time-averaged WSS. 5
14 1.5 Pa Figure 1-3: AAA with ILT. Time-averaged WSS and artery diameter plots at two points in time: when the aneurysm was first monitored and 29 months later. The normalised distance (N.D.) begins (0) at the proximal neck and ends (1) distal to the iliac bifurcation. (Doyle et al. 2013). When considering the low-flow theory, there are a couple of important WSS thresholds that have been related to particular anatomical behaviour: A low WSS, below 0.4 Pa, has been found to switch the endothelial cell phenotype from atheroprotective to atherogenic. This promotes lipid deposition and results in a high endothelial cell turnover (Sforza et al. 2009). In areas where the WSS is less than 0.36 Pa monocytes (a type of leukocyte) adhere to the endothelium (Hardman et al. 2013), and as the WSS tends towards zero, the 6
15 adhesion efficiency increases exponentially (Doyle et al. 2013). Once a monocyte has adhered to the endothelium it penetrates into the artery intima (Hardman et al. 2013). In the intima the monocyte acquires characteristics of the tissue macrophage (Figure 1-4). Macrophage cells serve many functions related to atherosclerosis, including the amplification of the local inflammatory response and degradation of the extracellular matrix (Libby 2002). Figure 1-4: When the endothelial cells undergo inflammatory activation, they increase their expression of various leukocyte adhesion molecules. The above images shows, the journey taken by a monocyte that is recruited into an artery wall. Once resident in the intima the monocyte acquires characteristics of the tissue macrophage (Libby 2002) Arterial Branching Structure Different arterial geometries alter the haemodynamic environment and influence the site specificity of aneurysmal disease. For example, vessel curvature, bifurcation angle, and limited branch diameter have significant influence on local haemodynamics (Norman & Powell 2010). Bifurcations typically cause greater WSS on the inner wall of the bifurcation and lower WSS on the outer wall (Sharp et al. 1982). Furthermore, arteries located proximal to sharp-angled bifurcations or hypoplastic arteries often encounter a rise in turbulence and wall stress (Stijntje et al. 2008). 7
16 1.1.3 Methods for Measuring Wall Shear Stress Time-resolved WSS vectors may be estimated from three-dimensional phase-contrast magnetic-resonance imaging (MRI) data and velocity mapping (Figure 1-5). However, even for relatively simple velocity profiles (absent of noise), MRI estimates of WSS cannot always be assumed to be linearly or even monotonically related to actual WSS. While distinguishing areas of low and moderate WSS may be feasible, high WSS values cannot be resolved (Petersson 2012). For this reason, CFD models have been developed to model the WSS distribution and flow field through patient-specific geometries, although, MRI data provides a useful means to estimate the boundary conditions for, or benchmark the results of CFD models (Papathanasopoulou 2003). a Figure 1-5: WSS vectors for decelerating flow at an artery bifurcation; (a) estimated directly from MRI; (b) predicted by CFD using MRI data as boundary conditions (Papathanasopoulou 2003). b 8
17 1.1.4 Computational Fluid Dynamics and Haemodynamics The strategy of CFD is to replace a continuous domain with a discrete domain using a grid (mesh). The governing partial differential equations (typically the Navier-Stokes equations) and boundary conditions are defined in terms of continuous variables, but are approximated in the domain in terms of discrete variables. The entire system is described by a large set of coupled, algebraic equations and solving it is a matrix-inversion problem. Most commercial CFD solvers (including STAR-CCM+) use the finite volume (FV) method to solve the conservation equations within the domain: the solution domain is subdivided into a finite number of cells and the conservation equations are applied to each cell. The centroid of each cell is the computational node, while quantities at cell surfaces are interpolated in terms of cell-center values (Ferziger & Perić 2002, p. 36). The FV method is conservative by construction, and can accommodate any grid. However, it is not without disadvantage; numerical methods higher than second-order are easier to implement in other solver methods, such as finite difference (FD) schemes (Ferziger & Perić 2002, p. 36). Furthermore, when doing CFD for intra-arterial haemodynamic flows there are a large number of CFD solvers, meshing methods and solution strategies (i.e. physical approximations and boundary treatments) available for consideration (Steinman 2013). In a 2012 bioengineering CFD challenge, the variability in CFD solutions for pressure and flow in a giant aneurysm were investigated by a wide variety of research groups (Steinman 2013). It found that the choice of CFD solver/solver-method used (i.e. FV, FD, spectral element, finite element) had less influence on the results than the meshing methods and solution strategies implemented. The results also demonstrated that the solution for the pressure drop was consistent across a wide range of CFD solvers and solution strategies. However, for one component of the challenge, the predicted pressure drops varied by up to 60% of the experimental result. Regarding this, the sensitivity of pressure drops to assumed/prescribed flow waveforms was stressed, as well as the general ability for CFD to under-or-overestimate pressure in the presence of flow biases. Furthermore, the same level of consistency found for the predicted pressure drop was not found for the flow pattern. However, the only concrete finding here, was a clear relationship between the time 9
18 accuracy of the solution and the prediction of flow instabilities. There remains no single way to approach intra-arterial haemodynamics, although there are commonly-used practices. Another recent, and large, inter-laboratory haemodynamic study examined a range of CFD approaches used to model flow in a nozzle with a sudden expansion (Stewart et al. 2012). Ultimately, twenty-eight groups from around the world submitted simulations and there was little uniformity between the CFD practices and methods used. The study found a large variation in predicted WSS distributions. It was suspected that variations of wall treatments, boundary conditions, viscous mesh spacing and convergence criteria contributed to the wide spread of results. The study identified a list of CFD best practices (which will be further discussed below), specifically: 1) The selection of material models and other problem specifications must be correctly identified and quantified. 2) If a turbulence model is used, the choice of the model and parameters used must be justified. 3) The boundary conditions must also be correctly identified and quantified. 4) Mesh cell types should be chosen with knowledge that different cell types have different numerical characteristics. 5) An anisotropic boundary-layer mesh should be implemented to re-solve the viscous flow effects. Regarding this, the mesh spacing should be justifiable via physical reasoning. 6) The solution must numerically converge. 7) A proper mesh refinement study should be performed (preferably by doubling and quadrupling the mesh density). Reasonable variables should be chosen for convergence monitoring, and there should be a logical rationale for choosing the best mesh. 8) If applicable, existing theoretical solutions should be used as bench-mark validation data (especially when experimental data or comparable models are unavailable). 10
19 The Navier-Stokes Equations The Navier-Stokes equations are the governing equations for a viscous, heat conducting fluid. They are obtained by applying Newton's second law to a fluid element (Equation ( )) and are supplemented by the mass conservation equation (Equation ( )) (Wesseling P 2001). The Navier-Stokes equations assume that the fluid behaves as a continuum, such that it is infinitely divisible and there are no atoms or molecules. The general forms of the Navier-Stokes are seen below in Cartesian tensor notation and the Einstein summation convention (Sodja 2007): [ ] ( ) ( ) [ ] ( ) where, ( ) is the component of the velocity at a point in three-dimensional space, [ ], and at time,. ( ) is the static pressure, is the fluid density, ( ) ( ) is the viscous stress tensor and represents external force. The variables with tildes are considered instantaneous quantities. 1) Material Models: The Navier-Stokes Equations for an Incompressible Newtonian Fluid Blood is a non-newtonian fluid, that is, its viscosity ( ) varies with shear rate (velocity gradient). However, the Newtonian model, constant viscosity ( ), is commonly used when approximating blood flow within large arteries (Sforza et al. 2009). Furthermore, most CFD-modelling approaches approximate blood flow as an incompressible fluid (Sforza et al. 2009), where a fluid may be considered as incompressible for small Mach numbers, less than 0.3 (Ferziger & Perić 2002, p. 2). These approximations provide welcome 11
20 simplifications to the Navier-Stokes equations (see Equations ( ) and ( )). Although, for some aneurysmal flows, the Newtonian approximation may not be entirely justified, as slow-flow regions present non-newtonian behaviour (Sforza et al. 2009). The Navier-Stokes equations for incompressible, Newtonian fluids are (Sodja 2007): [ ] ( ) ( ) ( ) When the flow is incompressible, the derivative of the density is zero, and the mass conservation equation, Equation ( ), simplifies to Equation ( ). Furthermore, the simplified form of the momentum equation, ( ), easily conveys the relationship between the viscous stresses, viscosity and the velocity gradient ( ( ) ). The WSS is the viscous stress induced at the wall due to the rate of change of velocity parallel to the wall, in the direction normal to the wall. 2) Modelling Turbulence and Viscous Flow Regimes` When using CFD techniques to model AAA, the size and time-dependence of the models makes the direct numerical simulation of the Navier-Stokes equations (for all cases) at all length and time-scales extremely computationally expensive (Sodja 2007). Alternatively, a viscous flow regime/model (i.e. laminar or turbulence) may be used as closure to a set of decomposed Navier-Stokes equations. In doing this the solution may then be solved, with stability, on a coarse grid. The flow in an AAA is undoubtedly turbulent during late systole (Les et al. 2010). However, laminar models are often used when doing CFD in AAA, as the time-dependent flow is predominantly characterised by Reynolds numbers of less than 2000 (developed turbulent flow is not present) (Patel 2011). The Reynolds number describes the ratio of inertial forces to viscous forces. Laminar flow occurs at low Reynolds numbers, where viscous forces are dominant, and there is smooth, constant fluid motion, while, turbulent 12
21 flow occurs at high Reynolds numbers, where the flow is dominated by inertial forces, which tend to produce flow instabilities (Patel 2011). When using a laminar model in CFD, turbulence is not accounted for, and the occurrence of physical instabilities cannot be relied on as an accurate indication of the transition to turbulence. In addition, numerical instabilities can arise from simulating laminar flows at Reynolds numbers that are too large. On the other hand, when using turbulence models in low-reynolds number simulations, the onset of turbulence in the viscous boundary layer cannot be predicted reliably by the turbulence model itself (CD-adapco 2014); a transitional flow model must be coupled with a turbulence model to estimate this. 3) Boundary Conditions In arteries, the flow and pressure waves are damped, dispersed and reflected due to change in vessel size, tissue properties or branch points. The solution to large-artery models is highly dependent on the outflow boundary conditions used to represent the down-stream vascular bed (Vignon-Clementel et al. 2006). In most three-dimensional models the flow distribution and/or pressure field is unknown. In order to overcome this, the solution may be coupled at the outflow boundaries with lumped-parameter or one-dimensional models of the respective downstream domain (Vignon-Clementel et al. 2006). Despite this, the majority of studies use prescribed pressure and flow boundary conditions, which can result in non-physiologic pressure and/or flow fields (Les et al. 2010). Fluid-structure interaction algorithms have been developed to incorporate wall compliance into vascular CFD models. However, it is a challenging problem because it depends on knowledge about the distribution of wall thickness, wall elasticity, and the intra-arterial pressure waveform. Many CFD studies of haemodynamics do not consider the vessel-wall compliance; its influence on in vivo intra-aneurysmal flow patterns is not entirely understood (Sforza et al. 2009). The pulsation of the wall has been measured using dynamic angiography images and non-rigid registration algorithms. When imposed as a boundary condition in CFD models the main characteristics of the flow patterns (such as the complexity and stability of the intra-aneurysmal flow pattern) are not significantly altered. However, velocity and WSS are affected by the wall motion (Sforza et al. 2009). 13
22 4) Selecting CFD Mesh Types Tetrahedral meshing was the first fully-automated volume meshing method. It is an unstructured meshing method, which means that the cells or control volumes may take any shape and there is no restriction on the number of neighbour elements or nodes (Ferziger & Perić 2002, p. 29). Tetrahedral meshes are often used when meshing AAA (Doyle et al. 2013; Les et al. 2010). However, they are not the only common option used for irregular geometries. Comparisons in many practical tests have verified that with polyhedral meshes, one needs about four-times fewer cells, half the memory and a fifth to a tenth of computing time than for tetrahedral meshes to reach solutions of the same accuracy (Perić 2004). In a polyhedral mesh the higher number of neighbouring cells allows for better approximation of local gradients and flow distribution. Furthermore, the major advantage of a polyhedral mesh is that the simplest approximations (linear interpolation, central difference, midpoint rule) are applicable and provide accuracy close to that of a structured mesh (i.e. a uniform hexahedral mesh) (Perić 2004). 5) Anisotropic Boundary-Layer Mesh Refinement To understand the purpose of boundary-layer mesh refinement, a clear understanding of the physical boundary layer and how it varies is imperative. When considering the velocity field, the viscous boundary layer is commonly defined as the region between the domain wall and the point at which the flow reaches the free-stream velocity (Abernathy 1968). Approaching a static, no-slip boundary, the fluid deceleration is transferred from one fluid layer to another via the viscosity (Abernathy 1968). Viscosity acts through the mechanism of molecular diffusion to spread out the vorticity as the flow propagates, such that, in an oscillatory flow field the viscous boundary layer remains thin and characterised by steep velocity gradients (Abernathy 1968). As the WSS is proportional to the velocity gradient at the wall, the accuracy with which the viscous boundary layer is captured by the mesh has direct implications on the flow phenomena associated with the site specificity of aneurysmal disease. To numerically resolve the viscous boundary layer the mesh near the wall is refined and structured into a number of sub-layers, such that there is a progressive decrease in the mesh size approaching the artery wall. The aim is to smoothly discretise the near-wall velocity accurately and predict the rapid change in gradient. Regarding this, the methodology used 14
23 for the progressive refinement of the boundary-layer mesh should have functional relevance to the velocity field (Sazonov & Nithiarasu 2012). Figure 1-6: Velocity boundary-layer transition on a flat plate. The turbulent boundary layer is made up of three distinct regions (Cotrell 2014). The nature of the boundary-layer refinement also depends on the development of turbulence in the domain (Figure 1-6). For laminar flow the boundary-layer fluid motion is highly ordered, while fluid motion in the turbulent boundary layer is highly irregular and is characterised by velocity fluctuations. These turbulent fluctuations result in increased fluid mixing causing thicker boundary layers with flatter velocity, concentration and temperature profiles (Cotrell 2014). The velocity gradient of a turbulent boundary layer is greater close to the wall, and therefore higher WSS may be generally expected in turbulent conditions (Abernathy 1968). A turbulent boundary layer is also less likely to separate. Boundary-layer separation is the occurrence of flow reversal at the wall relative to the free-stream flow direction; these zones encounter instances of zero WSS. Boundary-layer separation occurs when there is a large pressure gradient (Abernathy 1968); this may occur at regions of flow expansion, i.e. entering an aneurysm. Ultimately, the three-dimensional interchange between regions of high and low momentum in the turbulent boundary layer allows larger, more unfavourable pressure gradients to be withstood (Abernathy 1968). 15
24 6) Numerical Solution Convergence The solution of a CFD simulation is an iterative process. The solution values for mass, momentum and other quantities change from one iteration to next. Given that the physical assumptions, boundary conditions and mathematical models being used are sound; an appropriate number of iterations should be executed to converge the solution. There are plenty of examples where an un-converged solution does not obviously show non-physical results (CD-adapco 2014). In CFD the residuals are typically the normalised, iterative errors of the transport equations (mass and momentum). It is recommended that the numerical solution should converge via residual reductions and monitoring of physically-relevant fluid flow quantities. For steady flow simulations (typically time-independent problems) a residual drop of three orders of magnitude is usually sufficient for an engineering analysis (Stewart et al. 2012). However, this threshold should be considered alongside the method used to normalise the residuals. Furthermore, lower-order numerical schemes have a stabilising effect on the solution and tend to bring the residuals down (the discretisation error is more dissipative than dispersive) (CD-adapco 2014). For unsteady simulations, iterative convergence should occur within each time-step (implicit unsteady solver). Typically, if the time-step is large, then a greater number of inner iterations are required to converge the solution (CD-adapco 2014); as any time-dependent boundary conditions would have varied greatly. 7) Mesh Refinement A mesh refinement study investigates how the solution, notably variables of interest and importance (i.e. WSS), vary with the level of mesh refinement. In the CFD community, mesh discretisation error estimation is a requirement to publish numerical results in many journals (ASME Fluids Engineering Journal included) (Schwer 2008). Regarding this, the use of the Grid Convergence Index (GCI) method is encouraged (Celik et al. 2012). The GCI estimates the relative error between a grid solution and an unknown exact solution (Schwer 2008) and offers sufficient understanding of how well the mesh size is capturing the flow (or an aspect of it) (Mohamed et al. 2009). The solution on at least three different meshes is required to calculate the GCI. The formulae below (Equations ( ) and (1.6); Schwer 2008) calculates the GCI for a constant 16
25 grid refinement ratio ( ) (i.e. the increase in mesh density is the same between mesh refinements). ( ) ( ) Where, is the order of convergence and is the solution value for a particular mesh/grid ( denotes the finest grid solution). ( ) ( ) ( ) 8) Validation In the absence of patient-specific data, the unique geometries used in cardiovascular CFD make the validation of results difficult. A number of recommended practices are as follows: Ensure that the results are reasonable or intuitive from a fluid mechanics point of view. Compare the results with experimental results/waveforms found for patients afflicted with similar cardiovascular diseases. Use existing analytical solutions to the Navier-Stokes equations for similar geometries to benchmark results (e.g., steady and unsteady pipe flow) (Stewart et al. 2012). 17
26 2 Initial Work Before modelling the arterial geometries, the commercial CFD solver STAR-CCM+ (v9.07) (CD-adapco Group) was used to compare the numerical solution of the Navier- Stokes equations with the analytical solution for fluid flow through a straight, circular pipe section. The numerical solution was calculated for a number of different meshes. The results convey the mesh dependence of the steady, developed, laminar flow that was analysed. Although the flow addressed here is not characteristic of pulsatile flow through an artery, this study indirectly highlights the level of uncertainty that is expected when solving for laminar/viscous flow on relatively coarse meshes. Due to the geometric similarity to an artery branch, this pipe-flow study provides an assessment of the meshing methodology that is used for the arterial geometries modelled in Section 3. It contributes to an understanding of the effect that different meshing parameters have on the accuracy of the solution. STAR-CCM+ was chosen as the CFD solver as it, and other CD-adapco products have the unique ability to both create and solve upon arbitrary polyhedral meshes. The solver is capable of accepting any n-sided polyhedron; however, the mesher automatically creates high-quality polyhedral meshes (CD-adapco 2014). (The advantage of polyhedral meshes has already been discussed in Section 1). 2.1 Process The following subsections describe the model formation Geometry and Boundary Conditions The diameter of the cylinder is 8mm, which is similar to the size of an external iliac artery (Kahraman, et al. 2006). The length of the cylinder is 2mm. The sides of the cylinder are characterised by a no-slip, rigid wall boundary. The inlet and outlet boundaries are coupled by a fully-developed periodic interface (the flow that leaves the outlet enters the inlet). A constant mass flow rate ( ) is specified between these interface boundaries such that the 18
27 flow has a Reynolds number ( ) of The Reynolds number equation for flow in a pipe (Wood 1999) may be expressed as: ( ) where, is the cylinder diameter, is the cylinder area, and is the dynamic viscosity. The Reynolds number used is sufficiently large so that the velocity gradients are not an under-representation of those found in large arteries ( [ ]; Ahmed & Giddens 1983) Physical Approximations The fluid, blood, is considered to be incompressible and Newtonian with a density of 1050 kg/m 3 and dynamic viscosity of Pa s (Doyle et al. 2013). The steady, laminar flow model is used as the boundary conditions are time-independent and no flow instabilities are expected. The segregated flow solver is used as the fluid is incompressible; the pressure and velocity components are un-coupled and solved separately (CD-adapco 2014) Initial Conditions The initial condition for the velocity ( ) normal to the inlet/outlet boundaries is set to the Hagen-Poiseuille theory for fully-developed, steady, laminar flow in a straight, circular pipe (Schlichting 1979, p. 85): ( ) ( ) [ ] ( ) where, is the radius of the pipe, is the dynamic viscosity, and is a constant pressure gradient. 19
28 The free-stream velocity (for this flow theory) is half the maximum velocity (Schlichting 1979, p. 85); using this relationship the pressure gradient is calculated as: ( ) Equation ( ) is an exact solution of the Navier-Stokes equations that remains true while the Reynolds number is below 2300 (Schlichting 1979, p. 85). The initial pressure is set to 0 Pa; the reference pressure is 1 atm. If the numerical solution is perfectly accurate the solution will temporarily diverge from the parabolic Hagen- Poiseuille velocity distribution as the pressure/pressure gradient is corrected against the initial conditions Meshing A number of meshes were created to test the dependency of the velocity solution on both the surface size (circumferential cell count) and the number of structured boundary-layer cells, or prism layers. For each mesh constructed, the core-mesh is composed of polyhedral cells (Figure 2-1). The automated (or semi-automated) meshing process is as follows (Maley 2012): 1) The surface is re-meshed prior to volume generation. Executed according to the specified target surface size (and/or maximum/minimum surface size). 2) The automated volume-mesher then subtracts the total thickness of the prism-layer mesh (specified) from the boundary, creating an offset surface mesh. 3) The polyhedral mesh is created within the volume bounded by this offset surface mesh. Key parameters used to control the polyhedral mesh include the maximum cell size, mesh density and expansion rate. 4) The last step is the extrusion, and creation, of the prism-layer mesh from the offset surface toward the boundary. Key parameters used to control the prism-layer mesh are the aforementioned prism-layer thickness, number of prism layers and prismlayer stretching. 20
29 It should be noted that the irregular shape of polyhedral cells makes them inefficient for a pipe-flow problem like this where the flow is uniaxial (personal communication Ewing, P, CD-adapco support engineer). However, as a polyhedral mesh will be used for the arterial models (Section 3) it is applied here. Figure 2-1: Mesh scene showing the volume mesh (at the mid-plane) in blue and the cylinder surface (volume) mesh in grey for Mesh 1 (Table 2-1 and Table 2-2). Surface Mesh Size The target surface size, a dependent variable, is altered so that the circumferential surface cell count is 30, 45, 60 or 90. Polyhedral Mesh Parameters The maximum polyhedral cell size is kept constant at 5% of the radius ( ), the polyhedral expansion rate is 1.0 (default), and the polyhedral mesh density is 4.0. At this mesh density, the number of polyhedral cells constructed is 4.0 times the default quantity. 21
30 Boundary-Layer Mesh Refinement The thickness of the prism-layer mesh ( ) is specified as the distance from the boundary surface to the Hagen-Poiseuille free-stream velocity (half the maximum velocity magnitude): ( ) ( ) ( ( ) ) ( ) ( ) ( ) For this study, the number of prism layers is 5, 10 and 20. For each of these cases a linear prism-layer stretching parameter is set so that prism-layer mesh is progressively refined according to Hagen-Poiseuille theory. The goal is to have the same change in velocity across each prism layer so that the viscous boundary layer may be smoothly discretised. Equation ( ) below relates the velocity at the inner edge of the wall-adjacent prism layer ( ( )) to the velocity at the inner edge of the innermost prism layer, the free stream velocity. ( ( )) ( ) where, is the number of prism layers, and is the thickness of the wall-adjacent prism layer. Expanding we get: [ ( ( ) )] ( ) and solving gives, 22
31 ( ( ) ) ( ) To calculate the desired linear prism-layer stretching parameter (S), Equation (2.10) must be solved. This equation relates, to, previously denoted as the total prism-layer mesh thickness. ( ) ( ) To solve this equation an algorithm was written and implemented in MATLAB (The Mathworks Inc.); see Appendix A. List of Meshes and Meshing Parameters In total, six different meshes are created; their parameters are listed in Table 2-1 and Table 2-2. Mesh Table 2-1: Dynamic meshing parameters:, and S denote the target surface size, number of prism layers and prism-layer stretching, respectively. Mesh 1: ( ) Table 2-2: Constant meshing parameters:,, and denote polyhedral expansion rate, polyhedral mesh density, maximum polyhedral cell size and prism-layer mesh thickness, respectively. 23
32 2.1.5 Monitoring Steady State Convergence In STAR-CCM+ the absolute error in the solution of each transport equation is recorded at every cell. The root-mean-square of these values are calculated after each iteration; denoted as absolute residuals. The (default) residual of the solution is defined as the absolute residual at the current iteration divided by the maximum absolute residual that occurred within the first five iterations (CD-adapco 2014). The solution for each mesh is run until the difference between the maximum velocity and theoretical maximum velocity begins to show clear signs of asymptotic convergence, and the continuity (mass) and momentum residuals are below Results and Discussion The plots below contain the node-interpolated velocity profiles monitored across the diameter for the six different meshes. Figure 2-2 shows the results for meshes with 90, 60, 45 and 30 circumferential surface cells (CC) and 20 prism layers (meshes 1, 2, 3 and 4 (Table 2-1)), while, Figure 2-3 shows the results for meshes with 20, 10 and 5 prism layers (PL) when the circumferential surface cell count was fixed at 45 (meshes 3, 5 and 6 (Table 2-1)). 24
33 Velocity (m/s) Velocity (m/s) Velocity Profile for Different Surface Cell Sizes 90CC 60CC 45CC 30CC Theory R 0 R Distance Figure 2-2: Node-interpolated velocity profiles for meshes with 90, 60, 45 and 30 circumferential surface cells (CC) and 20 prism layers plotted against the distance from the centreline of the pipe. (R is the radius) Velocity Profile for Different Prism Layer Densities 20PL 10PL 5PL Theory R 0 R Distance Figure 2-3: Node-interpolated velocity profiles for meshes with 45 circumferential surface cells and 20, 10 and 5 prism layers (PL) plotted against the distance from the centreline of the pipe. (R is the radius). 25
34 For all meshes, the solution of velocity could not capture/maintain the Hagen-Poiseuille flow profile. Altering the number of prism layers resulted in a negligible change in the velocity profile, as shown in Figure 2-3. However, the transition to the polyhedral mesh from the prism layer mesh is slightly smoother for the solutions with 10 and 20 prism layers (more continuity in mesh size) compared with the solution with 5 prism layers. The variation from the theoretical maximum velocity is -6.17%, -7.80%, -9.51% and % for the meshes with 90, 60, 45 and 30 circumferential cells respectively, as shown in Figure 2-2. Furthermore, when 30 surface cells discretise the circumference there is a significant loss in smoothness, and the cross section noticeably resembles the polygon that it is, rather than a continuous circle. It is reasonable to assume that a further increase in the circumferential surface cell density would not significantly increase the accuracy of the solution unless it forced a decrease in the size of the polyhedral cells. Ultimately, a circumferential surface cell count of 45 provides an efficient solution, as doubling it provides only a marginal increase in the peak velocity and (more importantly) a negligible change in the near-wall velocity gradient. This is closely related to a sufficient discretisation of the initial circular exterior, rather than the inner volume. Thus, a similar level of surface refinement should be implemented in the arterial models, and the relationship between the polyhedral mesh density and the mesh discretisation error should be explored. 26
35 3 Arterial Modelling The commercial CFD solver STAR-CCM+ (v9.07) was used to calculate unsteady numerical solutions to the Navier-Stokes equations for pulsatile blood flow through two different arterial geometries. A mesh convergence study was undertaken, and both the timeaveraged magnitude of wall shear stress (TAWSS) and the oscillatory shear index (OSI) were investigated. These quantities were compared with physical stresses and flow phenomena associated with aneurysmal disease. 3.1 Process Geometries The healthy and diseased geometries were created from computed tomography (CT) scans (Figure 3-1). These geometries were provided as stereo-lithography (STL) files by the author s supervisor, Barry J. Doyle, and are the starting point of the arterial modelling process. The diseased geometry contains a large AAA, common IAA and internal IAA. The accuracy of a CT scan is limited by the pixel size and slice thickness (1mm and 3mm respectively), and consequently, a number of minor arteries were not included in the geometries. These include the inferior mesenteric artery connecting to the aorta and a number of arteries that branch from the internal iliac artery (see Figure 1-1, Section 1). 27
36 Healthy Geometry Diseased Geometry AAA Com. IAA Int. IA Ext. IA Int. IA Bifurcations Int. IAA Figure 3-1: Healthy and diseased geometries looking at the front (/side) of the body (partially labelled). Note the presence of the internal iliac artery (Int. IA) bifurcations in the healthy geometry, but in not the diseased geometry. Also, note the locality of the aneurysms in the diseased geometry. When the healthy geometry (STL file) was imported into STAR-CCM+ it had a concentration of manifold faces and edges at one of the iliac bifurcations. The volume was unable to mesh. These were manually fixed, so that the geometry closely resembled the other healthy iliac bifurcation (Figure 3-2). 28
37 Figure 3-2: Repairing manifold edges: before is at the top and after at the bottom Meshing For both the healthy and the diseased geometry the meshing procedure used is similar to the procedure used in Section 2. The core-mesh is constructed using polyhedral cells, a prismlayer mesh is used to discretise the boundary-layer region, and the surface is re-meshed prior to volume generation to control the surface size/circumferential surface mesh density. A mesh study is undertaken where the GCI is used to analyse the convergence of WSS parameters with mesh density. For both the healthy and the diseased geometries, the solution is calculated for three different mesh sizes, coarse, medium and fine. The number of prism layers and the polyhedral mesh density is doubled with each mesh refinement, while the surface size is fixed. The GCI is calculated according to the change in 29
38 mesh density within the prism layer and polyhedral mesh, such that the grid refinement ratio used is considered constant and equal to two. The following sub-sections discuss the inputs used by the surface, polyhedral, and prismlayer meshers in STAR-CCM+. Surface Mesh Size and Prism-Layer Thickness These meshing parameters are controlled within different regions (using STAR-CCM+ s Volumetric Controls ) so that the prism-layer thickness and surface size for each artery branch may be set relative to the average radius of the artery branch ( ). The average radius is estimated by relating the internal volume of each artery branch (away from the bifurcation regions) to the volume equation for a circular cylinder. Each artery branch has approximately 50 surface cells discretising its circumference and a prism-layer mesh thickness equivalent to the fluid boundary layer of fully developed laminar flow in a pipe (see Section 2). The bifurcation regions are assigned the mesh properties of the smallest proximal artery to increase the local mesh density, as these regions will encounter higher velocity gradients. Polyhedral Mesh Parameters The maximum polyhedral mesh size is kept constant at 10% of the estimated average artery radius ( ) and the polyhedral expansion rate is set to 1.0 (default). The polyhedral mesh density is set to 1.0, 2.0 or 4.0, for the coarse, medium and fine meshes. Boundary-Layer Mesh Refinement and Considerations The number of prism-layers is set to 5, 10 or 20, for the coarse, medium and fine meshes. Furthermore, as the flow is now oscillatory the fluid boundary-layer size is dynamic and there are higher velocity gradients close to the wall. Thus, the thickness of the prism-layer mesh used is excessive, although, the prism-layer cells provide a more efficient discretisation of the space than the polyhedral cells. To accommodate for the higher gradients close to the wall, the prism-layer stretching is increased compared with the values used in Section 2: the innermost prism layer is now twice the thickness of the layer proximal to the wall. That is: 30
39 ( ) ( ) where, is the linear prism-layer spacing parameter, and is the number of prism layers. Polyhedral and Prism-Layer Mesh Figures Figure 3-3 shows a vertical mesh threshold-section through the lower abdominal aorta and upper common iliac arteries of the diseased geometry. The purpose of the figure is to outline the refinement at the bifurcation regions, as well as show the refinement of the mesh relative to the arterial radius. The cross-section of the fine mesh for the diseased geometry is shown in Figure 3-4. This cross-section more closely shows the prism-layer mesh and progressive prism-layer mesh refinement. Figure 3-3: Diseased geometry mesh threshold demonstrating the relationship between cellsize and branch diameter, as well as the local refinement at bifurcation regions. 31
40 Figure 3-4: Diseased geometry: aortic cross section showing the fine mesh density. Note: for this mesh, the thickness of the prism-layer elements adjacent to the artery wall is within the range of 16 to 180 m, throughout the diseased geometry. Inlet and Outlet Extensions The inlet and outlets boundaries of both models are extended using the mesh extrusion feature in STAR-CCM+ (Figure 3-5) (forming prism-type cells). An inlet extension allows the flow profile to physically develop before entering the arterial geometry (Wood 1999) (Figure 3-6), while an outlet extension isolates any non-physical behavior near the boundary (i.e. uniform flow) from interfering with the rest of the flow field (areas of interest) (Hardman et al. 2013). The inlet extension length ( ) is calculated using Wood s unsteady flow method (Wood 1999): ( ) where, is the Reynolds number, is the inlet radius, and is a parameter of harmonic, pulsatile flow. The higher is, the more the velocity profile varies (is flattened) from the developed parabolic distribution seen in Section 2, and, the length required to develop the profile is 32
41 reduced. In the human aorta, is equal to approximately (Wood1999). The Reynolds number is estimated using the equation for flow in a pipe (Equation ( )). To ensure that the inlet blood flow is always well developed, the entrance length is calculated for peak flow conditions, 0.15 kg/s (Doyle et al. 2013) and. The result is an inlet extension length of 120 mm for both models. Furthermore, the outlets were extended by 11 times their respective diameter. An outlet extension greater than 10 times the outlet diameter is thought to be sufficient when doing large artery CFD (Hardman et al. 2013). Note, it is also encouraged/efficient to progressively elongate the cells in the mesh extensions when the flow near the boundaries is (or will be) uniaxial. This is done such that the last extruded layer is eight times the thickness of the first (Figure 3-5). Figure 3-5: Diseased inlet: Mesh extrusion. Note the progressive elongation of the extruded layers approaching the inlet boundary. The outlet boundary extensions can be seen in Figure 3-7 (p. 39). 33
42 A B C Figure 3-6: Inlet viscous flow development: The velocity profile at line-probe B is characteristic of the expected flow profile when ( ) (Wood 1999), before the downstream geometry/flow-field begins to influence direction of the developing flow (line-probe C). (These results were calculated using the diseased geometry and steady-flow solver at peak flow/systole (0.15 kg/s (see Figure 3-9)) using the fine mesh, and Newtonian and incompressible fluid approximations). Mesh Validity For both geometries, none of the cell faces in any of the meshes were invalid (i.e. faces pointing towards their centroid) and the aspect ratio of the volumes cells 1 is only found to approach zero (highly stretched) near the boundaries (where the mesh is purposely elongated along the axis of flow) (Figure 3-7). 1 The cell aspect ratio is used to identify regular polygonal cells from skewed or stretched cells. A value of 1 indicates that the cell is a regular polygon, and a value close to 0 would represent a highly stretched cell. (Cd- Adapco 2014) 34
43 Figure 3-7: Diseased geometry, fine mesh: Cell Aspect Ratio function across the volumerendered domain showing the general trend found regarding Cell Aspect Ratio. List of Meshes and Meshing Parameters The meshing parameters for both geometries are in Table 3-1, Table 3-2 and Table 3-3. As mentioned, the meshing methodology was specified relative to the average radius of each artery branch ( ), or the smallest proximal artery branch in the case of bifurcating regions. Mesh coarse medium fine Table 3-1: Dynamic meshing parameters:, and denote polyhedral mesh density, number of prism layers and prism-layer stretching, respectively. 35
44 Mesh All Meshes 1.0 ( ) Table 3-2: Constant meshing parameters/relationships:,, and denote the polyhedral expansion rate, surface size, maximum polyhedral cell size and prism-layer thickness. As mentioned, the surface size is a fiftieth of the estimated circumference, and the relative prism-layer thickness is that of the Hagen-Poiseuille flow, discussed in Section 2. Mesh Extrusion Type Extrusion Length Inlet 120 mm 8.0 Outlet 8.0 Layer Spacing Extrusion Type Average Normal Average Normal Table 3-3: Mesh extrusion parameters/relationships: is the outlet diameter; Layer Spacing is the thickness ratio of the last and first layers; and Extrusion Type is the direction of the extrusion Physical Approximations and Boundary Conditions For both models, the blood flow was approximated as laminar, and the blood was considered to be an incompressible, Newtonian fluid with a dynamic viscosity of Pa s and a density of 1050 kg/m 3. The walls of the arteries are characterised by no-slip, rigid wall boundary conditions. These are all common model assumptions when simulating the haemodynamics in large arteries (Doyle et al. 2013). The segregated flow model (uncoupled velocity and pressure equations) is implemented as the flow is considered to be incompressible, and the implicit unsteady solver is chosen; it is the only solver available with the segregated flow model in STAR-CCM+ (CD-adapco 2014). The default 2nd order upwind convection scheme is used by the segregated flow model to calculate the flux at the cell faces. 36
45 The Implicit Unsteady Model In the Implicit Unsteady approach, at each time-step, the solution is found by solving an equation, or system of equations, in terms of both the current state and future state of the system. Each physical time-step involves some number of inner iterations to converge the solution (CD-adapco 2014). STAR-CCM+ implements a SIMPLE algorithm (Semi- Implicit Method for Pressure Linked Equations; Ferziger & Perić 2002, pp ) when the flow is segregated to control the solution update and enforce mass conservation with each time-step. The temporal discretisation of the implicit solver is set to second order. This improves the time accuracy of the solution by calculating the solution with two time levels instead of one (first order). To understand the second-order temporal discretisation used by STAR-CCM+, consider the estimation of a transient gradient ( ): ( ) ( )[ ] [ ] ( ) ( ) where, n is the solution at the current time level and: ( ) ( ) This method gives more weight to the forward approximation, backward, than the Dynamic Time-stepping and the Convective Courant Number In STAR-CCM+, the convective Courant number is a field function that provides the local (cell) convective Courant number for a given time-step. The convective Courant number is the ratio of the physical time-step to the mesh convection scale. A solution is considered time accurate if the average convective Courant number is one in the area of interest (Nath 2014): this would mean that the fluid is convected approximately one cell length per time-step. When the solution is time-accurate the high-velocity flow patterns are captured by the model (Steinman 2013). 37
46 For both the healthy and the diseased geometry, a dynamic time-stepping system is implemented using user-defined field functions to ensure that the convective Courant number does not exceed one in the majority of cells in the iliac arteries (at any point in the cardiac cycle). During each time-step, at each cell, the required time-step ( ) for a desired Courant number ( ) is approximated using the following equation (Xu 2014): ( ) where, is an estimation of cell size using the cell volume is the local velocity magnitude., and Logically, due to the variation in the cell sizes and local (cell) velocity, there are a whole range of different values throughout the domain. Thus, the next time-step used by the solver is the average of the field across the cells spanning the iliac arteries (i.e. Figure 3-8). Where, the value of is set accordingly (0.1 for the healthy geometry; for the diseased geometry). 38
47 Figure 3-8: Volumetric domain over which the time-step for the diseased simulations is calculated. See Appendix B, Figure B-1 for results regarding this dynamic timestepping method (the convective Courant number field). Initial Conditions The blood is initialised with a small velocity so that the initial dynamic time-step is not infinite (see equation ( )): ( ) { } ( ) where, the global Cartesian coordinate Z is normal to inlet boundary. The initial pressure is 0 and the reference pressure is 1 atm. Inlet and Outlet Boundary Conditions 1) Flow Inlets A velocity inlet is used as the inlet boundary to the healthy geometry. The data used is taken from work done by Mills et al. (1970) on the pressure-flow relationship and vascular impedance in man. Twenty-three patients (some with heart disease) were studied using catheter-tip probes to investigate pressure-velocity relationships in the major arteries. The presence of the catheter-tip probe has a flattening effect on the profile and it is reasonable 39
48 Velocity (m/s) Mass Flow-rate (kg/s) to assume that the velocity waveforms obtained are proportional to the flow waveforms (Mills et al. 1970). Ironically, the healthy geometry used here is unhealthily narrow. The artery branches are of normal length, while the widths are unusually small. The inlet (abdominal aorta) diameter is 12.7 mm, compared with the average for the wider population of approximately 20 mm (Hirsch et al. 2006). For this reason the mass flow through the healthy geometry is significantly less than the norm. However, priority is given to the estimation of WSS using velocities of regular magnitude. A mass flow inlet is specified as the inlet boundary to the diseased model which uses an average waveform of 21 AAA patients previously examined using Doppler ultrasound by Fraser et al. (2008) Inlet Waveforms Diseased Inlet: AAA Mass Flow-rate Healthy Inlet: Velocity Time (s) Figure 3-9: Inlet Waveforms: healthy waveform period 1.0 s (Mills et al. 1970); disease waveform period 0.92 s (Frazer et al. 2008)
49 Mass Flow-rate (kg/s) For both models, the inlet waveforms (Figure 3-9) are digitised using the freeware java program Plot Digitizer. The digitised data is spline interpolated into STAR-CCM+ from a comma-separated-value file (a function of STAR-CCM+). 2) Flow Outlets For both geometries, the outlet boundary conditions are specified as mass-flow inlets that allow 15% of the flow through each internal iliac artery and 35% though each external iliac artery (common mass flow targets; Les et al. 2010). The field function applied at each outlet boundary is simply a scaled version of the inlet mass-flow field function (see Figure 3-10) (possible due to incompressibility) Healthy Geometry: Mass Flow-rates at the Inlet and Outlets Inlet External IAs Internal IA R1 Internal IA R2 Internal IA L1 Internal IA L Time (s) Figure 3-10: Healthy geometry: inlet and outlet boundary conditions. The inlet flowdirection convention (inflow: negative) is reversed here so that the mass flowrates in and out of the model may be easily compared. Note: in the healthy geometry the internal iliac arteries have two exists, designated: Internal IA R1 and R2 for the right side of the patient, and Internal IA L1 and L2 for the left side of the patient. In the healthy geometry, the presence of the internal iliac artery bifurcations means that the flow exiting each internal iliac artery is split between two downstream outlet boundaries (Figure 3-1). To estimate the proportion of internal iliac flow out these boundaries a steady 41
50 state model was run using the fine mesh mentioned in the above meshing section (Section 3.1.2). The peak velocity from Mills data (Figure 3-9) was used as the inlet boundary condition. All of the outlet boundaries were pressure outlets set to the field function of pressure. This is a very simplistic approach where the fluid exits the domain at the outlets according to the upstream resistance, as the pressure boundaries do not force a result Wall Shear Stress and the Oscillatory Shear Index Wall Shear Stress Monitoring and Grid Convergence Variables The spatial average and standard deviation of the WSS magnitude are monitored at the internal and external iliac arteries. The regions over which these parameters are monitored are specified as sections of the domain surface, shown below for the diseased geometry (Figure 3-11); see Appendix B, Figure B-2 for the healthy geometry surface sections. For the last cardiac cycle executed by the solver the monitor data is time-averaged and used as the grid solution values in the calculation of the GCI (Equation ( ), Section 1.1.4). Left Ext. IA Surf. Right Ext. IA Surf. Left Int. IA Surf. Right Int. IA Surf. Figure 3-11: Diseased geometry surface sections used to monitor WSS throughout the cardiac cycle. 42
51 The Time-Averaged Magnitude and Maximal Magnitude of Wall Shear Stress A field-mean monitor is used to discretely average the WSS magnitude field over the previous cardiac cycle/cycle duration. The time between samples ( ) is: ( ) where, is the period of the cardiac cycle defined at the inlet boundary. The field-mean monitor provides an approximation of the TAWSS integral (Equation ( )) (Soulis 2011) for all faces on the domain surface. ( ) where, is the instantaneous WSS magnitude, and is the instantaneous time. Furthermore, a field-maximum monitor is used to store the largest WSS magnitude that occurs at each cell, within a discretised data set described by the same sample period and sampling frequency (Equation ( )). Oscillatory Shear Index The OSI is a mechanical factor that measures the oscillation of the WSS vector. It relates the magnitude of the time-averaged WSS vector with the time-averaged magnitude of WSS (or TAWSS). The OSI quantifies the WSS vector deflection from predominant blood flow direction during the cardiac cycle. The OSI is calculated as (Soulis 2011): ( ) ( ) The OSI value can vary from 0.0, for no-cyclic variation of the WSS vector, to 0.5, for deflection of WSS direction. A field-sum monitor is used to sum the magnitude of 43
52 the WSS and the value of the WSS vector in all three global coordinates (X, Y, Z), again, using the same sampling frequency and over the same sample period as TAWSS monitor. The following user-defined field function (Equation ( )) is implemented in STAR- CCM+ to calculate the OSI. ( ([ ]) ) ( ) where, $ references a scalar function or variable, is a vector operation that calculates the magnitude of a vector, and,, and represent the field monitors. 3.2 Results and Discussion For both geometries (and all mesh densities), the spatial-averaged (Figure 3-12), standard deviation, minimum and maximum WSS magnitude traces for the external and internal iliac arteries showed negligible change between the second and third cardiac cycles (and thereafter). This was also found for the solution of the pressure field. Henceforth, the analysed results are taken from the third cardiac cycle, unless otherwise noted. This section of the report will begin by addressing how well the models relate to the CFD best practices identified in Section Following that, the Maximal WSS magnitude, TAWSS and OSI fields will be discussed alongside the theories for aneurysmal progression, before summarising the key limitations of the models. 44
53 Spatial-Averaged WSS (Pa) Healthy Geometry, 'fine' Mesh: Spatial-Averaged WSS Traces Right Ext. IA Surf. Left Ext. IA Surf. Right Int. IA Surf. Left Int. IA Surf Time (s) Figure 3-12: Spatial-Averaged WSS on the artery surface sections (discussed in Section 3.1.4) vs. Time (second and third cardiac cycles) for the healthy geometry ( fine mesh) Analysis of CFD Practices Used 1) Modelling Blood as a Newtonian, Incompressible Fluid The incompressible fluid assumption is appropriate here as the velocity in the models and in-vivo never approaches 0.3 Mach. The observed presence of slow flow (low shear rate) within aneurysmal regions (Figure 3-13) and near the outer-walls/shoulders of some bifurcations may promote localised non- Newtonian behaviour. This would raise the viscosity in these small regions, and by correlation, the WSS. However, the effect this has on the solution is thought to be of second order in arteries of this size (Steinman 2013). Figure 3-13 below shows the stagnation of near-wall flow within the diseased geometry at peak velocity. The boundary layer separates under the influence of the pressure gradient as it enters the internal IAA. 45
54 Boundary-Layer Separation; Slow Flow Region Flow Direction Figure 3-13: A cross section of the velocity vector field within the internal IAA at peak flow ( fine mesh). Vector magnitude is specified by colour. Note boundarylayer separation region is associated with low shear-rate (~WSS) and likely non-newtonian behaviour. 2) The Performance of the Laminar Flow Model As discussed in Section 1, the choice of the laminar flow model omits turbulence, and while the flow in an AAA is undoubtedly turbulent during late systole it largely becomes laminar again during diastole (Les et al. 2010). For both the healthy and the diseased geometry, the Reynolds number was estimated to be the largest (~2180) at peak flow/systole within the abdominal aorta of the diseased geometry (using Equation (2.1)). Thus, it is unlikely that the occurrence of turbulence in the iliac artery regions would be of great significance. Ultimately, the modelling of turbulence remains uncommon in large artery/aneurysm CFD (Steinman 2013) as it appears to have a short duration, and because most turbulence models assume fully-developed turbulent conditions (Les et al. 2010). 3) The Treatment of the Boundaries By implementing prescribed mass flow boundary conditions at the inlet and outlets the target flow rates were enforced, and therefore also the velocity field. This methodology is used for AAA models investigating WSS (Doyle et al. 2013). Inherently, the Navier-Stokes 46
55 equations are a set of highly-coupled partial differential equations (Sodja 2007), therefore in this case, the solution of the pressure field is calculated with respect to the velocity field. This is a recipe for a non-physiological pressure field when patient-specific flow waveforms are not used. However, a reasonable approximation of the velocity field and WSS is maintained. Furthermore, while having other implications, the rigid artery walls forgo the influence that the wall motion has on the local velocity and WSS. However, the general consensus is that this is another second-order effect in large artery models (Steinman 2013). 4) The Selection of a Polyhedral Mesh It is also not uncommon for polyhedral meshes to be used for aneurysmal flows (Steinman 2013). The polyhedral mesh was chosen in preference to its un-structured counter-part: the tetrahedral mesh. As mentioned in Section 1, a polyhedral mesh is thought to require fourtimes fewer cells, half the memory and a fifth to a tenth of computing time than a tetrahedral mesh to achieve the same solution (when using STAR-CCM+ as the solver). 5) The Refinement of the Prism-Layer Mesh The following figure shows the node-interpolated velocity plotted at cell centroids across the width of the left diseased external iliac artery (for the fine mesh) (Figure 3-14). The plot is at peak systole, in a region of high WSS. As desired, a reasonably smooth discretisation of the boundary-layer velocity occurs, although, the concentration of prismlayer cells close to the wall does not want to be any lower. If the prism-layer spacing parameter was increased slightly, higher velocity gradients might be caught. However, the observed spatial distributions of TAWSS for the coarse, medium and fine meshes already show little variation from one to the other (Figure B-3, Appendix B). 47
56 Figure 3-14: Node-interpolated velocity profile for a line passing through a high-flow region in the left external iliac artery of the diseased geometry ( fine mesh). 6) Numerical Convergence Due to the dynamic time-stepping function used, the variation in the flow field with each time-step was minimal. The reduced time-step during peak flow (early in the cardiac cycle) can be seen in the time-step trace below (Figure 3-15). Ultimately, only five internal iterations were required by the implicit unsteady solver to converge the solution at each time-step. Regarding this, the values which the residuals converged to, were between 10-2 and 10-4, varying over each cardiac cycle. Figure 3-15: Diseased geometry: fine mesh time-step trace for three cardiac cycles (T). The initial spike is attributable to the initial conditions. 48
57 7) The Mesh Refinement Analysis For both the healthy and the diseased model a mesh refinement study was undertaken using the recommended approach: doubling and quadrupling the mesh density, and monitoring the convergence of key variables (Stewart et al. 2012). In this case, the time-averaged, spatial-averaged WSS and time-averaged, standard-deviation of TAWSS were chosen as they represent aspects of the flow field that must be captured. From this data, the spatial discretisation error (in the areas of interest) was estimated using the GCI method, as it is considered a very robust method for reporting discretisation error (Celik et al. 2012). Looking at the results of the mesh refinement study: Increasing the density of the mesh led to higher velocity gradients being captured proximal to the arterial surface boundary. This was expected and can be seen as an increase in the time-averaged, spatial-averaged WSS values used to calculate the GCI (Figure 3-16 and Figure 3-18). The mean of the GCI values calculated from the time-averaged, spatial-averaged WSS were 2.12% for the healthy model, and 0.97% for the diseased model, while the GCI values calculated from the time-averaged, spatial standard deviation of WSS were even lower (see Figure 3-17 and Figure 3-19). In light of these results, the fine mesh solution for the WSS in the areas of interest seems to be sufficient. However, it should be noted that the mesh dependence of the velocity field elsewhere in the domains remains unchecked. 49
58 Diseased Iliac Ateries: Time-averaged, Spatial-averaged WSS vs. Cell Count 1.02 f/f mean Ext1, GCI: 1.33% Ext2, GCI: 1.86% Int1, GCI: 0.56% Int2, GCI: 0.14% Total Cell Count (Millions) Figure 3-16: Diseased geometry: normalised time-averaged, spatial-averaged WSS vs. the total cell count for each solution. f is the solution for a given mesh; f mean is the average of the three solutions: coarse, medium and fine. Diseased Iliac Ateries: Time-averaged, Spatial Standard Deviation of WSS vs. Cell Count 1.05 f/f mean Ext1, GCI: 0.25% Ext2, GCI: 0.97% Int1, GCI: 0.38% Int2, GCI: 0.39% Total Cell Count (Millions) Figure 3-17: Diseased geometry; normalised time-averaged, spatial standard deviation of WSS vs. the total cell count for each solution. f is the solution for a given mesh; f mean is the average of the three solutions: coarse, medium and fine. 50
59 Healthy Iliac Arteries: Time-averaged, Spatial-averaged WSS vs. Cell Count 1.01 f/f mean Ext1, GCI: 1.67% Ext2, GCI: 3.68% Int1, GCI: 1.71% Int2, GCI: 1.43% 1 2 Total Cell Count (Millions) Figure 3-18: Healthy geometry; normalised time-averaged, spatial-averaged WSS vs. the total cell count for each solution. f is the solution for a given mesh; f mean is the average of the three solutions: coarse, medium and fine. Healthy Iliac Arteries: Time-averaged, Spatial Standard Deviation of WSS vs. Cell Count 1.15 Ext1, GCI: 0.31% Ext2, GCI: 1.09e-4% Int1, GCI: 0.02% Int2, GCI: 1.75e-3% f/f mean Total Cell Count (Millions) Figure 3-19: Healthy geometry; normalised time-averaged, spatial standard deviation of WSS vs. the total cell count for each solution. f is the solution for a given mesh; f mean is the average of the three solutions: coarse, medium and fine. 51
60 The exact cell counts of each of the meshes are listed in the Table 3-4 below. Geometry Cell count: coarse Cell count: medium Cell count: fine Healthy 721,318 1,325,965 2,531,131 Diseased 866,755 1,580,059 3,001,171 Table 3-4: Exact cell counts for each mesh. Note: when solving the fine meshes it took approximately 2.5 days to calculate the solution for one cardiac cycle using 15 of 16 cores of a current generation Intel Xeon CPU. This is attributable to the fine time-steps used. 8) The Use of Bench-Mark Validation Data The magnitude of TAWSS found in the AAA region of the diseased geometry is within the same range as the results found by other CFD models and AAA geometries (Figure 1-3, Figure B-3 (Appendix B)). However, due to the scarcity of models containing IAAs, the comparability of WSS in those regions could not be checked. The lack of patient specific data is also unfortunate. When considering the comparison of the solution against the analytical Navier-Stokes solutions, the solution to unsteady pipe flow is the most comparable to flow within an artery branch. However, this has not been looked into. Instead, looking at the inability of the solution in Section 2 (steady pipe flow) to perfectly capture the velocity profile using a similar mesh density, it may be said that some aspects of the flow field are likely to be unresolved Maximal WSS and Time-Averaged Wall Shear Stress Fields For both geometries, the distribution of the maximal WSS and TAWSS is similar. For the diseased geometry, the maximal WSS and TAWSS functions saw focal increases within the patient s right-hand external iliac artery (Figure 3-20). Focal increases in these WSS parameters were also observed within the lower segments of the internal iliac arteries, where the flow should have branched off into a handful of minor arteries (see Section 1, Figure 1-1). Notably, these focal increases in WSS exceeded the WSS at nearby bifurcation regions; where the flow impinges on the artery wall and high WSS is expected (Norman & Powell 2010). The maximal WSS distribution for healthy geometry shows the elevated 52
61 WSS at the bifurcation regions (Figure B-3, Appendix B). Furthermore, the aneurysmal regions are predominantly characterised by low TAWSS and are void of any focal increases in WSS. The results do not show any affirmation of the high-flow theory for aneurysmal progression and, rather, they support the low-flow theory. Right Ext. IA: Focal Increase in WSS Figure 3-20: Diseased geometry (facing the right side of the patient): Maximal WSS that occurred over the 4 th cardiac cycle; fine mesh solution. The iliac artery regions of low TAWSS (below 0.4 Pa) are shown in Figure At 0.4 Pa the endothelial phenotype is known to change to atherogenic, and below 0.36 Pa, monocytes adhere to the endothelium, and are involved in the inflammation and degradation of the extracellular matrix (Section 1.1.2). 53
62 Aorta Right Com. IA Right Ext. IA Right Int. IA Left Com. IA Int. IAs Left Ext. IA Figure 3-21: Regions of TAWSS below 0.4 Pa; fine meshes. Top image: front/right side of the diseased patient geometry. Bottom image: front/left side of the healthy patient geometry. For both the healthy and the diseased geometries, there are no regions within the external iliac arteries where the TAWSS is below the threshold for monocyte adhesion. In the healthy geometry the TAWSS falls below 0.36 Pa at the outside shoulders of internal iliac arteries, where the common iliac arteries bifurcate, and in a number of places lower down the internal iliac arteries. Whereas, for the diseased geometry, only the aneurysmal regions contain areas of TAWSS low enough for monocyte adhesion to occur. Furthermore, while 54
63 there is uncertainty comparing the results of a model with strict quantitative thresholds, when the total distribution of WSS in iliac arteries is considered it is clear that the internal and common iliac arties experience lower WSS than the external iliac arteries. The TAWSS histograms of diseased geometry s non-aneurysmal (left-side) internal and external iliac arteries show this trend (Figure 3-22). Figure 3-22: Diseased geometry; fine mesh. Histograms showing the distribution of TAWSS across cells within specified surfaces (Figure 3-11). 55
64 3.2.3 Oscillatory Shear Index and Time-Averaged Wall Shear Stress For the healthy geometry, the abdominal aorta, common iliac artery back-sides and internal iliac artery shoulders all have large OSI values (>0.45). The greatest concentration of cells with high OSI occurs within the abdominal aorta. Figure 3-23: The OSI field for both the healthy and the diseased geometries; fine meshes. Left image: front/right side of the diseased patient geometry. Right image: front/left side of the healthy patient geometry. Even during systole, the aneurysmal regions that are afflicted by the lowest TAWSS seem to maintain oscillatory flow characteristics throughout the cardiac cycle (i.e. boundary-layer separation (Figure 3-13) and local flow circulation (Figure B-4, Appendix B)). In the presence of slow, oscillatory flow and associated high local particle residence times the likelihood that platelets and monocytes will adhere to the endothelium in areas of low WSS is increased. By observing the regions of low TAWSS (<0.36 Pa) limited by high OSI (>0.4) the resulting field (Figure 3-24) indicates the areas that are most susceptible to a degraded artery endothelium and monocyte adhesion. Ultimately, the overall distribution is similar to the regions of low TAWSS in Figure 3-21, while highlighting how the AAA dominates in terms of these haemodynamic extremes (supporting its prevalence). 56
65 Figure 3-24: Regions of low TAWSS (<0.36 Pa) limited by high OSI (>0.4); fine meshes. Left image: front/right side of the diseased patient geometry. Right image: front/left side of the healthy patient geometry Limitations The main limitations of this study are the lack of patient specific data and the absence of the minor arteries that attach to the internal iliac arteries (Figure 1-1). The absence of these arteries undermines the validity of the observations made. In reality the flow field within the lower iliac arteries would be different and the upstream flow field would be altered by a change in the impendence and boundary conditions representing the downstream vascular bed (Vignon-Clementel et al. 2006). Furthermore, the lack of patient-specific data retards the validation of the CFD models, while also forcing boundary conditions to rely on waveforms derived from other patients. By implementing the prescribed (non-patient-specific) mass-flow boundary conditions to the inlet and outlets of each geometry, target flow rates were enforced, the velocity fields were generalised and the downstream impedance was ignored. Regarding this, the solution of the pressure waveforms in abdominal aorta and common iliac arteries more closely resembles the time-shifted velocity waveform than the empirical pressure data (Mills et al. 1970); see Figure 3-25 below. 57
66 Pressure (gauge) (KPa) Healthy Geometry Aoric Pressure (Surface Average) vs. Pressure Data (From 23 Patients) (Probe) 5 Aortic Probe Pressure Data (Mills) Healthy Geometry: Aortic Plane Pressure 4 Reference Pressure(s) Time (s) Figure 3-25: This is a comparison between the pressure waveform found for the healthy geometry and the empirical pressure waveform (Mills et al. 1970). The inlet velocity boundary, and other boundaries used by the healthy geometry are derived from the same patient data. Variation is expected, however the modelled pressure more closely represents the flow boundary conditions applied. However, the flow boundary conditions are not solely responsible for all non-physiological phenomena that occur. Regarding the above pressure waveform, the rigid boundary condition used for the artery walls affects the nature of the pressure waves that reflect from the periphery, and prohibits the occurrence of pressure changes that would otherwise be induced from the local contraction and expansion of the lumen. However, more importantly this motion influences the WSS field (Sforza et al. 2009). Furthermore, the use of a viscous flow regime (laminar, transitional-flow or turbulence model) will never completely resolve the viscous flow features when turbulence is present (Les et al. 2010). While it was logical to use the laminar flow regime for flow that is thought to be largely laminar, the existence of turbulence in the geometries was not investigated in detail. Another issue, which could be investigated in future work, is the approximation of viscosity; the Newtonian assumption has its short-comings in regions of low shear rate (associated with regions of slow flow and low WSS). 58
67 4 Conclusions and Future Work The boundary conditions, viscous flow regime and fluid properties used in this study are often implemented when modelling haemodynamics in large arteries (Steinman 2013, Doyle et al. 2013). While some of these approximations are not entirely realistic, they provide an insight into the distribution and nature of the WSS. The distribution of TAWSS and maximal WSS throughout both the healthy and diseased geometries supports the lowflow theory for aneurysmal progression. The large OSI and low TAWSS found at the shoulders of the healthy internal iliac arteries may predispose these regions to both atherosclerosis and aneurysmal disease. Furthermore, these haemodynamic extremes do not occur within the external iliac arteries, but are present in the abdominal aorta, common iliac arteries and elsewhere in the internal iliac arteries. These results are in accordance with the arterial regions that exhibit aneurysmal disease. The key findings are listed below; it is important to remember that these findings are representative of two simplified arterial geometries: Aneurysmal-prone regions have no areas of elevated WSS (maximal or timeaveraged) associated with them and overall possess lower TAWSS than nonaneurysmal regions. Regions of low TAWSS (below the threshold for monocyte adhesion) are typically afflicted with elevated OSI, such that the common and internal iliac arteries appear susceptible to (low-flow) aneurysmal progression, while the external iliac arteries do not. This thesis has tackled an area of aneurysmal disease research which has not been thoroughly investigated. The modelling adhered to CFD best practices and provides a strong (and encouraging) foundation for future CFD investigation of iliac artery aneurysms. Improvements to the current CFD models or modelling methodology would include: 1) The inclusion of the minor arteries that branch from internal iliac arteries; which may be done using idealised geometries. 2) The investigation of the effect that different non-newtonian fluid approximations have on the WSS distributions, as well as any implications that the inclusion of the minor arteries have regarding the physical approximation of viscosity. 3) The assessment of the extent of turbulent development using a transitional-flow viscous regime. 59
68 4) Addressing the non-physiological manifestations attributable to the boundary conditions. The solution at the outflow boundaries may be coupled with onedimensional models of the respective transient downstream domain (Vignon-Clementel et al. 2010). However, a simpler alternative would be to implement impedance boundary conditions based on some approximated periodicity (Vignon-Clementel et al. 2006). 5) The investigation of vessel wall compliance. The CFD solver may also be coupled with a finite element analysis (FEA) solver to incorporate wall compliance into the models (CD-adapco 2014). In relation to the site specificity of aneurysmal disease, the end objective would be to accurately calculate the near-wall particle residence times of seeded monocyte-type particles. This would allow the patterns of cell deposition and the probability of recruitment into the vessel wall to be predicted (Hardman et al. 2013). The near-wall lift and drag forces must be included when considering the motion of monocytes and a WSS-limiter should be implemented to adjust the near-wall particle residence times according to monocyte adhesion efficiency (Hardman et al. 2013). In doing this, the mesh dependence of the entire flow field should be looked into, not just the mesh dependence of the viscous boundary layer. While this study was focused on the site specificity of aneurysmal disease in the iliac arteries, the inclusion of structural interaction (coupled CFD and FEA solvers) would also provide an understanding of the internal wall stresses. Currently, the development of noninvasive imaging, numerical techniques and software technologies have enabled patientspecific modelling to better inform clinicians (Doyle et al. 2009). Once there is a standardisation of cardiovascular-region-specific CFD practices, and a further increase in computing power, CFD models may be developed to predict stress distributions when a patient goes in to see the radiologist. Regarding this, the emergence of GPU-accelerated commercial CFD solvers (ANSYS Inc. 2014) is an encouraging development. 60
69 References ANSYS Inc. (2014). Platform-support-ansys Abernathy FH (1968). Fundamentals of Boundary Layers. National Committee for Fluid Mechanics Flims. Ahmed SA and Giddens DP (1983). "Velocity measurements in steady flow through axisymmetric stenoses at moderate Reynolds numbers." Journal of Biomechanical Engineering 16(7): Celik IB, Ghia U, Roache PJ, et al. (2008) Procedure for estimation and reporting of uncertainty due to discretization in CFD applications. Journal of Fluids Engineering 130: CD-adapco (2014). STAR-CCM+ User Guide. Cotrell D (2014). What is the Reynolds Analogy? The Steve Portal, Article Davidson JM, et al. (1985). "Longitudinal gradients of collagen and elastin gene expression in the porcine aorta." The Journal of Biological Chemistry 260: D Souza D (2013). "Histology of blood vessels." Retrieved 20/08, 2013, from Doyle BJ, Grace PA, Kavanagh EG, et al. (2009). Improved assessment and treatment of abdominal aortic aneurysms: the use of 3d reconstructions as a surgical guidance tool in endovascular repair Irish Journal of Medical Sciences 178: Doyle BJ, et al. (2013). From Detection to Rupture: A Serial Computational Fluid Dynamics Study of a Rapidly-Expanding, Patient-Specific, Ruptured Abdominal Aortic Aneurysm : Ferziger JH and Perić M (2002). Computational Methods for Fluid Dynamics, 3rd edn. Springer. Fitzgerald T (2014). "Human Gross Anatomy Atlas." Retrieved 1/05, 2014, from Frazer KH, et al. (2008). "Characterization of an Abdominal Aortic Velocity Waveform in Patients with Abdominal Aortic Aneurysm." Ultrasound in Med. & Biol. 34(1): Gapinski J, et al. (2007). "Comparison of three rheological models of shear flow behavior studied on blood samples from post-infarction patients." International Federation for Medical and Biological Engineering 45: 838. Glagov S, et al. (1988). "Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries." Archives of Pathology & Laboratory Medicine 112(10): Golledge J and Norman PE (2010). "Atherosclerosis and Abdominal Aortic Aneurysm: Cause, Response, or Common Risk Factors?" Arteriosclerosis, Thrombosis and Vascular Biology, American Heart Association 30:
70 Hardman D, et al. (2013). "On the prediction of monocyte deposition in abdominal aortic aneurysms using computational fluid dynamics." Journal of Engineering in Medicine 0(0): Hirsch AT, et al. (2006). "ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations." Journal of Vascular and Interventional Radiology 17(9): Kahraman H, et al. (2006). "The Diameters of the Aorta and Its Major Branches in Patients with Isolated Coronary Artery Ectasia." Texas Heart Institute Journal 33(4): Kirkwood M. (2013) Iliac Artery Aneurysm. 1 Latham RD, et al. (1985). "Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures." Circulation, American Heart Association 72: Les AS, et al. (2010). "Quantification of Hemodynamics in Abdominal Aortic Aneurysms During Rest and Exercise Using Magnetic Resonance Imaging and Computational Fluid Dynamics." Annals of Biomedical Engineering 38(4): Libby P (2002). "Inflammation in atherosclerosis." Natural Publishing Group 420: Maley K (2012). Best Practices: Volume Meshing. Star South East Asia Conference. Singapore, CD-adapco: 8-9. Martyn CN, et al. (1997). "Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension." The Lancet 350(9082): Meyer WW and Lind J (1972). "Calcifications of Iliac Arteries in Newborns and Infants." Archives of Disease in Childhood 47: Michel J, et al. (2011). "Novel Aspects of the Pathogenesis of Aneurysms of the Abdominal Aorta in Humans." Oxford Journals: Cardiovascular Research 90(1): Mills CJ, et al. (1970), "Pressure-flow relationships and vascular impedance in man," Cardiovascular Research, vol. 4, pp Mohamed SMA, et al. (2009). Grid Convergence Study for a Two-Dimensional Simulation of Flow Around a Square Cylinder at a Low Reynolds Number. Seventh International Conference on CFD in the Minerals and Process Industries CSIRO. Melbourne, Australia:
71 Nath A (2014). What is the difference between Courant number and Convective courant number in STAR-CCM+? The Steve Portal, Article Norman PE and Powell JT (2010). "Site Specificity of Aneurysmal Disease." Circulation, American Heart Association 121: Papathanasopoulou P, et al. (2003). "MRI measurement of time-resolved wall shear stress vectors in a carotid bifurcation model, and comparison with CFD predictions." Journal of Magnetic Resonance Imaging 17 (2): Patel V (2011). Impact of Geometry on Blood Flow Patterns in Abdominal Aortic Aneurysms, The State University of New Jersey. Masters in Science: Biomedical Engineering. Petersson S, Dyverfeldt D and Ebbers T (2012). "Assessment of the accuracy of MRI wall shear stress estimation using numerical simulations." Journal of Magnetic Resonance Imaging 36(1): Perić M (2004). Simulation of Flows in Complex Geometries: New Meshing and Solution Methods. NAFEMS Seminar: Simulation of Complex Flows (CFD) - Application and Trends. Niedernhausen/Wiesbaden, Germany. Sazonov I and Nithiarasu P (2012). Semi-automatic surface and volume mesh generation for subject-specific biomedical geometries. International Journal for Numerical Methods in Biomedical Engineering 28: Schlichting H (1979). Boundary-Layer Theory, 7th edn. McGraw-Hill. Schwer LE (2008). Is your mesh refined enough? Estimating discretization error using GCI, 7th LS-DYNA Anwenderforum 1(1): 50. Sforza DM, et al. (2009)."Hemodynamics of Cerebral Aneurysms." National Institute of Health, Annual Review Fluid Mech. 41: Sharp WV, et al. (1982). "Arterial occlusive disease: a function of vessel bifurcation angle." Surgery 91(6): Sodja J (2007). Turbulence Models in CFD, University of Ljubljana: Soulis JV, et al. (2011). Relative Residence Time and Oscillatory Shear Index of Non- Newtonian Flow Models in Aorta. Biomedical Engineering, th International Workshop. Kos, IEEE. Steinman DA (2013). "Variability of Computational Fluid Dynamics Solutions for Pressure and Flow in a Giant Aneurysm: The ASME 2012 Summer Bioengineering Conference CFD Challenge." Journal of Biomechanical Engineering 135(2): Stewart SF, et al. (2012). "Assessment of CFD Performance in Simulations of an Idealized Medical Device: Results of FDA s First Computational Interlaboratory Study." Cardiovascular Engineering and Technology 3(2): Stijntje E, et al. (2008). "Configuration of intracranial arteries and development of aneurysms." The Official Journal of American Academy Neurology 70(9):
72 Suh GY, et al. (2010). "Quantification of Particle Residence Time in Abdominal Aortic Aneurysms Using Magnetic Resonance Imaging and Computational Fluid Dynamics." Annals of Biomedical Engineering 39(2): Taylor CA, et al. (2002). "In Vivo Quantification of Blood Flow and Wall Shear Stress in the Human Abdominal Aorta During Lower Limb Exercise." Annals of Biomedical Engineering 30(3): Tong J, et al. (2011). "Effects of Age on the Elastic Properties of the Intraluminal Thrombus and the Thrombus-covered Wall in Abdominal Aortic Aneurysms: Biaxial Extension Behaviour and Material Modelling." European Journal of Vascular and Endovascular Surgery 42(2): VanderLaan PA, et al. (2004). "Site Specificity of Atherosclerosis, Site-Selective Responses to Atherosclerotic Modulators." Arteriosclerosis, Thrombosis and Vascular Biology. American Heart Association 24: Vignon-Clementel IE, et al. (2006). "Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries." Computer Methods in Applied Mechanics and Engineering 195(29 32): Vignon-Clementel IE, et al. (2010). " Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries." Computer Methods in Biomechanics and Biomedical Engineering 13(5): Wesseling P (2001). Principles of Computational Fluid Dynamic. Springer Series in Computational Mathematics. 29: Wood NB (1999). "Aspects of Fluid Dynamics Applied to the Larger Arteries." Journal of Theoretical Biology 199: Xu C (2014). Define the time-step with a field function using Courant number, The Steve Portal, Article
73 Appendices Appendix A MATLAB Script for Section 2 %@Lachlan Kelsey %Prism-layer stretching calculator for Hagen-Poiseuille steady pipe-flow theory %n is the number of boundary layers %K is the lump of constant terms out the front of Hagen-Poiseuille velocity eqn. %K is irrelevant %R is pipe radius %t1 is the finest prism layer thickness %plt is total prism layer mesh thickness = fluid boundary layer thickness %Velocity balance at boundary layer thickness/free-stream velocity: % (1/2)Vmax = n*k*(2*r*t1+t1^2) Solve for t1 %where Vmax = K*R^2 %Relationship between stretching, S, t1 and plt: %t1*(s^0 + S^ S^(n-1)) = plt %Where S is the parameter to solve! function S = PrismStretchParam(n) R = 4; plt = R - sqrt(1/2*r^2); %Outer radius (in mm) %prism layer mesh thickness %Velocity balance at free-stream t1 = R*((1+1/(2*n))^(1/2)-1); S = 1; RelError = 10000; %Initial Guess %Initialise Error %Loop while (abs(relerror) > ) %Set tolerance on solution sums = 0; for N=1:n sums = sums + S^(N-1); end AbsError = sums - plt/t1; RelError = AbsError/(plt/t1); S = S - S*RelError/100; %Reset Solution %Calculate Solution for S %Update Relative Error %Update S end %Check solution d = zeros(1,length(n)); d(1) = t1; %d is distance from wall 65
74 for i = 2:n d(i) = d(i-1) + d(1)*s^(i-1); end r = R - d; v = R^2 - r.^2; %velocity when K = 1; dv(1)= v(1); %change in velocity across each cell is dv dv(2:n)= v(2:n)-v(1:(n-1)); K = 1/(4*0.0035)*1093; %K for Re = 1500 bar(dv(1:n)*k) %Plot Solution 66
75 Appendix B Supplementary Figures Figure B-1: Diseased geometry (peak flow/systole); fine mesh. This image shows cells with convective Courant numbers greater than one; a few cells in the lower internal iliac arteries have convective Courant numbers that are slightly greater than one. However, the convective Courant number remains below one for all other regions of the geometry, throughout the cardiac cycle. In the healthy geometry the convective Courant number never exceeds one. 67
76 Figure B-2: Healthy geometry surface sections used to monitor WSS throughout the cardiac cycle. Mesh Density Coarse Healthy Geometry: TAWSS Diseased Geometry: TAWSS 68
77 Medium Fine Figure B-3: Comparison of the TAWSS field (in Pa) for the different mesh densities and both geometries (3rd cardiac cycles). 69
78 Figure B-4: Diseased patient geometry ( fine mesh): velocity streamlines showing the flow circulation in the internal iliac aneurysm during peak flow/systole. 70
Isogeometric Analysis of Fluid-Structure Interaction
 Isogeometric Analysis of Fluid-Structure Interaction Y. Bazilevs, V.M. Calo, T.J.R. Hughes Institute for Computational Engineering and Sciences, The University of Texas at Austin, USA e-mail: {bazily,victor,hughes}@ices.utexas.edu
Isogeometric Analysis of Fluid-Structure Interaction Y. Bazilevs, V.M. Calo, T.J.R. Hughes Institute for Computational Engineering and Sciences, The University of Texas at Austin, USA e-mail: {bazily,victor,hughes}@ices.utexas.edu
CPM Specifications Document Healthy Vertebral:
 CPM Specifications Document Healthy Vertebral: OSMSC 0078_0000, 0079_0000, 0166_000, 0167_0000 May 1, 2013 Version 1 Open Source Medical Software Corporation 2013 Open Source Medical Software Corporation.
CPM Specifications Document Healthy Vertebral: OSMSC 0078_0000, 0079_0000, 0166_000, 0167_0000 May 1, 2013 Version 1 Open Source Medical Software Corporation 2013 Open Source Medical Software Corporation.
COMPUTATIONAL FLUID DYNAMICS ANALYSIS OF ORIFICE PLATE METERING SITUATIONS UNDER ABNORMAL CONFIGURATIONS
 COMPUTATIONAL FLUID DYNAMICS ANALYSIS OF ORIFICE PLATE METERING SITUATIONS UNDER ABNORMAL CONFIGURATIONS Dr W. Malalasekera Version 3.0 August 2013 1 COMPUTATIONAL FLUID DYNAMICS ANALYSIS OF ORIFICE PLATE
COMPUTATIONAL FLUID DYNAMICS ANALYSIS OF ORIFICE PLATE METERING SITUATIONS UNDER ABNORMAL CONFIGURATIONS Dr W. Malalasekera Version 3.0 August 2013 1 COMPUTATIONAL FLUID DYNAMICS ANALYSIS OF ORIFICE PLATE
Coupling of STAR-CCM+ to Other Theoretical or Numerical Solutions. Milovan Perić
 Coupling of STAR-CCM+ to Other Theoretical or Numerical Solutions Milovan Perić Contents The need to couple STAR-CCM+ with other theoretical or numerical solutions Coupling approaches: surface and volume
Coupling of STAR-CCM+ to Other Theoretical or Numerical Solutions Milovan Perić Contents The need to couple STAR-CCM+ with other theoretical or numerical solutions Coupling approaches: surface and volume
Introduction to C omputational F luid Dynamics. D. Murrin
 Introduction to C omputational F luid Dynamics D. Murrin Computational fluid dynamics (CFD) is the science of predicting fluid flow, heat transfer, mass transfer, chemical reactions, and related phenomena
Introduction to C omputational F luid Dynamics D. Murrin Computational fluid dynamics (CFD) is the science of predicting fluid flow, heat transfer, mass transfer, chemical reactions, and related phenomena
CFD MODELING FOR PNEUMATIC CONVEYING
 CFD MODELING FOR PNEUMATIC CONVEYING Arvind Kumar 1, D.R. Kaushal 2, Navneet Kumar 3 1 Associate Professor YMCAUST, Faridabad 2 Associate Professor, IIT, Delhi 3 Research Scholar IIT, Delhi e-mail: arvindeem@yahoo.co.in
CFD MODELING FOR PNEUMATIC CONVEYING Arvind Kumar 1, D.R. Kaushal 2, Navneet Kumar 3 1 Associate Professor YMCAUST, Faridabad 2 Associate Professor, IIT, Delhi 3 Research Scholar IIT, Delhi e-mail: arvindeem@yahoo.co.in
1.2 Numerical Solutions of Flow Problems
 1.2 Numerical Solutions of Flow Problems DIFFERENTIAL EQUATIONS OF MOTION FOR A SIMPLIFIED FLOW PROBLEM Continuity equation for incompressible flow: 0 Momentum (Navier-Stokes) equations for a Newtonian
1.2 Numerical Solutions of Flow Problems DIFFERENTIAL EQUATIONS OF MOTION FOR A SIMPLIFIED FLOW PROBLEM Continuity equation for incompressible flow: 0 Momentum (Navier-Stokes) equations for a Newtonian
Driven Cavity Example
 BMAppendixI.qxd 11/14/12 6:55 PM Page I-1 I CFD Driven Cavity Example I.1 Problem One of the classic benchmarks in CFD is the driven cavity problem. Consider steady, incompressible, viscous flow in a square
BMAppendixI.qxd 11/14/12 6:55 PM Page I-1 I CFD Driven Cavity Example I.1 Problem One of the classic benchmarks in CFD is the driven cavity problem. Consider steady, incompressible, viscous flow in a square
Introduction to ANSYS CFX
 Workshop 03 Fluid flow around the NACA0012 Airfoil 16.0 Release Introduction to ANSYS CFX 2015 ANSYS, Inc. March 13, 2015 1 Release 16.0 Workshop Description: The flow simulated is an external aerodynamics
Workshop 03 Fluid flow around the NACA0012 Airfoil 16.0 Release Introduction to ANSYS CFX 2015 ANSYS, Inc. March 13, 2015 1 Release 16.0 Workshop Description: The flow simulated is an external aerodynamics
CFD Analysis of a Fully Developed Turbulent Flow in a Pipe with a Constriction and an Obstacle
 CFD Analysis of a Fully Developed Turbulent Flow in a Pipe with a Constriction and an Obstacle C, Diyoke Mechanical Engineering Department Enugu State University of Science & Tech. Enugu, Nigeria U, Ngwaka
CFD Analysis of a Fully Developed Turbulent Flow in a Pipe with a Constriction and an Obstacle C, Diyoke Mechanical Engineering Department Enugu State University of Science & Tech. Enugu, Nigeria U, Ngwaka
CFD simulations of blood flow through abdominal part of aorta
 CFD simulations of blood flow through abdominal part of aorta Andrzej Polanczyk, Aleksandra Piechota Faculty of Process and Enviromental Engineering, Technical University of Lodz, Wolczanska 13 90-94 Lodz,
CFD simulations of blood flow through abdominal part of aorta Andrzej Polanczyk, Aleksandra Piechota Faculty of Process and Enviromental Engineering, Technical University of Lodz, Wolczanska 13 90-94 Lodz,
ENERGY-224 Reservoir Simulation Project Report. Ala Alzayer
 ENERGY-224 Reservoir Simulation Project Report Ala Alzayer Autumn Quarter December 3, 2014 Contents 1 Objective 2 2 Governing Equations 2 3 Methodolgy 3 3.1 BlockMesh.........................................
ENERGY-224 Reservoir Simulation Project Report Ala Alzayer Autumn Quarter December 3, 2014 Contents 1 Objective 2 2 Governing Equations 2 3 Methodolgy 3 3.1 BlockMesh.........................................
Computational Fluid Dynamics (CFD) Simulation in Air Duct Channels Using STAR CCM+
 Available onlinewww.ejaet.com European Journal of Advances in Engineering and Technology, 2017,4 (3): 216-220 Research Article ISSN: 2394-658X Computational Fluid Dynamics (CFD) Simulation in Air Duct
Available onlinewww.ejaet.com European Journal of Advances in Engineering and Technology, 2017,4 (3): 216-220 Research Article ISSN: 2394-658X Computational Fluid Dynamics (CFD) Simulation in Air Duct
Isotropic Porous Media Tutorial
 STAR-CCM+ User Guide 3927 Isotropic Porous Media Tutorial This tutorial models flow through the catalyst geometry described in the introductory section. In the porous region, the theoretical pressure drop
STAR-CCM+ User Guide 3927 Isotropic Porous Media Tutorial This tutorial models flow through the catalyst geometry described in the introductory section. In the porous region, the theoretical pressure drop
Faculty of Mechanical and Manufacturing Engineering, University Tun Hussein Onn Malaysia (UTHM), Parit Raja, Batu Pahat, Johor, Malaysia
 Applied Mechanics and Materials Vol. 393 (2013) pp 305-310 (2013) Trans Tech Publications, Switzerland doi:10.4028/www.scientific.net/amm.393.305 The Implementation of Cell-Centred Finite Volume Method
Applied Mechanics and Materials Vol. 393 (2013) pp 305-310 (2013) Trans Tech Publications, Switzerland doi:10.4028/www.scientific.net/amm.393.305 The Implementation of Cell-Centred Finite Volume Method
Introduction to Computational Fluid Dynamics Mech 122 D. Fabris, K. Lynch, D. Rich
 Introduction to Computational Fluid Dynamics Mech 122 D. Fabris, K. Lynch, D. Rich 1 Computational Fluid dynamics Computational fluid dynamics (CFD) is the analysis of systems involving fluid flow, heat
Introduction to Computational Fluid Dynamics Mech 122 D. Fabris, K. Lynch, D. Rich 1 Computational Fluid dynamics Computational fluid dynamics (CFD) is the analysis of systems involving fluid flow, heat
CFD-1. Introduction: What is CFD? T. J. Craft. Msc CFD-1. CFD: Computational Fluid Dynamics
 School of Mechanical Aerospace and Civil Engineering CFD-1 T. J. Craft George Begg Building, C41 Msc CFD-1 Reading: J. Ferziger, M. Peric, Computational Methods for Fluid Dynamics H.K. Versteeg, W. Malalasekara,
School of Mechanical Aerospace and Civil Engineering CFD-1 T. J. Craft George Begg Building, C41 Msc CFD-1 Reading: J. Ferziger, M. Peric, Computational Methods for Fluid Dynamics H.K. Versteeg, W. Malalasekara,
Strömningslära Fluid Dynamics. Computer laboratories using COMSOL v4.4
 UMEÅ UNIVERSITY Department of Physics Claude Dion Olexii Iukhymenko May 15, 2015 Strömningslära Fluid Dynamics (5FY144) Computer laboratories using COMSOL v4.4!! Report requirements Computer labs must
UMEÅ UNIVERSITY Department of Physics Claude Dion Olexii Iukhymenko May 15, 2015 Strömningslära Fluid Dynamics (5FY144) Computer laboratories using COMSOL v4.4!! Report requirements Computer labs must
Application of Finite Volume Method for Structural Analysis
 Application of Finite Volume Method for Structural Analysis Saeed-Reza Sabbagh-Yazdi and Milad Bayatlou Associate Professor, Civil Engineering Department of KNToosi University of Technology, PostGraduate
Application of Finite Volume Method for Structural Analysis Saeed-Reza Sabbagh-Yazdi and Milad Bayatlou Associate Professor, Civil Engineering Department of KNToosi University of Technology, PostGraduate
Non-Newtonian Transitional Flow in an Eccentric Annulus
 Tutorial 8. Non-Newtonian Transitional Flow in an Eccentric Annulus Introduction The purpose of this tutorial is to illustrate the setup and solution of a 3D, turbulent flow of a non-newtonian fluid. Turbulent
Tutorial 8. Non-Newtonian Transitional Flow in an Eccentric Annulus Introduction The purpose of this tutorial is to illustrate the setup and solution of a 3D, turbulent flow of a non-newtonian fluid. Turbulent
computational Fluid Dynamics - Prof. V. Esfahanian
 Three boards categories: Experimental Theoretical Computational Crucial to know all three: Each has their advantages and disadvantages. Require validation and verification. School of Mechanical Engineering
Three boards categories: Experimental Theoretical Computational Crucial to know all three: Each has their advantages and disadvantages. Require validation and verification. School of Mechanical Engineering
Backward facing step Homework. Department of Fluid Mechanics. For Personal Use. Budapest University of Technology and Economics. Budapest, 2010 autumn
 Backward facing step Homework Department of Fluid Mechanics Budapest University of Technology and Economics Budapest, 2010 autumn Updated: October 26, 2010 CONTENTS i Contents 1 Introduction 1 2 The problem
Backward facing step Homework Department of Fluid Mechanics Budapest University of Technology and Economics Budapest, 2010 autumn Updated: October 26, 2010 CONTENTS i Contents 1 Introduction 1 2 The problem
Calculate a solution using the pressure-based coupled solver.
 Tutorial 19. Modeling Cavitation Introduction This tutorial examines the pressure-driven cavitating flow of water through a sharpedged orifice. This is a typical configuration in fuel injectors, and brings
Tutorial 19. Modeling Cavitation Introduction This tutorial examines the pressure-driven cavitating flow of water through a sharpedged orifice. This is a typical configuration in fuel injectors, and brings
COMPUTATIONAL AND EXPERIMENTAL INTERFEROMETRIC ANALYSIS OF A CONE-CYLINDER-FLARE BODY. Abstract. I. Introduction
 COMPUTATIONAL AND EXPERIMENTAL INTERFEROMETRIC ANALYSIS OF A CONE-CYLINDER-FLARE BODY John R. Cipolla 709 West Homeway Loop, Citrus Springs FL 34434 Abstract A series of computational fluid dynamic (CFD)
COMPUTATIONAL AND EXPERIMENTAL INTERFEROMETRIC ANALYSIS OF A CONE-CYLINDER-FLARE BODY John R. Cipolla 709 West Homeway Loop, Citrus Springs FL 34434 Abstract A series of computational fluid dynamic (CFD)
CHAPTER 1. Introduction
 ME 475: Computer-Aided Design of Structures 1-1 CHAPTER 1 Introduction 1.1 Analysis versus Design 1.2 Basic Steps in Analysis 1.3 What is the Finite Element Method? 1.4 Geometrical Representation, Discretization
ME 475: Computer-Aided Design of Structures 1-1 CHAPTER 1 Introduction 1.1 Analysis versus Design 1.2 Basic Steps in Analysis 1.3 What is the Finite Element Method? 1.4 Geometrical Representation, Discretization
Three Dimensional Numerical Simulation of Turbulent Flow Over Spillways
 Three Dimensional Numerical Simulation of Turbulent Flow Over Spillways Latif Bouhadji ASL-AQFlow Inc., Sidney, British Columbia, Canada Email: lbouhadji@aslenv.com ABSTRACT Turbulent flows over a spillway
Three Dimensional Numerical Simulation of Turbulent Flow Over Spillways Latif Bouhadji ASL-AQFlow Inc., Sidney, British Columbia, Canada Email: lbouhadji@aslenv.com ABSTRACT Turbulent flows over a spillway
Best Practices: Electronics Cooling. Ruben Bons - CD-adapco
 Best Practices: Electronics Cooling Ruben Bons - CD-adapco Best Practices Outline Geometry Mesh Materials Conditions Solution Results Design exploration / Optimization Best Practices Outline Geometry Solids
Best Practices: Electronics Cooling Ruben Bons - CD-adapco Best Practices Outline Geometry Mesh Materials Conditions Solution Results Design exploration / Optimization Best Practices Outline Geometry Solids
Transition Flow and Aeroacoustic Analysis of NACA0018 Satish Kumar B, Fred Mendonç a, Ghuiyeon Kim, Hogeon Kim
 Transition Flow and Aeroacoustic Analysis of NACA0018 Satish Kumar B, Fred Mendonç a, Ghuiyeon Kim, Hogeon Kim Transition Flow and Aeroacoustic Analysis of NACA0018 Satish Kumar B, Fred Mendonç a, Ghuiyeon
Transition Flow and Aeroacoustic Analysis of NACA0018 Satish Kumar B, Fred Mendonç a, Ghuiyeon Kim, Hogeon Kim Transition Flow and Aeroacoustic Analysis of NACA0018 Satish Kumar B, Fred Mendonç a, Ghuiyeon
Keywords: flows past a cylinder; detached-eddy-simulations; Spalart-Allmaras model; flow visualizations
 A TURBOLENT FLOW PAST A CYLINDER *Vít HONZEJK, **Karel FRAŇA *Technical University of Liberec Studentská 2, 461 17, Liberec, Czech Republic Phone:+ 420 485 353434 Email: vit.honzejk@seznam.cz **Technical
A TURBOLENT FLOW PAST A CYLINDER *Vít HONZEJK, **Karel FRAŇA *Technical University of Liberec Studentská 2, 461 17, Liberec, Czech Republic Phone:+ 420 485 353434 Email: vit.honzejk@seznam.cz **Technical
Optimizing Bio-Inspired Flow Channel Design on Bipolar Plates of PEM Fuel Cells
 Excerpt from the Proceedings of the COMSOL Conference 2010 Boston Optimizing Bio-Inspired Flow Channel Design on Bipolar Plates of PEM Fuel Cells James A. Peitzmeier *1, Steven Kapturowski 2 and Xia Wang
Excerpt from the Proceedings of the COMSOL Conference 2010 Boston Optimizing Bio-Inspired Flow Channel Design on Bipolar Plates of PEM Fuel Cells James A. Peitzmeier *1, Steven Kapturowski 2 and Xia Wang
Verification and Validation of Turbulent Flow around a Clark-Y Airfoil
 Verification and Validation of Turbulent Flow around a Clark-Y Airfoil 1. Purpose 58:160 Intermediate Mechanics of Fluids CFD LAB 2 By Tao Xing and Fred Stern IIHR-Hydroscience & Engineering The University
Verification and Validation of Turbulent Flow around a Clark-Y Airfoil 1. Purpose 58:160 Intermediate Mechanics of Fluids CFD LAB 2 By Tao Xing and Fred Stern IIHR-Hydroscience & Engineering The University
µ = Pa s m 3 The Reynolds number based on hydraulic diameter, D h = 2W h/(w + h) = 3.2 mm for the main inlet duct is = 359
 Laminar Mixer Tutorial for STAR-CCM+ ME 448/548 March 30, 2014 Gerald Recktenwald gerry@pdx.edu 1 Overview Imagine that you are part of a team developing a medical diagnostic device. The device has a millimeter
Laminar Mixer Tutorial for STAR-CCM+ ME 448/548 March 30, 2014 Gerald Recktenwald gerry@pdx.edu 1 Overview Imagine that you are part of a team developing a medical diagnostic device. The device has a millimeter
The Spalart Allmaras turbulence model
 The Spalart Allmaras turbulence model The main equation The Spallart Allmaras turbulence model is a one equation model designed especially for aerospace applications; it solves a modelled transport equation
The Spalart Allmaras turbulence model The main equation The Spallart Allmaras turbulence model is a one equation model designed especially for aerospace applications; it solves a modelled transport equation
Best Practices: Volume Meshing Kynan Maley
 Best Practices: Volume Meshing Kynan Maley Volume Meshing Volume meshing is the basic tool that allows the creation of the space discretization needed to solve most of the CAE equations for: CFD Stress
Best Practices: Volume Meshing Kynan Maley Volume Meshing Volume meshing is the basic tool that allows the creation of the space discretization needed to solve most of the CAE equations for: CFD Stress
A Workflow for Computational Fluid Dynamics Simulations using Patient-Specific Aortic Models
 2.8.8 A Workflow for Computational Fluid Dynamics Simulations using Patient-Specific Aortic Models D. Hazer 1,2, R. Unterhinninghofen 2, M. Kostrzewa 1, H.U. Kauczor 3, R. Dillmann 2, G.M. Richter 1 1)
2.8.8 A Workflow for Computational Fluid Dynamics Simulations using Patient-Specific Aortic Models D. Hazer 1,2, R. Unterhinninghofen 2, M. Kostrzewa 1, H.U. Kauczor 3, R. Dillmann 2, G.M. Richter 1 1)
Verification of Laminar and Validation of Turbulent Pipe Flows
 1 Verification of Laminar and Validation of Turbulent Pipe Flows 1. Purpose ME:5160 Intermediate Mechanics of Fluids CFD LAB 1 (ANSYS 18.1; Last Updated: Aug. 1, 2017) By Timur Dogan, Michael Conger, Dong-Hwan
1 Verification of Laminar and Validation of Turbulent Pipe Flows 1. Purpose ME:5160 Intermediate Mechanics of Fluids CFD LAB 1 (ANSYS 18.1; Last Updated: Aug. 1, 2017) By Timur Dogan, Michael Conger, Dong-Hwan
Computational Simulation of the Wind-force on Metal Meshes
 16 th Australasian Fluid Mechanics Conference Crown Plaza, Gold Coast, Australia 2-7 December 2007 Computational Simulation of the Wind-force on Metal Meshes Ahmad Sharifian & David R. Buttsworth Faculty
16 th Australasian Fluid Mechanics Conference Crown Plaza, Gold Coast, Australia 2-7 December 2007 Computational Simulation of the Wind-force on Metal Meshes Ahmad Sharifian & David R. Buttsworth Faculty
Investigation of cross flow over a circular cylinder at low Re using the Immersed Boundary Method (IBM)
 Computational Methods and Experimental Measurements XVII 235 Investigation of cross flow over a circular cylinder at low Re using the Immersed Boundary Method (IBM) K. Rehman Department of Mechanical Engineering,
Computational Methods and Experimental Measurements XVII 235 Investigation of cross flow over a circular cylinder at low Re using the Immersed Boundary Method (IBM) K. Rehman Department of Mechanical Engineering,
Simulation of Flow Development in a Pipe
 Tutorial 4. Simulation of Flow Development in a Pipe Introduction The purpose of this tutorial is to illustrate the setup and solution of a 3D turbulent fluid flow in a pipe. The pipe networks are common
Tutorial 4. Simulation of Flow Development in a Pipe Introduction The purpose of this tutorial is to illustrate the setup and solution of a 3D turbulent fluid flow in a pipe. The pipe networks are common
Verification and Validation in CFD and Heat Transfer: ANSYS Practice and the New ASME Standard
 Verification and Validation in CFD and Heat Transfer: ANSYS Practice and the New ASME Standard Dimitri P. Tselepidakis & Lewis Collins ASME 2012 Verification and Validation Symposium May 3 rd, 2012 1 Outline
Verification and Validation in CFD and Heat Transfer: ANSYS Practice and the New ASME Standard Dimitri P. Tselepidakis & Lewis Collins ASME 2012 Verification and Validation Symposium May 3 rd, 2012 1 Outline
Computational Study of Laminar Flowfield around a Square Cylinder using Ansys Fluent
 MEGR 7090-003, Computational Fluid Dynamics :1 7 Spring 2015 Computational Study of Laminar Flowfield around a Square Cylinder using Ansys Fluent Rahul R Upadhyay Master of Science, Dept of Mechanical
MEGR 7090-003, Computational Fluid Dynamics :1 7 Spring 2015 Computational Study of Laminar Flowfield around a Square Cylinder using Ansys Fluent Rahul R Upadhyay Master of Science, Dept of Mechanical
Pressure Losses Analysis in Air Duct Flow Using Computational Fluid Dynamics (CFD)
 International Academic Institute for Science and Technology International Academic Journal of Science and Engineering Vol. 3, No. 9, 2016, pp. 55-70. ISSN 2454-3896 International Academic Journal of Science
International Academic Institute for Science and Technology International Academic Journal of Science and Engineering Vol. 3, No. 9, 2016, pp. 55-70. ISSN 2454-3896 International Academic Journal of Science
CFD VALIDATION FOR SURFACE COMBATANT 5415 STRAIGHT AHEAD AND STATIC DRIFT 20 DEGREE CONDITIONS USING STAR CCM+
 CFD VALIDATION FOR SURFACE COMBATANT 5415 STRAIGHT AHEAD AND STATIC DRIFT 20 DEGREE CONDITIONS USING STAR CCM+ by G. J. Grigoropoulos and I..S. Kefallinou 1. Introduction and setup 1. 1 Introduction The
CFD VALIDATION FOR SURFACE COMBATANT 5415 STRAIGHT AHEAD AND STATIC DRIFT 20 DEGREE CONDITIONS USING STAR CCM+ by G. J. Grigoropoulos and I..S. Kefallinou 1. Introduction and setup 1. 1 Introduction The
ISSN(PRINT): ,(ONLINE): ,VOLUME-1,ISSUE-1,
 NUMERICAL ANALYSIS OF THE TUBE BANK PRESSURE DROP OF A SHELL AND TUBE HEAT EXCHANGER Kartik Ajugia, Kunal Bhavsar Lecturer, Mechanical Department, SJCET Mumbai University, Maharashtra Assistant Professor,
NUMERICAL ANALYSIS OF THE TUBE BANK PRESSURE DROP OF A SHELL AND TUBE HEAT EXCHANGER Kartik Ajugia, Kunal Bhavsar Lecturer, Mechanical Department, SJCET Mumbai University, Maharashtra Assistant Professor,
STAR-CCM+: Ventilation SPRING Notes on the software 2. Assigned exercise (submission via Blackboard; deadline: Thursday Week 9, 11 pm)
 STAR-CCM+: Ventilation SPRING 208. Notes on the software 2. Assigned exercise (submission via Blackboard; deadline: Thursday Week 9, pm). Features of the Exercise Natural ventilation driven by localised
STAR-CCM+: Ventilation SPRING 208. Notes on the software 2. Assigned exercise (submission via Blackboard; deadline: Thursday Week 9, pm). Features of the Exercise Natural ventilation driven by localised
NUMERICAL VISCOSITY. Convergent Science White Paper. COPYRIGHT 2017 CONVERGENT SCIENCE. All rights reserved.
 Convergent Science White Paper COPYRIGHT 2017 CONVERGENT SCIENCE. All rights reserved. This document contains information that is proprietary to Convergent Science. Public dissemination of this document
Convergent Science White Paper COPYRIGHT 2017 CONVERGENT SCIENCE. All rights reserved. This document contains information that is proprietary to Convergent Science. Public dissemination of this document
Optimization of under-relaxation factors. and Courant numbers for the simulation of. sloshing in the oil pan of an automobile
 Optimization of under-relaxation factors and Courant numbers for the simulation of sloshing in the oil pan of an automobile Swathi Satish*, Mani Prithiviraj and Sridhar Hari⁰ *National Institute of Technology,
Optimization of under-relaxation factors and Courant numbers for the simulation of sloshing in the oil pan of an automobile Swathi Satish*, Mani Prithiviraj and Sridhar Hari⁰ *National Institute of Technology,
Solution Recording and Playback: Vortex Shedding
 STAR-CCM+ User Guide 6663 Solution Recording and Playback: Vortex Shedding This tutorial demonstrates how to use the solution recording and playback module for capturing the results of transient phenomena.
STAR-CCM+ User Guide 6663 Solution Recording and Playback: Vortex Shedding This tutorial demonstrates how to use the solution recording and playback module for capturing the results of transient phenomena.
Using a Single Rotating Reference Frame
 Tutorial 9. Using a Single Rotating Reference Frame Introduction This tutorial considers the flow within a 2D, axisymmetric, co-rotating disk cavity system. Understanding the behavior of such flows is
Tutorial 9. Using a Single Rotating Reference Frame Introduction This tutorial considers the flow within a 2D, axisymmetric, co-rotating disk cavity system. Understanding the behavior of such flows is
McNair Scholars Research Journal
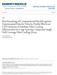 McNair Scholars Research Journal Volume 2 Article 1 2015 Benchmarking of Computational Models against Experimental Data for Velocity Profile Effects on CFD Analysis of Adiabatic Film-Cooling Effectiveness
McNair Scholars Research Journal Volume 2 Article 1 2015 Benchmarking of Computational Models against Experimental Data for Velocity Profile Effects on CFD Analysis of Adiabatic Film-Cooling Effectiveness
NUMERICAL SIMULATIONS OF FLOW THROUGH AN S-DUCT
 NUMERICAL SIMULATIONS OF FLOW THROUGH AN S-DUCT 1 Pravin Peddiraju, 1 Arthur Papadopoulos, 2 Vangelis Skaperdas, 3 Linda Hedges 1 BETA CAE Systems USA, Inc., USA, 2 BETA CAE Systems SA, Greece, 3 CFD Consultant,
NUMERICAL SIMULATIONS OF FLOW THROUGH AN S-DUCT 1 Pravin Peddiraju, 1 Arthur Papadopoulos, 2 Vangelis Skaperdas, 3 Linda Hedges 1 BETA CAE Systems USA, Inc., USA, 2 BETA CAE Systems SA, Greece, 3 CFD Consultant,
Numerical Investigation of Non-Newtonian Laminar Flow in Curved Tube with Insert
 Numerical Investigation of Non-Newtonian Laminar Flow in Curved Tube with Insert A. Kadyyrov 1 1 Research center for power engineering problems Federal government budgetary institution of science Kazan
Numerical Investigation of Non-Newtonian Laminar Flow in Curved Tube with Insert A. Kadyyrov 1 1 Research center for power engineering problems Federal government budgetary institution of science Kazan
Analysis Comparison between CFD and FEA of an Idealized Concept V- Hull Floor Configuration in Two Dimensions
 2010 NDIA GROUND VEHICLE SYSTEMS ENGINEERING AND TECHNOLOGY SYMPOSIUM MODELING & SIMULATION, TESTING AND VALIDATION (MSTV) MINI-SYMPOSIUM AUGUST 17-19 DEARBORN, MICHIGAN Analysis Comparison between CFD
2010 NDIA GROUND VEHICLE SYSTEMS ENGINEERING AND TECHNOLOGY SYMPOSIUM MODELING & SIMULATION, TESTING AND VALIDATION (MSTV) MINI-SYMPOSIUM AUGUST 17-19 DEARBORN, MICHIGAN Analysis Comparison between CFD
Steady Flow: Lid-Driven Cavity Flow
 STAR-CCM+ User Guide Steady Flow: Lid-Driven Cavity Flow 2 Steady Flow: Lid-Driven Cavity Flow This tutorial demonstrates the performance of STAR-CCM+ in solving a traditional square lid-driven cavity
STAR-CCM+ User Guide Steady Flow: Lid-Driven Cavity Flow 2 Steady Flow: Lid-Driven Cavity Flow This tutorial demonstrates the performance of STAR-CCM+ in solving a traditional square lid-driven cavity
Shape optimisation using breakthrough technologies
 Shape optimisation using breakthrough technologies Compiled by Mike Slack Ansys Technical Services 2010 ANSYS, Inc. All rights reserved. 1 ANSYS, Inc. Proprietary Introduction Shape optimisation technologies
Shape optimisation using breakthrough technologies Compiled by Mike Slack Ansys Technical Services 2010 ANSYS, Inc. All rights reserved. 1 ANSYS, Inc. Proprietary Introduction Shape optimisation technologies
Reproducibility of Complex Turbulent Flow Using Commercially-Available CFD Software
 Reports of Research Institute for Applied Mechanics, Kyushu University No.150 (71 83) March 2016 Reproducibility of Complex Turbulent Flow Using Commercially-Available CFD Software Report 3: For the Case
Reports of Research Institute for Applied Mechanics, Kyushu University No.150 (71 83) March 2016 Reproducibility of Complex Turbulent Flow Using Commercially-Available CFD Software Report 3: For the Case
ON THE NUMERICAL MODELING OF IMPINGING JET HEAT TRANSFER
 ON THE NUMERICAL MODELING OF IMPINGING JET HEAT TRANSFER Mirko Bovo 1,2, Sassan Etemad 2 and Lars Davidson 1 1 Dept. of Applied Mechanics, Chalmers University of Technology, Gothenburg, Sweden 2 Powertrain
ON THE NUMERICAL MODELING OF IMPINGING JET HEAT TRANSFER Mirko Bovo 1,2, Sassan Etemad 2 and Lars Davidson 1 1 Dept. of Applied Mechanics, Chalmers University of Technology, Gothenburg, Sweden 2 Powertrain
NUMERICAL STUDY OF BLOOD FLOW IN A VESSEL WITH INCREASING DEGREE OF STENOSIS USING DYNAMIC MESHES
 XIII CONGRESO INTERNACIONAL DE INGENIERÍA DE PROYECTOS Badajoz, 8-10 de julio de 2009 NUMERICAL STUDY OF BLOOD FLOW IN A VESSEL WITH INCREASING DEGREE OF STENOSIS USING DYNAMIC MESHES Ana Cristina Ferreira,
XIII CONGRESO INTERNACIONAL DE INGENIERÍA DE PROYECTOS Badajoz, 8-10 de julio de 2009 NUMERICAL STUDY OF BLOOD FLOW IN A VESSEL WITH INCREASING DEGREE OF STENOSIS USING DYNAMIC MESHES Ana Cristina Ferreira,
Turbulent Premixed Combustion with Flamelet Generated Manifolds in COMSOL Multiphysics
 Turbulent Premixed Combustion with Flamelet Generated Manifolds in COMSOL Multiphysics Rob J.M Bastiaans* Eindhoven University of Technology *Corresponding author: PO box 512, 5600 MB, Eindhoven, r.j.m.bastiaans@tue.nl
Turbulent Premixed Combustion with Flamelet Generated Manifolds in COMSOL Multiphysics Rob J.M Bastiaans* Eindhoven University of Technology *Corresponding author: PO box 512, 5600 MB, Eindhoven, r.j.m.bastiaans@tue.nl
Lab 9: FLUENT: Transient Natural Convection Between Concentric Cylinders
 Lab 9: FLUENT: Transient Natural Convection Between Concentric Cylinders Objective: The objective of this laboratory is to introduce how to use FLUENT to solve both transient and natural convection problems.
Lab 9: FLUENT: Transient Natural Convection Between Concentric Cylinders Objective: The objective of this laboratory is to introduce how to use FLUENT to solve both transient and natural convection problems.
SIMULATION OF FLOW AROUND KCS-HULL
 SIMULATION OF FLOW AROUND KCS-HULL Sven Enger (CD-adapco, Germany) Milovan Perić (CD-adapco, Germany) Robinson Perić (University of Erlangen-Nürnberg, Germany) 1.SUMMARY The paper describes results of
SIMULATION OF FLOW AROUND KCS-HULL Sven Enger (CD-adapco, Germany) Milovan Perić (CD-adapco, Germany) Robinson Perić (University of Erlangen-Nürnberg, Germany) 1.SUMMARY The paper describes results of
Andrew Carter. Vortex shedding off a back facing step in laminar flow.
 Flow Visualization MCEN 5151, Spring 2011 Andrew Carter Team Project 2 4/6/11 Vortex shedding off a back facing step in laminar flow. Figure 1, Vortex shedding from a back facing step in a laminar fluid
Flow Visualization MCEN 5151, Spring 2011 Andrew Carter Team Project 2 4/6/11 Vortex shedding off a back facing step in laminar flow. Figure 1, Vortex shedding from a back facing step in a laminar fluid
The viscous forces on the cylinder are proportional to the gradient of the velocity field at the
 Fluid Dynamics Models : Flow Past a Cylinder Flow Past a Cylinder Introduction The flow of fluid behind a blunt body such as an automobile is difficult to compute due to the unsteady flows. The wake behind
Fluid Dynamics Models : Flow Past a Cylinder Flow Past a Cylinder Introduction The flow of fluid behind a blunt body such as an automobile is difficult to compute due to the unsteady flows. The wake behind
Medical device design using Computational Fluid Dynamics (CFD)
 Medical device design using Computational Fluid Dynamics (CFD) Session: Summer 2016 IMPORTANT NOTE: This project has 8 deliverables, for each one timely work is expected. 1. General Design Specifications
Medical device design using Computational Fluid Dynamics (CFD) Session: Summer 2016 IMPORTANT NOTE: This project has 8 deliverables, for each one timely work is expected. 1. General Design Specifications
STUDY OF FLOW PERFORMANCE OF A GLOBE VALVE AND DESIGN OPTIMISATION
 Journal of Engineering Science and Technology Vol. 12, No. 9 (2017) 2403-2409 School of Engineering, Taylor s University STUDY OF FLOW PERFORMANCE OF A GLOBE VALVE AND DESIGN OPTIMISATION SREEKALA S. K.
Journal of Engineering Science and Technology Vol. 12, No. 9 (2017) 2403-2409 School of Engineering, Taylor s University STUDY OF FLOW PERFORMANCE OF A GLOBE VALVE AND DESIGN OPTIMISATION SREEKALA S. K.
STAR-CCM+: Wind loading on buildings SPRING 2018
 STAR-CCM+: Wind loading on buildings SPRING 2018 1. Notes on the software 2. Assigned exercise (submission via Blackboard; deadline: Thursday Week 3, 11 pm) 1. NOTES ON THE SOFTWARE STAR-CCM+ generates
STAR-CCM+: Wind loading on buildings SPRING 2018 1. Notes on the software 2. Assigned exercise (submission via Blackboard; deadline: Thursday Week 3, 11 pm) 1. NOTES ON THE SOFTWARE STAR-CCM+ generates
Fluent User Services Center
 Solver Settings 5-1 Using the Solver Setting Solver Parameters Convergence Definition Monitoring Stability Accelerating Convergence Accuracy Grid Independence Adaption Appendix: Background Finite Volume
Solver Settings 5-1 Using the Solver Setting Solver Parameters Convergence Definition Monitoring Stability Accelerating Convergence Accuracy Grid Independence Adaption Appendix: Background Finite Volume
Offshore Platform Fluid Structure Interaction (FSI) Simulation
 Offshore Platform Fluid Structure Interaction (FSI) Simulation Ali Marzaban, CD-adapco Murthy Lakshmiraju, CD-adapco Nigel Richardson, CD-adapco Mike Henneke, CD-adapco Guangyu Wu, Chevron Pedro M. Vargas,
Offshore Platform Fluid Structure Interaction (FSI) Simulation Ali Marzaban, CD-adapco Murthy Lakshmiraju, CD-adapco Nigel Richardson, CD-adapco Mike Henneke, CD-adapco Guangyu Wu, Chevron Pedro M. Vargas,
Life Sciences Applications: Modeling and Simulation for Biomedical Device Design SGC 2013
 Life Sciences Applications: Modeling and Simulation for Biomedical Device Design Kristian.Debus@cd-adapco.com SGC 2013 Modeling and Simulation for Biomedical Device Design Biomedical device design and
Life Sciences Applications: Modeling and Simulation for Biomedical Device Design Kristian.Debus@cd-adapco.com SGC 2013 Modeling and Simulation for Biomedical Device Design Biomedical device design and
Modeling External Compressible Flow
 Tutorial 3. Modeling External Compressible Flow Introduction The purpose of this tutorial is to compute the turbulent flow past a transonic airfoil at a nonzero angle of attack. You will use the Spalart-Allmaras
Tutorial 3. Modeling External Compressible Flow Introduction The purpose of this tutorial is to compute the turbulent flow past a transonic airfoil at a nonzero angle of attack. You will use the Spalart-Allmaras
Simulating Sinkage & Trim for Planing Boat Hulls. A Fluent Dynamic Mesh 6DOF Tutorial
 Simulating Sinkage & Trim for Planing Boat Hulls A Fluent Dynamic Mesh 6DOF Tutorial 1 Introduction Workshop Description This workshop describes how to perform a transient 2DOF simulation of a planing
Simulating Sinkage & Trim for Planing Boat Hulls A Fluent Dynamic Mesh 6DOF Tutorial 1 Introduction Workshop Description This workshop describes how to perform a transient 2DOF simulation of a planing
Potsdam Propeller Test Case (PPTC)
 Second International Symposium on Marine Propulsors smp 11, Hamburg, Germany, June 2011 Workshop: Propeller performance Potsdam Propeller Test Case (PPTC) Olof Klerebrant Klasson 1, Tobias Huuva 2 1 Core
Second International Symposium on Marine Propulsors smp 11, Hamburg, Germany, June 2011 Workshop: Propeller performance Potsdam Propeller Test Case (PPTC) Olof Klerebrant Klasson 1, Tobias Huuva 2 1 Core
DES Turbulence Modeling for ICE Flow Simulation in OpenFOAM
 2 nd Two-day Meeting on ICE Simulations Using OpenFOAM DES Turbulence Modeling for ICE Flow Simulation in OpenFOAM V. K. Krastev 1, G. Bella 2 and G. Campitelli 1 University of Tuscia, DEIM School of Engineering
2 nd Two-day Meeting on ICE Simulations Using OpenFOAM DES Turbulence Modeling for ICE Flow Simulation in OpenFOAM V. K. Krastev 1, G. Bella 2 and G. Campitelli 1 University of Tuscia, DEIM School of Engineering
CFD design tool for industrial applications
 Sixth LACCEI International Latin American and Caribbean Conference for Engineering and Technology (LACCEI 2008) Partnering to Success: Engineering, Education, Research and Development June 4 June 6 2008,
Sixth LACCEI International Latin American and Caribbean Conference for Engineering and Technology (LACCEI 2008) Partnering to Success: Engineering, Education, Research and Development June 4 June 6 2008,
NUMERICAL 3D TRANSONIC FLOW SIMULATION OVER A WING
 Review of the Air Force Academy No.3 (35)/2017 NUMERICAL 3D TRANSONIC FLOW SIMULATION OVER A WING Cvetelina VELKOVA Department of Technical Mechanics, Naval Academy Nikola Vaptsarov,Varna, Bulgaria (cvetelina.velkova1985@gmail.com)
Review of the Air Force Academy No.3 (35)/2017 NUMERICAL 3D TRANSONIC FLOW SIMULATION OVER A WING Cvetelina VELKOVA Department of Technical Mechanics, Naval Academy Nikola Vaptsarov,Varna, Bulgaria (cvetelina.velkova1985@gmail.com)
Lisa Michelle Dahl June 04, 2008 CHEM E Finlayson Mixing Properties of an Optimized SAR Mixer (2D and 3D Models)
 Mixing Properties of an Optimized SAR Mixer (2D and 3D Models) I. Introduction While mixing is the most common unit-operation in chemical industrial processes, optimizing mixing in microchemical processes
Mixing Properties of an Optimized SAR Mixer (2D and 3D Models) I. Introduction While mixing is the most common unit-operation in chemical industrial processes, optimizing mixing in microchemical processes
Adjoint Solver Workshop
 Adjoint Solver Workshop Why is an Adjoint Solver useful? Design and manufacture for better performance: e.g. airfoil, combustor, rotor blade, ducts, body shape, etc. by optimising a certain characteristic
Adjoint Solver Workshop Why is an Adjoint Solver useful? Design and manufacture for better performance: e.g. airfoil, combustor, rotor blade, ducts, body shape, etc. by optimising a certain characteristic
Reproducibility of Complex Turbulent Flow Using Commercially-Available CFD Software
 Reports of Research Institute for Applied Mechanics, Kyushu University, No.150 (60-70) March 2016 Reproducibility of Complex Turbulent Flow Using Commercially-Available CFD Software Report 2: For the Case
Reports of Research Institute for Applied Mechanics, Kyushu University, No.150 (60-70) March 2016 Reproducibility of Complex Turbulent Flow Using Commercially-Available CFD Software Report 2: For the Case
ONE DIMENSIONAL (1D) SIMULATION TOOL: GT-POWER
 CHAPTER 4 ONE DIMENSIONAL (1D) SIMULATION TOOL: GT-POWER 4.1 INTRODUCTION Combustion analysis and optimization of any reciprocating internal combustion engines is too complex and intricate activity. It
CHAPTER 4 ONE DIMENSIONAL (1D) SIMULATION TOOL: GT-POWER 4.1 INTRODUCTION Combustion analysis and optimization of any reciprocating internal combustion engines is too complex and intricate activity. It
THE EFFECTS OF THE PLANFORM SHAPE ON DRAG POLAR CURVES OF WINGS: FLUID-STRUCTURE INTERACTION ANALYSES RESULTS
 March 18-20, 2013 THE EFFECTS OF THE PLANFORM SHAPE ON DRAG POLAR CURVES OF WINGS: FLUID-STRUCTURE INTERACTION ANALYSES RESULTS Authors: M.R. Chiarelli, M. Ciabattari, M. Cagnoni, G. Lombardi Speaker:
March 18-20, 2013 THE EFFECTS OF THE PLANFORM SHAPE ON DRAG POLAR CURVES OF WINGS: FLUID-STRUCTURE INTERACTION ANALYSES RESULTS Authors: M.R. Chiarelli, M. Ciabattari, M. Cagnoni, G. Lombardi Speaker:
LES Analysis on Shock-Vortex Ring Interaction
 LES Analysis on Shock-Vortex Ring Interaction Yong Yang Jie Tang Chaoqun Liu Technical Report 2015-08 http://www.uta.edu/math/preprint/ LES Analysis on Shock-Vortex Ring Interaction Yong Yang 1, Jie Tang
LES Analysis on Shock-Vortex Ring Interaction Yong Yang Jie Tang Chaoqun Liu Technical Report 2015-08 http://www.uta.edu/math/preprint/ LES Analysis on Shock-Vortex Ring Interaction Yong Yang 1, Jie Tang
EXPERIMENTAL VALIDATION OF STAR-CCM+ FOR LIQUID CONTAINER SLOSH DYNAMICS
 EXPERIMENTAL VALIDATION OF STAR-CCM+ FOR LIQUID CONTAINER SLOSH DYNAMICS Brandon Marsell a.i. solutions, Launch Services Program, Kennedy Space Center, FL 1 Agenda Introduction Problem Background Experiment
EXPERIMENTAL VALIDATION OF STAR-CCM+ FOR LIQUID CONTAINER SLOSH DYNAMICS Brandon Marsell a.i. solutions, Launch Services Program, Kennedy Space Center, FL 1 Agenda Introduction Problem Background Experiment
Compressible Flow in a Nozzle
 SPC 407 Supersonic & Hypersonic Fluid Dynamics Ansys Fluent Tutorial 1 Compressible Flow in a Nozzle Ahmed M Nagib Elmekawy, PhD, P.E. Problem Specification Consider air flowing at high-speed through a
SPC 407 Supersonic & Hypersonic Fluid Dynamics Ansys Fluent Tutorial 1 Compressible Flow in a Nozzle Ahmed M Nagib Elmekawy, PhD, P.E. Problem Specification Consider air flowing at high-speed through a
Direct Numerical Simulation of a Low Pressure Turbine Cascade. Christoph Müller
 Low Pressure NOFUN 2015, Braunschweig, Overview PostProcessing Experimental test facility Grid generation Inflow turbulence Conclusion and slide 2 / 16 Project Scale resolving Simulations give insight
Low Pressure NOFUN 2015, Braunschweig, Overview PostProcessing Experimental test facility Grid generation Inflow turbulence Conclusion and slide 2 / 16 Project Scale resolving Simulations give insight
Computational Fluid Dynamics modeling of a Water Flow Over an Ogee Profile
 FINITE ELEMENT METHOD TECHNICAL REPORT Computational Fluid Dynamics modeling of a Water Flow Over an Ogee Profile ANSYS CFX 12 ENME 547 Dr. Sudak Matias Sessarego Written Report Due Date: Friday December
FINITE ELEMENT METHOD TECHNICAL REPORT Computational Fluid Dynamics modeling of a Water Flow Over an Ogee Profile ANSYS CFX 12 ENME 547 Dr. Sudak Matias Sessarego Written Report Due Date: Friday December
CFD modelling of thickened tailings Final project report
 26.11.2018 RESEM Remote sensing supporting surveillance and operation of mines CFD modelling of thickened tailings Final project report Lic.Sc.(Tech.) Reeta Tolonen and Docent Esa Muurinen University of
26.11.2018 RESEM Remote sensing supporting surveillance and operation of mines CFD modelling of thickened tailings Final project report Lic.Sc.(Tech.) Reeta Tolonen and Docent Esa Muurinen University of
Influence of mesh quality and density on numerical calculation of heat exchanger with undulation in herringbone pattern
 Influence of mesh quality and density on numerical calculation of heat exchanger with undulation in herringbone pattern Václav Dvořák, Jan Novosád Abstract Research of devices for heat recovery is currently
Influence of mesh quality and density on numerical calculation of heat exchanger with undulation in herringbone pattern Václav Dvořák, Jan Novosád Abstract Research of devices for heat recovery is currently
Coupled Analysis of FSI
 Coupled Analysis of FSI Qin Yin Fan Oct. 11, 2008 Important Key Words Fluid Structure Interface = FSI Computational Fluid Dynamics = CFD Pressure Displacement Analysis = PDA Thermal Stress Analysis = TSA
Coupled Analysis of FSI Qin Yin Fan Oct. 11, 2008 Important Key Words Fluid Structure Interface = FSI Computational Fluid Dynamics = CFD Pressure Displacement Analysis = PDA Thermal Stress Analysis = TSA
Supersonic Flow Over a Wedge
 SPC 407 Supersonic & Hypersonic Fluid Dynamics Ansys Fluent Tutorial 2 Supersonic Flow Over a Wedge Ahmed M Nagib Elmekawy, PhD, P.E. Problem Specification A uniform supersonic stream encounters a wedge
SPC 407 Supersonic & Hypersonic Fluid Dynamics Ansys Fluent Tutorial 2 Supersonic Flow Over a Wedge Ahmed M Nagib Elmekawy, PhD, P.E. Problem Specification A uniform supersonic stream encounters a wedge
Skåne University Hospital Lund, Lund, Sweden 2 Deparment of Numerical Analysis, Centre for Mathematical Sciences, Lund University, Lund, Sweden
 Volume Tracking: A New Method for Visualization of Intracardiac Blood Flow from Three-Dimensional, Time-Resolved, Three-Component Magnetic Resonance Velocity Mapping Appendix: Theory and Numerical Implementation
Volume Tracking: A New Method for Visualization of Intracardiac Blood Flow from Three-Dimensional, Time-Resolved, Three-Component Magnetic Resonance Velocity Mapping Appendix: Theory and Numerical Implementation
Tutorial 1. Introduction to Using FLUENT: Fluid Flow and Heat Transfer in a Mixing Elbow
 Tutorial 1. Introduction to Using FLUENT: Fluid Flow and Heat Transfer in a Mixing Elbow Introduction This tutorial illustrates the setup and solution of the two-dimensional turbulent fluid flow and heat
Tutorial 1. Introduction to Using FLUENT: Fluid Flow and Heat Transfer in a Mixing Elbow Introduction This tutorial illustrates the setup and solution of the two-dimensional turbulent fluid flow and heat
Multigrid Pattern. I. Problem. II. Driving Forces. III. Solution
 Multigrid Pattern I. Problem Problem domain is decomposed into a set of geometric grids, where each element participates in a local computation followed by data exchanges with adjacent neighbors. The grids
Multigrid Pattern I. Problem Problem domain is decomposed into a set of geometric grids, where each element participates in a local computation followed by data exchanges with adjacent neighbors. The grids
Simulation of In-Cylinder Flow Phenomena with ANSYS Piston Grid An Improved Meshing and Simulation Approach
 Simulation of In-Cylinder Flow Phenomena with ANSYS Piston Grid An Improved Meshing and Simulation Approach Dipl.-Ing. (FH) Günther Lang, CFDnetwork Engineering Dipl.-Ing. Burkhard Lewerich, CFDnetwork
Simulation of In-Cylinder Flow Phenomena with ANSYS Piston Grid An Improved Meshing and Simulation Approach Dipl.-Ing. (FH) Günther Lang, CFDnetwork Engineering Dipl.-Ing. Burkhard Lewerich, CFDnetwork
Good Practice in CFD. A rough guide.
 Good Practice in CFD. A rough guide. Prof. Neil W. Bressloff March 2018 Material covered Introduction External and internal flow The CFD process Geometry, meshing, simulation, post-processing The issues
Good Practice in CFD. A rough guide. Prof. Neil W. Bressloff March 2018 Material covered Introduction External and internal flow The CFD process Geometry, meshing, simulation, post-processing The issues
Turbulencja w mikrokanale i jej wpływ na proces emulsyfikacji
 Polish Academy of Sciences Institute of Fundamental Technological Research Turbulencja w mikrokanale i jej wpływ na proces emulsyfikacji S. Błoński, P.Korczyk, T.A. Kowalewski PRESENTATION OUTLINE 0 Introduction
Polish Academy of Sciences Institute of Fundamental Technological Research Turbulencja w mikrokanale i jej wpływ na proces emulsyfikacji S. Błoński, P.Korczyk, T.A. Kowalewski PRESENTATION OUTLINE 0 Introduction
CFD Analysis of 2-D Unsteady Flow Past a Square Cylinder at an Angle of Incidence
 CFD Analysis of 2-D Unsteady Flow Past a Square Cylinder at an Angle of Incidence Kavya H.P, Banjara Kotresha 2, Kishan Naik 3 Dept. of Studies in Mechanical Engineering, University BDT College of Engineering,
CFD Analysis of 2-D Unsteady Flow Past a Square Cylinder at an Angle of Incidence Kavya H.P, Banjara Kotresha 2, Kishan Naik 3 Dept. of Studies in Mechanical Engineering, University BDT College of Engineering,
MESHLESS SOLUTION OF INCOMPRESSIBLE FLOW OVER BACKWARD-FACING STEP
 Vol. 12, Issue 1/2016, 63-68 DOI: 10.1515/cee-2016-0009 MESHLESS SOLUTION OF INCOMPRESSIBLE FLOW OVER BACKWARD-FACING STEP Juraj MUŽÍK 1,* 1 Department of Geotechnics, Faculty of Civil Engineering, University
Vol. 12, Issue 1/2016, 63-68 DOI: 10.1515/cee-2016-0009 MESHLESS SOLUTION OF INCOMPRESSIBLE FLOW OVER BACKWARD-FACING STEP Juraj MUŽÍK 1,* 1 Department of Geotechnics, Faculty of Civil Engineering, University
Finite Element Method. Chapter 7. Practical considerations in FEM modeling
 Finite Element Method Chapter 7 Practical considerations in FEM modeling Finite Element Modeling General Consideration The following are some of the difficult tasks (or decisions) that face the engineer
Finite Element Method Chapter 7 Practical considerations in FEM modeling Finite Element Modeling General Consideration The following are some of the difficult tasks (or decisions) that face the engineer
Mesh Morphing and the Adjoint Solver in ANSYS R14.0. Simon Pereira Laz Foley
 Mesh Morphing and the Adjoint Solver in ANSYS R14.0 Simon Pereira Laz Foley 1 Agenda Fluent Morphing-Optimization Feature RBF Morph with ANSYS DesignXplorer Adjoint Solver What does an adjoint solver do,
Mesh Morphing and the Adjoint Solver in ANSYS R14.0 Simon Pereira Laz Foley 1 Agenda Fluent Morphing-Optimization Feature RBF Morph with ANSYS DesignXplorer Adjoint Solver What does an adjoint solver do,
Modeling Unsteady Compressible Flow
 Tutorial 4. Modeling Unsteady Compressible Flow Introduction In this tutorial, FLUENT s density-based implicit solver is used to predict the timedependent flow through a two-dimensional nozzle. As an initial
Tutorial 4. Modeling Unsteady Compressible Flow Introduction In this tutorial, FLUENT s density-based implicit solver is used to predict the timedependent flow through a two-dimensional nozzle. As an initial
