TG Autoantibody Assays
|
|
|
- Alaina Armstrong
- 6 years ago
- Views:
Transcription
1 TG Autoantibody Assays 24 May 2011 Page 1 of 14
2 Contents Making Label Plating Diluting Label Washing and adding PAS/IgA-Agarose...9 Washing Plates and Transferring 10 Getting Counts off the Beta counters..11 Calculating Results Transferring Results May 2011 Page 2 of 14
3 Differences from Std GADA/IA2A/ZnT8A IgA-Agarose is used as well as PAS PAS is added at 1.2ml/plate We incubate the PAS/IgA-Agarose with the label/serum for 1hr 30 mins. One standard curve per plate Preparing Radiolabel N.B. 35-S Methionine and other reagents are stored in the -70C freezer marked for storage of radioactive materials. Before starting ensure that the water bath is at 30C Wear laboratory coat and pair of medical examination gloves during all steps (Double gloving is recommended). Also wear finger monitor under gloves 1. To prepare label, mix the following reagents in the following order in a sterile 2.0ml Sarstedt tube (the stock 35-S methionine must be opened and added to the reaction mixture in the fume cupboard with fan running and sash lowered): Single (ul) Double (ul) Quad (ul) Reagent Defrost rapidly in Lysate hand, store on ice Mix well, since salts TNT buffer tend to precipitate SP6 polymerase Keep on ice Minus Met Thaw, store on ice S Methionine RNAsin Keep on ice Plasmid DNA* Thaw, store on ice dh2o Defrost lysate rapidly in hand * Plasmid DNA - the volume added is dependent on the concentration. 1ul is added when the conc. = 1mg/ml Change the amount of water added depending on the volume of plasmid added. Do not pipette less than 1ul of plasmid as it s not very accurate. The current TG plasmid DNA is at 1.45mg/ml. Therefore you need 1ul for a single and 1.5ul for a double. Final volume of: 50ul 100ul 200ul 2. Spin tube at 1500rpm (4 o C), then gently mix by tapping the tube. Place the tube in a floating holder and place that in the water bath at 30C for 1hr Fill in label worksheet in database$/antibodies/radac/stock/label2010.xls, including lot numbers and activity dates. 4. Approx. 20 mins before the end of incubation time (while the label is in the water bath), take a Nap5 column and leave to drain into a pot. When it has completely drained fill with LT-TBST+BSA and drain again repeat this 2 more times. 24 May 2011 Page 3 of 14
4 5. Take Eleven 2.0ml Sarstedt tubes and label them as follows: TC, 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 Also write TG on each tube, and add the date to tubes After the radiolabel has been in the waterbath for 1.5 hrs remove tube and place on ice. 7. Put 500ul of Denver buffer and 2ul of label into the first tube marked TC (for total count). 8. Load the full 50ul of label into the column, then add 50ul Denver buffer to the tube (to wash) and add this to the column, then load an additional 450ul Denver buffer to the column. Before each of these additions, check that the last addition has completely entered the column. This is made apparent by the top of the column giving a dull appearance as opposed to looking shiny when liquid is still resting upon the upper membrane. (This assumes a single label has been made. If a double is made instead, the total volume of the reaction is 100µl and 100µl of buffer will be needed to wash out the tube. You will then only require 350µl of buffer to reach the total void-volume of 550µl. If a quadruple is made, the total volume of the reaction is 200µl and 200µl of buffer will be needed to wash out the tube. You will then only require 150µl of buffer to reach the total void-volume of 550µl.) N.B. When collecting fractions, remember to make sure that the last drop at the end of the column is caught in the tube before removing it. Always swap tubes BEFORE adding the buffer for the next fraction. Also: As tubes labelled 1-4 should contain the products, put these on ice. 9. Collect the first fraction in the tube marked 0 (this should be clear), and avoid allowing any pink fluid from entering this tube. 10. The tube marked 1 will collect the first pink fraction when a further 300ul of buffer is added to the column. 11. From now until tube 5, each time you collect a new fraction; add 100ul of buffer to the column (for Quadruple, fraction 3 and 4 are divided into four 50ul fractions). 12. After collecting a fraction in tube 5 add 250ul of buffer collecting a fraction in tube After collecting a fraction in tube 6 add 500ul of buffer collecting fraction in tube After collecting a fraction in tube 7 add 750ul of buffer, collecting fraction in tube May 2011 Page 4 of 14
5 15. After collecting a fraction in tube 8 add 1000ul of buffer then collect the last fraction in tube 9. NB: the volume of buffer that is added each time is equal to that which will be collected The table below shows how much buffer is added to the column and which tube it will be collected into, note that the TC is not put through the column, and the 550ul collected into tube 0 is the void volume (i.e. the buffer that remains in the column until the label and additional buffer is loaded). Vol added (ul) Fraction 500 TC *550* When all 11 fractions have been collected, take 2ul from each tube and transfer to an OptiPlate. To each well add 200ul of microscint 40, vortex plate for 1 min, then load into the topcount. Count using total count protocol (see page 8). Ideally, there will be two peaks in the resulting data. The first peak relates to incorporated methionine and the second peak relates to free methionine. The incorporated methionine (i.e. the labelled antigen) fractions are what are required. To reduce background radioactivity, it is important to prevent free methionine from contaminating the incorporated methionine. To do this, aliquots from the first peak are only selected for use while the radioactive count is still falling. As soon as the count begins to rise (the start of the second peak) that aliquot and the one previous are disregarded. 16. Transfer the counts for the different fractions to the worksheet in label2010.xls. The spreadsheet will then give an estimate of the incorporation- this should be 10% or greater. This estimate will then be used to calculate the approximate volume of label needed to label each plate. For example 10% incorporation in a single reaction should provide sufficient label for at least 4 plates at 20,000cpm (approx. 125ul stock/plate). 17. Pool the fractions containing the labelled antigen (usually fractions 1-3), mix and then divide between the same fraction tubes (approximately 170ul per tube). Store these at -70C until use. 18. Discard all the other fractions in the radioactive sink, washing to waste with copious quantities of running water. Clear the working area and perform environmental and personal radioactive contamination monitoring with the appropriate mini-monitor. Record the monitoring results on the logsheet. 24 May 2011 Page 5 of 14
6 Plating Enter plate map in the appropriate workbook in the TG folder, e.g. TGBr13 file. This can be found in Antibodies folder on Database drive (//smh-srv2/database$). Each assay should include a standard curve and internal and external quality control samples. The standard curve goes on first, followed by 4 internal QCs and 2 external QCs (e.g.q0265), which are distributed randomly through the plate. TG Standards (1/05/98) 9 dilutions of PJB (20/4/98) in SG (28/04/98): S100 =1/2 S50 = 1/4 S25 = 1/8 S12.5 = 1/16 S6.25 = 1/32 S3.1 = 1/64 S1.6 = 1/128 S0.8 = 1/256 S0.4 = 1/512 (low Standard) TG QCs - Negative = SG (28/04/98) - Low Positive = LA (18/12/98) - Medium Positive = MH (4/12/98) - High Positive = High (PJB 10/11/98) Divide plate into halves, then sub-divide into 2 well segments and place in ice tray. Pipette 2µl of each sample into bottom of each pair of wells, using a clean pipette tip for every aliquot, thus allowing the tip box to become a guide for where the next aliquot should be dispensed. Each sample is plated in duplicate Black lines are drawn to indicate the sample duplicates A red line is drawn to divide the plate into 2 halves to aid plating Put plate in freezer if labelling in the future, or in the fridge if it will be labelled that day. In workbook copy the cells with the Codes for the Standards, QCs and Samples. Paste into Plated Template in TG folder. Insert correct Plate ID, e.g. 20/03/2008 A AL, copy and paste next to all CODEs. 24 May 2011 Page 6 of 14
7 Select and Copy the cells by selecting the relevant rows. Paste into the TG Plated table in the Plating Database (Access), which can be found in the Antibodies folder on the Database drive. Diluting Radiolabel for TG Assays 1. Measure 0.372g of EDTA in a 20ml sterilin tube and add 10mls of Denver buffer+0.1% BSA. This will make a 0.1M solution. The EDTA does not dissolve easily and will require about 30mins of gentle shaking (put on the vortex shaker). Once it has dissolved add ~200ul of 5M NaOH to bring the ph to 7.4 (check). 2. Label a 20ml sterilin tube with the date and label name (aliquots can be frozen at - 20C). 3. For each plate being assayed, use 2.375mls of Denver buffer+ 0.1% BSA and 125ul of the 0.1M EDTA solution. This equates to 50ul of 0.1M EDTA solution in each ml of dilution buffer, i.e.0.005m EDTA per ml of dilution buffer. 4. Assuming that % incorporation is 10%, add ~ 40µl radiolabel per plate (double label) (i.e. 40µl label to 2.5mls of buffer.) If diluting a small amount of label (10ml or less) add an additional µl of buffer to your total volume as you may run short otherwise (remember to add ~ 25ul of EDTA solution too). 5. When the label has been diluted, measure the total count (TC) by putting 25µl of the diluted label into a well of an OptiPlate, add 200µl of Microscint 40, cover with a sticky plate cover and shake for approximately 1 minute. If you put plate into the OLD topcount, then: Press F1 - this brings up the count directory Press F4 - this brings up the plate map Press F4 Press F2 Press F9 Press F1 Press F3 Press Alt F2 Press F4 Press F1 Press F10 Press F2 Press F1 Press F1 - this brings up the plate window - this clears the current cell - then go into the appropriate cell (position of where the label has been added in the plate) - this selects that cell to be read by the counter - this exits the plate window and returns to the plate map - this accepts the current plate map - this brings up the count control - this brings up the protocol window - type 4 in the box to select protocol 4 (this is the total count protocol) - this exits back to the count control screen - this turns the stacker on/off (should be off as only one plate is being loaded) - this loads and begins the count protocol - this exits back to the directory - this exits the directory 24 May 2011 Page 7 of 14
8 If you put plate into the New topcount, then: (there are short cut icons you can use which is quicker but this is the fool-proof route!) Go to Application then click on the Sample Mapping icon. This opens up another window. Then go to File, Open, Total Count file. Clear map by selecting cells and hitting delete on the keyboard. Identify the required wells by selecting the relevant cells, go to Edit, and select Fill Wells. Close the Sample Map window down. On the main instrument Top Count window select Total Count protocol instead of gada. The go to Control, Plate source and select Manual. N.B. There is a count delay of 1 minute in the total count protocol, and then it will count for a further 1 minute and then display the total count. 6. The ideal total count for all labels is 20,000 counts. However, there is a range that is acceptable: Lowest acceptable count: 19,000 Highest acceptable count: 21,000 Note: the New and Old Top Counts read slightly differently (counts come out higher on the old one) 7. If the label has a count between these values then it is ready to use, therefore: Using a stepper pipette, add 25µl of labelled antigen to each well and centrifuge. Cover plate with parafilm, mix on a shaking platform, put into ice tray and leave in the fridge to incubate for ~20hrs. If it is outside of these values it will need adjusting. The calculations required to work out how to increase / decrease the count can be found on the Count Calculations protocol. 8. If any adjustments have to made, the label will then need to be recounted following the same procedure as before. 9. Record the label making in the Counts book. 24 May 2011 Page 8 of 14
9 Washing/Adding PAS For each plate being assayed, take 1.2ml of 50% PAS suspension and transfer to a 20ml sterilin tube. Fill the tube with Denver+0.1% BSA buffer, centrifuge and pour off supernatant. After the first wash, add 1ml of 50% IgA-Agarose suspension to the tube and repeat the wash step 3 more times. For each plate you need 5mls of washed PAS/Agarose/Denver buffer, i.e. fill the tube to just above the 5ml line with buffer for 1 plate. Note: One sterilin tube can be used for up to 4 plates (final volume then being just over 20mls). Mix well and ensure that PAS/Agarose stays in suspension during pipetting by frequent mixing. Using a stepper pipette (with the end cut off), pipette 50µl of PAS/Agarose suspension into each well of the plate containing incubated serum and radiolabelled antigen. Centrifuge plates: prog 0 (1500 rpm, 3 4 o C). Place plate back into the fridge on a shaking platform (at 750rpm) for ~ 1.5 hrs. Washing and transferring the assay After plates have been on shaker for 1.5 hrs, remove from fridge and using the multidispenser fill the wells with Denver buffer (WITHOUT BSA). Centrifuge plates: prog 0 (1500 rpm, 3 4 o C) Aspirate with long aspirator, leaving a volume of ~50µl in each well. Repeat wash step 4 more times. Aspirate plates and leave in the fridge until ready to transfer. Add 100µl of ice-cold Denver buffer (+/-BSA doesn t matter) to each well using a stepper pipette. Only add buffer to the plate you are immediately going to transfer. Mix plate for 10-20secs on the shaking platform. Using a multi-channel pipette (yellow tips) transfer the samples to an optiplate. Mix samples with the pipette to ensure the PAS/Agarose is in suspension before transferring to the optiplate. Aim to re-shake plate every 3 columns (4 times a plate) to start off with. You can go for twice a plate when you re up to speed! 24 May 2011 Page 9 of 14
10 Go back to wells to remove any residue, wiggle the pipette about whilst sucking up. Check the tips for residue before discarding in the 35 S waste bin. If there appears to be residue in the tips, shake pipette (over the sink) to bring it down to the end of the tip and transfer it to the plate. After the transfer is complete, check the wells for residue by standing beneath the light and looking up through the bottom of the plate. If any PAS or liquid can be seen, mark the bottom with a red marker and transfer each well individually, adding 50µl buffer if necessary to the appropriate well. Leave Optiplates in the fridge while transferring any remaining plates. Then centrifuge Optiplates and aspirate excess buffer using the short aspirator. Do not leave un-aspirated plates in the fridge for longer than ~ 1hour (label starts unbinding from PAS/Agarose in excess buffer). Add 200µl Microscint 40 to each well and seal securely with a sticky lid. Vortex for ~3 mins to form an emulsion, then place in the topcount for counting. To load plates on the beta counter: Place the stacker rack at the front of the counter and lock into place Stack plates in the rack (plate 1 first and stop plate last) For the New Top Count also put metal weights on back and front stackers. o To count plates on the Old beta counter press: Alt F2 to get into the count window F4 to bring up protocols and select Protocol 1 F10 to switch on the stacker (only required if counting more than one plate) F2 to load the plate Remember to put repeat count protocol plate as last plate in the stack. Following a ten minute count delay plates will count for 5 minutes per well. o To count plates on the New beta counter: On the main instrument Top Count window select gada protocol instead of Top Count. The go to Control, Plate source and select Stacker. Start Assay Transferring Results from Old Beta counter to the worksheets A. To get data from Topcount to computer: Insert floppy disk. Press F7 - this takes you to DOS. At prompt type d then press return. At prompt type copy & then name of the last but one file followed by a:\....txt 24 May 2011 Page 10 of 14
11 E.g. copy gada a:\tga.txt A message should appear to say that the file has been copied. It may also ask if you want to overwrite, if it does then type y and press enter. To get back to the original main screen type x then press enter. B. To get the counter data into the computer: Insert the floppy disk containing the saved file.open the notepad file and Save As New Counts file in: Database$ Antibodies IA2, GAD, TG and ZnT8 Calculating Click Yes when asked if you want to replace the existing file. Open the Access database named Old Top Count Macro in the same folder. Select Macros then select Anna Old Top Count Macro to run from New counts file. Click Yes when asked if you want to replace the existing file. You should then be automatically taken into results\abres.xls Save As the date the plates were put on the counter in Antibodies \ IA2, GAD, TG and ZnT8 Calculating \Counts2010, e.g xls. Repeat to obtain the second set of counts, which will be in the last results file on the Beta counter. Instead of saving a new file, copy and paste the second set of counts in ABRES.XLS next to the first set saved in Counts2010. Transferring Results from New Beta counter to the worksheets 1. Open the one but last file in the gada folder in the Data folder on the counter s hard disk (there is a shortcut to Data on the desk top) e.g. gada1.160, use Notepad to open this file. 2. Use the Edit-Replace function to replace unk with 0 (this is a Zero!), Replace All. This should change the information in the Sample Type column from the format UNK001 to Save onto floppy disk. Repeat for the last file (second set of counts). 24 May 2011 Page 11 of 14
12 4. Insert floppy disk into computer, open the first file with Notepad and Save As New Counts file in Database$\Antibodies \ IA2, GAD, TG and ZnT8 Calculating. Click Yes when asked if you want to replace the existing file. 5. Open Anna s New Top-Count Macro in the same folder. 6. Select Macro and open Top Count Macro (New). When asked if you want to replace the existing file ABRES2.xls select yes. 7. Save As the date the plates were put on the counter, followed by New to indicate which counter it was counted on, e.g New.xls. Counts should be saved in the Counts2010 folder in the IA2, GAD, TG and ZnT8 Calculating folder. 8. Repeat steps 4-6 for the second set of counts, but copy and paste the information in the new Abres2 file next to the first set of counts just saved in Counts2010. Note. The macro will not work if the Sample Type column of the Notepad file includes any letters. Make sure you save the notepad file over the New counts.txt file otherwise you will run the macro on the old data. The Macro requires the Database to occupy the X drive on the computer. Additional information The results for all plates counted will be in the same file and need to be transferred separately from ABRES to the correct worksheets. There are 48 lines of data for each plate. Transfer is follows: Start on number Finish on number Plate 1: 1 95 Plate 2: Plate 3: Plate 4: May 2011 Page 12 of 14
13 These are guidelines not absolute rules. Threshold for positivity for TG= 1.31 Calculating Results 1. Take the counts out of the excel file saved in the Counts2010 folder, in the IA2, GAD, TG and ZnT8 Calculating folder in the Antibodies folder. Paste values into the correct space in the appropriate workbook e.g. TGBr Check the correct counts have appeared (in the top left of that plate s worksheet) for the standard curve. Right click on the graph and select data, selecting the counts (X- Value) and the Log2 units (Y-Value). 3. Check the graph the r 2 value should be above 0.95, if it is not identify any bad duplicates and delete them from the standard curve (on the top left of the worksheet s where the data is sourced from). 4. Once you are happy with the standard curve, change the Plate ID in the title of the graph. 5. Take the equation for the trendline off the graph and put it in the worksheet under the column Log 2 TGA. Make sure you apply this to all of the standards/samples/qcs. 6. Look at the %Error column. Investigate any errors over 30% further. If the error is on a standard ignore it because you should have dealt with it while drawing the graph. If the error is on a QC, check whether the QC is in a. If it s in ignore the duplicate. b. If it s out but deleting one of the duplicates brings it into range then mark as a RPT in the Units column. c. If it s out and deleting one of the duplicates doesn t bring it into range then mark as a RJT in the TGA Units column and RJT any samples in that range. If the error is on a Sample/ExQC look at the counts. i. If both of the duplicates are below the bottom of the standard curve (the counts for standard S0.4) ignore the error. ii. If one or both of the duplicates above the bottom of the standard curve than mark RPT in the TGA Units column. 7. Now go through the QCs and check that each is in range, ranges can be found for the QCs on the tab marked New TGA QC; they should tell you what to do if the QC is out. QCs that are out of range and any samples this affects are rejected by marking RJT in place of the units. 8. Mark on the worksheets Total Counts (from the Counts book) Assay Date (Day label was added) PAS lot IgA lot Label Date (Date Label was made) Now someone needs to check that 1-8 has all been done correctly. Then transfer the results into the database. 24 May 2011 Page 13 of 14
14 Transferring Results Once the Results have been calculated by someone else and you have checked they are done correctly: 1 Copy the TGA Units and CODE cells for all the assay results. 2 Paste these in the TGA results template.xls in the TG folder. 3 Identify the assay date from the worksheet and paste this into the results template under the date of assay heading and copy down for all the results. 4 Now select the three columns of information and sort by TGA Units. Any RPT or RJT values should sort to the bottom. 5 Copy the results (not RPT or RJT) by selecting the rows; paste these results into the Plating Database (Access) in the Antibodies folder, in the table named TG Results. 6 Now go into the TG Plated table and delete the plates you have just obtained results for. If you shut down Access it will automatically save changes. 7 Once you ve transferred the results from a plate, highlight it blue in the workbook. Trizma Base 2.422g Sodium Chloride 8.70g Denver Buffer (1 Litre) BSA dh2o 1g (not present in wash buffer) 1 litres HCl ~3.5mls (This should bring ph to check!) Tween ml 24 May 2011 Page 14 of 14
Standard IA2A, GADA and ZnT8A Assays
 Standard IA2A, GADA and ZnT8A Assays 24 May 2011 Page 1 of 14 Contents Making Standard Labels.....3 Plating.....6 Diluting Label........8 Washing PAS, Adding PAS....10 Washing Plates and Transferring 11
Standard IA2A, GADA and ZnT8A Assays 24 May 2011 Page 1 of 14 Contents Making Standard Labels.....3 Plating.....6 Diluting Label........8 Washing PAS, Adding PAS....10 Washing Plates and Transferring 11
Insulin Autoantibody Assays
 Insulin Autoantibody Assays 24 May 2011 Page 1 of 13 Contents Initial dilution of Insulin Label.. 3 IAA Plating.....4 ciaa Plating... 5 Diluting IAA Label........6 Diluting ciaa Label...... 7 Washing PAS,
Insulin Autoantibody Assays 24 May 2011 Page 1 of 13 Contents Initial dilution of Insulin Label.. 3 IAA Plating.....4 ciaa Plating... 5 Diluting IAA Label........6 Diluting ciaa Label...... 7 Washing PAS,
NCSBI Forensic Biology Section DNA SOP Effective Date: January 30, 2007 Title: ABI PRISM 7000: DNA Quantitation Revision 02
 1. Preparing the DNA Standards 1.1. Label eight microcentrifuge tubes to be used for dilution of standards (i.e. Std. 1, Std. 2, Std. 3, etc.) 1.2. Add required amount of TE/glycogen to each tube (See
1. Preparing the DNA Standards 1.1. Label eight microcentrifuge tubes to be used for dilution of standards (i.e. Std. 1, Std. 2, Std. 3, etc.) 1.2. Add required amount of TE/glycogen to each tube (See
Protein Quantification Kit (BCA Assay)
 Protein Quantification Kit (BCA Assay) Booklet Item NO. KTD3001 Product Name Protein Quantification Kit (BCA Assay) ATTENTION For laboratory research use only. Not for clinical or diagnostic use Version
Protein Quantification Kit (BCA Assay) Booklet Item NO. KTD3001 Product Name Protein Quantification Kit (BCA Assay) ATTENTION For laboratory research use only. Not for clinical or diagnostic use Version
Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: June 6, 2003.
 Policy # MI/SER/06/v01 Page 1 of 5 Section: Subject Title: Epstein Barr Virus Serology Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: June 6,
Policy # MI/SER/06/v01 Page 1 of 5 Section: Subject Title: Epstein Barr Virus Serology Issued by: LABORATORY MANAGER Original Date: March 14, 2001 Approved by: Laboratory Director Revision Date: June 6,
BioRobot 9604 Operating Procedure
 BioRobot 9604 Operating Procedure Note: An import table (directions given in number 20) must be made prior to a robot run. 1. Prior to operating the BioRobot 9604, perform all daily and weekly maintenance
BioRobot 9604 Operating Procedure Note: An import table (directions given in number 20) must be made prior to a robot run. 1. Prior to operating the BioRobot 9604, perform all daily and weekly maintenance
Nuclease-free water (e.g. ThermoFisher, cat # AM9937) Freshly-prepared 70% ethanol in nucleasefree water (optional)
 Before start checklist Materials Consumables Equipment Rapid Barcoding Sequencing Kit (SQK- RBK004) 1.5 ml Eppendorf DNA LoBind tubes Ice bucket with ice Flow Cell Priming Kit (EXP-FLP001) 0.2 ml thin-walled
Before start checklist Materials Consumables Equipment Rapid Barcoding Sequencing Kit (SQK- RBK004) 1.5 ml Eppendorf DNA LoBind tubes Ice bucket with ice Flow Cell Priming Kit (EXP-FLP001) 0.2 ml thin-walled
Policy # MI_SER_GAL Department of Microbiology. Page Quality Manual TABLE OF CONTENTS
 Department of Microbiology Version: 1.0 CURRENT 1 of 23 Prepared by QA Committee Issued by: Laboratory Manager Revision Date: 3/26/2018 Approved by Laboratory Director: Annual Review Date: 9/1/2018 Microbiologist-in-Chief
Department of Microbiology Version: 1.0 CURRENT 1 of 23 Prepared by QA Committee Issued by: Laboratory Manager Revision Date: 3/26/2018 Approved by Laboratory Director: Annual Review Date: 9/1/2018 Microbiologist-in-Chief
Tecan crystallisation robot user guide
 Tecan crystallisation robot user guide The TECAN crystallisation robot in RRB S9 provides an automated facility for setting up crystallisation trays. This user guide is meant as online support for trained
Tecan crystallisation robot user guide The TECAN crystallisation robot in RRB S9 provides an automated facility for setting up crystallisation trays. This user guide is meant as online support for trained
Register your instrument! PCR-Assistant. Software manual
 N) alanual Register your instrument! www.eppendorf.com/myeppendorf Software manual Copyright 2013 Eppendorf AG, Hamburg. No part of this publication may be reproduced without the prior permission of the
N) alanual Register your instrument! www.eppendorf.com/myeppendorf Software manual Copyright 2013 Eppendorf AG, Hamburg. No part of this publication may be reproduced without the prior permission of the
ab BCA protein assay kit reducing agent compatible (test tube)
 ab207004 BCA protein assay kit reducing agent compatible (test tube) Instructions for use: For measuring total protein concentration of pure proteins, extracts or lysates in the presence of reducing agents.
ab207004 BCA protein assay kit reducing agent compatible (test tube) Instructions for use: For measuring total protein concentration of pure proteins, extracts or lysates in the presence of reducing agents.
STANDARD OPERATING PROCEDURE
 Document No: LAB/023/2 Page 1 of 7 Uncontrolled copy if not printed on yellow paper. Title: STANDARD OPERATING PROCEDURE Use of the Magtration System 8Lx Document No: LAB/023 Version No: 2 CopyNo/Holder:
Document No: LAB/023/2 Page 1 of 7 Uncontrolled copy if not printed on yellow paper. Title: STANDARD OPERATING PROCEDURE Use of the Magtration System 8Lx Document No: LAB/023 Version No: 2 CopyNo/Holder:
VIAFLO 96 VIAFLO 384. Microplate Pipetting Easy and Affordable
 VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VITARIS AG Blegistrasse 9 CH-6340 Baar Tel.: 041 769 00 00 Fax: 041 769 00 01 E-mail: info@vitaris.com www.vitaris.com VIAFLO 96 VIAFLO 384
VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VITARIS AG Blegistrasse 9 CH-6340 Baar Tel.: 041 769 00 00 Fax: 041 769 00 01 E-mail: info@vitaris.com www.vitaris.com VIAFLO 96 VIAFLO 384
STAINING PROTOCOL FOR CYTOMETRY (Réf LabEx #1)
 STAINING PROTOCOL FOR CYTOMETRY (Réf LabEx #1) Aim To provide detailed description of all steps, material and methods used for fluorescent surface staining and preparation of whole blood cells for analysis
STAINING PROTOCOL FOR CYTOMETRY (Réf LabEx #1) Aim To provide detailed description of all steps, material and methods used for fluorescent surface staining and preparation of whole blood cells for analysis
BCA protein assay kit for low concentrations. For measuring total protein concentration of pure proteins, extracts or lysates.
 ab207002 BCA protein assay kit for low concentrations Instructions for use: For measuring total protein concentration of pure proteins, extracts or lysates. This product is for research use only and is
ab207002 BCA protein assay kit for low concentrations Instructions for use: For measuring total protein concentration of pure proteins, extracts or lysates. This product is for research use only and is
VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable
 VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VIAFLO 96 VIAFLO 384 Handheld microplate processing Have you reached the limits of productivity with traditional handheld pipettes? Would you
VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VIAFLO 96 VIAFLO 384 Handheld microplate processing Have you reached the limits of productivity with traditional handheld pipettes? Would you
VIAFLO 96 VIAFLO 384. Microplate Pipetting Easy and Affordable
 VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VIAFLO 96 VIAFLO 384 Handheld microplate processing Have you reached the limits of productivity with traditional handheld pipettes? Would you
VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VIAFLO 96 VIAFLO 384 Handheld microplate processing Have you reached the limits of productivity with traditional handheld pipettes? Would you
BD Multiwell AutoSampler Additional Features Tutorial
 BD Multiwell AutoSampler Additional Features Tutorial Introduction This tutorial provides step-by-step instructions on how to use the additional features available in BD Multiwell Plate Manager (MPM) software
BD Multiwell AutoSampler Additional Features Tutorial Introduction This tutorial provides step-by-step instructions on how to use the additional features available in BD Multiwell Plate Manager (MPM) software
QUICKStart Guide APT Versions 3.0 through 3.3
 QUICKStart Guide APT Versions 3.0 through 3.3 PCS QUICKStart Guide Powering On & Off...2-3 Seiko Printer...4-5 Consumable Components...6-7 Pipette Calibration Setup...8-11 Pipette Calibration...12-13 Completing
QUICKStart Guide APT Versions 3.0 through 3.3 PCS QUICKStart Guide Powering On & Off...2-3 Seiko Printer...4-5 Consumable Components...6-7 Pipette Calibration Setup...8-11 Pipette Calibration...12-13 Completing
fluidhandling 96 and 384 channel liquid handling Reagent dispensing Non-contact acoustic dispensing Accessories and consumables
 fluidhandling Xrd-384 96 and 384 channel liquid handling Reagent dispensing Non-contact acoustic dispensing Accessories and consumables Ats-100 Xpp-721 www.fluidx.co.uk Automated reagent dispenser for
fluidhandling Xrd-384 96 and 384 channel liquid handling Reagent dispensing Non-contact acoustic dispensing Accessories and consumables Ats-100 Xpp-721 www.fluidx.co.uk Automated reagent dispenser for
Ion 540 Kit Chef QUICK REFERENCE. Dilute the libraries. Prepare the libraries and consumables. Create a Planned Run. Catalog Numbers A27759, A30011
 QUICK REFERENCE Ion 540 Kit Chef Catalog Numbers A27759, A30011 Pub. No. MAN0014119 Rev. C.0 Note: For safety and biohazard guidelines, see the Safety appendix in the Ion 540 Kit Chef User Guide (Pub.
QUICK REFERENCE Ion 540 Kit Chef Catalog Numbers A27759, A30011 Pub. No. MAN0014119 Rev. C.0 Note: For safety and biohazard guidelines, see the Safety appendix in the Ion 540 Kit Chef User Guide (Pub.
Olink NPX Manager. User Guide
 Olink NPX Manager User Guide Table of contents INTRODUCTION 3 Process 3 Limitations 3 Requirements for analysis 3 List of abbreviations 3 TO CREATE A HEAT MAP CT CSV FILE 4 OLINK NPX MANAGER STEP BY STEP
Olink NPX Manager User Guide Table of contents INTRODUCTION 3 Process 3 Limitations 3 Requirements for analysis 3 List of abbreviations 3 TO CREATE A HEAT MAP CT CSV FILE 4 OLINK NPX MANAGER STEP BY STEP
PROTOCOL: GCP MS Analysis (MSA)
 PROTOCOL: GCP MS Analysis (MSA) Purpose Analyze and relatively quantify post translational modifications on core histone H3 with a fully targeted mass spectrometry assay. This protocol should be started
PROTOCOL: GCP MS Analysis (MSA) Purpose Analyze and relatively quantify post translational modifications on core histone H3 with a fully targeted mass spectrometry assay. This protocol should be started
VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable
 VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VIAFLO 96 VIAFLO 384 Handheld microplate processing Life science labs are confronted with a growing need to process more samples in parallel
VIAFLO 96 VIAFLO 384 Microplate Pipetting Easy and Affordable VIAFLO 96 VIAFLO 384 Handheld microplate processing Life science labs are confronted with a growing need to process more samples in parallel
FULLY AUTOMATED 2 MICROPLATE ANALYZER
 FULLY AUTOMATED 2 MICROPLATE ANALYZER The new Personal Lab,(Plab) is an integration between our unique extensive expertise and information gathered from thousands of installations. The new Plab is innovative
FULLY AUTOMATED 2 MICROPLATE ANALYZER The new Personal Lab,(Plab) is an integration between our unique extensive expertise and information gathered from thousands of installations. The new Plab is innovative
4 plate fully automated ELISA analyzer Reagent system. Open (Human tests predefined) Throughput (max.) Up to 7 plates / run Reaction / reading system
 Specifications & more > > System Specifications > > Consumables > > Accessories System Overview Cat. No. 16300 Analyzer type 4 plate fully automated ELISA analyzer Reagent system Open (Human tests predefined)
Specifications & more > > System Specifications > > Consumables > > Accessories System Overview Cat. No. 16300 Analyzer type 4 plate fully automated ELISA analyzer Reagent system Open (Human tests predefined)
Vial block. Power switch
 Powering On & Off The color-coded bars within the text throughout this Guide indicate the sections where specific instructions are given. Vial block Power switch Powering On Connect system according to
Powering On & Off The color-coded bars within the text throughout this Guide indicate the sections where specific instructions are given. Vial block Power switch Powering On Connect system according to
MINISCAN INSTRUCTIONS
 MINISCAN INSTRUCTIONS DR VIRGINIA GORDON Version 3, July 2011 General Hints on Running Mini-Scan Tests... 1 Total Glycerin Test (TG)... 3 GENERAL HINTS ON RUNNING MINI-SCAN TESTS PIPETTES To use the pipettes
MINISCAN INSTRUCTIONS DR VIRGINIA GORDON Version 3, July 2011 General Hints on Running Mini-Scan Tests... 1 Total Glycerin Test (TG)... 3 GENERAL HINTS ON RUNNING MINI-SCAN TESTS PIPETTES To use the pipettes
Appendix 1: DataStudio with ScienceWorkshop Sensors Tech Tips
 Appendix 1: DataStudio with ScienceWorkshop Sensors Tech Tips Section 1: Starting an experiment 1.1 Opening a file 1. Open the File menu and select Open Activity. 2. In the Open dialog box, navigate to
Appendix 1: DataStudio with ScienceWorkshop Sensors Tech Tips Section 1: Starting an experiment 1.1 Opening a file 1. Open the File menu and select Open Activity. 2. In the Open dialog box, navigate to
Aqueous GPC Instructions - Detailed
 Aqueous GPC Instructions - Detailed Waste Bottle Columns PL Datastream Injection Port GPC Computer UV Detector GPC Pump Eluent Bottle The Aqueous GPC Set Up RI Detector Important The points below must
Aqueous GPC Instructions - Detailed Waste Bottle Columns PL Datastream Injection Port GPC Computer UV Detector GPC Pump Eluent Bottle The Aqueous GPC Set Up RI Detector Important The points below must
Preliminary Lab Exercise Part 1 Calibration of Eppendorf Pipette
 Preliminary Lab Exercise Part 1 Calibration of Eppendorf Pipette Pipettes allow transfer of accurately known volume of solution from one container to another. Volumetric or transfer pipettes deliver a
Preliminary Lab Exercise Part 1 Calibration of Eppendorf Pipette Pipettes allow transfer of accurately known volume of solution from one container to another. Volumetric or transfer pipettes deliver a
CounterTrace. Users manual. Version 1.3.0
 CounterTrace Users manual Version 1.3.0 February 2004 Table of contents OVERVIEW...3 COUNTERTRACE SYSTEM COMPONENTS...4 COUNTERTRACE LOADING ADDITIVE...4 COUNTERTRACE SOFTWARE...4 COUNTERTRACE LOADING
CounterTrace Users manual Version 1.3.0 February 2004 Table of contents OVERVIEW...3 COUNTERTRACE SYSTEM COMPONENTS...4 COUNTERTRACE LOADING ADDITIVE...4 COUNTERTRACE SOFTWARE...4 COUNTERTRACE LOADING
SymBiot I Sample Workstation
 SymBiot I Sample Workstation Getting Started Guide DRAFT August 28, 2001 8:42 pm Symbfmgs.fm5 Copyright 1997, 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic
SymBiot I Sample Workstation Getting Started Guide DRAFT August 28, 2001 8:42 pm Symbfmgs.fm5 Copyright 1997, 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic
ClonePix TM 2 QUICK SET-UP INTRUCTIONS MANUAL SOFTWARE RELEASE
 ClonePix TM 2 QUICK SET-UP INTRUCTIONS MANUAL SOFTWARE RELEASE 1.2.80.1071 07MAN1181.A1 Effective Date: 30-Jan-11 ECO #: 3093 Contents What are Quick Set-Up Instructions?... 3 Starting up ClonePix 2...
ClonePix TM 2 QUICK SET-UP INTRUCTIONS MANUAL SOFTWARE RELEASE 1.2.80.1071 07MAN1181.A1 Effective Date: 30-Jan-11 ECO #: 3093 Contents What are Quick Set-Up Instructions?... 3 Starting up ClonePix 2...
PerkinElmer Life and Analytical Sciences, Inc. USING THE ALPHASCREEN OMNIBEADS CATALOG NUMBERS: D, M, R
 PerkinElmer Life and Analytical Sciences, Inc. USING THE ALPHASCREEN OMNIBEADS CATALOG NUMBERS: 6760626D, 6760626M, 6760626R For Laboratory Use Only Research Chemicals for Research Purposes Only 2 Precautions
PerkinElmer Life and Analytical Sciences, Inc. USING THE ALPHASCREEN OMNIBEADS CATALOG NUMBERS: 6760626D, 6760626M, 6760626R For Laboratory Use Only Research Chemicals for Research Purposes Only 2 Precautions
US IVD Bacterial Test Standard
 Instructions for Use US IVD Bacterial Test Standard Mass calibration standard containing a typical Escherichia coli DH5 alpha peptide and protein profile plus additional proteins. For quality control and
Instructions for Use US IVD Bacterial Test Standard Mass calibration standard containing a typical Escherichia coli DH5 alpha peptide and protein profile plus additional proteins. For quality control and
TECHNICAL MANUAL VERSION RoboSep The Fully Automated Cell Separator
 TECHNICAL MANUAL VERSION 2.3.1 RoboSep The Fully Automated Cell Separator i ii SAFETY INFORMATION AND WARNINGS This instrument is intended for research use only and must be operated by trained professionals.
TECHNICAL MANUAL VERSION 2.3.1 RoboSep The Fully Automated Cell Separator i ii SAFETY INFORMATION AND WARNINGS This instrument is intended for research use only and must be operated by trained professionals.
Unscrew and remove the cap from the waste tank
 Stanford Cytoflex HTS User Guide 11232015 Starting up 1. Check the sheath fluid level every time you use the cytometer. This ensures that you do not run out of sheath fluid during an experiment. Replenish
Stanford Cytoflex HTS User Guide 11232015 Starting up 1. Check the sheath fluid level every time you use the cytometer. This ensures that you do not run out of sheath fluid during an experiment. Replenish
Tutorial: FCAP Array Software with BD FACSArray Bioanalyzer
 Tutorial: FCAP Array Software with BD FACSArray Bioanalyzer After completing this tutorial you will be able to: Create an Experiment with the Experiment Wizard in FCAP Array software. Export the Experiment
Tutorial: FCAP Array Software with BD FACSArray Bioanalyzer After completing this tutorial you will be able to: Create an Experiment with the Experiment Wizard in FCAP Array software. Export the Experiment
Walk Lab Montana State University Fluorescent PCR Ribotyping for Clostridium difficile December 1, Amplification.
 Walk Lab Montana State University Fluorescent PCR Ribotyping for Clostridium difficile December 1, 2014 Amplification PCR reagents: -Promega MasterMix Promega (#M7502) -Primers from Bidet et al. 1999.
Walk Lab Montana State University Fluorescent PCR Ribotyping for Clostridium difficile December 1, 2014 Amplification PCR reagents: -Promega MasterMix Promega (#M7502) -Primers from Bidet et al. 1999.
JMJ-Group Microbiology BMB SDU
 JMJ-Group Microbiology BMB SDU Title: Daily use of FACS Aria II SOP number: SOPFACS0042_v01 Version number: 01 Date issued: 2013.01.16 Review date: 2014.12.01 Written by: Tina Kronborg and Michelle Madelung
JMJ-Group Microbiology BMB SDU Title: Daily use of FACS Aria II SOP number: SOPFACS0042_v01 Version number: 01 Date issued: 2013.01.16 Review date: 2014.12.01 Written by: Tina Kronborg and Michelle Madelung
Use the AccuSEQ Software v2.0 Custom Experiment mode
 QUICK REFERENCE Use the AccuSEQ Software v2.0 Custom Experiment mode Publication Part Number 4425585 Revision Date 25 January 2013 (Rev. B) Custom Experiments Note: For safety and biohazard guidelines,
QUICK REFERENCE Use the AccuSEQ Software v2.0 Custom Experiment mode Publication Part Number 4425585 Revision Date 25 January 2013 (Rev. B) Custom Experiments Note: For safety and biohazard guidelines,
Picus & Picus NxT Electronic Pipette User Manual
 Picus & Picus NxT Electronic Pipette User Manual Table of Contents 1. Introduction 3 1.1. Intended Use 4 1.2. Product Overview 4 1.2.1. Single and Multichannel Pipettes 4 1.2.2. Display 5 1.2.3. Optifit
Picus & Picus NxT Electronic Pipette User Manual Table of Contents 1. Introduction 3 1.1. Intended Use 4 1.2. Product Overview 4 1.2.1. Single and Multichannel Pipettes 4 1.2.2. Display 5 1.2.3. Optifit
Product Overview. your solutions
 Product Overview your solutions 2 CyBio - Product Overview your solutions Pipettors CyBi -Well Provides precise pipetting in 96-, 384- and 1536-well formats. Available with a variety of stackers and accessories
Product Overview your solutions 2 CyBio - Product Overview your solutions Pipettors CyBi -Well Provides precise pipetting in 96-, 384- and 1536-well formats. Available with a variety of stackers and accessories
Microsoft Excel 2007
 Learning computers is Show ezy Microsoft Excel 2007 301 Excel screen, toolbars, views, sheets, and uses for Excel 2005-8 Steve Slisar 2005-8 COPYRIGHT: The copyright for this publication is owned by Steve
Learning computers is Show ezy Microsoft Excel 2007 301 Excel screen, toolbars, views, sheets, and uses for Excel 2005-8 Steve Slisar 2005-8 COPYRIGHT: The copyright for this publication is owned by Steve
CyAn ADP Guide. Starting Up
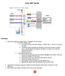 CyAn ADP Guide Starting Up 1. Check the sheath and waste fluids, if needed, fill and empty: 1. To fill the Sheath Tank: a. Unscrew the top of the tank labeled Sheath Tank, and set it on top of the tank.
CyAn ADP Guide Starting Up 1. Check the sheath and waste fluids, if needed, fill and empty: 1. To fill the Sheath Tank: a. Unscrew the top of the tank labeled Sheath Tank, and set it on top of the tank.
Appendix C. Vernier Tutorial
 C-1. Vernier Tutorial Introduction: In this lab course, you will collect, analyze and interpret data. The purpose of this tutorial is to teach you how to use the Vernier System to collect and transfer
C-1. Vernier Tutorial Introduction: In this lab course, you will collect, analyze and interpret data. The purpose of this tutorial is to teach you how to use the Vernier System to collect and transfer
Laboratory Line. Multipurpose Laboratory Equipment. > For all kinds of laboratories > Robust and versatile > Equipment in HUMAN quality
 Laboratory Line Multipurpose Laboratory Equipment > For all kinds of laboratories > Robust and versatile > Equipment in HUMAN quality Sample Preparation Flexible Solutions HuMax 4K Small capacity bench-top
Laboratory Line Multipurpose Laboratory Equipment > For all kinds of laboratories > Robust and versatile > Equipment in HUMAN quality Sample Preparation Flexible Solutions HuMax 4K Small capacity bench-top
ClonePix TM FL Quick Set-Up Instructions
 ClonePix TM FL Quick Set-Up Instructions Software Release: 1.2.13.971 07MAN1055.A2 Effective Date: 01-Jun-10 QUICK INSTRUCTIONS > CONTENTS Contents What are Quick Set Up Instructions?...3 Starting up ClonePix
ClonePix TM FL Quick Set-Up Instructions Software Release: 1.2.13.971 07MAN1055.A2 Effective Date: 01-Jun-10 QUICK INSTRUCTIONS > CONTENTS Contents What are Quick Set Up Instructions?...3 Starting up ClonePix
Thermo Scientific Samco Transfer Pipettes. Precisely what you need
 Thermo Scientific Samco Transfer Pipettes Precisely what you need Yesterday, today Thermo Scientific Samco transfer pipettes have been the industry leaders for over 35 years Design Grips easily with latex
Thermo Scientific Samco Transfer Pipettes Precisely what you need Yesterday, today Thermo Scientific Samco transfer pipettes have been the industry leaders for over 35 years Design Grips easily with latex
CH142: Instructions for the Vernier LabPro
 1 CH142: Instructions for the Vernier LabPro INTRODUCTION: The Vernier LabPro is a versatile data collection interface that can be used in combination with more than 45 sensors, and with a computer, TI
1 CH142: Instructions for the Vernier LabPro INTRODUCTION: The Vernier LabPro is a versatile data collection interface that can be used in combination with more than 45 sensors, and with a computer, TI
Introduction to Spreadsheets
 Introduction to Spreadsheets Spreadsheets are computer programs that were designed for use in business. However, scientists quickly saw how useful they could be for analyzing data. As the programs have
Introduction to Spreadsheets Spreadsheets are computer programs that were designed for use in business. However, scientists quickly saw how useful they could be for analyzing data. As the programs have
FACCalibur Users Guide
 FACCalibur Users Guide FACSCalibur Start Up Procedure If the Instrument is OFF: 1. Check the sheath and waste tanks. Open the fluidics drawer (front of instrument) and check the levels of sheath fluid
FACCalibur Users Guide FACSCalibur Start Up Procedure If the Instrument is OFF: 1. Check the sheath and waste tanks. Open the fluidics drawer (front of instrument) and check the levels of sheath fluid
VIAFLO Fixed and Adjustable Spacing Electronic Pipettes
 VIAFLO Fixed and Adjustable Spacing Electronic Pipettes VIAFLO Electronic Pipettes All VIAFLO pipettes function as repeaters, mixers, serial dilutors, manual pipettes, handheld sample processors and more!
VIAFLO Fixed and Adjustable Spacing Electronic Pipettes VIAFLO Electronic Pipettes All VIAFLO pipettes function as repeaters, mixers, serial dilutors, manual pipettes, handheld sample processors and more!
Pictoral Guide to Genotyping on the Ceq8000
 Pictoral Guide to Genotyping on the Ceq8000 The Beckman Ceq8000. available in beige Index............ 1 Overview........... 2 1. Getting started.......... 3 Logging on to the computer....... 3 Turning
Pictoral Guide to Genotyping on the Ceq8000 The Beckman Ceq8000. available in beige Index............ 1 Overview........... 2 1. Getting started.......... 3 Logging on to the computer....... 3 Turning
QAM-I-102 Operation and Calibration of the Autoanalyzer
 1. Applicability and Purpose a. This procedure applies to the operation and calibration of the LaChat Quikchem 8000 Automated Ion Analyzer. An equivalent system may be used with proper revision of this
1. Applicability and Purpose a. This procedure applies to the operation and calibration of the LaChat Quikchem 8000 Automated Ion Analyzer. An equivalent system may be used with proper revision of this
Thermo Scientific Finnpipette Novus Single and Multichannels
 Thermo Scientific Finnpipette Novus Single and Multichannels The first electronic pipette with a backlit graphical user interface in six languages Part of Thermo Fisher Scientific The new Thermo Scientific
Thermo Scientific Finnpipette Novus Single and Multichannels The first electronic pipette with a backlit graphical user interface in six languages Part of Thermo Fisher Scientific The new Thermo Scientific
Sartorius Biohit Picus Electronic Pipettes
 Sartorius Biohit Picus Electronic Pipettes Enjoy pipetting Achieve better results Learn to use it in a minute Description Picus electronic pipette is intended to be used in liquid handling applications
Sartorius Biohit Picus Electronic Pipettes Enjoy pipetting Achieve better results Learn to use it in a minute Description Picus electronic pipette is intended to be used in liquid handling applications
Instructions for How to Setup the Robot to Print
 Instructions for How to Setup the Robot to Print Introduction This document provides guidelines for setting up a Pat O. Brown arrayer * to print antigens onto glass slides. Pat Brown s website, http://cmgm.stanford.edu/pbrown/mguide/index.html,
Instructions for How to Setup the Robot to Print Introduction This document provides guidelines for setting up a Pat O. Brown arrayer * to print antigens onto glass slides. Pat Brown s website, http://cmgm.stanford.edu/pbrown/mguide/index.html,
Magnetic Immunoassay Reader Operator Manual
 Page 1 of 8 Magnetic Immunoassay Reader Operator Manual Table of contents 1 Introduction...1 2 Magnetic Immunoassay Reader...1 2.1 Contact information and spare parts...1 3 Contents of delivery...1 4 Technical
Page 1 of 8 Magnetic Immunoassay Reader Operator Manual Table of contents 1 Introduction...1 2 Magnetic Immunoassay Reader...1 2.1 Contact information and spare parts...1 3 Contents of delivery...1 4 Technical
Lab Notebook Example How to write a pre-lab and how to collect data and observations
 001 Lab Notebook Example How to write a pre-lab and how to collect data and observations * {Note anything in brackets are comments.} Purpose- The purpose of this experiment is to prepare 10 dilutions of
001 Lab Notebook Example How to write a pre-lab and how to collect data and observations * {Note anything in brackets are comments.} Purpose- The purpose of this experiment is to prepare 10 dilutions of
Intermediate Excel 2003
 Intermediate Excel 2003 Introduction The aim of this document is to introduce some techniques for manipulating data within Excel, including sorting, filtering and how to customise the charts you create.
Intermediate Excel 2003 Introduction The aim of this document is to introduce some techniques for manipulating data within Excel, including sorting, filtering and how to customise the charts you create.
Dionex AS-AP Sample Conductivity and ph Accessory Setup and Operation Guide
 Dionex AS-AP Sample Conductivity and ph Accessory Setup and Operation Guide Document No. 065470 Revision 02 February 2012 2012 by Thermo Fisher Scientific Inc. All rights reserved. Chromeleon is a registered
Dionex AS-AP Sample Conductivity and ph Accessory Setup and Operation Guide Document No. 065470 Revision 02 February 2012 2012 by Thermo Fisher Scientific Inc. All rights reserved. Chromeleon is a registered
Setup protocol for Firefly particles. On BD LSR II and LSRFortessa cytometers with single tube loader function
 Setup protocol for Firefly particles On BD LSR II and LSRFortessa cytometers with single tube loader function Version 1 Last Updated May 2016 Setup protocol for Firefly particles Contents Introduction
Setup protocol for Firefly particles On BD LSR II and LSRFortessa cytometers with single tube loader function Version 1 Last Updated May 2016 Setup protocol for Firefly particles Contents Introduction
DSX Automated ELISA System
 DSX Automated ELISA System Operator s Manual For Revelation 6.0 and above IMPORTANT Please read carefully before using the DSX ii Revision History Revision Date: September 19, 2005 12-16-2005 Part No.
DSX Automated ELISA System Operator s Manual For Revelation 6.0 and above IMPORTANT Please read carefully before using the DSX ii Revision History Revision Date: September 19, 2005 12-16-2005 Part No.
Universal Fit. Pipette Tips.
 Universal Fit Pipette Tips www.globescientific.com Universal Fit Pipette Tips Manufactured in a cleanroom using fully automated equipment No silicone additives, lubricants or heavy metals are used during
Universal Fit Pipette Tips www.globescientific.com Universal Fit Pipette Tips Manufactured in a cleanroom using fully automated equipment No silicone additives, lubricants or heavy metals are used during
Joe von Fischer Lab Michaelis-Menton Enzyme Kinetics Procedure
 1 Joe von Fischer Lab Michaelis-Menton Enzyme Kinetics Procedure Revised 2/7/13 by Claire Griebenow Preparation and Incubation I. Vial Prep 1. Label a set of 60mL vials. Refer to Appendix A for the correct
1 Joe von Fischer Lab Michaelis-Menton Enzyme Kinetics Procedure Revised 2/7/13 by Claire Griebenow Preparation and Incubation I. Vial Prep 1. Label a set of 60mL vials. Refer to Appendix A for the correct
4 Instrument Instructions for CE
 4 Instrument Instructions for CE Overview: the vial trays There are three instrumental steps in the experiment: a) Condition the capillary; b) Set up and, if necessary, optimize your method for a single
4 Instrument Instructions for CE Overview: the vial trays There are three instrumental steps in the experiment: a) Condition the capillary; b) Set up and, if necessary, optimize your method for a single
Nanosight NS300 NBTC User Instructions
 Nanosight NS300 NBTC User Instructions Preliminary notes: -The laser module is delicate and expensive. Handle it with care and do not leave it on the bench unattended. Only leave it in place in the instrument
Nanosight NS300 NBTC User Instructions Preliminary notes: -The laser module is delicate and expensive. Handle it with care and do not leave it on the bench unattended. Only leave it in place in the instrument
CEQ 8000 Series Fragment Analysis Training Guide
 TM CEQ 8000 Series Fragment Analysis Training Guide PN A16039-AB September 2005 Beckman Coulter, Inc., 4300 N. Harbor Blvd., Fullerton, CA 92835 Copyright 2005 Beckman Coulter, Inc., Printed in U.S.A.
TM CEQ 8000 Series Fragment Analysis Training Guide PN A16039-AB September 2005 Beckman Coulter, Inc., 4300 N. Harbor Blvd., Fullerton, CA 92835 Copyright 2005 Beckman Coulter, Inc., Printed in U.S.A.
(Updated 29 Oct 2016)
 (Updated 29 Oct 2016) 1 Class Maker 2016 Program Description Creating classes for the new school year is a time consuming task that teachers are asked to complete each year. Many schools offer their students
(Updated 29 Oct 2016) 1 Class Maker 2016 Program Description Creating classes for the new school year is a time consuming task that teachers are asked to complete each year. Many schools offer their students
Sample Storage STORAGE BOXES & RACKS
 Sample Storage STORAGE BOXES & RACKS Polypropylene Storage Boxes... 80 81, 85 Cardboard Storage Boxes... 82 Cryogenic Storage Boxes... 82 83 Centrifuge Tube Storage... 84... 85 Multi-Tube Racks... 86 88
Sample Storage STORAGE BOXES & RACKS Polypropylene Storage Boxes... 80 81, 85 Cardboard Storage Boxes... 82 Cryogenic Storage Boxes... 82 83 Centrifuge Tube Storage... 84... 85 Multi-Tube Racks... 86 88
Manual HPLC-4 Alliance-UV
 Manual HPLC-4 Alliance-UV MAKE SURE NEVER TO: 1. pump particles or air through the column. 2. inject when the injector is dry. 3. inject solutions containing particles. 4. apply large (sudden) pressure
Manual HPLC-4 Alliance-UV MAKE SURE NEVER TO: 1. pump particles or air through the column. 2. inject when the injector is dry. 3. inject solutions containing particles. 4. apply large (sudden) pressure
GEMINI. Compact Microplate Processor. System Guide
 GEMINI Compact Microplate Processor System Guide 1 Gemini System Guide Table of Contents 1. Introduction 3 2. User Levels 4 3. Instrument Components & Accessories 6 4. Basic Operation - Quick Guide: 1-Lane
GEMINI Compact Microplate Processor System Guide 1 Gemini System Guide Table of Contents 1. Introduction 3 2. User Levels 4 3. Instrument Components & Accessories 6 4. Basic Operation - Quick Guide: 1-Lane
Excel 2016 Basics for Mac
 Excel 2016 Basics for Mac Excel 2016 Basics for Mac Training Objective To learn the tools and features to get started using Excel 2016 more efficiently and effectively. What you can expect to learn from
Excel 2016 Basics for Mac Excel 2016 Basics for Mac Training Objective To learn the tools and features to get started using Excel 2016 more efficiently and effectively. What you can expect to learn from
TraceFinder Analysis Quick Reference Guide
 TraceFinder Analysis Quick Reference Guide This quick reference guide describes the Analysis mode tasks assigned to the Technician role in the Thermo TraceFinder 3.0 analytical software. For detailed descriptions
TraceFinder Analysis Quick Reference Guide This quick reference guide describes the Analysis mode tasks assigned to the Technician role in the Thermo TraceFinder 3.0 analytical software. For detailed descriptions
USER GUIDE TO BECKMAN COULTER FC500
 USER GUIDE TO BECKMAN COULTER FC500 Create a New Protocol Purpose: This procedure describes how to define the parameters and create rough analysis plots for a new experimental set-up. Some protocol templates
USER GUIDE TO BECKMAN COULTER FC500 Create a New Protocol Purpose: This procedure describes how to define the parameters and create rough analysis plots for a new experimental set-up. Some protocol templates
Setup protocol for Firefly particles. On BD FACSCanto cytometers with single tube loader function
 Setup protocol for Firefly particles On BD FACSCanto cytometers with single tube loader function Version 1 Last Updated May 2016 Setup protocol for Firefly particles Contents Introduction Cytometer requirements
Setup protocol for Firefly particles On BD FACSCanto cytometers with single tube loader function Version 1 Last Updated May 2016 Setup protocol for Firefly particles Contents Introduction Cytometer requirements
Premiere - Jazz Video Project Tutorial
 -Open Premiere and set up the Premiere Project -At the bottom left of the Project bin change the view from icon view to list view -Import all audio and video clips to the Project Bin window -Right Click
-Open Premiere and set up the Premiere Project -At the bottom left of the Project bin change the view from icon view to list view -Import all audio and video clips to the Project Bin window -Right Click
Thermo Scientific Samco Transfer Pipettes. Precisely what you need
 Thermo Scientific Samco Transfer Pipettes Precisely what you need Application Guide LIFE SCIENCE APPLICATIONS INDUSTRIAL, RESEARCH AND TESTING CLINICAL APPLICATIONS Top 9 Pipettes: 6, 3, 5, 20, 23, 24,
Thermo Scientific Samco Transfer Pipettes Precisely what you need Application Guide LIFE SCIENCE APPLICATIONS INDUSTRIAL, RESEARCH AND TESTING CLINICAL APPLICATIONS Top 9 Pipettes: 6, 3, 5, 20, 23, 24,
Read Me First (Excel 2007)
 Read Me First (Excel 2007) Concrete Mix Evaluator Before installing the CME program please go through these steps to configure your Excel 2007. Open a NEW BLANK Workbook and click on the "Developer " (A)
Read Me First (Excel 2007) Concrete Mix Evaluator Before installing the CME program please go through these steps to configure your Excel 2007. Open a NEW BLANK Workbook and click on the "Developer " (A)
MC2-ICE Integration with Prime Bid
 MC2-ICE Integration with Prime Bid The process of transferring costs from ICE to Prime Bid (integration) is based on exporting the information from an estimate (sections and costs) via custom Crystal Report
MC2-ICE Integration with Prime Bid The process of transferring costs from ICE to Prime Bid (integration) is based on exporting the information from an estimate (sections and costs) via custom Crystal Report
Computer Basics. Hardware. This class is designed to cover the following basics:
 Computer Basics This class is designed to cover the following basics: computer hardware software computer s operating system different kinds of storage devices you can use to save files using a mouse what
Computer Basics This class is designed to cover the following basics: computer hardware software computer s operating system different kinds of storage devices you can use to save files using a mouse what
Program installation. From the download page: choose the first option:
 Program installation From the download page: http://www.conferencevoting.com/download.htm, choose the first option: Right click on the downloaded file and choose Extract. If installing to another computer,
Program installation From the download page: http://www.conferencevoting.com/download.htm, choose the first option: Right click on the downloaded file and choose Extract. If installing to another computer,
815 Robotic USB Sample Processor XL
 815 Robotic USB Sample Processor XL Precise and reproducible results Automation for larger sample series Sample preparation and liquid handling Parallel sample preparation and analysis Robust, safe, reliable
815 Robotic USB Sample Processor XL Precise and reproducible results Automation for larger sample series Sample preparation and liquid handling Parallel sample preparation and analysis Robust, safe, reliable
ErgoOne Pipettes: Overview
 40 ErgoOne Pipettes: Overview Challenging in Performance and Price Everyday, pipettes are confronted with multiple tasks that must be exact and precise. Reliability is key in the laboratory and your pipette
40 ErgoOne Pipettes: Overview Challenging in Performance and Price Everyday, pipettes are confronted with multiple tasks that must be exact and precise. Reliability is key in the laboratory and your pipette
Introduction to Microsoft Excel
 Intro to Excel Introduction to Microsoft Excel OVERVIEW In this lab, you will become familiar with the general layout and features of Microsoft Excel spreadsheet computer application. Excel has many features,
Intro to Excel Introduction to Microsoft Excel OVERVIEW In this lab, you will become familiar with the general layout and features of Microsoft Excel spreadsheet computer application. Excel has many features,
Advanced Excel Macros : Data Validation/Analysis : OneDrive
 Advanced Excel Macros : Data Validation/Analysis : OneDrive Macros Macros in Excel are in short, a recording of keystrokes. Beyond simple recording, you can use macros to automate tasks that you will use
Advanced Excel Macros : Data Validation/Analysis : OneDrive Macros Macros in Excel are in short, a recording of keystrokes. Beyond simple recording, you can use macros to automate tasks that you will use
MAS 90/200 Intelligence Tips and Tricks Booklet Vol. 1
 MAS 90/200 Intelligence Tips and Tricks Booklet Vol. 1 1 Contents Accessing the Sage MAS Intelligence Reports... 3 Copying, Pasting and Renaming Reports... 4 To create a new report from an existing report...
MAS 90/200 Intelligence Tips and Tricks Booklet Vol. 1 1 Contents Accessing the Sage MAS Intelligence Reports... 3 Copying, Pasting and Renaming Reports... 4 To create a new report from an existing report...
The NGAL Test Application Note for Abbott Architect c8000
 The NGAL Test Application Note for Abbott Architect c8 General parameters Name: NGAL Assay number: *1 Assay version: 1 Assay type: Photometric Assay availability: Enabled Cal version: 1 Reaction definition
The NGAL Test Application Note for Abbott Architect c8 General parameters Name: NGAL Assay number: *1 Assay version: 1 Assay type: Photometric Assay availability: Enabled Cal version: 1 Reaction definition
Document Imaging User Guide
 Release 4.9 IMAGING TECHNOLOGY GROUP Document Imaging Systems Document Imaging User Guide IMAGING TECHNOLOGY GROUP IMIGIT tm Document Imaging User Guide Release 4.91 March 2007 Imaging Technology Group
Release 4.9 IMAGING TECHNOLOGY GROUP Document Imaging Systems Document Imaging User Guide IMAGING TECHNOLOGY GROUP IMIGIT tm Document Imaging User Guide Release 4.91 March 2007 Imaging Technology Group
Preview from Notesale.co.uk Page 2 of 61
 Modify a table Applying styles to tables; banding rows and columns; inserting total rows; removing styles from tables Filter and sort a table Filtering records; sorting data on multiple columns; changing
Modify a table Applying styles to tables; banding rows and columns; inserting total rows; removing styles from tables Filter and sort a table Filtering records; sorting data on multiple columns; changing
Tutorial EN
 Tutorial 8.102.8001EN Metrohm AG CH-9101 Herisau Switzerland Phone +41 71 353 85 85 Fax +41 71 353 89 01 info@metrohm.com www.metrohm.com Tutorial 8.102.8001EN 07.2009 ek Teachware Metrohm AG CH-9101
Tutorial 8.102.8001EN Metrohm AG CH-9101 Herisau Switzerland Phone +41 71 353 85 85 Fax +41 71 353 89 01 info@metrohm.com www.metrohm.com Tutorial 8.102.8001EN 07.2009 ek Teachware Metrohm AG CH-9101
Microsoft Office Excel
 Microsoft Office 2007 - Excel Help Click on the Microsoft Office Excel Help button in the top right corner. Type the desired word in the search box and then press the Enter key. Choose the desired topic
Microsoft Office 2007 - Excel Help Click on the Microsoft Office Excel Help button in the top right corner. Type the desired word in the search box and then press the Enter key. Choose the desired topic
Section 2 Customisation and Printing
 Level 6 Spreadsheet 6N4089 Section 2 Customisation and Printing Contents 1. Customise Toolbars and Create Custom Menus... 2 Recognise the Features Available on Toolbars... 2 Display or Hide the Ribbon...
Level 6 Spreadsheet 6N4089 Section 2 Customisation and Printing Contents 1. Customise Toolbars and Create Custom Menus... 2 Recognise the Features Available on Toolbars... 2 Display or Hide the Ribbon...
DNA Shearing with E220 Focused-ultrasonicator.
 Quick Guide: DNA Shearing with Focused-ultrasonicator. This Quick Guide provides DNA Shearing protocols when using -130, -50, -15, -500, or minitube and a Covaris Focused-ultrasonicator. Revision History
Quick Guide: DNA Shearing with Focused-ultrasonicator. This Quick Guide provides DNA Shearing protocols when using -130, -50, -15, -500, or minitube and a Covaris Focused-ultrasonicator. Revision History
Preprocessing Methods for the Maxprep Liquid Handler Technical Manual
 Preprocessing Methods for the Maxprep Liquid Handler Technical Manual Instructions for use of AS9100, AS9101, AS9200 and AS9201 TM529 11/17 Table of Contents 1 Description...1 2...2 2.1. Maxprep Liquid
Preprocessing Methods for the Maxprep Liquid Handler Technical Manual Instructions for use of AS9100, AS9101, AS9200 and AS9201 TM529 11/17 Table of Contents 1 Description...1 2...2 2.1. Maxprep Liquid
1 plate fully automated ELISA analyzer Reagent system. Open (Human tests predefined) Throughput (Max.) 1 plate /run Barcode reader
 Specifications & more Elisys Uno > > System Specifications > > Consumables > > Accessories System Overview Cat.. 17350 Analyzer type 1 plate fully automated ELISA analyzer Reagent system Open (Human tests
Specifications & more Elisys Uno > > System Specifications > > Consumables > > Accessories System Overview Cat.. 17350 Analyzer type 1 plate fully automated ELISA analyzer Reagent system Open (Human tests
DOING MORE WITH EXCEL: MICROSOFT OFFICE 2013
 DOING MORE WITH EXCEL: MICROSOFT OFFICE 2013 GETTING STARTED PAGE 02 Prerequisites What You Will Learn MORE TASKS IN MICROSOFT EXCEL PAGE 03 Cutting, Copying, and Pasting Data Basic Formulas Filling Data
DOING MORE WITH EXCEL: MICROSOFT OFFICE 2013 GETTING STARTED PAGE 02 Prerequisites What You Will Learn MORE TASKS IN MICROSOFT EXCEL PAGE 03 Cutting, Copying, and Pasting Data Basic Formulas Filling Data
Reconstitution Application Guide
 Reconstitution Application Guide For Research Use Only. Not for use in diagnostic procedures. This guide contains the following topics: About this guide on page 2 App description on page 3 Before you start
Reconstitution Application Guide For Research Use Only. Not for use in diagnostic procedures. This guide contains the following topics: About this guide on page 2 App description on page 3 Before you start
