YEAR Endorsed by: Training Grant is available under HRDF SBL Scheme
|
|
|
- Christine Benson
- 5 years ago
- Views:
Transcription
1 YEAR 2018 The course training program consists of 9 principal modules and 6 additional modules. These modules cover the essential principles of Good Manufacturing Practice (GMP). Participants are expected to gain an understanding of current requirements and future international trends within the pharmaceutical industry. Each participant will be assessed on their level of participation within classroom discussion, assignments and their level of competence in achieving the course objectives. Assignments will be case studies based on actual events that have occurred in the pharmaceutical industry. Training Grant is available under HRDF SBL Scheme Trainers This course has been developed by SeerPharma and trainers are provided by, SeerPharma (Singapore) Pte Ltd. All SeerPharma trainers hold higher education degrees with a minimum of a Bachelor s degree and have a number of years of industry experience in Quality Management or Production Management roles in major and multinational companies. They have experience in all international regulatory standards including FDA, EU, PIC/S, TGA and ISO. The trainer for each module will have specific expertise in that subject matter. SeerPharma is Australia s and Asia Pacific s premier training & consulting group offering integrated consulting, training and technical services to Australia and the Asia Pacific region to meet all international regulatory standards. Aims and Objectives The aim of the course is to provide an in-depth understanding of International GMP and the knowledge and know-how to be able to implement Good Manufacturing Practices in the work place. Organised by: Endorsed by: Malaysian Organisation of Pharmaceutical Industries National Pharmaceutical Regulatory Agency, MOH Presenter: Who Should Attend Key Personnel in any Aspect of GMP & Quality Management, Managers, Engineers, Executives, Quality Practitioners and any member of a pharmaceutical and related industry, those from Research and Development, Quality and Production will find this program relevant and beneficial to their job function. Certificates endorsed by the National Pharmaceutical Regulatory Agency, Ministry of Health, Malaysia will be awarded to participants upon successful completion of each module. For further details please visit
2 FUNDAMENTAL COURSE OUTLINE Those who are new to the pharmaceutical manufacturing industry or have recently transferred from other industries such as medical devices, electronics, food, research & development or cosmetics. This series is also recommended to those who have worked in the pharmaceutical industry with less than 3-5 years experience and are not familiar with International GMP standards such as PIC/s, EU or CFRs and wish to expand their current baseline knowledge of international GMP requirements and expectations. Module 1 International Good Manufacturing Practices, Quality Management Systems and GMP for Pharmaceutical Operations (23 25 January 2018) 3 day Course Aim: To provide an introduction to the regulations and Codes of Practice that governs the manufacture of therapeutic goods both nationally and internationally. To develop a broad understanding of the scope of Good Manufacturing Practices and Quality Management Systems applicable to drugs, devices and biologics and to provide a detailed analysis of the GMP requirements for manufacturing pharmaceuticals. Day2 AM QA Principles & International GMPs, updates of ASEAN Harmonisation focusing on GMP PM Quality Management, Quality Assurance & Quality Control AM Key Quality Assurance Systems and GMP Responsibilities for Managers & Supervisors PM GMP Principles for Manufacturing Operations AM GMP Principles for Packaging Operations includes control of printed packaging materials, line clearance and reconciliation of materials/products AM GMP Principles for Warehousing (related to manufacturing) PM Equipment Management All Personnel Module 2 Validation Principles and Practices (5-7 February 2018) 3 day Course Aim: This subject aims to introduce students to the validation principles covered in PIC/S, ICH, EU & FDA cgmps and to extend the principles to practical outcomes. AM Validation Principles & International Regulations PM Validation Master Plans and Validation Documents PM Equipment Qualification and Commissioning AM Introduction to Process Validation and Cleaning Validation PM Compiling URS against FDS documents PM Preparing DQ, IQ, OQ and PQ protocols AM Protocol Execution AM Deviation Management PM Final Summary Report QA, Engineers Production Behavourial GMP/Good Documentation Practices/Data Integrity (12 14 March 2018) 3 day Course Aim: To provide an introduction on the concepts of behavioural GMP and how they relate to human errors and incidents as well as develop methodologies for root cause analysis and failure investigation. This course helps quality managers and supervisors understand and identify personnel s mentality and common behaviours and cultural changes to minimise human errors. Behavioural GMP AM/PM Knowledge and Understanding GMP concepts, compliance and improvement Discipline Skills sources of human error and strategies to reduce human error in manufacturing Good Documentation Practices To understand the importance of creating documents and the need to maintain records in the industry to ensure compliance as well as traceability for processes and to prevent error. To understand the need for Good Documentation Practices to be applied throughout the manufacturing and supply chain. AM/PM Reasons and requirements for GMP documents and records Importance of document controls Current requirements for electronic records and signatories Handling of product complaints, recalls and CAPA All Personnel Data Integrity Data Integrity has become widely discussed as a global concern for the pharmaceutical industry. This section provides an introduction to develop a broad understanding of the scope of Data Integrity and how key principles may be applied in order to provide strategic elements necessary to ensure reliability and integrity of information and data throughout all aspects of a product s lifecycle. AM/PM The definition of Data Integrity within a Quality Management System Regulatory framework for Data Integrity and Document Control Practices Behavioural GMP and development of a quality culture to enable Data Integrity compliance Module 3 Contamination Control (26-28 March 2018) 3 day Course Aim: To develop a broad understanding of the types and sources of contamination; and to analyze and assess the major risks to pharmaceuticals and the practical control methods which are used to minimize and correct contamination problems. AM Introduction to Contamination Control and why it is critical to product quality PM Microbiological Aspects of Manufacturing including routes of contamination. Identify the key controls within a manufacturing facility AM Cleaning and Sanitation PM HVAC and Controlled Environments control & qualification AM Environmental Monitoring Programs QA and Production PM Control of Water Systems
3 FUNDAMENTAL COURSE OUTLINE Module 4 - Good (Quality Control) Laboratory Practices (G(QC)LPs) (23 24 April 2018) 2 day Course Aim: To facilitate the development of knowledge, and expertise in the regulations, quality standards and guidelines that govern the quality control of pharmaceuticals. AM Introduction to Good (Quality Control) Laboratory Practices (GLPs) PM Qualification and Calibration of Laboratory Equipment PM Analytical Method Validation AM Biological assays Validation and Control PM Basic Statistics for Quality Control Laboratories PM Pharmaceutical Sampling Plan & Pharmaceutical Stability Programs QA, QC Back to back with Practical Stability Study Application to Pharmaceuticals to Pharmaceuticals (25 26 April 2018) Module 5 Compliance with GMP for the Pharmaceutical Engineer (23 25 July 2018) 3 day Course Aim: To provide an introduction to the requirements of Good Manufacturing Practices for supporting design of facilities, equipment and processes in the pharmaceutical and related industries, and to develop a broad understanding of the scope of Good Engineering Practices and Good Manufacturing Practices. AM Facility Layout and Design Principles PM Design and Construction of Critical Utilities: inc. Water, Gases and Steam AM Water Systems: Design, Control & Validation PM HVAC Design, Control & Validation AM Qualification of Processing Equipment PM Planned Preventative Maintenance and Calibration Engineering Module 6 Good Distribution Practices (GDP) for the Regulated Industry (27 29 August 2018) 3 day Course Aim: To provide an introduction to the requirements of Good Distribution Practices (GDPs) for the therapeutic and medical device industries, also provide a better understanding of the concepts of validation and management for the handling, storage and distribution of pharmaceutical products. AM Relationship and integration with GMP PM Understanding the manufacturer s requirements AM Risk Management and continuous improvement in distribution PM Understanding GDPs for therapeutic products and Devices PM Understanding GDPs for medical devices AM Cold Chain Management regulatory updates for the cold chain investigation, current and future, maintenance, handling and packaging of cold chain products PM Validation of the supply chain QA, Warehousing, Distribution PM Introduction to the principles of warehouse design for product preservation Module 7 Good Aseptic Practices & Sterile Products (24 26 September 2018) 3 day Course Aim: This subject is designed to facilitate the development of knowledge and practical skills in the assessment of special risks associated with the manufacture of sterile pharmaceuticals and to develop and evaluate strategies and plans that will ensure acceptable sterility assurance levels. AM Assess the regulatory requirements for aseptic manufacturing processes in order to provide recommendations for their application to ensure compliance PM Evaluate the processing and compliance risks associated with aseptic processing and terminal sterilization AM Critically evaluate strategies for bioburden control PM Evaluate the available processes for sterilisation and depyrogenation AM Prepare risk based environmental assessments PM Understand the updates to ISO and -2:2015 QA and Production Module 8 Solid Dosage Manufacture Principles and Practices (22 24 October 2018) 3 day Course Aim: To provide an introduction to the GMP requirements for the formulation, scale up and optimization of Finished Solid Dose Forms and to develop a practical understanding of Process Mapping, Risk Analysis and Critical Control points, Validation requirements and Quality Plans as it applies to solid dose formulations. AM Granulation Technology and Control PM Blending and Milling Technology and Control AM Encapsulation Technology and Control PM Compression Technology and Control AM Coating Technology and Control PM Packaging Technology and Control Changed CONTENT Production and Engineering
4 ADVANCED COURSE OUTLINE Those who have already undergone the Fundamental GMP series or those with a strong GMP background and minimum 5 years of relevant experience. This series is recommended to those with supervisory management positions and wish to consolidate and specialise in areas of advanced GMP knowledge commensurate with their roles and responsibilities within their organisation. In addition, recommended for those whose duties require advanced GMP knowledge of international Quality by Design application as part of their job function, particularly those involved with Research and Development, Validation, Risk Management and site Quality Assurance oversight. Advanced Process Validation and Cleaning Validation (9 11 April 2018) 3 day Course Aim: To develop advanced understanding of Sterile and Non Sterile Process and Cleaning Validation in order to comply with contemporary regulatory expectations. Putting into perspective the interpretation of regulatory and industry guidance. AM Understanding ASEAN PV Guidance and SUPAC AM PIC/S Annex 15 and FDA Guidance on Process Validation PM Ongoing re-validation AM Process Performance Qualification AM Process capability analysis for process validation PM Developing a process validation rationale AM The process equipment train and cleanability AM PDE a scientific approach to cleaning validation PM Introduction to CIP principles and validation QA, QC and Production Practical Stability Study Application to Pharmaceuticals (25 26 April 2018) 2 day Course Aim: This course is designed to provide the quality professional with the key requirements for establishing and implementing a successful stability trial program. It will review the relevant ICH guidance documents and will include workshops to provide practical application of the key requirements for stability. It will develop techniques for planning new and on-going stability trials. AM ASEAN stability guidelines PM Preparation of stability protocol PM Bracketing and matrixing designs for new drug substances and products (ICH Q1D) AM Evaluation of stability data (ICH Q1E) PM Registration applications in climatic zones III and IV (ICH Q1F) PM FDA guideline on container/closure integrity QA, QC and Regulatory Back with High Demand Back to back with Module 5 - Good (Quality Control) Laboratory Practices (G(QC)LPs) (23 24 April 2018) Pharmaceutical CAPA and Problem Solving (7 9 May 2018) 3 day Course Aim: To learn about how to use the CAPA system not only to satisfy regulatory requirements but also to implement a closed loop problem solving system to help minimise quality issues and improve compliance. To help identify regulatory requirements and expectations related to failure investigation, root cause analysis (RCA) and CAPA. A brief discussion on controls such as pharmacovigilance for drug products, FSCA and AE reporting for medical devices. AM Defining CAPA PM Overview and Systematic application of the CAPA system as it applies to quality audits AM Relationships between CAPA and risk assessment/management PM Risk assessment/management as it applies to audit observations AM Application of CAPA to audit observation deficiencie PM How to perform Root Cause Analysis for a compliant CAPA All Personnel
5 ADVANCED COURSE OUTLINE Those who have already undergone the Fundamental GMP series or those with a strong GMP background and minimum 5 years of relevant experience. This series is recommended to those with supervisory management positions and wish to consolidate and specialise in areas of advanced GMP knowledge commensurate with their roles and responsibilities within their organisation. In addition, recommended for those whose duties require advanced GMP knowledge of international Quality by Design application as part of their job function, particularly those involved with Research and Development, Validation, Risk Management and site Quality Assurance oversight. Computer Systems for Regulated Environments/Data Integrity (25 27 June 2018) 3 day Course Aim: Computer Systems are an established and integral part of management in modern Life Science organisations. All users must ensure that their systems and software have been developed to best engineering practices in a quality assured and secure manner to comply with Regulatory Requirements and to be fit for Business Use. Data Integrity has become widely discussed as a global concern for the pharmaceutical industry. This section provides an introduction to develop a broad understanding of the scope of Data Integrity and how key principles may be applied in order to provide strategic elements necessary to ensure reliability and integrity of information and data throughout all aspects of a product s lifecycle. AM Understand how computer systems are regulated in the local and global regulatory environment AM Understanding the controls necessary to demonstrate data integrity PM Computer Systems Selection and Vendor Qualification AM Developing a risk-based approach to CSV AM Best practices for validation test execution, documentation and error handling PM Understanding how the CSV process fits into the company s Software Life Cycle (SLC), understand the types of, and elements of System Development Life Cycles (SDLC) and the use of GAMP 5 QA, QC and Engineering AM Understanding of the key components and principles of a software quality assurance (SQA) program and auditor expectations. PM Hands-on practice creating key validation deliverables, including requirements, test plan, test scripts and test summary PM Data Integrity: Risk Management in Pharmaceutical Operations (ICHQ9) (8-10 October 2018) 3 day Course Aim: To provide an introduction to the principles of risk management and its application in the pharmaceutical and related industries. To enable students to identify opportunities and apply risk principles within their GxP related operational areas. AM Principles of Risk Management and ICH Q9 PM Risk Management Techniques - FMEA, FTA, HACCP AM Risk Management to Compliance and Quality Assurance Management PM Applying Risk Management in Compliance AM Risk Management Principles in Validation Programs PM Applying Risk Management to Operations QA Module 9 GxP and Quality Auditing Practices (26 27 November 2018) 2 day Course Aim: To provide an introduction to auditing principles and practices, and to develop a broad understanding of the requirements and techniques for planning, conducting and reporting quality audits applicable to manufacturing systems for drugs, biologics and devices. AM GxP audit schedule, managing regulatory audits in an effective manner, what to expect from GMP licensing audits PM The role of supplier audits for actives, excipients and components in Vendor management PM Four fundamental steps of auditing explained in detail, tips on how to manage & facilitate audits in a constructive manner AM Utilisation of risk management in relation to prioritizing audits PM Auditing for data integrity QA
6 METHODOLOGY: Lectures, workshops, case studies and group activities. ASSESSMENT: A variety of assessment strategies will be used and may include assignments, classroom engagement, projects and presentations. Participants will be informed of the assessment method, date of assessment and percentage contribution at the start of the module. Registration Fee per participant per module: (The fee includes course materials, lunch and refreshments) MOPI Member 3 day Course 30 days before commencement of course RM2, days before commencement of course RM3, days before commencement of course RM3,300,00 Non-MOPI Member 3 day Course 30 days before commencement of course RM3, days before commencement of course RM3, days before commencement of course RM3, Foreign Participant 3 day Course 30 days before commencement of course USD $1, days before commencement of course USD $1, days before commencement of course USD $1, Registration fee is subjected to 6% GST Registration Fee per participant per module: (The fee includes course materials, lunch and refreshments) MOPI Member 2 day Course 30 days before commencement of course RM2, days before commencement of course RM2, days before commencement of course RM2,600,00 Non-MOPI Member 2 day Course 30 days before commencement of course RM2, days before commencement of course RM2, days before commencement of course RM2, Foreign Participant 2 day Course 30 days before commencement of course USD $ days before commencement of course USD $1, days before commencement of course USD $1, Training Venue: THE BOULEVARD St Giles Premier Hotel Hotel Address Mid Valley City Lingkaran Syed Putra Kuala Lumpur Malaysia Tel: Website: TIME SCHEDULE: 9.00 am 5.00 pm 8.30 am Registration 9.00 am AM Topic am Tea Break am AM Topic pm Lunch 1.25 pm PM Topic 3.00 pm Tea Break 3.15 pm PM Topic 5.00 pm End Optional Hotel accommodations: Cititel Mid Valley Tel: Website: Eastin Hotel, Petaling Jaya Tel: Website: Crystal Crown Hotel, Petaling Jaya Tel : Website: Armada PJ Hotel Tel: Website: BOOK YOUR SEAT NOW!!! For further enquiries, please contact: Mike/Janet, MOPI GLOBAL BUSINESS & CONVENTION CENTRE, MEZZANINE FLOOR, BLOCK A, NO. 8, JALAN 19/1, SECTION 19, PETALING JAYA, SELANGOER, WEST MALAYSIA Tel: Fax: mike@mopi.org.my and admin@mopi.org.my
7 ADMINISTRATION DETAILS: Important Notice: Payment is required with registration and must be received 2 weeks prior to the start of the relevant module to guarantee your place. Walkin participants will only be admitted on the basis of space availability at the course and with immediate full payment by banker s cheque in favour of the Malaysian Organisation of Pharmaceutical Industries. Registration will be treated as confirmed only upon receipt of payment in full. CANCELLATIONS & TRANSFERS: If a registrant is unable to attend, a substitute candidate is welcome at no extra charge. Please provide the name and the title of the substitute participant at least 2 working days prior to the relevant course. Notice of cancellation by fax/ is required 14 working days prior to commencement of each module and refund less RM500 as administration charge will be made. However a complete set of documentation will be sent to you. Regrettably, no refund can be made for cancellations received less than 10 working days prior to the commencement of each module. However a complete set of documentation will be sent to you. MOPI / SeerPharma reserves the right to cancel or reschedule the training modules. All efforts will be taken to inform participants of any change. MOPI /SeerPharma however will not be held liable for reimbursement of any claims or expenses should cancellation or rescheduling occur. REGISTRATION FORM Subject to Administration details MOPI Member Non-Member Foreign Please register the following participant(s) for the above program. (To be completed in BLOCK LETTERS) 1 Name Designation address Contact Number Vegetarian 2 Name Designation address Contact Number Vegetarian Enclosed cheque/bank draft No for RM being payment for participant(s) made in favour of the Malaysian Organisation of Pharmaceutical Industries. Select a course accordingly: Fundamental GMP Module Module 1 International Good Manufacturing Practices, Quality Management Systems and GMP for Pharmaceutical Operations January 2018 (Tues - Thu) 3 day Course Module 2 Validation Principles and Practices 5 7 February 2018 (Mon Wed) 3 day Course Behavourial GMP/ Good Documentation Practices/ Data Integrity March 2018 (Mon Wed) 3 day Course Advanced GMP Module Advanced Process Validation and Cleaning Validation 9 11 April 2018 (Mon Wed) 3 day Course Practical Stability Study Application to Pharmaceuticals April 2018 (Wed Thu) 2 day Course Pharmaceutical CAPA and Problem Solving 7 9 May 2018 (Mon Wed) 3 day Course Module 3 Contamination Control March 2018 (Mon Wed) 3 day Course Computer Systems for Regulated Environments/Data Integrity June (Mon Wed) 3 day Course Module 4 Good Quality Control Laboratory Practices (G(QC)LPs) April 2018 (Mon Tue) 2 day Course Risk Management in Pharmaceutical Operations (ICH Q9) 8 10 October (Mon Wed) 3 day Course Module 5 Compliance with GMP for the Pharmaceutical Engineer July 2018 (Mon Wed) 3 day Course Module 9 GxP and Quality Auditing Practices November 2018 (Mon Tue) 2 day Course Module 6 Good Distribution Practices (GDP) for the Regulated Industry August 2018 (Mon Wed) 3 day Course Module 7 Good Aseptic Practices & Sterile Products September 2018 (Mon Wed) 3 day Course Module 8 Solid Dosage Manufacture Principles and Practices October 2018 (Mon Wed) 3 day Course * * Dates and Instructors are subject to change depending on attendance feedbacks and instructor availability. In case of a change, updated dates and instructor profile will be advised to the organizer and the attendees prior to the start of each course Registration Submitted by: Name Designation Company Stamp (with Address, Telephone & Fax Number) Registration Fee per participant per course: (The fee includes course materials, lunch and refreshments) MOPI Member 3 day Course 30 days before commencement of course RM2, days before commencement of course RM3, days before commencement of course RM3,300,00 Non-MOPI Member 3 day Course 30 days before commencement of course RM3, days before commencement of course RM3, days before commencement of course RM3, Foreign Participant 3 day Course 30 days before commencement of course USD $1, days before commencement of course USD $1, days before commencement of course USD $1, Office Use Only Registration fee is subjected to 6% GST Registration Accepted on. Payment Accepted on..
Jointly Organised by: Presenter: Certificates endorsed by the National. Training Grant is available under HRDF SBL Scheme
 The course training program consists of 9 principal modules and 2 additional modules, each of 3 days duration. These modules cover the essential principles of Good Manufacturing Practice (GMP). Participants
The course training program consists of 9 principal modules and 2 additional modules, each of 3 days duration. These modules cover the essential principles of Good Manufacturing Practice (GMP). Participants
POST MASTER S CERTIFICATE IN VALIDATION SCIENCE
 POST MASTER S CERTIFICATE IN VALIDATION SCIENCE BACKGROUND Temple University s School of Pharmacy continues to be the leader in providing outstanding graduate-level courses in Regulatory Affairs and Quality
POST MASTER S CERTIFICATE IN VALIDATION SCIENCE BACKGROUND Temple University s School of Pharmacy continues to be the leader in providing outstanding graduate-level courses in Regulatory Affairs and Quality
Industry Guidelines for Computerized Systems Validation (GAMP, PDA Technical Reports)
 Training Course Computerized System Validation in the Pharmaceutical Industry Istanbul, 16-17 January 2003 Industry Guidelines for Computerized Systems Validation (GAMP, PDA Technical Reports) Wolfgang
Training Course Computerized System Validation in the Pharmaceutical Industry Istanbul, 16-17 January 2003 Industry Guidelines for Computerized Systems Validation (GAMP, PDA Technical Reports) Wolfgang
Touchstone Technologies, Inc. Course Catalog February 2017
 Touchstone Technologies, Inc. Course Catalog February 2017 Angela Bazigos ANGELA BAZIGOS 1 HR Courses Course Duration Audience Speaker 1. Basics of Project Management Webinar 90 mins Management Bazigos
Touchstone Technologies, Inc. Course Catalog February 2017 Angela Bazigos ANGELA BAZIGOS 1 HR Courses Course Duration Audience Speaker 1. Basics of Project Management Webinar 90 mins Management Bazigos
GMP/GDP auditor training course
 GMP/GDP auditor training course Frankfurt, November 20 th and 21 st 2018 INTRODUCTION Asociación Forum Auditorías (AFA) is providing 3 rd party audit services since 2005 to pharmaceutical companies. Since
GMP/GDP auditor training course Frankfurt, November 20 th and 21 st 2018 INTRODUCTION Asociación Forum Auditorías (AFA) is providing 3 rd party audit services since 2005 to pharmaceutical companies. Since
In-Vitro Diagnostic Directive (IVDD) and IVDD Technical File Preparation Workshop. 3-4 July 2014 TÜV SÜD
 In-Vitro Diagnostic Directive (IVDD) and IVDD Technical File Preparation Workshop 3-4 July 2014 TÜV SÜD Introduction The In-Vitro Diagnostic Directive (IVDD) 98/79/EC applies to all In-Vitro Diagnostic
In-Vitro Diagnostic Directive (IVDD) and IVDD Technical File Preparation Workshop 3-4 July 2014 TÜV SÜD Introduction The In-Vitro Diagnostic Directive (IVDD) 98/79/EC applies to all In-Vitro Diagnostic
Configuration, Installation, Commissioning, Troubleshooting, Operation & Maintenance
 ISO 9001:2008 Certified ISO 29990:2010 Certified (Certificate No:1007049195) ISO 29990 (Certificate No: 1078694951) Foundation Fieldbus Troubleshooting, Operation & Maintenance 29 May - 02 June 2016 Muscat
ISO 9001:2008 Certified ISO 29990:2010 Certified (Certificate No:1007049195) ISO 29990 (Certificate No: 1078694951) Foundation Fieldbus Troubleshooting, Operation & Maintenance 29 May - 02 June 2016 Muscat
TÜV Rheinland Functional Safety Program
 IICA Functional Safety Engineer Training Safety Instrumented Systems TÜV Rheinland Functional Safety Program IICA Functional Safety Engineer SIS Training TÜV Rheinland Functional Safety Program The IICA
IICA Functional Safety Engineer Training Safety Instrumented Systems TÜV Rheinland Functional Safety Program IICA Functional Safety Engineer SIS Training TÜV Rheinland Functional Safety Program The IICA
Tunku Abdul Rahman University College is conducting CIMA Certificate in Business Accounting (Cert BA).
 PUBLIC COURSE Tunku Abdul Rahman University College is conducting CIMA Certificate in Business Accounting (Cert BA). The CIMA Cert BA elevates people and businesses to success by developing skills and
PUBLIC COURSE Tunku Abdul Rahman University College is conducting CIMA Certificate in Business Accounting (Cert BA). The CIMA Cert BA elevates people and businesses to success by developing skills and
Advances in Well Testing and Wireline Formation Testing
 Advances in Well Testing and Wireline Formation Testing 8 9 December 2014 Kuala Lumpur Convention Center, Kuala Lumpur, Malaysia Why should you attend? To review and appreciate recent advances in the subject
Advances in Well Testing and Wireline Formation Testing 8 9 December 2014 Kuala Lumpur Convention Center, Kuala Lumpur, Malaysia Why should you attend? To review and appreciate recent advances in the subject
Global Impact for a Safe and Sustainable Future
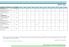 TRAINING CALENDAR FOR YEAR 2018 ISO 9001:2015 Quality Management System Foundation ISO 9001:2015 Quality Management System Internal Auditor ISO 9001:2015 QMS Auditor/Lead Auditor (IRCA Course Certification
TRAINING CALENDAR FOR YEAR 2018 ISO 9001:2015 Quality Management System Foundation ISO 9001:2015 Quality Management System Internal Auditor ISO 9001:2015 QMS Auditor/Lead Auditor (IRCA Course Certification
AL045: Laboratory Information Management System (LIMS)
 AL045: Laboratory Information Management System (LIMS) AL045 Rev.001 CMCT COURSE OUTLINE Page 1 of 5 Training Description: A Laboratory Information Management System (LIMS) is computer software that is
AL045: Laboratory Information Management System (LIMS) AL045 Rev.001 CMCT COURSE OUTLINE Page 1 of 5 Training Description: A Laboratory Information Management System (LIMS) is computer software that is
Criteria for SQF Training Courses
 Criteria for SQF Training Courses Licensed Course Criteria and Guidelines 9th Edition M A R C H 2 0 1 8 2018 Food Marketing Institute SQF Institute 2345 Crystal Drive, Suite 800 Arlington, VA 22202 USA
Criteria for SQF Training Courses Licensed Course Criteria and Guidelines 9th Edition M A R C H 2 0 1 8 2018 Food Marketing Institute SQF Institute 2345 Crystal Drive, Suite 800 Arlington, VA 22202 USA
IT TRAINING COURSE - JANUARY TO DECEMBER 2019
 IT TRAINING COURSE JANUARY TO DECEMBER In Collaboration with All courses are HRDF claimable is Microsoft WORD Microsoft Word (Fundamental & Discover The Techniques to A Professional Word Report 07 07 2
IT TRAINING COURSE JANUARY TO DECEMBER In Collaboration with All courses are HRDF claimable is Microsoft WORD Microsoft Word (Fundamental & Discover The Techniques to A Professional Word Report 07 07 2
With the successful completion of this course the participant will be able to:
 ISO 13485:2016 INTRODUCTION COURSE COURSE DURATION: 1 DAY Course Summary: The introduction course provides the participant with an oversight on the requirements of ISO 13485:2016 standard. Our course is
ISO 13485:2016 INTRODUCTION COURSE COURSE DURATION: 1 DAY Course Summary: The introduction course provides the participant with an oversight on the requirements of ISO 13485:2016 standard. Our course is
FMM Institute. Centre for Professional Development
 FMM Institute Centre for Professional Development In-House Training Available Course Description Participants will learn how to create and edit bullet slides, use PowerPoint s drawing tools, incorporate
FMM Institute Centre for Professional Development In-House Training Available Course Description Participants will learn how to create and edit bullet slides, use PowerPoint s drawing tools, incorporate
IT Audit Essentials. Date: 10 th 12 th March 2015 Time: 9 am to 5.30 pm Venue: Iverson Associates, Center Point Bandar Utama, Kuala Lumpur
 IT Audit Essentials Date: 10 th 12 th March 2015 Time: 9 am to 5.30 pm Venue: Iverson Associates, Center Point Bandar Utama, Kuala Lumpur IT Audit Essentials Workshop Overview ISACA Malaysia Chapter is
IT Audit Essentials Date: 10 th 12 th March 2015 Time: 9 am to 5.30 pm Venue: Iverson Associates, Center Point Bandar Utama, Kuala Lumpur IT Audit Essentials Workshop Overview ISACA Malaysia Chapter is
Advanced Systems, Inc. Registration Form. (Please complete all information)
 Registration Form (Please complete all information) To register by email, send a note to Advanced Systems, Inc., (mmoore@advancedsys.com) and indicate that you would like to register for the Field Sampling
Registration Form (Please complete all information) To register by email, send a note to Advanced Systems, Inc., (mmoore@advancedsys.com) and indicate that you would like to register for the Field Sampling
Advanced Systems, Inc. Course. Registration Form. (Please complete all information)
 MARCH 9 TO 11, 2015 Registration Form (Please complete all information) To register on-line for the Environmental Laboratory Assessments Basic Assessor Training. Course offered in Olathe, Kansas on March
MARCH 9 TO 11, 2015 Registration Form (Please complete all information) To register on-line for the Environmental Laboratory Assessments Basic Assessor Training. Course offered in Olathe, Kansas on March
What Disinfectants Do You Need - FDA or EPA Registered
 Microrite, Inc. brings you this unique learning experience in Understanding What Disinfectants Do You Need-FDA or EPA Registered; Part of Microrite s step-by-step webinar series. What Disinfectants Do
Microrite, Inc. brings you this unique learning experience in Understanding What Disinfectants Do You Need-FDA or EPA Registered; Part of Microrite s step-by-step webinar series. What Disinfectants Do
Risk Based EBRS Implementation using GAMP 5
 Risk Based EBRS Implementation using GAMP 5 Gilberto Rossi 1 ETIF Argentina Risk-based EBRS Implementation using GAMP 5 Risk management - why do we need it? Taking risk managing risk and mismanaging risk
Risk Based EBRS Implementation using GAMP 5 Gilberto Rossi 1 ETIF Argentina Risk-based EBRS Implementation using GAMP 5 Risk management - why do we need it? Taking risk managing risk and mismanaging risk
Workshop on Design for Compliance to IEC (3rd Edition) and EMC Standards & Requirements. 14 th - 15 th May 2015 TÜV SÜD
 Workshop on Design for Compliance to IEC 61010-1 (3rd Edition) and EMC Standards & Requirements 14 th - 15 th May 2015 TÜV SÜD Introduction The introduction of risk assessment to IEC 61010-1 (3rd Edition)
Workshop on Design for Compliance to IEC 61010-1 (3rd Edition) and EMC Standards & Requirements 14 th - 15 th May 2015 TÜV SÜD Introduction The introduction of risk assessment to IEC 61010-1 (3rd Edition)
Cleanroom Performance
 Cleanroom Performance Testing (CPT) Seminar March 4-6, 2019 Vancouver, WA Location: This Seminar is Designed for: This seminar is intended for individuals with a minimum of two years of cleanroom testing
Cleanroom Performance Testing (CPT) Seminar March 4-6, 2019 Vancouver, WA Location: This Seminar is Designed for: This seminar is intended for individuals with a minimum of two years of cleanroom testing
By Cornelia Wawretchek. The Drug Manufacturer s Guide to Site Master Files
 By Cornelia Wawretchek The Drug Manufacturer s Guide to Site Master Files ISBN: 978-3-943267-69-3 A Process Approach to Pharmaceutical Quality Systems A Guide to ICH Q10 Compliance Where a product trademark,
By Cornelia Wawretchek The Drug Manufacturer s Guide to Site Master Files ISBN: 978-3-943267-69-3 A Process Approach to Pharmaceutical Quality Systems A Guide to ICH Q10 Compliance Where a product trademark,
Facilitator. TIME SERIES ECONOMETRICS FOR THE PRACTITIONER (E-VIEWS) Time Series Econometrics- I
 TIME SERIES ECONOMETRICS FOR THE PRACTITIONER (E-VIEWS) Econometrics- I 11 12 April 2014 Econometrics II 18 19 April 2014 AND PANEL DATA ECONOMETRICS (STATA) Econometrics I 2 3 May 2014 Econometrics II
TIME SERIES ECONOMETRICS FOR THE PRACTITIONER (E-VIEWS) Econometrics- I 11 12 April 2014 Econometrics II 18 19 April 2014 AND PANEL DATA ECONOMETRICS (STATA) Econometrics I 2 3 May 2014 Econometrics II
Medical Device Seminar Bringing your medical products to United States of America (USA)
 Medical Device Seminar Bringing your medical products to United States of America (USA) 4 th June 2015 TÜV SÜD Introduction Healthcare improvements rely on an innovative and diverse medical device sector
Medical Device Seminar Bringing your medical products to United States of America (USA) 4 th June 2015 TÜV SÜD Introduction Healthcare improvements rely on an innovative and diverse medical device sector
FMM Institute. Centre for Professional Development
 FMM Institute Centre for Professional Development In-House Training Available Course Description PivotTables are powerful data analysis tools, yet most Excel users do not use them to their fullest potential.
FMM Institute Centre for Professional Development In-House Training Available Course Description PivotTables are powerful data analysis tools, yet most Excel users do not use them to their fullest potential.
ISPE Thailand 3 rd Seminar 2018
 ISPE Thailand 3 rd Seminar 2018 Contamination Risk Assessment in Pharmaceutical Cleanrooms & How to use Airflow Visualization (Smoke Studies) to troubleshoot Cleanroom Contamination issues 29 th 30 th
ISPE Thailand 3 rd Seminar 2018 Contamination Risk Assessment in Pharmaceutical Cleanrooms & How to use Airflow Visualization (Smoke Studies) to troubleshoot Cleanroom Contamination issues 29 th 30 th
Global Impact for a Safe and Sustainable Future
 ISO 9001:2015 Quality Management System Auditor Transition (IRCA) 2 800 13-14 - 7-8 - 6-7 - ISO 9001:2015 Quality Management System Foundation 2 700-11-12 5-6 - 5-6 ISO 9001:2015 Quality Management System
ISO 9001:2015 Quality Management System Auditor Transition (IRCA) 2 800 13-14 - 7-8 - 6-7 - ISO 9001:2015 Quality Management System Foundation 2 700-11-12 5-6 - 5-6 ISO 9001:2015 Quality Management System
MY PUBLIC ACCOUNTANT PRACTICE PROGRAM Preparation for practice success
 MY PUBLIC ACCOUNTANT PRACTICE PROGRAM 2017 Preparation for practice success tools for success The My Public Accountant (MPA) Practice Program is a fully interactive workshop which provides you with the
MY PUBLIC ACCOUNTANT PRACTICE PROGRAM 2017 Preparation for practice success tools for success The My Public Accountant (MPA) Practice Program is a fully interactive workshop which provides you with the
PMP Certification Preparatory Course
 PMP Certification Preparatory Course Why Project Management Professional (PMP ) Certification? In today's flexible organization, the boundaries between functions are becoming less defined. You are most
PMP Certification Preparatory Course Why Project Management Professional (PMP ) Certification? In today's flexible organization, the boundaries between functions are becoming less defined. You are most
Program Management Professionals (PgMP)
 An ISO 9001:2015 & ISO 29990:2010 Certified Company PgMP and PMI are registered marks of the Project of the Project Management Institute Inc. Program Management Examination Preparatory Course 02-06 December
An ISO 9001:2015 & ISO 29990:2010 Certified Company PgMP and PMI are registered marks of the Project of the Project Management Institute Inc. Program Management Examination Preparatory Course 02-06 December
Instructions for Exam Entry May 2012
 Instructions for Exam Entry May 2012 It is important that you read these instructions carefully before you complete your exam entry online. You can enter online for the May 2012 exams from 1 February 2012.
Instructions for Exam Entry May 2012 It is important that you read these instructions carefully before you complete your exam entry online. You can enter online for the May 2012 exams from 1 February 2012.
IMPLEMENTATION COURSE (MODULE 1) (ISO 9001:2008 AVAILABLE ON REQUEST)
 ISO 9001:2015 IMPLEMENTATION COURSE (MODULE 1) (ISO 9001:2008 AVAILABLE ON REQUEST) COURSE DURATION: 3 DAYS Course Summary: The implementation course provides the participant with an in-depth level of
ISO 9001:2015 IMPLEMENTATION COURSE (MODULE 1) (ISO 9001:2008 AVAILABLE ON REQUEST) COURSE DURATION: 3 DAYS Course Summary: The implementation course provides the participant with an in-depth level of
FMM Institute. Centre for Professional Development
 FMM Institute Centre for Professional Development In-House Training Available Course Description This course introduce participants to the more advanced features in Microsoft Access 2003. Tasks include
FMM Institute Centre for Professional Development In-House Training Available Course Description This course introduce participants to the more advanced features in Microsoft Access 2003. Tasks include
Cleanroom Performance
 Cleanroom Performance Testing (CPT) Seminar October 7-9, 2019 Gaithersburg, MD Location: This Seminar is Designed for: This seminar is intended for individuals with a minimum of two years of cleanroom
Cleanroom Performance Testing (CPT) Seminar October 7-9, 2019 Gaithersburg, MD Location: This Seminar is Designed for: This seminar is intended for individuals with a minimum of two years of cleanroom
DATA INTEGRITY (EMA AUGUST 2016)
 Data integrity Data integrity enables good decision-making by pharmaceutical manufacturers and regulatory authorities.it is a fundamental requirement of the pharmaceutical quality system described in EU
Data integrity Data integrity enables good decision-making by pharmaceutical manufacturers and regulatory authorities.it is a fundamental requirement of the pharmaceutical quality system described in EU
Cleanroom Microbiology for Non-Microbiologists
 Microrite, Inc. brings you this unique learning experience in understanding cleanroom microbiology concepts and techniques Cleanroom Microbiology for Non-Microbiologists A course designed specifically
Microrite, Inc. brings you this unique learning experience in understanding cleanroom microbiology concepts and techniques Cleanroom Microbiology for Non-Microbiologists A course designed specifically
HVAC DESIGN COURSE LEVEL 1 : ESSENTIAL TOOLS FOR HIGH PERFORMANCE BUILDING DESIGNERS
 HVAC DESIGN COURSE LEVEL 1 : ESSENTIAL TOOLS FOR HIGH PERFORMANCE BUILDING DESIGNERS DATE TIME VENUE : 4th to 6th August 2014 (Monday to Wednesday) : 9.00 am to 6.00pm (Registration at 8.15am) : Atlanta
HVAC DESIGN COURSE LEVEL 1 : ESSENTIAL TOOLS FOR HIGH PERFORMANCE BUILDING DESIGNERS DATE TIME VENUE : 4th to 6th August 2014 (Monday to Wednesday) : 9.00 am to 6.00pm (Registration at 8.15am) : Atlanta
Excel VBA for Absolute Beginners
 Applied Technology Group Sdn Bhd (1012178-W) W-5-3, Subang Square Business Centre, Jalan SS15/4G, 47500 Subang Jaya, Selangor, Malaysia. Tel: (+603) 5634 7905 Fax: (+603) 5637 9945 Email: admin@apptechgroups.net
Applied Technology Group Sdn Bhd (1012178-W) W-5-3, Subang Square Business Centre, Jalan SS15/4G, 47500 Subang Jaya, Selangor, Malaysia. Tel: (+603) 5634 7905 Fax: (+603) 5637 9945 Email: admin@apptechgroups.net
FMM Institute. Centre for Professional Development
 FMM Institute Centre for Professional Development In-House Training Available Course Description This course introduce participants to the more advanced features in Microsoft Access 2003. Tasks include
FMM Institute Centre for Professional Development In-House Training Available Course Description This course introduce participants to the more advanced features in Microsoft Access 2003. Tasks include
CERTIFICATE IN LUXEMBOURG COMPANY SECRETARIAL & GOVERNANCE PRACTICE
 CERTIFICATE IN LUXEMBOURG COMPANY SECRETARIAL & GOVERNANCE PRACTICE POLICY ILA asbl 19, rue de Bitbourg L-1273 Luxembourg TABLE OF CONTENTS Program Entry 3 Eligibility criteria 3 Training program 4 Application
CERTIFICATE IN LUXEMBOURG COMPANY SECRETARIAL & GOVERNANCE PRACTICE POLICY ILA asbl 19, rue de Bitbourg L-1273 Luxembourg TABLE OF CONTENTS Program Entry 3 Eligibility criteria 3 Training program 4 Application
Regulatory Compliance (Insurance)
 Regulatory Compliance (Insurance) Regulatory Compliance (Insurance) There is no denying that experience matched with the right training & education will help you achieve your goals and advance your career.
Regulatory Compliance (Insurance) Regulatory Compliance (Insurance) There is no denying that experience matched with the right training & education will help you achieve your goals and advance your career.
MEMBERS CIRCULAR 12 February 2015 Ref 003/02/15
 1 MEMBERS CIRCULAR 12 February 2015 Ref 003/02/15 Dear Members CERTIFICATION COURSE IN SHOPPING MALL MANAGEMENT: Marketing and Leasing Administration Operations and Maintenance Greetings! PPK Malaysia
1 MEMBERS CIRCULAR 12 February 2015 Ref 003/02/15 Dear Members CERTIFICATION COURSE IN SHOPPING MALL MANAGEMENT: Marketing and Leasing Administration Operations and Maintenance Greetings! PPK Malaysia
Example of QbD Application in Japan Yoshihiro Matsuda, Ph.D.
 Example of QbD Application in Japan Yoshihiro Matsuda, Ph.D. Senior Scientist (for Quality) Pharmaceuticals and Medical Devices Agency (PMDA) Aug 11, 2016 1 Agenda Introduction of PMDA QbD assessment experience
Example of QbD Application in Japan Yoshihiro Matsuda, Ph.D. Senior Scientist (for Quality) Pharmaceuticals and Medical Devices Agency (PMDA) Aug 11, 2016 1 Agenda Introduction of PMDA QbD assessment experience
Pre-registration Exam workshop 2017
 Pre-registration Exam workshop 2017 Programme TIME ITEM PRESENTER 09:00 09:30 Welcome and SAPC presentation SAPC (30 min) 09:30 10:30 Examination Paper 1 Facilitator (60 min) 10:30-10:45 SHORT BREAK 15
Pre-registration Exam workshop 2017 Programme TIME ITEM PRESENTER 09:00 09:30 Welcome and SAPC presentation SAPC (30 min) 09:30 10:30 Examination Paper 1 Facilitator (60 min) 10:30-10:45 SHORT BREAK 15
Vaccine data collection tool Oct Functions, Indicators & Sub-Indicators
 data collection tool Oct. 2011 A. National Regulatory System RS01: Legal framework for establishment of a regulatory system, mandate and enforcement power for each function RS01.01: Legislation or and
data collection tool Oct. 2011 A. National Regulatory System RS01: Legal framework for establishment of a regulatory system, mandate and enforcement power for each function RS01.01: Legislation or and
1. STRATEGIC PLANNING
 RAC (EU) EXAMINATION SUBJECTS & FORMAT The European RAC Examination is a knowledge-based examination addressing European Union laws, regulations, policies and guidelines affecting medical RAC devices,
RAC (EU) EXAMINATION SUBJECTS & FORMAT The European RAC Examination is a knowledge-based examination addressing European Union laws, regulations, policies and guidelines affecting medical RAC devices,
Financial Planning Institute of Southern Africa SETTING THE STANDARD. Continuous Professional Development (Cpd) Policy
 FPI FPI Financial Planning Institute of Southern Africa SETTING THE STANDARD Continuous Professional Development (Cpd) Policy Table of Contents Definitions 3-4 Introduction 4 Primary Responsibility 5 Mandatory
FPI FPI Financial Planning Institute of Southern Africa SETTING THE STANDARD Continuous Professional Development (Cpd) Policy Table of Contents Definitions 3-4 Introduction 4 Primary Responsibility 5 Mandatory
Program Management Professionals (PgMP)
 An ISO 9001:2015 & ISO 29990:2010 Certified Company Program Management 10-14 Dec 2017 Dubai, United Arab Emirates 27-JUN-17 Program Management Why Choose this Training Course? Program management is the
An ISO 9001:2015 & ISO 29990:2010 Certified Company Program Management 10-14 Dec 2017 Dubai, United Arab Emirates 27-JUN-17 Program Management Why Choose this Training Course? Program management is the
CBCI Certification Course (GPG)
 CBCI Certification Course (GPG) 5 Days with Examination Course Description This course offers a solid description of the methods, techniques and approaches used by business continuity (BC) professionals
CBCI Certification Course (GPG) 5 Days with Examination Course Description This course offers a solid description of the methods, techniques and approaches used by business continuity (BC) professionals
September 28 th -30 th, 2016 Kuala Lumpur, Malaysia
 Certification in ISO 31000 (3 day masterclasses) Become a certified ISO 31000 risk management professional September 28 th -30 th, 2016 Kuala Lumpur, Malaysia Contact: Ng Tien Seong, Email: ngseannts@gmail.com
Certification in ISO 31000 (3 day masterclasses) Become a certified ISO 31000 risk management professional September 28 th -30 th, 2016 Kuala Lumpur, Malaysia Contact: Ng Tien Seong, Email: ngseannts@gmail.com
October 21-23, 2013 Holly Springs, NC
 NEBB s Cleanroom Performance Testing Certified Professional Seminar and Exam October 21-23, 2013 Holly Springs, NC Introduction: NEBB, in continuance of its policy to enhance education and training within
NEBB s Cleanroom Performance Testing Certified Professional Seminar and Exam October 21-23, 2013 Holly Springs, NC Introduction: NEBB, in continuance of its policy to enhance education and training within
PECB Certified ISO Lead Implementer
 PECB Certified ISO 22301 Lead Implementer PECB Certified ISO 22301 Lead Implementer 5 Days with Examination Course Description This five day intensive course enables the participants to develop the necessary
PECB Certified ISO 22301 Lead Implementer PECB Certified ISO 22301 Lead Implementer 5 Days with Examination Course Description This five day intensive course enables the participants to develop the necessary
Process Safety Management Training
 IHS OPERATIONAL EXCELLENCE & RISK MANAGEMENT Process Safety Management Training Leverage state-of-the-art resources and practical knowhow to learn about proven management system concepts and techniques.
IHS OPERATIONAL EXCELLENCE & RISK MANAGEMENT Process Safety Management Training Leverage state-of-the-art resources and practical knowhow to learn about proven management system concepts and techniques.
Chartered Member Assessment
 Chartered Member Assessment CANDIDATE HANDBOOK 2015 CANDIDATE HANDBOOK 2015 2 Chartered Member Assessment Candidate Handbook 2015 The Chartered Member Assessment is a key criterion for entry to the category
Chartered Member Assessment CANDIDATE HANDBOOK 2015 CANDIDATE HANDBOOK 2015 2 Chartered Member Assessment Candidate Handbook 2015 The Chartered Member Assessment is a key criterion for entry to the category
Sample Site Selection Locations for RABS and Isolators
 Microrite, Inc. brings you this unique learning experience in Sample Site Selection Locations for RABS and Isolator; Part of Microrite s step-by-step webinar series. Sample Site Selection Locations for
Microrite, Inc. brings you this unique learning experience in Sample Site Selection Locations for RABS and Isolator; Part of Microrite s step-by-step webinar series. Sample Site Selection Locations for
Quality Management Systems (ISO 9001:2015 and ISO 29001) Lead Auditor training (EY/IMSA Q03)
 Quality Management Systems (ISO 9001:2015 and ISO 29001) Lead Auditor training (EY/IMSA Q03) Doha, 4 8 March 2018 IMSA is an IRCA/CQI Approved Training Provider Contents Section 1: About the program 04
Quality Management Systems (ISO 9001:2015 and ISO 29001) Lead Auditor training (EY/IMSA Q03) Doha, 4 8 March 2018 IMSA is an IRCA/CQI Approved Training Provider Contents Section 1: About the program 04
MHRA GMP Data Integrity Definitions and Guidance for Industry March Introduction:
 MHRA GMP Data Integrity Definitions and Guidance for Industry Introduction: Data integrity is fundamental in a pharmaceutical quality system which ensures that medicines are of the required quality. This
MHRA GMP Data Integrity Definitions and Guidance for Industry Introduction: Data integrity is fundamental in a pharmaceutical quality system which ensures that medicines are of the required quality. This
FMM Institute. Centre for Professional Development
 FMM Institute Centre for Professional Development In-House Training Available Course Description This course will cover in depth on VBA such as create userform application, design own toolbar, shortcut
FMM Institute Centre for Professional Development In-House Training Available Course Description This course will cover in depth on VBA such as create userform application, design own toolbar, shortcut
Client Services Procedure Manual
 Procedure: 85.00 Subject: Administration and Promotion of the Health and Safety Learning Series The Health and Safety Learning Series is a program designed and delivered by staff at WorkplaceNL to increase
Procedure: 85.00 Subject: Administration and Promotion of the Health and Safety Learning Series The Health and Safety Learning Series is a program designed and delivered by staff at WorkplaceNL to increase
Master the implementation and management of a Cybersecurity Program based on ISO/IEC 27032
 ISO/IEC 27032 Lead Manager 23rd - 27th October 2017 Hilton Hotel, Sandton, Johannesburg Master the implementation and management of a Program based on ISO/IEC 27032 Why should you attend? Manager training
ISO/IEC 27032 Lead Manager 23rd - 27th October 2017 Hilton Hotel, Sandton, Johannesburg Master the implementation and management of a Program based on ISO/IEC 27032 Why should you attend? Manager training
ISO LEAD AUDITOR TRAINING
 FINAL CERTIFICATION AWARDED BY PECB CANADA ISO 22301 LEAD AUDITOR TRAINING & CERTIFICATION (Business Continuity Management) Master the Audit of Business Continuity Management System (BCMS) based on ISO
FINAL CERTIFICATION AWARDED BY PECB CANADA ISO 22301 LEAD AUDITOR TRAINING & CERTIFICATION (Business Continuity Management) Master the Audit of Business Continuity Management System (BCMS) based on ISO
The Project Management Professional (PMP) Examination Preparatory Course
 An ISO 9001:2015 & ISO 29990:2010 Certified Company This course is registered with the Project Management Institute (PMI) R.E.P. Program* The Project Management Professional (PMP) 17-21 Sep 2017 Dubai,
An ISO 9001:2015 & ISO 29990:2010 Certified Company This course is registered with the Project Management Institute (PMI) R.E.P. Program* The Project Management Professional (PMP) 17-21 Sep 2017 Dubai,
IECEx Guide Guidance for Applications from Service Facilities seeking IECEx Certification
 IECEx Guide Guidance for Applications from Service Facilities seeking IECEx Certification INTERNATIONAL ELECTROTECHNICAL COMMISSION SCHEME FOR CERTIFICATION TO STANDARDS RELATING TO EQUIPMENT FOR USE IN
IECEx Guide Guidance for Applications from Service Facilities seeking IECEx Certification INTERNATIONAL ELECTROTECHNICAL COMMISSION SCHEME FOR CERTIFICATION TO STANDARDS RELATING TO EQUIPMENT FOR USE IN
Essentials of Freight Forwarding Program
 Program DESCRIPTION: Understanding how buyers and sellers manage risk with a variety of international payments, cargo insurance, properly completed commercial documents and export packaging leads students
Program DESCRIPTION: Understanding how buyers and sellers manage risk with a variety of international payments, cargo insurance, properly completed commercial documents and export packaging leads students
ICA CERTIFICATE IN COMPLIANCE. Leading Excellence in Banking. Wilmington Risk & Compliance
 ICA CERTIFICATE IN COMPLIANCE Leading Excellence in Banking Wilmington Risk & Compliance Global Reach BIBF plays a vital role in the training and development of human capital in the Middle East and North
ICA CERTIFICATE IN COMPLIANCE Leading Excellence in Banking Wilmington Risk & Compliance Global Reach BIBF plays a vital role in the training and development of human capital in the Middle East and North
RTO Policy 7: Credit Transfer
 RTO Policy 7: Credit Transfer 2 RTO POLICY 7: CREDIT TRANSFER OWNERSHIP This policy is the responsibility of CPA Australia s Registered Training Organisation () working group ( Working Group). Scope CPA
RTO Policy 7: Credit Transfer 2 RTO POLICY 7: CREDIT TRANSFER OWNERSHIP This policy is the responsibility of CPA Australia s Registered Training Organisation () working group ( Working Group). Scope CPA
Professional Evaluation and Certification Board Frequently Asked Questions
 Professional Evaluation and Certification Board Frequently Asked Questions 1. About PECB... 2 2. General... 2 3. PECB Official Training Courses... 4 4. Course Registration... 5 5. Certification... 5 6.
Professional Evaluation and Certification Board Frequently Asked Questions 1. About PECB... 2 2. General... 2 3. PECB Official Training Courses... 4 4. Course Registration... 5 5. Certification... 5 6.
Course Fees: 850 euro
 In conjuction with: Prishtinë: 19.02.2015. Offer: 2M Consulting & PECB, ISO 27001:2013 Lead Auditor Training Lecturer: Msc. CMC, Lekë Zogaj, Master ISO/IEC ISO 27001:2013 Convenient ISMS Lead Auditor Training
In conjuction with: Prishtinë: 19.02.2015. Offer: 2M Consulting & PECB, ISO 27001:2013 Lead Auditor Training Lecturer: Msc. CMC, Lekë Zogaj, Master ISO/IEC ISO 27001:2013 Convenient ISMS Lead Auditor Training
EMC & RF Workshop. 9 th November 2017 TÜV SÜD
 EMC & RF Workshop 9 th November 2017 TÜV SÜD Introduction Dealing with electromagnetic interference (EMI) can be one of the most problematic challenges in the design and development of electrical and electronic
EMC & RF Workshop 9 th November 2017 TÜV SÜD Introduction Dealing with electromagnetic interference (EMI) can be one of the most problematic challenges in the design and development of electrical and electronic
21 CFR PART 11 FREQUENTLY ASKED QUESTIONS (FAQS)
 21 CFR PART 11 FREQUENTLY ASKED QUESTIONS (S) The United States Food and Drug Administration (FDA) defines the criteria under which electronic records and electronic signatures are considered trustworthy,
21 CFR PART 11 FREQUENTLY ASKED QUESTIONS (S) The United States Food and Drug Administration (FDA) defines the criteria under which electronic records and electronic signatures are considered trustworthy,
Green Building/LEED AP Building Design & Construction Exam Preparation
 Green Building/LEED AP Building Design & Construction Exam Preparation Be among the first to master the new green building principles in the region February 16 17 and May 11 12, 2011 American University
Green Building/LEED AP Building Design & Construction Exam Preparation Be among the first to master the new green building principles in the region February 16 17 and May 11 12, 2011 American University
FOUNDATION CERTIFICATE IN INFORMATION SECURITY v2.0 INTRODUCING THE TOP 5 DISCIPLINES IN INFORMATION SECURITY SUMMARY
 FOUNDATION CERTIFICATE IN INFORMATION SECURITY v2.0 INTRODUCING THE TOP 5 DISCIPLINES IN INFORMATION SECURITY SUMMARY The Foundation Certificate in Information Security (FCIS) course is designed to provide
FOUNDATION CERTIFICATE IN INFORMATION SECURITY v2.0 INTRODUCING THE TOP 5 DISCIPLINES IN INFORMATION SECURITY SUMMARY The Foundation Certificate in Information Security (FCIS) course is designed to provide
Provider Monitoring Report. City and Guilds
 Provider Monitoring Report City and Guilds 22 May 2017 to 3 August 2017 Contents 1 Background 1 1.1 Scope 1 1.2 Provider Monitoring Report Timeline 2 1.3 Summary of Provider Monitoring Issues and Recommendations
Provider Monitoring Report City and Guilds 22 May 2017 to 3 August 2017 Contents 1 Background 1 1.1 Scope 1 1.2 Provider Monitoring Report Timeline 2 1.3 Summary of Provider Monitoring Issues and Recommendations
BCS Specialist Certificate in Change Management Syllabus
 BCS Specialist Certificate in Change Management Syllabus Version 2.0 April 2017 This qualification is not regulated by the following United Kingdom Regulators - Ofqual, Qualification in Wales, CCEA or
BCS Specialist Certificate in Change Management Syllabus Version 2.0 April 2017 This qualification is not regulated by the following United Kingdom Regulators - Ofqual, Qualification in Wales, CCEA or
CONTINUING PROFESSIONAL DEVELOPMENT RULES
 Independent Objective Authoritative The home for property professionals in Australia Australian Property Institute Limited CONTINUING PROFESSIONAL DEVELOPMENT RULES Reference Continuing Professional Development
Independent Objective Authoritative The home for property professionals in Australia Australian Property Institute Limited CONTINUING PROFESSIONAL DEVELOPMENT RULES Reference Continuing Professional Development
ISO9001:2015 LEAD IMPLEMENTER & LEAD AUDITOR
 ISO9001:2015 LEAD IMPLEMENTER & LEAD AUDITOR JPCANN ASSOCIATES LTD #58 NSAWAM ROAD, AVENOR JUNCTION, KOKOMLEMLE-ACCRA Office lines: +233 302 242 573 / +233 302 974 302 Mobile: +233 501 335 818 20 www.corptrainghana.com
ISO9001:2015 LEAD IMPLEMENTER & LEAD AUDITOR JPCANN ASSOCIATES LTD #58 NSAWAM ROAD, AVENOR JUNCTION, KOKOMLEMLE-ACCRA Office lines: +233 302 242 573 / +233 302 974 302 Mobile: +233 501 335 818 20 www.corptrainghana.com
Training Programmes Sri Lanka Accreditation Board for Conformity Assessment
 Training Programmes -2018 Sri Lanka Accreditation Board for Conformity Assessment General Instructions: All interested parties are requested to fill separate reservation form for each and submit to the
Training Programmes -2018 Sri Lanka Accreditation Board for Conformity Assessment General Instructions: All interested parties are requested to fill separate reservation form for each and submit to the
PMP Exam Preparation Training in Dubai. The Project Management Professional (PMP) September 2018 Dubai, United Arab Emirates
 An ISO 9001:2015 & ISO 29990:2010 Certified Company 17-SEP-17 The PMI Registered Education Provider logo and PMP are registered marks of the Project Management Institute, Inc. PMP Exam Preparation The
An ISO 9001:2015 & ISO 29990:2010 Certified Company 17-SEP-17 The PMI Registered Education Provider logo and PMP are registered marks of the Project Management Institute, Inc. PMP Exam Preparation The
of decisions, on which they receive immediate feedback. The module should typically take around 6 hours to complete.
 MICPA to ACCA FAQs 1. Why did MICPA and ACCA enter into this agreement? This Mutual Recognition Agreement (MRA) strengthens the already excellent relationship between the two bodies. It provides a route
MICPA to ACCA FAQs 1. Why did MICPA and ACCA enter into this agreement? This Mutual Recognition Agreement (MRA) strengthens the already excellent relationship between the two bodies. It provides a route
CMPIC s CM Training & Certification Courses
 CMPIC s CM Training & Courses CMPIC www.cmpic.com CMPIC Courses Why Choose CMPIC? Why choose CMPIC for your CM Training? CMPIC provides high quality, cost-effective, and the most up-to-date Configuration
CMPIC s CM Training & Courses CMPIC www.cmpic.com CMPIC Courses Why Choose CMPIC? Why choose CMPIC for your CM Training? CMPIC provides high quality, cost-effective, and the most up-to-date Configuration
Data Integrity and the FDA AFDO Education Conference
 Data Integrity and the FDA AFDO Education Conference June, 2018 OUR EXPERIENCE YOUR SUCCESS 1 Data Integrity What does it mean to you? 2 Data Integrity What does FDA say about data integrity No legal definition
Data Integrity and the FDA AFDO Education Conference June, 2018 OUR EXPERIENCE YOUR SUCCESS 1 Data Integrity What does it mean to you? 2 Data Integrity What does FDA say about data integrity No legal definition
Oil & Gas Industry Quality Management System Auditor/ Lead Auditor Training
 An Intensive 2-Week Training Course Oil & Gas Industry Quality Management System Auditor/ Lead Auditor Training 27 Oct - 07 Nov 2019, Dubai 24-OCT-18 This course is Designed, Developed, and will be Delivered
An Intensive 2-Week Training Course Oil & Gas Industry Quality Management System Auditor/ Lead Auditor Training 27 Oct - 07 Nov 2019, Dubai 24-OCT-18 This course is Designed, Developed, and will be Delivered
PMP Certification Preparatory Course
 PMP Certification Preparatory Course Client Relation Officer Ewa Kazimierczuk Tel. 508 018 380 ewa.kazimierczuk@pl.ey.com Dates: Warszawa, 8-10 October 2018 5-6 November 2018 Price: 5000 PLN net or 1140
PMP Certification Preparatory Course Client Relation Officer Ewa Kazimierczuk Tel. 508 018 380 ewa.kazimierczuk@pl.ey.com Dates: Warszawa, 8-10 October 2018 5-6 November 2018 Price: 5000 PLN net or 1140
CERTIFICATE IV IN COMPLIANCE & RISK MANAGEMENT
 10131NAT CERTIFICATE IV IN COMPLIANCE & RISK MANAGEMENT Associate GRC Institute (AGRCI) GRC Institute 10131NAT Certificate IV in Compliance and Risk Management Overview INTRODUCTION developed to provide
10131NAT CERTIFICATE IV IN COMPLIANCE & RISK MANAGEMENT Associate GRC Institute (AGRCI) GRC Institute 10131NAT Certificate IV in Compliance and Risk Management Overview INTRODUCTION developed to provide
INFORMATION SECURITY MANAGEMENT
 ISMS (ISO/IEC 27001:2005 to ISO/IEC 27001:2013) Transition Training Course (A17700) Two (2) Days It is recommended for ISMS registered Provisional Auditors, Auditors, Lead Auditors, Principal Auditors
ISMS (ISO/IEC 27001:2005 to ISO/IEC 27001:2013) Transition Training Course (A17700) Two (2) Days It is recommended for ISMS registered Provisional Auditors, Auditors, Lead Auditors, Principal Auditors
PRODUCT SAFETY PROFESSIONAL CERTIFICATION PROGRAM DETAILS. Overview
 Overview PRODUCT SAFETY PROFESSIONAL CERTIFICATION PROGRAM DETAILS The Product Safety Professional Certification Program at the Richard A. Chaifetz School of Business focuses on the theoretical as well
Overview PRODUCT SAFETY PROFESSIONAL CERTIFICATION PROGRAM DETAILS The Product Safety Professional Certification Program at the Richard A. Chaifetz School of Business focuses on the theoretical as well
BSB51615 Diploma of Quality Auditing. Information Pack
 BSB51615 Diploma of Quality Auditing Information Pack Confidently blast through your RTO audit! Become a skilled expert so you can be confident that you re always audit ready. Our BSB51615 Diploma of Quality
BSB51615 Diploma of Quality Auditing Information Pack Confidently blast through your RTO audit! Become a skilled expert so you can be confident that you re always audit ready. Our BSB51615 Diploma of Quality
European Code of Conduct on Data Centre Energy Efficiency
 EUROPEAN COMMISSION DIRECTORATE-GENERAL JOINT RESEARCH CENTRE Institute for Energy and Transport Renewable Energy Unit European Code of Conduct on Data Centre Energy Efficiency Introductory guide for applicants
EUROPEAN COMMISSION DIRECTORATE-GENERAL JOINT RESEARCH CENTRE Institute for Energy and Transport Renewable Energy Unit European Code of Conduct on Data Centre Energy Efficiency Introductory guide for applicants
Investigating Microbial Data Deviations
 Microrite, Inc. brings you this unique learning experience in Investigating Microbial Data Deviations; Part of Microrite s step-by-step webinar series. Investigating Microbial Data Deviations Since the
Microrite, Inc. brings you this unique learning experience in Investigating Microbial Data Deviations; Part of Microrite s step-by-step webinar series. Investigating Microbial Data Deviations Since the
Audit Report. Mineral Products Qualifications Council (MPQC) 31 March 2014
 Audit Report Mineral Products Qualifications Council (MPQC) 31 March 2014 Note Restricted or commercially sensitive information gathered during SQA Accreditation s quality assurance activities is treated
Audit Report Mineral Products Qualifications Council (MPQC) 31 March 2014 Note Restricted or commercially sensitive information gathered during SQA Accreditation s quality assurance activities is treated
Environmental Monitoring Program for Medical Devices per Current Guidances New AAMI and ISO EM Reference Documents and Standards
 Microrite, Inc. brings you this unique learning experience in Environmental Monitoring Program for Medical Devices per Current Guidances; Part of Microrite s step-by-step webinar series. Environmental
Microrite, Inc. brings you this unique learning experience in Environmental Monitoring Program for Medical Devices per Current Guidances; Part of Microrite s step-by-step webinar series. Environmental
ABB Limited. Table of Content. Executive Summary
 21 CFR Part 11 Electronic Records; Electronic Signatures Guidance for Industry Scope of Application Position Paper: A Summary and Interpretation of the Guidance Note: This document has been prepared based
21 CFR Part 11 Electronic Records; Electronic Signatures Guidance for Industry Scope of Application Position Paper: A Summary and Interpretation of the Guidance Note: This document has been prepared based
The forum will cover the key legislative amendments in the Companies (Amendment) Act 2014 and the practical applications and key filing requirements.
 The forum will cover the key legislative amendments in the Companies (Amendment) Act 2014 and the practical applications and key filing requirements. PROGRAMME AT A GLANCE Presentation on Key Legislative
The forum will cover the key legislative amendments in the Companies (Amendment) Act 2014 and the practical applications and key filing requirements. PROGRAMME AT A GLANCE Presentation on Key Legislative
CONTINUOUS PROFESSIONAL DEVELOPMENT (CPD) POLICY
 CONTINUOUS PROFESSIONAL DEVELOPMENT (CPD) POLICY SUMMARY: This defined as a framework that encourages continuous updating of professional knowledge, personal skills and competencies. DATE OF APPROVAL FOR
CONTINUOUS PROFESSIONAL DEVELOPMENT (CPD) POLICY SUMMARY: This defined as a framework that encourages continuous updating of professional knowledge, personal skills and competencies. DATE OF APPROVAL FOR
Understand European GMPs and the Role of the Qualified Person (QP)
 Understand European GMPs and the Role of the Qualified Person (QP) The Impact of the changing EU Directives and Guidelines on the Supply Chain Boston, MA, USA August 31 September 1, 2016 A conference organised
Understand European GMPs and the Role of the Qualified Person (QP) The Impact of the changing EU Directives and Guidelines on the Supply Chain Boston, MA, USA August 31 September 1, 2016 A conference organised
October 9-11, 2012 Lenexa, KS
 NEBB s Cleanroom Performance Testing Certified Professional Seminar and Exam October 9-11, 2012 Lenexa, KS Introduction: NEBB, in continuance of its policy to enhance education and training within the
NEBB s Cleanroom Performance Testing Certified Professional Seminar and Exam October 9-11, 2012 Lenexa, KS Introduction: NEBB, in continuance of its policy to enhance education and training within the
QP Current Practices, Challenges and Mysteries. Caitriona Lenagh 16 March 2012
 QP Current Practices, Challenges and Mysteries Caitriona Lenagh 16 March 2012 Agenda QP Roles and Responsibilities QP Current Practices Supply Chain Verification Study Specific Information Lot Specific
QP Current Practices, Challenges and Mysteries Caitriona Lenagh 16 March 2012 Agenda QP Roles and Responsibilities QP Current Practices Supply Chain Verification Study Specific Information Lot Specific
IPC Certification Scheme IPC QMS/EMS Auditors
 Page 1 of 16 International Personnel Certification Association I P C CERTIFICATION SCHEME IPC QUALITY/ENVIRONMENTAL MANAGEMENT SYSTEM AUDITORS ISSUE 1 Page 2 of 16 International Personnel Certification
Page 1 of 16 International Personnel Certification Association I P C CERTIFICATION SCHEME IPC QUALITY/ENVIRONMENTAL MANAGEMENT SYSTEM AUDITORS ISSUE 1 Page 2 of 16 International Personnel Certification
