A National Metadata Repository for Empirical Research in Germany
|
|
|
- Bennett Wilkins
- 5 years ago
- Views:
Transcription
1 A National Metadata Repository for Empirical Research in Germany 15 th International Open Forum on Metadata Jürgen Stausberg 1, Johannes Drepper 2, Matthias Löbe 3, Sylvie MN Ngouongo 1, Philippe Verplancke 4 1 IBE, Ludwig-Maximilians-Universität München, Germany 2 TMF - Technology, Methods, and Infrastructure for Networked Medical Research, Berlin, Germany 3 Institute for Medical Informatics (IMISE), Universität Leipzig, Germany 4 XClinical, München, Germany
2 Agenda Project Core packages of ISO/IEC Ed. 3 Information models and ISO/IEC Ed. 3 Contolled vocabularies and ISO/IEC Ed. 3 Pilot implementation
3 Objectives, scope Empirical medical research is based on the collection of observations stored in DBMS needs services for the maintenance of item collections and improvement of interoperability: Reuse of item`s definitions Quality improvement through harmonization and standardization Integration and use of controlled vocabularies as value lists for items Use of terminologies as buildings blocks for data elements MDR-Project in Germany launched in 2009 by the Federal Ministry of Education and Research to set up a national metadata repository for the support of empirical research. ISO/IEC Ed. 3 Information technology - Metadata Registries (MDR) part 3 Registry Metamodel and basic attributes
4 Oktober 2011: 89 Member organisations Disease-oriented Competence Networks in Medicine Coordinating Centres for Clinical Trials Networks for Rare Diseases German Clinical Trials Register German National Genome Research Network (NGFN) Fraunhofer Institute for Toxicology and Experimental Medicine Patient Organisations German National Cohort Pre-Test Phase Centralized Biobanks German Centres of Health Research
5 Use cases Single organization Maintain metadata - to specify a CRF or a catalogue of variables Import metadata - to establish a pool of metadata Mapping of metadata - to transform data from one model to another Queries for multiple versions of metadata - to support study series Multiple organizations Queries for multiple metadata - to support pooling of data Select projects - to identify projects covering specific variables Review of metadata - to harmonize variables and value lists National level Queries for multiple metadata - to support pooling of data Select projects - to identify projects covering specific variables Maintain standards - to harmonize CRFs and databases Improve standards - to identify gaps in data standards or terminologies
6 Core of the metamodel of ISO/IEC Ed. 3 object_class person characteristic sex concept layer * data_clement_concept person s sex * * * conceptual_domain karyotype <men women> 1 1 representation layer * data_element * value_domain person s sex <male female> <male female> ISO/IEC CD Information technology Metadata registries (MDR) Part 3: Registry metamodel and basic attributes. ISO/IEC JTC 1/SC 32 N 1851, Date: Available at * 1
7 Core of the metamodel of ISO/IEC Ed. 3 object_class person characteristic sex concept layer * data_clement_concept person s sex * * * conceptual_domain karyotype <men women> 1 1 representation layer * data_element * value_domain person s sex <male female> * 1 <male female>
8
9 Tested material in the metadata architecture ITEMS VOCABULARIES Layer 3: meta-metamodel CDISC ODM RIM ClaML Layer 2: metamodel Documentation scheme ISO MedDRA ICD-10-GM SNOMED CT Layer 1: model HIVNET register TNM LOINC Layer 0: information
10 Mapping to ISO/IEC V3 ITEMS VOCABULARIES METADATA REGISTRY Layer 3: meta-metamodel Layer 2: metamodel CDISC ODM Documentation scheme RIM ISO ClaML ISO/IEC 11179V3 MedDRA ICD-10-GM SNOMED CT Data base model Layer 1: model HIVNET register TNM LOINC CRF... Layer 0: information Data
11 Methods Mapping of model elements (metamodels) Import of models into an MDR-database
12 Documentation scheme/eav-model Disease registers no problem in general but in particular OIDs layout information of CRFs relationships between data_elements
13 Reference models EHR RIM of HL7 ISO Health informatics - Electronic health record communication no problem in general but inheritance complex information objects not explicitely mentioned
14 CRF -model 1
15 CRF -model 2 1 Context 3 Data_Elements Object_Class: hepatitis B 3 Characteristics: date of diagnosis, reason for diagnosis, first date of raised liver values 3 Value_Domains: 2 date, value list for reason for diagnosis 3 Permissible_Values, 3 Value_Meanings, 3 Conceptual_Domains 22 Designations, 1 Definition Not covered: position (4), constraint between reason for diagnosis and first date of raised liver values, order of the Data_Elements, oder of the Permissible_Values
16 Mapping CDISC ODM to ISO Ed. 3 CDISC Operational Data Model (ODM): XML-based standard that contains all metadata and clinical data of a clinical trial ODM item definitions are composed of meaning and representation Approach included the basic ODM build blocks for the specification of clinical items (ItemDef, MeasurementUnit, and CodeList) Results: Most elements could be mapped ODM doesn t have a counterpart for Data Element Concept Described_Conceptual_Domain/-Value_Domain is implicit in ODM ISO permits only a single Measurement Unit where ODM items can have many
17 Classifications and Terminologies ICD-10-GM: International Statistical Classification of Diseases and Related Health Problems 10th Revision German Modifications OPS: German procedure classification The TNM classification of malignant tumors A well-established standard for the classification of malignant tumors in oncology. the TNM-system classification provides three axes for postcoordination (T: local tumor, N: regional lymph nodes, M: metastasis). MedDRA: Medical Dictionary for Regulatory Activities The international medical terminology used to classify adverse events associated with the use of biopharmaceuticals and other medical products. SNOMED CT: Systematized Nomenclature of Medicine Clinical Terms
18 Material: Classifications and Terminologies - ClaML Classification Markup Language (ClaML) is attractive as interface standard for the import of classifications (ICD-10-GM, OPS) into the MDR. ClaML: EN 14463:2007, Standard for electronic representation of medical classification systems. Small excerpt of ClaML 18 <?xml version="1.0" encoding="utf-8"?> <!DOCTYPE ClaML SYSTEM "claml.dtd"[]> <ClaML version="2.0.0"> <Meta name="toplevelsort" value="i II III IV V VI VII VIII IX X XI XII XIII XIV XV XVI XVII XVIII XIX XX XXI XXII" /> <Meta name="lang" value="en" /> <Meta name="titlelong" value="international Statistical Classification of Diseases and Related Health Problems 10th Revision"/> <Identifier authority="who" uid="srfsfto be added later" /> <Title date=" " name="icd en" version="2008" /> <Class code="b27" kind="category"> <SuperClass code="b25-b34" /> <SubClass code="b27.0" /> <SubClass code="b27.1" /> <Rubric id="d " kind="preferred"> <Label xml:lang="en" xml:space="default">infectious mononucleosis</label> </Rubric> </Class> </ClaML>
19 Mapping : the results ClaML.root; ICD-10-GM; OPS; TNM; MedDRA; SNOMED-CT (Vocabularies) Classifications semantic ClaML-Classes / Vocabularies-Classes generalization Example of ICD-10 Intestinal infectious Diseases (A00-A09) 19 Hierarchy specialization is_a ClaML-Classes / MIE 2011, Sylvie Ngouongo Cholera Vocabularies-Classes (A00)
20 Classifications, ClaML some problems: representation of the code rules not covered coding standards exclusions, inclusions, special codes reconstruction not possible intermediate levels not supported direct use of classes not possible, doubling required
21 SNOMED CT core tables SNOMED CT ISO E3 Comment concept_system Value is the release of SNOMED CT. context Concept a) Value is the release of SNOMED CT. b) Value is Read Codes CTV3 as context for Read Codes. concept SNOMEDID definition Definition is associated with the context of the release of SNOMED CT. CTV3ID definition Definition is associated with the context Read Codes CTV3. IsPrimitive assertion Description designation Designation is associated with the context of the release of SNOMED CT. Term designation_sign DescriptionType designation_class Designation_class is an association nclass between designation and context. LanguageCode designation_language Relationships link RelationshipType relation_role The reverse relationship must be generated artificially. CharacteristicType assertion_formula Refinability Refinability is not covered by ISO/IEC E3. RelationshipGroup RelationshipGroup is not covered by ISO/IEC E3.
22 Terminological systems no problem in general reconstruction to some extent possible but SNOMED CT elements not covered relationship_type refinability for data entry Medical Dictionary for Regulatory Activities (MedDRA) versioning of coding systems and their relationship to the data description package TNM composition of concepts
23 Implementation ISO/IEC Ed. 3 view Data manager view
24
25
26
27
28 Conclusions ISO/IEC Ed. 3 is powerful and contributes many useful ideas for the definition of a national MDR. An extension of ISO/IEC Ed. 3 metamodel might be necessary to meet the predefined needs of a national MDR. The relationship between data elements and concepts should be further elaborated. The implementation for data managers requires a simplified view around the ISO/IEC Ed. 3 model. For clinical studies, ODM could be the simplified model.
29 Acknowledgements The presented work is part of the project MDR - Metadata Repository funded by the German Federal Ministry of Education and Research (BMBF).
National metadata repository for databases of registers and trials
 National metadata repository for databases of registers and trials IBE, Medical Faculty, Ludwig-Maximilians-Universität München, Germany Institute for Medical Informatics (IMISE), Universität Leipzig,
National metadata repository for databases of registers and trials IBE, Medical Faculty, Ludwig-Maximilians-Universität München, Germany Institute for Medical Informatics (IMISE), Universität Leipzig,
Integration of classifications and terminologies in metadata registries based on ISO/IEC 11179
 These sheets are only for use in connection with the speech. Integration of classifications and terminologies in metadata registries based on ISO/IEC 11179 Sylvie Ngouongo Jürgen Stausberg Ludwig-Maximilian-University
These sheets are only for use in connection with the speech. Integration of classifications and terminologies in metadata registries based on ISO/IEC 11179 Sylvie Ngouongo Jürgen Stausberg Ludwig-Maximilian-University
A ClaML-based Interface for the Import of Monohierarchical Classifications
 These sheets are only for use in connection with the speech. A ClaML-based Interface for the Import of Monohierarchical Classifications Sylvie Ngouongo Jürgen Stausberg Ludwig-Maximilian-University of
These sheets are only for use in connection with the speech. A ClaML-based Interface for the Import of Monohierarchical Classifications Sylvie Ngouongo Jürgen Stausberg Ludwig-Maximilian-University of
A Knowledge-Based System for the Specification of Variables in Clinical Trials
 A Knowledge-Based System for the Specification of Variables in Clinical Trials Matthias Löbe, Barbara Strotmann, Kai-Uwe Hoop, Roland Mücke Institute for Medical Informatics, Statistics and Epidemiology
A Knowledge-Based System for the Specification of Variables in Clinical Trials Matthias Löbe, Barbara Strotmann, Kai-Uwe Hoop, Roland Mücke Institute for Medical Informatics, Statistics and Epidemiology
ISO/IEC INTERNATIONAL STANDARD. Information technology Metadata registries (MDR) Part 3: Registry metamodel and basic attributes
 INTERNATIONAL STANDARD ISO/IEC 11179-3 Third edition 2013-02-15 Information technology Metadata registries (MDR) Part 3: Registry metamodel and basic attributes Technologies de l'information Registres
INTERNATIONAL STANDARD ISO/IEC 11179-3 Third edition 2013-02-15 Information technology Metadata registries (MDR) Part 3: Registry metamodel and basic attributes Technologies de l'information Registres
TIM: A Semantic Web Application for the Specification of Metadata Items in Clinical Research
 TIM: A Semantic Web Application for the Specification of Metadata Items in Clinical Research Matthias Löbe 1, Magnus Knuth 2, Roland Mücke 3 1 Clinical Trial Center Leipzig, University of Leipzig, Germany
TIM: A Semantic Web Application for the Specification of Metadata Items in Clinical Research Matthias Löbe 1, Magnus Knuth 2, Roland Mücke 3 1 Clinical Trial Center Leipzig, University of Leipzig, Germany
Summary of Contents LIST OF FIGURES LIST OF TABLES
 Summary of Contents LIST OF FIGURES LIST OF TABLES PREFACE xvii xix xxi PART 1 BACKGROUND Chapter 1. Introduction 3 Chapter 2. Standards-Makers 21 Chapter 3. Principles of the S2ESC Collection 45 Chapter
Summary of Contents LIST OF FIGURES LIST OF TABLES PREFACE xvii xix xxi PART 1 BACKGROUND Chapter 1. Introduction 3 Chapter 2. Standards-Makers 21 Chapter 3. Principles of the S2ESC Collection 45 Chapter
Health Information Exchange Content Model Architecture Building Block HISO
 Health Information Exchange Content Model Architecture Building Block HISO 10040.2 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary HISO 10040.1 Health Information
Health Information Exchange Content Model Architecture Building Block HISO 10040.2 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary HISO 10040.1 Health Information
Representation of ICD-10- Markup Language (ClaML)
 Representation of ICD-10- AM/ACHI using Classification Markup Language (ClaML) Robert Smith Western Sydney University Who are We? Australian Consortium for Classification Development (ACCD) University
Representation of ICD-10- AM/ACHI using Classification Markup Language (ClaML) Robert Smith Western Sydney University Who are We? Australian Consortium for Classification Development (ACCD) University
Mathematics Shape and Space: Polygon Angles
 a place of mind F A C U L T Y O F E D U C A T I O N Department of Curriculum and Pedagogy Mathematics Shape and Space: Polygon Angles Science and Mathematics Education Research Group Supported by UBC Teaching
a place of mind F A C U L T Y O F E D U C A T I O N Department of Curriculum and Pedagogy Mathematics Shape and Space: Polygon Angles Science and Mathematics Education Research Group Supported by UBC Teaching
Workshop 2. > Interoperability <
 Workshop 2 21 / 08 / 2011 > Interoperability < Heiko Zimmermann R&D Engineer, AHI CR Santec Heiko.Zimmermann@tudor.lu Interoperability definition Picture from NCI-Wiki (https://wiki.nci.nih.gov) 2 Interoperability
Workshop 2 21 / 08 / 2011 > Interoperability < Heiko Zimmermann R&D Engineer, AHI CR Santec Heiko.Zimmermann@tudor.lu Interoperability definition Picture from NCI-Wiki (https://wiki.nci.nih.gov) 2 Interoperability
Introduction to PTC Windchill ProjectLink 11.0
 Introduction to PTC Windchill ProjectLink 11.0 Overview Course Code Course Length TRN-4756-T 8 Hours In this course, you will learn how to participate in and manage projects using Windchill ProjectLink
Introduction to PTC Windchill ProjectLink 11.0 Overview Course Code Course Length TRN-4756-T 8 Hours In this course, you will learn how to participate in and manage projects using Windchill ProjectLink
Jena based Implementation of a ISO Metadata Registry. Gokce B. Laleci & A. Anil Sinaci
 Jena based Implementation of a ISO 11179 Metadata Registry Gokce B. Laleci & A. Anil Sinaci Agenda l l l l Motivation l l l A brief overview of SALUS Project SALUS Semantic Interoperability approach Role
Jena based Implementation of a ISO 11179 Metadata Registry Gokce B. Laleci & A. Anil Sinaci Agenda l l l l Motivation l l l A brief overview of SALUS Project SALUS Semantic Interoperability approach Role
ISO CTS2 and Value Set Binding. Harold Solbrig Mayo Clinic
 ISO 79 CTS2 and Value Set Binding Harold Solbrig Mayo Clinic ISO 79 Information technology - Metadata registries (MDR) Owning group is ISO/IEC JTC /SC 32 Organization responsible for SQL standard Six part
ISO 79 CTS2 and Value Set Binding Harold Solbrig Mayo Clinic ISO 79 Information technology - Metadata registries (MDR) Owning group is ISO/IEC JTC /SC 32 Organization responsible for SQL standard Six part
ISO/IEC INTERNATIONAL STANDARD. Information technology Metamodel framework for interoperability (MFI) Part 1: Reference model
 INTERNATIONAL STANDARD ISO/IEC 19763-1 First edition 2007-02-01 Information technology Metamodel framework for interoperability (MFI) Part 1: Reference model Technologies de l'information Cadre du métamodèle
INTERNATIONAL STANDARD ISO/IEC 19763-1 First edition 2007-02-01 Information technology Metamodel framework for interoperability (MFI) Part 1: Reference model Technologies de l'information Cadre du métamodèle
CEN/ISSS WS/eCAT. Terminology for ecatalogues and Product Description and Classification
 CEN/ISSS WS/eCAT Terminology for ecatalogues and Product Description and Classification Report Final Version This report has been written for WS/eCAT by Mrs. Bodil Nistrup Madsen (bnm.danterm@cbs.dk) and
CEN/ISSS WS/eCAT Terminology for ecatalogues and Product Description and Classification Report Final Version This report has been written for WS/eCAT by Mrs. Bodil Nistrup Madsen (bnm.danterm@cbs.dk) and
From Implementing CDISC Using SAS. Full book available for purchase here. About This Book... xi About The Authors... xvii Acknowledgments...
 From Implementing CDISC Using SAS. Full book available for purchase here. Contents About This Book... xi About The Authors... xvii Acknowledgments... xix Chapter 1: Implementation Strategies... 1 Why CDISC
From Implementing CDISC Using SAS. Full book available for purchase here. Contents About This Book... xi About The Authors... xvii Acknowledgments... xix Chapter 1: Implementation Strategies... 1 Why CDISC
ISO. International Organization for Standardization ISO/IEC JTC 1/SC 32. Data Management and Interchange WG 4. SQL Multimedia and Application Packages
 3 rd Working Draft for 1 st Edition ISO/IEC JTC 1/SC 32/WG 4: KMG-005-3WD-8-MRA-2010-05 ISO International Organization for Standardization ISO/IEC JTC 1/SC 32 Data Management and Interchange WG 4 SQL Multimedia
3 rd Working Draft for 1 st Edition ISO/IEC JTC 1/SC 32/WG 4: KMG-005-3WD-8-MRA-2010-05 ISO International Organization for Standardization ISO/IEC JTC 1/SC 32 Data Management and Interchange WG 4 SQL Multimedia
SNOMED Clinical Terms
 Representing clinical information using SNOMED Clinical Terms with different structural information models KR-MED 2008 - Phoenix David Markwell Laura Sato The Clinical Information Consultancy Ltd NHS Connecting
Representing clinical information using SNOMED Clinical Terms with different structural information models KR-MED 2008 - Phoenix David Markwell Laura Sato The Clinical Information Consultancy Ltd NHS Connecting
2.) ilit Welcome Screen
 1.) ilit Login Page a. Single Sign On (VPN) if you are logged in the VPN (getting emails, etc.), no password will be required when you launch I-Lit. You will be taken directly to the welcome screen. b.
1.) ilit Login Page a. Single Sign On (VPN) if you are logged in the VPN (getting emails, etc.), no password will be required when you launch I-Lit. You will be taken directly to the welcome screen. b.
Business Intelligence Roadmap HDT923 Three Days
 Three Days Prerequisites Students should have experience with any relational database management system as well as experience with data warehouses and star schemas. It would be helpful if students are
Three Days Prerequisites Students should have experience with any relational database management system as well as experience with data warehouses and star schemas. It would be helpful if students are
Health Information Exchange Clinical Data Repository Utility Services Architecture Building Block HISO
 Health Information Exchange Clinical Data Repository Utility Services Architecture Building Block HISO 10040.1 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary
Health Information Exchange Clinical Data Repository Utility Services Architecture Building Block HISO 10040.1 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary
CDASH MODEL 1.0 AND CDASHIG 2.0. Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams
 CDASH MODEL 1.0 AND CDASHIG 2.0 Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams 1 What is CDASH? Clinical Data Acquisition Standards Harmonization (CDASH) Standards for the collection
CDASH MODEL 1.0 AND CDASHIG 2.0 Kathleen Mellars Special Thanks to the CDASH Model and CDASHIG Teams 1 What is CDASH? Clinical Data Acquisition Standards Harmonization (CDASH) Standards for the collection
Terminology Harmonization
 Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
An introduction to metadata. Metadata registries for improved data management
 This afternoon An introduction to metadata Metadata registries for improved data management within the Highways Agency An introduction to metadata 1 My agenda What is metadata? Metadata standards What
This afternoon An introduction to metadata Metadata registries for improved data management within the Highways Agency An introduction to metadata 1 My agenda What is metadata? Metadata standards What
Implementing CDISC Using SAS. Full book available for purchase here.
 Implementing CDISC Using SAS. Full book available for purchase here. Contents About the Book... ix About the Authors... xv Chapter 1: Implementation Strategies... 1 The Case for Standards... 1 Which Models
Implementing CDISC Using SAS. Full book available for purchase here. Contents About the Book... ix About the Authors... xv Chapter 1: Implementation Strategies... 1 The Case for Standards... 1 Which Models
Annexure I: Contact Details:
 Ref: CO/IT-BPR/CSC Date:.09.2017 Annexure I: Contact Details: a) Name of the company b) Company s address in India c) Contact person d) Telephone no. e) Fax f) E-mail address g) Service tax registration
Ref: CO/IT-BPR/CSC Date:.09.2017 Annexure I: Contact Details: a) Name of the company b) Company s address in India c) Contact person d) Telephone no. e) Fax f) E-mail address g) Service tax registration
edify Requirements for Evidence-based Templates in Electronic Case Report Forms Marco Schweitzer, Stefan Oberbichler
 edify Requirements for Evidence-based Templates in Electronic Case Report Forms Marco Schweitzer, Stefan Oberbichler ehealth Research and Innovation Unit, UMIT - University for Health Sciences, Medical
edify Requirements for Evidence-based Templates in Electronic Case Report Forms Marco Schweitzer, Stefan Oberbichler ehealth Research and Innovation Unit, UMIT - University for Health Sciences, Medical
A Comprehensive Clinical Research Database based on CDISC ODM and i2b2
 e-health For Continuity of Care C. Lovis et al. (Eds.) 2014 European Federation for Medical Informatics and IOS Press. This article is published online with Open Access by IOS Press and distributed under
e-health For Continuity of Care C. Lovis et al. (Eds.) 2014 European Federation for Medical Informatics and IOS Press. This article is published online with Open Access by IOS Press and distributed under
Certificate Program. Introduction to Microsoft Excel 2013
 Certificate Program We offer online education programs designed to provide the workforce skills necessary to enter a new field or advance your current career. Our Online Career Training Programs in the
Certificate Program We offer online education programs designed to provide the workforce skills necessary to enter a new field or advance your current career. Our Online Career Training Programs in the
BSynchro [E-AUTHORIZATION USER GUIDE] BSYNCHRO
![BSynchro [E-AUTHORIZATION USER GUIDE] BSYNCHRO BSynchro [E-AUTHORIZATION USER GUIDE] BSYNCHRO](/thumbs/74/70571461.jpg) BSynchro 2014 BSYNCHRO [E-AUTHORIZATION USER GUIDE] This Document is intended as a user guide for providers who are planning to use the e-authorization system. Contents I. Introduction... 2 II. Purpose
BSynchro 2014 BSYNCHRO [E-AUTHORIZATION USER GUIDE] This Document is intended as a user guide for providers who are planning to use the e-authorization system. Contents I. Introduction... 2 II. Purpose
CDASH Standards and EDC CRF Library. Guang-liang Wang September 18, Q3 DCDISC Meeting
 CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
CDASH Standards and EDC CRF Library Guang-liang Wang September 18, 2014 2014 Q3 DCDISC Meeting 1 Disclaimer The content of this presentation does not represent the views of my employer or any of its affiliates.
Deliverable 4.4: Final specification of EHR4CR semantic interoperability solutions
 Electronic Health Records for Clinical Research Deliverable 4.4: Final specification of EHR4CR semantic interoperability solutions Version 1.0 Final 29/02/2016 Project acronym: EHR4CR Project full title:
Electronic Health Records for Clinical Research Deliverable 4.4: Final specification of EHR4CR semantic interoperability solutions Version 1.0 Final 29/02/2016 Project acronym: EHR4CR Project full title:
"Charting the Course... SharePoint 2007 Hands-On Labs Course Summary
 Course Summary Description This series of 33 hands-on labs allows students to explore the new features of Microsoft SharePoint Server, Microsoft Windows, Microsoft Office, including Microsoft Office Groove,
Course Summary Description This series of 33 hands-on labs allows students to explore the new features of Microsoft SharePoint Server, Microsoft Windows, Microsoft Office, including Microsoft Office Groove,
Models of Uses & Models of Meaning. Alan Rector. ode.org protege.stanford.org
 Models of Uses & Models of Meaning Alan Rector Information Management Group / Bio Health Informatics Forum Department of Computer Science, University of Manchester rector@cs.man.ac.uk co-ode ode-admin@cs.man.ac.uk
Models of Uses & Models of Meaning Alan Rector Information Management Group / Bio Health Informatics Forum Department of Computer Science, University of Manchester rector@cs.man.ac.uk co-ode ode-admin@cs.man.ac.uk
Kalaivani Ananthan Version 2.0 October 2008 Funded by the Library of Congress
 RUTGERS UNIVERSITY LIBRARIES OpenMIC User Manual Bibliographic Utility for analog and digital objects Kalaivani Ananthan Version 2.0 October 2008 Funded by the Library of Congress Table of Contents I.
RUTGERS UNIVERSITY LIBRARIES OpenMIC User Manual Bibliographic Utility for analog and digital objects Kalaivani Ananthan Version 2.0 October 2008 Funded by the Library of Congress Table of Contents I.
CHAPTER 7. Observations, Conclusions and Future Directions Observations 7.2. Limitations of the Model 7.3. Conclusions 7.4.
 CHAPTER 7 Observations, Conclusions and Future Directions 7.1. Observations 7.2. Limitations of the Model 7.3. Conclusions 7.4. Future work Domain-specific Ontology for Student s Information in Academic
CHAPTER 7 Observations, Conclusions and Future Directions 7.1. Observations 7.2. Limitations of the Model 7.3. Conclusions 7.4. Future work Domain-specific Ontology for Student s Information in Academic
Introduction to PTC Windchill PDMLink 11.0 for the Implementation Team
 Introduction to PTC Windchill PDMLink 11.0 for the Implementation Team Overview Course Code Course Length TRN-4752-T 16 Hours In this course, you will learn how to complete basic Windchill PDMLink functions.
Introduction to PTC Windchill PDMLink 11.0 for the Implementation Team Overview Course Code Course Length TRN-4752-T 16 Hours In this course, you will learn how to complete basic Windchill PDMLink functions.
CITY UNIVERSITY OF NEW YORK. Creating a New Project in IRBNet. i. After logging in, click Create New Project on left side of the page.
 CITY UNIVERSITY OF NEW YORK Creating a New Project in IRBNet i. After logging in, click Create New Project on left side of the page. ii. Enter the title of the project, the principle investigator s (PI)
CITY UNIVERSITY OF NEW YORK Creating a New Project in IRBNet i. After logging in, click Create New Project on left side of the page. ii. Enter the title of the project, the principle investigator s (PI)
Developing a national disease registry: the German approach to a rare disease registry
 Developing a national disease registry: the German approach to a rare disease registry T. Hartz @tobgerm University Medical Center Mainz, Germany Institute for Medical Biometry, Epidemiology and Informatics
Developing a national disease registry: the German approach to a rare disease registry T. Hartz @tobgerm University Medical Center Mainz, Germany Institute for Medical Biometry, Epidemiology and Informatics
Mechanism Design using Creo Parametric 3.0
 Mechanism Design using Creo Parametric 3.0 Overview Course Code Course Length TRN-4521-T 1 Day In this course, you will learn about creating mechanism connections, configuring the mechanism model, creating
Mechanism Design using Creo Parametric 3.0 Overview Course Code Course Length TRN-4521-T 1 Day In this course, you will learn about creating mechanism connections, configuring the mechanism model, creating
Introduction to Windchill PDMLink 10.2 for the Implementation Team
 Introduction to Windchill PDMLink 10.2 for the Implementation Team Overview Course Code Course Length TRN-4262-T 2 Days In this course, you will learn how to complete basic Windchill PDMLink functions.
Introduction to Windchill PDMLink 10.2 for the Implementation Team Overview Course Code Course Length TRN-4262-T 2 Days In this course, you will learn how to complete basic Windchill PDMLink functions.
ISO/IEC CD :200x(E) Title: Information technology - Framework for Metamodel interoperability Part 2: Reference model Project:
 Committee Draft ISO/IEC CD Date: 2005-06-30 Reference number: ISO/JTC 1/SC 32N1333 Supersedes document SC 32N1085 THIS DOCUMENT IS STILL UNDER STUDY AND SUBJECT TO CHANGE. IT SHOULD NOT BE USED FOR REFERENCE
Committee Draft ISO/IEC CD Date: 2005-06-30 Reference number: ISO/JTC 1/SC 32N1333 Supersedes document SC 32N1085 THIS DOCUMENT IS STILL UNDER STUDY AND SUBJECT TO CHANGE. IT SHOULD NOT BE USED FOR REFERENCE
A Justification-based Semantic Framework for Representing, Evaluating and Utilizing Terminology Mappings
 A Justification-based Semantic Framework for Representing, Evaluating and Utilizing Terminology Mappings Sajjad HUSSAIN a, Hong SUN b, Gokce B. Laleci ERTURKMEN c, Mustafa YUKSEL c, Charles MEAD d, Alasdair
A Justification-based Semantic Framework for Representing, Evaluating and Utilizing Terminology Mappings Sajjad HUSSAIN a, Hong SUN b, Gokce B. Laleci ERTURKMEN c, Mustafa YUKSEL c, Charles MEAD d, Alasdair
Smart Open Services for European Patients. Work Package 3.5 Semantic Services Definition Appendix E - Ontology Specifications
 24Am Smart Open Services for European Patients Open ehealth initiative for a European large scale pilot of Patient Summary and Electronic Prescription Work Package 3.5 Semantic Services Definition Appendix
24Am Smart Open Services for European Patients Open ehealth initiative for a European large scale pilot of Patient Summary and Electronic Prescription Work Package 3.5 Semantic Services Definition Appendix
Introduction to PTC Windchill PDMLink 11.0 for Heavy Users
 Introduction to PTC Windchill PDMLink 11.0 for Heavy Users Overview Course Code Course Length TRN-4751-T 16 Hours In this course, you will learn how to complete the day-to-day functions that enable you
Introduction to PTC Windchill PDMLink 11.0 for Heavy Users Overview Course Code Course Length TRN-4751-T 16 Hours In this course, you will learn how to complete the day-to-day functions that enable you
CDISC Standards End-to-End: Enabling QbD in Data Management Sam Hume
 CDISC Standards End-to-End: Enabling QbD in Data Management Sam Hume 1 Shared Health and Research Electronic Library (SHARE) A global electronic repository for developing, integrating
CDISC Standards End-to-End: Enabling QbD in Data Management Sam Hume 1 Shared Health and Research Electronic Library (SHARE) A global electronic repository for developing, integrating
Specification of a Cardiovascular Metadata Model: A Consensus Standard
 Specification of a Cardiovascular Metadata Model: A Consensus Standard Rebecca Wilgus, RN MSN 1 ; Dana Pinchotti 2 ; Salvatore Mungal 3 ; David F Kong, MD AM 1 ; James E Tcheng, MD 1 ; Brian McCourt 1
Specification of a Cardiovascular Metadata Model: A Consensus Standard Rebecca Wilgus, RN MSN 1 ; Dana Pinchotti 2 ; Salvatore Mungal 3 ; David F Kong, MD AM 1 ; James E Tcheng, MD 1 ; Brian McCourt 1
Introduction to Creo Elements/Direct 19.0 Modeling
 Introduction to Creo Elements/Direct 19.0 Modeling Overview Course Code Course Length TRN-4531-T 3 Day In this course, you will learn the basics about 3-D design using Creo Elements/Direct Modeling. You
Introduction to Creo Elements/Direct 19.0 Modeling Overview Course Code Course Length TRN-4531-T 3 Day In this course, you will learn the basics about 3-D design using Creo Elements/Direct Modeling. You
SNOMED CT Implementation Approaches. National Resource Centre for EHR Standards (NRCeS) C-DAC, Pune
 SNOMED CT Implementation Approaches National Resource Centre for EHR Standards (NRCeS) C-DAC, Pune SNOMED CT with no Health Record SNOMED CT is a powerful modern clinical terminology Comprehensive scope
SNOMED CT Implementation Approaches National Resource Centre for EHR Standards (NRCeS) C-DAC, Pune SNOMED CT with no Health Record SNOMED CT is a powerful modern clinical terminology Comprehensive scope
warwick.ac.uk/lib-publications
 Original citation: Zhao, Lei, Lim Choi Keung, Sarah Niukyun and Arvanitis, Theodoros N. (2016) A BioPortalbased terminology service for health data interoperability. In: Unifying the Applications and Foundations
Original citation: Zhao, Lei, Lim Choi Keung, Sarah Niukyun and Arvanitis, Theodoros N. (2016) A BioPortalbased terminology service for health data interoperability. In: Unifying the Applications and Foundations
Contents CHAPTER 1 CHAPTER 2. Recommended Reading. Chapter-heads. Electronic Funds Transfer) Contents PAGE
 Contents Foreword Recommended Reading Syllabus Chapter-heads iii v vii ix MODULE I : Technology in bank CHAPTER 1 Banking Environment and Technology u Introduction 3 u Evolution of Banking Technology over
Contents Foreword Recommended Reading Syllabus Chapter-heads iii v vii ix MODULE I : Technology in bank CHAPTER 1 Banking Environment and Technology u Introduction 3 u Evolution of Banking Technology over
Existing Healthcare Standards
 Existing Healthcare Standards Category Context (Information Model) Information Interchange Standard & Specific Elements ASN.1 Abstract Syntax Notation.1 ASTM E2369-05 Standard Specification for Continuity
Existing Healthcare Standards Category Context (Information Model) Information Interchange Standard & Specific Elements ASN.1 Abstract Syntax Notation.1 ASTM E2369-05 Standard Specification for Continuity
epsos Semantic Interoperability Semantic Days 2010 June 1 st, 2010 Stavanger, Norway
 epsos Semantic Interoperability ANA ESTELRICH,, ASIP Santé, epsos WP3.5 leader Semantic Days 2010 June 1 st, 2010 Stavanger, Norway epsos Europe wide large scale pilot on cross border interchange of patient
epsos Semantic Interoperability ANA ESTELRICH,, ASIP Santé, epsos WP3.5 leader Semantic Days 2010 June 1 st, 2010 Stavanger, Norway epsos Europe wide large scale pilot on cross border interchange of patient
M2 Glossary of Terms and Abbreviations
 M2 Glossary of Terms and Abbreviations 11 June 2015 M2: Electronic Standards for the Transfer of Regulatory Information Updated at ICH Expert Working Group meeting, Fukuoka, June 2015 Definitions... 2
M2 Glossary of Terms and Abbreviations 11 June 2015 M2: Electronic Standards for the Transfer of Regulatory Information Updated at ICH Expert Working Group meeting, Fukuoka, June 2015 Definitions... 2
Gramm Richardson - U.S. Department of Defense Elli Schwarz - SRA International, Inc.
 Gramm Richardson - U.S. Department of Defense Elli Schwarz - SRA International, Inc. 30 May, 2012 ISO/IEC 11179-3 Metamodel OWL Ontology 58 Classes 74 Datatype properties 113 Object properties Inspired
Gramm Richardson - U.S. Department of Defense Elli Schwarz - SRA International, Inc. 30 May, 2012 ISO/IEC 11179-3 Metamodel OWL Ontology 58 Classes 74 Datatype properties 113 Object properties Inspired
The SHARPn phenotyping funnel. Phenotype specific patient cohorts
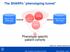 The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
Paper DS07 PhUSE 2017 CDISC Transport Standards - A Glance. Giri Balasubramanian, PRA Health Sciences Edwin Ponraj Thangarajan, PRA Health Sciences
 Paper DS07 PhUSE 2017 CDISC Transport Standards - A Glance Giri Balasubramanian, PRA Health Sciences Edwin Ponraj Thangarajan, PRA Health Sciences Agenda Paper Abstract CDISC Standards Types Why Transport
Paper DS07 PhUSE 2017 CDISC Transport Standards - A Glance Giri Balasubramanian, PRA Health Sciences Edwin Ponraj Thangarajan, PRA Health Sciences Agenda Paper Abstract CDISC Standards Types Why Transport
BMEGUI Tutorial 1 Spatial kriging
 BMEGUI Tutorial 1 Spatial kriging 1. Objective The primary objective of this exercise is to get used to the basic operations of BMEGUI using a purely spatial dataset. The analysis will consist in an exploratory
BMEGUI Tutorial 1 Spatial kriging 1. Objective The primary objective of this exercise is to get used to the basic operations of BMEGUI using a purely spatial dataset. The analysis will consist in an exploratory
"Charting the Course... Agile Database Design Techniques Course Summary
 Course Summary Description This course provides students with the skills necessary to design databases using Agile design techniques. It is based on the Scott Ambler book Agile Database Techniques: Effective
Course Summary Description This course provides students with the skills necessary to design databases using Agile design techniques. It is based on the Scott Ambler book Agile Database Techniques: Effective
Getting Started with Clinical Quality Language: Technical Implementation for Vendors
 Getting Started with Clinical Quality Language: Technical Implementation for Vendors Bryn Rhodes, ESAC James Bradley, The MITRE Corporation March 2018 The MITRE Corporation operates the Centers for Medicare
Getting Started with Clinical Quality Language: Technical Implementation for Vendors Bryn Rhodes, ESAC James Bradley, The MITRE Corporation March 2018 The MITRE Corporation operates the Centers for Medicare
Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata
 Paper SD02 Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata Dr. Philippe Verplancke, XClinical GmbH, Munich, Germany ABSTRACT define.xml is often created at the end of
Paper SD02 Study Composer: a CRF design tool enabling the re-use of CDISC define.xml metadata Dr. Philippe Verplancke, XClinical GmbH, Munich, Germany ABSTRACT define.xml is often created at the end of
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR
 Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeax 2, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester, MN, 55905, USA 2
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeax 2, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester, MN, 55905, USA 2
Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research
 Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research Ian Fore, D.Phil. Associate Director, Biorepository and Pathology Informatics Senior Program
Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research Ian Fore, D.Phil. Associate Director, Biorepository and Pathology Informatics Senior Program
Remote Access Guide. https://remote.lghealth.org
 Remote Access Guide https://remote.lghealth.org Created by: Joshua Steele Revision 1.0 7/14/2015 Table of Contents I. Remote Access using Internet Explorer... 2 II. Remote Access using Google Chrome...
Remote Access Guide https://remote.lghealth.org Created by: Joshua Steele Revision 1.0 7/14/2015 Table of Contents I. Remote Access using Internet Explorer... 2 II. Remote Access using Google Chrome...
Acquiring Experience with Ontology and Vocabularies
 Acquiring Experience with Ontology and Vocabularies Walt Melo Risa Mayan Jean Stanford The author's affiliation with The MITRE Corporation is provided for identification purposes only, and is not intended
Acquiring Experience with Ontology and Vocabularies Walt Melo Risa Mayan Jean Stanford The author's affiliation with The MITRE Corporation is provided for identification purposes only, and is not intended
CITY UNIVERSITY OF NEW YORK. i. Visit:
 CITY UNIVERSITY OF NEW YORK I. ACCESSING IRB NET (New Registration) i. Visit: https://www.irbnet.org/release/index.html ii. New users: Click on New Registration in the top right corner iii. Fill-out the
CITY UNIVERSITY OF NEW YORK I. ACCESSING IRB NET (New Registration) i. Visit: https://www.irbnet.org/release/index.html ii. New users: Click on New Registration in the top right corner iii. Fill-out the
Introduction to PTC Windchill MPMLink 11.0
 Introduction to PTC Windchill MPMLink 11.0 Overview Course Code Course Length TRN-4754-T 16 Hours In this course, you will learn how to complete basic Windchill MPMLink functions. You will learn about
Introduction to PTC Windchill MPMLink 11.0 Overview Course Code Course Length TRN-4754-T 16 Hours In this course, you will learn how to complete basic Windchill MPMLink functions. You will learn about
Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences, Chennai, India
 Paper CD15 PhUSE 2016 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences,
Paper CD15 PhUSE 2016 How to handle different versions of SDTM & DEFINE generation in a Single Study? Edwin Ponraj Thangarajan, PRA Health Sciences, Chennai, India Giri Balasubramanian, PRA Health Sciences,
Information technology Metadata registries (MDR)
 INTERNATIONAL STANDARD ISO/IEC 11179-3:2003 TECHNICAL CORRIGENDUM 1 Published 2004-04-01 INTERNATIONAL ORGANIZATION FOR STANDARDIZATION МЕЖДУНАРОДНАЯ ОРГАНИЗАЦИЯ ПО СТАНДАРТИЗАЦИИ ORGANISATION INTERNATIONALE
INTERNATIONAL STANDARD ISO/IEC 11179-3:2003 TECHNICAL CORRIGENDUM 1 Published 2004-04-01 INTERNATIONAL ORGANIZATION FOR STANDARDIZATION МЕЖДУНАРОДНАЯ ОРГАНИЗАЦИЯ ПО СТАНДАРТИЗАЦИИ ORGANISATION INTERNATIONALE
Copyright protected. Use is for Single Users only via a VHP Approved License. For information and printed versions please see
 TOGAF 9 Certified Study Guide 4th Edition The Open Group Publications available from Van Haren Publishing The TOGAF Series: The TOGAF Standard, Version 9.2 The TOGAF Standard Version 9.2 A Pocket Guide
TOGAF 9 Certified Study Guide 4th Edition The Open Group Publications available from Van Haren Publishing The TOGAF Series: The TOGAF Standard, Version 9.2 The TOGAF Standard Version 9.2 A Pocket Guide
ISO/IEC TR TECHNICAL REPORT. Information technology Procedures for achieving metadata registry (MDR) content consistency Part 1: Data elements
 TECHNICAL REPORT ISO/IEC TR 20943-1 First edition 2003-08-01 Information technology Procedures for achieving metadata registry (MDR) content consistency Part 1: Data elements Technologies de l'information
TECHNICAL REPORT ISO/IEC TR 20943-1 First edition 2003-08-01 Information technology Procedures for achieving metadata registry (MDR) content consistency Part 1: Data elements Technologies de l'information
TABLE OF CONTENTS CHAPTER NO. TITLE PAGE NO. ABSTRACT 5 LIST OF TABLES LIST OF FIGURES LIST OF SYMBOLS AND ABBREVIATIONS xxi
 ix TABLE OF CONTENTS CHAPTER NO. TITLE PAGE NO. ABSTRACT 5 LIST OF TABLES xv LIST OF FIGURES xviii LIST OF SYMBOLS AND ABBREVIATIONS xxi 1 INTRODUCTION 1 1.1 INTRODUCTION 1 1.2 WEB CACHING 2 1.2.1 Classification
ix TABLE OF CONTENTS CHAPTER NO. TITLE PAGE NO. ABSTRACT 5 LIST OF TABLES xv LIST OF FIGURES xviii LIST OF SYMBOLS AND ABBREVIATIONS xxi 1 INTRODUCTION 1 1.1 INTRODUCTION 1 1.2 WEB CACHING 2 1.2.1 Classification
R1 Test Case that tests this Requirement Comments Manage Users User Role Management
 2/19/2014 CDISC SHARE Requirements Page 1 of 23 Number Name Req ID Requirement Manage Users 2.1.1 User Role Manage Users 2.1.1 User Role Manage Users 2.1.1 User Role Manage Users 2.1.1 User Role Manage
2/19/2014 CDISC SHARE Requirements Page 1 of 23 Number Name Req ID Requirement Manage Users 2.1.1 User Role Manage Users 2.1.1 User Role Manage Users 2.1.1 User Role Manage Users 2.1.1 User Role Manage
Standards Development
 Thailand s ehealth & Health Information Standards Development Collaborating across countries to harmonize information system standards understanding country opportunities and developing strategies to deal
Thailand s ehealth & Health Information Standards Development Collaborating across countries to harmonize information system standards understanding country opportunities and developing strategies to deal
Course Outline. ProTech Professional Technical Services, Inc. Veritas Backup Exec 20.1: Administration. Course Summary.
 Course Summary Description The course is designed for the data protection professional tasked with architecting, implementing, backing up, and restoring critical data. This class covers how to back up
Course Summary Description The course is designed for the data protection professional tasked with architecting, implementing, backing up, and restoring critical data. This class covers how to back up
Excel Programming with VBA (Macro Programming) 24 hours Getting Started
 Excel Programming with VBA (Macro Programming) 24 hours Getting Started Introducing Visual Basic for Applications Displaying the Developer Tab in the Ribbon Recording a Macro Saving a Macro-Enabled Workbook
Excel Programming with VBA (Macro Programming) 24 hours Getting Started Introducing Visual Basic for Applications Displaying the Developer Tab in the Ribbon Recording a Macro Saving a Macro-Enabled Workbook
Administrator Guide. Oracle Health Sciences Central Designer 2.0. Part Number: E
 Administrator Guide Oracle Health Sciences Central Designer 2.0 Part Number: E37912-01 Copyright 2013, Oracle and/or its affiliates. All rights reserved. The Programs (which include both the software and
Administrator Guide Oracle Health Sciences Central Designer 2.0 Part Number: E37912-01 Copyright 2013, Oracle and/or its affiliates. All rights reserved. The Programs (which include both the software and
Supporting Patient Screening to Identify Suitable Clinical Trials
 Supporting Patient Screening to Identify Suitable Clinical Trials Anca BUCUR a,1, Jasper VAN LEEUWEN a, Njin-Zu CHEN a, Brecht CLAERHOUT b Kristof DE SCHEPPER b, David PEREZ-REY c, Raul ALONSO-CALVO c,
Supporting Patient Screening to Identify Suitable Clinical Trials Anca BUCUR a,1, Jasper VAN LEEUWEN a, Njin-Zu CHEN a, Brecht CLAERHOUT b Kristof DE SCHEPPER b, David PEREZ-REY c, Raul ALONSO-CALVO c,
Trials Units Information Systems. Data Standards EXECUTIVE SUMMARY. Data and Information Systems Project The DIMS Project Team
 Trials Units Information Systems Data Standards EXECUTIVE SUMMARY Data and Information Systems Project The DIMS Project Team Trials Units IS Data Standards (ES) Introduction 1 One of the deliverables of
Trials Units Information Systems Data Standards EXECUTIVE SUMMARY Data and Information Systems Project The DIMS Project Team Trials Units IS Data Standards (ES) Introduction 1 One of the deliverables of
Taming Rave: How to control data collection standards?
 Paper DH08 Taming Rave: How to control data collection standards? Dimitri Kutsenko, Entimo AG, Berlin, Germany Table of Contents Introduction... 1 How to organize metadata... 2 How to structure metadata...
Paper DH08 Taming Rave: How to control data collection standards? Dimitri Kutsenko, Entimo AG, Berlin, Germany Table of Contents Introduction... 1 How to organize metadata... 2 How to structure metadata...
Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies
 Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies Harold R. Solbrig 1, Guoqian Jiang 1 1 Mayo Clinic College of Medicine, Rochester, MN [solbrig.harold,
Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies Harold R. Solbrig 1, Guoqian Jiang 1 1 Mayo Clinic College of Medicine, Rochester, MN [solbrig.harold,
European Conference on Quality and Methodology in Official Statistics (Q2008), 8-11, July, 2008, Rome - Italy
 European Conference on Quality and Methodology in Official Statistics (Q2008), 8-11, July, 2008, Rome - Italy Metadata Life Cycle Statistics Portugal Isabel Morgado Methodology and Information Systems
European Conference on Quality and Methodology in Official Statistics (Q2008), 8-11, July, 2008, Rome - Italy Metadata Life Cycle Statistics Portugal Isabel Morgado Methodology and Information Systems
Semantic Technologies and CDISC Standards. Frederik Malfait, Information Architect, IMOS Consulting Scott Bahlavooni, Independent
 Semantic Technologies and CDISC Standards Frederik Malfait, Information Architect, IMOS Consulting Scott Bahlavooni, Independent Part I Introduction to Semantic Technology Resource Description Framework
Semantic Technologies and CDISC Standards Frederik Malfait, Information Architect, IMOS Consulting Scott Bahlavooni, Independent Part I Introduction to Semantic Technology Resource Description Framework
Organizational Track 1 p.m.-2 p.m. Maintenance and Updating Margo Imel, RHIT, MBA Terminology ManagerMapping, SNOMED, International Pat Wilson, RT
 Organizational Track 1 p.m.-2 p.m. Maintenance and Updating Margo Imel, RHIT, MBA Terminology ManagerMapping, SNOMED, International Pat Wilson, RT (R), CPC, Team Lead Health Data Dictionary 3M SNOMED CT
Organizational Track 1 p.m.-2 p.m. Maintenance and Updating Margo Imel, RHIT, MBA Terminology ManagerMapping, SNOMED, International Pat Wilson, RT (R), CPC, Team Lead Health Data Dictionary 3M SNOMED CT
From ODM to SDTM: An End-to-End Approach Applied to Phase I Clinical Trials
 PhUSE 2014 Paper PP05 From ODM to SDTM: An End-to-End Approach Applied to Phase I Clinical Trials Alexandre Mathis, Department of Clinical Pharmacology, Actelion Pharmaceuticals Ltd., Allschwil, Switzerland
PhUSE 2014 Paper PP05 From ODM to SDTM: An End-to-End Approach Applied to Phase I Clinical Trials Alexandre Mathis, Department of Clinical Pharmacology, Actelion Pharmaceuticals Ltd., Allschwil, Switzerland
From Integration to Interoperability: The Role of Public Health Systems in the Emerging World of Health Information Exchange
 From Integration to Interoperability: The Role of Public Health Systems in the Emerging World of Health Information Exchange Noam H. Arzt, PhD American Public Health Association Annual Meeting Session
From Integration to Interoperability: The Role of Public Health Systems in the Emerging World of Health Information Exchange Noam H. Arzt, PhD American Public Health Association Annual Meeting Session
Data Quality for Semantic Interoperable Electronic Health Records
 Data Quality for Semantic Interoperable Electronic Health Records Shivani Batra & Shelly Sachdeva Jaypee Institute of Information Technology University, Sector-128, Noida, India Workshop on Quality in
Data Quality for Semantic Interoperable Electronic Health Records Shivani Batra & Shelly Sachdeva Jaypee Institute of Information Technology University, Sector-128, Noida, India Workshop on Quality in
Controlled Medical Vocabulary in the CPR Generations
 Tutorials, B. Hieb, M.D. Research Note 5 November 2003 Controlled Medical Vocabulary in the CPR Generations A CMV capability becomes progressively more important as computer-based patient record systems
Tutorials, B. Hieb, M.D. Research Note 5 November 2003 Controlled Medical Vocabulary in the CPR Generations A CMV capability becomes progressively more important as computer-based patient record systems
24/18/2014 ICAAP 11 Bangkok, Thailand 22 November 2013
 Implementing WHO ehealth standards with peer support through the Asia ehealth Information Network Mr Mark Landry Team Leader Health Information, Evidence & Research World Health Organization Western Pacific
Implementing WHO ehealth standards with peer support through the Asia ehealth Information Network Mr Mark Landry Team Leader Health Information, Evidence & Research World Health Organization Western Pacific
CDISC Public Webinar Standards Updates and Additions. 26 Feb 2015
 CDISC Public Webinar Standards Updates and Additions 26 Feb 2015 CDISC 2014 Agenda SHARE Research Concepts Julie Evans, CDISC Anthony Chow, CDISC Rene Dahlheimer, CDISC Sam Hume, CDISC CDISC Education
CDISC Public Webinar Standards Updates and Additions 26 Feb 2015 CDISC 2014 Agenda SHARE Research Concepts Julie Evans, CDISC Anthony Chow, CDISC Rene Dahlheimer, CDISC Sam Hume, CDISC CDISC Education
METADATA REGISTRY, ISO/IEC 11179
 LLNL-JRNL-400269 METADATA REGISTRY, ISO/IEC 11179 R. K. Pon, D. J. Buttler January 7, 2008 Encyclopedia of Database Systems Disclaimer This document was prepared as an account of work sponsored by an agency
LLNL-JRNL-400269 METADATA REGISTRY, ISO/IEC 11179 R. K. Pon, D. J. Buttler January 7, 2008 Encyclopedia of Database Systems Disclaimer This document was prepared as an account of work sponsored by an agency
The Estonian ehealth experience strategy and results. Piret Simmo Estonian ehealth Foundation Standardization manager
 The Estonian ehealth experience strategy and results Piret Simmo Estonian ehealth Foundation Standardization manager 27.09.2011 Agenda Some history Legal environment ehealth Foundation Estonian central
The Estonian ehealth experience strategy and results Piret Simmo Estonian ehealth Foundation Standardization manager 27.09.2011 Agenda Some history Legal environment ehealth Foundation Estonian central
HEDIS 2018 Volume 2 Value Set Directory User Manual Version
 HEDIS 2018 Volume 2 Value Set Directory User Manual 2017-07-03 Version This HEDIS Volume 2 Value Set Directory (VSD) was developed by and is owned by the National Committee for Quality Assurance ( NCQA
HEDIS 2018 Volume 2 Value Set Directory User Manual 2017-07-03 Version This HEDIS Volume 2 Value Set Directory (VSD) was developed by and is owned by the National Committee for Quality Assurance ( NCQA
Note: Basic understanding of the CDISC ODM structure of Events, Forms, ItemGroups, Items, Codelists and MeasurementUnits is required.
 Paper CC-018 Exploring SAS PROC CDISC Model=ODM and Its Undocumented Parameters Elena Valkanova, Biostat International, Inc, Tampa, FL Irene Droll, XClinical GmbH, München, Germany ABSTRACT The CDISC Operational
Paper CC-018 Exploring SAS PROC CDISC Model=ODM and Its Undocumented Parameters Elena Valkanova, Biostat International, Inc, Tampa, FL Irene Droll, XClinical GmbH, München, Germany ABSTRACT The CDISC Operational
Overview Functionality Maintenance Future
 LEXICON Overview Functionality Maintenance Future OVERVIEW What is Lexicon Standard reference for terminology across VA Provides a basis for a common language of terminology so that all members of a healthcare
LEXICON Overview Functionality Maintenance Future OVERVIEW What is Lexicon Standard reference for terminology across VA Provides a basis for a common language of terminology so that all members of a healthcare
CROSS-REFERENCE TABLE ASME A Including A17.1a-1997 Through A17.1d 2000 vs. ASME A
 CROSS-REFERENCE TABLE ASME Including A17.1a-1997 Through A17.1d 2000 vs. ASME 1 1.1 1.1 1.1.1 1.2 1.1.2 1.3 1.1.3 1.4 1.1.4 2 1.2 3 1.3 4 Part 9 100 2.1 100.1 2.1.1 100.1a 2.1.1.1 100.1b 2.1.1.2 100.1c
CROSS-REFERENCE TABLE ASME Including A17.1a-1997 Through A17.1d 2000 vs. ASME 1 1.1 1.1 1.1.1 1.2 1.1.2 1.3 1.1.3 1.4 1.1.4 2 1.2 3 1.3 4 Part 9 100 2.1 100.1 2.1.1 100.1a 2.1.1.1 100.1b 2.1.1.2 100.1c
Editor s Draft. Outcome of Berlin Meeting ISO/IEC JTC 1/SC32 WG2 N1669 ISO/IEC CD :ED2
 ISO/IEC JTC 1/SC32 WG2 N1669 2012-06 ISO/IEC CD19763-1:ED2 ISO/IEC JTC 1/SC 32/WG 2 Secretariat: Information Technology Metamodel framework for interoperability (MFI) Part 1: Reference model, Second Edition
ISO/IEC JTC 1/SC32 WG2 N1669 2012-06 ISO/IEC CD19763-1:ED2 ISO/IEC JTC 1/SC 32/WG 2 Secretariat: Information Technology Metamodel framework for interoperability (MFI) Part 1: Reference model, Second Edition
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR
 Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeaux 2, Guohui Xiao 3, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester,
Developing A Semantic Web-based Framework for Executing the Clinical Quality Language Using FHIR Guoqian Jiang 1, Eric Prud Hommeaux 2, Guohui Xiao 3, and Harold R. Solbrig 1 1 Mayo Clinic, Rochester,
CG Transformer Division Laboratories, Crompton Greaves Ltd., Kanjurmarg (East), Mumbai, Maharashtra
 Last Amended on - Page 1 of 9 I. ELECTRICAL MATERIALS 1. Magnetic Materials a. Electrical Steel i) Surface Insulation Resistivity ASTM 717- (1995), IEC 60404-11(1996) IS 649-(1997) ii) Specific core loss
Last Amended on - Page 1 of 9 I. ELECTRICAL MATERIALS 1. Magnetic Materials a. Electrical Steel i) Surface Insulation Resistivity ASTM 717- (1995), IEC 60404-11(1996) IS 649-(1997) ii) Specific core loss
