Welcome to the Research Navigator IBC learning module for NYULMC users
|
|
|
- Barrie Booker
- 5 years ago
- Views:
Transcription
1 Welcome to the Research Navigator Institutional Biosafety Committee (IBC) Learning Module - Creation and Submission of Modifications Required for IBC approval OSR OFFICE OFFICE OF OFSCIENCE & RESEARCH
2 Welcome to the Research Navigator IBC learning module for NYULMC users In this module, you will learn how to create and submit IBC required modifications to secure approval in order to conduct research involving: recombinant DNA (rdna), infectious agents, non-human primate materials (including established cell lines), select agents or human gene transfer studies conducted at New York University and within the NYU Langone Medical Center. This request is known as a registration. Registrations are reviewed by the New York University Institutional Biosafety Committee. The IBC is responsible for providing review and oversight of these studies to ensure compliance with the NIH Guidelines and all of the Institution's policies. Registrations are submitted to the IBC through the online Research Navigator IBC module.
3 Washington Square/Dental School Users Please note that in addition to your NYU NET ID, a NYU Langone Medical Center Kerberos ID (KID) and password is required to login Navigator/IBC. Refer to the Welcome to Research Navigator Institutional Biosafety Committee (IBC) Learning Module Access from outside of the NYU Langone Medical Center training PowerPoint Presentation for specific instructions on how to obtain a KID.
4 Required training for IBC submissions. Environmental Health and Safety has developed a training course required for anyone submitting or participating in studies registered with the NYULMC IBC including those involving human gene transfer. This course entitled EH&S Recombinant DNA: Use and Safe Handling is located on idevelop. *** Washington Square/Dental School Users should contact Kechia.hester@nyu.edu for access to the rdna training course. The NIH s Office of Biotechnology Affairs (OBA) has issued citations to many institutions for insufficient training of rdna users on the NIH s rdna Guidelines. To satisfy this requirement, training is required once every three years. If you plan to submit a registration to the IBC, you are advised to take this short 10 minute course prior to submission. If you or your study staff have not successfully completed the course at the time of IBC submission, you will be reminded of the requirement prior to IBC approval. Contact mark.olmsted@nyumc.org in Environmental Health & Safety if you have any questions related to the training course.
5 Objectives After reviewing this PowerPoint Presentation, you will be able to: Create and submit IBC required modifications to a registration for research involving : rdna Other non-human gene transfer (HGT) studies
6 Log on to Research Navigator Enter your Kerberos ID and Password
7 Go to the IBC Module Select View My IBC Registrations
8 PI s Inbox Submitted registration that requires modification Notice that the state is modifications pending After logging in to Research Navigator IBC, select the registration in your Inbox that requires modification.
9 Selecting the registration link Current status Select to edit registration Select to IBC Select to attach documents such as rdna training certificate Select to view correspondence from IBC Select to submit changes to IBC after modifications have been made.
10 Viewing the correspondence letter Select this link to view letter from IBC. The letter lists the modifications required
11 Editing the registration form Select to edit the registration form and make any modifications that the IBC requires. After you have made the required modifications, be sure to select Finish at the bottom right corner of the registration form. Select the appropriate link to add supporting documents if required. Another box will open. Select the add button. Select the file on your computer then select ok to add.
12 Submitting the changes If you need to send an to the IBC Administrator, select this link. Once you ve completed all changes to the registration form, it is time to resubmit to the IBC. Be sure to select Submit Changes so the registration will be returned to the IBC. If any additional information is required, you will be notified. Be sure to check your Research Navigator IBC Inbox.
13 Saving your information As you create the new registration, use the Continue button to navigate directly to a particular section Selecting the Continue button automatically saves Selecting the Back button will NOT automatically save Select Save at the top or bottom of the form to ensure your data is saved
14 Saving your information Selecting the back button will NOT automatically save. Use the continue button to navigate directly to a particular section. Data is automatically saved. Select Save at the top or bottom to ensure your data is saved
15 IBC registration submission process PI Assurance After the PI selects submit application, a window will appear requiring the PI to attest the information submitted is accurate and complete. *If you ve added personnel, they will be required to Agree to Participate and you must attach their rdna course completion certificates (see Creating and Submitting PowerPoint training presentation available on the IBC website)*
16 IBC registration submission process Errors If there are errors on the registration document, they will appear after the PI agrees with the Assurance statement. These errors must be corrected in order to submit the registration.
17 After submission to the IBC After submission, the status changes to IBC Administrator Review. If there are any modifications needed, you will receive an from the IBC Administrator.
18 IBC registration submission process Successful Submission The PI will receive an automatic from indicating successful submission. After the registration has been submitted, information on the review status is located under the IBC Applications tab on your Home Folder page. Refer to this section frequently for information and any actions that you may be required to take to complete the review process. As you can see from this example, the registration is in the IBC Administrator Review state.
19 IBC registration submission process outcome of IBC review After review by the IBC, registrations are either: Approved or Modifications are required to the registration before approval - If the modifications are satisfactory to the IBC, the registration is approved.
20 Keys to Success Complete the IBC registration accurately and completely. Be sure to attach any documents as indicated on the registration. Ensure all study staff agree to participate and the rdna course completion certificates for the PI and study staff are attached. Submit the IBC registration prior to the submission deadlines posted on the IBC website. Frequently monitor your Research Navigator IBC inbox
21 Slide Title IBC Contact information IBC website: Text IBC address: IBC Director Natalie L. Mays Research Navigator information Research Navigator IBC module: If you experience any problems using Research Navigator IBC, please report the issue by opening a ticket online using MCIT Support & Services or by contacting the MCIT Help Desk at (x36868 Internal) or (toll free).
CREATION AND SUBMISSION OF AN ANNUAL CONTINUATION
 CREATION AND SUBMISSION OF AN ANNUAL CONTINUATION Research Navigator Institutional Biosafety Committee (IBC) Welcome to the Research Navigator IBC Learning Module for NYULMC Users In this module, you will
CREATION AND SUBMISSION OF AN ANNUAL CONTINUATION Research Navigator Institutional Biosafety Committee (IBC) Welcome to the Research Navigator IBC Learning Module for NYULMC Users In this module, you will
Welcome to the Research Navigator IBC learning module for users outside of NYU Langone Health
 WELCOME TO THE RESEARCH NAVIGATOR INSTITUTIONAL BIOSAFETY COMMITTEE (IBC) CREATION AND SUBMISSION OF NON-HUMAN GENE TRANSFER REGISTRATIONS LEARNING MODULE Welcome to the Research Navigator IBC learning
WELCOME TO THE RESEARCH NAVIGATOR INSTITUTIONAL BIOSAFETY COMMITTEE (IBC) CREATION AND SUBMISSION OF NON-HUMAN GENE TRANSFER REGISTRATIONS LEARNING MODULE Welcome to the Research Navigator IBC learning
eresearch IBC Staff Review Step-by-Step Procedures Core Committee Staff Home Page
 1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
1 Core Committee Staff Home Page 2 1. Verify Core Committee Staff is displayed on the home workspace. 2. Note that the Inbox is active. 3. Click the Name of the Registration to be reviewed. 3 4 6 5 4.
Safe Operating Procedure
 Safe Operating Procedure (Revised 2/10) NUgrant IBC PROTOCOL FORM INSTRUCTIONS (For assistance please contact EHS at (402) 472-4925 or visit our web site at http://ehs.unl.edu/) Contents Introduction...
Safe Operating Procedure (Revised 2/10) NUgrant IBC PROTOCOL FORM INSTRUCTIONS (For assistance please contact EHS at (402) 472-4925 or visit our web site at http://ehs.unl.edu/) Contents Introduction...
Logging In (Note you must be on campus or using a VPN connection)
 Logging In (Note you must be on campus or using a VPN connection) esirius is supported in Chrome and Firefox. There are still some minor issues with Firefox so we advise you to use Chrome The Chrome Icon
Logging In (Note you must be on campus or using a VPN connection) esirius is supported in Chrome and Firefox. There are still some minor issues with Firefox so we advise you to use Chrome The Chrome Icon
eibc Program User Guide
 eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eibc Program User Guide The University Of Iowa Environmental Health & Safety 122 Grand Avenue Court Iowa City, IA 52242-1000 Phone: 319-335-8501 Date Revised/Reviewed: 3/26/2018 Table of Contents 1. eibc
eirb User Manual for Research Staff
 eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
eirb User Manual for Research Staff Version 4 1 10.2016 Table of Contents eirb Access... 3 Log-in to eirb... 4 Overview of the eirb Submission and Review Process... 7 Create a New Application and Specify
2017 Gorman Certification
 Important things to know before beginning: 2017 Gorman Certification Please follow the steps provided. They will help you navigate through the process. If you encounter any errors after ensuring you followed
Important things to know before beginning: 2017 Gorman Certification Please follow the steps provided. They will help you navigate through the process. If you encounter any errors after ensuring you followed
ARC IRB Chair s Manual
 ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
ARC IRB Chair s Manual This guide serves to aid an IRB Chair in becoming familiar with the basic functions of the ARC system and how to review and approve an application. ARC IRB Chairs Manual Page 2 Table
IRBNet User Manual. University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB)
 University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
University of Denver Human Research Protection Program (HRPP) Institutional Review Board (IRB) IRBNet User Manual Office of Research Integrity and Education Mary Reed Building 222 INTRODUCTION The Office
istar User Reference Guide
 istar User Reference Guide Last Updated: 12/21/17 Table of Contents Questions and Issues 1 Getting Started 2 Dashboard 2 New Applications 4 Main Committee Page 5 Main Workspace page 6 Edit/View Application
istar User Reference Guide Last Updated: 12/21/17 Table of Contents Questions and Issues 1 Getting Started 2 Dashboard 2 New Applications 4 Main Committee Page 5 Main Workspace page 6 Edit/View Application
The most current protocol information should be entered. This includes incorporation of all past amendments and modifications.
 Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
Georgetown University Institutional Review Board Frequently Asked Questions (FAQs) about eric 1. What is eric? eric stands for Electronic Research and Institutional Compliance. This is an on-line IRB application
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803)
 eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
eirb Electronic Institutional Review Board User Guide For more information contact: Research Administration (803) 434 4899 Developed August 2008 by Research Development University of South Carolina 1 Introduction
Physician Time Study System. User Guide for Supporting Staff
 Physician Time Study System User Guide for Supporting Staff 1 Table of Contents 1. Introduction... 3 2. About your Support Staff Account... 3 3. Getting Started... 4 4. Your Main Navigation Area... 5 5.
Physician Time Study System User Guide for Supporting Staff 1 Table of Contents 1. Introduction... 3 2. About your Support Staff Account... 3 3. Getting Started... 4 4. Your Main Navigation Area... 5 5.
MSEDCL e-tendering Help-Contractor. MSEDCL e-tendering Contractor s Guide
 MSEDCL e-tendering Contractor s Guide 1 Table Of Contents Getting Started... 3 Getting Started... 3 Understanding MSEDCL e-tendering System... 3 Logging in... 4 Messages... 5 Sending Reply... 5 Tenders...
MSEDCL e-tendering Contractor s Guide 1 Table Of Contents Getting Started... 3 Getting Started... 3 Understanding MSEDCL e-tendering System... 3 Logging in... 4 Messages... 5 Sending Reply... 5 Tenders...
You may also scroll through the Table of Contents and click on the topic or question of interest.
 We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
We have posted our IRB process related FAQs in a searchable PDF format. You may search by using the CTRL/F key combination. Just type the search word in the box that appears. You may also scroll through
IRBNet Instructions for IBC, SCRO, IACUC Committees v
 Stony Brook University IRBNet Instructions for IBC, SCRO, IACUC Committees v. 04.23.14 Introduction: This document will explain, step by step, how to upload the required forms and documents you need to
Stony Brook University IRBNet Instructions for IBC, SCRO, IACUC Committees v. 04.23.14 Introduction: This document will explain, step by step, how to upload the required forms and documents you need to
Register by completing the form, or connecting via your GitHub or Google account.
 SDL Developer Portal Registration Guide In order to register an application on the SDL developer portal, you must first create both a developer and company profile. Developer Profile Registration To create
SDL Developer Portal Registration Guide In order to register an application on the SDL developer portal, you must first create both a developer and company profile. Developer Profile Registration To create
Animal Protocol Development Instructions for Researchers
 Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Animal Protocol Development Instructions for Researchers OFFICE OF THE VICE PRESIDENT FOR RESEARCH TOPAZ Elements - Protocol Development Instructions for Researchers Page 1 Topaz Elements Animal Protocol
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE
 Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Office of Research Integrity / Office of Sponsored Projects IACUC PROTOCOL SUBMISSION GUIDE Coeus Lite 4.5.1.Px Document Version 3 Updated May 2016 Table of Contents ABOUT COEUS... 3 o About the Coeus
Nova Scotia Health Authority Research Ethics Board. Researcher s (PI) User Manual
 Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
Nova Scotia Health Authority Research Ethics Board Researcher s (PI) User Manual The Researcher s Portal is available through the Login at the following URL: http://nsha-iwk.researchservicesoffice.com/romeo.researcher/login.aspx
University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Record Creation for Principal Investigators
 University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Record Creation for Principal Investigators WISPER - Record Creation for Principal Investigators Once a Pre-Proposal has been submitted
University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Record Creation for Principal Investigators WISPER - Record Creation for Principal Investigators Once a Pre-Proposal has been submitted
SUBMITTING AN IBC AMENDMENT
 The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
The submission of an amendment/modification to an approved IBC protocol requires the creation of a subsequent PACKAGE in a project. After an IBC application is approved, the project may require modifications
Introduction to webirb
 Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Introduction to webirb Training Course for Investigators and Study Staff V: 01.24.13 0 You will learn to 1. Navigate webirb 2. Create a new Study application 3. Respond to IRB Requests 4. Create an Amendment
Minor Volunteer Risk Management (RMA) Instructions
 All Washington Youth Soccer volunteers must register online to complete a national background check clearance. If you have participated in Founders Cup, Challenge Cup or Championships in the past 2 years,
All Washington Youth Soccer volunteers must register online to complete a national background check clearance. If you have participated in Founders Cup, Challenge Cup or Championships in the past 2 years,
Hello, and welcome to the presentation NHSN Enrollment Instructions for Long Term Care Facilities.
 Hello, and welcome to the presentation NHSN Enrollment Instructions for Long Term Care Facilities. This presentation will provide long term care facilities with instructions for NHSN enrollment. My name
Hello, and welcome to the presentation NHSN Enrollment Instructions for Long Term Care Facilities. This presentation will provide long term care facilities with instructions for NHSN enrollment. My name
NonProfit User Guide
 . NonProfit User Guide APRIL 5, 2018 BERMUDA COMMUNITY FOUNDATION 16 Wesley Street, Fourth Floor, Hamilton HM 11, Bermuda Contents Welcome to GiveBermuda.org...2 Registration/Creating an Account...2 Logging
. NonProfit User Guide APRIL 5, 2018 BERMUDA COMMUNITY FOUNDATION 16 Wesley Street, Fourth Floor, Hamilton HM 11, Bermuda Contents Welcome to GiveBermuda.org...2 Registration/Creating an Account...2 Logging
COVENTRY MEDICARE CERTIFICATION TRAINING CENTER
 1/1/2012 COVENTRY MEDICARE CERTIFICATION TRAINING CENTER 0 P a g e User Guide Coventry Medicare Certification Training Center User Guide Table of Contents Getting Started: Log In and User Registration...
1/1/2012 COVENTRY MEDICARE CERTIFICATION TRAINING CENTER 0 P a g e User Guide Coventry Medicare Certification Training Center User Guide Table of Contents Getting Started: Log In and User Registration...
Gateway Health Producer Onboarding Guide. Annual Recertification Process for Brokers
 Gateway Health Producer Onboarding Guide Annual Recertification Process for Brokers Table of Contents Contents Getting Started 1 Create Your Profile 2 Complete Your Onboarding Forms 4 Certification Training
Gateway Health Producer Onboarding Guide Annual Recertification Process for Brokers Table of Contents Contents Getting Started 1 Create Your Profile 2 Complete Your Onboarding Forms 4 Certification Training
CHAP LinQ User Guide. CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC Version 1.
 2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
2015 CHAP LinQ User Guide CHAP IT Department Community Health Accreditation Partner 1275 K Street NW Suite 800 Washington DC 2005 Version 1.1 CHAP LINQ USER GUIDE - OCTOBER 2015 0 Table of Contents ABOUT
eprotocol Investigator Role Manual
 eprotocol Investigator Role Manual Table of Contents Table of Contents Auto-Population of Stored User Data...17 5.4 Module Specific Work Flow Sections...18 1 Overview...4 1.1 Things to Remember...4 2 Creating
eprotocol Investigator Role Manual Table of Contents Table of Contents Auto-Population of Stored User Data...17 5.4 Module Specific Work Flow Sections...18 1 Overview...4 1.1 Things to Remember...4 2 Creating
eprotocol IBC Principal Investigator User Guide
 eprotocol IBC Principal Investigator User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software Version: 2.5.40.8 Document Version 2.9.2 2002-2016 Key Solutions, Inc. 2803
eprotocol IBC Principal Investigator User Guide Key Solutions, Inc. 2803 Lakeview Ct. Fremont, CA 94538 www.keyusa.com Software Version: 2.5.40.8 Document Version 2.9.2 2002-2016 Key Solutions, Inc. 2803
User s Guide: Sponsored Programs PreAward Module
 User s Guide: Sponsored Programs PreAward Module Adding a New Proposal NUgrant is a web portal through which you can route proposals, file disclosures of interest, and submit IRB or IACUC protocols. This
User s Guide: Sponsored Programs PreAward Module Adding a New Proposal NUgrant is a web portal through which you can route proposals, file disclosures of interest, and submit IRB or IACUC protocols. This
PCORI Online: Awardee User Guide Research Awards
 PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
PCORI Online: Awardee User Guide Research Awards Updated as of 1/31/18 1 Table of Contents Section 1: Introduction to PCORI Online... 3 1.1 Getting Started - Tips for Using PCORI Online... 4 1.2 Logging
Compliance Document Manager User Guide
 Compliance Document Manager User Guide Contents OVERVIEW... 3 SYSTEM REQUIREMENTS... 3 VENDORMATE PASSWORD REQUIREMENTS... 3 LOGIN... 4 THE HOME SCREEN... 5 BA Screening... 5 BA Oversight... 5 My Screening
Compliance Document Manager User Guide Contents OVERVIEW... 3 SYSTEM REQUIREMENTS... 3 VENDORMATE PASSWORD REQUIREMENTS... 3 LOGIN... 4 THE HOME SCREEN... 5 BA Screening... 5 BA Oversight... 5 My Screening
Guide for Researchers: Online Human Ethics Application Form
 Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Ethics & Integrity Research Office HUMAN RESEARCH ETHICS ONLINE APPLICATION October 2016/V1.03 Guide for Researchers: Online Human Ethics Application Form ENQUIRIES Senior Human Ethics Officer University
Version 2.1 June 12, 2018
 Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
Version 2.1 June 12, 2018 MAESTRO Review System login: https://maestro.research.nmsu.edu MAESTRO Help & Training: http://maestrohelp.research.nmsu.edu MAESTRO & IRB Questions and Helpdesk Support: Call
Instructions to complete CITI Human Subjects Training - New User
 Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
Instructions to complete CITI Human Subjects Training - New User To ensure compliance with federal regulations that all personnel engaged in research or advising research involving human subjects receive
College Deans, Department Chairs & Supervising Faculty
 Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
Revised: 8.26.09 Sam Houston State University Protection of Human Subjects Committee (PHSC/IRB) College Deans, Department Chairs & Supervising Faculty Sam Houston State University has developed a new online
IRBManager Faculty Adviser Role
 IRBManager Faculty Adviser Role Human Subjects Research Institutional Review Board irb@okstate.edu 405-744-3377 Page 1 Table of Contents ABOUT... 3 NOTIFICATION OF REVIEW... 3 LOGGING INTO YOUR IRBMANAGER
IRBManager Faculty Adviser Role Human Subjects Research Institutional Review Board irb@okstate.edu 405-744-3377 Page 1 Table of Contents ABOUT... 3 NOTIFICATION OF REVIEW... 3 LOGGING INTO YOUR IRBMANAGER
IRB News : Addition of a new application type for submitting reliance agreements
 In alignment with the NIH mandate for single IRB review that goes into effect in January 2018, HSERA is being updated to include a new reliance agreement specific application. This abbreviated online form
In alignment with the NIH mandate for single IRB review that goes into effect in January 2018, HSERA is being updated to include a new reliance agreement specific application. This abbreviated online form
(EHR) Incentive Program
 REGISTRATION USER GUIDE For Eligible Professionals Medicaid Electronic Health Record (EHR) Incentive Program DECEMBER 2010 (12.28.10 ver2) CONTENTS Step 1... Getting started 3 Step 2... Login instruction
REGISTRATION USER GUIDE For Eligible Professionals Medicaid Electronic Health Record (EHR) Incentive Program DECEMBER 2010 (12.28.10 ver2) CONTENTS Step 1... Getting started 3 Step 2... Login instruction
ACT Test Accessibility and Accommodations System (TAA) User Guide
 ACT Test Accessibility and Accommodations System (TAA) User Guide www.act.org ACT Test Accessibility and Accommodations System (TAA) User Guide Table of Contents Overview... 2 Introduction to the Test
ACT Test Accessibility and Accommodations System (TAA) User Guide www.act.org ACT Test Accessibility and Accommodations System (TAA) User Guide Table of Contents Overview... 2 Introduction to the Test
Welcome to the. Patient Portal!
 Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider and take control of your medical information. Using this quick reference guide,
Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider and take control of your medical information. Using this quick reference guide,
Step-by-Step Guide. Electronic Proposal Routing in Cayuse 424. Office of Research & Sponsored Programs (ORSP)
 Step-by-Step Guide Electronic Proposal Routing in Cayuse 424 Office of Research & Sponsored Programs (ORSP) Step # 1 Look for an email from Cayuse entitled Cayuse424 System Notification: Proposal Awaiting
Step-by-Step Guide Electronic Proposal Routing in Cayuse 424 Office of Research & Sponsored Programs (ORSP) Step # 1 Look for an email from Cayuse entitled Cayuse424 System Notification: Proposal Awaiting
Click IRB 7.3 IRB Ancillary Reviewer s Guide
 Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
Click IRB 7.3 IRB Ancillary Reviewer s Guide October 2013 Table of Contents Logging In... 3 Login issues... 3 Locating Your To-Do List... 4 Understanding My Inbox... 5 Viewing the Study Details... 6 Viewing
TABLE OF CONTENTS CHAPTER 1. PROTOCOL APPLICATION PROCESS OVERVIEW
 IBC Creating Protocol Applications TABLE OF CONTENTS CHAPTER 1. PROTOCOL APPLICATION PROCESS OVERVIEW... 1.1 CHAPTER 2. GENERAL INFORMATION... 2.1 2.1. STARTING AND LOGGING IN... 2.1 2.2. USING NAVIGATION
IBC Creating Protocol Applications TABLE OF CONTENTS CHAPTER 1. PROTOCOL APPLICATION PROCESS OVERVIEW... 1.1 CHAPTER 2. GENERAL INFORMATION... 2.1 2.1. STARTING AND LOGGING IN... 2.1 2.2. USING NAVIGATION
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review
 Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
Documentation for Non-Medical Research Ethics Board Researchers Full Board and Delegated Board Review July 23, 2013 Office of Research Ethics If you run into any difficulties or have questions about Romeo,
ecampus Submission Process
 ecampus Submission Process Progress Report Submission, and Installment Submission & Feedback 1 All Progress Reports and Installment Submissions are found on the Assignments Page. 2 Individual assignments
ecampus Submission Process Progress Report Submission, and Installment Submission & Feedback 1 All Progress Reports and Installment Submissions are found on the Assignments Page. 2 Individual assignments
The Lilly Safety Mailing Process
 The Lilly Safety Mailing Process 1 After this presentation you will be able to: Define Safety Mailings and the type of adverse events that trigger safety mailings. Define Principal Investigator (PI) and
The Lilly Safety Mailing Process 1 After this presentation you will be able to: Define Safety Mailings and the type of adverse events that trigger safety mailings. Define Principal Investigator (PI) and
Ferring Pharmaceuticals Inc. Educational Grant Applicant Working Guide
 Ferring Pharmaceuticals Inc. Educational Grant Applicant Working Guide 2014 Contents FERRING I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 4 III. HOW TO SUBMIT AN APPLICATION... 6 IV. HOW TO PROVIDE
Ferring Pharmaceuticals Inc. Educational Grant Applicant Working Guide 2014 Contents FERRING I. INTRODUCTION... 3 II. HOW TO REGISTER AND LOG-IN... 4 III. HOW TO SUBMIT AN APPLICATION... 6 IV. HOW TO PROVIDE
erequest How to apply guide
 Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
Overview is an application that assists UCB in request life cycle management. UCB has clear guidance in place on what they can support or sponsor. Online requests will go through an internal review and
USER MANUAL FOR AUTHOR.
 USER MANUAL FOR AUTHOR http://conference.binus.ac.id/ocs/index.php/icimtech/icimtech2017 Version : 1.0 May 2017 Table of Contents Create an Account... 2 Forgot Password... 6 Submit Paper... 9 Check Submitted
USER MANUAL FOR AUTHOR http://conference.binus.ac.id/ocs/index.php/icimtech/icimtech2017 Version : 1.0 May 2017 Table of Contents Create an Account... 2 Forgot Password... 6 Submit Paper... 9 Check Submitted
IACUC Proposal Entry Instructions
 IACUC Proposal Entry Instructions Version 1.0 October 10, 2016 Notes to Primary Investigator The following instructions are based on the IACUC Proposal form as currently found in iris. This instruction
IACUC Proposal Entry Instructions Version 1.0 October 10, 2016 Notes to Primary Investigator The following instructions are based on the IACUC Proposal form as currently found in iris. This instruction
PI Certification Quick Guide
 PI Certification Quick Guide 1 P age Table of Contents Certifications... 3 PI Self-Certification... 3 PI is Certifying via Link in Email Notification... 3 PI Goes Directly to the Proposal... 6 Certification
PI Certification Quick Guide 1 P age Table of Contents Certifications... 3 PI Self-Certification... 3 PI is Certifying via Link in Email Notification... 3 PI Goes Directly to the Proposal... 6 Certification
To the ETS Offset Notice Response Authorization Online Training Course
 Welcome To the ETS Offset Notice Response Authorization Online Training Course This process describes how an applicant can request authorization from the designated representative to create and submit
Welcome To the ETS Offset Notice Response Authorization Online Training Course This process describes how an applicant can request authorization from the designated representative to create and submit
NQF ONLINE MEASURE SUBMISSION FORM USERS GUIDE
 NQF ONLINE MEASURE SUBMISSION FORM USERS GUIDE VERSION 1.1 Guide Version 1.0 01/11 TABLE OF CONTENTS PART 1: TECHNICAL SUPPORT FOR SUBMISSION FORM TABLE OF CONTENTS... CREATING AN INDIVIDUAL ACCOUNT...
NQF ONLINE MEASURE SUBMISSION FORM USERS GUIDE VERSION 1.1 Guide Version 1.0 01/11 TABLE OF CONTENTS PART 1: TECHNICAL SUPPORT FOR SUBMISSION FORM TABLE OF CONTENTS... CREATING AN INDIVIDUAL ACCOUNT...
Getting Started with the Teacher Information Management System (TIMS)
 Getting Started with the Teacher Information Management System (TIMS) How Will This Presentation Help Me? This presentation is designed to help you: Access TIMS for the first time Introduce you to your
Getting Started with the Teacher Information Management System (TIMS) How Will This Presentation Help Me? This presentation is designed to help you: Access TIMS for the first time Introduce you to your
To access your My BackPack account, please click on this link:
 Dear Milwaukee Montessori School Families, Milwaukee Montessori School uses My BackPack for student and parent information. All new and returning families will need to complete the registration process
Dear Milwaukee Montessori School Families, Milwaukee Montessori School uses My BackPack for student and parent information. All new and returning families will need to complete the registration process
Insight 3.4: Release Date September 27, Help Desk/ Service Center # (If Available) Internal TFS # Description
 Insight 3.4: Release Date September 27, 2013 Internal TFS # Help Desk/ Service Center # (If Available) Description Agreements- Patient Care Implement Patient Care Details for BWH Patient Care Details are
Insight 3.4: Release Date September 27, 2013 Internal TFS # Help Desk/ Service Center # (If Available) Description Agreements- Patient Care Implement Patient Care Details for BWH Patient Care Details are
Electronic Statement of Deficiencies and Plan of Corrections
 Electronic Statement of Deficiencies and Plan of Corrections The purpose of this information sheet is to inform Federally Certified Skilled Nursing Facilities (SNFs) and Nursing Facilities (NFs) that:
Electronic Statement of Deficiencies and Plan of Corrections The purpose of this information sheet is to inform Federally Certified Skilled Nursing Facilities (SNFs) and Nursing Facilities (NFs) that:
Information Sheet for Scientific Approvers
 1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
1 Information Sheet for Scientific Approvers This document will provide you with the basic instructions for your role as a scientific approver in OSIRIS. All listed approvers have been designated by their
STEP BY STEP GUIDE TO TENCEL BRANDING
 STEP BY STEP GUIDE TO TENCEL BRANDING Most of KenDor s Lyocell fabrications are made from Lenzing certified yarns and therefore qualify for to be branded Lenzing proprietary Tencel marketing including
STEP BY STEP GUIDE TO TENCEL BRANDING Most of KenDor s Lyocell fabrications are made from Lenzing certified yarns and therefore qualify for to be branded Lenzing proprietary Tencel marketing including
MedicareBlue Online Training Center User Guide
 MedicareBlue Online Training Center User Guide Contents MedicareBlue Online Training Center Website URL... 2 Registration... 2 Login... 5 Forgot Password... 6 Forgot Username... 7 My Profile... 7 Changing
MedicareBlue Online Training Center User Guide Contents MedicareBlue Online Training Center Website URL... 2 Registration... 2 Login... 5 Forgot Password... 6 Forgot Username... 7 My Profile... 7 Changing
Welcome to the. Patient Portal!
 Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider, schedule and request appointments, take control of your medical information,
Welcome to the Patient Portal! You re about to find out just how easy it can be to communicate with your healthcare provider, schedule and request appointments, take control of your medical information,
New Online Process for Updating TN BME Committee on Physician Assistants Attachment 4 & 5 Authorization for Prescribing for PAs
 New Online Process for Updating TN BME Committee on Physician Assistants Attachment 4 & 5 Authorization for Prescribing for PAs As of May 2017, the Authorization for Prescribing for Physician Assistants
New Online Process for Updating TN BME Committee on Physician Assistants Attachment 4 & 5 Authorization for Prescribing for PAs As of May 2017, the Authorization for Prescribing for Physician Assistants
Participation in e-learning Course Avoiding Plagiarism
 To all graduate students Participation in e-learning Course Avoiding Plagiarism In compliance with the Guidelines for Responding to Misconduct in Research established by MEXT in August 2014, Nagoya University
To all graduate students Participation in e-learning Course Avoiding Plagiarism In compliance with the Guidelines for Responding to Misconduct in Research established by MEXT in August 2014, Nagoya University
Applying for PA Foundation Scholarships in SmarterSelect: A Guide for Applicants
 Applying for PA Foundation Scholarships in SmarterSelect: A Guide for Applicants The purpose of this guide is to walk you through the process of applying for PA Foundation scholarships using the SmarterSelect
Applying for PA Foundation Scholarships in SmarterSelect: A Guide for Applicants The purpose of this guide is to walk you through the process of applying for PA Foundation scholarships using the SmarterSelect
Lorin Muhlmann V0.5 Last updated 17/04/18
 ClubGRANTS Online Training Manual CONVENORS Contents Logging in... 4 Opening a grant round... 6 Completing an application... 10 Reviewing and assessing applications... 15 Completing and reviewing acquittal
ClubGRANTS Online Training Manual CONVENORS Contents Logging in... 4 Opening a grant round... 6 Completing an application... 10 Reviewing and assessing applications... 15 Completing and reviewing acquittal
Core Staff Review Approve with Contingencies
 Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Core Staff Review Approve with Contingencies Important Information In the event a submission is Approved with Contingencies, extra steps are required to complete the approval process. The basic steps to
Charlotte Housing Authority Applicant Portal Overview
 Topic Charlotte Housing Authority Table of Contents Page Login Page 3 Forgot Password 4 Registering- Currently on waiting list 6-10 Home Screen 11 Wait Listing Information Basic Details 12-13 Waiting List
Topic Charlotte Housing Authority Table of Contents Page Login Page 3 Forgot Password 4 Registering- Currently on waiting list 6-10 Home Screen 11 Wait Listing Information Basic Details 12-13 Waiting List
Qualitrac Provider Portal Guide. Page 1 of 19
 Qualitrac Provider Portal Guide Page 1 of 19 Page 2 of 19 Table of Contents Section 1: Provider Portal... 3 Section 2: Security Administrator Instructions... 13 Page 3 of 19 Section 1: Provider Portal
Qualitrac Provider Portal Guide Page 1 of 19 Page 2 of 19 Table of Contents Section 1: Provider Portal... 3 Section 2: Security Administrator Instructions... 13 Page 3 of 19 Section 1: Provider Portal
Wellington City Council Funding Portal Quick Reference Guide for Applicants
 Wellington City Council Funding Portal Quick Reference Guide for Applicants Before you Begin The intended audience for this document is a new user who is registering for the first time or for a returning
Wellington City Council Funding Portal Quick Reference Guide for Applicants Before you Begin The intended audience for this document is a new user who is registering for the first time or for a returning
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT
 Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
Rev: 06/2017 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR / STUDENT In This Document Protocol Application (Exempt and Expedited/Full Board Review)...2 NIH Certificate (Required)... 2 Logging In...
NYU Cayuse IRB Manual
 IRB NYU Cayuse IRB Manual prepared by the NYU UCAIHS (University Committee on Activities Involving Human Subjects) What is Cayuse? The Cayuse Research Suite is NYU s system to support the submission of
IRB NYU Cayuse IRB Manual prepared by the NYU UCAIHS (University Committee on Activities Involving Human Subjects) What is Cayuse? The Cayuse Research Suite is NYU s system to support the submission of
IRB Staff Approve Repository Application
 IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
IRB Staff Approve Repository Application Newly submitted Repository applications display on the Unassigned tab of the IRB Staff workspace. IRB Staff complete an initial review of newly submitted Repository
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018. I. Protocol Application & IRB Certification Tutorial...2
 STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
STREAMLYNE INITIAL GUIDE FOR PRINCIPAL INVESTIGATOR Rev. 10/2018 I. Protocol Application & IRB Certification Tutorial...2 II. Logging In (Password)... 2 III. Creating a New Protocol Record / Menu Bar...
IRB Committee Member Adverse Event (AE) Single Member Review
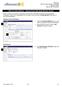 IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
IRB Committee Member AE Single IRB Committee Member Adverse Event (AE) Single When an Adverse Event (AE) is reported by the study team, IRB Staff will assign it to the appropriate reviewer(s). Once an
Before launching courses, here are some things you need to know.
 Quick Reference Guide How to Enroll into Online Health & Safety Courses Before launching courses, here are some things you need to know. 1. After a course has been completing, it can be re-launched again
Quick Reference Guide How to Enroll into Online Health & Safety Courses Before launching courses, here are some things you need to know. 1. After a course has been completing, it can be re-launched again
Module 6: Validating Decisions
 Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Table of Contents Module 6: Validating Decisions Module 6 Objective: Covers how Core Staff members process the decisions of the reviewers on each eresearch submission through a decision validation. Refer
Do Not enroll until after October 29th
 Do Not enroll until after October 29th Steps to complete 4 H Online initial enrollment for Nebraska 4 H 4 H Online will not work using Internet Explorer You must use Google Chrome or Firefox. To access
Do Not enroll until after October 29th Steps to complete 4 H Online initial enrollment for Nebraska 4 H 4 H Online will not work using Internet Explorer You must use Google Chrome or Firefox. To access
Sound United Reseller FAQs
 Sound United Reseller FAQs Why is Sound United instituting a Dealer Authorization Program? We are passionate about Audio and we will always go the extra mile to design, manufacture and sell the highest
Sound United Reseller FAQs Why is Sound United instituting a Dealer Authorization Program? We are passionate about Audio and we will always go the extra mile to design, manufacture and sell the highest
e-survey System User Manual for AML/CFT COMPLIANCE REPORT
 BANK NEGARA MALAYSIA CENTRAL BANK OF MALAYSIA e-survey System User Manual for AML/CFT COMPLIANCE REPORT VERSION 1.0 Table of Content BNM e-survey System User Manual for AML/CFT COMPLIANCE REPORT 1. Introduction...
BANK NEGARA MALAYSIA CENTRAL BANK OF MALAYSIA e-survey System User Manual for AML/CFT COMPLIANCE REPORT VERSION 1.0 Table of Content BNM e-survey System User Manual for AML/CFT COMPLIANCE REPORT 1. Introduction...
Signing Up Online for a MyUHCare Personal Health Record from a PC or Laptop
 Signing Up Online for a MyUHCare Personal Health Record from a PC or Laptop You can sign up online for a MyUHCare Personal Health Record (PHR) using a PC or laptop by following the steps below. 1. Navigate
Signing Up Online for a MyUHCare Personal Health Record from a PC or Laptop You can sign up online for a MyUHCare Personal Health Record (PHR) using a PC or laptop by following the steps below. 1. Navigate
IRBNet Picture Guide
 IRBNet Picture Guide Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org For any questions or concerns regarding your submission, visit CSUSM
IRBNet Picture Guide Instructions for On-line Submission of Research Protocols to the Institutional Review Board (IRB) www.irbnet.org For any questions or concerns regarding your submission, visit CSUSM
University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Update a Pre-Proposal for Full Submission
 University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Update a Pre-Proposal for Full Submission In order to obtain external funding for their research programs, sometimes investigators
University of Wisconsin - Milwaukee Grants Project Desk Reference WISPER Update a Pre-Proposal for Full Submission In order to obtain external funding for their research programs, sometimes investigators
Velos ecompliance. Researcher User Manual
 Velos ecompliance Researcher User Manual Table of Contents Disclaimer... 4 ecompliance Overview... 5 Contacts... 6 Administrator... 6 Super Users... 6 IT/Technical Support... 6 Quick Help... 7 Getting
Velos ecompliance Researcher User Manual Table of Contents Disclaimer... 4 ecompliance Overview... 5 Contacts... 6 Administrator... 6 Super Users... 6 IT/Technical Support... 6 Quick Help... 7 Getting
SUBMITTING A NEW IBC PROJECT APPLICATION (INITIAL)
 SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
SUBMITTING A After you have established and activated your username and password, you can begin to create an IBC application in IRBNet. To start building a new IBC application, you must first CREATE A
Electrolux Small Appliance Reseller FAQs
 Electrolux Small Appliance Reseller FAQs Why is Electrolux instituting a Reseller Authorization Program? Electrolux takes our brand and market position very seriously and desires to protect and promote
Electrolux Small Appliance Reseller FAQs Why is Electrolux instituting a Reseller Authorization Program? Electrolux takes our brand and market position very seriously and desires to protect and promote
WesternREM (WREM) Reviewer
 WesternREM (WREM) Reviewer REB Member Training Manual Helpdesk: 519-661-3036 Email: wrem@uwo.ca Table of Contents 1. About WesternREM... 3 1.1. Target Audience... 3 1.2. Internet Settings... 3 1.3. Technical
WesternREM (WREM) Reviewer REB Member Training Manual Helpdesk: 519-661-3036 Email: wrem@uwo.ca Table of Contents 1. About WesternREM... 3 1.1. Target Audience... 3 1.2. Internet Settings... 3 1.3. Technical
Supplier Profile Administration
 Supplier Profile Administration Uploading Certification(s) Document: Quality certificate and/or Environmental certificate* Entering Supplier Contacts Information *Uploading an Environmental Certificate
Supplier Profile Administration Uploading Certification(s) Document: Quality certificate and/or Environmental certificate* Entering Supplier Contacts Information *Uploading an Environmental Certificate
Online CDC service. HowTo guide for certifying organisations
 Online CDC service HowTo guide for certifying organisations Disclaimer While every reasonable effort has been made to ensure that this document is correct at the time of printing, the State of NSW, its
Online CDC service HowTo guide for certifying organisations Disclaimer While every reasonable effort has been made to ensure that this document is correct at the time of printing, the State of NSW, its
CPD Users Guide A step by step instruction guide for the CPD online diary
 CPD Users Guide A step by step instruction guide for the CPD online diary Table of Contents 1 Logging into the CPD diary... 2 2 Selecting your CPD Program... 4 3 Adding activities to the CPD diary... 5
CPD Users Guide A step by step instruction guide for the CPD online diary Table of Contents 1 Logging into the CPD diary... 2 2 Selecting your CPD Program... 4 3 Adding activities to the CPD diary... 5
Guide to the Distributor Zone
 Guide to the Distributor Zone Registering for a Distributor account Logging into the Distributor Zone Updating your profile Registering for a Challenge Registering a Participant Connecting a Participant
Guide to the Distributor Zone Registering for a Distributor account Logging into the Distributor Zone Updating your profile Registering for a Challenge Registering a Participant Connecting a Participant
umapps Using umapps 6/14/2017 Brought to you by: umtech & The Center for Teaching & Learning
 umapps Using umapps Center for Teaching and Learning (CTL) 100 Administration Bldg., Memphis, TN 38152 Phone: 901.678.8888 Email: itstrainers@memphis.edu Center for Teaching and Learning Website 6/14/2017
umapps Using umapps Center for Teaching and Learning (CTL) 100 Administration Bldg., Memphis, TN 38152 Phone: 901.678.8888 Email: itstrainers@memphis.edu Center for Teaching and Learning Website 6/14/2017
From the Online Tools list, scroll down to SBS Connect, and click on the Register for SBS Connect link. The SBS Connect login screen loads.
 SBS EXTERNAL HEALTHCARE REVIEW USER GUIDE Create New Account Register an Entity View Attachment Upload Attachment SBS CONNECT CREATE NEW ACCOUNT Before using SBS Connect for the first time, 1) create an
SBS EXTERNAL HEALTHCARE REVIEW USER GUIDE Create New Account Register an Entity View Attachment Upload Attachment SBS CONNECT CREATE NEW ACCOUNT Before using SBS Connect for the first time, 1) create an
Idaho Medicaid: Utilization Review Program. Orientation and System Training August 9, 2016
 Idaho Medicaid: Utilization Review Program Orientation and System Training August 9, 2016 Welcome Earlier this year a competitive RFP was issued to award a contract to an organization to provide Utilization
Idaho Medicaid: Utilization Review Program Orientation and System Training August 9, 2016 Welcome Earlier this year a competitive RFP was issued to award a contract to an organization to provide Utilization
IDF Appraisee Guide to the IDF Online Appraisal Form. Contents
 IDF Appraisee Guide to the IDF Online Appraisal Form The IDF Appraisee Guide is a step by step handbook on how to complete the IDF online appraisal form. This document contains detailed guidance on how
IDF Appraisee Guide to the IDF Online Appraisal Form The IDF Appraisee Guide is a step by step handbook on how to complete the IDF online appraisal form. This document contains detailed guidance on how
isite ICON Central Laboratories Secure Website for Site Information
 What is isite? isite is ICON Central Laboratories secure web portal that provides secure access to Site specific information, including laboratory results. You are able to view, download and print all
What is isite? isite is ICON Central Laboratories secure web portal that provides secure access to Site specific information, including laboratory results. You are able to view, download and print all
Partner Side SMART Guide
 Partner Side SMART Guide Table of Contents 1. Introduction... 3 2. Partner Registration Process... 3 3. Additional Form... 12 4. Scorecard... 13 5. View Buyer Profile... 14 Partner Side User Manual 31
Partner Side SMART Guide Table of Contents 1. Introduction... 3 2. Partner Registration Process... 3 3. Additional Form... 12 4. Scorecard... 13 5. View Buyer Profile... 14 Partner Side User Manual 31
