Building an Ancillary System for Cancer Registries from SDC CAP Templates
|
|
|
- Maud Dorsey
- 5 years ago
- Views:
Transcription
1 Building an Ancillary System for Cancer Registries from SDC CAP Templates Jennifer Seiffert, MLIS, CTR, Northrop Grumman, under contract to CDC s NPCR Sanjeev Baral, Northrop Grumman, under contract to CDC s NPCR Sandy Jones, CDC Richard Moldwin, MD, PhD, College of American Pathologists Joseph D. Rogers, MS, CDC NAACCR Annual Meeting June 12, 2018
2 Introduction: Purpose of Project 1. Using SDC biomarker forms, to demonstrate closer integration of electronic pathology reports with the central cancer registry s database using technology that is interoperable, flexible, easy to maintain, and based on current informatics best practices 2. To explore future extensions of this method as a potential technology solution for other parts of the NAACCR record
3 Introduction: A year ago.... Last year at NAACCR we demonstrated our proof of concept for registrars use of SDC XML CAP biomarker protocols from the Web Plus abstracting application to collect data on biomarkers for new data items in v18. This year we are expanding the scope to show how we are incorporating the SDC XML biomarker forms into the central registry database (CRS Plus) From laboratories reporting via emarc From registrars using Web Plus
4 Specific Goals for 2018 To further integrate Registry Plus applications (emarc, and CRS Plus) To create ancillary storage of electronic pathology reports and associate them with the registry s patient and tumor records To map selected values to NAACCR data items To add ability to view or query the path reports from within CRS Plus
5 Background: CAP Biomarker Templates
6 First Step: Creation of Biomarker Template by Pathologists at CAP Pathologists at College of American Pathologists (CAP) create a biomarker template based on expert knowledge of clinical needs and laboratory practices Templates are disease- and/or site-specific Include tests that are usually reported separately from the main anatomic pathology report Include test results, methods, and quality control information Templates are available in Word or PDF documents at CAP.ORG ecc (electronic checklists) are also available from CAP with a free license
7 CAP s Next Step: XML IT experts analyze template and create Structured Data Capture (SDC), structuring the template content into a series of questions and responses XML is the tool used to tag parts of template As questions or responses To indicate text to display online or in a printable report To create structure to accept the responses from an online form that pathologists can fill out Definitions and instructions for the XML are contained in separate text documents, for example: Stylesheet has instructions for rendering the XML for presentation as a web page by a browser XML Schema describes structure of the XML
8 CAP s Next Step: HTML IT experts and pathologists decide how form should appear when displayed on a computer screen HTML is the language used to tag the text in the document to ready the clinical contents for display in a browser The end user s browser reads and interprets the HTML tags along with a stylesheet to render it on screen Location of each piece of content on the screen Fonts, colors, other visual aspects What parts of form can accept data entered by user
9 HTML Example: ER Results, XML transformed to HTML rendered by Microsoft Edge Browser Everything in the Word version is contained in the SDC XML template
10 SDC Forms Are Made Available to Pathologists Through a Form Manager and Form Filler Pathologist fills out the form with appropriate responses The form has a Submit button at the bottom When the Submit button is pressed, pathologist s responses are written into the original XML and the XML file is sent over the internet, just like any secure web page, to pre-designated location(s)
11 Body Section - Review 2018 College of American Pathologists. All rights reserved
12 Where do the answers go?* 2018 College of American Pathologists. All rights reserved Optional to transmit This model is particularly well suited to ecc data representation and transmission. 12
13 Forms and Packages Form One SDC FormDesign XML file Package One or more SDC FormDesign templates (XML) One Demographic FormDesign template (optional) Maps to terminologies, data elements etc. Transforms and/or other formatting/styling artifacts Administrative data RELIABLE TRUSTED 2018 SCIENTIFIC College of American 13
14 The Receiving End [1] A receiving program needs to know how to handle (parse) generic SDC XML. The content doesn't matter, the computer doesn't care, as long as it's in SDC format. The user's content (responses) inside of SDC XML is relatively easy to consume and process. If the receiving software (e.g., emarc Plus or Web Plus) wants to place the data into existing NAACCR data fields, that can be handled.
15 The Receiving End [2] SMEs can map the SDC identifiers to specific NAACCR codes, and the software simply reads the SDC identifiers and copies the mapped NAACCR code data into the appropriate database fields. The maps are not difficult to create, and can be used by any SDC-based software. This is a simple task technically, made simplest if the NAACCR code data and CAP code data are congruent. New biomarker data items for 2018 have been purposely aligned with the CAP templates. More complex rules can also be created to map SDC identifiers to NAACCR codes. Eventually, we would want to use the SDC identifiers directly and phase out the need for mapping.
16 The Project
17 Use of SDC Biomarker Forms by Registrars We are using the SDC biomarker forms as the prototype for future integration of anatomic SDC pathology reports into the central registry data collection. Initial testing by registrars has been positive. Filling out the form is easy and relatively fast.
18 Advantages to the Cancer Registry Community [1] Maintenance will be much easier, because CAP is already doing the SME and IT work, and template contents are carefully vetted by committees of recognized experts. Interoperability with EHR vendors and others such as pathology software, AJCC, ASCO, and CMS Registry can process results from facility registrars the same way as from labs
19 Advantages to the Cancer Registry Community [2] Standardized data collection forms will be uniform, simplifying training and processing Infrastructure for creating, transmitting, and mapping the messages already exists and is in use for path lab reporting Most anatomic pathology and some major EHR vendors, such as Epic and Cerner, are already supporting this infrastructure for lab reporting
20 Advantages to the Cancer Registry Community[3] Will enable registries to quickly incorporate important new data elements without the usual time lag of several years without precluding the continued use of the current NAACCR format. Offers potential for increased flex- ibility, agility, in accommodating changes in cancer medicine
21 How to Integrate SDC Biomarker Forms into Registry Work Flow Need to make forms available via CDCprovided web service and/or DLL Incorporate access to XML data collection forms for biomarkers and prognostic factors from labs and registrars into registry software Map SDC identifiers to NAACCR items Submit biomarker and prognostic factor data to central registry in separate XML record, linked to routine NAACCR Type A (abstract) record by ID numbers
22 2018 College of American Pathologists. All rights reserved Location in our Project: Web Plus and emarc CAP, then CDC in the future Web Plus 22
23 Mapping SDC to NAACCR Items Table relates the unique ID of each relevant SDC response to NAACCR item number (usually a new SSDI) and value Some items not relevant to registries -- status of controls, methods, fixation time -- not mapped
24 Mapping SDC to NAACCR Items: Examples SDC ID Response (NAACCR item #, code) ER Positive (3271,1) % cells w/nuclear positivity 11-20% (3827,1) and (3826,R20) ER Negative (3827,0) and (3826,000) Cannot be determined (indeterminate) (3827,9) and (3826,XX9)
25 Beyond Mapping, Good News! Relevant data on form that is not mapped can still be collected, stored, and viewed or queried As soon as additional biomarkers and prognostic factors are added to the CAP forms, they can be incorporated into our central registry data sets Without time spent developing code sets no more SSDI Work Group! Without extensive re-programming of central registry software
26 Remember.... Pathologists at College of American Pathologists (CAP) create a biomarker template based on expert knowledge of clinical needs and laboratory practices Maintenance will be much easier, because CAP is already doing the SME and IT work, and template contents are carefully vetted by committees of recognized experts.
27 Integration into Central Registry Database (CRS Plus)
28 Two Use Cases for Completed SDC Biomarker Forms From emarc From Web Plus Lab Report Primary source document Mapped to NAACCR items creating secondary abstract with same information Registrar Report Secondary source document Values mapped to a complete NAACCR abstract
29 Laboratory Report Represents primary source for the information reported. Includes demographics. emarc creates a NAACCR Type A record and maps selected values from the lab report Type of Reporting Source = 3 (Laboratory only (hospitalaffiliated or independent)) Class of Case = 43 (PATHOLOGY or other lab specimens ONLY) Reporting Facility [NAACCR 540] = state-assigned number Unique ID assigned by emarc stored in????
30 Registrar Report (1) Represents secondary source for the information reported, which is being abstracted from a medical record during routine data collection in a reporting facility. Form remains in XML format
31 Registrar Report (2) Selected values mapped to NAACCR Type A record that is the primary abstract Type of Reporting Source = comes from Type A record Class of Case = not applicable Reporting Facility = not applicable Primary abstract s ID is added to SDC XML Unique patient ID of XML form as assigned by form filler is added to primary abstract
32 SDC Package as Submitted Demographic Patient Path Reporting Facility Path Ordering Facility Tests Performed Test names Test results in detail Methods
33 Procedure in Prep and CRS Plus Abstracts (NAACCR Type A records) with linked SDC forms (XML document) have 2 files Abstracts imported into Prep Plus where permanent AbsRefID is assigned to abstract and written to linked SDC form Abstract follows current Prep/CRS workflow for edits, linkage, and consolidation XML file (not edited) is stored in separate table but linked via AbsRef ID stored in both tables.
34 CRS Plus Data Structure w/sdc Forms
35 New Features Being Added to CRS Plus User interface changes needed for display of all data in the XML Need button to select view of SDC form We need to retain the unique ID assigned in emarc and add the AbsRef ID assigned in Prep Plus into SDC form XML.
36 Illustrative Case
37 Abstracted Data from Medical Record Year of Date of DX 2018 Primary Site C504 Histology/Behavior 8500/3 Summary of Predictive/Prognostic Markers Estrogen Receptor [SP1*] range 51-60% positive cells, intermediate intensity, Allred score 6 of 8 Progesterone Receptor [PR88*], 11% positive cells, weak intensity, Allred score 3 of 8 HER2 IHC (w/ HIER**) High overexpression (3+) Proliferation Index (Ki-67): 5% (Low prolif., <10%) * antibodies **heat-induced epitope retrieval (HIER), used with IHC
38 Partial Form with Values Selected by Registrar
39 Next steps Abstract and SDC form are submitted from Web Plus to the central registry which is using Prep Plus and CRS Plus
40 Tree Structure Patient Tumor Abstract/ Facility 1 Abstract/ Facility 2
41 Tree Structure Summarizes patient, tumor, and submitted abstracts/ facility records Pink text and designator e indicates that the second facility record is an electronic path report, in our case an SDC biomarker report
42
43
44 Voila! The biomarker form is re-created and displayed
45 Some Outstanding Issues (1) Need permanent Form Manager Need standard for populating Reporting Facility [540] and other items for lab only reports Use CLIA number? [NAACCR 7515]
46 Some Outstanding Issues (2) Deciding how the lab reports will be used in data item consolidation Decide about mapping from individual biomarker report to HER2 Overall Summary [3855] within individual reports
47 .... And Beyond! The Vision.... Wider implementation of SDC by lab vendors, EHR vendors, etc. Direct consumption of XML SDC path reports (cancer protocols and biomarker templates) by cancer registries Elimination of mapping to separate NAACCR codes New cancer registry data model
48 Laboratory Report (2) Both NAACCR abstract and XML form are linked by an ID number and sent to central cancer registry system The blank form has a unique id as does each instance of a form completed with a patient s data. The patient instance ID is versioned as more updates are submitted.
Jovanka N. Harrison, Ph.D., Chair For the NAACCR Pathology Data Work Group, and the NAACCR Supplement WG/Task Force
 Jovanka N. Harrison, Ph.D., Chair For the NAACCR Pathology Data Work Group, and the NAACCR Supplement WG/Task Force North American Association of Central Cancer Registries Annual Conference Austin, Texas
Jovanka N. Harrison, Ph.D., Chair For the NAACCR Pathology Data Work Group, and the NAACCR Supplement WG/Task Force North American Association of Central Cancer Registries Annual Conference Austin, Texas
Real-Time Reporting Task Force Report to the Board
 Real-Time Reporting Task Force Report to the Board June 2006 Ken Gerlach, MPH, CTR Toshi Abe, MSW, CTR Overview NAACCR Vision Primary Issues Process Review of Report to the Board Definition of Real-Time
Real-Time Reporting Task Force Report to the Board June 2006 Ken Gerlach, MPH, CTR Toshi Abe, MSW, CTR Overview NAACCR Vision Primary Issues Process Review of Report to the Board Definition of Real-Time
Transitioning Forward with EDITS50 Tools: EditWriter v5 and GenEDITS Plus v5
 Transitioning Forward with EDITS50 Tools: EditWriter v5 and GenEDITS Plus v5 Michelle Esterly Northrop Grumman Under Contract to NPCR Joseph D. Rogers, Team Lead Applications, Statistics, and Informatics
Transitioning Forward with EDITS50 Tools: EditWriter v5 and GenEDITS Plus v5 Michelle Esterly Northrop Grumman Under Contract to NPCR Joseph D. Rogers, Team Lead Applications, Statistics, and Informatics
Comparing a Standard (NAACCR Volume V) with a Draft Standard (HL7 version Implementation Guide: ELR Reporting to Public Health, Release 2)
 Comparing a Standard (NAACCR Volume V) with a Draft Standard (HL7 version 2.5.1 Implementation Guide: ELR Reporting to Public Health, Release 2) Jovanka Harrison, Ph.D., Lead for the NAACCR ELR Messaging
Comparing a Standard (NAACCR Volume V) with a Draft Standard (HL7 version 2.5.1 Implementation Guide: ELR Reporting to Public Health, Release 2) Jovanka Harrison, Ph.D., Lead for the NAACCR ELR Messaging
Collaborative Stage Data Collection System Version Implementation Guide for Registries and Vendors
 Collaborative Stage Data Collection System Version 02.05 Implementation Guide for Registries and Vendors Elaine N. Collins, RHIA, CTR Collaborative Stage Informatics Team Revised January 14, 2014 Revised
Collaborative Stage Data Collection System Version 02.05 Implementation Guide for Registries and Vendors Elaine N. Collins, RHIA, CTR Collaborative Stage Informatics Team Revised January 14, 2014 Revised
CAP Electronic Cancer Checklists (CAP ecc) Overview. An International Implementation of SNOMED CT
 CAP Electronic Cancer Checklists (CAP ecc) Overview An International Implementation of SNOMED CT 1 Outline Structured Reporting The CAP Cancer checklists Moving from paper form to application XML & the
CAP Electronic Cancer Checklists (CAP ecc) Overview An International Implementation of SNOMED CT 1 Outline Structured Reporting The CAP Cancer checklists Moving from paper form to application XML & the
Collaborative Stage Data Collection System. Version Implementation Guide for. Registries and Vendors. Elaine N. Collins, RHIA, CTR
 Collaborative Stage Data Collection System Version 02.05 Implementation Guide for Registries and Vendors Elaine N. Collins, RHIA, CTR Collaborative Stage Informatics Team Revised January 14, 2014 Informatics
Collaborative Stage Data Collection System Version 02.05 Implementation Guide for Registries and Vendors Elaine N. Collins, RHIA, CTR Collaborative Stage Informatics Team Revised January 14, 2014 Informatics
Central Cancer Registry: Documenting the Security of your Information Technology (IT) Infrastructure
 Central Cancer Registry: Documenting the Security of your Information Technology (IT) Infrastructure Joseph D. Rogers Team Lead Cancer Surveillance Branch Division of Cancer Prevention and Control National
Central Cancer Registry: Documenting the Security of your Information Technology (IT) Infrastructure Joseph D. Rogers Team Lead Cancer Surveillance Branch Division of Cancer Prevention and Control National
Site Specific Data Items (SSDI)
 Jennifer Ruhl Site Specific Data Items (SSDI) Developed by Jennifer Ruhl and the SSDI Taskforce What is an SSDI Site-Specific Data Item Based on primary site AJCC Chapter Summary Stage chapter Previously
Jennifer Ruhl Site Specific Data Items (SSDI) Developed by Jennifer Ruhl and the SSDI Taskforce What is an SSDI Site-Specific Data Item Based on primary site AJCC Chapter Summary Stage chapter Previously
Synoptic Reporting in Tumor Pathology Advantages of a Web-Based System
 Informatics / SYNOPTIC REPORTING IN TUMOR PATHOLOGY Synoptic Reporting in Tumor Pathology Advantages of a Web-Based System Zhenhong Qu, MD, PhD, 1 Shibu Ninan, MS, 1 Ahmed Almosa, MS, 1 K.G. Chang, MD,
Informatics / SYNOPTIC REPORTING IN TUMOR PATHOLOGY Synoptic Reporting in Tumor Pathology Advantages of a Web-Based System Zhenhong Qu, MD, PhD, 1 Shibu Ninan, MS, 1 Ahmed Almosa, MS, 1 K.G. Chang, MD,
The first approach to accessing pathology diagnostic information is the use of synoptic worksheets produced by the
 ABSTRACT INTRODUCTION Expediting Access to Critical Pathology Data Leanne Goldstein, City of Hope, Duarte, CA Rebecca Ottesen, City of Hope, Duarte, CA Julie Kilburn, City of Hope, Duarte, CA Joyce Niland,
ABSTRACT INTRODUCTION Expediting Access to Critical Pathology Data Leanne Goldstein, City of Hope, Duarte, CA Rebecca Ottesen, City of Hope, Duarte, CA Julie Kilburn, City of Hope, Duarte, CA Joyce Niland,
Structured Data Capture (SDC) System Manual
 Structured Data Capture (SDC) System Manual Jun 2018 Version 1.0 325 Waukegan Rd. Northfield, IL 60093 t: 800-323-4040 cap.org Version No 1.0. SDC User Guide 1 Table of Contents Structured Data Capture
Structured Data Capture (SDC) System Manual Jun 2018 Version 1.0 325 Waukegan Rd. Northfield, IL 60093 t: 800-323-4040 cap.org Version No 1.0. SDC User Guide 1 Table of Contents Structured Data Capture
Mapper Plus. Program Directors Meeting Atlanta April 16, 2009
 Mapper Plus Program Directors Meeting Atlanta April 16, 2009 Sanjeev Baral, Northrop Grumman contractor Wendy Scharber, Northrop Grumman contractor Sandy Thames, CDC Introduction Originally developed as
Mapper Plus Program Directors Meeting Atlanta April 16, 2009 Sanjeev Baral, Northrop Grumman contractor Wendy Scharber, Northrop Grumman contractor Sandy Thames, CDC Introduction Originally developed as
Terminology Harmonization
 Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
Guiding the Way to XML Data Exchange Implementation
 Guiding the Way to XML Data Exchange Implementation Isaac Hands, MPH Lead Software Architect, Kentucky Cancer Registry Chair, NAACCR XML Data Exchange Workgroup Representative-at-Large, NAACCR Board of
Guiding the Way to XML Data Exchange Implementation Isaac Hands, MPH Lead Software Architect, Kentucky Cancer Registry Chair, NAACCR XML Data Exchange Workgroup Representative-at-Large, NAACCR Board of
Frequently Asked Questions
 Frequently Asked Questions ISO 15189 Accreditation Program cap.org Contents ISO and the International Organization for Standardization What does ISO stand for? (page 3) What is the International Organization
Frequently Asked Questions ISO 15189 Accreditation Program cap.org Contents ISO and the International Organization for Standardization What does ISO stand for? (page 3) What is the International Organization
STANDARDS FOR AUTOMATED REPORTING
 North Carolina Central Cancer Registry STANDARDS FOR AUTOMATED REPORTING In accordance with the North American Association of Central Cancer Registries Record Layout Version 16.0 Effective 1/1/2016 January
North Carolina Central Cancer Registry STANDARDS FOR AUTOMATED REPORTING In accordance with the North American Association of Central Cancer Registries Record Layout Version 16.0 Effective 1/1/2016 January
I see it now! New Data Visualizations of U.S. Cancer Statistics
 I see it now! New Data Visualizations of U.S. Cancer Statistics Loria Pollack, MD, MPH CDC National Program of Cancer Registries On behalf of the CDC Cancer Data Visualization Workgroup NAACCR 2017 June
I see it now! New Data Visualizations of U.S. Cancer Statistics Loria Pollack, MD, MPH CDC National Program of Cancer Registries On behalf of the CDC Cancer Data Visualization Workgroup NAACCR 2017 June
05/12/2011 Revision: 04/19/2011 Revision:
 Collaborative Stage Version 2: 020000/020001/020100/020200 to Collaborative Stage Version 2: 020300/020301/020302 Revised Conversion Specifications/Release Notes 2/15/2011 Revised Conversion Specifications/Release
Collaborative Stage Version 2: 020000/020001/020100/020200 to Collaborative Stage Version 2: 020300/020301/020302 Revised Conversion Specifications/Release Notes 2/15/2011 Revised Conversion Specifications/Release
Interoperability. Doug Fridsma, MD PhD President & CEO, AMIA
 Interoperability Doug Fridsma, MD PhD President & CEO, AMIA Getting to Interoperability To get to interoperability (or to avoid information blocking) we need a common understanding of the problem Can t
Interoperability Doug Fridsma, MD PhD President & CEO, AMIA Getting to Interoperability To get to interoperability (or to avoid information blocking) we need a common understanding of the problem Can t
Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research
 Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research Ian Fore, D.Phil. Associate Director, Biorepository and Pathology Informatics Senior Program
Interoperability and Semantics in Use- Application of UML, XMI and MDA to Precision Medicine and Cancer Research Ian Fore, D.Phil. Associate Director, Biorepository and Pathology Informatics Senior Program
NCDB Vendor Webinar: NCDB Call for Data April 2019 and RQRS Updates
 NCDB Vendor Webinar: NCDB Call for Data April 2019 and RQRS Updates Topics to be Covered Today NCDB: 2019 Call for Data Submission Specifications Record layout and edits Mapping of NPI Data Items Facilitating
NCDB Vendor Webinar: NCDB Call for Data April 2019 and RQRS Updates Topics to be Covered Today NCDB: 2019 Call for Data Submission Specifications Record layout and edits Mapping of NPI Data Items Facilitating
Pathology Informatics Training and Education Workshop Lab InfoTech Summit April 9, 2008
 Pathology Informatics Training and Education Workshop Lab InfoTech Summit April 9, 2008 Walter H. Henricks, M.D. Cleveland Clinic Pathology Informatics Mission of pathology is to provide information necessary
Pathology Informatics Training and Education Workshop Lab InfoTech Summit April 9, 2008 Walter H. Henricks, M.D. Cleveland Clinic Pathology Informatics Mission of pathology is to provide information necessary
ALBERTA ADVERSE EVENT FOLLOWING IMMUNIZATION(AEFI) HL7 MESSAGING SPECIFICATION
 Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA ALBERTA ADVERSE EVENT FOLLOWING IMMUNIZATION(AEFI) HL7 MESSAGING SPECIFICATION MESSAGE STANDARD SUMMARY Status:
Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA ALBERTA ADVERSE EVENT FOLLOWING IMMUNIZATION(AEFI) HL7 MESSAGING SPECIFICATION MESSAGE STANDARD SUMMARY Status:
pathx pathx Laboratory Information System
 A LETTER FROM THE PRESIDENT. At PathX, we fully understand that your lab is focused on more than just patient reports and information processing. For most pathology labs, primary concerns include responsible
A LETTER FROM THE PRESIDENT. At PathX, we fully understand that your lab is focused on more than just patient reports and information processing. For most pathology labs, primary concerns include responsible
How to Place an Order
 How to Place an Order In this document, you will find information or step-by-step directions on the following topics: Add Items to Your Cart... 2 Add Programs, Publications, and Learning Items to Your
How to Place an Order In this document, you will find information or step-by-step directions on the following topics: Add Items to Your Cart... 2 Add Programs, Publications, and Learning Items to Your
Standards for Cancer Registries, Volume I. Data Exchange Standards and Record Descriptions
 Standards for Cancer Registries, Volume I Data Exchange Standards and Record Descriptions Version 16 November 2015 Edited by Lori A. Havener, CTR Sponsoring Organizations Canadian Partnership Against Cancer
Standards for Cancer Registries, Volume I Data Exchange Standards and Record Descriptions Version 16 November 2015 Edited by Lori A. Havener, CTR Sponsoring Organizations Canadian Partnership Against Cancer
Implementing Automated Pathology Report Case Identification In a Cancer Registry
 Implementing Automated Pathology Report Case Identification In a Cancer Registry Ann Griffin, PhD, CTR, UCSF Comprehensive Cancer Center San Francisco, California Chris Rogers C/NET Solutions Berkeley,
Implementing Automated Pathology Report Case Identification In a Cancer Registry Ann Griffin, PhD, CTR, UCSF Comprehensive Cancer Center San Francisco, California Chris Rogers C/NET Solutions Berkeley,
Alan Houser, C/NET Solutions, Public Health Institute Jennifer Seiffert, Northrop Grumman Mission Systems
 Publicly Available National Standard References in Online HTML Help Alan Houser, C/NET Solutions, Public Health Institute Jennifer Seiffert, Northrop Grumman Mission Systems DEPARTMENT OF HEALTH AND HUMAN
Publicly Available National Standard References in Online HTML Help Alan Houser, C/NET Solutions, Public Health Institute Jennifer Seiffert, Northrop Grumman Mission Systems DEPARTMENT OF HEALTH AND HUMAN
Introduction to XML. How it Works and What it Offers Us
 Introduction to XML How it Works and What it Offers Us Introduction NAACCR & XML Basics of the XML format What an XML structure offers NAACCR & XML The Flat File file sizes (full format) Type A Full Case
Introduction to XML How it Works and What it Offers Us Introduction NAACCR & XML Basics of the XML format What an XML structure offers NAACCR & XML The Flat File file sizes (full format) Type A Full Case
Ensuring Quality Terminology Mappings in Distributed SOA Environments
 Ensuring Quality Terminology Mappings in Distributed SOA Environments SOA in Healthcare Chicago Illinois April 16, 2008 Russell Hamm Informatics Consultant Apelon, Inc. 1 Outline Data standardization Goals
Ensuring Quality Terminology Mappings in Distributed SOA Environments SOA in Healthcare Chicago Illinois April 16, 2008 Russell Hamm Informatics Consultant Apelon, Inc. 1 Outline Data standardization Goals
Integrating the Healthcare Enterprise Patient Care Devices
 Integrating the Healthcare Enterprise Patient Care Devices Anything can be integrated Un-Interoperability: Highest Cause of Health IT project failures Base Standards The Hospital EHRs, CMMS, other ehealth
Integrating the Healthcare Enterprise Patient Care Devices Anything can be integrated Un-Interoperability: Highest Cause of Health IT project failures Base Standards The Hospital EHRs, CMMS, other ehealth
Specification of a Cardiovascular Metadata Model: A Consensus Standard
 Specification of a Cardiovascular Metadata Model: A Consensus Standard Rebecca Wilgus, RN MSN 1 ; Dana Pinchotti 2 ; Salvatore Mungal 3 ; David F Kong, MD AM 1 ; James E Tcheng, MD 1 ; Brian McCourt 1
Specification of a Cardiovascular Metadata Model: A Consensus Standard Rebecca Wilgus, RN MSN 1 ; Dana Pinchotti 2 ; Salvatore Mungal 3 ; David F Kong, MD AM 1 ; James E Tcheng, MD 1 ; Brian McCourt 1
Quality Data Model (QDM)
 Quality Data Model (QDM) Overview Document National Quality Forum 4/20/2011 QUALITY DATA MODEL (QDM): OVERVIEW TABLE OF CONTENTS Quality Data Model (QDM): Overview... 2 National Quality Forum: Overview
Quality Data Model (QDM) Overview Document National Quality Forum 4/20/2011 QUALITY DATA MODEL (QDM): OVERVIEW TABLE OF CONTENTS Quality Data Model (QDM): Overview... 2 National Quality Forum: Overview
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview
 Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
Digital Imaging and Communications in Medicine (DICOM) Part 1: Introduction and Overview Published by National Electrical Manufacturers Association 1300 N. 17th Street Rosslyn, Virginia 22209 USA Copyright
From Integration to Interoperability: The Role of Public Health Systems in the Emerging World of Health Information Exchange
 From Integration to Interoperability: The Role of Public Health Systems in the Emerging World of Health Information Exchange Noam H. Arzt, PhD American Public Health Association Annual Meeting Session
From Integration to Interoperability: The Role of Public Health Systems in the Emerging World of Health Information Exchange Noam H. Arzt, PhD American Public Health Association Annual Meeting Session
Title of Manual: Specimen Collection Document Number: GPA.SPC.52.0
 Page 1 of 17 I._PURPOSE This document is a guideline of procedures for Outreach 2.0 ordering and results application. It is intended to outline the features and functions of Outreach 2.0. All major functionality
Page 1 of 17 I._PURPOSE This document is a guideline of procedures for Outreach 2.0 ordering and results application. It is intended to outline the features and functions of Outreach 2.0. All major functionality
Exchanging Patient Demographics Information using ANSI/HL7 v2.8.2
 Exchanging Patient Demographics Information using ANSI/HL7 v2.8.2 Created by: National Resource Centre for EHR Standards, Centre for Development of Advanced Computing (C-DAC), Pune, India Published: August
Exchanging Patient Demographics Information using ANSI/HL7 v2.8.2 Created by: National Resource Centre for EHR Standards, Centre for Development of Advanced Computing (C-DAC), Pune, India Published: August
How to Place an Order
 How to Place an Order In this document, you will find information or step-by-step directions on the following topics: Add Items to Your Cart... 2 Add Programs, Publications, and Learning Items to Your
How to Place an Order In this document, you will find information or step-by-step directions on the following topics: Add Items to Your Cart... 2 Add Programs, Publications, and Learning Items to Your
Natural Language Processing
 Natural Language Processing NLP to Enhance Clinical Decision Support Peter Haug MD Intermountain Healthcare Testing a Series of NLP Systems Key Goal: : supporting clinical decision support systems. SPRUS
Natural Language Processing NLP to Enhance Clinical Decision Support Peter Haug MD Intermountain Healthcare Testing a Series of NLP Systems Key Goal: : supporting clinical decision support systems. SPRUS
Robert Snelick, NIST Sheryl Taylor, BAH. October 11th, 2012
 Test Tool Orientation for International Society for Disease Surveillance (ISDS): 2014 Edition 170.314(f)(3) Transmission to Public Health Agencies - Syndromic Surveillance Robert Snelick, NIST Sheryl Taylor,
Test Tool Orientation for International Society for Disease Surveillance (ISDS): 2014 Edition 170.314(f)(3) Transmission to Public Health Agencies - Syndromic Surveillance Robert Snelick, NIST Sheryl Taylor,
These are suggestions not policy. It is one approach that may help us understand
 Core Services List We propose to use NHIN terms to describe high-level services associated with data integration. The enclosed list is a sub-set of the longer and more extensive list of NHIN II objectives.
Core Services List We propose to use NHIN terms to describe high-level services associated with data integration. The enclosed list is a sub-set of the longer and more extensive list of NHIN II objectives.
DICOM Conformance Statement
 DICOM Conformance Statement TOPCON Corporation TOPCON SPECULAR MICROSCOPE SP-1P Version 1.10 Document Version: 1 Date: 2014-02-17 1 CONFORMANCE STATEMENT OVERVIEW This document declares conformance to
DICOM Conformance Statement TOPCON Corporation TOPCON SPECULAR MICROSCOPE SP-1P Version 1.10 Document Version: 1 Date: 2014-02-17 1 CONFORMANCE STATEMENT OVERVIEW This document declares conformance to
NAACCR XML Data Exchange Standard
 NAACCR XML Data Exchange Standard Implementation Guide Based on Specifications v1.3 of the NAACCR XML Standard Last updated on August 2017 Sponsoring Organizations Canadian Partnership Against Cancer Centers
NAACCR XML Data Exchange Standard Implementation Guide Based on Specifications v1.3 of the NAACCR XML Standard Last updated on August 2017 Sponsoring Organizations Canadian Partnership Against Cancer Centers
CAP Quick Guide for Proficiency Testing
 CAP Quick Guide for Proficiency Testing Definitions e-lab Solutions Suite (ELSS): The CAP s online portal to manage your laboratory improvement programs, including proficiency testing (PT), accreditation,
CAP Quick Guide for Proficiency Testing Definitions e-lab Solutions Suite (ELSS): The CAP s online portal to manage your laboratory improvement programs, including proficiency testing (PT), accreditation,
Workshop 2. > Interoperability <
 Workshop 2 21 / 08 / 2011 > Interoperability < Heiko Zimmermann R&D Engineer, AHI CR Santec Heiko.Zimmermann@tudor.lu Interoperability definition Picture from NCI-Wiki (https://wiki.nci.nih.gov) 2 Interoperability
Workshop 2 21 / 08 / 2011 > Interoperability < Heiko Zimmermann R&D Engineer, AHI CR Santec Heiko.Zimmermann@tudor.lu Interoperability definition Picture from NCI-Wiki (https://wiki.nci.nih.gov) 2 Interoperability
Accuracy made easy. Improving patient safety through lean workflow. Thermo Scientific Labelling and Tracking Solutions
 Thermo Scientific Labelling and Tracking Solutions Accuracy made easy Improving patient safety through lean workflow PrintMate AS Cassette Printer SlideMate AS Slide Printer LabWriter Software Primary
Thermo Scientific Labelling and Tracking Solutions Accuracy made easy Improving patient safety through lean workflow PrintMate AS Cassette Printer SlideMate AS Slide Printer LabWriter Software Primary
Covisint MIPS Quick Start User Guide
 Covisint MIPS Quick Start User Guide The Quick Start instructions explain the MIPS registration process, collecting and entering patient data online, and the submission process. Updated December 2017 Table
Covisint MIPS Quick Start User Guide The Quick Start instructions explain the MIPS registration process, collecting and entering patient data online, and the submission process. Updated December 2017 Table
DATA VALIDATION RESOURCES RCHC Data Group Webinar By Ben Fouts MPH November 14, 2017
 DATA VALIDATION RESOURCES RCHC Data Group Webinar By Ben Fouts MPH November 14, 2017 Agenda 1. Documents on new RCHC Peer Collaboration web page 2. Data Validation Resources (Introduction to the new version
DATA VALIDATION RESOURCES RCHC Data Group Webinar By Ben Fouts MPH November 14, 2017 Agenda 1. Documents on new RCHC Peer Collaboration web page 2. Data Validation Resources (Introduction to the new version
7/31/2012. NCI SEER CDC NPCR ACOS COC Other States Florida-Specific Collaborative Stage NAACCR EDITS Working Group
 Blank Field Checks Single Item Edit Valid Code Checks Single Item Edit Valid Date Checks Single Item Edit Inter-Field Edits Relationships Between Items Inter-Record Edits Relationships Between Cases CS
Blank Field Checks Single Item Edit Valid Code Checks Single Item Edit Valid Date Checks Single Item Edit Inter-Field Edits Relationships Between Items Inter-Record Edits Relationships Between Cases CS
Accuracy made easy. Improving patient safety through lean workflow. Thermo Scientific Labeling and Tracking Solutions
 Thermo Scientific Labeling and Tracking Solutions Accuracy made easy Improving patient safety through lean workflow PrintMate AS Cassette Printer SlideMate AS Slide Printer LabWriter Software Primary labeling
Thermo Scientific Labeling and Tracking Solutions Accuracy made easy Improving patient safety through lean workflow PrintMate AS Cassette Printer SlideMate AS Slide Printer LabWriter Software Primary labeling
Moving to the Web: A Glimpse of the Future
 Moving to the Web: A Glimpse of the Future Internet Communications for Cancer Registr James Harrison, M.D., Ph.D. Associate Director of Pathology Informatics Department of Pathology University of Pittsburgh
Moving to the Web: A Glimpse of the Future Internet Communications for Cancer Registr James Harrison, M.D., Ph.D. Associate Director of Pathology Informatics Department of Pathology University of Pittsburgh
ilabs User Guide ilabs Registration Process 1. ilabs registration requires the following information:
 ilabs User Guide ilabs Registration Process 1. ilabs registration requires the following information: an active institution email address your institutional role your PI s name, email address and telephone
ilabs User Guide ilabs Registration Process 1. ilabs registration requires the following information: an active institution email address your institutional role your PI s name, email address and telephone
Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies
 Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies Harold R. Solbrig 1, Guoqian Jiang 1 1 Mayo Clinic College of Medicine, Rochester, MN [solbrig.harold,
Harmonizing biocaddie Metadata Schemas for Indexing Clinical Research Datasets Using Semantic Web Technologies Harold R. Solbrig 1, Guoqian Jiang 1 1 Mayo Clinic College of Medicine, Rochester, MN [solbrig.harold,
Standards for Cancer Registries Volume V: Pathology Laboratory Electronic Reporting Supplement Version 1
 North American Association of Central Cancer Registries, Inc. Standards for Cancer Registries Volume V: Pathology Laboratory Electronic Reporting Supplement Version 1 Edited by Lori A. Havener Suggested
North American Association of Central Cancer Registries, Inc. Standards for Cancer Registries Volume V: Pathology Laboratory Electronic Reporting Supplement Version 1 Edited by Lori A. Havener Suggested
An Annotation Tool for Semantic Documents
 An Annotation Tool for Semantic Documents (System Description) Henrik Eriksson Dept. of Computer and Information Science Linköping University SE-581 83 Linköping, Sweden her@ida.liu.se Abstract. Document
An Annotation Tool for Semantic Documents (System Description) Henrik Eriksson Dept. of Computer and Information Science Linköping University SE-581 83 Linköping, Sweden her@ida.liu.se Abstract. Document
Clinical Optimization
 Clinical Optimization Learning Objectives Uses of the Alt Key User Preferences to customize Accuro for you Home Section Tips Shortcut Keys and their functions Virtual Chart tips Use of the ALT Key Alt+
Clinical Optimization Learning Objectives Uses of the Alt Key User Preferences to customize Accuro for you Home Section Tips Shortcut Keys and their functions Virtual Chart tips Use of the ALT Key Alt+
A Web-Based Protocol Tracking Management System For Clinical Research
 A Web-Based Protocol Tracking Management System For Clinical Research Huey Cheung a, Yang Fann b, Shaohua A. Wang a, Barg Upender a, Adam Frazin a Raj Lingam b, Sarada Chintala a, Frank Pecjak a, Gladys
A Web-Based Protocol Tracking Management System For Clinical Research Huey Cheung a, Yang Fann b, Shaohua A. Wang a, Barg Upender a, Adam Frazin a Raj Lingam b, Sarada Chintala a, Frank Pecjak a, Gladys
edify Requirements for Evidence-based Templates in Electronic Case Report Forms Marco Schweitzer, Stefan Oberbichler
 edify Requirements for Evidence-based Templates in Electronic Case Report Forms Marco Schweitzer, Stefan Oberbichler ehealth Research and Innovation Unit, UMIT - University for Health Sciences, Medical
edify Requirements for Evidence-based Templates in Electronic Case Report Forms Marco Schweitzer, Stefan Oberbichler ehealth Research and Innovation Unit, UMIT - University for Health Sciences, Medical
Clinical Optimization
 Clinical Optimization Learning Objectives Uses of the Alt Key User Preferences to customize Accuro for you Home Section Tips Shortcut Keys and their functions Virtual Chart tips Use of the ALT Key Alt+
Clinical Optimization Learning Objectives Uses of the Alt Key User Preferences to customize Accuro for you Home Section Tips Shortcut Keys and their functions Virtual Chart tips Use of the ALT Key Alt+
HRP Magenta chromogen for Dako Omnis. Simply revolutionary 'red'
 HRP Magenta chromogen for Dako Omnis Simply revolutionary 'red' A new color for the EnVision FLEX family on Dako Omnis Introducing HRP Magenta, a new chromogen available for your Dako Omnis solution that
HRP Magenta chromogen for Dako Omnis Simply revolutionary 'red' A new color for the EnVision FLEX family on Dako Omnis Introducing HRP Magenta, a new chromogen available for your Dako Omnis solution that
ALBERTA IMMUNIZATION HL7 MESSAGING SPECIFICATION
 Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA ALBERTA IMMUNIZATION HL7 MESSAGING SPECIFICATION MESSAGE STANDARD SUMMARY Status: Accepted in Draft Version:
Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA ALBERTA IMMUNIZATION HL7 MESSAGING SPECIFICATION MESSAGE STANDARD SUMMARY Status: Accepted in Draft Version:
CTSI Module 8 Workshop Introduction to Biomedical Informatics, Part V
 CTSI Module 8 Workshop Introduction to Biomedical Informatics, Part V Practical Tools: Data Processing & Analysis William Hsu, PhD Assistant Professor Medical Imaging Informatics Group Dept of Radiological
CTSI Module 8 Workshop Introduction to Biomedical Informatics, Part V Practical Tools: Data Processing & Analysis William Hsu, PhD Assistant Professor Medical Imaging Informatics Group Dept of Radiological
The SHARPn phenotyping funnel. Phenotype specific patient cohorts
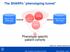 The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
Electronic Service Provider Standard
 Electronic Service Provider Standard Version: 1.6 Document ID: 3538 Copyright Notice Copyright 2018, ehealth Ontario All rights reserved No part of this document may be reproduced in any form, including
Electronic Service Provider Standard Version: 1.6 Document ID: 3538 Copyright Notice Copyright 2018, ehealth Ontario All rights reserved No part of this document may be reproduced in any form, including
CAP ecc XML Comparator User Guide
 CAP ecc XML Comparator User Guide November 2017 Version 1.0 325 Waukegan Rd. Northfield, IL 60093 t: 800-323-4040 cap.org Version No 1.0. CAP ecc XML Comparator User Guide 1 Table of Contents Introduction...2
CAP ecc XML Comparator User Guide November 2017 Version 1.0 325 Waukegan Rd. Northfield, IL 60093 t: 800-323-4040 cap.org Version No 1.0. CAP ecc XML Comparator User Guide 1 Table of Contents Introduction...2
Executive Summary for deliverable D6.1: Definition of the PFS services (requirements, initial design)
 Electronic Health Records for Clinical Research Executive Summary for deliverable D6.1: Definition of the PFS services (requirements, initial design) Project acronym: EHR4CR Project full title: Electronic
Electronic Health Records for Clinical Research Executive Summary for deliverable D6.1: Definition of the PFS services (requirements, initial design) Project acronym: EHR4CR Project full title: Electronic
ConCert FAQ s Last revised December 2017
 ConCert FAQ s Last revised December 2017 What is ConCert by HIMSS? ConCert by HIMSS is a comprehensive interoperability testing and certification program governed by HIMSS and built on the work of the
ConCert FAQ s Last revised December 2017 What is ConCert by HIMSS? ConCert by HIMSS is a comprehensive interoperability testing and certification program governed by HIMSS and built on the work of the
Healthcare Information Technology Standards Panel (HITSP) - FOR INFORMATION. HITSP-USHIK Quick Start Guide & USHIK Overview
 Document Number: HITSP 10 N 462 Date: February 3, 2010 TO: Healthcare Information Technology Standards Panel (HITSP) - FOR INFORMATION FROM: RE: Michelle Maas Deane HITSP Secretariat American National
Document Number: HITSP 10 N 462 Date: February 3, 2010 TO: Healthcare Information Technology Standards Panel (HITSP) - FOR INFORMATION FROM: RE: Michelle Maas Deane HITSP Secretariat American National
Building a Data Catalog
 Building a Data Catalog Promoting Data Reuse and Collaboration at an Academic Medical Center Kevin Read, MLIS, MAS Alisa Surkis, PhD, MLIS EXTERNAL DATASETS 2 EXTERNAL DATASETS INTERNAL DATASETS 3 NYU
Building a Data Catalog Promoting Data Reuse and Collaboration at an Academic Medical Center Kevin Read, MLIS, MAS Alisa Surkis, PhD, MLIS EXTERNAL DATASETS 2 EXTERNAL DATASETS INTERNAL DATASETS 3 NYU
M2 Glossary of Terms and Abbreviations
 M2 Glossary of Terms and Abbreviations 11 June 2015 M2: Electronic Standards for the Transfer of Regulatory Information Updated at ICH Expert Working Group meeting, Fukuoka, June 2015 Definitions... 2
M2 Glossary of Terms and Abbreviations 11 June 2015 M2: Electronic Standards for the Transfer of Regulatory Information Updated at ICH Expert Working Group meeting, Fukuoka, June 2015 Definitions... 2
Forcare B.V. Cross-Enterprise Document Sharing (XDS) Whitepaper
 Cross-Enterprise Document Sharing (XDS) Copyright 2010 Forcare B.V. This publication may be distributed in its unmodified whole with references to the author and company name. Andries Hamster Forcare B.V.
Cross-Enterprise Document Sharing (XDS) Copyright 2010 Forcare B.V. This publication may be distributed in its unmodified whole with references to the author and company name. Andries Hamster Forcare B.V.
IHE IT Infrastructure Technical Framework Supplement. Non-patient File Sharing (NPFSm) Rev. 1.1 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE IT Infrastructure Technical Framework Supplement 10 Non-patient File Sharing (NPFSm) HL7 FHIR STU 3 15 Using Resources at FMM Level 3-5 Rev. 1.1 Trial Implementation
Integrating the Healthcare Enterprise 5 IHE IT Infrastructure Technical Framework Supplement 10 Non-patient File Sharing (NPFSm) HL7 FHIR STU 3 15 Using Resources at FMM Level 3-5 Rev. 1.1 Trial Implementation
Leica BOND III. Fully automated IHC & ISH. Quality. Speed. Efficiency.
 Leica BOND III Fully automated IHC & ISH Quality. Speed. Efficiency. QUALITY Increase diagnostic confidence speed Provide answers sooner EFFICIENCY Help more patients and lower costs Connect Reduce work
Leica BOND III Fully automated IHC & ISH Quality. Speed. Efficiency. QUALITY Increase diagnostic confidence speed Provide answers sooner EFFICIENCY Help more patients and lower costs Connect Reduce work
LAB INFORMATION SYSTEMS MESSAGE STANDARD SUMMARY
 Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA LAB INFORMATION SYSTEMS MESSAGE STANDARD SUMMARY HL7 V2.4 LAB TEST RESULT DELIVERY SPECIFICATION AND HL7V3
Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA LAB INFORMATION SYSTEMS MESSAGE STANDARD SUMMARY HL7 V2.4 LAB TEST RESULT DELIVERY SPECIFICATION AND HL7V3
Analysis of the Central District Clinic
 Analysis of the Central District Clinic &6( Project 4 will require the creation of a database system to support the operation of a fictitious health clinic in downtown Seattle by studying how an organization
Analysis of the Central District Clinic &6( Project 4 will require the creation of a database system to support the operation of a fictitious health clinic in downtown Seattle by studying how an organization
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services MLN Matters Number: SE1620 Related CR Release Date: N/A Related CR Transmittal #: N/A Related Change Request (CR) #: N/A
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services MLN Matters Number: SE1620 Related CR Release Date: N/A Related CR Transmittal #: N/A Related Change Request (CR) #: N/A
IHE Endoscopy Technical Framework Supplement. Endoscopy Image Archiving (EIA) Rev. 1.1 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Endoscopy Technical Framework Supplement 10 Endoscopy Image Archiving (EIA) 15 Rev. 1.1 Trial Implementation 20 Date: November 28, 2018 Author: IHE Endoscopy
Integrating the Healthcare Enterprise 5 IHE Endoscopy Technical Framework Supplement 10 Endoscopy Image Archiving (EIA) 15 Rev. 1.1 Trial Implementation 20 Date: November 28, 2018 Author: IHE Endoscopy
ICD-10 Customer Testing Guidelines and Scenarios
 ICD-10 Customer Testing Guidelines and Scenarios Revision 2.0 Updated July 21, 2015 This document includes pre-release information and user interface designs that are in development for upcoming enhancements
ICD-10 Customer Testing Guidelines and Scenarios Revision 2.0 Updated July 21, 2015 This document includes pre-release information and user interface designs that are in development for upcoming enhancements
Request for Information Technical Response White Paper for Joint Operational Medicine Information Systems (JOMIS)
 Request for Information Technical Response White Paper for Joint Operational Medicine Information Systems (JOMIS) Ensuring Continuity of Data 5 May 2017 Prepared by: Northrop Grumman Systems Corporation
Request for Information Technical Response White Paper for Joint Operational Medicine Information Systems (JOMIS) Ensuring Continuity of Data 5 May 2017 Prepared by: Northrop Grumman Systems Corporation
NJIIS Immunization Registry
 NJIIS Immunization Registry This document, as well as the software dscribed in it, is provided under a software license agreement with STI Computer Services, Inc. Use of this software and all related documentation
NJIIS Immunization Registry This document, as well as the software dscribed in it, is provided under a software license agreement with STI Computer Services, Inc. Use of this software and all related documentation
University of Kentucky College of Nursing Electronic Practice Inquiry Project Submission Guide
 University of Kentucky College of Nursing Electronic Practice Inquiry Project Submission Guide UKnowledge (http://uknowledge.uky.edu/) is a digital collection of unique scholarship created by the University
University of Kentucky College of Nursing Electronic Practice Inquiry Project Submission Guide UKnowledge (http://uknowledge.uky.edu/) is a digital collection of unique scholarship created by the University
Standards Driven Innovation
 Standards Driven Innovation PhUSE Annual Conference 2014 Frederik Malfait IMOS Consulting GmbH, Hoffmann-La Roche AG Managing Standards 2 Data Standards Value Proposition Standards are increasingly mandated
Standards Driven Innovation PhUSE Annual Conference 2014 Frederik Malfait IMOS Consulting GmbH, Hoffmann-La Roche AG Managing Standards 2 Data Standards Value Proposition Standards are increasingly mandated
When Communities of Interest Collide: Harmonizing Vocabularies Across Operational Areas C. L. Connors, The MITRE Corporation
 When Communities of Interest Collide: Harmonizing Vocabularies Across Operational Areas C. L. Connors, The MITRE Corporation Three recent trends have had a profound impact on data standardization within
When Communities of Interest Collide: Harmonizing Vocabularies Across Operational Areas C. L. Connors, The MITRE Corporation Three recent trends have had a profound impact on data standardization within
FCDS IDEA User Accounts
 FCDS IDEA User Accounts 1.) Do I need an FCDS IDEA User Account? Yes, anyone accessing IDEA will need an FCDS IDEA User Account. 2.) How do I create an FCDS IDEA user account? Please follow the instructions
FCDS IDEA User Accounts 1.) Do I need an FCDS IDEA User Account? Yes, anyone accessing IDEA will need an FCDS IDEA User Account. 2.) How do I create an FCDS IDEA user account? Please follow the instructions
Integrating BigMatch into Automated Registry Record Linkage Operations
 Integrating BigMatch into Automated Registry Record Linkage Operations 2014 NAACCR Annual Conference June 25, 2014 Jason Jacob, MS, Isaac Hands, MPH, David Rust, MS Kentucky Cancer Registry Overview Record
Integrating BigMatch into Automated Registry Record Linkage Operations 2014 NAACCR Annual Conference June 25, 2014 Jason Jacob, MS, Isaac Hands, MPH, David Rust, MS Kentucky Cancer Registry Overview Record
Scheduling Book Set-Up & Check-In Preferences
 Clinical Informatics Scheduling Book Set-Up & Check-In Preferences 10/23/2018 Clinical Informatics 1 Scheduling Book Settings It is important that ALL settings in the Scheduling Book are set up correctly,
Clinical Informatics Scheduling Book Set-Up & Check-In Preferences 10/23/2018 Clinical Informatics 1 Scheduling Book Settings It is important that ALL settings in the Scheduling Book are set up correctly,
The Beau Biden Cancer Moonshot and the NCI Blue Ribbon Panel Recommendations. NAACCR Registry of the Future Presentation June 19, 2017
 The Beau Biden Cancer Moonshot and the NCI Blue Ribbon Panel Recommendations NAACCR Registry of the Future Presentation June 19, 2017 The Beau Biden Cancer Moonshot Accelerate progress in cancer, including
The Beau Biden Cancer Moonshot and the NCI Blue Ribbon Panel Recommendations NAACCR Registry of the Future Presentation June 19, 2017 The Beau Biden Cancer Moonshot Accelerate progress in cancer, including
Image analysis in IHC - overview, considerations and applications
 Image analysis in IHC - overview, considerations and applications Workshop in Diagnostic Immunohistochemistry Oud St. Jan/ Old St. John Brugge (Bruges), Belgium June 13th 15nd 2018 Rasmus Røge, MD, NordiQC
Image analysis in IHC - overview, considerations and applications Workshop in Diagnostic Immunohistochemistry Oud St. Jan/ Old St. John Brugge (Bruges), Belgium June 13th 15nd 2018 Rasmus Røge, MD, NordiQC
LiXuid Manuscript. Sean MacRae, Business Systems Analyst
 LiXuid Manuscript Sean MacRae, Business Systems Analyst Where are we heading? XML Workflows! Copy Edit XML Conversion Edit: Check Text Edit: Auth Proof Edit: Check Qs Source Files XML-working files And
LiXuid Manuscript Sean MacRae, Business Systems Analyst Where are we heading? XML Workflows! Copy Edit XML Conversion Edit: Check Text Edit: Auth Proof Edit: Check Qs Source Files XML-working files And
Electronic Health Reporting and Cancer Surveillance in the U.S.
 Electronic Health Reporting and Cancer Surveillance in the U.S. NAACCR 2006 Annual Meeting June 13, 2006 Sandy Thames, CDC Presentation Overview President s Health Initiative Electronic Health Records
Electronic Health Reporting and Cancer Surveillance in the U.S. NAACCR 2006 Annual Meeting June 13, 2006 Sandy Thames, CDC Presentation Overview President s Health Initiative Electronic Health Records
CancerCORE. A New Innovation in Central Cancer Registry Software. Presented by: Catherine Tapp, MPH & Chris Fisher Arkansas Central Cancer Registry
 CancerCORE A New Innovation in Central Cancer Registry Software Presented by: Catherine Tapp, MPH & Chris Fisher Arkansas Central Cancer Registry NAACCR Boston 2005 History Utilized several applications
CancerCORE A New Innovation in Central Cancer Registry Software Presented by: Catherine Tapp, MPH & Chris Fisher Arkansas Central Cancer Registry NAACCR Boston 2005 History Utilized several applications
Health Information Exchange: Can There Be ONE National Model?
 Health Information Exchange: Can There Be ONE National Model? Session 56, February 20, 2017 John P. Kansky, President & CEO, Indiana Health Information Exchange Keith W. Kelley, Vice President of Solution
Health Information Exchange: Can There Be ONE National Model? Session 56, February 20, 2017 John P. Kansky, President & CEO, Indiana Health Information Exchange Keith W. Kelley, Vice President of Solution
Efficiently Sharing All of a Patient s Data With All their Providers Completing the Model
 www.gdhealth.com Efficiently Sharing All of a Patient s Data With All their Providers Completing the Model Multi-Source Longitudinal Medical Record (MLMR) Thomas Pole and Robert Guajardo 6.13.2017 Our
www.gdhealth.com Efficiently Sharing All of a Patient s Data With All their Providers Completing the Model Multi-Source Longitudinal Medical Record (MLMR) Thomas Pole and Robert Guajardo 6.13.2017 Our
Your Test Your Workflow Your Freedom. Automated Staining Instrument. Freedom
 Flexibility Your Test Your Workflow Your Freedom Automated Staining Instrument Freedom www.biocare.net www.slidestainer.com Enjoy the Freedom of Maximum Flexibility & Productivity Reagent Rack Cold Station
Flexibility Your Test Your Workflow Your Freedom Automated Staining Instrument Freedom www.biocare.net www.slidestainer.com Enjoy the Freedom of Maximum Flexibility & Productivity Reagent Rack Cold Station
ehepqual- HCV Quality of Care Performance Measure Program
 NEW YORK STATE DEPARTMENT OF HEALTH AIDS INSTITUTE ehepqual- HCV Quality of Care Performance Measure Program USERS GUIDE A GUIDE FOR PRIMARY CARE AND HEPATITIS C CARE PROVIDERS * * For use with ehepqual,
NEW YORK STATE DEPARTMENT OF HEALTH AIDS INSTITUTE ehepqual- HCV Quality of Care Performance Measure Program USERS GUIDE A GUIDE FOR PRIMARY CARE AND HEPATITIS C CARE PROVIDERS * * For use with ehepqual,
HIE Participant Onboarding: Best Practices September 7, 2016
 HIE Participant Onboarding: Best Practices September 7, 2016 You have been automatically muted. Please use the Q&A panel to submit questions during the presentation Agenda Introductions Background Understanding
HIE Participant Onboarding: Best Practices September 7, 2016 You have been automatically muted. Please use the Q&A panel to submit questions during the presentation Agenda Introductions Background Understanding
Frequently Asked Questions (FAQs)
 Frequently Asked Questions (FAQs) General Questions Q: What is Medical Matrix Online (MMO)? A: Medical Matrix Online, the successor to the PC Matrix desktop application, is the premier source for occupational
Frequently Asked Questions (FAQs) General Questions Q: What is Medical Matrix Online (MMO)? A: Medical Matrix Online, the successor to the PC Matrix desktop application, is the premier source for occupational
A New GPU-Based Level Set Method for Medical Image Segmentation
 A New GPU-Based Level Set Method for Medical Image Segmentation Wenzhe Xue Research Assistant Radiology Department Mayo Clinic, Scottsdale, AZ Ph.D. Student Biomedical Informatics Arizona State University,
A New GPU-Based Level Set Method for Medical Image Segmentation Wenzhe Xue Research Assistant Radiology Department Mayo Clinic, Scottsdale, AZ Ph.D. Student Biomedical Informatics Arizona State University,
National Renal Administrator s Association Health Information Exchange. CROWNWeb Data Submission User s Guide
 National Renal Administrator s Association Health Information Exchange CROWNWeb Data Submission User s Guide Table of Contents 1 Overview... 3 1.1 Purpose... 3 1.2 Intended Audience... 3 2 NRAA HIE and
National Renal Administrator s Association Health Information Exchange CROWNWeb Data Submission User s Guide Table of Contents 1 Overview... 3 1.1 Purpose... 3 1.2 Intended Audience... 3 2 NRAA HIE and
