CDA and CCD for Patient Summaries
|
|
|
- Edwin Webster
- 6 years ago
- Views:
Transcription
1 CDA and CCD for Patient Summaries Bob Dolin, MD, FACP, FACMI, FHL7 Chair, Health Level Seven President and CMO,
2 What is the CDA? The CDA is a document markup standard for the structure and semantics of an exchanged "clinical document". A clinical document is a documentation of observations and other services with the following characteristics: Persistence Stewardship Potential for authentication Context Wholeness Human readability A CDA document is a defined and complete information object that can exist outside of a message, and can include text, images, sounds, and other multimedia content.
3 CDA Business Case CDA hits the sweet spot CDA encompasses all of clinical documents. A single standard for the entire EHR is too broad. Multiple standards and/or messages for each EHR function may be difficult to implement. CDA is just right. Implementation experience -CDA has been a normative standard since 2000, and has been balloted through HL7's consensus process. CDA is widely implemented. Gentle on-ramp to information exchange - CDA is straight-forward to implement, and provides a mechanism for incremental semantic interoperability. Improved patient care - CDA provides a mechanism for inserting evidence-based medicine directly into the process of care (via templates), making it easier to do the right thing. Lower costs CDA s top down strategy let s you implement once, and reuse many times for new scenarios.
4 CDA provides a gentle on-ramp to information exchange A minimally conformant CDA document: <ClinicalDocument> <typeid root=" " extension="pocd_hd000040"/> <id root=" "/> <code code=" " codesystem=" «/> <effectivetime value=" "/> <confidentialitycode code="n" codesystem=" "/> <recordtarget> <patientrole><id root=" "/></patientrole> </recordtarget> <author> <time value=" "/> <assignedauthor><id root=" "/></assignedauthor> </author> <custodian> <assignedcustodian> <representedcustodianorganization> <id root=" "/> </representedcustodianorganization> </assignedcustodian> </custodian> <legalauthenticator> <time value=" "/> <signaturecode code="s"/> <assignedentity><id root=" "/></assignedentity> </legalauthenticator> <component> <nonxmlbody> <text mediatype="text/plain ><reference value=" txt"/></text> </nonxmlbody> </component> </ClinicalDocument>
5 Key aspects of the CDA CDA documents are encoded in Extensible Markup Language (XML). CDA is derived from HL7's central Reference Information Model (RIM), thereby enabling data reusability - with lab or pharmacy messages, with claims attachments, clinical trials, etc. The CDA specification is richly expressive and flexible. Templates, conformance profiles, and implementation guides can be used to constrain the generic CDA specification.
6 CDA Guiding Principles Give priority to documents generated by clinicians involved in direct patient care. Minimize the technical barriers needed to implement the Standard. Promote longevity of all information encoded according to this architecture. Promote exchange that is independent of the underlying transfer or storage mechanism. Enable policy-makers to control their own information requirements without extension to this specification.
7 Major Components of a CDA Document <ClinicalDocument>... <structuredbody> <section> <text>...</text> <observation>...</observation> <substanceadministration> <supply>...</supply> </substanceadministration> <observation> <externalobservation>... </externalobservation> </observation> </section> <section> <section>...</section> </section> </structuredbody> </ClinicalDocument> External References Narrative Block E N T R I E S S E C T I O N S Header B O D Y D O C U M E N T
8 CDA, Release One Allergies and Adverse Reactions Penicillin - Hives Aspirin - Wheezing Codeine Itching and nausea <section> <caption> <caption_cd V=" " S= LOINC"/> Allergies and Adverse Reactions </caption> <list> <item><content ID= A1 >Penicillin - Hives</content></item> <item><content>aspirin - Wheezing</content></item> <item> <content>codeine Itching and nausea</content> </item> </list> <coded_entry> <coded_entry.value ORIGTXT= A1 V="DF-10074" S= SNOMED DN= Allergy to Penicillin /> </coded_entry> </section> ANSI/HL7 CDA R
9 CDA, Release Two <section> <code code=" " codesystem=" " codesystemname="loinc"/> <title>allergies and Adverse Reactions</title> <text> <list> <item><content ID="A1">Penicillin - Hives</content></item> <item>aspirin - Wheezing</item> <item>codeine - Itching and nausea</item> </list> </text> <entry> <observation classcode="obs" moodcode="evn"> <code code=" " codesystem=" " codesystemname="snomed CT" displayname="hives"> <originaltext><reference value="#a1"/></originaltext> </code> <entryrelationship typecode="mfst"> <observation classcode="obs" moodcode="evn"> <code code=" " codesystem=" " codesystemname="snomed CT" displayname="allergy to penicillin"/> </observation> </entryrelationship> </observation> </entry> </section>
10 CDA is based on a principle of Incremental Interoperability Incremental Interoperability means that an implementer can begin with a simple CDA, and then add structured data elements over time. CDA R2 consists of a single CDA XML Schema, and the architecture arises from the ability to apply one or more templates which serve to constrain the richness and flexibility of CDA. Professional society recommendations, national clinical practice guidelines, standardized data sets can be expressed as CDA templates. There are many kinds of templates that might be created. Two are particularly relevant for documents: Those that constrain the document sections based on the type of document (sectionlevel templates); Those that constrain the entries within document sections (entry-level templates).
11 ASTM CCR vs. HL7 CDA What if you could have both?!? (or, what if you could have your data elements, and send them in a common exchange framework too?)
12 ASTM CCR + HL7 CDA = CCD The primary use case for the ASTM CCR is to provide a snapshot in time containing a summary of the pertinent clinical, demographic, and administrative data for a specific patient. From the perspective of CDA, the ASTM CCR is a standardized data set that can be used to constrain CDA specifically for summary documents. The resulting specification is known as the Continuity of Care Document (CCD).
13 Continuity of Care Document (CCD) CCD maps the CCR elements into a CDA representation. <Results> <Result> <CCRDataObjectID> </CCRDataObjectID> <DateTime> <Type> <Text>Assessment Time</Text> </Type> <ExactDateTime> </ExactDateTime> </DateTime> <Type> <Text>Hematology</Text> </Type> <Description> <Text>CBC WO DIFFERENTIAL</Text> <Code> <Value> </Value> <CodingSystem>SNOMED CT</CodingSystem> </Code> </Description> <Status><Text>Final Results</Text></Status> <section> <templateid root=" " <code code=" codesystem=" " codesystemname="loinc"/> <title>laboratory results</title> <text> CBC (04/07/2000): HGB 13.2; WBC 6.7; PLT 123* </text> <entry> <organizer classcode="battery" moodcode="evn" <templateid root=" <id root=" " extension=" <code code=" " codesystem=" " codesystemname="snomed CT" displayname="cbc WO DIFFERENTIAL"/> <statuscode code="completed"/> <effectivetime value=" "/>
14 Continuity of Care Document (CCD) CCD sections include: Payers Advance Directives Support Functional Status Problems Family History Social History Alerts (e.g. Allergies, Adverse Events) Medications Medical Equipment Immunizations Vital Signs Results Procedures Encounters Plan of Care
15 CCD Implementation challenge Creation of an instance conforming to a particular CDA Implementation Guide may require knowledge of: CDA R2 base specification; HL7 Version 3 data type specification; CDA templates defined in the particular IG; CDA templates referenced by the particular IG; Terminology code lists defined/referenced by the particular IG; Validation of an instance conforming to a particular CDA IG may require: W3C Schema validation; Schematron validation;
16 A Solution Create an authoring schema that simplifies the creation and processing of a particular CDA IG: Clinically meaningful XML element and attribute names; 100% transformable into conformant CDA IG; Hides certain CDA complexities (such as moodcodes, fixed attributes, etc). We call this strategy: greencda greencda schemas are modular, corresponding to CDA templates.
17 An example build the greencda module Requirements greencda schema <result> <resultid> <resultdatetime> <resulttype> <resultstatus> <resultvalue> <resultinterpretation> <resultreferencerange> </result>
18 An example create a conformant instance greencda instance <result> <resultid> <resultdatetime> <resulttype> <resultstatus> <resultvalue> <resultinterpretation> <resultreferencerange> </result> Conformant CDA instance
19 Thank you!
CEF ehealth DSI 2018 Technical Boot Camp CDA Template Management
 CEF ehealth DSI 2018 Technical Boot Camp CDA Template Management 2018-04-25 to 26, Brussels ART-DECOR Challenge "Adherence to Standards ensures interoperability between different medical information systems"
CEF ehealth DSI 2018 Technical Boot Camp CDA Template Management 2018-04-25 to 26, Brussels ART-DECOR Challenge "Adherence to Standards ensures interoperability between different medical information systems"
Workshop 2. > Interoperability <
 Workshop 2 21 / 08 / 2011 > Interoperability < Heiko Zimmermann R&D Engineer, AHI CR Santec Heiko.Zimmermann@tudor.lu Interoperability definition Picture from NCI-Wiki (https://wiki.nci.nih.gov) 2 Interoperability
Workshop 2 21 / 08 / 2011 > Interoperability < Heiko Zimmermann R&D Engineer, AHI CR Santec Heiko.Zimmermann@tudor.lu Interoperability definition Picture from NCI-Wiki (https://wiki.nci.nih.gov) 2 Interoperability
CAQH CORE Webinar Series: Use & Adoption of Attachments in Healthcare Administration, Part IV
 CAQH CORE Webinar Series: Use & Adoption of Attachments in Healthcare Administration, Part IV Clinical Document Architecture (CDA) Basics -- Clinical Content (CDA Body) Thursday, January 18, 2018 2:00
CAQH CORE Webinar Series: Use & Adoption of Attachments in Healthcare Administration, Part IV Clinical Document Architecture (CDA) Basics -- Clinical Content (CDA Body) Thursday, January 18, 2018 2:00
CMS QRDA Submission Errors Eligible Hospitals / Critical Access Hospitals
 CMS QRDA Submission Errors Eligible Hospitals / Critical Access Hospitals November 5, 2014 Rick Geimer Lantana Consulting Group 1 Presenter Biography Rick Geimer Chief Technology Officer, Lantana Consulting
CMS QRDA Submission Errors Eligible Hospitals / Critical Access Hospitals November 5, 2014 Rick Geimer Lantana Consulting Group 1 Presenter Biography Rick Geimer Chief Technology Officer, Lantana Consulting
Electronic Health Information Standard based on CDA for Thai Medical System: focused on Medical Procedures in Medium-sized Hospitals (HOSxP)
 2015 12 th International Joint Conference on Computer Science and Software Engineering (JCSSE) Electronic Health Information Standard based on CDA for Thai Medical System: focused on Medical Procedures
2015 12 th International Joint Conference on Computer Science and Software Engineering (JCSSE) Electronic Health Information Standard based on CDA for Thai Medical System: focused on Medical Procedures
Florida HIE PLU Best Practices
 Florida HIE On-boarding Integration Florida HIE PLU Best Practices 1 Slide Use 1 of or 14 disclosure of data contained on this sheet or display screen is HARRIS subject PROPRIETARY to the restriction INFORMATION
Florida HIE On-boarding Integration Florida HIE PLU Best Practices 1 Slide Use 1 of or 14 disclosure of data contained on this sheet or display screen is HARRIS subject PROPRIETARY to the restriction INFORMATION
Implementing a Document Standard: Perspectives on Adoption, Interoperability, and Utilization
 Implementing a Document Standard: Perspectives on Adoption, Interoperability, and Utilization Health Level 7 Clinical Document Architecture: What is it? Who is using it? Why in ehealth? Scottish Health
Implementing a Document Standard: Perspectives on Adoption, Interoperability, and Utilization Health Level 7 Clinical Document Architecture: What is it? Who is using it? Why in ehealth? Scottish Health
Initial release of specifications for AHRQ Common Formats for Event Reporting Community Pharmacy Version 1.0 (CFER-CP V1.0)
 IMPLEMENTATION GUIDE AHRQ Common Formats for Event Reporting Community Pharmacy Version 1.0 Technical Specifications for Data Submission from PSO to PSOPPC AHRQ Common Formats for Event Reporting Community
IMPLEMENTATION GUIDE AHRQ Common Formats for Event Reporting Community Pharmacy Version 1.0 Technical Specifications for Data Submission from PSO to PSOPPC AHRQ Common Formats for Event Reporting Community
Andy Gregorowicz OSCON - July 23, 2008
 Andy Gregorowicz OSCON - July 23, 2008 My talk starts with a story of why Laika is important to me. This is my son Kevin... This is the top of his head... When he was an infant, his pediatrician would
Andy Gregorowicz OSCON - July 23, 2008 My talk starts with a story of why Laika is important to me. This is my son Kevin... This is the top of his head... When he was an infant, his pediatrician would
Health Information Exchange Content Model Architecture Building Block HISO
 Health Information Exchange Content Model Architecture Building Block HISO 10040.2 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary HISO 10040.1 Health Information
Health Information Exchange Content Model Architecture Building Block HISO 10040.2 To be used in conjunction with HISO 10040.0 Health Information Exchange Overview and Glossary HISO 10040.1 Health Information
APSR 2.0 Public Comment
 APSR 2.0 Public Comment Remarks 1 Comments on IHE platform Vol. 1, section 10.3: In table 10.3_1 the heading of the 1st column is referring to XD-LAB actor Change this heading to " APSR Actor Vol. 3, section
APSR 2.0 Public Comment Remarks 1 Comments on IHE platform Vol. 1, section 10.3: In table 10.3_1 the heading of the 1st column is referring to XD-LAB actor Change this heading to " APSR Actor Vol. 3, section
L32 Reference Document
 D O C U M E N T N U M B E R M T R 0 9 0 3 4 0 M I T R E T E C H N I C A L R E P O RT L32 Reference Document Lightweight C32 Implementation Kacey Oreal Shauni Deshmukh September 2009 The MITRE Corporation
D O C U M E N T N U M B E R M T R 0 9 0 3 4 0 M I T R E T E C H N I C A L R E P O RT L32 Reference Document Lightweight C32 Implementation Kacey Oreal Shauni Deshmukh September 2009 The MITRE Corporation
QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting. Next Page
 QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting Next Page QRDA Category I Conformance Statement Resource Overview As of Calendar Year 2016, Quality Reporting Document Architecture
QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting Next Page QRDA Category I Conformance Statement Resource Overview As of Calendar Year 2016, Quality Reporting Document Architecture
Most Common ecqm Submission Errors for Hospital QRDA Category-I Files
 Most Common ecqm Submission Errors for Hospital QRDA Category-I Files Jennifer Seeman Edaptive September 30, 2014 Objectives To provide education about electronically specified Clinical Quality Measures
Most Common ecqm Submission Errors for Hospital QRDA Category-I Files Jennifer Seeman Edaptive September 30, 2014 Objectives To provide education about electronically specified Clinical Quality Measures
HL7 Clinical Document Architecture Ambassador Briefing
 HL7 Clinical Document Architecture Ambassador Briefing May 2010 - IHIC Diego Kaminker HL7 Argentina KERN-IT SRL www.kern-it.com.ar 1 Tension of Documentation Extensible Markup Language (XML) Two extremes
HL7 Clinical Document Architecture Ambassador Briefing May 2010 - IHIC Diego Kaminker HL7 Argentina KERN-IT SRL www.kern-it.com.ar 1 Tension of Documentation Extensible Markup Language (XML) Two extremes
CAQH CORE Attachments Webinar Series Part 3
 CAQH CORE Attachments Webinar Series Part 3 Clinical Document Metadata Thursday, November 2, 2017 2:00 3:30 pm ET 2017 CAQH, All Rights Reserved. Logistics Presentation Slides & How to Participate in Today
CAQH CORE Attachments Webinar Series Part 3 Clinical Document Metadata Thursday, November 2, 2017 2:00 3:30 pm ET 2017 CAQH, All Rights Reserved. Logistics Presentation Slides & How to Participate in Today
IHE Pharmacy Technical Framework Supplement. Community Medication List (PML) Rev. 1.3 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Pharmacy Technical Framework Supplement 10 Community Medication List (PML) 15 Rev. 1.3 Trial Implementation 20 Date: October 11, 2017 Author: IHE Pharmacy Technical
Integrating the Healthcare Enterprise 5 IHE Pharmacy Technical Framework Supplement 10 Community Medication List (PML) 15 Rev. 1.3 Trial Implementation 20 Date: October 11, 2017 Author: IHE Pharmacy Technical
HL7 Implementation Guide for CDA Release 2: Questionnaire Response Document, Release 1
 CDAR2_IG_QRDOC_DSTU_R1_2014JAN HL7 Implementation Guide for CDA Release 2: Questionnaire Response Document, Release 1 Draft Standard for Trial Use January 2014 Sponsored by: Structured Documents Work Group
CDAR2_IG_QRDOC_DSTU_R1_2014JAN HL7 Implementation Guide for CDA Release 2: Questionnaire Response Document, Release 1 Draft Standard for Trial Use January 2014 Sponsored by: Structured Documents Work Group
HL7 Implementation Guide for CDA, Release 2 Clinical Oncology Treatment Plan and Summary May HL7 Draft Standard for Trial Use (DSTU) Ballot
 CDAR2_IG_CLONDATA_2013MAY HL7 Implementation Guide for CDA, Release 2 Clinical Oncology Treatment Plan and Summary May 2013 HL7 Draft Standard for Trial Use (DSTU) Ballot Sponsored by: Structured Documents
CDAR2_IG_CLONDATA_2013MAY HL7 Implementation Guide for CDA, Release 2 Clinical Oncology Treatment Plan and Summary May 2013 HL7 Draft Standard for Trial Use (DSTU) Ballot Sponsored by: Structured Documents
SNOMED CT, FHIR, and more
 SNOMED CT, FHIR, and more François Macary Phast-Services: manager of international projects IHE International: co-chair of Pathology and Laboratory Medicine (PaLM) committee Interop Santé: chair of HL7
SNOMED CT, FHIR, and more François Macary Phast-Services: manager of international projects IHE International: co-chair of Pathology and Laboratory Medicine (PaLM) committee Interop Santé: chair of HL7
QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting
 QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting Updated February 2017 Release Notes November 2016 Initial posting of this resource February 2017 Updated posting of this resource NOTE:
QRDA Category I Conformance Statement Resource CY 2016 ecqm Reporting Updated February 2017 Release Notes November 2016 Initial posting of this resource February 2017 Updated posting of this resource NOTE:
IHE Patient Care Coordination Technical Framework Supplement. Multiple Content Views (MCV) Rev Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination Technical Framework Supplement 10 Multiple Content Views 15 Rev. 1.2 - Trial Implementation 20 Date: November 2, 2018 Author: PCC Technical
Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination Technical Framework Supplement 10 Multiple Content Views 15 Rev. 1.2 - Trial Implementation 20 Date: November 2, 2018 Author: PCC Technical
IHE Patient Care Coordination Technical Framework Supplement. Multiple Content Views (MCV) Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination Technical Framework Supplement 10 Multiple Content Views 15 Trial Implementation 20 Date: August 28, 2014 Author: PCC Technical Committee
Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination Technical Framework Supplement 10 Multiple Content Views 15 Trial Implementation 20 Date: August 28, 2014 Author: PCC Technical Committee
Dansk profil for HL7 PHMR Data, datatyper og koder
 Dansk profil for HL7 PHMR Data, datatyper og koder Morten Bruun-Rasmussen mbr@mediq.dk 12. december 2013 HL7 v3. data types Basic data types Codes ANY CD Concepts Descriptor Booleans CE Coded with Equivalents
Dansk profil for HL7 PHMR Data, datatyper og koder Morten Bruun-Rasmussen mbr@mediq.dk 12. december 2013 HL7 v3. data types Basic data types Codes ANY CD Concepts Descriptor Booleans CE Coded with Equivalents
CDX Conformance Profile EMR System Conformance CDA Level 1
 CDX Conformance Profile - 001 EMR System Conformance CDA Level 1 20-Dec-2017 Version 3.3 Status: Final CDA Level 1 Conformance Profile 001 Page 1 of 33 Version Control Release Date Version Status / Comments
CDX Conformance Profile - 001 EMR System Conformance CDA Level 1 20-Dec-2017 Version 3.3 Status: Final CDA Level 1 Conformance Profile 001 Page 1 of 33 Version Control Release Date Version Status / Comments
QRDA Category I: Ballot Development
 QRDA Category I: Ballot Development HL7 Structured Documents Sub Workgroup for Developing the QRDA I Ballot for May Telecom: +1 770-657-9270 Participant Code: 310940 Web: https://www3.gotomeeting.com/join/412175430
QRDA Category I: Ballot Development HL7 Structured Documents Sub Workgroup for Developing the QRDA I Ballot for May Telecom: +1 770-657-9270 Participant Code: 310940 Web: https://www3.gotomeeting.com/join/412175430
Lab Results Interface (LRI) The Flexible Implementation Guide (IG) Standards and Interoperability Framework Initiative (ONC) Ken McCaslin, FHL7
 Lab Results Interface (LRI) The Flexible Implementation Guide (IG) Standards and Interoperability Framework Initiative (ONC) Ken McCaslin, FHL7 Director, Healthcare Standards Quest Diagnostics Chair Technical
Lab Results Interface (LRI) The Flexible Implementation Guide (IG) Standards and Interoperability Framework Initiative (ONC) Ken McCaslin, FHL7 Director, Healthcare Standards Quest Diagnostics Chair Technical
IPS TCON Minute in red Notes from the previous calls in green
 IPS TCON 2017-04-12 Minute in red Notes from the previous calls in green Agenda Madrid Meeting Header Medical Devices History of Past Illness Section EDQM Mapping (medication) High Level plan (time allowing)
IPS TCON 2017-04-12 Minute in red Notes from the previous calls in green Agenda Madrid Meeting Header Medical Devices History of Past Illness Section EDQM Mapping (medication) High Level plan (time allowing)
Discuss and finalize recommendations on Entity-Level Provider Directories (ELPDs):
 Agenda Discuss and finalize recommendations on Entity-Level Provider Directories (ELPDs): Users Uses/Functionality Directory Content Operating Requirements/Business Models Terminology Two TF calls to complete
Agenda Discuss and finalize recommendations on Entity-Level Provider Directories (ELPDs): Users Uses/Functionality Directory Content Operating Requirements/Business Models Terminology Two TF calls to complete
Workflow and Challenges
 Interactive Discussion Between Providers and Payers on Proposed Changes to Attachments Workflow and Challenges Erik Pupo, Deloitte Objectives of this session What are some common implementation challenges
Interactive Discussion Between Providers and Payers on Proposed Changes to Attachments Workflow and Challenges Erik Pupo, Deloitte Objectives of this session What are some common implementation challenges
HL7 CDA R2 Implementation Guide: Pharmacist Care Plan Document, Release 1 - US Realm Volume 1 Introductory Material September 2017
 CDAR2_IG_CCDA_MTM_CAREPLAN_R1_O1_2017SEP_Introductory_Material HL7 CDA R2 Implementation Guide: Pharmacist Care Plan Document, Release 1 - US Realm Volume 1 Introductory Material September 2017 1st Comment-Only
CDAR2_IG_CCDA_MTM_CAREPLAN_R1_O1_2017SEP_Introductory_Material HL7 CDA R2 Implementation Guide: Pharmacist Care Plan Document, Release 1 - US Realm Volume 1 Introductory Material September 2017 1st Comment-Only
HITSP/C32. December 13, 2007 Version 2.1. Healthcare Information Technology Standards Panel. Consumer Empowerment Technical Committee.
 December 13, 2007 Version 2.1 HITSP Summary Documents Using HL7 Continuity of Care Document (CCD) Component HITSP/C32 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Consumer
December 13, 2007 Version 2.1 HITSP Summary Documents Using HL7 Continuity of Care Document (CCD) Component HITSP/C32 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Consumer
Semantic Web and ehealth
 White Paper Series Semantic Web and ehealth January 2013 SEMANTIC IDENTITY OBSERVE REASON IMAGINE Publication Details Author: Renato Iannella Email: ri@semanticidentity.com Date: January 2013 ISBN: 1 74064
White Paper Series Semantic Web and ehealth January 2013 SEMANTIC IDENTITY OBSERVE REASON IMAGINE Publication Details Author: Renato Iannella Email: ri@semanticidentity.com Date: January 2013 ISBN: 1 74064
Terminology Harmonization
 Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
Terminology Harmonization Rob McClure, MD; Lisa Anderson, MSN, RN-BC; Angie Glotstein, BSN, RN November 14-15, 2018 Washington, DC Table of contents OVERVIEW OF CODE SYSTEMS AND TERMINOLOGY TOOLS USING
Maternal mhealth. Solution / Product and Technology Framework. Authors
 Maternal mhealth Solution / Product and Technology Framework VERSION 1.1, August 2014 Authors Chris Seebregts and Hannes Venter, Jembi Health Systems NPC Contributors Rhonwyn Cornell and Linda Taylor,
Maternal mhealth Solution / Product and Technology Framework VERSION 1.1, August 2014 Authors Chris Seebregts and Hannes Venter, Jembi Health Systems NPC Contributors Rhonwyn Cornell and Linda Taylor,
An Architectural Model for Extracting FHIR Resources From CDA Documents
 2016 12th International Conference on Signal-Image Technology & Internet-Based Systems An Architectural Model for Extracting FHIR Resources From CDA Documents Maria Mercorella, Mario Ciampi, Massimo Esposito,
2016 12th International Conference on Signal-Image Technology & Internet-Based Systems An Architectural Model for Extracting FHIR Resources From CDA Documents Maria Mercorella, Mario Ciampi, Massimo Esposito,
HL7 Clinical Document Architecture, Release 2
 30 DOLIN ET AL., HL7 CDA R2 Model Formulation n HL7 Clinical Document Architecture, Release 2 Original JAMIA Investigations ROBERT H. DOLIN, MD,LIORA ALSCHULER, SANDY BOYER, BSP,CALVIN BEEBE, FRED M. BEHLEN,
30 DOLIN ET AL., HL7 CDA R2 Model Formulation n HL7 Clinical Document Architecture, Release 2 Original JAMIA Investigations ROBERT H. DOLIN, MD,LIORA ALSCHULER, SANDY BOYER, BSP,CALVIN BEEBE, FRED M. BEHLEN,
Connected Health Summer School
 Connected Health Summer School Smart Environments for Smarter Living 27-30th June 2016 - Artimino, Firenze, Italy June 28 th 2016 Sungyoung Lee Dept. of Computer Engineering, Kyung Hee University, Korea
Connected Health Summer School Smart Environments for Smarter Living 27-30th June 2016 - Artimino, Firenze, Italy June 28 th 2016 Sungyoung Lee Dept. of Computer Engineering, Kyung Hee University, Korea
Smart Open Services for European Patients. Work Package Semantic Services
 24Am 5 Smart Open Services for European Patients Open ehealth initiative for a European large scale pilot of Patient Summary and Electronic Prescription 10 Work Package 3.5 - Semantic Services Appendix
24Am 5 Smart Open Services for European Patients Open ehealth initiative for a European large scale pilot of Patient Summary and Electronic Prescription 10 Work Package 3.5 - Semantic Services Appendix
Existing Healthcare Standards
 Existing Healthcare Standards Category Context (Information Model) Information Interchange Standard & Specific Elements ASN.1 Abstract Syntax Notation.1 ASTM E2369-05 Standard Specification for Continuity
Existing Healthcare Standards Category Context (Information Model) Information Interchange Standard & Specific Elements ASN.1 Abstract Syntax Notation.1 ASTM E2369-05 Standard Specification for Continuity
PS3.20. DICOM PS a - Imaging Reports using HL7 Clinical Document Architecture
 PS3.20 DICOM PS3.20 2018a - Imaging Reports using HL7 Clinical Document Architecture Page 2 PS3.20: DICOM PS3.20 2018a - Imaging Reports using HL7 Clinical Document Architecture Copyright 2018 NEMA DICOM
PS3.20 DICOM PS3.20 2018a - Imaging Reports using HL7 Clinical Document Architecture Page 2 PS3.20: DICOM PS3.20 2018a - Imaging Reports using HL7 Clinical Document Architecture Copyright 2018 NEMA DICOM
S&I Framework Initiatives
 S&I Framework Initiatives HIT Standards Committee Update August 17, 2011 Introduction and Context The Standards & Interoperability (S&I) Framework: Creates a collaborative, coordinated, incremental standards
S&I Framework Initiatives HIT Standards Committee Update August 17, 2011 Introduction and Context The Standards & Interoperability (S&I) Framework: Creates a collaborative, coordinated, incremental standards
Slide 1 Welcome to Networking and Health Information Exchange, Health Data Interchange Standards. This is lecture b.
 HEALTH DATA EXCHANGE AND PRIVACY AND SECURITY Audio Transcript Component 9 Unit 5 Lecture B Networking and Health Information Exchange Slide 1 Welcome to Networking and Health Information Exchange, Health
HEALTH DATA EXCHANGE AND PRIVACY AND SECURITY Audio Transcript Component 9 Unit 5 Lecture B Networking and Health Information Exchange Slide 1 Welcome to Networking and Health Information Exchange, Health
Beyond Regional Health Information Exchange in China: A Practical and Industrial-Strength Approach
 Beyond Regional Health Information Exchange in China: A Practical and Industrial-Strength Approach Shengping Liu, PhD 1, Baoyao Zhou, PhD 1, Guotong Xie, MS 1, Jing Mei, PhD 1, Haifeng Liu, PHD 1, Changsheng
Beyond Regional Health Information Exchange in China: A Practical and Industrial-Strength Approach Shengping Liu, PhD 1, Baoyao Zhou, PhD 1, Guotong Xie, MS 1, Jing Mei, PhD 1, Haifeng Liu, PHD 1, Changsheng
IHE Patient Care Coordination (PCC) Technical Framework Supplement. CDA Document Summary Sections (CDA-DSS) Revision 1.1 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Sections (CDA-DSS) 15 Revision 1.1 Trial Implementation 20 Date: September
Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Sections (CDA-DSS) 15 Revision 1.1 Trial Implementation 20 Date: September
HL7 Implementation Guide for CDA Release 2.0 CDA Header. (DK CDA Header) Draft for Trial Use. Release 1.1
 HL7 Implementation Guide for CDA Release 2.0 CDA Header (DK CDA Header) Draft for Trial Use Release 1.1 November 15 th 2017 Revision History Release Author Date Notes 1.0 MedCom 19.05.2015 DK CDA Header
HL7 Implementation Guide for CDA Release 2.0 CDA Header (DK CDA Header) Draft for Trial Use Release 1.1 November 15 th 2017 Revision History Release Author Date Notes 1.0 MedCom 19.05.2015 DK CDA Header
User Manual. phr.mtbc.com
 User Manual Table of Contents Introduction Appointments Appointment History Claims History CCDA Report Demographics Health History Lab Reports Online Payment Secure Messages Health Recommendation Patient
User Manual Table of Contents Introduction Appointments Appointment History Claims History CCDA Report Demographics Health History Lab Reports Online Payment Secure Messages Health Recommendation Patient
Interoperability. Doug Fridsma, MD PhD President & CEO, AMIA
 Interoperability Doug Fridsma, MD PhD President & CEO, AMIA Getting to Interoperability To get to interoperability (or to avoid information blocking) we need a common understanding of the problem Can t
Interoperability Doug Fridsma, MD PhD President & CEO, AMIA Getting to Interoperability To get to interoperability (or to avoid information blocking) we need a common understanding of the problem Can t
IHE Patient Care Coordination (PCC) Technical Framework Supplement. CDA Document Summary Section (CDA-DSS) Revision 1.0 Draft for Public Comment
 Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Section (CDA-DSS) 15 Revision 1.0 Draft for Public Comment 20 Date: May
Integrating the Healthcare Enterprise 5 IHE Patient Care Coordination (PCC) Technical Framework Supplement 10 CDA Document Summary Section (CDA-DSS) 15 Revision 1.0 Draft for Public Comment 20 Date: May
ALBERTA ADVERSE EVENT FOLLOWING IMMUNIZATION(AEFI) HL7 MESSAGING SPECIFICATION
 Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA ALBERTA ADVERSE EVENT FOLLOWING IMMUNIZATION(AEFI) HL7 MESSAGING SPECIFICATION MESSAGE STANDARD SUMMARY Status:
Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA ALBERTA ADVERSE EVENT FOLLOWING IMMUNIZATION(AEFI) HL7 MESSAGING SPECIFICATION MESSAGE STANDARD SUMMARY Status:
Quality Data Model (QDM)
 Quality Data Model (QDM) Overview Document National Quality Forum 4/20/2011 QUALITY DATA MODEL (QDM): OVERVIEW TABLE OF CONTENTS Quality Data Model (QDM): Overview... 2 National Quality Forum: Overview
Quality Data Model (QDM) Overview Document National Quality Forum 4/20/2011 QUALITY DATA MODEL (QDM): OVERVIEW TABLE OF CONTENTS Quality Data Model (QDM): Overview... 2 National Quality Forum: Overview
Guidance for Creation of an NHS 111 Repeat Caller Query
 Document filename: Document1 Directorate / Programme NHS111 Project NHS111 Document Reference Project Manager Tony Yates Status Final Owner Tony Yates Version 1.0 Author David Barnet Version issue date
Document filename: Document1 Directorate / Programme NHS111 Project NHS111 Document Reference Project Manager Tony Yates Status Final Owner Tony Yates Version 1.0 Author David Barnet Version issue date
LAB INFORMATION SYSTEMS MESSAGE STANDARD SUMMARY
 Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA LAB INFORMATION SYSTEMS MESSAGE STANDARD SUMMARY HL7 V2.4 LAB TEST RESULT DELIVERY SPECIFICATION AND HL7V3
Health Information Messaging Specification HEALTH INFORMATION STANDARDS COMMITTEE FOR ALBERTA LAB INFORMATION SYSTEMS MESSAGE STANDARD SUMMARY HL7 V2.4 LAB TEST RESULT DELIVERY SPECIFICATION AND HL7V3
CMS QRDA Implementation Guide Changes for CY 2017 Hospital Quality Reporting
 CMS QRDA Implementation Guide Changes for CY 2017 Hospital Quality Reporting Yan Heras, PhD Principal Informaticist, Enterprise Science and Computing (ESAC), Inc. Artrina Sturges, EdD Project Lead, Inpatient
CMS QRDA Implementation Guide Changes for CY 2017 Hospital Quality Reporting Yan Heras, PhD Principal Informaticist, Enterprise Science and Computing (ESAC), Inc. Artrina Sturges, EdD Project Lead, Inpatient
Clinical Decision Support
 Clinical Decision Support Charles Jaffe, MD, PhD Chief Executive Officer Orlando February 21, 2011 Clinical Decision Support Clinical decision support (CDS) systems apply information technology to address,
Clinical Decision Support Charles Jaffe, MD, PhD Chief Executive Officer Orlando February 21, 2011 Clinical Decision Support Clinical decision support (CDS) systems apply information technology to address,
IHE Eye Care Technical Framework Supplement. C-CDA Based General Eye Evaluation (GEE) Trial Implementation. Integrating the Healthcare Enterprise
 Integrating the Healthcare Enterprise IHE Eye Care Technical Framework Supplement C-CDA Based General Eye Evaluation (GEE) Trial Implementation September 3, 2014 IHE Eye Care Technical Committee eyecare@ihe.net
Integrating the Healthcare Enterprise IHE Eye Care Technical Framework Supplement C-CDA Based General Eye Evaluation (GEE) Trial Implementation September 3, 2014 IHE Eye Care Technical Committee eyecare@ihe.net
The Estonian ehealth experience strategy and results. Piret Simmo Estonian ehealth Foundation Standardization manager
 The Estonian ehealth experience strategy and results Piret Simmo Estonian ehealth Foundation Standardization manager 27.09.2011 Agenda Some history Legal environment ehealth Foundation Estonian central
The Estonian ehealth experience strategy and results Piret Simmo Estonian ehealth Foundation Standardization manager 27.09.2011 Agenda Some history Legal environment ehealth Foundation Estonian central
The use of standard content specifications in a national health interoperability framework
 electronic Journal of Health Informatics http://www.ejhi.net 2010; Vol 5(1): e3 The use of standard content specifications in a national health interoperability framework Sam Heard CHIME, University College
electronic Journal of Health Informatics http://www.ejhi.net 2010; Vol 5(1): e3 The use of standard content specifications in a national health interoperability framework Sam Heard CHIME, University College
EHR Connectivity Integration Specification
 EHR Connectivity Integration Specification HeC Contact information Name Phone Email Title/Role Jeremy Smith (315) 671 2241 x320 jsmith@healtheconnections.org Manager, HIE Integration OVERVIEW This document
EHR Connectivity Integration Specification HeC Contact information Name Phone Email Title/Role Jeremy Smith (315) 671 2241 x320 jsmith@healtheconnections.org Manager, HIE Integration OVERVIEW This document
Information Modeling Service-Oriented Architecture. Galen Mulrooney (contractor to VHA) June 3, 2009 V 1.1
 Information Modeling Service-Oriented Architecture Galen Mulrooney (contractor to VHA) Galen.Mulrooney@va.gov June 3, 2009 V 1.1 1 Service Oriented Architecture Definitions Service Oriented Architecture
Information Modeling Service-Oriented Architecture Galen Mulrooney (contractor to VHA) Galen.Mulrooney@va.gov June 3, 2009 V 1.1 1 Service Oriented Architecture Definitions Service Oriented Architecture
A Deep Dive in the Use of LOINC Codes. C-CDA and LOINC
 A Deep Dive in the Use of LOINC Codes C-CDA and LOINC Rick Geimer Lantana Consulting Group 2018 Summer Forum Tuesday, July 31 Disclaimer Conference presentations are intended for educational purposes only
A Deep Dive in the Use of LOINC Codes C-CDA and LOINC Rick Geimer Lantana Consulting Group 2018 Summer Forum Tuesday, July 31 Disclaimer Conference presentations are intended for educational purposes only
Presenter(s): Dave Venier. Topic Rosetta Interoperability (C-CDA and IHE) Level 200
 Presenter(s): Dave Venier Topic Rosetta Interoperability (C-CDA and IHE) Level 200 Safe Harbor Provisions/Legal Disclaimer This presentation may contain forward-looking statements within the meaning of
Presenter(s): Dave Venier Topic Rosetta Interoperability (C-CDA and IHE) Level 200 Safe Harbor Provisions/Legal Disclaimer This presentation may contain forward-looking statements within the meaning of
Representing clinical information using SNOMED Clinical Terms with different structural information models
 Representing clinical information using SNOMED Clinical Terms with different structural information models David Markwell, MB, BS [a], Laura Sato MSc [b], Edward Cheetham MB ChB [c] a) The Clinical Information
Representing clinical information using SNOMED Clinical Terms with different structural information models David Markwell, MB, BS [a], Laura Sato MSc [b], Edward Cheetham MB ChB [c] a) The Clinical Information
A NOVEL ALGORITHM FOR CLEANICAL ROUTINE USING SYSTEM INTEGRATION OF PACS AND CBIR
 International Journal of Computer Science Engineering and Information Technology Research (IJCSEITR) ISSN(P): 2249-6831; ISSN(E): 2249-7943 Vol. 4, Issue 4, Aug 2014, 15-20 TJPRC Pvt. Ltd. A NOVEL ALGORITHM
International Journal of Computer Science Engineering and Information Technology Research (IJCSEITR) ISSN(P): 2249-6831; ISSN(E): 2249-7943 Vol. 4, Issue 4, Aug 2014, 15-20 TJPRC Pvt. Ltd. A NOVEL ALGORITHM
LOINC for Claims Attachments
 LOINC for Claims Attachments Swapna Abhyankar, MD Associate Director for Content Development 1 Outline Overview of LOINC Content How to access, and staying current LOINC for claims attachments How to find
LOINC for Claims Attachments Swapna Abhyankar, MD Associate Director for Content Development 1 Outline Overview of LOINC Content How to access, and staying current LOINC for claims attachments How to find
Structured Documentation of Physiological Models and Behavioural Patterns containing Uncertainty
 Structured Documentation of Physiological Models and Behavioural Patterns containing Uncertainty Myriam Lipprandt OFFIS - Institute for Information Technology Escherweg 2, D-26121 Oldenburg, Germany Email:
Structured Documentation of Physiological Models and Behavioural Patterns containing Uncertainty Myriam Lipprandt OFFIS - Institute for Information Technology Escherweg 2, D-26121 Oldenburg, Germany Email:
SNOMED CT Implementation Approaches. National Resource Centre for EHR Standards (NRCeS) C-DAC, Pune
 SNOMED CT Implementation Approaches National Resource Centre for EHR Standards (NRCeS) C-DAC, Pune SNOMED CT with no Health Record SNOMED CT is a powerful modern clinical terminology Comprehensive scope
SNOMED CT Implementation Approaches National Resource Centre for EHR Standards (NRCeS) C-DAC, Pune SNOMED CT with no Health Record SNOMED CT is a powerful modern clinical terminology Comprehensive scope
Digital Imaging and Communications in Medicine (DICOM) Supplement 101: HL7 Structured Document Object References
 Digital Imaging and Communications in Medicine (DICOM) Supplement 0: HL7 Structured Document Object References Prepared by: DICOM Standards Committee, Working Group 20 and Working Group 8 300 N. 7th Street
Digital Imaging and Communications in Medicine (DICOM) Supplement 0: HL7 Structured Document Object References Prepared by: DICOM Standards Committee, Working Group 20 and Working Group 8 300 N. 7th Street
Galen Mulrooney J P Systems, Inc. Sydney, NSW January 15, 2011 v. 1.0
 Galen Mulrooney J P Systems, Inc. gem@jpsys.com Sydney, NSW January 15, 2011 v. 1.0 Service Oriented Architecture (SOA) is a paradigm for organizing and utilizing distributed capabilities that may be under
Galen Mulrooney J P Systems, Inc. gem@jpsys.com Sydney, NSW January 15, 2011 v. 1.0 Service Oriented Architecture (SOA) is a paradigm for organizing and utilizing distributed capabilities that may be under
Information Architecture. It s utility with the NHS landscape. Presented by Richard Kavanagh, Head of
 Information Architecture It s utility with the NHS landscape Presented by Richard Kavanagh, Head of Information Architecture for Interoperability An insight into current work to support a more capable
Information Architecture It s utility with the NHS landscape Presented by Richard Kavanagh, Head of Information Architecture for Interoperability An insight into current work to support a more capable
FHIR Overview. HL7 FHIR Connectathon20 January 12, 2019
 FHIR Overview HL7 FHIR Connectathon20 January 12, 2019 Presenter: Richard Ettema FHIR Certified Implementer Lead Consultant, AEGIS.net, Inc. richard.ettema@aegis.net 2018 AEGIS.net, Inc., HL7 International.
FHIR Overview HL7 FHIR Connectathon20 January 12, 2019 Presenter: Richard Ettema FHIR Certified Implementer Lead Consultant, AEGIS.net, Inc. richard.ettema@aegis.net 2018 AEGIS.net, Inc., HL7 International.
HHS/CMS Unified Agenda - NPRM
 Attachment Regulation Panel - Durwin Day (HCSC), Tony Benson (BCBSAL), Christol Green (Anthem) August, 29, 10:45 12:00 Durwin Day, Health Information Manager, HCSC HL7 Attachment WG Co-chair, PUG Co-chair
Attachment Regulation Panel - Durwin Day (HCSC), Tony Benson (BCBSAL), Christol Green (Anthem) August, 29, 10:45 12:00 Durwin Day, Health Information Manager, HCSC HL7 Attachment WG Co-chair, PUG Co-chair
NextGen UD2 Upgrade Enhancements
 NextGen UD2 Upgrade Enhancements Summary NextGen EHR Enhancements May 23, 2016: Workflow Module Patient Information Bar Alerts Medication Module Allergy Module Encounter/Category View Filters NG Share
NextGen UD2 Upgrade Enhancements Summary NextGen EHR Enhancements May 23, 2016: Workflow Module Patient Information Bar Alerts Medication Module Allergy Module Encounter/Category View Filters NG Share
IHE Pharmacy Technical Framework Supplement. Community Prescription (PRE) Rev. 1.8 Trial Implementation
 Integrating the Healthcare Enterprise 5 IHE Pharmacy Technical Framework Supplement 10 Community Prescription (PRE) 15 Rev. 1.8 Trial Implementation 20 Date October 11, 2017 Author IHE Pharmacy Technical
Integrating the Healthcare Enterprise 5 IHE Pharmacy Technical Framework Supplement 10 Community Prescription (PRE) 15 Rev. 1.8 Trial Implementation 20 Date October 11, 2017 Author IHE Pharmacy Technical
Implementation Guide for CDA Release 2.0. Patient Authored Note (PAN) Document. (Universal Realm) And. (US Realm)
 Implementation Guide for CDA Release 2.0 Patient Authored Note (PAN) Document (Universal Realm) And (US Realm) Draft version: November 2012 Primary Editor: Virinder Batra Intuit Health virinder_batra@intuit.com
Implementation Guide for CDA Release 2.0 Patient Authored Note (PAN) Document (Universal Realm) And (US Realm) Draft version: November 2012 Primary Editor: Virinder Batra Intuit Health virinder_batra@intuit.com
A Linked Data Translation Approach to Semantic Interoperability
 A Data Translation Approach to Semantic Interoperability November 12, 2014 Dataversity Webinar Rafael M Richards MD MS Physician Informaticist Veterans Health Administratioan U.S. Department of Veterans
A Data Translation Approach to Semantic Interoperability November 12, 2014 Dataversity Webinar Rafael M Richards MD MS Physician Informaticist Veterans Health Administratioan U.S. Department of Veterans
Module 2: Health Information Exchange Services
 Module 2: Health Information Exchange Services Introduction In this module, Health Information Exchange (HIE) will be introduced. This system is designed to provide patient information for THR facilities
Module 2: Health Information Exchange Services Introduction In this module, Health Information Exchange (HIE) will be introduced. This system is designed to provide patient information for THR facilities
SNOMED Clinical Terms
 Representing clinical information using SNOMED Clinical Terms with different structural information models KR-MED 2008 - Phoenix David Markwell Laura Sato The Clinical Information Consultancy Ltd NHS Connecting
Representing clinical information using SNOMED Clinical Terms with different structural information models KR-MED 2008 - Phoenix David Markwell Laura Sato The Clinical Information Consultancy Ltd NHS Connecting
Section I: Best Available Vocabulary/Code Set/Terminology Standards and Implementation Specifications
 Section I: Best Available Vocabulary/Code Set/Terminology s and s I-A: Allergies Interoperability Need: Representing patient allergic reactions / SNOMED-CT Final Production No Free N/A SNOMED-CT may not
Section I: Best Available Vocabulary/Code Set/Terminology s and s I-A: Allergies Interoperability Need: Representing patient allergic reactions / SNOMED-CT Final Production No Free N/A SNOMED-CT may not
ART-DECOR Introduction anddemos
 ART-DECOR Introduction anddemos ehealth Member States Expert Group (ehmseg) SemanticTask Force February 23, 2017 Dr Kai U. Heitmann, MD, FHL7 About Kai Dr Kai U. Heitmann, MD, FHL7 Heitmann Consulting
ART-DECOR Introduction anddemos ehealth Member States Expert Group (ehmseg) SemanticTask Force February 23, 2017 Dr Kai U. Heitmann, MD, FHL7 About Kai Dr Kai U. Heitmann, MD, FHL7 Heitmann Consulting
Usage of Dates/Times/Timezones/Intervals in CDA Documents. Draft Version, 20-Nov 2012 (Brett Esler / Grahame Grieve)
 Usage of Dates/Times/Timezones/Intervals in CDA Documents Draft Version, 20-Nov 2012 (Brett Esler / Grahame Grieve) Overview As part of CDA Implementation Guides for continuity of care document types there
Usage of Dates/Times/Timezones/Intervals in CDA Documents Draft Version, 20-Nov 2012 (Brett Esler / Grahame Grieve) Overview As part of CDA Implementation Guides for continuity of care document types there
ICD-10 Customer Testing Guidelines and Scenarios
 ICD-10 Customer Testing Guidelines and Scenarios Revision 2.0 Updated July 21, 2015 This document includes pre-release information and user interface designs that are in development for upcoming enhancements
ICD-10 Customer Testing Guidelines and Scenarios Revision 2.0 Updated July 21, 2015 This document includes pre-release information and user interface designs that are in development for upcoming enhancements
Integrating the Healthcare Enterprise Patient Care Devices
 Integrating the Healthcare Enterprise Patient Care Devices Anything can be integrated Un-Interoperability: Highest Cause of Health IT project failures Base Standards The Hospital EHRs, CMMS, other ehealth
Integrating the Healthcare Enterprise Patient Care Devices Anything can be integrated Un-Interoperability: Highest Cause of Health IT project failures Base Standards The Hospital EHRs, CMMS, other ehealth
Standards Development
 Thailand s ehealth & Health Information Standards Development Collaborating across countries to harmonize information system standards understanding country opportunities and developing strategies to deal
Thailand s ehealth & Health Information Standards Development Collaborating across countries to harmonize information system standards understanding country opportunities and developing strategies to deal
Content Testing Program
 Content Testing Program Effective February 5, 2018 Acknowledgements Many thanks are due to the ehealth Exchange Content Testing Workgroup members and Co-chairs, Omar Bouhaddou, Chief Health Informatics
Content Testing Program Effective February 5, 2018 Acknowledgements Many thanks are due to the ehealth Exchange Content Testing Workgroup members and Co-chairs, Omar Bouhaddou, Chief Health Informatics
Implementation Guide. Consolidated Clinical Documentation Architecture (C-CDA) Documents for Clinical Data Repository (CDR)
 Implementation Guide Consolidated Clinical Documentation Architecture (C-CDA) Documents for Clinical Data Repository (CDR) Revised: November 2017 Version 2.2 Table of Contents 1. DOCUMENT CHANGE HISTORY...
Implementation Guide Consolidated Clinical Documentation Architecture (C-CDA) Documents for Clinical Data Repository (CDR) Revised: November 2017 Version 2.2 Table of Contents 1. DOCUMENT CHANGE HISTORY...
HITSP/IS06. January 25, 2010 Version 2.0. Healthcare Information Technology Standards Panel. Population Perspective Technical Committee.
 January 25, 2010 Version 2.0 HITSP/IS06 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Technical Committee DOCUMENT CHANGE HISTORY Version Number Description of Change Name
January 25, 2010 Version 2.0 HITSP/IS06 Submitted to: Healthcare Information Technology Standards Panel Submitted by: Technical Committee DOCUMENT CHANGE HISTORY Version Number Description of Change Name
Ensuring Quality Terminology Mappings in Distributed SOA Environments
 Ensuring Quality Terminology Mappings in Distributed SOA Environments SOA in Healthcare Chicago Illinois April 16, 2008 Russell Hamm Informatics Consultant Apelon, Inc. 1 Outline Data standardization Goals
Ensuring Quality Terminology Mappings in Distributed SOA Environments SOA in Healthcare Chicago Illinois April 16, 2008 Russell Hamm Informatics Consultant Apelon, Inc. 1 Outline Data standardization Goals
Moving EHR-S FM Components
 Moving EHR-S FM Components This document describes the way components, sections, headers, functions and/ or conformance criteria, of the EHR-S FM can be moved. In a Functional Profile it is likely that
Moving EHR-S FM Components This document describes the way components, sections, headers, functions and/ or conformance criteria, of the EHR-S FM can be moved. In a Functional Profile it is likely that
Data Quality Attributes
 Data Quality Improvement Unit 11.2b: Data Quality Attributes 1 Data Quality Attributes 11 2 Consistency of Data Value of the data should be reliable and the same across applications 3 1 Consistency of
Data Quality Improvement Unit 11.2b: Data Quality Attributes 1 Data Quality Attributes 11 2 Consistency of Data Value of the data should be reliable and the same across applications 3 1 Consistency of
Interoperability, critical element for an ehealth Strategy
 Interoperability, critical element for an ehealth Strategy Forum e-zdrowia / ehealth Forum Gdansk - September 14-15, 2017 Charles Parisot, IHE-Services Chair IHE International Board Member, GE Healthcare
Interoperability, critical element for an ehealth Strategy Forum e-zdrowia / ehealth Forum Gdansk - September 14-15, 2017 Charles Parisot, IHE-Services Chair IHE International Board Member, GE Healthcare
The SHARPn phenotyping funnel. Phenotype specific patient cohorts
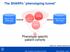 The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
The SHARPn phenotyping funnel Mayo Clinic EHR data QDMs CEMs Intermountain EHR data DRLs Phenotype specific patient cohorts [Welch et al., JBI 2012; 45(4):763-71] 2012 MFMER slide-1 Algorithm Development
ISO/IEEE 11073, HL7 Medical Devices WG and NIST
 ISO/IEEE 11073, HL7 Medical Devices WG and NIST NIST Medical Device Connectivity Test Tooling Semantic Interoperability of Medical Devices HL7/IEEE WG Meetings (Healthcare Devices WG @ Cambridge, Mass)
ISO/IEEE 11073, HL7 Medical Devices WG and NIST NIST Medical Device Connectivity Test Tooling Semantic Interoperability of Medical Devices HL7/IEEE WG Meetings (Healthcare Devices WG @ Cambridge, Mass)
HL7 V3 User Model. The V3 Editing Team. April 9, Ockham Information Services LLC
 HL7 V3 User Model The V3 Editing Team April 9, 2007 Ockham Information Services LLC Contents Profile orientation Profile drafts Use case drafts Document map prototypes The need for profiles Documents require
HL7 V3 User Model The V3 Editing Team April 9, 2007 Ockham Information Services LLC Contents Profile orientation Profile drafts Use case drafts Document map prototypes The need for profiles Documents require
Health Level 7 (HL7) Implementation Guide
 Health Level 7 (HL7) Implementation Guide Current and Future States Craig Newman Why do we need a national IG? Immunization related data is exchanged between a wide variety of systems 50+ public health
Health Level 7 (HL7) Implementation Guide Current and Future States Craig Newman Why do we need a national IG? Immunization related data is exchanged between a wide variety of systems 50+ public health
IPS. New Orleans WGM Q3 SDWG
 IPS New Orleans WGM 2018-01-29 Q3 SDWG Topics IPS status overview Project status PSS update status Ballot reconciliation THE IPS PROJECT 3 The IPS Project HL7 Int. CEN/TC 251 agreement (April, 2017) Vision
IPS New Orleans WGM 2018-01-29 Q3 SDWG Topics IPS status overview Project status PSS update status Ballot reconciliation THE IPS PROJECT 3 The IPS Project HL7 Int. CEN/TC 251 agreement (April, 2017) Vision
Open Health Tools UI Platform The MITRE Corporation. All rights Reserved. Approved for Public Release: XXXXX. Distribution Unlimited.
 Open Health Tools UI Platform Approved for Public Release: XXXXX. Distribution Unlimited. Agenda UI Problem Open Health Tool UI Platform Collaboration OHT UI Platform Architecture Enabling Innovation UI
Open Health Tools UI Platform Approved for Public Release: XXXXX. Distribution Unlimited. Agenda UI Problem Open Health Tool UI Platform Collaboration OHT UI Platform Architecture Enabling Innovation UI
HISO :2015 edischarge Messaging Interim Standard
 HISO 10011.4:2015 edischarge Messaging Interim Standard July 2015 Document information HISO 10011.4:2015 edischarge Messaging Standard is an interim standard for the New Zealand health and disability sector
HISO 10011.4:2015 edischarge Messaging Interim Standard July 2015 Document information HISO 10011.4:2015 edischarge Messaging Standard is an interim standard for the New Zealand health and disability sector
DICOM Structured Reporting
 inforad 891 DICOM Structured Reporting Part 1. Overview and Characteristics 1 Rada Hussein, MSc Uwe Engelmann, PhD Andre Schroeter, MSc Hans-Peter Meinzer, PhD Supplement 23 to (DICOM) is an introduction
inforad 891 DICOM Structured Reporting Part 1. Overview and Characteristics 1 Rada Hussein, MSc Uwe Engelmann, PhD Andre Schroeter, MSc Hans-Peter Meinzer, PhD Supplement 23 to (DICOM) is an introduction
Approaching semantic interoperability in Health Level Seven
 Approaching semantic interoperability in Health Level Seven Robert H Dolin, 1 Liora Alschuler 2 Perspective 1 Semantically Yours, LLC, USA 2 Alschuler Associates, LLC, USA Correspondence to Dr Robert H
Approaching semantic interoperability in Health Level Seven Robert H Dolin, 1 Liora Alschuler 2 Perspective 1 Semantically Yours, LLC, USA 2 Alschuler Associates, LLC, USA Correspondence to Dr Robert H
