FiloQuant manual V1.0 Table of Contents
|
|
|
- Andrew Rice
- 5 years ago
- Views:
Transcription
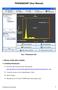
1 FiloQuant manual V1.0 Table of Contents 1) FiloQuant aims and distribution license...2 2) Installation...3 3) FiloQuant, step-by-step instructions (single images)...4 1: Choose the region of interest to analyze...4 2: Brightness / Contrast adjustment...4 3: Parameters to detect the cell edge...4 4: Validation of filopodia-free cell edge detection...5 5: Parameters to detect filopodia...6 6: Validation of filopodia detection...6 7: Validation of filopodia detection...7 8: Parameters used to measure the length of the cell edge...7 9: Validation of contour detection : Results...8 4) FiloQuant, semi-automated analyses ) FiloQuant automated analyses, live-cell imaging and filopodia tracking
2 1) FiloQuant aims and distribution license FiloQuant is a user friendly and modifiable tool for automated detection and quantification of filopodia properties such as length and density. We developed FiloQuant as a plugin for the freely available, and popular, ImageJ with inter operating systems compatibility. FiloQuant was designed with four goals in mind: First, we aimed to make this software as easy to use as possible. To this end we created versions of FiloQuant containing step-by-step user validation of the various processing stages to help users to achieve optimal settings for filopodia detection. Second, this software was designed to simplify and speed up the analysis of filopodia properties. To this end semi-automated as well as a fully-automated versions of FiloQuant are provided. Using the semi-automated version of FiloQuant, users can analyze rapidly a large number of images while keeping control over the settings used to analyze each image and modify these settings on the fly to improve the accuracy of detection. Using the automated version of FiloQuant, users can choose the settings for analyzing a large number of images at once (batch analysis). This latter version of FiloQuant is especially useful for screening purposes or to analyse filopodia properties and dynamics from live-cell imaging data. Third, we aimed to make FiloQuant as broadly usable/flexible as possible, with no limitation in terms of cell geometry or imaging modality. We successfully used FiloQuant with images (acquired on different microscopes) of cells migrating collectively or as single cells in various environments including 2D fibronectin and 3D cell-derived matrices. In addition, we tested the ability of FiloQuant to detect filopodia in neurons, which have a more complex morphology. Finally, although this software was found to effectively identify filopodia in many different types of images, we appreciate that others might require extra functionalities or the ability to included FiloQuant within larger analysis routines. To facilitate easy modification of FiloQuant, we wrote this software using the simple ImageJ macro language that we also fully annotated, which can therefore be edited with limited coding knowledge. FiloQuant was created by Guillaume Jacquemet and Alexandre Carisey. This program is free software; you can redistribute it and/or modify it under the terms of the GNU General Public License as published by the Free Software Foundation ( This program is distributed in the hope that it will be useful, but WITHOUT ANY WARRANTY; without even the implied warranty of MERCHANTABILITY or FITNESS FOR A PARTICULAR PURPOSE. See the GNU General Public License for more details. 2
3 2) Installation We recommend the use of the Fiji distribution of ImageJ ( to use with FiloQuant as Fiji already contains all the necessary dependencies required by FiloQuant. To run FiloQuant in ImageJ, users need to install the following dependencies: Enhanced Local Contrast (CLAHE.class; Skeletonize3D.jar ( AnalyzeSkeleton.jar ( and Temporal-Color Code ( Installation of FiloQuant can be easily done in Fiji using the FiloQuant ImageJ update site: 1) In Fiji, click on Help Update 2) then Manage update sites 3) and add my site 4) In the field ImageJ Wiki account input: FiloQuant then click OK. Close the Manage update sites window and, in the ImageJ Updater window, click on Apply changes. The three version of FiloQuant can then be found under plugin FiloQuant. 3
4 3) FiloQuant, step-by-step instructions (single images) The single image or semi-automated version of FiloQuant contains step-by-step user validation of the various processing steps to help users achieve optimal settings for filopodia detection. Below you will find detailed instructions related to the various steps. 1: Choose the region of interest to analyze Outline the region of interest (ROI) to be analyzed by drawing a square/other shape around the area on the initial image, then click ok. If you want to use the whole image, just click OK 2: Brightness / Contrast adjustment To calculate optimal contrast settings, you can select an ROI by drawing a square and then click OK. Alternatively, to use the whole image for auto contrast calculations, just click OK 3: Parameters to detect the cell edge Input the parameters that FiloQuant will use to generate the filopodia-free cell edge: - Threshold for cell edge: intensity value to be used for the intensity based thresholding (input range:1-255). - Number of iterations for Open: Open is the process used by FiloQuant to shave off the filopodia. Input here the number of times you want to perform this operation (input range: 1-100). - Number of cycle for Erode Dilate*: Alternative method to shave off filopodia. Input here the number of time you want to perform this operation (input range: 1-100). *We recommend to use Open first. Fill holes on edges? Tick this box if you want FiloQuant to fill the holes at the image boundary Fill holes? Tick this box if you want FiloQuant to fill the hole in the middle of the image. Input the parameters to modify edge length measurement? Tick this box if you want to control how the edges are measured. 4
5 Fill holes on edges? box ticked No holes filled Fill holes? box ticked Number of iterations for Open = 0Number of iterations for Open = 5 Number of iterations for Open = 20 4: Validation of filopodia-free cell edge detection Is the threshold correct? - If you are happy with the threshold, click OK - If you are not happy with the threshold, untick the box and click OK. You will then be able to remodify the parameters to detect the filopodiafree cell edge. Repeat until you are satisfied with the parameters 5
6 5: Parameters to detect filopodia - Threshold for filopodia: intensity value to be used for the intensity based thresholding (input range: 1255). - Filopodia minimum size: Input the minimum size (in pixels) of structures to be considered analysis. further for - Filopodia repair cycles: Input the number of times you want to perform the close operation to try to restore broken filopodia. We recommend 0 or 1. Inputting 0 will disable the option. - Use convolve to improve filopodia detection? Tick this box if you want to use a standard convolution kernel to help with filopodia detection. We recommend this option as we found it to be very powerful in extracting faint filopodia. - Use local contrast enhancement to improve filopodia detection? Tick this box if you want to use this option to improve the detection of faint filopodia. This option needs to be disabled if the image is noisy. - Filopodia detection: maximum distance from the cell edge? Input the maximum distance (in pixels) that filopodia are allowed to be from the cell edge to be considered further for analysis. Inputting 0 will disable this option. 6: Validation of filopodia detection Is the threshold correct? - If you are happy with the threshold, click OK - If you are not happy with the threshold, untick the box and click OK. You will then be able to remodify the parameters for filopodia detection. Repeat until you are satisfied with the parameters. Note: filopodia filtering in function of size happens at a later stage and cannot be validated at this step. 6
7 7: Validation of filopodia detection - If you are happy with the filopodia detection (in purple), click OK. - If you are not happy with the filopodia detection, untick the box and click OK. You will be able to restart the analyses again. Repeat until you are satisfied with the parameters 8: Parameters used to measure the length of the cell edge Note: this window becomes available only if the box Input the parameters to modify edge length measurement? is ticked (in step 3). Otherwise default parameters are used. These options can be used to smooth the edges before their measurement. Number of iterations for Close: Input here the number of times you want to perform this operation (input range: 1-100). Number of iterations for Erode: Input here the number of times you want to perform this operation (input range: 1-100). Number of iterations for Dilate: Input here the number of times you want to perform this operation (input range: 1-100). 7
8 Values : 0, 0, 0 Values: 10, 10, 10 Values, 50, 50, 50 9: Validation of contour detection Is the contour detection correct? - If you are happy with the contour, click OK - If you are not happy with the contour, untick the box and click OK. You will then be able to re-modify the parameters for contour detection. Repeat until you are satisfied with the parameters. 10: Results - The result can be found in the same location as the original image - Result includes: a results.csv file containing the length of all the detected filopodia in one column and the length of all the detected edges in another column. a settings.csv file containing all the settings used for the analysis of this particular image. This file can be found in the folder intermediate files. The following images: The result image. Detected filopodia are in purple. 8 The cell edge The image used to detect filopodia
9 The detected filopodia The contour detection Note: The initial thresholding of the cell edge can sometimes be challenging. It is possible during step 1 and 2 to manually outline the cell using the ImageJ freehand tool and then use the fill function. 9
10 4) FiloQuant, semi-automated analyses Using the semi-automated version of FiloQuant, users can analyze rapidly a large number of images while keeping control over the settings used to analyze each image and modify these settings on the fly to improve the accuracy of detection. The analysis process is very similar to the one described for the single image analysis except that the user is prompted to choose the location of the folder containing the images to analyse and a folder where the results can be saved. 1) Choose the input folder Choose the folder containing the images to be analysed. Images need to be in a.tiff or.tif format 2) Choose the output folder Choose the folder where you want to save the results 3) Perform analyses as described for the single image above 4) Results can be found in the output folder 10
11 5) FiloQuant automated analyses, live-cell imaging and filopodia tracking Using the automated version of FiloQuant, users can choose the settings for analyzing a large number of images at once (batch analysis). This latter version of FiloQuant is especially useful for screening purposes and/or to analyse filopodia properties and dynamics from live-cell imaging data. 1) Choose the input folder Choose the folder containing the images and/or stacks to be analyzed. - CRITICAL: Make sure that there are no spaces in the file names or in the file paths - Images need to be in a.tiff or.tif format 2) Choose the output folder Choose the folder where you want to save the results 3) Input all the parameters to be used Example of a time projection of detected filopodia created by FiloQuant when the Batch mode: stack analysis option is enabled. 4) Results can be found in the output folder 11
12 Parameters that FiloQuant will use to generate the filopodia-free cell edge: - Threshold for cell edge: intensity value to be used for the intensity based thresholding (input range:1-255). - Number of iterations for Open: Open is the process used by FiloQuant to shave off the filopodia. Input here the number of times you want to perform this operation (input range: 1-100). - Number of cycle for Erode Dilate*: Alternative method to shave off filopodia. Input here the number of time you want to perform this operation (input range: 1-100). *We recommend to use Open first. - Fill holes on edges? Tick this box if you want FiloQuant to fill the holes at the image boundary - Fill holes? Tick this box if you want FiloQuant to fill the hole in the middle of the image. - Input the parameters to modify edge length measurement? Tick this box if you want to control how the edges are measured. Parameters to detect filopodia: - Threshold for filopodia: intensity value to be used for the intensity based thresholding (input range: 1255). - Filopodia minimum size: Input the minimum size (in pixels) of structures to be considered further for analysis. - Filopodia repair cycles: Input the number of times you want to perform the close operation to try to restore broken filopodia. We recommend 0 or 1. Inputting 0 will disable the option. - Use convolve to improve filopodia detection? Tick this box if you want to use a standard convolution kernel to help with filopodia detection. We recommend this option as we found it to be very powerful in extracting faint filopodia. - Use local contrast enhancement to improve filopodia detection? Tick this box if you want to use this option to improve the detection of faint filopodia. This option needs to be disabled if the image is noisy. - Filopodia detection: maximum distance from the cell edge? Input the maximum distance (in pixels) that filopodia are allowed to be from the cell edge to be considered further for analysis. Inputting 0 will disable this option. Contour measurement: - Number of iterations for Close: Input here the number of times you want to perform this operation (input range: 1-100). - Number of iterations for Erode: Input here the number of times you want to perform this operation (input range: 1-100). - Number of iterations for Dilate: Input here the number of times you want to perform this operation (input range: 1-100). Batch Mode: - Batch mode: stack analysis? Tick this box if the folder you want to analyse contains stacks of images rather than single images, e.g. live-cell imaging data. This option will: 12 organise the result data differently (more suitable for stacks) generate a time projection of the detected filopodia generate a tracking file of the detected filopodia that can be further analyzed using automated tracking software such as TrackMate.
3. Open ImageJ, select Plugin Macro Run and then select NeuronRead, which will start immediately.
 NeuronRead Tutorial Setup The NeuronRead 1 macro runs on ImageJ software and is supported by three main plugins: MorphoLibJ, Skeletonize3D and Analyze Skeleton. For the smoothest and fastest application
NeuronRead Tutorial Setup The NeuronRead 1 macro runs on ImageJ software and is supported by three main plugins: MorphoLibJ, Skeletonize3D and Analyze Skeleton. For the smoothest and fastest application
9 Tumor Blood Vessels: 3-D Tubular Network Analysis
 Chap. 9 2015/8/20 14:02 page 1 1 9 Tumor Blood Vessels: 3-D Tubular Network Analysis Chap. 9 2015/8/20 14:02 page 2 2 Chap. 9 2015/8/20 14:02 page 3 3 Contents 9 Tumor Blood Vessels: 3-D Tubular Network
Chap. 9 2015/8/20 14:02 page 1 1 9 Tumor Blood Vessels: 3-D Tubular Network Analysis Chap. 9 2015/8/20 14:02 page 2 2 Chap. 9 2015/8/20 14:02 page 3 3 Contents 9 Tumor Blood Vessels: 3-D Tubular Network
Supplementary File 2 TopCap Manual
 Supplementary File 2 TopCap Manual TopCap: a Tool to Quantify Soil Surface Topology and Subsurface Structure Amin Garbout, Craig J. Sturrock, Elena Armenise, Sujung Ahn, Robert W. Simmons, Stefan Doerr,
Supplementary File 2 TopCap Manual TopCap: a Tool to Quantify Soil Surface Topology and Subsurface Structure Amin Garbout, Craig J. Sturrock, Elena Armenise, Sujung Ahn, Robert W. Simmons, Stefan Doerr,
EE795: Computer Vision and Intelligent Systems
 EE795: Computer Vision and Intelligent Systems Spring 2012 TTh 17:30-18:45 WRI C225 Lecture 04 130131 http://www.ee.unlv.edu/~b1morris/ecg795/ 2 Outline Review Histogram Equalization Image Filtering Linear
EE795: Computer Vision and Intelligent Systems Spring 2012 TTh 17:30-18:45 WRI C225 Lecture 04 130131 http://www.ee.unlv.edu/~b1morris/ecg795/ 2 Outline Review Histogram Equalization Image Filtering Linear
CS443: Digital Imaging and Multimedia Binary Image Analysis. Spring 2008 Ahmed Elgammal Dept. of Computer Science Rutgers University
 CS443: Digital Imaging and Multimedia Binary Image Analysis Spring 2008 Ahmed Elgammal Dept. of Computer Science Rutgers University Outlines A Simple Machine Vision System Image segmentation by thresholding
CS443: Digital Imaging and Multimedia Binary Image Analysis Spring 2008 Ahmed Elgammal Dept. of Computer Science Rutgers University Outlines A Simple Machine Vision System Image segmentation by thresholding
[ ] Review. Edges and Binary Images. Edge detection. Derivative of Gaussian filter. Image gradient. Tuesday, Sept 16
![[ ] Review. Edges and Binary Images. Edge detection. Derivative of Gaussian filter. Image gradient. Tuesday, Sept 16 [ ] Review. Edges and Binary Images. Edge detection. Derivative of Gaussian filter. Image gradient. Tuesday, Sept 16](/thumbs/89/98496315.jpg) Review Edges and Binary Images Tuesday, Sept 6 Thought question: how could we compute a temporal gradient from video data? What filter is likely to have produced this image output? original filtered output
Review Edges and Binary Images Tuesday, Sept 6 Thought question: how could we compute a temporal gradient from video data? What filter is likely to have produced this image output? original filtered output
Morphological Compound Operations-Opening and CLosing
 Morphological Compound Operations-Opening and CLosing COMPSCI 375 S1 T 2006, A/P Georgy Gimel farb Revised COMPSCI 373 S1C -2010, Patrice Delmas AP Georgy Gimel'farb 1 Set-theoretic Binary Operations Many
Morphological Compound Operations-Opening and CLosing COMPSCI 375 S1 T 2006, A/P Georgy Gimel farb Revised COMPSCI 373 S1C -2010, Patrice Delmas AP Georgy Gimel'farb 1 Set-theoretic Binary Operations Many
Colin Paul Updated 14 November Preparation of publication-quality videos using ImageJ
 Preparation of publication-quality videos using ImageJ Statements made in scientific papers are often much easier to understand if they are supplemented by representative videos. In the best case, these
Preparation of publication-quality videos using ImageJ Statements made in scientific papers are often much easier to understand if they are supplemented by representative videos. In the best case, these
Neural Circuit Tracer
 2014 Neural Circuit Tracer Software for automated tracing of neurites from light microscopy stacks of images User Guide Version 4.0 Contents 1. Introduction... 2 2. System requirements and installation...
2014 Neural Circuit Tracer Software for automated tracing of neurites from light microscopy stacks of images User Guide Version 4.0 Contents 1. Introduction... 2 2. System requirements and installation...
Biomedical Image Analysis. Mathematical Morphology
 Biomedical Image Analysis Mathematical Morphology Contents: Foundation of Mathematical Morphology Structuring Elements Applications BMIA 15 V. Roth & P. Cattin 265 Foundations of Mathematical Morphology
Biomedical Image Analysis Mathematical Morphology Contents: Foundation of Mathematical Morphology Structuring Elements Applications BMIA 15 V. Roth & P. Cattin 265 Foundations of Mathematical Morphology
A Tutorial Guide to Tribology Plug-in
 Supplementary Material A Tutorial Guide to Tribology Plug-in Tribology An ImageJ Plugin for surface topography analysis of laser textured surfaces. General Description This plugin presents an easy-to-use
Supplementary Material A Tutorial Guide to Tribology Plug-in Tribology An ImageJ Plugin for surface topography analysis of laser textured surfaces. General Description This plugin presents an easy-to-use
09/11/2017. Morphological image processing. Morphological image processing. Morphological image processing. Morphological image processing (binary)
 Towards image analysis Goal: Describe the contents of an image, distinguishing meaningful information from irrelevant one. Perform suitable transformations of images so as to make explicit particular shape
Towards image analysis Goal: Describe the contents of an image, distinguishing meaningful information from irrelevant one. Perform suitable transformations of images so as to make explicit particular shape
C E N T E R A T H O U S T O N S C H O O L of H E A L T H I N F O R M A T I O N S C I E N C E S. Image Operations II
 T H E U N I V E R S I T Y of T E X A S H E A L T H S C I E N C E C E N T E R A T H O U S T O N S C H O O L of H E A L T H I N F O R M A T I O N S C I E N C E S Image Operations II For students of HI 5323
T H E U N I V E R S I T Y of T E X A S H E A L T H S C I E N C E C E N T E R A T H O U S T O N S C H O O L of H E A L T H I N F O R M A T I O N S C I E N C E S Image Operations II For students of HI 5323
Morphological Image Processing
 Morphological Image Processing Introduction Morphology: a branch of biology that deals with the form and structure of animals and plants Morphological image processing is used to extract image components
Morphological Image Processing Introduction Morphology: a branch of biology that deals with the form and structure of animals and plants Morphological image processing is used to extract image components
Rat Spinal Cord Image Archive (RatSCIA) Tutorial: Motoneuron Quantification with ImageJ
 Rat Spinal Cord Image Archive (RatSCIA) Tutorial: Motoneuron Quantification with ImageJ Background The Rat Spinal Cord Image Archive (RatSCIA) is an image library of spinal cord motoneurons to use in analyzing
Rat Spinal Cord Image Archive (RatSCIA) Tutorial: Motoneuron Quantification with ImageJ Background The Rat Spinal Cord Image Archive (RatSCIA) is an image library of spinal cord motoneurons to use in analyzing
4. TROUBLESHOOTING PREVIOUS VERSIONS RUN LOLITRACK ALWAYS AS ADMIN WIBU SOFTWARE PROTECTION... 30
 Version 4.2.0 CONTENTS 1. GETTING STARTED... 2 2. TYPICAL APPLICATIONS... 4 3. USER GUIDE... 5 3.1 SINGLE OBJECT MODE... 7 3.2 SINGLE ARENA MODE... 12 3.3 EVENT RECORDER... 19 3.4 BATCH TRACKING... 21
Version 4.2.0 CONTENTS 1. GETTING STARTED... 2 2. TYPICAL APPLICATIONS... 4 3. USER GUIDE... 5 3.1 SINGLE OBJECT MODE... 7 3.2 SINGLE ARENA MODE... 12 3.3 EVENT RECORDER... 19 3.4 BATCH TRACKING... 21
Edges and Binary Images
 CS 699: Intro to Computer Vision Edges and Binary Images Prof. Adriana Kovashka University of Pittsburgh September 5, 205 Plan for today Edge detection Binary image analysis Homework Due on 9/22, :59pm
CS 699: Intro to Computer Vision Edges and Binary Images Prof. Adriana Kovashka University of Pittsburgh September 5, 205 Plan for today Edge detection Binary image analysis Homework Due on 9/22, :59pm
CS334: Digital Imaging and Multimedia Edges and Contours. Ahmed Elgammal Dept. of Computer Science Rutgers University
 CS334: Digital Imaging and Multimedia Edges and Contours Ahmed Elgammal Dept. of Computer Science Rutgers University Outlines What makes an edge? Gradient-based edge detection Edge Operators From Edges
CS334: Digital Imaging and Multimedia Edges and Contours Ahmed Elgammal Dept. of Computer Science Rutgers University Outlines What makes an edge? Gradient-based edge detection Edge Operators From Edges
Binary Image Processing. Introduction to Computer Vision CSE 152 Lecture 5
 Binary Image Processing CSE 152 Lecture 5 Announcements Homework 2 is due Apr 25, 11:59 PM Reading: Szeliski, Chapter 3 Image processing, Section 3.3 More neighborhood operators Binary System Summary 1.
Binary Image Processing CSE 152 Lecture 5 Announcements Homework 2 is due Apr 25, 11:59 PM Reading: Szeliski, Chapter 3 Image processing, Section 3.3 More neighborhood operators Binary System Summary 1.
Image Processing. Filtering. Slide 1
 Image Processing Filtering Slide 1 Preliminary Image generation Original Noise Image restoration Result Slide 2 Preliminary Classic application: denoising However: Denoising is much more than a simple
Image Processing Filtering Slide 1 Preliminary Image generation Original Noise Image restoration Result Slide 2 Preliminary Classic application: denoising However: Denoising is much more than a simple
Image Processing. Traitement d images. Yuliya Tarabalka Tel.
 Traitement d images Yuliya Tarabalka yuliya.tarabalka@hyperinet.eu yuliya.tarabalka@gipsa-lab.grenoble-inp.fr Tel. 04 76 82 62 68 Noise reduction Image restoration Restoration attempts to reconstruct an
Traitement d images Yuliya Tarabalka yuliya.tarabalka@hyperinet.eu yuliya.tarabalka@gipsa-lab.grenoble-inp.fr Tel. 04 76 82 62 68 Noise reduction Image restoration Restoration attempts to reconstruct an
Babu Madhav Institute of Information Technology Years Integrated M.Sc.(IT)(Semester - 7)
 5 Years Integrated M.Sc.(IT)(Semester - 7) 060010707 Digital Image Processing UNIT 1 Introduction to Image Processing Q: 1 Answer in short. 1. What is digital image? 1. Define pixel or picture element?
5 Years Integrated M.Sc.(IT)(Semester - 7) 060010707 Digital Image Processing UNIT 1 Introduction to Image Processing Q: 1 Answer in short. 1. What is digital image? 1. Define pixel or picture element?
IDL Tutorial. Working with Images. Copyright 2008 ITT Visual Information Solutions All Rights Reserved
 IDL Tutorial Working with Images Copyright 2008 ITT Visual Information Solutions All Rights Reserved http://www.ittvis.com/ IDL is a registered trademark of ITT Visual Information Solutions for the computer
IDL Tutorial Working with Images Copyright 2008 ITT Visual Information Solutions All Rights Reserved http://www.ittvis.com/ IDL is a registered trademark of ITT Visual Information Solutions for the computer
IMAGE STUDIO LITE. Tutorial Guide Featuring Image Studio Analysis Software Version 3.1
 IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
Manual for Microfilament Analyzer
 Manual for Microfilament Analyzer Eveline Jacques & Jan Buytaert, Michał Lewandowski, Joris Dirckx, Jean-Pierre Verbelen, Kris Vissenberg University of Antwerp 1 Image data 2 image files are needed: a
Manual for Microfilament Analyzer Eveline Jacques & Jan Buytaert, Michał Lewandowski, Joris Dirckx, Jean-Pierre Verbelen, Kris Vissenberg University of Antwerp 1 Image data 2 image files are needed: a
Detection of Edges Using Mathematical Morphological Operators
 OPEN TRANSACTIONS ON INFORMATION PROCESSING Volume 1, Number 1, MAY 2014 OPEN TRANSACTIONS ON INFORMATION PROCESSING Detection of Edges Using Mathematical Morphological Operators Suman Rani*, Deepti Bansal,
OPEN TRANSACTIONS ON INFORMATION PROCESSING Volume 1, Number 1, MAY 2014 OPEN TRANSACTIONS ON INFORMATION PROCESSING Detection of Edges Using Mathematical Morphological Operators Suman Rani*, Deepti Bansal,
Counting Particles or Cells Using IMAQ Vision
 Application Note 107 Counting Particles or Cells Using IMAQ Vision John Hanks Introduction To count objects, you use a common image processing technique called particle analysis, often referred to as blob
Application Note 107 Counting Particles or Cells Using IMAQ Vision John Hanks Introduction To count objects, you use a common image processing technique called particle analysis, often referred to as blob
1. In the first step, the polylines are created which represent the geometry that has to be cut:
 QCAD/CAM Tutorial Caution should be exercised when working with hazardous machinery. Simulation is no substitute for the careful verification of the accuracy and safety of your CNC programs. QCAD/CAM or
QCAD/CAM Tutorial Caution should be exercised when working with hazardous machinery. Simulation is no substitute for the careful verification of the accuracy and safety of your CNC programs. QCAD/CAM or
PHASEQUANT (User Manual)
 PHASEQUANT (User Manual) ROI Pane Toolbar Threshold Bar Canvas Threshold Display Training Pane Buttons Fig 1. PhaseQuant GUI 1. INSTALLATION AND LOADING 1.1 Installing PhaseQuant Download the zip file
PHASEQUANT (User Manual) ROI Pane Toolbar Threshold Bar Canvas Threshold Display Training Pane Buttons Fig 1. PhaseQuant GUI 1. INSTALLATION AND LOADING 1.1 Installing PhaseQuant Download the zip file
SOP.8. Automated Analysis
 Circulating Tumor Cells TheRapeutic APheresis: a novel biotechnology enabling personalized therapy for all cancer patients SOP.8. Automated Analysis Scoring CTC with ICY Introduction This Standard Operating
Circulating Tumor Cells TheRapeutic APheresis: a novel biotechnology enabling personalized therapy for all cancer patients SOP.8. Automated Analysis Scoring CTC with ICY Introduction This Standard Operating
The walkthrough is available at /
 The walkthrough is available at https://downloads.openmicroscopy.org/presentations/2018/gbi-sydney / Description We will demonstrate a number of features of the OMERO platform using an OMERO server based
The walkthrough is available at https://downloads.openmicroscopy.org/presentations/2018/gbi-sydney / Description We will demonstrate a number of features of the OMERO platform using an OMERO server based
Cell Tracking via Proposal Generation & Selection
 Cell Tracking via Proposal Generation & Selection Saad Ullah Akram, Juho Kannala, Lauri Eklund and Janne Heikkilä saad.akram@oulu.fi 2 Overview Introduction Importance Challenges: detection & tracking
Cell Tracking via Proposal Generation & Selection Saad Ullah Akram, Juho Kannala, Lauri Eklund and Janne Heikkilä saad.akram@oulu.fi 2 Overview Introduction Importance Challenges: detection & tracking
Documentation to image analysis
 Documentation to image analysis The documentation below provides a detailed explanation and pointers towards the use of the MS-ECS-2D update site. It is made available in conjunction with the publication
Documentation to image analysis The documentation below provides a detailed explanation and pointers towards the use of the MS-ECS-2D update site. It is made available in conjunction with the publication
please download and install.
 Scripting using ImageJ Macro - a quick 1 hour tutorial - We use Fiji. http://fiji.sc please download and install. Kota Miura @ CMCI EMBL 2-1 Why do we write macro? Aim: Students acquire ImageJ macro programming
Scripting using ImageJ Macro - a quick 1 hour tutorial - We use Fiji. http://fiji.sc please download and install. Kota Miura @ CMCI EMBL 2-1 Why do we write macro? Aim: Students acquire ImageJ macro programming
Image Processing Fundamentals. Nicolas Vazquez Principal Software Engineer National Instruments
 Image Processing Fundamentals Nicolas Vazquez Principal Software Engineer National Instruments Agenda Objectives and Motivations Enhancing Images Checking for Presence Locating Parts Measuring Features
Image Processing Fundamentals Nicolas Vazquez Principal Software Engineer National Instruments Agenda Objectives and Motivations Enhancing Images Checking for Presence Locating Parts Measuring Features
JAVA DIP - OPEN SOURCE LIBRARIES
 JAVA DIP - OPEN SOURCE LIBRARIES http://www.tutorialspoint.com/java_dip/open_source_libraries.htm Copyright tutorialspoint.com In this chapter, we explore some of the free image processing libraries that
JAVA DIP - OPEN SOURCE LIBRARIES http://www.tutorialspoint.com/java_dip/open_source_libraries.htm Copyright tutorialspoint.com In this chapter, we explore some of the free image processing libraries that
3-D. Here red spheres show the location of gold nanoparticles inside/around a cell nucleus.
 3-D The CytoViva 3-D System allows the user can locate objects of interest in a 3-D space. It does this by acquiring multiple Z planes and performing our custom software routines to locate and observe
3-D The CytoViva 3-D System allows the user can locate objects of interest in a 3-D space. It does this by acquiring multiple Z planes and performing our custom software routines to locate and observe
Image Processing Guideline for TMU 7T MRI
 Image Processing Guideline for TMU 7T MRI Chia Feng Lu Laboratory of NeuroImage Biomarker Analysis, Translational Imaging Research Center, TMU 08/18/2015, version 1.0 Section 1: Installation of ImageJ
Image Processing Guideline for TMU 7T MRI Chia Feng Lu Laboratory of NeuroImage Biomarker Analysis, Translational Imaging Research Center, TMU 08/18/2015, version 1.0 Section 1: Installation of ImageJ
Guidelines for the MorphoLeaf application.
 Guidelines for the MorphoLeaf application. (Version 1.0, December 2015) Presentation of MorphoLeaf 2 Installation of MorphoLeaf 3 Some advice to start with optimal leaf images 4 1. Importing leaf images
Guidelines for the MorphoLeaf application. (Version 1.0, December 2015) Presentation of MorphoLeaf 2 Installation of MorphoLeaf 3 Some advice to start with optimal leaf images 4 1. Importing leaf images
Michal Kuneš
 The Open Microscopy Environment A DataBase for the storage and manipulation of image data Michal Kuneš xkunes@utia.cas.cz ZOI UTIA, ASCR, Friday seminar 13.12.2013 OMERO http://www.openmicroscopy.org/site/support/omero4/users/index.html
The Open Microscopy Environment A DataBase for the storage and manipulation of image data Michal Kuneš xkunes@utia.cas.cz ZOI UTIA, ASCR, Friday seminar 13.12.2013 OMERO http://www.openmicroscopy.org/site/support/omero4/users/index.html
Deep Learning. Deep Learning provided breakthrough results in speech recognition and image classification. Why?
 Data Mining Deep Learning Deep Learning provided breakthrough results in speech recognition and image classification. Why? Because Speech recognition and image classification are two basic examples of
Data Mining Deep Learning Deep Learning provided breakthrough results in speech recognition and image classification. Why? Because Speech recognition and image classification are two basic examples of
Using References in NIS-Elements
 Using References in NIS-Elements Overview This technical note describes general usage of References in NIS-Elements for image processing and analysis. The Reference feature in NIS-Elements refers to a
Using References in NIS-Elements Overview This technical note describes general usage of References in NIS-Elements for image processing and analysis. The Reference feature in NIS-Elements refers to a
BioImageXD Getting started
 BioImageXD Getting started Updated 2012-07-01 by Pasi Kankaanpää Introduction Welcome to BioImageXD, free open source software for biomedical image post-processing, visualization and analysis. This guide
BioImageXD Getting started Updated 2012-07-01 by Pasi Kankaanpää Introduction Welcome to BioImageXD, free open source software for biomedical image post-processing, visualization and analysis. This guide
Fast and accurate automated cell boundary determination for fluorescence microscopy
 Fast and accurate automated cell boundary determination for fluorescence microscopy Stephen Hugo Arce, Pei-Hsun Wu &, and Yiider Tseng Department of Chemical Engineering, University of Florida and National
Fast and accurate automated cell boundary determination for fluorescence microscopy Stephen Hugo Arce, Pei-Hsun Wu &, and Yiider Tseng Department of Chemical Engineering, University of Florida and National
82 REGISTRATION OF RETINOGRAPHIES
 82 REGISTRATION OF RETINOGRAPHIES 3.3 Our method Our method resembles the human approach to image matching in the sense that we also employ as guidelines features common to both images. It seems natural
82 REGISTRATION OF RETINOGRAPHIES 3.3 Our method Our method resembles the human approach to image matching in the sense that we also employ as guidelines features common to both images. It seems natural
GENERAL AUTOMATED FLAW DETECTION SCHEME FOR NDE X-RAY IMAGES
 GENERAL AUTOMATED FLAW DETECTION SCHEME FOR NDE X-RAY IMAGES Karl W. Ulmer and John P. Basart Center for Nondestructive Evaluation Department of Electrical and Computer Engineering Iowa State University
GENERAL AUTOMATED FLAW DETECTION SCHEME FOR NDE X-RAY IMAGES Karl W. Ulmer and John P. Basart Center for Nondestructive Evaluation Department of Electrical and Computer Engineering Iowa State University
Digital Image Processing Fundamentals
 Ioannis Pitas Digital Image Processing Fundamentals Chapter 7 Shape Description Answers to the Chapter Questions Thessaloniki 1998 Chapter 7: Shape description 7.1 Introduction 1. Why is invariance to
Ioannis Pitas Digital Image Processing Fundamentals Chapter 7 Shape Description Answers to the Chapter Questions Thessaloniki 1998 Chapter 7: Shape description 7.1 Introduction 1. Why is invariance to
3D-Modeling with Microscopy Image Browser (im_browser) (pdf version) Ilya Belevich, Darshan Kumar, Helena Vihinen
 3D-Modeling with Microscopy Image Browser (im_browser) (pdf version) Ilya Belevich, Darshan Kumar, Helena Vihinen Dataset: Huh7.tif (original), obtained by Serial Block Face-SEM (Gatan 3View) Huh7_crop.tif
3D-Modeling with Microscopy Image Browser (im_browser) (pdf version) Ilya Belevich, Darshan Kumar, Helena Vihinen Dataset: Huh7.tif (original), obtained by Serial Block Face-SEM (Gatan 3View) Huh7_crop.tif
Lecture 19 - Applied Image Analysis
 Lecture 19 - Applied Image Analysis Graeme Ball Three Questions 1. Why should you be interested in image processing & analysis? 2. What are the key concepts & techniques? 3. How can you apply them most
Lecture 19 - Applied Image Analysis Graeme Ball Three Questions 1. Why should you be interested in image processing & analysis? 2. What are the key concepts & techniques? 3. How can you apply them most
MetaMorph Standard Operation Protocol Basic Application
 MetaMorph Standard Operation Protocol Basic Application Contents Basic Navigation and Image Handling... 2 Opening Images... 2 Separating Multichannel Images... 2 Cropping an Image... 3 Changing an 8 bit
MetaMorph Standard Operation Protocol Basic Application Contents Basic Navigation and Image Handling... 2 Opening Images... 2 Separating Multichannel Images... 2 Cropping an Image... 3 Changing an 8 bit
3D Accounting and Measuring Plugin
 3D Accounting and Measuring Plugin v. 1.0.1 Manual Laetz, E.M.J. 1, Rühr, P.T. 1, Bartolomaeus, T. 2, Preisfeld, A. 3 & Wägele, H. 1 May 2016 1 Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Bonn,
3D Accounting and Measuring Plugin v. 1.0.1 Manual Laetz, E.M.J. 1, Rühr, P.T. 1, Bartolomaeus, T. 2, Preisfeld, A. 3 & Wägele, H. 1 May 2016 1 Zoologisches Forschungsmuseum Alexander Koenig (ZFMK), Bonn,
Lecture 6: Segmentation by Point Processing
 Lecture 6: Segmentation by Point Processing Harvey Rhody Chester F. Carlson Center for Imaging Science Rochester Institute of Technology rhody@cis.rit.edu September 27, 2005 Abstract Applications of point
Lecture 6: Segmentation by Point Processing Harvey Rhody Chester F. Carlson Center for Imaging Science Rochester Institute of Technology rhody@cis.rit.edu September 27, 2005 Abstract Applications of point
Synoptics Limited reserves the right to make changes without notice both to this publication and to the product that it describes.
 GeneTools Getting Started Although all possible care has been taken in the preparation of this publication, Synoptics Limited accepts no liability for any inaccuracies that may be found. Synoptics Limited
GeneTools Getting Started Although all possible care has been taken in the preparation of this publication, Synoptics Limited accepts no liability for any inaccuracies that may be found. Synoptics Limited
Manual for Constructing Research Trails (Sciences)
 Grit Laudel and Jochen Gläser Manual for Constructing Research Trails (Sciences) (updated July 2015) 1. Download publications from the Web of Science 1.1 Search in the ISI databases Note!: A major problem
Grit Laudel and Jochen Gläser Manual for Constructing Research Trails (Sciences) (updated July 2015) 1. Download publications from the Web of Science 1.1 Search in the ISI databases Note!: A major problem
How to use MPLABX to program and debug PICsimLab
 How to use MPLABX to program and debug PICsimLab Luis Claudio Gambôa Lopes http://sourceforge.net/projects/picsim/ November 2, 2015 Contents 1 Installing the Necessary Tools 2 1.1
How to use MPLABX to program and debug PICsimLab Luis Claudio Gambôa Lopes http://sourceforge.net/projects/picsim/ November 2, 2015 Contents 1 Installing the Necessary Tools 2 1.1
SE06: In-Sight Explorer New Tools for Defect Detection - Hands On Lab Werner Solution Expo April 8 & 9
 SE06: In-Sight Explorer New Tools for Defect Detection - Hands On Lab Werner Solution Expo April 8 & 9 Learning Goals: At the end of this lab, the student should have familiarity with the most common settings
SE06: In-Sight Explorer New Tools for Defect Detection - Hands On Lab Werner Solution Expo April 8 & 9 Learning Goals: At the end of this lab, the student should have familiarity with the most common settings
/5 Stacks. Displays the slice that follows the currently displayed slice. As a shortcut, press the > key.
 20-02-2018 1/5 Stacks Stacks This submenu contains commands that work with stacks. Add Slice Inserts a blank slice after the currently displayed slice. Hold down the Alt key to add the slice before the
20-02-2018 1/5 Stacks Stacks This submenu contains commands that work with stacks. Add Slice Inserts a blank slice after the currently displayed slice. Hold down the Alt key to add the slice before the
Physics MRI Research Centre UNIFIT VERSION User s Guide
 Physics MRI Research Centre UNIFIT VERSION 1.24 User s Guide Note: If an error occurs please quit UNIFIT and type:.reset in the IDL command line, and restart UNIFIT. Last Update November 2016 by Katie
Physics MRI Research Centre UNIFIT VERSION 1.24 User s Guide Note: If an error occurs please quit UNIFIT and type:.reset in the IDL command line, and restart UNIFIT. Last Update November 2016 by Katie
SECTION 5 IMAGE PROCESSING 2
 SECTION 5 IMAGE PROCESSING 2 5.1 Resampling 3 5.1.1 Image Interpolation Comparison 3 5.2 Convolution 3 5.3 Smoothing Filters 3 5.3.1 Mean Filter 3 5.3.2 Median Filter 4 5.3.3 Pseudomedian Filter 6 5.3.4
SECTION 5 IMAGE PROCESSING 2 5.1 Resampling 3 5.1.1 Image Interpolation Comparison 3 5.2 Convolution 3 5.3 Smoothing Filters 3 5.3.1 Mean Filter 3 5.3.2 Median Filter 4 5.3.3 Pseudomedian Filter 6 5.3.4
Lecture 7: Morphological Image Processing
 I2200: Digital Image processing Lecture 7: Morphological Image Processing Prof. YingLi Tian Oct. 25, 2017 Department of Electrical Engineering The City College of New York The City University of New York
I2200: Digital Image processing Lecture 7: Morphological Image Processing Prof. YingLi Tian Oct. 25, 2017 Department of Electrical Engineering The City College of New York The City University of New York
Morphological Image Processing
 Morphological Image Processing Morphology Identification, analysis, and description of the structure of the smallest unit of words Theory and technique for the analysis and processing of geometric structures
Morphological Image Processing Morphology Identification, analysis, and description of the structure of the smallest unit of words Theory and technique for the analysis and processing of geometric structures
PAPARA(ZZ)I User Manual
 PAPARA(ZZ)I 2.0 - User Manual June 2016 Authors: Yann Marcon (yann.marcon@awi.de) Autun Purser (autun.purser@awi.de) PAPARA(ZZ)I Program for Annotation of Photographs and Rapid Analysis (of Zillions and
PAPARA(ZZ)I 2.0 - User Manual June 2016 Authors: Yann Marcon (yann.marcon@awi.de) Autun Purser (autun.purser@awi.de) PAPARA(ZZ)I Program for Annotation of Photographs and Rapid Analysis (of Zillions and
Numerical Propagation Manual
 Numerical Propagation Manual Numerical Propagation Manual 1 Numerical Propagation Manual Version 1.2 Juan Pablo Piedrahita Quintero, Raúl Andrés Castañeda Quintero and Jorge Iván García Sucerquia Monday,
Numerical Propagation Manual Numerical Propagation Manual 1 Numerical Propagation Manual Version 1.2 Juan Pablo Piedrahita Quintero, Raúl Andrés Castañeda Quintero and Jorge Iván García Sucerquia Monday,
DETAILED INSTRUCTIONS FOR RUNNING BINARY_TRAVERSER
 DETAILED INSTRUCTIONS FOR RUNNING BINARY_TRAVERSER These instructions are also available at http://www.geo.umass.edu/climate/lewis/analysis/ Install ImageJ from http://rsb.info.nih.gov/ij/download.html.
DETAILED INSTRUCTIONS FOR RUNNING BINARY_TRAVERSER These instructions are also available at http://www.geo.umass.edu/climate/lewis/analysis/ Install ImageJ from http://rsb.info.nih.gov/ij/download.html.
Processing of binary images
 Binary Image Processing Tuesday, 14/02/2017 ntonis rgyros e-mail: argyros@csd.uoc.gr 1 Today From gray level to binary images Processing of binary images Mathematical morphology 2 Computer Vision, Spring
Binary Image Processing Tuesday, 14/02/2017 ntonis rgyros e-mail: argyros@csd.uoc.gr 1 Today From gray level to binary images Processing of binary images Mathematical morphology 2 Computer Vision, Spring
Digital Image Processing COSC 6380/4393
 Digital Image Processing COSC 6380/4393 Lecture 21 Nov 16 th, 2017 Pranav Mantini Ack: Shah. M Image Processing Geometric Transformation Point Operations Filtering (spatial, Frequency) Input Restoration/
Digital Image Processing COSC 6380/4393 Lecture 21 Nov 16 th, 2017 Pranav Mantini Ack: Shah. M Image Processing Geometric Transformation Point Operations Filtering (spatial, Frequency) Input Restoration/
Using Microsoft Word. Working With Objects
 Using Microsoft Word Many Word documents will require elements that were created in programs other than Word, such as the picture to the right. Nontext elements in a document are referred to as Objects
Using Microsoft Word Many Word documents will require elements that were created in programs other than Word, such as the picture to the right. Nontext elements in a document are referred to as Objects
Files Used in This Tutorial. Background. Feature Extraction with Example-Based Classification Tutorial
 Feature Extraction with Example-Based Classification Tutorial In this tutorial, you will use Feature Extraction to extract rooftops from a multispectral QuickBird scene of a residential area in Boulder,
Feature Extraction with Example-Based Classification Tutorial In this tutorial, you will use Feature Extraction to extract rooftops from a multispectral QuickBird scene of a residential area in Boulder,
A Procedure for the 3D Reconstruction of Biological Organs from 2D Image Sequences
 A Procedure for the 3D Reconstruction of Biological Organs from 2D Image Sequences Kirana Kumara P Centre for Product Design and Manufacturing Indian Institute of Science Bangalore, 560 012 India Ashitava
A Procedure for the 3D Reconstruction of Biological Organs from 2D Image Sequences Kirana Kumara P Centre for Product Design and Manufacturing Indian Institute of Science Bangalore, 560 012 India Ashitava
Image J An introduction to image processing And more
 Image J An introduction to image processing And more What is Image NOT J? Photoshop (not an artistic tool) Powerpoint (not a presentation tool) Instagram (not easy to understand) Maya, Blender (not a 3D
Image J An introduction to image processing And more What is Image NOT J? Photoshop (not an artistic tool) Powerpoint (not a presentation tool) Instagram (not easy to understand) Maya, Blender (not a 3D
Features included in isolution Lite, i-solution, isolution DT
 Features included in isolution Lite, i-solution, isolution DT Include: Live Measurement and Overlay Settings Users can perform measurements on the live preview image, using the crosshair or grid masks
Features included in isolution Lite, i-solution, isolution DT Include: Live Measurement and Overlay Settings Users can perform measurements on the live preview image, using the crosshair or grid masks
CS534: Introduction to Computer Vision Edges and Contours. Ahmed Elgammal Dept. of Computer Science Rutgers University
 CS534: Introduction to Computer Vision Edges and Contours Ahmed Elgammal Dept. of Computer Science Rutgers University Outlines What makes an edge? Gradient-based edge detection Edge Operators Laplacian
CS534: Introduction to Computer Vision Edges and Contours Ahmed Elgammal Dept. of Computer Science Rutgers University Outlines What makes an edge? Gradient-based edge detection Edge Operators Laplacian
Last update: May 4, Vision. CMSC 421: Chapter 24. CMSC 421: Chapter 24 1
 Last update: May 4, 200 Vision CMSC 42: Chapter 24 CMSC 42: Chapter 24 Outline Perception generally Image formation Early vision 2D D Object recognition CMSC 42: Chapter 24 2 Perception generally Stimulus
Last update: May 4, 200 Vision CMSC 42: Chapter 24 CMSC 42: Chapter 24 Outline Perception generally Image formation Early vision 2D D Object recognition CMSC 42: Chapter 24 2 Perception generally Stimulus
3D Processing and Analysis with ImageJ
 3D Processing and Analysis with ImageJ Rapid development of technologies yielding multidimensional data LSCM / Video Electron tomography IRM / CT scan Thomas Boudier, Associate Professor, Cellular Imaging
3D Processing and Analysis with ImageJ Rapid development of technologies yielding multidimensional data LSCM / Video Electron tomography IRM / CT scan Thomas Boudier, Associate Professor, Cellular Imaging
QGIS LAB SERIES GST 102: Spatial Analysis Lab 7: Raster Data Analysis - Density Surfaces
 QGIS LAB SERIES GST 102: Spatial Analysis Lab 7: Raster Data Analysis - Density Surfaces Objective Learn Density Analysis Methods Document Version: 2014-07-11 (Beta) Contents Introduction...2 Objective:
QGIS LAB SERIES GST 102: Spatial Analysis Lab 7: Raster Data Analysis - Density Surfaces Objective Learn Density Analysis Methods Document Version: 2014-07-11 (Beta) Contents Introduction...2 Objective:
Digital Image Processing
 Digital Image Processing Third Edition Rafael C. Gonzalez University of Tennessee Richard E. Woods MedData Interactive PEARSON Prentice Hall Pearson Education International Contents Preface xv Acknowledgments
Digital Image Processing Third Edition Rafael C. Gonzalez University of Tennessee Richard E. Woods MedData Interactive PEARSON Prentice Hall Pearson Education International Contents Preface xv Acknowledgments
BioIRC solutions. CFDVasc manual
 BioIRC solutions CFDVasc manual Main window of application is consisted from two parts: toolbar - which consist set of button for accessing variety of present functionalities image area area in which is
BioIRC solutions CFDVasc manual Main window of application is consisted from two parts: toolbar - which consist set of button for accessing variety of present functionalities image area area in which is
Neuron Morpho plugin for ImageJ Version May 25, 2007
 Neuron Morpho plugin for ImageJ Version 1.1.6 - May 25, 2007 Giampaolo D Alessandro Department of Mathematics, University of Southampton, Southampton, SO17 1BJ, England, UK 1 Introduction The Neuron Morpho
Neuron Morpho plugin for ImageJ Version 1.1.6 - May 25, 2007 Giampaolo D Alessandro Department of Mathematics, University of Southampton, Southampton, SO17 1BJ, England, UK 1 Introduction The Neuron Morpho
Adaptive active contours (snakes) for the segmentation of complex structures in biological images
 Adaptive active contours (snakes) for the segmentation of complex structures in biological images Philippe Andrey a and Thomas Boudier b a Analyse et Modélisation en Imagerie Biologique, Laboratoire Neurobiologie
Adaptive active contours (snakes) for the segmentation of complex structures in biological images Philippe Andrey a and Thomas Boudier b a Analyse et Modélisation en Imagerie Biologique, Laboratoire Neurobiologie
Artifacts and Textured Region Detection
 Artifacts and Textured Region Detection 1 Vishal Bangard ECE 738 - Spring 2003 I. INTRODUCTION A lot of transformations, when applied to images, lead to the development of various artifacts in them. In
Artifacts and Textured Region Detection 1 Vishal Bangard ECE 738 - Spring 2003 I. INTRODUCTION A lot of transformations, when applied to images, lead to the development of various artifacts in them. In
Cornell Spectrum Imager (CSI) Open Source Spectrum Analysis with ImageJ Tutorial
 Cornell Spectrum Imager (CSI) Open Source Spectrum Analysis with ImageJ Tutorial Electron Microscopy Summer School 2017 Why CSI Current Software Black box Expensive Steep learning curve Cornell Spectrum
Cornell Spectrum Imager (CSI) Open Source Spectrum Analysis with ImageJ Tutorial Electron Microscopy Summer School 2017 Why CSI Current Software Black box Expensive Steep learning curve Cornell Spectrum
Comparative Neuroanatomy Tutorial: Hippocampal Quantification with ImageJ
 Comparative Neuroanatomy Tutorial: Hippocampal Quantification with ImageJ Background The Comparative Neuroanatomy Module is based on an image library of coronal brain sections from various mammalian species.
Comparative Neuroanatomy Tutorial: Hippocampal Quantification with ImageJ Background The Comparative Neuroanatomy Module is based on an image library of coronal brain sections from various mammalian species.
AutoCount Server. A c c o u n t B i l l i n g S t o c k P O S P a y r o l l. ISV/Software Solutions
 AutoCount Server A c c o u n t B i l l i n g S t o c k P O S P a y r o l l ISV/Software Solutions Introduction AutoCount server is mandatory to install in server pc for AutoCount Accounting 2.0. It is
AutoCount Server A c c o u n t B i l l i n g S t o c k P O S P a y r o l l ISV/Software Solutions Introduction AutoCount server is mandatory to install in server pc for AutoCount Accounting 2.0. It is
Image Segmentation Techniques for Object-Based Coding
 Image Techniques for Object-Based Coding Junaid Ahmed, Joseph Bosworth, and Scott T. Acton The Oklahoma Imaging Laboratory School of Electrical and Computer Engineering Oklahoma State University {ajunaid,bosworj,sacton}@okstate.edu
Image Techniques for Object-Based Coding Junaid Ahmed, Joseph Bosworth, and Scott T. Acton The Oklahoma Imaging Laboratory School of Electrical and Computer Engineering Oklahoma State University {ajunaid,bosworj,sacton}@okstate.edu
Olympus DeltaVision Microscope Start-Up and Shut-Down Instructions.
 DeltaVision Instructions: 1. Start-up (Olympus DV) 2. Basic operation (Olympus DV) 2.1 set-up 2.2 designing and running an experiment 3. Transferring files via FTP 4. Deconvolving files 5. File conversion
DeltaVision Instructions: 1. Start-up (Olympus DV) 2. Basic operation (Olympus DV) 2.1 set-up 2.2 designing and running an experiment 3. Transferring files via FTP 4. Deconvolving files 5. File conversion
Albert M. Vossepoel. Center for Image Processing
 Albert M. Vossepoel www.ph.tn.tudelft.nl/~albert scene image formation sensor pre-processing image enhancement image restoration texture filtering segmentation user analysis classification CBP course:
Albert M. Vossepoel www.ph.tn.tudelft.nl/~albert scene image formation sensor pre-processing image enhancement image restoration texture filtering segmentation user analysis classification CBP course:
Zeiss Efficient Navigation (ZEN) Blue Edition Standard Operation Protocol
 Faculty Core Facility ZEN BLUE 2.3 SOP A-1 Zeiss Efficient Navigation (ZEN) Blue Edition Standard Operation Protocol Faculty Core Facility ZEN BLUE 2.3 SOP A-2 A. Content Overview. 3 Start up. 4 Display
Faculty Core Facility ZEN BLUE 2.3 SOP A-1 Zeiss Efficient Navigation (ZEN) Blue Edition Standard Operation Protocol Faculty Core Facility ZEN BLUE 2.3 SOP A-2 A. Content Overview. 3 Start up. 4 Display
WormBox. A tool set for automating measurements with Fiji/ImageJ. Bruno C. Vellutini & Fernando P.L Marques version 0.9 beta. Denis J.
 WormBox version 0.9 beta Denis J. Machado A tool set for automating measurements with Fiji/ImageJ Bruno C. Vellutini & Fernando P.L Marques 2011 - https://github.com/nelas/wormbox/zipball/masterabout WormBox:
WormBox version 0.9 beta Denis J. Machado A tool set for automating measurements with Fiji/ImageJ Bruno C. Vellutini & Fernando P.L Marques 2011 - https://github.com/nelas/wormbox/zipball/masterabout WormBox:
Programming for Image Analysis/Processing
 Computer assisted Image Analysis VT04 Programming for Image Analysis/Processing Tools and guidelines to write your own IP/IA applications Why this lecture? Introduction To give an overview of What is needed
Computer assisted Image Analysis VT04 Programming for Image Analysis/Processing Tools and guidelines to write your own IP/IA applications Why this lecture? Introduction To give an overview of What is needed
Image Shift Correction in Microscopic Time Lapse Sequences
 Image Shift Correction in Microscopic Time Lapse Sequences 1. General Information It is of importance to ensure mechanical stability of your live cell imaging set-up during a time lapse experiment. Vibration
Image Shift Correction in Microscopic Time Lapse Sequences 1. General Information It is of importance to ensure mechanical stability of your live cell imaging set-up during a time lapse experiment. Vibration
GoTo [ File ] [ Import KNIME workflow ] Select archive file: and import the CountNuclei.zip example workflow.
![GoTo [ File ] [ Import KNIME workflow ] Select archive file: and import the CountNuclei.zip example workflow. GoTo [ File ] [ Import KNIME workflow ] Select archive file: and import the CountNuclei.zip example workflow.](/thumbs/75/71593469.jpg) Manual KNIME Image Processing :: First steps This manual aims to provide first insights in KNIME Image Processing. The image processing workflow Count nuclei is described in detail and can be downloaded
Manual KNIME Image Processing :: First steps This manual aims to provide first insights in KNIME Image Processing. The image processing workflow Count nuclei is described in detail and can be downloaded
Morphological Image Processing
 Morphological Image Processing Ranga Rodrigo October 9, 29 Outline Contents Preliminaries 2 Dilation and Erosion 3 2. Dilation.............................................. 3 2.2 Erosion..............................................
Morphological Image Processing Ranga Rodrigo October 9, 29 Outline Contents Preliminaries 2 Dilation and Erosion 3 2. Dilation.............................................. 3 2.2 Erosion..............................................
Digital image processing
 Digital image processing Morphological image analysis. Binary morphology operations Introduction The morphological transformations extract or modify the structure of the particles in an image. Such transformations
Digital image processing Morphological image analysis. Binary morphology operations Introduction The morphological transformations extract or modify the structure of the particles in an image. Such transformations
N.Priya. Keywords Compass mask, Threshold, Morphological Operators, Statistical Measures, Text extraction
 Volume, Issue 8, August ISSN: 77 8X International Journal of Advanced Research in Computer Science and Software Engineering Research Paper Available online at: www.ijarcsse.com A Combined Edge-Based Text
Volume, Issue 8, August ISSN: 77 8X International Journal of Advanced Research in Computer Science and Software Engineering Research Paper Available online at: www.ijarcsse.com A Combined Edge-Based Text
Digital Image Processing COSC 6380/4393
 Digital Image Processing COSC 6380/4393 Lecture 6 Sept 6 th, 2017 Pranav Mantini Slides from Dr. Shishir K Shah and Frank (Qingzhong) Liu Today Review Logical Operations on Binary Images Blob Coloring
Digital Image Processing COSC 6380/4393 Lecture 6 Sept 6 th, 2017 Pranav Mantini Slides from Dr. Shishir K Shah and Frank (Qingzhong) Liu Today Review Logical Operations on Binary Images Blob Coloring
morphology on binary images
 morphology on binary images Ole-Johan Skrede 10.05.2017 INF2310 - Digital Image Processing Department of Informatics The Faculty of Mathematics and Natural Sciences University of Oslo After original slides
morphology on binary images Ole-Johan Skrede 10.05.2017 INF2310 - Digital Image Processing Department of Informatics The Faculty of Mathematics and Natural Sciences University of Oslo After original slides
Feature Analyst Quick Start Guide
 Feature Analyst Quick Start Guide River Extraction River extractions are much like road extractions, in that you are trying to identify a continuous object running through other features within your image.
Feature Analyst Quick Start Guide River Extraction River extractions are much like road extractions, in that you are trying to identify a continuous object running through other features within your image.
Crop Counting and Metrics Tutorial
 Crop Counting and Metrics Tutorial The ENVI Crop Science platform contains remote sensing analytic tools for precision agriculture and agronomy. In this tutorial you will go through a typical workflow
Crop Counting and Metrics Tutorial The ENVI Crop Science platform contains remote sensing analytic tools for precision agriculture and agronomy. In this tutorial you will go through a typical workflow
Neurite-J 1.0. ImageJ plug-in. User manual. April, 2014
 Neurite-J 1.0 ImageJ plug-in User manual April, 2014 Content 1. About Neurite-J 1.0... 2 2. Neurite-J installation... 2 3. How to use Neurite-J... 2 3.1 User interface... 2 3.2 Before start... 3 3.3 Image
Neurite-J 1.0 ImageJ plug-in User manual April, 2014 Content 1. About Neurite-J 1.0... 2 2. Neurite-J installation... 2 3. How to use Neurite-J... 2 3.1 User interface... 2 3.2 Before start... 3 3.3 Image
Feature Extraction. Contents. 1. Installing Feature Extraction. 2. Using Feature Extraction
 1 Feature Extraction Contents 1. Installing Feature Extraction 2. Using Feature Extraction Adding an image to a view Converting image to grid Resample a grid Connecting to Arc/Info Server Calculating the
1 Feature Extraction Contents 1. Installing Feature Extraction 2. Using Feature Extraction Adding an image to a view Converting image to grid Resample a grid Connecting to Arc/Info Server Calculating the
