java -jar picard.jar CollectInsertSizeMetrics I=aln_sorted.bam O=out.metrics HISTOGRAM_FILE=chartoutput.pdf VALIDATION_STRINGENCY=LENIENT
|
|
|
- Alexandrina Holland
- 5 years ago
- Views:
Transcription
1 Supplementary Note 1 Pre-Processing and Alignment Commands Trimmomatic Command java -jar trimmomatic-0.32.jar PE -threads 15 -phred33 /Volumes/Drobo_Storage/Raw_Data_and_Trimmomatic_Files/First_Six_Samples_Raw_Data/Ra w_seq_files/als1_1_sequence.txt.gz /Volumes/Drobo_Storage/Raw_Data_and_Trimmomatic_Files/First_Six_Samples_Raw_Data/Ra w_seq_files/als1_2_sequence.txt.gz Spinal_Samples/ALS1/ALS1_forward_paired.fq Spinal_Samples/ALS1/ALS1_forward_unpaired.fq Spinal_Samples/ALS1/ALS1_reverse_paired.fq Spinal_Samples/ALS1/ALS1_reverse_unpaired.fq ILLUMINACLIP:TruSeq3-PE- 2.fa:2:30:10:8:true TRAILING:20 MINLEN:50 BWA Commands head ALS1_forward_paired.fq > 1.txt head ALS1_reverse_paired.fq > 2.txt bwa aln hg19tr.fa 1.txt > 1.sai bwa aln hg19tr.fa 2.txt > 2.sai bwa sampe hg19tr.fa 1.sai 2.sai 1.txt 2.txt > aln.sam samtools view -Sb aln.sam > aln.bam samtools sort aln.bam aln_sorted java -jar picard.jar CollectInsertSizeMetrics I=aln_sorted.bam O=out.metrics HISTOGRAM_FILE=chartoutput.pdf VALIDATION_STRINGENCY=LENIENT # This command will generate a file called out.metrics in the picard folder containing mean_insert size and standard deviation values # Subtract the total length of your reads (in our case 2X150, so 300 total) from the mean_insert size i.e = -102 for the Tophat r value. Tophat Command tophat -p 2 -o /Volumes/Drobo_Storage/Tophat_Output/ALS1_150_ARemoved_AllReads -G hg19/annotation/genes/genes.gtf --library-type fr-firststrand -r mate-std-dev transcriptome-index=hg19.transcriptome/hg19tr genome Spinal_Samples/ALS1/ALS1_forward_paired.fq Spinal_Samples/ALS1/ALS1_reverse_paired.fq
2 Collect RNA-Seq Metrics Command java -jar picard.jar CollectRnaSeqMetrics REF_FLAT=/Volumes/Drobo_Storage/Homo_sapiens_UCSC_hg19/Homo_sapiens/UCSC/hg19/ Annotation/Genes/refFlat.txt.gz RIBOSOMAL_INTERVALS= intervallist.txt STRAND_SPECIFICITY=SECOND_READ_TRANSCRIPTION_STRAND INPUT=accepted_hits.bam OUTPUT=Stats GATK Point Mutation Analysis Commands STAR --runmode genomegenerate --genomedir /Volumes/Drobo_Storage/STARgenome -- genomefastafiles wtie2index/genome.fa --runthreadn 10 STAR --genomedir /Volumes/Drobo_Storage/STARgenome/ --readfilesin /Volumes/Drobo_Storage/Raw_Data_and_Trimmomatic_Files/Processed_Samples/ALS1/150Bp _AllReads_Adaptors_Removed/ALS1_forward_paired.fq /Volumes/Drobo_Storage/Raw_Data_and_Trimmomatic_Files/Processed_Samples/ALS1/150Bp _AllReads_Adaptors_Removed/ALS1_reverse_paired.fq --runthreadn 15 STAR --runmode genomegenerate --genomedir /Volumes/Drobo_Storage/STARgenome_2ndpass/ --genomefastafiles wtie2index/genome.fa --sjdbfilechrstartend /Volumes/Drobo_Storage/STAR_Alignments/PAT_ALS1/SJ.out.tab --sjdboverhang runthreadn 15 STAR --genomedir /Volumes/Drobo_Storage/STARgenome_2ndpass/ --readfilesin /Volumes/Drobo_Storage/Raw_Data_and_Trimmomatic_Files/Processed_Samples/ALS1/150Bp _AllReads_Adaptors_Removed/ALS1_forward_paired.fq /Volumes/Drobo_Storage/Raw_Data_and_Trimmomatic_Files/Processed_Samples/ALS1/150Bp _AllReads_Adaptors_Removed/ALS1_reverse_paired.fq --runthreadn 15 java -jar picard.jar AddOrReplaceReadGroups I=Aligned.out.sam O=rg_added_sorted.bam SO=coordinate RGID=ALS1 RGLB=ALS1 RGPL=ILLUMINA RGPU=NextSeq500 RGSM=ALS1 java -Xmx50g -jar picard.jar MarkDuplicates I=rg_added_sorted.bam O=deduped.bam CREATE_INDEX=true VALIDATION_STRINGENCY=SILENT M=output.metrics java -jar GenomeAnalysisTK.jar -T SplitNCigarReads -R wtie2index/genome.fa -I deduped.bam -o split.bam -rf ReassignOneMappingQuality -RMQF RMQT 60 -U ALLOW_N_CIGAR_READS java -jar GenomeAnalysisTK.jar -T BaseRecalibrator -R wtie2index/genome.fa -I split.bam -knownsites /Volumes/Drobo_Storage/Homo_sapiens_UCSC_hg19/Homo_sapiens/UCSC/hg19/Annotation/E verything_else/variation/dbsnp_138.hg19.vcf -nct 14 -o recal_data.table java -jar GenomeAnalysisTK.jar -T BaseRecalibrator -R
3 wtie2index/genome.fa -I split.bam -knownsites /Volumes/Drobo_Storage/Homo_sapiens_UCSC_hg19/Homo_sapiens/UCSC/hg19/Annotation/E verything_else/variation/dbsnp_138.hg19.vcf -BQSR recal_data.table -nct 14 -o Final_recal_data.table java -jar GenomeAnalysisTK.jar -T PrintReads -R wtie2index/genome.fa -I split.bam -BQSR Final_recal_data.table -o Recal_split.bam java -jar GenomeAnalysisTK.jar -T HaplotypeCaller -D /Volumes/Drobo_Storage/Homo_sapiens_UCSC_hg19/Homo_sapiens/UCSC/hg19/Annotation/E verything_else/variation/dbsnp_138.hg19.vcf -R wtie2index/genome.fa -I Recal_split.bam -dontusesoftclippedbases -stand_call_conf stand_emit_conf o output.vcf java -jar GenomeAnalysisTK.jar -T VariantFiltration -R wtie2index/genome.fa -V output.vcf -window 35 -cluster 3 -filtername FS -filter "FS >30.0" - filtername QD -filter "QD <2.0" -o final_output.vcf HTSeq Count Command # From within a Tophat Output Folder # samtools sort -n accepted_hits.bam accepted_hits.sorted samtools view accepted_hits.sorted.bam htseq-count -s reverse - /Volumes/Drobo_Storage/Homo_sapiens_UCSC_hg19/Homo_sapiens/UCSC/hg19/Annotation/G enes/genes.gtf > PAT_ALS1_Count.txt Cufflinks, Cuffmerge, and Cuffdiff Commands Cufflinks Command cufflinks -o /Volumes/Drobo_Storage/Cufflinks_Cuffdiff_Output/Postmortem_Spinals_Cufflinks_Output_No_ M/PAT_ALS1_no_novel_highdata1 --library-type fr-firststrand -p 1 -G hg19/annotation/genes/genes.gtf -b hg19/sequence/bowtie2index/genome.fa -M hg19/sequence/bowtie2index/rrna.trna.mt.gtf -u --compatible-hits-norm --max-bundle-frags /Volumes/Drobo_Storage/Tophat_Output/Postmortem_Spinal_Samples/PAT_ALS1_150_ARemo ved_allreads/accepted_hits.bam Cuffmerge Command cuffmerge -p 14 -o CTLvPAT_M -g hg19/annotation/genes/genes.gtf -s
4 hg19/sequence/wholegenomefasta/genome.fa assemblies.txt Cuffdiff2 Command cuffdiff --library-type fr-firststrand -o /Volumes/Drobo_Storage/PATvCTL_No_M_compared -p 14 - b hg19/sequence/bowtie2index/genome.fa -u -L CTL,PAT -M hg19/sequence/bowtie2index/rrna.trna.mt.gtf -max-bundle-frags v /Volumes/Drobo_Storage/CTLvPAT_No_M/merged.gtf /Volumes/Drobo_Storage/Tophat_Output/CTL_ALS6_150_ARemoved_AllReads/accepted_hits.b am,/volumes/drobo_storage/tophat_output/ctl_als8_150_aremoved_allreads/accepted_hi ts.bam,/volumes/drobo_storage/tophat_output/ctl_als16_150_aremoved_allreads/accept ed_hits.bam,/volumes/drobo_storage/tophat_output/ctl_als22_150_aremoved_allreads/a ccepted_hits.bam,/volumes/drobo_storage/tophat_output/ctl_als23_150_aremoved_allre ads/accepted_hits.bam,/volumes/drobo_storage/tophat_output/ctl_als24_150_aremoved_ AllReads/accepted_hits.bam,/Volumes/Drobo_Storage/Tophat_Output/CTL_ALS25_150_ARemo ved_allreads/accepted_hits.bam,/volumes/drobo_storage/tophat_output/ctl_als27_150_ar emoved_allreads/accepted_hits.bam /Volumes/Drobo_Storage/Tophat_Output/PAT_ALS1_150_ARemoved_AllReads/accepted_hits.b am,/volumes/drobo_storage/tophat_output/pat_als2_150_aremoved_allreads/accepted_hi ts.bam,/volumes/drobo_storage/tophat_output/pat_als3_150_aremoved_allreads/accepte d_hits.bam,/volumes/drobo_storage/tophat_output/pat_als4_150_aremoved_allreads/acc epted_hits.bam,/volumes/drobo_storage/tophat_output/pat_als9_150_aremoved_allreads/ accepted_hits.bam,/volumes/drobo_storage/tophat_output/pat_als10_150_aremoved_allr eads/accepted_hits.bam,/volumes/drobo_storage/tophat_output/pat_als14_150_aremoved _AllReads/accepted_hits.bam Calculating p-values using Benjamini-Hochberg correction #Copy the p-value column in gene_exp.diff output file. Paste them into first column of Excel file, and save as All_P.csv. Open R, then # Data <- read.csv(file="all_p.csv",header=true,stringsasfactors=false) FDR <- p.adjust(data[,1],method="bh") write.csv(fdr, file="fdr.csv") #Paste these values back into the gene_exp.diff file. These are FDR corrected p values. DeSeq2 Commands source(" bioclite("deseq2") bioclite("pasilla") library(deseq2) library(pasilla) library(biobase) setwd("/users/bennett_lab/desktop/deseq2/individual_count_files_postmortem") getwd() directory = "/Users/Bennett_Lab/Desktop/DESeq2/Individual_Count_Files_Postmortem/" samplefiles <- grep("pat",list.files(directory),value=true)
5 samplecondition <- sub("(.*pat).*","\\1",samplefiles) sampletable <- data.frame(samplename = samplefiles, filename = samplefiles, condition = samplecondition) ddshtseq <- DESeqDataSetFromHTSeqCount(sampleTable = sampletable, directory = directory, design = ~ condition) dds <- DESeq(ddsHTSeq) res <- results(dds) write.csv(res, file="res.csv") edger Commands setwd("/users/bennett_lab/desktop/edger/postmortem Spinal Samples/") source(" bioclite("edger") library(edger) x <- read.delim("edger.txt",row.names="symbol") group <- factor(c(2,2,2,2,2,2,2,1,1,1,1,1,1,1,1)) y <- DGEList(counts=x,group=group) keep <- rowsums(cpm(y)>1) >= 7 y <- y[keep,] y$samples$lib.size <- colsums(y$counts) y$samples y <- calcnormfactors(y) y <- estimatecommondisp(y) y <- estimatetagwisedisp(y) et <- exacttest(y) toptags(et) et$table write.csv(et$table, "DEGList.csv") #Copy the Pvalue column in the DEGList.csv output file. Paste them into first column of Excel file, and save as All_P.csv. Open R, then # Data <- read.csv(file="all_p.csv",header=true,stringsasfactors=false) FDR <- p.adjust(data[,1],method="bh") write.csv(fdr, file="fdr.csv") #Paste these adjusted p values back into the DEGList.csv file. These are FDR corrected p values. WGCNA Commands setwd("/users/bennett_lab/desktop/wgcna") getwd() source(" bioclite("impute") install.packages("wgcna") library(wgcna); options(stringsasfactors = FALSE) ALLOW_WGCNA_THREADS=6
6 lnames=load(file="allsubjects-data.input.rdata") # This loads the variables datexpr (including information from All_Sample_Data.csv ) and dattraits (including information from dattraits.csv). #### powers = c(c(1:10), seq(from = 12, to=24, by=2)) datexpr[, c(1:13301)] <- sapply(datexpr[, c(1:13301)], as.numeric) sft= picksoftthreshold(datexpr, powervector = powers, verbose = 5, networktype ="signed", corfnc= "bicor", coroptions=list(maxpoutliers=0.1)) sizegrwindow(9, 5) par(mfrow = c(1,2)); cex1 = 0.9; plot(sft$fitindices[,1], -sign(sft$fitindices[,3])*sft$fitindices[,2], xlab="soft Threshold (power)",ylab="scale Free Topology Model Fit,signed R^2",type="n", main = paste("scale independence")); text(sft$fitindices[,1], -sign(sft$fitindices[,3])*sft$fitindices[,2], labels=powers,cex=cex1,col="red"); abline(h=0.80,col="red") plot(sft$fitindices[,1], sft$fitindices[,5], xlab="soft Threshold (power)",ylab="mean Connectivity", type="n", main = paste("mean connectivity")) text(sft$fitindices[,1], sft$fitindices[,5], labels=powers, cex=cex1,col="red") adjacency = adjacency(datexpr, power=24, corfnc="bicor", type="signed", coroptions = "use = 'p', maxpoutliers = 0.1") TOM= TOMsimilarity(adjacency) disstom = 1-TOM genetree = hclust(as.dist(disstom), method = "average"); sizegrwindow(12,9) plot(genetree, xlab="", sub="", main = "Gene clustering on TOM-based dissimilarity", labels = FALSE, hang = 0.04); minmodulesize = 30; dynamicmods = cutreedynamic(dendro = genetree, distm = disstom, deepsplit = 2, pamrespectsdendro = FALSE, minclustersize = minmodulesize); table(dynamicmods) dynamiccolors = labels2colors(dynamicmods) table(dynamiccolors) sizegrwindow(8,6) plotdendroandcolors(genetree, dynamiccolors, "Dynamic Tree Cut", dendrolabels = FALSE, hang = 0.03, addguide = TRUE, guidehang = 0.05, main = "Gene dendrogram and module colors") MEList = moduleeigengenes(datexpr, colors = dynamiccolors) MEs = MEList$eigengenes MEDiss = 1-cor(MEs); METree = hclust(as.dist(mediss), method = "average");
7 sizegrwindow(7, 6) plot(metree, main = "Clustering of module eigengenes", xlab = "", sub = "") MEDissThres = 0.25 abline(h=medissthres, col = "red") merge = mergeclosemodules(datexpr, dynamiccolors, cutheight = MEDissThres, verbose = 3) mergedcolors = merge$colors; mergedmes = merge$newmes; plotdendroandcolors(genetree, cbind(dynamiccolors, mergedcolors), c("dynamic Tree Cut", "Merged dynamic"), dendrolabels = FALSE, hang = 0.03, addguide = TRUE, guidehang = 0.05) modulecolors = mergedcolors colororder = c("grey", standardcolors(50)); modulelabels = match(modulecolors, colororder)-1; MEs = mergedmes; ngenes = ncol(datexpr); nsamples = nrow(datexpr); MEs0 = moduleeigengenes(datexpr, modulecolors)$eigengenes MEs = ordermes(mes0) moduletraitcor = cor(mes, dattraits, use = "p"); moduletraitpvalue = corpvaluestudent(moduletraitcor, nsamples); sizegrwindow(10,6) textmatrix = paste(signif(moduletraitcor, 2), "\n(", signif(moduletraitpvalue, 1), ")", sep = ""); dim(textmatrix) = dim(moduletraitcor) par(mar = c(6, 8.5, 3, 3)); labeledheatmap(matrix = moduletraitcor, xlabels = names(dattraits), ylabels = names(mes), ysymbols = names(mes), colorlabels = FALSE, colors = greenwhitered(50), textmatrix = textmatrix, setstdmargins = FALSE, cex.text = 0.5, zlim = c(-1,1), main = paste("module-trait relationships")) ##### This is for modular membership scores ##### datkme = signedkme(datexpr, MEs, outputcolumnname = "kme") write.csv(datkme, file="datkme.csv") ##### This is for gene significance scores ##### Disease = as.data.frame(dattraits$disease.status) GS.Disease = as.numeric(cor(datexpr, Disease, use = "p")) write.csv(gs.disease, file="gs.disease.csv") ##### This is for intramodular connectivity scores #####
8 IMConnectivity = intramodularconnectivity(adjacency, modulecolors, scalebymax = FALSE) write.csv(imconnectivity, file="imconnectivity.csv") To retrieve module genes for IPA Core Analyses: module = "black" probes = names(datexpr) inmodule = (modulecolors==module); modprobes = probes[inmodule];
Clustering using WGCNA
 Clustering using WGCNA Overview: The WGCNA package (in R) uses functions that perform a correlation network analysis of large, high-dimensional data sets (RNAseq datasets). This unbiased approach clusters
Clustering using WGCNA Overview: The WGCNA package (in R) uses functions that perform a correlation network analysis of large, high-dimensional data sets (RNAseq datasets). This unbiased approach clusters
Supplemental Data. Cañas et al. Plant Cell (2017) /tpc
 Supplemental Method 1. WGCNA script. #Microarray and Trait data load getwd() workingdir = "C:/Users/..." setwd(workingdir) library(wgcna) library(flashclust) options(stringsasfactors = FALSE) femdata =
Supplemental Method 1. WGCNA script. #Microarray and Trait data load getwd() workingdir = "C:/Users/..." setwd(workingdir) library(wgcna) library(flashclust) options(stringsasfactors = FALSE) femdata =
Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice
 Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice 2.b Step-by-step network construction and module detection Peter Langfelder and Steve
Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice 2.b Step-by-step network construction and module detection Peter Langfelder and Steve
Reads Alignment and Variant Calling
 Reads Alignment and Variant Calling CB2-201 Computational Biology and Bioinformatics February 22, 2016 Emidio Capriotti http://biofold.org/ Institute for Mathematical Modeling of Biological Systems Department
Reads Alignment and Variant Calling CB2-201 Computational Biology and Bioinformatics February 22, 2016 Emidio Capriotti http://biofold.org/ Institute for Mathematical Modeling of Biological Systems Department
Exome sequencing. Jong Kyoung Kim
 Exome sequencing Jong Kyoung Kim Genome Analysis Toolkit The GATK is the industry standard for identifying SNPs and indels in germline DNA and RNAseq data. Its scope is now expanding to include somatic
Exome sequencing Jong Kyoung Kim Genome Analysis Toolkit The GATK is the industry standard for identifying SNPs and indels in germline DNA and RNAseq data. Its scope is now expanding to include somatic
Exercise 1 Review. --outfiltermismatchnmax : max number of mismatch (Default 10) --outreadsunmapped fastx: output unmapped reads
 Exercise 1 Review Setting parameters STAR --quantmode GeneCounts --genomedir genomedb -- runthreadn 2 --outfiltermismatchnmax 2 --readfilesin WTa.fastq.gz --readfilescommand zcat --outfilenameprefix WTa
Exercise 1 Review Setting parameters STAR --quantmode GeneCounts --genomedir genomedb -- runthreadn 2 --outfiltermismatchnmax 2 --readfilesin WTa.fastq.gz --readfilescommand zcat --outfilenameprefix WTa
Exercise 1. RNA-seq alignment and quantification. Part 1. Prepare the working directory. Part 2. Examine qualities of the RNA-seq data files
 Exercise 1. RNA-seq alignment and quantification Part 1. Prepare the working directory. 1. Connect to your assigned computer. If you do not know how, follow the instruction at http://cbsu.tc.cornell.edu/lab/doc/remote_access.pdf
Exercise 1. RNA-seq alignment and quantification Part 1. Prepare the working directory. 1. Connect to your assigned computer. If you do not know how, follow the instruction at http://cbsu.tc.cornell.edu/lab/doc/remote_access.pdf
RPGC Manual. You will also need python 2.7 or above to run our home-brew python scripts.
 Introduction Here we present a new approach for producing de novo whole genome sequences--recombinant population genome construction (RPGC)--that solves many of the problems encountered in standard genome
Introduction Here we present a new approach for producing de novo whole genome sequences--recombinant population genome construction (RPGC)--that solves many of the problems encountered in standard genome
Simulation studies of module preservation: Simulation study of weak module preservation
 Simulation studies of module preservation: Simulation study of weak module preservation Peter Langfelder and Steve Horvath October 25, 2010 Contents 1 Overview 1 1.a Setting up the R session............................................
Simulation studies of module preservation: Simulation study of weak module preservation Peter Langfelder and Steve Horvath October 25, 2010 Contents 1 Overview 1 1.a Setting up the R session............................................
Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice. 1. Data input and cleaning
 Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice 1. Data input and cleaning Peter Langfelder and Steve Horvath February 13, 2016 Contents
Tutorial for the WGCNA package for R II. Consensus network analysis of liver expression data, female and male mice 1. Data input and cleaning Peter Langfelder and Steve Horvath February 13, 2016 Contents
REPRODUCIBLE NGS WORKFLOWS WITH NEXTFLOW. Paolo Di Tommaso NGS'17 - Workshop, 5 April 2017
 REPRODUCIBLE NGS WORKFLOWS WITH NEXTFLOW Paolo Di Tommaso NGS'17 - Workshop, 5 April 2017 AGENDA Common problems with genomic pipelines Coffee break Quick overview of Nextflow framework How write a Nextflow
REPRODUCIBLE NGS WORKFLOWS WITH NEXTFLOW Paolo Di Tommaso NGS'17 - Workshop, 5 April 2017 AGENDA Common problems with genomic pipelines Coffee break Quick overview of Nextflow framework How write a Nextflow
Benchmarking of RNA-seq aligners
 Lecture 17 RNA-seq Alignment STAR Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Based on this analysis the most reliable
Lecture 17 RNA-seq Alignment STAR Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Benchmarking of RNA-seq aligners Based on this analysis the most reliable
Tutorial for the WGCNA package for R: III. Using simulated data to evaluate different module detection methods and gene screening approaches
 Tutorial for the WGCNA package for R: III. Using simulated data to evaluate different module detection methods and gene screening approaches 8. Visualization of gene networks Steve Horvath and Peter Langfelder
Tutorial for the WGCNA package for R: III. Using simulated data to evaluate different module detection methods and gene screening approaches 8. Visualization of gene networks Steve Horvath and Peter Langfelder
Our data for today is a small subset of Saimaa ringed seal RNA sequencing data (RNA_seq_reads.fasta). Let s first see how many reads are there:
 Practical Course in Genome Bioinformatics 19.2.2016 (CORRECTED 22.2.2016) Exercises - Day 5 http://ekhidna.biocenter.helsinki.fi/downloads/teaching/spring2016/ Answer the 5 questions (Q1-Q5) according
Practical Course in Genome Bioinformatics 19.2.2016 (CORRECTED 22.2.2016) Exercises - Day 5 http://ekhidna.biocenter.helsinki.fi/downloads/teaching/spring2016/ Answer the 5 questions (Q1-Q5) according
Cyverse tutorial 1 Logging in to Cyverse and data management. Open an Internet browser window and navigate to the Cyverse discovery environment:
 Cyverse tutorial 1 Logging in to Cyverse and data management Open an Internet browser window and navigate to the Cyverse discovery environment: https://de.cyverse.org/de/ Click Log in with your CyVerse
Cyverse tutorial 1 Logging in to Cyverse and data management Open an Internet browser window and navigate to the Cyverse discovery environment: https://de.cyverse.org/de/ Click Log in with your CyVerse
RNA-Seq Analysis With the Tuxedo Suite
 June 2016 RNA-Seq Analysis With the Tuxedo Suite Dena Leshkowitz Introduction In this exercise we will learn how to analyse RNA-Seq data using the Tuxedo Suite tools: Tophat, Cuffmerge, Cufflinks and Cuffdiff.
June 2016 RNA-Seq Analysis With the Tuxedo Suite Dena Leshkowitz Introduction In this exercise we will learn how to analyse RNA-Seq data using the Tuxedo Suite tools: Tophat, Cuffmerge, Cufflinks and Cuffdiff.
Reference guided RNA-seq data analysis using BioHPC Lab computers
 Reference guided RNA-seq data analysis using BioHPC Lab computers This document assumes that you already know some basics of how to use a Linux computer. Some of the command lines in this document are
Reference guided RNA-seq data analysis using BioHPC Lab computers This document assumes that you already know some basics of how to use a Linux computer. Some of the command lines in this document are
Goal: Learn how to use various tool to extract information from RNAseq reads. 4.1 Mapping RNAseq Reads to a Genome Assembly
 ESSENTIALS OF NEXT GENERATION SEQUENCING WORKSHOP 2014 UNIVERSITY OF KENTUCKY AGTC Class 4 RNAseq Goal: Learn how to use various tool to extract information from RNAseq reads. Input(s): magnaporthe_oryzae_70-15_8_supercontigs.fasta
ESSENTIALS OF NEXT GENERATION SEQUENCING WORKSHOP 2014 UNIVERSITY OF KENTUCKY AGTC Class 4 RNAseq Goal: Learn how to use various tool to extract information from RNAseq reads. Input(s): magnaporthe_oryzae_70-15_8_supercontigs.fasta
High-throughout sequencing and using short-read aligners. Simon Anders
 High-throughout sequencing and using short-read aligners Simon Anders High-throughput sequencing (HTS) Sequencing millions of short DNA fragments in parallel. a.k.a.: next-generation sequencing (NGS) massively-parallel
High-throughout sequencing and using short-read aligners Simon Anders High-throughput sequencing (HTS) Sequencing millions of short DNA fragments in parallel. a.k.a.: next-generation sequencing (NGS) massively-parallel
Identification of consensus modules in Adenocarcinoma data
 Identification of consensus modules in Adenocarcinoma data Peter Langfelder and Steve Horvath June 9, 01 Contents 1 Overview 1 Setting up the R session 1 Loading of data Scale-free topology analysis Identification
Identification of consensus modules in Adenocarcinoma data Peter Langfelder and Steve Horvath June 9, 01 Contents 1 Overview 1 Setting up the R session 1 Loading of data Scale-free topology analysis Identification
Next Generation Sequence Alignment on the BRC Cluster. Steve Newhouse 22 July 2010
 Next Generation Sequence Alignment on the BRC Cluster Steve Newhouse 22 July 2010 Overview Practical guide to processing next generation sequencing data on the cluster No details on the inner workings
Next Generation Sequence Alignment on the BRC Cluster Steve Newhouse 22 July 2010 Overview Practical guide to processing next generation sequencing data on the cluster No details on the inner workings
Tumor-Specific NeoAntigen Detector (TSNAD) v2.0 User s Manual
 Tumor-Specific NeoAntigen Detector (TSNAD) v2.0 User s Manual Zhan Zhou, Xingzheng Lyu and Jingcheng Wu Zhejiang University, CHINA March, 2016 USER'S MANUAL TABLE OF CONTENTS 1 GETTING STARTED... 1 1.1
Tumor-Specific NeoAntigen Detector (TSNAD) v2.0 User s Manual Zhan Zhou, Xingzheng Lyu and Jingcheng Wu Zhejiang University, CHINA March, 2016 USER'S MANUAL TABLE OF CONTENTS 1 GETTING STARTED... 1 1.1
Sequence Analysis Pipeline
 Sequence Analysis Pipeline Transcript fragments 1. PREPROCESSING 2. ASSEMBLY (today) Removal of contaminants, vector, adaptors, etc Put overlapping sequence together and calculate bigger sequences 3. Analysis/Annotation
Sequence Analysis Pipeline Transcript fragments 1. PREPROCESSING 2. ASSEMBLY (today) Removal of contaminants, vector, adaptors, etc Put overlapping sequence together and calculate bigger sequences 3. Analysis/Annotation
Clustering. Dick de Ridder 6/10/2018
 Clustering Dick de Ridder 6/10/2018 In these exercises, you will continue to work with the Arabidopsis ST vs. HT RNAseq dataset. First, you will select a subset of the data and inspect it; then cluster
Clustering Dick de Ridder 6/10/2018 In these exercises, you will continue to work with the Arabidopsis ST vs. HT RNAseq dataset. First, you will select a subset of the data and inspect it; then cluster
CORE Year 1 Whole Genome Sequencing Final Data Format Requirements
 CORE Year 1 Whole Genome Sequencing Final Data Format Requirements To all incumbent contractors of CORE year 1 WGS contracts, the following acts as the agreed to sample parameters issued by NHLBI for data
CORE Year 1 Whole Genome Sequencing Final Data Format Requirements To all incumbent contractors of CORE year 1 WGS contracts, the following acts as the agreed to sample parameters issued by NHLBI for data
Sentieon DNA Pipeline for Variant Detection Software-only solution, over 20 faster than GATK 3.3 with identical results
 Sentieon DNA Pipeline for Variant Detection Software-only solution, over 20 faster than GATK 3.3 with identical results Jessica A. Weber 1, Rafael Aldana 5, Brendan D. Gallagher 5, Jeremy S. Edwards 2,3,4
Sentieon DNA Pipeline for Variant Detection Software-only solution, over 20 faster than GATK 3.3 with identical results Jessica A. Weber 1, Rafael Aldana 5, Brendan D. Gallagher 5, Jeremy S. Edwards 2,3,4
The QoRTs Analysis Pipeline Example Walkthrough
 The QoRTs Analysis Pipeline Example Walkthrough Stephen Hartley National Human Genome Research Institute National Institutes of Health March 30, 2017 QoRTs v1.2.0 JunctionSeq v1.5.4 Contents 1 Overview
The QoRTs Analysis Pipeline Example Walkthrough Stephen Hartley National Human Genome Research Institute National Institutes of Health March 30, 2017 QoRTs v1.2.0 JunctionSeq v1.5.4 Contents 1 Overview
WM2 Bioinformatics. ExomeSeq data analysis part 1. Dietmar Rieder
 WM2 Bioinformatics ExomeSeq data analysis part 1 Dietmar Rieder RAW data Use putty to logon to cluster.i med.ac.at In your home directory make directory to store raw data $ mkdir 00_RAW Copy raw fastq
WM2 Bioinformatics ExomeSeq data analysis part 1 Dietmar Rieder RAW data Use putty to logon to cluster.i med.ac.at In your home directory make directory to store raw data $ mkdir 00_RAW Copy raw fastq
A Tutorial: Genome- based RNA- Seq Analysis Using the TUXEDO Package
 A Tutorial: Genome- based RNA- Seq Analysis Using the TUXEDO Package The following data and software resources are required for following the tutorial. Data: ftp://ftp.broad.mit.edu/pub/users/bhaas/rnaseq_workshop/rnaseq_workshop_dat
A Tutorial: Genome- based RNA- Seq Analysis Using the TUXEDO Package The following data and software resources are required for following the tutorial. Data: ftp://ftp.broad.mit.edu/pub/users/bhaas/rnaseq_workshop/rnaseq_workshop_dat
Sentieon DNA pipeline for variant detection - Software-only solution, over 20 faster than GATK 3.3 with identical results
 Sentieon DNA pipeline for variant detection - Software-only solution, over 0 faster than GATK. with identical results Sentieon DNAseq Software is a suite of tools for running DNA sequencing secondary analyses.
Sentieon DNA pipeline for variant detection - Software-only solution, over 0 faster than GATK. with identical results Sentieon DNAseq Software is a suite of tools for running DNA sequencing secondary analyses.
SNP Calling. Tuesday 4/21/15
 SNP Calling Tuesday 4/21/15 Why Call SNPs? map mutations, ex: EMS, natural variation, introgressions associate with changes in expression develop markers for whole genome QTL analysis/ GWAS access diversity
SNP Calling Tuesday 4/21/15 Why Call SNPs? map mutations, ex: EMS, natural variation, introgressions associate with changes in expression develop markers for whole genome QTL analysis/ GWAS access diversity
RNASeq2017 Course Salerno, September 27-29, 2017
 RNASeq2017 Course Salerno, September 27-29, 2017 RNA- seq Hands on Exercise Fabrizio Ferrè, University of Bologna Alma Mater (fabrizio.ferre@unibo.it) Hands- on tutorial based on the EBI teaching materials
RNASeq2017 Course Salerno, September 27-29, 2017 RNA- seq Hands on Exercise Fabrizio Ferrè, University of Bologna Alma Mater (fabrizio.ferre@unibo.it) Hands- on tutorial based on the EBI teaching materials
Analysing re-sequencing samples. Malin Larsson WABI / SciLifeLab
 Analysing re-sequencing samples Malin Larsson Malin.larsson@scilifelab.se WABI / SciLifeLab Re-sequencing Reference genome assembly...gtgcgtagactgctagatcgaaga...! Re-sequencing IND 1! GTAGACT! AGATCGG!
Analysing re-sequencing samples Malin Larsson Malin.larsson@scilifelab.se WABI / SciLifeLab Re-sequencing Reference genome assembly...gtgcgtagactgctagatcgaaga...! Re-sequencing IND 1! GTAGACT! AGATCGG!
Falcon Accelerated Genomics Data Analysis Solutions. User Guide
 Falcon Accelerated Genomics Data Analysis Solutions User Guide Falcon Computing Solutions, Inc. Version 1.0 3/30/2018 Table of Contents Introduction... 3 System Requirements and Installation... 4 Software
Falcon Accelerated Genomics Data Analysis Solutions User Guide Falcon Computing Solutions, Inc. Version 1.0 3/30/2018 Table of Contents Introduction... 3 System Requirements and Installation... 4 Software
Evaluate NimbleGen SeqCap RNA Target Enrichment Data
 Roche Sequencing Technical Note November 2014 How To Evaluate NimbleGen SeqCap RNA Target Enrichment Data 1. OVERVIEW Analysis of NimbleGen SeqCap RNA target enrichment data generated using an Illumina
Roche Sequencing Technical Note November 2014 How To Evaluate NimbleGen SeqCap RNA Target Enrichment Data 1. OVERVIEW Analysis of NimbleGen SeqCap RNA target enrichment data generated using an Illumina
Analysing re-sequencing samples. Anna Johansson WABI / SciLifeLab
 Analysing re-sequencing samples Anna Johansson Anna.johansson@scilifelab.se WABI / SciLifeLab Re-sequencing Reference genome assembly...gtgcgtagactgctagatcgaaga... Re-sequencing IND 1 GTAGACT AGATCGG GCGTAGT
Analysing re-sequencing samples Anna Johansson Anna.johansson@scilifelab.se WABI / SciLifeLab Re-sequencing Reference genome assembly...gtgcgtagactgctagatcgaaga... Re-sequencing IND 1 GTAGACT AGATCGG GCGTAGT
Data: ftp://ftp.broad.mit.edu/pub/users/bhaas/rnaseq_workshop/rnaseq_workshop_dat a.tgz. Software:
 A Tutorial: De novo RNA- Seq Assembly and Analysis Using Trinity and edger The following data and software resources are required for following the tutorial: Data: ftp://ftp.broad.mit.edu/pub/users/bhaas/rnaseq_workshop/rnaseq_workshop_dat
A Tutorial: De novo RNA- Seq Assembly and Analysis Using Trinity and edger The following data and software resources are required for following the tutorial: Data: ftp://ftp.broad.mit.edu/pub/users/bhaas/rnaseq_workshop/rnaseq_workshop_dat
Testing for Differential Expression
 Testing for Differential Expression Objectives Once we've obtained abundance counts for our genes/exons/transcripts, we are usually interested in identifying those genes/exons/transcripts that are differentially
Testing for Differential Expression Objectives Once we've obtained abundance counts for our genes/exons/transcripts, we are usually interested in identifying those genes/exons/transcripts that are differentially
srap: Simplified RNA-Seq Analysis Pipeline
 srap: Simplified RNA-Seq Analysis Pipeline Charles Warden October 30, 2017 1 Introduction This package provides a pipeline for gene expression analysis. The normalization function is specific for RNA-Seq
srap: Simplified RNA-Seq Analysis Pipeline Charles Warden October 30, 2017 1 Introduction This package provides a pipeline for gene expression analysis. The normalization function is specific for RNA-Seq
Standard output. Some of the output files can be redirected into the standard output, which may facilitate in creating the pipelines:
 Lecture 18 RNA-seq Alignment Standard output Some of the output files can be redirected into the standard output, which may facilitate in creating the pipelines: Filtering of the alignments STAR performs
Lecture 18 RNA-seq Alignment Standard output Some of the output files can be redirected into the standard output, which may facilitate in creating the pipelines: Filtering of the alignments STAR performs
From the Schnable Lab:
 From the Schnable Lab: Yang Zhang and Daniel Ngu s Pipeline for Processing RNA-seq Data (As of November 17, 2016) yzhang91@unl.edu dngu2@huskers.unl.edu Pre-processing the reads: The alignment software
From the Schnable Lab: Yang Zhang and Daniel Ngu s Pipeline for Processing RNA-seq Data (As of November 17, 2016) yzhang91@unl.edu dngu2@huskers.unl.edu Pre-processing the reads: The alignment software
SweGen: A whole-genome data resource of genetic variability in a cross-section of the Swedish population
 Supplementary Material and Methods SweGen: A whole-genome data resource of genetic variability in a cross-section of the Swedish population Adam Ameur, Johan Dahlberg, Pall Olason, Francesco Vezzi, Robert
Supplementary Material and Methods SweGen: A whole-genome data resource of genetic variability in a cross-section of the Swedish population Adam Ameur, Johan Dahlberg, Pall Olason, Francesco Vezzi, Robert
halvade Documentation
 halvade Documentation Release 1.1.0 Dries Decap Mar 12, 2018 Contents 1 Introduction 3 1.1 Recipes.................................................. 3 2 Installation 5 2.1 Build from source............................................
halvade Documentation Release 1.1.0 Dries Decap Mar 12, 2018 Contents 1 Introduction 3 1.1 Recipes.................................................. 3 2 Installation 5 2.1 Build from source............................................
Variation among genomes
 Variation among genomes Comparing genomes The reference genome http://www.ncbi.nlm.nih.gov/nuccore/26556996 Arabidopsis thaliana, a model plant Col-0 variety is from Landsberg, Germany Ler is a mutant
Variation among genomes Comparing genomes The reference genome http://www.ncbi.nlm.nih.gov/nuccore/26556996 Arabidopsis thaliana, a model plant Col-0 variety is from Landsberg, Germany Ler is a mutant
Bash for SLURM. Author: Wesley Schaal Pharmaceutical Bioinformatics, Uppsala University
 Bash for SLURM Author: Wesley Schaal Pharmaceutical Bioinformatics, Uppsala University wesley.schaal@farmbio.uu.se Lab session: Pavlin Mitev (pavlin.mitev@kemi.uu.se) it i slides at http://uppmax.uu.se/support/courses
Bash for SLURM Author: Wesley Schaal Pharmaceutical Bioinformatics, Uppsala University wesley.schaal@farmbio.uu.se Lab session: Pavlin Mitev (pavlin.mitev@kemi.uu.se) it i slides at http://uppmax.uu.se/support/courses
323 lines (286 sloc) 15.7 KB. 7d7a6fb
 323 lines (286 sloc) 15.7 KB 7d7a6fb sed 's/ercc-/ercc_/g' ERCC92.fa > ERCC92.patched.fa with open('homo_sapiens_assembly38.fasta', 'r') as fasta: contigs = fasta.read() contigs = contigs.split('>') contig_ids
323 lines (286 sloc) 15.7 KB 7d7a6fb sed 's/ercc-/ercc_/g' ERCC92.fa > ERCC92.patched.fa with open('homo_sapiens_assembly38.fasta', 'r') as fasta: contigs = fasta.read() contigs = contigs.split('>') contig_ids
Understanding and Pre-processing Raw Illumina Data
 Understanding and Pre-processing Raw Illumina Data Matt Johnson October 4, 2013 1 Understanding FASTQ files After an Illumina sequencing run, the data is stored in very large text files in a standard format
Understanding and Pre-processing Raw Illumina Data Matt Johnson October 4, 2013 1 Understanding FASTQ files After an Illumina sequencing run, the data is stored in very large text files in a standard format
Goal: Learn how to use various tool to extract information from RNAseq reads.
 ESSENTIALS OF NEXT GENERATION SEQUENCING WORKSHOP 2017 Class 4 RNAseq Goal: Learn how to use various tool to extract information from RNAseq reads. Input(s): Output(s): magnaporthe_oryzae_70-15_8_supercontigs.fasta
ESSENTIALS OF NEXT GENERATION SEQUENCING WORKSHOP 2017 Class 4 RNAseq Goal: Learn how to use various tool to extract information from RNAseq reads. Input(s): Output(s): magnaporthe_oryzae_70-15_8_supercontigs.fasta
all M 2M_gt_15 2M_8_15 2M_1_7 gt_2m TopHat2
 Pairs processed per second 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 72,318 418 1,666 49,495 21,123 69,984 35,694 1,9 71,538 3,5 17,381 61,223 69,39 55 19,579 44,79 65,126 96 5,115 33,6 61,787
Pairs processed per second 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 6, 4, 2, 72,318 418 1,666 49,495 21,123 69,984 35,694 1,9 71,538 3,5 17,381 61,223 69,39 55 19,579 44,79 65,126 96 5,115 33,6 61,787
RNAseq analysis: SNP calling. BTI bioinformatics course, spring 2013
 RNAseq analysis: SNP calling BTI bioinformatics course, spring 2013 RNAseq overview RNAseq overview Choose technology 454 Illumina SOLiD 3 rd generation (Ion Torrent, PacBio) Library types Single reads
RNAseq analysis: SNP calling BTI bioinformatics course, spring 2013 RNAseq overview RNAseq overview Choose technology 454 Illumina SOLiD 3 rd generation (Ion Torrent, PacBio) Library types Single reads
Input files: Trim reads: Create bwa index: Align trimmed reads: Convert sam to bam: Sort bam: Remove duplicates: Index sorted, no-duplicates bam:
 Input files: 11B-872-3.Ac4578.B73xEDMX-2233_palomero-1.fq 11B-872-3.Ac4578.B73xEDMX-2233_palomero-2.fq Trim reads: java -jar trimmomatic-0.32.jar PE -threads $PBS_NUM_PPN -phred33 \ [...]-1.fq [...]-2.fq
Input files: 11B-872-3.Ac4578.B73xEDMX-2233_palomero-1.fq 11B-872-3.Ac4578.B73xEDMX-2233_palomero-2.fq Trim reads: java -jar trimmomatic-0.32.jar PE -threads $PBS_NUM_PPN -phred33 \ [...]-1.fq [...]-2.fq
RNA-seq. Manpreet S. Katari
 RNA-seq Manpreet S. Katari Evolution of Sequence Technology Normalizing the Data RPKM (Reads per Kilobase of exons per million reads) Score = R NT R = # of unique reads for the gene N = Size of the gene
RNA-seq Manpreet S. Katari Evolution of Sequence Technology Normalizing the Data RPKM (Reads per Kilobase of exons per million reads) Score = R NT R = # of unique reads for the gene N = Size of the gene
Maize genome sequence in FASTA format. Gene annotation file in gff format
 Exercise 1. Using Tophat/Cufflinks to analyze RNAseq data. Step 1. One of CBSU BioHPC Lab workstations has been allocated for your workshop exercise. The allocations are listed on the workshop exercise
Exercise 1. Using Tophat/Cufflinks to analyze RNAseq data. Step 1. One of CBSU BioHPC Lab workstations has been allocated for your workshop exercise. The allocations are listed on the workshop exercise
Statistical analysis of RNA-Seq data
 Practical Statistical analysis of RNA-Seq data (solutions) Ignacio González Plateforme Bioinformatique INRA Toulouse Plateforme Biostatistique IMT Université Toulouse III Toulouse, 20/21 novembre 2014
Practical Statistical analysis of RNA-Seq data (solutions) Ignacio González Plateforme Bioinformatique INRA Toulouse Plateforme Biostatistique IMT Université Toulouse III Toulouse, 20/21 novembre 2014
Differential gene expression analysis
 Differential gene expression analysis Overview In this exercise, we will analyze RNA-seq data to measure changes in gene expression levels between wild-type and a mutant strain of the bacterium Listeria
Differential gene expression analysis Overview In this exercise, we will analyze RNA-seq data to measure changes in gene expression levels between wild-type and a mutant strain of the bacterium Listeria
11/8/2017 Trinity De novo Transcriptome Assembly Workshop trinityrnaseq/rnaseq_trinity_tuxedo_workshop Wiki GitHub
 trinityrnaseq / RNASeq_Trinity_Tuxedo_Workshop Trinity De novo Transcriptome Assembly Workshop Brian Haas edited this page on Oct 17, 2015 14 revisions De novo RNA-Seq Assembly and Analysis Using Trinity
trinityrnaseq / RNASeq_Trinity_Tuxedo_Workshop Trinity De novo Transcriptome Assembly Workshop Brian Haas edited this page on Oct 17, 2015 14 revisions De novo RNA-Seq Assembly and Analysis Using Trinity
version /1/2011 Source code Linux x86_64 binary Mac OS X x86_64 binary
 Cufflinks RNA-Seq analysis tools - Getting Started 1 of 6 14.07.2011 09:42 Cufflinks Transcript assembly, differential expression, and differential regulation for RNA-Seq Site Map Home Getting started
Cufflinks RNA-Seq analysis tools - Getting Started 1 of 6 14.07.2011 09:42 Cufflinks Transcript assembly, differential expression, and differential regulation for RNA-Seq Site Map Home Getting started
TopHat, Cufflinks, Cuffdiff
 TopHat, Cufflinks, Cuffdiff Andreas Gisel Institute for Biomedical Technologies - CNR, Bari TopHat TopHat TopHat TopHat is a program that aligns RNA-Seq reads to a genome in order to identify exon-exon
TopHat, Cufflinks, Cuffdiff Andreas Gisel Institute for Biomedical Technologies - CNR, Bari TopHat TopHat TopHat TopHat is a program that aligns RNA-Seq reads to a genome in order to identify exon-exon
1. Quality control software FASTQC:
 ITBI2017-2018, Class-Exercise5, 1-11-2017, M-Reczko 1. Quality control software FASTQC: https://www.bioinformatics.babraham.ac.uk/projects/download.html#fastqc Documentation: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/help/
ITBI2017-2018, Class-Exercise5, 1-11-2017, M-Reczko 1. Quality control software FASTQC: https://www.bioinformatics.babraham.ac.uk/projects/download.html#fastqc Documentation: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/help/
By Ludovic Duvaux (27 November 2013)
 Array of jobs using SGE - an example using stampy, a mapping software. Running java applications on the cluster - merge sam files using the Picard tools By Ludovic Duvaux (27 November 2013) The idea ==========
Array of jobs using SGE - an example using stampy, a mapping software. Running java applications on the cluster - merge sam files using the Picard tools By Ludovic Duvaux (27 November 2013) The idea ==========
Centre (CNIO). 3rd Melchor Fernández Almagro St , Madrid, Spain. s/n, Universidad de Vigo, Ourense, Spain.
 O. Graña *a,b, M. Rubio-Camarillo a, F. Fdez-Riverola b, D.G. Pisano a and D. Glez-Peña b a Bioinformatics Unit, Structural Biology and BioComputing Programme, Spanish National Cancer Research Centre (CNIO).
O. Graña *a,b, M. Rubio-Camarillo a, F. Fdez-Riverola b, D.G. Pisano a and D. Glez-Peña b a Bioinformatics Unit, Structural Biology and BioComputing Programme, Spanish National Cancer Research Centre (CNIO).
Galaxy workshop at the Winter School Igor Makunin
 Galaxy workshop at the Winter School 2016 Igor Makunin i.makunin@uq.edu.au Winter school, UQ, July 6, 2016 Plan Overview of the Genomics Virtual Lab Introduce Galaxy, a web based platform for analysis
Galaxy workshop at the Winter School 2016 Igor Makunin i.makunin@uq.edu.au Winter school, UQ, July 6, 2016 Plan Overview of the Genomics Virtual Lab Introduce Galaxy, a web based platform for analysis
Short Read Sequencing Analysis Workshop
 Short Read Sequencing Analysis Workshop Day 8: Introduc/on to RNA-seq Analysis In-class slides Day 7 Homework 1.) 14 GABPA ChIP-seq peaks 2.) Error: Dataset too large (> 100000). Rerun with larger maxsize
Short Read Sequencing Analysis Workshop Day 8: Introduc/on to RNA-seq Analysis In-class slides Day 7 Homework 1.) 14 GABPA ChIP-seq peaks 2.) Error: Dataset too large (> 100000). Rerun with larger maxsize
Short tutorial on studying module preservation: Preservation of female mouse liver modules in male data
 Short tutorial on studying module preservation: Preservation of female mouse liver modules in male data Peter Langfelder and Steve Horvath October 1, 0 Contents 1 Overview 1 1.a Setting up the R session............................................
Short tutorial on studying module preservation: Preservation of female mouse liver modules in male data Peter Langfelder and Steve Horvath October 1, 0 Contents 1 Overview 1 1.a Setting up the R session............................................
Decrypting your genome data privately in the cloud
 Decrypting your genome data privately in the cloud Marc Sitges Data Manager@Made of Genes @madeofgenes The Human Genome 3.200 M (x2) Base pairs (bp) ~20.000 genes (~30%) (Exons ~1%) The Human Genome Project
Decrypting your genome data privately in the cloud Marc Sitges Data Manager@Made of Genes @madeofgenes The Human Genome 3.200 M (x2) Base pairs (bp) ~20.000 genes (~30%) (Exons ~1%) The Human Genome Project
Gene Expression Data Analysis. Qin Ma, Ph.D. December 10, 2017
 1 Gene Expression Data Analysis Qin Ma, Ph.D. December 10, 2017 2 Bioinformatics Systems biology This interdisciplinary science is about providing computational support to studies on linking the behavior
1 Gene Expression Data Analysis Qin Ma, Ph.D. December 10, 2017 2 Bioinformatics Systems biology This interdisciplinary science is about providing computational support to studies on linking the behavior
Differential Expression
 Differential Expression Data In this practical, as before, we will work with RNA-Seq data from Arabidopsis seeds that matured at standard temperature (ST, 22 C day/18 C night) or at high temperature (HT,
Differential Expression Data In this practical, as before, we will work with RNA-Seq data from Arabidopsis seeds that matured at standard temperature (ST, 22 C day/18 C night) or at high temperature (HT,
Halvade: scalable sequence analysis with MapReduce
 Bioinformatics Advance Access published March 26, 2015 Halvade: scalable sequence analysis with MapReduce Dries Decap 1,5, Joke Reumers 2,5, Charlotte Herzeel 3,5, Pascal Costanza, 4,5 and Jan Fostier
Bioinformatics Advance Access published March 26, 2015 Halvade: scalable sequence analysis with MapReduce Dries Decap 1,5, Joke Reumers 2,5, Charlotte Herzeel 3,5, Pascal Costanza, 4,5 and Jan Fostier
RNA-Seq. Joshua Ainsley, PhD Postdoctoral Researcher Lab of Leon Reijmers Neuroscience Department Tufts University
 RNA-Seq Joshua Ainsley, PhD Postdoctoral Researcher Lab of Leon Reijmers Neuroscience Department Tufts University joshua.ainsley@tufts.edu Day four Quantifying expression Intro to R Differential expression
RNA-Seq Joshua Ainsley, PhD Postdoctoral Researcher Lab of Leon Reijmers Neuroscience Department Tufts University joshua.ainsley@tufts.edu Day four Quantifying expression Intro to R Differential expression
Meta-analysis of aging methylation data sets Validation success of various meta-analysis methods in selecting genes
 Meta-analysis of aging methylation data sets Validation success of various meta-analysis methods in selecting genes Peter Langfelder and Steve Horvath June 27, 2012 Contents 1 Overview 1 2 Setting up the
Meta-analysis of aging methylation data sets Validation success of various meta-analysis methods in selecting genes Peter Langfelder and Steve Horvath June 27, 2012 Contents 1 Overview 1 2 Setting up the
Galaxy Platform For NGS Data Analyses
 Galaxy Platform For NGS Data Analyses Weihong Yan wyan@chem.ucla.edu Collaboratory Web Site http://qcb.ucla.edu/collaboratory Collaboratory Workshops Workshop Outline ü Day 1 UCLA galaxy and user account
Galaxy Platform For NGS Data Analyses Weihong Yan wyan@chem.ucla.edu Collaboratory Web Site http://qcb.ucla.edu/collaboratory Collaboratory Workshops Workshop Outline ü Day 1 UCLA galaxy and user account
Package ArrayExpressHTS
 Package ArrayExpressHTS April 9, 2015 Title ArrayExpress High Throughput Sequencing Processing Pipeline Version 1.16.0 Author Angela Goncalves, Andrew Tikhonov Maintainer Angela Goncalves ,
Package ArrayExpressHTS April 9, 2015 Title ArrayExpress High Throughput Sequencing Processing Pipeline Version 1.16.0 Author Angela Goncalves, Andrew Tikhonov Maintainer Angela Goncalves ,
Colorado State University Bioinformatics Algorithms Assignment 6: Analysis of High- Throughput Biological Data Hamidreza Chitsaz, Ali Sharifi- Zarchi
 Colorado State University Bioinformatics Algorithms Assignment 6: Analysis of High- Throughput Biological Data Hamidreza Chitsaz, Ali Sharifi- Zarchi Although a little- bit long, this is an easy exercise
Colorado State University Bioinformatics Algorithms Assignment 6: Analysis of High- Throughput Biological Data Hamidreza Chitsaz, Ali Sharifi- Zarchi Although a little- bit long, this is an easy exercise
Package dynamictreecut
 Package dynamictreecut November 18, 2013 Version 1.60-2 Date 2013-11-16 Title Methods for detection of clusters in hierarchical clustering dendrograms. Author Peter Langfelder
Package dynamictreecut November 18, 2013 Version 1.60-2 Date 2013-11-16 Title Methods for detection of clusters in hierarchical clustering dendrograms. Author Peter Langfelder
New High Performance Computing Cluster For Large Scale Multi-omics Data Analysis. 28 February 2018 (Wed) 2:30pm 3:30pm Seminar Room 1A, G/F
 New High Performance Computing Cluster For Large Scale Multi-omics Data Analysis 28 February 2018 (Wed) 2:30pm 3:30pm Seminar Room 1A, G/F The Team (Bioinformatics & Information Technology) Eunice Kelvin
New High Performance Computing Cluster For Large Scale Multi-omics Data Analysis 28 February 2018 (Wed) 2:30pm 3:30pm Seminar Room 1A, G/F The Team (Bioinformatics & Information Technology) Eunice Kelvin
Expander 7.2 Online Documentation
 Expander 7.2 Online Documentation Introduction... 2 Starting EXPANDER... 2 Input Data... 3 Tabular Data File... 4 CEL Files... 6 Working on similarity data no associated expression data... 9 Working on
Expander 7.2 Online Documentation Introduction... 2 Starting EXPANDER... 2 Input Data... 3 Tabular Data File... 4 CEL Files... 6 Working on similarity data no associated expression data... 9 Working on
Package ssizerna. January 9, 2017
 Type Package Package ssizerna January 9, 2017 Title Sample Size Calculation for RNA-Seq Experimental Design Version 1.2.9 Date 2017-01-05 Maintainer Ran Bi We propose a procedure for
Type Package Package ssizerna January 9, 2017 Title Sample Size Calculation for RNA-Seq Experimental Design Version 1.2.9 Date 2017-01-05 Maintainer Ran Bi We propose a procedure for
Ensembl RNASeq Practical. Overview
 Ensembl RNASeq Practical The aim of this practical session is to use BWA to align 2 lanes of Zebrafish paired end Illumina RNASeq reads to chromosome 12 of the zebrafish ZV9 assembly. We have restricted
Ensembl RNASeq Practical The aim of this practical session is to use BWA to align 2 lanes of Zebrafish paired end Illumina RNASeq reads to chromosome 12 of the zebrafish ZV9 assembly. We have restricted
EpiGnome Methyl Seq Bioinformatics User Guide Rev. 0.1
 EpiGnome Methyl Seq Bioinformatics User Guide Rev. 0.1 Introduction This guide contains data analysis recommendations for libraries prepared using Epicentre s EpiGnome Methyl Seq Kit, and sequenced on
EpiGnome Methyl Seq Bioinformatics User Guide Rev. 0.1 Introduction This guide contains data analysis recommendations for libraries prepared using Epicentre s EpiGnome Methyl Seq Kit, and sequenced on
Sentieon Documentation
 Sentieon Documentation Release 201808.03 Sentieon, Inc Dec 21, 2018 Sentieon Manual 1 Introduction 1 1.1 Description.............................................. 1 1.2 Benefits and Value..........................................
Sentieon Documentation Release 201808.03 Sentieon, Inc Dec 21, 2018 Sentieon Manual 1 Introduction 1 1.1 Description.............................................. 1 1.2 Benefits and Value..........................................
Package dynamictreecut
 Package dynamictreecut June 13, 2014 Version 1.62 Date 2014-05-07 Title Methods for detection of clusters in hierarchical clustering dendrograms. Author Peter Langfelder and
Package dynamictreecut June 13, 2014 Version 1.62 Date 2014-05-07 Title Methods for detection of clusters in hierarchical clustering dendrograms. Author Peter Langfelder and
RNA sequence practical
 RNA sequence practical Michael Inouye Baker Heart and Diabetes Institute Univ of Melbourne / Monash Univ Summer Institute in Statistical Genetics 2017 Integrative Genomics Module Seattle @minouye271 www.inouyelab.org
RNA sequence practical Michael Inouye Baker Heart and Diabetes Institute Univ of Melbourne / Monash Univ Summer Institute in Statistical Genetics 2017 Integrative Genomics Module Seattle @minouye271 www.inouyelab.org
Services Performed. The following checklist confirms the steps of the RNA-Seq Service that were performed on your samples.
 Services Performed The following checklist confirms the steps of the RNA-Seq Service that were performed on your samples. SERVICE Sample Received Sample Quality Evaluated Sample Prepared for Sequencing
Services Performed The following checklist confirms the steps of the RNA-Seq Service that were performed on your samples. SERVICE Sample Received Sample Quality Evaluated Sample Prepared for Sequencing
NGS Sequence data. Jason Stajich. UC Riverside. jason.stajich[at]ucr.edu. twitter:hyphaltip stajichlab
![NGS Sequence data. Jason Stajich. UC Riverside. jason.stajich[at]ucr.edu. twitter:hyphaltip stajichlab NGS Sequence data. Jason Stajich. UC Riverside. jason.stajich[at]ucr.edu. twitter:hyphaltip stajichlab](/thumbs/74/70633133.jpg) NGS Sequence data Jason Stajich UC Riverside jason.stajich[at]ucr.edu twitter:hyphaltip stajichlab Lecture available at http://github.com/hyphaltip/cshl_2012_ngs 1/58 NGS sequence data Quality control
NGS Sequence data Jason Stajich UC Riverside jason.stajich[at]ucr.edu twitter:hyphaltip stajichlab Lecture available at http://github.com/hyphaltip/cshl_2012_ngs 1/58 NGS sequence data Quality control
Hinri Kerstens. NGS pipeline using Broad's Cromwell
 Hinri Kerstens NGS pipeline using Broad's Cromwell Introduction Princess Máxima Center is a organization fully specialized in pediatric oncology. By combining the best possible research and care, we will
Hinri Kerstens NGS pipeline using Broad's Cromwell Introduction Princess Máxima Center is a organization fully specialized in pediatric oncology. By combining the best possible research and care, we will
Automated Bioinformatics Analysis System on Chip ABASOC. version 1.1
 Automated Bioinformatics Analysis System on Chip ABASOC version 1.1 Phillip Winston Miller, Priyam Patel, Daniel L. Johnson, PhD. University of Tennessee Health Science Center Office of Research Molecular
Automated Bioinformatics Analysis System on Chip ABASOC version 1.1 Phillip Winston Miller, Priyam Patel, Daniel L. Johnson, PhD. University of Tennessee Health Science Center Office of Research Molecular
m6aviewer Version Documentation
 m6aviewer Version 1.6.0 Documentation Contents 1. About 2. Requirements 3. Launching m6aviewer 4. Running Time Estimates 5. Basic Peak Calling 6. Running Modes 7. Multiple Samples/Sample Replicates 8.
m6aviewer Version 1.6.0 Documentation Contents 1. About 2. Requirements 3. Launching m6aviewer 4. Running Time Estimates 5. Basic Peak Calling 6. Running Modes 7. Multiple Samples/Sample Replicates 8.
Evaluate NimbleGen SeqCap Epi Target Enrichment Data
 Sequencing Solutions Technical Note April 2014 How To Evaluate NimbleGen SeqCap Epi Target Enrichment Data 1. OVERVIEW Analysis of NimbleGen SeqCap Epi target enrichment data generated using an Illumina
Sequencing Solutions Technical Note April 2014 How To Evaluate NimbleGen SeqCap Epi Target Enrichment Data 1. OVERVIEW Analysis of NimbleGen SeqCap Epi target enrichment data generated using an Illumina
General instructions:
 R Tutorial: Geometric Interpretation of Gene Co-Expression Network Analysis, Applied to Female Mouse Liver Microarray Data Jun Dong, Steve Horvath Correspondence: shorvath@mednet.ucla.edu, http://www.ph.ucla.edu/biostat/people/horvath.htm
R Tutorial: Geometric Interpretation of Gene Co-Expression Network Analysis, Applied to Female Mouse Liver Microarray Data Jun Dong, Steve Horvath Correspondence: shorvath@mednet.ucla.edu, http://www.ph.ucla.edu/biostat/people/horvath.htm
Preservation of protein-protein interaction networks Simple simulated example
 Preservation of protein-protein interaction networks Simple simulated example Peter Langfelder and Steve Horvath May, 0 Contents Overview.a Setting up the R session............................................
Preservation of protein-protein interaction networks Simple simulated example Peter Langfelder and Steve Horvath May, 0 Contents Overview.a Setting up the R session............................................
Calling variants in diploid or multiploid genomes
 Calling variants in diploid or multiploid genomes Diploid genomes The initial steps in calling variants for diploid or multi-ploid organisms with NGS data are the same as what we've already seen: 1. 2.
Calling variants in diploid or multiploid genomes Diploid genomes The initial steps in calling variants for diploid or multi-ploid organisms with NGS data are the same as what we've already seen: 1. 2.
Introduction to Read Alignment. UCD Genome Center Bioinformatics Core Tuesday 15 September 2015
 Introduction to Read Alignment UCD Genome Center Bioinformatics Core Tuesday 15 September 2015 From reads to molecules Why align? Individual A Individual B ATGATAGCATCGTCGGGTGTCTGCTCAATAATAGTGCCGTATCATGCTGGTGTTATAATCGCCGCATGACATGATCAATGG
Introduction to Read Alignment UCD Genome Center Bioinformatics Core Tuesday 15 September 2015 From reads to molecules Why align? Individual A Individual B ATGATAGCATCGTCGGGTGTCTGCTCAATAATAGTGCCGTATCATGCTGGTGTTATAATCGCCGCATGACATGATCAATGG
Visualization using CummeRbund 2014 Overview
 Visualization using CummeRbund 2014 Overview In this lab, we'll look at how to use cummerbund to visualize our gene expression results from cuffdiff. CummeRbund is part of the tuxedo pipeline and it is
Visualization using CummeRbund 2014 Overview In this lab, we'll look at how to use cummerbund to visualize our gene expression results from cuffdiff. CummeRbund is part of the tuxedo pipeline and it is
Differential Expression with DESeq2
 Differential Expression with DESeq2 Drosophila melanogaster This document and the data used in this example can be found at: https://software.rc.fas.harvard.edu/ngsdata/workshops/2015_march or on the cluster
Differential Expression with DESeq2 Drosophila melanogaster This document and the data used in this example can be found at: https://software.rc.fas.harvard.edu/ngsdata/workshops/2015_march or on the cluster
Our typical RNA quantification pipeline
 RNA-Seq primer Our typical RNA quantification pipeline Upload your sequence data (fastq) Align to the ribosome (Bow>e) Align remaining reads to genome (TopHat) or transcriptome (RSEM) Make report of quality
RNA-Seq primer Our typical RNA quantification pipeline Upload your sequence data (fastq) Align to the ribosome (Bow>e) Align remaining reads to genome (TopHat) or transcriptome (RSEM) Make report of quality
AgroMarker Finder manual (1.1)
 AgroMarker Finder manual (1.1) 1. Introduction 2. Installation 3. How to run? 4. How to use? 5. Java program for calculating of restriction enzyme sites (TaqαI). 1. Introduction AgroMarker Finder (AMF)is
AgroMarker Finder manual (1.1) 1. Introduction 2. Installation 3. How to run? 4. How to use? 5. Java program for calculating of restriction enzyme sites (TaqαI). 1. Introduction AgroMarker Finder (AMF)is
Mapping NGS reads for genomics studies
 Mapping NGS reads for genomics studies Valencia, 28-30 Sep 2015 BIER Alejandro Alemán aaleman@cipf.es Genomics Data Analysis CIBERER Where are we? Fastq Sequence preprocessing Fastq Alignment BAM Visualization
Mapping NGS reads for genomics studies Valencia, 28-30 Sep 2015 BIER Alejandro Alemán aaleman@cipf.es Genomics Data Analysis CIBERER Where are we? Fastq Sequence preprocessing Fastq Alignment BAM Visualization
High-throughput sequencing: Alignment and related topic. Simon Anders EMBL Heidelberg
 High-throughput sequencing: Alignment and related topic Simon Anders EMBL Heidelberg Established platforms HTS Platforms Illumina HiSeq, ABI SOLiD, Roche 454 Newcomers: Benchtop machines: Illumina MiSeq,
High-throughput sequencing: Alignment and related topic Simon Anders EMBL Heidelberg Established platforms HTS Platforms Illumina HiSeq, ABI SOLiD, Roche 454 Newcomers: Benchtop machines: Illumina MiSeq,
Functional Genomics Research Stream. Computational Meeting: March 29, 2012 RNA-seq Analysis Pipeline
 Functional Genomics Research Stream Computational Meeting: March 29, 2012 RNA-seq Analysis Pipeline CHAPTER 2 Prepare Whole Transcriptome Libraries Fragment the whole transcriptome RNA 100 500 µg poly(a)
Functional Genomics Research Stream Computational Meeting: March 29, 2012 RNA-seq Analysis Pipeline CHAPTER 2 Prepare Whole Transcriptome Libraries Fragment the whole transcriptome RNA 100 500 µg poly(a)
Clustering. RNA-seq: What is it good for? Finding Similarly Expressed Genes. Data... And Lots of It!
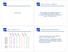 RNA-seq: What is it good for? Clustering High-throughput RNA sequencing experiments (RNA-seq) offer the ability to measure simultaneously the expression level of thousands of genes in a single experiment!
RNA-seq: What is it good for? Clustering High-throughput RNA sequencing experiments (RNA-seq) offer the ability to measure simultaneously the expression level of thousands of genes in a single experiment!
