C+D Manufacturer Documentation. External Document for use by Manufacturers registered on C+D. Authors: Amy Sharp & Eva Kovatsova
|
|
|
- Dominick Edwin Grant
- 5 years ago
- Views:
Transcription
1 C+D Manufacturer Documentation External Document for use by Manufacturers registered on C+D Authors: Amy Sharp & Eva Kovatsova
2 Contents Introduction... 2 Logging in for the first time... 3 Manufacturer login... 3 Your Home Page Updating manufacturer information... 6 Setting up a user/contact... 9 Request/edit a brand or generic Adding a Brand/Generic Requesting/editing a product Browse Brand/Navigate the tree view Deleting a product using the tree view Adding/editing a product request Frequently asked questions Minimum Requirments for Products Quick Rules when inputting products
3 Introduction This documentation was created to assist with input of manufacturer details and product requests on C+D s website ( As part of this there will also be a frequently asked questions section. We hope you find this documentation of use, as always the C+D team are available on info@cddata.co.uk. 2
4 Logging in for the first time When the new website launched, we launched a whole new login system. Your username is your address, these are now unique to each user. Another change is we can no longer access your user account and we cannot view your passwords. If you are already set up but cannot locate the automated . In the username field type your address and request a forgotten password. Manufacturer login If you are an existing manufacturer you can access your account using the Sign in section. If you need to reset your password, please use the forgotten password question. If you do not receive the link, please see FAQ s for help. 3
5 Your Home Page. Entity Summary breakdown. Saved, not yet submitted This is where any requests which have not been submitted for approval can be found. This is designed to save the application if there is missing information. This allows the user to continue applications at a later date. If you save an item in the saved not submitted folder you can remove it using the red bin icon. This will not affect any product, but any items changed and not submitted will be discarded. allocated. If a product request stays in the saved and not submitted it will not be approved, and a PIP 4
6 Pending approval Any requests which have been submitted to C+D s admin staff can be viewed here. This will not allow you to edit the request. Items missing If a C+D admin notices items/information missing from any product listing, a note can be left on the product. These requests can be viewed in this folder. Reserved Any product which has been approved but has a future effective date can be viewed in this folder. This also includes a section for any brands when no products have the status in-use. In-Use This section is any brand or product which is live on the system. This means the products are viewable to all subscribers. Deleted Any product or brand which has been deleted can be viewed in this section. Declined Any request which has been declined by admin can be viewed here. Users can also make the edits and resubmit the product request without the need to start again. 5
7 Updating manufacturer information This box displays on the home page under quick links. This will show you the basic information, including the primary contacts details. To view/edit you manufacturer contact details press the edit manufacturer details. 6
8 When editing the company name, please note we use Ltd and Plc instead of limited/public limited. The address should be a generic company . For example info@/sales@. This will be printed in the price book. The company s house registration can be found online at: s/companies-house Manufacturer/distributor flags. The main difference here is that only companies with the manufacturer flag ticked can edit products. If this needs to be changed please info@cddata.co.uk. The address should be head office or the main factory details. The phone number should be a generic call center number. This will get printed in the price book and viewable to all subscribers. 7
9 Orders phone number, should not be the same as generic phone number. This applies to the orders address. Types of products and company details is a section which allows the user can put information about the manufacturer/product lines. The contacts section will display each contacts/users information. The number and should be direct. The address here, is the username. They should also be unique, the information stored in this section is viewable to only the manufacturer and admin staff. 8
10 Setting up a user/contact There are two primary ways a manufacturer can add a user to the system. The first way is the primary contact can login and edit manufacturer details. On the right of the screen you will see the below box. Fill out this section and select add contact at the bottom. The second one is only to be used if the primary contact has left, or is unknown. Contact info@cddata.co.uk, with all the details which it requests on the form. Important information! All changes must be submitted. This includes changes which are to be viewed by subscribers or if they are a permanent change which needs to be reflected in the data. 9
11 Request/edit a brand or generic Using the quick links section on the home page, click on the manage brands section. This will also show the user all registered brands under the manufacturer This section will not allow the user to manage the products. It is purely for brands/generics. To add a new brand or generic use the add brand button. Do not add duplicate brands/generics. These fields will allow the user to filter the brands/generics If the brand has a distributer use the pencil icon to add them to the brand. To delete the entire brand/generic (And all products attached to the brand/generic), use the red bin icon 10
12 Do s and Don ts of brand/generic requests Do s Don ts Request all new brands and generics. Put full product details when adding a brand/generic name. For example Paracetamol tablets film-coated 30mg 60. Use capital letters in brand requests, for Duplicate Brand/Generic requests. example EXAMPLE BRAND. Generics should be submitted in the Use the section to make products assigned following manner, Example Generic. to the brand live from reserved, Do not request a brand if you are taking over Do not input products until your from another manufacturer. brand/generic has been accepted. info@cddata.co.uk to request a brand transfer. Contact info@cddata.co.uk if you would like Do not use special characters in the brand to make a brand name change. name. Contact info@cddata.co.uk if you need any Do not put the manufacturer name into the help. brand request. Adding a Brand/Generic The name should contain only the brand/generic name Use the drop down to select the type of request (Brand/Generic) For the brand to be added to the tree view, the request must be submitted. R 11
13 Important information Adding in a branded generic. A branded generic is a product which has a brand name for legal reasons, but is prescribed as a generic. This is common among modified release/prolonged release products. These should be requested in the format: Ingredient Name (Brand Name) 12
14 Requesting/editing a product The manage products section, is used to view, edit, add and delete products. This will only show products which have been approved. The first thing which must be done is a brand selected. Use the drop down function to pick from all brands/generics which have been allocated to the manufacturer 13
15 2 Browse Brand/Navigate the tree view. The tree view will show the user, all the products which have been assigned to the brand. The black triangle means there is a sub group. To expand this grouping as shown click on the on the triangle The number shown displays how many products have been allocated to that specific group. To view these products click on the group. Please note this is not an indication there are no products in group structure. If the top group displays (0), and there is a black triangle always expand the listing. Important information The grouping structure can only be edited by the C+D admin staff. Deleting a product using the tree view To delete an individual product use the red bin 14
16 Adding/editing a product request Use the add product button for new product requests. The top line can be used to filter the products in the selected group Use the blue pencil to edit an existing product The effective date allows C+D admin staff to set up/approve products in order. For new requests it is the estimated launch date, or the date of application if the product is to go live on approval If the user was updating the product, this is the field is when the C+D team would aim to approve the request on. The product status has 3 possible options. In use means the product is currently available on the U.K. market. Reserved means a product is due for release at a future time. Once the effective date is reached the C+D will contact manufacturers as a reminder to update the product lines. Deleted products are not available on the U.K. market. Product descriptions must be vague, there should be enough information for a viewer to know what the product is. Descriptions are limited to 50 characters. 15
17 Do s and Don ts of Product Descriptions Do s Do put the form in the description. For example tablets or capsules. Do put the group information in. This helps us align your products. Don ts Don t put product use or marketing information in the description. Do not put the size in the description. Do not put the strength in the description. Do not put all capitals in the description. Do not put the brand name in the description. Descriptions Abbreviations In the product description users may see some abbreviations. These are standard throughout all product applications. Some common ones are; f/c film coated, s/f sugar free, s/c sugar coated, c/f colour free m/r modified release p/r prolonged release 16
18 Free text is a field users can input any information which might help the admins approve the product requests. This field is always deleted upon approval. Search tags can be inputted by manufacturers. A feature of C+D s website means subscribers can search for products. Keywords can increase the visibility of products. Size and Quantity need to be filled out. If the products are tablets, the quantity is the correct field. If there is a size like medium or 10 ml use the size field. Order number is a manufacturer code which a subscriber can use to order products. Fridge line indicator should be ticked for all products which need to be stored in a temperature controlled area. This is used for the correct storage of medications If the product is CE marked please provide the CE number. If the product has a COSHH (Control of substances hazardous to health) please ensure this box is selected. 17
19 C+D s platform allows for manufacturers to input product images. This is a free service and may increase the visibility online. The image will also show on the EPOS systems of pharmacies Formulations table, displays the active ingredient and strength. This is mandatory for all licensed products (P, POM and GSL) info@cddata.co.uk if the ingredient is missing 18
20 Product dimensions refer the product in the packaging. These are mandatory and are used by pharmacists to assist with stock control. Outer dimensions refer to the packaging the consumer packs come in. Shipper details refer to the packaging the outers are transported in. Outers per shipper is the number of outers in the shipper. 19
21 Retail price is the recommended retail price set by manufacturers. This must include VAT, and mark-up. The trade price displays what price it will cost the pharmacy to purchase your product. This must exclude VAT. If the product has a NHS list price this must be displayed in the trade price. Trade price should display the total cost per outer. If a single unit trade price is 1 pound, but the minimum consumer packs a pharmacy can buy in is 10, the trade price should display 10 pounds. Product Classification is used to group the products. It can also be used to search, for example searching for plasters. Medicines will always have medical category. OTC and Appliances have their own categories. Always select the one which is best fit for the product. Legal codes are mandatory. They are used by the pharmacy when dealing with customers. If there is a legal code there must be a PL number. We list PL, EU and THR codes. Please ensure when inputting the correct license type is stated. For example PL12345/1235, THR12345/1234 or EU/1/12/123/123 20
22 Breakdown of legal Classifications for Medicines. General Sales List (GSL): General sales list subject to control under the misuse of drugs act. All GSL products will have a product license number, if this is not provided we cannot list the product as GSL. An important thing to note is this often gets confused with a product being for general sale. Medical Device (MD): Medical devices will most likely have a CE code. A Medical devices are regulated by the Medicines & Healthcare products Regulatory Agency (MHRA), and more information can be found online. Pharmacy Only Products (P): Products which are sold under the supervision of a pharmacist. These products will also have a Product License number and this must recorded. These will have a product License number which needs to be recorded. Pharmacy Only (PO): These vary slightly from P line products. These are GSL products but can only be purchased through a pharmacy. As these are GSL we do need to get the product license number. Pharmacy Only Subject to Control under The Misuse of Drugs Act 1971 (P CDI): These are also known as a schedule 5 pharmacy only product. These products are however exempt from restrictions under the regulations however pharmacy s need to keep the invoices for two years. Prescription Only Medicine (POM): These products can only be supplied to those with a practitioner s prescription. A Product License code is required. Prescription Only Medicines subject to control under The Misuse of Drugs Act 1971 (POM CD): These medicines are under the full control of The Misuse of Drugs Act 1971 and its schedule 2 opiates and major stimulants regulations. A product License code is required. Prescription Only Medicines subject to control under The Misuse of Drugs Act 1971 (POM CDAN): subject to full control under the Misuse of Drugs Act 1971 expect the prescriptions and labelling requirements (expect those under the Medicines Act 1977). 21
23 Records are not required to be kept by retailers. These are under schedule 4 part ll. A product license code is required. Prescription Only Medicines subject to control under The Misuse of Drugs Act 1971 (POM CDBE): These are exempt from most restrictions and regulations, and has no safe custody requirements. (Schedule 4 part l-benzodiazepine Tranquilisers). A product license code is required. Prescription Only Medicines subject to control under The Misuse of Drugs Act 1971 (POM CDI): These are exempt from most restrictions, however invoices are required to be kept for two years. These are under (schedule 5, Prescription only products). A product license code is required. Prescription Only Medicines subject to control under The Misuse of Drugs Act 1971 (POM CDNR): The only exemption on this category is records are not required to be kept on CD register. However invoices are to be kept for two years. (Schedule 3 barbiturates) If the product can only be dispensed through a hospital pharmacy please tick the hospital only tick box. GTIN 13 is mandatory for all products. The only exception is for companies which do not hold a GS1 license. Admin notes is used by Admins to inform manufacturers for missing information. Please provide this information as soon as possible If the product requires a PIP code submit the product. Save the product if you need to get more information. For the minimum acceptable requirements please see the end of this guide. 22
24 Frequently asked questions My company has an account, but I cannot access it? The first thing we need to know is who the last known person able to login was. If this person has left and a new primary contact needs to be set up, you need to info@cddata.co.uk. For a new contact to be created we must have the users direct address, telephone number and job role. If your details have been created previously, you can follow the logging in for the first time steps. I have not received the forgotten password link. The forgotten password is sent from no-reply@ubm.com. Please check to ensure you are inputting the correct address. Also check your spam/junk, and ensure you have contacted your system administrators to ensure it is not being affected by a filter. If you have done the above but still have not received the address, please contact info@cddata.co.uk. Does my product meet the requirements for a PIP code? We do not issue PIP codes for products which are temporary. Products must be available for at least 6 months. As a result of this we do not list gifts or seasonal products. We do not list promotional packs, or any display units. If I meet the requirements how long does it take for a PIP code to be allocated? We aim to have all requests processed 48 hours from the time of submission. During the busy periods this may increase the time taken. When does a PIP code change? A PIP code is assigned to the product. This means that changes to its packaging, barcodes or name do not always constitute a change of PIP code. However if the formulation 23
25 (active ingredient and strengths) or its pack size (quantity of tablets for example) change it does require a change of PIP code. I have logged in but cannot see my products. Some products might be in sub groups. This allows better management of products. If you see a black triangle as shown below, click to expand the listings. Will my changes be in the next month s price book? This is depends on the when the changes are due to go into effect. To ensure the price books are sent to pharmacy s at the start of each month, the product file is run normally on the second week of the month before. This means if you want your price change to go into effect on the 1 st of October the file would have already been sent, and the books printed. However it will be made available to pharmacies on the date the change has been approved. These are made available via the change reports which can be downloaded online. What s the difference between save and submit? A saved request we cannot view. The main function of this is to provide the manufacturers a way of editing or creating products without submitting. For example if you are missing information you will no longer have to lose your edits or request. 24
26 The submit function sends any edits to the C+D admin staff for approval. Only approved requests will be viewable by subscribers. This excludes edits to manufacturer contacts, only admin can view this section, but new contacts do need to be approved. I cannot see the full product description in the description field. The reason you may not see the full description might be in the grouping structure. This allows us to create variant products, which reduce the amount of space used in the print publications. It also allows us to better manage your product records. A black tick here indicates a variant group. Items can only variants if they meet certain criteria. - The prince must be the same in both trade and retail. - The VAT must be the same in all products - All products must match in form, for example colostomy and Ileostomy bags would need to be in separate groups. What is the difference between products per outer and quantity/size? Quantity is how many items are in the consumer pack, for example a pack of 24 tablets, will have 24 in the quantity. Products per outer is the minimum consumer units a pharmacy can buy in. 25
27 For example a Shampoo might be 400ml and a consumer can buy one item as a minimum. But the company may sell them to the pharmacy as a pack of six, 400ml shampoos. In this case the Products per outer should be six, and the size should be 400ml. What VAT option should I select? Standard VAT is applicable to most medicines. Low rate can be applied to items such as female menstrual products. Zero rated tax can be applied to items such as baby products. Which VAT you pick is linked to the individual product not the manufacturer. What PL number should I use? This depends on what authority licensed the product. PL and THR licenses are issued by the MHRA, and must meet their requirements. EU numbers are issued by the European Union, and must meet their requirements. 26
28 Minimum Requirments for Products 27
29 Quick Rules when inputting products If the product has a legal code, it must have a PL number. o If the product has a legal code of Medical Device it must have a CE mark. o If the product has a legal code it must have a formulation table. A formulation table must have the strength of the active ingredient. The product has a trade price it must have a products per outer. o If products per outer is more than one, the trade price must reflect this. Retail prices always display the single unit price Branded medicines must have the basic NHS list price in the trade price column The Description must say the form (Tablets, capsules, Eye Drops). Product image should be on a white background. They should also be of a front facing pack. GTIN s are mandatory unless you do not have a GS1 license. In this stance please info@cddata.co.uk, or add into free text when making the request 28
LiiV Handbook. Version 2.1. Supplier information in the VARA register. This handbook describes pharmaceutical companies work in LiiV
 LiiV Handbook Supplier information in the VARA register Version 2.1 This handbook describes pharmaceutical companies work in LiiV Contents 1. Introduction... 4 1.1 Information owner... 4 2. How does LiiV
LiiV Handbook Supplier information in the VARA register Version 2.1 This handbook describes pharmaceutical companies work in LiiV Contents 1. Introduction... 4 1.1 Information owner... 4 2. How does LiiV
Foodstuffs NZ Ltd. Data express Basics. A guide to getting started with Data express in the Foodstuffs exchange
 Foodstuffs NZ Ltd Data express Basics A guide to getting started with Data express in the Foodstuffs exchange Foodstuffs ecommerce 6/23/2016 DATA EXPRESS BASICS WHAT YOU NEED TO KNOW TO GET STARTED WITH
Foodstuffs NZ Ltd Data express Basics A guide to getting started with Data express in the Foodstuffs exchange Foodstuffs ecommerce 6/23/2016 DATA EXPRESS BASICS WHAT YOU NEED TO KNOW TO GET STARTED WITH
TIS HELP FOR INDEPENDENT OPERATORS CONTENTS
 TIS HELP FOR INDEPENDENT OPERATORS CONTENTS 1 INTRODUCTION... 3 1.1 TIE... 3 1.2 Account set up in TIS... 3 1.3 VAT number (EU only)... 3 1.4 Business license number (China only)... 3 1.5 Access levels...
TIS HELP FOR INDEPENDENT OPERATORS CONTENTS 1 INTRODUCTION... 3 1.1 TIE... 3 1.2 Account set up in TIS... 3 1.3 VAT number (EU only)... 3 1.4 Business license number (China only)... 3 1.5 Access levels...
Managing Product Master Data
 Managing Product Master Data Table of Contents Managing Product Master Data 3 Product Information 4 Adding Product Master Data 5 Updating Products 27 Accessing Product Master Data 28 Exporting Product
Managing Product Master Data Table of Contents Managing Product Master Data 3 Product Information 4 Adding Product Master Data 5 Updating Products 27 Accessing Product Master Data 28 Exporting Product
Fulfillment User Guide FULFILLMENT
 Fulfillment User Guide FULFILLMENT TABLE OF CONTENTS I. System Requirements II. Logging In III. Launchpad a. Home b. Profile c. Settings IV. Dashboard Tab a. Actionable Insights b. Open Orders V. Transactions
Fulfillment User Guide FULFILLMENT TABLE OF CONTENTS I. System Requirements II. Logging In III. Launchpad a. Home b. Profile c. Settings IV. Dashboard Tab a. Actionable Insights b. Open Orders V. Transactions
New Item Submission System (NISS)
 New Item Submission System (NISS) Agent/Supplier User Guide 1 of 36 Table of Contents INTRODUCTION... 3 Overview... 3 Support... 3 NISS at a Glance... 4 To Access NISS... 4 PASSWORD... 6 First Time Use...
New Item Submission System (NISS) Agent/Supplier User Guide 1 of 36 Table of Contents INTRODUCTION... 3 Overview... 3 Support... 3 NISS at a Glance... 4 To Access NISS... 4 PASSWORD... 6 First Time Use...
PRESCRIPTION MONITORING PROGRAM (PMP)
 PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
Enhanced new user experience with simple to use navigation and better buying experience. Trade accounts will see current order status, and history
 NEW FEATURES AT ATLANTIC.REXEL.CA What s New? Enhanced new user experience with simple to use navigation and better buying experience Updated search functionality Trade accounts will see current order
NEW FEATURES AT ATLANTIC.REXEL.CA What s New? Enhanced new user experience with simple to use navigation and better buying experience Updated search functionality Trade accounts will see current order
Mozy Rewards Training Guide: North America
 Mozy Rewards Training Guide: North America 1 WELCOME This training guide is designed to help you navigate the Mozy Rewards portal, answer commonly asked questions and help you learn how to: Navigate the
Mozy Rewards Training Guide: North America 1 WELCOME This training guide is designed to help you navigate the Mozy Rewards portal, answer commonly asked questions and help you learn how to: Navigate the
Frooition Implementation guide
 Frooition Implementation guide Version: 2.0 Updated: 14/12/2016 Contents Account Setup: 1. Software Checklist 2. Accessing the Frooition Software 3. Completing your Account Profile 4. Updating your Frooition
Frooition Implementation guide Version: 2.0 Updated: 14/12/2016 Contents Account Setup: 1. Software Checklist 2. Accessing the Frooition Software 3. Completing your Account Profile 4. Updating your Frooition
Welcome to the Cub Cadet Dealer Community. Any questions please
 Welcome to the Cub Cadet Dealer Community Any questions please email dealercommunitysupport@cubcadet.com Go to www.mtdcommunity.com Initial Log In - Enter User ID & Password you received in the email -
Welcome to the Cub Cadet Dealer Community Any questions please email dealercommunitysupport@cubcadet.com Go to www.mtdcommunity.com Initial Log In - Enter User ID & Password you received in the email -
Student Guide to Neehr Perfect Go!
 Student Guide to Neehr Perfect Go! I. Introduction... 1 II. Quick Facts... 1 III. Creating your Account... 1 IV. Applying Your Subscription... 4 V. Logging in to Neehr Perfect... 6 VI. Activities... 6
Student Guide to Neehr Perfect Go! I. Introduction... 1 II. Quick Facts... 1 III. Creating your Account... 1 IV. Applying Your Subscription... 4 V. Logging in to Neehr Perfect... 6 VI. Activities... 6
TIS HELP VCCS TECHNICAL INFORMATION SHOP (TIS) INSTRUCTION FOR INDEPENDENT OPERATORS
 VCCS TECHNICAL INFORMATION SHOP (TIS) INSTRUCTION FOR INDEPENDENT OPERATORS CONTENTS 1 INTRODUCTION... 3 1.1 Account set up... 3 1.1.1 Independent operators with TIE access... 3 1.2 Login for registered
VCCS TECHNICAL INFORMATION SHOP (TIS) INSTRUCTION FOR INDEPENDENT OPERATORS CONTENTS 1 INTRODUCTION... 3 1.1 Account set up... 3 1.1.1 Independent operators with TIE access... 3 1.2 Login for registered
This document provides an overview of the enhancements and support issue fixes in this Minfos release.
 Release Notes Minfos 3.11.1 Welcome to Minfos 3.11.1 This document provides an overview of the enhancements and support issue fixes in this Minfos release. Highlights of this release: New Script Enquiry
Release Notes Minfos 3.11.1 Welcome to Minfos 3.11.1 This document provides an overview of the enhancements and support issue fixes in this Minfos release. Highlights of this release: New Script Enquiry
Using SystmOnline - A Guide for Patients
 Using SystmOnline - A Guide for Patients About SystmOnline SystmOnline allows you to do things like booking appointments and ordering repeat prescriptions at a time convenient to you. SystmOnline is also
Using SystmOnline - A Guide for Patients About SystmOnline SystmOnline allows you to do things like booking appointments and ordering repeat prescriptions at a time convenient to you. SystmOnline is also
How to use Novixus.com. Step by Step guide to using the new novixus.com website
 How to use Novixus.com Step by Step guide to using the new novixus.com website Requirements to use new site Browsers supported: How to Check your version 1. Internet Explorer 9 and higher 2. Chrome Version
How to use Novixus.com Step by Step guide to using the new novixus.com website Requirements to use new site Browsers supported: How to Check your version 1. Internet Explorer 9 and higher 2. Chrome Version
YourStore A GUIDE TO
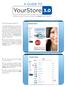 A GUIDE TO YourStore 3.0 Selling contact lenses online has never been easier! This is the homepage of YourStore 3.0. 1. The header displays just your company name as its default. The rectangular space
A GUIDE TO YourStore 3.0 Selling contact lenses online has never been easier! This is the homepage of YourStore 3.0. 1. The header displays just your company name as its default. The rectangular space
Introduction to SystmOnline. Contents
 Introduction to SystmOnline SystmOnline is the name of our online services. We offer the facility for you to request prescriptions online, book or cancel appointments, and much more. This short guide is
Introduction to SystmOnline SystmOnline is the name of our online services. We offer the facility for you to request prescriptions online, book or cancel appointments, and much more. This short guide is
ENTERING GREAT TASTE 2018 ONLINE INSTRUCTIONS
 ENTERING GREAT TASTE 2018 ONLINE INSTRUCTIONS Please visit www.gff.co.uk/gta Click on Enter Here (in red box) or Enter Awards (appears top right of web page) 1. Registration for MyGuild All entrants to
ENTERING GREAT TASTE 2018 ONLINE INSTRUCTIONS Please visit www.gff.co.uk/gta Click on Enter Here (in red box) or Enter Awards (appears top right of web page) 1. Registration for MyGuild All entrants to
Table of contents. Portal User Guide. 1. How to get started. 2. How to create a quote. 3. How to order Paxton10 products
 Portal User Guide Portal User Guide Table of contents 1. How to get started What is the Paxton10 portal? Registering for your account Logging in The homepage My account My account opening a credit account
Portal User Guide Portal User Guide Table of contents 1. How to get started What is the Paxton10 portal? Registering for your account Logging in The homepage My account My account opening a credit account
In partnership with. PO Convert User Guide - Service-Type Purchase Orders
 In partnership with PO Convert User Guide - Service-Type Purchase Orders Imperial College phases out paper invoices with electronic invoicing Imperial College London has implemented a series of changes
In partnership with PO Convert User Guide - Service-Type Purchase Orders Imperial College phases out paper invoices with electronic invoicing Imperial College London has implemented a series of changes
User Guide for Patients
 Creating a My Health Online Account User Guide for Patients Before you can create a My Health Online account you must register for this service at your GP practice. Your practice will provide you with
Creating a My Health Online Account User Guide for Patients Before you can create a My Health Online account you must register for this service at your GP practice. Your practice will provide you with
Logging Into ipart Enter the Store ID
 Logging Into ipart Enter the Store ID Using your computers web browser, navigate to the ipart homepage URL: https://ipart.amador.ca which will look similar to the image above. Enter the Store ID requested
Logging Into ipart Enter the Store ID Using your computers web browser, navigate to the ipart homepage URL: https://ipart.amador.ca which will look similar to the image above. Enter the Store ID requested
MyDispense User Guide
 V1.3 Page 1 MyDispense User Guide 1 About MyDispense MyDispense is an online pharmacy simulation that allows you to develop and to practice your dispensing skills. It provides a safe environment in which
V1.3 Page 1 MyDispense User Guide 1 About MyDispense MyDispense is an online pharmacy simulation that allows you to develop and to practice your dispensing skills. It provides a safe environment in which
Key Contacts Guide to the University Supplier Database
 Key Contacts Guide to the University Supplier Database Table of Contents Requesting Access... 3 Search Only... 3 Access to request new supplier, amend or reactivate... 3 Logging on and Searching Suppliers
Key Contacts Guide to the University Supplier Database Table of Contents Requesting Access... 3 Search Only... 3 Access to request new supplier, amend or reactivate... 3 Logging on and Searching Suppliers
This section covers printing requirements for the supply of medicinal products and appliances under a patient group directive (Non-FP10).
 Overprint Specification For: Non-FP10 Supply Forms This section covers printing requirements for the supply of medicinal products and appliances under a patient group directive (Non-FP10). Pharmacy Stamp
Overprint Specification For: Non-FP10 Supply Forms This section covers printing requirements for the supply of medicinal products and appliances under a patient group directive (Non-FP10). Pharmacy Stamp
Unit 4 Agresso 571 Web Services. Journal Registration
 Unit 4 Agresso 571 Web Services Journal Registration Author S J Price June 2018 CONTENTS Starting Agresso Web... 3 Login Screen... 3 Journal Registration... 4 Registered Journals enquiry... 9 Journal Registration
Unit 4 Agresso 571 Web Services Journal Registration Author S J Price June 2018 CONTENTS Starting Agresso Web... 3 Login Screen... 3 Journal Registration... 4 Registered Journals enquiry... 9 Journal Registration
SilverScript Enrollment Portal 2018 Agent User Guide
 SilverScript Enrollment Portal 08 Agent User Guide Your resource to efficiently navigate our award-winning website Step-by-step instructions to maximize productivity Agent Portal User Guide Logging In
SilverScript Enrollment Portal 08 Agent User Guide Your resource to efficiently navigate our award-winning website Step-by-step instructions to maximize productivity Agent Portal User Guide Logging In
Controlled Substances
 45.75.1 POLICY This policy addresses obtaining, using, storing, recordkeeping, dispensing, and disposing of controlled substances at WSU. This policy provides information and procedures to enable individuals
45.75.1 POLICY This policy addresses obtaining, using, storing, recordkeeping, dispensing, and disposing of controlled substances at WSU. This policy provides information and procedures to enable individuals
Sales Order Processing
 Windows Print Management System Sales Order Processing Sales Order Processing Contents Sales Order Processing Contents Sales Order Processing Contents... 1.1 Introduction to SOP... 2.1 Stock Type... 3.1
Windows Print Management System Sales Order Processing Sales Order Processing Contents Sales Order Processing Contents Sales Order Processing Contents... 1.1 Introduction to SOP... 2.1 Stock Type... 3.1
Customer Portal User Guide. https://customerportal.dfds.com 11/06/2015
 Customer Portal User Guide https://customerportal.dfds.com 11/06/2015 Table of Contents 1. Introduction 3 2. Getting Started 3 3. Search & Overview 3 4. Downloading Documentation 5 5. Create Booking 6
Customer Portal User Guide https://customerportal.dfds.com 11/06/2015 Table of Contents 1. Introduction 3 2. Getting Started 3 3. Search & Overview 3 4. Downloading Documentation 5 5. Create Booking 6
Registration and account management. NPDA User Guide: How to register to use the NPDA data capture system
 NPDA User Guide: How to register to use the NPDA data capture system Register an account on the system PLEASE NOTE: If you have already registered to submit data to the NPDA in 2017, your login details
NPDA User Guide: How to register to use the NPDA data capture system Register an account on the system PLEASE NOTE: If you have already registered to submit data to the NPDA in 2017, your login details
OptumRx Quick Reference Guide
 OptumRx Our website, www.optumrx.com is a fast, safe and secure way to manage your prescription benefits online. This quick reference guide illustrates how to use the tools and features that will help
OptumRx Our website, www.optumrx.com is a fast, safe and secure way to manage your prescription benefits online. This quick reference guide illustrates how to use the tools and features that will help
Online Catalogue and Ordering. Guidance Notes
 Online Catalogue and Ordering Guidance Notes Version 2.6 June 2017 Contents General information... 5 What s new?... 5 Key to icons... 5 System messages... 6 Abbreviations and glossary... 6 Examples used
Online Catalogue and Ordering Guidance Notes Version 2.6 June 2017 Contents General information... 5 What s new?... 5 Key to icons... 5 System messages... 6 Abbreviations and glossary... 6 Examples used
SPS Commerce WebForms Reference Guide
 SPS Commerce WebForms Reference Guide Table of Contents Table of Contents... i Introduction to the WebForms System... 1 Welcome... 1 What is WebForms?... 1 Requirements What do I need to get started?...
SPS Commerce WebForms Reference Guide Table of Contents Table of Contents... i Introduction to the WebForms System... 1 Welcome... 1 What is WebForms?... 1 Requirements What do I need to get started?...
ProScript User Guide. Chronic Medication Service (CMS) Version Release Date 21/10/2010 Author Rx Systems
 User Guide Chronic Medication Service (CMS) Version 5.0.4 Release Date 21/10/2010 Author Rx Systems Table of Contents Objectives... 3 Introduction... 4 Getting Started CMS Main Screen... 5 Registering
User Guide Chronic Medication Service (CMS) Version 5.0.4 Release Date 21/10/2010 Author Rx Systems Table of Contents Objectives... 3 Introduction... 4 Getting Started CMS Main Screen... 5 Registering
Risk Management Programme (RMP) UK & Ireland electronic Prescription Authorisation Form (epaf) User Guide
 Risk Management Programme (RMP) UK & Ireland electronic Prescription Authorisation Form (epaf) User Guide 1. RMP UK & Ireland - epaf Head Pharmacist Self-registering in the epaf system 1.1 Starting self-registration
Risk Management Programme (RMP) UK & Ireland electronic Prescription Authorisation Form (epaf) User Guide 1. RMP UK & Ireland - epaf Head Pharmacist Self-registering in the epaf system 1.1 Starting self-registration
OTC Direct Limited Customer account application / amendment form
 OTC Direct Limited Customer account application / amendment form. 2017 Please find attached all forms needed to open an account with OTC Direct Ltd. Please fax all forms to 0800 169 6622 or email to sales@otc-direct-ltd.com
OTC Direct Limited Customer account application / amendment form. 2017 Please find attached all forms needed to open an account with OTC Direct Ltd. Please fax all forms to 0800 169 6622 or email to sales@otc-direct-ltd.com
Alabama PDMP AWA E. User Support Manual
 Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
Alabama PDMP AWA E User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Pre-Loaded User Access... 4 3 Registration... 5 3.1 Registration Process... 6 3.2 Registering as a Delegate... 10 3.3 Email
Electrolux Small Appliance Reseller FAQs
 Electrolux Small Appliance Reseller FAQs Why is Electrolux instituting a Reseller Authorization Program? Electrolux takes our brand and market position very seriously and desires to protect and promote
Electrolux Small Appliance Reseller FAQs Why is Electrolux instituting a Reseller Authorization Program? Electrolux takes our brand and market position very seriously and desires to protect and promote
ENTERING GREAT TASTE 2016 ONLINE INSTRUCTIONS
 ENTERING GREAT TASTE 2016 ONLINE INSTRUCTIONS - Go to www.gff.co.uk/gta - Click on Enter Here (in red box) or Access MyGuild (top right of web page) 1) Registration for MyGuild If you have already registered
ENTERING GREAT TASTE 2016 ONLINE INSTRUCTIONS - Go to www.gff.co.uk/gta - Click on Enter Here (in red box) or Access MyGuild (top right of web page) 1) Registration for MyGuild If you have already registered
Course Collect user manual 11/08/2014 V1.0
 11/08/2014 V1.0 Copyright Published by: UCAS Rosehill New Barn Lane Cheltenham GL52 3LZ UCAS 2014 All rights reserved. UCAS is a registered trade mark. UCAS, a company limited by guarantee, is registered
11/08/2014 V1.0 Copyright Published by: UCAS Rosehill New Barn Lane Cheltenham GL52 3LZ UCAS 2014 All rights reserved. UCAS is a registered trade mark. UCAS, a company limited by guarantee, is registered
MyDispense User Guide
 MyDispense User Guide 1 About MyDispense MyDispense is an online pharmacy simulation that allows you to develop and to practise your dispensing skills. It provides a safe environment in which you may make
MyDispense User Guide 1 About MyDispense MyDispense is an online pharmacy simulation that allows you to develop and to practise your dispensing skills. It provides a safe environment in which you may make
Welcome to Care Wisconsin s Provider Authorization Portal Training. The Authorization Portal is a web-based portal which allows you, the provider,
 Welcome to Care Wisconsin s Provider Authorization Portal Training. The Authorization Portal is a web-based portal which allows you, the provider, ready access to specific service authorization information
Welcome to Care Wisconsin s Provider Authorization Portal Training. The Authorization Portal is a web-based portal which allows you, the provider, ready access to specific service authorization information
General Use. Searching for Assets (All users) Browsing for Assets (All users) Viewing and Downloading an Asset (All Users)
 User Guide Rev1.1 Table of Contents General Use... 2 Searching for Assets (All users)... 2 Browsing for Assets (All users)... 2 Viewing and Downloading an Asset (All Users)... 2 Downloading Large Files
User Guide Rev1.1 Table of Contents General Use... 2 Searching for Assets (All users)... 2 Browsing for Assets (All users)... 2 Viewing and Downloading an Asset (All Users)... 2 Downloading Large Files
What is a Prescription Drug Monitoring Program?
 What is a Prescription Drug Monitoring Program? A prescription drug monitoring program (PDMP) is a state program that collects controlled substance prescription records from dispensers (e.g., pharmacies)
What is a Prescription Drug Monitoring Program? A prescription drug monitoring program (PDMP) is a state program that collects controlled substance prescription records from dispensers (e.g., pharmacies)
ODS Licensing System. Manual
 EUROPEAN COMMISSION DIRECTORATE-GENERAL CLIMATE ACTION Directorate C - Mainstreaming Adaptation and Low Carbon Technology CLIMA.C.2 - Transport and Ozone ODS Licensing System Manual PART II REGISTRATION
EUROPEAN COMMISSION DIRECTORATE-GENERAL CLIMATE ACTION Directorate C - Mainstreaming Adaptation and Low Carbon Technology CLIMA.C.2 - Transport and Ozone ODS Licensing System Manual PART II REGISTRATION
MetaTrader 4 for Android. User Manual
 MetaTrader 4 for Android User Manual LOG IN After downloading and installing the terminal from the Google Play store you will see the Metatrader 4 icon added to your app list. Tap the Metatrader 4 icon
MetaTrader 4 for Android User Manual LOG IN After downloading and installing the terminal from the Google Play store you will see the Metatrader 4 icon added to your app list. Tap the Metatrader 4 icon
Implementation of the Minor Ailment Service Produced by NES Pharmacy
 Implementation of the Minor Ailment Service Produced by NES Pharmacy Using emas Software SECTION 3 Using emas Software NHS pharmacists in Scotland can apply for a printed copy of this pack, please contact:
Implementation of the Minor Ailment Service Produced by NES Pharmacy Using emas Software SECTION 3 Using emas Software NHS pharmacists in Scotland can apply for a printed copy of this pack, please contact:
BUSINESS ACCOUNT MANAGEMENT SYSTEMS (BAMS) AND TRAVEL MANAGEMENT COMPANIES (BAMS)
 BUSINESS ACCOUNT MANAGEMENT SYSTEMS (BAMS) AND TRAVEL MANAGEMENT COMPANIES (BAMS) USER GUIDE Contents Contents Contents Contents 2 1. Introduction 3 2. Account Activation 4 3. BAMS 5 3.1 Logon 5 3.2 TMC
BUSINESS ACCOUNT MANAGEMENT SYSTEMS (BAMS) AND TRAVEL MANAGEMENT COMPANIES (BAMS) USER GUIDE Contents Contents Contents Contents 2 1. Introduction 3 2. Account Activation 4 3. BAMS 5 3.1 Logon 5 3.2 TMC
Creating a Listing Access to Listing Search Listings Contacting Users/Message Center Edit/Update Listing
 Creating a Listing What is the process for creating a listing? What information is required to complete a listing? What information will be visible to other users? About how long does it take to complete
Creating a Listing What is the process for creating a listing? What information is required to complete a listing? What information will be visible to other users? About how long does it take to complete
Codex Portal Supplier Guide
 Codex Portal Supplier Guide August 2016 Codex Global, Atlantic House, 351 Oxford Street, London W1C 2JF t +44 (0)20 7647 9555 f +44 (0)20 7900 6060 e info@codexglobal.net www.codexglobal.net Contents 1.
Codex Portal Supplier Guide August 2016 Codex Global, Atlantic House, 351 Oxford Street, London W1C 2JF t +44 (0)20 7647 9555 f +44 (0)20 7900 6060 e info@codexglobal.net www.codexglobal.net Contents 1.
New BoundTree.com User Guide Fall Version 6
 New BoundTree.com User Guide Fall 2016 Version 6 Table of Contents Overview Navigating the Home Page Creating an Account Logging into an Existing Account Forgot Your Password? Reviewing Your Account Editing
New BoundTree.com User Guide Fall 2016 Version 6 Table of Contents Overview Navigating the Home Page Creating an Account Logging into an Existing Account Forgot Your Password? Reviewing Your Account Editing
DSWR User Guide. In effect from January 29 th,, BCLDB Direct Sales Web Reporting User Guide Page 1
 DSWR User Guide In effect from January 29 th,, 2017 BCLDB Direct Sales Web Reporting User Guide Page 1 Contents Introduction... 4 Before You Get Started... 4 Registering for the DSWR Application... 5 Log-in...
DSWR User Guide In effect from January 29 th,, 2017 BCLDB Direct Sales Web Reporting User Guide Page 1 Contents Introduction... 4 Before You Get Started... 4 Registering for the DSWR Application... 5 Log-in...
Major Appliance Recycling Roundtable ONLINE REGISTRATION & REPORTING SYSTEM INSTRUCTIONS
 Major Appliance Recycling Roundtable ONLINE REGISTRATION & REPORTING SYSTEM INSTRUCTIONS Authored By: Major Appliance Recycling Roundtable Table of Contents Introduction... 3 Section 1- MARR Registration
Major Appliance Recycling Roundtable ONLINE REGISTRATION & REPORTING SYSTEM INSTRUCTIONS Authored By: Major Appliance Recycling Roundtable Table of Contents Introduction... 3 Section 1- MARR Registration
A PPG guide to using SystmOnline in Woodbridge Medical Practice.
 A PPG guide to using SystmOnline in Woodbridge Medical Practice. Table of Contents What is SystmOnline?... 2 How to access SystmOnline... 3 Detailed Instructions and guide.... 6 Appointments... 7 Book
A PPG guide to using SystmOnline in Woodbridge Medical Practice. Table of Contents What is SystmOnline?... 2 How to access SystmOnline... 3 Detailed Instructions and guide.... 6 Appointments... 7 Book
Supplier Reference Guide (QRG) Table of Contents
 Supplier Onboarding Supplier Reference Guide (QRG) Table of Contents Supplier Checklist... 2 New Supplier Onboarding Steps... 3 Introduction... 4 Registration... 6 Certification... 16 Acceptance... 35
Supplier Onboarding Supplier Reference Guide (QRG) Table of Contents Supplier Checklist... 2 New Supplier Onboarding Steps... 3 Introduction... 4 Registration... 6 Certification... 16 Acceptance... 35
Online Ordering Manual
 Online Ordering Manual for the Pay-LESS website www.paylessoffice.com Customer Log In... 2-3 Finding Your Account Number... 4 Searching for Products... 5-6 Quick Order... 7-8 Product Comparison... 9-10
Online Ordering Manual for the Pay-LESS website www.paylessoffice.com Customer Log In... 2-3 Finding Your Account Number... 4 Searching for Products... 5-6 Quick Order... 7-8 Product Comparison... 9-10
User Guide. CKS Software Version 8 Pharmacy Program. Document Version: UCS Technology Services PTY (LTD) Pharmacy Program Manual (V3.10.
 User Guide CKS Software Version 8 Pharmacy Program Document Version: 3.10.11 UCS Technology Services PTY (LTD) Pharmacy Program Manual (V3.10.11) 1 Module 1 : Pharmacy Program In this module you are shown
User Guide CKS Software Version 8 Pharmacy Program Document Version: 3.10.11 UCS Technology Services PTY (LTD) Pharmacy Program Manual (V3.10.11) 1 Module 1 : Pharmacy Program In this module you are shown
Welcome to Shopfront. Your distributor will supply your user name, password, and the website address for your login page.
 User Guide Table of Contents Login... 3 Choose a Location... 4 Home Page... 5 Header Bar... 6 My Catalog... 6 Menu Bar... 7 My Profile... 8 Contact Us... 9 Change Location... 10 Shopping Lists... 11 Quick
User Guide Table of Contents Login... 3 Choose a Location... 4 Home Page... 5 Header Bar... 6 My Catalog... 6 Menu Bar... 7 My Profile... 8 Contact Us... 9 Change Location... 10 Shopping Lists... 11 Quick
SIAM R3.0 USER GUIDE
 SIAM R3.0 USER GUIDE Document Reference: 8295 September 2016 Revision: 3 Version Date Author Changes Number 1 Mar 2015 John Lindsay 2 Jun Sam Unsuspending a SIM card description updated. 2016 Smith 3 Sep
SIAM R3.0 USER GUIDE Document Reference: 8295 September 2016 Revision: 3 Version Date Author Changes Number 1 Mar 2015 John Lindsay 2 Jun Sam Unsuspending a SIM card description updated. 2016 Smith 3 Sep
Training Guide for Practitioners
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program July 2014 RxSentry is a proprietary system for prescription monitoring provided by
Using Lloyd s Direct Reporting. User Guide
 Using Lloyd s Direct Reporting User Guide AUGUST 2013 2 Contents CONTENTS 2 LLOYD S DIRECT REPORTING 3 ABOUT THIS SERVICE 3 FURTHER HELP AND SUPPORT 3 USER GUIDE 4 ACCESSING LLOYD S DIRECT REPORTING 4
Using Lloyd s Direct Reporting User Guide AUGUST 2013 2 Contents CONTENTS 2 LLOYD S DIRECT REPORTING 3 ABOUT THIS SERVICE 3 FURTHER HELP AND SUPPORT 3 USER GUIDE 4 ACCESSING LLOYD S DIRECT REPORTING 4
Member guide. How to use the new Constructionline platform
 Member guide How to use the new Constructionline platform Contents About this guide... 3 1. Joining Constructionline... 4 1.1 Select plan & pay... 4 1.2 Create buyer profile... 10 1.3 Create supplier profile...
Member guide How to use the new Constructionline platform Contents About this guide... 3 1. Joining Constructionline... 4 1.1 Select plan & pay... 4 1.2 Create buyer profile... 10 1.3 Create supplier profile...
Chapter 6 Data Entry. Tasks in Data Entry. Window Elements in Data Entry
 Chapter 6 Data Entry Tasks in Data Entry Starting Data Entry Searching for a Prescriber in Data Entry Searching for a Product in Data Entry Using the Generic Substitution Window Entering Data Entry Detail
Chapter 6 Data Entry Tasks in Data Entry Starting Data Entry Searching for a Prescriber in Data Entry Searching for a Product in Data Entry Using the Generic Substitution Window Entering Data Entry Detail
Quick Reference Guide: SafeShop 3 Web Store. User Manual
 : SafeShop 3 Web Store User Manual TABLE OF CONTENTS REGISTRATION.2 SAFESHOP WEBSTORE. 5 1. FIND MY TRANSACTION:.5 2. STAGED ORDERS:.6 3. MANAGE ORDERS: 7 4. MANAGE SALES...9 5. MANAGE CREDIT/CHARGEBACK
: SafeShop 3 Web Store User Manual TABLE OF CONTENTS REGISTRATION.2 SAFESHOP WEBSTORE. 5 1. FIND MY TRANSACTION:.5 2. STAGED ORDERS:.6 3. MANAGE ORDERS: 7 4. MANAGE SALES...9 5. MANAGE CREDIT/CHARGEBACK
Administrator Manual. Version 10
 Administrator Manual Version 10 Table of Contents Administrator Option Screen 3 Sales Screen 6 Search Sales for finding past sales 7 Pay Account for Paying outstanding accounts 10 Paid in or Out Petty
Administrator Manual Version 10 Table of Contents Administrator Option Screen 3 Sales Screen 6 Search Sales for finding past sales 7 Pay Account for Paying outstanding accounts 10 Paid in or Out Petty
BEEDS portal Bank of England Electronic Data Submission portal. User guide. Credit unions Version 1.2
 BEEDS portal Bank of England Electronic Data Submission portal User guide Credit unions Version 1.2 May 2018 Contents Document versions 3 1. Introduction 4 a. Bank of England contact details 4 2. General
BEEDS portal Bank of England Electronic Data Submission portal User guide Credit unions Version 1.2 May 2018 Contents Document versions 3 1. Introduction 4 a. Bank of England contact details 4 2. General
University of Cambridge International Examinations Teacher Support Site User Guide for Coordinators
 University of Cambridge International Examinations Teacher Support Site User Guide for Coordinators Getting started Log into the Teacher Support Site http://teachers.cie.org.uk/ Click the Administer Users
University of Cambridge International Examinations Teacher Support Site User Guide for Coordinators Getting started Log into the Teacher Support Site http://teachers.cie.org.uk/ Click the Administer Users
Provider Portal User Guide. For the Provider Portal External Use
 Provider Portal User Guide For the Provider Portal External Use IT Department Issued January 2017 mynexus 2017. All rights reserved. Version 1.4 Revised 07122017 Contents Getting Started with the Portal...
Provider Portal User Guide For the Provider Portal External Use IT Department Issued January 2017 mynexus 2017. All rights reserved. Version 1.4 Revised 07122017 Contents Getting Started with the Portal...
Training Guide for Practitioners. Washington State Department of Health Washington State Prescription Monitoring Program
 Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Training Guide for Practitioners Washington State Department of Health Washington State Prescription Monitoring Program April 2017 Training Guide for Practitioners Contents Contents 1 Document Overview...
Administrator User Guide
 QA Ltd. Administrator User Guide MY QA PORTAL Contents Introduction... 3 Logging into myqa... 4 Homepage and Administration Options.... 6 Users and Hierarchy... 7 Finding Users in myqa... 9 Enabling an
QA Ltd. Administrator User Guide MY QA PORTAL Contents Introduction... 3 Logging into myqa... 4 Homepage and Administration Options.... 6 Users and Hierarchy... 7 Finding Users in myqa... 9 Enabling an
daa isupplier User Guide
 daa isupplier User Guide December 2017 Contents Prerequisites... 3 Introduction... 4 1.1 Registration... 6 1.2 Login... 6 1.3 Basic Overview of the isupplier Portal Homepage... 7 1.4 Purchase Order Acceptance
daa isupplier User Guide December 2017 Contents Prerequisites... 3 Introduction... 4 1.1 Registration... 6 1.2 Login... 6 1.3 Basic Overview of the isupplier Portal Homepage... 7 1.4 Purchase Order Acceptance
Paperless Pipeline Agent Manual
 Paperless Pipeline Agent Manual 1 of 20 The Basics Logging In A welcome email with your username and password has been sent to you. To log in, go to: app.paperlesspipeline.com and enter your login information.
Paperless Pipeline Agent Manual 1 of 20 The Basics Logging In A welcome email with your username and password has been sent to you. To log in, go to: app.paperlesspipeline.com and enter your login information.
Retail Fashion Expert User Guide Independent Customer
 Retail Fashion Expert User Guide Independent Customer Training Material Document Version: 1.1 Software Version: 1.0 Last Revised: MAY21-10 Expert: Customer Service 204.982.5587 2008 Nygård International
Retail Fashion Expert User Guide Independent Customer Training Material Document Version: 1.1 Software Version: 1.0 Last Revised: MAY21-10 Expert: Customer Service 204.982.5587 2008 Nygård International
Easy does it. Making florists successful
 Getting started This step-by-step guide is intended to get you started on eflorist. Further in-depth instruction is available through the Help system on eflorist itself. In addition, there is also a dedicated
Getting started This step-by-step guide is intended to get you started on eflorist. Further in-depth instruction is available through the Help system on eflorist itself. In addition, there is also a dedicated
How to Create and Edit Offers
 How to Create and Edit Offers Instructions, explanations and support for creating and editing offers as well as entering product-related data. Last update 29 July 2016 Table of Contents 1. General Information...
How to Create and Edit Offers Instructions, explanations and support for creating and editing offers as well as entering product-related data. Last update 29 July 2016 Table of Contents 1. General Information...
QUALNET CLAIM USER GUIDE MAY Warranty Policy, appendix 2 : Claim registration in Qualnet for Montluel factory product
 QUALNET CLAIM USER GUIDE MAY 2017 Warranty Policy, appendix 2 : Claim registration in Qualnet for Montluel factory product Access and Internet explorer version compatibility - Qualnet Access : - http://cfrmoa0e.eu.carrier.utc.com/redirect_qualnetv2016.asp
QUALNET CLAIM USER GUIDE MAY 2017 Warranty Policy, appendix 2 : Claim registration in Qualnet for Montluel factory product Access and Internet explorer version compatibility - Qualnet Access : - http://cfrmoa0e.eu.carrier.utc.com/redirect_qualnetv2016.asp
Just Listed/Just Sold FAQ
 Just Listed/Just Sold FAQ General... 3 Where is my Agent Profile Information?... 3 What is the Just Listed/Just Sold Postcard service?... 3 How do I sign up for this service?... 3 Who will receive the
Just Listed/Just Sold FAQ General... 3 Where is my Agent Profile Information?... 3 What is the Just Listed/Just Sold Postcard service?... 3 How do I sign up for this service?... 3 Who will receive the
Carroll Tire Online USER GUIDE Version 2b June 2009
 Carroll Tire Online USER GUIDE Version 2b June 2009 Page 1 of 33 TABLE OF CONTENTS 1 WELCOME TO CARROLL TIRE ONLINE...4 1.1 ABOUT THIS GUIDE...4 1.2 WHAT CAN I DO AT CARROLL TIRE ONLINE?...4 2 CTO QUICK
Carroll Tire Online USER GUIDE Version 2b June 2009 Page 1 of 33 TABLE OF CONTENTS 1 WELCOME TO CARROLL TIRE ONLINE...4 1.1 ABOUT THIS GUIDE...4 1.2 WHAT CAN I DO AT CARROLL TIRE ONLINE?...4 2 CTO QUICK
Welcome to the Kantar Retail UK online Purchase Order approval and Expenses tracking system
 Handbook Welcome to the Kantar Retail UK online Purchase Order approval and Expenses tracking system System Purpose The purpose of the system is twofold: 1. To raise, track and approve Purchase Orders
Handbook Welcome to the Kantar Retail UK online Purchase Order approval and Expenses tracking system System Purpose The purpose of the system is twofold: 1. To raise, track and approve Purchase Orders
Need help? Call: / DOCMAIL: PRINT DRIVER USER GUIDE
 Need help? Call: 01761 409701 / 409702 DOCMAIL: PRINT DRIVER USER GUIDE February 2018 1 GETTING STARTED Display settings Please be aware that to allow for the Print Driver to function efficiently, there
Need help? Call: 01761 409701 / 409702 DOCMAIL: PRINT DRIVER USER GUIDE February 2018 1 GETTING STARTED Display settings Please be aware that to allow for the Print Driver to function efficiently, there
Arkansas Prescription Drug Monitoring Program. User Support Manual
 Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
Arkansas Prescription Drug Monitoring Program User Support Manual 1 Contents 1 What Is a Requestor?... 4 2 Registration... 4 2.1 Registration Process... 5 2.2 Registering as a Delegate... 9 2.3 Email Verification...
DataCar Portal. User guide. DataCar Portal. User Guide TABLE OF CONTENTS
 User Guide TABLE OF CONTENTS 1. INTRODUCTION... 2 2. LOGGING IN TO DATACAR PORTAL... 2 3. DATACAR PORTAL MENUS... 3 3.1 home page... 3 3.2 My Applications... 4 3.3 News... 5 3.4 My Reports... 6 3.5. Surveys...
User Guide TABLE OF CONTENTS 1. INTRODUCTION... 2 2. LOGGING IN TO DATACAR PORTAL... 2 3. DATACAR PORTAL MENUS... 3 3.1 home page... 3 3.2 My Applications... 4 3.3 News... 5 3.4 My Reports... 6 3.5. Surveys...
Mission Seek Instruction Manual for Basic Partnership Level
 Mission Seek Instruction Manual for Basic Partnership Level Index Registering your Agency (This step is for new partners only) 2 & 3 Login after your Agency is registered.4 Account Pending and Verifying
Mission Seek Instruction Manual for Basic Partnership Level Index Registering your Agency (This step is for new partners only) 2 & 3 Login after your Agency is registered.4 Account Pending and Verifying
Q: The barcode on the item is not scanning or the product is not found, what should I do to complete the transaction for my customer?
 FAQ s Q: The barcode on the item is not scanning or the product is not found, what should I do to complete the transaction for my customer? First, scan the Scrubs and Beyond sticker ticket. If the sticker
FAQ s Q: The barcode on the item is not scanning or the product is not found, what should I do to complete the transaction for my customer? First, scan the Scrubs and Beyond sticker ticket. If the sticker
MANUAL OF SUBMISSION OF APPLICATIONS FOR CERTIFICATES OF A PHARMACEUTICAL PRODUCT WHO MODEL AND STATEMENTS OF PHARMACEUTICAL PRODUCTS
 MANUAL OF SUBMISSION OF APPLICATIONS FOR CERTIFICATES OF A PHARMACEUTICAL PRODUCT WHO MODEL AND STATEMENTS OF PHARMACEUTICAL PRODUCTS GLOSSARY... 2 INTRDUCTION... 3 Objective... 3 DEFINITIONS... 3 Certificate
MANUAL OF SUBMISSION OF APPLICATIONS FOR CERTIFICATES OF A PHARMACEUTICAL PRODUCT WHO MODEL AND STATEMENTS OF PHARMACEUTICAL PRODUCTS GLOSSARY... 2 INTRDUCTION... 3 Objective... 3 DEFINITIONS... 3 Certificate
Table of Contents Brainshark. All rights reserved.
 Table of Contents Administrator Reference Guide... 2 Introduction... 2 Topics... 2 Folders... 3 Manage Folders... 3 Edit Folder... 3 Edit Folder Properties... 3 Assign Folder Permissions (Viewer, Author,
Table of Contents Administrator Reference Guide... 2 Introduction... 2 Topics... 2 Folders... 3 Manage Folders... 3 Edit Folder... 3 Edit Folder Properties... 3 Assign Folder Permissions (Viewer, Author,
Using the Telstra T-Suite Management Console. Customer Administrator s Reference Manual
 Using the Telstra T-Suite Management Console Customer Administrator s Reference Manual June 2011 Registering With TMC Notice Pivot Path is a registered trademark of Jamcracker, Inc. Registered trademark
Using the Telstra T-Suite Management Console Customer Administrator s Reference Manual June 2011 Registering With TMC Notice Pivot Path is a registered trademark of Jamcracker, Inc. Registered trademark
LCMS User Manual Last Updated: October 2011
 LCMS User Manual Last Updated: October 2011 LCMS User Manual Page 1 Contents 1. Introduction... 3 1.1 Introduction to the system... 3 1.2 Help Icons... 3 1.3 Accessing LCMS... 3 1.4 Registering with LCMS...
LCMS User Manual Last Updated: October 2011 LCMS User Manual Page 1 Contents 1. Introduction... 3 1.1 Introduction to the system... 3 1.2 Help Icons... 3 1.3 Accessing LCMS... 3 1.4 Registering with LCMS...
Mobile Working for Windows
 Mobile Working for Windows Training Guide for OOH Contents Introduction... 3 Logging onto Mobile Working... 3 Using the Home Screen... 6 Connectivity Status... 7 Assigning Vehicles... 7 Cases... 8 Viewing
Mobile Working for Windows Training Guide for OOH Contents Introduction... 3 Logging onto Mobile Working... 3 Using the Home Screen... 6 Connectivity Status... 7 Assigning Vehicles... 7 Cases... 8 Viewing
MADE4TRADE USER GUIDE
 1 MADE4TRADE USER GUIDE 2 Table of contents CREATING AN ACCOUNT... 3 MAIN NAVIGATION... 4 ORDERING A PRODUCT... 5 ADHOC ORDERS...15 DELIVERY TRACKING...21 EVENTS PROCESS... 2123 EVENT PRE-ORDERING/ CALL-OFF
1 MADE4TRADE USER GUIDE 2 Table of contents CREATING AN ACCOUNT... 3 MAIN NAVIGATION... 4 ORDERING A PRODUCT... 5 ADHOC ORDERS...15 DELIVERY TRACKING...21 EVENTS PROCESS... 2123 EVENT PRE-ORDERING/ CALL-OFF
USER GUIDE FOR SUPPLIERS. OpusCapita Business Network
 USER GUIDE FOR SUPPLIERS OpusCapita Business Network Contents 1. Introduction... 3 2. Finalizing registration and changing your password... 4 2.1 Finalize your registration... 4 2.2 Change your forgotten
USER GUIDE FOR SUPPLIERS OpusCapita Business Network Contents 1. Introduction... 3 2. Finalizing registration and changing your password... 4 2.1 Finalize your registration... 4 2.2 Change your forgotten
y o u r u s e r g u i d e
 your user guide www.thermofisher.com.au 2 key benefits Easy to do business Support at hand Partners in process See your prices and stock availability in real-time with information available 24/7 Search
your user guide www.thermofisher.com.au 2 key benefits Easy to do business Support at hand Partners in process See your prices and stock availability in real-time with information available 24/7 Search
Brill s Editorial Manager (EM) Manual for Authors Contents
 Brill s Editorial Manager (EM) Manual for Authors Contents 1. Introduction... 2 2. Getting Started: Creating an Account... 2 2.1 Creating an Account Using Your ORCID Record... 3 3. Logging into EM... 4
Brill s Editorial Manager (EM) Manual for Authors Contents 1. Introduction... 2 2. Getting Started: Creating an Account... 2 2.1 Creating an Account Using Your ORCID Record... 3 3. Logging into EM... 4
Supplier Invoice Submission Guide. English
 Supplier Invoice Submission Guide English Date: May 2 nd, 2017 1 Table of Contents How to submit an invoice through the SWIM... 3 How to access the SWIM... 3 Submitting a PO invoice... 4 Creating an invoice...
Supplier Invoice Submission Guide English Date: May 2 nd, 2017 1 Table of Contents How to submit an invoice through the SWIM... 3 How to access the SWIM... 3 Submitting a PO invoice... 4 Creating an invoice...
In-State Tobacco Products Wholesale Dealer s Report
 In-State Tobacco Products Wholesale Dealer s Report Logging Into EDS Log in with the user id and password provided through the EDS registration process and click on the Login button. If you have not registered,
In-State Tobacco Products Wholesale Dealer s Report Logging Into EDS Log in with the user id and password provided through the EDS registration process and click on the Login button. If you have not registered,
Registration closed circuit
 Registration closed circuit Practical overview 2 Topics Regulatory background and scope Objectives of the closed circuit Modification Royal Decree: obligations for users (Proposal) Registration process
Registration closed circuit Practical overview 2 Topics Regulatory background and scope Objectives of the closed circuit Modification Royal Decree: obligations for users (Proposal) Registration process
Student Learning Center
 Student Learning Center Pass Assured, LLC www.passassured.com Contents Overview... 2 Login... 3 Message Center... 3 Section Tutorials... 5 Study Aids... 8 Videos and Law... 9 Quizzes and Section Tests...
Student Learning Center Pass Assured, LLC www.passassured.com Contents Overview... 2 Login... 3 Message Center... 3 Section Tutorials... 5 Study Aids... 8 Videos and Law... 9 Quizzes and Section Tests...
Adding Automated Dispensing Devices in Facility Maintenance...53 Adding Products to a Device in Facility Maintenance...54 Modifying an Automated Dispe
 Contents 1 Home Page Administration...1 Home Page Administration Overview... 1 Managing Home Page Messages... 2 Adding and Editing Home Page Messages...3 Removing a Home Page Message... 5 Managing News
Contents 1 Home Page Administration...1 Home Page Administration Overview... 1 Managing Home Page Messages... 2 Adding and Editing Home Page Messages...3 Removing a Home Page Message... 5 Managing News
