1. Getting Started: Brief Step-By-Step Guide (PDF File)
|
|
|
- Cora Hubbard
- 6 years ago
- Views:
Transcription
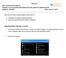
1 Page 1 of 5 1. Getting Started: Brief Step-By-Step Guide (PDF File) Leica SP2 confocal microscope is controlled via software LCS (Leica Confocal Software). The latest version is V2.5. Bellowing is a brief step-by-step guide to help new users get started smoothly. For detailed description about choosing related parameters, please refer to corresponding section in Part 3 of this guide, Operation and Optimization: Settings in detail. 1. Check your specimen under conventional fluorescent microscope, confirm the staining, and make a plan in advance on which laser or how many lasers you are going to use before you go to confocal microscope. 2. Turn on Power switch (2) which starts up computer and provides power supply for scanner and microscope controller. 3. While computer is booting up: Turn on Scanner: button (3a) Turn on Microscope controller: button (3b) Note: 3a and 3b must be on at least half a minute before launching LCS program. So, it is better to turn them on during computer boot-up. The time needed for computer boot-up is enough for these two hardware to be ready. 4. Turn on laser lines of your choice: Figure out which laser line or lines you are going to use before turning all of the lasers on. Beware: each turn-on of laser costs life-time of the laser and is expensive. If you need 458/476/488/514 nm line, turn on Argon Laser: First, Argon Laser Fan (3c) on. without cooling fan, Argon laser will not work. After fan on, turn on Argon laser Power key (3d)-left. If you need 633 nm line, turn on He-Ne laser 633 by Power key (3d)-right. This laser does not need cooling fan. If you need 568 line, turn on krypton laser 568 in a separate box on the floor. Same as Argon laser, Kr 568 has cooling fan button and its power key. 5. Turn on HBO mercury arc lamp on the granite table which is for conventional FL Microscopy. 6. Log in into your WinNT account with password. 7. Click Leica Confocal Software icon, wait for Initialization of Hardware Note: Scanner 3a and microscope controller 3b must be ON at least 30 sec before launching the program. Otherwise, the initialization will fail and it takes extra long time to end an unsuccessful initialization. LCS has several function modules. The default module at start is Acquisition module. The user interface of Acquisition Module looks like the screen-shot below:
2 Page 2 of 5 8. Click Beam icon This launch a parameter setting panel. Drag it to the center of the window. The screen shot looks like below: 9. you need choose a field of view under convention microscope before scanning. Click and press microscope control icon MicCtrl, choose visual from the pull-down menu. Look at microscope stand front panel, be sure Shutter light is off. Put your slide UPSIDE DOWN on microscope stage. For Objectives from 40 x on, use oil. On the left side of microscope, there are two buttons for changing
3 Page 3 of 5 objectives. Objectives can also be changed from LSC software by Obj icon. Note: to toggle between oil and dry OBJ, you have to press simultaneously two arrow keys at same time on the front panel of the microscope stand, or use Obj icon and confirm the change when being asked. On the right side of the microscope stand, there are two buttons for fast focus up and down adjusting in addition to the focus knob. Choose filter-set for conventional fluorescence microscope, 3 filter-set available: I3 for blue excitation, green emission (FITC, Alexa 488, etc.), N2.1 for green excitation, red emission (Rhodamine, etc.). Y5 for red Excitation, long red emission (CY5, Alexa 633, etc.). Click MicCtrl again, choose Scan this time to switch back to confocal mode. 10. In LCS window, click one of the pre-settings from the list for different fluorescent dyes. The upper part of the list under Leica is protected from being changed. The lower part of the list under User holds all user modified and saved settings By a single click on a pre-setting, you get all ten basic acquisition related parameters done, including laser, beam splitter, PMT and spectro-detecting range, LUT for PMT, etc. You can later modify them. 11. Set other six scan related parameters: scan Mode; Scan Speed; Scan Format; Pinhole size, Zoom factor and image AVERAGE respectively. If this is the first scan after you launch the program, there are default values for these parameters, which usually you don't need change before scanning. These values are: Scan mode: xyz Scan speed: 400 Hz Scan format: 512 x 512 Pinhole: 1 Airy unit Zoom factor: 1 x Image average: 1 If this is the later scan after specimen or field change, check these parameters and be sure return some of these values, especially zoom factor to 1 x, average to Click Continuous to begin scanning, while in scan, adjust other four in-line parameters: Gain, Offset of a given detecting channel to get optimal signal intensity, adjust Z-level to get a slice showing the specific structure of your interesting. These three parameters are the key players when you get a black image screen. Pinhole can also be adjusted to value other than 1 airy unit if desired. All these four can be changed through a 7-knob-Control- Box.
4 Page 4 of 5 The function of each knob can be defined at your will. The saved knob definition can be loaded from icon: 13. The image will appear on image viewer at the right-side monitor. You can toggle image display mode among single channel, tiled (two or more channels), OVL overlay, Gallery, or play series. This can be done by clicking respective icon on the vertical tool bar on the left side of the image viewer window. 14. Scale bar, grids and coordinates can be superimposed on the image by checking scale, grids or coordinates box in the Function Overview panel on the lower-left side panel of the LCS main window below Experiment overview. please note, like color overlay, these overlaid marker are not saved by default, Export has to be used to fix these markers on image. See more at item 18: Data saved. 15. If you are interested in a specific region or cell rather than the whole field, you can make a region of interesting, scan only on that ROI by using Zoom In 16. If you are satisfied with the result, stop continuous scan. Usually, it is difficult or unnecessary to get a clear background simply by adjusting gain and offset alone at the cost of signal intensity. Instead, you can tolerate some background noise to maintain reasonable intensity and use image AVERAGE to improve SNR and quality of your image. 4-8 times average works for most cases. 17. Click Single Scan or Series scan (if applicable) to get the final data acquired Save and manipulating acquired data files: It is better to understand some details about how LCS handle acquired data. Experiment overview at upper-left side panel of the LCS main window is an important area for data file handling. Please pay close attention to it during data acquisition. All images acquired are listed under Experiment overview. Only Single scan or Series scan really acquires image data thus has separate entry under Experiment overview window. Continuous scan is used for image optimization thus image acquired through it are constantly updated without fixed entry under experiment overview, and can not be saved excepting for the latest one. All entries here are held in PC's memory, not on disk. Windows crash will lead loss of them if not saved. Save them as soon as possible. The entries can be re-named, deleted. It is strongly recommended that you save them before manipulating on them, then re-save after manipulation. It takes only
5 Page 5 of 5 several seconds but helps to avoid accidental data loss. The more entries listed in the window, the higher risk you have for Windows crash during the manipulation. Rename *.lei itself is not recommended since it save a whole duplicated copy of all the images with a new folder name. Multiple experiment overview windows can be opened by File/New or Save As command. Each experiment is represented by a folder icon and a *.lei name, and each has a image viewer. But all windows consume memory and lead to diminished computer resource. Delete Experiment from the experiments list (the same thing as close image viewer window on the right-side monitor) removes data from memory, but not from disk if you have saved them already. 19. Data saved by LCS: The saved data on hard disk are organized by experiments, i.e., the *.lei entries. Each entry is saved as a separate folder with TIF color image files for all detecting channel you have used. The image overlay of different channels or overlay of scale bar and grids on top of image, however, are not saved by default. To save them, you have to select them by rightmouse-click, choose "Export, Export selection" from the pop-up context menu. This will add an extra color image entry under the experiment overview panel, then they can be saved. The color overlay or scale bar superimposing can be done later at any time if you re-load the original data into LCS program, providing the *.lei file remain untouched. Saved together with TIF image files, the *.lei file is an important file which holds information of your confocal session and make the data active to LCS program as if you have just acquired them. Keep them within the folder if you want re-load and re-play or modify the image within LCS program. 20. File naming convention: When you click save, you will be prompted to enter a name. Please bear in mind: what you enter here is not a single file name, instead, it is the folder name for the experiment and it is also the first common part of file name for all images under the same.lei entry. After the first part, there are image-name, series number, channel number, TIF extension. All these can make very long file name if not named carefully. So, the first part doesn't need to be specific, just use a category name and as short as possible. After the experiment is saved, modify the second part "image-xx" to a short specific name. Other parts can not be modified. Long file name will be truncated if viewed under Mac OS before OS X. 21. After confirm the saved files on the hard disk, you can turn off the system in a reversed order as you turn it on. Quit Leica control software. Turn off HBO mercury lamp, Turn off scanner. Turn off all laser keys. Don,t turn off Fan at this moment!. Let them running at least five minutes after the laser key off. Don 긔 t forget Kr laser on the floor. Clean microscope objectives and stage, make working log during the 5 min time. Finally, turn off Leica microscope controller, turn off fans. Turn off PC. This is just a quick flow-chart. Personal guide on advanced function can be obtained on request.
LSM510 Confocal Microscope Standard Operation Protocol Basic Operation
 LSM510 Confocal Microscope Standard Operation Protocol Basic Operation Please make sure that the COMPRESSED AIR has been TURNED ON prior to the use of the equipment. Kindly inform the administrator if
LSM510 Confocal Microscope Standard Operation Protocol Basic Operation Please make sure that the COMPRESSED AIR has been TURNED ON prior to the use of the equipment. Kindly inform the administrator if
LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes
 LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes Brief Operating Manual Release 4.2 January 2007 Contents Page Starting the System...3 Setting the microscope...6 Configuring the beam path
LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes Brief Operating Manual Release 4.2 January 2007 Contents Page Starting the System...3 Setting the microscope...6 Configuring the beam path
Manual ANGSTROM grid confocal microscope
 Manual ANGSTROM grid confocal microscope Switching on the system 1) Start by switching on the different components of the microscope system. When using live cell imaging also start the temperature and
Manual ANGSTROM grid confocal microscope Switching on the system 1) Start by switching on the different components of the microscope system. When using live cell imaging also start the temperature and
Basic Users Guide for the LSM 510 Confocal Microscope
 Basic Users Guide for the LSM 510 Confocal Microscope Ian Jones and Adam Westmacott Department of Biology & Biochemistry, University of Bath, Bath, UK Updated 01 October 2003 This guide describes the basic
Basic Users Guide for the LSM 510 Confocal Microscope Ian Jones and Adam Westmacott Department of Biology & Biochemistry, University of Bath, Bath, UK Updated 01 October 2003 This guide describes the basic
Confocal Microscope - Radiance. Basic Instructions. (Stefanie Reichelt) The Radiance. Confocal Scanning Microscope. designed and developed by
 Confocal Microscope - Radiance Basic Instructions (Stefanie Reichelt) The Radiance Confocal Scanning Microscope designed and developed by Brad Amos (MRC-LMB) Commercialised by Bio-Rad Inc. (1998 2004)
Confocal Microscope - Radiance Basic Instructions (Stefanie Reichelt) The Radiance Confocal Scanning Microscope designed and developed by Brad Amos (MRC-LMB) Commercialised by Bio-Rad Inc. (1998 2004)
Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b
 Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b User Guide Biomedicum Imaging Unit (BIU) University of Helsinki www.biu.helsinki.fi 11.11.2016 1 GENERAL...1
Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b User Guide Biomedicum Imaging Unit (BIU) University of Helsinki www.biu.helsinki.fi 11.11.2016 1 GENERAL...1
Multi-Photon Training
 Multi-Photon Training Overview This training will take approximately 4 hours. The first 2 hours will be spent learning one-on-one with your trainer, while the following 2 hours will be your opportunity
Multi-Photon Training Overview This training will take approximately 4 hours. The first 2 hours will be spent learning one-on-one with your trainer, while the following 2 hours will be your opportunity
Indiana Center for Biological Microscopy. BioRad MRC 1024 MP Confocal & Multi-Photon Microscope
 Indiana Center for Biological Microscopy BioRad MRC 1024 MP Confocal & Multi-Photon Microscope Microscope and the Attached Accessories A: B: C: D: E: F: G: H: Mercury Lamp Transmission Light Kr/Ar Laser
Indiana Center for Biological Microscopy BioRad MRC 1024 MP Confocal & Multi-Photon Microscope Microscope and the Attached Accessories A: B: C: D: E: F: G: H: Mercury Lamp Transmission Light Kr/Ar Laser
Zeiss LSM 510 Meta inverted confocal manual
 Power Up Zeiss LSM 510 Meta inverted confocal manual *adapted from the Duke University Light Microscopy Core Facility manual 1. Turn on the mercury lamp (if required) and sign in the log book, recording
Power Up Zeiss LSM 510 Meta inverted confocal manual *adapted from the Duke University Light Microscopy Core Facility manual 1. Turn on the mercury lamp (if required) and sign in the log book, recording
Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy
 Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy 7 May 2007 Michael Hooker Microscopy Facility Michael Chua microscopy@unc.edu 843-3268 6007 Thurston Bowles Wendy Salmon wendy_salmon@med.unc.edu
Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy 7 May 2007 Michael Hooker Microscopy Facility Michael Chua microscopy@unc.edu 843-3268 6007 Thurston Bowles Wendy Salmon wendy_salmon@med.unc.edu
Nikon A1-B Confocal Operating Manual. Start-up. Microscope
 Nikon A1-B Confocal Operating Manual Start-up 1. Turn on Excite 120 LED power supply. a. No need for cool down as for mercury bulb b. Open shutter by pushing down contol knob. c. Adjust intensity by turning
Nikon A1-B Confocal Operating Manual Start-up 1. Turn on Excite 120 LED power supply. a. No need for cool down as for mercury bulb b. Open shutter by pushing down contol knob. c. Adjust intensity by turning
PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual
 PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual -Turn on the mercury lamp if you need to see the fluorescence through the eye pieces. Check that its shutter is on the open
PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual -Turn on the mercury lamp if you need to see the fluorescence through the eye pieces. Check that its shutter is on the open
Short instructions. Olympus FV1000
 Olympus FV1000-1- Short instructions Short instructions. Olympus FV1000 Version 140416 This short instruction will guide you through the turn on and off sequence of the microscope system. For a detailed
Olympus FV1000-1- Short instructions Short instructions. Olympus FV1000 Version 140416 This short instruction will guide you through the turn on and off sequence of the microscope system. For a detailed
Start-up system. Nikon Wide Field Microscope Ee1078. Complete manual
 Start-up system Switch on the power socket located on the floor Switch devices on in the order of: o Mercury lamp control-unit switch on and ignite o Halogen lamp o Incubator control-box o Microscope stand
Start-up system Switch on the power socket located on the floor Switch devices on in the order of: o Mercury lamp control-unit switch on and ignite o Halogen lamp o Incubator control-box o Microscope stand
Spinning Disk Protocol
 Spinning Disk Protocol 1) System Startup F Please note our sign-up policy You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time
Spinning Disk Protocol 1) System Startup F Please note our sign-up policy You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time
Samples Carolina sample slides (pollen, algae, ). Clean off oil with lens paper then OpticPad around lens (metal not glass) when done.
 Bi/BE 227 Winter 2018 Assignment #1 Widefield and confocal laser scanning microscopy Schedule: Jan 3: Lecture Jan 3-12: Students get trained on how to use scopes, start on assignment Jan 3-17: Carrying
Bi/BE 227 Winter 2018 Assignment #1 Widefield and confocal laser scanning microscopy Schedule: Jan 3: Lecture Jan 3-12: Students get trained on how to use scopes, start on assignment Jan 3-17: Carrying
Olympus DeltaVision Microscope Start-Up and Shut-Down Instructions.
 DeltaVision Instructions: 1. Start-up (Olympus DV) 2. Basic operation (Olympus DV) 2.1 set-up 2.2 designing and running an experiment 3. Transferring files via FTP 4. Deconvolving files 5. File conversion
DeltaVision Instructions: 1. Start-up (Olympus DV) 2. Basic operation (Olympus DV) 2.1 set-up 2.2 designing and running an experiment 3. Transferring files via FTP 4. Deconvolving files 5. File conversion
The Paper Project Guide to Confocal Imaging at Home & in the Classroom
 The Paper Project Guide to Confocal Imaging at Home & in the Classroom http://lifesciences.asu.edu/paperproject The Paper Project Guide to Confocal Imaging at Home & in the Classroom CJ Kazilek & Dennis
The Paper Project Guide to Confocal Imaging at Home & in the Classroom http://lifesciences.asu.edu/paperproject The Paper Project Guide to Confocal Imaging at Home & in the Classroom CJ Kazilek & Dennis
Confocal Application Notes April 2010
 April 2010 Motorized Stage Applications Mark & Find Prepared by Louise Bertrand Applications and Technical Support Group, Leica Microsystems, Inc In this issue of Leica Confocal Application Notes, we describe
April 2010 Motorized Stage Applications Mark & Find Prepared by Louise Bertrand Applications and Technical Support Group, Leica Microsystems, Inc In this issue of Leica Confocal Application Notes, we describe
DSU Start-Up instructions
 DSU Start-Up instructions Always: - start with the 10x objective - properly center the stage around the current objective before changing to another objective - when done, leave the 10x objective in standby
DSU Start-Up instructions Always: - start with the 10x objective - properly center the stage around the current objective before changing to another objective - when done, leave the 10x objective in standby
COLOCALISATION. Alexia Ferrand. Imaging Core Facility Biozentrum Basel
 COLOCALISATION Alexia Ferrand Imaging Core Facility Biozentrum Basel OUTLINE Introduction How to best prepare your samples for colocalisation How to acquire the images for colocalisation How to analyse
COLOCALISATION Alexia Ferrand Imaging Core Facility Biozentrum Basel OUTLINE Introduction How to best prepare your samples for colocalisation How to acquire the images for colocalisation How to analyse
COLOCALISATION. Alexia Loynton-Ferrand. Imaging Core Facility Biozentrum Basel
 COLOCALISATION Alexia Loynton-Ferrand Imaging Core Facility Biozentrum Basel OUTLINE Introduction How to best prepare your samples for colocalisation How to acquire the images for colocalisation How to
COLOCALISATION Alexia Loynton-Ferrand Imaging Core Facility Biozentrum Basel OUTLINE Introduction How to best prepare your samples for colocalisation How to acquire the images for colocalisation How to
LSM 510 Frequently Asked Questions
 LSM 510 Frequently Asked Questions you to the top of this Click on the question below to view the answer - CTRL HOME will bring page! Q: I cannot get the motorized functions of the microscope to operate
LSM 510 Frequently Asked Questions you to the top of this Click on the question below to view the answer - CTRL HOME will bring page! Q: I cannot get the motorized functions of the microscope to operate
Renishaw invia Raman Microscope (April 2006)
 Renishaw invia Raman Microscope (April 2006) I. Starting the System 1. The main system unit is ON all the time. 2. Switch on the Leica microscope and light source for reflective bright field (BF) imaging.
Renishaw invia Raman Microscope (April 2006) I. Starting the System 1. The main system unit is ON all the time. 2. Switch on the Leica microscope and light source for reflective bright field (BF) imaging.
Confocal 6 Guide. Switching On Components of the system should be switched on in the following order: 1. Lasers Argon laser (488nm and 514nm)
 Confocal 6 Guide The system is kept at 37⁰C. If you require the system at a lower temperature book at least one hour before the beginning of your session to allow the system to cool down. It is your responsibility
Confocal 6 Guide The system is kept at 37⁰C. If you require the system at a lower temperature book at least one hour before the beginning of your session to allow the system to cool down. It is your responsibility
XRADIA microxct Manual
 XRADIA microxct Manual Multiscale CT Lab Table of Contents 1. Introduction and Basics 1.1 Instrument Parts 1.2 Powering up the system 1.3 Preparing your sample 2. TXM Controller 2.1 Starting up 2.2 Finding
XRADIA microxct Manual Multiscale CT Lab Table of Contents 1. Introduction and Basics 1.1 Instrument Parts 1.2 Powering up the system 1.3 Preparing your sample 2. TXM Controller 2.1 Starting up 2.2 Finding
Jasco FP-6500 Spectrofluorimeter Updated November 14, 2017
 Jasco FP-6500 Spectrofluorimeter Updated November 14, 2017 Instrument instructions can be found at: http://academic.bowdoin.edu/chemistry/resources/instructions.shtml If you have any problems with the
Jasco FP-6500 Spectrofluorimeter Updated November 14, 2017 Instrument instructions can be found at: http://academic.bowdoin.edu/chemistry/resources/instructions.shtml If you have any problems with the
Wide Guy: Inverted Widefield Microscope
 Wide Guy: Inverted Widefield Microscope Kyle Marchuk Adam Fries Jordan Briscoe August 2017 Contents 1 Introduction 2 2 Initial Setup 3 2.1 Hardware Startup........................................... 3
Wide Guy: Inverted Widefield Microscope Kyle Marchuk Adam Fries Jordan Briscoe August 2017 Contents 1 Introduction 2 2 Initial Setup 3 2.1 Hardware Startup........................................... 3
FACScan 4 and 5 Color Upgrade Rainbow Users Guide
 FACScan 4 and 5 Color Upgrade Rainbow Users Guide For Research Use Only 1 Table of Contents Overview... 3 Hardware Specifications... 3 Rainbow Software... 4 Rainbow Preferences Window... 5 Setting up the
FACScan 4 and 5 Color Upgrade Rainbow Users Guide For Research Use Only 1 Table of Contents Overview... 3 Hardware Specifications... 3 Rainbow Software... 4 Rainbow Preferences Window... 5 Setting up the
Olympus IX-70 Imaging Protocol
 Olympus IX-70 Imaging Protocol 1) System Startup F Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for
Olympus IX-70 Imaging Protocol 1) System Startup F Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for
Olympus IX-70 Imaging Protocol
 Olympus IX-70 Imaging Protocol 1) System Startup Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused
Olympus IX-70 Imaging Protocol 1) System Startup Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused
TN425: A study of fluorescence standards confirms that OptiGrid confocal images are suitable for quantitative microscopy
 TN425: A study of fluorescence standards confirms that OptiGrid confocal images are suitable for quantitative microscopy Introduction The OptiGrid converts the illumination system of a conventional wide
TN425: A study of fluorescence standards confirms that OptiGrid confocal images are suitable for quantitative microscopy Introduction The OptiGrid converts the illumination system of a conventional wide
Olympus BX51 on Aura. Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope
 Olympus BX51 on Aura Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope Contents Start-up Preparing for Imaging Part I General Part II Transmitted Brightfield Part III Transmitted
Olympus BX51 on Aura Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope Contents Start-up Preparing for Imaging Part I General Part II Transmitted Brightfield Part III Transmitted
Prism Starter Guide 1.0 Hoskins Lab Last Modified 03/14/2017 Chris DeCiantis
 Start Up: Upon entering the laser room turn on the wall mounted Laser Power Button by pulling it away from the wall. Turn on Shutter controllers (toggle switch on back of unit). There should be a U in
Start Up: Upon entering the laser room turn on the wall mounted Laser Power Button by pulling it away from the wall. Turn on Shutter controllers (toggle switch on back of unit). There should be a U in
ConfoCor 2 Fluorescence Correlation Microscope
 ConfoCor 2 Fluorescence Correlation Microscope Operating Manual Release 3.2 INTRODUCTION Carl Zeiss ConfoCor 2 Knowledge of this manual is required for the operation of the instrument. Would you therefore
ConfoCor 2 Fluorescence Correlation Microscope Operating Manual Release 3.2 INTRODUCTION Carl Zeiss ConfoCor 2 Knowledge of this manual is required for the operation of the instrument. Would you therefore
Table of Contents. Important Information... 4 Product Description... 4 Computer Requirements Windows Based PCs Mac OS X Based PCs...
 Table of Contents Important Information... 4 Product Description... 4 Computer Requirements... 5 Windows Based PCs... 5 Mac OS X Based PCs... 5 Package Contents... 6 Product Overview... 7 Product Specifications...
Table of Contents Important Information... 4 Product Description... 4 Computer Requirements... 5 Windows Based PCs... 5 Mac OS X Based PCs... 5 Package Contents... 6 Product Overview... 7 Product Specifications...
Zeiss Z.1 Lightsheet. Quick-Start Guide
 Zeiss Z.1 Lightsheet Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 Chamber and Sample preparation Part 2 Image Acquisition Shut-down Start-up: Turn on the Microscope / Computers Turn
Zeiss Z.1 Lightsheet Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 Chamber and Sample preparation Part 2 Image Acquisition Shut-down Start-up: Turn on the Microscope / Computers Turn
User Guide for Preferences of PROGRES GRYPHAX software
 User Guide for Preferences of PROGRES GRYPHAX software General description: PROGRES GRYPHAX software is a modular and platform independent software for state of the art microscopy. It contains camera control
User Guide for Preferences of PROGRES GRYPHAX software General description: PROGRES GRYPHAX software is a modular and platform independent software for state of the art microscopy. It contains camera control
SPOT 5.3 BASIC Software
 SPOT 5.3 BASIC Software Imaging Software for Microscopy and Macro-Photography IF YOU ALSO PURCHASED SPOT ADVANCED SOFTWARE OR ANY SPOT ADVANCED SOFTWARE MODULE: This guide and the activation codes below
SPOT 5.3 BASIC Software Imaging Software for Microscopy and Macro-Photography IF YOU ALSO PURCHASED SPOT ADVANCED SOFTWARE OR ANY SPOT ADVANCED SOFTWARE MODULE: This guide and the activation codes below
Zeiss AxioImager.Z2 Fluorescence Protocol
 Zeiss AxioImager.Z2 Fluorescence Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
Zeiss AxioImager.Z2 Fluorescence Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
with an incubator for study of live
 Light microscopy Support medium is glass. Choose this route for confocal imaging. Do you want to quickly check your cells for green, red or blue fluorescence or using phase contrast? (If so, refer to microscope
Light microscopy Support medium is glass. Choose this route for confocal imaging. Do you want to quickly check your cells for green, red or blue fluorescence or using phase contrast? (If so, refer to microscope
Virtual Frap User Guide
 Virtual Frap User Guide http://wiki.vcell.uchc.edu/twiki/bin/view/vcell/vfrap Center for Cell Analysis and Modeling University of Connecticut Health Center 2010-1 - 1 Introduction Flourescence Photobleaching
Virtual Frap User Guide http://wiki.vcell.uchc.edu/twiki/bin/view/vcell/vfrap Center for Cell Analysis and Modeling University of Connecticut Health Center 2010-1 - 1 Introduction Flourescence Photobleaching
Leica TCS STED. The Fast Track to Superresolution Technical Documentation
 Leica TCS STED The Fast Track to Superresolution Technical Documentation 8 9 6 7 Inverted research microscope Leica DMI6000 CS Scan head Laser and power supply Computer table Air damped optical table 6
Leica TCS STED The Fast Track to Superresolution Technical Documentation 8 9 6 7 Inverted research microscope Leica DMI6000 CS Scan head Laser and power supply Computer table Air damped optical table 6
Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL
 Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL 1 TABLE OF CONTENTS ANATOMY OF THE ZEISS AXIOIMAGE MICROSCOPE 3 INITIALISATION PROCEDURE 4 LEFT SIDE OF MICROSCOPE (DETAILED VIEW)
Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL 1 TABLE OF CONTENTS ANATOMY OF THE ZEISS AXIOIMAGE MICROSCOPE 3 INITIALISATION PROCEDURE 4 LEFT SIDE OF MICROSCOPE (DETAILED VIEW)
Leica TCS SPE. Spectacular Imaging! Technical Documentation
 Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve A Reliable System Affordable Excellence The high resolution spectral confocal Leica TCS SPE
Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve A Reliable System Affordable Excellence The high resolution spectral confocal Leica TCS SPE
How to scan images and then import them into iphoto (Mac OS X)
 How to scan images and then import them into iphoto (Mac OS X) Using the Cyberview X application as a Stand alone scanning interface. Perform these steps after the software and hardware have been installed
How to scan images and then import them into iphoto (Mac OS X) Using the Cyberview X application as a Stand alone scanning interface. Perform these steps after the software and hardware have been installed
Work Instructions for Confocal Laser Scanning Microscopy
 Contents 0.1 To be Verified Before Switch ON......................... 2 0.2 Switch ON Protocol-Single Photon Mode Confocal Laser Scanning Microscope 2 0.3 Switch ON Protocol - Multi Photon Mode Confocal
Contents 0.1 To be Verified Before Switch ON......................... 2 0.2 Switch ON Protocol-Single Photon Mode Confocal Laser Scanning Microscope 2 0.3 Switch ON Protocol - Multi Photon Mode Confocal
OIC. User Guide to Confocal Microscope: Zeiss LSM Pascal
 User Guide to Confocal Microscope: Zeiss LSM Pascal This guide does not cover all the details in documentation that comes with the Zeiss micro-scope. Please do find time to go through the original documentation
User Guide to Confocal Microscope: Zeiss LSM Pascal This guide does not cover all the details in documentation that comes with the Zeiss micro-scope. Please do find time to go through the original documentation
User s Guide to the LMD Laser Micro-dissection. System
 User s Guide to the LMD-6000 Laser Micro-dissection System Page 1 Glen MacDonald October 31, 2018 Start-up Procedure for Leica LMD-6000. 1. Turn on mercury lamp by pressing the rocker switch; a. beige
User s Guide to the LMD-6000 Laser Micro-dissection System Page 1 Glen MacDonald October 31, 2018 Start-up Procedure for Leica LMD-6000. 1. Turn on mercury lamp by pressing the rocker switch; a. beige
How To Title: Scan and File into ICS Purpose: To scan and file information into the patient s medical record Audience: All Staff Date: June 21, 2013
 There are two ways to import medical records into ICS: A. Scan paper document(s) utilizing a scanner B. Import documents from a file, folder or Right Fax Acquiring images through a scanner: 1. Check that
There are two ways to import medical records into ICS: A. Scan paper document(s) utilizing a scanner B. Import documents from a file, folder or Right Fax Acquiring images through a scanner: 1. Check that
IMAGE STUDIO LITE. Tutorial Guide Featuring Image Studio Analysis Software Version 3.1
 IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
Multi Time Series Rev
 Multi Time Series Rev. 4.0.12 The Multi Time Series program is designed to provide flexible programming of automated time dependent experiments. The basic programming unit is a single Time Series Block
Multi Time Series Rev. 4.0.12 The Multi Time Series program is designed to provide flexible programming of automated time dependent experiments. The basic programming unit is a single Time Series Block
MiBio Reference Manual
 MiBio Reference Manual www.microtek.com Copyright 2012 by Microtek International, Inc. All rights reserved. Trademarks Microtek, ScanMaker, ArtixScan, ScanWizard and ColoRescue are trademarks or registered
MiBio Reference Manual www.microtek.com Copyright 2012 by Microtek International, Inc. All rights reserved. Trademarks Microtek, ScanMaker, ArtixScan, ScanWizard and ColoRescue are trademarks or registered
Last class. This class. Single molecule imaging Deconvolution. FLIM Confocal
 FLIM, Confocal Last class Single molecule imaging Deconvolution This class FLIM Confocal FLIM Fluorescence Lifetime IMaging FLIM Fluorescence lifetime imaging Easier and more accurate quantitation Much
FLIM, Confocal Last class Single molecule imaging Deconvolution This class FLIM Confocal FLIM Fluorescence Lifetime IMaging FLIM Fluorescence lifetime imaging Easier and more accurate quantitation Much
Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System
 Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System Use Protocols: A. Reservation and Starting Up B. Sample Observation (No Image Capture) Transmitted
Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System Use Protocols: A. Reservation and Starting Up B. Sample Observation (No Image Capture) Transmitted
Renderize Live Overview
 Renderize Live Overview The Renderize Live interface is designed to offer a comfortable, intuitive environment in which an operator can create projects. A project is a savable work session that contains
Renderize Live Overview The Renderize Live interface is designed to offer a comfortable, intuitive environment in which an operator can create projects. A project is a savable work session that contains
Fluorescence Microscope with Spinning Disk Confocal and Total Internal Reflection Fluorescence Modules
 Open Tender Notification for the procurement of Fluorescence Microscope with Spinning Disk Confocal and Total Internal Reflection Fluorescence Modules at the Indian Institute of Science, Bangalore (Last
Open Tender Notification for the procurement of Fluorescence Microscope with Spinning Disk Confocal and Total Internal Reflection Fluorescence Modules at the Indian Institute of Science, Bangalore (Last
Confocal vs. Deconvolution
 Confocal vs. Deconvolution Cesare Covino ALEMBIC Advanced Light and Electron Bio-Imaging Center Istituto Scientifico San Raffaele (Milano) www.hsr.it/research/alembic Fluorescence high contrast high sensibility
Confocal vs. Deconvolution Cesare Covino ALEMBIC Advanced Light and Electron Bio-Imaging Center Istituto Scientifico San Raffaele (Milano) www.hsr.it/research/alembic Fluorescence high contrast high sensibility
Model FP-6500 Spectrofluorometer Instruction Manual. FP-6500 for Windows
 Model FP-6500 Spectrofluorometer Instruction Manual FP-6500 for Windows P/N: 0302-9999 April 2000 Contents Safety Considerations...i Regulatory Statements... iii Preface... iv Installation Conditions...v
Model FP-6500 Spectrofluorometer Instruction Manual FP-6500 for Windows P/N: 0302-9999 April 2000 Contents Safety Considerations...i Regulatory Statements... iii Preface... iv Installation Conditions...v
User Operation Manual
 User Operation Manual Manual Version: 1.3 For Single Cell Bioanalyzer (SCB-402) Doc# 102-SCB-402-1.3 Table of Contents 1. General Information... 1 1.1 How to Use This Manual... 1 1.2 Operation Safety...
User Operation Manual Manual Version: 1.3 For Single Cell Bioanalyzer (SCB-402) Doc# 102-SCB-402-1.3 Table of Contents 1. General Information... 1 1.1 How to Use This Manual... 1 1.2 Operation Safety...
Operating Procedure for Horiba Raman Microscope
 Operating Procedure for Horiba Raman Microscope SAFETY Be aware of Laser radiation at all times! Do not remove the covers of the instrument. Components are supplied with 110V electric source. Do not touch
Operating Procedure for Horiba Raman Microscope SAFETY Be aware of Laser radiation at all times! Do not remove the covers of the instrument. Components are supplied with 110V electric source. Do not touch
LEXT 3D Measuring LASER Microscope
 LEXT 3D Measuring LASER Microscope Warning: This instrument may only be operated by those who have been trained by AAF staff and have read and signed the AAF laboratory policies. A) STARTUP 1. Computer
LEXT 3D Measuring LASER Microscope Warning: This instrument may only be operated by those who have been trained by AAF staff and have read and signed the AAF laboratory policies. A) STARTUP 1. Computer
Leica TCS STED CW. The Fast Track to Superresolution. Leica TCS STED CW
 The Fast Track to Superresolution content Motivation Concept Realisation Applications - why do researchers need the TCS STED CW? - what is the TCS STED CW based on? - how does the TCS STED CW work? - what
The Fast Track to Superresolution content Motivation Concept Realisation Applications - why do researchers need the TCS STED CW? - what is the TCS STED CW based on? - how does the TCS STED CW work? - what
FACSLSRFortessa SORP QUICK REFERENCE GUIDE
 FACSLSRFortessa SORP QUICK REFERENCE GUIDE INSTRUMENT: 1. The computer is left on at all times. Note: If not Username: Administrator Password: BDIS 2. Unlock the screen with your PPMS account (UTSW username
FACSLSRFortessa SORP QUICK REFERENCE GUIDE INSTRUMENT: 1. The computer is left on at all times. Note: If not Username: Administrator Password: BDIS 2. Unlock the screen with your PPMS account (UTSW username
UHP-M Ultra-High Power UV-Visible LED Light Source Fluorescence Microscopy Ver. 07
 Introduction UHP-M Ultra-High Power UV-Visible LED Light Source Fluorescence Microscopy Ver. 07 Prizmatix UHP-M Dual-LED light engine is designed to substitute the Metal-Halide and Mercury lamps in routine
Introduction UHP-M Ultra-High Power UV-Visible LED Light Source Fluorescence Microscopy Ver. 07 Prizmatix UHP-M Dual-LED light engine is designed to substitute the Metal-Halide and Mercury lamps in routine
Colocalization in Confocal Microscopy. Quantitative Colocalization Analysis
 Quantitative Colocalization Analysis 1 Quantitative Colocalization Analysis Colocalization of fluorescently labeled molecules (immunostainings, fluorescent proteins, etc.) is often used as the first indicator
Quantitative Colocalization Analysis 1 Quantitative Colocalization Analysis Colocalization of fluorescently labeled molecules (immunostainings, fluorescent proteins, etc.) is often used as the first indicator
Computer Instructions
 Mary Washington Elder Study Computer Instructions October 2, 2014 Alan Zirkle Alan Zirkle (540) 373-6448 h (540) 845-6030 c az@azirkle.com Getting Help UMW Audio Visual Technician Joshua Jones has been
Mary Washington Elder Study Computer Instructions October 2, 2014 Alan Zirkle Alan Zirkle (540) 373-6448 h (540) 845-6030 c az@azirkle.com Getting Help UMW Audio Visual Technician Joshua Jones has been
TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ)
 TISSUEFAXS CONFOCAL TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ) TissueFAXS Confocal provides a unique combination of digital slide scanning and laser
TISSUEFAXS CONFOCAL TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ) TissueFAXS Confocal provides a unique combination of digital slide scanning and laser
fmri/dti analysis using Dynasuite
 fmri/dti analysis using Dynasuite Contents 1 Logging in 2 Finding patient session 3 Viewing and adjusting images 4 Checking brain segmentation 5 Checking image registration 6 Seeing fmri results 7 Saving
fmri/dti analysis using Dynasuite Contents 1 Logging in 2 Finding patient session 3 Viewing and adjusting images 4 Checking brain segmentation 5 Checking image registration 6 Seeing fmri results 7 Saving
UICapture Macintosh Training Session
 UICapture Macintosh Training Session Today s Session 1. UICapture Overview (PowerPoint) 2. Exercise (Hands On) 3. Individual Work Time Before You Begin 1. (For individuals working on their own) If you
UICapture Macintosh Training Session Today s Session 1. UICapture Overview (PowerPoint) 2. Exercise (Hands On) 3. Individual Work Time Before You Begin 1. (For individuals working on their own) If you
BA310 Epi-LED FL. New innovative Fluorescence microscope
 BA310 Epi-LED FL New innovative Fluorescence microscope BA BA310 Epi-LED FL New innovative Fluorescence microscope BA310 Epi-LED FL is designed specifically for the daily routine work in the demanding
BA310 Epi-LED FL New innovative Fluorescence microscope BA BA310 Epi-LED FL New innovative Fluorescence microscope BA310 Epi-LED FL is designed specifically for the daily routine work in the demanding
IMAGING PLATFORMS IN THE FACULTY OF MEDICINE. Guo Jing Lab Manager Faculty Core Facilty June
 IMAGING PLATFORMS IN THE FACULTY OF MEDICINE Guo Jing Lab Manager Faculty Core Facilty June 27 2011 http://www.med.hku.hk/corefac/ Mission Training and education Basic operation Advanced application Imaging
IMAGING PLATFORMS IN THE FACULTY OF MEDICINE Guo Jing Lab Manager Faculty Core Facilty June 27 2011 http://www.med.hku.hk/corefac/ Mission Training and education Basic operation Advanced application Imaging
Visitron Spinning Disk Unit 1
 Startup Procedure and Notes (Last update: Sept. 2014) 6 4 2 5 1 3 7 8 9 1. Switch on the fluorescence illumination for widefield viewing (HXP lamp) 2. Switch on power-bar on the left side of the shelf
Startup Procedure and Notes (Last update: Sept. 2014) 6 4 2 5 1 3 7 8 9 1. Switch on the fluorescence illumination for widefield viewing (HXP lamp) 2. Switch on power-bar on the left side of the shelf
UNWRAP User Document. Figure 1. Workflow of the UNWRAP application.
 UNWRAP User Document This MATLAB application takes a set of confocal microscope z-stack images of fluorescently labeled cells in a 3D cylindrical geometry and creates an unwrapped 2D image from the tube
UNWRAP User Document This MATLAB application takes a set of confocal microscope z-stack images of fluorescently labeled cells in a 3D cylindrical geometry and creates an unwrapped 2D image from the tube
Creators Basic Guide to Using UICapture (Mac)
 Creators Basic Guide to Using UICapture (Mac) Download the software from http://helpdesk.its.uiowa.edu/software/signin.htm After downloading the software and setting up an account with your local IT support,
Creators Basic Guide to Using UICapture (Mac) Download the software from http://helpdesk.its.uiowa.edu/software/signin.htm After downloading the software and setting up an account with your local IT support,
UNC Flow Cytometry Core Facility LSR II Operation 2018
 Reference: Startup BD FACSDiva 6 Online Course BD Training & elearning 1. Check Sheath and Waste containers: Empty Waste container in the sink Add bleach to the black mark ( ~10% final concentration).
Reference: Startup BD FACSDiva 6 Online Course BD Training & elearning 1. Check Sheath and Waste containers: Empty Waste container in the sink Add bleach to the black mark ( ~10% final concentration).
Lab 5 Microscopy. Introduction. Carrying the Microscope. Depth of Focus. Parts of the Compound Microscope. Magnification
 Lab 5 Microscopy Introduction The microscope is an instrument that contains one or more lenses and is used to view objects that are too small be seen with the unaided eye. A magnifying glass is a simple
Lab 5 Microscopy Introduction The microscope is an instrument that contains one or more lenses and is used to view objects that are too small be seen with the unaided eye. A magnifying glass is a simple
Leica TCS SPE. Spectacular Imaging! Technical Documentation
 Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve Built-in Reliability Affordable Excellence The new high resolution spectral confocal Leica TCS
Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve Built-in Reliability Affordable Excellence The new high resolution spectral confocal Leica TCS
Verity Central Quick Reference Manual. Document ID A04
 Verity Central Quick Reference Manual Document ID 6620-003-A04 Welcome to Verity Central. This Quick Reference Manual is intended to be used in tandem with the Verity Central Technical Reference Manual,
Verity Central Quick Reference Manual Document ID 6620-003-A04 Welcome to Verity Central. This Quick Reference Manual is intended to be used in tandem with the Verity Central Technical Reference Manual,
Leica 3D Disto PROJECTOR in stair case
 Leica 3D Disto PROJECTOR in stair case Workflow description: Measurement Stair Design Projection of fixing points Preparing the measurement 1 mark 1 point on each wall of the stair case Measurement of
Leica 3D Disto PROJECTOR in stair case Workflow description: Measurement Stair Design Projection of fixing points Preparing the measurement 1 mark 1 point on each wall of the stair case Measurement of
DOC-103-D idirect User Manual SOFTWARE VERSION 1.10
 idirect User Manual SOFTWARE VERSION 1.10 1.0 Introduction 4.0 idirect Overview 1.1 Contact RCL P.1 1.2 ipad Resources P.1 1.3 Minimum Requirements P.1 1.4 Required Equipment P.2 1.5 Spotlight Limits P.2
idirect User Manual SOFTWARE VERSION 1.10 1.0 Introduction 4.0 idirect Overview 1.1 Contact RCL P.1 1.2 ipad Resources P.1 1.3 Minimum Requirements P.1 1.4 Required Equipment P.2 1.5 Spotlight Limits P.2
Cadrage Director s Viewfinder USER MANUAL
 For ios & Android User Manual February 2018 Current App Versions: ios: 3.0.1 Android: 4.0 www.cadrage.at Table of contents GET STARTED QUICKLY 4 BASIC FUNCTIONALITY 6 Introduction 6 The Main View 6 Prime
For ios & Android User Manual February 2018 Current App Versions: ios: 3.0.1 Android: 4.0 www.cadrage.at Table of contents GET STARTED QUICKLY 4 BASIC FUNCTIONALITY 6 Introduction 6 The Main View 6 Prime
The MetroloJ plugin. Fabrice P. Cordelières, Cédric Matthews
 The MetroloJ plugin Fabrice P. Cordelières, Cédric Matthews Beta version, April 20, 2009 Contents 1 How to install the plugin? 3 2 Generate PSF report 4 2.1 How to generate the images?.........................
The MetroloJ plugin Fabrice P. Cordelières, Cédric Matthews Beta version, April 20, 2009 Contents 1 How to install the plugin? 3 2 Generate PSF report 4 2.1 How to generate the images?.........................
Setup protocol for Firefly particles. On BD LSR II and LSRFortessa cytometers with single tube loader function
 Setup protocol for Firefly particles On BD LSR II and LSRFortessa cytometers with single tube loader function Version 1 Last Updated May 2016 Setup protocol for Firefly particles Contents Introduction
Setup protocol for Firefly particles On BD LSR II and LSRFortessa cytometers with single tube loader function Version 1 Last Updated May 2016 Setup protocol for Firefly particles Contents Introduction
Publishing Electronic Portfolios using Adobe Acrobat 5.0
 Step-by-Step Publishing Electronic Portfolios using Adobe Acrobat 5.0 2002, Helen C. Barrett Here is the process we will use to publish a digital portfolio using Adobe Acrobat. The portfolio will include
Step-by-Step Publishing Electronic Portfolios using Adobe Acrobat 5.0 2002, Helen C. Barrett Here is the process we will use to publish a digital portfolio using Adobe Acrobat. The portfolio will include
Science is hard. Flow cytometry should be easy.
 Science is hard. Flow cytometry should be easy. CFlow User Guide TABLE OF CONTENTS 1 INTRODUCTION TO CFLOW... 1 1.1 Installing CFlow... 1 1.2 Starting CFlow... 1 1.3 CFlow Workspace... 2 1.4 Opening a
Science is hard. Flow cytometry should be easy. CFlow User Guide TABLE OF CONTENTS 1 INTRODUCTION TO CFLOW... 1 1.1 Installing CFlow... 1 1.2 Starting CFlow... 1 1.3 CFlow Workspace... 2 1.4 Opening a
NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes
 NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes bh FLIM systems for the confocal and the multiphoton versions of the Leica SP2 and SP5 microscopes are available since 2002 [4]. These
NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes bh FLIM systems for the confocal and the multiphoton versions of the Leica SP2 and SP5 microscopes are available since 2002 [4]. These
CVI SPECTRAL PRODUCTS. 111 Highland Drive Putnam CT, (860) SM32Pro
 CVI SPECTRAL PRODUCTS 111 Highland Drive Putnam CT, 06260 (860) 928-5834 SM32Pro 2.8.28 Table of Contents Warranty and Liability 1 Quick Start Installation Guide 2 System Requirements 3 Requirements for
CVI SPECTRAL PRODUCTS 111 Highland Drive Putnam CT, 06260 (860) 928-5834 SM32Pro 2.8.28 Table of Contents Warranty and Liability 1 Quick Start Installation Guide 2 System Requirements 3 Requirements for
Volocity ver (2013) Standard Operation Protocol
 Faculty Core Facility Volocity 6.3.0 (2013) SOP A-1 Volocity ver. 6.3.0 (2013) Standard Operation Protocol Faculty Core Facility Volocity 6.3.0 (2013) SOP A-2 A. Content Overview. 3 Start up. 3 Change
Faculty Core Facility Volocity 6.3.0 (2013) SOP A-1 Volocity ver. 6.3.0 (2013) Standard Operation Protocol Faculty Core Facility Volocity 6.3.0 (2013) SOP A-2 A. Content Overview. 3 Start up. 3 Change
Automation Engine. Getting Started
 Getting Started 05-2017 Contents 1. Installing Server and Clients... 4 2. Changing the Language used in the Pilot... 5 3. Starting or Updating the Pilot... 6 4. The Pilot's Main Window... 7 5. Concepts
Getting Started 05-2017 Contents 1. Installing Server and Clients... 4 2. Changing the Language used in the Pilot... 5 3. Starting or Updating the Pilot... 6 4. The Pilot's Main Window... 7 5. Concepts
CCD Image Acquisition Tutorial
 CCD Image Acquisition Tutorial Gatan, Inc. 5933 Coronado Lane, Pleasanton, CA 94588 Tel: (925) 463-0200 Fax: (925) 463-0204 May 2001 1. Introduction This document will guide users of Gatan CCD camera through
CCD Image Acquisition Tutorial Gatan, Inc. 5933 Coronado Lane, Pleasanton, CA 94588 Tel: (925) 463-0200 Fax: (925) 463-0204 May 2001 1. Introduction This document will guide users of Gatan CCD camera through
You might think of Windows XP as a set of cool accessories, such as
 Controlling Applications under Windows You might think of Windows XP as a set of cool accessories, such as games, a calculator, and an address book, but Windows is first and foremost an operating system.
Controlling Applications under Windows You might think of Windows XP as a set of cool accessories, such as games, a calculator, and an address book, but Windows is first and foremost an operating system.
Prizmatix. Optical Specifications. Mic-LED-635 spectrum. BLCC-02 Benchtop LED Current Controller Specifications
 Mic-LED-635 High Power Collimated Red LED Light Source for Fluorescence Microscopy Featuring Prizmatix Modular Design for Multi-Wavelength and Fiberoptic Setup Ver. 01 Introduction The compact Mic-LED-635,
Mic-LED-635 High Power Collimated Red LED Light Source for Fluorescence Microscopy Featuring Prizmatix Modular Design for Multi-Wavelength and Fiberoptic Setup Ver. 01 Introduction The compact Mic-LED-635,
Setting up Micro-Magellan for Device control
 Setting up Micro-Magellan for Device control From the plug-in menu, select Micro-Magellan and the unpopulated window opens Select Configure device control The window below will open, select devices to
Setting up Micro-Magellan for Device control From the plug-in menu, select Micro-Magellan and the unpopulated window opens Select Configure device control The window below will open, select devices to
OPTIKA. fluo Series Upright and inverted epi-fluorescence microscopes
 OPTIKA M I C R O S C O P E S I T A L Y Upright and inverted epi-fluorescence microscopes FLUO B-383LD1 / B-383LD2 / B-383FL / B-500TiFL / XDS-2FL / XDS-3FL / XDS-3FL4 / SZP-FLUO / B-1000FL-LED / B-1000FL-HBO
OPTIKA M I C R O S C O P E S I T A L Y Upright and inverted epi-fluorescence microscopes FLUO B-383LD1 / B-383LD2 / B-383FL / B-500TiFL / XDS-2FL / XDS-3FL / XDS-3FL4 / SZP-FLUO / B-1000FL-LED / B-1000FL-HBO
UHP-M Ultra-High Power UV-Visible LED Light Source Fluorescence Microscopy Ver. 04
 Introduction UHP-M Ultra-High Power UV-Visible LED Light Source Fluorescence Microscopy Ver. 04 Prizmatix UHP-M Dual-LED light engine is designed to substitute the Metal-Halide and Mercury lamps in routine
Introduction UHP-M Ultra-High Power UV-Visible LED Light Source Fluorescence Microscopy Ver. 04 Prizmatix UHP-M Dual-LED light engine is designed to substitute the Metal-Halide and Mercury lamps in routine
LASER SHOW SYSTEM USER MANUAL
 LASER SHOW SYSTEM BI-214 GREEN LASER USER MANUAL 1. Unpacking Thanks for purchasing our laser. Please read our user manual carefully before use. Keep user manual for future reference and operate according
LASER SHOW SYSTEM BI-214 GREEN LASER USER MANUAL 1. Unpacking Thanks for purchasing our laser. Please read our user manual carefully before use. Keep user manual for future reference and operate according
BD Influx Standard Operation Protocol - Basic Operation
 Optimizing the drop phase BD Influx Standard Operation Protocol - Basic Operation 1. Verify the 2-tube holder with dummy 15ml tube is in place on the sort stage 2. Select 2 tube holder 2 way sort as the
Optimizing the drop phase BD Influx Standard Operation Protocol - Basic Operation 1. Verify the 2-tube holder with dummy 15ml tube is in place on the sort stage 2. Select 2 tube holder 2 way sort as the
A Guide to Processing Photos into 3D Models Using Agisoft PhotoScan
 A Guide to Processing Photos into 3D Models Using Agisoft PhotoScan Samantha T. Porter University of Minnesota, Twin Cities Fall 2015 Index 1) Automatically masking a black background / Importing Images.
A Guide to Processing Photos into 3D Models Using Agisoft PhotoScan Samantha T. Porter University of Minnesota, Twin Cities Fall 2015 Index 1) Automatically masking a black background / Importing Images.
Use stage 16 embryos see Pereanu et al., 2007 for morphological staging criteria. First start Vaa3d (http://vaa3d.org/; Peng et al., 2010).
 Image acquisition. Co- stain with anti- Even- skipped 2B8 (1:50, often this needs to be pre- absorbed several times for optimal staining], or 3C10 (1:50) available at DSHB. Use stage 16 embryos see Pereanu
Image acquisition. Co- stain with anti- Even- skipped 2B8 (1:50, often this needs to be pre- absorbed several times for optimal staining], or 3C10 (1:50) available at DSHB. Use stage 16 embryos see Pereanu
