Basic Users Guide for the LSM 510 Confocal Microscope
|
|
|
- Cecil Berry
- 6 years ago
- Views:
Transcription
1 Basic Users Guide for the LSM 510 Confocal Microscope Ian Jones and Adam Westmacott Department of Biology & Biochemistry, University of Bath, Bath, UK Updated 01 October 2003 This guide describes the basic operation of the confocal microscope for routine immunocyto- and immunohisto-chemistry (single and multiple fluorescent labelling). It is not intended to be definitive users manual and certainly does not cover all techniques, such as line scans and DIC imaging. Further information and advice can be obtained from the Zeiss manual in the confocal room or from experienced confocal users within the department. The confocal has 3 single photon lasers (Ar, HeNe 543 and HeNe 633) which permit the user to select excitation wavelengths of 458, 488, 514, 543, 568 and 633nm. The LSM software provides a wide range of image processing functions, including standard 2D / 3D (stereo, projection) functions, processing of voxels and 3D measurement functions and parameters.
2 Quick Start Guide Action Page 1. Start up the system (if not already on) 3 2. Switch on required lasers 3 3. Set track laser configurations 4 4. Set microscope configuration 5 5. Orientate and focus sample using microscope (with transmitted or fluorescence illumination) 6 6. Set confocal scan configurations and scan image 6 7. Creation of Z-stacks 8 8. Creating a database 9 9. Putting system into standby mode or shutting down 10 2
3 1. Start Up if the system is switched off (if left on, go to 1.h. or 2.a.) a. Turn system on using the control switch (large black button to the left of the microscope marked Remote Control ). This should switch on the microscope and laser control unit. Turn on the PC using button on top right of tower case (on floor under monitors). b. Switch on the air conditioning unit using the infra-red control (the air conditioning must be on if the lasers are in use to avoid overheating). c. Switch on the fluorescent lamp unit (box to the left of the microscope marked 2788 ). d. Check that the transmitted light unit is on (box to the left of the microscope marked SNT 12V 100W) and the dial is turned up to around 10. e. When prompted press Ctrl+Alt+Del to log on to the computer. f. Press Return at the username / password prompt (do not enter any values). g. Double click on the LSM 510 icon to start confocal operating software. The LSM 510 operating mode panel will appear: h. For scanning, click on Scan New Images and Start Expert Mode (the LSM programme will initialise and open the main menu bar). For analysis of existing images, please use the confocal workstation in 0.44 to keep the confocal microscope free for scanning only (will also save money!). 2. Switch on required lasers a. Click on the Acquire button on the main menu bar and then the Laser button in the subfolder: 3
4 This will open the laser control window: There are three lasers currently connected to the confocal microscope: i. Argon Laser = 488nm (used for FITC, CY2, AlexaFluor488, etc) ii. HeNe1 Laser = 543nm (used for rhodamine, Texas Red, CY3, AlexaFluor543, etc) iii. HeNe2 Laser = 633nm (used for CY5, etc) b. To switch on the Argon laser, highlight the laser name in the control window and click the Standby button. The laser will start to warm up and after one minute a Ready message will appear. Now click on the On button and scroll the laser intensity to 75%. The HeNe lasers can be switched on directly by highlighting them individually in the control window and clicking the On button(s). 3. Set track laser configurations a. Choose the required laser path settings (e.g. single FITC or double FITC + Rhodamine) using one of the pre-programmed track configurations: Click on the Config button in the Acquire subfolder on the main menu bar. The configuration control window will appear: 4
5 For single fluorochrome visualisation, click on Single Track, and for multiple fluorochrome visualisation, click on Multi Track. In either case, click on the Config icon on right side of window and choose the appropriate track configuration from the resulting drop-down and finally click Apply : 4. Set microscope configuration a. To set up the microscope, click on Acquire on the main menu bar and then the Micro button in the subfolder. This will open the Axiovert control window: b. Choose the appropriate objective lens by clicking on objective icon and selecting from the resulting drop-down menu (note, there are two 63X lenses, one for oil-immersion and one water-immersion choose the correct one!). It is good practise to lower the objective lens to its bottom position using the focussing wheels on the side of the microscope prior to installing a new sample. To save time, there is a course adjustment button on the left of the microscope by the focussing wheel (small black button). This is an on-off button so must be pressed again to return to fine focussing. 5
6 c. If necessary, place a small drop of oil on the objective lens (make sure that it is an oilimmersion lens first!) then place the slide to be analysed upside-down on the stage and secure in place using the sliding clamps. Align the sample on the slide over the objective lens using stage control wheels on the left of the microscope. 5. Orientate and focus sample using microscope (with transmitted or fluorescence illumination) a. To view specimens directly down the microscope itself, pull out the lower of the two sliders on the right hand face of the microscope (there is a diagram by the sliders indicating slider position for different viewing modes). b. For transmitted light, click the Transmitted light button at the top of the Axiovert control window (see previous page). Switch light on and use the neighbouring scroll bar to vary the light intensity accordingly. Use the focus wheels to raise the objective lens until the specimen is in focus. c. For fluorescence imaging, switch off the transmitted light by clicking the button in the Axiovert control window (if required) and choose the appropriate filter from the Reflector drop down menu (in the same window): i. Fset09 = 488nm (used for FITC, CY2, AlexaFluor488, etc) ii. Fset15 = 543nm (used for used for rhodamine, Texas Red, CY3, AlexaFluor543, etc). (note, fluorochromes excited by the HeNe2 laser (e.g. CY5) cannot be viewed directly down the microscope). 6. Set confocal scan configurations and scan image a. Push in the bottom slider on the right side of the microscope (if not already in) to prepare the microscope for scanning mode. b. To scan an image, click on the Scan button in the Acquire subfolder on the main menu bar. The scan control window will appear: 6
7 For a fast scan, click Fast XY on the right hand side of the scan control window (an image window will appear). To view the individual channels of a multi-track image, click Split XY in the image window (return to a single image by clicking XY ). Ensure that your sample is in focus using the focus wheel and work fast as the fluorescence may bleach with time. c. To optimise the image, click on Channels in the scan control window and click on the relevant channel that you wish to alter (Ch1, Ch2 or Ch3), then click Find on the right hand side of the window. The software will automatically determine the optimal settings for your sample. d. If necessary, further optimise the image by adjusting the scroll bars in the scan control window ( Channels sub-folder): i. Detector gain sets brightness/contrast ii. Ampl. offset adjusts background iii. Ampl. gain alters amplification factor e. Normally there is no need to change the excitation of the track or the pinhole setting as these have been optimised for standard samples. On occasion, however, this may be necessary (e.g. for weakly fluorescent samples) and these parameters can be altered using the scroll bars in the scan control window ( Channels sub-folder). f. For multi-tracking, repeat steps d - f for each of the remaining channels in use. g. To further improve image quality, open the Mode sub-folder in the scan control window (see page 6) and then click on the Frame button. Choose an appropriate frame size (512x512 pixels for quick scan, 1024x1024 pixels for high-quality scan), a low scan speed (5 or 6) and 7
8 the number of averages (1 for quick scan, 4 or 8 for high-quality scan). Magnify, move and rotate the image if required using the buttons at the bottom of the window. The other functions in this window usually do not require altering for a standard frame scan. h. When the image has been optimised, click Stop in the scan control window and then on Single to generate a single image. To store this image in your database, click on the Save As button on the right of the image window (see section 8 for guide to creating a database). The image can be annotated (size bar, etc) using the workstation in 0.44 (or you can download a free basic image manipulation programme from the Zeiss web site and do this on your own PC). i. Images can also be exported as graphics files (JPEG, TIF, etc). For this, select the image to be exported and click Export on the main menu bar. 7. Creation of Z-stacks a. Click on the Z-Stack button in the scan control window (this will open the Z settings panel) and then click on Fast XY. Use the focus wheel to focus on the top of the sample, where the Z-stack is to start, and click on Mark First in the Z settings panel. Now focus on the bottom of the image, where the Z-stack is to end, and click on Mark Last. b. Click on Z-Slice in the Z settings panel to set the optimal interval and pinhole diameter(s) for the stack. If necessary, change the image pixel size and number of averages in the Mode sub-folder, then click on Start to create the stack. 8
9 c. To create a 3D projection of the Z-stack, open the 3D View folder on the main menu bar and click on Projection. In the projection window, select the image to be projected, the first angle, number of projections and difference angle, then click Apply. The projection will appear in a new image window and can be animated by clicking on the appropriate button in the image window. 8. Creating a database a. To create a new database for saving scanned images to, click on File on the main menu bar and then the New button in the subfolder. Databases should preferably be created either on a removable disk (e.g. MO disk) or on an external computer (connected to via network neighbourhood) and not the confocal hard disks. Enter a database name, destination folder and click Create. 9
10 b. To open an existing database, click on File on the main menu bar and then the Open button in the subfolder. 9. Putting system into standby mode or shutting system down a. Make sure that any oil is removed from the oil-immersion objectives using the lens tissue provided. Tidy the workspace around the microscope and fill out the log sheets with your name, confocal usage time, lab number, grant code and any comments. b. If the confocal is booked after you (or within one hour), close all windows except the Main menu bar and Laser subfolder and place the Argon laser in Standby Mode. Do not switch the Argon laser off otherwise then next user must wait 35 minutes before it can be switched back on again. If no-one is booked to use the confocal for at least an hour, switch off the argon laser and the fluorescent and transmitted lamp units (see 1.b. and 1.c.). c. If you are the last user for the day, switch off the lasers, close all windows, turn off the fluorescent and transmitted lamp units and shutdown the computer. After 5 minutes (to allow the lasers to cool down), the system can be switched off using the main remote switch (see 1.a.). Finally, cover the microscope, switch off the air conditioning and lock the room as you leave. 10
OIC. User Guide to Confocal Microscope: Zeiss LSM Pascal
 User Guide to Confocal Microscope: Zeiss LSM Pascal This guide does not cover all the details in documentation that comes with the Zeiss micro-scope. Please do find time to go through the original documentation
User Guide to Confocal Microscope: Zeiss LSM Pascal This guide does not cover all the details in documentation that comes with the Zeiss micro-scope. Please do find time to go through the original documentation
LSM510 Confocal Microscope Standard Operation Protocol Basic Operation
 LSM510 Confocal Microscope Standard Operation Protocol Basic Operation Please make sure that the COMPRESSED AIR has been TURNED ON prior to the use of the equipment. Kindly inform the administrator if
LSM510 Confocal Microscope Standard Operation Protocol Basic Operation Please make sure that the COMPRESSED AIR has been TURNED ON prior to the use of the equipment. Kindly inform the administrator if
LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes
 LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes Brief Operating Manual Release 4.2 January 2007 Contents Page Starting the System...3 Setting the microscope...6 Configuring the beam path
LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes Brief Operating Manual Release 4.2 January 2007 Contents Page Starting the System...3 Setting the microscope...6 Configuring the beam path
Indiana Center for Biological Microscopy. BioRad MRC 1024 MP Confocal & Multi-Photon Microscope
 Indiana Center for Biological Microscopy BioRad MRC 1024 MP Confocal & Multi-Photon Microscope Microscope and the Attached Accessories A: B: C: D: E: F: G: H: Mercury Lamp Transmission Light Kr/Ar Laser
Indiana Center for Biological Microscopy BioRad MRC 1024 MP Confocal & Multi-Photon Microscope Microscope and the Attached Accessories A: B: C: D: E: F: G: H: Mercury Lamp Transmission Light Kr/Ar Laser
1. Getting Started: Brief Step-By-Step Guide (PDF File)
 Page 1 of 5 1. Getting Started: Brief Step-By-Step Guide (PDF File) Leica SP2 confocal microscope is controlled via software LCS (Leica Confocal Software). The latest version is V2.5. Bellowing is a brief
Page 1 of 5 1. Getting Started: Brief Step-By-Step Guide (PDF File) Leica SP2 confocal microscope is controlled via software LCS (Leica Confocal Software). The latest version is V2.5. Bellowing is a brief
Nikon A1-B Confocal Operating Manual. Start-up. Microscope
 Nikon A1-B Confocal Operating Manual Start-up 1. Turn on Excite 120 LED power supply. a. No need for cool down as for mercury bulb b. Open shutter by pushing down contol knob. c. Adjust intensity by turning
Nikon A1-B Confocal Operating Manual Start-up 1. Turn on Excite 120 LED power supply. a. No need for cool down as for mercury bulb b. Open shutter by pushing down contol knob. c. Adjust intensity by turning
Confocal Microscope - Radiance. Basic Instructions. (Stefanie Reichelt) The Radiance. Confocal Scanning Microscope. designed and developed by
 Confocal Microscope - Radiance Basic Instructions (Stefanie Reichelt) The Radiance Confocal Scanning Microscope designed and developed by Brad Amos (MRC-LMB) Commercialised by Bio-Rad Inc. (1998 2004)
Confocal Microscope - Radiance Basic Instructions (Stefanie Reichelt) The Radiance Confocal Scanning Microscope designed and developed by Brad Amos (MRC-LMB) Commercialised by Bio-Rad Inc. (1998 2004)
Zeiss LSM 510 Meta inverted confocal manual
 Power Up Zeiss LSM 510 Meta inverted confocal manual *adapted from the Duke University Light Microscopy Core Facility manual 1. Turn on the mercury lamp (if required) and sign in the log book, recording
Power Up Zeiss LSM 510 Meta inverted confocal manual *adapted from the Duke University Light Microscopy Core Facility manual 1. Turn on the mercury lamp (if required) and sign in the log book, recording
Confocal 6 Guide. Switching On Components of the system should be switched on in the following order: 1. Lasers Argon laser (488nm and 514nm)
 Confocal 6 Guide The system is kept at 37⁰C. If you require the system at a lower temperature book at least one hour before the beginning of your session to allow the system to cool down. It is your responsibility
Confocal 6 Guide The system is kept at 37⁰C. If you require the system at a lower temperature book at least one hour before the beginning of your session to allow the system to cool down. It is your responsibility
Zeiss Elyra Microscope Standard Operation Protocol Basic Operation
 Machine Startup Zeiss Elyra Microscope Standard Operation Protocol Basic Operation 1. Turn on the Incubator power to warm up the incubator for a couple of hours prior to sample acquisition as image could
Machine Startup Zeiss Elyra Microscope Standard Operation Protocol Basic Operation 1. Turn on the Incubator power to warm up the incubator for a couple of hours prior to sample acquisition as image could
LSM 510 Frequently Asked Questions
 LSM 510 Frequently Asked Questions you to the top of this Click on the question below to view the answer - CTRL HOME will bring page! Q: I cannot get the motorized functions of the microscope to operate
LSM 510 Frequently Asked Questions you to the top of this Click on the question below to view the answer - CTRL HOME will bring page! Q: I cannot get the motorized functions of the microscope to operate
Olympus IX-70 Imaging Protocol
 Olympus IX-70 Imaging Protocol 1) System Startup F Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for
Olympus IX-70 Imaging Protocol 1) System Startup F Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for
Olympus IX-70 Imaging Protocol
 Olympus IX-70 Imaging Protocol 1) System Startup Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused
Olympus IX-70 Imaging Protocol 1) System Startup Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused
Multi-Photon Training
 Multi-Photon Training Overview This training will take approximately 4 hours. The first 2 hours will be spent learning one-on-one with your trainer, while the following 2 hours will be your opportunity
Multi-Photon Training Overview This training will take approximately 4 hours. The first 2 hours will be spent learning one-on-one with your trainer, while the following 2 hours will be your opportunity
Samples Carolina sample slides (pollen, algae, ). Clean off oil with lens paper then OpticPad around lens (metal not glass) when done.
 Bi/BE 227 Winter 2018 Assignment #1 Widefield and confocal laser scanning microscopy Schedule: Jan 3: Lecture Jan 3-12: Students get trained on how to use scopes, start on assignment Jan 3-17: Carrying
Bi/BE 227 Winter 2018 Assignment #1 Widefield and confocal laser scanning microscopy Schedule: Jan 3: Lecture Jan 3-12: Students get trained on how to use scopes, start on assignment Jan 3-17: Carrying
Manual ANGSTROM grid confocal microscope
 Manual ANGSTROM grid confocal microscope Switching on the system 1) Start by switching on the different components of the microscope system. When using live cell imaging also start the temperature and
Manual ANGSTROM grid confocal microscope Switching on the system 1) Start by switching on the different components of the microscope system. When using live cell imaging also start the temperature and
PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual
 PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual -Turn on the mercury lamp if you need to see the fluorescence through the eye pieces. Check that its shutter is on the open
PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual -Turn on the mercury lamp if you need to see the fluorescence through the eye pieces. Check that its shutter is on the open
Work Instructions for Confocal Laser Scanning Microscopy
 Contents 0.1 To be Verified Before Switch ON......................... 2 0.2 Switch ON Protocol-Single Photon Mode Confocal Laser Scanning Microscope 2 0.3 Switch ON Protocol - Multi Photon Mode Confocal
Contents 0.1 To be Verified Before Switch ON......................... 2 0.2 Switch ON Protocol-Single Photon Mode Confocal Laser Scanning Microscope 2 0.3 Switch ON Protocol - Multi Photon Mode Confocal
Zeiss AxioImager.Z2 Fluorescence Protocol
 Zeiss AxioImager.Z2 Fluorescence Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
Zeiss AxioImager.Z2 Fluorescence Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
Start-up system. Nikon Wide Field Microscope Ee1078. Complete manual
 Start-up system Switch on the power socket located on the floor Switch devices on in the order of: o Mercury lamp control-unit switch on and ignite o Halogen lamp o Incubator control-box o Microscope stand
Start-up system Switch on the power socket located on the floor Switch devices on in the order of: o Mercury lamp control-unit switch on and ignite o Halogen lamp o Incubator control-box o Microscope stand
DSU Start-Up instructions
 DSU Start-Up instructions Always: - start with the 10x objective - properly center the stage around the current objective before changing to another objective - when done, leave the 10x objective in standby
DSU Start-Up instructions Always: - start with the 10x objective - properly center the stage around the current objective before changing to another objective - when done, leave the 10x objective in standby
Spinning Disk Protocol
 Spinning Disk Protocol 1) System Startup F Please note our sign-up policy You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time
Spinning Disk Protocol 1) System Startup F Please note our sign-up policy You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time
ConfoCor 2 Fluorescence Correlation Microscope
 ConfoCor 2 Fluorescence Correlation Microscope Operating Manual Release 3.2 INTRODUCTION Carl Zeiss ConfoCor 2 Knowledge of this manual is required for the operation of the instrument. Would you therefore
ConfoCor 2 Fluorescence Correlation Microscope Operating Manual Release 3.2 INTRODUCTION Carl Zeiss ConfoCor 2 Knowledge of this manual is required for the operation of the instrument. Would you therefore
IMAGING PLATFORMS IN THE FACULTY OF MEDICINE. Guo Jing Lab Manager Faculty Core Facilty June
 IMAGING PLATFORMS IN THE FACULTY OF MEDICINE Guo Jing Lab Manager Faculty Core Facilty June 27 2011 http://www.med.hku.hk/corefac/ Mission Training and education Basic operation Advanced application Imaging
IMAGING PLATFORMS IN THE FACULTY OF MEDICINE Guo Jing Lab Manager Faculty Core Facilty June 27 2011 http://www.med.hku.hk/corefac/ Mission Training and education Basic operation Advanced application Imaging
Prism Starter Guide 1.0 Hoskins Lab Last Modified 03/14/2017 Chris DeCiantis
 Start Up: Upon entering the laser room turn on the wall mounted Laser Power Button by pulling it away from the wall. Turn on Shutter controllers (toggle switch on back of unit). There should be a U in
Start Up: Upon entering the laser room turn on the wall mounted Laser Power Button by pulling it away from the wall. Turn on Shutter controllers (toggle switch on back of unit). There should be a U in
Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL
 Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL 1 TABLE OF CONTENTS ANATOMY OF THE ZEISS AXIOIMAGE MICROSCOPE 3 INITIALISATION PROCEDURE 4 LEFT SIDE OF MICROSCOPE (DETAILED VIEW)
Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL 1 TABLE OF CONTENTS ANATOMY OF THE ZEISS AXIOIMAGE MICROSCOPE 3 INITIALISATION PROCEDURE 4 LEFT SIDE OF MICROSCOPE (DETAILED VIEW)
The Paper Project Guide to Confocal Imaging at Home & in the Classroom
 The Paper Project Guide to Confocal Imaging at Home & in the Classroom http://lifesciences.asu.edu/paperproject The Paper Project Guide to Confocal Imaging at Home & in the Classroom CJ Kazilek & Dennis
The Paper Project Guide to Confocal Imaging at Home & in the Classroom http://lifesciences.asu.edu/paperproject The Paper Project Guide to Confocal Imaging at Home & in the Classroom CJ Kazilek & Dennis
TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ)
 TISSUEFAXS CONFOCAL TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ) TissueFAXS Confocal provides a unique combination of digital slide scanning and laser
TISSUEFAXS CONFOCAL TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ) TissueFAXS Confocal provides a unique combination of digital slide scanning and laser
Wide Guy: Inverted Widefield Microscope
 Wide Guy: Inverted Widefield Microscope Kyle Marchuk Adam Fries Jordan Briscoe August 2017 Contents 1 Introduction 2 2 Initial Setup 3 2.1 Hardware Startup........................................... 3
Wide Guy: Inverted Widefield Microscope Kyle Marchuk Adam Fries Jordan Briscoe August 2017 Contents 1 Introduction 2 2 Initial Setup 3 2.1 Hardware Startup........................................... 3
Transmitted light Illuminator VIS-LED, color temperature. Epifluorescence lamp module X-Cite 120PC Q (Excelitas
 ELYRA PS.1 Multi-functional fluorescence inverted widefield microscope enabling live-cell imaging, TIRF or HILO illuminion and two super-resolution techniques: structured illuminion microscopy (SIM) and
ELYRA PS.1 Multi-functional fluorescence inverted widefield microscope enabling live-cell imaging, TIRF or HILO illuminion and two super-resolution techniques: structured illuminion microscopy (SIM) and
Leica TCS STED. The Fast Track to Superresolution Technical Documentation
 Leica TCS STED The Fast Track to Superresolution Technical Documentation 8 9 6 7 Inverted research microscope Leica DMI6000 CS Scan head Laser and power supply Computer table Air damped optical table 6
Leica TCS STED The Fast Track to Superresolution Technical Documentation 8 9 6 7 Inverted research microscope Leica DMI6000 CS Scan head Laser and power supply Computer table Air damped optical table 6
with an incubator for study of live
 Light microscopy Support medium is glass. Choose this route for confocal imaging. Do you want to quickly check your cells for green, red or blue fluorescence or using phase contrast? (If so, refer to microscope
Light microscopy Support medium is glass. Choose this route for confocal imaging. Do you want to quickly check your cells for green, red or blue fluorescence or using phase contrast? (If so, refer to microscope
Primary Use. Operating Principle
 Primary Use The Leica DVM6 is an optical microscope that has the ability observe samples at a high magnification at a high resolution. The microscope allows users to view their sample with up to a 2350x
Primary Use The Leica DVM6 is an optical microscope that has the ability observe samples at a high magnification at a high resolution. The microscope allows users to view their sample with up to a 2350x
Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy
 Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy 7 May 2007 Michael Hooker Microscopy Facility Michael Chua microscopy@unc.edu 843-3268 6007 Thurston Bowles Wendy Salmon wendy_salmon@med.unc.edu
Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy 7 May 2007 Michael Hooker Microscopy Facility Michael Chua microscopy@unc.edu 843-3268 6007 Thurston Bowles Wendy Salmon wendy_salmon@med.unc.edu
Bosch Institute Advanced Microscopy Facility Workshops Summer/Autumn 2016
 Bosch Institute Advanced Microscopy Facility Workshops Summer/Autumn 2016 Presented by Dr Louise Cole and Dr Cathy Payne, Advanced Microscopy Facility, Bosch Institute, School of Medical Sciences, The
Bosch Institute Advanced Microscopy Facility Workshops Summer/Autumn 2016 Presented by Dr Louise Cole and Dr Cathy Payne, Advanced Microscopy Facility, Bosch Institute, School of Medical Sciences, The
Multi Time Series Rev
 Multi Time Series Rev. 4.0.12 The Multi Time Series program is designed to provide flexible programming of automated time dependent experiments. The basic programming unit is a single Time Series Block
Multi Time Series Rev. 4.0.12 The Multi Time Series program is designed to provide flexible programming of automated time dependent experiments. The basic programming unit is a single Time Series Block
TN425: A study of fluorescence standards confirms that OptiGrid confocal images are suitable for quantitative microscopy
 TN425: A study of fluorescence standards confirms that OptiGrid confocal images are suitable for quantitative microscopy Introduction The OptiGrid converts the illumination system of a conventional wide
TN425: A study of fluorescence standards confirms that OptiGrid confocal images are suitable for quantitative microscopy Introduction The OptiGrid converts the illumination system of a conventional wide
Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System
 Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System Use Protocols: A. Reservation and Starting Up B. Sample Observation (No Image Capture) Transmitted
Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System Use Protocols: A. Reservation and Starting Up B. Sample Observation (No Image Capture) Transmitted
Dorigny resources MICROSCOPES - BIOPHORE. Confocal Microscope Zeiss LSM 700
 Dorigny resources Contributed by Yannick KREMPP Friday, 13 July 2007 Last Updated Monday, 30 October 2017 MICROSCOPES - BIOPHORE Confocal Microscope Zeiss LSM 700 This Zeiss LSM700 confocal is mounted
Dorigny resources Contributed by Yannick KREMPP Friday, 13 July 2007 Last Updated Monday, 30 October 2017 MICROSCOPES - BIOPHORE Confocal Microscope Zeiss LSM 700 This Zeiss LSM700 confocal is mounted
What should I know about the 3D Restoration module? Improvision Technical Note No. 142 Last updated: 28 June, 2000
 What should I know about the 3D Restoration module? Improvision Technical Note No. 142 Last updated: 28 June, 2000 Topic This technical note discusses some aspects of iterative deconvolution and how to
What should I know about the 3D Restoration module? Improvision Technical Note No. 142 Last updated: 28 June, 2000 Topic This technical note discusses some aspects of iterative deconvolution and how to
Zeiss Z.1 Lightsheet. Quick-Start Guide
 Zeiss Z.1 Lightsheet Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 Chamber and Sample preparation Part 2 Image Acquisition Shut-down Start-up: Turn on the Microscope / Computers Turn
Zeiss Z.1 Lightsheet Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 Chamber and Sample preparation Part 2 Image Acquisition Shut-down Start-up: Turn on the Microscope / Computers Turn
Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b
 Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b User Guide Biomedicum Imaging Unit (BIU) University of Helsinki www.biu.helsinki.fi 11.11.2016 1 GENERAL...1
Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b User Guide Biomedicum Imaging Unit (BIU) University of Helsinki www.biu.helsinki.fi 11.11.2016 1 GENERAL...1
INFINITY-CORRECTED TUBE LENSES
 INFINITY-CORRECTED TUBE LENSES For use with Infinity-Corrected Objectives Available in Focal Lengths Used by Thorlabs, Nikon, Leica, Olympus, and Zeiss Designs for Widefield and Laser Scanning Applications
INFINITY-CORRECTED TUBE LENSES For use with Infinity-Corrected Objectives Available in Focal Lengths Used by Thorlabs, Nikon, Leica, Olympus, and Zeiss Designs for Widefield and Laser Scanning Applications
Microscopy Specs. Media refractive index
 Zeiss AxioImager A2 EC Plan-Neofluar (420320-9901-000000) EC Plan-Neofluar (420330-9901-000000) Plan-Apochromat (420640-9900-000000) Plan-Apochromat (420650-9901-000000) Plan-Apochromat Korr (420660-9970-000)
Zeiss AxioImager A2 EC Plan-Neofluar (420320-9901-000000) EC Plan-Neofluar (420330-9901-000000) Plan-Apochromat (420640-9900-000000) Plan-Apochromat (420650-9901-000000) Plan-Apochromat Korr (420660-9970-000)
Setting up Micro-Magellan for Device control
 Setting up Micro-Magellan for Device control From the plug-in menu, select Micro-Magellan and the unpopulated window opens Select Configure device control The window below will open, select devices to
Setting up Micro-Magellan for Device control From the plug-in menu, select Micro-Magellan and the unpopulated window opens Select Configure device control The window below will open, select devices to
User s Guide to the LMD Laser Micro-dissection. System
 User s Guide to the LMD-6000 Laser Micro-dissection System Page 1 Glen MacDonald October 31, 2018 Start-up Procedure for Leica LMD-6000. 1. Turn on mercury lamp by pressing the rocker switch; a. beige
User s Guide to the LMD-6000 Laser Micro-dissection System Page 1 Glen MacDonald October 31, 2018 Start-up Procedure for Leica LMD-6000. 1. Turn on mercury lamp by pressing the rocker switch; a. beige
Bosch Institute Advanced Microscopy Facility Workshops Summer/Autumn 2017
 Bosch Institute Advanced Microscopy Facility Workshops Summer/Autumn 2017 Presented by Dr Louise Cole, Advanced Microscopy Facility, Bosch Institute, School of Medical Sciences, The University of Sydney.
Bosch Institute Advanced Microscopy Facility Workshops Summer/Autumn 2017 Presented by Dr Louise Cole, Advanced Microscopy Facility, Bosch Institute, School of Medical Sciences, The University of Sydney.
LECO LM247AT Microhardness Tester & Amh43 Software
 Instrument Operation Instructions Department of Materials Science & Engineering Equipment: Location: Access: Contact: LECO LM247AT Microhardness Tester and Control Software 3355 Hoover Hall Open for undergraduate
Instrument Operation Instructions Department of Materials Science & Engineering Equipment: Location: Access: Contact: LECO LM247AT Microhardness Tester and Control Software 3355 Hoover Hall Open for undergraduate
Fluorescence Microscope with Spinning Disk Confocal and Total Internal Reflection Fluorescence Modules
 Open Tender Notification for the procurement of Fluorescence Microscope with Spinning Disk Confocal and Total Internal Reflection Fluorescence Modules at the Indian Institute of Science, Bangalore (Last
Open Tender Notification for the procurement of Fluorescence Microscope with Spinning Disk Confocal and Total Internal Reflection Fluorescence Modules at the Indian Institute of Science, Bangalore (Last
Optical Ptychography Imaging
 Optical Ptychography Imaging Summer Project Annafee Azad Supervisors: Dr Fucai Zhang Prof Ian Robinson Summer 2014 23 October 2014 Optical Ptychography Imaging P a g e 2 Abstract This report details a
Optical Ptychography Imaging Summer Project Annafee Azad Supervisors: Dr Fucai Zhang Prof Ian Robinson Summer 2014 23 October 2014 Optical Ptychography Imaging P a g e 2 Abstract This report details a
Technology and equipment at the Bioimaging Center
 Technology and equipment at the Bioimaging Center Light microscopy / Widefields Name Type Applications and equipment LEICA AF6000LX Nikon BioStation Timelaps imaging, CO2 and temperature controlled (14
Technology and equipment at the Bioimaging Center Light microscopy / Widefields Name Type Applications and equipment LEICA AF6000LX Nikon BioStation Timelaps imaging, CO2 and temperature controlled (14
The Microscope. Lab Exercise. Contents. Light Microscope. Objectives. Scanning Electron Microscope. Introduction. Transmission Electron Microscope
 Lab Exercise The Microscope Contents Objectives 1 Introduction 1 Activity.1 Care of Microscopes 2 Activity.2 Parts of the Microscope 3 Activity.3 Viewing Images 4 Activity.4 Magnification of Images 4 Resutls
Lab Exercise The Microscope Contents Objectives 1 Introduction 1 Activity.1 Care of Microscopes 2 Activity.2 Parts of the Microscope 3 Activity.3 Viewing Images 4 Activity.4 Magnification of Images 4 Resutls
Optical Sectioning. Bo Huang. Pharmaceutical Chemistry
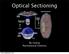 Optical Sectioning Bo Huang Pharmaceutical Chemistry Approaches to 3D imaging Physical cutting Technical difficulty Highest resolution Highest sensitivity Optical sectioning Simple sample prep. No physical
Optical Sectioning Bo Huang Pharmaceutical Chemistry Approaches to 3D imaging Physical cutting Technical difficulty Highest resolution Highest sensitivity Optical sectioning Simple sample prep. No physical
IMAGE STUDIO LITE. Tutorial Guide Featuring Image Studio Analysis Software Version 3.1
 IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
Working Copy For Review Purposes Only!
 Working Copy For Review Purposes Only! Version #1.5 08/29/06 Aaron Holland Up-dated D.Turnbull 10-18-2006 (MarkerSOP3.doc) Up-dated D. Turnbull 12-11-06 (MarkerSOP4.doc) Up-dated D.Turnbull 05-12-2008
Working Copy For Review Purposes Only! Version #1.5 08/29/06 Aaron Holland Up-dated D.Turnbull 10-18-2006 (MarkerSOP3.doc) Up-dated D. Turnbull 12-11-06 (MarkerSOP4.doc) Up-dated D.Turnbull 05-12-2008
Renishaw invia Raman Microscope (April 2006)
 Renishaw invia Raman Microscope (April 2006) I. Starting the System 1. The main system unit is ON all the time. 2. Switch on the Leica microscope and light source for reflective bright field (BF) imaging.
Renishaw invia Raman Microscope (April 2006) I. Starting the System 1. The main system unit is ON all the time. 2. Switch on the Leica microscope and light source for reflective bright field (BF) imaging.
NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes
 NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes bh FLIM systems for the confocal and the multiphoton versions of the Leica SP2 and SP5 microscopes are available since 2002 [4]. These
NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes bh FLIM systems for the confocal and the multiphoton versions of the Leica SP2 and SP5 microscopes are available since 2002 [4]. These
Biology 105 Laboratory 1: Microscope
 Biology 105 Laboratory 1: Microscope A. The Compound Microscope The microscope is a delicate, precision instrument. It must be treated gently, as the slightest bump or jar may damage the alignment of its
Biology 105 Laboratory 1: Microscope A. The Compound Microscope The microscope is a delicate, precision instrument. It must be treated gently, as the slightest bump or jar may damage the alignment of its
Carestream Vita user quick guide. Software version 3.2 From April 2012
 Carestream Vita user quick guide Software version 3.2 From April 2012 1 Carestream Vita user quick guide Software version 3.2 from April 2012 1. To switch your Vita on Press the power button on the PC
Carestream Vita user quick guide Software version 3.2 From April 2012 1 Carestream Vita user quick guide Software version 3.2 from April 2012 1. To switch your Vita on Press the power button on the PC
XRADIA microxct Manual
 XRADIA microxct Manual Multiscale CT Lab Table of Contents 1. Introduction and Basics 1.1 Instrument Parts 1.2 Powering up the system 1.3 Preparing your sample 2. TXM Controller 2.1 Starting up 2.2 Finding
XRADIA microxct Manual Multiscale CT Lab Table of Contents 1. Introduction and Basics 1.1 Instrument Parts 1.2 Powering up the system 1.3 Preparing your sample 2. TXM Controller 2.1 Starting up 2.2 Finding
Operating Procedure for Horiba Raman Microscope
 Operating Procedure for Horiba Raman Microscope SAFETY Be aware of Laser radiation at all times! Do not remove the covers of the instrument. Components are supplied with 110V electric source. Do not touch
Operating Procedure for Horiba Raman Microscope SAFETY Be aware of Laser radiation at all times! Do not remove the covers of the instrument. Components are supplied with 110V electric source. Do not touch
3-D. Here red spheres show the location of gold nanoparticles inside/around a cell nucleus.
 3-D The CytoViva 3-D System allows the user can locate objects of interest in a 3-D space. It does this by acquiring multiple Z planes and performing our custom software routines to locate and observe
3-D The CytoViva 3-D System allows the user can locate objects of interest in a 3-D space. It does this by acquiring multiple Z planes and performing our custom software routines to locate and observe
Use of the Binocular Microscope
 Use of the Binocular Microscope Before you begin this learning module be sure that you have the following materials in front of you: A microscope A packet of lens paper and Kimwipes A glass slide of a
Use of the Binocular Microscope Before you begin this learning module be sure that you have the following materials in front of you: A microscope A packet of lens paper and Kimwipes A glass slide of a
Volocity ver (2013) Standard Operation Protocol
 Faculty Core Facility Volocity 6.3.0 (2013) SOP A-1 Volocity ver. 6.3.0 (2013) Standard Operation Protocol Faculty Core Facility Volocity 6.3.0 (2013) SOP A-2 A. Content Overview. 3 Start up. 3 Change
Faculty Core Facility Volocity 6.3.0 (2013) SOP A-1 Volocity ver. 6.3.0 (2013) Standard Operation Protocol Faculty Core Facility Volocity 6.3.0 (2013) SOP A-2 A. Content Overview. 3 Start up. 3 Change
Quick Start Guide ZEN 2 core. Imaging Software
 Quick Start Guide ZEN 2 core Imaging Software Table of Contents 1 Welcome 03 1.1 Introduction 04 1.2 Workflows 05 1.3 User Roles 06 1.4 Starting the Software and Logging In 07 2 Creating a Job Template
Quick Start Guide ZEN 2 core Imaging Software Table of Contents 1 Welcome 03 1.1 Introduction 04 1.2 Workflows 05 1.3 User Roles 06 1.4 Starting the Software and Logging In 07 2 Creating a Job Template
Look Ahead to Get Ahead
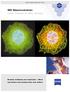 Microscopy from Carl Zeiss Archive 3D Deconvolution Z-stack 3D Deconvolution Look Ahead to Get Ahead Mark & Find Multichannel Timelapse Greater brilliance and resolution More convenient and precise than
Microscopy from Carl Zeiss Archive 3D Deconvolution Z-stack 3D Deconvolution Look Ahead to Get Ahead Mark & Find Multichannel Timelapse Greater brilliance and resolution More convenient and precise than
Higher -o-o-o- Past Paper questions o-o-o- 3.2 Refraction
 Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.2 Refraction 2000 Q27 A student is investigating the effect that a semicircular glass block has on a ray of monochromatic light. She observes that
Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.2 Refraction 2000 Q27 A student is investigating the effect that a semicircular glass block has on a ray of monochromatic light. She observes that
Independent Resolution Test of
 Independent Resolution Test of as conducted and published by Dr. Adam Puche, PhD University of Maryland June 2005 as presented by (formerly Thales Optem Inc.) www.qioptiqimaging.com Independent Resolution
Independent Resolution Test of as conducted and published by Dr. Adam Puche, PhD University of Maryland June 2005 as presented by (formerly Thales Optem Inc.) www.qioptiqimaging.com Independent Resolution
EPFL SV PTBIOP BIOP COURSE 2015 OPTICAL SLICING METHODS
 BIOP COURSE 2015 OPTICAL SLICING METHODS OPTICAL SLICING METHODS Scanning Methods Wide field Methods Point Scanning Deconvolution Line Scanning Multiple Beam Scanning Single Photon Multiple Photon Total
BIOP COURSE 2015 OPTICAL SLICING METHODS OPTICAL SLICING METHODS Scanning Methods Wide field Methods Point Scanning Deconvolution Line Scanning Multiple Beam Scanning Single Photon Multiple Photon Total
Short instructions. Olympus FV1000
 Olympus FV1000-1- Short instructions Short instructions. Olympus FV1000 Version 140416 This short instruction will guide you through the turn on and off sequence of the microscope system. For a detailed
Olympus FV1000-1- Short instructions Short instructions. Olympus FV1000 Version 140416 This short instruction will guide you through the turn on and off sequence of the microscope system. For a detailed
PANalytical X-Ray Diffractometer with PW3830 Generator. Standard Operating Procedure. Revised on 02/15/2007
 PANalytical X-Ray Diffractometer with PW3830 Generator Standard Operating Procedure Revised on 02/15/2007 Booking of XRD time 1. Please book the XRD schedule first come/first serve in advance on the sheets
PANalytical X-Ray Diffractometer with PW3830 Generator Standard Operating Procedure Revised on 02/15/2007 Booking of XRD time 1. Please book the XRD schedule first come/first serve in advance on the sheets
Olympus cellsens TIRF Guide
 Startup Power up the system as described in the "Startup & Environmental Control" guide, including power strip #2 ("TIRF"). ON For each laser line needed, turn the key on the control unit right and press
Startup Power up the system as described in the "Startup & Environmental Control" guide, including power strip #2 ("TIRF"). ON For each laser line needed, turn the key on the control unit right and press
Great Bay Community College Document Number: Corporate Drive Revision Number: 3
 Page 1 of 6 Approvals: Preparer: Kari Britt Date 03Aug10 Reviewer: Sonia Wallman Date 03Aug10 1. Purpose: Operation of the Leica DME microscope. 2. Scope: Applies to the proper usage of the Leica DME microscope.
Page 1 of 6 Approvals: Preparer: Kari Britt Date 03Aug10 Reviewer: Sonia Wallman Date 03Aug10 1. Purpose: Operation of the Leica DME microscope. 2. Scope: Applies to the proper usage of the Leica DME microscope.
Zeiss Universal SIM. A structured illumination system based on a Zeiss Universal microscope stand. by Nidhogg
 Zeiss Universal SIM A structured illumination system based on a Zeiss Universal microscope stand. by Nidhogg Structured illumination Structured illumination applied to fluorescence microscopy is a super
Zeiss Universal SIM A structured illumination system based on a Zeiss Universal microscope stand. by Nidhogg Structured illumination Structured illumination applied to fluorescence microscopy is a super
Heit/Kerfoot/Dikeakos. Leica DMI6000 B. User Manual
 Heit/Kerfoot/Dikeakos Leica DMI6000 B User Manual WARNING This microscope is a multi lab resource that many individuals are dependent on. As such the following rules must be followed in order to minimize
Heit/Kerfoot/Dikeakos Leica DMI6000 B User Manual WARNING This microscope is a multi lab resource that many individuals are dependent on. As such the following rules must be followed in order to minimize
CFIM MICROSCOPY COURSE TIMETABLE PRINCIPLES OF MICROSCOPY MONDAY 6 TH OF JANUARY 2014 FRIDAY 10 TH OF JANUARY 2014
 MICROSCOPY COURSE TIMETABLE PRINCIPLES OF MICROSCOPY MONDAY 6 TH OF JANUARY 2014 FRIDAY 10 TH OF JANUARY 2014 CONFOCAL AND FLUORESCENCE MICROSCOPY MONDAY 20 TH OF JANUARY 2014 FRIDAY 24 TH OF JANUARY 2014
MICROSCOPY COURSE TIMETABLE PRINCIPLES OF MICROSCOPY MONDAY 6 TH OF JANUARY 2014 FRIDAY 10 TH OF JANUARY 2014 CONFOCAL AND FLUORESCENCE MICROSCOPY MONDAY 20 TH OF JANUARY 2014 FRIDAY 24 TH OF JANUARY 2014
LEXT 3D Measuring LASER Microscope
 LEXT 3D Measuring LASER Microscope Warning: This instrument may only be operated by those who have been trained by AAF staff and have read and signed the AAF laboratory policies. A) STARTUP 1. Computer
LEXT 3D Measuring LASER Microscope Warning: This instrument may only be operated by those who have been trained by AAF staff and have read and signed the AAF laboratory policies. A) STARTUP 1. Computer
USER GUIDE TO BECKMAN COULTER FC500
 USER GUIDE TO BECKMAN COULTER FC500 Create a New Protocol Purpose: This procedure describes how to define the parameters and create rough analysis plots for a new experimental set-up. Some protocol templates
USER GUIDE TO BECKMAN COULTER FC500 Create a New Protocol Purpose: This procedure describes how to define the parameters and create rough analysis plots for a new experimental set-up. Some protocol templates
Leica TCS SPE. Spectacular Imaging! Technical Documentation
 Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve A Reliable System Affordable Excellence The high resolution spectral confocal Leica TCS SPE
Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve A Reliable System Affordable Excellence The high resolution spectral confocal Leica TCS SPE
IN5132/IN5142/IN5134/IN5134a IN5144/IN5144a/IN5135/IN5145 User's Manual (detailed) Instant Stack Guide
 Projector IN5132/IN5142/IN5134/IN5134a IN5144/IN5144a/IN5135/IN5145 User's Manual (detailed) Instant Stack Guide Thank you for purchasing this product. Features This projector can be used in conjunction
Projector IN5132/IN5142/IN5134/IN5134a IN5144/IN5144a/IN5135/IN5145 User's Manual (detailed) Instant Stack Guide Thank you for purchasing this product. Features This projector can be used in conjunction
Lightsheet Z.1. Light Sheet Fluorescence Microscopy by Carl Zeiss. Fabrice Schmitt, Sales Manager Carl ZEISS France
 Lightsheet Z.1 Light Sheet Fluorescence Microscopy by Carl Zeiss Fabrice Schmitt, Sales Manager Carl ZEISS France 12.12.2012 Light Sheet Fluorescence Microscopy (LSFM) Principle The Principle of Light
Lightsheet Z.1 Light Sheet Fluorescence Microscopy by Carl Zeiss Fabrice Schmitt, Sales Manager Carl ZEISS France 12.12.2012 Light Sheet Fluorescence Microscopy (LSFM) Principle The Principle of Light
Confocal Microscopy Imaging of Single Emitter Fluorescence and Hanbury Brown, and Twiss Setup for Photon Antibunching. Abstract
 James Maslek 10/26/12 Confocal Microscopy Imaging of Single Emitter Fluorescence and Hanbury Brown, and Twiss Setup for Photon Antibunching Abstract The purpose of this experiment was to observe fluorescence
James Maslek 10/26/12 Confocal Microscopy Imaging of Single Emitter Fluorescence and Hanbury Brown, and Twiss Setup for Photon Antibunching Abstract The purpose of this experiment was to observe fluorescence
Olympus BX51 on Aura. Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope
 Olympus BX51 on Aura Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope Contents Start-up Preparing for Imaging Part I General Part II Transmitted Brightfield Part III Transmitted
Olympus BX51 on Aura Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope Contents Start-up Preparing for Imaging Part I General Part II Transmitted Brightfield Part III Transmitted
CoSMoS Starter Guide 1.4 Hoskins Lab Last Modified 03/03/2017 Chris DeCiantis
 Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
H.-J. Jordan (NanoFocus Messtechnik GmbH), R. Brodmann (Brodmann Marketing & Vertrieb)
 Highly accurate surface measurement by means of white light confocal microscopy Hochgenaue Oberflächenmessung mit Hilfe von konfokalen Weißlichttechniken H.-J. Jordan (NanoFocus Messtechnik GmbH), R. Brodmann
Highly accurate surface measurement by means of white light confocal microscopy Hochgenaue Oberflächenmessung mit Hilfe von konfokalen Weißlichttechniken H.-J. Jordan (NanoFocus Messtechnik GmbH), R. Brodmann
LIGHT SHEET MICROSCOPE. Alphα NEW GENERATION. Ground Breaking Technology For Selective Plane Illumination Microscopy
 LIGHT SHEET MICROSCOPE NEW GENERATION 3 Alphα Ground Breaking Technology For Selective Plane Illumination Microscopy Alphα 3 GET THE MOST OUT OF LIGHT SHEET IMAGING Optimal SPIM Architecture SHARP OPTICAL
LIGHT SHEET MICROSCOPE NEW GENERATION 3 Alphα Ground Breaking Technology For Selective Plane Illumination Microscopy Alphα 3 GET THE MOST OUT OF LIGHT SHEET IMAGING Optimal SPIM Architecture SHARP OPTICAL
DV2. Alignment Procedure. Install DV2 on Microscope NOTE: PLEASE READ THE ENTIRE PROCEDURE BEFORE YOU BEGIN ALIGNMENT OF THE DV2. Alignment Procedure
 H I G H - P E R F O R M A N C E E M C C D & C C D C A M E R A S F O R L I F E S C I E N C E S DV2 This document provides a straightforward, step-by-step outline of the alignment procedure for the Photometrics
H I G H - P E R F O R M A N C E E M C C D & C C D C A M E R A S F O R L I F E S C I E N C E S DV2 This document provides a straightforward, step-by-step outline of the alignment procedure for the Photometrics
Panalytical MRD X-Ray Diffraction SOP
 Panalytical MRD X-Ray Diffraction SOP Table of Contents 1.0 Safety 2.0 Training 3.0 Sample Preparation 4.0 Pre-Operation 5.0 Sample Height Adjustment 6.0 Running Programs 7.0 Sample Unloading 8.0 Post-Operation
Panalytical MRD X-Ray Diffraction SOP Table of Contents 1.0 Safety 2.0 Training 3.0 Sample Preparation 4.0 Pre-Operation 5.0 Sample Height Adjustment 6.0 Running Programs 7.0 Sample Unloading 8.0 Post-Operation
Contents. Preparation for Software Installation Recommended Configuration Installing Motic Images Plus 2.0 Mac OS X...
 Contents Preparation for Software Installation... 1 Recommended Configuration... 1 Installing... 1 Precise Calibration... 2 The Menus... 3 File Menu...3 Edit Menu...6 View Menu...6 Image Menu...7 Paint
Contents Preparation for Software Installation... 1 Recommended Configuration... 1 Installing... 1 Precise Calibration... 2 The Menus... 3 File Menu...3 Edit Menu...6 View Menu...6 Image Menu...7 Paint
Last class. This class. Single molecule imaging Deconvolution. FLIM Confocal
 FLIM, Confocal Last class Single molecule imaging Deconvolution This class FLIM Confocal FLIM Fluorescence Lifetime IMaging FLIM Fluorescence lifetime imaging Easier and more accurate quantitation Much
FLIM, Confocal Last class Single molecule imaging Deconvolution This class FLIM Confocal FLIM Fluorescence Lifetime IMaging FLIM Fluorescence lifetime imaging Easier and more accurate quantitation Much
The Danish Stem Cell Center (DanStem) Danish Stem Cell Center Flow Cytometry Core Facility. LSR Fortessa Guide
 Danish Stem Cell Center Flow Cytometry Core Facility LSR Fortessa Guide LSR Fortessa Overview The Danish Stem Cell Center (DanStem) BD FACSFlow Supply System Fluidics Cart Sheath container Waste container
Danish Stem Cell Center Flow Cytometry Core Facility LSR Fortessa Guide LSR Fortessa Overview The Danish Stem Cell Center (DanStem) BD FACSFlow Supply System Fluidics Cart Sheath container Waste container
NANOSPEC 4150 STANDARD OPERATING PROCEDURES
 NANOSPEC 4150 STANDARD OPERATING PROCEDURES Version: 1.0 JAN 2016 UNIVERSITY OF TEXAS AT ARLINGTON Nanotechnology Research Center TABLE OF CONTENTS 1.0 INTRODUCTION.. 3 2.0 HARDWARE....... 3 3.0 OPERATING
NANOSPEC 4150 STANDARD OPERATING PROCEDURES Version: 1.0 JAN 2016 UNIVERSITY OF TEXAS AT ARLINGTON Nanotechnology Research Center TABLE OF CONTENTS 1.0 INTRODUCTION.. 3 2.0 HARDWARE....... 3 3.0 OPERATING
SUSS SB6E BONDER SOP. October 2013
 SUSS SB6E BONDER SOP October 2013 The SUSS SB6 bonder is capable of silicon fusion, anodic, and eutectic bonding. There are two separate heads; one for anodic bonding, and another for fusion bonding. Definitions
SUSS SB6E BONDER SOP October 2013 The SUSS SB6 bonder is capable of silicon fusion, anodic, and eutectic bonding. There are two separate heads; one for anodic bonding, and another for fusion bonding. Definitions
Panalytical XPert Powder X-Ray Diffraction SOP
 Revision 1 2/06/2018 Panalytical XPert Powder SOP Page 1 of 11 Panalytical XPert Powder X-Ray Diffraction SOP NOTE: Latest revisions are in Blue. Table of Contents 1.0 Safety 2.0 Training 3.0 Sample Preparation
Revision 1 2/06/2018 Panalytical XPert Powder SOP Page 1 of 11 Panalytical XPert Powder X-Ray Diffraction SOP NOTE: Latest revisions are in Blue. Table of Contents 1.0 Safety 2.0 Training 3.0 Sample Preparation
Non-Descanned FLIM Systems for Olympus FV-1000 and FV-300 Multiphoton Microscopes
 Non-Descanned FLIM Systems for Olympus FV-1000 and FV-300 Multiphoton Microscopes Abstract. Recently multiphoton versions of the Olympus FV 1000 and FV 300 laser scanning microscopes have become available.
Non-Descanned FLIM Systems for Olympus FV-1000 and FV-300 Multiphoton Microscopes Abstract. Recently multiphoton versions of the Olympus FV 1000 and FV 300 laser scanning microscopes have become available.
CoSMoS Starter Guide 1.6 Hoskins Lab Last Modified 03/30/2017 Chris DeCiantis
 Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Chapter 3. Experimental Procedure
 Chapter 3 Experimental Procedure 33 3.1 Burner Systems Startup 3.1.1 Instrumentation power up The instrumentation of the burner including the PC need to be turned on, in order to provide safe ignition
Chapter 3 Experimental Procedure 33 3.1 Burner Systems Startup 3.1.1 Instrumentation power up The instrumentation of the burner including the PC need to be turned on, in order to provide safe ignition
Hand sectioning a mushroom
 Hand sectioning a mushroom 1)cut a wedge from cap that includes the gills 2) Hold the section tightly in your fingers and try to shave off thin longitudinal sections of the gills and cap 3) Float the section
Hand sectioning a mushroom 1)cut a wedge from cap that includes the gills 2) Hold the section tightly in your fingers and try to shave off thin longitudinal sections of the gills and cap 3) Float the section
Olympus cellsens Live/TIRF Unit
 Version 4 (July 2017) Startup (Widefield Mode) Switch on power strips #1-4 (behind the monitor/lasers) in the order as they are labeled. Switch on #2 (TIRF lasers) only if you need one or more lasers for
Version 4 (July 2017) Startup (Widefield Mode) Switch on power strips #1-4 (behind the monitor/lasers) in the order as they are labeled. Switch on #2 (TIRF lasers) only if you need one or more lasers for
SmartSoft AES Operator s Guide
 SmartSoft AES Operator s Guide Part No. 702064 Rev. A Physical Electronics USA, PHI, SMART-Tool, SmartSoft, MultiPak and Watcher are trademarks of ULVAC-PHI, Inc. All other trademarks are the property
SmartSoft AES Operator s Guide Part No. 702064 Rev. A Physical Electronics USA, PHI, SMART-Tool, SmartSoft, MultiPak and Watcher are trademarks of ULVAC-PHI, Inc. All other trademarks are the property
