PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual
|
|
|
- Chloe Atkinson
- 5 years ago
- Views:
Transcription
1 PCIC Plant Cell Imaging Center (BTI, room 104B) CONFOCAL LEICA SP5 user manual -Turn on the mercury lamp if you need to see the fluorescence through the eye pieces. Check that its shutter is on the open position, and that the intensity is not adjusted beyond the second setting. Startup procedure: -Remove the dust cover from the microscope -On the main switch board, switch on PC/microscope (button Nº1). Wait for 15 seconds. (The computer will turn on automatically). -Switch on the Scanner power button (Nº2, on the main switch board). Wait for 15 seconds. Check that the fan is working properly. -Switch on the Laser power button (Nº3, on the main switch board). It may be already on, in which case leave it that way. -Turn the laser key (Nº4, on the main switch board) from OFF to ON. -Fill out the log book. -On PC: login as your first name and password is PCICuser! STARTING THE LAS AF SOFTWARE: -After login, wait for about 45 seconds (important). -After 30 seconds, right click on the LAS AF icon on the desktop and press open -On the starting screen, check that the configuration is OK: MACHINE should be picked (If you need the resonant scanner, mark the box Activate the resonant scanner ). Then click OK. -When the window Microscope stand pops up, say NO unless you are doing advanced techniques (such as Multiple Field, mosaic, etc.). PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 1/16
2 In the configuration tab (1), select the laser window (2). Turn on the laser(s) you need (3). By checking the box next to laser means starting up the laser. Adjust the Argon laser to approximately 30 % (4) if you use it (You can increase the power if you are doing bleaching, or if your signal is really too weak for example).!!! If lasers were turned off by the previous user recently, make sure the Argon laser was left off for at least 2 hours before you turn it back on. HeNeLasers have to rest at least one hour before being back on. The 405 nm laser needs to rest only about 15 minutes. The DPSS 561nm can be turned back on immediately. SLIDE OBSERVATION THROUGH THE EYEPIECES: First select the 10 X objective to identify your sample, and check if you are in focus when the stage moves to its preset Z=0 position (more details on next page). Although it is possible to change the objectives manually, our SP5 is equipped with an automated objective turret. To use this function, go back in the Acquire tab and select the appropriate objective (see the supplementary information for details on available objectives, and correct ring settings). Avoid switching manually the objectives, as you may inadvertently rotate the objective ring, or even worse, the objective itself. Be careful not to put oil on water objectives! If this happens, contact Mamta. Use ONLY Leica OIL for oil immersion. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 2/16
3 Lower the stage by pressing the Down Z axis button of the stage knob (D). Place your sample under the microscope. Be very gentle when you place your slide on the stage: it is very delicate. Press the Up button of the stage knob until it stops automatically. Look down the eyepiece and turn the X/Y or Z axis wheels of the stage knob to identify your sample (you can adjust the speed of movement (X/Y and Z) on the right). Fine focus along the Z axis by turning the knob on the side of the microscope stand. Check the Z position on the XYZ Screen (see below). If you are too far from the preset Z=0, you must reset this parameter so that you can safely switch to higher magnification objective. The left 4 th xyz icon is used to do the stage limit settings. To move the stage up, press and hold the top arrow icon until it reaches the previously set zero position. To go down, press and hold the lower down arrow icon. To reset the top position, when you are at 10X (never set with higher mag objective), press set with the top arrow (left side) of set/clear limits. For lower stage settings use the right side of the set/clear limits. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 3/16
4 Left side 2 nd icon: To see if you are in TL or IL mode and which filter set you are using You can change filter cubes and also switch different modes of TL from #2 icon. Third icon (objective): to check where you are and to switch objective if you turned off the software or when you need to move to 10X after closing software by mistake before switching to 10X. Always switch objective through LASAF software (recommended). PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 4/16
5 TRANSMITTED LIGHT: To use transmitted light (TL), check that the transmitted light lever (behind the microscope stand) is directed towards the eyepieces. Note: Adjusting the microscope for an optimal brightfield image (Köhler illumination) is more complicated than for fluorescence. Although we regularly check that the settings are optimal, you may need further readjustment. Please ask for help the first few times. By pressing the buttons on the left side of the microscope stand, you can switch to TL. You can also pick between various TL modes: brightfield, polarized and DIC. The last button allows you to visualize fluorescence and DIC together (not available). If the objective (eg. 10X) is not equipped for a specific illumination mode (e.g. DIC), an exclamation mark will appear on the touch pad. The resolution in X and Y is determined by the distance from the center of the Airy disk to the first dark ring. This is referred to as Airy 1. This distance depends on the wavelength of light (λ) and the Numerical Aperture of the lens (NA). For a confocal system, the pinhole radius is set somewhat smaller than Airy 1 and thus the X Y resolution equation is: Rxy = 0.8 λ / 2 NA PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 5/16
6 On the left side of the stand, underneath the stage, push the TL/IL button until TL appears on the touch pad. Look through the eyepieces and adjust the brightness with the INT buttons. You can adjust the shape and size of the illuminated area with the FD button. Fine adjustment of the rotation of the Wollaston prism for DIC imaging is performed by rotating the thumbscrew located right above the objectives. FLUORESCENCE: By pressing the buttons on the right side of the microscope stand, you can switch to fluorescence mode. You can choose the appropriate filter cube with the cube button. Its name will appear on the touch pad. Filter cubes available: A = UV I3 = Green LP GFP = Green BP N21 = Red N3 = Red BP See supplementary information for more details. The last button is the fluorescence shutter. On the left side of the stand, underneath the stage, push the TL/IL button until fluo appears on the touch pad. Look through the eyepieces and adjust the brightness with the INT buttons. You can adjust the shape and size of the illuminated area with the FD button. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 6/16
7 CONFOCAL ACQUISITION ADJUSTING THE PARAMETERS FOR PMT1, PMT3 and PMT5 Otherwise activate the UV or Visible icons, and adjust the required laser lines (3). For PMT, use between 5 and 50 % depending on the line and your sample. For HyD, do not use more than 20% of laser. Then activate and adjust the required PMT s/hyd s by checking the Active box (4). Use first the PMTs located just under the spectral band you want to detect. You can download the emission spectra of numerous dyes to help you adjust your settings (5). This has no impact on your image collection, and the display of any spectra from the list will not affect the data collection. Make sure the detection wavelengths don t cover any laser line! (and is set atleast 10 nm higher than the laser line) Move the cursor slowly to prevent damage to the mechanical part. Go to the Acquire Tab of the LAS AF software. You can expand any menu on the left part of the settings screen by clicking on the black arrows. In the first panel, pick the desired acquisition mode (1). Standard mode is XYZ. If you want to use preset adjustments or have already saved yours, load them from the scrolling list (2). If you want to use transmitted light, reposition the transmitted light lever (see page 3) towards the confocal position. Click on Additional channels in the acquisition tab, then select SCAN-BF or SCAN-DIC (1). Then activate the transmitted light PMT by checking the Active box. When you switch from using the software to viewing through the eye pieces, always make sure that the transmitted light PMT is inactivated, and click on the TL/IL button on the microscope stand before using any other buttons. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 7/16
8 Start preview scanning by clicking on LIVE (bottom of the left screen). In the image window (right screen), activate the multi-panel view (1). Click on the Quick-LUT icon to obtain a false color optimization screen (2). Click on the panel of the first fluorophore. Gain adjustment: Increase Smart gain until just a few single blue dots appear (saturated pixels=value 255). Offset adjustment: Reduce the Smart offset level until very few green dots appear (Green pixels= no signal=0). Sets automatically in HyD detectors. Repeat with the other channels. Check the settings by moving along the Z axis (you can use the button bar). Save the settings. To adjust the TL channel: exit the Q-LUT mode and adjust gain and offset in the standard mode. NOTE: In case of PMTs, for proper gain adjustment, you may want to increase your smart gain value up to V. A smart gain value lower than 400V means that you can lower the laser line intensity, and readjust your smart gain. A smart gain between would suggest increasing the laser line intensity. REMEMBER: Enhancing the laser line intensity will bleach your fluorophore faster, but increasing the gain does not affect your sample. Therefore, in order to protect your sample, it is better to keep the laser line intensity as low as possible, and increase the gain instead. For HyDs, offset is set automatically, but gain can be adjusted between 10 and 500 based on LUT. In the XY panel (left screen), set the image format, scan speed and zoom factor (3). Faster scans require zooming. You can use the arrows to move the imaged area. You can also zoom specifically on an area by activating the zoom box (3), then drawing a rectangle around your region of interest (the rectangle drawing tool will appear on the right screen once you select the zoom option). Adjust the averaging to improve the image definition (4): - For live imaging: use line averaging (8 or 16) - For fixed samples: line or frame averaging - For weak samples, you can also use the accumulating function for PMT or use HyD) NOTE: if you change the scan speed or the zoom factor, or if you switch objectives, you may need to readjust gain and offset. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 8/16
9 ADJUSTING THE PARAMETERS FOR Hybrid Detectors HyD (old PMT2 and PMT4): If weakly fluorescent samples, you may want to use HyD detector to collect your signal. Do not use high laser with HyD detector: use as low laser as possible (1%-max 20%) Old PMT2 and PMT4 are now HYD detector which provides: Large Dynamic range Improved cell viability High speed Imaging Single photon counting Low dark noise Exquisite contrast Gain adjustment: can go from Increase Smart gain until just a few single blue dots appear (saturated pixels=value 255). Offset adjustment: Sets automatically in HyD Leica HyD has 3 modes of collection: 1) Standard mode (most commonly used) 2) BrightR (rarely used, non-linear gain applied to structure) 3) Photon Counting Mode (good for quantitative imaging; works best with dim sample) Z stack Expand the Z-stack panel and activate the live viewing mode. Move along the Z axis with the appropriate wheel of the button bar. Click the red arrow next to the Begin field to mark the start position. Then define the end position by rotating the wheel and clicking the end arrow. Define the number of slices by either entering the number of steps, or by choosing a defined z-step size. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 9/16
10 Sequential scan To avoid crosstalk between fluorophores, you can perform a sequential scan. Click on the Sequential icon (1). Define the number of successive sequential scans by clicking on the - and + icons (2). Decide when the scanning modes should be switched: - For live imaging: use between lines. This mode does not provide the full range of scanning options. - For fixed samples: use between frames or stacks. For each individual scan, make sure only one laser line will be activated (3), and adjust accordingly the corresponding PMT or HyD (4). Adjust separately scan 1, then scan 2, etc. You can control the settings by using the Live function. ACQUIRING AND SAVING DATA When you are satisfied with your settings, click on Capture image to acquire one image, or Start if you want to acquire an image sequence (such as a Z-stack). Save your files regularly by going into the experiments sub-tab. Place the mouse arrow over your file and right click to display the menu and save the images. Saving the images in the.lif format (LEICA image format) will allow you to import acquisition parameters during future experiments, as well as having an access to detailed information regarding the parameters used for the acquisition of the data (see below). You can also export single images or the entire experiment as Tiff, rename the file, etc. Save your files in D:/users/your full name. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 10/16
11 Your old files (from 2008) are all saved under d:\images\your lab folder\your folder NOTE: do not go beyond 2 Giga for a single experiment. TO REAPPLY SETTINGS FROM PREVIOUS EXPERIMENTS Open an experiment. Right-click on the image name in the file list or the image window itself. Open Properties and click on Apply settings at the bottom of the window. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 11/16
12 TRANSFER YOUR DATA TO THE SERVER For this you have to map a network drive: MAP A NETWORK DRIVE (do it after you login as you on confocal PC) Double Click on MY COMPUTER Top Menu Bar TOOLS MAP A NETWORK DRIVE Z \\ \PCIC\ Check Reconnect at Logon Click Connect Using a Different User Name User Name: PCIC_user Password: enter password (contact PCIC if you forgot the password.) OK Finish New window showing the Users Data folder on the Workstation will appear CREATE A SHORTUCT ON YOUR DESKTOP: My Computer Scroll to bottom on screen You will see your mapped network drive Right Click on it (rename it if you want for eg. Data transfer to Server ) Create Shortcut On Desktop (it creates an icon shortcut to users data or an icon Data transfer to Server ) When you need to transfer your data to the Workstation use the shortcut on your desktop (which is as before shortcut to users ) Please note it may take a few minutes before the two computers are able to communicate. Copy (or drag) your files from the D: drive to this folder. TO RETRIEVE YOUR DATA FROM THE SERVER VIA YOUR COMPUTER On a PC: Go to My Computer and right click. Choose "map network drive" In the window that comes up type the following: Drive: Z Folder: \\ \PCIC\ Check Reconnect at Logon Click Connect Using a Different User Name User Name: PCIC_user Password: enter password (contact PCIC if you forgot the password.) OK Finish New window showing the Users Data folder on the Workstation will appear We will regularly erase files from the server and the confocal computer, so don't wait to transfer your files to your own computer... PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 12/16
13 On a Mac: From the Finder select "Connect to server" from the GO menu (or press command K) Type smb: \\ \PCIC\ In the address box / Connect In the dialog box that appears, leave confocal in the workgroup or domain box. Enter user name and password (contact PCIC if you forgot it). You can then download an entire folder. You should be able to connect. If it still doesn't -- Apple Menu System Preferences Sharing Services tab -- make sure personal file sharing is checked Firewall tab -- make sure personal file sharing is checked then try again If it still doesn't work, contact Elaine (eev1@cornell.edu). TO PROCESS YOUR FILES The workstation 1 hosts an offline version of the LAS AF software, as well as Image J and Image-Pro Plus version 6.3. Image-Pro Plus is a powerful and customizable image processing and analysis software. The workstation 2 has Photoshop and two free softwares (LAS AF Lite and Image J) allowing limited manipulations of your Leica files.!!! It is extremely important that you keep an intact copy of all your original files: - Some journals request the access to the unmodified, original data before publication - If you open and save a.lif file in LAS AF Lite or the offline full version of LAS AF, you may not be able to open this file and reapply its settings on the confocal machine (depends on the confocal version and the offline and the Lite versions). On your own computer: To install LAS AF Lite or Image J on your computer, follow the instructions on the PCIC website (look under software). PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 13/16
14 TROUBLESHOOT:. -I am not sure if the fluorescence comes from my fluorophore More info on how to perform a lambda scan can be provided upon request. Contact PCIC. -I turned on everything according to the manual, but nothing is working!!! Check that the microscope control box is on (E, page 1) -The fluorescence is too dim, although I adjusted gain and contrast 3 factors can affect the brightness: the laser line power (see supplementary information for details), the laser intensity (change the % in the laser configuration tab, but keep it as low as possible) and the pinhole size (should be set to 1 Airy). If you increase the pinhole size, you will have a brighter image, but it will affect the definition of your image). Also, more info on how to use the Enhanced Data Transfer Mode or frame accumulation for dim samples can be provided upon request. Contact PCIC. -The fluorescence of my sample is bleaching You can reduce the number of frame/line averaging, or increase the scan speed (it will decrease the definition of your image). Also, more info on how to use the Enhanced Data Transfer Mode for dim samples can be provided upon request. Contact PCIC. -I am using live samples and some components are moving too rapidly More info on how to use the bidirectional scan or the resonant scanner for high-speed imaging can be provided upon request. Contact PCIC. Need some help? Imaging help can be provided by Mamta Srivastava from 9 am to 1 pm. For questions call or come between 9 am and 1 pm at the PCIC, or send an to pcic@cornell.edu PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 14/16
15 SUPPLEMENTARY INFORMATION: Filter Excitation filter Dichroic mirror Emission filter Filter cubes: cube A BP LP 425 I3 BP LP 515 GFP BP 470/ BP N2,1 BP LP 590 N3 BP 546/ BP Configuration of the lasers: Laser Wavelength Suitable for Near UV Diode 405 nm CFP Argon 458 nm 476 nm 488 nm 496 nm 514 nm GFP YFP DPSS (Diode-Pumped Solid State) 561 nm HeNe Orange (Helium-Neon) 594 nm HeNe red (Helium-Neon) 633 nm Relative Argon laser power: Source: Laser Line (nm) % of the strongest Objectives: Type 10X Dry 20X Water 40X Dry 40X Oil 63X Oil 63X Water 100 X Oil Magnif. (N.A.) 10X (0.40) 20X (0.70) 40X (0.85) 40X ( ) 63X ( ) 63X (1.20) 100X (1.44) Type (Ref #) HC PL APO CS ( ) HC PL APO CS ( ) HCX PL APO CS ( ) HCX PL APO CS ( ) HCX PL APO CS ( ) HCX PL APO ( ) HCX PL APO ( ) Cover Glass Free working distance DIC Resol. max (nm) in xy/ in z (488nm laser) mm No 488 / DRY only Correction with / without 0.26 with water and 0.17 Yes / 1284 OIL=oil GLYC= glycerol 0.17= with cover glass, water immersion Yes 229.6/464.1 DRY only Yes / Oil only Yes / Oil only Immersion ring setting W= no cover glass, salted buffer (ca. 3% NaCl) 0= no cover glass, with water immersion Yes / Water only, correction for coverglass thickness Yes 135.6/ Oil only Max speed of the regular scanner: about 5 frames / second (512/512 pixels) Max speed of the bi-directional resonant scanner: about 25 frames / second (512/512 pixels) PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 15/16
16 SHUTDOWN PROCEDURE: First, check the Microsoft Outlook calendar on the PCIC workstation No. 1. If someone booked within one hour after you (DO NOT UNCHECK THE BOX NEXT TO LASER) -Save your images and leave the objective at 10X (do not rotate the objective manually). -Exit the program. -Transfer your data to the server (see above) -Log off and fill out the logbook -Lower the stage and clean objectives with fresh lens cleaning tissue (NO Kimwipes!) -clear up the desk and the working area If the next booking is later today (>1h but <3h later) -Save your images and leave the objective at 10X (do not rotate the objective manually). -In the laser configuration tab (see page 2), uncheck all lasers except the Argon. Slide down the argon power to 0%. -Exit the program. -Transfer your data to the server (see above) -Log off and fill out the logbook -Turn off the mercury lamp (see page 1, A) - Lower the stage and clean objectives with fresh lens cleaning tissue (NO Kimwipes!) -Put the dust cover on the microscope -clear up the desk and the working area If no one booked after you (last user of the day or >3h later) -Save your images and leave the objective at 10X (do not rotate the objective manually). -Lower the stage -In the laser configuration tab (see page 2), uncheck all lasers. -Exit the program. -Transfer your data to the server (see above) -Turn off the computer via the Start menu of Windows -Turn off the mercury lamp (A) -Turn the laser key (Nº4, on the main switch board) from ON to OFF -Keep the Laser power button (Nº3, on the main switch board) ON for at least 15 minutes -Switch off the Scanner power button (Nº2, on the main switch board). -Switch off the PC/microscope button (Nº1). -Log off and Fill out the logbook -Clean objectives with fresh lens cleaning tissue (NO Kimwipes!) -Clear up the desk and the working area -Put the dust cover on the microscope -15 minutes after shut down, turn off the laser power button (Nº3). Important: after hours, make sure the next person is coming (contact by or phone). If nobody comes, perform a total shutdown. PCIC SP5 confocal microscope user manual, Hélène Javot updated by Mamta Srivastava 2/11/2017, page 16/16
Multi-Photon Training
 Multi-Photon Training Overview This training will take approximately 4 hours. The first 2 hours will be spent learning one-on-one with your trainer, while the following 2 hours will be your opportunity
Multi-Photon Training Overview This training will take approximately 4 hours. The first 2 hours will be spent learning one-on-one with your trainer, while the following 2 hours will be your opportunity
LSM510 Confocal Microscope Standard Operation Protocol Basic Operation
 LSM510 Confocal Microscope Standard Operation Protocol Basic Operation Please make sure that the COMPRESSED AIR has been TURNED ON prior to the use of the equipment. Kindly inform the administrator if
LSM510 Confocal Microscope Standard Operation Protocol Basic Operation Please make sure that the COMPRESSED AIR has been TURNED ON prior to the use of the equipment. Kindly inform the administrator if
Indiana Center for Biological Microscopy. BioRad MRC 1024 MP Confocal & Multi-Photon Microscope
 Indiana Center for Biological Microscopy BioRad MRC 1024 MP Confocal & Multi-Photon Microscope Microscope and the Attached Accessories A: B: C: D: E: F: G: H: Mercury Lamp Transmission Light Kr/Ar Laser
Indiana Center for Biological Microscopy BioRad MRC 1024 MP Confocal & Multi-Photon Microscope Microscope and the Attached Accessories A: B: C: D: E: F: G: H: Mercury Lamp Transmission Light Kr/Ar Laser
1. Getting Started: Brief Step-By-Step Guide (PDF File)
 Page 1 of 5 1. Getting Started: Brief Step-By-Step Guide (PDF File) Leica SP2 confocal microscope is controlled via software LCS (Leica Confocal Software). The latest version is V2.5. Bellowing is a brief
Page 1 of 5 1. Getting Started: Brief Step-By-Step Guide (PDF File) Leica SP2 confocal microscope is controlled via software LCS (Leica Confocal Software). The latest version is V2.5. Bellowing is a brief
Nikon A1-B Confocal Operating Manual. Start-up. Microscope
 Nikon A1-B Confocal Operating Manual Start-up 1. Turn on Excite 120 LED power supply. a. No need for cool down as for mercury bulb b. Open shutter by pushing down contol knob. c. Adjust intensity by turning
Nikon A1-B Confocal Operating Manual Start-up 1. Turn on Excite 120 LED power supply. a. No need for cool down as for mercury bulb b. Open shutter by pushing down contol knob. c. Adjust intensity by turning
Confocal 6 Guide. Switching On Components of the system should be switched on in the following order: 1. Lasers Argon laser (488nm and 514nm)
 Confocal 6 Guide The system is kept at 37⁰C. If you require the system at a lower temperature book at least one hour before the beginning of your session to allow the system to cool down. It is your responsibility
Confocal 6 Guide The system is kept at 37⁰C. If you require the system at a lower temperature book at least one hour before the beginning of your session to allow the system to cool down. It is your responsibility
LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes
 LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes Brief Operating Manual Release 4.2 January 2007 Contents Page Starting the System...3 Setting the microscope...6 Configuring the beam path
LSM 5 MP, LSM 510 and LSM 510 META Laser Scanning Microscopes Brief Operating Manual Release 4.2 January 2007 Contents Page Starting the System...3 Setting the microscope...6 Configuring the beam path
Basic Users Guide for the LSM 510 Confocal Microscope
 Basic Users Guide for the LSM 510 Confocal Microscope Ian Jones and Adam Westmacott Department of Biology & Biochemistry, University of Bath, Bath, UK Updated 01 October 2003 This guide describes the basic
Basic Users Guide for the LSM 510 Confocal Microscope Ian Jones and Adam Westmacott Department of Biology & Biochemistry, University of Bath, Bath, UK Updated 01 October 2003 This guide describes the basic
Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b
 Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b User Guide Biomedicum Imaging Unit (BIU) University of Helsinki www.biu.helsinki.fi 11.11.2016 1 GENERAL...1
Leica TCS SP8 X Confocal Microscope and Leica Application Suite X software DRAFT VERSION Room B123b User Guide Biomedicum Imaging Unit (BIU) University of Helsinki www.biu.helsinki.fi 11.11.2016 1 GENERAL...1
Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL
 Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL 1 TABLE OF CONTENTS ANATOMY OF THE ZEISS AXIOIMAGE MICROSCOPE 3 INITIALISATION PROCEDURE 4 LEFT SIDE OF MICROSCOPE (DETAILED VIEW)
Cell Imaging Unit UIC Bárbara Fekete ZEISS AXIOIMAGER MICROSCOPE MANUAL 1 TABLE OF CONTENTS ANATOMY OF THE ZEISS AXIOIMAGE MICROSCOPE 3 INITIALISATION PROCEDURE 4 LEFT SIDE OF MICROSCOPE (DETAILED VIEW)
User s Guide to the LMD Laser Micro-dissection. System
 User s Guide to the LMD-6000 Laser Micro-dissection System Page 1 Glen MacDonald October 31, 2018 Start-up Procedure for Leica LMD-6000. 1. Turn on mercury lamp by pressing the rocker switch; a. beige
User s Guide to the LMD-6000 Laser Micro-dissection System Page 1 Glen MacDonald October 31, 2018 Start-up Procedure for Leica LMD-6000. 1. Turn on mercury lamp by pressing the rocker switch; a. beige
Olympus IX-70 Imaging Protocol
 Olympus IX-70 Imaging Protocol 1) System Startup Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused
Olympus IX-70 Imaging Protocol 1) System Startup Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused
Olympus IX-70 Imaging Protocol
 Olympus IX-70 Imaging Protocol 1) System Startup F Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for
Olympus IX-70 Imaging Protocol 1) System Startup F Please note our sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for
Manual ANGSTROM grid confocal microscope
 Manual ANGSTROM grid confocal microscope Switching on the system 1) Start by switching on the different components of the microscope system. When using live cell imaging also start the temperature and
Manual ANGSTROM grid confocal microscope Switching on the system 1) Start by switching on the different components of the microscope system. When using live cell imaging also start the temperature and
Zeiss AxioImager.Z2 Fluorescence Protocol
 Zeiss AxioImager.Z2 Fluorescence Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
Zeiss AxioImager.Z2 Fluorescence Protocol 1) System Startup Please note put sign-up policy. You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge
Olympus BX51 on Aura. Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope
 Olympus BX51 on Aura Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope Contents Start-up Preparing for Imaging Part I General Part II Transmitted Brightfield Part III Transmitted
Olympus BX51 on Aura Introduction to the NRI-MCDB Microscopy Facility BX51 Upright Microscope Contents Start-up Preparing for Imaging Part I General Part II Transmitted Brightfield Part III Transmitted
DSU Start-Up instructions
 DSU Start-Up instructions Always: - start with the 10x objective - properly center the stage around the current objective before changing to another objective - when done, leave the 10x objective in standby
DSU Start-Up instructions Always: - start with the 10x objective - properly center the stage around the current objective before changing to another objective - when done, leave the 10x objective in standby
Confocal Microscope - Radiance. Basic Instructions. (Stefanie Reichelt) The Radiance. Confocal Scanning Microscope. designed and developed by
 Confocal Microscope - Radiance Basic Instructions (Stefanie Reichelt) The Radiance Confocal Scanning Microscope designed and developed by Brad Amos (MRC-LMB) Commercialised by Bio-Rad Inc. (1998 2004)
Confocal Microscope - Radiance Basic Instructions (Stefanie Reichelt) The Radiance Confocal Scanning Microscope designed and developed by Brad Amos (MRC-LMB) Commercialised by Bio-Rad Inc. (1998 2004)
Start-up system. Nikon Wide Field Microscope Ee1078. Complete manual
 Start-up system Switch on the power socket located on the floor Switch devices on in the order of: o Mercury lamp control-unit switch on and ignite o Halogen lamp o Incubator control-box o Microscope stand
Start-up system Switch on the power socket located on the floor Switch devices on in the order of: o Mercury lamp control-unit switch on and ignite o Halogen lamp o Incubator control-box o Microscope stand
Leica TCS SPE. Spectacular Imaging! Technical Documentation
 Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve Built-in Reliability Affordable Excellence The new high resolution spectral confocal Leica TCS
Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve Built-in Reliability Affordable Excellence The new high resolution spectral confocal Leica TCS
Samples Carolina sample slides (pollen, algae, ). Clean off oil with lens paper then OpticPad around lens (metal not glass) when done.
 Bi/BE 227 Winter 2018 Assignment #1 Widefield and confocal laser scanning microscopy Schedule: Jan 3: Lecture Jan 3-12: Students get trained on how to use scopes, start on assignment Jan 3-17: Carrying
Bi/BE 227 Winter 2018 Assignment #1 Widefield and confocal laser scanning microscopy Schedule: Jan 3: Lecture Jan 3-12: Students get trained on how to use scopes, start on assignment Jan 3-17: Carrying
Olympus DeltaVision Microscope Start-Up and Shut-Down Instructions.
 DeltaVision Instructions: 1. Start-up (Olympus DV) 2. Basic operation (Olympus DV) 2.1 set-up 2.2 designing and running an experiment 3. Transferring files via FTP 4. Deconvolving files 5. File conversion
DeltaVision Instructions: 1. Start-up (Olympus DV) 2. Basic operation (Olympus DV) 2.1 set-up 2.2 designing and running an experiment 3. Transferring files via FTP 4. Deconvolving files 5. File conversion
Leica TCS SPE. Spectacular Imaging! Technical Documentation
 Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve A Reliable System Affordable Excellence The high resolution spectral confocal Leica TCS SPE
Leica TCS SPE Spectacular Imaging! Technical Documentation Leica TCS SPE Spectacular Imaging Easy to Achieve A Reliable System Affordable Excellence The high resolution spectral confocal Leica TCS SPE
OIC. User Guide to Confocal Microscope: Zeiss LSM Pascal
 User Guide to Confocal Microscope: Zeiss LSM Pascal This guide does not cover all the details in documentation that comes with the Zeiss micro-scope. Please do find time to go through the original documentation
User Guide to Confocal Microscope: Zeiss LSM Pascal This guide does not cover all the details in documentation that comes with the Zeiss micro-scope. Please do find time to go through the original documentation
Zeiss LSM 510 Meta inverted confocal manual
 Power Up Zeiss LSM 510 Meta inverted confocal manual *adapted from the Duke University Light Microscopy Core Facility manual 1. Turn on the mercury lamp (if required) and sign in the log book, recording
Power Up Zeiss LSM 510 Meta inverted confocal manual *adapted from the Duke University Light Microscopy Core Facility manual 1. Turn on the mercury lamp (if required) and sign in the log book, recording
Operating Procedure for Horiba Raman Microscope
 Operating Procedure for Horiba Raman Microscope SAFETY Be aware of Laser radiation at all times! Do not remove the covers of the instrument. Components are supplied with 110V electric source. Do not touch
Operating Procedure for Horiba Raman Microscope SAFETY Be aware of Laser radiation at all times! Do not remove the covers of the instrument. Components are supplied with 110V electric source. Do not touch
The Paper Project Guide to Confocal Imaging at Home & in the Classroom
 The Paper Project Guide to Confocal Imaging at Home & in the Classroom http://lifesciences.asu.edu/paperproject The Paper Project Guide to Confocal Imaging at Home & in the Classroom CJ Kazilek & Dennis
The Paper Project Guide to Confocal Imaging at Home & in the Classroom http://lifesciences.asu.edu/paperproject The Paper Project Guide to Confocal Imaging at Home & in the Classroom CJ Kazilek & Dennis
Wide Guy: Inverted Widefield Microscope
 Wide Guy: Inverted Widefield Microscope Kyle Marchuk Adam Fries Jordan Briscoe August 2017 Contents 1 Introduction 2 2 Initial Setup 3 2.1 Hardware Startup........................................... 3
Wide Guy: Inverted Widefield Microscope Kyle Marchuk Adam Fries Jordan Briscoe August 2017 Contents 1 Introduction 2 2 Initial Setup 3 2.1 Hardware Startup........................................... 3
Zeiss Elyra Microscope Standard Operation Protocol Basic Operation
 Machine Startup Zeiss Elyra Microscope Standard Operation Protocol Basic Operation 1. Turn on the Incubator power to warm up the incubator for a couple of hours prior to sample acquisition as image could
Machine Startup Zeiss Elyra Microscope Standard Operation Protocol Basic Operation 1. Turn on the Incubator power to warm up the incubator for a couple of hours prior to sample acquisition as image could
Spinning Disk Protocol
 Spinning Disk Protocol 1) System Startup F Please note our sign-up policy You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time
Spinning Disk Protocol 1) System Startup F Please note our sign-up policy You must inform the facility at least 24 hours beforehand if you can t come; otherwise, you will receive a charge for unused time
Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy
 Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy 7 May 2007 Michael Hooker Microscopy Facility Michael Chua microscopy@unc.edu 843-3268 6007 Thurston Bowles Wendy Salmon wendy_salmon@med.unc.edu
Introductory Guide to Light Microscopy - Biomedical Confocal Microscopy 7 May 2007 Michael Hooker Microscopy Facility Michael Chua microscopy@unc.edu 843-3268 6007 Thurston Bowles Wendy Salmon wendy_salmon@med.unc.edu
Leica TCS STED. The Fast Track to Superresolution Technical Documentation
 Leica TCS STED The Fast Track to Superresolution Technical Documentation 8 9 6 7 Inverted research microscope Leica DMI6000 CS Scan head Laser and power supply Computer table Air damped optical table 6
Leica TCS STED The Fast Track to Superresolution Technical Documentation 8 9 6 7 Inverted research microscope Leica DMI6000 CS Scan head Laser and power supply Computer table Air damped optical table 6
TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ)
 TISSUEFAXS CONFOCAL TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ) TissueFAXS Confocal provides a unique combination of digital slide scanning and laser
TISSUEFAXS CONFOCAL TissueFAXS SL Confocal high throughput configuration (actual appearance of the product may differ) TissueFAXS Confocal provides a unique combination of digital slide scanning and laser
LEXT 3D Measuring LASER Microscope
 LEXT 3D Measuring LASER Microscope Warning: This instrument may only be operated by those who have been trained by AAF staff and have read and signed the AAF laboratory policies. A) STARTUP 1. Computer
LEXT 3D Measuring LASER Microscope Warning: This instrument may only be operated by those who have been trained by AAF staff and have read and signed the AAF laboratory policies. A) STARTUP 1. Computer
Renishaw invia Raman Microscope (April 2006)
 Renishaw invia Raman Microscope (April 2006) I. Starting the System 1. The main system unit is ON all the time. 2. Switch on the Leica microscope and light source for reflective bright field (BF) imaging.
Renishaw invia Raman Microscope (April 2006) I. Starting the System 1. The main system unit is ON all the time. 2. Switch on the Leica microscope and light source for reflective bright field (BF) imaging.
Primary Use. Operating Principle
 Primary Use The Leica DVM6 is an optical microscope that has the ability observe samples at a high magnification at a high resolution. The microscope allows users to view their sample with up to a 2350x
Primary Use The Leica DVM6 is an optical microscope that has the ability observe samples at a high magnification at a high resolution. The microscope allows users to view their sample with up to a 2350x
Zeiss Z.1 Lightsheet. Quick-Start Guide
 Zeiss Z.1 Lightsheet Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 Chamber and Sample preparation Part 2 Image Acquisition Shut-down Start-up: Turn on the Microscope / Computers Turn
Zeiss Z.1 Lightsheet Quick-Start Guide Contents Start-up Preparing for Imaging Part 1 Chamber and Sample preparation Part 2 Image Acquisition Shut-down Start-up: Turn on the Microscope / Computers Turn
NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes
 NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes bh FLIM systems for the confocal and the multiphoton versions of the Leica SP2 and SP5 microscopes are available since 2002 [4]. These
NDD FLIM Systems for Leica SP2 MP and SP5 MP Multiphoton Microscopes bh FLIM systems for the confocal and the multiphoton versions of the Leica SP2 and SP5 microscopes are available since 2002 [4]. These
Prism Starter Guide 1.0 Hoskins Lab Last Modified 03/14/2017 Chris DeCiantis
 Start Up: Upon entering the laser room turn on the wall mounted Laser Power Button by pulling it away from the wall. Turn on Shutter controllers (toggle switch on back of unit). There should be a U in
Start Up: Upon entering the laser room turn on the wall mounted Laser Power Button by pulling it away from the wall. Turn on Shutter controllers (toggle switch on back of unit). There should be a U in
Short instructions. Olympus FV1000
 Olympus FV1000-1- Short instructions Short instructions. Olympus FV1000 Version 140416 This short instruction will guide you through the turn on and off sequence of the microscope system. For a detailed
Olympus FV1000-1- Short instructions Short instructions. Olympus FV1000 Version 140416 This short instruction will guide you through the turn on and off sequence of the microscope system. For a detailed
ECLIPSE LV Series Support Tools
 M374E 07.1.NF.2 (3/3) ECLIPSE LV Series Support Tools (Setup software for ECLIPSE LV series microscopes) Software Manual Introduction Thank you for purchasing the Nikon products. This manual describes
M374E 07.1.NF.2 (3/3) ECLIPSE LV Series Support Tools (Setup software for ECLIPSE LV series microscopes) Software Manual Introduction Thank you for purchasing the Nikon products. This manual describes
CoSMoS Starter Guide 1.4 Hoskins Lab Last Modified 03/03/2017 Chris DeCiantis
 Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System
 Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System Use Protocols: A. Reservation and Starting Up B. Sample Observation (No Image Capture) Transmitted
Biology Shared Instrumentation Facility Olympus IX70 Inverted Microscope and Simple PCI Imaging System Use Protocols: A. Reservation and Starting Up B. Sample Observation (No Image Capture) Transmitted
CoSMoS Starter Guide 1.6 Hoskins Lab Last Modified 03/30/2017 Chris DeCiantis
 Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Contents Start Up:... 2 Turn on Shutter controllers... 2 Turn on Lasers... 2 Turn on other equipment:... 2 Load Slide onto Sample Holder:... 2 Turn on Computer/ Start Labview/Glimpse:... 3 Shutters Activate.vi
Hand sectioning a mushroom
 Hand sectioning a mushroom 1)cut a wedge from cap that includes the gills 2) Hold the section tightly in your fingers and try to shave off thin longitudinal sections of the gills and cap 3) Float the section
Hand sectioning a mushroom 1)cut a wedge from cap that includes the gills 2) Hold the section tightly in your fingers and try to shave off thin longitudinal sections of the gills and cap 3) Float the section
Epilog Laser Cutter Instructions (Only the Essentials)
 Epilog Laser Cutter Instructions (Only the Essentials) How to export a file for SKETCHUP put it on the server, open it in Illustrator, and Prepare it for the Epilog Laser Cutter 1. In Sketchup: Draw a
Epilog Laser Cutter Instructions (Only the Essentials) How to export a file for SKETCHUP put it on the server, open it in Illustrator, and Prepare it for the Epilog Laser Cutter 1. In Sketchup: Draw a
Last class. This class. Single molecule imaging Deconvolution. FLIM Confocal
 FLIM, Confocal Last class Single molecule imaging Deconvolution This class FLIM Confocal FLIM Fluorescence Lifetime IMaging FLIM Fluorescence lifetime imaging Easier and more accurate quantitation Much
FLIM, Confocal Last class Single molecule imaging Deconvolution This class FLIM Confocal FLIM Fluorescence Lifetime IMaging FLIM Fluorescence lifetime imaging Easier and more accurate quantitation Much
Jasco FP-6500 Spectrofluorimeter Updated November 14, 2017
 Jasco FP-6500 Spectrofluorimeter Updated November 14, 2017 Instrument instructions can be found at: http://academic.bowdoin.edu/chemistry/resources/instructions.shtml If you have any problems with the
Jasco FP-6500 Spectrofluorimeter Updated November 14, 2017 Instrument instructions can be found at: http://academic.bowdoin.edu/chemistry/resources/instructions.shtml If you have any problems with the
Microscopy Specs. Media refractive index
 Zeiss AxioImager A2 EC Plan-Neofluar (420320-9901-000000) EC Plan-Neofluar (420330-9901-000000) Plan-Apochromat (420640-9900-000000) Plan-Apochromat (420650-9901-000000) Plan-Apochromat Korr (420660-9970-000)
Zeiss AxioImager A2 EC Plan-Neofluar (420320-9901-000000) EC Plan-Neofluar (420330-9901-000000) Plan-Apochromat (420640-9900-000000) Plan-Apochromat (420650-9901-000000) Plan-Apochromat Korr (420660-9970-000)
Heit/Kerfoot/Dikeakos. Leica DMI6000 B. User Manual
 Heit/Kerfoot/Dikeakos Leica DMI6000 B User Manual WARNING This microscope is a multi lab resource that many individuals are dependent on. As such the following rules must be followed in order to minimize
Heit/Kerfoot/Dikeakos Leica DMI6000 B User Manual WARNING This microscope is a multi lab resource that many individuals are dependent on. As such the following rules must be followed in order to minimize
Confocal Application Notes April 2010
 April 2010 Motorized Stage Applications Mark & Find Prepared by Louise Bertrand Applications and Technical Support Group, Leica Microsystems, Inc In this issue of Leica Confocal Application Notes, we describe
April 2010 Motorized Stage Applications Mark & Find Prepared by Louise Bertrand Applications and Technical Support Group, Leica Microsystems, Inc In this issue of Leica Confocal Application Notes, we describe
University of Minnesota Nano Fabrication Center Standard Operating Procedure
 Equipment Name: University of Minnesota Nano Fabrication Center Coral Name: hs-scope Revision Number: 1.5 Model: HS200A Revisionist: M. Fisher Location: Bay 1 Date: 9/12/2013 1 Description The Hyphenated
Equipment Name: University of Minnesota Nano Fabrication Center Coral Name: hs-scope Revision Number: 1.5 Model: HS200A Revisionist: M. Fisher Location: Bay 1 Date: 9/12/2013 1 Description The Hyphenated
The Microscope. Lab Exercise. Contents. Light Microscope. Objectives. Scanning Electron Microscope. Introduction. Transmission Electron Microscope
 Lab Exercise The Microscope Contents Objectives 1 Introduction 1 Activity.1 Care of Microscopes 2 Activity.2 Parts of the Microscope 3 Activity.3 Viewing Images 4 Activity.4 Magnification of Images 4 Resutls
Lab Exercise The Microscope Contents Objectives 1 Introduction 1 Activity.1 Care of Microscopes 2 Activity.2 Parts of the Microscope 3 Activity.3 Viewing Images 4 Activity.4 Magnification of Images 4 Resutls
Spectrometer Visible Light Spectrometer V4.4
 Visible Light Spectrometer V4.4 Table of Contents Package Contents...3 Trademarks...4 Manual Driver and Application installation...5 Manual Application Installation...6 First Start of the Application...8
Visible Light Spectrometer V4.4 Table of Contents Package Contents...3 Trademarks...4 Manual Driver and Application installation...5 Manual Application Installation...6 First Start of the Application...8
Exercise Guide. Published: August MecSoft Corpotation
 VisualCAD Exercise Guide Published: August 2018 MecSoft Corpotation Copyright 1998-2018 VisualCAD 2018 Exercise Guide by Mecsoft Corporation User Notes: Contents 2 Table of Contents About this Guide 4
VisualCAD Exercise Guide Published: August 2018 MecSoft Corpotation Copyright 1998-2018 VisualCAD 2018 Exercise Guide by Mecsoft Corporation User Notes: Contents 2 Table of Contents About this Guide 4
Proudly serving laboratories worldwide since 1979 CALL for Refurbished & Certified Lab Equipment Leica DM
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +847.913.0777 for Refurbished & Certified Lab Equipment Leica DM4000 6000 Brilliant, Easy Imaging at the Speed of Light! The New Generation
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +847.913.0777 for Refurbished & Certified Lab Equipment Leica DM4000 6000 Brilliant, Easy Imaging at the Speed of Light! The New Generation
Lab 4 - The Microscope
 Lab 4 - The Microscope Part A: The parts of the compound microscope. Obtain a microscope as indicated by your instructor. Always carry the microscope with one hand under the base and the other hand holding
Lab 4 - The Microscope Part A: The parts of the compound microscope. Obtain a microscope as indicated by your instructor. Always carry the microscope with one hand under the base and the other hand holding
IMAGING PLATFORMS IN THE FACULTY OF MEDICINE. Guo Jing Lab Manager Faculty Core Facilty June
 IMAGING PLATFORMS IN THE FACULTY OF MEDICINE Guo Jing Lab Manager Faculty Core Facilty June 27 2011 http://www.med.hku.hk/corefac/ Mission Training and education Basic operation Advanced application Imaging
IMAGING PLATFORMS IN THE FACULTY OF MEDICINE Guo Jing Lab Manager Faculty Core Facilty June 27 2011 http://www.med.hku.hk/corefac/ Mission Training and education Basic operation Advanced application Imaging
Motic Images Plus 3.0 ML Software. Windows OS User Manual
 Motic Images Plus 3.0 ML Software Windows OS User Manual Motic Images Plus 3.0 ML Software Windows OS User Manual CONTENTS (Linked) Introduction 05 Menus and tools 05 File 06 New 06 Open 07 Save 07 Save
Motic Images Plus 3.0 ML Software Windows OS User Manual Motic Images Plus 3.0 ML Software Windows OS User Manual CONTENTS (Linked) Introduction 05 Menus and tools 05 File 06 New 06 Open 07 Save 07 Save
2 SELECTING AND ALIGNING
 2 SELECTING AND ALIGNING Lesson overview In this lesson, you ll learn how to do the following: Differentiate between the various selection tools and employ different selection techniques. Recognize Smart
2 SELECTING AND ALIGNING Lesson overview In this lesson, you ll learn how to do the following: Differentiate between the various selection tools and employ different selection techniques. Recognize Smart
DV2. Alignment Procedure. Install DV2 on Microscope NOTE: PLEASE READ THE ENTIRE PROCEDURE BEFORE YOU BEGIN ALIGNMENT OF THE DV2. Alignment Procedure
 H I G H - P E R F O R M A N C E E M C C D & C C D C A M E R A S F O R L I F E S C I E N C E S DV2 This document provides a straightforward, step-by-step outline of the alignment procedure for the Photometrics
H I G H - P E R F O R M A N C E E M C C D & C C D C A M E R A S F O R L I F E S C I E N C E S DV2 This document provides a straightforward, step-by-step outline of the alignment procedure for the Photometrics
Standard Operating Procedure for the Horiba FluroMax-4
 Standard Operating Procedure for the Horiba FluroMax-4 Adapted from Horiba Operations Manual Created by Michael Delcau, Modified by Brian Lamp The Fluoromax is capable of making a variety of measurements.
Standard Operating Procedure for the Horiba FluroMax-4 Adapted from Horiba Operations Manual Created by Michael Delcau, Modified by Brian Lamp The Fluoromax is capable of making a variety of measurements.
MiBio Reference Manual
 MiBio Reference Manual www.microtek.com Copyright 2012 by Microtek International, Inc. All rights reserved. Trademarks Microtek, ScanMaker, ArtixScan, ScanWizard and ColoRescue are trademarks or registered
MiBio Reference Manual www.microtek.com Copyright 2012 by Microtek International, Inc. All rights reserved. Trademarks Microtek, ScanMaker, ArtixScan, ScanWizard and ColoRescue are trademarks or registered
XRADIA microxct Manual
 XRADIA microxct Manual Multiscale CT Lab Table of Contents 1. Introduction and Basics 1.1 Instrument Parts 1.2 Powering up the system 1.3 Preparing your sample 2. TXM Controller 2.1 Starting up 2.2 Finding
XRADIA microxct Manual Multiscale CT Lab Table of Contents 1. Introduction and Basics 1.1 Instrument Parts 1.2 Powering up the system 1.3 Preparing your sample 2. TXM Controller 2.1 Starting up 2.2 Finding
Gallios TM Quick Reference
 Gallios TM Quick Reference Purpose: The purpose of this Quick Reference is to provide a simple step by step outline of the information needed to perform various tasks on the system. We begin with basic
Gallios TM Quick Reference Purpose: The purpose of this Quick Reference is to provide a simple step by step outline of the information needed to perform various tasks on the system. We begin with basic
Application for Inverted Research Microscope ECLIPSE Ti2 Series Ti2 Control Ver Instruction Manual (for Android)
 M705E 17.6.Nx.5 Application for Inverted Research Microscope ECLIPSE Ti2 Series Ti2 Control Ver.1.1.0 Instruction Manual (for Android) Introduction Introduction Thank you for purchasing a Nikon product.
M705E 17.6.Nx.5 Application for Inverted Research Microscope ECLIPSE Ti2 Series Ti2 Control Ver.1.1.0 Instruction Manual (for Android) Introduction Introduction Thank you for purchasing a Nikon product.
New Olympus FV3000RS Laser Scanning Confocal System. Quotation Number V2
 New Olympus FV3000RS Laser Scanning Confocal System Quotation Number 00056013 V2 Quotation Date: 08/08/2018 Revised Date: 07/09/2018 Quotation number: 00056013 Quotation Expiry: 07/11/2018 Macquarie University
New Olympus FV3000RS Laser Scanning Confocal System Quotation Number 00056013 V2 Quotation Date: 08/08/2018 Revised Date: 07/09/2018 Quotation number: 00056013 Quotation Expiry: 07/11/2018 Macquarie University
Table of Contents. Important Information... 4 Product Description... 4 Computer Requirements Windows Based PCs Mac OS X Based PCs...
 Table of Contents Important Information... 4 Product Description... 4 Computer Requirements... 5 Windows Based PCs... 5 Mac OS X Based PCs... 5 Package Contents... 6 Product Overview... 7 Product Specifications...
Table of Contents Important Information... 4 Product Description... 4 Computer Requirements... 5 Windows Based PCs... 5 Mac OS X Based PCs... 5 Package Contents... 6 Product Overview... 7 Product Specifications...
Image Analysis begins with loading an image into GenePix Pro, and takes you through all the analysis steps required to extract data from the image.
 GenePix Pro 4.0 Tutorial 25 GenePix Pro 4.0 Tutorial This tutorial guides you through loading your first array and scanning your first array image. It also leads you through a basic array analysis, and
GenePix Pro 4.0 Tutorial 25 GenePix Pro 4.0 Tutorial This tutorial guides you through loading your first array and scanning your first array image. It also leads you through a basic array analysis, and
Click Install View Touch. Installation starts. Click Next. Click Finish.
 1. Please read the instructions carefully. Improper installation may cause permanent damages, which may not be covered by the warranty. 2. Check all the parts in the package against the following parts
1. Please read the instructions carefully. Improper installation may cause permanent damages, which may not be covered by the warranty. 2. Check all the parts in the package against the following parts
Quick Set-up Guide for the Biomedx Configured Olympus CX43
 Quick Set-up Guide for the Biomedx Configured Olympus CX43 Page 1 of 15 Your microscope is supplied with an Olympus manual, please refer to that for more complete information on microscope operation. This
Quick Set-up Guide for the Biomedx Configured Olympus CX43 Page 1 of 15 Your microscope is supplied with an Olympus manual, please refer to that for more complete information on microscope operation. This
ConfoCor 2 Fluorescence Correlation Microscope
 ConfoCor 2 Fluorescence Correlation Microscope Operating Manual Release 3.2 INTRODUCTION Carl Zeiss ConfoCor 2 Knowledge of this manual is required for the operation of the instrument. Would you therefore
ConfoCor 2 Fluorescence Correlation Microscope Operating Manual Release 3.2 INTRODUCTION Carl Zeiss ConfoCor 2 Knowledge of this manual is required for the operation of the instrument. Would you therefore
Use of the Binocular Microscope
 Use of the Binocular Microscope Before you begin this learning module be sure that you have the following materials in front of you: A microscope A packet of lens paper and Kimwipes A glass slide of a
Use of the Binocular Microscope Before you begin this learning module be sure that you have the following materials in front of you: A microscope A packet of lens paper and Kimwipes A glass slide of a
SENSITIVITY DIFFERENTLY TO SEE THINGS THE SPEED AND. Opera Phenix High Content Screening System
 THE SPEED AND SENSITIVITY TO SEE THINGS DIFFERENTLY Opera Phenix High Content Screening System For research use only. Not for use in diagnostic procedures. HIGH CONTENT SCREENING WITHOUT THE COMPROMISE
THE SPEED AND SENSITIVITY TO SEE THINGS DIFFERENTLY Opera Phenix High Content Screening System For research use only. Not for use in diagnostic procedures. HIGH CONTENT SCREENING WITHOUT THE COMPROMISE
Polarization Microscope. Omano OM349P Series. User Guide
 Polarization Microscope Omano OM349P Series User Guide Table of Contents Getting Started... 3 Components... 3 Technical Specifications... 5 Setup Instructions... 6 Basic Operation... 7 Changing the Light
Polarization Microscope Omano OM349P Series User Guide Table of Contents Getting Started... 3 Components... 3 Technical Specifications... 5 Setup Instructions... 6 Basic Operation... 7 Changing the Light
All-In-One. digital inverted microscope. High-quality Imaging Has Never Been Easier. evos-ca.com
 All-In-One digital inverted microscope High-quality Imaging Has Never Been Easier evos-ca.com Fluorescence Imaging Is Now Easier Than Ever ALL-IN-ONE, digital inverted fluorescence microscope The easiest
All-In-One digital inverted microscope High-quality Imaging Has Never Been Easier evos-ca.com Fluorescence Imaging Is Now Easier Than Ever ALL-IN-ONE, digital inverted fluorescence microscope The easiest
Windows XP. A Quick Tour of Windows XP Features
 Windows XP A Quick Tour of Windows XP Features Windows XP Windows XP is an operating system, which comes in several versions: Home, Media, Professional. The Windows XP computer uses a graphics-based operating
Windows XP A Quick Tour of Windows XP Features Windows XP Windows XP is an operating system, which comes in several versions: Home, Media, Professional. The Windows XP computer uses a graphics-based operating
Introduction to Microsoft Office PowerPoint 2010
 Introduction to Microsoft Office PowerPoint 2010 TABLE OF CONTENTS Open PowerPoint 2010... 1 About the Editing Screen... 1 Create a Title Slide... 6 Save Your Presentation... 6 Create a New Slide... 7
Introduction to Microsoft Office PowerPoint 2010 TABLE OF CONTENTS Open PowerPoint 2010... 1 About the Editing Screen... 1 Create a Title Slide... 6 Save Your Presentation... 6 Create a New Slide... 7
IMAGE STUDIO LITE. Tutorial Guide Featuring Image Studio Analysis Software Version 3.1
 IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
IMAGE STUDIO LITE Tutorial Guide Featuring Image Studio Analysis Software Version 3.1 Notice The information contained in this document is subject to change without notice. LI-COR MAKES NO WARRANTY OF
OPTIKA. fluo Series Upright and inverted epi-fluorescence microscopes
 OPTIKA M I C R O S C O P E S I T A L Y Upright and inverted epi-fluorescence microscopes FLUO B-383LD1 / B-383LD2 / B-383FL / B-500TiFL / XDS-2FL / XDS-3FL / XDS-3FL4 / SZP-FLUO / B-1000FL-LED / B-1000FL-HBO
OPTIKA M I C R O S C O P E S I T A L Y Upright and inverted epi-fluorescence microscopes FLUO B-383LD1 / B-383LD2 / B-383FL / B-500TiFL / XDS-2FL / XDS-3FL / XDS-3FL4 / SZP-FLUO / B-1000FL-LED / B-1000FL-HBO
Learning the Pro/ENGINEER Interface
 2 Learning the Pro/ENGINEER Interface This chapter introduces the Pro/ENGINEER interface tools: the menus, the dashboards, the selection tools and the viewing controls. As you go through this chapter,
2 Learning the Pro/ENGINEER Interface This chapter introduces the Pro/ENGINEER interface tools: the menus, the dashboards, the selection tools and the viewing controls. As you go through this chapter,
SolidWorks Intro Part 1b
 SolidWorks Intro Part 1b Dave Touretzky and Susan Finger 1. Create a new part We ll create a CAD model of the 2 ½ D key fob below to make on the laser cutter. Select File New Templates IPSpart If the SolidWorks
SolidWorks Intro Part 1b Dave Touretzky and Susan Finger 1. Create a new part We ll create a CAD model of the 2 ½ D key fob below to make on the laser cutter. Select File New Templates IPSpart If the SolidWorks
SolidWorks 2½D Parts
 SolidWorks 2½D Parts IDeATe Laser Micro Part 1b Dave Touretzky and Susan Finger 1. Create a new part In this lab, you ll create a CAD model of the 2 ½ D key fob below to make on the laser cutter. Select
SolidWorks 2½D Parts IDeATe Laser Micro Part 1b Dave Touretzky and Susan Finger 1. Create a new part In this lab, you ll create a CAD model of the 2 ½ D key fob below to make on the laser cutter. Select
HCImage Olympus IX3 Microscope Guide
 HCImage Olympus IX3 Microscope Guide 2017 Hamamatsu Corporation. All rights reserved. This guide, as well as the software described in it, is covered under license agreement and may be used or copied only
HCImage Olympus IX3 Microscope Guide 2017 Hamamatsu Corporation. All rights reserved. This guide, as well as the software described in it, is covered under license agreement and may be used or copied only
Optical Sectioning. Bo Huang. Pharmaceutical Chemistry
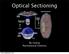 Optical Sectioning Bo Huang Pharmaceutical Chemistry Approaches to 3D imaging Physical cutting Technical difficulty Highest resolution Highest sensitivity Optical sectioning Simple sample prep. No physical
Optical Sectioning Bo Huang Pharmaceutical Chemistry Approaches to 3D imaging Physical cutting Technical difficulty Highest resolution Highest sensitivity Optical sectioning Simple sample prep. No physical
CyAn ADP Guide. Starting Up
 CyAn ADP Guide Starting Up 1. Check the sheath and waste fluids, if needed, fill and empty: 1. To fill the Sheath Tank: a. Unscrew the top of the tank labeled Sheath Tank, and set it on top of the tank.
CyAn ADP Guide Starting Up 1. Check the sheath and waste fluids, if needed, fill and empty: 1. To fill the Sheath Tank: a. Unscrew the top of the tank labeled Sheath Tank, and set it on top of the tank.
Olympus cellsens TIRF Guide
 Startup Power up the system as described in the "Startup & Environmental Control" guide, including power strip #2 ("TIRF"). ON For each laser line needed, turn the key on the control unit right and press
Startup Power up the system as described in the "Startup & Environmental Control" guide, including power strip #2 ("TIRF"). ON For each laser line needed, turn the key on the control unit right and press
Fluorimeter User s Booklet
 Fluorimeter User s Booklet 1) Verify that the instrument power and the computer are both off. Then turn on the lamp. The red indicator light will glow when the lamp is on. Turn on lamp switch here. This
Fluorimeter User s Booklet 1) Verify that the instrument power and the computer are both off. Then turn on the lamp. The red indicator light will glow when the lamp is on. Turn on lamp switch here. This
Dorigny resources MICROSCOPES - BIOPHORE. Confocal Microscope Zeiss LSM 700
 Dorigny resources Contributed by Yannick KREMPP Friday, 13 July 2007 Last Updated Monday, 30 October 2017 MICROSCOPES - BIOPHORE Confocal Microscope Zeiss LSM 700 This Zeiss LSM700 confocal is mounted
Dorigny resources Contributed by Yannick KREMPP Friday, 13 July 2007 Last Updated Monday, 30 October 2017 MICROSCOPES - BIOPHORE Confocal Microscope Zeiss LSM 700 This Zeiss LSM700 confocal is mounted
Independent Resolution Test of
 Independent Resolution Test of as conducted and published by Dr. Adam Puche, PhD University of Maryland June 2005 as presented by (formerly Thales Optem Inc.) www.qioptiqimaging.com Independent Resolution
Independent Resolution Test of as conducted and published by Dr. Adam Puche, PhD University of Maryland June 2005 as presented by (formerly Thales Optem Inc.) www.qioptiqimaging.com Independent Resolution
Zeiss Efficient Navigation (ZEN) Blue Edition Standard Operation Protocol
 Faculty Core Facility ZEN BLUE 2.3 SOP A-1 Zeiss Efficient Navigation (ZEN) Blue Edition Standard Operation Protocol Faculty Core Facility ZEN BLUE 2.3 SOP A-2 A. Content Overview. 3 Start up. 4 Display
Faculty Core Facility ZEN BLUE 2.3 SOP A-1 Zeiss Efficient Navigation (ZEN) Blue Edition Standard Operation Protocol Faculty Core Facility ZEN BLUE 2.3 SOP A-2 A. Content Overview. 3 Start up. 4 Display
ANDOR/OLYMPUS SPINNING DISK USER GUIDE
 ANDOR/OLYMPUS SPINNING DISK USER GUIDE CONTENTS Overview... 2 Turn off sequence:... 2 Turn on sequence:... 2 Getting started... 2 Multi Dimensional Acquisition (MDA)... 3 FRAP... 3 Focus motors and long-term
ANDOR/OLYMPUS SPINNING DISK USER GUIDE CONTENTS Overview... 2 Turn off sequence:... 2 Turn on sequence:... 2 Getting started... 2 Multi Dimensional Acquisition (MDA)... 3 FRAP... 3 Focus motors and long-term
CS Multimedia and Communications REMEMBER TO BRING YOUR MEMORY STICK TO EVERY LAB! Lab 02: Introduction to Photoshop Part 1
 CS 1033 Multimedia and Communications REMEMBER TO BRING YOUR MEMORY STICK TO EVERY LAB! Lab 02: Introduction to Photoshop Part 1 Upon completion of this lab, you should be able to: Open, create new, save
CS 1033 Multimedia and Communications REMEMBER TO BRING YOUR MEMORY STICK TO EVERY LAB! Lab 02: Introduction to Photoshop Part 1 Upon completion of this lab, you should be able to: Open, create new, save
Application for Inverted Research Microscope ECLIPSE Ti2 Series Ti2Control Ver Instruction Manual (for Android)
 M705E 17.3.Nx.4 Application for Inverted Research Microscope ECLIPSE Ti2 Series Ti2Control Ver.1.0.2 Instruction Manual (for Android) Introduction Introduction Thank you for purchasing a Nikon product.
M705E 17.3.Nx.4 Application for Inverted Research Microscope ECLIPSE Ti2 Series Ti2Control Ver.1.0.2 Instruction Manual (for Android) Introduction Introduction Thank you for purchasing a Nikon product.
Work Instructions for Confocal Laser Scanning Microscopy
 Contents 0.1 To be Verified Before Switch ON......................... 2 0.2 Switch ON Protocol-Single Photon Mode Confocal Laser Scanning Microscope 2 0.3 Switch ON Protocol - Multi Photon Mode Confocal
Contents 0.1 To be Verified Before Switch ON......................... 2 0.2 Switch ON Protocol-Single Photon Mode Confocal Laser Scanning Microscope 2 0.3 Switch ON Protocol - Multi Photon Mode Confocal
XnView Image Viewer. a ZOOMERS guide
 XnView Image Viewer a ZOOMERS guide Introduction...2 Browser Mode... 5 Image View Mode...14 Printing... 22 Image Editing...26 Configuration... 34 Note that this guide is for XnView version 1.8. The current
XnView Image Viewer a ZOOMERS guide Introduction...2 Browser Mode... 5 Image View Mode...14 Printing... 22 Image Editing...26 Configuration... 34 Note that this guide is for XnView version 1.8. The current
INFINITY-CORRECTED TUBE LENSES
 INFINITY-CORRECTED TUBE LENSES For use with Infinity-Corrected Objectives Available in Focal Lengths Used by Thorlabs, Nikon, Leica, Olympus, and Zeiss Designs for Widefield and Laser Scanning Applications
INFINITY-CORRECTED TUBE LENSES For use with Infinity-Corrected Objectives Available in Focal Lengths Used by Thorlabs, Nikon, Leica, Olympus, and Zeiss Designs for Widefield and Laser Scanning Applications
Publishing Electronic Portfolios using Adobe Acrobat 5.0
 Step-by-Step Publishing Electronic Portfolios using Adobe Acrobat 5.0 2002, Helen C. Barrett Here is the process we will use to publish a digital portfolio using Adobe Acrobat. The portfolio will include
Step-by-Step Publishing Electronic Portfolios using Adobe Acrobat 5.0 2002, Helen C. Barrett Here is the process we will use to publish a digital portfolio using Adobe Acrobat. The portfolio will include
Blender Lesson Ceramic Bowl
 Blender Lesson Ceramic Bowl This lesson is going to show you how to create a ceramic looking bowl using the free program Blender. You will learn how to change the view, add, delete, scale and edit objects
Blender Lesson Ceramic Bowl This lesson is going to show you how to create a ceramic looking bowl using the free program Blender. You will learn how to change the view, add, delete, scale and edit objects
Leica DMI4000 B Leica DMI6000 B. Automated Inverted Microscopes for Life Sciences
 Leica DMI4000 B Leica DMI6000 B Automated Inverted Microscopes for Life Sciences Leica Design by Christophe Apothéloz 3 Intelligence Intelligent Imaging At the Touch of a Button Let there be light Change
Leica DMI4000 B Leica DMI6000 B Automated Inverted Microscopes for Life Sciences Leica Design by Christophe Apothéloz 3 Intelligence Intelligent Imaging At the Touch of a Button Let there be light Change
