Motion management for MRI-guided abdominal radiotherapy. Bjorn Stemkens
|
|
|
- Barbra Hopkins
- 5 years ago
- Views:
Transcription
1 Motion management for MRI-guided abdominal radiotherapy Bjorn Stemkens
2 Colophon Cover: The Vitruvian man demonstrating the radial k-space trajectory. The magnifying glass resembles the MRL; clear visualization of internal anatomy leading to a converging radiation beam. Design: B. Stemkens, Vitruvian man designed by Freepik. Motion management for MRI-guided abdominal radiotherapy PhD thesis, Utrecht University, the Netherlands All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means without the prior written permission from the author. The copyright of the papers that have been published or have been accepted for publication has been transferred to the respective journals. For one project (Chapter 3) funding was provided in part by the Royal Netherlands Academy of Arts and Sciences (KNAW) Ter Meulen grant. Manuscript Layout: B. Stemkens Typeset in: L A TEX Printed by: ProefschriftMaken ISBN: Copyright c Bjorn Stemkens c Elsevier (Chapter 2) c Wiley (Chapters 3 and 6) c IOP Publishing Ltd (Chapters 4 and 5)
3 Motion management for MRI-guided abdominal radiotherapy Het controleren van beweging voor MRI-gestuurde radiotherapie in het abdomen (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de rector magnificus, prof.dr. G.J. van der Zwaan, ingevolge het besluit van het college voor promoties in het openbaar te verdedingen op 5 oktober 2017 des middags te 2.30 uur door Bjorn Stemkens geboren op 30 maart 1989 te Helmond
4 Promotor: Copromotoren: Prof.dr.ir. J.J.W. Lagendijk Dr.ir. H.N. Tijssen Dr.ir. C.A.T. van den Berg Financial support for publication of this thesis was kindly provided by Philips, Modus Medical Devices Inc., Elekta and Chipsoft.
5 For those about to rock, we salute you!
6
7 Contents List of Acronyms ix 1 General introduction 1 2 Optimizing 4D-MRI data sampling for respiratory motion analysis of pancreatic tumors 13 3 Adaptive bulk-motion exclusion for improved robustness of abdominal MR imaging 25 4 Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy 41 5 Dose accumulation in free-breathing MR-guided renal-cell carcinoma SBRT 65 6 Maximizing SNR in simultaneous multi-slice body imaging 83 7 Summary and Discussion Samenvatting 113 Bibliography 117 List of Publications 129 Acknowledgements 133 Curriculum vitae 137 vii
8
9 List of Acronyms bssfp CAIPIRINHA CBCT CS CT CTV DCE DVF DWI EBRT ED EPID FFT FID FOV g-factor GLM GRAPPA GTV IGRT IMRT ITV kv Linac LISS MLC MRI MRL MS MV NUFFT OAR PET balanced Steady-State Free Precession Controlled Aliasing In Parallel Imaging Results In Higher Acceleration Cone-Beam Computed Tomography Compressed Sensing Computed Tomography Clinical Target Volume Dynamic Contrast Enhanced Deformable Vector Field Diffusion Weighted Imaging External Beam Radiation Therapy Electron Density Electronic Portal Imaging Device Fast Fourier Transform Free-Induction Decay Field-Of-View Geometry factor General Linear Model Generalized Autocalibrating Partially Parallel Acquisitions Gross Tumor Volume Image-Guided Radiation Therapy Intensity Modulated Radiation Therapy Internal Target Volume kilo Voltage Linear Accelerator Linearly Increasing Slice Shifts Multi-Leaf Collimator Magnetic Resonance Imaging Magnetic Resonance Linear Accelerator Multi-Slice Mega Voltage Non-Uniform Fast Fourier Transform Organ At Risk Positron Emission Tomography ix
10 PI PSA PTV RF SBRT SENSE SMASH SMS SNR SOS SPECT TCP TSE TV VMAT Parallel Imaging Power Spectrum Analysis Planning Target Volume Radio Frequency Stereotactic Body Radiation Therapy Sensitivity Encoding Simultaneous Acquisition of Spatial Harmonics Simultaneous Multi-Slice Signal-to-Noise Ratio Stack-Of-Stars Single-Photon Emission Computed Tomography Tumor Control Probability Turbo Spin Echo Total Variation Volumetric Arc Therapy
11 CHAPTER 1 General introduction 1.1 Radiotherapy Radiotherapy, or radiation therapy, is one of the main cancer therapies. It is a complex, but captivating treatment modality that uses high-energy ionizing radiation to damage and kill malignant cancer cells. The goal of radiotherapy is to deliver lethal doses to tumorous cells, whilst sparing healthy tissue as much as possible. In standard external beam radiotherapy (EBRT), which is the radiotherapy technique highlighted in this thesis, mega voltage (MV) photons are generated by a linear accelerator, or linac. These photons interact with matter in the human body, generating secondary electrons that damage the DNA [1]. In case this damage is irreparable, these cells are eliminated. Since a higher dose to the tumor results in higher tumor control probability (TCP), ideally high doses are delivered to the tumor [1]. A favorable hallmark is the diminished repair ability of tumorous cells, making them more sensitive to radiation. Although normal tissue is also damaged by the radiation dose, EBRT is generally delivered in multiple fractions (fractionation) [2] to allow normal tissue to recover. As an example, glioblastoma multiforme, an aggressive type of brain cancer, is treated with 30 fractions of 2 Gy spread over 6 consecutive weeks at our institution. Inevitably, radiotherapy has to balance between delivering sufficiently high doses to the tumor, and limiting the radiation dose to the surrounding healthy tissue to reduce adverse effects as much as possible. Radiotherapy treatment process As preparation for the treatment, pre-treatment imaging scans of the patient are acquired. On these scans, relevant anatomical structures are delineated and material properties of the tissues that are used in the dose calculations, such as the electron densities (ED), are defined. The radiation oncologist manually delineates the visible tumor, called the Gross Tumor Volume (GTV), and the 1
12 Chapter 1 General introduction surrounding organs at risk (OARs) [3]. Since most tumors have microscopic tumor spread, the GTV is often expanded to create the Clinical Target Volume (CTV) [3]. An additional margin is added to the CTV, the so-called Planning Target Volume (PTV) [3], to account for all geometric inaccuracies and uncertainties, such as patient setup errors, delineations inaccuracies and tumor position. Based on these delineations and predefined dose prescriptions and constraints, a radiation treatment plan is generated that aims to deliver the prescribed dose to the tumor, while minimizing dose to the OARs. Since the same radiation plan is used for up to 35 fractions (i.e. 7 weeks), the PTV margin should also account for patient weight loss, tumor shrinkage or growth, and other variations between fractions. Since tumor margins are designed to prevent underdosage and derived from patient population statistics, nearby OARs are sometimes irradiated with considerable doses [4, 5]. Radiotherapy of abdominal tumors, such as liver, renal, and pancreatic tumors, is further hindered by physiological-induced motion, such as respiratory, cardiac and peristaltic motion. Respiration, which is the prime focus of this thesis, induces quasi-periodic tumors displacements, which can be up to 40 mm in cranio-caudal direction [6, 7], and is therefore the main source of motion in the abdomen. To deliver adequate dose to the target, an additional margin encompassing this internal motion, the Internal Target Volume (ITV) [8], is commonly used on top of the CTV. On the day of treatment, the plan is sent to the linac where the patient is carefully positioned. Fixation devices, such as thermoplastic masks for head-and-neck tumors [9], arm supports for breast tumor [10], and corsets for abdominal tumors [11, 12], are often used to minimize motion to a certain extent. Stereotactic Body Radiotherapy Most tumor types encountered in the abdomen are considered to be radioresistant to conventional EBRT [13 15]. In combination with high radio-sensitivity of healthy surrounding tissue, these tumors are challenging to irradiate accurately. To get a high TCP, Stereotactic Body Radiotherapy (SBRT) gained interest in recent years in treating abdominal tumors. This treatment method uses a small number of high dose fractions and steep dose gradients to enable tight margins [1, 16]. Precise targeting and accurate motion management is therefore the basis for good treatment outcomes in abdominal tumors irradiated with SBRT. This thesis outlines some concepts and solutions for more precise and accurate targeting and motion management. 1.2 Imaging in radiotherapy To achieve the required accuracy in SBRT, image guidance is essential. Besides providing crucial information for accurate delineations, imaging is frequently used in modern day radiotherapy to direct and monitor the treatment, a process called image-guided radiotherapy (IGRT) [17, 18]. The increased imaging opportunities in IGRT have the potential to improve accuracy of the radiation field, reduce dose exposure to OARs and manage respiratory motion precisely. However, precise 2
13 1.2 Imaging in radiotherapy imaging lies at the basis of successful IGRT, especially for abdominal tumors that are affected by respiratory motion. kv- and MV-based IGRT In the pre-treatment, or simulation phase, imaging is used to define the target volume and delineate the OARs. In addition to Computed Tomography (CT) that is generally always acquired, other imaging modalities, including Positron Emission Tomography (PET), Single-Photon Emission Computed Tomography (SPECT), and Magnetic Resonance Imaging (MRI) can be used. Additionally, these complementary techniques can reflect tumor tissue characteristics, such as cell density, tissue metabolism and proliferation rate. Next, at the start of each treatment fraction the patient is positioned such that the tumor is at the same location as the tumor location in the treatment plan. In addition to skin tattoo alignment, in-room radiographic imaging is used to verify and, whenever necessary, refine the position using repositioning and couch shifts. In this prebeam phase different kilo voltage (kv) and MV-based imaging modalities are used. The Electronic Portal Imaging Device (EPID), mounted diametrically with relation to the MV source, uses the treatment beam to acquire MV transmission images [19]. Due to the high energy, EPID imaging has low contrast and can therefore only differentiate bone, air and implanted markers from soft tissue (see Figure 1.1). Cone-beam CT (CBCT), on the other hand, uses a radiation source in the kv range, mounted perpendicular to the MV source, to acquire CT-like images, by rotating the gantry around the patient [20]. The higher contrast in these lower energy images facilitates setup correction based on bones, markers, and, to some extent, soft tissue landmarks. However, as can be seen in Figure 1.1 image quality is generally limited. The increased accuracy in IGRT facilitates a PTV reduction and consequently decreases dose to the OARs, while increasing the TCP. However, respiratory motion can severely impair this increased accuracy. First, respiratory motion during image acquisition can cause artifacts, leading to deceptive structures and misinterpretation of images [21 23]. Even if imaging is fast enough to minimize motion artifacts, it is often unclear what respiratory state is represented in the images. Moreover, this motion state may not represent the motion during treatment, leading to increased uncertainty. To reduce this uncertainty, the respiratory behavior of the tumor can be characterized using phase-resolved imaging, such as 4D-CT, in which the fourth dimension reflects the different respiratory phases [24]. The phase-resolved respiratory motion information can be used to generate the aforementioned ITV margin, or for other respiratory motion management techniques like the mid-ventilation [25] or midposition concepts [26]. Alternatively, it can be taken into account through 4D planning strategies [27, 28]. Second, respiratory motion can change between fractions (inter-fraction) or within a fraction (intra-fraction) [29]. Changes from abdominal to thoracic breathing and vice versa, respiratory frequency variations, and amplitude fluctuations can 3
14 Chapter 1 General introduction EPID CBCT CT MRI Figure 1.1: Four modalities that are used in image-guided radiotherapy. Portal imaging, acquired in the pelvic region, and cone-beam CT of the abdomen, both acquired just prior to treatment, display limited soft-tissue contrast. The lower row exemplifies pre-treatment CT and MRI of the same abdominal patient showing increased image quality and superior soft-tissue contrast in MRI. all result in differences in the mean abdominal tumor position, or baseline, leading to systemtic errors [30 32]. Phase-resolved 4D-CBCT or fluoroscopic imaging is often used to detect the inter-fraction variations, but due to the limited contrast in these imaging modalities, restricted to changes of implanted fiducial markers [33]. Intra-fraction motion compensation is even more complicated, but is especially relevant in hypo-fractionated treatments, such as SBRT, as (baseline) errors are less likely to cancel out over the limited number of fractions. Gated radiation delivery can minimize motion errors by switching off the treatment beam when the target is outside a predefined motion window [34]. A more time-efficient strategy is tracking, which constantly aligns the radiation beam with the moving target by dynamically adapting the collimator aperture using the multi-leaf collimators (MLC). The required feedback signal can be extracted from MV imaging [35], or a combination of kv and MV imaging [36]. However, the disadvantage of these methods is the fact that they use markers or surrogates, either inside or outside 4
15 1.3 MRI in radiotherapy the body, to provide the required motion signal for abdominal tumors. The limited number of markers do not provide motion information of OARs, are invasive and therefore increase patient stress, risk of perforation and contamination, are not suitable for all tumors sites, and may migrate from the expected implanted position [37]. Direct visualization of the internal organ motion could provide accurate motion signals, leading to better treatments [38, 39]. Whilst many different imaging modalities and techniques are proposed for the aforementioned imaging tasks, combining all these data requires accurate (nonrigid) registration to a common reference frame. This registration process introduces uncertainties that propagate as a systematic error throughout the treatment [40, 41]. Ideally, a single imaging modality is used that has the ability to provide imaging data for all stages within the radiotherapy treatment workflow. 1.3 MRI in radiotherapy MRI can provide imaging data for all processes of the radiotherapy treatment. Its use in radiotherapy was first demonstrated in 1987 by [42] and the first MRI simulator dedicated for radiotherapy was proposed in 1996 [43]. With its superior soft-tissue contrast and multi-contrast abilities both tumorous tissue and OARs can clearly be visualized in the abdomen, which aids target definition. Moreover, opposed to CT, MRI can visualize the underlying anatomy in any desired orientation in one, two, or three dimensions and does not use ionizing radiation. Lastly, physiological motion, including respiratory [44], cardiac [45], and peristaltic motion [46], can be mapped using dedicated MR sequences. Concluding, MRI would be an excellent imaging modality for all stages of IGRT. Consequently, the use of MRI in radiotherapy has become more common and combining MRI with radiotherapy treatment gained large interest in recent years [47]. MRI principles MRI is a sophisticated, but intriguing imaging technique. Despite numerous parameters that can be tuned, there is an inherent trade-off between acquisition speed, resolution and signal-to-noise ratio (SNR), which has to be optimized for the desired application. Here, the basic principles for understanding the ideas and methods presented in this thesis are outlined. For a more comprehensive review and physical background the reader is referred to [21, 48, 49] and the references therein. Most clinically used MR acquisitions fill k-space in a line-by-line manner on a rectilinear grid, so-called Cartesian sampling. Advantages of Cartesian sampling include the direct use of a subsequent inverse FFT and therefore fast reconstruction, reduced sensitivity to gradient imperfections, and the fact that most acceleration algorithms have been designed for Cartesian sampling. Since MRI is generally slow, numerous techniques have been developed to accelerate acquisitions. Parallel imaging (PI) is a robust acceleration method that acquires a reduced amount of 5
16 Chapter 1 General introduction Cartesian Radial k y k x φ k r k y k y k x k x G x G x G y G y Figure 1.2: Illustration of Cartesian and radial filling of k-space alongside the required gradient waveforms for acquiring these trajectories. The white dots represent the acquired k-space points. k-space data using multi-channel receive coil arrays that circumvent the anatomy. The unavoidable undersampling artifacts, which appears as aliasing in Cartesian acquisitions, are resolved using reconstruction techniques like simultaneous acquisition of spatial harmonics (SMASH) [50], sensitivity enconding (SENSE) [51] and generalized autocalibrating partially parallel acquisitions (GRAPPA) [52]. These techniques use additional encoding power from differences in spatial coil sensitivities in a multi-channel coil array to resolve aliasing and reconstruct an unaliased image. Since less data is acquired, defined as the reduction factor R, the SNR is decreased by a factor SNR PI = SNR full g R, (1.1) where SNR full is the SNR for a fully sampled data set and g is the geometry factor. This g-factor is equal to or greater than one and expresses the spatially varying noise amplification. From Equation 1.1 it can be deduced that an acceleration of R=2, results in an SNR penalty of at least 29%. To alleviate this SNR loss, simultaneous multi-slice (SMS) [53] and Controlled Aliasing in Parallel Imaging Results In Higher Acceleration (CAIPIRINHA) [54, 55] have been introduced. These techniques sample multiple slices simultaneously and thereby mitigate the R SNR penalty [53], exploit coil sensitivity variations more efficiently by controlling the aliasing [55], or a combination of both [54] to increase the effective SNR. Although these techniques can increase SNR per slice for accelerated scans significantly, the increased number of parameters that have to be defined in these methods makes it increasingly difficult to take full advantage of its potential SNR 6
17 1.3 MRI in radiotherapy T2 T1 Contrast-enhanced Diffusion Figure 1.3: Different contrast weightings in MR images acquired in the same patient, each showing unique tumor and OARs characteristics. gain. Moreover, since 3D acquisitions are relatively slow compared to physiological movements, even when high acceleration factors are used, motion-induced artifacts can appear. Alternative sampling methods to fill k-space include radial, spiral, and propeller sampling. Due to technical improvements in hardware and software these non- Cartesian sampling methods re-gained interest in recent years. Because of its reduced sensitivity to motion artifacts, incoherent undersampling artifacts, and self-navigation opportunities, radial sampling is used extensively throughout this thesis. In a radial acquisition k-space is sampled along radial spokes, contrary to parallel rows (see Figure 1.2). The azimuthal angle φ between subsequent spokes can be chosen arbitrary. In this thesis either uniform sampling, for which φ is defined as π divided by the number of spokes [56], or Golden angle sampling, in which φ = , is used [57]. Disadvantages of radial sampling include the increased sensitivity to system imperfections, such as gradient imperfections, and the fact that data has to be regridded onto a rectilinear grid prior to applying an inverse FFT, thus increasing reconstruction time. MRI for simulation The use of MRI in the radiation treatment planning has shown a rapid growth in the past decade. By detailed imaging of the tumor, its micro-environment, and its surroundings, a precise delineation of the tumor and OARs can be established. Besides common T 1 - and T 2 -weighted images, functional MRI, such as diffusion weighted (DWI) and dynamic contrast enhanced (DCE) imaging, has been used to discriminate between healthy and tumorous tissue (see Figure 1.3). Similar techniques also proved to be useful in assessing tumor response after radiotherapy. Examples include determining the response of esophageal [58] and rectal [59] tumors, and quantification of radiation-induced changes in the OARs for headand-neck patients [60]. Abdominal MR imaging is challenging, due to physiological and bulk motion, resulting in degraded image quality and non-diagnostic images. To reduce motionrelated artifacts, numerous techniques have been proposed. Commonly used clinical solutions include breath-holds, respiratory triggering, or respiratory gating, using either 1D-MR navigators or external respiratory bellows as surrogate signal. However, many (older) patients are unable to sustain a breath hold long enough 7
18 Chapter 1 General introduction 2008 Prototype 2012 Prototype 2017 Clinical Prototype Figure 1.4: Overview of the different prototypes of the MRL system developed at the radiotherapy department of the University Medical Center Utrecht. (often > 15 seconds). Because of its reduced sensitivity to respiratory motion, radial sampling is a good alternative for abdominal imaging [61]. Alternatively, a 4D-MRI can be acquired that captures (i.e. characterizes) motion, instead of suppress it, similar to 4D-CT. Unfortunately, such acquisitions are not directly available on clinical MR scanners and therefore an active field of research. Ideally, a 4D-MRI acquisition is fast, has good contrast between different structures and sufficient spatial resolution to resolve the motion accurately. Moreover, it should cover an adequately large 3D field-of-view (FOV) and be consistent over a large cohort of patients. In conclusion, robust abdominal imaging remains an unsolved problem but is key to accurate delineations and tumor characterization. MRL Motivated by the high flexibility, superior soft-tissue contrast, and real-time imaging feedback, the idea of a hybrid MRI-linac was first proposed in 1999 [62]. Despite numerous technological difficulties, such as magnetic coupling between the MRI and the linac gun, beam transmission through the cryostat and RF interference, the first prototypes were already developed around 2008 (see Figure 1.4). Currently, there are four different designs with various magnetic field strengths, orientations and radiation sources that have shown proof-of-principles or are clinically introduced. The system proposed by the Cross Center Institute (Edmonton, Alberta, Canada) is based on a biplanar 0.6T magnet with a perpendicular or parallel 6 MV linac waveguide [63]. A functional prototype with a 0.2 T permanent magnet was built and tested in 2008 [64]. The Australian MRI-linac also uses a 6 MV linac system, but has a custom designed 1T open-bore magnet [65]. This system produced the first images in 2016 [66]. The MRIdian system (ViewRay, Mountain View, CA, United States) consists of a split 0.35T cylindrical magnet, combined with 3 60 Co sources, mounted at 120 angles around the patient [67]. Despite the relatively low magnetic field, it has 8
19 1.4 Imaging on the MRL Brain Head-neck Kidney Liver Transversal Coronal Sagittal Figure 1.5: Example of different anatomical images acquired on the MRL at the University Medical Center Utrecht. been successfully used in several hospitals since 2014 [68]. In 2017, a linac-based system was presented at ESTRO 36. The research described in this thesis is conducted for the MRI-linac (MRL) machine, designed at the radiotherapy department in Utrecht in collaboration with Philips and Elekta [69, 70]. This machine combines a whole-body 1.5T cylindrical MRI scanner (Ingenia, Philips, Best, the Netherlands), with a 7 MV linac (Elekta AB, Stockholm, Sweden) mounted on a ring-shaped gantry and was unveiled at ESTRO 36 as the Unity system (see Figure 1.4). A beam passage path, created by splitting the gradient coil and the main magnet, allows for an irradiation field of 23 cm in feet-head direction at the isocenter of the system. Proof-of-principle images were acquired with and without radiation in 2009 [71], while gating [72] and tracking [73] possibilities were shown in 2011 and 2012, respectively. At the University Medical Center Utrecht, patients and volunteers have been imaged on this system since early 2017 (see Figure 1.5) and the first patients were treated in May Imaging on the MRL The MRL offers the opportunity to acquire diagnostic quality MR images just before treatment, so-called pre-beam imaging, and during radiation, a process called beam-on imaging. While the pre-beam imaging can be used for positioning, plan adaptation and motion characterization, the beam-on imaging yields necessary information for gating and tracking. This increased amount of imaging information may lead to less uncertainties and therefore allows more conformal 9
20 Chapter 1 General introduction dose distributions with smaller margins. Imaging on the MRL can be divided into three different parts that have their own specific requirements. Pre-beam imaging While a single pre-treatment CT provides a snapshot of the anatomy, pre-beam imaging can produce a daily set of high spatial resolution anatomical images to determine the daily anatomy. The pre-treatment delineations can subsequently be transferred to this daily anatomy using deformable image registration. This information can then be used to apply virtual couch shifts [74], or completely re-optimized the plan [75, 76], a process called adaptive radiotherapy [77]. This does not only require high-resolution imaging with sufficient contrast, but also fast planning tools, as this needs to be performed while the patient is on the treatment table. Besides set-up imaging, the pre-beam imaging phase can additionally be used for motion characterization for moving tumors, such as abdominal tumors. Since the pre-beam imaging time is limited, imaging should be in the order of seconds to a few minutes, including the reconstruction, and be very robust, since re-acquisitions are unrealistic. Ideally, sufficient contrast is present to facilitate deformable registrations or re-delineation, and the tumor and OARs motion is quantified over a large FOV in three dimensions with high geometric fidelity. Beam-on imaging Beam-on imaging has to provide adequate motion information for online gating and tracking. This requires a sufficiently fast acquisition, reconstruction, and post-processing to quantify motion with minimal latency. Since a respiratory cycle typically takes between three and ten seconds, tracking requires sub-second temporal resolution in the order of a few hundred ms. Ultimately, the imaging should be volumetric and near real-time with sufficient contrast and resolution. As this is currently not possible, acquisitions that have sufficient SNR and spatiotemporal resolution are limited to 2D and therefore lack volumetric coverage. Imaging for dose calculations Since imaging data can be acquired during treatment, the accumulated dose could be calculated after each treatment fraction, by correlating this imaging data with the linac output. Knowledge of the 3D position of every voxel over the entire treatment time is prerequisite. However, constraints on reconstruction time are less stringent, as these calculations can be performed after each fraction. Differences between the accumulated dose and planned dose can then be accounted for in the next fraction. 1.5 Scientific contributions and thesis outline This thesis aims to present strategies for MR-guided abdominal radiotherapy. These include MR methods for robust abdominal acquisitions and accurate 4D 10
21 1.5 Scientific contributions and thesis outline motion characterization for pre-treatment and pre-beam imaging, fast MR methods for beam-on imaging, and MRI-based procedures to calculate the accumulated dose. Chapter 2 introduces a volumetric radial stack-of-stars (SOS) acquisition scheme for pre-treatment and pre-beam 4D-MRI motion characterization. It is shown that this sampling method is beneficial for reformatting 3D data into a phaseresolved 4D-MRI compared to conventional Cartesian sampling. Moreover, to sort the acquired data, several respiratory motion surrogate signal are evaluated and it is shown that internal surrogates are more robust than external ones. This acquisition may replace 4D-CT in the future for robust motion characterization. This radial SOS sampling method is used in Chapter 3 for robust free-breathing abdominal imaging in the presence of bulk motion. The free-induction decay (FID) is used as internal surrogate signal to automatically identify and exclude bulk motion-corrupted data in real time. It is shown that this increases image quality, reduces artifacts and results in an overall increase of acquisition robustness. Chapter 4 focuses on generating volumetric MR images with high spatio-temporal resolution, so-called volumetric cine-mri. Using the 4D-MRI method outlined in Chapter 2, a respiratory motion model is generated by parameterizing its underlying motion. This model is subsequently used to generate high spatio-temporal resolution 3D MR volumes by filling in missing volumetric information of fast 2D beam-on cine-mr images. To ensure its correctness, the accuracy of the 4D-MRI and 2D cine-mr images is validated using phantom measurements. In Chapter 5 these volumetric cine-mris are used to calculate the accumulated dose of abdominal tumor treatments. It is shown that precise imaging with sufficient temporal resolution is required for accurate dose tracking algorithms. Both fast and slow variations in breathing can cause significant deviations from the planned dose and should be taken into account. The model introduced in Chapter 4 uses a limited number of 2D slices. By increasing the number of slices, the resulting volumetric cine-mr images are better defined and more information is available for gating and tracking. SMS and CAIPIRINHA are promising technique, but rarely used in the abdomen. Chapter 6 investigates the potential acceleration factors that can be achieved using these methods in the abdomen. By optimizing specific parameters, high acceleration factors can be accomplished, resulting in fast pre-beam imaging and more beam-on imaging data. Lastly, the most important findings of this thesis are summarized in Chapter 7. Moreover the benefits and potential of the techniques described in the thesis for MRI-guided abdominal radiotherapy are discussed and future perspectives are outlined. 11
22
23 CHAPTER 2 Optimizing 4D-MRI data sampling for respiratory motion analysis of pancreatic tumors Stemkens, Bjorn Tijssen, Rob H.N. Denis de Senneville, Baudouin Heerkens, Hanne D. van Vulpen, Marco Lagendijk, Jan J.W. van den Berg, Cornelis A.T. The following chapter is based on: Optimizing 4-Dimensional Magnetic Resonance Imaging data sampling for respiratory motion analysis of pancreatic tumors, 2015, International Journal Of Radiation Oncology Biology Physics; 91:
24 Chapter 2 4D-MRI for respiratory motion analysis Abstract Purpose: To determine the optimum sampling strategy for retrospective reconstruction of 4D-MR data for non-rigid motion characterization of tumor and organs at risk for radiotherapy purposes. Methods: For optimization, we compared two surrogate signals (external respiratory bellows and internal MR navigators), and two MR sampling strategies (Cartesian and radial) in terms of image quality and robustness. Using the optimized protocol, six pancreatic cancer patients were scanned to calculate the 4D motion. ROI analysis was performed to characterize the respiratory-induced motion of the tumor and organs at risk simultaneously. Results: The MRI navigator was found to be a more reliable surrogate for pancreatic motion than the respiratory bellows signal. Radial sampling is most benign for undersampling artifacts and intra-view motion. Motion characterization revealed inter-organ and inter-patient variation, as well as heterogeneity within the tumor. Conclusions: A robust 4D-MRI method, based on clinically available protocols, is presented and successfully applied to characterize the abdominal motion in a small number of pancreatic cancer patients. 14
25 2.1 Introduction 2.1 Introduction Radiotherapy treatment of pancreatic cancer is challenging due to motion, primarily induced by respiration. Conventionally, margins are used to ensure full dose coverage of the target at the cost of extra radiation to nearby organs at risk (OARs). Prior knowledge about patient specific motion, however, may lead to more conformal radiation plans [29]. Currently, 4D-CT is the method of choice for motion characterization in radiotherapy. Unfortunately, CT has limited soft tissue contrast and 4D-CT often suffers from volume inconsistencies (e.g. duplicating, incomplete, or overlapping structures) caused by discrepancies between the breathing frequency and the pitch [22, 23]. Magnetic Resonance Imaging (MRI), on the other hand, has superior soft tissue contrast, allowing better differentiation between tumor and surrounding tissue. Moreover, the flexibility of image acquisition in MRI could be used to overcome some of the image artifacts typical of 4D-CT. Previous authors have reported MRI based motion characterization using 2Dcine MRI [6, 44, 78, 79]. However, these methods solely provide tumor motion characteristics, and do not provide information of the OARs. To construct high resolution 4D-MR data, both prospective gated imaging and retrospective binning have been explored in previous studies. In prospective gated imaging, 3D volumes are acquired at different breathing depths [80], determined for example by a 1D-MRI navigator that records the diaphragm position [81]. However, prospective gating may prolong scan time and the probabilistic information about the duration of each respiratory phase is not captured. Consequently, retrospective binning methods have gained interest. To bin the acquired data into appropriate respiratory phases, a number of respiratory surrogates have been investigated, such as respiratory bellows [82], image based body area [83], or 2D navigator slices [84]. Cai et al. [83] used 2D axial slices for retrospective slice stacking, which is closely related to 4D-CT and may therefore suffer from similar volume inconsistencies similar to 4D-CT. To address these disadvantages, Von Siebenthal et al. [84] and Tryggestad et al. [82] employed retrospective stacking of 2D sagittal slices. While Von Siebenthal et al. used a custom 2D acquisition, Tryggestad et al. used clinical sequences and a novel two-pass sorting approach. In lieu of these multi-slice 2D methods, volumetric 3D acquired MRI scans, in which the entire volume is excited each time, have been used by Buerger et al. [85] for retrospective binning since they inherently have a higher signal-to-noise ratio (SNR) and no through-plane motion. However, the last three methods either require a custom read-out or long acquisition/reconstruction times, which is undesirable for clinical practice. Ideally, one would like to benefit from the higher SNR of 3D acquisitions while still employing clinically available scan protocols. The goal of this study is therefore to develop a robust 4D-MRI method, based on 3D acquired MR data, to characterize respiratory induced motion of pancreatic tumors and its surrounding OARs using clinically available scan protocols within a clinically acceptable timeframe. Two motion surrogates, which serve as input for the binning process, are assessed for their agreement with respiratory induced 15
26 Chapter 2 4D-MRI for respiratory motion analysis organ motion. Moreover, two MRI sampling strategies are explored in terms of robustness and image quality in multiple volunteers. The final resulting technique is applied to six pancreatic cancer patients. To demonstrate the applicability of this technique to radiotherapy, motion of both tumor and OARs are characterized using a 3D optical flow (OF) algorithm, because of its short processing time and minimal user intervention [86]. 2.2 Methods We performed two different experiments in which the surrogate signal and MRI sampling strategy were independently optimized. The optimized protocol was then used to scan six pancreatic cancer patients. 3D motion was calculated for both the tumor and the duodenum over the complete respiratory cycle. All experiments were conducted on a 1.5T Philips Achieva MR scanner (Philips Healthcare, Best, The Netherlands). Processing was performed using Matlab (The Mathworks, Natick, MA). Surrogate signal comparison: correlation with pancreatic motion Six healthy subjects were scanned using a fast, coronal, 2D transient balanced steady-state gradient echo (balanced Turbo Field Echo, btfe) sequence with radial read-out (α = 30 o, TE/TR = 1.3/2.6 ms, FOV = 294 x 294 mm 2, pixelsize = 1.5 x 1.5 mm 2, slice thickness = 7 mm, bandwidth = 1580 Hz/px, turbo-factor = 128, 500 dynamics (i.e. number of consecutive repeats of the 2D sequence), temporal frequency = 2.6 Hz). The imaging plane was angulated parallel to the spine to sample along the principal axis of pancreatic motion, thereby reducing through-plane motion. The image acquisition was interleaved with a 1D MRI navigator, which was positioned at the dome of the right hemi-diaphragm and processed online using the vendors reconstruction method. The respiratory bellows, provided by the MRI vendor, were placed on the upper abdomen. While the navigator is inherently synchronized with the motion in the 2D images, the bellows signal required minor manual synchronization, due to imperfect temporal synchronization with the MR acquisition. Pixel-wise non-rigid displacement was calculated on the 2D time series using a 2D OF algorithm [87 90]. In short, the OF algorithm calculates non-rigid displacement between two imaging frames based on intensity gradients with an additional constraint on motion smoothness to model elastic organ deformation. To assess the agreement of the two surrogates with the pancreatic motion, we performed a general linear model (GLM) analysis, which is a least-squares fit to the data described by Y = Xβ + η. Here, Y is the pixel-based cranio-caudal (CC) motion, obtained from the pixel-by-pixel vectorfield calculated by the 2D OF algorithm, β the parameter estimates, X the surrogate signal and η represents the residuals. This analysis was performed on each pixel independently. The root mean square of the residuals was calculated and plotted in a spatial residual error map to find the surrogate with the lowest residuals. Furthermore, power spectrum 16
27 2.2 Methods Table 2.1: Acquisition parameters for the in-vivo experiments for 2 different in-plane sampling strategies, with the number of healthy volunteers scanned using the corresponding protocol. Parameter Cartesian Radial Time to echo [ms] Time to repetition [ms] Flip angle [ o ] FOV [mm 3 ] 330 x 250 x x 330 x 96 Matrix Size 152 x 126 x x 152 x 24 Dynamics T acq [min] 10:13.7 8:35.1 Bandwidth [Hz/px] No. of subjects 5 10 analysis (PSA) was performed on the surrogate signals and the pancreatic motion to get insight in the agreement in frequency range of the surrogates with the pancreatic motion. In-vivo experiments Eleven healthy volunteers provided written informed consent to be scanned according to institutional rules to test the feasibility and robustness of the proposed 4D-MRI technique. A clinically available 3D btfe with Cartesian or radial inplane sampling was used, utilizing the parameters described in Table 2.1. Turbo direction was along kz and a shot length equal to the amount of slices, thus acquiring all kz lines within a single shot (see Figure 2.1a). Prior to each shot, a 1D MR navigator, placed at the dome of the right hemi-diaphragm, was acquired. 25 dynamics (i.e. the number of repeats of a 3D volume acquisition) were acquired for the radial protocol as a concession between imaging time and filling. For the Cartesian protocol 50 dynamics were acquired to assure sufficient data were acquired at the center of k-space. Four volunteers were scanned with both radial and Cartesian protocol. Due to time constraints, six volunteers were scanned using solely the radial protocol (from which two had a smaller FOV of 330 x 330 x 64 mm 3 ), whereas one subject was scanned using only the Cartesian protocol. Reconstruction was performed offline on an 8-core CPU computer using Recon- Frame (Gyrotools, Zurich, CH), which is a commercial Matlab-based MRI reconstruction software package with the ability to operate on the MRI scanner. Using the respiratory waveform derived from the navigator, k-space data were sorted into ten respiratory phases using phase binning (i.e. equal-time based binning), similar to 4D-CT phase binning. Complex averaging was performed to increase SNR, when certain k-space segments were acquired multiple times. In case no k-lines were assigned, the lines were left blank. Image quality was assessed both visually and using the structural similarity (SSIM) index [91], which calculates the similarity between two images as a ratio between 0 (no similarity) and 1 (perfect similarity). SSIM values were calculated between undersampled images and the full reference (i.e. 50 dynamics for Cartesian, 25 dynamics for radial). 17
28 Chapter 2 4D-MRI for respiratory motion analysis (a) (b) (c) r 1,1 r 2,1 3D images Registered images I2 D2 Reg 2 kz D3 I1 r 1,n I3 Reg 3 r 2,1 D10 r 1,1 r 1,n k-space read-out r 2,n K 1 K 10 kz kz 10 respiratory phases (k-spaces) Respiratory phases Graphical overview phase binning method I10 Reg 10 Calculate 4D deformation field and registered images Figure 2.1: Workflow of the in vivo experiments. (a) Cartesian and radial trajectories with turbo direction along kz. First, readout lines r 1,1 r 1,n are acquired within 1 turbo field echo shot. In the next shot, r 2,1 r 2,n are acquired, etc. (b) Graphic overview of the phase binning method for a respiratory signal. (c) After reconstruction, 3-dimensional images are registered to the reference volume, which results in a 4-dimensional deformation field. 4D non-rigid motion analysis of tumor and OAR Six patients with an unresectable pancreas tumor were scanned using the radial protocol described in Table 2.1 according to institutional rules after written informed consent. After phase binning and image reconstruction, non-rigid displacement between the respiratory phases was estimated using a multi-threaded optimized 3D OF algorithm [86]. All respiratory phases were registered to one reference phase (the first, exhale phase) as can be seen in Figure 2.1c. The tumor and OARs were delineated on the reconstructed reference volume, and the mean motion within each delineated structure was calculated for all respiratory phases to construct motion trajectories of the tumor and duodenum over ten respiratory phases. 2.3 Results Surrogate signal comparison: correlation with pancreatic motion Figure 2.2a shows the residual maps of the GLM analysis using either the respiratory bellows (upper row) or the MRI navigator (lower row) as surrogate signal to model the motion within the abdomen for three out of the six subjects. The inhomogeneous residual maps indicate motion heterogeneity over the FOV. Moreover, the liver and pancreas show better correlation with the surrogates than the intestines. It can be seen that the residuals within the delineated pancreas are significantly higher for the bellows in subject one and two, whereas subject three demonstrates comparable values. The residual error within the pancreas over 18
29 2.3 Results (a) Subj. 1 Residual error maps of GLMs Subj. 2 Subj. 3 Power Spectra (b) 0.1 Subj. 1 Respiratory bellows Navigator Bellows Pancreas motion 0.05 Subj. 2 Respiratory bellows Navigator Navigator Pancreas motion Frequency (Hz) Figure 2.2: (a) Residual errors of the general linear model (GLM) after regression with both surrogates in 3 representative subjects with the pancreas delineated. (b) Power spectra displaying the power (x-axis) at different frequencies (y-axis) of the pancreatic motion, navigator, and bellows signal (results shown for subjects 1 and 2). Respiratory peaks are visible between 0.25 and 0.32 Hz for all modalities, but low-frequency peaks only in the pancreatic motion and navigator spectrum. Moreover, peaks induced by cardiac motion are revealed at 1 Hz for subject 2 in the bellows spectrum. the six subjects is 2.4 ± 1.1 times higher for the bellows compared to when the navigator is used. Moreover, the respiratory bellows signal required manual synchronization. Figure 2.2b displays the power spectra of the pancreatic motion, and the spectra of the two surrogates for the first two subjects shown in panel a. Aside from large respiratory peaks between 0.2 and 0.32 Hz in all spectra, the power spectrum of the respiratory bellows of subject 2 displays additional peaks close to 1 Hz, owing to cardiac motion. Moreover, low frequencies ( 0.05 Hz) are present in the spectra of the pancreatic motion and the navigator for both subjects, which may indicate slow frequency position drifts or noise of the respiratory motion. These peaks are absent in the spectra of the bellows, which could be the result of filtering of this signal by the scanner. In-vivo experiments Figure 2.3a displays the reconstructed images of maximum exhale (phase 1) for Cartesian and radial sampling using an increasing amount of dynamics (i.e. decreasing amount of undersampling after data reordering). The Cartesian sampled images reveal ghosting artifacts in all reconstructions as a result of undersampling. Radially sampled images show minor streaking artifacts when 10 dynamics are used, which become less pronounced when more dynamics are included, as a result of less undersampling). SSIM values between 10 and 20 dynamics and the full reference are 0.69 ± and 0.81 ± for Cartesian and 0.76 ± and 0.93 ± for radial between all subjects. This shows that radial sampling is more benign for undersampling, revealing fewer artifacts. Moreover, the large 19
30 Chapter 2 4D-MRI for respiratory motion analysis Radial Cartesian Exhale 10 dynamics 20 dynamics 25 dynamics 10 dynamics 20 dynamics 25 dynamics Inhale 50 dynamics 50 dynamics 25 dynamics Figure 2.3: Resulting images for Cartesian and radial in-plane sampling. (a) Reconstructions of exhale phase (phase 1) using an increasing number of dynamics, and (b) fully sampled reconstructions of inhale phase (phase 5). standard deviation for Cartesian sampled images demonstrates the large variety in image quality, which is primarily caused by the unpredictability of sorting lines close to the k-space center to all the respiratory phases. When these lines are absent, this will severely deteriorate image quality, causing severe ghosting artifacts. Figure 2.3b displays the reconstructed images for inhale (phase 5), which is the most difficult image to reconstruct due to large intra-phase variation. Despite 100% filling, the Cartesian sampled images still experience ghosting artifacts, as a result of intra-view motion. The image quality of the radially sampled image, on the other hand, is comparable to the first phase. These images demonstrate sufficient SNR and contrast for delineations and motion analysis (see Figure e1 ( for reconstructed images of all phases using radial sampling). 4D non-rigid motion analysis of tumor and OAR Figure 2.4 shows a coronal and sagittal slice with the vectorfield displayed on top in panels a-b. Panel c shows the cranio-caudal (CC) displacement between the inhale and exhale phase (i.e. the reference phase) for one patient. The motion trajectories of the tumor and duodenum are shown in panels d-e for three other patients. Each trajectory displays the average displacement over the entire tumor/duodenum. The vectorfields demonstrate the motion heterogeneity within the FOV. Figure 2.4c reveals large CC motion for the tumor and the OARs, whereas the motion around the spine and closer to the body contour is significantly less. Substantial differences in CC motion are observed for the two kidneys. AP motion is considerably less, with the largest anterior motion at the location of the psoas muscle, affecting both kidneys. LR motion is marginal in all structures. Furthermore, heterogeneity of motion is observed within the tumor (arrow). As the tumor shown here was unresectable, the heterogeneity could result from ingrowth into blood vessels that immobilize the tumor at this location. 20
31 2.3 Results (a) Coronal slice with vectorfield (c) CC-motion on axial slice 10 R L 8 6 (b) Sagittal slice with vectorfield 4 2 A P 0 2 (d) 0 Coronal Cran (e) 0 Sagittal 4 CC motion (mm) -2-4 CC motion (mm) Pancreas Caud Duodenum Left LR motion (mm) Right Anterior AP motion (mm) Posterior Figure 2.4: Vector fields displayed on top of (a) coronal and (b) sagittal slices of the first patient alongside corresponding (c) cranio-caudal (CC) motion with the tumor (arrow) and other structures delineated. (d) Coronal and (e) sagittal motion of tumor and duodenum for 3 patients. This phenomenon was present in four patients. Motion trajectories over all respiratory phases exhibit hysteresis effects for both the tumor and the duodenum (Figure 2.4d-e). Table 2.2 lists the displacement between ex- and inhale for the tumor and duodenum in CC, AP and LR direction for the six patients. Additionally, the magnitude of the resulting 3D vector (i.e. total motion) is noted. Moreover, the average motion and standard deviation are displayed in the two bottom rows. Duodenum motion was found to be larger in amplitude in CC and AP direction and overall motion compared to tumor motion. Total processing time, which consisted of acquisition (8:35), rebinning/reconstruction (2:44), and 4D motion characterization using optical flow (1:39), was 12:58 minutes for each patient. 21
32 Chapter 2 4D-MRI for respiratory motion analysis Table 2.2: Average displacement between ex- and inhale for the tumor and the duodenum in cranio-caudal (CC), antero-posterior (AP), and lefteright (LR) direction, along with the total displacement (ie magnitude of 3D vector) for the 6 patients. CC (cranial = positive) AP (posterior = positive) LR (right = positive) Total displacement Patient no. Tumor Duodenum Tumor Duodenum Tumor Duodenum Tumor Duodenum Avg SD
33 2.4 Discussion 2.4 Discussion In this paper, a novel 4D-MRI method was presented using only clinically available scan protocols. The total imaging and processing time was less than 15 minutes, which makes MRI based motion characterization of tumor and OARs clinically feasible. We have shown a 1D MRI navigator correlated better with pancreatic motion than external respiratory bellows. Moreover, synchronization between the acquired image data and the MR navigator is more robust than for the bellows, which require manual preprocessing, as mentioned previously [82]. It is noteworthy, however, that the acquisition of the MR navigator requires some additional scanning time (±15% increase in scanning time). Additionally, the low frequency drifts observed in the power spectra may have a significant influence on image quality if amplitude binning is used, since these drifts influence the amplitude of the respiratory waveform. It was demonstrated that radial in-plane sampling is superior to Cartesian sampling. In radial sampling the center of k-space is sampled for every read-out line, which makes the sequence more robust against image artifacts resulting from undersampling (i.e. incompletely filled phases) or intra-view motion (i.e. intra-phase amplitude variation) [92, 93]. For quantification of SSIM values, the full reference was defined as an image with no image artifacts, as a result of undersampling. Since radial sampling is more benign for undersampling, only 25 dynamics were required (i.e. 7% undersampling), whereas Cartesian sampled images showed sufficient image quality after 50 dynamics (i.e. 1% undersampling). Finally, a demonstration of 4D motion analyses was shown in six patients. We were able to detect inter-organ and inter-patient variation, which shows the clinical importance of patient specific motion models. Motion heterogeneity was apparent within the tumors, which may be caused by fixation of the tumor to blood vessels, due to ingrowth, which was confirmed by a radiologist. The calculated patient specific 4D motion trajectories can be used for optimizing the radiation plan for both the GTV and the OARs in abdominal tumors. This can be used to transition from a population-based ITV [94] to a patient-specific ITV. Moreover, the ability to perform motion characterization of OARs, such as the duodenum, allows for better definition of planning organs at risk volumes (PRVs) [95], which will aid reducing toxicity. Further, with the development of MR-Linac systems (e.g. [70]), the presented techniques can be used to calculate the daily variation in motion, online, just prior to treatment and used for online tracking models and beam steering. Although the technique is only used for pancreatic tumors in this study, we believe it can be used for other abdominal tumors, which suffer from respiratory motion, e.g. liver and kidney tumors. This study has some limitations. Higher frequency induced motion (e.g. pulsatile motion) will not be visualized by retrospective respiratory binning, which is a limitation of any retrospective method. Furthermore, retrospective binning represents the motion of an average breathing cycle, whereas inter- and intra-cycle variation is not modeled, which may vary substantially (as was seen in the 2D 23
34 Chapter 2 4D-MRI for respiratory motion analysis data). Moreover, a thorough validation of the 3D OF algorithm is required for clinical implementation. Ideally, a comparison with a gold standard is preferred. Although this is a topic of active research, such a validation is beyond the scope of this article. Previous studies, however, have examined the accuracy of the OF algorithm on 2D MR images [87 89, 96], and in 3D on 4D-CT data [86] using manual tracking and found an accuracy of mm and 1.1 mm in 2D and 3D respectively. To visually assess if 3D MRI data is registered with similar accuracy, all images were registered to the reference phase and visually inspected for residual motion, which revealed small residual errors, indicating good motion estimations (see Figure e2, Additionally, motion vectors of consecutive phases were added in the complete FOV for all patients, resulting in a mean residual error of 0.51 mm. Since this trajectory comprises a full respiratory cycle, the error should be close to zero. Lastly, the OF results were compared to displacement results obtained by Elastix [97]. Elastix is a widely used ITK-based non-rigid registration package, employing B-spline registration. The motion trajectories of the tumor and duodenum revealed similar motion paths, with an average difference below 0.5 mm. On top of that, manual tracking of the tumor in ex- and inhale revealed similar values as summarized in Table 2.2 for the change in center of gravity between ex- and inhale. Future studies will mainly focus on investigating the accuracy of the OF algorithm, the overall accuracy of the 4D-MR technique and the comparison with 4D-CT. 2.5 Conclusion A way to estimate 3D motion for ten respiratory phases based on retrospectively binning of k-space data using solely clinically scanning protocols has been presented. Respiratory binning based on internal MR navigators, in combination with radial in-plane sampling was found to be the most robust approach for generating 4D-MRI data. Using a fast 3D optical flow algorithm, we were able to calculate 4D motion for all abdominal structures within 13 minutes after starting acquisition. 24
35 CHAPTER 3 Adaptive bulk-motion exclusion for improved robustness of abdominal MR imaging Stemkens, Bjorn Benkert, Thomas Chandarana, Hersh Bittman, Mark E. van den Berg, Cornelis A.T. Lagendijk, Jan J.W. Sodickson, Daniel K. Tijssen, Rob H.N. Block, Kai Tobias The following chapter is based on: Adaptive bulk-motion exclusion for improved robustness of abdominal MR imaging, Accepted for publication in NMR in Biomedicine 25
36 Chapter 3 Adaptive bulk-motion exclusion Abstract Purpose: Non-Cartesian free-breathing MR sequences have shown great promise for robust abdominal examination, but these sequences can have difficulty coping with bulk patient motion (i.e. voluntary or involuntary patient movement resulting in translation, rotation or elastic deformations of the body). This work describes a data-consistencydriven image stabilization technique, which detects and excludes bulk movements during data acquisition. Methods: Bulk motion is detected by identifying changes in the signalintensity distribution across different elements of a multi-channel receive coil array. A short free-induction decay signal is acquired after excitation and used as measure to determine alterations in the load distribution. The technique was implemented on a clinical MR scanner and evaluated in the abdomen. In addition, motion-resolved images were reconstructed to demonstrate the possibility for further reduction of respiratory motion. Neck and knee images were acquired to show the applicability to other body areas. Results: Data corrupted by bulk motion was successfully detected and excluded from image reconstruction. An overall increase in image sharpness and reduction of streaking and shine-through artifacts were observed in an abdominal volunteer study as well as in neck and knee examinations. Conclusions: The proposed technique enables automatic real-time detection and exclusion of bulk motion during free-breathing abdominal MR examinations without user interaction. It may help to improve the reliability of pediatric examinations without use of sedation. 26
37 3.1 Introduction 3.1 Introduction Motion poses a key challenge for diagnostic three-dimensional (3D) MR examination of the abdomen. Both physiological motion, such as respiratory and cardiac motion, and bulk motion, caused by bulk body movements or coughing, can impair the image quality and result in non-diagnostic images [98 100]. Various strategies have been proposed to handle physiological motion-related artifacts. Common approaches for avoiding respiratory-motion artifacts include breath-hold [101] and respiration-gated acquisitions [80, 102]. However, many patients are unable to suspend respiration long enough to acquire sufficient data (often 15 seconds). Moreover, pediatric patients may not be able to hold their breath at all, and they can perform sudden movements. Therefore, general anesthesia or deep sedation is often necessary to obtain diagnostic images in these patients [103]. Disadvantages of sedation include health risks [104] as well as increased logistical complexity and costs. As an alternative, gated or triggered acquisitions can be employed, using either respiratory bellows or navigators, which result in significantly reduced scan efficiency. Furthermore, respiratory bellows cannot differentiate respiratory- and non-respiratory-related motion. Navigators, on the other hand, may affect the contrast of the imaging volume or interrupt the steady state. A comprehensive review of motion-compensation techniques can be found in Zaitsev et al. [99], van Heeswijk et al. [100], and MacLaren et al. [105]. In recent years, free-breathing approaches based on non-cartesian k-space acquisition have become popular [61, ]. Residual respiration artifacts can additionally be removed by weighting the data with the amount of motion [109, 110] or by reconstructing multiple respiratory phases [111]. However, these techniques cannot correct for bulk motion, which includes voluntary and involuntary patient movement resulting in translation, rotation, or elastic deformation of the body [100]. One possibility to remove bulk motion is to exclude corrupted data retrospectively [112]. Because the duration and number of intervals with bulk motion is not known in advance, k-space has to be acquired with significant oversampling to provide sufficient k-space coverage. This prolongs the scan time unnecessarily if no (or only short) intervals of bulk motion occur, or results in residual undersampling artifacts if more bulk motion than expected occurs. Prospective methods [113, 114] either reacquire motion-corrupted data [113] or stop the acquisition when patient motion occurs [114], based on motion information derived from navigator scans. Intermediate navigator acquisition, however, prolongs the scan time and may not be compatible with all sequence types. The aim of this work was to develop a novel time-efficient adaptive, data-consistencydriven image stabilization technique to detect and exclude bulk movements. This is achieved by calculating a consistency measure for the acquired data in realtime and continuing the acquisition until sufficient consistent data is acquired. Without user interaction, the technique automatically detects motion-free windows and stops the scan if sufficient data have been acquired. The method has been tested in five healthy volunteers. While the proposed technique efficiently rejects data corrupted by bulk motion, 27
38 Chapter 3 Adaptive bulk-motion exclusion it intentionally does not detect respiratory motion, which remains visible in the motion-stabilized images. Residual respiratory motion can be removed in a second processing step by applying the previously described XD-GRASP approach [111] to the motion-stabilized data, which is shown for a free-breathing abdominal scan. To demonstrate the techniques generalizability, additional head/neck and knee examinations were performed. 3.2 Methods Overall strategy The strategy of the proposed technique is to obtain a consistent data window that is not corrupted by bulk motion and that contains sufficient samples to reconstruct images with diagnostic quality. To this end, k-space samples are acquired continuously while a real-time mechanism evaluates whether bulk motion is occurring or not. Several possibilities exist to extract motion from the acquired data, commonly referred to as self-navigation techniques. One option is to detect motion from non-phase-encoded k-space center lines [111, 115]. While this can be accomplished efficiently for stack-of-stars sequences [111] and other non-cartesian 3D trajectories, for Cartesian 3D sampling this requires acquisition of additional nonphase-encoded k-space lines, which prolongs scan time [115]. Alternatively, free induction decay (FID) navigators can be acquired, which sample the center of k-space immediately after the radiofrequency (RF) excitation without spatial encoding [ ]. This has minimal impact on the echo time (TE), requires no additional RF pulses, and can be generalized to many different k-space trajectories. The FID approach is used in the current work to derive a motion signal. It has been shown previously that variations in the signal-intensity ratio between different receive coils allow identification of bulk motion [115]. When bulk motion is detected, the data are rejected until the subject holds still. The scan is stopped automatically when a pre-defined window of non-corrupted consecutive k-space lines has been acquired. Data affected by bulk motion are discarded because it cannot be assumed that the absolute position of the patient before and after bulk motion will be identical. If no such data window can be found within a user-specified maximal scan time (e.g., when the subject moves continuously), the best available window (i.e. longest window without bulk motion) is identified and used for image reconstruction. The entire procedure is schematically shown in Figure 3.1. It is important to note that if the subject does not move during the exam, the technique does not prolong scan time relative to a non-stabilized exam. Implementation Data are acquired using a T 1 -weighted radial 3D gradient-echo sequence with both RF and gradient spoiling [61]. A stack-of-stars scheme with Cartesian encoding along the kz-plane and radial sampling in the kx-ky plane is used for 28
39 3.2 Methods During continuous data acquistion After completion of scan MRI Scanner Real-time system Reconstruction system Host FID data Calculation consistency measure Window borders Dicoms Data acquisition Radial projections + FID data Stop signal Consistent window detected Maximal scan time reached Triggers to send stop signal Display PACS transfer Radial projections Data acquisition RF/ADC FID Echo Gx/Gy Gz Sum of FID Correlation coefficient Reference line 1 Coil channel 26 Calculation consistency measure Projection number Sum of FID Sum of FID No bulk motion 1 Coil channel 26 Bulk motion 1 Coil channel 26 Figure 3.1: Schematic overview of the workflow for the proposed bulk motion exclusion technique. A radial stack-of-stars 3D sequence has been modified to acquire a FID signal after RF excitation. The FID data are sent to the real-time processing system, which calculates the consistency of the current projection with a reference projection based on the correlation coefficient. Once sufficient motionfree data have been acquired, or if the maximum scan time has been reached, a stop signal is sent to the scanner. Data from the accepted window are then reconstructed on the scanner. volumetric k-space coverage. After spectral fat suppression, all partitions for one radial angle are acquired with centric reordering before the readout scheme is repeated for subsequent radial projections. Golden-angle ordering with an angular increment of [57] is used for continuous data acquisition. To obtain motion information, 32 samples of the FID signal are acquired after rewinding the slice-selection gradient, which increases the echo time by less than 0.5 ms. This combination of FID sampling and golden-angle radial sampling may prove to be a robust method for real-time motion compensation. The entire data acquisition scheme is shown in Figure 3.1. The FID signals are transferred to the real-time control component of the Siemens image calculation (ICE) framework of the scanner where the processing and detection of bulk motion has been implemented. To reduce the latency of the data transfer, only the FID signals from the center k-space partition are sent. The first and last four samples of each FID signal are discarded to account for pre- and post-ringing. The remaining samples are summed and, for each projection, the 29
40 Chapter 3 Adaptive bulk-motion exclusion summed values from the different coils are concatenated into a projection vector of length N coil. Motion is detected by calculating the correlation coefficient between a reference projection vector and all other projection vectors [115]. The correlation coefficient CC is defined as CC x,y = 1 N coil 1 Ncoil i=1 ((x i x) (y i ȳ)) Ncoil i=1 (x i x) 2 1 N coil 1 Ncoil i=1 (y i ȳ) 2, (3.1) where x i and y i denote the i-th entry of the current projection vector x and reference projection y. x and ȳ are the mean values of both vectors. A low correlation coefficient implies that the load distribution of the coil elements has changed. This indicates that the patient position has been altered between the projections. Since it is not known beforehand if and when bulk motion occurs, the reference projection vector, which is defined as the projection with highest overall correlation to all previously acquired projections, is dynamically updated after each projection. The calculation of all correlation coefficients is computationally demanding and cannot always be done in real time. In the current implementation, the reference projection is, therefore, only calculated within a sliding window containing the previous 100 projections. Outlier projections, which indicate bulk motion, are identified using a user-defined acceptance threshold for the correlation coefficient. Imaging studies All volunteer scans for this study were approved by the Institutional Review Board (IRB) and were HIPAA compliant. Written informed consent was obtained for all imaging studies. Abdominal imaging Six healthy volunteers (3 male, 3 female) were scanned on a 3T scanner (MAGNE- TOM Skyra, Siemens Healthineers, Germany) equipped with a 24-element spine coil array in combination with an 18-element body coil array. For each volunteer, four separate free-breathing scans were performed during which the subjects were asked to perform different motion tasks (Table 3.1). Additionally, a scan without bulk motion was acquired as reference. Data were acquired with the described 3D radial stack-of-stars sequence. The maximum scan time was set to 5:11 min, which corresponds to a total of 1500 projections. The length of the acceptance window was set to 400 projections (1:23 min) to fulfill the Nyquist-criterion for radial sampling. A threshold of was used to differentiate between regular breathing motion and bulk motion. This value was chosen empirically after evaluating results with different thresholds (an example is shown in Supporting Figure S1). For all scans, the liver was covered with 72 transversal slices using the following sequence parameters: field-of-view (FOV) = 350 x 350 x 216 mm 3, slice resolution = 72%, flip angle = 12, TR/TE = 3.35/1.71 ms, readout bandwidth 30
41 3.2 Methods Table 3.1: Motion instructions given to the volunteers for the four different scans. The scan was stopped automatically when a user-defined number of consistent projections were acquired. Types of motion for the four separate volunteer scans Free-breathing ( 45 sec) Bulk motion ( 30 sec) Free-breathing (until scan stops automatically) Bulk motion ( 30 sec) Free-breathing (until scan stops automatically) Free-breathing ( 45 sec) Change position Free-breathing (until scan stops automatically) Random motion, up to the subject ( 45 sec) Free-breathing (until scan stops automatically) = 890 Hz/px. Using a matrix size of 256 x 256 pixels, the resulting resolution was 1.4 x 1.4 x 3.0 mm 3. Neck and knee imaging To demonstrate the applicability of the technique to other body areas, both a neck scan and a knee scan were performed in one volunteer each. For the neck scan, the subject was asked to perform random head motion during the scan. A 3T scanner (MAGNETOM Skyra, Siemens Healthineers, Germany) equipped with a 20-element combined head/neck coil array was used to acquire data with the radial stack-of-stars sequence. The length of the acceptance window was set to 400 projections (1:47 min), while the maximum scan time was set to 6:42 min (1500 projections). 72 transversal slices with the following imaging parameters were used to cover the neck area: field-of-view (FOV) = 200 x 200 x 216 mm 3, matrix size = 256 x 256 pixels, resolution = 0.8 x 0.8 x 3.0 mm 3, slice resolution = 72%, flip angle = 12, TR/TE = 4.40/2.25 ms, readout bandwidth = 650 Hz/px. For the knee scan, the subject was instructed not to move during the first 40 sec of the scan, then to move the knee for 50 sec, and to suspend motion for the remainder of the scan. A radial stack-of-stars dataset was acquired using a 3T scanner (MAGNETOM Prisma, Siemens Healthineers, Germany), equipped with a 15-element knee coil array (QED, Cleveland, Ohio, USA). The following imaging parameters were used to cover the knee with 72 transversal slices: fieldof-view (FOV) = 190 x 190 x 144 mm 3, matrix size = 256 x 256 pixels, resolution = 0.7 x 0.7 x 2.0 mm 3, slice resolution = 72%, flip angle = 12, TR/TE = 4.51/2.30 ms, readout bandwidth = 670 Hz/px. The maximum scan time was set to 6:48 min (1500 projections), while the length of the acceptance window was set to 400 projections (1:49 min). Because breathing motion does not occur in a knee exam and because bulk movements are expected to be less severe, the correlation values are expected to be higher than for abdominal scans. Therefore, the threshold value was increased to
42 Chapter 3 Adaptive bulk-motion exclusion Image reconstruction After real-time detection of the motion-free data window, the window borders are transferred to the reconstruction pipeline. Final images are reconstructed using only the projections from within this data window. Image reconstruction is performed using custom-developed modules in the ICE framework. First, phase encoding along the slice direction is removed by applying an inverse fast Fourier transformation (FFT). After density compensation with a Ram-Lak filter, images are then reconstructed with a non-uniform FFT, which uses a Kaiser-Bessel window as interpolation kernel for the gridding step. Because the processing has been implemented into the scanner framework, built-in post-processing steps, such as image-distortion correction, are performed automatically, while additional corrections, such as gradient delay corrections, are not implemented. A sum-of-squares algorithm was used to combine signals from different coil channels and inhomogeneities in the receive profiles were compensated by enabling prescan normalize. Calculated correlation coefficients and the borders of the accepted window are written into a text file, which is exported for optional visualization of the motion curves. To demonstrate the performance of the proposed image-stabilization technique, the automatically reconstructed images are compared with two additional reconstructions using 1) the same window length, but starting from the first projection (i.e. the first 400 projections), and 2) all acquired projections, corresponding to the same maximum scan duration. To show the compatibility of the proposed method with additional respiratory motion compensation, the XD-GRASP approach [111] was applied to the accepted data window from one volunteer dataset. The FID navigator data allows detection not only of bulk motion but also of respiratory motion. This information was used to sort the data into four respiratory states, ranging from end-expiration to end-inspiration. Each frame then consisted of 100 projections, which would result in visible undersampling artifacts in standard regridding reconstructions. Therefore, an iterative reconstruction was performed that minimizes the total variation along the respiratory dimension to suppress radial undersampling artifacts. As final step, the respiratory phases were registered using a non-rigid 3D optical flow algorithm [88] and averaged to generate one final high-quality image. Validation To validate the correlation measure for detection of bulk motion, the four abdominal scans of one additional volunteer were acquired and recorded with a video camera (PowerShot SX170IS, Canon Inc., Tokyo, Japan), which was mounted outside the bore. Start of the MR acquisition was determined using the audio signal. Bulk motion was detected from the videos by calculating the sum of absolute differences (SAD) between consecutive frames. When no motion or only slight motion occurs between frames, such as regular breathing, the SAD value is very small. Bulk motion, in contrast, results in a high SAD value. For each scan, the SAD values were plotted over time and visually compared with the corresponding plot of the correlation coefficients. 32
43 3.3 Results Correlation coefficient [a.u.] Correlation coefficient [a.u.] FID motion detection Video motion detection Time [sec] 50 Time [sec] Motion detection validation (a) FB - bulk motion - FB (b) Bulk motion - FB Sum-of-absolute differences [a.u.] Sum-of-absolute differences [a.u.] Correlation coefficient [a.u.] Time [sec] (c) Change position (d) Random Correlation coefficient [a.u.] Time [sec] Figure 3.2: Plots of the correlation coefficients calculated from the FID navigator (blue) and the sum-of-absolute differences (SAD) from the video (orange) for the four scans from one volunteer. When bulk momotiontion occurred, the correlation decreased, whereas the SAD increased. Note that, as a result of noise and respiratory motion, the SAD values are nonzero even when no bulk motion occurs Sum-of-absolute differences [a.u.] Sum-of-absolute differences [a.u.] 3.3 Results Validation Figure 3.2 summarizes the results from the video validation for one of the volunteers. For all scans, the motion curve detected with the proposed technique (blue) shows high agreement with the motion detected on video (orange). When bulk motion occurs, the correlation coefficient decreases while the SAD increases due to the larger differences between the video frames (see Supporting Video S1 in Supplementary Material). Because of noise and respiration, SAD values ranged between 0.1 and 0.2 even in intervals without bulk motion. Figure 3.2c shows plots from the scan when the volunteer changed position. The two positions are clearly distinguishable from the FID motion curve, whereas the video-based detection only indicates the moment when the subject moved. Figure 3.2d shows different periods of free breathing and bulk motion. The initial peaks around 40 sec were caused by coughing. The last peak around 300 sec was caused by head 33
44 Chapter 3 Adaptive bulk-motion exclusion motion, which was, appropriately, not identified by the FID navigators because the RF pulse did not excite this part of the body. Because no consistent window of 400 projections was detected, the scan was stopped after 311 sec and the best window, between 50 and 100 sec, was used for image reconstruction. 3D abdominal imaging with bulk motion Figure 3.3 compares images reconstructed from the first 400 projections (orange), all acquired projections (red), and only the accepted projections (green) with the reference free-breathing acquisition without bulk motion for a volunteer that changed position once. Additionally, the time course of the correlation coefficient is shown (Figure 3.3b). A major reduction of shine-through artifacts can be seen with the proposed technique. Although the reconstruction using all projections shows fewer streaking artifacts and higher SNR, the images are non-diagnostic due to the two overlapping positions of the subject. These artifacts are removed with the proposed technique, which shows comparable image quality to the reference free-breathing acquisition. Figure 3.4 shows results from another subject that started moving after 36 seconds (175 projections), followed by normal free-breathing after 65 seconds (317 projections). Images from the initial 400 projections show substantial motion artifacts in both transversal and sagittal view. The artifact strength is somewhat reduced in the transversal view when all 717 projections are used, but is worse in the sagittal view. Best image quality is achieved if only the accepted projections are used, with similar image quality to the reference reconstruction in the transversal view. Respiratory motion remains visible, resulting in blurring at the top of the diaphragm on the sagittal view, which appears to be slightly less in the reference free-breathing acquisition without bulk motion. Motion-resolved 3D abdominal imaging Figure 3.5 shows images with and without additional respiratory motion compensation. In the motion-averaged image (Figure 3.5a), blurring is observed at the tip of the liver in the coronal and sagittal views, as well as around the liver vessels on the transversal view. The individual respiratory phases from the XD- GRASP reconstruction show much sharper edges and contours in all views and phases (Figure 3.5b). Residual undersampling artifacts are visible, which are not completely removed by the motion-resolved reconstruction. After averaging the non-rigidly registered volumes, these artifacts are largely removed. The respiratory signal extracted from the FID signal correlated well with the self-navigation signal extracted from the center of k-space. 3D head/neck and knee imaging Figure 3.6a shows results from the head/neck scan during which the volunteer moved her head twice. The rotations are clearly noticeable in the correlation plot around projection number 180 and 330. Although the movements and corresponding artifacts are more subtle than for abdominal bulk motion, reduced 34
45 3.4 Discussion (a) First 400 projections All projections Proposed technique No bulk motion, free-breathing (b) Correlation coefficient Projection number Figure 3.3: Results from a healthy volunteer that changed position once. (a) Reconstructions from the first 400 projections (orange), all projections (red), and the accepted projections (green). Additionally, the reference free-breathing reconstruction is shown that was acquired in a separate bulk-motion-free scan. (b) Correlation coefficients and the corresponding data windows from (a). artifacts are observed with the proposed technique, indicated by the white arrows. Results from the knee scan are shown in Figure 3.6b. The correlation plot clearly reveals motion between projections 150 and 340. When these data are included in the reconstruction, image quality is severely degraded, and artifacts are clearly visible as indicated by the white arrows. With the proposed technique, a motion-free interval was identified and improved image quality without motion artifacts was obtained. 3.4 Discussion While a variety of techniques for motion-robust abdominal imaging have been proposed in literature, occurrence of bulk motion still poses an unresolved problem and can result in non-diagnostic image quality or misinterpretation of images. This work describes a novel technique to detect and exclude non-respiratory bulk 35
46 Chapter 3 Adaptive bulk-motion exclusion (a) First 400 projections All projections Proposed technique No bulk motion (FB) (b) Correlation coefficient Projection number Figure 3.4: Results from another volunteer that started with free-breathing, followed by bulk motion and normal breathing without bulk motion after 330 projections. (a) Reconstructed images in transversal and sagittal orientation and (b) corresponding data windows for the initial 400 projections (orange), all projections (red), or the accepted projections (green). Note that the proposed method does not correct for respiratory motion, resulting in residual blurring at the diaphragm, similar to the free-breathing reference reconstruction. motion from continuously acquired 3D scans, based on measuring variations in the signal-intensity distribution between different elements of a multi-channel receive coil. Using recordings of volunteer scans, it has been validated that the variations in FID intensity ratios correlate with bulk motion (see Supporting Video S1 in Supplementary Material). Five volunteers were scanned and asked to perform different types of motion, including free breathing, bulk motion, position changes, coughing, moving the coil or arms, and rolling around. For all motion types, the proposed technique was able to detect motion-free data windows. The technique has several advantages over previously described methods for retrospective bulk-motion correction [112, 120]. First, it is fully automatic and does not require user interaction. Since a minimum number of projections is always acquired, images can be reconstructed directly on the scanner without need for complex processing such as compressed sensing [121] or registration algorithms [120]. Second, the technique is time efficient, as no assumptions are made regarding if, when, and how much bulk motion might occur during the scan. This is important 36
47 3.4 Discussion Motion averaged (b) Phase 1 Motion resolved reconstruction Phase 2 Phase 3 (c) Phase 4 Sum of registered images Sagittal Coronal Axial (a) Figure 3.5: Respiration-resolved reconstruction for one volunteer. (a) Motionaveraged reconstructed volumes from the bulk-motion-stabilized data with blurring due to respiratory motion. These images show residual blurring as a consequence of respiratory motion. (b) Motion-resolved images for the four respiratory phases with increased sharpness in all planes. Residual undersampling artifacts are observed, as visible in the inferior positions of the coronal view. The red line is displayed for visual feedback to show that the different respiratory phases do indeed represent different diaphragmatic positions and stages of the respiratory cycle. (c) Averaged image after registration to a fixed respiratory phase, which is largely artifact-free. because some patients may start moving when the scan starts (due to acoustic noise onset), whereas others may move at a later time (due to discomfort). If no bulk motion occurs, only the minimum scan time is used, which may not be the case for retrospective bulk-motion removal [112]. Third, to ensure that the scan time is not unreasonably prolonged in presence of ongoing bulk motion, a maximum allowed scan time can be defined. The longest bulk motion-free window is then used for reconstruction. If the number of projections in this window is smaller than the required minimum number of projections, the threshold is slightly reduced until a window with sufficient data is found. This mechanism was implemented because images with too few projections would be affected by undersampling artifacts. Lastly, another advantage of the proposed technique is that it does not reacquire data [113] but continues the golden-angle acquisition until sufficient consecutive motion-free data have been recorded. This results in higher scan efficiency than reacquisition of the data. In the current implementation, only consecutively acquired profiles are accepted for reconstruction. This restriction could be relaxed by using all data except for motion-corrupted profiles, which would result in improved time efficiency. However, the body position of the patient before and after bulk motion cannot be assumed to be identical, especially in abdominopelvic applications (as seen in Figure 3.2 and Figure 3.3), even for somewhat mild bulk motion, such as coughing. Therefore, reconstruction from non-consecutive data would increase blurring and result in an overlay of different patient positions, which has previously been reported for body [112] and head [120] imaging. Nonetheless, for gentle move37
48 Chapter 3 Adaptive bulk-motion exclusion Correlation coefficient (a) Neck results Projection number (b) Knee results First 400 projections All projections Proposed technique First 400 projections All projections Proposed technique Correlation coefficient Projection number Figure 3.6: Results from the neck (a) and knee (b) scan. The technique automatically selected reconstruction windows without motion-corrupted projections (green). Compared to the non-stabilized reconstructions (red, orange), blurring as well as streaking (indicated by the white arrows) is decreased and overall image quality is improved. ments in relatively stable anatomies, such as tongue motion in the neck area, only the projections affected by bulk motion can be removed to improve scan efficiency. The acceptance threshold parameter was chosen heuristically, based on retrospective processing of several in-vivo data sets. For the abdominal and the neck scans, a value of was found to be a reasonable tradeoff between exclusion of bulk motion and acceptance of respiratory motion. As is visible in Supporting Figure S1, a threshold value between and gives comparable image quality, suggesting that the method is not very sensitive to the exact value of the threshold parameter. It is important to note, however, that the threshold value can depend on the application and type of motion. For example, a higher threshold was required for the neck and knee examination. Thus, it might be necessary to select a fixed threshold value for each distinct application. For some of the abdominal acquisitions, the number of accepted projections was greater than the required 400 projections. This can be explained by the fact that the reference projection is dynamically updated and likewise the correlation coefficients. For example, the reference projection for an acquisition that starts with free breathing followed by bulk motion and free-breathing, will be one of the projections in the first free-breathing part for a certain period. Only when sufficient consistent data is found in the second free-breathing part, will the reference change to one of these projections. It may happen that already more than 400 projections were acquired at that moment, especially when the first free-breathing period was relatively long. This can be improved by increasing the sliding window length that is used to calculate the reference projection or by averaging consecutive accepted projections into a mean reference projection. 38
49 3.6 Conclusion The proposed method is versatile and generalizable. In this initial study, a golden-angle radial stack-of-stars T1-weighted 3D gradient-echo sequence was used. Other continuous sampling techniques could be employed as well, such as Variable Density sampling and Radial view ordering (VDRad) [110], Goldenstep Cartesian [122], blipped multi-echo 3D radial stack-of-stars [123], or 3D radial [124]. Some of these trajectories have inherent self-navigation capabilities. However, due to use of the FID navigator, the proposed method can also be applied to Cartesian sampling strategies [116, 125]. A further benefit of the FID navigator is that it extracts motion from the entire excited volume independently from the scan orientation, allowing it to be used also for coronal, sagittal, and oblique acquisitions [126]. Moreover, the FID signal is not affected by gradient imperfections, such as gradient delays, while the self-navigation extracted from the center of k-space will have an additional dependency on the radial angle as a result of such imperfections [56]. Furthermore, it can detect both bulk motion and respiration, which can be compensated for in a subsequent step via retrospective sorting or weighting of the data, as demonstrated in the XD-GRASP example (Figure 3.5). Additionally, the FID signal provides high temporal resolution as it is acquired prior to each read-out event. Conventional self-navigation techniques often provide lower temporal resolution, which can be insufficient for large imaging volumes, or relatively slow sampling methods, such as radial stack-of-stars Turbo Spin Echo acquisitions. Lastly, this technique may be useful for online feedback in MR-guided radiotherapy treatment [127], by automatically detecting bulk motion in real-time for gated treatments. A limitation of the approach is that it cannot be applied to all clinical imaging protocols. While it is straightforward to apply the technique to T 2 -weighted sequences, it cannot be applied for dynamic contrast-enhanced acquisitions because rejection of data and scan continuation is not a viable strategy when images at defined enhancement phases are required. A further limitation of the current work is that the method has been tested in only a small number of volunteer scans. Evaluation in a patient study is necessary before conclusions about the effectiveness in routine clinical application can be drawn. 3.5 Conclusion This work describes a time-efficient method for automatic detection and exclusion of bulk motion during volumetric abdominal MR examination. Bulk motion is detected by correlating FID signals across different coil elements using a realtime implementation. The acquisition is continued until sufficient consecutive data without bulk motion has been acquired. Images from the motion-free period are reconstructed on the scanner without additional user interaction. The technique promises to improve the robustness of abdominal MRI scans and may be of particular value for clinical examination of pediatric patients without use of sedation. 39
50 Chapter 3 Adaptive bulk-motion exclusion 3.6 Acknowledgments The authors would like to acknowledge support from the US National Institutes of Health (NIH R01 EB018308, NIH P41 EB017183), and from the Royal Netherlands Academy of Arts and Sciences (KNAW) Ter Meulen grant. 40
51 CHAPTER 4 Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy Stemkens, Bjorn Tijssen, Rob H.N. Denis de Senneville, Baudouin Lagendijk, Jan J.W. van den Berg, Cornelis A.T. The following chapter is based on: Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy, 2016, Physics in Medicine and Biology; 61:
52 Chapter 4 3D cine-mri Abstract Purpose: Respiratory motion introduces substantial uncertainties in abdominal radiotherapy for which traditionally large margins are used. The MR-Linac will open up the opportunity to acquire high resolution MR images just prior to radiation and during treatment. However, volumetric MRI time series are not able to characterize 3D tumor and organ-at-risk motion with sufficient temporal resolution. In this study we propose a method to estimate 3D deformation vector fields (DVFs) with high spatial and temporal resolution based on fast 2D imaging and a subject-specific motion model based on respiratory correlated MRI. Methods: In a pre-beam phase, a retrospectively sorted 4D-MRI is acquired, from which the motion is parameterized using a principal component analysis. This motion model is used in combination with fast 2D cine-mr images, which are acquired during radiation, to generate full field-of-view 3D DVFs with a temporal resolution of 476 ms. Results: The geometrical accuracies of the input data (4D-MRI and 2D multi-slice acquisitions) and the fitting procedure were determined using an MR-compatible motion phantom and found to be mm on average. The framework was tested on seven healthy volunteers for both the pancreas and the kidney. The calculated motion was independently validated using one of the 2D slices, with an average error of 1.45 mm. Conclusions: The calculated 3D DVFs can be used retrospectively for treatment simulations, plan evaluations, or to determine the accumulated dose for both the tumor and organs-at-risk on a subject-specific basis in MR-guided radiotherapy. 42
53 4.1 Introduction 4.1 Introduction Respiratory-induced motion hampers the application of highly conformal radiotherapy in the abdomen and thorax due to blurring of the dose. Traditionally, mobile tumors are treated with large margins, thus restricting the dose being applied. These margins are calculated using margin recipes, based on population motion averages and homogenous doses [5]. In stereotactic body radiotherapy (SBRT), stricter margins should be used. However, subject-specific motion, which can change over the course of a radiation treatment or between treatments [29], and inhomogeneous dose distributions within the tumor, introduces large variations in the definitive tumor dose coverage and organs-at-risk (OARs) doses. In case cone-beam CT position verification with sufficient contrast and/or implanted fiducial markers is available, SBRT is allowed by employing for example the midvent position [25] and/or internal target volume (ITV) technique [128], which are determined using pre-treatment 4D-CT or 4D-MRI. Nevertheless, the exact accumulated doses in the tumor and the OARs are unknown, since the motion during radiation is unidentified. Accurate motion estimations for both the tumor and the OARs during radiation would allow for this information. The recent introduction of MR-Linac (MRL) systems will enable MRI guidance during treatment (i.e. beam-on imaging) for tracking or gating and high quality MR imaging just prior to treatment (i.e. pre-beam imaging) [64, 67, 69]. Current MR imaging, however, is not fast enough to acquire 3D volumetric images with sufficient signal-to-noise ratio (SNR), spatial and temporal resolution, 3D coverage, and contrast for treatment guidance in the abdomen. Typically, the acquisition time for fast 3D volumetric images is in the order of seconds, while multiple 2D slices (up to ten) can be acquired within one second [79, 129]. To facilitate subsecond 3D MR imaging, the acquisition of multiple 2D slices can be combined with a patient-specific 3D motion model that can be acquired in a pre-beam phase. Pre-beam imaging involves both anatomical imaging to track day-to-day variations, and motion characterization scans. Linking the pre-beam imaging and beam-on imaging opens up the opportunity to retrospectively calculate the accumulated dose in both tumor and OARs, based on the organ motion and the given dose. In the context of radiotherapy, several groups pioneered the use of motion models that link fast 2D imaging during radiation treatment with patient-specific characterization of the 4D motion to calculate 3D volumes with sufficient spatial and temporal resolution to track the tumor. They used a 4D-CT motion model of the lung, calculated through a principal component analysis (PCA) in combination with fast online kv, MV or X-ray imaging [ ]. In the field of PET-MRI, several groups worked on MRI-based motion models [ ]. Fayad et al. [138] employed the generalized reconstruction by inversion of coupled system (GRICS) approach [139] for respiratory motion correction in simultaneous PET-MRI acquisitions of the lung by simultaneously estimating the physiological motion and reconstructed MR images, using a single 1D MRI navigator. King et al. [135], on the other hand, used an MRI motion model based on fast 3D volumetric imaging, in combination with a fast 2D MR images for motion correction of PET-data 43
54 Chapter 4 3D cine-mri in simultaneous PET-MRI lung acquisitions. Although these frameworks worked well for retrospective motion correction of PET data in the lung, abdominal applications will be challenging due to low and unfavorable contrast in the 2D and 3D images. A comprehensive review of existing respiratory motion models has recently been published [140]. The goal of this study is to generate 3D MR volumes with high spatio-temporal resolution, which can be used for treatment simulations, plan evaluations, or dose accumulation mapping in MR-guided radiotherapy of abdominal tumors (e.g. tumors in the pancreas, kidney and liver). We propose to use a patient-specific, retrospectively sorted 4D-MRI acquisition with high SNR and beneficial contrast to build a motion model in pre-beam phase, just prior to treatment. The motion, calculated from this 4D-MRI, is parameterized using a principal component analysis (PCA). Subsequently, this model is used to register a 3D reference volume to fast 2D multi-slice (MS) cine-mr images that are acquired during treatment, thereby updating the weights of the parameterized motion. The resulting 3D deformation vector fields (DVFs) have the same subsecond temporal resolution as the 2D-MS acquisition (less than 500 ms) and identical spatial resolution and coverage as the reconstructed 4D-MRI. For validation, the additional, orthogonallypositioned, interleaved acquired 2D images were used to observe the correctness of the motion characterization in the corresponding 2D plane. The added value of this approach is the fact that the combination of these orthogonal 2D cine MR images provides the imaging, necessary for 3D motion information of the centroid of the tumor during MR-guided radiation therapy. The proposed method is tested and validated in this work for future implementation on the MR-Linac. The main contributions of this work include: The proposed method extends previous CT and MRI-based methods for the lung into the abdominal area by optimization of the different steps for abdominal tumors, solely based on input from pre-beam and beam-on MR imaging. Phantom measurements are used to verify the geometrical accuracy of the 4D-MRI and 2D MR acquisitions, as well as the fitting procedure described above. The proposed framework is tested in seven healthy volunteers on both the pancreas and the kidneys, while the accuracy is independently determined using one of the two MR images. 4.2 Methods Framework The proposed framework is graphically shown in Figure 4.1. The framework consists of three parts, which are individually discussed in separate paragraphs; 1) The pre-beam imaging and generation of the subject-specific motion model using a 4D-MRI acquisition, 2) 2D cine MR imaging during radiation therapy for 44
55 4.2 Methods Pre-treatment imaging and model formation x-y-z-φ x-y-z-φ 4D-MRI acquisition Register 4D deformation vector fields 5 0 Displacement [mm] -5 PCA CC x-y-z-pc 0 LR -2 First two Principal Components for each voxel AP 1 st PC 2 nd PC Imaging during treatment 3D ref. volume Retrospective 4D motion calculation Final output x-y-z-t 2D cine-mri acquisition Warp 3D reference volume to 2D image using motion model... Time Full FOV 3D motion estimations Yes Warp 3D volume with weights w Calculate gradient-based similarity Converged to optimal? Update weighting w No Figure 4.1: Graphical overview of the workflow. In pre-beam imaging phase (green), a 4D-MRI is acquired on which non-rigid registration is performed. This motion information is parameterized using a principal components analysis from which only the first two components are used. During treatment (blue), two 2D slices are acquired in an interleaved fashion. The full field-of-view 3D motion information is calculated, by optimizing the weight of the eigenvectors through an iterative loop, in which a 3D reference volume is warped within the constraints of the model until it matches the incoming 2D slice (red). fast feedback of the tumor position and 3) Linking the patient-specific motion model to the fast 2D images by fitting a 3D reference volume to the 2D images and updating the weights for the motion model (called the fitting procedure). To simulate the online MRL setting, sequential 4D-MRI and 2D-MS cine data were acquired on a 1.5T MRI-RT scanner (Ingenia, Philips Healthcare, Best, the Netherlands) using a 28-channel body coil. All processing steps were performed in Matlab (The Mathworks, Natick, MA). 45
56 Chapter 4 3D cine-mri Pre-treatment imaging and model formation First, a retrospectively sorted 4D-MRI was acquired using a 3D, navigator-interleaved sequence, based on a previously described method by our group [141]. In brief, this 4D-MRI method acquires 3D-MRI data continuously using a clinically available 3D stack-of-stars balanced turbo field echo (btfe) sequence. Interleaved with the radial blades (shot length 130 ms), a 1D-navigator was acquired, which provided the information for retrospectively sorting the k-space data. This retrospective reordening was performed based on common linear phase binning, in which the respiratory cycle is divided into ten bins of equal time periods between subsequent exhale peaks. This respiratory correlated 4D-MRI method does not require an external breathing surrogate, has a moderate acquisition and reconstruction time, and, due to the balanced sequence, acquires 3D volumes with a high SNR efficiency [142] and beneficial abdominal contrast. Patient-specific PCA model: Non-rigid registration was performed on these ten 3D volumes using a 3D optical flow algorithm with the exhale phase as a reference volume I ref [143, 144]. This resulted in a set of 3D DVFs, denoted as DV F n (x, y, z) with n = [1, 2,..., N] the respiratory phases (N = 10 in this case) and x, yandz the spatial coordinates. A parameterized subject-specific motion model was build from these DVFs. A PCA was used to find an orthonormal basis that depicts the underlying pattern of the motion cycle. This model allowed for scaling of the parameterized motion and could therefore detect respiratory motion with higher amplitudes that were not encountered during construction of the model (i.e. during the 4D-MRI acquisition) when the model was applied. The PCA model was constructed in a similar way to the PCA model described by Zhang et al. [130] and used by others [131, 133, 135, 145]. In summary, a matrix of concatenated motion vector X = [X 1,, X N ] is formed in which ( X n = DV Fn x (1, 1, 1), DV Fn y (1, 1, 1), DV Fn(1, z 1, 1),, DV F x n (X, Y, Z), DV F y n (X, Y, Z), DV F z n(x, Y, Z)) T. (4.1) In here X, Y and Z are the matrix sizes in x, y, and z direction and DV F x,y,z n (x, y, z) are 3D motion vectors. Next, the zero sample mean of the matrix X is calculated by subtracting the sample mean µ from the vectors X φ : ˆX n = X n µ. (4.2) From this, the matrix B = [ ˆX 1,, ˆX N ] was constructed. The matrix B was used to calculate the eigenvalues λ and eigenvectors through a singular value decomposition (SVD) B = UΣV 1. (4.3) The matrix U consists of ten 3D eigenvectors for every voxel. Using a linear combination of these eigenvectors (or basis vectors), the 3D spatial transformation for each voxel was calculated through a small set of weights w: 46 ˆp = µ + Uw. (4.4)
57 4.2 Methods In this work, the first two eigencomponents were used as was also proposed by [130] and [133] to represent lung motion. This significantly reduces the dimensionality of the vector space. Moreover, this ensured that noise contributions from the model were minimized, since these are described by higher principal components. The Bayesian Information Criterion (BIC) [146] was used to demonstrate that the first two eigencomponents were sufficient to describe the motion in the 4D-MRI. The BIC is used to compare models with a different number of parameters (here eigencomponents), with low BIC-scores indicating a favourable model. Low BIC-scores are favourable as those models resolve the motion correctly, weighted by the total number of eigencomponents as penalty. Imaging during treatment Two fast, orthogonally-positioned, 2D slices were acquired in an interleaved fashion (sagittal, coronal, sagittal, coronal, etc.) during radiation (acquisition time is less than 250 ms per slice, thus at least two coronal and sagittal slices per second). These slices were positioned on the target (i.e. tumor) and can be angulated in any direction to sample the primary axis of motion. These two slices provided 3D MR imaging on the intersection of these two slices, typically the centroid of the tumor, and can therefore be used for fast feedback of the tumor position during MRL beam-on imaging. In the future, we want to track the tumor using 2D deformation vector fields. In order to do that, pre-treatment delineations will be transferred to the daily anatomy through registration of the pre-treatment 3D anatomical MR volumes to the daily anatomy and subsequently tracked using a fast deformable image registration method. Retrospective 4D motion calculation The subject-specific respiratory model was linked to the 2D slices by registering the 3D volume I ref to the 2D data through a linear combination of the first two principal components. By warping the 3D reference volume I ref in such a way that it corresponds to the incoming coronal 2D slice a vector of optimized weights w opt was calculated: w opt = arg max w Sim(I 2D, I 3D,w ). (4.5) In here, I 2D corresponds to the coronal 2D slice and I 3D,w to the warped 3D reference volume. The image similarity Sim was solely calculated on the overlapping 2D anatomy in the coronal slice. The coronal slice was used, opposed to the sagittal slice, since more anatomical information was available on the coronal slice. To allow for differences in contrast between the 3D reference volume (i.e the contrast of the 4D-MRI acquisition) and the 2D slices, an image similarity index, based on the image gradients was used [147] defined as: ( ) L M 2D,3D (x, y) cos(2 θ 2D,3D (x, y) l=1 Sim(I 2D, I 3D,w ) =. (4.6) L M 2D,3D (x, y) l=1 47
58 Chapter 4 3D cine-mri In here, M 2D,3D (x, y) is the product of the image gradient magnitudes and θ 2D,3D (x, y) the difference between the image gradient directions of the 2D coronal slice and the overlapping 2D anatomy in the warped 3D reference volume. L is the total number of voxels in the overlapping 2D anatomy. This image similarity index was found to be more robust for differenes in contrast between the 2D image and 3D volume, compared to other methods, such as the normalized cross correlation (NCC). A pattern search algorithm was used to maximize the similarity [148]. When converged, a full 3D DVF was calculated for each 2D acquisition time point by applying the optimized eigenvalues w opt to the model using Eq To assess the accuracy of the calculated motion on each time point, displacements on both slices of the 2D cine-mri acquisition were also estimated using a 2D optical flow algorithm [149] and compared with the estimated motion from the motion model using the root-mean-squared error (RMSE) and the normalized RMSE (NRMSE). Phantom experiments Geometrical accurateness of the imaging is vital for MR-guided radiotherapy [127]. Within the proposed framework, there are three steps that can introduce geometric errors (see Figure 4.2); 1) a positional error within the 4D-MRI (ε 1 ), 2) positional errors within the 2D-MS input data (ε 2 ), and 3) small misalignments after registering the 3D reference volume to the 2D data (ε 3 ). The positional errors are defined as differences between the absolute position of an object and reconstructed position of this object on the MR images. To determine these errors, the geometrical accuracies of individual steps of the framework were determined using phantom measurements [150]. A torso-shaped, MRI-compatible motion phantom (Quasar, Modus Medical Devices Inc., London, Canada) was used, which consists of two static compartments and one linearly moving cylinder (see Figure 4.3). The static parts were filled with CuSO 4 -doped water (0.5 g/l), while the moving compartment was filled with CuSO 4 -doped agarose gel (3%). A Ping-Pong (PP) ball, which simulated a moving tumor, was filled with CuSO 4 -doped water and additional MnCl 2 and inserted in the center of the moving cylinder. The moving part, which can produce cranio-caudal (CC) motion, was controlled by software provided by the vendor. The displacement is independently measured using a position encoder. Using the software user-defined motion patterns were played out. 48
59 4.2 Methods Table 4.1: Acquisition parameters for the 4D-MRI and 2D-MS phantom and in-vivo acquisitions. Imaging parameters 4D and 3D-MRI (phantom) 2D-MS (phantom) 4D-MRI (in-vivo) 2D-MS (in-vivo) Acquisition type 3D RF-spoiled GE 2D RF-spoiled GE 3D balanced GE 2D balanced GE TE/TR [ms] 1.45/ / / /3.0 Flip angle [ o ] FOV [mm] 330 x 330 x x x 330 x x 350 Slice thickness [mm] Matrix Size [mm] 152 x 152 x x x 152 x x 148 Bandwidth [Hz] SENSE - 2 (AP) 1.5 (CC) 2 (AP) Shot length [ms] Tvol [sec] Tacq [min:sec] 8:22.4 (4D), :16.5 8:35.0 1:59.0 (3D) GE = Gradient Echo 49
60 Chapter 4 3D cine-mri error ε 1 error ε 3 4D-MRI acquisition error ε 2 Fitting of the model to the 2D coronal slice 2D cine-mri acquisition Figure 4.2: Graphical overview of the three different geometrical errors that can be assessed using phantom experiments. ε 1 is the geometrical error in the 4D-MRI acquisition, ε 2 is geometrical error in the 2D-MS acquisition, and ε 3 is the error introduced in the fitting procedure. First, the geometrical error of the respiratory correlated 4D-MRI was calculated (ε 1 ). Ideally, the respiratory-correlated 4D-MRI depicts the average position in each phase, based on all the different positions within this phase. We hypothesize that this will be the case for the employed respiratory-correlated 4D-MRI, since radial in-plane sampling is used. This sampling method acquires the center of k-space for every read-out line, therefore reducing motion-related artifacts [92]. Since each phantom position contributes equally to the reconstructed position of the object, the average position will be depicted after sorting. To test this hypothesis, static 3D volumes (see imaging parameters in Table 4.1) were acquired at seven equidistant positions, serving as reference volumes and compared to the images of the individual respiratory phases after reconstruction of the 4D-MRI data set. Additionally, B0 maps were acquired at the seven positions. Various respiration motion patterns were simulated during the acquisition of the 4D-MRI data sets (see Table 4.2); three sine waveforms with varying amplitude and one respiratory bellow trace, obtained from in-vivo scans, were used in four separate 4D-MRI acquisitions (parameters in Table 4.1) in order to test the correspondence of the respiratory-correlated volumes with the reference volumes. The absolute geometrical correct (GC) positions were determined for the four different 4D-MRI acquisitions by calculating the average amplitude for every phase after phase binning. Boxplots were calculated for the different phases, representing the intra-phase amplitude variations, based on the input motion pattern. To 50
61 4.2 Methods (a) Motion phantom (b) Set-up on MR-table (c) Transversal Coronal Sagittal Figure 4.3: (a) Image of the MRI-compatible motion phantom before the different compartments were filled. The piezo-electric motor can be seen in the left cylinder. (b) Set-up of the phantom on the MR table with filled compartments. (c) Example images of the phantom with the moving cylinder in the center and the ping-pong ball visible in the middle of the cylinder in the transversal, coronal and sagittal view. determine ε 1, the position of the PP ball was extracted from the different 4D-MRI phases using two methods; 1) An automatic detection of the PP ball using a 1D profile in CC direction (called profile-determined positions) and 2) the center of mass (COM) of the ball was calculated after manual delineation on all phases of the four 4D-MRI acquisitions. Moreover, the PP ball was delineated on the static 3D volumes. These 3D delineation were used to determine the GC positions of the ball within the images by interpolating the delineations to the corresponding GC positions. The differences in COM between these (static) GC positions and the 4D-MRI positions represents the positional error of the 4D-MRI ε 1. Next, the geometrical error of the 2D-MS acquisition was assessed (ε 2 ). Nine 2D-MS data sets were acquired (imaging parameters in Table 4.1), while the cylinder was moving using the motion patterns defined in Table 4.2. These motion patterns represent all expected motions within the abdomen. Rigid registration was performed on the coronal slice using an affine registration within the Elastix toolbox [97] to obtain the translation in the images. The RMSE and NRMSE between this estimated translation and the user-defined motion patterns were calculated and serve as measure for the geometric fidelity (ε 2 ) of the 2D-MS acquisition. Last, the error for the fitting procedure was calculated (ε 3 ). Four PCA motion models were constructed from the four 4D-MRI acquisitions that were used to determine ε 1. Combinations of these models with the different 2D-MS acquisitions were used to test the robustness of the framework against variations in amplitude and frequency between the construction of the model (i.e. 4D-MRI acquisition) and applying the model (2D-MS acquisition), simulating variations in breathing between the pre-beam phase and the radiation treatment. The frequency sweep waveform was used to determine the highest frequency (and therefore speed) at which the location was still accurately depicted by the framework. Since the phantom only moves in CC direction, rigid registration was performed on the 4D-MRI acquisitions (opposed to non-rigd registration) and only one principal component (instead of two) was used. The RMSE and NRMSE between the 51
62 Chapter 4 3D cine-mri Table 4.2: Overview of the user-defined phantom motion patterns played out during acquisition of the 4D-MRI and 2D-MS data sets. 4D-MRI acquisition Sine, 0.23 Hz, ± 10 mm Sine, 0.23 Hz, ± 15 mm Sine, 0.23 Hz, increasing amplitude (5-14 mm) In-vivo extracted motion (normal breathing) 2D-MS acquisition Sine, 0.23 Hz, ± 10 mm Sine, 0.23 Hz, ± 15 mm Sine, 0.23 Hz, increasing amplitude (5-14 mm) In-vivo extracted motion, normal breathing (2x) In-vivo extracted motion, shallow breathing (2x) In-vivo extracted motion, deep breathing Frequency sweep sine, Hz, ± 15 mm estimated motion and the input motion patterns were calculated as measure for the error ε 3 for the fitting procedure. In-vivo experiments Several in-vivo experiments were conducted to demonstrate the feasibility of the proposed method for MR-guided radiotherapy in both the pancreas and the kidneys. The robustness of the method was determined by testing the method on different breathing scenarios. Additionally, the fitting procedure was validated in these in-vivo scans. This was only performed once for every subject and is an extra validation step that is not part of the regular framework. Seven healthy volunteers were scanned with informed consent. For each subject a 4D-MRI (T acq = 8m35s) and three 2D-MS data sets (T acq = 1m59s) were acquired using the parameters described in Table 4.1. The subjects were not instructed before the 4D-MRI acquisition, and therefore expected to breathe normally. The two slices of the 2D-MS acquisition (sagittal and coronal) were positioned on the pancreas (no angulations). To identify the robustness and validity of the framework, all subjects were asked to breath normally, shallowly, and deeply during the acquisition of the three 2D cine data sets, respectively. These different respiratory challenges simulated extreme differences in both respiratory frequency and amplitude between the model formation and were used to identify the constraints of the proposed framework. Lastly, for six of the seven subjects, an additional 2D-MS data set was acquired in which the slices were angulated 45 around the CC-axis to image both kidneys, while the subjects were breathing normally. After non-rigid registration of the 4D-MRI data (using the exhale volume as reference), the PCA motion model was constructed using the first two eigencomponents. As an additional validation step, the validity of the fitting procedure was determined on these in-vivo acquired data using several tests. A leave-one-out approach was used to generate the ten phase volumes, using the PCA model and the reference, exhale 3D volume. The PCA model should be able to reproduce each of the ten acquired phases. Differences between the acquired 4D-MRI and the PCA generated phase volumes reflects the amount of information lost due to the dimensionality reduction, since only two components were used. Although 52
63 4.3 Results the error for the fitting procedure ε 3 was already calculated using phantom measurements, this error will be different for the in-vivo experiments (ε 3) due to differences between the phantom and the in-vivo data (e.g. number of principal components, contrast, and (non)-rigid registration). First, the NCC between the calculated 3D volumes and the ten 4D-MRI volumes were calculated to measure the similarity between the warped 3D volumes and the 3D phase volumes in the 4D-MRI. Second, the pancreas was manually delineated on the reference volume, once for every subject, and the motion of this region-of-interest (ROI) was calculated for the 4D-MRI data and on the warped 3D volumes. The RMSE and NRMSE between these motion patterns was calculated as a measure for the invivo ε 3. Last, the BIC score was calculated to determine the optimal number of eigencomponents for every subject. These analyses will determine the validity of the model, which is important, since only a small number of eigencomponents were used. Next, the regular framework was executed on each of the 2D-MS acquisitions from the different breathing scenarios (i.e. the 3D reference volume was fitted to the 2D slices using the motion model). Since 250 2D images were acquired, this resulted in 250 3D volumes for each data set. Spatial RMSE maps were calculated using the difference between the motion on the 2D slices and the calculated motion in the corresponding planes. For the coronal slice these maps quantify the correctness of the fit between the 3D and the 2D images, whereas the maps on the sagittal slice independently quantify the model s accuracy and applicability outside the slice that was used as an input. The pancreatic motion on the calculated 3D volumes and the 2D slices were compared using the RMSE and NRMSE for the three respiratory challenges (normal, shallow and deep breathing). Last, for the six kidney scans, the framework was applied to the right kidney and independently checked on the contralateral, left kidney. After delineation of the kidneys, spatial RMSE maps were constructed for both slices and the RMSEs and NRMSEs between the kidney motion calculated using the framework and on the 2D slices were calculated. 4.3 Results Phantom results The differences between the positions determined by the 1D-navigator and the prescribed motion patterns were less than 1 mm for all acquisitions. Differences in the B0 maps, acquired at seven equidistant positions, were approximately 2 Hz, which demonstrates that geometric distortions arising from B0 variations are negligible. Figures 4.4a-d displays the COM positions within the different respiratory phases in blue, the profile-determined positions in green and the median positions calculated using the waveforms as red horizontal lines for the four 4D-MRI acquisitions. The automatically and manually determined positions correspond well with the defined waveforms for most of the phases, with the largest difference for phase 5 in 53
64 Chapter 4 3D cine-mri (a) 15 4D-MRI motion trajectory over the ten respiratory phases COM positions CC profile determined ball positions Part of the waveform Sine 10 mm (b) Sine 15 mm 15 Amplitude [mm] Amplitude [mm] Phase Number (c) Sine increasing amplitude Phase Number (d) In-vivo extracted waveform Amplitude [mm] Amplitude [mm] Phase Number Phase Number Figure 4.4: Motion trajectories of the four 4D-MRI acquisitions (a-d). In blue the center-of-mass positions of the ball are plotted, based on the manual delineations. In green the positions of the ball determined using the CC profile are plotted. The boxplots represent the intra-phase amplitude variation, with the red line the median position. Figure 4.4d just below 2 mm. In general, differences between the blue (COM) and green (profile-determined) lines were small. For the exhale phases 7-10 the positions resolved from the reconstructed volumes were, on average, slightly higher than the median positions, which were determined using the input waveforms. The average differences in COM between the determined positions in the 4D- MRI and the median positions from the actual positions over the ten phases for the four 4D-MRI acquisitions, indicated by ε 1, were 1.12, 1.24, 1.10 and 1.29 mm, respectively. The intraphase amplitude distributions, based on the input motion patters, are displayed using boxplots. The largest variation for the first two 4D-MRI acquisitions (Figures 4.4a and b) can be observed for the mid-inhale and mid-exhale phases (i.e. phase 3 and 8), since at those phases the speed of the phantom was largest, inducing large intraphase variations. Moreover, for the exhale and inhale positions (i.e. phase 1, 10 and 5, 6), the variations of the amplitude were small. For Figure 4.4c, the boxplots are similar for all the phases, since the amplitude variation is similar for all the phases. For the last 4D-MRI (Figure 4.4d), the intraphase amplitude variation is very large, primarily for the end-inhale phase (i.e. phase 5) as a result of significant differences in amplitude and phase between respiratory cycles for this waveform. Figure 4.5a displays coronal views of the reconstructed 3D volumes of the differ- 54
65 4.3 Results (a) Reconstructed phantom images and corresponding reference positions Reference Positions 4D-MRI inhale 4D-MRI exhale (b) Reconstructed phantom images for different waveforms for phase 3 Sine 10 mm Sine 15 mm Sine Variable amplitude (c) Reconstructed phantom images for different waveforms at 5mm Sine 10 mm Sine Variable ampltidue In-vivo waveform Figure 4.5: (a) Coronal views of the ten reconstructed respiratory phases of the 4D-MRI acquired while the phantom was moving using the first sinusoidal waveform, alongside the respective position in the sinusoidal waveform. In the left column, five coronal views of the corresponding reference positions are displayed. The red lines represent the center of the imaging volume. (b) Coronal views of the mid-inhale phase 3 of the first three reconstructed 4D-MRIs. Since these were acquired while the phantom was moving in a sinusoidal fashion, the mid-inhale phase should represent the same position. (c) Coronal views of different phases of three 4D-MRI acquisitions that represent an identical position (at +5 mm). Phase 2 for the first and third 4D-MRI acquisition alongside end-inhale phase 5 of the last 4D-MRI are shown. ent phases of the 4D-MRI using the first waveform (corresponding to Figure 4.4a) alongside the reference positions in the left column. Since a sine waveform was used, the in- and exhale GC positions were identical. The red line represents the center of the imaging volume. The positions in the 4D-MRI correspond well with the reference positions. Figure 4.5b shows the reconstructed volume of the mid-inhale position for the first three 4D-MRI acquisitions, corresponding to the same position (0 mm). The positions of the PP ball are comparable. However, more blurring (and ghosting to some extent) of the PP ball is observed for the second acquisition, which may be explained by the larger amplitude variation within this phase (see Figure 4.4b). Figure 4.5c presents the reconstructed volumes of phase 2 for the first and third 4D-MRI, and phase 5 of the last 4D-MRI acquisition. Although the intraphase variation in the last image was very large (see Figure 4.4d), a visual comparison of the reconstructed volume compares well 55
66 Chapter 4 3D cine-mri (a) 15 Amplitude [mm] RMSE = 1.00 RMSE = (c) Time [s] Time [s] Cranio-caudal motion (d) Cranio-caudal motion Estimated motion Estimated motion 10 Waveform 10 Waveform Amplitude [mm] Estimated motion by the model and the corresponding RMSE ε 3 Cranio-caudal motion Estimated motion Waveform (b) RMSE = 1.25 RMSE = Time [s] Time [s] Amplitude [mm] Amplitude [mm] Estimated motion Waveform Cranio-caudal motion Figure 4.6: Calculated motion by the four PCA-models (red) and the in-vivo waveform that was played out (blue). The RMSE values represents the geometrical error for the fitting step ε 3 in mm. with the other reconstructed volumes, but was more blurred than the first two reconstructed volumes. The estimated motion on the 2D-MS slices after registration showed good overlap with the input waveforms, with an overall average RMSE of 1.16 ±0.75 mm (NRMSE = 5.1% ±2.0%), which equals the average ε 2 for all 2D-MS acquisitions. Figures 4.6a-d show the estimated motion using the presented framework (red) and the input motion pattern (blue) for one of the in-vivo extracted waveforms. The four plots originate from the four motion models, calculated from the different 4D-MRI acquisitions. A good overlap can be seen for all four models, with a few overestimations at end-exhales for the last three models. The average RMSEs for the four 4D-MRI acquisitions with the first eight 2D-MS acquisitions were 1.23±0.27 mm, 1.85±0.36 mm, 1.44±0.13 mm, and 1.61±0.15 mm mm respectively, which equals NRMSE values below 8% for ε 3. This demonstrates that the framework is robust against differences in motion frequency and amplitude, between the model formation and utilization of the model by scaling the eigenvector, based on the motion in the 2D images. Fitting on the last 2D-MS acquisition (acquired while the speed of the phantom increased using a frequency sweep waveform) showed that the model performed well up to periodic motions with a frequency of 0.6 Hz, exceedings speeds of 50 cm/s, which is approximately a factor two higher than the expected respiratory frequencies for free-breathing patients. 56
67 4.3 Results (a) 5 Calculated pancreatic motion trajectories Amplitude [mm] CC AP LR Model 2D Sagittal 2D Coronal Time [s] (b) Sagittal Coronal (c) Histogram of CC differences 2D input slices 3D Model output RMSE maps 6 4 [mm] 2 0 Counts [-] Counts [-] 25 Coronal 20 Mean 15 difference Sagittal Difference [mm] Difference [mm] 3 Figure 4.7: In-vivo pancreas results for one of the subjects. (a) Motion trajectories within the delineated pancreas in CC, AP and LR direction, calculated by the model (red), on the sagittal slice (green) and on the coronal slice (black). (b) 2D anatomical images, corresonding output from the model and the RMSE maps in CC direction for the sagittal and coronal view. The pancreas is delineated in red. The black arrow represent an increased RMSE, originating from peristaltic motion. (c) Histograms of the differences in pancreatic motion in CC direction between the model and the motion on the 2D slices. In-vivo results Assessment of ε 3 on the in-vivo acquired data demonstrated a significant increase in NCC after registrating the 3D volumes to the ten respiratory phases within the 4D-MRI. The average NCC over all the phases and subjects was 0.91 ± 0.036, while before registration the NCC between the ten phases and the reference volumes was 0.84 ± The RMSE between the pancreatic motion trajectories calculated using the model and motion trajectories from the 4D-MRI over all phases was on average 0.69, 0.20 and 0.27 mm in CC, AP and LR direction, respectively. BIC analysis indicated that one eigencomponent was the most favorable trade-off between variance reduction and risk of overfitting as only ten phases were used to construct the PCA-model. However, since hysteresis effects can only be modeled with more than one eigencomponent, the remaining results were generated using two eigencomponents. Figure 4.7 illustrates in-vivo results from the pancreas for one of the subjects. Figure 4.7a displays the pancreatic motion in cranio-caudal, antero-posterior and 57
68 Chapter 4 3D cine-mri left-right direction calculated by the model (red) and calculated on the sagittal slice (CC and AP in green) and the coronal slice (CC and LR in black). As can be seen in the first plot, the CC motion calculated by the model accurately aligned with the motion calculated on the 2D slices (RMSE = 1.04 and 0.71 for the sagittal and coronal slice). On the sagittal slice an additional, higher frequency modulation can be observed, which may originate from a pulsating vessel posterior to the pancreas. The second plot shows that, despite the fact that the model is fitted on a coronal slice, the AP motion was still modeled satisfactory. The last plot illustrates a very small drift in lateral motion (±1mm), which is not completely resolved by the model. Figure 4.7b shows the RMSE maps in the sagittal and coronal plane alongside the 2D input slices and the corresponding model output views with the manually delineated pancreas in red. Small, slightly darker bands can be seen in the 2D input slices, due to spin history effects (i.e. cross-talk), which are not present in the model output views. The RMSE was small in the center of the FOV, with an average RMSE within the delineated pancreas of 0.83 mm for this subject. Increased values were observed in the cranio-caudal borders. Moreover, a slightly higher RMSE was observed in the right of the coronal slice (indicated by the black arrow). As can be concluded from the anatomical views, at these positions intestines were present. Motion of these intestines were both respiratory- and peristaltic-induced, which is not captured the 4D-MRI acquisition. RMSE values in CC direction for the pancreas were 1.28 mm (± 0.47 mm), 1.15 mm (± 0.55 mm), 2.34 mm (± 1.44 mm) for normal, shallow and deep-breathing, corresponding to NRMSEs between 10 and 15%. For the AP and LR direction, the RMSE values were all below 1 mm. Figure 4.7c reveals the differences in pancreatic motion estimated by the model and the 2D input images as histograms. For both the coronal and sagittal slice the median error is close to zero, suggesting that no systematic error is introduced by the motion model. Figure 4.8 exhibits the results for the kidneys from a different subject. Figure 4.8a gives an anatomical view of the positioning of the 2D slices and one of reconstructed slices from the 4D-MRI. Both 2D slices image one of the kidneys and cross approximately through the pancreas. Figure 4.8b shows the RMSE maps of the two slices alongside the anatomical views. The model was fitted on the right kidney, which shows low RMSE values in the posterior part of the anatomy and around the kidney. Increased RMSEs were again found in the cranio-caudal borders. The contralateral, left kidney displays low RMSEs in the posterior part of the anatomy, close to the kidney, but increased values towards the anterior part. This may be a result of local intestine motion, which was observed in the 2D slices, but not correctly modeled by the framework. Figure 4.8c plots the motion calculated using the model (red) and estimated on the 2D slice on the contralateral kidney. Although the weights were calculated on the other kidney, the motion trajectories nicely overlap for the contralateral kidney in CC direction. However, the oblique motion, which is a combination of anterior-posterior and lateral motion, was underestimated to some extent. The average RMSE on the contralateral kidney for all six subjects was 0.95 mm (± 0.32 mm). 58
69 4.4 Discussion (a) Anatomical view (b) Right Kidney RMSE maps Left kidney [mm] 0 (c) Amplitude [mm] Oblique CC Motion trajectories on contralateral left kidney Model SI motion Oblique motion Time [s] Figure 4.8: In-vivo kidney results for a typical subject. (a) Anatomical view illustrating the orientation of the two 2D slices. (b) RMSE maps of the differences in CC motion for both slices, with the kidneys delineated in red. The model was fitted on the right kidney. (c) Motion trajectories of the contralateral, left kidney determined by the model (red) and on the 2D slice in CC direction (green) and oblique direction (black). Table 4.3: Overview of the geometrical errors in the phantom and in-vivo experiments. The RMSE is tablated in mm, alongside the NRMSE in percentages between brackets. CC AP LR phantom ε (4.2%) ε (5.1%) ε (6.8%) in-vivo ε (11.7%) 0.20 (19.4%) 0.27 (19.6%) pancreas normal breathing 1.28 (12.7%) 0.47 (16.4%) 0.61 (17.7%) pancreas shallow breathing 1.15 (12.1%) 0.58 (20.0%) 0.64 (18.0%) pancreas deep breathing 2.34 (15.0%) 0.73 (17.7%) 0.90 (20.0%) kidney 0.95 (10.5%) 4.4 Discussion In this study we have introduced a PCA-based motion model to calculate high spatio-temporal 3D MRI volumes for MR-guided radiotherapy in the abdomen, using a retrospectively sorted 4D-MRI acquisition and fast 2D-MS cine MR images, which can accurately detect changes in motion amplitude, frequency and drift. The geometric accuracy was quantified on both phantom and in-vivo data (see Table 4.3). Subsequently, the framework was succesfully tested on seven healthy volunteers. This study extends previous studies on subject-specific motion models of the lung [ , 135] to the abdomen, through the use of dedicated MR sequences. 59
70 Chapter 4 3D cine-mri From the phantom experiments it was concluded that the geometric errors of the input data of the model (mean ε 1 = 1.21 mm and mean ε 2 = 1.16 mm) were small, which is vital for correct motion estimations. Figure 4.4 showed that the amplitude variation within the different phases can be large, leading to more blurring in the reconstructed volumes. This was a result of significant differences in amplitude and phase between respiratory cycles in combination with phase binning for which the data was sorted into ten equal time bins. Moreover, since the waveforms were only sampled between subsequent shots ( 130 ms), they were discretized. Decreasing the shot lenght or sampling the respiratory waveform for every read-out line (for example by using the noise variance [126]) may decrease the amplitude variation. Nonetheless, the positions of the PP ball were correctly identified with errors that were smaller than the voxelsize. Moreover, the fitting error ε 3 was identified to be 1.53 mm for the phantom data, which demonstrates that the framework can accurately manage changes in frequency and amplitude between the model formation and utilization of the model. Since this was validated with the gold standard input waveform, ε 3 represents the overall accuracy of the framework for the phantom measurements. The phantom measurements provided a quantitative assessment of the geometrical accuracy of the different parts of the framework. However, the motion applied by the phantom is a simplification of the complex in-vivo motion, since it can only produce rigid motion in CC-direction. Moreover, due to the limited size of the phantom in CC-direction, the 4D-MRI and 2D-MS acquisitions were adopted to decrease image artifacts induced by the sharp transitions between the phantom and air (e.g. Gibbs ringing). Therefore, the validity of the model, when two eigencomponents were used, was additionally evaluated in the 4D-MRI in-vivo data through ε 3, which gave comparable results to the phantom experiments. Furthermore, the accuracy of the motion model partly depends on the accuracy of the deformable registration method. The optical flow method was previously validated on 2D [144] and 3D [86, 141] in-vivo and phantom data, with sub-voxelsize errors of 0.33 to 1.1 mm, which was in the order of ε 3 (see Table 4.3). These small errors are supported by the small differences in pancreatic CC motion between the 2D coronal and sagittal slice in the presented data from this study. Lastly, the influence of cross-talk effects (dark bands) on the 2D images was found to be negligible, due to the multi-resolution registration scheme and the smoothing term in the optical flow method. This was validated using additional simulations (results not shown) in which artificial cross-talk effects were simulated showing maximum motion amplitude errors of only 1.3%. In-vivo pancreas data showed good results for normal and shallow breathing in all seven subjects, with RMSEs slightly higher than the registration error for the optical flow algorithm (RMSE = 1.28 and 1.15 mm). However, for deep breathing RMSEs were higher (RMSE = mm), primarily induced by underestimation of the inhale peaks. One subject had a significantly higher RMSE (5.43 mm). Investigation of the data showed that this subject s breathing amplitude during deep breathing exceeded 40 mm, which was underestimated by the motion model. The underestimation of the inhale peaks may be a consequence of incorrect warping of the exhale reference volume with very large amplitude motion. 60
71 4.4 Discussion Additionally, this may be a result of a change in breathing pattern when the subjects were asked to breathe deeply or a result of the fact that only two eigencomponents were used to warp the 3D volume. Since the model was constructed from a 4D-MRI acquisition during which the subjects were not instructed, a difference in the way the subjects breathe may cause the model to be less accurate for deep breathing. The RMSE maps showed small errors in the center of the field-of-view (FOV), but increased values in the cranio-caudal borders. This was a consequence of the fact that no anatomical information from outside the FOV was available to correctly warp these areas. Moreover, the motion after registration may not be completely resolved, since anatomy is moving in and out at the cranio-caudal borders, leading to inaccurate motion estimates. However, high local similarity (i.e. small RMSE) is crucial in high dose areas, such as the tumor and nearby structures, and to a lesser extent for low dose areas. For this study, the FOV is CC direction was limited as a compromise between scan time and coverage. Nevertheless, the radiation window of the MR-Linac is 200 mm at the isocenter in CC direction [71] and to fully exploit this window, the FOV of the 4D-MRI should be extended in CC direction. The histograms of the differences indicated that no systematic errors were found. This is an important finding in the context of MR-guided radiotherapy, since small random errors might cancel out over a radiation treatment. Furthermore, for the kidney data the model was applied on the right kidney, while the accuracy of the model was independently assessed on the left kidney. A good overlap in CC direction and low RMSEs, but a slight underestimation of oblique motion was observed, which demonstrates the framework s ability to determine the motion throughout the entire anatomy. The RMSEs found in the phantom experiments (1.53 mm on average, corresponding to NRMSE of 6.8%) are comparable to XCAT simulations and phantom experiments, based on 4D-CT from Mishra et al. (RMSE = 0.73 mm, NRMSE = 9.5%) [131] and Li et al. (RMSE = 1.2 and 2.4 mm for XCAT and 0.65 and 0.40 mm for phantom motion, corresponding to NRMSEs of 7-8%) [133]. Similarly, in-vivo results showed comparable results to 4D-CT methods in the lung by Zhang et al. (errors less than 2.0 mm) [130], Mishra et al. (RMSE in 2D of 0.90 ± 0.65mm) [131] and Fayad et al. (mean model errors between 1.35 and 2.21 mm for different motion scenarios) [134]. The PCA model is a parameterized representation of the abdominal motion, which can handle changes in amplitude and frequency, such as irregular breathing or motion drifts, to a certain extent, which is unique to this method. However, non-respiratory-induced motion, such as cardiac or bowel motion, are difficult to model correctly, as they are not well depicted in the respiratory resolved 4D- MRI. Moreover, since the PCA eigenvectors are fixed after model formation, large changes in breathing patterns (e.g. change from abdominal to chest breathing) may be difficult to model, since the motion is not accurately represented by a linear combination of these eigenvectors. In this work the sagittal slice was used as an independent measure for the accuracy of the calculated motion. However, this slice can also be used in addition to the coronal slice to increase the amount of anatomical information for fitting 61
72 Chapter 4 3D cine-mri the model and increase the temporal resolution. Moreover, the direction of the eigenvectors in the 4D-MRI can be used to position the 2D slices in such a way that they span the first three principal components and therefore a 3D orthogonal basis. During beam-on imaging, these orientations can be verified and updated to consistently sample the primary axis of motion. Moreover, these slices can be used to detect changes in breathing pattern to monitor the validity of the model during beam-on imaging. The acquisition of the 2D images can start immediately after the 4D-MRI acquisition. The processing of the 4D-MRI and construction of the model can be performed on a retrospective basis for dose accumulation purposes. This way the computing resources can readily be used for feedback of the tumor centroid using the 2D images for e.g. gating or tracking. Although it took approximately 3 seconds to calculate the weights w for every 2D slice, a dedicated GPU code may allow for 3D motion estimations that are fast enough for real-time feedback in combination with motion prediction techniques, such as an artificial neural network [151]. Alternatively, a pre-treatment 4D-MRI acquisition can be used. However, since during the same session the breathing pattern is less likely to change compared to day-to-day variation, the model may be sub-optimal. To test this, the reproducibility of the PCA-models have to be determined, which will be subject of future studies. The way the framework is designed allows for the individual optimization of the three parts (i.e. 4D-MRI, 2D-MS acquisition and fitting). Several techniques for abdominal 4D-MRI were recently proposed with various contrasts, spatial resolutions and FOVs (e.g. [141, ]). However, the acquisition time of these implementations all exceed 8 minutes. Since the processing of the 4D-MRI can be performed retrospectively, an iterative compressed sensing reconstruction can be used, in combination with a highly undersampled 4D-MRI acquisition, leading to a decreased acquisition time [155]. Unfortunately, these techniques can not provide real-time feedback of the tumor during radiation. Moreover, improvements on the 2D and 3D registration may lead to even smaller errors [156]. Furthermore, the number of 2D slices can be increased, without sacrifying acquisition time, to increase the anatomical information within the fitting procedure using specialized acceleration techniques, such as simultaneously multi-slice techniques [54, 157]. The 3D motion fields that were calculated by the framework have the same temporal resolution as the 2D-MS acquisition (i.e. 476 ms per time point) and similar spatial resolution to the reconstructed 4D-MRI (i.e. 1.9 x 1.9 x 2.0 mm 3 ). These high spatio-temporal resolution 3D deformation vector fields can be used to calculate the accumulated dose during MR-guided radiotherapy, which was recently shown for low spatio-temporal resolution motion fields for gated MR-guided radiotherapy [158]. This enables plan adaptation for subsequent fractions, based on the deposited dose that is different to the planned dose, due to changes in motion amplitude, frequency and drifts, which all can be detected by the presented framework. Also, pre-treatment motion characterization can be used to evaluate at forehand the dose of plans using subject-specific 3D motion. Moreover, different radiation strategies, such as margins, gating or tracking can be simulated to establish the optimal treatment for each patient or improve the margins. Although the outlook of this study is on MR-guided radiotherapy in the abdomen, 62
73 4.5 Conclusion the 3D motion information can also be used for 3D target tracking in MR-guided High Intensity Focused Ultrasound (e.g. [159]) in the abdomen or for retrospective compensation of PET data, acquired on an integrated PET/MRI scanner (e.g. [160]). 4.5 Conclusion A framework is presented, which calculates high spatio-temporal resolution 3D motion fields during the complete breathing activity, based on a pre-treatment 4D-MRI, beam-on 2D-MS acquisitions, and a PCA-based motion model. This enables the detection of changes in motion amplitude, frequency and drifts. The calculated 3D motion fields, that have the same temporal resolution as the 2D- MS acquisition (i.e. 476 ms), can be used to calculate the accumulated dose in the tumor and OARs or optimize the radiation plan on a subject-specific basis in MR-guided radiation treatments. The geometrical accuracies were calculated using an MRI-compatible motion phantom and found to be 1.21, 1.16 and 1.53 mm for the 4D-MRI, 2D-MS acquisition, and the fitting procedure, respectively. The framework was successfully tested on seven healthy subjects in both the pancreas and kidney and independently analyzed using an additional 2D slice with an average error of 1.45 mm. 63
74
75 CHAPTER 5 Dose accumulation in free-breathing MR-guided renal-cell carcinoma SBRT Stemkens, Bjorn Glitzner, Markus Kontaxis, Charis Denis de Senneville, Baudouin Prins, Fieke M. Crijns, Sjoerd P.M. Kerkmeijer, Linda G.W. Lagendijk, Jan J.W. van den Berg, Cornelis A.T. Tijssen, Rob H.N. The following chapter is based on: Effect of intra-fraction motion on the accumulated dose for free-breathing MR-guided stereotactic body radiation therapy of renal-cell carcinoma, Accepted for publication in Physics in Medicine and Biology 65
76 Chapter 5 MRI-guided dose accumulation Abstract Purpose: Stereotactic body radiation therapy (SBRT) has shown great promise in increasing local control rates for renal-cell carcinoma (RCC). Characterized by steep dose gradients and high fraction doses, these hypo-fractionated treatments are, however, prone to dosimetric errors as a result of variations in intra-fraction respiratory-induced motion, such as drifts and amplitude alterations. This may lead to significant variations in the deposited dose. This study aims to develop a method for calculating the accumulated dose for MRI-guided SBRT of RCC in the presence of intra-fraction respiratory variations and determine the effect of such variations on the deposited dose. Methods: RCC SBRT treatments were simulated while the underlying anatomy was moving, based on motion information from three motion models with increasing complexity: 1) STATIC, in which static anatomy was assumed, 2) AVG-RESP, in which 4D-MRI phase-volumes were time-weighted, and 3) PCA, a method that generates 3D volumes with sufficient spatio-temporal resolution to capture respiration and intra-fraction variations. Five RCC patients and two volunteers were included and treatments delivery was simulated, using motion derived from subject-specific MR imaging. Results: Motion was most accurately estimated using the PCA method with root-mean-squared errors of 2.7, 2.4, 1.0 mm for STATIC, AVG- RESP and PCA, respectively. The heterogeneous patient group demonstrated relatively large dosimetric differences between the STATIC and AVG-RESP, and the PCA reconstructed dose maps, with hotspots up to 40% of the D99 and an underdosed GTV in three out of the five patients. Conclusions: With the proposed pipeline the influence of intra-fraction motion variations on the accumulated dose was calculated and found to be significant. This shows the potential importance of including these intra-fraction motion variations into dose calculations. 66
77 5.1 Introduction 5.1 Introduction Stereotactic body radiation therapy (SBRT) is increasingly used in radiotherapy treatment for abdominal tumors, such as liver [13], pancreatic [14], and renal tumors [15] to increase local control rates. Recently, it was demonstrated that a few high-dose fractions (or even a single fraction) is preferred in renal-cell carcinoma (RCC) treatments as conventional fractionated radiation schemes are ineffective and give poor outcomes [15]. SBRT is characterized by hypo-fractionation, steep dose gradients, tight margins, and high fraction doses [161]. Consequently, small geometric errors can quickly lead to underdosage of the target or increased dose to organs at risk (OARs). Traditionally, margin concepts, such as the internal target volume (ITV) [128], mid-ventilation [25], or mid-position [26] approaches, are used for mobile tumors. Margins are determined using either population-based metrics or individual measurements. Within such margin recipes, respiratory motion is expressed as a random error [5]. However, intra-fraction respiratory variations, such as in/exhale amplitude variations, can change significantly within a treatment fraction [7, 29, 162, 163]. Another important factor that limits the use of populationbased margins are drifts. Von Siebenthal et al., for example, found drifts up to 12.8 mm in the liver [7]. For hypo-fractionated treatments, such as SBRT, these variations can lead to more systematic type of errors and therefore have a greater dosimetric effect (compared to fractionated treatments where they are represented as random errors). In order to determine this effect, the deposited dose should be tracked to retrospectively calculate the accumulated dose accurately. However, this requires high spatio-temporal 3D anatomical information about the tumor and all OARs during radiation therapy, which is not readily available on current radiotherapy systems. Hitherto, primarily Computed Tomography (CT) has been used to track the deposited dose. Either 4D-CT or interpolated in- and exhale 3D-CTs have been used to create multiple phase volumes to determine organ motion and calculate the dose for each respiratory phase (e.g. [ ]). These phase volumes are subsequently time-weighted using the respiratory probability density function (PDF), extracted from models or patient measurements, to calculate the total accumulated dose. However, since these studies used a small number of discretized phase volumes (2-10), only the average respiratory motion is included. Consequently, changes in respiration during treatment, such as intra-fraction baseline variation, motion drifts due to muscle relaxation, and in/exhale amplitude variations, are not taken into account. These time-weighted approaches might therefore be an oversimplified representation of reality. Others used Magnetic Resonance Imaging (MRI) to simulate the accumulated dose [158, 168, 169]. The recent introduction of hybrid MRI-Linac (MRL) systems [63, 65, 68, 70, 127] opens up the opportunity to acquire the necessary MR information just prior to (i.e. pre-beam), and during (i.e. beam-on) treatment. Unfortunately, the acquisition of 3D MRI volumes is generally too slow to capture respiratory motion and intra-fraction respiratory variations in three dimensions. MRI-based dose reconstruction studies therefore focused on breath-hold, or gated treatments. Although motion induced by normal 67
78 Chapter 5 MRI-guided dose accumulation breathing was not included in these MRI-based dose reconstruction studies, [158] demonstrated that motion drifts give rise to considerable dose differences. Ideally, the breathing motion is included into these MR-based methods to characterize the effect of breathing and motion drifts on the dose. The purpose of this study is to show the technical feasibility of merging the CT and MRI-based methods for evaluating the effect of intra-fraction motion variations on the dose in free-breathing treatments. To achieve this, a pipeline was developed to simulate free-breathing MR-guided RCC SBRT treatments, while the underlying anatomy was moving, based on motion information from different motion models. To demonstrate the dosimetric effects of different aspects of motion (such as drift and frequency/amplitude variations), three motion models were implemented with increasing complexity: 1) No motion characterization during treatment (STATIC), 2) time-weighted average motion (AVG-RESP), and 3) a method that characterizes 3D motion with sufficient spatio-temporal resolution to capture both respiration and intra-fraction respiratory variabilities, such as amplitude variations and motion drifts, based on a previously published MRI-based motion model (PCA) [170]. The different reconstructed doses are calculated and compared to determine the effect of intra-fraction motion variations. 5.2 Methods A pipeline was established for calculating accumulated doses, using motion information from deformable vector fields (DVFs), derived from MR imaging. Two healthy volunteers and five patients were included in this study after written informed consent was obtained. The volunteers provided pilot data to show the validity of the models for dose accumulation applications. An overview of the pipeline is graphically shown in Figure 5.1. First, MR images, including 4D-MRI and multi-2d cine MRI, are acquired to simulate an on-line MR-Linac treatment. From these MRI data 3D motion was calculated, using three different motion models. Bulk density pseudo CTs (pcts) were constructed for every time point on which 3D motion information was available (every 360 ms), by warping a reference pct using the reciprocal 3D DVFs. A clinically acceptable plan was generated and delivered in-silico. Lastly, the delivered dose was reconstructed by applying Monte-Carlo (MC) dose calculations on the warped pcts, which were subsequently warped back to the reference state and summed over time to generate the accumulated dose maps. All MRI data were acquired on a 1.5T MRI-RT scanner (Ingenia, Philips, Best, the Netherlands) to simulate the online MR-Linac setting. All processing steps were performed in Matlab (The Mathworks, Natick, MA). MR imaging Three MR sequences were used in this study. Due to time restrictions, the patients were scanned with a slightly modified and shorter protocol, relative to the healthy volunteers. 68
79 5.2 Methods Volumetric 3D anatomical imaging Delineations Radiation plan pct Delivery Dynamic volumetric volumet- imaging Dynamic volumetric imaging Dynamic ric imaging Dose reconstruction Machine parameters Machine parameters Machine parameters Accumulated dose 3D reference volume Reference pseudo CT Accumulate dose DVF t -1 DVF t DVF t -1 Σ DVF t Dynamic 3D volume Dynamic pseudo CT Reconstructed dose segment Figure 5.1: Schematic overview of the proposed methods. First, imaging data is acquired (green), including anatomical scans and scans to calculate dynamic volumetric images using the different motion models. Next, delineations are made and a pseudo CT is generated (blue). Then, a radiation plan is generated and delivered on an emulator to log the machine parameters. These are used, in combination with the fast volumetric imaging to reconstruct the accumulated dose. Forward and backward deformation vector fields (DVFs) are calculated from the dynamic volumes, which are subsequently used to reconstruct individual dose segments on the dynamic grid that are warped back and summed on the reference grid to yield to accumulated dose. First, a T 2 -weighted Turbo Spin Echo (TSE) acquisition (resolution = 0.6 x 0.6 x 3.5 mm 3, T acq = 2 min) was performed for delineation purposes. This sequences was respiratory-triggered to acquire slices in exhale phase. In an MR-Linac setting, this acquisition could be performed in a simulation phase, for delineation purposes to generate a plan, as well as in the pre-beam phase, for daily anatomical imaging. Next, 4D-MR imaging was performed using a modified version of a previously described implementation by our group [141] (see parameters in Table 5.1). This respiratory-correlated 4D-MRI does not require external respiratory surrogates, acquires full 3D volumes with high SNR and contrast between organs, and has a moderate acquisition and reconstruction time. In short, this 4D-MRI implementation acquires 3D-MRI data continuously using a 3D stack-ofstars balanced steady-state free precession (bssfp) sequence with radial in-plane 69
80 Chapter 5 MRI-guided dose accumulation Table 5.1: Acquisition parameters for the 4D-MRI and 2D cine-mri acquisition for the volunteers and patients. For the 4D-MRI T vol is defined as the time to acquire a Nyquist-sampled 3D volume, while for the M2D cine acquisition it is defined as the time to acquire both slices. Imaging parameters 4D-MRI (volunteer) 4D-MRI (patient) M2D cine MRI (volunteer/patient) Acquisition type 3D bssfp 3D bssfp 2D bssfp TE/TR [ms] 1.45/ / /3.0 Flip angle [ o ] FOV [mm] 300 x 300 x x x x 347 Slice thickness [mm] Matrix Size 160 x 160 x x x x 148 Bandwidth [Hz/px] SENSE factor 1.5 (CC) - 2 Partial Fourier (CC) 0.75 T vol [sec] T acq [min:sec] 5:26.2 3:22.4-5:03.1 6:54.0 (volunteer) 5:06.0 (patient) sampling and Cartesian sampling along the k z direction. Instead of acquiring a navigator interleaved with the radial projections (as was done in the original implementation), a self-gating signal was extracted by taking a 1D Fourier transformed projection profile along the k z direction through the central k x = k y = 0 points [155]. Retrospective binning was performed using standard phase binning, in which the respiratory cycle is divided into ten respiratory bins of equal time periods between subsequent exhale peaks. For patients, golden-angle radial sampling [57] was used in combination with a compressed sensing reconstruction to reduce the acquisition time, while keeping similar image quality [171, 172]. In order to fully include both kidneys for all patients, the field-of-view (FOV) was adapted for every patient, resulting in different matrix sizes and consequently different acquisition times. The reconstructed 4D-MRI has a resolution of 1.9 x 1.9 x 2.0 mm 3. Last, two fast 2D cine-mri slices with similar contrast to the 4D-MRI were acquired in an interleaved fashion (see parameters in Table 5.1). For patients the sagittal and coronal slice were positioned through the tumor, while for volunteers the sagittal and coronal slice were rotated 45 around the cranio-caudal (CC) axis, to image both kidneys. Using the parameters from Table 5.1, both slices were acquired in 360 ms, which equals the temporal resolution of the 3D motion estimations. Motion approximation Three motion models were implemented, which are schematically presented in Figure 5.2: 1) STATIC, 2) AVG-RESP, and 3) PCA. For all methods, the reconstructed 4D-MRI was non-rigidly registered using a 3D optical flow algo- 70
81 5.2 Methods rithm [143, 144] with the exhale phase as a reference volume. The utilized optical flow algorithm was previously validated on 2D [144,173] and 3D [86,141,170] invivo and phantom data. Since both the forward and backward DVFs are used, inverse consistency was ensured for all DVFs [158]. For the first method (Figure 5.2a), static anatomy was assumed (STATIC). This simulates the current clinicial workflow in which no 3D motion information is available during treatment. The 4D-MRI DVFs were used to construct a midposition (mid-pos) 3D reference volume for treatment planning [26]. This mid-pos volume was constructed by applying the inverse of the mean motion DVF to the exhale volume. The same transformation was used to warp the T 2 -weighted anatomical scan to mid-pos for delineation. The second method (AVG-RESP, see Figure 5.2b) additionally determines the respiratory phase from the 2D cine-mr images for every time-point and subsequently uses the corresponding 4D-MRI phase volume (and the concomitant DVFs) as the current motion state. Effectively, this generates a time-weighted 4D-MRI in which every phase is accurately weighted based on the individual respiratory phase during radiation (beam-on time). This simulation is analogous to the method previously described by [ ]. The last method (PCA, see Figure 5.2c) uses a previously described image-based motion model [170]. In short, this method generates a motion model, on a voxelby-voxel basis, from the 4D-MRI by performing a principal component analysis (PCA) on the 4D-MRI DVFs to create a parameterized model of the underlying pattern of the motion cycle. Subsequently, for every set of 2D cine-mr images PCA weights are calculated that scale the model, creating individual 3D volumes for every set of 2D images. These weights are calculate by warping the 3D midpos volume in such a way that it corresponds to the 2D cine-mri slices. The advantage of this model is the fact that it allows for scaling of the parameterized motion and it can therefore handle drifts and inter-cycle variations, such as varying exhale and inhale amplitudes. All three motion models generated high spatial (1.9 x 1.9 x 2.0 mm 3 ) and temporal (every 360 ms) resolution DVFs. Lastly, 2D non-rigid registration was performed on the two 2D cine-mri slices using an optical flow algorithm. These 2D DVFs were used to quantitatively compare the accuracy of the three motion methods. Pseudo CT generation Electron density (ED) information is required for the fluence optimization in a radiation plan, which is traditionally extracted from a CT scan. However, since the volunteers and patients in this study did not receive a CT scan, pseudo CTs (pcts) were generated, based on a two compartment model [158, 169, 174]. In this model, the automatically generated body contour on the anatomical midpos volume was filled with Hounsfield Units (HUs) equal to the HUs of water HU water = 0. Other voxels were set to air, HU air = 2900, which resulted in the reference pct ref. Note that the lungs are not in the FOV. pct ref was subsequently used to generate a pct for every cine-mri time point 71
82 Chapter 5 MRI-guided dose accumulation a) 4D-MRI 4D-MRI DVF Mid-pos b) 4D-MRI 2D cine 4D-MRI DVF Determine resp. phase Mid-pos 4D-MRI phase 4D-MRI volume 4D-MRI phase phase volume volume 4D-MRI 4D-MRI phase phase volume volume Time c) 4D-MRI 4D-MRI DVF Mid-pos 2D cine Update PCA-weights Individual 3D volume Individual 3D Individual Individual volume3d Individual 3D volumes 3D volume volume Time Figure 5.2: Schematic overview of the three motion models (a) STATIC that assumes static motion, (b) AVG-RESP that models the average breathing motion and (c) PCA that includes motion drifts and ex- and inhale amplitude variations. t, pct t. The 3D DVFs, generated by either of the three motion models, were used to warp pct ref to the dynamic grid on which each of the segments were delivered (see Figure 5.1). Plan generation and delivery For patients the renal tumor was delineated by a radiation oncologist, while for volunteers a fictitious lesion was placed in the right or left kidney. Moreover, all OARs were delineated, which included the spleen, stomach, spinal cord, duodenum, liver, pancreas, small and large bowel, aorta, and both kidneys by a radiation oncologist. Based on clinical constraints from Table 5.2 [175, 176], a 5x7 Gy step-and-shoot intensity modulated radiotherapy (IMRT) plan was generated [15], in which a 1.5T magnetic field was taken into account [75], based on the mid-pos anatomy. A GTV-PTV margin of 3 mm was used, according to our clinical standard [176]. Next, one fraction of the plan was delivered on a linac emulator (Elekta AB, 72
83 Table 5.2: Dose descriptions for the target and the OARs. Planning dose constraints Anatomical Site Constraint Value GTV V 35 99% D max 52.5 Gy PTV V % V % Aorta D max 35 Gy Heart D max 38 Gy V 32 < 15 cc Chest Wall D max Gy V 40 < 10 cc Skin V 36.5 < 10 cc V 27.5 < 5 cc Myelum V 23 < 0.35 cc V 14.5 < 1.2 cc Ipsilateral Kidney D mean 11.3 Gy Both Kidneys V 16.8 < 67% Ureter D max 42 Gy Duodenum D max 32 Gy V 18 < 5 cc V 12.5 < 10 cc Small Bowel D max 35 Gy V 19.5 < 5 cc Large Bowel D max 38 Gy V 25 < 20 cc Esophagus V 27.5 < 5 cc V 35 < cc Stomach D max 32 Gy V 18 < 10 cc Liver D mean 18 Gy V 21 < 700 cc Pancreas D max 21 Gy Spleen D mean 11.5 Gy 5.2 Methods Stockholm, Sweden) and the machine parameters (e.g. gantry angle, dose rate, leaf positions) were logged for every cine-mri time point t in order to describe the synchronized machine state with the anatomy [158]. Dose reconstruction An MC-based dose calculation was used to reconstruct the dose at each time point t on the corresponding pct t, yielding a dose map D t for every time point. Finally, these individual dose maps were warped back to the reference state (midpos) and summed for all t time points T to generate the reconstructed dose 73
84 Chapter 5 MRI-guided dose accumulation D recon [ ]: T D recon = DV F t D t. (5.1) t=1 Motion accuracy and plan evaluation The accuracy of the three motion methods was determined by comparing GTV motion on the 2D cine-mr images with the GTV calculated by the different motion models. Both the root-mean-squared error (RMSE) and the normalized RMSE (NRMSE) were calculated and used as quantitative accuracy assessments. Dose volume histogram (DVH) points and integral parameters (e.g. Dmax, Dmean, D99) were analyzed for all three methods. Moreover, dose difference maps were calculated to highlight local differences and the maximum and minimum dose differences were reported. 5.3 Results Figure 5.3a illustrates the different motion trajectories that can be generated by the three motion models. While STATIC assumes no motion and therefore only represents one position (essentially the reference position), AVG-RESP is able to capture hysteresis, but no inter-cycle variations. The PCA method, on the other hand, can fully characterize hysteresis and inter-cycle variations, including drifts, and amplitude variations. This is demonstrated by the cranio-caudal (CC) motion of the (fictitious) GTV of one of the healthy subject (subject one) that is plotted in Figure 5.3b. The GTV motion calculated on the 2D cine-mr images (coronal slice) is plotted by the dotted black line and serves as the reference motion, while the GTV motion determined by the three motion models are depicted in blue (STATIC), green (AVG-RESP) and red (PCA) lines. For this subject a clear caudal drift of approximately 2 mm is observed, which is correctly captured by the PCA model, despite the fact that some of the inhale peaks are over/under estimated. Since the AVG-RESP model only uses predefined DVFs (from the 4D-MRI), the GTV motion trajectory is very repetitive and does not include any drift or amplitude variations, leading to increased RMSE and NRMSE values. Figure 5.4 displays an example of the reconstructed mid-pos volumes including the delineations for the anatomical T 2 -TSE and 4D-MRI acquisitions in the transversal, coronal and sagittal view for one of the patients. The delineations were made on the mid-pos T 2 -weighted scans (left) and were transferred to the 4D-MRI reconstructed mid-pos volume (right). Although the contrast in these scans is different, most delineated structures can be identified on both volumes. Some blurring is observed in the 4D-MRI for the small (green) and large bowel (yellow), since these organs experience peristaltic motion, which was not resolved by the respiratory-correlated 4D-MRI. Figure 5.5 shows the CC GTV motion for three different patients, with varying motion amplitudes, frequencies and respiratory variability. The lines are color-coded similar to Figure 5.3. For patient one a considerable offset between 74
85 5.3 Results (a) STATIC AVG-RESP PCA End-exhale End-exhale End-inhale End-inhale (b) Amplitude [mm] Cranio-caudal GTV motion trajectory over time (Subject one) -4 2D-cine MRI STATIC -6 AVG-RESP PCA Time [sec] RMSE NRMSE (mm) (%) STATIC AVG-RESP 1.30 PCA Figure 5.3: (a) An illustration of the motion trajectories that can be produced by the three different motion models; STATIC assumes no motion, AVG-RESP is able to capture hysteresis, but no inter-cycle variations, PCA is able to capture hysteresis, inter-cycle motion, and slow drifts. (b) Cranio-caudal GTV motion trajectories for one of the healthy volunteers. GTV motion as determined on the 2D cine-mr images are displayed in black (dashed line), while GTV motion determined by STATIC (blue dashed-dotted), AVG-RESP (green dashed), and PCA (red solid) are plotted by the other lines. The root-mean-squared error (RMSE) and normalized root-mean-squared error (NRMSE) are reported for all three methods. the 2D cine-mr GTV motion and GTV motion calculated using STATIC and AVG-RESP is observed. This was caused by a body shift in cranial direction of approximately 2.5 mm between the 4D-MRI (i.e. setup imaging) and 2D cine (i.e. images during treatment) acquisitions. While a shift between patient setup and treatment is not captured using STATIC or AVG-RESP, it was correctly modeled by the PCA motion model. Patient three shows large variations in amplitude and frequency, as well as small drifts. Although the AVG-RESP methods represents the average amplitude correctly, these variations are only correctly modeled by the PCA method. Nevertheless, some large inhale amplitude peaks are underestimated to some extent by the PCA method. Patient four, on the other hand, displays a more regular breathing pattern. However, some variations in inhale amplitude and exhale positions are still observed that are only captured by the PCA method to a large extent. Moreover, the AVG-RESP method underestimates the motion amplitude structurally by 40%. Over all subjects (patients and healthy volunteers) the average RMSEs for STATIC, AVG-RESP and PCA were 2.69 ± 0.68, 2.37 ± 0.78 and 1.09 ± 0.47 mm, corresponding to NRMSEs of 24%, 21% and 9%, respectively. Figure 5.6 shows the reconstructed dose maps and dose differences for patient one, corresponding to Figure 5.5a. Figure 5.6a shows the reconstructed dose maps with the GTV and PTV delineation for the three methods on top of the anatomical T 2 -weighted images. Here, STATIC corresponds to the planned dose. 75
86 Chapter 5 MRI-guided dose accumulation Anatomical T2-TSE mid-pos 4D-MRI mid-pos Sagittal Coronal Trannsversal Figure 5.4: Mid-pos transversal, coronal and sagittal view of the anatomical T2-TSE and 4D-MRI mid-pos volumes with the delineated structures (GTV in red, PTV in blue). Figure 5.6b displays the dose differences between the AVG-RESP reconstructed dose and the planned dose and the PCA-based reconstructed dose and the planned dose. While the former dose difference map shows merely minor variations, with some cold spots just outside the PTV, the latter dose map presents distinct hotand cold spots caudal and cranial of the GTV. The maximum and minimum dose differences are 2.9 and -4.8 Gy, respectively. Note that the planned dose in the tumor was 7 Gy for this single fraction. These large differences are caused by the 2.5 mm cranial body shift, leading to increased dose caudal of the target. Figure 5.7 demonstrates the reconstructed dose maps and dose difference maps for patient four (matching Figure 5.5c), plotted on top of the mid-pos 4D-MRI volume. Although the differences are smaller compared to Figure 5.6, primarily the PCA dose difference map shows significant variations and distinct cold- and hotspots, with a maximum and minimum difference of 1.3 and -1.6 Gy, respectively. While some spots are visible in the AVG-RESP dose difference map, they are smaller, with lower magnitude and at slightly different locations, as a result of different underlying moving anatomy. The reconstructed dose maps of the other patients can be found in the supplementary materials. Figure 5.8 shows the DVHs of four patients for the different reconstructed doses (STATIC in dashed-dotted lines, AVG-RESP in dashed lines, and PCA in solid 76
87 5.3 Results (a) Patient 1 6 Amplitude [mm] 0 Cranio-caudal GTV motion trajectory over time Time [sec] (b) Patient 3 5 Amplitude [mm] (c) Patient 4 Time [sec] 5 Amplitude [mm] Time [sec] 2D-cine MRI STATIC AVG-RESP PCA 2D-cine MRI STATIC AVG-RESP PCA 2D-cine MRI STATIC AVG-RESP PCA Figure 5.5: Cranio-caudal GTV motion trajectories for three patients. GTV motion as determined on the 2D cine-mr images are displayed in black (dashed line), while GTV motion determined by STATIC (blue dashed-dotted), AVG- RESP (green dashed), and PCA (red solid) are plotted by the other lines. lines) for the GTV and multiple OARs. The PTV structure is also shown to provide additional insight into the differences between the three methods, although clinically one would not assess the given dose on PTV structures. The three motion models demonstrate small absolute differences for the OARs, as they are all situated in the low dose regions for the investigated treatment plans. As expected, the largest differences are observed for the PTV structures, resulting from the differences in estimated positions in the vicinity of the steep dose gradients. In three out of the five patients, the PCA method estimated an underdosage to the GTV, which was not shown by the AVG-RESP method. The largest differences were observed in patient one, where the D99 in the GTV was reduced from 7.6 Gy for STATIC, to 7.5 Gy for AVG-RESP, and only 6.1 Gy for the PCA method. For patient three and four small underdoses of 6.8 Gy and 6.9 Gy were observed, respectively. Moreover, in patient four, the PCA-based method also showed a lower dose to the ipsilater kidney, but a higher low dose region. To evaluate the dose constraints from Table 5.2 on the reconstructed dose maps, the doses were multiplied by the number of fractions (five). While this is a simplification, since it is unlikely that the motion is identical for all fractions, verifying the dose constraints provides valuable controls for considering accepting of adapting the plan. For all patients the constraints were met in the planned dose. However, the V 35 < 99% GTV constraint was not met in three patients in the PCA case, and for one patient in the AVG-RESP case. Moreover, the Dmax constraint for the small bowel was not met in the AVG-RESP reconstruction, 77
88 Chapter 5 MRI-guided dose accumulation (a) Reconstructed dose maps STATIC AVG-RESP PCA 7 Transversal GTV PTV Sagittal Coronal 4 Gy 0 (b) Dose difference maps PCA - STATIC Coronal AVG-RESP - STATIC 3 Sagittal 0 Gy -3 Figure 5.6: (a) Shows the different reconstructed dose maps on top of the T2 weighted mid-pos volume for patient one. (b) shows the dose difference maps between the planned dose (STATIC) and the reconstructed dose using one of the AVG-RESP or PCA method, zoomed in on the tumor region. The GTV (grey) and PTV (black) are delineated on all views. whereas it was within the constraints for the PCA reconstructions. 5.4 Discussion While different strategies for calculating accumulated doses have been demonstrated in literature, this is the first study that evaluates the effect of inter-cycle respiratory motion variations on the accumulated dose in free-breathing abdominal SBRT treatments. For this purpose, a dose accumulation pipeline was developed, solely based on MR imaging and three motion models were compared. Using the GTV motion in the 2D cine-mr images as reference, it was demonstrated that the PCA method describes this motion most accurately, with NRMSEs lower than 10%. Reconstructed dose maps revealed large dissimilarities between the different methods and between patients, induced by intra-fraction motion variations that 78
89 5.4 Discussion Reconstructed dose maps (a) STATIC AVG-RESP PCA 7 Transversal GTV PTV Sagittal Coronal 4 Gy 0 (b) Dose difference maps (zoomed in) Coronal AVG-RESP - STATIC PCA - STATIC 1.5 Sagittal 0 Gy -1.5 Figure 5.7: (a) Shows the different reconstructed dose maps for patient four, overlayed on the 4D-MRI mid-pos volume. (b) shows the zoomed dose difference maps. The GTV (grey) and PTV (black) are delineated on all views. were only captured by the PCA method. The advantage of the proposed pipeline over previously described methods for calculating the accumulated dose is the increased accuracy of the utilized DVFs. By incorporating variations over the entire fraction in the PCA method, RMSEs in the calculated motion were reduced from 2.69 mm (STATIC) and 2.37 mm (AVG-RESP) to 1.09 mm. This provides some evidence that the calculated DVFs, and therefore the calculated dose, is most accurately reconstructed with the PCA method. As can be seen in Figure 5.5, the GTV motion differs largely between the patients, both in frequency and amplitude. This large variety consequently leads to large variations in the reconstructed doses. In general, the PCA reconstructed dose maps showed larger differences with the planned dose than the AVG-RESP reconstructed doses. This was expressed either as distinct hot79
90 (d) Dose-volume histogram comparison (Patient 2) Chapter 5 MRI-guided dose accumulation (a) 100 Volume [%] Dose-volume histogram comparison (Patient 1) STATIC AVG-RESP PCA GTV PTV Spleen Kidney Ipsilateral Adrenal Ipsilateral Dose [Gy] (b) Dose-volume histogram comparison (Patient 2) 100 Volume [%] STATIC AVG-RESP PCA GTV PTV Spleen Kidney Ipsilateral Pancreas Chest wall Skin Aorta Small bowel Large bowel Dose [Gy] (c) Dose-volume histogram comparison (Patient 3) 100 Volume [%] STATIC AVG-RESP PCA GTV PTV Kidney Ipsilateral Adrenal Ipsilateral Skin Dose [Gy] (d) Dose-volume histogram comparison (Patient 4) 100 Volume [%] STATIC AVG-RESP PCA GTV PTV Kidney Ipsilateral Duodenum Small bowel Dose [Gy] Figure 5.8: Dose-volume histograms of the GTV, PTV, and a number of OARs for the three motion models STATIC (dash-dotted lines), AVG-RESP (dashed lines), and PCA (solid lines) for patient 1 (a), patient 2 (b), patient 3 (c), and patient 4 (d). and cold-spots in the dose difference maps, where these spots were absent in the AVG-RESP dose difference maps, or the positions of such spots were different in the two dose difference maps. These deviations can solely be explained by the differences in calculated motion, and therefore the DVFs that are used in the dose reconstruction. For example, when the GTV moves more caudal than expected, as a result of drifts or large inhale amplitudes, the accumulated dose on the cranial side of the GTV will be higher, while the caudal side is estimated to receive less dose. If this motion is significantly large, this leads to underdosage of parts of the GTV, and overdosage to OARs that are cranial of the GTV/PTV. Note that the dose difference maps from patient one express large variations, as a result of a structural difference in GTV position. While this is ideally captured and corrected by re-positioning the patient prior to treatment, this body shift occurred after the 4D-MRI acquisition that was acquired just prior to the interleaved 2D cine images. On a regular linac, this discrepancy can only be detected just prior to and/or after the treatment using online imaging, while an MRL can provide the necessary online imaging feedback to detect this in real time during treatment. The dose differences found in this study are, in general, larger than previously reported for free-breathing treatments [ ] and gated treatments [158]. This may indicate that the influence of intra-fraction motion variations on the accumulated dose can be significant and should be taken into account. While patient three showed larger amplitude motion and larger inter-cycle variations, the dose 80
91 5.4 Discussion differences were smaller compared to patient four and five, leading to the conclusion that more systematic errors, such as structurally lower mid-positions or motion drifts, induce the largest dose differences. Although the results depend on the treatment choice (mid-pos in this case), radiation plan and PTV margin, the dosimetric impact may be similar for other treatments. For example, neither an ITV-based plan nor a slightly larger PTV margin, encompasses the GTV continually when a drift, or a structurally lower/higher GTV position occurs, leading to distinct cold- and hotspots. The AVG-RESP method, which relies heavily on the quality of the 4D-MRI (or 4D-CT in other studies), showed smaller dose differences than the PCA method. This may be caused by an underestimation of the motion in the 4D-MRI, as was observed for patient two and four. The utilized 4D-MRI has been validated previously using phantom experiments, where it was shown that all respiratory phases display the average position within a phase bin [170]. Nevertheless, it has been reported by others that phase-binned 4D reconstructions (both 4D-MRI and 4D-CT) can underestimate abdominal motion [12, 172]. Therefore, using a motion model that parameterizes the motion, instead of using a small number of discretized phase volumes, increases the accuracy of the estimated motion. In this study a two compartment pct model was used. Although, it has been shown by [177] that this is a good approximation, this pct can be improved by using electron density information converted from Hounsfield units in a CT or by using more compartments. Unfortunately, the patients in this study did not receive a planning CT, since they were scheduled for surgery. Moreover, the number of patients in this feasibility study is limited. However, since a large inter-patient variations was observed for the motion and reconstructed doses, the results provide a relatively broad range of scenarios. Lastly, only one fraction, out of a five fraction SBRT scheme, was simulated, since the motion information was extracted from a single MRI examination. The large differences between the PCA-based reconstructed dose and the planned dose, might cancel out to a certain extent by using more fractions. However, [15] concluded that higher doses in less (or even a single) fractions give the best local control in RCC with a maximum of five fractions. The large differences shown for this one simulated fraction are therefore relevant for clinical SBRT RCC treatments that want to maximize local control. The results from this study indicate that accurate intrafraction motion estimation is essential to precisely calculate the accumulated dose in such hypo-fractionated treatments. The largest discrepancies between the reconstructed dose and the planned dose appeared to originate from slow variations in the motion, such as drifts, and in/exhale amplitude variations. Consequently, such variations lead to a slightly different effective average tumor position (i.e. the tumor mid-pos). Since this leads to systematic errors, such slow variations should be accounted for, either during planning by employing larger margins, or during treatment, by gating or tracking. To determine the optimum treatment technique, the effect of patientspecific respiratory changes on the deposited dose could be simulated as a pretreatment step. While retrospective dose calculations after treatment require 81
92 Chapter 5 MRI-guided dose accumulation online imaging capabilities, pre-treatment simulations can be performed using a single MR-sim session. Alternatively, since gating may have low radiation efficiency, especially when drifts occur, and tracking is technically challenging, tumor trailing can be employed that adapts the gating window accordingly to capture baseline drifts [178]. By dynamically updating the mid-pos volume and recalculating the plan [75], the low frequency motion variations may have a reduced effect on the accumulated dose. Although the PCA method from this study takes a few seconds to calculate a new dynamic 3D volume, it may be fast enough to update the mid-pos volume with low temporal resolution (e.g. every minute). Since the retrospective dose calculations in this study are not time-critical, the computational time is less relevant for this work. Future studies will, however, focus on speeding up the PCA calculation, utilizing the PCA model to update the mid-pos volume, and calculating the accumulated dose for multiple fractions. In this paper, only the dose accumulation of static (i.e. margin-based) delivery techniques was investigated. The presented dose accumulation pipeline, however, can be directly applied to tracked delivery techniques on an MR-linac, offering great potential to track not only the dose to the target [65, 73, 179], but also the OARs, something that is currently not feasible on kv- or optical-based systems [180]. Since the PCA method is able to deal with non-rigid deformations, it opens great perspective for organs underlying complex deformations, such as the liver and pancreas. 5.5 Conclusion A pipeline to evaluate the dosimetric effect of intra-fraction motion variations in renal-cell carcinoma was described, solely based on MR imaging. The PCAbased motion model characterizes the motion most accurately, since drifts and inter-cycle respiratory variations, which appear to be the most important factors for precise dose calculations, are captured correctly. In the presented patient sampling, these variations demonstrated relatively large dosimetric differences compared to the planned dose and reconstructed dose maps using time-weighted average breathing volumes. It demonstrates the potential importance of including intra-fraction motion variations in dose calculations, which may be incorporated using online MRI guidance in combination with real-time treatment planning. 82
93 CHAPTER 6 Maximizing SNR in simultaneous multi-slice body imaging Stemkens, Bjorn Sbrizzi, Alessandro Andreychenko, Anna A. Crijns, Sjoerd P.M. Lagendijk, Jan J.W. van den Berg, Cornelis A.T. Tijssen, Rob H.N. The following chapter is based on: An optimization framework to maximize signal-to-noise ratio in simultaneous multi-slice body imaging, 2016, NMR in Biomedicine; 29:
94 Chapter 6 Optimizing SMS body imaging Abstract Purpose: Parallel imaging is essential for the acceleration of abdominal and pelvic 2D multi-slice imaging in order to reduce scan time and mitigate motion artifacts. CAIPIRINHA (Controlled Aliasing In Parallel Imaging Results IN Higher Acceleration) accelerated imaging has been shown to significantly increase the signal-to-noise ratio (SNR) compared to in-plane parallel imaging with similar acceleration. We hypothesize that for CAIPIRINHA-accelerated abdominal imaging the consistency of image quality and SNR is more difficult to achieve due to the subject-specific coil sensitivity profiles, caused by 1) flexible coil placement, 2) variations in anatomy, and 3) variations in scan coverage along the superior-inferior direction. Methods: To test this hypothesis, a mathematical framework is introduced that calculates the (retained) SNR for in-plane and simultaneous MS (SMS)-accelerated acquisitions. Moreover, this framework was used to optimize the sampling pattern by maximizing the local SNR within a region-of-interest (ROI) through non-linear, RF-induced CAIPIRINHA slice shifts. The framework was evaluated on 14 healthy subjects and the optimized sampling pattern was compared with in-plane acceleration and CAIPIRINHA acceleration with linear slice shifts that are primarily used in brain imaging. Results: We demonstrate that the FOV in cranio-caudal direction, the coil positioning, and the individual anatomy have a large impact on the image SNR (changes up to 50% for varying coil positions and 40% differences between subjects) and image artifacts for simultaneous multi-slice acceleration. Consequently, sampling patterns have to be optimized for acquisitions employing different FOVs and ideally on an individual basis. Optimization of the sampling pattern, which exploits non-linear shifts between slices, showed a considerable SNR increase (10 30%) for higher acceleration factors. Conclusion: The framework outlined in this paper can be used to optimize sampling patterns for a broad range of accelerated body acquisitions on an individual basis. 84
95 6.1 Introduction 6.1 Introduction 2D multi-slice (MS) sequences are widely used acquisition methods for diagnostic MRI examinations in the abdominal and pelvic region. Combinations of 2D MS sequences with varying contrasts (e.g. T 1, T 2, balanced, or diffusion weighted) play a key role in diagnostic and oncologic imaging, e.g. for the detection, characterization and delineations of tumors in the abdominal [181] and pelvic area [182]. Besides its value in diagnostic radiology examinations, 2D MS acquisitions have large potential in real-time tracking for MR-guided interventions, such as MR-guided radiotherapy [127]. To accelerate these acquisitions, parallel imaging (PI) techniques are commonly employed. They exploit the spatial encoding power of radio frequency (RF) receiver coil arrays to unfold aliased images. Conventional PI techniques, such as simultaneous acquisition of spatial harmonics (SMASH) [50], sensitivity encoding (SENSE) [51] or generalized autocalibrating partially parallel acquisitions (GRAPPA) [52] reduce the number of acquired phase-encoding lines, leading to aliasing artifacts. These are unfolded in the reconstruction process, which requires sufficient receiver coil sensitivity variation. How well the aliased signals can be unfolded can be expressed by the geometry factor (g-factor) [51]. This factor expresses the spatially varying noise amplification and is determined by the differences in coil sensitivities for aliased pixels. In-plane PI techniques are usually constrained to moderate reduction factors (up to four for coil arrays with a typically large number (16-32) of coil elements). First, this is caused by an SNR loss due to Fourier averaging as a result of a reduced amount of acquired data. Second, the coil array has insufficient encoding power for these higher acceleration factors, as the reconstruction becomes ill-conditioned, leading to high g-factors. To mitigate this SNR loss, simultaneous MS (SMS) [53] and CAIPIRINHA (Controlled Aliasing In Parallel Imaging Results IN Higher Acceleration Factors) [54] acceleration is used to accelerate 2D brain imaging [183, 184]. SMS and CAIPIR- INHA excite multiple slices simultaneously by using multiband RF-pulses. The collapsed slices are unfolded during reconstruction, similar to in-plane PI. Compared to in-plane PI the excited volume is S-times larger, where S equals the SMS acceleration factor (i.e. number of simultaneously excited slices), resulting in a factor S higher SNR [54], analogous to the SNR advantage of 3D over 2D acquisitions. However, encoding power in the through-plane direction is generally limited due to the coil s design and therefore the SMS reconstruction becomes illconditioned, resulting in high g-factor type noise. CAIPIRINHA mitigates this limitation by shifting simultaneously excited slices relative to each other in a controlled, coherent fashion, thereby exploiting sensitivity variation in the coil array more efficiently, enabling simultaneous sampling of closely spaced slices. The slice shifts are induced by means of modified phase encoding patterns for each slice through RF [54] or gradient phase modulation [185]. Compared to both in-plane PI and through-plane PI (SMS), the g-factor in CAIPIRINHA is decreased, since the variation of coil sensitivities is exploited in both in-plane and through-plane direction. However, selecting unfavorable shifting patterns can have a large effect on the g-factor and directly influences the resulting image quality. 85
96 Chapter 6 Optimizing SMS body imaging Accelerated body imaging may also profit from these benefits, as was shown by [54]. Previous studies on accelerated body imaging focused on evaluation of image quality of clinical scans acquired using 2D-CAIPIRINHA [55] in comparison to standard PI sampling for 3D volumetric imaging [ ]. They showed superior image quality, clinical feasibility, and reduced unfolding artifacts using the CAIPIRINHA sampling pattern. However, Deshpande et al. [186] found that inter-subject anatomical variations resulted in increased artifacts for larger patients. Moreover, Weavers et al. [191] demonstrated that individual optimization of the 2D-CAIPIRINHA kernel was required for 3D subtraction contrast-enhanced MR angiograpy to minimize noise. However, these studies only examined 2D- CAIPIRINHA for acceleration of 3D acquisitions. In the present work, we are interested in the acceleration of 2D MS acquisitions for clinical abdominal protocols. Apart from the original implementation by Breuer et al., very few body applications use CAIPIRINHA, since large variations in anatomy and the use of flexible coils considerably change the coil s sensitivity patterns between subjects, which may lead to inconsistencies in image quality between subjects and acquisitions. Throughout the rest of this article CAIPIR- INHA refers to MS-CAIPIRINHA for SMS acquisitions. Since the coil sensitivity patterns are subject-dependent and directly influence the resulting image SNR, inter-subject variations may greatly affect the image quality. These inter-subject variations, such as anatomy, coil positioning, and field-of-view (FOV) variations, are much larger than in brain applications and therefore vary the image quality substantially. Consequently, individual optimization of the CAIPIRINHA sampling pattern may be required. In this study, we will focus on RF-induced phase modulation, since this provides the freedom to shift each slice independently, whereas gradient-induced phase modulation is linearly dependent in the slice direction and therefore constrained to linear phase ramps in the through-plane direction. The purpose of this paper is twofold. First, a mathematical description for the SNR of a SMS experiment is provided that employs both in-plane and throughplane acceleration. This model is then used to evaluate different acceleration sampling patterns for several clinical situations to assess the effect of inter-subject variations on the resulting image quality. Second, this model is used to calculate the optimal sampling pattern for a given acceleration factor, which maximizes the local SNR. This is achieved by shifting each simultaneously excited slice independently, allowing for non-linear slice shifts, opposed to linearly dependent slice shifts mostly used in CAIPIRINHA-accelerated imaging thus far. Optimization results in an SNR increase up to 40% at the cost of complex shifting patterns for CAIPIRINHA. We demonstrate that the sampling patterns that influence SNR depend on the FOV, individual anatomy and coil positioning and that these factors should be taken into account for scans employing SMS techniques in abdominal exams. Since the results are coil and sequence-specific, the code for this framework is made available online to evaluate other coils and acquisition protocols. 86
97 6.2 Methods x-y-z-coil x-y-z Define fixed parameters: FOV PE R tot Sens. maps ROI Standard sampling scheme Low resolution sensitivity maps Fully sampled 2D-MS anatomical data Pattern search optimization loop Optimized sampling scheme Simulated SNR maps and images Calculate SNR maps for all slices Calculate mean 10% min SNR Converged to optimal? Yes Define new optimization parameters No Figure 6.1: Graphical overview of the workflow for the optimization. First, coil sensitivity information is acquired and sensitivity maps are calculated. Moreover, 2D MS anatomical data are acquired to simulate accelerated images. The fixed parameters (imaging requirements) are defined and alongside a standard, initial sampling scheme fed into the optimization loop, which calculates retained SNR maps for all SMS slices. Within this optimization, the mean of the 10% lowest SNR is calculated within the ROI and compared with previous values. If SNR converges to a maximum, the optimum sampling scheme and retained SNR maps are returned. 6.2 Methods We performed various experiments to simulate different acceleration methods in clinically relevant settings. We compared in-plane and CAIPIRINHA acceleration with linearly increasing slice-shifts (referred to as LISS-CAIPIRINHA) with the optimized sampling pattern that provided the theoretically maximum SNR. For each subject, a standard SENSE reference scan (3D spoiled gradient echo [TE/TR = 0.57/8 ms, voxel size = 7.5 x 7.5 x 7.5 mm 3, flip angle = 7 ]) was acquired to determine the coil sensitivity maps (see Figure. 6.1). Moreover, 2D MS anatomical data were acquired to simulate in-plane and CAIPIRINHA acceleration and to calculate SNR maps for visualization of aliasing artifacts. ReconFrame (Gyro- Tools LLC, Winterhur, CH) was used for resampling and reconstruction of sensitivity maps and anatomical data. All simulations and analyses were performed in Matlab (The Mathworks, Natick, MA). To optimize the balance between in-plane and SMS acceleration, and the FOV shift for each slice, a signal model is required that incorporates all these factors. 87
98 Chapter 6 Optimizing SMS body imaging Signal model Starting from the SENSE matrix formulation of an accelerated 2D MS experiment [51], a generalized signal model was derived. f n (x, y, z) = S R 1 s=1 r=0 ( C n x, y + η(φ s) R ( ρ x, y + η(φ s) R + r FOV ) PE, z + (s 1) R ) + r FOV PE, z + (s 1) R. (6.1) In this equation, f n is the signal in the folded image of the n th coil at location (x, y, z), C n the sensitivity profile per coil, ρ the signal in a specific voxel, R the inplane acceleration factor, and FOV PE the full FOV in the phase encoding direction (in this case, the y-direction). S is the SMS acceleration factor (i.e. number of simultaneous excited slices), the slice distance between the simultaneously excited slices, and η(φ s ) the FOV shift induced by the phase modulation vector φ = (φ 1,, φ S ). φ s is the steepness of the linear phase ramp along the phase encoding lines for the s-th slice defined in fraction of 2π as described by [54]. For instance, φ = [0 π], results in image shifts of η = [0 FOV/2] for the two simultaneously excited slices. Based on this generalized signal model, the g-factor is calculated, which is subsequently used to determine the expected SNR in an accelerated SMS acquisition using SNR acc = SNR full S. (6.2) g general Rtot Here g general is the generalized g-factor, SNR full is the SNR of a fully encoded (nonaccelerated) single slice acquisition and S the SMS acceleration factor. The total acceleration factor R tot is defined as the combination of the SMS acceleration factor S and the in-plane acceleration factor R R tot = S R, (6.3) with R tot S. To quantitatively compare 2D MS acquisitions, that use inplane, CAIPIRINHA or CAIPIRINHA with additional in-plane acceleration, the retained SNR is defined as SNR retained = SNR acc SNR full. (6.4) Quality metric For the detection of small structures within the abdominal area, such as lesions or tumors, sufficient image quality has to be guaranteed. Since image quality depends on the expected SNR, which is affected by the used acceleration method (as can be derived from Eq. 6.2), an SNR quality metric was employed to estimate the resulting image quality. For robustness and to minimize the influence of noise in the sensitivity data, the mean of the relative SNR values that lie below the 88
99 6.2 Methods 10 th percentile (SNR 10 ) was used as quality metric for all acceleration methods. Moreover, a region-of-interest (ROI) specific SNR 10 was calculated to assure a threshold SNR within a pre-defined part of the image, which is important in the detection and characterization of small structures. Optimization parameters For the optimization of the acceleration parameters, the SNR 10 is maximized by penalizing low SNR values, to minimize image quality degradation within this ROI. The goal of the optimization is therefore: p opt = arg max SNR 10 ( p). (6.5) p Here, p = (S, R,, η(φ s )) is the vector of optimization parameters and p opt the vector of optimized parameters. Since a threshold SNR is required, the optimization aims to maximize low SNR values, while preserving high SNR values. It is expected that this selection criterion ensures superior SNR and image quality in the selected ROI. The SNR was calculated using Eq. 6.4 where SNR full was set to 1, which resembles a fully-sampled single slice acquisition (i.e. R tot = 1). Prior to this calculation, noise prewhitening was performed using the noise covariance matrix Ψ to decorrelate the noise within the coil sensitivity maps, as described in [192]. Several fixed parameters were required as input for the optimization problem (see Figure 6.1). A fixed FOV was determined, based on the anatomical acquisition, and a predetermined total acceleration factor R tot. Moreover, a predefined ROI was used for local optimization. Given these fixed parameters, the optimal balance between SMS acceleration S and in-plane acceleration R, and FOV shift per slice η(φ s ) (if S > 1) were calculated, based on the coil sensitivity matrix C. A pattern search [148] algorithm was used within Matlab (using the patternsearch function) to calculate the set of optimization parameters that resulted in the highest SNR 10, for the given set of acquisition parameters. The pattern search is a direct-search optimization method and does not require the gradient of the objective function, which in this case is not differentiable. Retained SNR maps (i.e. SNR calculated using Eq. 6.4) were calculated for all simultaneously excited slices (e.g. for S = 3, three retained SNR maps were calculated). To keep calculation time at a minimum, no SNR maps were calculated for the other sets of slices within the FOV. Moreover, all simultaneously excited slices are equally spaced over the FOV. Lastly, the optimizer was allowed to vary S and R independently between 1 and R tot ), while R tot R tot,desired. In-vivo experiments Abdominal data were acquired for three different simulation experiments to evaluate the acceleration methods and to demonstrate the performance of the optimization algorithm for clinically relevant situations. Written informed consent was obtained from all subjects before the examination using an institutionally 89
100 Chapter 6 Optimizing SMS body imaging approved protocol. Coil sensitivity maps were calculated using the parameters described in Table 6.1. Table 6.1: Table with the acquisition parameters. Experiment name FOV experiment Coil position experiment Inter-subject variation experiment FOV (mm 3 ) 350x280x x210x x280x Size C (voxels) 66x48x50 66x40x30 66x48x43-50 Maximum acceleration factor Number of subjects out of 12 are also included in the FOV experiment Fourteen subjects were scanned on a 1.5T Philips Achieva (Philips Healthcare, Best, The Netherlands) using the vendor s standard 16-channel abdominal coil array. Triggered axial 2D MS turbo spin-echo images were acquired for anatomical reference based on a clinical protocol (TR/TE = 493/68 ms, voxel size = 1 x 1.3 x 3.5 mm 3, flip angle = 90, bandwidth = 396 Hz, SENSE = 2) with phase encoding in anterior-posterior (AP) direction. Two subjects from the first experiment were also included in the last experiment. For all experiments an ellipsoid encompassing the pancreas was delineated and used as ROI, because of its central location within the abdomen. In the first experiment the effect of changes in the FOV in the through-plane direction was examined, since in clinical practice the FOV in this direction is frequently adjusted, both the size and the position of the FOV. This is a direct result of variations in the patients anatomy and spread of the disease in the through-plane direction. This changes the relative differences in coil sensitivities, and due to the coil array s shape (two rings in CC direction of 2x4 (APxLR) elements), this may have a significant influence on the SNR when CAIPIRINHA is used. To address this, we reduced the FOV along the z-direction for two subjects retrospectively and investigated the differences in SNR 10 for two LISS- CAIPIRINHA sampling patterns (i.e. [0 F OV 2 0] and [0 F OV 2F OV 3 3 ]) and the optimized phase modulation scheme for R tot = 3. Moreover, in-plane acceleration was simulated on the corresponding three slices and the SNR 10 was averaged over these slices. This analysis was only performed for R tot = 3, since for this acceleration factor the largest variation was expected. Next, the impact of variations in the coil setup were investigated. Two data sets were acquired for which the anterior part of the coil was shifted 5 cm in cranial direction with respect to the posterior part for the second scan to simulate clinical setup variations in positioning the coils. Using the optimization tool the SNR 10 was maximized and compared with in-plane and LISS-CAIPIRINHA acceleration. The LISS-CAIPIRINHA simulations used linear slice shifts, which showed good 90
101 6.3 Results results in previous experiments [193] and are described in Table 6.2. The sampling parameters were optimized on one set of S simultaneously excited slices. Using these parameters, the acquisition of the entire FOV was simulated and the SNR 10 was calculated on each set of slices. Last, inter-subject variation was examined by comparing SNR 10 values for twelve subjects for in-plane, LISS-CAIPIRINHA, and the optimized sampling pattern. A two-sided paired Student s t-test was used to determine the significance of the SNR 10 increase after optimization. Inter-subject variation was examined before and after optimization by calculating the coefficient of variation of SNR 10, which is defined as the ratio between the standard deviation to the mean. 6.3 Results Figure 6.2 demonstrates the different simultaneously excited slices with their corresponding phase modulation and FOV-shifts for R tot = 3, the resulting folded image, and one of the retained SNR maps with the matching unfolded slice for LISS-CAIPIRINHA (with FOV shifts [0 F OV 2F OV 3 3 ]) and after optimization. An increase of SNR is clearly visible in the retained SNR maps and unfolded images after optimization. This is confirmed by the difference between the two unfolded images, which reveals an area of increased noise in the center of the body. Figures 6.3a and b display one of the resulting retained SNR maps for R tot = 4 for another subject using CAIPIRINHA (i.e. S = 4, R = 1) before and after optimization, respectively. Lower SNR values are present using the LISS- CAIPIRINHA pattern, as a result of overlapping simultaneously excited slices and insufficient encoding power of the array coil. This is primarily noticeable in the center of the FOV. After optimization, low SNR values are suppressed over the entire FOV. This is confirmed by Figure 6.3c, which presents the cumulative histograms within the ROI for both SNR maps. These histograms illustrate higher SNR values within the ROI after optimization, with an SNR 10 of 0.65 before and 0.71 after optimization. These cumulative histograms display a typical shape of the curves, which indicate an overall SNR increase within the ROI. Figure 6.4 shows the SNR 10 as a function of the FOV in through-plane direction Table 6.2: Table with LISS-CAIPIRINHA slice shifts for each slice for acceleration factors up to R tot = 8. Acceleration factor FOV shift for each slice 2 [0 F OV 2 ] 3 [0 F OV 2F OV 3 3 ] 4 [0 F OV 2 0 F OV 2 ] 6 [0 F OV 2F OV F OV 3 8 [0 F OV F OV 3F OV F OV 4 2F OV 3 ] F OV 3F OV 2 4 ] 91
102 Chapter 6 Optimizing SMS body imaging LISS-CAIPIRINHA 0π phase ramp 0 FOV shift 2 3π phase ramp 1 FOV shift 3 4 3π phase ramp 2 FOV shift 3 Folded Images Retained SNR maps A 1 Unfolded images 0.7 Optimized 0π phase ramp 0 FOV shift B 1.22π phase ramp 0,27π phase ramp 0.61 FOV shift FOV shift Folded Images Retained SNR maps Unfolded images Figure 6.2: Example for Rtot = 3 of a LISS-CAIPIRINHA acquisition (a) and the acquisition with the optimized sampling pattern (b). The different simultaneously excited slices are shown with their phase modulation value and FOV shift for each slice alongside the folded images. Moreover, retained SNR maps are shown next to the unfolded images, which both demonstrate decreased noise amplification after optimization. (i.e. CC direction) for in-plane, LISS-CAIPIRINHA with two sampling schemes 2F OV (i.e. [0 F OV 0] and [0 F OV ]), and the optimized sampling scheme for an acceleration factor of Rtot = 3. Optimized parameters solely used CAIPIRINHA and no extra in-plane acceleration (i.e. S = Rtot = 3 and R = 1). This was calculated by the optimizer and not imposed by the user. As expected, in-plane SENSE reconstruction is not affected by the changing FOV in z-direction, since it only uses in-plane sensitivity encoding power for unfolding. SMS acceleration shows considerably higher SNR compared to in-plane acceleration, but the SNR decreases for smaller FOVs, which is a result of lower encoding power along SI in this reduced FOV. For a larger FOV, there is a clear SNR benefit for using a FOV shift of [0 FOV 0], which is very close to optimal. This can be explained 2 by a larger variation in sensitivity profile between the multiple slices, resulting in increased encoding power. However, for an FOV 90 mm a FOV shift of 2FOV F OV 0] scheme. The optimization algorithm [0 FOV 3 3 ]) outperforms the [0 2 automatically calculates the scheme resulting in the highest SNR. This increases SNR primarily for smaller FOVs (up to 15%), which is interesting for acquisitions with an FOV in slice direction between 70 and 110 mm, such as several prostate acquisitions. Moreover, comparison of both subjects reveals significant differences in SNR10 for standard CAIPIRINHA and the optimized schemes as a result of anatomical differences. Figures 6.5a and b display the mean SNR10 for Rtot = 3 and 4 obtained with in-plane, LISS-CAIPIRINHA and the optimized sampling patterns for both subjects. The mean SNR10, which is defined as the average over the different slices, is displayed alongside an errorbar representing ± one standard deviation. The standard deviation was calculated for the SNR10 values within the ROI over the 92
103 6.3 Results (a) (b) Optimized LISS-CAIPIRINHA Retained SNR maps (c) Counts (normalized) Cumulative histogram LISS-CAIPI (SNR 10 = 0.65) Optimized (SNR 10 = 0.71) Retained SNR Figure 6.3: Schematic overview of the calculation of the SNR 10 selection criterion (R tot = 4 is shown as an example). First retained SNR maps are calculated using the acceleration parameters before (a) and after (b) optimization. Then, the mean of the SNR values within the predefined elliptical ROI that lie below the 10 th percentile are calculated, which equals the SNR 10 quality metric. The cumulative histograms of the SNR values within the ROI are shown in (c) before (black) and after (red) optimization alongside their SNR 10 values. SNR Subject 1 Subject FOV (mm) [0 FOV 2FOV 3 3 ] [0 FOV 2 0] Optimized In-plane FOV (mm) Figure 6.4: SNR 10 for an increasing FOV size in through-plane direction for inplane, LISS-CAIPIRINHA with different FOV-shifts, and the optimized sampling patterns with an acceleration factor of R tot = 3 for two subjects. different slices. This way, the standard deviation is an indication of the robustness of the acceleration method over the FOV. The blue data points represent the first scan, whereas the red data points display the results for the second scan after the anterior coil was re-positioned. For both subjects in-plane acceleration shows comparable results for both scans, with little variation over the FOV. LISS-CAIPIRINHA, on the other hand, is largely influenced by the position of the anterior coil, primarily for the first subject, which shows severely reduced SNR 10 values. Moreover, the larger errorbars indicate an increased variation over the FOV, as a results of varying coil sensitivity profiles in CC direction. Optimization is able to recover the reduced SNR 10 values to some extent and increases SNR 10 for the second scan considerably (5 70%) for R tot 3. Furthermore, the variation over the FOV is noticeably smaller. This indicates that the optimized scheme produces more robust and consistent image SNR compared 93
104 Chapter 6 Optimizing SMS body imaging (a) SNR Subject 1 In-plane (1 st ) In-plane (2 nd ) LISS-CAIPIRINHA (1 st ) LISS-CAIPIRINHA (2 nd ) Optimized (2 nd ) SNR 10 (b) Subject 2 ** (c) Retained SNR maps *In-plane **LISS-CAIPIRINHA *** Retained SNR R tot = 3 R tot = R tot = 3 R tot = 4 * ***Optimized 0 R = 1 Q = 4 = 25.4 mm η = FOV [ ] Figure 6.5: Comparison between the SNR 10 for two coil positions (blue and red) for R tot = 3 and 4. SNR 10 is calculated before (blue) and after (red) shifting the coil for in-plane (circle) and LISS-CAIPIRINHA (square) acceleration. The optimized sampling pattern (triangle) is only calculated after shifting the coil. The errorbar represents ± one standard deviation of the SNR 10 over the FOV in z-direction. For subject 1 (a) a significant drop in SNR 10 is observed after shifting the coil for LISS-CAIPIRINHA, which is partly recovered after optimization. Subject 2 (b) displays less differences in SNR 10 between the coil positions. (c) Retained SNR maps of one of the four slices for the three acceleration methods corresponding to the measurements indicated with the stars, alongside the optimized sampling parameters. to LISS-CAIPIRINHA for the accidental variation of coil sensitivity as a consequence of sub-optimal coil positioning. Figure 6.5c shows the retained SNR maps corresponding to the data points highlighted with stars in Figure 6.5b for in-plane, LISS-CAIPIRINHA and the optimized values alongside the optimized sampling pattern. SNR values within the entire ROI increased substantially for CAIPIRINHA, which rise even more after optimization. Figure 6.6 plots the SNR 10 for an increasing acceleration factor obtained with in-plane, LISS-CAIPIRINHA, and the optimized parameters for all twelve subjects on an individual basis. The average time for optimization on an 8-core CPU computer using the Matlab implementation ranged typically between 8 and 95 seconds for R tot = 2 and R tot = 8, respectively. In-plane acceleration displays considerably lower SNR values with an SNR 10 < 0.1 for R tot 6. LISS- CAIPIRINHA, on the other hand, reveals a significantly higher SNR, but more inter-subject variation. For R tot = 2, optimizing the acceleration parameters increases the SNR marginally, since the optimized schemes are very similar to the LISS-CAIPIRINHA sampling scheme. However, for R tot 3, a significant SNR increase can be observed with p-values of P < The coefficient of variation decreased for all acceleration factors after optimization, indicating less variation in SNR 10 after optimization (CV = 0.14 before and CV = 0.11 after optimization for all subjects and acceleration factors). Additionally, for R tot = 3 the SNR 10 values for CAIPIRINHA with a [0 F OV 2 0] FOV shift are displayed. It can be observed that these values are very similar to the SNR 10 values after optimization 94
105 6.4 Discussion ** ** ** ** ** In-plane LISS-CAIPIRINHA Optimized SNR ** ** ** ** 0.0 R tot = 2 R tot = 3 R tot = 4 R tot = 6 R tot = 8 Figure 6.6: SNR 10 for an increasing acceleration factor obtained by in-plane (circles), LISS-CAIPIRINHA (squares) and after optimization (triangles) for all twelve subjects. The stars indicate a significant change in SNR 10. For R tot = 3, two standard CAIPIRINHA sampling pattern are shown ([0 F OV 2F OV 3 3 ] and [0 F OV 2 0]). The optimized patterns use solely CAIPIRINHA acceleration (i.e. R tot = S x 1). as a result of the large FOV used in these scans and therefore close to optimum. 6.4 Discussion In this work we have introduced a framework to evaluate and optimize CAIPIR- INHA accelerated 2D MS abdominal acquisitions. The framework combines inplane acceleration, and SMS acceleration using CAIPIRINHA to optimize the local SNR on a individual basis by minimizing noise amplification. This is achieved by controlling the aliased regions through appropriate phase modulation of the different simultaneously excited slices. The method was applied on fourteen subjects for different clinical situations that can be encountered in abdominal imaging. The results of the three experiments show that the SNR of CAIPIRINHA-accelerated images is highly influenced by the size of the FOV in SI direction, the coil setup, and the individual anatomy. Mainly for an FOV of 80 to 120 mm (which is typical for various prostate and cardiac exams), this may result in a sub-optimal SNR. For larger FOVs, on the other hand, standard, linear phase modulation schemes produce high SNR values, since larger variations in coil sensitivities enable appropriate unfolding with minimal noise amplification due to large inter-slice distances. This is confirmed by Figure 6.4, which illustrates that [0 F OV 2 0] are suitable FOV-shifts for large FOVs in CC direction despite the fact that the first and third slice fold on top of each other. Moreover, the position of the flexible coil array affects the image quality as can be seen in Figure 6.5 resulting from altered coil sensitivities due 95
106 Chapter 6 Optimizing SMS body imaging to re-positioning of the anterior part of the coil. For the first subject this significantly reduced the SNR, which may indicate that the FOV was fully encompassed by one of the coil s rings, omitting the sensitivity variation of the other ring for unfolding and therefore reducing the overall encoding power. However, for the second subject there was a small increase in SNR. This demonstrates that the relative coil sensitivity differences change in an unpredictable way, induced by the coil position and the anatomy. Since abdominal coils are flexible, opposed to rigid head coils, the position of the coil relative to the acquired FOV may be different for each subject. Figure 6.6 illustrates the relatively large variation in SNR 10 between subjects, predominantly for R tot = 3 and R tot = 4, which can be explained by differences in coil sensitivity profiles and coil position between these subjects. For higher field strengths (> 1.5T), these differences may be even larger. In-plane acceleration shows less inter-subject variation, but an overall lower SNR, which is consistent with previous results [54]. Generally, larger subjects had a lower SNR 10 for all techniques, since the quantity of aliased pixels is increased, whereas smaller subject have less overlap in aliasing slices and hence lower noise enhancement, which was also observed by Deshpande et al [186]. Although these results are specific to the coil array employed in this study, they suggest that one should be careful in selecting the phase modulation scheme for CAIPIRINHA in abdominal exams. Optimization of the acceleration parameters can increase the SNR significantly (11 42%) for R tot 3 and reduce inter-subject variation (see Figure 6.6). This can have a large impact on the resulting image quality and contrast (e.g. for tumor visibility for delineations), as can be seen in Figure 6.2 for a LISS and optimized CAIPIRINHA situation. Moreover, as illustrated by Figure 6.4, the optimal sampling pattern is influenced by the FOV in CC direction. Previous work on the optimization of acceleration parameters focused on 3D volumetric imaging [186, 191, 194] for different parts of the body. This work extends these previous studies to accelerated 2D MS body imaging. To verify that the optimized parameters are still preferred over the standard CAIPIRINHA values, the SNR 10 was calculated over the entire FOV for all fourteen subjects in the inter-subject variation experiment. For all subjects, the optimized parameters outperformed the standard acquisition parameters for acceleration factors R tot 3 throughout the entire FOV. The optimized acceleration parameters that give the theoretically maximum SNR include FOV shifts that demand complex phase modulation schemes (e.g. the shifts in Figure 6.2 or Figure 6.5c). This requires a tailored multiband RF pulse for each phase encoding line. Although these schemes may be impractical, since many tailored RF pulses are required, they provide insight into choosing appropriate LISS-CAIPIRINHA sampling patterns. Figure 6.7 shows the SNR 10 for different FOV shifts for R tot = 3 (slice one has no slice shift). When the sampling schemes are constrained to multiples of FOV 2 to reduce the number of required RF-pulses, the SNR 10 values at the location of the crosses are found. The red cross depicts the optimized sampling scheme that was calculated by the optimization framework, which resulted in the highest SNR
107 6.4 Discussion FOV x SNR 10 for various FOV shifts x x x x 1 3FOV/4 x x x x x 0.8 FOV shift slice 3 FOV/2 FOV/4 x x x x x x x x x x SNR 10 0 x x x x 0 FOV/4 FOV/2 3FOV/4 FOV shift slice 2 x FOV 0 Figure 6.7: SNR 10 for different phase modulation values for two slices calculated for R tot = 3 (FOV in slice direction is 175 mm). The black crosses represent the SNR 10 values found if phase cycling is constraint to multiples of FOV 2. The red cross represents the optimized values that was found by the optimization framework. As for LISS-CAIPIRINHA and the optimized parameters, the number of SMS was constrained to R tot, resulting in high SMS acceleration factors for high R tot. T 2 Turbo Spin Echo (TSE) sequences in the body are often acquired with SENSE = 2 to decrease the echo train length. To simulate this, the framework can be constrained to lower SMS factors in combination with higher in-plane acceleration. Since in-plane and SMS acceleration are complementary strategies, calculating the best acceleration scheme can increase the SNR significantly for R = 2 and S > 1. Moreover, multi-band RF pulses are limited to moderate acceleration factors by the peak power of the RF amplifiers. Higher acceleration factors can be achieved by constraining the SMS factors in the framework or reducing the peak power through multi-transmit excitation [195], optimized RF excitation phase schedules [196], or numerically designed RF-pulses [197]. Constraining the SMS factor, in combination with higher in-plane acceleration, also decreases the specific absorption rate (SAR), which is primarily beneficial for TSE sequences that deposit a substantial amount of RF energy. Furthermore, multiband RF pulses with imperfect phase modulation over the phase encoding lines induce inter-slice cross talk and lower signal within the slice [198]. These imperfections can be quantified using a quick calibration scan over a few read-out lines. Subsequently, this information can be used within the optimization framework to account for these imperfections. In this study, the SNR 10 is used as a quality metric to ensure a threshold SNR in a specified ROI and reduce outlier sensitivity. For the optimization, using 97
108 Chapter 6 Optimizing SMS body imaging this selection criterion, a decrease of low SNR values within the entire ROI is observed (see Figure 6.3). Consequently, the SNR outside this ROI may become lower. Inspection of the SNR outside the ROI showed, in general, higher values after optimization than using LISS-CAIPIRINHA sampling, although this is subject-specific. Furthermore, the size and position of the ROI will alter the optimized sampling schemes and resulting SNR. Again, this is subject-specific. When a large ROI, encompassing the entire anatomy was used for optimization, and subsequently re-optimized on a smaller ROI around the pancreas, liver or either kidney, the SNR 10 increased for one subject up to 30% for R tot 3, whereas another subject showed only SNR 10 -increases up to 10%. In this work only cartesian sampled images were considered from a clinical perspective. Further improvements in acceleration may be obtained using noncartesian sampling schemes, since encoding power in all three spatial dimensions can be used for unfolding [ ]. A similar approach as presented in this paper could be taken to optimize non-cartesian sampling schemes, although the signal equation (Eq. 6.1) would have to be adjusted accordingly to allow for these types of read-outs. Moreover, this framework was designed for RF-induced phase modulation for the different slices, since this can be used for a wide variety of acquisitions and is flexible in phase modulation schemes [54]. For gradient-induced phase modulation, such as blipped-caipi [185], these schemes are linearly dependent in slice direction and therefore constrained to linear phase ramps in the through-plane direction. However, Zhu et al. [202] recently showed incoherent (non-linear) aliasing for echo planar imaging using pseudorandom gradient blips in an attempt to minimize the maximum g-factor over the entire slice. Furthermore, phase-constrained reconstructions have been shown to decrease the g-factor by RF-induced relative phases between different slices (e.g. [203]). This requires an individual spatial phase map for optimum results. Therefore, this is not taken into account for optimization in this framework. However, the framework can be extended to include these parameters. Although the framework was only evaluated on abdomen data, it can be generalized to other imaging regions. It is expected that regions with large inter-subject variation (such as neck or prostate imaging) or very specific imaging regions (such as the visual cortex for retinotopic mapping) can benefit greatly from an individual ROI-specific optimization. Finally, this study only considered integer values for the in-plane acceleration R. Since all the optimized sampling patterns only used SMS acceleration (i.e. Q = R tot ) and no extra in-plane acceleration, it is expected that non-integer in-plane acceleration will not increase the SNR significantly. Non-integer values of R where R is reduced to R < 1 (and therefore S > R tot ), on the other hand, may result in higher SNR values. However, this would require the FOV in PE direction to be 1/R larger with very high SMS-factors, resulting in challenging RF-pulses and therefore beyond the scope of this article. The presented results indicate that abdomen-specific variations largely affect the resulting SNR for CAIPIRINHA-accelerated acquisitions. These factors are difficult to control in clinical practice. The implemented framework opens up the opportunity to test different sampling schemes and compare them to the optimized pattern, which provides the maximum achievable SNR, given the input param- 98
109 6.5 Conclusion eters. For certain conditions, mainly large FOVs, LISS-CAIPIRINHA sampling schemes can achieve results close to optimal, since encoding power is sufficient. Moreover, for R tot = 2, LISS-CAIPIRINHA sampling showed good results with minimal noise amplification. However, for smaller FOVs and higher acceleration factors, optimal results are more difficult to accomplish using standard schemes and individual optimization may be required. This can be facilitated through an online implementation of the optimization. Once the coil sensitivities are mapped and the geometrical parameters are defined, the software calculates the optimal acceleration parameters, which can be constrained to practically feasible phase modulation schemes. Figures 6.5 and 6.6 demonstrate that this would make the exam more robust in terms of SNR on an individual basis 6.5 Conclusion CAIPIRINHA is a viable alternative to in-plane PI for acceleration of abdominal 2D MS acquisitions with less SNR loss. In this study, we demonstrated that abdomen-specific variations in coil positioning, FOV, and subject-specific coil sensitivity patterns have a large impact on the SNR performance of CAIPIRINHA in 2D abdominal acquisitions. An optimization algorithm can be used to examine a range of LISS-CAIPIRINHA and compare them to the optimized scheme in terms of retained SNR. This information can be used to determine the most robust phase modulation for a specific acquisition. In conclusion, care should be taken in selecting the sampling patterns, based on prior knowledge of the resulting image quality. 99
110
111 CHAPTER 7 Summary and Discussion The use of MRI in radiotherapy increased significantly the past decade, fueled by an ever-growing desire towards more conformal and better targeted treatments. This lead to a gradual shift from a CT-based workflow, with its known limitations in soft-tissue contrast, to MR-guided radiation treatments for potentially better patient outcomes [41]. MR-guided radiotherapy has the potential to boost successful treatment outcomes by decreasing uncertainties in tumor detection, location and shape, through unprecedented soft-tissue imaging. With the introduction of hybrid MRL machines, the opportunity to acquire MR images just prior to and during treatment is now a reality. For abdominal tumors this could induce smaller margins for more conformal dose distributions using precise pre-treatment imaging, tracked or gated dose delivery by means of real-time image feedback of the tumor and OARs positions, and accurate dose calculations throughout a large FOV. To maximize this potential for abdominal radiotherapy, accurate MR image guidance in all stages of treatment is essential. A general challenge for these tumors is physiological-induced motion. This thesis describes various acquisition, reconstruction and post-processing methods for managing this motion in all steps of the treatment. 7.1 Pre-treatment and pre-beam imaging Successful MR-guided radiotherapy starts with robust pre-treatment and prebeam imaging to visualize the tumor and all OARs, and characterize their respiratoryinduced motion excursions. Pre-treatment imaging for abdominal MR-guided treatments could comprise of a comprehensive, fast, and robust imaging protocol in which several anatomical images are acquired, as well as functional images. Functional imaging includes contrast-enhanced and diffusion-weighted imaging, but also the generation of pcts for MR-only treatment planning (e.g. [204]). Additionally, motion can be characterized using a dedicated 4D-MRI acquisition. Since the accuracy, and thus the margins, of an MR-guided treatment is largely 101
112 Chapter 7 Summary and Discussion determined by the geometrical accuracy of pre-treatment imaging, it is of vital importance to generate precise and conclusive MR images. For these images it is not only important to provide sufficient contrast between tumor and surrounding tissue for delineations, but also depict a representative motion state for treatment planning. Pre-beam imaging has to fulfill similar conditions, but is much more time-critical. Pre-beam imaging will start with a fast anatomical scan for position verification, after which the contours are transferred to the anatomy of the day. If necessary, fast replanning tools can generate a new plan, optimized for the current anatomy. In the meantime, the motion of the tumor and OARs has to be characterized using fast 4D-MRI acquisitions. 4D-MRI Similar to 4D-CT, 4D-MRI can be used to provide fundamental information for treatment planning of abdominal tumors, including individual ITV margins and the generation of mid-vent/-pos volumes. Additionally, a 4D-MRI can provide unique information about the motion of the OARs for better definition of PRVs due to superior soft-tissue contrast, be used to transfer all MRI acquisitions to the same respiratory state for better delineations, and as input for motion models to simulate treatment possibilities. Driven by these advantages, many different approaches have been taken in the last decade to generate 4D-MRI data for abdominal and thoracic tumors. Most early work focused on sorting 2D images in different respiratory phases in image space [7,82,83,205]. Although this approach is easy, it is prone to long acquisition times, volume inconsistencies, such as missing slices, overlapping structures, and stitching artifacts similar to 4D-CT [206], and limited resolution in through-plane direction. 3D acquisitions, on the other hand, do not suffer from volume inconsistencies, can be acquired with isotropic voxels, and have an intrinsic higher SNR and should therefore be used to generate robust 4D-MRIs. In Chapter 2 the volumetric radial stack-of-stars (SOS) acquisition was introduced as a more robust alternative to Cartesian sampling for reconstruction of phase-resolved 4D-MRI data. This trajectory is both more forgiving to undersampling and less affected by respiratory-induced artifacts [92] after sorting data into respiratory phases. This technique was tested in several healthy volunteers and pancreatic cancer patients and abdominal motion was calculated using fast non-rigid registration, using a 3D optical flow algorithm. The observed motion of the pancreatic tumors and duodenum, which is the primary OAR for these tumors, was mainly in CC direction and was larger for the duodenum compared to the tumor. The bssfp radial SOS acquisition exhibited valuable contrast between structures, good spatial resolution, and a sufficiently large 3D FOV for these tumors. In combination with a fast 3D optical flow algorithm, 4D motion for all abdominal structures was calculated within 13 minutes after starting the acquisition. This can directly be exploited as pre-treatment motion characterization method for treatment planning, and be used as starting point for a faster pre-beam 4D-MRI implementation To sort the data, a respiratory surrogate signal is required. Chapter 2 showed 102
113 7.1 Pre-treatment and pre-beam imaging that internal surrogates are better than external surrogates. Using quantitative analyses, it was concluded that respiratory bellows have higher errors and are unable to capture low frequency motion, opposed to a 1D-MR navigator. These navigators are therefore reliable, internal, respiratory motion surrogates, although it increases scan time by approximately 15%. Ideally, these surrogates are acquired with minimal or no extra scanning time. Examples of robust internal surrogates include the FID signal that is used in Chapter 3, self-navigation [155], which is used in Chapter 5, low-frequency k-space points [207], or the thermal noise variance [126]. Since most of these surrogates are compatible, a combination of surrogates could be a powerful solution for providing robust respiration signals, both for pre-treatment/-beam imaging and beam-on imaging. Sorting the data can be performed in a number of ways, such as phase, or amplitude binning [22]. In phase binning, data is sorted into a number of equal time length bins for each respiratory cycle, similarly to what most CT scanners do. The rationale is that the bins hold probabilistic information, which is interesting for probabilistic planning techniques, but phase binning increases the intra-bin amplitude variations (i.e. the difference in amplitude at which data was acquired within one respiratory bin). This can induce blurring and increases artifacts in the reconstructed bin volumes for 3D acquisitions. Amplitude binning, on the other hand, sorts data in equal amplitude bins and therefore decreases intra-bin variations, possibly increasing image quality, but requires longer scanning to fill all the bins. To keep the probabilistic information, variable amplitude bins could be used, or a hybrid between amplitude and phase binning, such as relative amplitude binning [208]. Since 3D acquisitions are generally slow, compressed sensing (CS) [209] techniques have recently been introduced for a faster 4D-MRI generation [152,172,210]. This can significantly reduce acquisition time (e.g. down to 40 seconds for a large FOV encompassing the lungs and liver [210]), but increases reconstruction times tremendously (i.e. in the order of several minutes to multiple hours). Although this might not be a problem for pre-treatment imaging, pre-beam imaging would benefit from a 4D-MRI with fast acquisition and reconstruction. Albeit it has been shown that a planning 4D-CT is often not representative for respiratory motion during a 3-5 week treatment [39, 211], the durability of a 4D-MRI (i.e. how long the estimated motion is valid) is an unstudied field. Moreover, since respiration can change on an inter- and intra-fraction basis, for example when the patient switches from chest to belly breathing or vice versa, ideally the 4D-MRI is acquired before each fraction. A moderate acquisition time (i.e. 1-3 minutes) with a fast, parallelized reconstruction in the order of seconds is most likely the best compromise. Moreover, CS reconstructions employ regularization in space and/or over the different respiratory phases. Such regularized reconstructions may result in underestimated motion patterns [172]. Validation of these techniques before clinical introduction is therefore essential, but not straightforward. Before 4D- MRI can be adopted widely, the geometrical accurateness, such as the correctness of the body outline and the represented positions in the different phases, has to be determined (as was shown in Chapter 4). MR-compatible 4D motion phantoms can provide some validation [212]. Such phantoms should be able to represent respiratory tumor motion as good as possible, including respiratory variations, 103
114 Chapter 7 Summary and Discussion Exhale Inhale Lung Liver Figure 7.1: Liver and lung examples of an accelerated 4D-MRI with moderately regularized compressed sensing reconstruction that takes approximately 2 minutes to acquire. The white line is plotted to demonstrate the difference in anatomical positions between exhale and inhale. but also remain highly reproducible and controllable. Moreover, it should be relatively easy to handle and flexible to simulate different types of tumors and tissue characteristics and ideally be used for dosimetry in all four dimensions. The ideal 4D-MRI remains an active field of research, but the radial SOS proves to be a versatile acquisition with many benefits. Combining the radial SOS trajectory with moderately regularized CS reconstruction is a viable possibility for a fast and accurate 4D-MRI with moderate reconstruction times. In the past year such implementation was tested at our institute with different contrasts and on different anatomical sites, including pancreas, liver, kidney and lung with promising results (see Figure 7.1). To possibly leverage contrast between tumor and nearby tissue, 4D-MRIs with different contrasts, like a mixed T 2 / T 1 (see Chapter 2), fat-suppressed T 1 (see Figure 7.1), or pure T 2 [213], should be developed as certain tumors may benefit from different contrasts. Alternatively, a multi-echo read-out can be employed, opening the way for ultra-short echo time (UTE) imaging and Dixon reconstructions [123]. This does not only provide excellent fat-suppressed images, but can also be used to generate 4D-pCT data sets [214], expediting dynamic MR-only workflows. Robust abdominal imaging Abdominal imaging has long been an obstacle in MRI due to respiratory-induced motion, leading to artifacts. Breath-holds and gated or triggered scans, using external respiratory bellows or internal MR navigators are able to reduce these artifacts to a large extent. However, patients are often unable to maintain a sufficiently long breath-hold. Moreover, this definite breath-hold time limits the acquisition time and therefore spatial resolution, coverage, or SNR. Furthermore, triggered and gated scans can have low efficiencies, leading to significantly increased scan times. Recently, 3D free-breathing approaches have shown great 104
115 7.2 Beam-on imaging potential for abdominal imaging [61]. This removes the need for breath-holds, thereby making the acquisition more efficient and more consistent. However, these free-breathing techniques fail when bulk motion occurs. For some exams this leads to non-diagnostic image quality or misinterpretation of the images. Rescanning of these patients is often the only solution, which is not an option during pre-beam imaging. In Chapter 3 the radial SOS acquisition was extended to a robust motion compensation technique for preparatory MRI acquisitions. This was accomplished by identifying bulk motion through changes in the signal intensity distribution across elements of a multi-channel receive array using the FID signal. Using video recordings it was validated that variations in FID intensity ratios can detect bulk motion. The online, fully automated implementation enables a continued acquisition until sufficient data without bulk motion has been acquired, after which bulk-motion-free images are reconstructed on the scanner. This technique has the potential to greatly improve the robustness of abdominal MRI scans, and decrease the need for rescanning, reducing imaging time and cost. Moreover, respiratory-induced motion can additionally be characterized by generating a phase-resolved 4D-MRI, similar to Chapter 2, or be discarded by reconstructing volumes in a specified respiratory state in a post-processing step for sharper image contours and edges with reduced artifacts. Apart from increasing robustness for (uncooperative) elderly patients, this technique may even allow scanning pediatric patients without sedation. Due to its generalizability it can be employed for a large range of image sequences and even different anatomical locations. 7.2 Beam-on imaging Respiratory-induced motion during treatment hampers the effectiveness of highly conformal SBRT in the abdomen due to blurring of the dose. One of the key features of the MRL is the ability to visualize the tumor and all OARs during treatment and adapt treatment delivery accordingly. The first step to realize this adaptive workflow is by generating high temporal resolution images. As MRI is currently too slow to acquire full 3D volumes in a large FOV with sufficient temporal resolution, intermediate steps have to be taken to aid radiation adaptations based on MR imaging. Only a limited amount of 2D slices can be acquired with sufficient temporal resolution. Two orthogonally oriented slices, positioned on the tumor and acquired in an interleaved fashion can provide the necessary 3D motion of the tumor centroid [79, 90]. However, this does not provide any information about the OARs and may not cover the entire tumor. To increase spatial coverage without compromising temporal resolution, several promising options were explored in this thesis. Motion models Chapter 4 introduces a model-based approach to solve the problem of slow volumetric MR acquisitions. To generate these 3D cine-mr volumes, a pre-beam 4D-MRI is acquired, similar to the 4D-MRI introduced in Chapter 2, from which the motion is calculated through non-rigid registration, and parameterized using 105
116 Chapter 7 Summary and Discussion a PCA. This parameterized model is used in combination with beam-on M2D cine-mr imaging to synthesize full FOV 3D cine-mr volumes with sub-second temporal resolution. The beam-on imaging can readily be used to track the tumor centroid for fast imaging feedback. Since geometrical accurateness is vital for MR-guided radiotherapy, the geometrical accuracy of the different input data and processing steps are determined using measurements on an MR-compatible motion phantom. The geometrical error for the 4D-MRI, 2D cine-mr imaging, and the fitting procedure were 1.21 mm, 1.16 mm, and 1.53 mm, respectively. In-vivo data were acquired for both the pancreas and kidneys. While one of the M2D cine-mr images served as input for the model, the other slice was used to independently quantify the model s accuracy and applicability outside the slice that was used as input. Root-mean-squared errors (RMSE) were generally below 2 mm in all directions for the pancreas and kidney. The generated high spatio-temporal resolution 3D MR volumes can be used for treatment simulations, plan evaluation, and dose accumulation. Although the generation of 3D volumes using the motion model is currently not fast enough to provide real-time feedback (i.e. in the order of several seconds depending on the FOV), optimizing the reconstruction pipeline may reduce the calculation times significantly, enabling rapid image-based gating and tracking. Multi-slice CAIPIRINHA Beam-on imaging that has been described in this thesis mainly used two 2D slices, acquired in an interleaved fashion. To enable tracking of multiple structures, such as the tumor and several OARs, and possibly improve the accurateness of the PCA model described in Chapter 4, multiple slices can be acquired simultaneously using CAIPIRINHA-accelerated acquisitions. Since the image quality consistency of such acquisitions might be difficult in the abdomen, due to subject-specific coil sensitivity profiles, a mathematical framework is described in Chapter 6 that enables optimization of the CAIPIRINHA sampling pattern. In different simulation experiments it was shown that the FOV in through-plane direction, the coil positioning, and the individual anatomy have a large impact on the image SNR and artifacts. Optimizing sampling patterns on an individual basis can increase SNR up to 30%. This framework is not only useful in acquiring more images with high SNR in a pre-treatment and pre-beam phase [215], but can also be used to acquire multiple images during treatment with sufficient SNR for tracking purposes and increase the number of input slices to our motion model. Apart from exciting two parallel slices as described in Chapter 4, orthogonal acquisitions have also been explored more recently [216] Undersampled 3D imaging Acquiring many 2D slices is an intuitive approach to sample as much of the required imaging volume as possible. However, since the same slices are sampled throughout the entire treatment, the incoherence over time is low, leading to a low information density. Contrary to looking at a subset of images of the entire 106
117 7.3 Dose accumulation 3D FOV, acquiring an undersampled part of the full 3D k-space may be favorable, since temporal incoherence is increased. Some 3D acquisitions, including the radial SOS acquisition, have possibilities for multi-temporal resolution reconstructions. They can provide both fast imaging for tracking purposes, and undersampled 3D information at a lower temporal resolution for sanity checks and dose reconstructions. One of the advantages lies in the fact that temporal and spatial resolution are interchangeable in these acquisitions. Moreover, undersampled reconstructions are a very active field of research and hence it is expected that reconstruction will become much faster in the upcoming years [217]. Tracking on beam-on imaging The increased amount of image information per unit of time can significantly reduce uncertainties in the positions of the tumor and OARs. In order to provide feedback to the linac, the position of the tumor has to be encoded. In this thesis the optical flow algorithm was used extensively, because it produces a voxelby-voxel deformable vector field, is robust in different acquisitions, such as EPI [149] and radial [89], and can be implemented on a GPU architecture to reduce computational times to 15 ms for a single 2D slice [159]. Alternatively, since we are primarily interested in the tumor motion during treatment, other algorithms that only focus on a single structures can be used, such as a particle filter [218] or MOSSE tracker [6] to reduce calculation times even further. Based on the output of the tracking algorithms, the treatment can be adapted by temporary blocking the beam when the tumor is outside a predefined gating window, or by dynamically changing the MLC leaves to track the tumor [179, 180, 219]. A dynamic type of gating, in which the gating window is adapted if drifts occur, may prove to be the perfect combination of gating and tracking. 7.3 Dose accumulation While tracking methods can increase target coverage, the dosimetric impact of 3D deformation of the target and OARs are not taken into account. The previously introduced motion model was used in Chapter 5 to calculate the accumulated dose in SBRT renal-cell carcinoma treatments. To show the added value of the high spatio-temporal resolution of this model, it was compared to other state of the art dose accumulation models that are unable to capture intra-fraction respiratory motion variations, such as drifts, and in- and exhale amplitude variations. The accurateness of the motion models was measured by comparing the GTV motion, as calculated by the models, with the GTV motion on the 2D cine-mr imaging. The proposed motion model was the only one to correctly characterize drifts and amplitude variations, represented by the lowest RMSE of 1.09 mm, compared to 2.69 mm and 2.37 mm for the other models, respectively. These motion estimates were fed into a dose accumulation algorithm by deforming bulk density pcts for five RCC patients. Within a single 7 Gy SBRT fraction, it was concluded that the GTV was underdosed for three out of the five patients, when inter respiratory cycle variations were included. Moreover, distinct hotspots of overdosage were observed just outside the PTV margin that may lead to critically 107
118 Chapter 7 Summary and Discussion high doses to OARs on these anatomical locations. The outlined pipeline is a practical tool to determine the accumulated dose and shows the importance of including intra-fraction motion variations into dose calculations for abdominal tumors. Moreover, it may prove its value for treatment simulation and to test different treatment adaptation methods, primarily for highly mobile tumors such as the liver, kidneys and pancreas. Lastly, it can be combined with very fast plan adaptation tools, such as the adaptive sequencer [75], to adapt the plan online. 7.4 Synergy with other MR-guided applications Hadron therapy is, besides MR-guided radiotherapy, another important development in radiotherapy in the last two decades. In hadron therapy, protons or heavy ion, such as carbon, are used for radiation. Contrary to photon beams that have a slow dose fall off and relatively large dose deposition on the entry site, hadron beams increase to a sharp maximum (so called Bragg peak), followed by a rapid drop-off [220]. Although the sharp dose drop-off can potentially reduce radiation to OARs and therefore adverse effects, the high level of uncertainty in range, dose calculation, and biology limits the usefulness of the Bragg peak. Without knowing where the target is at all times during treatment, the effect of geometric uncertainties and intrafractional motion is even more considerable for hadron therapy than photon therapy. Robust plan optimization has been proposed to account for random range and setup uncertainties [221]. Unfortunately, the effect of respiratory motion on dose distributions is more difficult to account for. A small drift, for example, can result in a significant target dose degradation and, more severely, a serious dose increase to surrounding tissue, much larger than the results presented in Chapter 5 for photon therapy. Accurate imaging that could visualize the tumor and surrounding tissue during treatment, would reduce uncertainties in hadron therapy enormously. MR-guided proton therapy, or hadron therapy in general, is therefore of potentially great value [222]. A different treatment that has successfully been combined with MRI guidance is High Intensity Focused Ultrasound (HIFU), in which tumors are ablated by thermal energy deposition of an ultrasound beam. Although mainly used for the ablation of uterine fibriods [223], it has also been proposed for moving targets, such as liver [224, 225], and kidney tumors [225]. The techniques introduced in this thesis can readily be used in MR-HIFU, although anatomical imaging or cine imaging has to be interleaved with dynamic temperature mapping to provide feedback for treatment monitoring. Similarly, tracking methods developed for MR-HIFU can directly be translated to MR-guided radiotherapy. Recently, integrated whole-body MR-PET scanners have been introduced. These hybrid scanners are able to simultaneously acquire PET and MR images with perfect spatial and temporal correlation. This would, for example, be beneficial in visualizing individual lymph nodes and detecting the active lymph nodes, for individual lymph node boosts. Respiratory motion induces a blurring of the PET signal, similarly to the radiation dose [226]. Using the MRI, respiratory motion 108
119 7.5 Future Perspectives Early enhancement Late enhancement Exhale Inhale Exhale Inhale Sagittal Coronal Transversal Figure 7.2: Five dimensional reconstruction showing different enhancement and respiratory phases in the transversal, coronal and sagittal view. can be mapped and used to reconstruct a motion-resolved 4D-PET volume, or a motion-compensated 3D-PET volume. Similarly to Chapter 4, motion models have been proposed for these tasks (e.g. [135]), as well as tagged MR acquisition [227] and the generalized reconstruction by inversion of coupled systems framework [139]. An interesting opportunity lies in the joint reconstruction of MR and PET data [228]. In conclusion, MR-guided radiotherapy can benefit from the developments in hadron therapy, MR-HIFU, and MR-PET and vice versa. This symbiotic relationship can be used in a range of applications from the generation of attenuation maps, and the development of radio transparent coils, to calculating motion models and tracking algorithms for motion compensation and characterization. 7.5 Future Perspectives Within the MRI community several techniques emerged the last couple of years that may prove very useful for abdominal imaging. Motivated by improvements in soft- and hardware, alongside a drive for increased value proposition in MR imaging, different trends are becoming visible, which could also be adopted into MR-guided abdominal treatments. A trend is seen in sparser data sampling, but denser dimension reconstructions. This trend was initialized by the development of CS [209], in which quasi-random undersampling and nonlinear reconstructions are used to reconstruct sparsely sampled images. Exploiting more incoherence in the acquisition and sparsity 109
120 Chapter 7 Summary and Discussion in the reconstruction achieves better image reconstructions. One way to get more sparsity and correlations is to encode more dimensions. Apart from the three spatial dimensions, different physiological or functional dimensions can be encoded, such as respiratory motion [216], cardiac motion [229], contrastenhancement [171], or flow [230]. Moreover, correlations can be exploited in multiple dimensions, thereby creating five dimensional [155], or even six dimensional data sets [230] from a single acquisition. An example of a five dimensional data set, in which respiratory motion and contrast enhancement are encoded using a continuous 3D radial SOS acquisition, can be seen in Figure 7.2. Not only can this add valuable information in the pre-treatment and pre-beam imaging phase in less time, it may potentially also result in more robust free-breathing imaging, since respiratory and cardiac motion can be encoded, rather than suppressed [107]. Moreover, for beam-on imaging sparsity can be exploited for multi-scale reconstruction, both spatially and temporally to provide the necessary imaging information for tracking and plan adaptation. To improve the performance of CS, the reconstruction is often combined with parallel imaging (PI) methods. Since PI relies on the variations in coil sensitivity profiles, it benefits from an increased number of coil elements. Although abdominal MR imaging is nowadays often performed using coil arrays with 16 to 32 elements, the MRL has an eight-channel coil array to minimize interference with the radiation beam. To optimally use advances in image reconstruction, a radio-transparent coil array with 32 to 64 coil channels should be designed. Such design should not only take radio transparency into account, but also noise correlation, PI performance, and skin dose effect, as a result of the electron return effect [231]. Simulations using for example the framework outlined in Chapter 6 are an essential part of the design process. In order to seamlessly integrate these novel reconstructions into the treatment pipeline, an automated reconstruction framework is required [61]. Since CS uses an iterative, non-linear reconstruction, long reconstruction times are typical and an online reconstruction on the scanner is often not feasible. Besides optimizing reconstruction algorithms in terms of speed, robustness and efficiently, the use of modern hardware, such as GPUs, and an offline reconstruction server with sufficient computational resources to automatically reconstruct multi-dimensional data and save images onto a DICOM storage server is required. This also expedites clinical introduction of advanced acquisition/reconstruction techniques as the images can directly be viewed by clinicians. A consequence of this trend is the fact that a continuous, high-density data stream is acquired, from which several parameters and dimensions can be determined, leading to more information per unit of time. For MR-guided radiotherapy, where images are acquired during every stage of the treatment, this means a massive amount of data. To efficiently handle all this data, machine learning can play a key role. In different parts of an MR-guided treatment, machine learning can assist. First, the reconstruction of vastly undersampled multi-dimensional MR data, which can take minutes to hours using CS algorithms, can severely be accelerated using deep learning [232]. Since training the reconstruction model, which is modeled as a set of filter kernels and activation functions, is the time- 110
121 7.6 Concluding remarks consuming step, applying it may only take a fraction of a second. Second, once images are available, machine learning can be employed to facilitate OARs and possibly tumor delineations [233], automatically generate pcts, or calculate motion in a time-series using supervised learning of optical flow estimations [234]. Lastly, machine learning can aid the treatment planning, or predict the outcomes of therapy, based on pre-treatment imaging and patient characteristics [235]. All these examples share the underlying assumptions that they can be accelerated significantly and made more robust with the use of machine learning, an attractive feature for the many time-critical processes in an adaptive workflow on the MRL. Abdominal MRL treatment Taken these trends into account, an abdominal MRL treatment may comprise of the following steps. In the pre-treatment phase, one, or a few free-breathing continuous acquisitions will be performed, after which the necessary image contrasts, pct, and quantitative parameters are reconstructed in a single representative motion state, such as the mid-position. In combination with machine learning, a robust SBRT plan is created. Just prior to treatment, a fast scan is acquired to visualize the anatomy of the day and characterize the motion, which is immediately taken into account into an optimized plan-of-the-day. Beam-on imaging involves acquiring as much data as possible and reconstructing with minimal latency. Based on the extracted motion gating or tracking is performed. Simultaneously, the accumulated dose is calculated and any necessary adaptation of the plan are performed online. 7.6 Concluding remarks The developments in MR-guided radiotherapy will drive a paradigm shift in radiotherapy. For the first time we have the ability to visualize both the tumor and OARs before and during treatment in real time. A constant drive towards faster, more comprehensive imaging is required to fully exploit the opportunities of the MRL. In this thesis methods were presented for pre-treatment, pre-beam, and beam-on MR imaging, as well as dose calculations for abdominal MR-guided radiotherapy. These techniques will directly add valuable information for robust pre-treatment imaging, treatment simulation and planning, day-to-day plan adjustments, and ultimately can be used for real-time image-guidance and online plan adaptation. In combination with developments in MRI, image-guided therapies and image analysis, the outlined methods will advance MR-guided abdominal radiotherapy into clinical use. 111
122
123 CHAPTER 8 Samenvatting Het doel van radiotherapie is om kankercellen te doden door het DNA te beschadigen met behulp van een hoge dosis ioniserende straling. Hierbij moet worden gezorgd dat gezond omringend weefsel zo veel mogelijk wordt gespaard. Naast fractionering, waarbij de totale dosis in kleinere fracties wordt gegeven om gezond weefsel in staat te stellen het DNA te herstellen, worden verschillende beeldvormende technieken gebruikt om de tumor beter te kunnen visualiseren. Hierdoor kunnen kritische gezonde organen zoveel mogelijk worden gespaard. In dit proefschrift staat het gebruik van MRI voor radiotherapie centraal. Door het superieure weke delen contrast en de hoge flexibiliteit kan zowel de tumor als het omliggend weefsel uitstekend worden gevisualiseerd. Dankzij deze eigenschappen kan de positie van de tumor in real time worden bepaald. Hierdoor is het gebruik van MRI voor radiotherapie de afgelopen jaren flink gestegen, wat uiteindelijk heeft geleid tot de ontwikkeling van de hybride MR-Linac. Dit apparaat, dat MRI beelden kan acquireren voor de behandeling en zelfs tijdens de behandeling, is ontwikkeld binnen de afdeling radiotherapie van het UMC Utrecht, in samenwerking met Philips en Elekta. In mei 2017 zijn de eerste patiënten in Utrecht behandeld met deze revolutionaire techniek. Het is de verwachting dat MRI-gestuurde radiotherapie in de toekomst bij veel meer patiënten behandelingsresultaten kan verbeteren door onzekerheden in tumor detectie en locatie te minimaliseren. Bij tumoren in de buik kan dit leiden tot kleinere marges voor een meer conforme dosisverdeling, tracked of gated dosis afgifte door middel van real-time beeld feedback van de tumor positie en nauwkeurige dosis berekeningen in het hele abdomen. Om deze mogelijkheden te realiseren is nauwkeurige MRI-beeldgeleiding in alle stadia van de behandeling van essentieel belang. Een algemene uitdaging voor deze tumoren is fysiologisch geïnduceerde beweging. Voor een veilige, klinische introductie van MRIgeleide stereotactische behandelingen van abdominale tumoren, zijn robuuste en nauwkeurige MRI-technieken cruciaal. Dit proefschrift beschrijft diverse acquisi- 113
124 Chapter 8 Samenvatting tie, reconstructie en post-processing methoden voor het controleren van deze beweging voor en tijdens de behandeling. Succesvolle MRI-geleide radiotherapie begint met robuuste beeldvorming in de pre-treatment (simulatie voor de behandeling) en pre-beam (net voor radiatie) fase om de tumor en alle kritieke organen te visualiseren en hun respiratoir geïnduceerde beweging te karakteriseren. In Hoofdstuk 2 wordt de radiële stack-of-stars (SOS) acquisitie geïntroduceerd als een robuust alternatief voor Cartesische sampling voor het genereren van een 4D-MRI om de ademhaling te karakteriseren. Deze radiële sampling is meer vergevingsgezind voor undersampling en wordt minder beïnvloed door ademhalingsgeïnduceerde artefacten na het sorteren van de data in de ademhalingsfasen. Deze techniek is getest bij verscheidene gezonde vrijwilligers en patiënten met alvleesklierkanker waarbij de beweging werd berekend met behulp van een snelle, non-rigide registratie. De waargenomen beweging van de alvleeskliertumoren was hoofdzakelijk in de cranio-caudale richting en was groter voor het duodenum in vergelijking met de tumor. Met behulp van de radiële SOS acquisitie en de snelle 3D-registratie werd 4Dbeweging voor alle organen in de buik berekend binnen 13 minuten na aanvang van de beeldopname. Deze techniek kan direct gebruikt worden om de beweging te karakteriseren en te gebruiken in de behandelplannen. In Hoofdstuk 3 wordt dezelfde radiële SOS acquisitie uitgebreid naar een robuuste techniek voor bewegingscompensatie voor abdominale MRI. Foutieve acquisitie lijnen, die zijn opgenomen ten tijden van beweging (d.w.z. bulk bewegingen door bijvoorbeeld discomfort of hoesten), worden met deze techniek gedetecteerd en automatisch verwijderd door variaties in de spoellading te bepalen aan de hand van veranderingen in het FID signaal. De online implementatie zorgt ervoor dat de scanner automatisch stopt wanneer voldoende data is geacquireerd. Additionele respiratoire beweging kan verder worden gefilterd of er kan een 4D-MRI van worden gemaakt, zoals beschreven in Hoofdstuk 2. Deze techniek heeft de potentie om MRI scans van het abdomen robuuster te maken, zodat scans niet hoeven te worden herhaald of patiënten terug moeten komen voor een extra scansessie. Dit is niet alleen waardevol voor niet-coöperatieve ouderen, maar ook voor pediatrische patiënten, waar anesthesie kan worden verminderd of zelfs helemaal weg worden gelaten. Ademhaling tijdens de behandeling belemmert de effectiviteit van conforme radiotherapie als gevolg van blurring van de dosis. Om echter inzicht te krijgen in het effect van ademhalingsbeweging op de dosisverdeling van de tumor en de kritieke organen, is 3D beeldvorming met adequate temporele resolutie vereist. Helaas zijn MRI-acquisities over het algemeen relatief traag en is de snelle acquisitie van zulke 3D-beelden een probleem. Hoofdstuk 4 introduceert een bewegingsmodel om dit probleem op te lossen. Met behulp van een pre-beam 4D-MRI, zoals beschreven in Hoofdstuk 2, wordt een model opgesteld door de beweging te parametriseren. Dit model wordt in combinatie met snelle beam-on 2D cine-mr imaging gebruikt om 3D cine-mr volumes te genereren met sub-seconde temporele resolutie. Deze hoog resolute 114
125 8 Samenvatting beelden kunnen direct worden gebruikt voor feedback van de positie van de tumoren en al het omringend weefsel. Dit is waardevol voor simulatie van de behandeling, om verschillende plannen te evalueren en om de geaccumuleerde dosis te berekenen. Het eerder geïntroduceerde bewegingsmodel wordt in Hoofdstuk 5 gebruikt om de geaccumuleerde dosis van een gehypofractioneerde nierkanker behandeling te berekenen. De waarde van de hoge temporele resolutie is aangetoond door de geaccumuleerde dosis te vergelijken met andere modellen die deze hoge temporele resolutie niet hebben. Het voorgestelde model kan de beweging van de tumor het beste karakteriseren, waarbij zelfs drift kan worden gevisualiseerd. Met behulp van dit model is aangetoond dat voor drie van vijf patiënten de tumor onvoldoende dosis kreeg, wat niet kan worden gemodelleerd door de andere modellen. Daarnaast stijgt de dosis in enkele kritieke organen rond de tumor wat tot onvoorziene bijwerkingen kan leiden. Met behulp van de voorgestelde techniek kan nauwkeurig de afgegeven dosis worden berekend. Hierbij kunnen alle aspecten van de ademhaling worden meegenomen, zoals variatie tussen verschillende ademhalingsteugen. Dit is niet alleen waardevol voor online dosis accumulatie op de MR-Linac, maar ook voor behandelingssimulaties gebaseerd op een pre-treatment MRI van tumoren die veel bewegen, zoals in de lever, nieren en pancreas. Beam-on imaging dat tot dusver is beschreven in dit proefschrift maakte gebruik van twee orthogonale 2D slices. Om tracking van meerdere structuren mogelijk te maken, zoals de tumor en kritiek omliggend weefsel, kunnen meerdere slices tegelijk worden geacquireerd met behulp van CAIPIRINHA-versnelde acquisities. Het is echter moeilijk om consistent goede beeldkwaliteit te kunnen garanderen in het abdomen, door patiënt-specifieke variatie, zoals de spoelgevoeligheid. Hoofdstuk 6 beschrijft een mathematisch model om CAIPIRINHA sampling patronen te optimaliseren. In verschillende experimenten is aangetoond dat het FOV in de cranio-caudale richting, de positie van de spoel en patient-specifieke anatomie allen grote invloed hadden op de SNR en het artefact level. Het optimaliseren van het sampling patroon met behulp van het mathematisch model kan de SNR met 30% verhogen. Het model kan niet alleen worden gebruikt om SNR te verhogen van MRI acquisitie in de pre-treatment en pre-beam fase, maar ook om het aantal slices dat wordt gebruikt tijdens beam-on imaging te vergroten voor tracking of als input voor een bewegingsmodel. In dit proefschrift zijn meerdere methoden beschreven voor pre-treatment, prebeam en beam-on MR imaging, alsmede een methode om de geaccumuleerde dosis te kunnen berekenen voor MRI-gestuurde radiotherapie in het abdomen. Deze technieken kunnen direct waardevolle informatie toevoegen voor robuuste pretreatment MRI, behandelingssimulatie en planning, dag-tot-dag aanpassingen en kunnen uiteindelijk worden gebruikt voor real-time beeldgestuurde behandelingen en online planadaptaties. 115
126
127 Bibliography [1] Khan FM, Gibbons JP, The Physics of Radiation Therapy. Lippincott Williams & Wilkins, fifth ed., [2] Joiner M, van der Kogel A, Basic Clinical Radiobiology. Hodder Arnold, fourth ed., [3] International Commission on Radiation Units and Measurements, ICRU report 50: Prescribing, Recording and Reporting Photon Beam therapy, Technical report, [4] Van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000; 47: [5] van Herk M. Errors and margins in radiotherapy. Semin. Radiat. Oncol. 2004; 14: [6] Heerkens HD, van Vulpen M, van den Berg CAT, Tijssen RHN, Crijns SPM, Molenaar IQ, van Santvoort HC, Reerink O, Meijer GJ. MRI-based tumor motion characterization and gating schemes for radiation therapy of pancreatic cancer. Radiother. Oncol. 2014; 111: [7] Von Siebenthal M, Szekely G, Gamper U, Boesiger P, Lomax A, Cattin P. 4D MR imaging of respiratory organ motion and its variability. Phys. Med. Biol. 2007; 52:1547. [8] International Commission on Radiation Units and Measurements, ICRU report 62: Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50), Technical report, [9] Gilbeau L, OctavePrignot M, Loncol T, Renard L, Scalliet P, Grégoire V. Comparison of setup accuracy of three different thermoplastic masks for the treatment of brain and head and neck tumors. Radiother. Oncol. 2001; 58: [10] Mitine C, Dutreix A, van der Schueren E. Tangential breast irradiation: influence of technique of set-up on transfer errors and reproducibility. Radiother. Oncol. 1991; 22: [11] Stemkens B, Tijssen R, Denis de Senneville B, Lagendijk J, van den Berg C. TH-A-BRF-07: Retrospective Reconstruction of 3D Radial MRI Data to Evaluate the Effect of Abdominal Compression On 4D Abdominal Organ Motion. Med. Phys. 2014; 41:538. [12] Heerkens HD, Reerink O, Intven MPW, Hiensch RR, Van Den Berg CAT, Crijns SPM, Vulpen MV, Meijer GJ. Pancreatic tumor motion reduction by use of a custom abdominal corset. Phys. Imaging Radiat. 2017; 2:7 10. [13] Feng M, BenJosef E. Radiation Therapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2011; 21: [14] Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, Hodul PJ, Malafa MP, Meredith KL, Hoffe SE, Shridhar R. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 2013; 86: [15] De Meerleer G, Khoo V, Escudier B, Joniau S, Bossi A, Ost P, Briganti A, Fonteyne V, Van Vulpen M, Lumen N, Spahn M, Mareel M. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014; 15: [16] Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF, Tome Wa, Verellen D, Wang L, Yin FF. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010; 37:4078. [17] Xing L, Thorndyke B, Schreibmann E, Yang Y, Li TF, Kim GY, Luxton G, Koong A. Overview of image-guided radiation therapy. Med. Dosim. 2006; 31: [18] Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in imageguided radiotherapy. Nat. Rev. Cancer 2007; 7: [19] Langmack KA. Portal imaging. Br. J. Radiol. 2001; 74: [20] Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002; 53:
128 Appendix BIBLIOGRAPHY [21] Bernstein MA, King KF, Zhou XJ, Handbook of MRI pulse Sequences. Academic Press, first ed., [22] Abdelnour AF, Nehmeh SA, Pan T, Humm JL, Vernon P, Schöder H, Rosenzweig KE, Mageras GS, Yorke E, Larson SM, Erdi YE. Phase and amplitude binning for 4D-CT imaging. Phys. Med. Biol. 2007; 52: [23] Yamamoto T, Langner U, Loo BW, Shen J, Keall PJ. Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 2008; 72: [24] Rietzel E, Pan T, Chen GT. Four-dimensional computed tomography: image formation and clinical protocol. Med Phys 2005; 32: [25] Wolthaus JW, Schneider C, Sonke JJ, van Herk M, Belderbos JS, Rossi MM, Lebesque JV, Damen EM. Mid-ventilation CT scan construction from four-dimensional respiration-correlated CT scans for radiotherapy planning of lung cancer patients. Int. J. Radiat. Oncol. 2006; 65: [26] Wolthaus JWH, Sonke JJ, van Herk M, Belderbos JSA, Rossi MMG, Lebesque JV, Damen EMF. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2008; 70: [27] Unkelbach J, Oelfke U. Inclusion of organ movements in IMRT treatment planning via inverse planning based on probability distributions. Phys. Med. Biol. 2004; 49: [28] Rietzel E, Chen GTY, Choi NC, Willet CG. Four-dimensional image-based treatment planning: Target volume segmentation and dose calculation in the presence of respiratory motion. Int. J. Radiat. Oncol. Biol. Phys. 2005; 61: [29] Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, Kapatoes JM, Low Da, Murphy MJ, Murray BR, Ramsey CR, Van Herk MB, Vedam SS, Wong JW, Yorke E. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 2006; 33: [30] Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, V. Lebesque J, Miyasaka K. Precise and Real-Time Measurement of 3D Tumor Motion in Lung Due To Breathing and Heartbeat, Measured During Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002; 53: [31] Juhler Nøttrup T, Korreman SS, Pedersen AN, Aarup LR, Nyström H, Olsen M, Specht L. Intraand interfraction breathing variations during curative radiotherapy for lung cancer. Radiother. Oncol. 2007; 84: [32] Sonke JJ, Lebesque J, van Herk M. Variability of Four-Dimensional Computed Tomography Patient Models. Int. J. Radiat. Oncol. Biol. Phys. 2008; 70: [33] Sonke JJ, Zijp L, Remeijer P, van Herk M. Respiratory correlated cone beam CT. Med. Phys. 2005; 32: [34] Hugo GD, Agazaryan N, Solberg TD. The effects of tumor motion on planning and delivery of respiratory-gated IMRT. Med. Phys. 2003; 30: [35] Poulsen PR, Schmidt ML, Keall P, Worm ES, Fledelius W, Hoffmann L. A method of dose reconstruction for moving targets compatible. Med. Phys. 2012; 39: [36] Cho B, Poulsen PR, Sloutsky A, Sawant A, Keall PJ. First Demonstration of Combined kv/mv Image-Guided Real-Time Dynamic Multileaf-Collimator Target Tracking. Int. J. Radiat. Oncol. Biol. Phys. 2009; 74: [37] Delouya G, Carrier JF, BéliveauNadeau D, Donath D, Taussky D. Migration of intraprostatic fiducial markers and its influence on the matching quality in external beam radiation therapy for prostate cancer. Radiother. Oncol. 2010; 96: [38] Stemkens B, Tijssen RH, Denis de Senneville B, Lagendijk JJW, Van den Berg CAT. OC-0337: Assessment of fast external and internal motion surrogates to supplement MRI based tracking of the pancreas. Radiother. Oncol. 2013; 106:S132. [39] Minn AY, Schellenberg D, Maxim P, Suh Y, McKenna S, Cox B, Dieterich S, Xing L, Graves E, Goodman KA, Chang D, Koong AC. Pancreatic Tumor Motion on a Single Planning 4D-CT Does Not Correlate With Intrafraction Tumor Motion During Treatment. Am. J. Clin. Oncol. 2009; 32: [40] Nyholm T, Nyberg M, Karlsson MG, Karlsson M. Systematisation of spatial uncertainties for comparison between a MR and a CT-based radiotherapy workflow for prostate treatments. Radiat. Oncol. 2009; 4: [41] Nyholm T, Jonsson J. Counterpoint: Opportunities and Challenges of a Magnetic Resonance Imaging-Only Radiotherapy Work Flow. Semin. Radiat. Oncol. 2014; 24:
129 Appendix BIBLIOGRAPHY [42] Fraass BA, McShan DL, Diaz R, Ten Haken RK, Aisen A, Gebrarski S, Glazer G, Lichter A. Integration of magnetic resonance imaging into radiation therapy treatment planning: I. Technial considerations. Int. J. Radiat. Oncol. Biol. Phys. 1987; 13: [43] Mizowaki T, Nagata Y, Okajima K, Muruta R, Yamamoto M, Kokubo M, Hiraoka M, Abe M. Development of an MR simulator: Experimental verification of geometric distortion and clinical application. Ther. Radiol. 1996; 199: [44] Feng M, Balter JM, Normolle D, Adusumilli S, Cao Y, Chenevert TL, Benjosef E. Characterization of Pancreatic Tumor Motion Using Cine- MRI: Surrogates for Tumor Position Should be Used with Caution. Int. J. Radiat. Oncol. Biol. Phys. 2009; 74: [45] Park J, Metaxas D, Young AA, Axel L. Deformable models with parameter functions for cardiac motion analysis from tagged MRI data. IEEE Trans. Med. Imaging 1996; 15: [46] Froehlich JM, Patak MA, Von Weymarn C, Juli CF, Zollikofer CL, Wentz KU. Small bowel motility assessment with magnetic resonance imaging. J. Magn. Reson. Imaging 2005; 21: [47] Ménard C, van der Heide UA. Introduction: Magnetic Resonance Imaging Comes of Age in Radiation Oncology. Semin. Radiat. Oncol. 2014; 24: [48] Haacke EM, Brown RW, Thompson MR, Venkatesan R, Magnetic Resonance Imaging. John Wiley and Sons Inc., first ed., [49] McRobbie DW, Moore EA, Graves MJ, Prince MR, MRI From Picture to Proton. Cambridge University Press, second ed., [50] Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): Fast imaging with radiofrequency coil arrays. Magn. Reson. Med. 1997; 38: [51] Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 1999; 42: [52] Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 2002; 47: [53] Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J. Magn. Reson. Imaging 2001; 13: [54] Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled Aliasing in Parallel Imaging Results in Higher Acceleration ( CAIPIRINHA ) for Multi-Slice Imaging. Magn. Reson. Med. 2005; 53: [55] Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled Aliasing in Volumetric Parallel Imaging ( 2D CAIPIRINHA ). Magn. Reson. Med. 2006; 55: [56] Block KT. Advanced Methods for Radial Data Sampling in Magnetic Resonance Imaging. PhD thesis, University of Gottingen, [57] Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans. Med. Imaging 2007; 26: [58] Van Rossum PSN, Van Hillegersberg R, Lever FM, Lips IM, Van Lier ALHMW, Meijer GJ, Van Leeuwen MS, Van Vulpen M, Ruurda JP. Imaging strategies in the management of oesophageal cancer: What s the role of MRI? Eur. Radiol. 2013; 23: [59] Intven M, Reerink O, Philippens MEP. Diffusion-weighted MRI in locally advanced rectal cancer: Pathological response prediction after neo-adjuvant radiochemotherapy. Strahlentherapie und Onkol. 2013; 189: [60] Houweling AC, Schakel T, Van Den Berg CAT, Philippens MEP, Roesink JM, Terhaard CHJ, Raaijmakers CPJ. MRI to quantify early radiation-induced changes in the salivary glands. Radiother. Oncol. 2011; 100: [61] Block KT, Chandarana H, Milla S, Bruno M, Mulholland T, Fatterpekar G, Hagiwara M, Grimm R, Geppert C, Kiefer B, Sodickson DK. Towards Routine Clinical Use of Radial Stackof-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J. Korean Soc. Magn. Reson. Med. 2014; 18: [62] Lagendijk JJW, Bakker CJG. MRI guided radiotherapy: a MRI based linear accelerator. In: Radiother. Oncol., p. S60. [63] Fallone BG. The Rotating Biplanar Linac-Magnetic Resonance Imaging System. Semin. Radiat. Oncol. 2014; 24: [64] Fallone BG, Murray B, Rathee S, Stanescu T, Steciw S, Vidakovic S, Blosser E, Tymofichuk D. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-mr system. Med. Phys. 2009; 36:
130 Appendix BIBLIOGRAPHY [65] Keall PJ, Barton M, Crozier S. The Australian Magnetic Resonance Imaging-Linac Program. Semin. Radiat. Oncol. 2014; 24: [66] Liney GP, Dong B, Begg J, Vial P, Zhang K, Lee F, Walker A, Rai R, Causer T, Alnaghy SJ, Oborn BM, Holloway L, Metcalfe P, Barton M, Crozier S, Keall P. Technical Note: Experimental results from a prototype high-field inline MRI-linac. Med. Phys. 2016; 43: [67] Dempsey J, Benoit D, Fitzsimmons J, Haghighat A, Li J, Low D, Mutic S, Palta J, Romeijn H, Sjoden G. A Device for Realtime 3D Image-Guided IMRT. Int. J. Radiat. Oncol. 2005; 63:S202. [68] Mutic S, Dempsey JF. The ViewRay System: Magnetic Resonance-Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014; 24: [69] Lagendijk JJW, Raaymakers BW, van Vulpen M. The Magnetic Resonance Imaging-Linac System. Semin. Radiat. Oncol. 2014; 24: [70] Lagendijk JJW, Raaymakers BW, Raaijmakers AJE, Overweg J, Brown KJ, Kerkhof EM, van der Put RW, Hårdemark B, van Vulpen M, van der Heide UA. MRI/linac integration. Radiother. Oncol. 2008; 86: [71] Raaymakers BW, Lagendijk JJW, Overweg J, Kok JGM, Raaijmakers aje, Kerkhof EM, van der Put RW, Meijsing I, Crijns SPM, Benedosso F, van Vulpen M, de Graaff CHW, Allen J, Brown KJ. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys. Med. Biol. 2009; 54:N229 N237. [72] Crijns SPM, Kok JGM, Lagendijk JJW, Raaymakers BW. Towards MRI-guided linear accelerator control: gating on an MRI accelerator. Phys. Med. Biol. 2011; 56: [73] Crijns SPM, Raaymakers BW, Lagendijk JJW. Proof of concept of MRI-guided tracked radiation delivery: tracking one-dimensional motion. Phys. Med. Biol. 2012; 57: [74] Bol GH, Lagendijk JJW, Raaymakers BW. Virtual couch shift (VCS): accounting for patient translation and rotation by online IMRT re-optimization. Phys. Med. Biol. 2013; 58: [75] Kontaxis C, Bol GH, Lagendijk JJW, Raaymakers BW. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys. Med. Biol. 2015; 60: [76] Kontaxis C, Bol GH, Lagendijk JJW, Raaymakers BW. Towards adaptive IMRT sequencing for the MR-linac. Phys. Med. Biol. 2015; 60: [77] Martinez AA, Yan D, Lockman D, Brabbins D, Kota K, Sharpe M, Jaffray DA, Vicini F, Wong J. Improvement in dose escalation using the process of adaptive radiotherapy combined with three-dimensional conformal or intensity-modulated beams for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001; 50: [78] Bussels B, Goethals L, Feron M, Bielen D, Dymarkowski S, Suetens P, Haustermans K. Respiration-induced movement of the upper abdominal organs: a pitfall for the threedimensional conformal radiation treatment of pancreatic cancer. Radiother. Oncol. 2003; 68: [79] Bjerre T, Crijns S, af Rosenschöld PM, Aznar M, Specht L, Larsen R, Keall P. Threedimensional MRI-linac intra-fraction guidance using multiple orthogonal cine-mri planes. Phys. Med. Biol. 2013; 58: [80] Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989; 173: [81] Wang Y, Riederer J, Grimm RC, Riederer SJ, Ehman RL. Navigator-Echo-based real-time respiratory gating and Triggering for Reduction of respiration effect in three-dimensional Coronary MR Angiography. Card. Radiol. 1996; 198: [82] Tryggestad E, Flammang A, HanOh S, Hales R, Herman J, McNutt T, Roland T, Shea SM, Wong J. Respiration-based sorting of dynamic MRI to derive representative 4D-MRI for radiotherapy planning. Med. Phys. 2013; 40: [83] Cai J, Chang Z, Wang Z, Segars WP, Yin FF. Four-dimensional magnetic resonance imaging (4D-MRI) using image-based respiratory surrogate: A feasibility study. Med. Phys. 2011; 38: [84] von Siebenthal M, Székely G, Gamper U, Boesiger P, Lomax A, Cattin P. 4D MR imaging of respiratory organ motion and its variability. Phys. Med. Biol. 2007; 52: [85] Buerger C, Clough RE, King AP, Schaeffter T, Prieto C. Nonrigid Motion Modeling of the Liver From. IEEE Trans. Med. Imaging 2012; 31: [86] Østergaard Noe K, De Senneville BD, Elstrøm UV, Tanderup K, Sørensen TS. Acceleration and validation of optical flow based deformable registration for image-guided radiotherapy. Acta Oncol. 2008; 47: [87] Senneville BDD, Ries M, Maclair G, Moonen C. MR-guided thermotherapy of abdominal organs using a robust PCA-based motion descriptor. IEEE Trans. Med. Imaging 2011; 30:
131 Appendix BIBLIOGRAPHY [88] Roujol S, Ries M, Moonen C, Senneville BDD. Automatic Non-Rigid Calibration of Image Registration for Real Time Interventional MRI of mobile organs. IEEE Trans. Med. Imaging 2011; 30: [89] Stemkens B, Tijssen RHN, van den Berg CAT, Lagendijk JJW, Moonen CTW, Ries M, de Senneville BD. Optical flow analysis on undersampled radial acquisitions for real-time tracking of the pancreas in MR guided radiotherapy. In: Proc Int Soc Magn Reson Med, p [90] Stemkens B. MR-methods for real-time motion characterization of the pancreas. Master of science thesis, University of Technology Eindhoven, [91] Wang Z, Bovik A, Sheikh H, Simoncelli E. Image Quality Assessment: From Error Visibility to Structural Similarity. IEEE Trans. Image Process. 2004; 13: [92] Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn. Reson. Med. 1992; 28: [93] Scheffler K, Hennig J. Reduced circular field-of-view imaging. Magn. Reson. Med. 1998; 40: [94] Underberg RWM, Lagerwaard FJ, Cuijpers JP, Slotman BJ, van Sörnsen de Koste JR, Senan S. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004; 60: [95] McKenzie A, van Herk M, Mijnheer B. Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother. Oncol. 2002; 62: [96] de Senneville BD, Mougenot C, Moonen CTW. Real-time adaptive methods for treatment of mobile organs by MRI-controlled high-intensity focused ultrasound. Magn. Reson. Med. 2007; 57: [97] Klein S, Staring M, Murphy K, Viergever M, Pluim J. Elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010; 29: [98] Wood ML, Henkelman RM. MR image artifacts from periodic motion. Med. Phys. 1985; 12: [99] Zaitsev M, Maclaren J, Herbst M. Motion Artifacts in MRI: A Complex Problem With Many Partial Solutions. J. Magn. Reson. Imaging 2015; 42: [100] van Heeswijk RB, Bonanno G, Coppo S, Coristine A, Kober T, Stuber M. Motion compensation strategies in magnetic resonance imaging. Crit. Rev. Biomed. Eng. 2012; 40: [101] Wood ML, Henkelman RM. Suppression of respiratory motion artifacts in magnetic resonance imaging. Med. Phys. 1986; 13: [102] Ehman R, McNamara M, Pallack M, Hricak H, Higgins C. Magnetic Resonance Imaging with Respiratory Gatung : Techniques and Advantages. Am. J. Roentgenol. 1984; 143: [103] SchulteUentrop L, Goepfert MS. Anaesthesia or sedation for MRI in children. Curr. Opin. Anaesthesiol. 2010; 23: [104] Cote CJ, Karl HW, Notterman DA, Weinberg JA. Adverse Sedation Events in Pediatrics : Analysis of Medications Used for Sedation. Pediatrics 2000; 105: [105] Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: A review. Magn. Reson. Med. 2013; 69: [106] Cheng JY, Zhang T, Ruangwattanapaisarn N, Alley MT, Uecker M, Pauly JM, Lustig M, Vasanawala SS. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. J. Magn. Reson. Imaging 2015; 42: [107] Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-Breathing Radial 3D Fat-Suppressed T1-Weighted Gradient Echo Sequence. Invest. Radiol. 2011; 46: [108] Chandarana H, Feng L, Block TK, Rosenkrantz AB, Lim RP, Babb JS, Sodickson DK, Otazo R. Free-Breathing Contrast-Enhanced Multiphase MRI of the Liver Using a Combination of Compressed Sensing, Parallel Imaging, and Golden-Angle Radial Sampling. Invest Radiol. 2013; 48:1 17. [109] Johnson KM, Block WF, Reeder SB, Samsonov A. Improved least squares MR image reconstruction using estimates of k-space data consistency. Magn. Reson. Med. 2012; 67: [110] Cheng JY, Zhang T, Ruangwattanapaisarn N, Alley MT, Uecker M, Pauly JM, Lustig M, Vasanawala SS. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. J. Magn. Reson. Imaging 2015; 42: [111] Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. J. Magn. Reson. Imaging 2017; 45:
132 Appendix BIBLIOGRAPHY [112] Higano NS, Hahn AD, Tkach JA, Cao X, Walkup LL, Thomen RP, Merhar SL, Kingma PS, Fain SB, Woods JC. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn. Reson. Med. 2017; 77: [113] Sachs TS, Meyer CH, Irarrazabal P, Hu BS, Nishimura DG, Macovski A. The diminishing variance algorithm for real-time reduction of motion artifacts in MRI. Magn. Reson. Med. 1995; 34: [114] Li G, Zaitsev M, Buchert M, Raithel E, Paul D, Korvink JG, Hennig J. Improving the robustness of 3D turbo spin echo imaging to involuntary motion. Magma Magn. Reson. Mater. Physics, Biol. Med. 2015; 28: [115] Hu P, Hong S, Moghari MH, Goddu B, Goepfert L, Kissinger KV, Hauser TH, Manning WJ, Nezafat R. Motion correction using coil arrays (MOCCA) for free-breathing cardiac cine MRI. Magn. Reson. Med. 2011; 66: [116] Brau ACS, Brittain JH. Generalized self-navigated motion detection technique: Preliminary investigation in abdominal imaging. Magn. Reson. Med. 2006; 55: [117] Kober T, Marques JP, Gruetter R, Krueger G. Head motion detection using FID navigators. Magn. Reson. Med. 2011; 66: [118] Kober T, Gruetter R, Krueger G. Prospective and retrospective motion correction in diffusion magnetic resonance imaging of the human brain. Neuroimage 2012; 59: [119] Buehrer M, Curcic J, Boesiger P, Kozerke S. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn. Reson. Med. 2008; 60: [120] Anderson AG, Velikina J, Block W, Wieben O, Samsonov A. Adaptive retrospective correction of motion artifacts in cranial MRI with multicoil three-dimensional radial acquisitions. Magn. Reson. Med. 2013; 69: [121] Li H, Haltmeier M, Zhang S, Frahm J, Munk A. Aggregated motion estimation for real-time MRI reconstruction. Magn. Reson. Med. 2013; 1048: [122] Prieto C, Doneva M, Usman M, Henningsson M, Greil G, Schaeffter T, Botnar RM. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J. Magn. Reson. Imaging 2015; 41: [123] Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-Breathing Volumetric Fat/Water Separation by Combining Radial Sampling, Compressed Sensing, and Parallel Imaging. Magn. Reson. Med. 2016;. [124] Piccini D, Littmann A, NiellesVallespin S, Zenge MO. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 2011; 66: [125] Crowe ME, Larson AC, Zhang Q, Carr J, White RD, Li D, Simonetti OP. Automated rectilinear self-gated cardiac cine imaging. Magn. Reson. Med. 2004; 52: [126] Andreychenko A, Raaijmakers AJE, Sbrizzi A, Crijns SPM, Lagendijk JJW, Luijten PR, van den Berg CAT. Thermal noise variance of a receive radiofrequency coil as a respiratory motion sensor. Magn. Reson. Med. 2017; 77: [127] Lagendijk JJW, Raaymakers BW, Van den Berg CAT, Moerland MA, Philippens ME, van Vulpen M. MR guidance in radiotherapy. Phys. Med. Biol. 2014; 59:R349 R369. [128] International Commission on Radiation Units and Measurements, Report 83: Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT), Technical report, [129] Yun J, Wachowicz K, Mackenzie M, Rathee S, Robinson D, Fallone BG. First demonstration of intrafractional tumor-tracked irradiation using 2D phantom MR images on a prototype linac- MR. Med. Phys. 2013; 40: [130] Zhang Q, Pevsner A, Hertanto A, Hu YC, Rosenzweig KE, Ling CC, Mageras GS. A patientspecific respiratory model of anatomical motion for radiation treatment planning. Med. Phys. 2007; 34: [131] Mishra P, Li R, Mak RH, Rottmann J, Bryant JH, Williams CL, Berbeco RI, Lewis JH. An initial study on the estimation of time-varying volumetric treatment images and 3D tumor localization from single MV cine EPID images. Med. Phys. 2014; 41: [132] Cai W, Hurwitz MH, Williams CL, Dhou S, Berbeco RI, Seco J, Mishra P, Lewis JH. 3D delivered dose assessment using a 4DCT-based motion model. Med. Phys. 2015; 42: [133] Li R, Lewis JH, Jia X, Zhao T, Liu W, Wuenschel S, Lamb J, Yang D, Low Da, Jiang SB. On a PCA-based lung motion model. Phys. Med. Biol. 2011; 56: [134] Fayad H, Pan T, Pradier O, Visvikis D. Patient specific respiratory motion modeling using a 3D patient s external surface. Med. Phys. 2012; 39:
133 Appendix BIBLIOGRAPHY [135] King AP, Buerger C, Tsoumpas C, Marsden PK, Schaeffter T. Thoracic respiratory motion estimation from MRI using a statistical model and a 2-D image navigator. Med. Image Anal. 2012; 16: [136] Dikaios N, IzquierdoGarcia D, Graves MJ, Mani V, Fayad ZA, Fryer TD. MRI-based motion correction of thoracic PET: Initial comparison of acquisition protocols and correction strategies suitable for simultaneous PET/MRI systems. Eur. Radiol. 2012; 22: [137] Fürst S, Grimm R, Hong I, Souvatzoglou M, Casey ME, Schwaiger M, Nekolla SG, Ziegler SI. Motion Correction Strategies for Integrated PET/MR. J. Nucl. Med. 2015; 56: [138] Fayad H, Odille F, Schmidt H, Würslin C, Küstner T, Feblinger J, Visvikis D. The use of a generalized reconstruction by inversion of coupled systems (GRICS) approach for generic respiratory motion correction in PET/MR imaging. Phys. Med. Biol. 2015; 60: [139] Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magn. Reson. Med. 2008; 60: [140] Mcclelland JR, Hawkes DJ, Schaeffter T, King AP. Respiratory motion models : A review. Med. Image Anal. 2013; 17: [141] Stemkens B, Tijssen RHN, Denis de Senneville B, Heerkens HD, Vulpen MV, Lagendijk JJW, van den Berg CAT. Optimizing 4-Dimensional Magnetic Resonance Imaging Data Sampling for Respiratory Motion Analysis of Pancreatic Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2015; 91: [142] Miller KL, Tijssen RH, Stikov N, Okell TW. Steady-state MRI: methods for neuroimaging. Imaging Med. 2011; 3: [143] Horn BK, Schunck BG. Determining optical flow. Artif. Intell. 1981; 17: [144] Roujol S, Ries M, Quesson B, Moonen C, Denis de Senneville B. Real-time MR-thermometry and dosimetry for interventional guidance on abdominal organs. Magn. Reson. Med. 2010; 63: [145] Preiswerk F, De Luca V, Arnold P, Celicanin Z, Petrusca L, Tanner C, Bieri O, Salomir R, Cattin PC. Model-guided respiratory organ motion prediction of the liver from 2D ultrasound. Med. Image Anal. 2014; 18: [146] Kass RE, Raftery AE. Bayes Factors. J. Am. Stat. Assoc. 1995; 90: [147] Sun Y, Jolly Mp, Moura JMF. Integrated registration of dynamic renal perfusion MR images. In: IEEE Int. Conf. Image Process., pp [148] Torczon V. On the convergence of pattern search algorithms. SIAM J. Optim. 1997; 7:1 25. [149] Roujol S, BenoisPineau J, Denis de Senneville B, Ries M, Quesson B, Moonen C. Robust realtime-constrained estimation of respiratory motion for interventional MRI on mobile organs. IEEE Trans. Inf. Technol. Biomed. 2012; 16: [150] Yun J, Yip E, Wachowicz K, Rathee S, Mackenzie M, Robinson D, Fallone BG. Evaluation of a lung tumor autocontouring algorithm for intrafractional tumor tracking using low-field MRI: A phantom study. Med. Phys. 2012; 39:1481. [151] Yun J, Mackenzie M, Rathee S, Robinson D, Fallone BG. An artificial neural network (ANN)- based lung-tumor motion predictor for intrafractional MR tumor tracking. Med. Phys. 2012; 39: [152] Deng Z, Pang J, Yang W, Yue Y, Sharif B, Tuli R, Li D, Fraass B, Fan Z. Four-dimensional MRI using three-dimensional radial sampling with respiratory self-gating to characterize temporal phase-resolved respiratory motion in the abdomen. Magn. Reson. Med. 2015;. [153] Celicanin Z, Bieri O, Preiswerk F, Cattin P, Scheffler K, Santini F. Simultaneous acquisition of image and navigator slices using CAIPIRINHA for 4D MRI. Magn. Reson. Med. 2015; 73: [154] Paganelli C, Summers P, Bellomi M, Baroni G, Riboldi M. Liver 4DMRI: A retrospective image-based sorting method. Med. Phys. 2015; 42: [155] Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn. Reson. Med. 2016; 75: [156] Zachiu C, Papadakis N, Ries M, Moonen C, Denis de Senneville B. An improved optical flow tracking technique for real-time MR-guided beam therapies in moving organs. Phys. Med. Biol. 2015; 60: [157] Stemkens B, Sbrizzi A, Andreychenko AA, Crijns SPM, Lagendijk JJW, Van den Berg CAT, Tijssen RHN. An optimization framework to maximize signal-to-noise ratio in simultaneous multi- slice body imaging. NMR Biomed. 2016; 29:
134 Appendix BIBLIOGRAPHY [158] Glitzner M, Crijns SPM, Senneville BDD, Kontaxis C, Prins FM, Lagendijk JJW, Raaymakers BW. On-line MR imaging for dose validation of abdominal radiotherapy. Phys. Med. Biol. 2015; [159] Ries M, de Senneville BD, Roujol S, Berber Y, Quesson B, Moonen C. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn. Reson. Med. 2010; 64: [160] Grimm R, Furst S, Souvatzoglou M, Forman C, Hutter J, Dregely I, Ziegler S, Kiefer B, Block T, Nekolla S. Self-Gated MRI Motion Modeling for Respiratory Motion Compensation in Integrated PET/MRI. Med. Image Anal. 2014; 19: [161] Potters L, Steinberg M, Rose C, Timmerman R, Ryu S, Hevezi JM, Welsh J, Mehta M, Larson DA, Janjan NA. American Society for Therapeutic Radiology and Oncology and American College of Radiology Practice Guideline for the performance of stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2004; 60: [162] Langen KM, Jones DTL. Organ motion and its management. Int. J. Radiat. Oncol. Biol. Phys. 2001; 50: [163] Suh Y, Dieterich S, Cho B, Keall PJ. An analysis of thoracic and abdominal tumour motion for stereotactic body radiotherapy patients. Phys. Med. Biol. 2008; 53: [164] Brock KK, McShan DL, Ten Haken RK, Hollister SJ, Dawson La, Balter JM. Inclusion of organ deformation in dose calculations. Med. Phys. 2003; 30: [165] Rosu M, Chetty IJ, Balter JM, Kessler ML, McShan DL, Ten Haken RK. Dose reconstruction in deforming lung anatomy: dose grid size effects and clinical implications. Med. Phys. 2005; 32: [166] Wu QJ, Thongphiew D, Wang Z, Chankong V, Yin FF. The impact of respiratory motion and treatment technique on stereotactic body radiation therapy for liver cancer. Med. Phys. 2008; 35: [167] Velec M, Moseley JL, Eccles CL, Craig T, Sharpe MB, Dawson LA, Brock KK. Effect of breathing motion on radiotherapy dose accumulation in the abdomen using deformable registration. Int. J. Radiat. Oncol. Biol. Phys. 2011; 80: [168] Kerkhof EM, Raaymakers BW, van Vulpen M, Zonnenberg BA, Bosch JLHR, van Moorselaar RJA, Lagendijk JJW. A new concept for non-invasive renal tumour ablation using real-time MRI-guided radiation therapy. BJU Int. 2011; 107: [169] Stam MK, van Vulpen M, Barendrecht MM, Zonnenberg Ba, Crijns SPM, Lagendijk JJW, Raaymakers BW. Dosimetric feasibility of MRI-guided external beam radiotherapy of the kidney. Phys. Med. Biol. 2013; 58: [170] Stemkens B, Tijssen RHN, de Senneville BD, Lagendijk JJW, van den Berg CAT. Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy. Phys. Med. Biol. 2016; 61: [171] Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn. Reson. Med. 2014; 72: [172] Mickevicius NJ, Paulson E. Investigation of undersampling and reconstruction algorithm dependence on respiratory correlated 4D-MRI for online MR-guided radiation therapy. Phys. Med. Biol. 2017; 62: [173] Senneville BDD, Hamidi AE, Moonen C. A Direct PCA-Based Approach for Real-Time Description of Physiological Organ Deformations. IEEE Trans. Med. Imaging 2015; 34: [174] Kerkhof EM, Balter JM, Vineberg K, Raaymakers BW. Treatment plan adaptation for MRIguided radiotherapy using solely MRI data: a CT-based simulation study. Phys. Med. Biol. 2010; 55:N433 N440. [175] Swaminath A, Chu W. Stereotactic body radiotherapy for the treatment of medically inoperable primary renal cell carcinoma : Current evidence and future directions. Can. Urol. Assoc. 2015; 9: [176] Chang JH, Cheung P, Erler D, Sonier M, Korol R, Chu W. Stereotactic Ablative Body Radiotherapy for Primary Renal Cell Carcinoma in Non-surgical Candidates: Initial Clinical Experience. Clin. Oncol. 2016; 28:e109 e114. [177] Kerkhof EM, Balter JM, Vineberg K, Raaymakers BW. Treatment plan adaptation for MRIguided radiotherapy using solely MRI data: a CT-based simulation study. Phys. Med. Biol. 2010; 55:N433 N440. [178] Trofimov A, Vrancic C, Chan TCY, Sharp GC, Bortfeld T. Tumor trailing strategy for intensitymodulated radiation therapy. Med. Phys. 2008; 35:
135 Appendix BIBLIOGRAPHY [179] Fast MF, Nill S, Bedford JL, Oelfke U. Dynamic tumor tracking using the Elekta Agility MLC. Med. Phys. 2014; 41: [180] Fast MF, Kamerling CP, Ziegenhein P, Menten MJ, Bedford JL, Nill S, Oelfke U. Assessment of MLC tracking performance during hypofractionated prostate radiotherapy using real-time dose reconstruction. Phys. Med. Biol. 2016; 61: [181] Schima W. MRI of the pancreas: tumours and tumour-simulating processes. Cancer Imaging 2006; 6: [182] Groenendaal G, Moman MR, Korporaal JG, van Diest PJ, van Vulpen M, Philippens MEP, van der Heide UA. Validation of functional imaging with pathology for tumor delineation in the prostate. Radiother. Oncol. 2010; 94: [183] Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One 2010; 5:e [184] Feinberg DA, Setsompop K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J. Magn. Reson. 2013; 229: [185] Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g- factor penalty. Magn. Reson. Med. 2012; 67: [186] Deshpande V, Nickel D, Kroeker R, Kannengiesser S, Laub G. Optimized Caipirinha acceleration patterns for routine clinical 3D imaging. In: Proc. Intl. Soc. Mag. Reson. Med. 20, p [187] Wright KL, Harrell MW, Jesberger JA, Landeras L, Nakamoto DA, Thomas S, Nickel D, Kroeker R, Griswold MA, Gulani V. Clinical evaluation of CAIPIRINHA: comparison against a GRAPPA standard. J. Magn. Reson. Imaging 2014; 39: [188] Yu MH, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Clinical application of controlled aliasing in parallel imaging results in a higher acceleration (CAIPIRINHA)-volumetric interpolated breathhold (VIBE) sequence for gadoxetic acid-enhanced liver MR imaging. J. Magn. Reson. Imaging 2013; 38: [189] Riffel P, Attenberger UI, Kannengiesser S, Nickel MD, Arndt C, Meyer M, Schoenberg SO, Michaely HJ. Highly accelerated T1-weighted abdominal imaging using 2-dimensional controlled aliasing in parallel imaging results in higher acceleration: a comparison with generalized autocalibrating partially parallel acquisitions parallel imaging. Invest. Radiol. 2013; 48: [190] Michaely HJ, Morelli JN, Budjan J, Riffel P, Nickel D, Kroeker R, Schoenberg SO, Attenberger UI. CAIPIRINHA-Dixon-TWIST (CDT)-Volume-Interpolated Breath-Hold Examination (VIBE): A New Technique for Fast Time-Resolved Dynamic 3-Dimensional Imaging of the Abdomen With High Spatial Resolution. Invest. Radiol. 2013; 48: [191] Weavers PT, Borisch EA, Riederer SJ. Selection and evaluation of optimal two-dimensional CAIPIRINHA kernels applied to time-resolved three-dimensional CE-MRA. Magn. Reson. Med. 2015; 73: [192] Hansen MS, Kellman P. Image reconstruction: An overview for clinicians. J. Magn. Reson. Imaging 2015; 41: [193] Stemkens B, Tijssen RH, Andreychenko A, Crijns SPM, Sbrizzi A, Lagendijk JJW, van den Berg CAT. Optimizing CAIPIRINHA multi-band acquisition scheme for 2D multi-slice experiments in the abdomen. In: Proc. Intl. Soc. Mag. Reson. Med. 22, p [194] Weavers PT, Borisch Ea, Johnson CP, Riederer SJ. Acceleration apportionment: A method of improved 2D SENSE acceleration applied to 3D contrast-enhanced MR angiography. Magn. Reson. Med. 2013; 680: [195] Poser BA, Anderson RJ, Guérin B, Setsompop K, Deng W, Mareyam A, Serano P, Wald LL, Stenger VA. Simultaneous multislice excitation by parallel transmission. Magn. Reson. Med. 2014; 71: [196] Wong E. Optimized phase schedules for minimizing peak RF power in simultaneous multi-slice RF excitation pulses. In: Proc. Intl. Soc. Mag. Reson. Med. 20, p [197] Sbrizzi A, Poser BA, Tse DHY, Hoogduin H, Luijten PR, van den Berg CAT. RF peak power reduction in CAIPIRINHA excitation by interslice phase optimization. NMR Biomed. 2015; 28: [198] Sbrizzi A, Stemkens B, Crijns SPM, van den Berg CAT, Lagendijk JJW, Luijten PR, Tijssen RHN, Andreychenko A. Quantification of imperfect phase cycling in muliband imaging: Mathematical model and proof of principle. In: Proc. Intl. Soc. Mag. Reson. Med. 22, p
136 Appendix BIBLIOGRAPHY [199] Yutzy SR, Seiberlich N, Duerk JL, Griswold MA. Improvements in multislice parallel imaging using radial CAIPIRINHA. Magn. Reson. Med. 2011; 65: [200] Zahneisen B, Poser BA, Ernst T, Stenger VA. Three-dimensional Fourier encoding of simultaneously excited slices: generalized acquisition and reconstruction framework. Magn. Reson. Med. 2014; 71: [201] Bilgic B, Gagoski Ba, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave-CAIPI for highly accelerated 3D imaging. Magn. Reson. Med. 2015; 73: [202] Zhu K, Dougherty RF, Pauly JM, Kerr AB. Multislice acquisition with incoherent aliasing (MICA). In: Proc. Intl. Soc. Mag. Reson. Med. 22, p [203] Blaimer M, Choli M, Jakob PM, Griswold MA, Breuer FA. Multiband phase-constrained parallel MRI. Magn. Reson. Med. 2013; 69: [204] Kapanen M, Tenhunen M. T1/T2*-weighted MRI provides clinically relevant pseudo-ct density data for the pelvic bones in MRI-only based radiotherapy treatment planning. Acta Oncol. (Madr). 2013; 52: [205] Tokuda J, Morikawa S, Haque Ha, Tsukamoto T, Matsumiya K, Liao H, Masamune K, Dohi T. Adaptive 4D MR imaging using navigator-based respiratory signal for MRI-guided therapy. Magn. Reson. Med. 2008; 59: [206] Yamamoto T, Langner U, Loo Jr BW, Shen J, Keall PJ. Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 2008; 72: [207] Hui C, Wen Z, Stemkens B, Tijssen RHN, van den Berg CAT, Hwang KP, Beddar S. 4D MR imaging using robust internal respiratory signal. Phys. Med. Biol. 2016; 61: [208] Stemkens B, Tijssen RH, Denis de Senneville B, Lagendijk JJW, Van den Berg CAT. Optimal MRI sampling and binning for online 4D retrospective respiratory motion analysis of the abdomen. In: Proc. Intl. Soc. Mag. Reson. Med. 22, p [209] Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007; 58: [210] Rank CM, Heußer T, Buzan MTA, Wetscherek A, Freitag MT, Dinkel J, Kachelrieß M. 4D Respiratory Motion-Compensated Image Reconstruction of Free-Breathing Radial MR Data with Very High Undersampling. Magn. Reson. Med. 2017; 77: [211] Lens E, van der Horst A, Kroon PS, van Hooft JE, Dávila Fajardo R, Fockens P, van Tienhoven G, Bel A. Differences in respiratory-induced pancreatic tumor motion between 4D treatment planning CT and daily cone beam CT, measured using intratumoral fiducials. Acta Oncol. 2014; 53: [212] Stemkens B, Tijssen RHN, Lagendijk JJW, van den Berg CAT. Validation of a 4D-MRI motion framework using an MR-compatible motion phantom. In: Proc. Intl. Soc. Mag. Reson. Med, p [213] Benkert T, Mugler III JP, Stemkens B, Sodickson DK, Chandarana H, Block TK. Free-breathing T2-weighted abdominal examination using radial 3D fast spin-echo imaging. In: Proc. Intl. Soc. Mag. Reson. Med. 25, p [214] Maspero M, Seevinck PR, Schubert G, Hoesl MAU, van Asselen B, Viergever MA, Lagendijk JJW, Meijer GJ, van den Berg CAT. Quantification of confounding factors in MRI-based dose calculations as applied to prostate IMRT. Phys. Med. Biol. 2017; 62: [215] Stemkens B, Andreychenko A, Crijns SP, Sbrizzi A, Lagendijk JJW, van den Berg CAT, Tijssen RHN. MO-G-18C-08: Increasing throughput of a clinical MR protocol for prostate cancer with a factor of two using multiband imaging. Med. Phys. 2014; 41:441. [216] Mickevicius NJ, Paulson ES. Simultaneous orthogonal plane imaging. Magn. Reson. Med. 2016; Preprint. [217] Schaetz S, Uecker M. A Multi-GPU Programming Library for Real-Time Applications. In: ICA3PP, pp [218] Bourque AE, Bedwani S, Filion É, Carrier Jf. A particle filter based autocontouring algorithm for lung tumor tracking using dynamic magnetic resonance imaging A particle filter based autocontouring algorithm for lung tumor tracking using dynamic magnetic resonance imaging. Med. Phys. 2016; 43: [219] Glitzner M, Crijns SPM, Denis de Senneville B, Lagendijk JJW, Raaymakers BW. On the suitability of Elekta s Agility 160 MLC for tracked radiation delivery: closed-loop machine performance. Phys. Med. Biol. 2015; 60: [220] Olsen DR, Bruland ØS, Frykholm G, Norderhaug IN. Proton therapy - A systematic review of clinical effectiveness. Radiother. Oncol. 2007; 83:
137 Appendix BIBLIOGRAPHY [221] Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med. Phys. 2012; 39: [222] Raaymakers BW, Raaijmakers AJE, Lagendijk JJW. Feasibility of MRI guided proton therapy: magnetic field dose effects. Phys. Med. Biol. 2008; 53: [223] Kim YS, Keserci B, Partanen A, Rhim H, Lim HK, Park MJ, K??hler MO. Volumetric MR- HIFU ablation of uterine fibroids: Role of treatment cell size in the improvement of energy efficiency. Eur. J. Radiol. 2012; 81: [224] Wijlemans JW, Bartels LW, Deckers R, Ries M, Mali WPTM, Moonen CTW, Van Den Bosch MAAJ. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) ablation of liver tumours. Cancer Imaging 2012; 12: [225] Quesson B, Laurent C, Maclair G, De Senneville BD, Mougenot C, Ries M, Carteret T, Rullier A, Moonen CT. Real-time volumetric MRI thermometry of focused ultrasound ablation in vivo: A feasibility study in pig liver and kidney. NMR Biomed. 2011; 24: [226] Catana C. Motion Correction Options in PET/MRI. Semin. Nucl. Med. 2015; 45: [227] Guérin B, Cho S, Chun SY, Zhu X, Alpert NM, El Fakhri G, Reese T, Catana C. Nonrigid PET motion compensation in the lower abdomen using simultaneous tagged-mri and PET imaging. Med. Phys. 2011; 38: [228] Ehrhardt MJ, Thielemans K, Pizarro L, Atkinson D, Ourselin S, Hutton BF, Arridge SR. Joint reconstruction of PET-MRI by exploiting structural similarity. Inverse Probl. 2015; 31: [229] Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn. Reson. Med. 2010; 64: [230] Cheng JY, Zhang T, Pauly JM, Vasanawala S. Feasibility of ultra-high-dimensional flow imaging for rapid pediatric cardiopulmonary MRI. J. Cardiovasc. Magn. Reson. 2016; 18:217. [231] Raaijmakers AJE, Raaymakers BW, Lagendijk JJW. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissueair interfaces in a lateral magnetic field due to returning electrons. Phys. Med. Biol. 2005; 50: [232] Hammernik K, Knoll F, Sodickson DK, Pock T. Learning a Variational Model for Compressed Sensing MRI Reconstruction. In: Proc. Intl. Soc. Mag. Reson. Med, p [233] Bernard G, Verleysen M, Lee J. SU-C-18A-03 : Automatic Organ at Risk Delineation with Machine Learning Techniques Purpose. Med. Phys. 2014; 41:101. [234] Lee EK, Kurokawa YK, Tu R, George SC, Khine M. Machine learning plus optical flow: a simple and sensitive method to detect cardioactive drugs. Sci. Rep. 2015; 5: [235] El Naqa I, Li R, Murphy MJ, Machine Learning in Radiation Oncology. Springer, first ed.,
138
139 List of Publications Published papers B. Stemkens, T. Benkert, H. Chandarana, M.E. Bittman, C.A.T. van den Berg, J.J.W. Lagendijk, D.K. Sodickson, R.H.N. Tijssen, K.T. Block, Adaptive bulk-motion exclusion for improved robustness of abdominal MR imaging, NMR in Biomed., 2017; Epub ahead of print B. Stemkens, M. Glitzner, C. Kontaxis, B. Denis de Senneville, F.M. Prins, S.P.M. Crijns, L.G.W. Kerkmeijer, J.J.W. Lagendijk, C.A.T. van den Berg, R.H.N. Tijssen, Effect of intra-fraction motion on the accumulated dose for free-breathing MR-guided stereotactic body radiation therapy of renal-cell carcinoma, Phys. Med. Biol., 2017; Epub ahead of print C. Kontaxis, G.H. Bol, B. Stemkens, M. Glitzner, F.M. Prins, L.G.W. Kerkmeijer, J.J.W. Lagendijk, B.W. Raaymakers, Towards fast online intrafraction replanning for free-breathing stereotactic body radiation therapy with the MR-linac, Phys. Med. Biol., 2017; Epub ahead of print B. Stemkens, R.H.N. Tijssen, B. Denis de Senneville, J.J.W. Lagendijk, C.A.T. van den Berg, Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy, Phys. Med. Biol., 2016; 61(14): C. Hui, Z. Wen, B. Stemkens, R.H.N. Tijssen, C.A.T. van den Berg, K-P Hwang, S. Beddar, 4D MR imaging using robust internal respiratory signal, Phys. Med. Biol., 2016; 61(9): B. Stemkens, A. Sbrizzi, A. Andreychenko, S.P.M. Crijns, J.J.W. Lagendijk, C.A.T. van den Berg, R.H.N. Tijssen An optimization framework to maximize signal-to-noise ratio in simultaneous multi-slice body imaging, NMR in Biomed., 2016; 29(3): B. Stemkens, R.H.N. Tijssen, B. Denis de Senneville, H.D. Heerkens, M. van Vulpen, J.J.W. Lagendijk, CA.T. van den Berg, Optimizing 4D Magnetic Resonance Imaging data sampling for respiratory motion analysis of pancreatic tumors, Int. J. Radiat. Oncol. Biol. Phys., 2015;91(3): Submitted T. Bruijnen, B. Stemkens, C.H.J. Terhaard, J.J.W. Lagendijk, C.P.J. Raaijmakers, R.H.N. Tijssen, Intrafraction motion quantification of head-and-neck tumors using cine Magnetic Reconance Imaging, Int. J. Radiat. Oncol. Biol. Phys., submitted Proceedings B. Stemkens, C.A.T. van den Berg, T. Bruijnen, F.M. Prins, L.G.W. Kerkmeijer, J.J.W. Lagendijk, R.H.N. Tijssen, A dual-purpose MR acquisition for combined 4D-MRI These authors contributed equally 129
140 Appendix List of Publications and dynamic contrast enhanced imaging, In: Proc. 59th AAPM, 2017 (oral presentation) C. Kontaxis, G.H. Bol, B. Stemkens, M. Glitzner, F.M. Prins, L.G.W. Kerkmeijer, J.J.W. Lagendijk, B.W. Raaymakers, Fast online intrafraction replanning for the MRlinac, In: Proc. 5th MR in RT Symposium, 2017 (oral presentation) B. Stemkens, T. Benkert, H. Chandarana, C.A.T. van den Berg, J.J.W. Lagendijk, D.K. Sodickson, R.H.N. Tijssen, K.T. Block, Real-time prospective bulk motion exclusion for robust 3D free-breathing abdominal imaging, In: Proc. 25th ISMRM, 2017 (e-poster) T. Bruijnen, B. Stemkens, J.J.W. Lagendijk, C.A.T. van den Berg, R.H.N. Tijssen, The efficacy of existing k-space correction methods for 2D golden angle radial sampling on clinical 1.5T and 3T systems, In: Proc. 25th ISMRM, 2017 (e-poster) T. Benkert, J.P. Mugler III, B. Stemkens, D.K. Sodickson, H. Chandarana, K.T. Block, Free-breathing T2-weighted abdominal examination using radial 3D fast spinecho imaging, In: Proc. 25th ISMRM, 2017 (traditional poster) T. Bruijnen, R.H.N. Tijssen, M.E.P. Philippens, C.H.J. Terhaard, T. Schakel, J.J.W. Lagendijk, C.P.J. Raaijmakers, B. Stemkens, Intra-fraction motion quatification of head-and-neck tumors using dynamic MRI, In: Proc. 36th ESTRO, 2017 (e-poster) B. Stemkens, M. Glitzner, C. Kontaxis, B. Denis de Senneville, F. Prins, S.P.M. Crijns, L. Kerkmeijer, J.J.W. Lagendijk, C.A.T. van den Berg, R.H.N. Tijssen, The effects of inter-cycle respiratory motion variation on dose accumulation in single fraction MRguided SBRT treatment or renal cell carcinoma, In: Proc. 58th AAPM, 2016 (SNAP oral presentation) B. Stemkens, R.H.N. Tijssen, J.J.W. Lagendijk, C.A.T. van den Berg, Validation of a 4D-MRI motion framework using an MR-compatible motion phantom, In: Proc. 24th ISMRM, 2016 (traditional poster) C. Kontaxis, B. Stemkens, G.H. Bol, J.J.W. Lagendijk, B.W. Raaymakers, Towards inter- and intrafraction plan adaptation using MR-derived three-dimensional deformation fields, In: Proc. 18th ICCR, 2016 (oral presentation) T. Bruijnen, B. Stemkens, M.E.P. Philippens, L.P.W. Canjels, R.H.N. Tijssen, T. Schakel, C.H.J. Terhaard, J.J.W. Lagendijk, C.P.J. Raaijmakers, Intra-fraction motion characterization of head-and-neck tumors using cine-mri, In: Proc. 35th ESTRO, 2016 (poster discussion) L.P.W. Canjels, M.E.P. Philippens, T. Bruijnen, B. Stemkens, D.C.P. Cobben, S. Sharouni, J.J.W. Lagendijk, A.L.H.M.W. van Lier, R.H.N. Tijssen, Assessment of respiratory and cardiac motion to supplement MRI based tracking of hilar lymph nodes, In: Proc. 35th ESTRO, 2016 (poster) B. Stemkens, R.H.N. Tijssen, B. Denis de Senneville, J.J.W. Lagendijk, C.A.T. Van den Berg, A data-driven 4D-MRI motion model to estimate full field-of-view abdominal motion from 2D image navigators during MR-Linac treatment, In: Proc. 57th AAPM, 2015 (oral presentation) C. Hui, Z. Wen, B. Stemkens, R.H.N. Tijssen, C.A.T. van den Berg, S. Beddar, Achieving 4D MRI in regular breathing cycle with extended acquisition time of dynamic MR images, In: Proc. 57th AAPM, 2015 (SNAP oral presentation) Z. Wen, C. Hui, B. Stemkens, R.H.N. Tijssen, C.A.T. van den Berg, S. Beddar, Sorting 2D dynamic MR images using internal respiratory signal for 4D MRI, In: Proc. 57th AAPM, 2015 (campus e-poster) 130
141 Appendix List of Publications B. Stemkens, R.H.N. Tijssen, B. Denis de Senneville, J.J.W. Lagendijk, C.A.T. Van den Berg, Estimating dynamic 3D abdominal motion for radiation dose accumulation mapping using a PCA-based model and 2D navigators, In: Proc. 23rd ISMRM, 2015 (e-poster) T. Schakel, B. Stemkens, H. Hoogduin, M. Philippens, Fat suppression for DW-FSE sequences using an integrated multi-acquisition Dixon method, In: Proc. 23rd ISMRM, 2015 (e-poster) S.P.M. Crijns, A. Sbrizzi, B. Stemkens, C.A.T. Van den Berg, P. Luijten, J.J.W. Lagendijk, A. Andreychenko, Simultaneous multi-slice imaging with chemical shift separation, In: Proc. 23rd ISMRM, 2015 (oral presentation) H.D. Heerkens, O. Reerink, M. van Vulpen, R.H. Tijssen, B. Stemkens, S.P.M. Crijns, R.R. Hiensch, C.A.T. Van den Berg, G.J. Meijer, Use of a custom abdominal corset to reduce pancreatic tumor motion during stereotactic radiotherapy, In: Proc. 3rd ESTRO Forum, 2015 (poster) B. Stemkens, R.H.N. Tijssen, B. Denis de Senneville, J.J.W. Lagendijk, C.A.T. van den Berg, Retrospective reconstruction of 3D radial MRI data to evaluate the effect of abdominal compression on 4D abdominal organ motion, In: Proc. 56th AAPM, 2014 (oral presentation) B. Stemkens, A. Andreychenko, S.P.M. Crijns, A. Sbrizzi, J.J.W. Lagendijk, C.A.T. van den Berg, R.H.N. Tijssen, Increasing throughput of a clinical MR protocol for prostate cancer with a factor of two using multiband imaging, In: Proc. 56th AAPM, 2014 (oral presentation) S.P.M. Crijns, B. Stemkens, A. Sbrizzi, J.J.W. Lagendijk, C.A.T. van den Berg, A. Andreychenko, Accelerated Water/fat Separation in MRI for Radiotherapy Planning Using Multi-Band Imaging Techniques, In: Proc. 56th AAPM, 2014 (oral presentation) B. Stemkens, R.H. Tijssen, A. Andreychenko, S.P.M. Crijns, A. Sbrizzi, J.J.W. Lagendijk, C.A.T. van den Berg, Optimizing CAIPIRINHA multi-band acquisition scheme for 2D multi-slice experiments in the abdomen, In: Proc. 22nd ISMRM, 2014 (oral presentation) B. Stemkens, R.H. Tijssen, B. Denis de Senneville, J.J.W. Lagendijk, C.A.T. van den Berg, Optimal MRI sampling and binning for online 4D retrospective respiratory motion analysis of the abdomen, In: Proc. 22nd ISMRM, 2014 (e-poster) A. Sbrizzi, B. Stemkens, S.P.M. Crijns, C.A.T. van den Berg, J.J.W. Lagendijk, P.R. Luijten, R.H.N. Tijssen, A. Andreychenko, Quantification of imperfect phase cycling in multi-band imaging: Mathematical model and proof of principle, In: Proc. 22nd ISMRM, 2014 (poster) S.P.M. Crijns, B. Stemkens, A. Sbrizzi, J.J.W. Lagendijk, P. Luijten, C.A.T. van den Berg, A. Andreychenko, A Single Point, Echo Time Independent Water/fat Separation Method, In: Proc. 22nd ISMRM, 2014 (e-poster) A. Andreychenko, S.P.M. Crijns, A. Raaijmakers, B. Stemkens, P. Luijten, J.J.W. Lagendijk, C.A.T van den Berg, Noise variance of an RF receive array reflects respiratory motion: a novel respiratory motion predictor, In: Proc. 22nd ISMRM, 2014 (power poster) B. Stemkens, R.H.N. Tijssen, H.D. Heerkens, B. Denis de Senneville, G.J. Meijer, M. van Vulpen, J.J.W. Lagendijk, C.A.T. van den Berg, 4D motion analysis using retrospective binning of MR images in the abdomen, In: Proc. 33rd ESTRO, 2014 (oral 131
142 Appendix List of Publications presentation) B. Stemkens, R.H.N. Tijssen, C.A.T. Van den Berg, J.J.W. Lagendijk, C.T.W. Moonen, M. Ries, B. Denis de Senneville, Optical flow analysis on undersampled radial acquisitions for real-time tracking of the pancreas in MR guided radiotherapy, In: Proc. 21st ISMRM, 2013 (e-poster) B. Stemkens, R.H.N. Tijssen, B. Denis de Senneville, J.J.W. Lagendijk, C.A.T. van den Berg, Assessment of fast external and internal motion surrogates to supplement MRI based tracking of the pancreas, In: Proc. 2nd ESTRO Forum, 2013 (oral presentation) Invited presentations, awards and nominations 4D MR imaging (developments in conventional and real time imaging), 4D workshop, Groningen, 2016 Motion management for abdominal MRI-guided radiotherapy, Memorial Sloan Kettering Cancer Center Radiotherapy Physics Seminar, New York, 2016 Outstanding Reviewer Award, Physics in Medicine and Biology, 2016 Best poster award, ISMRM Benelux annual meeting, Maastricht, 2014 IMDI talent nominee for best Master thesis, ZonMW,
143 Acknowledgements Alone we can do so little; together we can do so much. Helen Keller Dit proefschrift is tot stand gekomen met directe en indirecte hulp van velen die ik hier graag wil bedanken. Allereerst de man die mij het meest heeft geholpen bij dit proefschrift. Rob, na een leuk en succesvol master afstudeerproject heb ik geen moment spijt gehad van deze promotie onder jouw supervisie. Ik ben enorm dankbaar dat je altijd tijd maakte om mij dingen uit te leggen, een document door te nemen of gewoon even wat te bespreken, zowel op professioneel maar ook zeker op persoonlijk vlak. Als mijn daily supervisor heb je aardig wat uurtjes moeten spenderen om weer eens een abstract, manuscript of deel van mijn proefschrift door te nemen, maar ik kreeg altijd waardevol commentaar binnen een paar uur of dagen. Ik vind het mooi om te zien hoe je van klifio naar onmisbare klinisch fysicus en project leider van RESOLVE bent gegroeid. Ik heb enorm veel respect voor hoe jij het werk als klinisch fysicus combineert met de begeleiding van (inmiddels) meerdere PhD studenten en je dagelijkse reis van en naar Neerkant. Ik hoop in de toekomst nog veel met je samen te kunnen werken. Nico, als mijn tweede co-promotor was jouw werk wat meer op de achtergrond, maar desondanks enorm waardevol. Ik vond de meetings met z n drieën altijd ontzettend leuk en leerzaam en jij kwam altijd wel met nieuwe ideeën aanzetten die allereerst vrij absurd klonken maar bij nader inzien toch wel (deels) erg nuttig bleken. Je onmiskenbare enthousiasme is aanstekelijk en ik bewonder hoe jij het voor elkaar bokst om zoveel PhD studenten te begeleiden en projecten in goede banen te leiden. Uiteraard wil ik je ook bedanken voor de tafelvoetbaltafel die de 16:00 middagpauze helemaal heeft teruggebracht voor de onderzoekers. Jan, als mijn promotor hoefde je je weinig te bemoeien met de dagelijkse gebeurtenissen van mijn onderzoek, maar je keek altijd kritisch naar mijn werk en gaf telkens nuttige tips. Jouw tomeloze energie en inzet omtrent het MRL-project is ongeëvenaard en heeft uiteindelijk tot de eerste patiëntenbehandeling geleid in mei van dit jaar. Nu op naar real-time tracking van tumoren in het abdomen! Dear committee members, prof. dr. Bas Raaymakers, prof. dr. Peter Luijten, prof. dr. Chrit Moonen, prof. dr. Tufve Nyholm, and dr. Hersh Chandarana, I am very grateful for your evaluation of this thesis. Many thanks for your efforts. 133
144 Appendix Acknowledgements Jean-Paul en Tim, Koning kantoorhumor en Het wonderkind van de radiotherapie, mijn paranimfen en maatjes tijdens de verschillende congressen van de afgelopen jaren. Ik heb veel van jullie geleerd op het wetenschappelijk gebied, maar ook op het gebied van useless knowledge. Ik ben daarom bijzonder blij jullie aan mijn zijde te hebben tijdens mijn verdediging. Christel, Matteo, Michaëla en Tom. Als mijn kamergenootjes op Q2 heb ik een enorm leuke tijd met jullie gehad. Naast discussies over allerlei werkgerelateerde onderwerpen was er ook voldoende tijd voor een geintje en voor het bespreken van andere onderwerpen. Ik heb veel van jullie geleerd en jullie hopelijk ook wat van mij. Alle collega OiO, AiO s en andere onderzoekers waar ik de afgelopen jaren direct of indirect mee heb samengewerkt, zowel van de afdeling radiotherapie als radiologie, wil ik graag bedanken. Zonder de gezamelijke discussies en het delen van inzichten of code was dit proefschrift een stuk minder interessant geweest. Door de goede sfeer ben ik altijd met veel plezier naar mijn werk gegaan. Ik heb ook altijd genoten van de (reisjes voor/na de) congressen, OiO-uitjes, pauzes, tafelvoetbalwedstrijden en borrels die ik met jullie heb kunnen doen. Markus, Charis, Tristan, Maxence, Stefan, Luca, Stan, Frank, Kimmy, Mariska, Hans, Filipa, Joris, Sophie, Ellis, Anna, Christoph, Mark, Robin, Pim, and all others that I m forgetting, thank y all! Thanks to all co-authors of the different articles and abstracts that we worked on. Your efforts really helped me in improving the articles that eventually led to this thesis. Een extra bedankje voor Hanne en Fieke, de AiO s die de patiënten hebben geïncludeerd voor verschillende studies die in dit proefschrift zijn beschreven, zodat ik de verschillende technieken in patiënten kon testen. Daarnaast wil ik ook nog Sjoerd, Anna en Alessandro bedanken voor de fijne samenwerking bij het CAIPIRINHA-project. Ik vond deze samenwerking in het begin van mijn promotie erg leuk en ik was er trots op dat we in zo n korte tijd de gehele implementatie rond hadden. Ook wil ik nog alle onderzoekers van de MRI, tracking en RESOLVE meeting bedanken voor de interessante, leerzame en leuke besprekingen de afgelopen jaren. A big thank you goes to Baudouin, for helping me a lot during my Master s thesis and especially the first years of my PhD with the registration tools, programming problems and ideas for publications. I hope we can continue this collaboration for the upcoming years! A huge thanks to all my temporary colleagues from CBI/CAI 2 R in New York and especially to dr. Thomas Benkert and dr. Tobias Block. Because of your warm welcome (straight to the bar), I immediately enjoyed New York. I really enjoyed working with two Young Investigator Award winners and learned a lot. I had a great time with all the CBI people in the office, at the foosball table and at all the places we went for food, drinks, sports and music. I truly had a blast in New York, thank you all! Daarnaast wil ik alle MRI laboranten bedanken die de patiënten voor de verschillende studies hebben gescand, terwijl ik allerlei aanwijzingen in hun oor aan het schreeuwen was. Ook alle ICT-medewerkers (inclusief Gijs en Alexis) die 134
145 Appendix Acknowledgements me hebben geholpen met computergerelateerde problemen verdienen hier een bedankje. Tevens wil ik alle laboranten, artsen, klinische fysici, fysisch medewerkers en andere medewerkers van de afdeling bedanken voor de gezellige pauzes, dagjes uit en borrels. En de radio robbies van Geuken United kunnen natuurlijk niet ontbreken in dit dankwoord na alle leuke wedstrijden, derde helften en ski-vakanties! Naast al mijn collega s wil ik ook graag mijn vrienden uit Eindhoven en Meijel bedanken voor de mooie avonden, concerten en weekendjes weg die we hebben gehad! Ook al hebben jullie niet direct bijgedragen aan dit proefschrift, ik vond het altijd erg fijn om (met een biertje in de hand) lekker over andere dingen te kunnen praten. Geen gezeik, gewoon vrienden onder elkaar zoals het hoort! Heerlijk! Papa Jos en mama Tonny, mijn super ouders. Hoewel ik al een tijdje niet meer bij jullie woon, voelt het toch altijd als thuiskomen wanneer ik bij jullie over de vloer kom. Jullie oprechte interesse in mijn onderzoek en het feit dat jullie altijd voor me klaar staan stel ik enorm op prijs. Ook wil ik mijn zus Kim en haar man Ramon bedanken voor alle tips en advies de afgelopen jaren op elk vlak. En omdat jullie allemaal niet altijd snapte waar ik nou eigenlijk mee bezig was (en voor alle anderen die het tot hier vol hebben gehouden), een kleine samenvatting: De MRL combineert MRI met een versneller voor bestraling. Hierdoor kun je MRI plaatjes maken tijdens de bestraling, wat veel onzekerheden weg kan nemen en waardoor we gerichter kunnen bestralen. Met name voor tumoren in de buik kan dit enorme voordelen opleveren, omdat we rekening kunnen houden met ademhalingsbeweging en dus geen gezond weefsel bestralen. De methoden in dit proefschrift zijn oplossingen voor enkele problemen om de bestralingen in de buik accurater te maken. En dan de belangrijkste plek voor de belangrijkste persoon. Lieve Merel, jij hebt me de afgelopen jaren altijd enorm gesteund, met name als ik het zwaar had. Het feit dat er meer dan een jaar vele tijdszones ons scheidden door onze reislust, heeft er alleen maar voor gezorgd dat we elkaars aanwezigheid nog meer waarderen. Dat we daarna een huis kopen, terwijl ik dit proefschrift aan het schrijven ben laat zien dat we een goed team zijn. Ik ben enorm gelukkig met ons eigen huisje in Eindhoven en ik kijk er naar uit wat de komende jaren ons zal brengen! Bjorn 135
146
147 Curriculum vitae Bjorn Stemkens was born on March 30, 1989 in Helmond, the Netherlands. After primary school in Meijel, he attended the St. Willibrord Gymnasium in Deurne where he received his VWO diploma in Subsequently, he studied Biomedical Engineering at the Eindhoven University of Technology (TU/e). During his Medical Engineering Master program he did an internship on combining functional PET and MR parameters from a hybrid MR-PET system for detecting changes in tumor characteristics in glioblastoma patients at the A.A. Martinos Center for Biomedical Imaging in Boston. He conducted his Master s thesis at the University Medical Center Utrecht, where he focused on real-time MRI tracking for MRI-guided pancreatic treatments. Captivated by MRI-guided radiotherapy, he started his PhD at the same hospital after obtaining his Master s degree in During his PhD he went to the Center for Biomedical Imaging/CAI2 R in New York for a research project on the automated detection and exclusion of bulk motion for robust abdominal imaging. The most important results of his doctoral research are presented in this thesis. He is currently a post-doctoral researcher at the UMC Utrecht, continuing his research and developments around the MRL project with a focus on clinical introduction of MRI methods and applications for MRI-guided radiotherapy. 137
MR-guided radiotherapy: Vision, status and research at the UMC Utrecht. Dipl. Ing. Dr. Markus Glitzner
 MR-guided radiotherapy: Vision, status and research at the UMC Utrecht Dipl. Ing. Dr. Markus Glitzner About myself Training Medizintechnik TU Graz PhD UMC Utrecht Clinical work Software implementation
MR-guided radiotherapy: Vision, status and research at the UMC Utrecht Dipl. Ing. Dr. Markus Glitzner About myself Training Medizintechnik TU Graz PhD UMC Utrecht Clinical work Software implementation
7/31/2011. Learning Objective. Video Positioning. 3D Surface Imaging by VisionRT
 CLINICAL COMMISSIONING AND ACCEPTANCE TESTING OF A 3D SURFACE MATCHING SYSTEM Hania Al-Hallaq, Ph.D. Assistant Professor Radiation Oncology The University of Chicago Learning Objective Describe acceptance
CLINICAL COMMISSIONING AND ACCEPTANCE TESTING OF A 3D SURFACE MATCHING SYSTEM Hania Al-Hallaq, Ph.D. Assistant Professor Radiation Oncology The University of Chicago Learning Objective Describe acceptance
Brilliance CT Big Bore.
 1 2 2 There are two methods of RCCT acquisition in widespread clinical use: cine axial and helical. In RCCT with cine axial acquisition, repeat CT images are taken each couch position while recording respiration.
1 2 2 There are two methods of RCCT acquisition in widespread clinical use: cine axial and helical. In RCCT with cine axial acquisition, repeat CT images are taken each couch position while recording respiration.
Image Guidance and Beam Level Imaging in Digital Linacs
 Image Guidance and Beam Level Imaging in Digital Linacs Ruijiang Li, Ph.D. Department of Radiation Oncology Stanford University School of Medicine 2014 AAPM Therapy Educational Course Disclosure Research
Image Guidance and Beam Level Imaging in Digital Linacs Ruijiang Li, Ph.D. Department of Radiation Oncology Stanford University School of Medicine 2014 AAPM Therapy Educational Course Disclosure Research
Image Quality Assessment and Quality Assurance of Advanced Imaging Systems for IGRT. AAPM Penn-Ohio Chapter Sep 25, 2015 Soyoung Lee, PhD
 Image Quality Assessment and Quality Assurance of Advanced Imaging Systems for IGRT AAPM Penn-Ohio Chapter Sep 25, 2015 Soyoung Lee, PhD 1 Outline q Introduction q Imaging performances in 4D-CBCT Image
Image Quality Assessment and Quality Assurance of Advanced Imaging Systems for IGRT AAPM Penn-Ohio Chapter Sep 25, 2015 Soyoung Lee, PhD 1 Outline q Introduction q Imaging performances in 4D-CBCT Image
Tumor motion during liver SBRT
 Tumor motion during liver SBRT - projects at Aarhus University Hospital - Per Poulsen, Esben Worm, Walther Fledelius, Morten Høyer Aarhus University Hospital, Denmark SBRT: Stereotactic Body Radiation
Tumor motion during liver SBRT - projects at Aarhus University Hospital - Per Poulsen, Esben Worm, Walther Fledelius, Morten Høyer Aarhus University Hospital, Denmark SBRT: Stereotactic Body Radiation
UvA-DARE (Digital Academic Repository) Motion compensation for 4D PET/CT Kruis, M.F. Link to publication
 UvA-DARE (Digital Academic Repository) Motion compensation for 4D PET/CT Kruis, M.F. Link to publication Citation for published version (APA): Kruis, M. F. (2014). Motion compensation for 4D PET/CT General
UvA-DARE (Digital Academic Repository) Motion compensation for 4D PET/CT Kruis, M.F. Link to publication Citation for published version (APA): Kruis, M. F. (2014). Motion compensation for 4D PET/CT General
VALIDATION OF DIR. Raj Varadhan, PhD, DABMP Minneapolis Radiation Oncology
 VALIDATION OF DIR Raj Varadhan, PhD, DABMP Minneapolis Radiation Oncology Overview Basics: Registration Framework, Theory Discuss Validation techniques Using Synthetic CT data & Phantoms What metrics to
VALIDATION OF DIR Raj Varadhan, PhD, DABMP Minneapolis Radiation Oncology Overview Basics: Registration Framework, Theory Discuss Validation techniques Using Synthetic CT data & Phantoms What metrics to
Is deformable image registration a solved problem?
 Is deformable image registration a solved problem? Marcel van Herk On behalf of the imaging group of the RT department of NKI/AVL Amsterdam, the Netherlands DIR 1 Image registration Find translation.deformation
Is deformable image registration a solved problem? Marcel van Herk On behalf of the imaging group of the RT department of NKI/AVL Amsterdam, the Netherlands DIR 1 Image registration Find translation.deformation
State-of-the-Art IGRT
 in partnership with State-of-the-Art IGRT Exploring the Potential of High-Precision Dose Delivery and Real-Time Knowledge of the Target Volume Location Antje-Christin Knopf IOP Medical Physics Group Scientific
in partnership with State-of-the-Art IGRT Exploring the Potential of High-Precision Dose Delivery and Real-Time Knowledge of the Target Volume Location Antje-Christin Knopf IOP Medical Physics Group Scientific
Development and Optimization of Fourdimensional Magnetic Resonance Imaging (4D-MRI) for Radiation Therapy
 Development and Optimization of Fourdimensional Magnetic Resonance Imaging (4D-MRI) for Radiation Therapy by Yilin Liu Medical Physics Graduate Program Duke University Date: Approved: Jing Cai, co-advisor
Development and Optimization of Fourdimensional Magnetic Resonance Imaging (4D-MRI) for Radiation Therapy by Yilin Liu Medical Physics Graduate Program Duke University Date: Approved: Jing Cai, co-advisor
Motion Artifacts and Suppression in MRI At a Glance
 Motion Artifacts and Suppression in MRI At a Glance Xiaodong Zhong, PhD MR R&D Collaborations Siemens Healthcare MRI Motion Artifacts and Suppression At a Glance Outline Background Physics Common Motion
Motion Artifacts and Suppression in MRI At a Glance Xiaodong Zhong, PhD MR R&D Collaborations Siemens Healthcare MRI Motion Artifacts and Suppression At a Glance Outline Background Physics Common Motion
Abbie M. Diak, PhD Loyola University Medical Center Dept. of Radiation Oncology
 Abbie M. Diak, PhD Loyola University Medical Center Dept. of Radiation Oncology Outline High Spectral and Spatial Resolution MR Imaging (HiSS) What it is How to do it Ways to use it HiSS for Radiation
Abbie M. Diak, PhD Loyola University Medical Center Dept. of Radiation Oncology Outline High Spectral and Spatial Resolution MR Imaging (HiSS) What it is How to do it Ways to use it HiSS for Radiation
Estimating 3D Respiratory Motion from Orbiting Views
 Estimating 3D Respiratory Motion from Orbiting Views Rongping Zeng, Jeffrey A. Fessler, James M. Balter The University of Michigan Oct. 2005 Funding provided by NIH Grant P01 CA59827 Motivation Free-breathing
Estimating 3D Respiratory Motion from Orbiting Views Rongping Zeng, Jeffrey A. Fessler, James M. Balter The University of Michigan Oct. 2005 Funding provided by NIH Grant P01 CA59827 Motivation Free-breathing
Position accuracy analysis of the stereotactic reference defined by the CBCT on Leksell Gamma Knife Icon
 Position accuracy analysis of the stereotactic reference defined by the CBCT on Leksell Gamma Knife Icon WHITE PAPER Introduction An image guidance system based on Cone Beam CT (CBCT) is included in Leksell
Position accuracy analysis of the stereotactic reference defined by the CBCT on Leksell Gamma Knife Icon WHITE PAPER Introduction An image guidance system based on Cone Beam CT (CBCT) is included in Leksell
REAL-TIME ADAPTIVITY IN HEAD-AND-NECK AND LUNG CANCER RADIOTHERAPY IN A GPU ENVIRONMENT
 REAL-TIME ADAPTIVITY IN HEAD-AND-NECK AND LUNG CANCER RADIOTHERAPY IN A GPU ENVIRONMENT Anand P Santhanam Assistant Professor, Department of Radiation Oncology OUTLINE Adaptive radiotherapy for head and
REAL-TIME ADAPTIVITY IN HEAD-AND-NECK AND LUNG CANCER RADIOTHERAPY IN A GPU ENVIRONMENT Anand P Santhanam Assistant Professor, Department of Radiation Oncology OUTLINE Adaptive radiotherapy for head and
TomoTherapy Related Projects. An image guidance alternative on Tomo Low dose MVCT reconstruction Patient Quality Assurance using Sinogram
 TomoTherapy Related Projects An image guidance alternative on Tomo Low dose MVCT reconstruction Patient Quality Assurance using Sinogram Development of A Novel Image Guidance Alternative for Patient Localization
TomoTherapy Related Projects An image guidance alternative on Tomo Low dose MVCT reconstruction Patient Quality Assurance using Sinogram Development of A Novel Image Guidance Alternative for Patient Localization
White Pixel Artifact. Caused by a noise spike during acquisition Spike in K-space <--> sinusoid in image space
 White Pixel Artifact Caused by a noise spike during acquisition Spike in K-space sinusoid in image space Susceptibility Artifacts Off-resonance artifacts caused by adjacent regions with different
White Pixel Artifact Caused by a noise spike during acquisition Spike in K-space sinusoid in image space Susceptibility Artifacts Off-resonance artifacts caused by adjacent regions with different
Lab Location: MRI, B2, Cardinal Carter Wing, St. Michael s Hospital, 30 Bond Street
 Lab Location: MRI, B2, Cardinal Carter Wing, St. Michael s Hospital, 30 Bond Street MRI is located in the sub basement of CC wing. From Queen or Victoria, follow the baby blue arrows and ride the CC south
Lab Location: MRI, B2, Cardinal Carter Wing, St. Michael s Hospital, 30 Bond Street MRI is located in the sub basement of CC wing. From Queen or Victoria, follow the baby blue arrows and ride the CC south
Use of MRI in Radiotherapy: Technical Consideration
 Use of MRI in Radiotherapy: Technical Consideration Yanle Hu, PhD Department of Radiation Oncology, Mayo Clinic Arizona 04/07/2018 2015 MFMER slide-1 Conflict of Interest: None 2015 MFMER slide-2 Objectives
Use of MRI in Radiotherapy: Technical Consideration Yanle Hu, PhD Department of Radiation Oncology, Mayo Clinic Arizona 04/07/2018 2015 MFMER slide-1 Conflict of Interest: None 2015 MFMER slide-2 Objectives
Volumetric Cine Imaging for On-board Target Localization in Radiation Therapy. Wendy Beth Harris. Graduate Program in Medical Physics Duke University
 Volumetric Cine Imaging for On-board Target Localization in Radiation Therapy by Wendy Beth Harris Graduate Program in Medical Physics Duke University Date: Approved: Lei Ren, Supervisor Fang-Fang Yin,
Volumetric Cine Imaging for On-board Target Localization in Radiation Therapy by Wendy Beth Harris Graduate Program in Medical Physics Duke University Date: Approved: Lei Ren, Supervisor Fang-Fang Yin,
ADVANCING CANCER TREATMENT
 The RayPlan treatment planning system makes proven, innovative RayStation technology accessible to clinics that need a cost-effective and streamlined solution. Fast, efficient and straightforward to use,
The RayPlan treatment planning system makes proven, innovative RayStation technology accessible to clinics that need a cost-effective and streamlined solution. Fast, efficient and straightforward to use,
ADVANCING CANCER TREATMENT
 3 ADVANCING CANCER TREATMENT SUPPORTING CLINICS WORLDWIDE RaySearch is advancing cancer treatment through pioneering software. We believe software has un limited potential, and that it is now the driving
3 ADVANCING CANCER TREATMENT SUPPORTING CLINICS WORLDWIDE RaySearch is advancing cancer treatment through pioneering software. We believe software has un limited potential, and that it is now the driving
The MSKCC Approach to IMRT. Outline
 The MSKCC Approach to IMRT Spiridon V. Spirou, PhD Department of Medical Physics Memorial Sloan-Kettering Cancer Center New York, NY Outline Optimization Field splitting Delivery Independent verification
The MSKCC Approach to IMRT Spiridon V. Spirou, PhD Department of Medical Physics Memorial Sloan-Kettering Cancer Center New York, NY Outline Optimization Field splitting Delivery Independent verification
Slide 1. Technical Aspects of Quality Control in Magnetic Resonance Imaging. Slide 2. Annual Compliance Testing. of MRI Systems.
 Slide 1 Technical Aspects of Quality Control in Magnetic Resonance Imaging Slide 2 Compliance Testing of MRI Systems, Ph.D. Department of Radiology Henry Ford Hospital, Detroit, MI Slide 3 Compliance Testing
Slide 1 Technical Aspects of Quality Control in Magnetic Resonance Imaging Slide 2 Compliance Testing of MRI Systems, Ph.D. Department of Radiology Henry Ford Hospital, Detroit, MI Slide 3 Compliance Testing
Respiratory Motion Compensation for Simultaneous PET/MR Based on Strongly Undersampled Radial MR Data
 Respiratory Motion Compensation for Simultaneous PET/MR Based on Strongly Undersampled Radial MR Data Christopher M Rank 1, Thorsten Heußer 1, Andreas Wetscherek 1, and Marc Kachelrieß 1 1 German Cancer
Respiratory Motion Compensation for Simultaneous PET/MR Based on Strongly Undersampled Radial MR Data Christopher M Rank 1, Thorsten Heußer 1, Andreas Wetscherek 1, and Marc Kachelrieß 1 1 German Cancer
Image Acquisition Systems
 Image Acquisition Systems Goals and Terminology Conventional Radiography Axial Tomography Computer Axial Tomography (CAT) Magnetic Resonance Imaging (MRI) PET, SPECT Ultrasound Microscopy Imaging ITCS
Image Acquisition Systems Goals and Terminology Conventional Radiography Axial Tomography Computer Axial Tomography (CAT) Magnetic Resonance Imaging (MRI) PET, SPECT Ultrasound Microscopy Imaging ITCS
Lucy Phantom MR Grid Evaluation
 Lucy Phantom MR Grid Evaluation Anil Sethi, PhD Loyola University Medical Center, Maywood, IL 60153 November 2015 I. Introduction: The MR distortion grid, used as an insert with Lucy 3D QA phantom, is
Lucy Phantom MR Grid Evaluation Anil Sethi, PhD Loyola University Medical Center, Maywood, IL 60153 November 2015 I. Introduction: The MR distortion grid, used as an insert with Lucy 3D QA phantom, is
Role of Parallel Imaging in High Field Functional MRI
 Role of Parallel Imaging in High Field Functional MRI Douglas C. Noll & Bradley P. Sutton Department of Biomedical Engineering, University of Michigan Supported by NIH Grant DA15410 & The Whitaker Foundation
Role of Parallel Imaging in High Field Functional MRI Douglas C. Noll & Bradley P. Sutton Department of Biomedical Engineering, University of Michigan Supported by NIH Grant DA15410 & The Whitaker Foundation
Initial Clinical Experience with 3D Surface Image Guidance
 Initial Clinical Experience with 3D Surface Image Guidance Amanda Havnen-Smith, Ph.D. Minneapolis Radiation Oncology Ridges Radiation Therapy Center Burnsville, MN April 20 th, 2012 Non-funded research
Initial Clinical Experience with 3D Surface Image Guidance Amanda Havnen-Smith, Ph.D. Minneapolis Radiation Oncology Ridges Radiation Therapy Center Burnsville, MN April 20 th, 2012 Non-funded research
New Technology Allows Multiple Image Contrasts in a Single Scan
 These images were acquired with an investigational device. PD T2 T2 FLAIR T1 MAP T1 FLAIR PSIR T1 New Technology Allows Multiple Image Contrasts in a Single Scan MR exams can be time consuming. A typical
These images were acquired with an investigational device. PD T2 T2 FLAIR T1 MAP T1 FLAIR PSIR T1 New Technology Allows Multiple Image Contrasts in a Single Scan MR exams can be time consuming. A typical
Module 5: Dynamic Imaging and Phase Sharing. (true-fisp, TRICKS, CAPR, DISTAL, DISCO, HYPR) Review. Improving Temporal Resolution.
 MRES 7005 - Fast Imaging Techniques Module 5: Dynamic Imaging and Phase Sharing (true-fisp, TRICKS, CAPR, DISTAL, DISCO, HYPR) Review Improving Temporal Resolution True-FISP (I) True-FISP (II) Keyhole
MRES 7005 - Fast Imaging Techniques Module 5: Dynamic Imaging and Phase Sharing (true-fisp, TRICKS, CAPR, DISTAL, DISCO, HYPR) Review Improving Temporal Resolution True-FISP (I) True-FISP (II) Keyhole
Single Breath-hold Abdominal T 1 Mapping using 3-D Cartesian Sampling and Spatiotemporally Constrained Reconstruction
 Single Breath-hold Abdominal T 1 Mapping using 3-D Cartesian Sampling and Spatiotemporally Constrained Reconstruction Felix Lugauer 1,3, Jens Wetzl 1, Christoph Forman 2, Manuel Schneider 1, Berthold Kiefer
Single Breath-hold Abdominal T 1 Mapping using 3-D Cartesian Sampling and Spatiotemporally Constrained Reconstruction Felix Lugauer 1,3, Jens Wetzl 1, Christoph Forman 2, Manuel Schneider 1, Berthold Kiefer
8/3/2016. Image Guidance Technologies. Introduction. Outline
 8/3/26 Session: Image Guidance Technologies and Management Strategies Image Guidance Technologies Jenghwa Chang, Ph.D.,2 Department of Radiation Medicine, Northwell Health 2 Hofstra Northwell School of
8/3/26 Session: Image Guidance Technologies and Management Strategies Image Guidance Technologies Jenghwa Chang, Ph.D.,2 Department of Radiation Medicine, Northwell Health 2 Hofstra Northwell School of
3/27/2012 WHY SPECT / CT? SPECT / CT Basic Principles. Advantages of SPECT. Advantages of CT. Dr John C. Dickson, Principal Physicist UCLH
 3/27/212 Advantages of SPECT SPECT / CT Basic Principles Dr John C. Dickson, Principal Physicist UCLH Institute of Nuclear Medicine, University College London Hospitals and University College London john.dickson@uclh.nhs.uk
3/27/212 Advantages of SPECT SPECT / CT Basic Principles Dr John C. Dickson, Principal Physicist UCLH Institute of Nuclear Medicine, University College London Hospitals and University College London john.dickson@uclh.nhs.uk
CHAPTER 9: Magnetic Susceptibility Effects in High Field MRI
 Figure 1. In the brain, the gray matter has substantially more blood vessels and capillaries than white matter. The magnified image on the right displays the rich vasculature in gray matter forming porous,
Figure 1. In the brain, the gray matter has substantially more blood vessels and capillaries than white matter. The magnified image on the right displays the rich vasculature in gray matter forming porous,
Deviceless respiratory motion correction in PET imaging exploring the potential of novel data driven strategies
 g Deviceless respiratory motion correction in PET imaging exploring the potential of novel data driven strategies Presented by Adam Kesner, Ph.D., DABR Assistant Professor, Division of Radiological Sciences,
g Deviceless respiratory motion correction in PET imaging exploring the potential of novel data driven strategies Presented by Adam Kesner, Ph.D., DABR Assistant Professor, Division of Radiological Sciences,
Magnetic Resonance Elastography (MRE) of Liver Disease
 Magnetic Resonance Elastography (MRE) of Liver Disease Authored by: Jennifer Dolan Fox, PhD VirtualScopics Inc. jennifer_fox@virtualscopics.com 1-585-249-6231 1. Overview of MRE Imaging MRE is a magnetic
Magnetic Resonance Elastography (MRE) of Liver Disease Authored by: Jennifer Dolan Fox, PhD VirtualScopics Inc. jennifer_fox@virtualscopics.com 1-585-249-6231 1. Overview of MRE Imaging MRE is a magnetic
Artefakt-resistente Bewegungsschätzung für die bewegungskompensierte CT
 Artefakt-resistente Bewegungsschätzung für die bewegungskompensierte CT Marcus Brehm 1,2, Thorsten Heußer 1, Pascal Paysan 3, Markus Oehlhafen 3, and Marc Kachelrieß 1,2 1 German Cancer Research Center
Artefakt-resistente Bewegungsschätzung für die bewegungskompensierte CT Marcus Brehm 1,2, Thorsten Heußer 1, Pascal Paysan 3, Markus Oehlhafen 3, and Marc Kachelrieß 1,2 1 German Cancer Research Center
Development and validation of clinically feasible 4D-MRI in radiotherapy
 MSc. Applied Physics Thesis Development and validation of clinically feasible 4D-MRI in radiotherapy Daniel Tekelenburg By: Daniel Ruphay Tekelenburg In partial fulfilment of the requirements for the degree
MSc. Applied Physics Thesis Development and validation of clinically feasible 4D-MRI in radiotherapy Daniel Tekelenburg By: Daniel Ruphay Tekelenburg In partial fulfilment of the requirements for the degree
8/3/2016. MR-guided RT: Commissioning and Quality Control. Topics for MR-guided RT system commissioning & QC
 MR-guided RT: Commissioning and Quality Control T. Stanescu, PhD, MCCPM Medical Physicist, Princess Margaret Cancer Centre, UHN Assistant Professor, Radiation Oncology, University of Toronto Affiliated
MR-guided RT: Commissioning and Quality Control T. Stanescu, PhD, MCCPM Medical Physicist, Princess Margaret Cancer Centre, UHN Assistant Professor, Radiation Oncology, University of Toronto Affiliated
Classification of Subject Motion for Improved Reconstruction of Dynamic Magnetic Resonance Imaging
 1 CS 9 Final Project Classification of Subject Motion for Improved Reconstruction of Dynamic Magnetic Resonance Imaging Feiyu Chen Department of Electrical Engineering ABSTRACT Subject motion is a significant
1 CS 9 Final Project Classification of Subject Motion for Improved Reconstruction of Dynamic Magnetic Resonance Imaging Feiyu Chen Department of Electrical Engineering ABSTRACT Subject motion is a significant
Design and performance characteristics of a Cone Beam CT system for Leksell Gamma Knife Icon
 Design and performance characteristics of a Cone Beam CT system for Leksell Gamma Knife Icon WHITE PAPER Introduction Introducing an image guidance system based on Cone Beam CT (CBCT) and a mask immobilization
Design and performance characteristics of a Cone Beam CT system for Leksell Gamma Knife Icon WHITE PAPER Introduction Introducing an image guidance system based on Cone Beam CT (CBCT) and a mask immobilization
ROBUST OPTIMIZATION THE END OF PTV AND THE BEGINNING OF SMART DOSE CLOUD. Moe Siddiqui, April 08, 2017
 ROBUST OPTIMIZATION THE END OF PTV AND THE BEGINNING OF SMART DOSE CLOUD Moe Siddiqui, April 08, 2017 Agenda Background IRCU 50 - Disclaimer - Uncertainties Robust optimization Use Cases Lung Robust 4D
ROBUST OPTIMIZATION THE END OF PTV AND THE BEGINNING OF SMART DOSE CLOUD Moe Siddiqui, April 08, 2017 Agenda Background IRCU 50 - Disclaimer - Uncertainties Robust optimization Use Cases Lung Robust 4D
FOREWORD TO THE SPECIAL ISSUE ON MOTION DETECTION AND COMPENSATION
 Philips J. Res. 51 (1998) 197-201 FOREWORD TO THE SPECIAL ISSUE ON MOTION DETECTION AND COMPENSATION This special issue of Philips Journalof Research includes a number of papers presented at a Philips
Philips J. Res. 51 (1998) 197-201 FOREWORD TO THE SPECIAL ISSUE ON MOTION DETECTION AND COMPENSATION This special issue of Philips Journalof Research includes a number of papers presented at a Philips
Fast Dynamic MRI for Radiotherapy
 1 Fast Dynamic MRI for Radiotherapy KE SHENG, PH.D., FAAPM DEPARTMENT OF RADIATION ONCOLOGY UNIVERSITY OF CALIFORNIA, LOS ANGELES Disclosures I receive research grants from Varian Medical Systems I am
1 Fast Dynamic MRI for Radiotherapy KE SHENG, PH.D., FAAPM DEPARTMENT OF RADIATION ONCOLOGY UNIVERSITY OF CALIFORNIA, LOS ANGELES Disclosures I receive research grants from Varian Medical Systems I am
IMSURE QA SOFTWARE FAST, PRECISE QA SOFTWARE
 QA SOFTWARE FAST, PRECISE Software for accurate and independent verification of monitor units, dose, and overall validity of standard, IMRT, VMAT, SRS and brachytherapy plans no film, no phantoms, no linac
QA SOFTWARE FAST, PRECISE Software for accurate and independent verification of monitor units, dose, and overall validity of standard, IMRT, VMAT, SRS and brachytherapy plans no film, no phantoms, no linac
G Practical Magnetic Resonance Imaging II Sackler Institute of Biomedical Sciences New York University School of Medicine. Compressed Sensing
 G16.4428 Practical Magnetic Resonance Imaging II Sackler Institute of Biomedical Sciences New York University School of Medicine Compressed Sensing Ricardo Otazo, PhD ricardo.otazo@nyumc.org Compressed
G16.4428 Practical Magnetic Resonance Imaging II Sackler Institute of Biomedical Sciences New York University School of Medicine Compressed Sensing Ricardo Otazo, PhD ricardo.otazo@nyumc.org Compressed
1. Learn to incorporate QA for surface imaging
 Hania Al-Hallaq, Ph.D. Assistant Professor Radiation Oncology The University of Chicago ***No disclosures*** 1. Learn to incorporate QA for surface imaging into current QA procedures for IGRT. 2. Understand
Hania Al-Hallaq, Ph.D. Assistant Professor Radiation Oncology The University of Chicago ***No disclosures*** 1. Learn to incorporate QA for surface imaging into current QA procedures for IGRT. 2. Understand
8/4/2016. Emerging Linac based SRS/SBRT Technologies with Modulated Arc Delivery. Disclosure. Introduction: Treatment delivery techniques
 Emerging Linac based SRS/SBRT Technologies with Modulated Arc Delivery Lei Ren, Ph.D. Duke University Medical Center 2016 AAPM 58 th annual meeting, Educational Course, Therapy Track Disclosure I have
Emerging Linac based SRS/SBRT Technologies with Modulated Arc Delivery Lei Ren, Ph.D. Duke University Medical Center 2016 AAPM 58 th annual meeting, Educational Course, Therapy Track Disclosure I have
ICARO Vienna April Implementing 3D conformal radiotherapy and IMRT in clinical practice: Recommendations of IAEA- TECDOC-1588
 ICARO Vienna April 27-29 2009 Implementing 3D conformal radiotherapy and IMRT in clinical practice: Recommendations of IAEA- TECDOC-1588 M. Saiful Huq, Ph.D., Professor and Director, Dept. of Radiation
ICARO Vienna April 27-29 2009 Implementing 3D conformal radiotherapy and IMRT in clinical practice: Recommendations of IAEA- TECDOC-1588 M. Saiful Huq, Ph.D., Professor and Director, Dept. of Radiation
(a Scrhon5 R2iwd b. P)jc%z 5. ivcr3. 1. I. ZOms Xn,s. 1E IDrAS boms. EE225E/BIOE265 Spring 2013 Principles of MRI. Assignment 8 Solutions
 EE225E/BIOE265 Spring 2013 Principles of MRI Miki Lustig Assignment 8 Solutions 1. Nishimura 7.1 P)jc%z 5 ivcr3. 1. I Due Wednesday April 10th, 2013 (a Scrhon5 R2iwd b 0 ZOms Xn,s r cx > qs 4-4 8ni6 4
EE225E/BIOE265 Spring 2013 Principles of MRI Miki Lustig Assignment 8 Solutions 1. Nishimura 7.1 P)jc%z 5 ivcr3. 1. I Due Wednesday April 10th, 2013 (a Scrhon5 R2iwd b 0 ZOms Xn,s r cx > qs 4-4 8ni6 4
Robust Lung Ventilation Assessment
 Fifth International Workshop on Pulmonary Image Analysis -75- Robust Lung Ventilation Assessment Sven Kabus 1, Tobias Klinder 1, Tokihiro Yamamoto 2, Paul J. Keall 3, Billy W. Loo, Jr. 4, and Cristian
Fifth International Workshop on Pulmonary Image Analysis -75- Robust Lung Ventilation Assessment Sven Kabus 1, Tobias Klinder 1, Tokihiro Yamamoto 2, Paul J. Keall 3, Billy W. Loo, Jr. 4, and Cristian
Spiral CT. Protocol Optimization & Quality Assurance. Ge Wang, Ph.D. Department of Radiology University of Iowa Iowa City, Iowa 52242, USA
 Spiral CT Protocol Optimization & Quality Assurance Ge Wang, Ph.D. Department of Radiology University of Iowa Iowa City, Iowa 52242, USA Spiral CT Protocol Optimization & Quality Assurance Protocol optimization
Spiral CT Protocol Optimization & Quality Assurance Ge Wang, Ph.D. Department of Radiology University of Iowa Iowa City, Iowa 52242, USA Spiral CT Protocol Optimization & Quality Assurance Protocol optimization
Module 4. K-Space Symmetry. Review. K-Space Review. K-Space Symmetry. Partial or Fractional Echo. Half or Partial Fourier HASTE
 MRES 7005 - Fast Imaging Techniques Module 4 K-Space Symmetry Review K-Space Review K-Space Symmetry Partial or Fractional Echo Half or Partial Fourier HASTE Conditions for successful reconstruction Interpolation
MRES 7005 - Fast Imaging Techniques Module 4 K-Space Symmetry Review K-Space Review K-Space Symmetry Partial or Fractional Echo Half or Partial Fourier HASTE Conditions for successful reconstruction Interpolation
Compressed Sensing for Rapid MR Imaging
 Compressed Sensing for Rapid Imaging Michael Lustig1, Juan Santos1, David Donoho2 and John Pauly1 1 Electrical Engineering Department, Stanford University 2 Statistics Department, Stanford University rapid
Compressed Sensing for Rapid Imaging Michael Lustig1, Juan Santos1, David Donoho2 and John Pauly1 1 Electrical Engineering Department, Stanford University 2 Statistics Department, Stanford University rapid
Automated Image Analysis Software for Quality Assurance of a Radiotherapy CT Simulator
 Automated Image Analysis Software for Quality Assurance of a Radiotherapy CT Simulator Andrew J Reilly Imaging Physicist Oncology Physics Edinburgh Cancer Centre Western General Hospital EDINBURGH EH4
Automated Image Analysis Software for Quality Assurance of a Radiotherapy CT Simulator Andrew J Reilly Imaging Physicist Oncology Physics Edinburgh Cancer Centre Western General Hospital EDINBURGH EH4
Patient Set-ups and Tumor Localizations
 Patient Set-ups and Tumor Localizations Amy S. Harrison Patient Positioning Prior to starting any localization or simulation procedure patients need to be positioned and immobilized Patients disease location
Patient Set-ups and Tumor Localizations Amy S. Harrison Patient Positioning Prior to starting any localization or simulation procedure patients need to be positioned and immobilized Patients disease location
UNIVERSITY OF SOUTHAMPTON
 UNIVERSITY OF SOUTHAMPTON PHYS2007W1 SEMESTER 2 EXAMINATION 2014-2015 MEDICAL PHYSICS Duration: 120 MINS (2 hours) This paper contains 10 questions. Answer all questions in Section A and only two questions
UNIVERSITY OF SOUTHAMPTON PHYS2007W1 SEMESTER 2 EXAMINATION 2014-2015 MEDICAL PHYSICS Duration: 120 MINS (2 hours) This paper contains 10 questions. Answer all questions in Section A and only two questions
Fast Imaging Trajectories: Non-Cartesian Sampling (1)
 Fast Imaging Trajectories: Non-Cartesian Sampling (1) M229 Advanced Topics in MRI Holden H. Wu, Ph.D. 2018.05.03 Department of Radiological Sciences David Geffen School of Medicine at UCLA Class Business
Fast Imaging Trajectories: Non-Cartesian Sampling (1) M229 Advanced Topics in MRI Holden H. Wu, Ph.D. 2018.05.03 Department of Radiological Sciences David Geffen School of Medicine at UCLA Class Business
Parallel Imaging. Marcin.
 Parallel Imaging Marcin m.jankiewicz@gmail.com Parallel Imaging initial thoughts Over the last 15 years, great progress in the development of pmri methods has taken place, thereby producing a multitude
Parallel Imaging Marcin m.jankiewicz@gmail.com Parallel Imaging initial thoughts Over the last 15 years, great progress in the development of pmri methods has taken place, thereby producing a multitude
Supplementary methods
 Supplementary methods This section provides additional technical details on the sample, the applied imaging and analysis steps and methods. Structural imaging Trained radiographers placed all participants
Supplementary methods This section provides additional technical details on the sample, the applied imaging and analysis steps and methods. Structural imaging Trained radiographers placed all participants
Financial disclosure. Onboard imaging modality for IGRT
 Tetrahedron Beam Computed Tomography Based On Multi-Pixel X- Ray Source and Its Application in Image Guided Radiotherapy Tiezhi Zhang, Ph.D. Advanced X-ray imaging Lab Financial disclosure Patent royalty
Tetrahedron Beam Computed Tomography Based On Multi-Pixel X- Ray Source and Its Application in Image Guided Radiotherapy Tiezhi Zhang, Ph.D. Advanced X-ray imaging Lab Financial disclosure Patent royalty
An Automated Image-based Method for Multi-Leaf Collimator Positioning Verification in Intensity Modulated Radiation Therapy
 An Automated Image-based Method for Multi-Leaf Collimator Positioning Verification in Intensity Modulated Radiation Therapy Chenyang Xu 1, Siemens Corporate Research, Inc., Princeton, NJ, USA Xiaolei Huang,
An Automated Image-based Method for Multi-Leaf Collimator Positioning Verification in Intensity Modulated Radiation Therapy Chenyang Xu 1, Siemens Corporate Research, Inc., Princeton, NJ, USA Xiaolei Huang,
GE Healthcare CLINICAL GALLERY. Discovery * MR750w 3.0T. This brochure is intended for European healthcare professionals.
 GE Healthcare CLINICAL GALLERY Discovery * MR750w 3.0T This brochure is intended for European healthcare professionals. NEURO PROPELLER delivers high resolution, motion insensitive imaging in all planes.
GE Healthcare CLINICAL GALLERY Discovery * MR750w 3.0T This brochure is intended for European healthcare professionals. NEURO PROPELLER delivers high resolution, motion insensitive imaging in all planes.
Thoracic target volume delineation using various maximum-intensity projection computed tomography image sets for stereotactic body radiation therapy
 Texas Medical Center Library DigitalCommons@TMC UT GSBS Dissertations and Theses (Open Access) Graduate School of Biomedical Sciences 8-2010 Thoracic target volume delineation using various maximum-intensity
Texas Medical Center Library DigitalCommons@TMC UT GSBS Dissertations and Theses (Open Access) Graduate School of Biomedical Sciences 8-2010 Thoracic target volume delineation using various maximum-intensity
MRI Physics II: Gradients, Imaging
 MRI Physics II: Gradients, Imaging Douglas C., Ph.D. Dept. of Biomedical Engineering University of Michigan, Ann Arbor Magnetic Fields in MRI B 0 The main magnetic field. Always on (0.5-7 T) Magnetizes
MRI Physics II: Gradients, Imaging Douglas C., Ph.D. Dept. of Biomedical Engineering University of Michigan, Ann Arbor Magnetic Fields in MRI B 0 The main magnetic field. Always on (0.5-7 T) Magnetizes
8/11/2009. Common Areas of Motion Problem. Motion Compensation Techniques and Applications. Type of Motion. What s your problem
 Common Areas of Motion Problem Motion Compensation Techniques and Applications Abdominal and cardiac imaging. Uncooperative patient, such as pediatric. Dynamic imaging and time series. Chen Lin, PhD Indiana
Common Areas of Motion Problem Motion Compensation Techniques and Applications Abdominal and cardiac imaging. Uncooperative patient, such as pediatric. Dynamic imaging and time series. Chen Lin, PhD Indiana
THE WIRELESS PHANTOM PERFORM ACCURATE PATIENT QA IN LESS TIME THAN EVER!
 THE WIRELESS PHANTOM PERFORM ACCURATE PATIENT QA IN LESS TIME THAN EVER! Confidence in complex treatments Modern radiation therapy uses complex plans with techniques such as IMRT, VMAT and Tomotherapy.
THE WIRELESS PHANTOM PERFORM ACCURATE PATIENT QA IN LESS TIME THAN EVER! Confidence in complex treatments Modern radiation therapy uses complex plans with techniques such as IMRT, VMAT and Tomotherapy.
Dynamic Autocalibrated Parallel Imaging Using Temporal GRAPPA (TGRAPPA)
 Magnetic Resonance in Medicine 53:981 985 (2005) Dynamic Autocalibrated Parallel Imaging Using Temporal GRAPPA (TGRAPPA) Felix A. Breuer, 1 * Peter Kellman, 2 Mark A. Griswold, 1 and Peter M. Jakob 1 Current
Magnetic Resonance in Medicine 53:981 985 (2005) Dynamic Autocalibrated Parallel Imaging Using Temporal GRAPPA (TGRAPPA) Felix A. Breuer, 1 * Peter Kellman, 2 Mark A. Griswold, 1 and Peter M. Jakob 1 Current
Clinical Importance. Aortic Stenosis. Aortic Regurgitation. Ultrasound vs. MRI. Carotid Artery Stenosis
 Clinical Importance Rapid cardiovascular flow quantitation using sliceselective Fourier velocity encoding with spiral readouts Valve disease affects 10% of patients with heart disease in the U.S. Most
Clinical Importance Rapid cardiovascular flow quantitation using sliceselective Fourier velocity encoding with spiral readouts Valve disease affects 10% of patients with heart disease in the U.S. Most
Intraoperative Prostate Tracking with Slice-to-Volume Registration in MR
 Intraoperative Prostate Tracking with Slice-to-Volume Registration in MR Sean Gill a, Purang Abolmaesumi a,b, Siddharth Vikal a, Parvin Mousavi a and Gabor Fichtinger a,b,* (a) School of Computing, Queen
Intraoperative Prostate Tracking with Slice-to-Volume Registration in MR Sean Gill a, Purang Abolmaesumi a,b, Siddharth Vikal a, Parvin Mousavi a and Gabor Fichtinger a,b,* (a) School of Computing, Queen
8/3/2017. Contour Assessment for Quality Assurance and Data Mining. Objective. Outline. Tom Purdie, PhD, MCCPM
 Contour Assessment for Quality Assurance and Data Mining Tom Purdie, PhD, MCCPM Objective Understand the state-of-the-art in contour assessment for quality assurance including data mining-based techniques
Contour Assessment for Quality Assurance and Data Mining Tom Purdie, PhD, MCCPM Objective Understand the state-of-the-art in contour assessment for quality assurance including data mining-based techniques
Shadow casting. What is the problem? Cone Beam Computed Tomography THE OBJECTIVES OF DIAGNOSTIC IMAGING IDEAL DIAGNOSTIC IMAGING STUDY LIMITATIONS
 Cone Beam Computed Tomography THE OBJECTIVES OF DIAGNOSTIC IMAGING Reveal pathology Reveal the anatomic truth Steven R. Singer, DDS srs2@columbia.edu IDEAL DIAGNOSTIC IMAGING STUDY Provides desired diagnostic
Cone Beam Computed Tomography THE OBJECTIVES OF DIAGNOSTIC IMAGING Reveal pathology Reveal the anatomic truth Steven R. Singer, DDS srs2@columbia.edu IDEAL DIAGNOSTIC IMAGING STUDY Provides desired diagnostic
Object Identification in Ultrasound Scans
 Object Identification in Ultrasound Scans Wits University Dec 05, 2012 Roadmap Introduction to the problem Motivation Related Work Our approach Expected Results Introduction Nowadays, imaging devices like
Object Identification in Ultrasound Scans Wits University Dec 05, 2012 Roadmap Introduction to the problem Motivation Related Work Our approach Expected Results Introduction Nowadays, imaging devices like
BME I5000: Biomedical Imaging
 1 Lucas Parra, CCNY BME I5000: Biomedical Imaging Lecture 4 Computed Tomography Lucas C. Parra, parra@ccny.cuny.edu some slides inspired by lecture notes of Andreas H. Hilscher at Columbia University.
1 Lucas Parra, CCNY BME I5000: Biomedical Imaging Lecture 4 Computed Tomography Lucas C. Parra, parra@ccny.cuny.edu some slides inspired by lecture notes of Andreas H. Hilscher at Columbia University.
New Technology in Radiation Oncology. James E. Gaiser, Ph.D. DABR Physics and Computer Planning Charlotte, NC
 New Technology in Radiation Oncology James E. Gaiser, Ph.D. DABR Physics and Computer Planning Charlotte, NC Technology s s everywhere From the imaging chain To the planning system To the linac To QA..it..it
New Technology in Radiation Oncology James E. Gaiser, Ph.D. DABR Physics and Computer Planning Charlotte, NC Technology s s everywhere From the imaging chain To the planning system To the linac To QA..it..it
Respiratory Motion Estimation using a 3D Diaphragm Model
 Respiratory Motion Estimation using a 3D Diaphragm Model Marco Bögel 1,2, Christian Riess 1,2, Andreas Maier 1, Joachim Hornegger 1, Rebecca Fahrig 2 1 Pattern Recognition Lab, FAU Erlangen-Nürnberg 2
Respiratory Motion Estimation using a 3D Diaphragm Model Marco Bögel 1,2, Christian Riess 1,2, Andreas Maier 1, Joachim Hornegger 1, Rebecca Fahrig 2 1 Pattern Recognition Lab, FAU Erlangen-Nürnberg 2
Using a research real-time control interface to go beyond dynamic MLC tracking
 in partnership with Using a research real-time control interface to go beyond dynamic MLC tracking Dr. Simeon Nill Joint Department of Physics at The Institute of Cancer Research and the Royal Marsden
in partnership with Using a research real-time control interface to go beyond dynamic MLC tracking Dr. Simeon Nill Joint Department of Physics at The Institute of Cancer Research and the Royal Marsden
RADIOMICS: potential role in the clinics and challenges
 27 giugno 2018 Dipartimento di Fisica Università degli Studi di Milano RADIOMICS: potential role in the clinics and challenges Dr. Francesca Botta Medical Physicist Istituto Europeo di Oncologia (Milano)
27 giugno 2018 Dipartimento di Fisica Università degli Studi di Milano RADIOMICS: potential role in the clinics and challenges Dr. Francesca Botta Medical Physicist Istituto Europeo di Oncologia (Milano)
The team. Disclosures. Ultrasound Guidance During Radiation Delivery: Confronting the Treatment Interference Challenge.
 Ultrasound Guidance During Radiation Delivery: Confronting the Treatment Interference Challenge Dimitre Hristov Radiation Oncology Stanford University The team Renhui Gong 1 Magdalena Bazalova-Carter 1
Ultrasound Guidance During Radiation Delivery: Confronting the Treatment Interference Challenge Dimitre Hristov Radiation Oncology Stanford University The team Renhui Gong 1 Magdalena Bazalova-Carter 1
M R I Physics Course
 M R I Physics Course Multichannel Technology & Parallel Imaging Nathan Yanasak, Ph.D. Jerry Allison Ph.D. Tom Lavin, B.S. Department of Radiology Medical College of Georgia References: 1) The Physics of
M R I Physics Course Multichannel Technology & Parallel Imaging Nathan Yanasak, Ph.D. Jerry Allison Ph.D. Tom Lavin, B.S. Department of Radiology Medical College of Georgia References: 1) The Physics of
Advanced Targeting Using Image Deformation. Justin Keister, MS DABR Aurora Health Care Kenosha, WI
 Advanced Targeting Using Image Deformation Justin Keister, MS DABR Aurora Health Care Kenosha, WI History of Targeting The advance of IMRT and CT simulation has changed how targets are identified in radiation
Advanced Targeting Using Image Deformation Justin Keister, MS DABR Aurora Health Care Kenosha, WI History of Targeting The advance of IMRT and CT simulation has changed how targets are identified in radiation
Novel methods and tools for MR Commissioning and Quality Control
 Novel methods and tools for MR Commissioning and Quality Control T. Stanescu, PhD, MCCPM Medical Physicist, RMP, Princess Margaret Cancer Centre Assistant Professor, Radiation Oncology, University of Toronto
Novel methods and tools for MR Commissioning and Quality Control T. Stanescu, PhD, MCCPM Medical Physicist, RMP, Princess Margaret Cancer Centre Assistant Professor, Radiation Oncology, University of Toronto
IMRT site-specific procedure: Prostate (CHHiP)
 IMRT site-specific procedure: Prostate (CHHiP) Scope: To provide site specific instructions for the planning of CHHIP IMRT patients Responsibilities: Radiotherapy Physicists, HPC Registered Therapy Radiographers
IMRT site-specific procedure: Prostate (CHHiP) Scope: To provide site specific instructions for the planning of CHHIP IMRT patients Responsibilities: Radiotherapy Physicists, HPC Registered Therapy Radiographers
PURE. ViSION Edition PET/CT. Patient Comfort Put First.
 PURE ViSION Edition PET/CT Patient Comfort Put First. 2 System features that put patient comfort and safety first. Oncology patients deserve the highest levels of safety and comfort during scans. Our Celesteion
PURE ViSION Edition PET/CT Patient Comfort Put First. 2 System features that put patient comfort and safety first. Oncology patients deserve the highest levels of safety and comfort during scans. Our Celesteion
Tomographic Reconstruction
 Tomographic Reconstruction 3D Image Processing Torsten Möller Reading Gonzales + Woods, Chapter 5.11 2 Overview Physics History Reconstruction basic idea Radon transform Fourier-Slice theorem (Parallel-beam)
Tomographic Reconstruction 3D Image Processing Torsten Möller Reading Gonzales + Woods, Chapter 5.11 2 Overview Physics History Reconstruction basic idea Radon transform Fourier-Slice theorem (Parallel-beam)
C a t p h a n / T h e P h a n t o m L a b o r a t o r y
 C a t p h a n 5 0 0 / 6 0 0 T h e P h a n t o m L a b o r a t o r y C a t p h a n 5 0 0 / 6 0 0 Internationally recognized for measuring the maximum obtainable performance of axial, spiral and multi-slice
C a t p h a n 5 0 0 / 6 0 0 T h e P h a n t o m L a b o r a t o r y C a t p h a n 5 0 0 / 6 0 0 Internationally recognized for measuring the maximum obtainable performance of axial, spiral and multi-slice
MRI Hot Topics Motion Correction for MR Imaging. medical
 MRI Hot Topics Motion Correction for MR Imaging s medical Motion Correction for MR Imaging Kyle A. Salem, PhD Motion Artifacts in a Clinical Setting Patient motion is probably the most common cause of
MRI Hot Topics Motion Correction for MR Imaging s medical Motion Correction for MR Imaging Kyle A. Salem, PhD Motion Artifacts in a Clinical Setting Patient motion is probably the most common cause of
A Novel Iterative Thresholding Algorithm for Compressed Sensing Reconstruction of Quantitative MRI Parameters from Insufficient Data
 A Novel Iterative Thresholding Algorithm for Compressed Sensing Reconstruction of Quantitative MRI Parameters from Insufficient Data Alexey Samsonov, Julia Velikina Departments of Radiology and Medical
A Novel Iterative Thresholding Algorithm for Compressed Sensing Reconstruction of Quantitative MRI Parameters from Insufficient Data Alexey Samsonov, Julia Velikina Departments of Radiology and Medical
Advanced Imaging Trajectories
 Advanced Imaging Trajectories Cartesian EPI Spiral Radial Projection 1 Radial and Projection Imaging Sample spokes Radial out : from k=0 to kmax Projection: from -kmax to kmax Trajectory design considerations
Advanced Imaging Trajectories Cartesian EPI Spiral Radial Projection 1 Radial and Projection Imaging Sample spokes Radial out : from k=0 to kmax Projection: from -kmax to kmax Trajectory design considerations
Ch. 4 Physical Principles of CT
 Ch. 4 Physical Principles of CT CLRS 408: Intro to CT Department of Radiation Sciences Review: Why CT? Solution for radiography/tomography limitations Superimposition of structures Distinguishing between
Ch. 4 Physical Principles of CT CLRS 408: Intro to CT Department of Radiation Sciences Review: Why CT? Solution for radiography/tomography limitations Superimposition of structures Distinguishing between
Sparse sampling in MRI: From basic theory to clinical application. R. Marc Lebel, PhD Department of Electrical Engineering Department of Radiology
 Sparse sampling in MRI: From basic theory to clinical application R. Marc Lebel, PhD Department of Electrical Engineering Department of Radiology Objective Provide an intuitive overview of compressed sensing
Sparse sampling in MRI: From basic theory to clinical application R. Marc Lebel, PhD Department of Electrical Engineering Department of Radiology Objective Provide an intuitive overview of compressed sensing
Coverage based treatment planning to accommodate organ deformable motions and contouring uncertainties for prostate treatment. Huijun Xu, Ph.D.
 Coverage based treatment planning to accommodate organ deformable motions and contouring uncertainties for prostate treatment Huijun Xu, Ph.D. Acknowledgement and Disclosure Dr. Jeffrey Siebers Dr. DJ
Coverage based treatment planning to accommodate organ deformable motions and contouring uncertainties for prostate treatment Huijun Xu, Ph.D. Acknowledgement and Disclosure Dr. Jeffrey Siebers Dr. DJ
Photon beam dose distributions in 2D
 Photon beam dose distributions in 2D Sastry Vedam PhD DABR Introduction to Medical Physics III: Therapy Spring 2014 Acknowledgments! Narayan Sahoo PhD! Richard G Lane (Late) PhD 1 Overview! Evaluation
Photon beam dose distributions in 2D Sastry Vedam PhD DABR Introduction to Medical Physics III: Therapy Spring 2014 Acknowledgments! Narayan Sahoo PhD! Richard G Lane (Late) PhD 1 Overview! Evaluation
Comprehensive treatment planning for brachytherapy. Advanced planning made easy
 Comprehensive treatment planning for brachytherapy Advanced planning made easy Oncentra Brachy offers a variety of smart tools that facilitate many of the repetitive tasks for you. In contemporary brachytherapy,
Comprehensive treatment planning for brachytherapy Advanced planning made easy Oncentra Brachy offers a variety of smart tools that facilitate many of the repetitive tasks for you. In contemporary brachytherapy,
Motion artifact detection in four-dimensional computed tomography images
 Motion artifact detection in four-dimensional computed tomography images G Bouilhol 1,, M Ayadi, R Pinho, S Rit 1, and D Sarrut 1, 1 University of Lyon, CREATIS; CNRS UMR 5; Inserm U144; INSA-Lyon; University
Motion artifact detection in four-dimensional computed tomography images G Bouilhol 1,, M Ayadi, R Pinho, S Rit 1, and D Sarrut 1, 1 University of Lyon, CREATIS; CNRS UMR 5; Inserm U144; INSA-Lyon; University
IMRT and VMAT Patient Specific QA Using 2D and 3D Detector Arrays
 IMRT and VMAT Patient Specific QA Using 2D and 3D Detector Arrays Sotiri Stathakis Outline Why IMRT/VMAT QA AAPM TG218 UPDATE Tolerance Limits and Methodologies for IMRT Verification QA Common sources
IMRT and VMAT Patient Specific QA Using 2D and 3D Detector Arrays Sotiri Stathakis Outline Why IMRT/VMAT QA AAPM TG218 UPDATE Tolerance Limits and Methodologies for IMRT Verification QA Common sources
GE Healthcare. Agile Ultrasound. The Next Revolution in Ultrasound Imaging
 Agile Ultrasound The Next Revolution in Ultrasound Imaging Abstract Diagnostic use of ultrasound has greatly expanded over the past couple of decades because it offers many advantages as an imaging modality.
Agile Ultrasound The Next Revolution in Ultrasound Imaging Abstract Diagnostic use of ultrasound has greatly expanded over the past couple of decades because it offers many advantages as an imaging modality.
Compressed Sensing Reconstructions for Dynamic Contrast Enhanced MRI
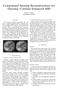 1 Compressed Sensing Reconstructions for Dynamic Contrast Enhanced MRI Kevin T. Looby klooby@stanford.edu ABSTRACT The temporal resolution necessary for dynamic contrast enhanced (DCE) magnetic resonance
1 Compressed Sensing Reconstructions for Dynamic Contrast Enhanced MRI Kevin T. Looby klooby@stanford.edu ABSTRACT The temporal resolution necessary for dynamic contrast enhanced (DCE) magnetic resonance
