HHS Public Access Author manuscript Comput Vis Image Underst. Author manuscript; available in PMC 2017 October 01.
|
|
|
- Vernon Noah Lawrence
- 5 years ago
- Views:
Transcription
1 Modeling 4D Pathological Changes by Leveraging Normative Models Bo Wang a,b,, Marcel Prastawa c, Andrei Irimia d, Avishek Saha e, Wei Liu a,b, S.Y. Matthew Goh d, Paul M. Vespa f, John D. Van Horn d, and Guido Gerig g a Scientific Computing and Imaging Institute, University of Utah, 72 Central Campus Drive, Salt Lake City, UT USA b School of Computing, University of Utah, 50 S., Central Campus Drive, Salt Lake City, UT USA c Icahn School of Medicine at Mount Sinai, 1468 Madison Avenue, New York, NY USA d The Institute for Neuroimaging and Informatics, Keck School of Medicine, University of Southern California, 2001 North Soto Street, Los Angeles CA USA e Yahoo Labs, 701 1st Ave, Sunnyvale, CA USA f Brain Injury Research Center, Department of Neurosurgery, David Geffen School of Medicine, University of California, Los Angeles, CA USA g Tandon School of Engineering, Department of Computer Science and Engineering, NYU, USA Abstract HHS Public Access Author manuscript Published in final edited form as: Comput Vis Image Underst October ; 151: doi: /j.cviu With the increasing use of efficient multimodal 3D imaging, clinicians are able to access longitudinal imaging to stage pathological diseases, to monitor the efficacy of therapeutic interventions, or to assess and quantify rehabilitation efforts. Analysis of such four-dimensional (4D) image data presenting pathologies, including disappearing and newly appearing lesions, represents a significant challenge due to the presence of complex spatio-temporal changes. Image analysis methods for such 4D image data have to include not only a concept for joint segmentation of 3D datasets to account for inherent correlations of subject-specific repeated scans but also a mechanism to account for large deformations and the destruction and formation of lesions (e.g., edema, bleeding) due to underlying physiological processes associated with damage, intervention, and recovery. In this paper, we propose a novel framework that provides a joint segmentation-registration framework to tackle the inherent problem of image registration in the presence of objects not present in all images of the time series. Our methodology models 4D changes in pathological anatomy across time and and also provides an explicit mapping of a healthy normative template to a subject s image data with pathologies. Since atlas-moderated segmentation methods cannot Corresponding author. bowang@cs.utah.edu (Bo Wang a,b ). Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
2 Wang et al. Page 2 explain appearance and locality pathological structures that are not represented in the template atlas, the new framework provides different options for initialization via a supervised learning approach, iterative semisupervised active learning, and also transfer learning, which results in a fully automatic 4D segmentation method. We demonstrate the effectiveness of our novel approach with synthetic experiments and a 4D multimodal MRI dataset of severe traumatic brain injury (TBI), including validation via comparison to expert segmentations. However, the proposed methodology is generic in regard to different clinical applications requiring quantitative analysis of 4D imaging representing spatiotemporal changes of pathologies. Keywords Image segmentation; brain parcellation; medical imaging 1. Introduction Quantitative studies in longitudinal image data from traumatic brain injury (TBI), autism, and Huntington s disease, for example, are important for assessment of treatment efficacy, monitoring disease progression, or making predictions on outcome. In this paper, we will use the term pathological anatomy to indicate image data that represent pathologies and lesions and therefore differ from normative anatomical templates that encode healthy anatomy. The modeling of four-dimensional (4D) pathological anatomy is essential to understand the complex dynamics of pathologies and enables other analyses such as structural pathology (Irimia et al., 2011) and brain connectivity (Irimia et al., 2012). Modeling pathological changes is a challenging task because of the difficulties in localizing multiple lesions at specific time points and estimating deformations across time points with changing lesion patterns. Such modeling involves solving interdependent segmentation and image registration since registration needs to know about areas of pathology but 4D segmentation requires an intrasubject mapping across time points. Furthermore, subject-specific registration across time points is not sufficient to segment pathological anatomy if we follow the well-established concept of atlas-driven segmentation, which includes probabilistic tissue priors for a Bayesian maximum posterior classification. Such a scheme requires an explicit registration from a normative template atlas to each subject s image time point, with the additional advantage of using anatomical labeling of subregions as used in brain connectivity analysis, for example. The main goal of this paper therefore is the development of a new 4D pathological anatomy modeling framework that simultaneously solves segmentation of each time point and also estimates nonlinear deformations to a normative atlas space and across time points. We will use severe TBI as our clinical driving problem to demonstrate the different components of the novel method and to validate results in comparison to human expert segmentations. TBI is not only a critical problem in healthcare that impacts approximately 1.7 million people in the United States every year (Faul et al., 2010), but also one of the most challenging tasks for quantitative image-based interpretation and analysis. The varying causes (falls, car
3 Wang et al. Page 3 accidents, etc.) and degrees of TBI present highly heterogeneous multifocal patterns of lesions with largely variable morphometry. Spatio-temporal analysis of serial TBI imaging is motivated by the clinical need for improved insight and quantitative data from therapeutic intervention and rehabilitation that change brain neuroanatomy and function with a reduction/cessation of symptoms. 2. Related Work / Previous Work 4D pathological anatomy modeling is closely related to longitudinal image analysis, a research area of increasing interest to the scientific community due to the availability and use of longitudinal imaging in medical research and clinical practice. Researchers have proposed different methods for longitudinal image/shape analysis (Datar et al., 2009; Fletcher, 2012; Niethammer et al., 2011b; Fishbaugh et al., 2013; Hong et al., 2012). These image regression methods were developed for image data resembling normal anatomy with small-scale temporal deformations. However, these methods are not designed for pathological anatomy presenting nondiffeomorphic deformations and topological changes due to spatio-temporal lesion evolution. In the following sections, we discuss previous work related to pathological brain MR image analysis, organized by the type of problem to be solved D/4D Segmentation One class of work focuses on 3D volume data segmentation (Menze et al., 2010; Geremia et al., 2011; Bauer et al., 2011; Gao et al., 2012; Ledig et al., 2015). Menze et al. (2010) presented a generative model for brain tumor segmentation using multimodal MR images. Geremia et al. (2011) proposed to use random forests for automatic segmentation of multiple sclerosis (MS) lesions in multimodal MR images. Bauer et al. (2011) presented an automatic segmentation method based on support vector machine classification with smoothness constraints. Gao et al. (2012) developed a robust statistics-based interactive multiobject segmentation tool. Ledig et al. (2015) proposed a method based on Gaussian mixture modeling (GMM) with patch-based spatially and temporally varying constraints to enforce temporal coupling. All these methods use different classifiers with different image-derived features as input or even incorporate temporal information for pixel classification, but they are not designed for longitudinal pathological anatomy studies due to the lack of intra- and intersubject registration. Atlas-based segmentation has been demonstrated to be a powerful solution in the case of segmenting healthy or normal-looking brain MR images (Leemput et al., 1999). In scenarios where pathological structures are present in the subject image but not in the atlas, this method could be extended to a GMM-based approach where an affine-registered atlas and user initialization are combined (Prastawa et al., 2003) Intrasubject registration A second class of methods is concerned with registration across time points (Chitphakdithai and Duncan, 2010; Niethammer et al., 2011a; Ou et al., 2011; Lou et al., 2013). Chitphakdithai and Duncan (2010) proposed a registration method accommodating the
4 Wang et al. Page 4 missing correspondences for pre-operative and post-resection brain images. Niethammer et al. (2011a) presented a registration framework for TBI images using geometric metamorphosis that maps TBI over time using known, presegmented lesion boundaries defined manually. Ou et al. (2011) proposed a generic deformable registration method using attribute matching and mutual-saliency weighting. Lou et al. (2013) presented a deformable registration method for intra-time point multimodal image registration. However, these image-registration methods describe deformations but do not provide segmentation of anatomical structures Pathological anatomy growth model A third class of methods focuses on modeling the growth of pathological anatomy such as tumor or glioma (Kyriacou et al., 1999; Cuadra et al., 2004; Zacharaki et al., 2008; Menze et al., 2011; Gooya et al., 2012). Kyriacou et al. (1999) proposed a finite element-based biomechanical model for registering a normal atlas to a patient image. Cuadra et al. (2004) developed an atlas-based segmentation method using a lesion growth model. Zacharaki et al. (2008) proposed a multiresolution method that utilized a principal component analysis (PCA)-based model of tumor growth for deformable registration of brain tumor images. Gooya et al. (2012) presented a segmentation and registration method for MR images of glioma patients using a tumor growth model. Menze et al. (2011) proposed a generative model of tumor growth and image appearance that relies on modeling the pathophysiological process and data likelihood. These methods provide a segmentation of pathological anatomy or mapping from an atlas to the pathological data but do not include intrasubject registration for a full 4D modeling of healthy and pathological structures Joint segmentation and registration A fourth class of methods is joint segmentation and registration (Pohl et al., 2006; Parisot et al., 2014; Kwon et al., 2014; Liu et al., 2014). Pohl et al. (2006) proposed a Bayesian framework for joint segmentation and registration for normal brain MR images. Parisot et al. (2014) proposed a tumor segmentation and registration method to solve brain tumor segmentation and atlas to patient image registration simultaneously by using a Markov random field model with a sparse grid. Kwon et al. (2014) developed a deformable tumor registration tool for preoperative and postrecurrence brain MR scans. Liu et al. (2014) proposed a low-rank image decomposition-based atlas registration method to map a healthy brain atlas to a patient image. These methods approach problems similar to those discussed in this paper but focus on one subproblem such as atlas registration (Liu et al., 2014), intrasubject registration (Kwon et al., 2014), and tumor segmentation with atlas registration (Parisot et al., 2014) rather than a combined, joint framework Novel methodological framework Although significant progress has been demonstrated in various aspects of methods for longitudinal pathological image analysis, we propose a solution that includes the remaining open issues related to multimodal image analysis and longitudinal modeling. The processing framework includes explicit mapping from a normative probabilistic template representing healthy anatomy to each image of the subject s image time series and multimodal segmentation and extends early work (Wang et al., 2013b) in which we focused on a
5 Wang et al. Page 5 3. Method pathological anatomy modeling framework with transfer learning-based image appearance model estimation by adding user initialization and user interaction as alternative approaches to estimate the image appearance model. We also present a study of manual expert annotation comparison by having three human experts perform manual segmentation using the latest version of ITK-SNAP (Yushkevich et al., 2006), which is to our knowledge the only tool available to take multimodal data as input for performing manual segmentation Motivation and challenges Image registration schemes often assume preservation of topology and point-to-point correspondences and therefore apply diffeomorphic registration schemes, assumptions that are clearly violated in the presence of newly appearing and disappearing lesion patterns. Fig. 1 shows a toy example with two classes of changes between different time points. The first class of changes is described by the expanding or shrinking of existing structures, which is simply geometric change. The second class of changes is given by disappearing or newly appearing structures, resulting in changes of topology D modeling of pathological anatomy The previous example illustrates the core motivation for the proposed methodology, which is a concept to provide nonlinear registration and 4D segmentation in the presence of changes in topology. We propose a processing scheme that constructs 4D models of pathological anatomy including a prior from a normative template and describes changes at different time points to provide a complete 4D segmentation of normal tissue and pathologies. Fig. 2 shows a conceptual overview of our framework. We model anatomical changes over time as a combination of diffeomorphic image deformation and nondiffeomorphic changes of probabilities for lesion categories, accounting for temporally smooth deformations and abrupt changes due to lesions appearing and disappearing over time, for example. Specifically, the spatial prior point t is modeled as (1) for each class c at time where A is the tissue class probability that is initially associated with the healthy template, φ t is the diffeomorphic deformation from time to the atlas, and Q t is the nondiffeomorphic probabilistic change for time t. The method estimates a common subject-specific atlas A for all time points. Fig. 3 shows a conceptual view of the two components. Given the model and 4D multimodal images I t at time points t, we estimate model parameters that minimize the following functional:
6 Wang et al. Page 6 where F represents the data functional (the negative total log-likelihood) and R represents the regularity terms: T denotes the number of observed time points, C denotes the number of tissue classes, p(i t c, ) is the image likelihood function for class c with parameter, A (0) is the initial atlas A obtained from the healthy template, and d(id, ) is the distance to the identity transform. R 1 enforces the sparsity of Q, R 2 prevents extreme deviations in A from the initial model, and R 3 enforces the smoothness of the deformations φ t. These regularization functionals are weighted by user-defined parameters α, β, γ, respectively. The model parameters {A, φ t, Q t } are obtained via gradient descent and the initial image likelihoods of the multimodal input image data, p(i c, t ) are obtained via an initialization scheme as discussed later. We use multimodal image intensities as our feature vector, I (x) t = [I 1 (x), I 2 (x),, I k (x)]. t 3.3. Image appearance model Given multimodal images I t = {I(x 1 ),, I(x N )} t at time point t with N voxels indexed by positions x and M t number of channels, we estimate the parameters of p(i c, t maximize the log-likelihood function for each time point t as where C t is the number of classes at time point t, and time t. (5) (3) ) that (2) is the spatial prior for class c at In order to estimate the image appearance model, we provide flexible choices for initialization via different machine learning approaches (see section 3.5). Depending on the machine learning approaches we use for image appearance modeling, p(i t (x) ) is either p(i t (x) ), the multivariate Gaussian probability distribution with mean and (4)
7 Wang et al. Page 7 covariance, for user initialization (3.5.1) and iterative user interaction (3.5.2), or the kernel density model parameterized by the kernel bandwidths for each class θ t = {h c=1,, h c=c } for transfer learning (3.5.3) Model parameter estimation We perform model parameter estimation by minimizing the overall objective function (Eq. 2) with respect to each parameter using alternating gradient descent. In particular, we use these gradient equations to optimize the data functional F: where Dφ denotes the determinant of the Jacobian of φ. The updates show that Q t moves to the data likelihood specific to time t, A moves to the average data likelihood over time, and φ t deforms A to match the boundaries between data and atlas. Please refer to Appendix A for the derivations of Equations 6-8. Constraints are enforced using projected gradient descent (Prastawa et al., 2012)). The image likelihood model p(i t c, (6) (8) (7) ) obtained from the image appearance model is fitted to the input image data using gradient descent update, which finds the parameter 1. that best matches data to the current atlas. The algorithm for model parameter estimation is shown in Algorithm
8 Wang et al. Page Machine learning-based image appearance model estimation Standard atlas-based methods are not suitable to estimate the image appearance model for pathological data since the lesion locations and appearance are not represented in the atlas. For example, standard normative brain atlases contain priors of white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) but cannot explain lesions such as edema, necrosis, and bleeding. The method proposed here includes the segmentation and modeling of pathology but requires an initialization of the multimodal appearance parameters. We propose several options for such initialization that may be suitable for different scenarios and application domains. Semisupervised machine learning methods offer semiautomatic solutions by asking the user to provide training data through initialization or interaction. Transfer learning offers a fully automatic solution by leveraging labeled data in one domain to learn a classifier for data in another domain where training data is not available. We discuss a supervised learning method in Subsection 3.5.1, a semisupervised learning method in Subsection 3.5.2, and a transfer learning method in Subsection User initialization A common procedure for supervised classification is to let a user define regions of interest of the different types of pathology categories in an affineregistered normative atlas, for example by drawing spherical regions for lesion types (Prastawa et al., 2004; Wang et al., 2012). The expert input, number of lesion types, and an affine-registered atlas are then used to initialize the parameters of the multivariate Gaussian probability distribution ( and ), and spatial prior via these user-input spheres S t for each tissue/lesion class c = 1,, k t, k l,, K where c = 1,, k t are normal tissue classes and c = k l,, K are lesion classes. We use the K-means algorithm (Lloyd, 1982) to get initial estimates and for each lesion class c = k l,, K. For normal tissue classes c = 1,, k t, we use the affine-registered atlas (masked by user input S t ) to estimate and. The initial priors for each class are obtained by modifying the standard atlas using S t. We assume that lesions are found in a subset of normal tissue classes L c = 1,, k t, which depends on the specific applications, so that the initial prior for each lesion class becomes chosen to be a small value (0.001 for example). The transformed to ensure that, for c = k l,, K, where w is a uniform weight for lesions at each location. of other classes are linearly Iterative user interaction: ActiveCut A second way to estimate the image appearance model is to use iterative user interaction following an ActiveCut scheme (Wang et al., 2014). Given a user-defined bounding box, the hard-constraint map is set to normal outside the box and uncertain inside. Then, we initialize an α map such that α = 1 (lesion) in the uncertain region of the hard-constraint, and α = 0 elsewhere. The expectationmaximization (EM) algorithm learns θ of both foreground and background GMMs given α, and then a graph-cut step (Rother et al., 2004) updates only the α for voxels within the uncertain region. This update strategy of graph-cuts prevents false-positive detections. Upon EM and graph-cuts convergence, there may be some false-negative voxels in normal
9 Wang et al. Page 9 regions. We group these voxels into spatially coherent objects and find the one with the highest score computed from certain criteria. The algorithm either automatically adds the candidate to lesions or queries the user for an answer, depending on its confidence in the candidate. We then update the hard-constraint map to reflect the knowledge learned from the new object, and a new EM and graph-cuts iteration starts. Objects already accepted or rejected in previous steps are recorded so they will be excluded from future queries. This learning process repeats until the user stops the algorithm, and the image appearance parameters are then used to initialize to the 4D segmentation scheme as discussed previously Transfer learning A third approach is the use of transfer learning following Wang et al. (2013b), a procedure that does not require any user input and thus results in a fully automatic processing scheme. We compute the image appearance model p(i c, t ) using domain adaptation, where we adapt an appearance model from a known domain (here tumor image database with labelled data) to the input domain (TBI images). We use a simulator (Prastawa et al., 2009) to generate a large collection of synthetic tumor images that resemble TBI images, and make use of the rich information in this database to automatically compute the likelihood density models, which are then transferred to the TBI domain. We select a tumor image from the database that has the smallest earth mover s distance (Pele and Werman, 2009) compared to the input TBI images. We then obtain training samples in the known or source domain as a subset of the completely segmented tumor data, with representing the tumor intensities, representing the discrete segmentations, representing the probabilistic segmentations, and representing the coordinates in the tumor image domain. The transfer of learned appearance models is accomplished via domain adaptation that incorporates importance weighting. We weight intensity observations I using the weights with being the density in the source domain. In practice, w is estimated using KLIEP (Kullback-Leibler Importance Estimation Procedure) (Sugiyama et al., 2008), which minimizes the Kullback-Leibler divergence between the density of the input domain and the weighted density of the source domain KL(p(I) w(i) (I)). Fig. 4 shows a toy example of applying KLIEP to estimate the weight. We observe the estimated weights are close to the true weights. Using the estimated weights w, we compute the density parameter that maximizes the data likelihood in the tumor domain: We use the kernel density model for the image appearance, parameterized by the kernel bandwidths for each class. The image likelihood in the TBI domain is modeled in the same fashion, where we initialize the TBI parameter θ using the domain adapted tumor parameter.
10 Wang et al. Page Results 4.1. Synthetic data analysis Proof of concept of the new method is based on a synthetic longitudinal multimodal image data with two time points with pathologies. We simulate images with 4 channels (Ch1 to Ch4) and 5 classes (C1 to C5) with class 4 and 5 mimicking behavior of two different new classes that are not represented in the atlas. In order to simulate the topological change problem, we create two types of changes of the fourth and fifth classes (C4 and C5). At the top of both time points, we simulate a deforming structure that changes shape. In the left of the images of time 1 and the bottom of the images of time 2, we simulate disappearing and newly appearing structures. Gaussian noise is added to the synthetic images for the purpose of simulation. In addition to this longitudinal synthetic dataset, we also create a normative atlas that contains the pair of multimodal images and priors for the first three classes (C1 - C3) (Fig. 5). We use the simulated dataset to illustrate the mechanism of the proposed algorithm, shown in Fig. 6. We observe that the initial estimates of posterior probabilities at each time point (P t=1 and P t=2 ) look like the mixture of the original atlas and data likelihood at the specific time point. The final estimation of the posteriors P t=1 and P t=2 becomes much more similar to the data likelihood at the specific time points, illustrating that the proposed method is able to account for topological changes across time although lesions are not modeled in the normative template Model parameter analysis We apply our framework to multimodal image data of three TBI subjects. Each subject was scanned at two time points: an acute scan at 3 days and a chronic scan 6 months later. The image data of each subject include T1, T2, FLAIR, and GRE modalities. Acute and chronic images of Subject I are shown in Fig. 7 where nonhemorrhagic lesions (edema / swelling) are shown as hyperintense regions in FLAIR while hemorrhagic lesions (bleeding) are shown as hypointense regions in T2 and GRE. The estimated 4D spatial priors for TBI Subject II are illustrated in Fig. 8, incorporating template deformation to match image boundaries and nondiffeomorphic changes due to lesions. Subject II provides an interesting example of the complexity of longitudinal pathology progression. The acute scan reveals gross pathology in the left frontal region, which results in considerable atrophy in this region at the chronic stage. However, the subject s chronic scan features an additional large lesion in the mid-frontal region due to the occurrence of a large abscess between the acute and chronic scans. This is an excellent example of the dynamic and complex longitudinal changes that can occur in TBI patients Qualitative and quantitative evaluation of manual segmentation Validation has been performed by a comparison of our results to expert segmentations. We asked three experts familiar with TBI lesion appearance to perform manual annotation using ITK-SNAP (Yushkevich et al., 2006), a new tool that provides simultaneous display of
11 Wang et al. Page 11 multimodality data along with user-guided 2D and 3D segmentation. We also explored the variability of human experts via qualitative and quantitative comparison. Fig. 9 shows the axial view of edema manual segmentation of three human experts for the acute time point data of one subject. We clearly observe that the manual segmentations of three human experts show some variability regarding lesion boundaries but also the number of lesions. The manual segmentations of the first two rows cover the major site of edema; however, the result in the third row includes two additional lesions apart from the major site (top-left and middle-right in the third row images). Moreover, even if the results of the first two rows look similar, the volumes are significantly different. Fig. 10 shows the acute lesion volume averages of three subjects with standard deviation bars. We observe a relatively large standard deviation that reflects the significant variability and disagreement of manual segmentation by different human experts. These qualitative and quantitative results provide evidence that TBI lesion segmentation is a difficult task for experts even using new tools that offer multimodality displays Qualitative and quantitative evaluation of automatic segmentation As introduced in the method section, we provide three possibilities for initialization of image appearance models: user initialization via regions of interest, iterative user interaction (ActiveCut), and fully automatic transfer learning. In order to illustrate the performance of the proposed method, we compare our method to 1) appearance model estimation without transfer learning, which uses the same training dataset as our method of learning with transfer learning; 2) appearance model learning with transfer learning but without an atlas; 3) the GrabCut segmentation without iterative updates but allowing the foreground to be segmented as background classes. The three methods are used as the baseline algorithms to compare with our three image appearance model learning methods. Since we have three independent manual segmentations for each subject, each human expert s manual segmentations are used to compare with the segmentation results of different methods. We validate the performance of different methods by comparing the segmentation to the selected manual segmentation. We use the Dice coefficient as our comparison metric, which measures the volumetric overlap of two binary segmentations and lies in [0, 1]. Table 1 shows the comparisons of the results of our methods and three baseline algorithms against the three independent manual segmentations. The Dice coefficient values are relatively low due to the complex shapes of the small lesions, but the importance is the difference between the baseline methods and ours. In general, we observe that the performance of our method varies with different image appearance model estimations. The Dice values increase from fully automatic transfer learning-based initialization to semiautomatic user initialization, to active learning-based user interaction. The growth of Dice values is reasonable as the algorithm gets increasingly better training data. We may not conclude that the transfer learning-based initialization would be poor since it provides a fully automatic solution with the cost that the segmentation accuracy may be less optimal. On the other hand, even if the result of the active learning-based method is the best in terms of segmentation accuracy, it requires users to do iterative interaction for each dataset. Such a
12 Wang et al. Page 12 procedure may be appropriate for a small sample set but may not be suitable for large population studies. Fig. 11 shows the qualitative comparison between GrabCut and our active learning-based method (ActiveCut). Without user interaction, the baseline algorithm detected a large number of false-positive voxels due to the ambiguity between lesion and non-brain tissue or fluid Result of brain parcellation 5. Conclusions The proposed method has the advantage of providing a mapping from a normative template to a specific subject. In Fig. 12, we show the brain parcellation label image, provided by the International Consortium for Brain Mapping (ICBM), mapped to the acute (first row) and chronic (second row) time points of one TBI subject using affine registration and our method. We observe that our method produces more accurate mapping from healthy brain atlas to individual time points of a TBI subject. In Fig. 13, we illustrate another result of mapping the ICBM parcellation label image to a TBI subject with both 2D and 3D visualizations. The mapping of a normal anatomy to a pathological anatomy will be potentially important to compare type, locality, and spatial extent of brain damage in the context of anatomically relevant regions with associated brain function information. We have presented a new framework that models 4D changes in pathological anatomy over time and provides explicit deformable mapping from a healthy template to subjects with pathology. The novel idea for registration of images with lesions is the estimation of spatial class priors as a combination of diffeomorphic atlas deformation and nondiffeomorphic changes of lesion probabilities. This concept enables intrasubject registration of longitudinal images with spatially and temporally varying lesion patterns but also a mapping of a normative atlas to subject images. To overcome the short-comings of atlas-based segmentation, which cannot be applied to images with new structures that are not represented in the template, our framework uses semisupervised learning approaches to get training data for Gaussian mixture models by user initialization or active learning-based user interaction. We also discuss the use of a transfer learning method that results in a fully automatic 4D segmentation method. The feasibility of our approach is demonstrated with synthetic data and 4D multimodal MR data of patients with severe TBI. The novel joint segmentation-registration method for longitudinal image data provides not only change trajectories of lesions but also simultaneous segmentation of normal-appearing tissue classes. We thus provide 4D spatiotemporal models of pathological anatomy and information on tissue fate profiles and lesion changes, which will be relevant for clinical assessment of therapeutic intervention but are not yet available in clinical practice. We include a preliminary validation by comparing automatic or automated segmentation to the results of three expert raters. Even with experts familiar with reading multimodal MR
13 Wang et al. Page 13 images of TBI subjects and applying the updated ITK-SNAP tool designed for user-guided multimodal image analysis, we observe relatively large inter-rater variability. Such disagreement between raters will represent a fundamental limitation of the proposed quantitative analysis as there is no clear notion of a ground truth for lesions. We have performed validation limited to three subjects where multimodal longitudinal data was available. As part of our multicenter collaboration, we plan to extend validation and testing to a larger cohort. Our proposed method has potential applications for brain mapping, but now in the presence of large pathologies, as it registers a normative template to each individual time point, the template-based parcellation of each subject facilitates analysis of structural brain connectivity with diffusion imaging. Progress on connectivity analysis has so far been held up by the fact that well-established processing pipelines do not work on image data presenting mass-occupying lesions and infiltrations. Researchers therefore had to go through tedious and time-consuming manual editing, requiring hours to days per dataset, in order to pre-process image data. In the future, we plan to demonstrate the use of our framework for conducting regional analysis similar to the one performed by Irimia et al. (2012), where TBI subjects are labeled appropriately without manual editing. The spatiotemporal model obtained through our framework also provides measures of change in normal-appearing tissue, e.g., loss of gray matter and reduction of cortical thickness. Such measures will provide quantitative parameters for monitoring the trajectory of TBI rehabilitation in addition to lesion delineation, as shown in our previous work (Wang et al., 2013a) on identifying biomarkers for patient recovery. 6. Acknowledgements Supported by grants: National Alliance for Medical Image Computing (NA-MIC) U54 EB (GG) and the Utah Science Technology and Research (USTAR) initiative at the University of Utah. Appendix A: Derivation of Update Equations We can rewrite F (A, φ t, Q t ) as (A.1) which is defined in subject space I t. Alternatively, we can define this functional in the atlas space A, 2) (A.
14 Wang et al. Page 14 References The updated for Q is obtained through the derivative of the functional in subject space I t, The update for A is obtained through a change of coordinates, (A.3) (A.4) The update for φ is obtained through the functional derivative of φ in subject space I t, (A.5) Bauer S, Nolte L-P, Reyes M. Fully automatic segmentation of brain tumor images using support vector machine classification in combination with hierarchical conditional random field regularization. Medical Image Computing and Computer-Assisted Intervention MICCAI. 2011; 2011: Chitphakdithai N, Duncan JS. Non-rigid registration with missing correspondences in preoperative and postresection brain images. MICCAI. 2010: Cuadra MB, Pollo C, Bardera A, Cuisenaire O, Villemure J-G, Thiran J. Atlas-based segmentation of pathological MR brain images using a model of lesion growth. Medical Imaging, IEEE Transactions on. 2004; 23(10): Datar, M.; Cates, J.; Fletcher, PT.; Gouttard, S.; Gerig, G.; Whitaker, R. Medical Image Computing and Computer-Assisted Intervention MICCAI Springer; Particle based shape regression of open surfaces with applications to developmental neuroimaging; p Faul, M.; Xu, L.; Wald, M.; Coronado, V. Traumatic brain injury in the united states: Emergency department visits, hospitalizations and deaths CDC, National Center for Injury Prevention and Control; Atlanta (GA): Fishbaugh, J.; Prastawa, M.; Gerig, G.; Durrleman, S. Information Processing in Medical Imaging. Springer; Geodesic shape regression in the framework of currents; p Fletcher PT. Geodesic regression and the theory of least squares on riemannian manifolds. International Journal of Computer Vision. 2012:1 15. Gao Y, Kikinis R, Bouix S, Shenton M, Tannenbaum A. A 3D interactive multi-object segmentation tool using local robust statistics driven active contours. Medical image analysis. 2012; 16(6): [PubMed: ] Geremia E, Clatz O, Menze BH, Konukoglu E, Criminisi A, Ayache N. Spatial decision forests for ms lesion segmentation in multi-channel magnetic resonance images. NeuroImage. 2011; 57(2): [PubMed: ] Gooya A, Pohl KM, Bilello M, Cirillo L, Biros G, Melhem ER, Davatzikos C. Glistr: Glioma image segmentation and registration. IEEE Trans. Med. Imaging. 2012; 31(10): [PubMed: ]
15 Wang et al. Page 15 Hong, Y.; Joshi, S.; Sanchez, M.; Styner, M.; Niethammer, M. Medical Image Computing and Computer-Assisted Intervention MICCAI Springer; Metamorphic geodesic regression; p Irimia A, Chambers MC, Alger JR, Filippou M, Prastawa M, Wang B, Hovda DA, Gerig G, Toga AW, Kikinis R, Vespa PM, Horn JDV. Comparison of acute and chronic traumatic brain injury using semi-automatic multimodal segmentation of MR volumes. J. Neurotrauma. Nov Irimia A, Wang B, Aylward S, Prastawa M, Pace D, Gerig G, Hovda D, Kikinis R, Vespa P, Van Horn J. Neuroimaging of structural pathology and connectomics in traumatic brain injury: Toward personalized outcome prediction. NeuroImage: Clinical. 2012; 1(1):1 17. [PubMed: ] Kwon D, Niethammer M, Akbari H, Bilello M, Davatzikos C, Pohl K. Portr: Pre-operative and postrecurrence brain tumor registration. IEEE Trans. Med. Imaging Kyriacou SK, Davatzikos C, Zinreich SJ, Bryan RN. Nonlinear elastic registration of brain images with tumor pathology using a biomechanical model. Medical Imaging, IEEE Transactions on. 1999; 18(7): Ledig C, Heckemann RA, Hammers A, Lopez JC, Newcombe VF, Makropoulos A, Lötjönen J, Menon DK, Rueckert D. Robust whole-brain segmentation: Application to traumatic brain injury. Medical image analysis. 2015; 21(1): [PubMed: ] Leemput KV, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE TMI. 1999: Liu, X.; Niethammer, M.; Kwitt, R.; McCormick, M.; Aylward, S. Low-rank to the rescue atlasbased analyses in the presence of pathologies. Proceedings of the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI); Lloyd SP. Least squares quantization in pcm. IEEE Transactions on Information Theory. 1982; 28: Lou Y, Irimia A, Vela P, Chambers M, Van Horn J, Vespa P, Tannenbaum A. Multimodal deformable registration of traumatic brain injury MR volumes via the Bhattacharyya distance. IEEE Trans. Med. Imaging. 2013; 60(9): Menze, BH.; Leemput, KV.; Honkela, A.; Konukoglu, E.; Weber, M.; Ayache, N.; Golland, P. A generative approach for image-based modeling of tumor growth. Information Processing in Medical Imaging - 22nd International Conference, IPMI 2011; p Menze, BH.; Van Leemput, K.; Lashkari, D.; Weber, M-A.; Ayache, N.; Golland, P. Medical Image Computing and Computer-Assisted Intervention MICCAI Springer; A generative model for brain tumor segmentation in multi-modal images; p Niethammer M, Hart GL, Pace DF, Vespa PM, Irimia A, Horn JDV, Aylward SR. Geometric metamorphosis. MICCAI (2). 2011a: Niethammer, M.; Huang, Y.; Vialard, F-X. Medical Image Computing and Computer-Assisted Intervention MICCAI Springer; 2011b. Geodesic regression for image time-series; p Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Medical Image Analysis. 2011; 15(4): [PubMed: ] Parisot S III, Chemouny S, Duffau H, Paragios N. Concurrent tumor segmentation and registration with uncertainty-based sparse non-uniform graphs. Medical Image Analysis. 2014; 18(4): W. W. [PubMed: ] Pele O, Werman M. Fast and robust earth mover s distances. Computer vision, 2009 IEEE 12th international conference on. IEEE. 2009: Pohl KM, Fisher J, Grimson WEL, Kikinis R, Wells WM. A bayesian model for joint segmentation and registration. NeuroImage. 2006; 31(1): [PubMed: ] Prastawa M, Awate S, Gerig G. Building spatiotemporal anatomical models through joint segmentation, registration, and 4D-atlas estimation. MMBIA. 2012: [PubMed: ] Prastawa M, Bullitt E, Gerig G. Simulation of brain tumors in MR images for evaluation of segmentation efficacy. Medical image analysis. 2009; 13(2): [PubMed: ] Prastawa M, Bullitt E, Ho S, Gerig G. A brain tumor segmentation framework based on outlier detection. Medical Image Analysis. 2004; 8(3): [PubMed: ]
16 Wang et al. Page 16 Prastawa M, Bullitt E, Moon N, Van Leemput K, Gerig G. Automatic brain tumor segmentation by subject specific modification of atlas priors. Academic radiology. 2003; 10(12): [PubMed: ] Rother C, Kolmogorov V, Blake A. Grabcut: Interactive foreground extraction using iterated graph cuts. ACM TOG. 2004; 23: Sugiyama M, Nakajima S, Kashima H, Von Buenau P, Kawanabe M. Direct importance estimation with model selection and its application to covariate shift adaptation. Advances in NIPS. 2008; 20: Wang, B.; Liu, W.; Prastawa, M.; Irimia, A.; Vespa, PM.; van Horn, JD.; Fletcher, PT.; Gerig, G. 4D active cut: An interactive tool for pathological anatomy modeling. Biomedical Imaging (ISBI), 2014 IEEE 11th International Symposium on. IEEE; p Wang, B.; Prastawa, M.; Awate, SP.; Irimia, A.; Chambers, MC.; Vespa, PM.; Van Horn, JD.; Gerig, G. Segmentation of serial MRI of TBI patients using personalized atlas construction and topological change estimation. Biomedical Imaging (ISBI), th IEEE International Symposium on. IEEE; p Wang B, Prastawa M, Irimia A, Chambers MC, Sadeghi N, Vespa PM, Van Horn JD, Gerig G. Analyzing imaging biomarkers for traumatic brain injury using 4d modeling of longitudinal mri. Biomedical Imaging (ISBI), 2013 IEEE 10th International Symposium on. IEEE. 2013a: Wang, B.; Prastawa, M.; Saha, A.; Awate, SP.; Irimia, A.; Chambers, MC.; Vespa, PM.; Van Horn, JD.; Pascucci, V.; Gerig, G. Multimodal Brain Image Analysis. Springer; 2013b. Modeling 4D changes in pathological anatomy using domain adaptation: Analysis of tbi imaging using a tumor database; p Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3d active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006; 31(3): [PubMed: ] Zacharaki EI, Shen D, Lee S-K, Davatzikos C. ORBIT: a multiresolution framework for deformable registration of brain tumor images. Medical Imaging, IEEE Transactions on. 2008; 27(8):
17 Wang et al. Page 17 Figure 1. Motivation and challenges in 4D pathological anatomy modeling. Given a time series of image data, we show deformable lesions that can be modeled by diffeomorphic registration (green) but also disappearing and newly appearing lesions across time. The normative template (left) does not provide prior information on lesion type and locality.
18 Wang et al. Page 18 Figure 2. Conceptual overview of the proposed framework. To provide a spatial context for the 4D modeling, the method maps a healthy template to input images of a subject at different time points. For initialization of the image appearance model, we provide different machine learning approaches from semisupervised methods such as user initialization or iterative user interaction to fully automatic transfer learning.
19 Wang et al. Page 19 Figure 3. Illustration of the basic concept. Class-specific posteriors at a specific time point t are modeled as a combination of a diffeomorphic deformation of a normative template and a nondiffeomorphic probability.
20 Wang et al. Page 20 Figure 4. Toy example to illustrate estimation of weights using KLIEP.
21 Wang et al. Page 21 Figure 5. Axial view of synthetic dataset for modal parameter analysis. Top to bottom: Multimodal images at time point 1, time point 2, and the co-registered atlas that represent only healthy classes but not pathology. Simulated pathology includes a deformable lesion (positioned at top within images), an appearing lesion (bottom location in images), and a disappearing lesion (left location in images). Please note that the atlas (bottom) does not provide spatial priors for lesions.
22 Wang et al. Page 22 Figure 6. Estimated 4D anatomical priors for synthetic data. First row shows the initial atlas A (0) in the template space, with the Ch1 channel image shown as a reference. Second and fourth rows show initial estimation (iteration 1 of the alternating gradient decent) of the personalized atlas P t = A φ t + Q t for time points 1 and 2, shown together with input channel Ch3. Third and fifth rows show the final estimation (iteration 16 of the alternating gradient decent) of the personalized atlas P t for both time points, with the input Ch4 channel image shown as a reference.
23 Wang et al. Page 23 Figure 7. Axial views of acute and chronic images of Subject I.
24 Wang et al. Page 24 Figure 8. Estimated 4D anatomical priors for TBI Subject II. First row shows the initial atlas A (0) in the template space, with the healthy T1 image as a reference. Second and third rows show the personalized atlas P t = A φ t + Q t for acute and chronic stages, with input T2 images shown. Our method is able to account for changes in the left-frontal and mid-frontal regions across time.
25 Wang et al. Page 25 Figure 9. Axial view of manual segmentation by three human experts for acute nonhemorrhagic lesions (NHL) of Subject III.
26 Wang et al. Page 26 Figure 10. Mean and standard deviation of manual annotations from three human experts for acute hemorrhagic lesion (HL) and nonhemorrhagic lesion (NHL) of subjects I, II, and III.
27 Wang et al. Page 27 Figure 11. Comparison between GrabCut and our active learning-based method. Left: our method in 3D space and axial view. Right: GrabCut. Light blue: edema. Brown: bleeding. Note the large false-positive detection of GrabCut in the CSF region.
28 Wang et al. Page 28 Figure 12. Example of brain parcellation labels mapped to the acute (first row) and chronic (second row) time points of Subject I.
29 Wang et al. Page 29 Figure 13. Example of brain parcellation labels mapped to the acute (left) and chronic (right) time points of Subject II. For each time point, we show the input T1 image and the overlaid parcellation labels. Our method generates parcellation maps that match tissue boundaries and account for lesions.
30 Wang et al. Page 30 Table 1 Dice values comparing the acute NHL segmentation result of the six methods to the manual segmentations of the three human experts. Method I: no transfer learning and no atlas; Method II: transfer learning without atlas; Method III: transfer learning and atlas; Method IV: user initialization; Method V: GrabCut; Method VI: active learning-based user interaction (ActiveCut). Method I, II, V are baseline algorithms, the other three are the proposed methods for image appearance model learning. Subject Human Expert Noninteractive Method Interactive I II III IV V VI I II III
Modeling 4D Changes in Pathological Anatomy using Domain Adaptation: Analysis of TBI Imaging using a Tumor Database
 Modeling 4D Changes in Pathological Anatomy using Domain Adaptation: Analysis of TBI Imaging using a Tumor Database Bo Wang 1,2,, Marcel Prastawa 1,2, Avishek Saha 1,2, Suyash P. Awate 1,2, Andrei Irimia
Modeling 4D Changes in Pathological Anatomy using Domain Adaptation: Analysis of TBI Imaging using a Tumor Database Bo Wang 1,2,, Marcel Prastawa 1,2, Avishek Saha 1,2, Suyash P. Awate 1,2, Andrei Irimia
Modeling 4D Changes in Pathological Anatomy Using Domain Adaptation: Analysis of TBI Imaging Using a Tumor Database
 Modeling 4D Changes in Pathological Anatomy Using Domain Adaptation: Analysis of TBI Imaging Using a Tumor Database Bo Wang 1,2,, Marcel Prastawa 1,2, Avishek Saha 1,2, Suyash P. Awate 1,2, Andrei Irimia
Modeling 4D Changes in Pathological Anatomy Using Domain Adaptation: Analysis of TBI Imaging Using a Tumor Database Bo Wang 1,2,, Marcel Prastawa 1,2, Avishek Saha 1,2, Suyash P. Awate 1,2, Andrei Irimia
Low-Rank to the Rescue Atlas-based Analyses in the Presence of Pathologies
 Low-Rank to the Rescue Atlas-based Analyses in the Presence of Pathologies Xiaoxiao Liu 1, Marc Niethammer 2, Roland Kwitt 3, Matthew McCormick 1, and Stephen Aylward 1 1 Kitware Inc., USA 2 University
Low-Rank to the Rescue Atlas-based Analyses in the Presence of Pathologies Xiaoxiao Liu 1, Marc Niethammer 2, Roland Kwitt 3, Matthew McCormick 1, and Stephen Aylward 1 1 Kitware Inc., USA 2 University
Automatic MS Lesion Segmentation by Outlier Detection and Information Theoretic Region Partitioning Release 0.00
 Automatic MS Lesion Segmentation by Outlier Detection and Information Theoretic Region Partitioning Release 0.00 Marcel Prastawa 1 and Guido Gerig 1 Abstract July 17, 2008 1 Scientific Computing and Imaging
Automatic MS Lesion Segmentation by Outlier Detection and Information Theoretic Region Partitioning Release 0.00 Marcel Prastawa 1 and Guido Gerig 1 Abstract July 17, 2008 1 Scientific Computing and Imaging
Segmenting Glioma in Multi-Modal Images using a Generative-Discriminative Model for Brain Lesion Segmentation
 Segmenting Glioma in Multi-Modal Images using a Generative-Discriminative Model for Brain Lesion Segmentation Bjoern H. Menze 1,2, Ezequiel Geremia 2, Nicholas Ayache 2, and Gabor Szekely 1 1 Computer
Segmenting Glioma in Multi-Modal Images using a Generative-Discriminative Model for Brain Lesion Segmentation Bjoern H. Menze 1,2, Ezequiel Geremia 2, Nicholas Ayache 2, and Gabor Szekely 1 1 Computer
Segmenting Glioma in Multi-Modal Images using a Generative Model for Brain Lesion Segmentation
 Segmenting Glioma in Multi-Modal Images using a Generative Model for Brain Lesion Segmentation Bjoern H. Menze 1,2, Koen Van Leemput 3, Danial Lashkari 4 Marc-André Weber 5, Nicholas Ayache 2, and Polina
Segmenting Glioma in Multi-Modal Images using a Generative Model for Brain Lesion Segmentation Bjoern H. Menze 1,2, Koen Van Leemput 3, Danial Lashkari 4 Marc-André Weber 5, Nicholas Ayache 2, and Polina
Ischemic Stroke Lesion Segmentation Proceedings 5th October 2015 Munich, Germany
 0111010001110001101000100101010111100111011100100011011101110101101012 Ischemic Stroke Lesion Segmentation www.isles-challenge.org Proceedings 5th October 2015 Munich, Germany Preface Stroke is the second
0111010001110001101000100101010111100111011100100011011101110101101012 Ischemic Stroke Lesion Segmentation www.isles-challenge.org Proceedings 5th October 2015 Munich, Germany Preface Stroke is the second
The Anatomical Equivalence Class Formulation and its Application to Shape-based Computational Neuroanatomy
 The Anatomical Equivalence Class Formulation and its Application to Shape-based Computational Neuroanatomy Sokratis K. Makrogiannis, PhD From post-doctoral research at SBIA lab, Department of Radiology,
The Anatomical Equivalence Class Formulation and its Application to Shape-based Computational Neuroanatomy Sokratis K. Makrogiannis, PhD From post-doctoral research at SBIA lab, Department of Radiology,
Geometric Metamorphosis
 Geometric Metamorphosis Marc Niethammer 12, Gabriel L. Hart 3, Danielle F. Pace 3, Paul M. Vespa 5, Andrei Irimia 4, John D. Van Horn 4, and Stephen R. Aylward 3 1 University of North Carolina (UNC), Chapel
Geometric Metamorphosis Marc Niethammer 12, Gabriel L. Hart 3, Danielle F. Pace 3, Paul M. Vespa 5, Andrei Irimia 4, John D. Van Horn 4, and Stephen R. Aylward 3 1 University of North Carolina (UNC), Chapel
Assessment of Reliability of Multi-site Neuroimaging via Traveling Phantom Study
 Assessment of Reliability of Multi-site Neuroimaging via Traveling Phantom Study Sylvain Gouttard 1, Martin Styner 2,3, Marcel Prastawa 1, Joseph Piven 3, and Guido Gerig 1 1 Scientific Computing and Imaging
Assessment of Reliability of Multi-site Neuroimaging via Traveling Phantom Study Sylvain Gouttard 1, Martin Styner 2,3, Marcel Prastawa 1, Joseph Piven 3, and Guido Gerig 1 1 Scientific Computing and Imaging
NIH Public Access Author Manuscript Proc SPIE. Author manuscript; available in PMC 2013 December 30.
 NIH Public Access Author Manuscript Published in final edited form as: Proc SPIE. 2013 March 12; 8669:. doi:10.1117/12.2006682. Longitudinal Intensity Normalization of Magnetic Resonance Images using Patches
NIH Public Access Author Manuscript Published in final edited form as: Proc SPIE. 2013 March 12; 8669:. doi:10.1117/12.2006682. Longitudinal Intensity Normalization of Magnetic Resonance Images using Patches
Registration of Pathological Images
 Registration of Pathological Images Xiao Yang 1, Xu Han 1, Eunbyung Park 1, Stephen Aylward 3, Roland Kwitt 4, Marc Niethammer 1,2 1 UNC Chapel Hill/ 2 Biomedical Research Imaging Center, Chapel Hill,
Registration of Pathological Images Xiao Yang 1, Xu Han 1, Eunbyung Park 1, Stephen Aylward 3, Roland Kwitt 4, Marc Niethammer 1,2 1 UNC Chapel Hill/ 2 Biomedical Research Imaging Center, Chapel Hill,
Measuring longitudinal brain changes in humans and small animal models. Christos Davatzikos
 Measuring longitudinal brain changes in humans and small animal models Christos Davatzikos Section of Biomedical Image Analysis University of Pennsylvania (Radiology) http://www.rad.upenn.edu/sbia Computational
Measuring longitudinal brain changes in humans and small animal models Christos Davatzikos Section of Biomedical Image Analysis University of Pennsylvania (Radiology) http://www.rad.upenn.edu/sbia Computational
An Introduction To Automatic Tissue Classification Of Brain MRI. Colm Elliott Mar 2014
 An Introduction To Automatic Tissue Classification Of Brain MRI Colm Elliott Mar 2014 Tissue Classification Tissue classification is part of many processing pipelines. We often want to classify each voxel
An Introduction To Automatic Tissue Classification Of Brain MRI Colm Elliott Mar 2014 Tissue Classification Tissue classification is part of many processing pipelines. We often want to classify each voxel
Knowledge-Based Segmentation of Brain MRI Scans Using the Insight Toolkit
 Knowledge-Based Segmentation of Brain MRI Scans Using the Insight Toolkit John Melonakos 1, Ramsey Al-Hakim 1, James Fallon 2 and Allen Tannenbaum 1 1 Georgia Institute of Technology, Atlanta GA 30332,
Knowledge-Based Segmentation of Brain MRI Scans Using the Insight Toolkit John Melonakos 1, Ramsey Al-Hakim 1, James Fallon 2 and Allen Tannenbaum 1 1 Georgia Institute of Technology, Atlanta GA 30332,
Assessment of Reliability of Multi-site Neuroimaging Via Traveling Phantom Study
 Assessment of Reliability of Multi-site Neuroimaging Via Traveling Phantom Study Sylvain Gouttard 1, Martin Styner 2,3, Marcel Prastawa 1, Joseph Piven 3, and Guido Gerig 1 1 Scientific Computing and Imaging
Assessment of Reliability of Multi-site Neuroimaging Via Traveling Phantom Study Sylvain Gouttard 1, Martin Styner 2,3, Marcel Prastawa 1, Joseph Piven 3, and Guido Gerig 1 1 Scientific Computing and Imaging
Image Registration Driven by Combined Probabilistic and Geometric Descriptors
 Image Registration Driven by Combined Probabilistic and Geometric Descriptors Linh Ha 1, Marcel Prastawa 1, Guido Gerig 1, John H. Gilmore 2, Cláudio T. Silva 1, Sarang Joshi 1 1 Scientific Computing and
Image Registration Driven by Combined Probabilistic and Geometric Descriptors Linh Ha 1, Marcel Prastawa 1, Guido Gerig 1, John H. Gilmore 2, Cláudio T. Silva 1, Sarang Joshi 1 1 Scientific Computing and
Low-rank Atlas Image Analyses in the Presence of Pathologies
 JOURNAL OF L A TEX CLASS FILES, VOL. 11, NO. 4, DECEMBER 2014 1 Low-rank Atlas Image Analyses in the Presence of Pathologies Xiaoxiao Liu, Marc Niethammer, Roland Kwitt, Nikhil Singh, Matt McCormick, Stephen
JOURNAL OF L A TEX CLASS FILES, VOL. 11, NO. 4, DECEMBER 2014 1 Low-rank Atlas Image Analyses in the Presence of Pathologies Xiaoxiao Liu, Marc Niethammer, Roland Kwitt, Nikhil Singh, Matt McCormick, Stephen
Fuzzy Multi-channel Clustering with Individualized Spatial Priors for Segmenting Brain Lesions and Infarcts
 Fuzzy Multi-channel Clustering with Individualized Spatial Priors for Segmenting Brain Lesions and Infarcts Evangelia Zacharaki, Guray Erus, Anastasios Bezerianos, Christos Davatzikos To cite this version:
Fuzzy Multi-channel Clustering with Individualized Spatial Priors for Segmenting Brain Lesions and Infarcts Evangelia Zacharaki, Guray Erus, Anastasios Bezerianos, Christos Davatzikos To cite this version:
ADAPTIVE GRAPH CUTS WITH TISSUE PRIORS FOR BRAIN MRI SEGMENTATION
 ADAPTIVE GRAPH CUTS WITH TISSUE PRIORS FOR BRAIN MRI SEGMENTATION Abstract: MIP Project Report Spring 2013 Gaurav Mittal 201232644 This is a detailed report about the course project, which was to implement
ADAPTIVE GRAPH CUTS WITH TISSUE PRIORS FOR BRAIN MRI SEGMENTATION Abstract: MIP Project Report Spring 2013 Gaurav Mittal 201232644 This is a detailed report about the course project, which was to implement
MR IMAGE SEGMENTATION
 MR IMAGE SEGMENTATION Prepared by : Monil Shah What is Segmentation? Partitioning a region or regions of interest in images such that each region corresponds to one or more anatomic structures Classification
MR IMAGE SEGMENTATION Prepared by : Monil Shah What is Segmentation? Partitioning a region or regions of interest in images such that each region corresponds to one or more anatomic structures Classification
Joint Tumor Segmentation and Dense Deformable Registration of Brain MR Images
 Joint Tumor Segmentation and Dense Deformable Registration of Brain MR Images Sarah Parisot 1,2,3, Hugues Duffau 4, Stéphane Chemouny 3, Nikos Paragios 1,2 1. Center for Visual Computing, Ecole Centrale
Joint Tumor Segmentation and Dense Deformable Registration of Brain MR Images Sarah Parisot 1,2,3, Hugues Duffau 4, Stéphane Chemouny 3, Nikos Paragios 1,2 1. Center for Visual Computing, Ecole Centrale
Context-sensitive Classification Forests for Segmentation of Brain Tumor Tissues
 Context-sensitive Classification Forests for Segmentation of Brain Tumor Tissues D. Zikic, B. Glocker, E. Konukoglu, J. Shotton, A. Criminisi, D. H. Ye, C. Demiralp 3, O. M. Thomas 4,5, T. Das 4, R. Jena
Context-sensitive Classification Forests for Segmentation of Brain Tumor Tissues D. Zikic, B. Glocker, E. Konukoglu, J. Shotton, A. Criminisi, D. H. Ye, C. Demiralp 3, O. M. Thomas 4,5, T. Das 4, R. Jena
NIH Public Access Author Manuscript Proc Soc Photo Opt Instrum Eng. Author manuscript; available in PMC 2014 October 07.
 NIH Public Access Author Manuscript Published in final edited form as: Proc Soc Photo Opt Instrum Eng. 2014 March 21; 9034: 903442. doi:10.1117/12.2042915. MRI Brain Tumor Segmentation and Necrosis Detection
NIH Public Access Author Manuscript Published in final edited form as: Proc Soc Photo Opt Instrum Eng. 2014 March 21; 9034: 903442. doi:10.1117/12.2042915. MRI Brain Tumor Segmentation and Necrosis Detection
Multi-Atlas Segmentation of the Cardiac MR Right Ventricle
 Multi-Atlas Segmentation of the Cardiac MR Right Ventricle Yangming Ou, Jimit Doshi, Guray Erus, and Christos Davatzikos Section of Biomedical Image Analysis (SBIA) Department of Radiology, University
Multi-Atlas Segmentation of the Cardiac MR Right Ventricle Yangming Ou, Jimit Doshi, Guray Erus, and Christos Davatzikos Section of Biomedical Image Analysis (SBIA) Department of Radiology, University
Methods for data preprocessing
 Methods for data preprocessing John Ashburner Wellcome Trust Centre for Neuroimaging, 12 Queen Square, London, UK. Overview Voxel-Based Morphometry Morphometry in general Volumetrics VBM preprocessing
Methods for data preprocessing John Ashburner Wellcome Trust Centre for Neuroimaging, 12 Queen Square, London, UK. Overview Voxel-Based Morphometry Morphometry in general Volumetrics VBM preprocessing
Attribute Similarity and Mutual-Saliency Weighting for Registration and Label Fusion
 Attribute Similarity and Mutual-Saliency Weighting for Registration and Label Fusion Yangming Ou, Jimit Doshi, Guray Erus, and Christos Davatzikos Section of Biomedical Image Analysis (SBIA) Department
Attribute Similarity and Mutual-Saliency Weighting for Registration and Label Fusion Yangming Ou, Jimit Doshi, Guray Erus, and Christos Davatzikos Section of Biomedical Image Analysis (SBIA) Department
Graphical Models, Bayesian Method, Sampling, and Variational Inference
 Graphical Models, Bayesian Method, Sampling, and Variational Inference With Application in Function MRI Analysis and Other Imaging Problems Wei Liu Scientific Computing and Imaging Institute University
Graphical Models, Bayesian Method, Sampling, and Variational Inference With Application in Function MRI Analysis and Other Imaging Problems Wei Liu Scientific Computing and Imaging Institute University
CHAPTER 2. Morphometry on rodent brains. A.E.H. Scheenstra J. Dijkstra L. van der Weerd
 CHAPTER 2 Morphometry on rodent brains A.E.H. Scheenstra J. Dijkstra L. van der Weerd This chapter was adapted from: Volumetry and other quantitative measurements to assess the rodent brain, In vivo NMR
CHAPTER 2 Morphometry on rodent brains A.E.H. Scheenstra J. Dijkstra L. van der Weerd This chapter was adapted from: Volumetry and other quantitative measurements to assess the rodent brain, In vivo NMR
Spatio-Temporal Registration of Biomedical Images by Computational Methods
 Spatio-Temporal Registration of Biomedical Images by Computational Methods Francisco P. M. Oliveira, João Manuel R. S. Tavares tavares@fe.up.pt, www.fe.up.pt/~tavares Outline 1. Introduction 2. Spatial
Spatio-Temporal Registration of Biomedical Images by Computational Methods Francisco P. M. Oliveira, João Manuel R. S. Tavares tavares@fe.up.pt, www.fe.up.pt/~tavares Outline 1. Introduction 2. Spatial
Machine Learning for Medical Image Analysis. A. Criminisi
 Machine Learning for Medical Image Analysis A. Criminisi Overview Introduction to machine learning Decision forests Applications in medical image analysis Anatomy localization in CT Scans Spine Detection
Machine Learning for Medical Image Analysis A. Criminisi Overview Introduction to machine learning Decision forests Applications in medical image analysis Anatomy localization in CT Scans Spine Detection
Optimal MAP Parameters Estimation in STAPLE - Learning from Performance Parameters versus Image Similarity Information
 Optimal MAP Parameters Estimation in STAPLE - Learning from Performance Parameters versus Image Similarity Information Subrahmanyam Gorthi 1, Alireza Akhondi-Asl 1, Jean-Philippe Thiran 2, and Simon K.
Optimal MAP Parameters Estimation in STAPLE - Learning from Performance Parameters versus Image Similarity Information Subrahmanyam Gorthi 1, Alireza Akhondi-Asl 1, Jean-Philippe Thiran 2, and Simon K.
Supplementary methods
 Supplementary methods This section provides additional technical details on the sample, the applied imaging and analysis steps and methods. Structural imaging Trained radiographers placed all participants
Supplementary methods This section provides additional technical details on the sample, the applied imaging and analysis steps and methods. Structural imaging Trained radiographers placed all participants
Comparison Study of Clinical 3D MRI Brain Segmentation Evaluation
 Comparison Study of Clinical 3D MRI Brain Segmentation Evaluation Ting Song 1, Elsa D. Angelini 2, Brett D. Mensh 3, Andrew Laine 1 1 Heffner Biomedical Imaging Laboratory Department of Biomedical Engineering,
Comparison Study of Clinical 3D MRI Brain Segmentation Evaluation Ting Song 1, Elsa D. Angelini 2, Brett D. Mensh 3, Andrew Laine 1 1 Heffner Biomedical Imaging Laboratory Department of Biomedical Engineering,
Learning Algorithms for Medical Image Analysis. Matteo Santoro slipguru
 Learning Algorithms for Medical Image Analysis Matteo Santoro slipguru santoro@disi.unige.it June 8, 2010 Outline 1. learning-based strategies for quantitative image analysis 2. automatic annotation of
Learning Algorithms for Medical Image Analysis Matteo Santoro slipguru santoro@disi.unige.it June 8, 2010 Outline 1. learning-based strategies for quantitative image analysis 2. automatic annotation of
Non-rigid Image Registration using Electric Current Flow
 Non-rigid Image Registration using Electric Current Flow Shu Liao, Max W. K. Law and Albert C. S. Chung Lo Kwee-Seong Medical Image Analysis Laboratory, Department of Computer Science and Engineering,
Non-rigid Image Registration using Electric Current Flow Shu Liao, Max W. K. Law and Albert C. S. Chung Lo Kwee-Seong Medical Image Analysis Laboratory, Department of Computer Science and Engineering,
Knowledge-Based Segmentation of Brain MRI Scans Using the Insight Toolkit
 Knowledge-Based Segmentation of Brain MRI Scans Using the Insight Toolkit John Melonakos 1, Ramsey Al-Hakim 1, James Fallon 2 and Allen Tannenbaum 1 1 Georgia Institute of Technology, Atlanta GA 30332,
Knowledge-Based Segmentation of Brain MRI Scans Using the Insight Toolkit John Melonakos 1, Ramsey Al-Hakim 1, James Fallon 2 and Allen Tannenbaum 1 1 Georgia Institute of Technology, Atlanta GA 30332,
Subcortical Structure Segmentation using Probabilistic Atlas Priors
 Subcortical Structure Segmentation using Probabilistic Atlas Priors Sylvain Gouttard 1, Martin Styner 1,2, Sarang Joshi 3, Brad Davis 2, Rachel G. Smith 1, Heather Cody Hazlett 1, Guido Gerig 1,2 1 Department
Subcortical Structure Segmentation using Probabilistic Atlas Priors Sylvain Gouttard 1, Martin Styner 1,2, Sarang Joshi 3, Brad Davis 2, Rachel G. Smith 1, Heather Cody Hazlett 1, Guido Gerig 1,2 1 Department
A Nonparametric Model for Brain Tumor Segmentation and Volumetry in Longitudinal MR Sequences
 A Nonparametric Model for Brain Tumor Segmentation and Volumetry in Longitudinal MR Sequences Esther Alberts 1,2,6, Guillaume Charpiat 3, Yuliya Tarabalka 4, Thomas Huber 1, Marc-André Weber 5, Jan Bauer
A Nonparametric Model for Brain Tumor Segmentation and Volumetry in Longitudinal MR Sequences Esther Alberts 1,2,6, Guillaume Charpiat 3, Yuliya Tarabalka 4, Thomas Huber 1, Marc-André Weber 5, Jan Bauer
Where are we now? Structural MRI processing and analysis
 Where are we now? Structural MRI processing and analysis Pierre-Louis Bazin bazin@cbs.mpg.de Leipzig, Germany Structural MRI processing: why bother? Just use the standards? SPM FreeSurfer FSL However:
Where are we now? Structural MRI processing and analysis Pierre-Louis Bazin bazin@cbs.mpg.de Leipzig, Germany Structural MRI processing: why bother? Just use the standards? SPM FreeSurfer FSL However:
Joint Segmentation and Registration for Infant Brain Images
 Joint Segmentation and Registration for Infant Brain Images Guorong Wu 1, Li Wang 1, John Gilmore 2, Weili Lin 1, and Dinggang Shen 1(&) 1 Department of Radiology and BRIC, University of North Carolina,
Joint Segmentation and Registration for Infant Brain Images Guorong Wu 1, Li Wang 1, John Gilmore 2, Weili Lin 1, and Dinggang Shen 1(&) 1 Department of Radiology and BRIC, University of North Carolina,
Automatic Vascular Tree Formation Using the Mahalanobis Distance
 Automatic Vascular Tree Formation Using the Mahalanobis Distance Julien Jomier, Vincent LeDigarcher, and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab, Department of Radiology The University
Automatic Vascular Tree Formation Using the Mahalanobis Distance Julien Jomier, Vincent LeDigarcher, and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab, Department of Radiology The University
The organization of the human cerebral cortex estimated by intrinsic functional connectivity
 1 The organization of the human cerebral cortex estimated by intrinsic functional connectivity Journal: Journal of Neurophysiology Author: B. T. Thomas Yeo, et al Link: https://www.ncbi.nlm.nih.gov/pubmed/21653723
1 The organization of the human cerebral cortex estimated by intrinsic functional connectivity Journal: Journal of Neurophysiology Author: B. T. Thomas Yeo, et al Link: https://www.ncbi.nlm.nih.gov/pubmed/21653723
Comparison of Vessel Segmentations Using STAPLE
 Comparison of Vessel Segmentations Using STAPLE Julien Jomier, Vincent LeDigarcher, and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab, The University of North Carolina at Chapel Hill, Department
Comparison of Vessel Segmentations Using STAPLE Julien Jomier, Vincent LeDigarcher, and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab, The University of North Carolina at Chapel Hill, Department
White Matter Lesion Segmentation (WMLS) Manual
 White Matter Lesion Segmentation (WMLS) Manual 1. Introduction White matter lesions (WMLs) are brain abnormalities that appear in different brain diseases, such as multiple sclerosis (MS), head injury,
White Matter Lesion Segmentation (WMLS) Manual 1. Introduction White matter lesions (WMLs) are brain abnormalities that appear in different brain diseases, such as multiple sclerosis (MS), head injury,
An ITK Filter for Bayesian Segmentation: itkbayesianclassifierimagefilter
 An ITK Filter for Bayesian Segmentation: itkbayesianclassifierimagefilter John Melonakos 1, Karthik Krishnan 2 and Allen Tannenbaum 1 1 Georgia Institute of Technology, Atlanta GA 30332, USA {jmelonak,
An ITK Filter for Bayesian Segmentation: itkbayesianclassifierimagefilter John Melonakos 1, Karthik Krishnan 2 and Allen Tannenbaum 1 1 Georgia Institute of Technology, Atlanta GA 30332, USA {jmelonak,
Automatic Segmentation of Neonatal Brain MRI
 Automatic Segmentation of Neonatal Brain MRI 1 Marcel Prastawa, 2 John Gilmore, 3 Weili Lin, and 1,2 Guido Gerig Department of 1 Computer Science, 2 Psychiatry, and 3 Radiology University of North Carolina,
Automatic Segmentation of Neonatal Brain MRI 1 Marcel Prastawa, 2 John Gilmore, 3 Weili Lin, and 1,2 Guido Gerig Department of 1 Computer Science, 2 Psychiatry, and 3 Radiology University of North Carolina,
Methodological progress in image registration for ventilation estimation, segmentation propagation and multi-modal fusion
 Methodological progress in image registration for ventilation estimation, segmentation propagation and multi-modal fusion Mattias P. Heinrich Julia A. Schnabel, Mark Jenkinson, Sir Michael Brady 2 Clinical
Methodological progress in image registration for ventilation estimation, segmentation propagation and multi-modal fusion Mattias P. Heinrich Julia A. Schnabel, Mark Jenkinson, Sir Michael Brady 2 Clinical
Learning-based Neuroimage Registration
 Learning-based Neuroimage Registration Leonid Teverovskiy and Yanxi Liu 1 October 2004 CMU-CALD-04-108, CMU-RI-TR-04-59 School of Computer Science Carnegie Mellon University Pittsburgh, PA 15213 Abstract
Learning-based Neuroimage Registration Leonid Teverovskiy and Yanxi Liu 1 October 2004 CMU-CALD-04-108, CMU-RI-TR-04-59 School of Computer Science Carnegie Mellon University Pittsburgh, PA 15213 Abstract
Atlas Based Segmentation of the prostate in MR images
 Atlas Based Segmentation of the prostate in MR images Albert Gubern-Merida and Robert Marti Universitat de Girona, Computer Vision and Robotics Group, Girona, Spain {agubern,marly}@eia.udg.edu Abstract.
Atlas Based Segmentation of the prostate in MR images Albert Gubern-Merida and Robert Marti Universitat de Girona, Computer Vision and Robotics Group, Girona, Spain {agubern,marly}@eia.udg.edu Abstract.
Tissue Tracking: Applications for Brain MRI Classification
 Tissue Tracking: Applications for Brain MRI Classification John Melonakos a and Yi Gao a and Allen Tannenbaum a a Georgia Institute of Technology, 414 Ferst Dr, Atlanta, GA, USA; ABSTRACT Bayesian classification
Tissue Tracking: Applications for Brain MRI Classification John Melonakos a and Yi Gao a and Allen Tannenbaum a a Georgia Institute of Technology, 414 Ferst Dr, Atlanta, GA, USA; ABSTRACT Bayesian classification
Population Modeling in Neuroscience Using Computer Vision and Machine Learning to learn from Brain Images. Overview. Image Registration
 Population Modeling in Neuroscience Using Computer Vision and Machine Learning to learn from Brain Images Overview 1. Part 1: Theory 1. 2. Learning 2. Part 2: Applications ernst.schwartz@meduniwien.ac.at
Population Modeling in Neuroscience Using Computer Vision and Machine Learning to learn from Brain Images Overview 1. Part 1: Theory 1. 2. Learning 2. Part 2: Applications ernst.schwartz@meduniwien.ac.at
Automatic Optimization of Segmentation Algorithms Through Simultaneous Truth and Performance Level Estimation (STAPLE)
 Automatic Optimization of Segmentation Algorithms Through Simultaneous Truth and Performance Level Estimation (STAPLE) Mahnaz Maddah, Kelly H. Zou, William M. Wells, Ron Kikinis, and Simon K. Warfield
Automatic Optimization of Segmentation Algorithms Through Simultaneous Truth and Performance Level Estimation (STAPLE) Mahnaz Maddah, Kelly H. Zou, William M. Wells, Ron Kikinis, and Simon K. Warfield
Rigid and Deformable Vasculature-to-Image Registration : a Hierarchical Approach
 Rigid and Deformable Vasculature-to-Image Registration : a Hierarchical Approach Julien Jomier and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab The University of North Carolina at Chapel
Rigid and Deformable Vasculature-to-Image Registration : a Hierarchical Approach Julien Jomier and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab The University of North Carolina at Chapel
NIH Public Access Author Manuscript Proc IEEE Int Symp Biomed Imaging. Author manuscript; available in PMC 2014 January 15.
 NIH Public Access Author Manuscript Published in final edited form as: Proc IEEE Int Symp Biomed Imaging. 2013 December 31; 2013: 1396 1399. doi:10.1109/isbi. 2013.6556794. MODELING LONGITUDINAL MRI CHANGES
NIH Public Access Author Manuscript Published in final edited form as: Proc IEEE Int Symp Biomed Imaging. 2013 December 31; 2013: 1396 1399. doi:10.1109/isbi. 2013.6556794. MODELING LONGITUDINAL MRI CHANGES
Subcortical Structure Segmentation using Probabilistic Atlas Priors
 Subcortical Structure Segmentation using Probabilistic Atlas Priors Sylvain Gouttard a, Martin Styner a,b, Sarang Joshi c, Rachel G. Smith a, Heather Cody a, Guido Gerig a,b a Department of Psychiatry,
Subcortical Structure Segmentation using Probabilistic Atlas Priors Sylvain Gouttard a, Martin Styner a,b, Sarang Joshi c, Rachel G. Smith a, Heather Cody a, Guido Gerig a,b a Department of Psychiatry,
PROSTATE CANCER DETECTION USING LABEL IMAGE CONSTRAINED MULTIATLAS SELECTION
 PROSTATE CANCER DETECTION USING LABEL IMAGE CONSTRAINED MULTIATLAS SELECTION Ms. Vaibhavi Nandkumar Jagtap 1, Mr. Santosh D. Kale 2 1 PG Scholar, 2 Assistant Professor, Department of Electronics and Telecommunication,
PROSTATE CANCER DETECTION USING LABEL IMAGE CONSTRAINED MULTIATLAS SELECTION Ms. Vaibhavi Nandkumar Jagtap 1, Mr. Santosh D. Kale 2 1 PG Scholar, 2 Assistant Professor, Department of Electronics and Telecommunication,
Automatic Generation of Training Data for Brain Tissue Classification from MRI
 MICCAI-2002 1 Automatic Generation of Training Data for Brain Tissue Classification from MRI Chris A. Cocosco, Alex P. Zijdenbos, and Alan C. Evans McConnell Brain Imaging Centre, Montreal Neurological
MICCAI-2002 1 Automatic Generation of Training Data for Brain Tissue Classification from MRI Chris A. Cocosco, Alex P. Zijdenbos, and Alan C. Evans McConnell Brain Imaging Centre, Montreal Neurological
Correspondence Detection Using Wavelet-Based Attribute Vectors
 Correspondence Detection Using Wavelet-Based Attribute Vectors Zhong Xue, Dinggang Shen, and Christos Davatzikos Section of Biomedical Image Analysis, Department of Radiology University of Pennsylvania,
Correspondence Detection Using Wavelet-Based Attribute Vectors Zhong Xue, Dinggang Shen, and Christos Davatzikos Section of Biomedical Image Analysis, Department of Radiology University of Pennsylvania,
Efficient population registration of 3D data
 Efficient population registration of 3D data Lilla Zöllei 1, Erik Learned-Miller 2, Eric Grimson 1, William Wells 1,3 1 Computer Science and Artificial Intelligence Lab, MIT; 2 Dept. of Computer Science,
Efficient population registration of 3D data Lilla Zöllei 1, Erik Learned-Miller 2, Eric Grimson 1, William Wells 1,3 1 Computer Science and Artificial Intelligence Lab, MIT; 2 Dept. of Computer Science,
Shape-based Diffeomorphic Registration on Hippocampal Surfaces Using Beltrami Holomorphic Flow
 Shape-based Diffeomorphic Registration on Hippocampal Surfaces Using Beltrami Holomorphic Flow Abstract. Finding meaningful 1-1 correspondences between hippocampal (HP) surfaces is an important but difficult
Shape-based Diffeomorphic Registration on Hippocampal Surfaces Using Beltrami Holomorphic Flow Abstract. Finding meaningful 1-1 correspondences between hippocampal (HP) surfaces is an important but difficult
Hybrid Spline-based Multimodal Registration using a Local Measure for Mutual Information
 Hybrid Spline-based Multimodal Registration using a Local Measure for Mutual Information Andreas Biesdorf 1, Stefan Wörz 1, Hans-Jürgen Kaiser 2, Karl Rohr 1 1 University of Heidelberg, BIOQUANT, IPMB,
Hybrid Spline-based Multimodal Registration using a Local Measure for Mutual Information Andreas Biesdorf 1, Stefan Wörz 1, Hans-Jürgen Kaiser 2, Karl Rohr 1 1 University of Heidelberg, BIOQUANT, IPMB,
MEDICAL IMAGE COMPUTING (CAP 5937) LECTURE 10: Medical Image Segmentation as an Energy Minimization Problem
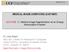 SPRING 07 MEDICAL IMAGE COMPUTING (CAP 97) LECTURE 0: Medical Image Segmentation as an Energy Minimization Problem Dr. Ulas Bagci HEC, Center for Research in Computer Vision (CRCV), University of Central
SPRING 07 MEDICAL IMAGE COMPUTING (CAP 97) LECTURE 0: Medical Image Segmentation as an Energy Minimization Problem Dr. Ulas Bagci HEC, Center for Research in Computer Vision (CRCV), University of Central
AN AUTOMATED SEGMENTATION FRAMEWORK FOR BRAIN MRI VOLUMES BASED ON ADAPTIVE MEAN-SHIFT CLUSTERING
 AN AUTOMATED SEGMENTATION FRAMEWORK FOR BRAIN MRI VOLUMES BASED ON ADAPTIVE MEAN-SHIFT CLUSTERING Sam Ponnachan 1, Paul Pandi 2,A.Winifred 3,Joselin Jose 4 UG Scholars 1,2,3,4 Department of EIE 1,2 Department
AN AUTOMATED SEGMENTATION FRAMEWORK FOR BRAIN MRI VOLUMES BASED ON ADAPTIVE MEAN-SHIFT CLUSTERING Sam Ponnachan 1, Paul Pandi 2,A.Winifred 3,Joselin Jose 4 UG Scholars 1,2,3,4 Department of EIE 1,2 Department
Automatic segmentation of the cortical grey and white matter in MRI using a Region Growing approach based on anatomical knowledge
 Automatic segmentation of the cortical grey and white matter in MRI using a Region Growing approach based on anatomical knowledge Christian Wasserthal 1, Karin Engel 1, Karsten Rink 1 und André Brechmann
Automatic segmentation of the cortical grey and white matter in MRI using a Region Growing approach based on anatomical knowledge Christian Wasserthal 1, Karin Engel 1, Karsten Rink 1 und André Brechmann
maximum likelihood estimates. The performance of
 International Journal of Computer Science and Telecommunications [Volume 2, Issue 6, September 2] 8 ISSN 247-3338 An Efficient Approach for Medical Image Segmentation Based on Truncated Skew Gaussian Mixture
International Journal of Computer Science and Telecommunications [Volume 2, Issue 6, September 2] 8 ISSN 247-3338 An Efficient Approach for Medical Image Segmentation Based on Truncated Skew Gaussian Mixture
NIH Public Access Author Manuscript Proc IEEE Int Symp Biomed Imaging. Author manuscript; available in PMC 2014 November 15.
 NIH Public Access Author Manuscript Published in final edited form as: Proc IEEE Int Symp Biomed Imaging. 2013 April ; 2013: 748 751. doi:10.1109/isbi.2013.6556583. BRAIN TUMOR SEGMENTATION WITH SYMMETRIC
NIH Public Access Author Manuscript Published in final edited form as: Proc IEEE Int Symp Biomed Imaging. 2013 April ; 2013: 748 751. doi:10.1109/isbi.2013.6556583. BRAIN TUMOR SEGMENTATION WITH SYMMETRIC
Norbert Schuff VA Medical Center and UCSF
 Norbert Schuff Medical Center and UCSF Norbert.schuff@ucsf.edu Medical Imaging Informatics N.Schuff Course # 170.03 Slide 1/67 Objective Learn the principle segmentation techniques Understand the role
Norbert Schuff Medical Center and UCSF Norbert.schuff@ucsf.edu Medical Imaging Informatics N.Schuff Course # 170.03 Slide 1/67 Objective Learn the principle segmentation techniques Understand the role
Comparison of Vessel Segmentations using STAPLE
 Comparison of Vessel Segmentations using STAPLE Julien Jomier, Vincent LeDigarcher, and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab The University of North Carolina at Chapel Hill, Department
Comparison of Vessel Segmentations using STAPLE Julien Jomier, Vincent LeDigarcher, and Stephen R. Aylward Computer-Aided Diagnosis and Display Lab The University of North Carolina at Chapel Hill, Department
Semantic Context Forests for Learning- Based Knee Cartilage Segmentation in 3D MR Images
 Semantic Context Forests for Learning- Based Knee Cartilage Segmentation in 3D MR Images MICCAI 2013: Workshop on Medical Computer Vision Authors: Quan Wang, Dijia Wu, Le Lu, Meizhu Liu, Kim L. Boyer,
Semantic Context Forests for Learning- Based Knee Cartilage Segmentation in 3D MR Images MICCAI 2013: Workshop on Medical Computer Vision Authors: Quan Wang, Dijia Wu, Le Lu, Meizhu Liu, Kim L. Boyer,
Multi-Sectional Views Textural Based SVM for MS Lesion Segmentation in Multi-Channels MRIs
 56 The Open Biomedical Engineering Journal, 2012, 6, 56-72 Open Access Multi-Sectional Views Textural Based SVM for MS Lesion Segmentation in Multi-Channels MRIs Bassem A. Abdullah*, Akmal A. Younis and
56 The Open Biomedical Engineering Journal, 2012, 6, 56-72 Open Access Multi-Sectional Views Textural Based SVM for MS Lesion Segmentation in Multi-Channels MRIs Bassem A. Abdullah*, Akmal A. Younis and
Preprocessing II: Between Subjects John Ashburner
 Preprocessing II: Between Subjects John Ashburner Pre-processing Overview Statistics or whatever fmri time-series Anatomical MRI Template Smoothed Estimate Spatial Norm Motion Correct Smooth Coregister
Preprocessing II: Between Subjects John Ashburner Pre-processing Overview Statistics or whatever fmri time-series Anatomical MRI Template Smoothed Estimate Spatial Norm Motion Correct Smooth Coregister
PBSI: A symmetric probabilistic extension of the Boundary Shift Integral
 PBSI: A symmetric probabilistic extension of the Boundary Shift Integral Christian Ledig 1, Robin Wolz 1, Paul Aljabar 1,2, Jyrki Lötjönen 3, Daniel Rueckert 1 1 Department of Computing, Imperial College
PBSI: A symmetric probabilistic extension of the Boundary Shift Integral Christian Ledig 1, Robin Wolz 1, Paul Aljabar 1,2, Jyrki Lötjönen 3, Daniel Rueckert 1 1 Department of Computing, Imperial College
STIC AmSud Project. Graph cut based segmentation of cardiac ventricles in MRI: a shape-prior based approach
 STIC AmSud Project Graph cut based segmentation of cardiac ventricles in MRI: a shape-prior based approach Caroline Petitjean A joint work with Damien Grosgeorge, Pr Su Ruan, Pr JN Dacher, MD October 22,
STIC AmSud Project Graph cut based segmentation of cardiac ventricles in MRI: a shape-prior based approach Caroline Petitjean A joint work with Damien Grosgeorge, Pr Su Ruan, Pr JN Dacher, MD October 22,
A Review on Label Image Constrained Multiatlas Selection
 A Review on Label Image Constrained Multiatlas Selection Ms. VAIBHAVI NANDKUMAR JAGTAP 1, Mr. SANTOSH D. KALE 2 1PG Scholar, Department of Electronics and Telecommunication, SVPM College of Engineering,
A Review on Label Image Constrained Multiatlas Selection Ms. VAIBHAVI NANDKUMAR JAGTAP 1, Mr. SANTOSH D. KALE 2 1PG Scholar, Department of Electronics and Telecommunication, SVPM College of Engineering,
Hierarchical Multi structure Segmentation Guided by Anatomical Correlations
 Hierarchical Multi structure Segmentation Guided by Anatomical Correlations Oscar Alfonso Jiménez del Toro oscar.jimenez@hevs.ch Henning Müller henningmueller@hevs.ch University of Applied Sciences Western
Hierarchical Multi structure Segmentation Guided by Anatomical Correlations Oscar Alfonso Jiménez del Toro oscar.jimenez@hevs.ch Henning Müller henningmueller@hevs.ch University of Applied Sciences Western
Characterizing growth patterns in longitudinal MRI using image contrast
 Characterizing growth patterns in longitudinal MRI using image contrast Avantika Vardhan a,b, Marcel Prastawa a,c, Clement Vachet a, Joseph Piven d for IBIS *, Guido Gerig a,b,c a Scientific Computing
Characterizing growth patterns in longitudinal MRI using image contrast Avantika Vardhan a,b, Marcel Prastawa a,c, Clement Vachet a, Joseph Piven d for IBIS *, Guido Gerig a,b,c a Scientific Computing
Free-Form B-spline Deformation Model for Groupwise Registration
 Free-Form B-spline Deformation Model for Groupwise Registration The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Balci
Free-Form B-spline Deformation Model for Groupwise Registration The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Balci
Supervised Learning for Image Segmentation
 Supervised Learning for Image Segmentation Raphael Meier 06.10.2016 Raphael Meier MIA 2016 06.10.2016 1 / 52 References A. Ng, Machine Learning lecture, Stanford University. A. Criminisi, J. Shotton, E.
Supervised Learning for Image Segmentation Raphael Meier 06.10.2016 Raphael Meier MIA 2016 06.10.2016 1 / 52 References A. Ng, Machine Learning lecture, Stanford University. A. Criminisi, J. Shotton, E.
Regional Manifold Learning for Deformable Registration of Brain MR Images
 Regional Manifold Learning for Deformable Registration of Brain MR Images Dong Hye Ye, Jihun Hamm, Dongjin Kwon, Christos Davatzikos, and Kilian M. Pohl Department of Radiology, University of Pennsylvania,
Regional Manifold Learning for Deformable Registration of Brain MR Images Dong Hye Ye, Jihun Hamm, Dongjin Kwon, Christos Davatzikos, and Kilian M. Pohl Department of Radiology, University of Pennsylvania,
Modified Expectation Maximization Method for Automatic Segmentation of MR Brain Images
 Modified Expectation Maximization Method for Automatic Segmentation of MR Brain Images R.Meena Prakash, R.Shantha Selva Kumari 1 P.S.R.Engineering College, Sivakasi, Tamil Nadu, India 2 Mepco Schlenk Engineering
Modified Expectation Maximization Method for Automatic Segmentation of MR Brain Images R.Meena Prakash, R.Shantha Selva Kumari 1 P.S.R.Engineering College, Sivakasi, Tamil Nadu, India 2 Mepco Schlenk Engineering
Quantitative MRI of the Brain: Investigation of Cerebral Gray and White Matter Diseases
 Quantities Measured by MR - Quantitative MRI of the Brain: Investigation of Cerebral Gray and White Matter Diseases Static parameters (influenced by molecular environment): T, T* (transverse relaxation)
Quantities Measured by MR - Quantitative MRI of the Brain: Investigation of Cerebral Gray and White Matter Diseases Static parameters (influenced by molecular environment): T, T* (transverse relaxation)
Classification of Subject Motion for Improved Reconstruction of Dynamic Magnetic Resonance Imaging
 1 CS 9 Final Project Classification of Subject Motion for Improved Reconstruction of Dynamic Magnetic Resonance Imaging Feiyu Chen Department of Electrical Engineering ABSTRACT Subject motion is a significant
1 CS 9 Final Project Classification of Subject Motion for Improved Reconstruction of Dynamic Magnetic Resonance Imaging Feiyu Chen Department of Electrical Engineering ABSTRACT Subject motion is a significant
Automatic Registration-Based Segmentation for Neonatal Brains Using ANTs and Atropos
 Automatic Registration-Based Segmentation for Neonatal Brains Using ANTs and Atropos Jue Wu and Brian Avants Penn Image Computing and Science Lab, University of Pennsylvania, Philadelphia, USA Abstract.
Automatic Registration-Based Segmentation for Neonatal Brains Using ANTs and Atropos Jue Wu and Brian Avants Penn Image Computing and Science Lab, University of Pennsylvania, Philadelphia, USA Abstract.
PET Image Reconstruction using Anatomical Information through Mutual Information Based Priors
 2005 IEEE Nuclear Science Symposium Conference Record M11-354 PET Image Reconstruction using Anatomical Information through Mutual Information Based Priors Sangeetha Somayajula, Evren Asma, and Richard
2005 IEEE Nuclear Science Symposium Conference Record M11-354 PET Image Reconstruction using Anatomical Information through Mutual Information Based Priors Sangeetha Somayajula, Evren Asma, and Richard
Norbert Schuff Professor of Radiology VA Medical Center and UCSF
 Norbert Schuff Professor of Radiology Medical Center and UCSF Norbert.schuff@ucsf.edu 2010, N.Schuff Slide 1/67 Overview Definitions Role of Segmentation Segmentation methods Intensity based Shape based
Norbert Schuff Professor of Radiology Medical Center and UCSF Norbert.schuff@ucsf.edu 2010, N.Schuff Slide 1/67 Overview Definitions Role of Segmentation Segmentation methods Intensity based Shape based
Automatic Generation of Training Data for Brain Tissue Classification from MRI
 Automatic Generation of Training Data for Brain Tissue Classification from MRI Chris A. COCOSCO, Alex P. ZIJDENBOS, and Alan C. EVANS http://www.bic.mni.mcgill.ca/users/crisco/ McConnell Brain Imaging
Automatic Generation of Training Data for Brain Tissue Classification from MRI Chris A. COCOSCO, Alex P. ZIJDENBOS, and Alan C. EVANS http://www.bic.mni.mcgill.ca/users/crisco/ McConnell Brain Imaging
Classification of Structural Images via High-Dimensional Image Warping, Robust Feature Extraction, and SVM
 Classification of Structural Images via High-Dimensional Image Warping, Robust Feature Extraction, and SVM Yong Fan, Dinggang Shen, and Christos Davatzikos Section of Biomedical Image Analysis, Department
Classification of Structural Images via High-Dimensional Image Warping, Robust Feature Extraction, and SVM Yong Fan, Dinggang Shen, and Christos Davatzikos Section of Biomedical Image Analysis, Department
Computational Neuroanatomy
 Computational Neuroanatomy John Ashburner john@fil.ion.ucl.ac.uk Smoothing Motion Correction Between Modality Co-registration Spatial Normalisation Segmentation Morphometry Overview fmri time-series kernel
Computational Neuroanatomy John Ashburner john@fil.ion.ucl.ac.uk Smoothing Motion Correction Between Modality Co-registration Spatial Normalisation Segmentation Morphometry Overview fmri time-series kernel
Ensemble registration: Combining groupwise registration and segmentation
 PURWANI, COOTES, TWINING: ENSEMBLE REGISTRATION 1 Ensemble registration: Combining groupwise registration and segmentation Sri Purwani 1,2 sri.purwani@postgrad.manchester.ac.uk Tim Cootes 1 t.cootes@manchester.ac.uk
PURWANI, COOTES, TWINING: ENSEMBLE REGISTRATION 1 Ensemble registration: Combining groupwise registration and segmentation Sri Purwani 1,2 sri.purwani@postgrad.manchester.ac.uk Tim Cootes 1 t.cootes@manchester.ac.uk
EMSegment Tutorial. How to Define and Fine-Tune Automatic Brain Compartment Segmentation and the Detection of White Matter Hyperintensities
 EMSegment Tutorial How to Define and Fine-Tune Automatic Brain Compartment Segmentation and the Detection of White Matter Hyperintensities This documentation serves as a tutorial to learn to customize
EMSegment Tutorial How to Define and Fine-Tune Automatic Brain Compartment Segmentation and the Detection of White Matter Hyperintensities This documentation serves as a tutorial to learn to customize
ANALYSIS OF PULMONARY FIBROSIS IN MRI, USING AN ELASTIC REGISTRATION TECHNIQUE IN A MODEL OF FIBROSIS: Scleroderma
 ANALYSIS OF PULMONARY FIBROSIS IN MRI, USING AN ELASTIC REGISTRATION TECHNIQUE IN A MODEL OF FIBROSIS: Scleroderma ORAL DEFENSE 8 th of September 2017 Charlotte MARTIN Supervisor: Pr. MP REVEL M2 Bio Medical
ANALYSIS OF PULMONARY FIBROSIS IN MRI, USING AN ELASTIC REGISTRATION TECHNIQUE IN A MODEL OF FIBROSIS: Scleroderma ORAL DEFENSE 8 th of September 2017 Charlotte MARTIN Supervisor: Pr. MP REVEL M2 Bio Medical
8/3/2017. Contour Assessment for Quality Assurance and Data Mining. Objective. Outline. Tom Purdie, PhD, MCCPM
 Contour Assessment for Quality Assurance and Data Mining Tom Purdie, PhD, MCCPM Objective Understand the state-of-the-art in contour assessment for quality assurance including data mining-based techniques
Contour Assessment for Quality Assurance and Data Mining Tom Purdie, PhD, MCCPM Objective Understand the state-of-the-art in contour assessment for quality assurance including data mining-based techniques
Image Processing with Nonparametric Neighborhood Statistics
 Image Processing with Nonparametric Neighborhood Statistics Ross T. Whitaker Scientific Computing and Imaging Institute School of Computing University of Utah PhD Suyash P. Awate University of Pennsylvania,
Image Processing with Nonparametric Neighborhood Statistics Ross T. Whitaker Scientific Computing and Imaging Institute School of Computing University of Utah PhD Suyash P. Awate University of Pennsylvania,
GLIRT: Groupwise and Longitudinal Image Registration Toolbox
 Software Release (1.0.1) Last updated: March. 30, 2011. GLIRT: Groupwise and Longitudinal Image Registration Toolbox Guorong Wu 1, Qian Wang 1,2, Hongjun Jia 1, and Dinggang Shen 1 1 Image Display, Enhancement,
Software Release (1.0.1) Last updated: March. 30, 2011. GLIRT: Groupwise and Longitudinal Image Registration Toolbox Guorong Wu 1, Qian Wang 1,2, Hongjun Jia 1, and Dinggang Shen 1 1 Image Display, Enhancement,
Statistical Modeling of Neuroimaging Data: Targeting Activation, Task-Related Connectivity, and Prediction
 Statistical Modeling of Neuroimaging Data: Targeting Activation, Task-Related Connectivity, and Prediction F. DuBois Bowman Department of Biostatistics and Bioinformatics Emory University, Atlanta, GA,
Statistical Modeling of Neuroimaging Data: Targeting Activation, Task-Related Connectivity, and Prediction F. DuBois Bowman Department of Biostatistics and Bioinformatics Emory University, Atlanta, GA,
Neuroimaging and mathematical modelling Lesson 2: Voxel Based Morphometry
 Neuroimaging and mathematical modelling Lesson 2: Voxel Based Morphometry Nivedita Agarwal, MD Nivedita.agarwal@apss.tn.it Nivedita.agarwal@unitn.it Volume and surface morphometry Brain volume White matter
Neuroimaging and mathematical modelling Lesson 2: Voxel Based Morphometry Nivedita Agarwal, MD Nivedita.agarwal@apss.tn.it Nivedita.agarwal@unitn.it Volume and surface morphometry Brain volume White matter
Prototype of Silver Corpus Merging Framework
 www.visceral.eu Prototype of Silver Corpus Merging Framework Deliverable number D3.3 Dissemination level Public Delivery data 30.4.2014 Status Authors Final Markus Krenn, Allan Hanbury, Georg Langs This
www.visceral.eu Prototype of Silver Corpus Merging Framework Deliverable number D3.3 Dissemination level Public Delivery data 30.4.2014 Status Authors Final Markus Krenn, Allan Hanbury, Georg Langs This
ABSTRACT 1. INTRODUCTION 2. METHODS
 Finding Seeds for Segmentation Using Statistical Fusion Fangxu Xing *a, Andrew J. Asman b, Jerry L. Prince a,c, Bennett A. Landman b,c,d a Department of Electrical and Computer Engineering, Johns Hopkins
Finding Seeds for Segmentation Using Statistical Fusion Fangxu Xing *a, Andrew J. Asman b, Jerry L. Prince a,c, Bennett A. Landman b,c,d a Department of Electrical and Computer Engineering, Johns Hopkins
Sampling-Based Ensemble Segmentation against Inter-operator Variability
 Sampling-Based Ensemble Segmentation against Inter-operator Variability Jing Huo 1, Kazunori Okada, Whitney Pope 1, Matthew Brown 1 1 Center for Computer vision and Imaging Biomarkers, Department of Radiological
Sampling-Based Ensemble Segmentation against Inter-operator Variability Jing Huo 1, Kazunori Okada, Whitney Pope 1, Matthew Brown 1 1 Center for Computer vision and Imaging Biomarkers, Department of Radiological
